Abstract
Background: Coronavirus disease 2019 (COVID-19) is an emerging infectious disease that has spread worldwide.
Methods: This was a retrospective case series involving 218 patients admitted to three tertiary hospitals in the Loudi, Shaoyang, and Xiangtan areas of China from January 21 to June 27, 2020, who were confirmed by RT-PCR to have SARS-CoV-2. The patients' clinical characteristics, laboratory results, treatments, and prognoses based on clinical classification were recorded. Poor outcome was defined as admission to an ICU, the use of mechanical ventilation, or death.
Results: The patients were classified into four clinical groups based on disease severity, namely mild (10/218, 5%), moderate (146/218, 67%), severe (24/218, 11%), or critical (14/218, 6%); 24 (11%) asymptomatic cases were also included in the study. The most common symptoms were self-reported cough (162/218, 74%), fever (145/218, 67%), sputum production (99/218, 45%), and fatigue (77/218, 35%). Among the 218 patients, 192 (88%) received lopinavir/ritonavir and interferon-alpha inhalation, and 196 (90%) patients received traditional Chinese medicine. Among the severe and critical patients, 25 (11%) were admitted to an ICU with or without mechanical ventilation, and one patient died. The presence of diabetes [relative risk (RR), 3.0; 95% CI, 1.3–6.8; p = 0.007) or other comorbidities (RR, 5.9; 95% CI, 1.9–17.8; p = 0.002) was independently associated with poor outcome. To date, 20 (9%) patients have retested positive for SARS-CoV-2 RNA after recovering and being discharged.
Conclusion: The majority of patients in this case series were clinically classified as having moderate COVID-19. Older patients tended to present with greater levels of clinical severity. The prognosis for patients who were elderly or had diabetes or other chronic comorbidities was relatively poor.
Keywords: COVID-19, clinical classification, clinical characteristics, treatment, prognosis
Introduction
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and has rapidly spread across the world since first emerging in December 2019 (1). By April 17, 2020, COVID-19 had been discovered in 212 countries or territories, affecting 2,074,529 individuals and causing 139,378 deaths (2). The pandemic continues to escalate rapidly (3, 4). Typical symptoms are fever, cough, fatigue, and sputum production (5–7). However, a few patients with SARS-CoV-2 develop severe pneumonia, pulmonary edema, acute respiratory distress syndrome (ARDS), multiple organ failure, or even death (8–10).
In this retrospective case series, 218 patients testing positive for SARS-CoV-2 were clinically classified (mild, moderate, severe, or critical) according to the guidelines of the Diagnosis and Treatment Protocol for COVID-19 (trial version 7) issued by the National Health Commission of the People's Republic of China (11). Asymptomatic patients, who acquire and can transmit the coronavirus that causes COVID-19 (12, 13), were also included in this study.
These clinical classifications of COVID-19 are characterized by different clinical features and provide an objective basis for treatment and prognosis. To date, there have been no studies reporting COVID-19 treatment and outcomes based on clinical classification. Here, we comprehensively explored the clinical features, treatment, and prognosis of 218 confirmed SARS-CoV-2-infected patients in three top-tier hospitals in the Hunan province of China.
Materials and Methods
Study Design and Participants
This multicenter, retrospective, and observational study was conducted on COVID-19 patients who were diagnosed in the Hunan province of China. Clinicians collected the patients who met the study inclusion criteria across three tertiary hospitals in the cities of Shaoyang, Loudi, and Xiangtan. The authors of this paper include the physicians who either supervised patient care or directly provided patient care for all of the patients included in the study to ensure complete follow-through for all cases.
We retrospectively analyzed COVID-19 patients who had been diagnosed during the period of January 21 to June 27, 2020, according to the WHO interim guidance. Real-time, reverse-transcription polymerase chain reaction (RT-PCR) tests for SARS-CoV-2 nucleic acids were performed on nasopharyngeal swabs from suspected patients to confirm the diagnosis. A confirmed case of COVID-19 was defined as having a positive result from the RT-PCR assay of a nasopharyngeal swab. Only laboratory-confirmed cases were included in the analysis. Suspected patients showing negative results after multiple tests during hospitalization were excluded. Where the typical symptoms, signs, and imaging manifestations were present, combined with a PaO2/FiO2 ratio <300 mmHg [based on the Berlin definition (14)], the patients were diagnosed as having ARDS. This study was approved by the ethics committee of each participating hospital. Written informed consent was obtained from all patients.
Clinical Classification
In this retrospective study, the whole disease course was examined for each patient. The clinical classification of the patients was based on the clinical conditions present during the most severe stage of COVID-19 based on the guidelines outlined in the Diagnosis and treatment protocol for COVID-19 (trial version 7) released by the National Health Commission of the People's Republic of China on March 3, 2020 (http://www.nhc.gov.cn/xcs/zhengcwj/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml) (11). According to their clinical symptoms, signs, and chest imaging manifestations, the patients were classified as being mild, moderate, severe, or critical COVID-19 cases (see Supplementary Material for further details).
Data Collection
Data on the clinical characteristics, treatment, and prognosis of the 218 confirmed COVID-19 patients were collected at Shaoyang Central Hospital, Loudi Central Hospital, and Xiangtan Central Hospital in the Hunan province. The information of interest included age, sex, exposure history, smoking history, chronic diseases (including diabetes), symptoms from onset to hospital admission, laboratory tests on admission, coexisting infections, treatment, and living status. The data regarding the PaO2/FiO2 ratios were analyzed when the patients were monitored in the ICU.
Treatment
The patient treatment venue was determined based on the severity of each patient's disease according to the Diagnosis and treatment protocol for COVID-19 (11). Suspected and confirmed cases were isolated and treated at designated hospitals with effective isolation, protection, and prevention conditions. Suspected cases were treated in isolation or together in a single room. Confirmed cases were treated in isolation or together in a single room. In the absence of pathogen-specific interventions, patient management largely depended on supportive treatment.
Most patients were provided with effective oxygen therapy, including a nasal catheter, mask oxygenation, and nasal high-flow oxygen therapy. Lopinavir/ritonavir, interferon-alpha inhalation, and arbidol were used as antiviral therapies. Moxifloxacin and other antibiotics were used to fight against bacterial infections where present. Glucocorticoids were used for short periods when patients showed rapidly progressive deterioration.
Patients who met the following criteria were admitted to the ICU for comprehensive treatment and care at an early stage: (1) severe cases with respiratory distress (≥30 breaths/min) and chest imaging showing >50% of lung area with obvious lesion progression within 24–48 h; and (2) all critical cases.
In addition, patients were treated with traditional Chinese medicine (Qingfei Paidu decoction, Lianhuaqingwen capsules, Huoxiangzhengqi liquid, and/or Xuebijing injection) according to the national guidelines. The full treatment protocol used for the COVID-19 patients is described in detail in the Supplementary Materials.
Discharge
When a patient's body temperature had returned to normal for more than 3 days, respiratory symptoms were significantly improved, pulmonary imaging showed obvious absorption of inflammation, and two consecutive SARS-CoV-2 nucleic acid tests were negative using respiratory tract samples (sampling interval of at least 24 h), he or she was discharged from the hospital. After discharge, the patients were required to quarantine and monitor their health for 14 days and requested to come back to the hospital for follow-up exams every 2–4 weeks.
Prognosis
All patients were traced from hospital admission to presenting prognosis. The primary outcome was “cured and discharged,” and a poor outcome was defined as admission to an ICU, the use of mechanical ventilation, or death. This analysis method was referenced from other retrospective studies on viral pneumonia, such as SARS (15, 16). Time to discharge, time to death, and time to a poor outcome were analyzed using survival analysis (details in Statistical Analysis) tracing all patients from hospital admission to presenting prognosis.
Statistical Analysis
Data are presented as the mean ± standard deviation (SD) or median ± interquartile range (IQR) for continuous variables and as a number (%) for categorical variables. Differences in measurement data among the asymptomatic, mild, moderate, severe, and critical cases were compared with analysis of variance using the least significant difference post-hoc test. The Chi-square test and Fisher's exact test were used for categorical variables. Kaplan–Meier plots were used to analyze the survival data. Differences among groups of time-to-event data were determined using the Cox proportional hazards model, with graphical and statistical checks for the proportionality of hazards. Given that there were only 25 patients with poor outcomes in our study, we considered only three binary variables in the multiple regression model as a priori hypotheses: age of 60 years or older, diabetes, and other comorbidities. We used SPSS (version 26.0) for all analyses. For all analyses, p < 0.05 was considered statistically significant.
Results
Demographics
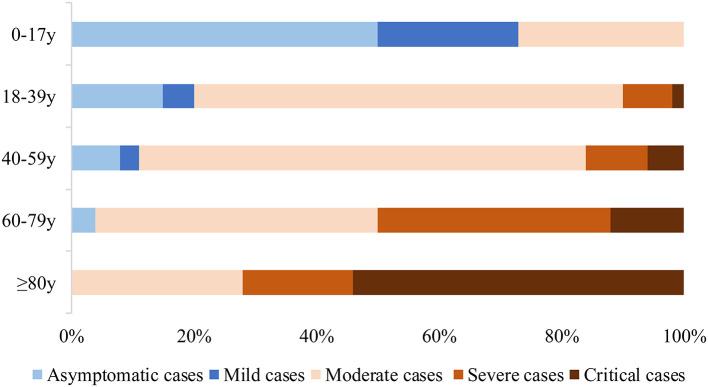
A total of 218 patients were confirmed during the study period. The patients' demographic details and comorbidities are listed in Table 1. Age was correlated with the clinical classification of COVID-19 severity (Figure 1). The median age of the patients was 43 years (IQR 32–52), with 14 (6%) patients <18 years of age and 6 (3%) ≥80 years old; 122 (56%) were male. A total of 100 patients (46%) had known exposure to COVID-19, and 111 patients (51%) had recently traveled to Wuhan, China. There were four (2%) patients who had neither traveled recently to Wuhan nor had known exposure to confirmed COVID-19 patients who were nevertheless diagnosed with COVID-19, and the route of transmission in these cases might have been the use of public transportation. As for their personal medical history, 23 (11%) patients had a history of smoking, 38 (17%) had cardiovascular disease, 27 (12%) had diabetes, 14 (6%) had chronic pulmonary disease, 13 (6%) had liver disease, 10 (5%) had nutritional deficiency diseases, six (3%) had cerebrovascular disease, four (2%) had chronic renal diseases, two (1%) had cancer, and two (1%) had autoimmune diseases.
Table 1.
Demographics and baseline characteristics of patients with COVID-19.
| All patient (n = 218) | Asymptomatic cases (n = 24) | Mild cases (n = 10) | Moderate cases (n = 146) | Severe cases (n = 24) | Critical cases (n = 14) | P-value | |
|---|---|---|---|---|---|---|---|
| Age, years | 42.9 (32.0–52.3) | 32.0 (16.3–44.8)c, d, e | 23.6 (11.8–34.3)c, d, e | 42.3 (32.0–50.0)a, b, d, e | 55.9 (46.3–67.0)a, b, c | 59.5 (42.3–76.5)a, b, c | 0.000* |
| Age range, years | |||||||
| 0–17 | 14 (6%) | 6 (25%) | 4 (40%) | 4 (3%) | 0 | 0 | 0.000** |
| 18–39 | 82 (38%) | 11 (46%) | 5 (50%) | 60 (41%) | 4 (17%) | 2 (14%) | |
| 40–59 | 86 (39%) | 6 (25%) | 1 (10%) | 65 (45%) | 9 (37%) | 5 (36%) | |
| 60–79 | 30 (14%) | 1 (4%) | 0 | 15 (10%) | 10 (42%) | 4 (29%) | |
| ≥80 | 6 (3%) | 0 | 0 | 2 (1%) | 1 (4%) | 3 (21%) | |
| Sex | |||||||
| Male | 122 (56%) | 16 (67%) | 6 (60%) | 77 (53%) | 14 (58%) | 9 (64%) | 0.691 |
| Female | 96 (44%) | 8 (33%) | 4 (40%) | 69 (47%) | 10 (42%) | 5 (36%) | |
| Exposure | |||||||
| Exposure to Wuhan | 111 (51%) | 12 (50%) | 3 (30%) | 76 (52%) | 13 (54%) | 7 (50%) | 0.768 |
| Exposure to patients† | 100 (46%) | 18 (75%) | 7 (70%) | 59 (40%) | 8 (33%) | 8 (57%) | 0.006** |
| Use of public transportation‡ | 4 (2 %) | 0 | 0 (%) | 3 (2%) | 0 | 1 (7%) | 0.535 |
| Current smoking | 23 (11%) | 2 (8%) | 2 (20%) | 14 (10%) | 3 (13%) | 2 (14%) | 0.902 |
| Chronic medical illness | |||||||
| Cardiovascular disease | 38 (17%) | 3 (13%) | 0 | 17 (12%) | 13 (54%) | 5 (36%) | 0.000** |
| Diabetes | 27 (12%) | 3 (13%) | 0 | 12 (8%) | 10 (42%) | 2 (14%) | 0.001** |
| Chronic pulmonary disease | 14 (6%) | 0 | 0 | 5 (3%) | 4 (17%) | 5 (36%) | 0.000** |
| Liver disease | 13 (6%) | 1 (4%) | 0 | 10 (7%) | 2 (8%) | 0 | 0.909 |
| Malnutrition§ | 10 (5%) | 0 | 1 (10%) | 6 (4%) | 1 (4%) | 2 (14%) | 0.193 |
| Cerebrovascular disease | 6 (3%) | 0 | 0 | 2 (1%) | 1 (4%) | 3 (21%) | 0.014** |
| Chronic renal diseases | 4 (2%) | 0 | 0 | 1 (1%) | 1 (4%) | 2 (14%) | 0.031** |
| Cancer | 2 (1%) | 0 | 0 | 2 (1%) | 0 | 0 | 1.000 |
| Autoimmune disease | 2 (1%) | 0 | 0 | 1 (1%) | 0 | 1 (7%) | 0.256 |
Data are median (IQR), n (%), or mean (SD), unless otherwise specified.
ANOVA was used for group comparisons with LSD for post-hoc tests.
avs. Asymptomatic cases (p < 0.05), bvs. Mild cases (p < 0.05), cvs. Moderate cases (p < 0.05), dvs. Severe cases (p < 0.05), evs. Critical cases (p < 0.05).
Statistical analysis was performed with the chi-square test or Fisher's exact test.
Patients who have confirmed SARS-CoV-2 infection or are highly suspected of being infected.
Without exposure to Wuhan and diagnosed patients.
In this cohort, 3 patients suffer from undernutrition and 7 are overweight.
Figure 1.
Clinical classification (including asymptomatic cases) and age distribution of patients with COVID-19.
Disease Course
The patients' COVID-19 onset symptoms are shown in Table 2. Common clinical features included cough (162/218, 74%), fever (145/218, 67%), sputum production (99/218, 45%), and fatigue (77/218, 35%). Only 3% (6/218) of patients had nasal congestion and rhinorrhea. On admission, 39% (86/218) of patients had a recorded temperature of ≥38.1°C. No lung lesions were identified in the computed tomography (CT) scans of asymptomatic and mild cases. In moderate cases, the main imaging changes were ground-glass opacities and local patchy shadowing. In severe cases, the principal abnormality visible on CT scans was diffuse patchy shadowing. In critical cases, pulmonary consolidation and diffuse patchy shadowing were more common (Table 3). Several of the characteristic chest CT features of COVID-19 observed in the moderate, severe, and critical cases are shown in Figure 2. Although there was a notable degree of variability in the pattern of the infiltrates (ground-glass, local, diffuse, pulmonary consolidation), most patients had ground-glass opacities.
Table 2.
Symptoms (at the time of admission), comorbidities, treatments, and prognosis of patients with COVID-19.
| All patients (n = 218) | Asymptomatic cases (n = 24) | Mild cases (n = 10) | Moderate cases (n = 146) | Severe cases (n = 24) | Critical cases (n = 14) | P-value | |
|---|---|---|---|---|---|---|---|
| Symptoms† | |||||||
| Fever | 145 (67%) | 0 | 2 (20%) | 108 (74%) | 23 (96%) | 12 (86%) | 0.000** |
| <37.3°C | 73 (33%) | 24 (100%) | 8 (80%) | 38 (26%) | 1 (4%) | 2 (14%) | |
| 37.3–38.0°C | 59 (27%) | 0 | 2 (20%) | 45 (31%) | 8 (33%) | 4 (29%) | |
| 38.1–39°C | 70 (32%) | 0 | 0 | 55 (38%) | 11 (46%) | 4 (29%) | |
| >39°C | 16 (7%) | 0 | 0 | 8 (5%) | 4 (17%) | 4 (29%) | |
| Cough | 162 (74%) | 0 | 10 (100%) | 117 (80%) | 21 (88%) | 14 (100%) | 0.000** |
| Sputum production | 99 (45%) | 0 | 4 (40%) | 68 (47%) | 15 (63%) | 12 (86%) | 0.018** |
| Fatigue | 77 (35%) | 0 | 1 (10%) | 55 (38%) | 12 (50%) | 9 (64%) | 0.006** |
| Shortness of breath | 42 (19%) | 0 | 0 | 16 (11%) | 16 (67%) | 10 (71%) | 0.000** |
| Myalgia | 41 (19%) | 0 | 0 | 32 (22%) | 6 (25%) | 3 (21%) | 0.407 |
| Chills | 39 (18%) | 0 | 0 | 23 (16%) | 9 (38%) | 7 (50%) | 0.001** |
| Headache | 28 (13%) | 0 | 1 (10%) | 18 (12%) | 4 (17%) | 5 (36%) | 0.125 |
| Sore throat | 25 (11%) | 0 | 1 (10%) | 20 (14%) | 2 (8%) | 2 (14%) | 0.942 |
| Diarrhea | 16 (7%) | 0 | 1 (10%) | 11 (8%) | 3 (13%) | 1 (7%) | 0.722 |
| Nasal congestion and rhinorrhea | 6 (3%) | 0 | 0 | 6 (4%) | 0 | 0 | 1.000 |
| Complications | |||||||
| ARDS | 14 (6%) | 0 | 0 | 0 | 0 | 14 (100%) | 0.000** |
| Liver dysfunction | 40 (18%) | 0 | 1 (10%) | 23 (16%) | 9 (38%) | 7 (50%) | 0.000** |
| Acute kidney injury | 10 (5%) | 0 | 0 | 4 (3%) | 1 (4%) | 5 (36%) | 0.001** |
| Acquired pneumonia | 20 (9%) | 0 | 0 | 3 (2%) | 4 (17%) | 13 (93%) | 0.000** |
| Septic shock | 4 (2%) | 0 | 0 | 0 | 0 | 4 (29%) | 0.000** |
| Treatment | |||||||
| Oxygen treatment‡ | 156 (72%) | 0 | 3 (30%) | 115 (79%) | 24 (100%) | 14 (100%) | 0.000** |
| Mechanical ventilation | 16 (7%) | 0 | 0 | 0 | 2 (8%) | 14 (100%) | 0.000** |
| Non-invasive | 9 (4%) | 0 | 0 | 0 | 2 (8%) | 7 (50%) | |
| Invasive | 7 (3%) | 0 | 0 | 0 | 0 | 7 (50%) | |
| Prone position ventilation | 14 (6%) | 0 | 0 | 0 | 0 | 14 (100%) | 0.000** |
| Renal replacement therapy | 5 (2%) | 0 | 0 | 11 (46%) | 3 (21%) | 0.000** | |
| Convalescent plasma | 4 (2%) | 0 | 0 | 0 | 0 | 4 (17%) | 0.000** |
| Stem cell treatment | 3 (1%) | 0 | 0 | 0 | 0 | 3 (21%) | 0.000** |
| Lopinavir/ritonavir | 192 (88%) | 19 (79%) | 7 (70%) | 133 (91%) | 20 (83%) | 13 (93%) | 0.172 |
| Interferon alpha inhalation | 192 (88%) | 18 (75%) | 7 (70%) | 131 (90%) | 23 (96%) | 13 (93%) | 0.059 |
| Arbidol | 126 (58%) | 9 (38%) | 3 (30%) | 83 (57%) | 18 (75%) | 13 (93%) | 0.001** |
| Antibiotics | 115 (53%) | 6 (25%) | 1 (10%) | 71 (49%) | 23 (96%) | 14 (100%) | 0.000** |
| Chinese medicine§ | 196 (90%) | 21 (88%) | 9 (90%) | 133 (91%) | 20 (83%) | 13 (93%) | 0.714 |
| Qingfei Paidu decoction | 114 (52%) | 17 (71%) | 6 (60%) | 75 (51%) | 9 (38%) | 7 (50%) | 0.220 |
| Lianhuaqingwen capsule | 66 (30%) | 3 (13%) | 2 (20%) | 47 (32%) | 10 (42%) | 4 (29%) | 0.203 |
| Huoxiangzhengqi liquid | 6 (3%) | 0 | 0 | 6 (4%) | 0 | 0 | 0.822 |
| Xuebijing injection | 26 (12%) | 0 | 0 | 11 (8%) | 7 (29%) | 8 (33%) | 0.000** |
| Corticosteroid | 47 (22%) | 0 | 0 | 17 (12%) | 18 (75%) | 12 (86%) | 0.000** |
| Gamma globulin | 33 (15%) | 0 | 0 | 13 (9%) | 11 (46%) | 9 (64%) | 0.000** |
| Prognosis | |||||||
| Discharge from hospital | 217 (99.5%) | 24 (100%) | 10 (100%) | 146 (100%) | 24 (100%) | 13 (93%) | 0.000** |
| Death | 1 (0.5%) | 0 | 0 | 0 | 0 | 1 (7%) | |
| The hospitalization days of discharged patients | 12.2 ± 6.2 | 7.1 ± 2.8c, d, e | 8.6 ± 5.0d, e | 12.1 ± 5.8a, d, e | 16.1 ± 5.5a, b, c, e | 20.5 ± 6.0a, b, c, d | 0.000* |
Data are mean (SD) or n (%).
ANOVA was used for group comparisons with LSD for post-hoc tests.
avs. Asymptomatic cases (p < 0.05), bvs. Mild cases (p < 0.05), cvs. Moderate cases (p < 0.05), dvs. Severe cases (p < 0.05), evs. Critical cases (p < 0.05).
Statistical analysis was performed with the chi-square test or Fisher's exact test.
In part of this series, asymptomatic cases were not included in the statistics.
Oxygen therapy includes nasal catheter, mask oxygenation and nasal high-flow oxygen therapy.
A small number of patients were given two or more kind of Chinese medicine.
Table 3.
Chest CT/X-ray features of patients with COVID-19 at the most severe stage.
| Distribution of pulmonary lesions | All patients (n = 218) | Asymptomatic cases (n = 24) | Mild cases (n = 10) | Moderate cases (n = 146) | Severe cases (n = 24) | Critical cases (n = 14) |
|---|---|---|---|---|---|---|
| No lesion | 37 (17%) | 24 (100%) | 10 (100%) | 3 (2%) | 0 | 0 |
| Ground-glass opacities | 65 (30%) | 0 | 0 | 65 (45%) | 0 | 0 |
| Local patchy shadowing | 77 (35%) | 0 | 0 | 76 (52%) | 1 (4%) | 0 |
| Diffuse patchy shadowing | 30 (14%) | 0 | 0 | 2 (1%) | 21 (88%) | 7 (50%) |
| Pulmonary consolidation | 9 (4%) | 0 | 0 | 0 | 2 (8%) | 7 (50%) |
A total of 205- chest CT cases included in this table. Other 13 patients had chest X-ray.
Figure 2.
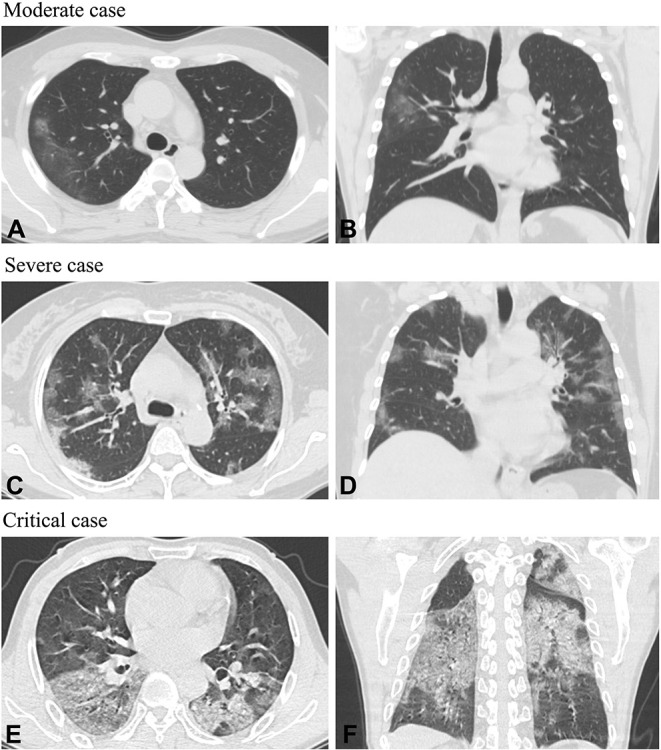
Axial planes and coronal chest CT scans in patients with COVID-19. Moderate case: (A,B) Chest CT images of a 41-year-old man showed a ground-glass lesion in the right lobe on the 3rd day following a fever. Severe case: (C,D) Chest CT images of a 55-year-old woman showed bilateral multifocal ground-glass opacities on the 8th day after having chills, cough, and expectoration. Critical case: (E,F) Chest CT images of a 61-year-old man showed diffuse patchy shadowing and mixed consolidation on the 13th day after having cough, expectoration, and fever.
Laboratory Indices
Laboratory indices on admission are shown in Table 4. With increasing grades of disease severity based on clinical classification, the proportion of lymphocytes gradually decreased (p = 0.001). Elevated D-dimer levels were significantly associated with disease severity (p < 0.000), with high D-dimer levels in the severe (0.76 ± 1.22 μg/mL) and critical (1.76 ± 3.34 μg/mL) groups. With increasing grades of disease severity, the level of lactate dehydrogenase gradually increased (p = 0.000).
Table 4.
Initial laboratory results of patients with COVID-19.
| All patients (n = 218) | Asymptomatic cases (n = 24) | Mild cases (n = 10) | Moderate cases (n = 146) | Severe cases (n = 24) | Critical cases (n = 14) | P-value | |
|---|---|---|---|---|---|---|---|
| Hematologic | |||||||
| Leucocytes (×109/L; reference range 3.69–9.16) | 5.92 ± 3.23 | 6.22 ± 2.06 | 5.19 ± 1.40 | 5.72 ± 3.18 | 6.39 ± 3.89 | 7.24 ± 4.30 | 0.407 |
| Lymphocytes (×109/L; reference range 0.8–4.0) | 1.25 ± 0.61 | 1.68 ± 0.79d, e | 1.95 ± 0.67d, e | 1.26 ± 0.55d, e | 0.90 ± 0.40a, b, c | 0.76 ± 0.33a, b, c | 0.001* |
| Coagulation function | |||||||
| APTT (s; reference range 23.0–40.0) | 33.64 ± 13.51 | 36.49 ± 13.70e | 34.86 ± 7.76e | 31.64 ± 7.45e | 32.54 ± 4.70e | 50.37 ± 38.1a, b, c, d | 0.001* |
| D-dimer (μg/ml; reference range 0.0–0.7) | 0.45 ± 1.06 | 0.31 ± 0.18e | 0.212 ± 0.083e | 0.29 ± 0.22e | 0.76 ± 1.22e | 1.76 ± 3.34a, b, c, d | 0.000* |
| Biochemistry | |||||||
| Alanine aminotransferase (U/L; reference range 0–40.0) | 27.88 ± 21.62 | 23.54 ± 16.75 | 18.99 ± 6.92 | 27.62 ± 20.16 | 36.90 ± 34.88 | 24.76 ± 11.76 | 0.173 |
| Aspartate aminotransferase (U/L; reference range 0–40.0) | 27.75 ± 13.55 | 19.36 ± 7.77c, d, e | 22.80 ± 6.58d | 27.37 ± 13.11a, d | 34.08 ± 17.86a, b, c | 33.44 ± 11.23a | 0.004* |
| Serum creatinine (μmol/L; reference range 53.0–115·0) | 72.39 ± 56.64 | 65.81 ± 21.27 | 64.83 ± 10.07 | 67.47 ± 37.33 | 102.47 ± 137.40 | 84.66 ± 21.84 | 0.082 |
| Serum urea (mmol/L; reference range 2.86–7.14) | 4.21 ± 3.02 | 3.89 ± 1.39 | 3.79 ± 0.48 | 4.04 ± 3.40 | 4.95 ± 2.09 | 5.27 ± 1.75 | 0.440 |
| Lactate dehydrogenase (U/L; reference range 114.0–240.0) | 236.77 ± 216.84 | 167.47 ± 47.54e | 172.71 ± 41.18e | 212.07 ± 76.62e | 292.90 ± 85.76e | 510.06 ± 733.24a, b, c, d | 0.000* |
| C-reactive protein (mg/L; reference range 0–3.0) | 18.57 ± 33.82 | 1.64 ± 2.34d, e | 0.95 ± 0.73d, e | 13.46 ± 23.58d, e | 38.70 ± 51.53a, b, c, e | 66.01 ± 54.34a, b, c, d | 0.000* |
| Erythrocyte sedimentation rate (mm/h; reference range 0–20.0) | 41.09 ± 31.72 | 14.00 ± 19.76c, d, e | 14.60 ± 21.9d, e | 43.03 ± 30.24a | 50.56 ± 31.69a, b | 61.00 ± 37.98a, b | 0.000* |
| PaO2/FiO2 (mm Hg; reference range 400–500)† | NA | NA | NA | NA | NA | 176 ± 49 | ·· |
Data are n (%), n/N (%), mean (SD), and median (IQR).
ANOVA was used for group comparisons with LSD for post-hoc tests.
avs. Asymptomatic cases (p < 0.05), bvs. Mild cases (p < 0.05), cvs. Moderate cases (p < 0.05), dvs. Severe cases (p < 0.05), evs. Critical cases (p < 0.05).
We analyzed the data when patients were monitored in ICU.
Treatment
The chief method of patient management was through symptomatic treatment. Regardless of severity, the vast majority of patients received antiviral treatment. Several patients had bacterial infections and were also given antibiotics. In detail, among the 218 patients, 192 (88%) patients received lopinavir/ritonavir, 192 (88%) patients received interferon-alpha inhalation, 126 (58%) patients received arbidol, 115 (53%) patients received antibiotics, 47 (22%) patients received corticosteroids, 33 (15%) patients received gamma globulin, four (2%) patients received convalescent plasma, and three (1%) patients received umbilical cord mesenchymal stem cell treatment. For respiratory support, 156 (72%) patients were treated with oxygen treatment (including a nasal catheter, mask oxygenation, and/or nasal high-flow oxygen therapy), 16 (7%) with mechanical ventilation, and 14 (6%) with prone position ventilation. Five (2%) patients required renal replacement therapy. Most distinctive is that the majority of cases (196/218, 90%) received traditional Chinese medicine, which is a different treatment approach from that used in other countries. Among these Chinese medicines, the Qingfei Paidu decoction (114/218, 52%) and Lianhuaqingwen capsules (66/218, 30%) were the most frequently used. Huoxiangzhengqi liquid was used only in patients with gastrointestinal discomfort, while the Xuebijing injection was mainly used for severe and critical patients (Table 2).
Prognosis
There was one death in our cohort of 218 hospitalized COVID-19 patients. This patient had diabetes, hypertension, and severe obesity. As of March 14, most individuals (217/218 [99.5%]) had recovered and were discharged from the hospital. Among the patients who survived, the median hospital stay was 12.2 days (IQR 8–16 days). There were 25 (11%) patients who developed serious conditions during hospitalization, including pulmonary aggravation requiring oxygen ventilation or transfer to an ICU, and 13 patients did not receive steroids during the early stage of the disease but were treated with corticosteroids at a later stage. Nine (<1%) patients had rapid disease progression.
Among the whole cohort, 11% of patients (25/218) were admitted to the ICU and 7% (16/218) received mechanical ventilation. Of the 6% of patients (14/218) diagnosed with ARDS, all belonged to the critical group of cases. Among the 16 patients who received mechanical ventilation, one (6%) died, and the remaining 15 (94%) were discharged before March 14, 2020. Overall, 25 patients in our cohort met the criteria for a poor outcome (death or ICU admission with or without mechanical ventilation). The majority of these poor outcomes occurred within 10 days of hospitalization.
Table 5 shows summaries of the age, sex, clinical classification, and initial laboratory results of patients classified as having a poor prognosis. Univariate analysis of these data showed that advanced age, disease severity (based on clinical classification), an increased activated partial thromboplastin time (APTT), a higher erythrocyte sedimentation rate, and elevated levels of lactate dehydrogenase and C-reaction protein were significantly associated with poor outcome. Lymphopenia was also significantly associated with poor outcome.
Table 5.
Analysis of poor outcome and clinical features.
| Univariate analysis, mean (IQR) | |||
|---|---|---|---|
| Variable | No poor outcome (n = 193) | Poor outcome† (n = 25) | P-value |
| Age, y | 40.9 (30.0–50.0) | 58.4 (49.0–67.5) | 0.000 |
| Men, % | 107 (55%) | 15 (60%) | 0.831 |
| Clinical classification | 0.000 | ||
| Critical cases | 0 | 14 (56%) | ·· |
| Severe cases | 13 (7%) | 11 (44%) | ·· |
| Moderate cases | 146 (76%) | 0 | ·· |
| Mild cases | 10 (5%) | 0 | ·· |
| Asymptomatic cases | 24 (12%) | 0 | ·· |
| Leucocytes, × 109/L | 5.87 ± 3.19 | 6.34 ± 3.50 | 0.493 |
| Lymphocytes, × 109/L | 1.31 ± 0.61 | 0.80 ± 0.34 | 0.000 |
| APTT, s | 23.89 ± 16.71 | 39.17 ± 33.99 | 0.045 |
| ALT, U/L | 27.81 ± 22.57 | 28.40 ± 13.37 | 0.899 |
| AST, U/L | 27.12 ± 13.78 | 32.22 ± 11.02 | 0.078 |
| Scr, μmol/L | 71.78 ± 59.78 | 77.00 ± 21.26 | 0.679 |
| LDH, U/L | 211.08 ± 77.18 | 433.43 ± 574.54 | 0.000 |
| CRP, mg/L | 12.89 ± 24.69 | 58.84 ± 56.48 | 0.000 |
| ESR, mm/h | 38.51 ± 30.58 | 62.43 ± 34.03 | 0.001 |
|
Univariate analysis‡ Relative risk (95% CI) of poor outcome§ |
P-value | ||
| Age≥60 y | 3.6 (1.6–8.0) | 0.001 | |
| Diabetes | 5.9 (2.7–13.0) | 0.000 | |
| Other comorbid disease | 8.9 (3.0–26.0) | 0.000 | |
|
Multivariable analysis‡ Relative risk (95% CI) of poor outcome§ |
P-value | ||
| Age≥60 y | 1.9 (0.8–4.2) | 0.134 | |
| Diabetes | 3.0 (1.3–6.8) | 0.007 | |
| Other comorbid disease | 5.9 (1.9–17.8) | 0.002 | |
Data are median (IQR), n (%), or mean (SD).
APTT, Activated partial thromboplastin time; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; Scr, Serum creatinine; LDH, Lactate dehydrogenase; CRP, C-reaction protein; ESR, Erythrocyte sedimentation rate.
Defined as death or intensive care unit admission with or without mechanical ventilation.
Results are from Cox proportional hazards model.
Reference group is younger than 60 years, with no diabetes, and no other comorbid disease (chronic pulmonary disease, cardiovascular disease, chronic renal diseases, cerebrovascular disease, liver disease, cancer, malnutrition, or autoimmune disease).
Following univariate analysis (Table 5), the Cox proportional hazards model showed that the risk of a poor outcome was increased for those aged 60 years or older [relative risk (RR), 3.6; 95% CI, 1.6–8.0; p = 0.001]. The presence of any comorbid disease (other than diabetes) was found to increase the risk of a poor outcome (RR, 8.9; 95% CI, 3.0–26.0; p = 0.000), as was the presence of diabetes (RR, 5.9; 95% CI, 2.7–13.0; p = 0.000).
Multivariable Cox proportional hazards analysis was performed with the a priori hypothesis that age and comorbid diseases were independently associated with a poor outcome (Table 5). In the model including diabetes, other comorbid diseases, and an age ≥60 years, no significant association was found between advanced age and poor outcome (RR, 1.9; 95% CI, 0.8–4.2; p = 0.134). However, diabetes alone or with other diseases (RR, 3.0; 95% CI, 1.3–6.8; p = 0.007) and any comorbid diseases other than diabetes (cardiovascular disease, chronic pulmonary disease, and other chronic diseases; RR, 5.9; 95% CI, 1.9–17.8; p = 0.002) were independently associated with a poor outcome.
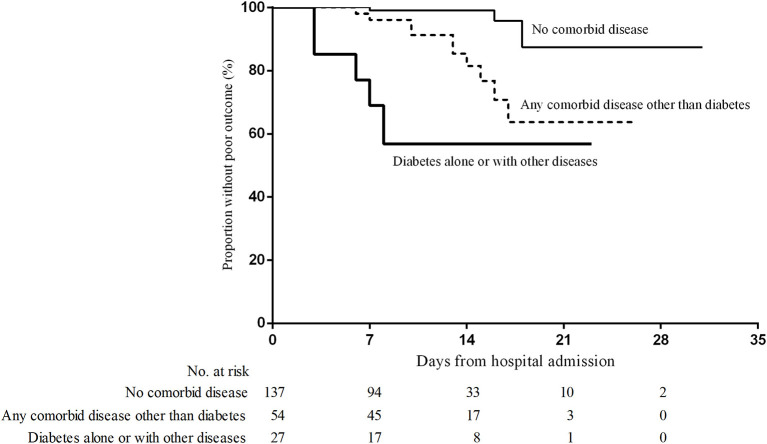
Despite age ≥60 years, diabetes, and other chronic diseases all being positively associated with a poor outcome, a comparison of the parameter estimates as well as the standard errors in the single and multivariable models indicated that collinearity was not apparent. The standard error for the age parameter was only marginally larger in the multivariable models than in the univariate regression model of age alone. Figure 3 shows the Kaplan–Meier survival curves for these three groups defined by the presence and absence of diabetes and other chronic comorbidities.
Figure 3.
Time from admission to poor outcome based on the presence of a comorbid disease. Poor outcome was defined as death or intensive care unit admission with or without mechanical ventilation.
Follow-Up
To date, 20 patients (20/218, 9%) have retested positive for SARS-CoV-2 RNA in nasopharyngeal swabs after having recovered and being discharged. Among these, 18 were classified into the moderate disease group and two were classified into the mild group upon their first admission. These patients showed relatively mild symptoms or were asymptomatic during follow-up. Thus, far, no critical or severe cases have retested positive after being discharged.
Discussion
Here, the characteristics of a cohort of 218 COVID-19 patients were summarized based on clinical classification of disease severity. This study reflects China's initial experience as the first country to respond to the virus. These findings have important clinical, infection control, and public health implications.
Most patients were clinically classified as moderate cases and had a good prognosis. The median age of the patients increased with the clinical classification of disease severity. Continued vigilance is, therefore, warranted for this high-risk group. The prognosis for the elderly and patients with diabetes and other chronic comorbidities was poor. We attempted to analyze the role of each comorbid disease in COVID-19; however, the number of patients was too small to perform statistical analyses when subgrouping each comorbid disease separately. As for diabetes, previous studies have shown that diabetes can affect the prognosis of patients with viral pneumonia and it should, therefore, be analyzed separately from other comorbid diseases (15). In the univariate analysis performed here and in a previous study by Chen et al. (17), diabetes was found to be associated with poor COVID-19 outcome. For this reason, we analyzed data from the diabetes patients separately from those with other comorbid diseases.
The hallmark laboratory findings of our study indicated that elevated levels of lactate dehydrogenase, C-reaction protein, and D-dimer, as well as an increased erythrocyte sedimentation rate, were positively correlated with clinical classification. Thus, these factors may be involved in disease progression and should receive further attention.
Asymptomatic cases comprised 11% of our cohort, suggesting that there may be a large number of asymptomatic patients in the general population who have not been tested and are transmitting the virus (18). In agreement with a report by Guan et al. who studied a cohort of 1,099 COVID-19 patients in China (6), the most common symptoms reported here were cough, fever, sputum production, and fatigue. Cough was the first symptom reported by many patients (74%). Only 3% of patients had nasal congestion and rhinorrhea, which may assist in differentiating this disease from the common cold.
Most patients had positive CT images. CT imaging has been observed to show multiple ground-glass opacities and even infiltration in both lungs as COVID-19 progresses (19, 20). In severe cases, pulmonary consolidation may be found (19). Chest CT is very important for COVID-19 diagnosis and patient management. Therefore, if medical conditions permit, it is recommended that patients undergo follow-up CT (20).
Currently, no standard treatment has been recommended for coronavirus infection besides careful supportive care (11, 21–23). Given the retrospective nature of our study, it was difficult to determine whether there was any therapeutic benefit conferred by the treatment regimens used for COVID-19, particularly the antibiotic and corticosteroid treatments (24). Treatment with lopinavir/ritonavir was previously reported to show potential in the treatment of SARS, and it can be supposed that this treatment may be beneficial in the treatment of COVID-19 (25).
Recent reports suggest that patients recover from COVID-19 when they receive combined traditional Chinese and Western medicine (23). In our cohort, 53% of patients received antibacterial agents, 88% received antiviral therapy, and 22% received methylprednisolone. Furthermore, 90% received Chinese medicine treatment. The favorable outcome observed for most cases in this cohort may support a COVID-19 treatment approach comprising a combination of traditional Chinese medicine and modern therapies (26). Notably, the most common Chinese medicines, the Lianhuaqingwen capsule and Qingfei Paidu decoction, have proven to be effective in viral pneumonia (27, 28), whereas the Xuebijing injection has been used for severe pneumonia for many years (29).
In agreement with Guan et al. (6), only 7% of the patients in our cohort required mechanical ventilation. Furthermore, we observed a low crude mortality rate (0.5%). This may be related to early nucleic acid detection in close contacts, as well as the relatively low incidence and adequate medical resources found in Hunan province (2). Cases with an exposure history tended to have a milder clinical classification, which may be owing to the vigilance of patients and healthcare workers in seeking early diagnosis and treatment.
Age, lymphocytes, lactate dehydrogenase, C-reaction protein, and erythrocyte sedimentation rate were all associated with the clinical classification. In our multivariable Cox proportional hazards model, diabetes and other chronic comorbid conditions were independently associated with poor prognosis, although an age of 60 years and older was not. Larger sample studies are needed to further elucidate which patients are at most risk of death or requiring admission to an ICU (8).
Currently, the RT-PCR is the standard test for the diagnosis of COVID-19 (11, 30). Notably, the infection appears to be transmitted during the incubation period of the index patient, in whom the illness is brief and non-specific (31). Asymptomatic cases in this study comprised 11% of the patients, all of whom were potential sources of SARS-CoV-2 infection (32, 33). To increase the positive rate of nucleic acid testing, we recommend that sputum and nasopharyngeal swabs be retained as much as possible (11). We further recommend that RT-PCR be repeated twice or more for suspected cases and close contacts as early as possible. This can facilitate early diagnosis, early isolation, and early treatment, and help to reduce the spread of disease (34).
The main strength of our study lies in the application of a new method for clinical classification. Zhang et al. studied the clinical and laboratory characteristics of 140 community-infected COVID-19 patients (35). They compared the data between only severe and non-severe groups, which were defined according to clinical severity. Here, the clinical classification of COVID-19 was performed by referencing the Diagnosis and treatment protocol for COVID-19 (trial version 7) (11), which is the latest version of the clinical practice guidelines and has stricter criteria. In this way, the classification and category distribution of groups were described comprehensively and systematically. Using this approach, we found that the moderate cases were the most common. In contrast, the proportions of severe and critical cases were relatively small. In the context of the high prevalence of SARS-CoV-2, the current clinical classification is particularly significant for the guidance of patient management and treatment. Further, our pilot results showed that most of the patients who retested positive for SARS-CoV-2 were from the moderate and mild groups. As such, our classification approach may have implications for clinical monitoring, treatment, and prognosis.
Our study had several limitations. One was the relatively low number of patients and critical cases included. A larger sample size with a greater proportion of critical cases is necessary for future investigations. Moreover, our study was not a randomized controlled trial but rather a retrospective study. Multiple drugs were used, making it difficult to evaluate the effectiveness of a single treatment. Hence, randomized, controlled, multicenter clinical trials are needed to confirm the present findings. As a retrospective observation, the main focus of this study was the nucleic acids present in swabs from the respiratory system. Testing stool nucleic acid is a valuable complementary tool to better understand COVID-19 progression and transmission. Future projects investigating the clinical longitudinal changes in COVID-19 should take the stool nucleic acid test into consideration.
In conclusion, despite the widespread implications of COVID-19, most patients have a favorable clinical prognosis. The COVID-19 epidemic has placed enormous strain on the health and economic status of nations. The excellent spirit of international collaboration among clinicians, researchers, and government agencies needs to continue in an effort to better control and treat COVID-19 (36–38).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shaoyang Central Hospital; Ethics Committee of Loudi Central Hospital; Ethics Committee of Xiangtan Central Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
XY and YZ contributed to the study concept and design of this study. XY, XH, DP, YF, ZF, DL, YX, SZ, FC, and WL contributed to the acquisition, analysis, interpretation of data, and the drafting of the paper. YZ contributed to the review and the revision of the manuscript. All authors give final approval to this manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all patients involved in the study.
Footnotes
Funding. This study was funded by the Program of Hunan Science and Technology Department (2020SK3011).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.00485/full#supplementary-material
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus Disease (COVID-2019) Situation Reports. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed April 18, 2020)
- 3.Thompson R. Pandemic potential of 2019-nCoV. Lancet Infect Dis. (2020) 20:280. 10.1016/S1473-3099(20)30068-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. (2020) 395:470–3. 10.1016/S0140-6736(20)30185-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, Zhu F, Liu X. J., Zhang, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z, Shi L, Wang Y, Zhang J, Huang LC, Zhang, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–2. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C, Chen X, Cai Y, Xia J, Zhou X. S., Xu, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. (2020) 180:1–11. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Health Commission of the People's Republic of China Diagnosis and Treatment Protocol for COVID-19 (trial version 7). Available online at: http://www.nhc.gov.cn/xcs/zhengcwj/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml (in Chinese) (accessed March 3, 2020).
- 12.Pan X, Chen D, Xia Y, Wu X, Li T, Ou X, et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. (2020) 20:410–1. 10.1016/S1473-3099(20)30114-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Tian S, Lou J, Chen Y. Familial cluster of COVID-19 infection from an asymptomatic. Crit Care. (2020) 24:119. 10.1186/s13054-020-2817-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. (2012) 307:2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 15.Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. (2003) 289:2801–9. 10.1001/jama.289.21.JOC30885 [DOI] [PubMed] [Google Scholar]
- 16.Gomersall CD, Joynt GM, Lam P, Li T, Yap F, Lam D, et al. Short-term outcome of critically ill patients with severe acute respiratory syndrome. Intensive Care Med. (2004) 30:381–7. 10.1007/s00134-003-2143-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Yang D, Cheng B, Chen J, Peng A, Yang C, et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. (2020) 43:1399–407. 10.2337/dc20-0660 [DOI] [PubMed] [Google Scholar]
- 18.Yan X, Han X, Fan Y, Fang Z, Long D, Zhu Y. Duration of SARS-CoV-2 viral RNA in asymptomatic carriers. Crit Care. (2020) 24:245. 10.1186/s13054-020-02952-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. (2020) 20:425–34. 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee EYP, Ng MY, Khong PL. COVID-19 pneumonia: what has CT taught us? Lancet Infect Dis. (2020) 20:384–5. 10.1016/S1473-3099(20)30134-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. (2020) 20:398–400. 10.1016/S1473-3099(20)30141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. (2020) 20:400–2. 10.1016/S1473-3099(20)30132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. (2020) 14:64–8. 10.5582/bst.2020.01030 [DOI] [PubMed] [Google Scholar]
- 24.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. (2020) 395:473–5. 10.1016/S0140-6736(20)30317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. (2004) 59:252–6. 10.1136/thorax.2003.012658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang DH, Wu KL, Zhang X, Deng SQ, Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J Integr Med. (2020) 18:152–8. 10.1016/j.joim.2020.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Runfeng L, Yunlong H, Jicheng H, Weiqi P, Qinhai M, Yongxia S, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. (2020) 156:104761. 10.1016/j.phrs.2020.104761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo E, Zhang D, Luo H, Liu B, Zhao K, Zhao Y, et al. Treatment efficacy analysis of traditional Chinese medicine for novel coronavirus pneumonia (COVID-19): an empirical study from Wuhan, Hubei Province, China. Chin Med. (2020) 15:34. 10.1186/s13020-020-00317-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song Y, Yao C, Yao Y, Han H, Zhao X, Yu K, et al. XueBiJing injection versus placebo for critically Ill patients with severe community-acquired pneumonia: a randomized controlled trial. Crit Care Med. (2019) 47:e735–43. 10.1097/CCM.0000000000003842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. (2020) 25:2000045 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. (2020) 395:514–23. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. (2020) 382:970–1. 10.1056/NEJMc2001468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. (2020) 323:1406–7. 10.1001/jama.2020.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellewell J, Abbott S, Gimma A, Bosse NI, Jarvis CI, Russell TW, et al. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. (2020) 8:e488–96. 10.1016/S2214-109X(20)30074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. (2020) 75:1730–41. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 36.Calisher C, Carroll D, Colwell R, Corley RB, Daszak P, Drosten C, et al. Statement in support of the scientists, public health professionals, and medical professionals of China combatting COVID-19. Lancet. (2020) 395:e42–3. 10.1016/S0140-6736(20)30418-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Z, Li S, Tian S, Li H, Kong LQ. Full spectrum of COVID-19 severity still being depicted. Lancet. (2020) 395:947–8. 10.1016/S0140-6736(20)30308-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heymann DL. Data sharing and outbreaks: best practice exemplified. Lancet. (2020) 395:469–70. 10.1016/S0140-6736(20)30184-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.