Abstract
Objective
To demonstrate proof-of-concept for a quantitative MRI method using histographic analysis to assess bone marrow oedema and fat metaplasia in the sacroiliac joints.
Materials and methods
Fifty-three adolescents aged 12–23 with known or suspected sacroiliitis were prospectively recruited and underwent quantitative MRI (qMRI) scans, consisting of chemical shift-encoded (at 3 T) and diffusion-weighted imaging (at 1.5 T), plus conventional MRI (at 1.5 T) and clinical assessment. qMRI scans produced proton-density fat fraction (PDFF) and apparent diffusion coefficient (ADC) maps of the sacroiliac joints (SIJs), which were analysed using an in-house software tool enabling partially automated ROI definition and histographic analysis. Logistic regression and receiver operating characteristic (ROC) analyses assessed the predictive performance of ADC- and PDFF-based parameters in identifying active inflammation (oedema) and structural damage (fat metaplasia).
Results
ADC-based parameters were associated with increased odds of oedema (all p < 0.05); ROC-AUC was higher for histographic parameters representing the upper end of the ADC distribution than for simple averages. Similarly, PDFF-based parameters were associated with increased odds of fat metaplasia (all p < 0.05); ROC area-under-the-curve was higher for histographic parameters representing the upper end of the PDFF distribution than for simple averages. Both ADC- and PDFF-based histographic parameters demonstrated excellent inter- and intra-observer agreement (ICC > 0.9).
Conclusions
ADC-based parameters can differentiate patients with bone marrow oedema from those without, whilst PDFF-based parameters can differentiate patients with fat metaplasia from those without. Histographic analysis might improve performance compared with simple averages such as the mean and median and offers excellent agreement within and between observers.
Key Points
• Quantitative MRI with histographic analysis can identify bone marrow oedema (an active inflammatory lesion) and fat metaplasia (a ‘chronic’ inflammatory lesion) in patients with spondyloarthritis.
• The use of histographic analysis might improve the performance of quantitative MRI for detecting bone marrow oedema and fat metaplasia compared with simple averages such as the mean and median.
• Bone marrow oedema and fat metaplasia are known to be of diagnostic and prognostic significance, and the proposed method could support clinical decisions around biologic (and other) therapies in spondyloarthritis.
Electronic supplementary material
The online version of this article (10.1007/s00330-020-06785-x) contains supplementary material, which is available to authorized users.
Keywords: Magnetic resonance imaging, Inflammation, Arthritis, Spine
Introduction
Spondyloarthritis (SpA) encompasses a group of immune-mediated inflammatory diseases characterised by spinal pain, stiffness and damage which commonly affect young people and have poor long-term health outcomes [1]. Diagnosis of SpA is often difficult due to the complex nature of pain in young patients [2], and delays in diagnosis and treatment are common [3]. Identification of bone marrow oedema on MRI is of importance for showing inflammation of the sacroiliac joints and supports diagnosis of axial SpA [4–7]. This directly influences the decision to treat patients with disease-modifying or biologic drugs [5].
Unfortunately, the definition of active inflammation on MRI is based on subjective criteria and is heavily dependent on the expertise and opinion of the scan reader [8–11]. ‘Conventional’ MR images used to detect inflammation—typically short inversion time inversion recovery (STIR) and T1-weighted spin echo images—produce complex image contrast that depends on multiple tissue properties, including T1, T2, proton density, perfusion and diffusion [11–13], which may confound the identification and quantification of oedema. These factors can lead to a lack of consistency between observers and scanners/hospitals [7, 14]. Therefore, there is a need for a method which can simply and objectively assess skeletal inflammation on MRI scans to support diagnostic and therapeutic decisions.
Previous studies have investigated the use of diffusion-weighted imaging (DWI) and chemical shift-encoded MRI (CSE-MRI) as objective methods for assessing bone marrow oedema, with promising initial results [12, 13, 15]. Using DWI, it has been shown that apparent diffusion coefficient (ADC) measurements are increased in areas of marrow oedema, probably due to an expansion of the extracellular space [13, 15–17]. Using CSE-MRI, it has been shown that proton density fat fraction (PDFF) measurements are reduced in areas of oedema compared with normal marrow, due to increased water content [12]. CSE-MRI can also be used to assess the severity of fat metaplasia, defined as a focal increase in content in areas of previous inflammation (with diagnostic and prognostic significance), in a quantitative fashion [12]. Previous studies measuring ADC in subchondral bone have typically relied on manual placement of regions-of-interest (ROIs) within the subchondral bone [15, 16, 18] which introduces substantial methodological subjectivity. Furthermore, these studies have relied on mean ADC measurements, which may perform poorly in patients with mixed active and chronic inflammation due to neutralisation of opposing effects [19]. There is currently no validated tool for quantifying proton density fat fraction (PDFF) in the sacroiliac joints.
We describe a new analysis tool which enables a more complete and consistent assessment of subchondral bone and derives a series of histographic parameters from both ADC and PDFF maps, aiming to isolate and separately quantify the active and chronic components of the inflammatory process. We aimed to demonstrate proof-of-principle for this tool in a prospective study of young people with SpA.
Methods
This study received ethical approval from the Queen Square Research Ethics Committee, London, UK (Research Ethics Committee reference 15/LO/1475). All participants gave written informed consent prior to study entry.
Study design and participants
A prospective cross-sectional study was performed at a single specialist tertiary referral centre for adolescents and young adults with inflammatory arthritis. Fifty-three consecutive patients meeting the eligibility criteria (mean age, 18 years; age range, 12–23 years) were prospectively recruited between July 2016 and December 2018 (31 males, mean age 18 years, and 22 females, mean age 17 years). Patients were included if they were referred for an MRI scan of the sacroiliac joints for suspicion of sacroiliitis or for monitoring of known sacroiliitis and were excluded if they had a contraindication to MRI scanning. All patients with known, pre-existing sacroiliitis had a clinical diagnosis of either non-radiographic axial SpA or enthesitis-related arthritis [18–21]. The sample size was fixed and based on logistical constraints. Patients were classified according to the presence or absence of bone marrow oedema and fat metaplasia using established criteria, based on conventional MRI scans, as described below.
Image acquisition
All subjects underwent both quantitative and conventional MRI scans on the same visit. Quantitative CSE-MR images were acquired on a 3-T Philips Ingenia scanner (Ingenia, Philips) using an investigational version of the Philips mDixon Quant acquisition and post-processing pipeline, as described previously [12]. The images were acquired using a multi-echo gradient echo acquisition with bipolar readout (TE1 1.17 ms, ΔTE 1.6 ms, TR 25 ms, flip angle 3°, matrix size 320 × 320, pixel spacing 1.76 × 1.76 mm, bandwidth 394 Hz/Px) and PDFF maps were generated using complex fitting incorporating T2* decay and a 10-peak model of human adipose tissue [12]. Images were acquired coronal to the long axis of the sacroiliac joint [12]. DW images were acquired on a 1.5-T Siemens Avanto scanner (Avanto, Siemens) using b values of 0, 50, 100, 300 and 600 s/mm2 with spectrally attenuated inversion recovery (SPAIR) fat suppression and echo planar imaging readout (TE = 89 ms, TR = 3600 ms, 4 averages, 8 mm slices, matrix size 144 × 192, FOV 237 × 316 mm, bandwidth 1447 Hz/Px), with images acquired axial to the sacroiliac joint [13, 15]. Conventional MRI consisted of T2-weighted STIR images, T1-weighted turbo spin echo images and fat-suppressed post-contrast T1-weighted turbo spin images acquired coronal to the sacroiliac joint (see Supplementary Information for sequence parameters) [11, 12].
Image analysis
Histographic parameters were obtained from the PDFF and ADC maps used an in-house software tool known as BEACH (Bone Edema and Adiposity Characterisation with Histograms) as shown in Figs. 1, 2 and 3, and as described in detail in the Supplementary Information. This method generates a series of histographic parameters for both ADC and PDFF.
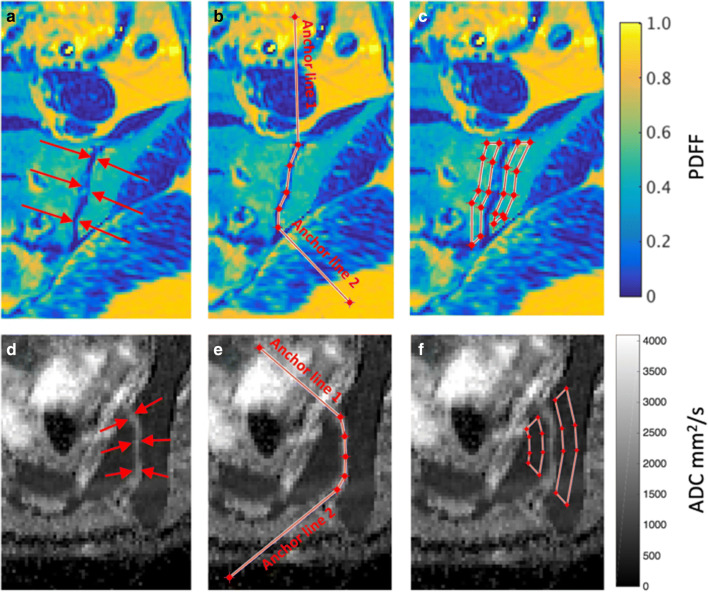
Fig. 1.
Definition of polygonal ROIs on subchondral bone. The observer is asked to define the line of the sacroiliac joint and ‘anchor lines’ are added to define the angles made by the joint with the anterior and posterior cortex of the bone, thus enabling the automatically propagated ROIs to better fit the subchondral bone
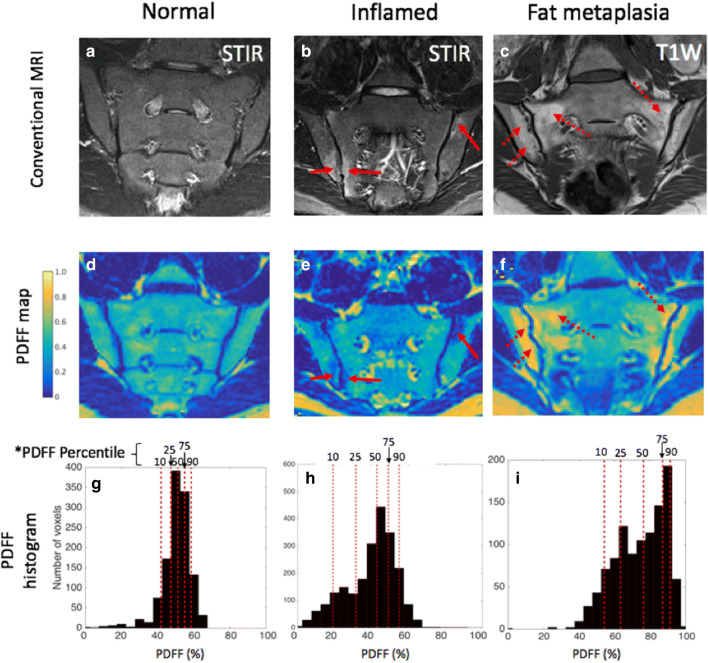
Fig. 2.
Examples of histograms generated using the BEACH tool. Conventional MR images (a–c), PDFF maps (d–f) and PDFF histograms (g–i) are shown. In the normal patient’s histogram (g), PDFF values are clustered around 50%, corresponding to normal marrow. In the patient with inflammation, a number of low-PDFF pixels have emerged in the histogram (h). In the patient with fat metaplasia, there is an upward shift in PDFF values, with a large number of high-PDFF pixels (i)
Fig. 3.
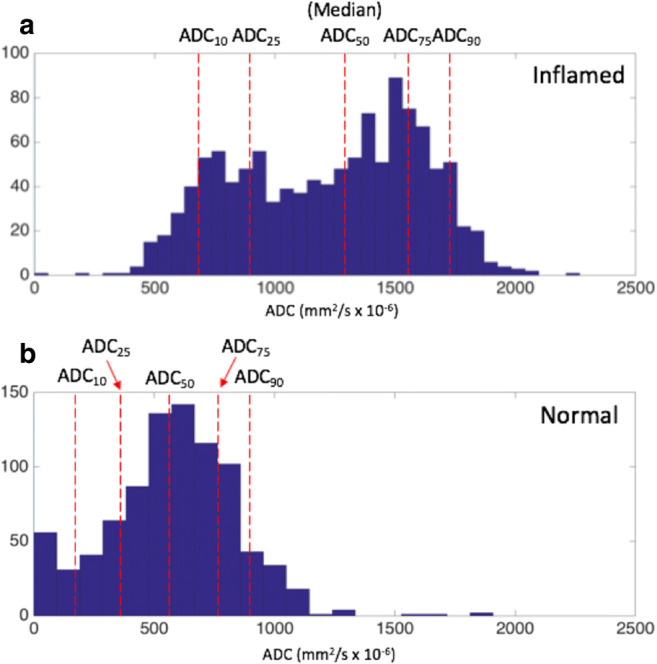
Examples of ADC histograms in patients with sacroiliitis (a) and control patients (b). The red lines indicate the 10th, 25th, 50th, 75th and 90th percentiles of the ADC distribution
The BEACH tool operates as follows. The observer is prompted to define the line of the sacroiliac joint using a single series of connected straight lines—an open polygon (Fig. 1). ‘Anchor lines’ are used to define the angle made by the joint with the cortical surface, at both the top and bottom of the joint, enabling the shape of the polygonal ROIs to be closely matched to subchondral bone. The software automatically generates a pair of polygonal ROIs in the subchondral bone either side of the joint (Fig. 1, Supplementary Figure S1). This is repeated for both sacroiliac joints covering the entire fibrocartilaginous part of the joint. For the ADC maps, all slices where the fibrocartilaginous joint was visible were included, whereas alternate slices were used for the PDFF maps due to the smaller slice thickness. For each patient, pixel values from the total volume of defined subchondral bone (i.e. from all ROIs) are analysed histographically. For both PDFF and ADC, we measured the 10th, 25th, 50th, 75th, and 90th centiles of the distribution (designated PDFF10, PDFF25… and ADC10, ADC25.. etc., as shown in Figs. 2 and 3). For each quantitative score, the mean of the two observers’ measurements was used for analysis.
The BEACH analysis was performed by two radiology residents (NS and AD, with 2 and 1 year of experience in MR imaging) who received a detailed training session (from TB). Both residents were blinded to all clinical information and to the qualitative radiological scores.
Scoring of conventional MRI
Each subject’s set of conventional MR images was scored by two experienced musculoskeletal radiologists (KR and MHC) with 10 and over 25 years of MRI experience, both blinded to clinical diagnosis, to treatment and to the quantitative image data. Images were read on a research workstation. For each patient, observers assigned a qualitative score between 0 and 72 for the extent/severity of bone marrow oedema [22]. The patient was deemed to have active inflammation if the mean bone marrow oedema score from the two readers was ≥ 2, as per the Assessment of SpondyloArthritis Internal Society (ASAS) criteria [9, 23, 24]. Structural lesions consisting of fat metaplasia, erosions and joint ankylosis were assessed using a structural visual scoring system [25]. Patients with a score of ≥ 3 were deemed to be positive for the presence of fat metaplasia [25, 26].
Clinical scores
Symptoms were assessed using a dedicated research questionnaire (see Supplementary Information). We report here the Bath Ankylosing Spondylitis Disability Index (BASDAI) and Bath Ankylosing Spondylitis Functional Index (BASFI), in addition to C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
Statistical analysis
Quantitative parameters derived from ADC and PDFF maps were compared between groups with and without inflammation/fat metaplasia using logistic regression (∝ = 0.05) and receiver-operating characteristic analyses. The optimal operating point for the ROC analysis was defined as the cut-point with the highest positive likelihood ratio (LR+) producing sensitivity and specificity greater than 70%. ROC-AUC values for percentile measurements were compared against the median using the method of DeLong et al [27], implemented using the roccomp function in Stata (∝ = 0.05). To evaluate whether combinations of parameters could improve prediction, multiple logistic regression was performed using combinations of ADC-based and PDFF-based parameters. Likelihood ratio testing was used to test whether combinations of explanatory variables provided an improved fit. Linear regression was used to evaluate the relationship between the qualitative scores and the best-performing qMRI parameters from the ROC analysis. Spearman correlation was used to evaluate the relationship between clinical scores and radiological scores. Inter- and intra-observer variability was assessed using the Bland-Altman 95% limits of agreement and the intra-class correlation coefficient.
Results
Detection of inflammation
Fifteen of 53 patients (24.5%) had sufficient bone marrow oedema to meet the ASAS criteria for active inflammation. The inflamed group included 12 males and 3 females. The gender difference between the inflamed and uninflamed groups was not significant (p = 0.065). There was no significant age difference between the inflamed and uninflamed groups (p = 0.43).
Comparisons of quantitative parameters between inflamed and uninflamed SIJs are shown in Figs. 4 and 5, and the results of the corresponding logistic regression and ROC analyses are shown in Table 1.
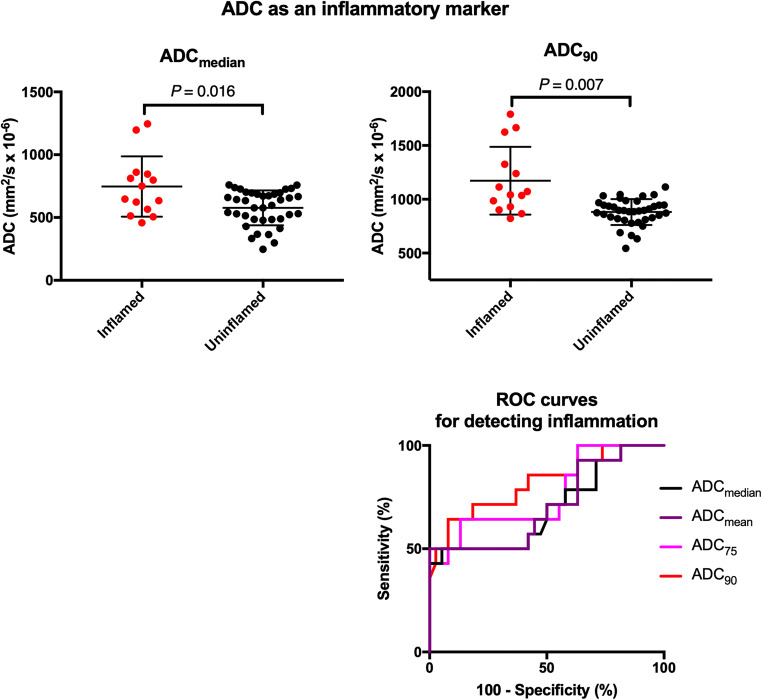
Fig. 4.
ADC as an inflammatory marker. Representative BEACH parameters (ADCmedian and ADC90) are compared between inflamed and uninflamed groups. The displayed p values were obtained by logistic regression. ROC curves for all relevant parameters are shown in the bottom right
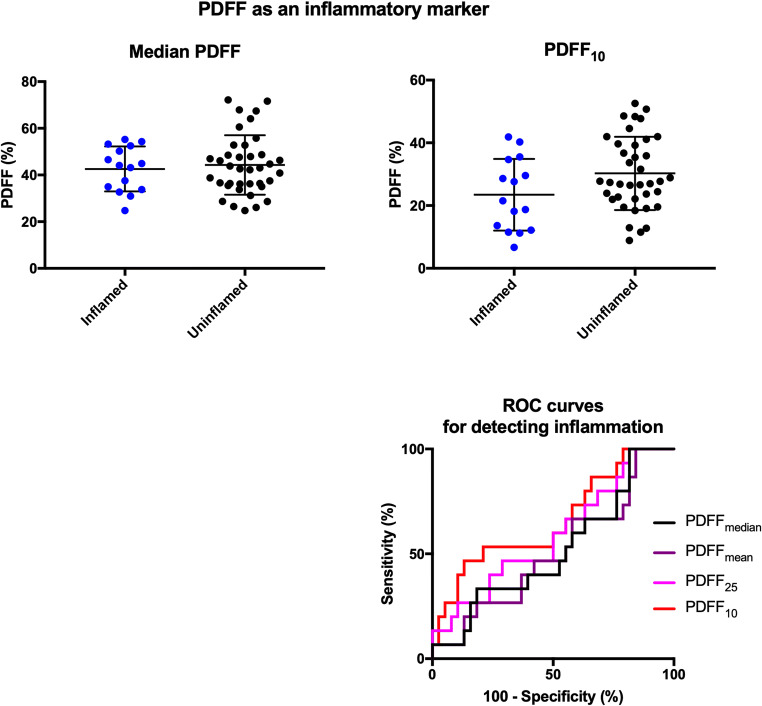
Fig. 5.
PDFF as an inflammatory marker. Representative BEACH parameters (PDFFmedian and PDFF10) are compared between inflamed and uninflamed groups. The displayed p values were obtained by logistic regression. ROC curves for all relevant parameters are shown in the bottom right
Table 1.
Comparison of inflammatory parameters between inflamed and non-inflamed patients. ADC75, ADC90, etc. refer to the 75th and 90th percentiles of ADC measurements in the defined ROI. Estimates from each group are displayed as mean (95% CI). Odds ratio (OR) and p values (*) were derived from logistic regression. The highest ROC AUC value for the evaluation of inflammation is shown in italics. Sensitivity and specificity values for the optimal cutoff values (far right) are provided in the main “Results” section. The right-hand p values (**) relate to the comparison of ROC AUC with the median value
| Inflammation | |||||||
|---|---|---|---|---|---|---|---|
| Present (n = 15) | Absent (n = 38) | OR (95% CI) | *p= | ROC AUC (95% CI) | Optimal cutoff | **p= | |
| ADCmean | 758 (629–887) | 578 (535–621) | 1.006 (1.001–1.011) | 0.011 | 0.709 (0.530–0.888) | – | 0.249 |
| ADCmedian | 747 (621–872) | 577 (534–620) | 1.006 (1.001–1.011) | 0.016 | 0.695 (0.512–0.877) | – | – |
| ADC75 | 964 (813–1115) | 733 (695–770) | 1.008 (1.002–1.014) | 0.011 | 0.763 (0.602–0.924) | – | 0.073 |
| ADC90 | 1172 (1007–1337) | 882 (842–921) | 1.010 (1.002–1.017) | 0.007 | 0.819 (0.676–0.962) | 986 | 0.072 |
| PDFFmean | 42.3 (37.2–47.4) % | 43.9 (40.0–47.8) % | 0.988 (0.934–1.041) | 0.650 | 0.505 (0.329–0.682) | – | 0.640 |
| PDFFmedian | 42.6 (37.8–47.6) % | 44.3 (40.2–48.4) % | 0.988 (0.937–1.040) | 0.639 | 0.514 (0.337–0.691) | – | – |
| PDFF25 | 32.8 (27.5–38.1) % | 37.1 (33.4–40.8) % | 0.964 (0.910–1.021) | 0.215 | 0.589 (0.414–0.763) | – | 0.010 |
| PDFF10 | 23.5 (17.8–29.2) % | 30.3 (26.6–34.0) % | 0.948 (0.895–1.004) | 0.067 | 0.657 (0.485–0.829) | – | 0.001 |
All ADC-based parameters were associated with significantly increased odds of inflammation. Parameters which sampled the upper end of the ADC distribution (i.e. ADC75 and ADC90) performed best for distinguishing inflamed from uninflamed SIJs; ADC90 produced an AUC value of 0.819 (0.676–0.962; p = 0.072 when compared with ADCmedian). The optimal cutoff for ADC90 was 986 mm2/s (sensitivity 71.4%, specificity 81.6%). Cutoffs for ADCmean, ADCmedian and ADC75 did not meet pre-specified performance thresholds.
PDFF-based parameters performed poorly as measures of inflammation with no significant difference between inflamed and uninflamed SIJs. Nonetheless, performance increased for parameters sampling the lower end of the distribution (AUC = 0.657 for PDFF10, 0.514 for PDFFmedian).
Detection of fat metaplasia
Thirty of 53 patients (56.6%) met the criteria for fat metaplasia. Patients with fat metaplasia were significantly older than those without fat metaplasia (mean ages (95% CI) were 19.6 (18.5–20.7) and 17.6 (16.5–18.7) respectively (p = 0.046)). There was no significant difference in gender between patients with and without fat metaplasia (p = 0.56).
Comparisons of quantitative parameters between patients with and without fat metaplasia are shown in Fig. 6, and the results of the corresponding logistic regression and ROC analyses are shown in Table 2.
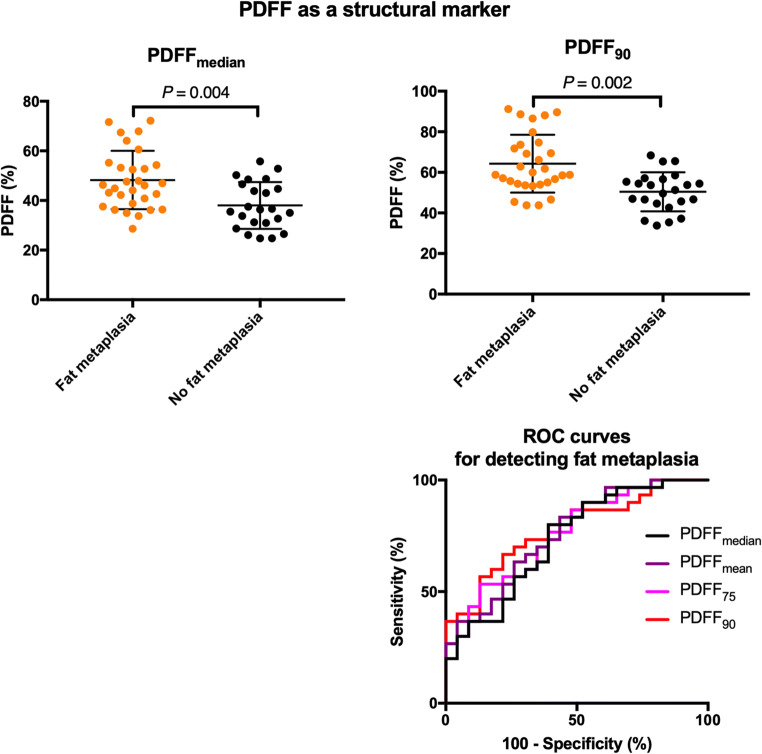
Fig. 6.
PDFF as a structural marker. Representative BEACH parameters (PDFFmedian and PDFF90) are compared between patients with and without fat metaplasia. The displayed p values were obtained by logistic regression. ROC curves for all relevant parameters are shown in the bottom right
Table 2.
Comparison of structural parameters between patients with and without fat metaplasia. PDFF75, PDFF90, etc. refer to the 75th and 90th percentiles of ADC measurements in the defined ROI. Estimates are displayed as mean (95% CI). Odds ratio (OR) and p values (*) were derived from logistic regression. The highest ROC AUC value for the evaluation of fat metaplasia is shown in italics. Sensitivity and specificity values for the optimal cutoff values (far right) are provided in the main “Results” section. The right-hand p values (**) relate to the comparison of ROC AUC with the median value
| Fat metaplasia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Present (n = 30) | Absent (n = 23) | OR (95% CI) | *p= | ROC AUC (95% CI) | Optimal cutoff | **p= | ||
| PDFFmean | 48.2 (43.9–52.5) % | 37.4 (33.7–41.1) % | 1.110 (1.035–1.119) | 0.003 | 0.759 (0.629–0.889) | – | 0.061 | |
| PDFFmedian | 48.2 (44.1–52.3) % | 38.0 (34.0–41.9) % | 1.100 (1.030–1.174) | 0.004 | 0.735 (0.597–0.872) | – | – | |
| PDFF75 | 57.1 (52.0–62.2) % | 44.5 (48.2–40.8) % | 1.105 (1.034–1.182) | 0.003 | 0.759 (0.630–0.888) | – | 0.254 | |
| PDFF90 | 64.3 (59.2–69.4) % | 50.4 (46.5–54.3) % | 1.111 (1.037–1.189) | 0.002 | 0.780 (0.656–0.903) | 55.7% | 0.263 | |
| ADCmean | 606 (541–671) | 652 (571–732) | 0.999 (0.996–1.002) | 0.380 | 0.567 (0.407–0.726) | – | 0.669 | |
| ADCmedian | 602 (535–669) | 648 (572–724) | 0.999 (0.996–1.002) | 0.380 | 0.572 (0.413–0.731) | – | – | |
PDFF-based parameters were associated with increased odds of fat metaplasia, and the separation between patients with and without fat metaplasia was improved for parameters which specifically sampled the upper end of the PDFF distribution (i.e. PDFF75 and PDFF90). The best performing parameter, PDFF90, had an AUC of 0.780 (0.656–0.903; p = 0.263 when compared with PDFFmedian). The optimal operating point for PDFF90 was 55.7%, producing a sensitivity of 70% and a specificity of 73.9%.
There were no significant differences in ADCmean or ADCmedian between patients with and without fat metaplasia.
Prediction of inflammation and fat using combinations of parameters
Multiple logistic regression using both ADC90 and FF90 or ADC90 and FFmedian as predictor variables did not significantly improve the model fit compared with simple logistic regression using ADC90 as a single predictor (p = 0.41 and 0.81, respectively). Similarly, the combination of FF90 and ADC90 or FF90 and ADCmedian did not improve the model fit compared with using FF90 alone (p = 0.86 and 0.73, respectively).
Relationship between BEACH parameters and qualitative MRI scores
The relationship between visual scores of inflammation/fat metaplasia and qMRI parameters is shown in Supplementary Figure S2. There were significant positive relationships between ADC90 and the visual inflammation score (slope = 15.33, p < 0.0001) and between PDFF90 and the fat metaplasia score (slope = 1.05, p < 0.0001).
Relationship between MRI and symptoms
Scatterplots showing the relationship between BASDAI scores and visual and quantitative scores of inflammation and fat metaplasia are shown in Supplementary Figure S3.
There was no significant correlation between visual scores of inflammation and any clinical score (p = 0.45, 0.48, 0.14 and 0.49 for BASDAI, BASFI, CRP and ESR) or between ADC90 parameters and clinical scores (for ADC90 p = 0.48, 0.37, 0.19 and 0.63).
There was a significant negative relationship between fat metaplasia visual scores and clinical symptoms (p = 0.004 and 0.006 for BASDAI and BASFI), and a similar relationship was observed for the corresponding qMRI parameter PDFF90 (p = 0.03 and 0.01 for BASDAI and BASFI). There was no significant relationship between either visual or quantitative fat metaplasia scores and CRP or ESR (all p > 0.05).
Inter- and intra-observer agreement
Inter- and intra-observer agreement statistics for qMRI parameters and visual scores are shown in Table 3. Inter-observer and intra-observer agreement were excellent for all assessed qMRI parameters. Inter-observer agreement was excellent for visual inflammation scores, although the 95% limits of agreement (0.6 ± 6.4) were relatively wide compared with the ASAS definition of active inflammation (score of ≥ 2 diagnostic for active inflammation). Inter-observer agreement was poorer for fat metaplasia scores with an ICC value of 0.544.
Table 3.
Inter-observer and intra-observer variability statistics for selected (most relevant) parameters. The intra-class correlation coefficient and Bland-Altman limits of agreement are shown
| Inter-observer variability | Intra-observer variability (observer 1) | Intra-observer variability (observer 2) | ||||
|---|---|---|---|---|---|---|
| ICC | 95% LoA | ICC | 95% LoA | ICC | 95% LoA | |
| ADCmean | 0.962 | 8.4 ± 103 mm2/s | 0.949 | − 27.6 ± 128 mm2/s | 0.949 | − 0.19 ± 112 mm2/s |
| ADCmedian | 0.943 | 8.4 ± 111 mm2/s | 0.954 | − 20.6 ± 124 mm2/s | 0.898 | 11 ± 156 mm2/s |
| ADC90 | 0.918 | − 10.1 ± 188 mm2/s | 0.918 | − 38.1 ± 210 mm2/s | 0.961 | 3.9 ± 123 mm2/s |
| PDFFmean | 0.982 | − 1.03 ± 4.42% | 0.994 | 0.15 ± 2.40% | 0.986 | − 1.08 ± 3.69% |
| PDFFmedian | 0.974 | − 0.97 ± 5.08% | 0.989 | 0.34 ± 3.20% | 0.968 | − 0.87 ± 5.45% |
| PDFF90 | 0.986 | − 0.31 ± 4.70% | 0.990 | − 0.41 ± 3.60% | 0.968 | 0.05 ± 6.52% |
| Visual inflammation score | 0.944 | 0.6 ± 6.4 | – | – | – | – |
| Visual fat metaplasia score | 0.534 | − 6.8 ± 18.3 | – | – | – | – |
Discussion
We describe a quantitative, partially automated method for measurement of bone marrow oedema and fat metaplasia based on histographic analysis of quantitative MR images. We show that histogram-based qMRI parameters enable separation of patients according to the presence of oedema and fat metaplasia, both of which are of importance for the diagnosis and management of SpA. The proposed tool offers a simple and potentially repeatable means to quantify inflammation and fat and could be incorporated into picture archiving and communications system (PACS) systems relatively easily. Such a tool could be of value for monitoring inflammation over time and for guiding clinical decisions around initiation and changes of biologic and other therapies. Importantly, ADC-based and PDFF-based parameters provide discrete information regarding oedema and fat metaplasia and could therefore inform on the relative burden of active inflammation versus structural damage.
We found that ADC measurements produced superior performance to PDFF measurements for separating patients with and without inflammation. This suggests that increases in diffusivity are an important part of the inflammatory process in the bone marrow, rather than changes in water content per se. However, previous studies have shown substantial differences in PDFF between normal and inflamed marrow [12], and it may be that the discrepant observations in this study are due to the variability in the composition of normal bone marrow [28]. This could be investigated further by comparing the composition of the inflamed subchondral bone with normal bone marrow.
Our results showed that PDFF90 enabled separation of patients with and without fat metaplasia. Fat metaplasia can contribute to diagnosis [6, 7] and is also a prognostic factor, since patients with fat metaplasia are more likely to fuse their sacroiliac joints [29–31].
Interestingly, the 90th percentiles of ADC and PDFF yielded more accurate separation of inflamed and non-inflamed joints and joints with and without fat metaplasia compared with simple averages, although this difference did not reach statistical significance. This suggests that percentiles measuring the extremes of the distribution might be better ‘targeted’ to areas of oedema (for ADC) or fat metaplasia (for PDFF) than mean or median measurements, which may be ‘contaminated’ by non-inflamed or non-fatty sites, respectively, to a greater extent.
Importantly, the inter- and intra-observer variability for both ADC- and PDFF-based parameters was good or excellent. Inter-observer variability was excellent for visual scoring of bone marrow oedema, but substantially poorer for scoring of fat metaplasia. Given the known inconsistencies in radiologists’ interpretation in spondyloarthritis in clinical practice [14], a more consistent measurement could be a major advantage. However, formal studies are needed to assess repeatability and reproducibility across sites and MRI vendors.
We did not find a strong relationship between inflammation on MRI and symptoms in this study, likely reflecting the complex and multidimensional nature of pain in SpA [32]. There was a negative relationship between the severity of fat metaplasia and symptom scores. This suggests that fat metaplasia, a post-inflammatory phenomenon [30, 33], is more common in patients already on treatment with well-controlled symptoms.
A strength of our study is that the control subjects (i.e. those without inflammation) were patients where MRI was clinically indicated and thus likely to have either biomechanical back pain or quiescent inflammatory arthritis. Consequently, the reported statistics for separating patients with and without inflammation are likely to be realistic in a real-world clinical setting (this point is emphasised in the QUADAS-2 quality criteria [34]). By contrast, the use of healthy controls can artificially inflate sensitivity and specificity statistics and give a misleading impression of diagnostic performance. An additional strength is that the histographic parameters used are relatively simple and likely to offer superior performance to more complex metrics based on maximum likelihood estimation. Nonetheless, future work could explore the use of more complex analysis methods, such as Gaussian mixture modeling, to identify discrete subpopulations of pixels within the ROI.
A limitation of this study is that the diagnostic performance reported is not likely to be sufficient for the current use in clinical practice. This may be partially due to the variations in the composition of normal marrow in young patients, where the marrow may be partially ossified and contains varying proportions of water and fat. This factor may bias ADC and PDFF measurements and could have weakened the separation of inflamed and non-inflamed patients. In the future, the BEACH tool could be extended to isolate ossified bone, potentially improving performance. Similarly, the proportion of red and yellow marrow in ossified bone may vary between individuals. The use of variable thresholds depending on the composition of the normal ‘background’ marrow might help to improve the technique for detecting inflammation. ADC measurements can also suffer from poor reproducibility across sites, partly due to the difficulty of achieving high-quality fat suppression [19]. A final limitation is that the proposed tool is only partially automated; further methodological development is required to achieve full automation.
In conclusion, we describe a method for quantifying bone marrow oedema and fat metaplasia in patients with SpA, based on histographic analysis. ADC-based parameters can objectively differentiate patients with bone marrow oedema from those without, whilst PDFF-based parameters can differentiate patients with fat metaplasia from those without. Histographic analysis might improve performance compared with simple averages such as the mean and median and offers excellent agreement within and between observers.
Electronic supplementary material
(DOCX 1819 kb)
Generation of polygonal ROIs
The observer is prompted to define the line of the sacroiliac joint using a single series of connected straight lines - an open polygon – as shown in Fig. S1. This open polygon includes ‘anchor lines’ (shown in blue), which define the angle made by the joint with the cortical surface, at both the top and bottom of the joint (Fig. S1), and the line of the joint itself. The first anchor line is placed perpendicular to the surface of the bone at the upper end of the sacroiliac joint, in order to define the angle α between the bone surface (labeled s) and the joint itself. Next, the observer places a series of joint lines (also shown in blue, labeled j1… jn), which trace the course of the synovial part of the sacroiliac joint. Finally, the observer places a second anchor line defining the angle β formed between the joint and the cortical surface (in the case of axial diffusion-weighted images, the ligamentous part of the joint is not including in the scoring so the angle β is formed between the synovial and ligamentous parts of the joint, rather than at the cortical surface). A series of lines (shown in red on Fig. S1a) with width w1 are then automatically generated and placed such that each line is perpendicular to the preceding joint line. In this study, we used w1 = 14 pixels (equal to 21.8 mm for PDFF maps and 23.4 mm for ADC maps), chosen empirically. The coordinates of these lines are used to form a polygonal ROI including both the subchondral bone and joint space (on Fig. S1b). This procedure is repeated to generated a further set of shorter perpendicular lines, with width w2, which define the width of the joint space itself (not shown). Here, we used w2 = 6 pixels (equal to 9.36 mm for PDFF maps and 10.02 mm for ADC maps). Finally, the coordinates of these two sets of lines are used to generate two separate polygonal areas A1 and A2 adjacent to the joint, which comprise the total region-of-interest A for a single SIJ (Fig. S1c).
Clinical Scores
For each patient, symptoms were assessed using a dedicated research questionnaire designed by an adolescent and young adult rheumatologist (XX), which included the Bath Ankylosing Spondylitis Disability Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), Health Activity Questionnaire (HAQ), Work Productivity Impairment Questionnaire plus Classroom Impairment Questions (WPAI+CIQ), Patient Global Assessment, Jenkin’s Sleep scale and Margolis Pain Diagram. Additionally, all patients were clinically examined by a specialist adolescent rheumatologist at the time of scan referral to determine the presence of swollen, restricted or active joints, and of enthesitis.
Conventional MRI
Conventional MRI consisted of T2-weighted STIR images acquired coronal to the sacroiliac joint using a turbo inversion recovery magnitude (TIRM) sequence (TE 77 ms, TR 3880 ms, TI 150 ms, 2 averages, matrix size 256 × 192, FOV 200x200mm, 4 mm slices, bandwidth 132 Hz/Px, echo train length 11) and T1-weighted turbo spin echo images (TE 9.2 ms, TR 687 ms, 2 averages, matrix size 256 × 243, FOV 200x200mm, 3 mm slices, bandwidth 171 Hz/Px, echo train length 5), with fat-suppressed T1-weighted turbo spin echo images (TE 9.2 ms, TR 792 ms, 2 averages, matrix size 256 × 230, FOV 200x200mm, 3 mm slices, bandwidth 171 Hz/Px, echo train length 5) acquired after gadolinium injection, performed on the same 1.5 T Siemens Avanto scanner, again coronal to the sacroiliac joint [11, 12].
Abbreviations
- ADC
Apparent diffusion coefficient
- AUC
Area under the curve
- BASDAI
Bath Ankylosing Spondylitis Disability Index
- BASFI
Bath Ankylosing Spondylitis Functional Index
- BEACH
Bone edema and adiposity characterisation
- BRC
Biomedical Research Centre
- CRP
C-reactive protein
- CSE-MRI
Chemical shift-encoded MRI
- DWI
Diffusion-weighted imaging
- ESR
Erythrocyte sedimentation rate
- MRI
Magnetic resonance imaging
- NIHR
National Institute for Health Research
- OR
Odds ratio
- PACS
Picture archiving and communications system
- PDFF
Proton density fat fraction
- qMRI
Quantitative MRI
- ROC
Receiver operating characteristic
- ROI
Region-of-interest
- SIJ
Sacroiliac joint
- SpA
Spondyloarthritis
- STIR
Short inversion time inversion recovery
- TNFi
Tumour necrosis factor inhibitor
Funding information
This work was undertaken at UCLH/UCL, which receives funding from the UK Department of Health’s the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) funding scheme. The views expressed in this publication are those of the authors and not necessarily those of the UK Department of Health. TJPB was supported by Arthritis Research UK Grant 21,369. CF was also supported by Arthritis Research UK. MHC is supported by the NIHR University College London Hospitals Biomedical Research Centre.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Professor Margaret A. Hall-Craggs.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Eighteen of the 53 subjects included in this work were included in a prior publication (developing the PDFF-mapping methodology underpinning this work, but without the BEACH analysis performed here) [Bray et al, Magn Reson Med 2018;79(2)] and in a separate study investigating the use of quantitative susceptibility mapping (an entirely separate method from those investigated here) [Bray et al Magn Reson Med 2019;81(5)].
Methodology
• Prospective
• Observational
• Performed at one institution
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390:73–84. doi: 10.1016/S0140-6736(16)31591-4. [DOI] [PubMed] [Google Scholar]
- 2.Wedderkopp N, Leboeuf-Yde C, Bo Andersen L, Froberg K, Steen Hansen H (2003) Back pain in children: no association with objectively measured level of physical activity. Spine (Phila Pa 1976) 28:2019–2024 [DOI] [PubMed]
- 3.Dincer U, Cakar E, Kiralp MZ, Dursun H. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol. 2008;27:457–462. doi: 10.1007/s10067-007-0727-6. [DOI] [PubMed] [Google Scholar]
- 4.Weiss PF, Xiao R, Biko DM, Johnson AM, Chauvin NA (2015) Detection of inflammatory sacroiliitis in children with magnetic resonance imaging: is gadolinium contrast enhancement necessary? Arthritis Rheumatol 67:2250–2256 [DOI] [PMC free article] [PubMed]
- 5.Fisher C, Ioannou Y, Hall-Craggs MA, Sen D. Enthesitis related arthritis; a new era of understanding. Ann Paediatr Rheumatol. 2012;1:8. doi: 10.5455/apr.011520120733. [DOI] [Google Scholar]
- 6.Bray TJP, Jones A, Bennett AN, et al. Recommendations for acquisition and interpretation of MRI of the spine and sacroiliac joints in the diagnosis of axial spondyloarthritis in the UK. Rheumatology (Oxford) 2019;58:1831–1838. doi: 10.1093/rheumatology/kez173. [DOI] [PubMed] [Google Scholar]
- 7.Jones A, Bray TJP, Mandl P, Hall-Craggs MA, Marzo-Ortega H, Machado PM (2019) Performance of magnetic resonance imaging in the diagnosis of axial spondyloarthritis: a systematic literature review. Rheumatology (Oxford) 58:1955–1965 [DOI] [PMC free article] [PubMed]
- 8.Baraliakos X, Braun J. Opinion: perspectives on imaging in axial spondyloarthritis. Nat Rev Rheumatol. 2013;9:498–502. doi: 10.1038/nrrheum.2013.83. [DOI] [PubMed] [Google Scholar]
- 9.Rudwaleit M, Jurik AG, Hermann KGA, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68:1520–1527. doi: 10.1136/ard.2009.110767. [DOI] [PubMed] [Google Scholar]
- 10.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 11.Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. 2009;68:ii1–i44. doi: 10.1136/ard.2008.097774. [DOI] [PubMed] [Google Scholar]
- 12.Bray TJP, Bainbridge A, Punwani S, Ioannou Y, Hall-Craggs MA (2018) Simultaneous quantification of bone edema/adiposity and structure in inflamed bone using chemical shift-encoded MRI in spondyloarthritis. Magn Reson Med 79:1031–1042 [DOI] [PMC free article] [PubMed]
- 13.Bray TJP, Vendhan K, Ambrose N, et al. Diffusion-weighted imaging is a sensitive biomarker of response to biologic therapy in enthesitis-related arthritis. Rheumatology (Oxford) 2017;56:399–407. doi: 10.1093/rheumatology/kex390.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett AN, Marzo-Ortega H, Kaur-Papadakis D, Rehman A. The use of magnetic resonance imaging in axial spondyloarthritis: time to bridge the gap between radiologists and rheumatologists. J Rheumatol. 2017;44:780–785. doi: 10.3899/jrheum.161337. [DOI] [PubMed] [Google Scholar]
- 15.Vendhan K, Bray TJP, Atkinson D, et al. A diffusion-based quantification technique for assessment of sacroiliitis in adolescents with enthesitis-related arthritis. Br J Radiol. 2016;89:20150775. doi: 10.1259/bjr.20150775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaspersic N, Sersa I, Jevtic V, Tomsic M, Praprotnik S (2008) Monitoring ankylosing spondylitis therapy by dynamic contrast-enhanced and diffusion-weighted magnetic resonance imaging. Skeletal Radiol 37:123–131 [DOI] [PubMed]
- 17.Gezmis E, Donmez FY, Agildere M. Diagnosis of early sacroiliitis in seronegative spondyloarthropathies by DWI and correlation of clinical and laboratory findings with ADC values. Eur J Radiol. 2013;82:2316–2321. doi: 10.1016/j.ejrad.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 18.Bozgeyik Z, Ozgocmen S, Kocakoc E. Role of diffusion-weighted MRI in the detection of early active sacroiliitis. AJR Am J Roentgenol. 2008;191:980–986. doi: 10.2214/AJR.07.3865. [DOI] [PubMed] [Google Scholar]
- 19.Dietrich O, Geith T, Reiser MF, Baur-Melnyk A (2017) Diffusion imaging of the vertebral bone marrow. NMR Biomed 30. 10.1002/nbm.3333 [DOI] [PubMed]
- 20.Rudwaleit M, Landewé R, Van Der Heijde D et al (2009) The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 68:770–776 [DOI] [PubMed]
- 21.Petty RE, Southwood TR, Manners P et al (2004) International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31:390–392 [PubMed]
- 22.Maksymowych WP, Inman RD, Salonen D et al (2005) Spondyloarthritis research consortium of Canada magnetic resonance imaging index for assessment of sacroiliac joint inflammation in ankylosing spondylitis. Arthritis Rheum 53:703–709 [DOI] [PubMed]
- 23.Aydin SZ, Maksymowych WP, Bennett AN, McGonagle D, Emery P, Marzo-Ortega H (2012) Validation of the ASAS criteria and definition of a positive MRI of the sacroiliac joint in an inception cohort of axial spondyloarthritis followed up for 8 years. Ann Rheum Dis 71:56–60 [DOI] [PubMed]
- 24.Lambert RGW, Bakker PAC, van der Heijde D, et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: update by the ASAS MRI working group. Ann Rheum Dis. 2016;75:1958–1963. doi: 10.1136/annrheumdis-2015-208642. [DOI] [PubMed] [Google Scholar]
- 25.Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen S (2015) Development and preliminary validation of the spondyloarthritis research consortium of Canada magnetic resonance imaging sacroiliac joint structural score. J Rheumatol 42:79–86 [DOI] [PubMed]
- 26.Bakker PAC, Van Den Berg R, Lenczner G, et al. Can we use structural lesions seen on MRI of the sacroiliac joints reliably for the classification of patients according to the ASAS axial spondyloarthritis criteria? Data from the DESIR cohort. Ann Rheum Dis. 2017;76:392–398. doi: 10.1136/annrheumdis-2016-209405. [DOI] [PubMed] [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 28.Bray TJP, Vendhan K, Roberts J, et al. Association of the apparent diffusion coefficient with maturity in adolescent sacroiliac joints. J Magn Reson Imaging. 2016;44:556–564. doi: 10.1002/jmri.25209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandl P, Navarro-Compan V, Terslev L, et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis. 2015;74:1327–1339. doi: 10.1136/annrheumdis-2014-206971. [DOI] [PubMed] [Google Scholar]
- 30.Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen J (2014) Fat metaplasia and backfill are key intermediaries in the development of sacroiliac joint ankylosis in patients with ankylosing spondylitis. Arthritis Rheumatol 66:2958–2967 [DOI] [PubMed]
- 31.Kang KY, Kim IJ, Yoon MA, Hong YS, Park S-H, Ju JH (2015) Fat metaplasia on sacroiliac joint magnetic resonance imaging at baseline is associated with spinal radiographic progression in patients with axial spondyloarthritis. PLoS One 10:e0135206 [DOI] [PMC free article] [PubMed]
- 32.Stinson JN, Luca NJC, Jibb LA. Assessment and management of pain in juvenile idiopathic arthritis. Pain Res Manag. 2012;17:391–396. doi: 10.1155/2012/237258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiowchanwisawakit P, Lambert RGW, Conner-Spady B, Maksymowych WP. Focal fat lesions at vertebral corners on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis. Arthritis Rheum. 2011;63:2215–2225. doi: 10.1002/art.30393. [DOI] [PubMed] [Google Scholar]
- 34.Whiting PF, Rutjes AWS, Westwood ME et al (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1819 kb)