Abstract
Purpose
Fosfomycin is now widely used to treat methicillin-resistant S. aureus due to its unique antibacterial activity. However, fosfomycin-resistant S. aureus has rapidly emerged, it is urgent to find new treatments to eliminate fosfomycin-resistant S. aureus infection. The purpose of this study was to analyze the activity of cryptanshinone, a traditional Chinese medicine monomer, in combination with fosfomycin against fosfomycin-sensitive S. aureus (FSSA) and fosfomycin-resistant S. aureus (FRSA).
Methods
The MICs of fosfomycin and/or cryptanshinone were determined by agar dilution assay and checkerboard microdilution assay. Furthermore, synergistic effects from fosfomycin and/or cryptanshinone were analyzed by the time-kill assay in vitro.
Results
The combination of fosfomycin and cryptotanshinone had a synergistic effect on most (71.43%) of the FRSA and had a partial (28.57%) synergistic effect on a small part. In addition, time sterilization curve verified synergistic activity between cryptanshinone and fosfomycin against FSSA and FRSA, especially when fosfomycin was added for a second time.
Conclusion
These data suggest that cryptanshinone combined with fosfomycin could be a novel treatment for FRSA and provide a new direction for the treatment of bacterial infections in the future.
Keywords: traditional Chinese medicine, cryptanshinone, fosfomycin, synergistic effect, Staphylococcus aureus
Introduction
Staphylococcus aureus (S. aureus), as an important cause of many diseases such as abscess and epifolliculitis, was first isolated from a surgical abscess by Ogston in 1880.1 Many researches have shown that the mortality rate of patients infected with S. aureus in the United States is as high as 18%.2 Significant morbidity and mortality show S. aureus infection as an increasingly serious problem in clinical practice.3 With the widespread use of antibiotics in clinical practice, the drug-resistance of S. aureus is becoming more and more serious,4 especially methicillin-resistant S. aureus (MRSA), which has become a major problem in clinical treatment worldwide.5 Therefore, how to properly treat drug-resistant S. aureus infection is an urgent problem for medical workers.
The rise of drug-resistant S. aureus has also led to a significant decline for its treatments.6 At present, the commonly used drugs are teicoplanin, tigecycline, vancomycin, linezolid, and fosfomycin.7,8 Fosfomycin is one of the bactericidal antibiotics discovered in Streptomyces in 1969, which could act on S. aureus by inhibiting cell wall synthesis.9,10 As a common antibiotic, fosfomycin could be used alone or in combination with other antibiotics to treat the infections caused by MRSA.11 However, with the extensive use of fosfomycin against S. aureus, fosfomycin-resistant Staphylococcus aureus (FRSA) has also emerged. Relevant data showed that the resistance rate of fosfomycin has reached 30%.12 Among the new promising strategies, in this context, combination therapies between fosfomycin and other drugs are used to treat bacterial infections.13
Recently, many studies have indicated that fosfomycin combined with daptomycin, linezolid vancomycin or imipenem can be used as an alternative to the treatment of MRSA infection.11,12,14,15 MRSA reduced PBP2A expression in the presence of fosfomycin, thereby increasing sensitivity to β-lactam.11 However, there are few reports on the treatment of infections caused by FRSA. In Japan, Kouda, many scholars found that the combination of imipenem, cilastatin and cephalosporin has a significant synergistic effect on FRSA.16 Thus, there is an urgent need to develop new antibacterial compounds that synergize with fosfomycin, aiming to solve the problem of S. aureus resistance.
Traditional Chinese Medicine (TCM) has its own unique advantages in the treatment of infectious diseases. At present, there are many researches on developing new drugs from TCM in China and abroad. Cryptotanshinone, a TCM monomer with antibacterial, anti-inflammatory and anticancer effects extracted from Salvia miltiorrhizais, is a natural compound.17 In terms of Chinese medicine, cryptotanshinone has the effects of promoting blood circulation and removing blood stasis, menstrual pain relief, cooling blood and carbuncle. Cryptotanshinone exhibits bacterial activity against most Gram-positive bacteria, including S. aureus, Gram-negative bacteria and other microorganisms.18 Currently, there is no study on the combination of cryptanshinone and fosfomycin to restore the sensitivity of fosfomycin against FRSA.
The aim of this study was to evaluate the activity of cryptotanshinone combined with fosfomycin against FSSA and FRSA, which would provide a better treatment scheme for FRSA infection clinically and new ideas for the treatment of other drug-resistant infections in the clinic as well.
Materials and Methods
Bacterial Strains
Seven clinical FRSA strains were isolated during 2011 and 2012 from Lishui Central Hospital in Zhejiang Province, and the reference fosfomycin-susceptible Staphylococcus aureus (FSSA) ATCC 25,923 strain was obtained from ATCC company.
MurA, GlpT, UhpT, fosA, fosB and fosC Genes Amplification and Sequencing
To investigate the effects of MurA, GlpT, UhpT, fosA, fosB and fosC genes on FRSA, any genetic variation was analyzed accordingly. The genes were amplified using primers listed in Table 1 of the reference.19 All the PCR products were sequenced by TSINGKE (Chengdu, China) and then verified by sequence alignment with their corresponding genes in NCBI Nucleotide Database.
Table 1.
Fosfomycin MICs and FSSA and FRSA Strains Description
| Pathogen | Strain | Reference/Source | Description | Fosfomycin MIC (μg/mL) |
|---|---|---|---|---|
| S. aureus | ATCC25923 | American Type Culture Collection | Fosfomycin-susceptible reference strain | 2 |
| #25 | Lishui central hospital, zhejiang province | Clinical isolate with a G790A base substitution of MurA, and C299T base substitution of GlpT and G1044T base substitution of UhpT | 128 | |
| #28 | Lishui central hospital, zhejiang province | Clinical isolate with a G790A base substitution of MurA, and C299T base substitution of GlpT and G1044T base substitution of UhpT | 64 | |
| #47 | Lishui central hospital, zhejiang province | Clinical isolate with a G790A base substitution of MurA, and C299T base substitution of GlpT and G1044T base substitution of UhpT | 64 | |
| #87 | Lishui central hospital, zhejiang province | Clinical isolate with a G790A base substitution of MurA, and C299T base substitution of GlpT and G1044T base substitution of UhpT | 64 | |
| #99 | Lishui central hospital, zhejiang province | Clinical isolate with a G790A base substitution of MurA, and C299T base substitution of GlpT and G1044T base substitution of UhpT | 64 | |
| #122 | Lishui central hospital, zhejiang province | Clinical isolate with a G248 base deletion of GlpT | 2048 | |
| #133 | Lishui central hospital, zhejiang province | Clinical isolate with a G248 base deletion of GlpT | 2048 |
In vitro Susceptibility Testing
MIC of fosfomycin and cryptanshinone against FSSA and FRSA were determined by agar dilution assay according to American Clinical and Laboratory Standards Institute (CLSI) recommendations.20 The MIC of fosfomycin in combination with cryptanshinone was determined by checkerboard microdilution assay in accordance with the recommendations of the CLSI as well. For MIC measurement, the initial inoculation quantity of each strain was 5×105 CFU/mL in presence of fosfomycin, cryptanshinone, or fosfomycin plus cryptanshinone. After inoculation, the strains were incubated for 16~18 hours at 37°C. S. aureus ATCC 25,923 was used as the control.
The effect of the drug combination was evaluated by Fractional Inhibitory Concentration index (FICI).21 A and B stand for two antibacterial drugs: ∑FIC=FICA+FICB, and FICA= MIC(A in the presence of B)/MIC(A alone); FICB= MIC(B in the presence of A)/MIC(B alone). The FIC index is then evaluated using the following methods: synergy, FICI≤0.5; additive, FICI>0.5–1; indifference, FICI>1 to <2; antagonism, FICI≥2.
Time-Kill Curve Assays
In the presence of fosfomycin, cryptanshinone, fosfomycin plus cryptanshinone, the initial inoculation of S. aureus ATCC 25,923 and FOF-R #122 strains in Mueller-Hinton Broth culture medium was 5×105 CFU/mL. Each experiment has a non-medicated bacterial-containing medium as a control. Under some conditions, cryptotanshinone was added after one hour of bacterial inoculation in order to avoid the antagonism of cryptotanshinone and fosfomycin. Similarly, fosfomycin was re-added after 4 hours of bacterial inoculation because the half-life of fosfomycin was 3 to 5 hours. The tubes under different conditions were then placed in a shaking table at 37°C for cultivation. Samples were taken at 0, 2, 4, 8, and 24 hours and appropriately diluted; 100 μL of the diluted bacterial solution was uniformly applied to Mueller-Hinton Agar medium and incubated at 37°C for 24 hours. After counting the monoclone, the logarithm of the viable cells (CFU/mL) can be obtained. Judging criteria for joint effects:13 Compared with the single drug with stronger activity, the bacterial colony number decreased by more than 2 log10 CFU/mL in the combination group, which was considered to be synergy; similarly, a decrease in bacterial colonies less than 2 log10 CFU/mL was considered to be irrelevant; the increase of bacterial colony number was greater than 2 log10 CFU/mL, which was considered to be antagonistic.
Statistical Analysis
Each set of data was expressed as mean ± SEM and a t-test was used to determine the difference between the means. Each experiment was carried out three times. P < 0.05 indicates that the difference was statistically significant.
Results
Resistance Mechanism and MIC of Fosfomycin
The MIC of FRSA ranged from 64 to 2048 μg/mL, while the MIC of fosfomycin-sensitive S. aureus was 2 μg/mL. The analysis of the MurA, GlpT, UhpT, fosA, fosB and fosC sequences showed that fosA, fosB and fosC genes were absent in all 7 fosfomycin-resistant strains. The MurA, GlpT and UhpT of 5 low-level fosfomycin-resistant strains all contained the same type of mutation sites, including G790A mutation on MurA, C299T mutation on GlpT and G1044T mutation on UhpT. The other 2 strains of fosfomycin with high resistance only contained G248 deletion of GlpT gene (Table 1).
In vitro Activity of Cryptotanshinone Combination with Fosfomycin Against FSSA and FRSA
The MIC of cryptotanshinone alone and the combination of fosfomycin on FSSA and FRSA were determined by broth dilution method and checkerboard dilution method, as shown in Table 2. Cryptotanshinone alone for FSSA and FRSA showed a range of MIC from 8~128 μg/mL. When cryptotanshinone combined with fosfomycin, 5 of 7 resistant strains showed synergistic effect, and 2 strains have partial synergistic effect.
Table 2.
Determination of MIC of Cryptotanshinone and Fosfomycin Alone or in Combination Against FSSA and FRSA
| Pathogen | Strain | Agent | MIC Alone (μg/mL) | MIC Combination (μg/mL) | FIC | FICI | Outcome |
|---|---|---|---|---|---|---|---|
| S. aureus | ATCC25923 | Cryptotanshinone | 8 | 2 | 0.25 | 0.3125 | Synergistic |
| Fosfomycin | 2 | 0.125 | 0.0625 | ||||
| #25 | Cryptotanshinone | 8 | 1 | 0.125 | 0.625 | Partialsynergy | |
| Fosfomycin | 128 | 64 | 0.5 | ||||
| #28 | Cryptotanshinone | 8 | 1 | 0.125 | 0.375 | Synergistic | |
| Fosfomycin | 64 | 16 | 0.25 | ||||
| #47 | Cryptotanshinone | 8 | 1 | 0.125 | 0.375 | Synergistic | |
| Fosfomycin | 64 | 16 | 0.25 | ||||
| #87 | Cryptotanshinone | 8 | 1 | 0.125 | 0.375 | Synergistic | |
| Fosfomycin | 64 | 16 | 0.25 | ||||
| #99 | Cryptotanshinone | 8 | 0.5 | 0.0625 | 0.5625 | Partialsynergy | |
| Fosfomycin | 128 | 32 | 0.5 | ||||
| #122 | Cryptotanshinone | 128 | 32 | 0.25 | 0.3125 | Synergistic | |
| Fosfomycin | 2048 | 128 | 0.0625 | ||||
| #133 | Cryptotanshinone | 128 | 32 | 0.25 | 0.3125 | Synergistic | |
| Fosfomycin | 2048 | 128 | 0.0625 |
Time-Killing Curves
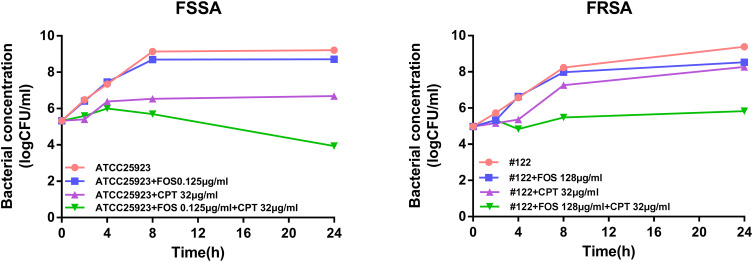
Through time-bactericidal experiment, the sterilization abilities of cryptotanshinone combined with fosfomycin and single drug were measured against FSSA and FRSA. The strain ATCC 25,923 treated with 32 μg/mL cryptotanshinone in combination with 0.125 μg/mL fosfomycin showed higher synergistic activity, with bacterial cell count decreased by 4.77 log10 CFU/mL compared to fosfomycin alone at 24h (P<0.05).(Figure 1). Combination of 32 μg/mL cryptotanshinone with 128 μg/mL fosfomycin of #122 strain showed higher synergistic activity compared with fosfomycin alone. The colony count of the combination group was significantly lower than that of the single group (P<0.05). The bacterial cell count in the combination group decreased by 2.70 log10 CFU/mL at 24h compared with fosfomycin alone (Figure 1).
Figure 1.
Early addition of cryptotanshinone potentiates the fosfomycin activity against FSSA and FRSA strains. Time-kill curves of S. aureus ATCC 25,923 and #122 strains in presence of cryptotanshinone, and fosfomycin alone or in combination with cryptotanshinone for 24 h. FOS, fosfomycin, CPT, cryptotanshinone.
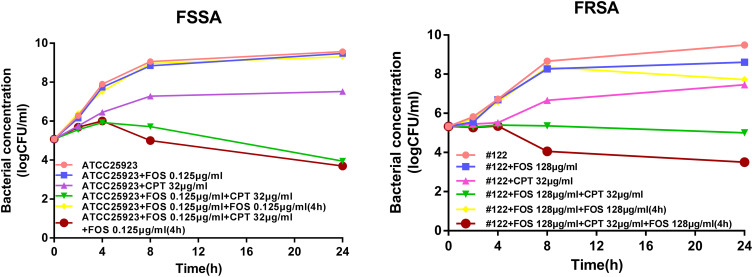
Since the half-life of fosfomycin in vitro is 2.5 h,22 we did not rule out the possibility of degradation of fosfomycin in the culture medium during the time sterilization curve test, thus affecting the experimental results. Therefore, in order to maintain the concentration of fosfomycin in the medium, we added 0.125 μg/mL fosfomycin again in the 2 μg/mL cryptotanshinone combined with 0.125 μg/mL fosfomycin group, as well as the 0.125 μg/mL fosfomycin alone group after incubation for 4 h in the following experiment. This approach decreased the bacterial cell count of ATCC 25,923 strain by 5.60 log10 CFU/mL with respect to fosfomycin plus fosfomycin at 4 h (P<0.05) (Figure 2). In addition to #122 strain, the addition of fosfomycin for a second time increased the synergy of cryptotanshinone and fosfomycin plus fosfomycin which decreased the bacterial cell count by 4.22 log10 CFU/mL with respect to fosfomycin plus fosfomycin at 4 h (P<0.05) (Figure 2).
Figure 2.
Cryptotanshinone potentiates the fosfomycin activity against FSSA and FRSA strains after second time addition of fosfomycin. Time-kill curves of S. aureus ATCC 25,923 and #122 strains in presence of cryptotanshinone and fosfomycin alone, or in combination with or without addition of fosfomycin for second time 4 h after bacterial addition. FOS, fosfomycin, CPT, cryptotanshinone.
Discussion
At present, the treatment of S. aureus infection is a thorny problem worldwide. In particular, after the emergence of MRSA, some antibiotics commonly used for S. aureus infection also appeared to be resistant, such as FRSA.23 Addressing the drug resistance of S. aureus infection is critical, and combination therapy is one of the breakthroughs.
In this study, according to the drug susceptibility test, the MIC of fosfomycin on 7 drug-resistant strains ranged from 64 to 2048 μg/mL, while the MIC of sensitive strains was 2 μg/mL. The resistance value of fosfomycin was much higher than that found by Fu et al,24 which indicated that the phenomenon of highly resistant strains has appeared gradually, suggesting that fosfomycin should be used reasonably in the future.
Then, we investigated the mechanism of fosfomycin resistance and found that none of the plasmid-mediated fosfomycin-modified protein fosA, fosB and fosC were detected in the 7 strains of FRSA. In general, enzymes encoded by these three genes modify fosfomycin to make it resistant to drugs.25 The fosA and fosC genes are generally not present in positive bacteria, while fosB is often detected in positive bacteria.24 For example, French Etienne and other scholars26 isolated 18 strains containing fosB gene in 39 strains of FRSA, with a positive rate of 46.15%; Fu et al24 collected and tested the positive rate of fosB gene in China Huashan Hospital, with a positive rate of 13.43%. However, the positive rate of fosB gene in the drug-resistant strains in this study is zero, which is inconsistent with the previous results, indicating that the positive rate of fosB gene may also be affected by different geographical regions and the presence of the fosB gene may not absolutely lead to fosfomycin resistance.27
For the chromosomal resistance gene MurA, GlpT and UhpT, there are four mutations in 7 strains of fosfomycin-resistant strains, namely G790A mutation on MurA, C299T mutation and G248 deletion on GlpT, and G1044T mutation on UhpT, respectively. In contrast, FSSA ATCC 25,923 has the C299T mutation on GlpT only. Our data showed that the C299T mutation on GlpT was not related to fosfomycin resistance, which was consistent with the phenomenon studied by Fu et al.24 In addition, due to the deletion of G248 on GlpT, the MIC of fosfomycin against S. aureus increased from 2 to 2048 μg/mL, indicating that the loss of G248 on GlpT may lead to higher levels of resistance to fosfomycin. Of note, Fu et al also found that strains containing GlpT mutations showed high levels of resistance.19 Some researchers have found that when GlpT or UhpT proteins are mutated, bacterial uptake of fosfomycin decreases, leading to drug resistance.28 In addition, the G790A mutation on MurA and the G1044T mutation on UhpT may cause moderate resistance to fosfomycin, which increases the MIC of fosfomycin from 2 to 128 due to mutations in both. Studies have shown that mutations in the fosfomycin-targeted protein MurA decrease the affinity of fosfomycin to MurA protein, resulting in bacterial resistance.29 Our findings are also consistent with the previous results.28,30,31
Next, this study tested the in vitro activity of cryptotanshinone combined with fosfomycin against FSSA and FRSA, and found that the combination of the two drugs can produce synergistic effects on drug-resistant strains. Moreover, the effective concentration of fosfomycin can be reduced by 2~16 times compared with the treatment alone. Compared with the results of Hao Chen et al,32 the concentration of fosfomycin required for the combination of fosfomycin and cryptotanshinone was lower in this study. The combination of fosfomycin and cryptotanshinone has a synergistic effect on most (71.43%) of the FRSA, and has a partial (28.57%) synergistic effect on a small part, indicating that fosfomycin combined with cryptanshinone could effectively target fosfomycin-resistant S. aureus. In addition, FICI is greater than or equal to 0.375 for low-level FRSA, whereas FICI is equal to 0.3125 for high-level FRSA. Thus, the drug combination can produce a better synergistic effect on the high level of fosfomycin-resistant S. aureus.
On the other hand, in order to verify the synergistic effect of the combination of the two drugs, time-kill assay is essential. The combination of fosfomycin and cryptanshinone increased the activity of fosfomycin against S. aureus, especially fosfomycin-resistant S. aureus at 24 h in the study. In the time-sterilization curve, the reduction in bacterial colony number was greater than 2log10 CFU/mL compared with the fosfomycin alone group, indicating that the combination of fosfomycin and cryptotanshinone can produce a synergistic effect, which confirms our previous conclusion. The combination of fosfomycin and cryptotanshinone has stronger antibacterial activity than single drug. According to our results, fosfomycin and cryptanshinone had no obvious inhibitory effect on the growth of bacteria when treated alone, while the antibacterial effect was significant after combined treatment. One of the reasons could be the inhibitory effect of fosfomycin on the synthesis of bacterial cell wall in the early stage,33 which affected the growth of bacteria, so that cryptanshinone could better enter the bacteria and play a role.
What is more, when fosfomycin was added again at the 4th hour for the group with fosfomycin resistance S. aureus, compared with the group without adding fosfomycin, the number of bacterial colonies decreased significantly, indicating that the half-life of fosfomycin still had a certain effect on the activity of the combined treatment. Moreover, the addition of fosfomycin can reduce the impact on the combination due to half-life.
Finally, the combination of cryptotanshinone and fosfomycin may help fosfomycin in destroying the cell wall of FRSA,34,35 and inhibit the synthesis of its protein and nucleic acid, resulting in the loss of normal bacterial function. There are limited reports on the combination of cryptotanshinone and other antibiotics.17,34 In addition, we confirmed that for the first time the possibility of combining cryptanshinone with fosfomycin to fight against fosfomycin-resistant S. aureus in the study, thus promoting the pharmacodynamic advantage of fosfomycin against drug-resistant bacteria infection.
Conclusions
Cryptotanshinone has potential effect of fosfomycin against FSSA and FRSA. Thus, cryptotanshinone can be used as a natural source for the development of new functional drugs in combination with fosfomycin, aiming to eliminate fosfomycin-resistant S. aureus. The results of this study can provide new ideas for the clinical treatment of S. aureus infection.
Acknowledgments
This work was supported by the Thousand Talents Plan of Sichuan Province, China (No. 1060) and the Key R&D Projects of Sichuan Province, China (No. 2018SZ0061) and the National Natural Science Foundation of China (No.31870135). Author order was determined on the basis of seniority.
Ethics
The ethics Review Committee of Chengdu Medical College approved the use of the bacterial isolates in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ogston A. Micrococcus poisoning. J Anat Physiol. 1882;16:526–567. [PMC free article] [PubMed] [Google Scholar]
- 2.Kourtis AP, Hatfield K, Baggs J, et al. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections − United States. Morb Mortal Wkly Rep. 2019;68:214–219. doi: 10.15585/mmwr.mm6809e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones M, Jernigan JA, Evans ME, et al. Vital signs: trends in Staphylococcus aureus infections in veterans affairs medical centers — United States, 2005–2017. Morb Mortal Wkly Rep. 2019;68:220–224. doi: 10.15585/mmwr.mm6809e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harkins CP, Pichon B, Doumith M, et al. Methicillin-resistant Staphylococcus aureus emerged long before the introduction of methicillin into clinical practice. Genome Biol. 2017;18:130. doi: 10.1186/s13059-017-1252-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Che Hamzah AM, Yeo CC, Puah SM, et al. Infections in Malaysia: a review of antimicrobial resistance and characteristics of the clinical isolates, 1990–2017. Antibiotics. 2019;8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Lai XX, Zhang L, et al. [Distribution and drug resistance of pathogens in infected organ donors from donation after the citizen death]. Zhonghua Yi Xue Za Zhi. 2018;98:181–185. doi: 10.3760/cma.j.issn.0376-2491.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 7.Li L, Dai JX, Xu L, et al. Antimicrobial resistance and pathogen distribution in hospitalized burn patients: a multicenter study in Southeast China. Medicine. 2018;97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis PO, Heil EL, Covert KL, et al. Treatment strategies for persistent methicillin-resistant Staphylococcus aureus bacteraemia. J Clin Pharm Therap. 2018;43:614–625. doi: 10.1111/jcpt.12743 [DOI] [PubMed] [Google Scholar]
- 9.Hendlin D, Stapley EO, Jackson M, et al. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969;166:122–123. [DOI] [PubMed] [Google Scholar]
- 10.Falagas ME, Vouloumanou EK, Samonis G, et al. Fosfomycin. Clin Microbiol Rev. 2016;29:321–347. doi: 10.1128/CMR.00068-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richter DC, Brenner T, Brinkmann A, et al. [Infections due to multidrug-resistant pathogens: pathogens, resistance mechanisms and established treatment options]. Anaesthesist. 2019;68:711–730. German. [DOI] [PubMed] [Google Scholar]
- 12.Wu D, Chen Y, Sun L, et al. Prevalence of fosfomycin resistance in methicillin-resistant Staphylococcus aureus isolated from patients in a university hospital in China from 2013 to 2015. Jpn J Infect Dis. 2018;71:312–314. doi: 10.7883/yoken.JJID.2018.013 [DOI] [PubMed] [Google Scholar]
- 13.Ayerbe-Algaba R, Gil-Marqués ML, Jiménez-Mejías ME, et al. Synergistic activity of niclosamide in combination with colistin against colistin-susceptible and colistin-resistant and colistin-resistant Acinetobacter baumannii and Klebsiella pneumoniae. Front Cell Infect Microbiol. 2018;8:348. doi: 10.3389/fcimb.2018.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chai D, Liu X, Wang R, et al. Efficacy of linezolid and fosfomycin in catheter-related biofilm infection caused by methicillin-resistant Staphylococcus aureus. Biomed Res Int. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuenyongviwat V, Ingviya N, Pathaburee P, et al. Inhibitory effects of vancomycin and fosfomycin on methicillin-resistant Staphylococcus aureus from antibiotic-impregnated articulating cement spacers. Bone Joint Res. 2017;6:132–136. doi: 10.1302/2046-3758.63.2000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouda M. [In vitro effects of combinations of antibiotics against highly-fosfomycin-resistant, methicillin-resistant Staphylococcus aureus. With special reference to efficacies of combinations of imipenem/cilastatin and cephems]. Jpn J Antibiot. 1993;46:142–153. Japanese. [PubMed] [Google Scholar]
- 17.Teng Z, Li M, Shi D, et al. Synergistic interactions of cryptotanshinone and aminoglycoside antibiotics against Staphylococcus aureus in vitro. J Glob Antimicrob Resist. 2018;13:264–265. doi: 10.1016/j.jgar.2018.05.013 [DOI] [PubMed] [Google Scholar]
- 18.Feng H, Xiang H, Zhang J, et al. Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cryptotanshinone. J Biomed Biotechnol. 2009;2009:617509. doi: 10.1155/2009/617509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Z, Ma Y, Chen C, et al. Prevalence of fosfomycin resistance and mutations in murA, glpT, and uhpT in methicillin-resistant Staphylococcus aureus strains isolated from blood and cerebrospinal fluid samples. Front Microbiol. 2015;6:1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin JH, Kim MN, Jang SJ, et al. Detection of amphotericin B resistance in Candida haemulonii and closely related species by use of the Etest, Vitek-2 yeast susceptibility system, and CLSI and EUCAST broth microdilution methods. J Clin Microbiol. 2012;50:1852–1855. doi: 10.1128/JCM.06440-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fratini F, Mancini S, Turchi B, et al. A novel interpretation of the fractional inhibitory concentration index: the case origanum vulgare L. and Leptospermum scoparium J. R. et G. Forst essential oils against Staphylococcus aureus strains. Microbiol Res. 2017;195:11–17. doi: 10.1016/j.micres.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 22.Noel A, Attwood M, Bowker K, et al. The pharmacodynamics of fosfomycin against Staphylococcus aureus studied in an in vitro model of infection. Int J Antimicrob Agents. 2020;56(1):105985. doi: 10.1016/j.ijantimicag.2020.105985 [DOI] [PubMed] [Google Scholar]
- 23.Shi J, Mao NF, Wang L, et al. Efficacy of combined vancomycin and fosfomycin against methicillin-resistant Staphylococcus aureus in biofilms in vivo. PLoS One. 2014;9:e113133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Z, Liu Y, Chen C, et al. Characterization of fosfomycin resistance gene, fosB, in methicillin-resistant Staphylococcus aureus isolates. PLoS One. 2016;11:e0154829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito R, Tomich AD, McElheny CL, et al. Inhibition of fosfomycin resistance protein FosA by phosphonoformate (Foscarnet) in multidrug-resistant gram-negative pathogens. Antimicrob Agents Chemother. 2017;61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etienne J, Gerbaud G, Fleurette J, et al. Characterization of staphylococcal plasmids hybridizing with the fosfomycin resistance gene fosB. FEMS Microbiol Lett. 1991;68:119–122. doi: 10.1111/j.1574-6968.1991.tb04580.x [DOI] [PubMed] [Google Scholar]
- 27.DiCicco M, Weese S, Neethirajan S, et al. Fosfomycin susceptibility of canine methicillin-resistant Staphylococcus pseudintermedius isolates. Res Vet Sci. 2014;96:251–253. doi: 10.1016/j.rvsc.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 28.Takahata S, Ida T, Hiraishi T, et al. Molecular mechanisms of fosfomycin resistance in clinical isolates of Escherichia coli. Int J Antimicrob Agents. 2010;35:333–337. doi: 10.1016/j.ijantimicag.2009.11.011 [DOI] [PubMed] [Google Scholar]
- 29.Kahan FM, Kahan JS, Cassidy PJ, et al. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci. 1974;235:364–386. [DOI] [PubMed] [Google Scholar]
- 30.Roberts AA, Sharma SV, Strankman AW, et al. Mechanistic studies of FosB: a divalent-metal-dependent bacillithiol-S-transferase that mediates fosfomycin resistance in Staphylococcus aureus. Biochem J. 2013;451:69–79. doi: 10.1042/BJ20121541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fillgrove KL, Pakhomova S, Newcomer ME, et al. Mechanistic diversity of fosfomycin resistance in pathogenic microorganisms. J Am Chem Soc. 2003;125:15730–15731. doi: 10.1021/ja039307z [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Li L, Liu Y, et al. In vitro activity and post-antibiotic effects of linezolid in combination with fosfomycin against clinical isolates of. Infect Drug Resist. 2018;11:2107–2115. doi: 10.2147/IDR.S175978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gobernado M. [Fosfomycin]. Revista espanola de quimioterapia. 2003;16:15–40. [PubMed] [Google Scholar]
- 34.Cha JD, Lee JH, Choi KM, et al. Synergistic effect between cryptotanshinone and antibiotics against clinic methicillin and vancomycin-resistant Staphylococcus aureus. Evid Based Complement Alternat Med. 2014;2014:1–16. doi: 10.1155/2014/450572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Liu L, Luo Y, et al. Cryptotanshinone activates p38/JNK and inhibits Erk1/2 leading to caspase-independent cell death in tumor cells. Cancer Prev Res. 2012;5:778–787. doi: 10.1158/1940-6207.CAPR-11-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]