Abstract
Objectives
We aimed to evaluate optic chiasm (OC) measures as potential imaging marker for anterior optic pathway damage assessment in the context of neuromyelitis optica spectrum disorders (NMOSD).
Materials and method
This cross-sectional study included 39 patients exclusively with aquaporin 4-IgG seropositive NMOSD of which 25 patients had a history of optic neuritis (NMOSD-ON) and 37 age- and sex-matched healthy controls (HC). OC heights, width, and area were measured using standard 3D T1-weighted MRI. Sensitivity of these measures to detect neurodegeneration in the anterior optic pathway was assessed in receiver operating characteristics analyses. Correlation coefficients were used to assess associations with structural measures of the anterior optic pathway (optic nerve dimensions, retinal ganglion cell loss) and clinical measures (visual function and disease duration).
Results
OC heights and area were significantly smaller in NMOSD-ON compared to HC (NMOSD-ON vs. HC p < 0.0001). An OC area smaller than 22.5 mm2 yielded a sensitivity of 0.92 and a specificity of 0.92 in separating chiasms of NMOSD-ON from HC. OC area correlated well with structural and clinical measures in NMOSD-ON: optic nerve diameter (r = 0.4, p = 0.047), peripapillary retinal nerve fiber layer thickness (r = 0.59, p = 0.003), global visual acuity (r = − 0.57, p = 0.013), and diseases duration (r = − 0.5, p = 0.012).
Conclusion
Our results suggest that OC measures are promising and easily accessible imaging markers for the assessment of anterior optic pathway damage.
Key Points
• Optic chiasm dimensions were smaller in neuromyelitis optica spectrum disorder patients compared to healthy controls.
• Optic chiasm dimensions are associated with retinal measures and visual dysfunction.
• The optic chiasm might be used as an easily accessible imaging marker of neurodegeneration in the anterior optic pathway with potential functional relevance.
Keywords: Optic neuritis, Optic chiasm, Magnetic resonance imaging, Neuromyelitis optica
Introduction
Neuromyelitis optic spectrum disorders (NMOSDs) are inflammatory autoimmune CNS diseases that preferentially target the optic nerves and are frequently associated with serum autoantibodies to aquaporin-4 [1, 2]. Optic pathway degeneration following optic neuritis (ON) [3, 4] results in atrophy involving the entire visual pathway [5–10]. At the optic chiasma (OC), fibers from the left and the right optic nerve merge and form the site of highest axonal density. Direct damage or other pathophysiological effects affecting the optic nerves may accumulate in the OC, making it a promising target for the assessment of anterior optic pathway damage.
Measures of optic pathway degeneration are an important outcome measure of ON, since they are related to impaired visual function and reduction of vision-related quality of life [11–14]. Optic pathway dimensions as assessed by MRI have been used as a surrogate marker of inflammatory damage and atrophy of the optic nerve and anatomically connected tracts [3, 4, 15–18]. It has been suggested that MRI is sensitive to axonal loss as a cause of optic nerve atrophy [17–20]. Optical coherence tomography (OCT) reveals damage to the retinal axons and ganglion cells by means of measuring peripapillary retinal nerve fiber layer (pRNFL) thickness and combined ganglion cell-inner plexiform layer (GCIPL) volume. OCT measures have been successfully used as surrogate markers of optic nerve atrophy [16, 17, 19, 21–25], being associated to MRI-detected macro- and microstructural optic pathway atrophy and visual function [16, 17, 20, 26–28].
Although MRI is used as part of the routine clinical workup of NMOSD patients [29, 30], no method to evaluate optic pathway damage has been established. In addition to the accumulation of damage within the anterior optic pathway, the OC appears particularly promising as a potential imaging marker, since it would simplify evaluation by reducing the region of interest from multiple structures to one. Only few studies have focused on the assessment of physiologic OC dimensions [31–33] and their changes in optic atrophy [18], while quantitative correlation analysis to visual function and optic pathway degeneration has not been performed.
The aim of this study was to assess whether neurodegenerative changes in the anterior optic pathway are detectable by assessing OC measures. We hypothesized that the OC assessment in standard 3D-T1w images is sensitive to anterior optic pathway damage. To test this hypothesis, we used NMOSD as a model for optic pathway damage and compared different OC measures (area, width, left, central, right, and total height) between aquaporin 4-IgG (AQP4-IgG) seropositive NMOSD patients with and without history of ON (NMOSD-ON and NMOSD-NON) and healthy controls (HC). In addition, we investigated the association of OC measures with optic nerve diameter, visual acuity, pRNFL thickness, and GCIPL volume in NMOSD-ON.
Material and methods
Study population
Data of 78 NMOSD patients acquired from an ongoing longitudinal prospective observational cohort study at the NeuroCure Clinical Research Center, Charité-Universitätsmedizin Berlin (recruited from May 2013 to January 2018) were screened for eligibility. All patients (i) were 18 years or older and (ii) had a diagnosis of AQP4-IgG seropositive NMOSD according to the current panel criteria [29] and (iii) either had a last ON attack at least 5 months prior to MRI or had no history of ON. AQP4-IgG status was determined by a cell-based assay (Euroimmun, Lübeck, Germany). Patients with AQP4-IgG seronegative (n = 25) antibody status, unknown antibody status and/or incomplete clinical data (n = 10), lacking MRI data (n = 3), or ON within 5 months prior to MRI (n = 1) were excluded.
Thirty-nine patients exclusively with AQP4-IgG seropositive NMOSD and 37 age- and sex-matched HC subjects were included in this study (Table 1). All HC subjects were 18 years or older and had ophthalmologic testing and no history of neurological or ophthalmological diseases.
Table 1.
Demographics and clinical characteristics
| HC | NMOSD-NON | NMOSD-ON | p | |
|---|---|---|---|---|
| Number | 37 | 14 | 25 | – |
| Age, years (mean, SD) | 47.8 (12.6) | 53.8 (12.5) | 48.09 (14.9) | 0.33 |
| Sex (F/M) (% female) | 30/7 (81%) | 14/0 (100%) | 21/4 (84%) | 0.22 |
| Disease duration, years (median, range) | – | – | 7.1 (3–34.1) | |
| Number of ON (median, range) | – | – | 2 (1–12) | – |
| Time since first ON, years (median, range) | – | – | 6.9 (3.4–32.2) | – |
| Time since last ON, years (median, range) | – | – | 4.9 (0.5–13.2) | – |
| ON involvement, bilateral/unilateral | – | – |
11/14 (8 right/6 left) |
– |
| Optic nerve diameter, mm (mean, SD) | 8.37 (0.50) | 8.13 (0.90) | 7.06 (1.23) | < 0.001 |
| pRNFL, μm (mean, SD) | 91.99 (15.10) | 97.96 (10.13) | 67.57 (17.92) | < 0.001 |
| GCIPL mm3 (mean, SD) | 1.83 (0.28) | 1.88 (0.14) | 1.49 (0.25) | < 0.001 |
| Visual acuity, logMAR (mean, SD) | − 0.01 (0.21) | −0.15 (0.21) | 0.30 (0.63) | 0.04 |
| Visual Functional System Score (median, range) | – | 0 (0–2) | 1 (0–6) | < 0.001 |
| EDSS (median, range) | – | 3 (1–7) | 4 (0–6.5) | 0.71 |
p values are groupwise comparisons. HC = healthy controls; NMOSD-ON = neuromyelitis optica patients with history of optic neuritis; NMOSD-NON = neuromyelitis optica patients without history of optic neuritis; ON = optic neuritis; pRNFL = peripapillary retinal nerve fiber layer; GCIPL = combined ganglion cell-inner plexiform layer; logMAR = logarithm of the minimum angle of resolution; EDSS = Expanded Disability Status Scale
This study was approved by the local ethics committee (Ethikkommission der Charité–Universitätsmedizin Berlin; EA1/131/09) and conducted according the declaration of Helsinki and applicable German law. All participants gave written informed consent.
MRI acquisition
MRI data were acquired on the same 3-T scanner (MAGNETOM Trio, A Tim System, Siemens) at the Berlin Center for Advanced Neuroimaging using a volumetric high-resolution T1-weighted MPRAGE sequence (RT = 1900 ms, TE = 3.03 ms, TI = 900 ms, FOV = 256 × 256 mm2, matrix 256 × 256, slice thickness 1 mm). OC measurements were performed on reconstructed 3D MPRAGE MR images.
Optic chiasm measures
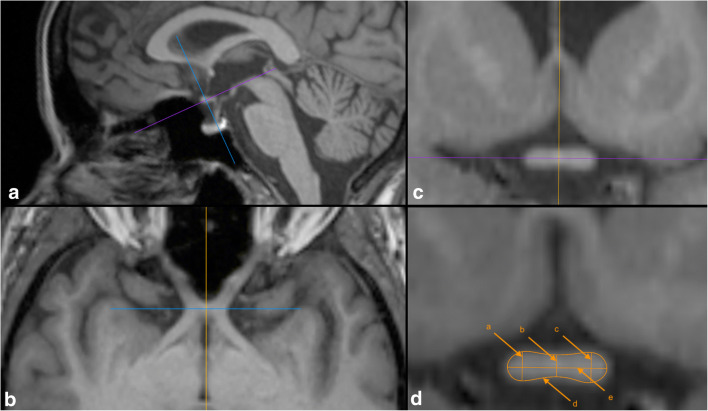
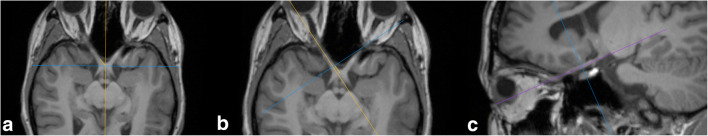
After training with a neuroradiologist with more than 8 years of experience (M.S.), OC and optic nerve measurements were performed by V.J. (radiology trainee), blinded to clinical data, using a standardized protocol: First, the central point of the OC was determined on all 3 planes. The axes of the planes were reoriented to the course of the optic pathway, so that they were perpendicular to the orientation of the individual OC in the central point. On the individually reoriented transversal plane, OC area, heights, and width were measured. Optic nerve diameters were measured in the cisternal segment 7 mm anterior to the previously defined central point of the OC perpendicular to the optic nerve course (Figs. 1 and 2). All measurements were performed using region-of-interest software from Horos, version 3.3.2 (https://www.horosproject.org). Width was defined as the diameter along the adjusted frontal axis. Heights were measured perpendicularly to that diameter: central height at the middle, the lateral heights at the maximal diameter left and right to the center. For interrater reliability analysis, J.V. and M.C. (trainee) measured OC dimensions within 10 randomly selected HC and intraclass correlations (ICC) were calculated.
Fig. 1.
Optic chiasm measurement. Panels a, b, and c illustrate how axes were adjusted perpendicularly to the orientation of the OC in the center point. Panel d shows a sample OC measurement (a = right, b = central, c = left height, d = area, e = width)
Fig. 2.
Optic nerve measurement. Panels a, b, and c illustrate how axes were adjusted perpendicularly to the orientation of the optic nerve
Clinical assessment
Neurological disability was on the Expanded Disability Status Scale (EDSS), including the visual functional system score according to the Neurostatus definitions [34]. Raters were under the supervision of board-certified neurologists. The global neurological examination also included assessment of ON history using clinical criteria. Visual acuity was tested monocularly under phototopic conditions using retroilluminated Early Treatment in Diabetes Retinopathy Study charts at a 4-m distance. The logarithm of the minimum angle of resolution (logMAR) served as a measure of visual function. Visual acuity data was included only from patients where best correction was used (n = 30).
Optical coherence tomography measures
All OCT data were acquired on a spectral domain OCT device (Spectralis, Heidelberg Engineering) with automated real-time function. No pupil dilatation was used. We report the OCT acquisition settings and scanning protocol according to the APOSTEL recommendations [35]: The pRNFL thickness was measured using 3.4-mm ring scans around the optic nerve head (12°, 1536 A-scans, 9 ≤ ART ≤ 100). The GCIPL volume was measured using a 6-mm diameter cylinder around the fovea from a macular volume scan (25°×30°, 61 vertical B-scans, 768 A-scans per B-scan, ART = 15). Segmentation of the pRNFL and the intraretinal layers in the macular scan was performed semi-automatically using software provided by the optical coherence tomography manufacturer (Eye Explorer 1.9.10.0 with viewing module 6.0.9.0; Heidelberg Engineering). Quality was evaluated according to the OSCAR-IB criteria [36, 37].
Two patients did not have OCT data. Eight eyes from six NMOSD-ON had to be excluded due to incidental findings or quality reasons. Only the macular scan from two additional NMOSD-ON eyes was excluded due to quality reasons.
Statistics
Proportional group differences were tested with χ2 test for sex and with ANOVA test for age. For comparison of ordinal and continuous measurements, groupwise comparison was performed using Kruskal-Wallis and ANOVA tests, respectively. Group comparison of OC dimensions was corrected for multiple comparison using the Holm-Bonferroni method. The variations of the individual metrics were compared within the HC group using a coefficient of variation (CoV) computed according to
and served as an indicator of variation in the individual OC measures. Sensitivity to optic nerve atrophy of individual OC measures was evaluated with receiver operating characteristic (ROC) analysis, including area under the curve (AUC) comparison using DeLong method [38]. Association analysis of individual OC measures with the T2 lesion load, the SIENAX V-scaling [39] factor for head size and gender as potential influencing factors, mean optic nerve diameter, mean pRNFL thickness, mean GCIPL volume, visual function (mean logMAR), and disease duration was performed with the Pearson correlation test, association with the number of ON attacks with the Spearman test.
Statistical analyses were performed using R software, version 3.5.1. (http://www.r-project.org/) with the tidyverse [40], ggpubr [41], and pROC packages [42]. Statistical significance was set at a p value < 0.05.
Results
Demographics
Table 1 shows the demographic and clinical characteristics of the cohort. No significant differences of sex distribution, age, and physical disability were found between groups. Optic nerve diameters were different in NMOSD-ON compared to HC (p < 0.0001), NMOSD-ON compared to NMOSD-NON (p < 0.01), but not in NMOSD-NON compared to HC (p > 0.05).
Group comparison and receiver operating characteristics
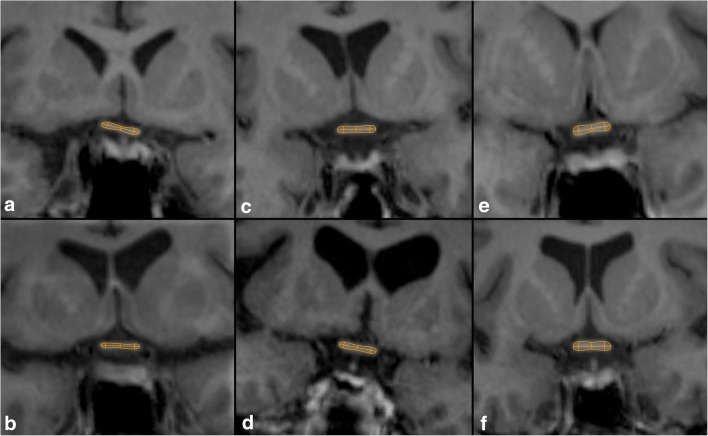
Figure 3 shows the OC of NMOSD-ON, NMOSD-NON, and HC to illustrate the reduction of OC dimensions in NMOSD.
Fig. 3.
Difference in optic chiasm dimensions. Shown are OCs of NMOSD-ON (a, b), NMOSD-NON (c, d), and HC (e, f)
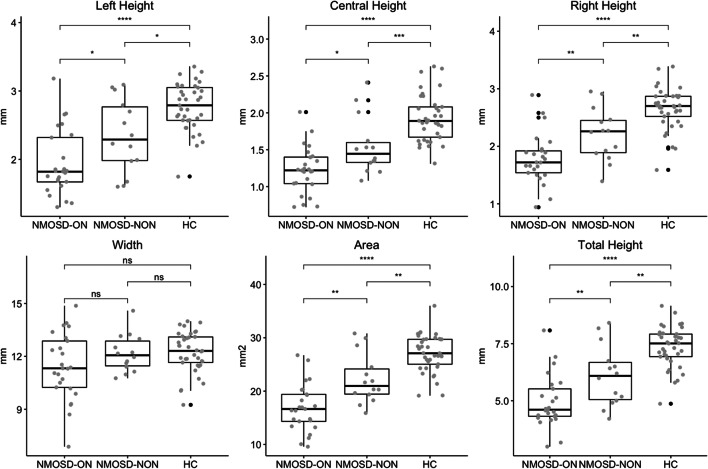
All OC measures except width were significantly smaller in all group comparisons (NMOSD-ON vs. HC: p < 0.0001; NMOSD-NON vs. HC: p < 0.01; and NMO-ON vs. NMO-NON: p < 0.03), as shown in Table 2 and Fig. 4. When correcting for multiple comparisons (corrected p = 0.003), this remained significant for all measures for HC vs. NMOSD-ON, for all measures except left height for HC vs. NMOSD-NON and for area for NMOSD-NON vs. NMOSD-ON.
Table 2.
Optic chiasm measures
| Measure | HC | NMOSD-NON | NMOSD-ON | HC vs. NMOSD-NON | HC vs. NMOSD-ON | NMOSD-NON vs. NMOSD-ON | CoV | ICC |
|---|---|---|---|---|---|---|---|---|
| Left height (CI, mm) |
2.77 (0.35) |
2.34 (0.53) |
1.94 (0.48) |
t = 2.77 p = 0.01 |
t = 7.49 p < 0.0001 |
t = 2.36 p = 0.03 |
0.12 | 0.71 |
| Central height (CI, mm) |
1.93 (0.32) |
1.55 (0.39) |
1.22 (0.32) |
t = 3.27 p < 0.001 |
t = 8.63 p < 0.0001 |
t = 2.70 p = 0.013 |
0.16 | 0.51 |
| Right height (CI, mm) |
2.65 (0.36) |
2.20 (0.46) |
1.79 (0.43) |
t = 3.31 p = 0.003 |
t = 8.23 p < 0.0001 |
t = 2.79 p = 0.008 |
0.14 | 0.77 |
| Width (CI, mm) |
12.23 (1.15) |
12.17 (1.05) |
11.43 (1.87) |
t = 0.17 p = 0.56 |
t = 1.91 p = 0.059 |
t = 1.59 p = 0.18 |
0.09 | 0.95 |
| Area (CI, mm2) |
27.07 (3.50) |
22.26 (4.65) |
16.89 (4.44) |
t = 3.51 p = 0.003 |
t = 9.61 p < 0.0001 |
t = 3.51 p = 0.001 |
0.13 | 0.89 |
| Total height (CI, mm) |
7.35 (0.90) |
6.09 (1.33) |
4.94 (1.14) |
t = 3.27 p = 0.003 |
t = 8.86 p < 0.0001 |
t = 2.73 p = 0.009 |
0.12 | 0.76 |
Shown are means of OC measures. p and t-values are derived from t tests. Corrected p = 0.003. CI = 95% confidence interval; HC = healthy controls; NMOSD-ON = neuromyelitis optica patients with history of optic neuritis; NMOSD-NON = neuromyelitis optica patients without history of optic neuritis; CoV = coefficient of variation; ICC = intraclass correlations
Fig. 4.
Optic chiasm measures. Group differences in optic chiasm heights, width, and area between neuromyelitis optica spectrum disorders patients with optic neuritis (NMOSD-ON), without optic neuritis (NMOSD-NON), and healthy controls (HC). ns: p > 0.05, * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, ****p ≤ 0.0001
OC measures were not associated with the T2 lesion load (r = − 0.35, p > 0.09), the number of ON attacks (r = − 0.31, p > 0.13), the number of ON attacks per side (r = − 0.09, p > 0.13), the SIENAX V-scaling factor, or gender (r < 0.08, p > 0.23). Thus, no correction for head size or sex was performed.
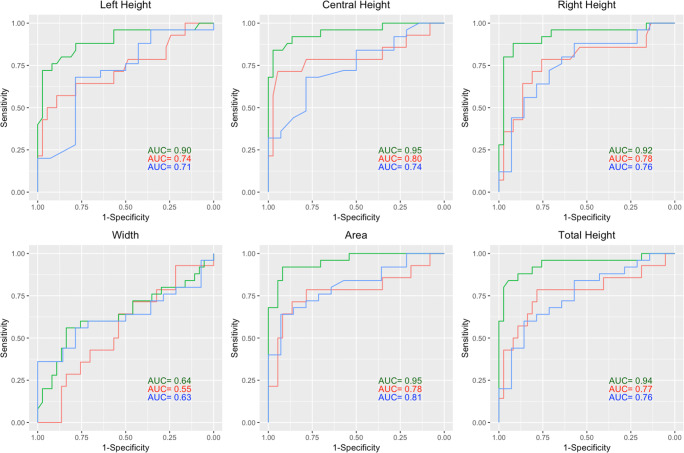
A ROC analysis was conducted to test the ability of the OC to predict the presence of damage in the anterior optic pathway, namely to differentiate between groups. OC area and OC heights have comparable AUC values for each group (NMOSD-ON vs. HC: AUC > 0.92; NMOSD-NON vs. HC: AUC > 0.74; NMO-ON vs. NMO-NON: AUC > 0.71), whereas width has lower AUC values, as shown in Table 3 and Fig. 5. AUC comparison using DeLong method and variation comparison using the CoV of the best performing measures revealed no significant difference. An OC area smaller than 22.5 mm2 yielded a sensitivity of 0.92 and a specificity of 0.92 in separating chiasms of NMOSD-ON from HC.
Table 3.
Receiver operating characteristics analysis
| HC vs. NMOSD-ON | Area | Width |
Left Height |
Central Height |
Right Height |
Total Height |
| AUC | 0.95 | 0.64 | 0.90 | 0.95 | 0.92 | 0.94 |
| 95% CI | 0.91–1.00 | 0.49–0.79 | 0.81–0.98 | 0.89–1.00 | 0.85–1.00 | 0.87–1.00 |
| p value AUC comparison | – | < 0.01 | 0.02 | 0.78 | 0.16 | 0.40 |
| HC vs. NMOSD-NON | Area | Width |
Left Height |
Central Height |
Right Height |
Total Height |
| AUC | 0.78 | 0.55 | 0.74 | 0.80 | 0.78 | 0.77 |
| 95% CI | 0.57–0.92 | 0.38–0.73 | 0.52–0.88 | 0.59–0.95 | 0.62–0.94 | 0.60–0.94 |
| p value AUC comparison | – | 0.08 | 0.31 | 0.49 | 0.93 | 0.77 |
| NMOSD-ON vs. NMOSD-NON | Area | Width |
Left Height |
Central Height |
Right Height |
Total Height |
| AUC | 0.81 | 0.63 | 0.71 | 0.74 | 0.76 | 0.76 |
| 95% CI | 0.69–0.95 | 0.46–0.80 | 0.54–0.89 | 0.59–0.90 | 0.57–0.90 | 0.60–0.92 |
| p value AUC comparison | – | 0.08 | 0.06 | 0.19 | 0.29 | 0.14 |
Shown are area under the curve (AUC), 95% confidence interval (CI), and p value for AUC comparison using area as reference. HC = healthy controls; NMOSD-ON = neuromyelitis optica patients with history of optic neuritis; NMOSD-NON = neuromyelitis optica patients without history of optic neuritis
Fig. 5.
Receiver operating characteristic for optic chiasm measures. Each line represents one group differentiation: green lines HC vs. NMOSD-ON, red lines HC vs. NMOSD-NON, blue lines NMOSD-ON vs. NMOSD-NON; ON = optic neuritis
Associations with structural and clinical measures
Table 4 summarizes the association analysis within the NMOSD-ON group. Higher OC measures were associated with bigger optic nerve diameter, better visual acuity, and better OCT measures. This was most prominent for OC area: Higher values significantly correlated with bigger optic nerve diameter (r = 0.4, p = 0.047), better logMAR (r = − 0.57, p = 0.013), thicker pRNFL (r = 0.59, p = 0.003), bigger GCIPL (r = 0.55, p = 0.007), and shorter disease duration (r = − 0.5, p = 0.012). Within OC heights, only central height was significantly associated with GCIPL (r = 0.46, p = 0.028).
Table 4.
Associations of OC measures and visual acuity, optic nerve diameter and optical coherence tomography measures for NMOSD-ON patients
| Mean | Area | Width | Left Height |
Central Height |
Right Height |
Total Height |
|---|---|---|---|---|---|---|
| Visual acuity (logMAR) |
r = − 0.57 p = 0.013 |
r = − 0.53 p = 0.023 |
r = − 0.05 p = 0.84 |
r = − 0.43 p = 0.079 |
r = − 0.21 p = 0.41 |
r = − 0.22 p = 0.39 |
| Optic nerve diameter |
r = 0.4 p = 0.047 |
r = 0.75 p < 0.001 |
r = − 0.06 p = 0.78 |
r = − 0.09 p = 0.68 |
r = 0.09 p = 0.66 |
r = − 0.03 p = 0.87 |
| pRNFL thickness |
r = 0.59 p = 0.003 |
r = 0.54 p = 0.008 |
r = 0.15 p = 0.49 |
r = 0.38 p = 0.07 |
r = 0.30 p = 0.16 |
r = 0.28 p = 0.19 |
| GCIPL volume |
r = 0.55 p = 0.007 |
r = 0.33 p = 0.13 |
r = 0.19 p = 0.39 |
r = 0.46 p = 0.028 |
r = 0.34 p = 0.11 |
r = 0.33 p = 0.12 |
r values are Pearson’s correlation coefficients. NMOSD-ON = neuromyelitis optica patients with history of optic neuritis; logMAR = logarithm of the minimum angle of resolution; pRNFL = peripapillary retinal nerve fiber layer; GCIPL = combined ganglion cell-inner plexiform layer
Discussion
We evaluated OC measures as imaging marker of anterior optic pathway damage. We demonstrated significant group differences between NMOSD patients and HC and strong associations of OC measures with structural and clinical measures. Our data show that OC assessment in standard 3D-T1w images is sensitive to anterior optic pathway damage. Hence, OC measures are easily accessible and sensitive markers of anterior optic pathway damage.
OC dimension values for HC presented in our study are similar to recently published data [33]. A previous study by Wagner et al [31], however, reported slightly higher OC width and area values. This study excluded OC height due to a high degree of variance. Notably, unlike our study, Wagner et al did not account for the transverse course of the optic pathway and did not define precise measure locations. Thus, the coronal plane would be at varying angles (not perpendicular) to the course of the optic pathway at which OC assessment results in higher values and variance. Furthermore, our study employed a higher resolution MRI sequence, which may have an impact on values and variance.
OC heights and area showed differences between all groups. This is in line with earlier investigations suggesting that optic nerve dimensions discriminate ON patients from controls [16]. OC assessment, as suggested in our study, only requires a standard and broadly available MRI sequence (3D T1-weighted MPRAGE) and assesses a rather fixed structure less vulnerable to motion artifacts compared to the previously used orbital optic nerves [3, 4, 16, 17]. Note that in this study optic nerve diameters were measured in the cisternal segment 7 mm anterior to the OC, since contrast heterogeneity and motion artifacts in the orbital part rendered orbital assessment difficult in 25% of the patients. The observation that smaller OC dimensions were also found in NMOSD-NON (compared to HC and NMOSD-ON) supports microstructural changes in the optic pathway independent of ON [26], which have been described in NMOSD [5, 27, 43, 44]. In concordance with a study by Harrigan et al [16], the optic nerve diameter was smaller in patients with a history of ON compared to HC but not in patients without ON. Although this should not be overstated in consideration of the small NMOSD-NON sample size, this might indicate that microstructural changes independent of ON, including anterograde degeneration originating from anterior optic pathway damage but also retrograde degeneration originating from posterior optic pathway damage, might accumulate in the OC.
The AUC values obtained from ROC analyses for OC area and heights indicate that OC measures are sensitive to anterior optic pathway damage. On the descriptive level, OC left height shows slightly poorer ROC performance than right. This might rather be caused by an asymmetric severity of atrophy predominantly affecting fibers contributing to the right side of the OC (8 ON right vs. 6 ON left), of handedness or other asymmetries present in our study, than by a different sensitivity of the individual measure. This and the observation that no association between ON attacks per side and OC heights was found highlight the fact that OC assessment accumulates pathophysiologic processes of both sides and does not provide information on the origin of the fibers.
The observed associations between higher OC area and better visual acuity (r = − 0.57), thicker pRNFL (r = 0.53) and bigger GCIPL (r = 0.55) in NMOSD are similar to the associations reported on optic nerve dimensions (r = − 0.50, r = 0.66, r = 0.59) in MS [17]. The degree of association between pRNFL and anterior optic pathway dimensions in MRI might be equally limited in NMOSD and MS, since axonal loss is not the only substrate of neuronal atrophy and myelin loss, gliosis, and changes in water content also contribute to MRI-detected changes after ON [45]. Alongside findings suggesting that axonal loss is a major substrate of MRI-detected optic pathway atrophy after ON [17–20], the association between pRNFL (a surrogate for retinal axons) and OC measures implies that they are sensitive to atrophic changes of the anterior optic pathway. The association of OC area with visual function suggests a role for the OC as an imaging marker of neurodegenerative damage in the optic pathway with potential functional relevance.
Despite MRI’s broad availability, no standardized MRI method for evaluation of optic pathway degeneration in standard scans is available. One major problem in optic nerve assessment is defining standardized measurement locations along the variable course of the nerve, which has high inter-individual variability even in healthy populations [3, 4, 15, 17]. Several methods to measure optic nerve dimensions have been put forward [16, 17, 32]. These methods typically involve dedicated orbital MRI fat-saturated acquisition sequences along the axis of the optic nerve [3] additional to the commonly acquired sequences and extend the scan time for each patient. Others involve complex imaging post-processing procedures [16] and, thus, may be difficult to implement in the routine clinical workup. Moreover, motion artifacts from eye movements and contrast reduction in the posterior region of the optic nerve, due to thinning of the CSF filled sub-arachnoid space, render MRI-based optic nerve assessment technically difficult [16]. This limits the accessibility of optic nerve measurements using MRI in the clinical setting.
The OC is less vulnerable to motion artifacts and consistently surrounded by CSF. It is less variable in morphology, bigger in dimensions and, thus, a simple target for MR investigations. OC assessment, as suggested in our study, only requires a broadly available MRI sequence (3D T1-weighted). While it does not provide information on the origin of the fibers and evaluation of focal optic nerve damage might better be achieved by direct measurement at the sight of inflammation, it accumulates neurodegeneration from both sides of the anterior optic pathway causing observable impairment. Thus, it extends the amount of information from a single measurement in the conventional and clinical standard scan, which can be used for monitoring of disease progression or therapeutic effectiveness.
Despite the low total number of subjects included in the study owing to the low prevalence of the disease, a significant difference in OC measures was shown within a relatively large homogeneous cohort exclusively consisting of AQP4-IgG-positive NMOSD patients. Separate gender analysis could not be conducted due to the high proportion of female patients. This is in line with the strong female preponderance in NMOSD [46]. OC width was measured along straight lines, which may not account for curved width. In future investigations, curved lines could be drawn; however, only few participants showed recognizable deviation from straight lines and, thus, we do not expect that this would drastically change the presented results.
Our data provide a strong rationale for future, larger studies on OC measures in ON, including in NMOSD patients with acute ON, in which inflammation might result in an increase in OC dimensions, as well as in patients with inflammatory diseases such as MS and myelin oligodendrocyte glycoprotein antibody associated disease (MOGAD) [47]. Finally, studies on the influence of susceptibility artifacts on scanners with different field strength and resolution seem justified and the application of advanced quantitative imaging methods such as DTI could reveal insights into the relationship between anterior and posterior optic pathway neurodegeneration.
Conclusion
Our study represents an initial and thorough assessment of OC measures to evaluate optic pathway degeneration using standard MRI and shows that the OC area is suitable and reliable. This simple method extends the amount of information that can be obtained from conventional and clinically available scans. Our results suggest that the OC might evolve into an easily accessible imaging marker of neurodegeneration in the anterior optic pathway with potential functional relevance.
Acknowledgments
MR imaging for this study was performed at the Berlin Center for Advanced Neuroimaging. The authors thank the participants of the study, Susan Pikol, Cynthia Kraut, and Charlotte Bereuter for their excellent technical support.
Abbreviations
- AQP4-IgG
Aquaporin 4-IgG
- AUC
Area under the curve
- CI
95% confidence interval
- CoV
Coefficient of variation
- EDSS
Expanded Disability Status Scale
- GCIPL
Combined ganglion cell-inner plexiform layer
- HC
Healthy controls
- ICC
Intraclass correlations
- logMAR
Logarithm of the minimum angle of resolution
- NMOSD
Neuromyelitis optica spectrum disorders
- NMOSD-ON
Neuromyelitis optica patients with history of optic neuritis
- NMOSD-NON
Neuromyelitis optica patients without history of optic neuritis
- OC
Optic chiasm
- OCT
Optical coherence tomography
- ON
Optic neuritis
- pRNFL
Peripapillary retinal nerve fiber layer
- ROC
Receiver operating characteristics
Funding information
Open Access funding provided by Projekt DEAL. The authors state that this work has not received any funding.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Friedemann Paul.
Conflict of interest
The authors of this manuscript declare relationships with the following companies:
NS has received travel grants from Biogen Idec and sanofi-aventis/Genzyme.
SA received travel grants from Celgene GmbH, unrelated to this project.
FCO was employed by Nocturne, unrelated to this project.
HZ received research grants from Novartis, unrelated to this project.
KR was supported by the German Ministry of Education and Research (BMBF/KKNMS, Competence Network Multiple Sclerosis) and has received research support from Novartis and Merck Serono as well as speaking fees and travel grants from Guthy Jackson Charitable Foundation, Bayer Healthcare, Biogen Idec, Merck Serono, sanofi-aventis/Genzyme, Teva Pharmaceuticals, Roche, and Novartis.
JBS has received travel grants and speaking fees from Bayer Healthcare, Biogen Idec, Merck Serono, sanofi-aventis/Genzyme, Teva Pharmaceuticals, and Novartis.
FP declares that he has received research grants and speaker’s honoraria from Bayer Healthcare, Teva Pharmaceuticals, Genzyme, Merck and Co., Novartis, and MedImmune. He is also a member of the steering committee for the OCTIMS study (run by Novartis).
AUB is cofounder and shareholder of technology start-ups Motognosis and Nocturne. He is named as inventor on several patent applications describing MS serum biomarkers, perceptive visual computing for motor function assessment and retinal image analysis.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported in PMID 31127016.
Methodology
• Retrospective
• Observational
• Performed at one institution
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alexander U. Brandt and Michael Scheel contributed equally to this article.
References
- 1.Jarius S, Wildemann B, Paul F. Neuromyelitis optica: clinical features, immunopathogenesis and treatment. Clin Exp Immunol. 2014;176(2):149–164. doi: 10.1111/cei.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waters P, Reindl M, Saiz A, et al. Multicentre comparison of a diagnostic assay: aquaporin-4 antibodies in neuromyelitis optica. J Neurol Neurosurg Psychiatry. 2016;87(9):1005–1015. doi: 10.1136/jnnp-2015-312601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hickman SJ, Brex PA, Brierley CM, et al. Detection of optic nerve atrophy following a single episode of unilateral optic neuritis by MRI using a fat-saturated short-echo fast FLAIR sequence. Neuroradiology. 2001;43:123–128. doi: 10.1007/s002340000450. [DOI] [PubMed] [Google Scholar]
- 4.Hickman SJ, Toosy AT, Jones SJ, et al. A serial MRI study following optic nerve mean area in acute optic neuritis. Brain. 2004;127:2498–2505. doi: 10.1093/brain/awh284. [DOI] [PubMed] [Google Scholar]
- 5.Tian DC, Su L, Fan M, et al. Bidirectional degeneration in the visual pathway in neuromyelitis optica spectrum disorder (NMOSD) Mult Scler. 2018;24:1585–1593. doi: 10.1177/1352458517727604. [DOI] [PubMed] [Google Scholar]
- 6.Tur C, Goodkin O, Altmann DR, et al. Longitudinal evidence for anterograde trans-synaptic degeneration after optic neuritis. Brain. 2016;139:816–828. doi: 10.1093/brain/awv396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabilondo I, Martinez-Lapiscina EH, Martinez-Heras E, et al. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol. 2014;75:98–107. doi: 10.1002/ana.24030. [DOI] [PubMed] [Google Scholar]
- 8.Sinnecker T, Oberwahrenbrock T, Metz I, et al. Optic radiation damage in multiple sclerosis is associated with visual dysfunction and retinal thinning~an ultrahigh-field MR pilot study. Eur Radiol. 2015;25:122–131. doi: 10.1007/s00330-014-3358-8. [DOI] [PubMed] [Google Scholar]
- 9.Kuchling J, Backner Y, Oertel FC et al (2018) Comparison of probabilistic tractography and tract-based spatial statistics for assessing optic radiation damage in patients with autoimmune inflammatory disorders of the central nervous system. Neuroimage Clin 19:538–550 [DOI] [PMC free article] [PubMed]
- 10.Kuchling J, Brandt AU, Paul F, Scheel M. Diffusion tensor imaging for multilevel assessment of the visual pathway: possibilities for personalized outcome prediction in autoimmune disorders of the central nervous system. EPMA J. 2017;8:279–294. doi: 10.1007/s13167-017-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merle H, Olindo S, Bonnan M, et al. Natural history of the visual impairment of relapsing neuromyelitis optica. Ophthalmology. 2007;114:810–815. doi: 10.1016/j.ophtha.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt F, Zimmermann H, Mikolajczak J, et al. Severe structural and functional visual system damage leads to profound loss of vision-related quality of life in patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord. 2017;11:45–50. doi: 10.1016/j.msard.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Stiebel-Kalish H, Hellmann MA, Mimouni M, et al. Does time equal vision in the acute treatment of a cohort of AQP4 and MOG optic neuritis? Neurol Neuroimmunol Neuroinflamm. 2019;6:e572. doi: 10.1212/NXI.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beekman J, Keisler A, Pedraza O, et al. Neuromyelitis optica spectrum disorder: patient experience and quality of life. Neurol Neuroimmunol Neuroinflamm. 2019;6:e580. doi: 10.1212/NXI.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickman SJ, Brierley CM, Brex PA, et al. Continuing optic nerve atrophy following optic neuritis: a serial MRI study. Mult Scler. 2002;8:339–342. doi: 10.1191/1352458502ms809oa. [DOI] [PubMed] [Google Scholar]
- 16.Harrigan RL, Smith AK, Lyttle B, et al. Quantitative characterization of optic nerve atrophy in patients with multiple sclerosis. Mult Scler J Exp Transl Clin. 2017;3:2055217317730097. doi: 10.1177/2055217317730097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trip SA, Schlottmann PG, Jones SJ, et al. Optic nerve atrophy and retinal nerve fibre layer thinning following optic neuritis: evidence that axonal loss is a substrate of MRI-detected atrophy. Neuroimage. 2006;31:286–293. doi: 10.1016/j.neuroimage.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 18.Parravano JG, Toledo A, Kucharczyk W. Dimensions of the optic nerves, chiasm, and tracts: MR quantitative comparison between patients with optic atrophy and normals. J Comput Assist Tomogr. 1993;17:688–690. doi: 10.1097/00004728-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Manogaran P, Vavasour IM, Lange AP et al (2016) Quantifying visual pathway axonal and myelin loss in multiple sclerosis and neuromyelitis optica. Neuroimage Clin 11:743–750 [DOI] [PMC free article] [PubMed]
- 20.Manogaran P, Hanson JV, Olbert ED, et al. Optical coherence tomography and magnetic resonance imaging in multiple sclerosis and Neuromyelitis Optica Spectrum disorder. Int J Mol Sci. 2016;17:e1894. doi: 10.3390/ijms17111894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trip SA, Schlottmann PG, Jones SJ, et al. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58:383–391. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- 22.Oertel FC, Zimmermann HG, Brandt AU, Paul F. Novel uses of retinal imaging with optical coherence tomography in multiple sclerosis. Expert Rev Neurother. 2018;19:31–43. doi: 10.1080/14737175.2019.1559051. [DOI] [PubMed] [Google Scholar]
- 23.Oberwahrenbrock T, Traber GL, Lukas S, et al. Multicenter reliability of semiautomatic retinal layer segmentation using OCT. Neurol Neuroimmunol Neuroinflamm. 2018;5:e449. doi: 10.1212/NXI.0000000000000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorr J, Wernecke KD, Bock M, et al. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PLoS One. 2011;6:e18132. doi: 10.1371/journal.pone.0018132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayadi N, Dörr J, Motamedi S. Temporal visual resolution and disease severity in MS. Neurol Neuroimmunol Neuroinflamm. 2018;5(5):e49. doi: 10.1212/NXI.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oertel FC, Zimmermann H, Paul F, Brandt AU. Optical coherence tomography in neuromyelitis optica spectrum disorders: potential advantages for individualized monitoring of progression and therapy. EPMA J. 2018;9:21–33. doi: 10.1007/s13167-017-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oertel FC, Kuchling J, Zimmermann H, et al. Microstructural visual system changes in AQP4-antibody-seropositive NMOSD. Neurol Neuroimmunol Neuroinflamm. 2017;4:e334. doi: 10.1212/NXI.0000000000000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett JL, de Seze J, Lana-Peixoto M, et al. Neuromyelitis optica and multiple sclerosis: seeing differences through optical coherence tomography. Mult Scler. 2015;21:678–688. doi: 10.1177/1352458514567216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HJ, Paul F, Lana-Peixoto MA, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015;84:1165–1173. doi: 10.1212/WNL.0000000000001367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner AL, Murtagh FR, Hazlett KS, Arrington JA. Measurement of the normal optic chiasm on coronal MR images. AJNR Am J Neuroradiol. 1997;18:723–726. [PMC free article] [PubMed] [Google Scholar]
- 32.Avery RA, Mansoor A, Idrees R, et al. Quantitative MRI criteria for optic pathway enlargement in neurofibromatosis type 1. Neurology. 2016;86:2264–2270. doi: 10.1212/WNL.0000000000002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasmann-Kellner B, Schafer T, Krick CM, Ruprecht KW, Reith W, Schmitz BL. Anatomical differences in optic nerve, chiasma and tractus opticus in human albinism as demonstrated by standardized clinical and MRI evaluation. Klin Monbl Augenheilkd. 2003;220:334–344. doi: 10.1055/s-2003-39427. [DOI] [PubMed] [Google Scholar]
- 34.Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis. Neurology. 33:1444–1452 [DOI] [PubMed]
- 35.Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86:2303–2309. doi: 10.1212/WNL.0000000000002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One. 2012;7:e34823. doi: 10.1371/journal.pone.0034823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schippling S, Balk L, Costello F, et al. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler. 2015;21:163–170. doi: 10.1177/1352458514538110. [DOI] [PubMed] [Google Scholar]
- 38.Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med. 2013;4:627–635. [PMC free article] [PubMed] [Google Scholar]
- 39.Smith SM, ThangY JM, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 40.Wickham H (2017) Tidyverse: easily Install and Load the “Tidyverse.”. Available via https://cranr-project.org/package. Accessed 10 Oct 2018
- 41.Kassambara A (2017) ggpubr: “ggplot2” Based Publication Ready Plots. Available via https://cran.r-project.org/web/packages/ggpubr/index.html. Accessed 10 Oct 10 2018
- 42.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akaishi T, Kaneko K, Himori N, et al. Subclinical retinal atrophy in the unaffected fellow eyes of multiple sclerosis and neuromyelitis optica. J Neuroimmunol. 2017;313:10–15. doi: 10.1016/j.jneuroim.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Oertel FC, Havla J, Roca-Fernandez A, et al. Retinal ganglion cell loss in neuromyelitis optica: a longitudinal study. J Neurol Neurosurg Psychiatry. 2018;89:1259–1265. doi: 10.1136/jnnp-2018-318382. [DOI] [PubMed] [Google Scholar]
- 45.Noval S, Contreras I, Muñoz S, Oreja-Guevara C, Manzano B, Rebolleda G. Optical coherence tomography in multiple sclerosis and neuromyelitis optica: an update. Mult Scler Int. 2011;2011:472790. doi: 10.1155/2011/472790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gold SM, Willing A, Leypoldt F, Paul F, Friese MA. Sex differences in autoimmune disorders of the central nervous system. Semin Immunopathol. 2019;41:177–188. doi: 10.1007/s00281-018-0723-8. [DOI] [PubMed] [Google Scholar]
- 47.Borisow N, Mori M, Kuwabara S, Scheel M, Paul F. Diagnosis and treatment of NMO spectrum disorder and MOG-encephalomyelitis. Front Neurol. 2018;9:888. doi: 10.3389/fneur.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]