Abstract
In this study, we firstly reported the production and the structural characterization of a novel hetero-exopolysaccharide namely EPS-K2 from the extremely halophilc Halomonas smyrnensis K2. Results revealed that EPS-K2 was mainly composed of three monosaccharides including mannose (66.69%), glucose (19.54%) and galactose (13.77%). EPS-K2 showed high thermostability with a degradation temperature around 260 °C, which could make it a suitable candidate for application in thermal processes. Moreover, EPS-K2 showed attractive functional properties. In fact, it exhibited potent antioxidant activity in a dose-dependent manner as assessed in analyses of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging, iron chelating and DNA protection ability. Furthermore, EPS-K2 showed strong adhesion inhibition activity against Enterococcus faecalis (75.52 ± 3.35%) and Escherichia coli (61.95 ± 2.48%) at 1 g/l concentration, as well as a high biofilm disruption activity especially against E. coli (70.73 ± 2.78%), at 2 g/l concentration. According to its biotechnological properties, EPS-K2 could be exploited as functional ingredient in food, biomedicine, and pharmaceutical industries.
Keywords: Hetero-exopolysaccharide, Chemical characterization, Thermostability, In vitro biological properties
Introduction
Hypersaline environments are typical extreme habitats characterized by their high salt concentrations, where living conditions are very difficult (Poli et al. 2017, 2019). Therefore, to survive in these noxious environments, many halophiles produce exopolysaccharides (EPS) as protection and adaptation strategies. EPS are high-molecular-weight carbohydrate polymers and are mainly composed by monosaccharides and their derivatives. They are secreted into the external environment to maintain the osmotic environment within the microbial cell and to colonize the bacteria to protect them and increase their survival instincts (Insulkar et al. 2018). During the last decades, EPS from halophilic bacteria have been attracting substantial interest due to their structural and functional diversity. Although numerous halophilic bacteria possess the ability to produce EPS with greatly-varying composition, structure and function (Casillo et al. 2018; Finore et al. 2019; Nicolaus et al. 2010), most of these EPS are hetero-polysaccharides and are commonly composed of mannose and glucose (Wang et al. 2019). The wide chemical and structural diversity of EPS makes them suitable for various applications in several industrial fields, such as food, pharmaceutical, biomedical, cosmetics, bioremediation and wastewater treatment (Wang et al. 2019). They may function as gelling, stabilizing, emulsifying and viscosifying agents (Arias et al. 2003; Li et al. 2001; Radchenkova et al. 2018). Moreover, some halophilic EPS possess beneficial health effects such as antioxidant, anti-cancer and anti-cytotoxicity (Arun et al. 2017; Poli et al. 2009; Ruiz–Ruiz et al. 2011; Sarilmiser and Öner 2014). In addition, halophilic EPS can be used in bioremediation as bioflocculants and heavy metal binding agents (Llamas et al. 2012; Mata et al. 2008; Sam et al. 2011).
At present, numerous halophilic Halomonas species have been identified as EPS producers (Freitas et al. 2017; Martínez-Cánovas et al. 2004; Poli et al. 2007, 2009). According to the available literature, Halomonas smyrnensis AAD6T has been reported to produce levan as a fructan type EPS constituted by β-(2 → 6)-fructofuranosyl residues (Erginer et al. 2016; Erkorkmaz and Toksoy 2016; Poli et al. 2009; Sam et al. 2011; Sarilmiser and Öner 2014; Sogutcu et al. 2012). This biopolymer is the catalytic product of levansucrase activity with sucrose as a substrate. Levan has been considered as an industrially important biopolymer due to its low viscosity, high solubility in oil and water, strong adhesivity, good biocompatibility and film-forming ability (Tohme et al. 2018).
To the best of our knowledge, this is the first study describing the production and the characterization of new EPS from the extremely halophilic Halomonas smyrnensis strain K2 isolated from the solar saltern of Kerkennah in Tunisia. Moreover, prospective biotechnological properties of EPS-K2 were described throughout this study to evaluate its potential applications in various industrial fields.
Materials and methods
Microorganism
Halomonas smyrnensis strain K2 (LT986497), a Gram-negative bacterium with rod-shaped appearance (Fig. 1), was used throughout the present study as a potential EPS-producing microorganism. It was isolated from soil samples collected from the solar saltern of Kerkennah located in Tunisia (Joulak et al. 2019). Glycerol stocks were maintained at − 80 °C and working stock cultures were maintained at 4 °C.
Fig. 1.
Microscopic observation of H. smyrnensis K2 cells cultivated in semi-complex medium using phase-contrast microscopy (bar marker, 10 µm)
EPS production
EPS production was carried out according to Joulak et al. (2019). Briefly, the strain was inoculated in semi complex medium (SCM) composed of (g/l): NaCl, 50; MgCl2·6H2O, 13; MgSO4·7H2O, 9; KCl, 1.3; CaCl2·2H2O, 0.2; NaBr, 0.15; NaHCO3, 0.05; yeast extract, 0.3; peptone, 0.2 and glucose, 10. After 4 days of incubation at 30 °C at 150 rpm, broth culture was centrifuged at 10,000 rpm for 20 min. The supernatant was precipitated by adding 1.5 volumes of cold absolute ethanol, kept at 4 °C overnight and then centrifuged (10,000 rpm, 30 min, 4 °C). The obtained EPS was dissolved in distilled water, dialyzed against several runs of distilled water for 3 days using dialysis tubes (12.00-14.00 kDa as molecular weight cut off), freeze-dried and then weighted.
Kinetics of EPS production
All kinetics of EPS production were studied in SCM. Microbial growth, EPS production and glucose consumption were monitored in batch cultures in 1 L-Erlenmeyer flasks containing 250 ml of culture medium (3 replicate flasks per experiment). Fermentation broths were collected at different time intervals from 0 to 96 h. The cell dry weight (CDW) was estimated in grams of dried cells per liter of culture medium. The yield of EPS was assessed as grams of freeze-dried EPS produced per liter of cell-free fermentation broth (Choudhury et al. 2011). The net carbohydrate content of freeze-dried EPS samples, which were recovered from the cell-free fermentation broths taken at regular time intervals, was estimated by the phenol–sulphuric acid method (Dubois et al. 1956). The residual carbon source (glucose) was calculated using the glucose oxidase technique (Southgate 1976).
Structural characterization of EPS
Monosaccharide composition analysis
EPS-K2 (5 mg) was hydrolyzed with 0.5 M trifluoroacetic acid (TFA) for 2 h at 120 °C, then analyzed by high performance anion exchange column with amperometric detection (HPAE-PAD, DIONEX), with CarboPacPA1column eluted as previously reported (Casillo et al. 2017; Finore et al. 2016).
Determination of total carbohydrate, protein and uronic acid contents
Total carbohydrate content was assessed colorimetrically by the phenol–sulfuric acid method using glucose as standard (Dubois et al. 1956). Protein content was determined by the Bradford test using bovine serum albumin (BSA) as standard (Bradford 1976). Uronic acid content was estimated according to Blumenkrantz and Asboe-Hansen (1973), using glucuronic acid as standard.
Molecular weight determination
The molecular weight of EPS-K2 was measured by gel permeation chromatography (GPC) using a GPC Max Viscotek system with a TDA 305 detector (Refractive Index, Low Angle Light Scattering, Right Angle Light Scattering and Viscometer), UV detector equipped with pre-column TSK (Tosoh Corporation), PolySep-GFC-P5000 and PolySep-GFC-P3000 (Phenomenex). The EPS sample was prepared and eluted in H2O (0.2% NaN3 and 0.1 M NaNO3). After complete dissolution, the sample was filtered on nylon membranes of 0.22 μm. The injection volume was 100 μl, the flow rate was 0.6 ml/min. The chosen method of analysis was a triple point, calibrated with a polyethylene oxide (PEO) standard, provided by Viscotek, having a narrow molecular weight distribution. The measurements, carried out at 40 °C according to the temperatures of columns and detectors, were run for 50 min in duplicate.
FT-IR spectroscopy
EPS-K2 was analyzed by FT-IR spectroscopy and the spectrum was recorded with a light source in the middle infrared range (650–4000 cm−1) in an Agilent Cary 630 FT-IR spectrometer.
NMR spectroscopy
1H and 13C NMR spectra of EPS-K2 were performed on a Bruker AMX-300 MHz (1H NMR) and 400 MHz (13C NMR) at 70 °C. For 1H analysis, the sample EPS-K2 was exchanged twice with D2O with an intermediate lyophilizing step and then dissolved in 700 µl D2O. Chemical shifts were reported in parts per million relative to sodium 2,2,3,3-d 4-(trimethylsilyl) propanoate for 1H and CDCl3 for 13C-NMR spectra (Caruso et al. 2019).
Thermogravimetrical analysis
Thermogravimetrical analysis (TGA) was performed using TGA/DTA Perkin-Elmer, Pyris Diamond TGA/DTA equipped with a gas station. The EPS-K2 sample (4–5 mg) was heated from 30 °C up to 600 °C at a heating rate of 20 °C/min under nitrogen.
Anti-biofilm activity of EPS
The anti-biofilm ability of EPS-K2 was evaluated as previously detailed by Abid et al. (2018) through determining the pre- and post-adhesion treatments of EPS samples. Percentages of microbial adhesion inhibition were determined according to the following equation:
where Ac corresponds to the absorbance at 595 nm of the well with EPS at concentration c and A0 represents the absorbance of the wells without EPS. Negative controls contained only EPS dissolved in PBS. Assays were carried out three times and results were expressed as the average of three independent assays.
Antioxidant activity of EPS
DDPH radical scavenging activity
The DPPH radical scavenging capacity of EPS-K2 was evaluated by dissolving different concentrations of EPS (0, 2.5, 5, 7.5 and 10 g/l) in distilled water (Di Donato et al. 2017; Lama et al. 2014). An aliquot of 500 µl from each sample prepared at these concentrations was added to 375 µl of 99% ethanol and 125 µl of the ethanolic solution of DPPH as a free radical source. After 60 min of incubation in the dark at room temperature, the absorbance was measured at 517 nm using UV mini 1240, UV/VIS spectrophotometer (SHIMDZU, Kyoto, Japan). It was assumed that a lower absorbance value of the mixture indicated higher radical-scavenging activity. The radical scavenging activity (RSA) was evaluated according to the following equation:
where Acontrol is the absorbance of the control (DPPH solution without EPS), Ablank is the absorbance of the blank (without DPPH solution) and Asample is the absorbance of the sample (DPPH solution with EPS). Butylated hydroxyanisole (BHA) was used as a standard antioxidant. The results are the mean of three independent experiments performed in triplicate.
Reducing power assay
The ability of EPS-K2 to reduce iron (III) was assessed according to the method described by Oyaizu (1986) with little modifications. Diluted sample at different concentrations was mixed with 2.5 ml of sodium phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of 1% (w/v) potassium ferricyanide solution K3Fe(CN)6. The mixed reaction was incubated for 30 min at 50 °C. After incubation, the solution was cooled at room temperature, mixed with 2.5 ml of 10% (w/v) trichloroacetic acid, and centrifuged at 5000 rpm for 10 min. Thereafter, the supernatant (2.5 ml) was mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% (w/v) ferric chloride. After 10 min of incubation, the absorbance was measured at 700 nm against a blank. Higher absorbance values of the reaction mixture denoted higher ability to reduce iron. The control was prepared in the same manner, except that distilled water was used instead of EPS, and BHA was used as a standard antioxidant. Values presented are the mean of triplicate experiments.
Fe2+ chelating ability
The ferrous ion-chelating potential of EPS-K2 was measured according the method of Decker and Welch (1990). Diluted sample (0.1 ml) at different concentrations was mixed with 0.1 ml of 2 mM FeCl2·4H2O and 0.2 ml of 5 mM 3-(2-pyridyl)-5,6-bis (4-phenyl-sulphonic acid)—1,2,4-triazine (ferrozine). After an incubation of 10 min, the absorbance of the reaction mixture was measured at 562 nm. The obtained complex of Fe2+/ferrozine exhibited a strong absorbance at 562 nm. A high Fe2+ chelating ability in the sample results in a low absorbance. The ferrous ion-chelating activity exhibited by the sample was calculated by the following equation:
where Acontrol is the absorbance of the control (ferrozine without EPS), Ablank is the absorbance of the blank (without ferrozine) and Asample is the absorbance of the EPS and the standard (EDTA with ferrozine). Ethylenediaminetetraacetic acid (EDTA) was used as a standard antioxidant. Values presented are the mean of triplicate experiments.
DNA damage protection assay
DNA damage inhibition potential of EPS-K2 was investigated as described by Lee et al. (2002). The genomic DNA (0.5 µg/well) was initially pre-incubated for 10 min at room temperature with 10 µl of EPS-K2 at the concentrations of 5, 7.5 and 10 g/l. Oxidative damage was induced by the addition of 10 µl of Fenton’s reagent (30 mM H2O2, 50 µM ascorbic acid and 80 µM FeCl3) and the mixture was then incubated for 5 min at 37 °C. Finally, all samples were analyzed by gel electrophoresis run on a 1% (w/v) agarose gel containing ethidium bromide and photographed.
Statistical analysis
All experiments were performed at least three times. The results were represented as the averages with their respective standard deviations. ANOVA analysis and Duncan test were used to determine statistically significant differences (p < 0.05) between averages at confident level of 95%, using the software IBM SPSS V. 21.
Results and discussion
Kinetics of bacterial growth and EPS production
The extremely halophilic bacterium Halomonas smyrnensis K2 that optimally grows at 15% (w/v) of NaCl, secreted crude EPS (EPS-K2) with a production yield around 74 mg/l after 96 h of incubation at 30 °C (Fig. 2). The time courses of bacterial growth (CDW), EPS production and glucose consumption during the cultivation of H. smyrnensis K2 in SCM were studied. In general, EPS production by H. smyrnensis K2 exhibited fermentation kinetics: started early during the exponential growth phase, then increased concomitantly with the rise bacterial cells entering into stationary phase when the major part of glucose substrate was consumed. Similarly, bacterial cells grew exponentially for the first 72 h to reach a maximum of 1.2 g CDW/l, then remained constant during the subsequent 24 h. The EPS production began with no lag phase and increased during the exponential growth phase to reach a maximum of 74 mg/l concomitantly with their growth maximum, and then plateaued during the stationary growth phase. Deshmukh et al. (2017) reported that the marine moderately halophilic bacterium H. smyrnensis SVD III exhibited a much higher EPS yield (10.5 g/l), using 3% (w/v) of glucose as substrate. As reported in previous studies, there is a strong correlation between bacterial growth and EPS production, suggesting that EPS could be considered as primary metabolite, since their production is cell-growth associated (Abid et al. 2018; Joulak et al. 2019). During the entire fermentation, glucose concentration in the culture broth decreased from 9.6 to 2.0 g/l simultaneously with the cell growth increase from 0.35 to 1.20 g/l. Moreover, EPS production was negatively correlated with the glucose concentration in the culture medium. The net carbohydrate content of freeze-dried EPS recovered from the cell-free fermentation broths was monitored and results showed that it increased during the entire bacterial cultivation to reach a maximum of 34.37% at 96 h (Fig. 2). Similar fermentation kinetic trends were observed for H. smyrnensis AAD6T, isolated from soil samples from Camalti Saltern area (Turkey); it produced 1.073 g/l of EPS when grown in the presence of sucrose as carbon source (Sarilmiser et al. 2015). In the same vein, H. smyrnensis strain SVD III, isolated from ocean water collected in Deobaug in West Coast of Maharashtra (India), produced maximum EPS after 7 days of incubation at 45 °C with a yield 24 times higher than of H. smyrnensis AAD6T (Deshmukh et al. 2017).
Fig. 2.
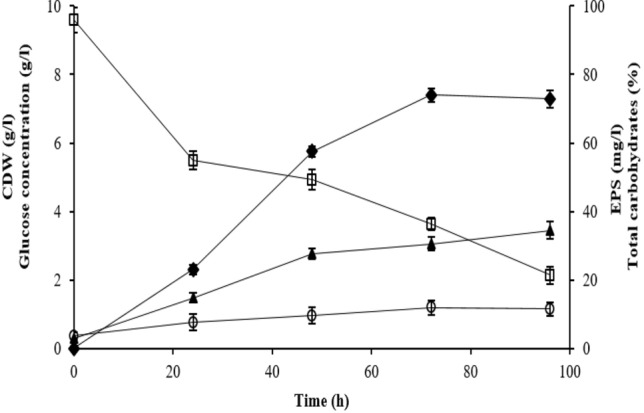
Growth profile and EPS production by H. smyrnensis K2. (empty circle) CDW (g/l); (empty square) glucose consumption (g/l); (filled diamond) EPS-K2 production (mg/l); (filled triangle) total carbohydrates (%). Results are the mean of triplicate measurements
Structural composition of EPS-K2
The chemical composition of EPS is extremely important and could determine its functional properties and define its potential use for industrial applications. The results indicated that the crude EPS-K2 was mainly composed of polysaccharides (34.47%). Other non-carbohydrate compounds such as uronic acid (1.01%) and protein (0.75%) were also detected. According to its monosaccharide profile (Fig. 3), crude EPS-K2 is hetero-polysaccharide with mannose being the most abundant monosaccharide (66.69%), followed by glucose (19.54%) and galactose (13.77%). It has been reported that H. smyrnensis AAD6T was able to synthesize levan as a long linear homopolymeric EPS of β-(2-6)-linked fructose residues, while the EPS from H. smyrnensis SVD III revealed heptasaccharide nature (Deshmukh et al. 2017; Sarilmiser et al. 2015).
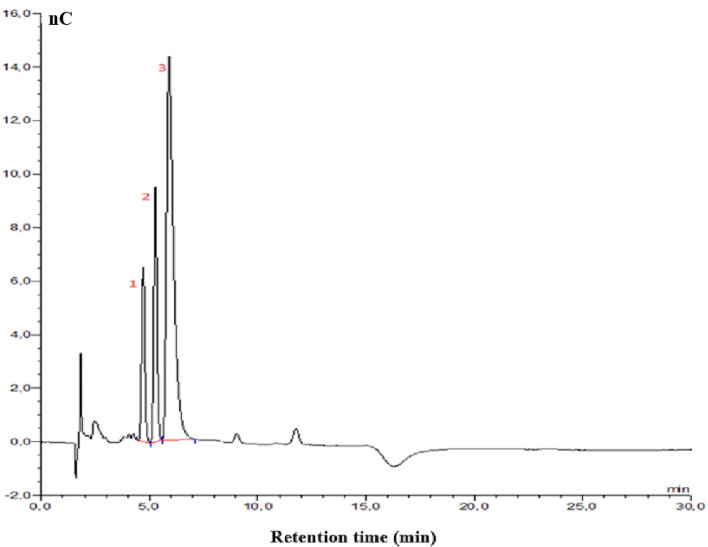
Fig. 3.
HPAE-PAD analysis of monosaccharide composition of EPS-K2 with 1: galactose (RT 4.71 min), 2: glucose (RT 5.27 min) and 3: mannose (RT 5.91 min)
Molecular size analysis
Figure 4 reports the SEC curve of EPS-K2, where the green line corresponds to the right angle light scattering signal (RALS), and the red line related to the refractive index signal (RI). The chromatogram highlights three elution peaks in RI detector: the first two peaks are relative to polymeric species, while the last peak (19.5 ml) corresponds to salts (sodium nitrate and sodium azide) perceived in the eluent. RI and RALS signals indicate three different polymeric species. The first species is relative to the peak at 14 ml (representing about 60% of the overall sample) with Mw of 400 kDa, the second is related to the shoulder at 14.5 ml (50 kDa) and the last one at 16.5 ml with no detectable Mw due to low viscosity suggesting a high ramification degree. Comparing RI and RALS and low viscosity could indicate differences in terms of ramification between polymeric species. The elution peak at 14 ml shows good response in RI and RALS detectors indicating a higher degree of linear polymer against ramification. In the shoulder peak at 14.5 ml, one can observe the absence of symmetry between RI and RALS possibly due to an increase of ramifications. Finally, the peak at 16.5 ml shows RI response but none RALS indicating a higher degree of ramification. The presence of different species is later confirmed by TGA/DTG.
Fig. 4.
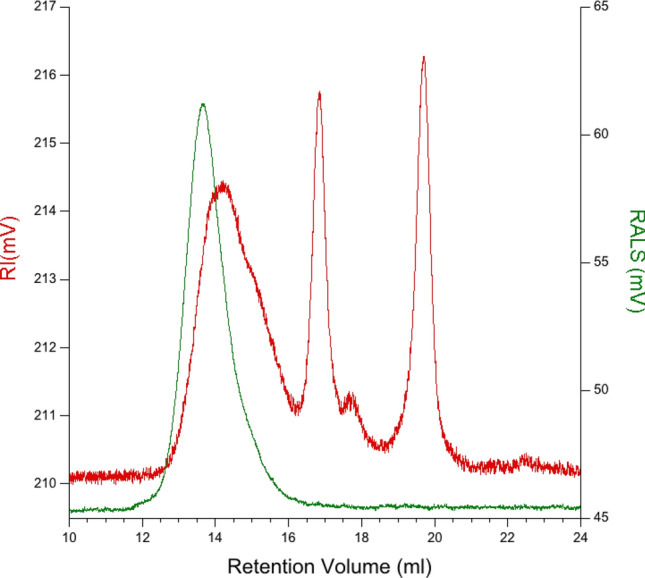
Molecular weight determination of EPS-K2 using gel permeation chromatography (GPC) (RALS: green line, RI: red line)
FT-IR spectrum analysis
FT-IR is a potent and very useful tool for deciphering the structure and the presence of functional groups in EPS (Sran et al. 2019). The FT-IR spectrum of EPS-K2 sample is presented in Fig. 5. A strong and wide band was observed at 3374 cm−1 which corresponds to the stretching vibration of the hydroxyl groups of carbohydrates (O–H) and another band at around 2930 cm−1 associated with a C-H stretching band (Rani et al. 2017; Yu et al. 2016). The band at 1645 cm−1 was attributed to the stretching vibrations of C=O that corresponds to the characteristic absorption of polysaccharides (Bremer and Geesey 1991). Additionally, the band at 1645 cm−1 was attributed to C–N, according to Singh et al. (2011), which could reflect the presence of protein in the EPS sample. The presence of uronic acid was detected by the absorbance band at around 1418 cm−1 (O–C=O) binding. Typical bands of polysaccharides at 1200 cm−1 and 1067 cm−1 that are within the so called fingerprint region (1200–950 cm−1) related to C–O–C glycosidic linkage were also detected (Shang et al. 2013). Likewise, Deshmukh et al. (2017) have described the dominance of glycosidic linkages in the polysaccharide at 1122.54 cm−1 and 1076.14 cm−1 as shown in the FTIR spectrum of the heptasaccharide produced by H. smyrnensis SVD III.
Fig. 5.
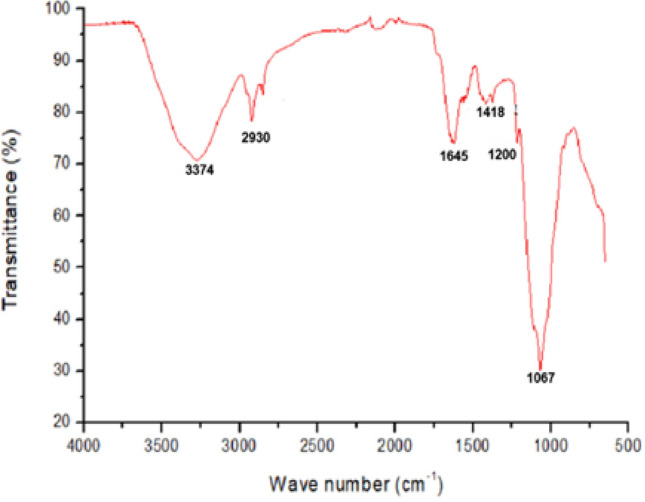
Fourier-transform infrared spectrum of EPS-K2
1H NMR and 13C NMR spectroscopy
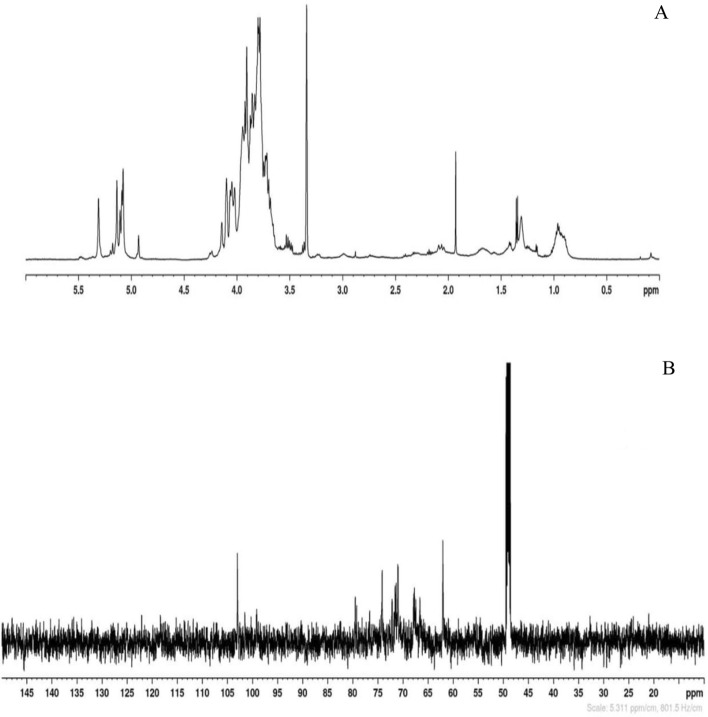
The NMR analysis of EPS-K2 confirmed the heterogeneous structure of the polysaccharide as already suggested by the determination of the chemical composition and the molecular size. The chemical analysis revealed the presence of only three monomer sugars (i.e., mannose, glucose and galactose): in the anomeric region of the 1H-NMR spectrum inter alia, three more intense signals at 5.31, 5.14 and 5.07 ppm were identified together with less intense signals at 5.20, 5.18, 5.12, 5.09 and 4.93 ppm (Fig. 6a). In the anomeric region of the 13C-NMR spectrum, one main signal at 103.0 ppm was followed by two less intense resonances at 101.5 and 100.3 ppm (Fig. 6b). The complexity of the NMR spectra coupled with the evidences from the chemical composition, the TGA and the molecular size analyses, allowed us to confirm that EPS-K2 is a mixture of polysaccharides made of three monomer sugars, all in α-configuration and likely linked by different types of glycosidic linkages.
Fig. 6.
1H NMR (A) and 13C NMR (B) spectra of EPS-K2. Chemical shifts were reported in ppm relative to sodium 2,2,3,3-d 4-(trimethylsilyl) propanoate for 1H and CDCl3 for 13C-NMR spectra
Thermogravimetric analysis (TGA)
Thermogravimetric analysis is a thermal analysis in which the mass of the sample is measured over time as the temperature changes. It can be used to evaluate the thermal stability of the material, which can be interesting for potential application of the biopolymer in packaging or other industrial use (Borrachero et al. 2018). During the current experiment, the EPS sample was heated at a heat flow from 20 to 600 °C under nitrogen while measuring the weight loss to determine the melting temperature at which the polysaccharide degrades and loses its potential use. Figure 7 reports both profiles of the TGA and the first derivative TGA (DTG) curves. Firstly, a step around 60 °C was observed that could be related to the loss of water. A second step, related to the degradation of bigger part of the sample, was centered between 210 and 350 °C. By analyzing the DTG profile, in the temperature range from 200 to 400 °C three peaks could be observed indicating multiplicity in the polysaccharide structures in terms of ramification (Yang et al. 2018). Therefore, it can be assumed that EPS-K2 can be processed at temperature below 200 °C without any thermal degradation risk.
Fig. 7.
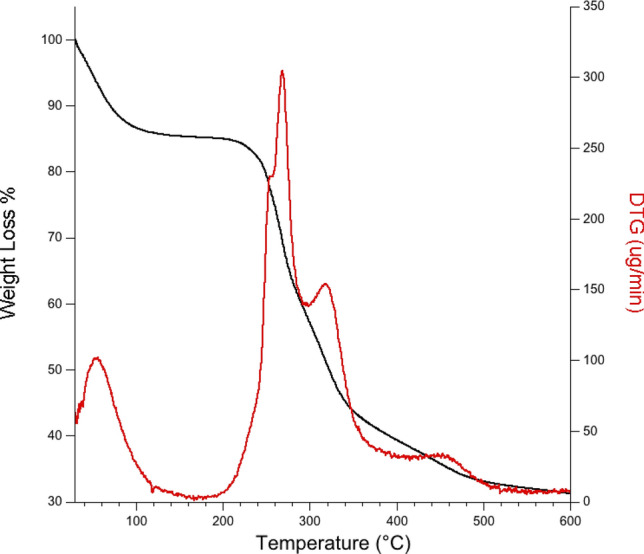
Thermogravimetrical analysis of EPS-K2 by: TGA traces of EPS-K2 (TG (µg) black line; DTG (µg/min) red line)
Biological activities of EPS-K2
Anti-biofilm activity
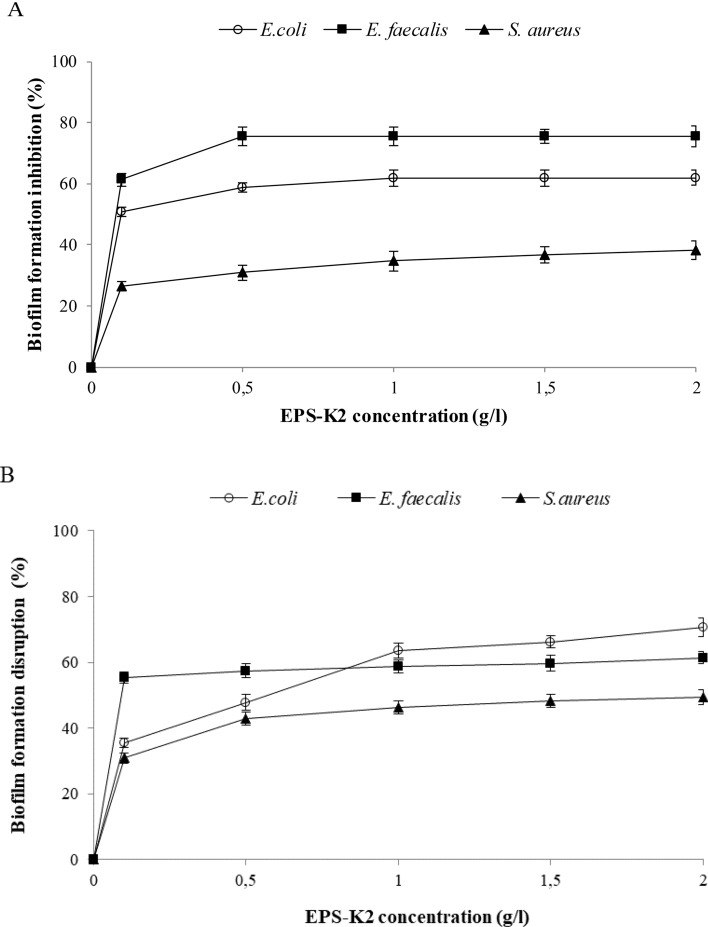
Since bacterial EPS are recently considered as new biocontrol agent to inhibit biofilm development by pathogens (Abid et al. 2018; Spanò et al. 2016), the anti-biofilm activity of EPS-K2 was tested against the Gram-negative bacterium Escherichia coli (ATCC 25922) and the Gram-positive bacteria Enterococcus faecalis (ATCC 25912) and Staphylococcus aureus (ATCC 25923) by the determination of pre-and post-adhesion treatments. Figure 8a shows that the inhibition of biofilm formation increased, in a concentration-dependent manner, with increasing the EPS-K2 concentration from 0.5 to 2 g/l. Furthermore, EPS-K2 showed the maximum adhesion inhibition against both E. faecalis (75.52 ± 3.35%) and E. coli (61.95 ± 2.48%) at 1 g/l, while it showed the highest anti-biofilm activity against S. aureus (38.38 ± 3.03%) at 2 g/l. The ability of EPS-K2 to disrupt pre-formed biofilms through removing the attached pathogenic strains from the surface was also studied (Fig. 8b). The disruptive activity increased concomitantly with the increase of EPS-K2 concentration. The highest biofilm disruption activity of EPS-K2 was observed at 2 g/l against E. coli (70.73 ± 2.78%), followed by E. faecalis (61.42 ± 1.90%) and S. aureus (49.40 ± 2.28%).
Fig. 8.
Effect of EPS-K2 concentrations on (a) biofilm formation inhibition (%) and (b) biofilm disruption (%) against Staphylococcus aureus, Enterococcus faecalis and Escherichia coli. All values are mean ± standard deviation (± SD) of triplicate measurements
According to Rendueles et al. (2014), the antibiofilm activity of bacterial EPS is likely to be mediated by mechanisms other than growth inhibition. Three hypothetical nonbiocidal modes of action were proposed. In fact, most antibiofilm EPS act as surfactants that modify the physical characteristics of bacterial cells and abiotic surfaces. Some studies indicate that EPS might act as signaling molecules that modulate gene expression of recipient bacteria (Kim et al. 2009). Another possible mode of action is competitive inhibition of multivalent carbohydrate–protein interactions (Wittschier et al. 2007).
The present findings are in agreement with those of Jiang et al. (2011), which proved that EPS (A101) from marine bacterium Vibrio sp. QY101 was not only able to inhibit biofilm formation by a wide range of Gram-negative and Gram-positive bacteria but also disrupt pre-formed biofilm of some strains. It could also be seen that EPS-K2 showed an important effect on both inhibition and disruption of the formed biofilm. Thus, EPS produced by H. smyrnensis K2 could be effective in the treatment and prevention of infections related to biofilms produced by pathogenic bacteria (Rendueles et al. 2014).
Antioxidant activities
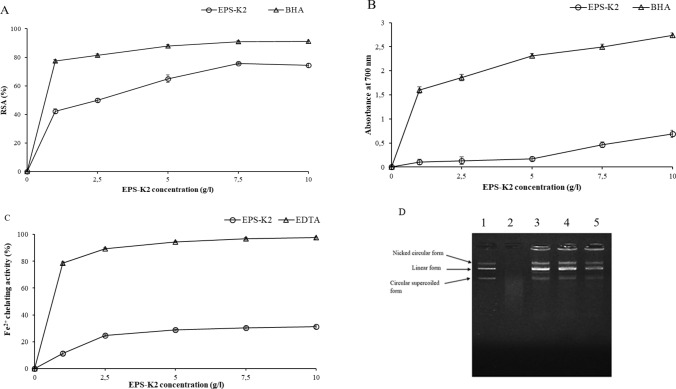
Removal of hydroxyl radicals is necessary because of their extremely reactive nature. It involves highly harmful species capable of damaging almost every molecule found in living cells, thereby leading to aging, cancer, inflammation and several chronic diseases (Vidhyalakshmi et al. 2016). The DPPH free radical scavenging assay is extensively used to evaluate the antioxidant capacities of different compounds. The RSA of EPS-K2, tested at different concentrations, is shown in Fig. 9a. EPS-K2 possessed a strong RSA in a concentration-dependent manner. The RSA increased significantly (p (4.36 10−10) < 0.05) with increasing concentration of EPS-K2. At 1 g/l, EPS-K2 exhibited a RSA of 42.25 ± 1.59%. By adding 2.5 and 5 g/l of EPS-K2, a further increase of RSA to 49.86 ± 1.25 and 65.00 ± 2.59%, respectively, was observed. Finally, the maximum RSA was obtained for EPS concentration of 7.5 g/l, EPS-K2 (75.56 ± 1.74%), above which RSA remains almost constant. This finding is encouraging in comparison with those reported by Arun et al. (2017) on EPS from Halolactibacillus miurensis that exhibited a maximum RSA of 84% at 10 g/l.
Fig. 9.
Antioxidant activities of EPS-K2 at different concentrations (0, 2.5, 5, 7.5 and 10 g/l): a DPPH radical scavenging activity; b Reducing power; c Fe2+ chelating activity. BHA was used as standard. All values are expressed as mean ± SD of three measurements. d DNA protection assay: Lane 1: Native plasmid DNA, lane 2: plasmid DNA incubated with Fenton’s reagent, lane 3, 4 and 5: plasmid DNA incubated with Fenton’s reagent and 5, 7.5 and 10 g/l of EPS-K2, respectively. (empty circle): EPS-K1; (empty triangle): reference antioxidant
The reducing power of a compound is generally associated with the presence of reductants whose antioxidant action is based on the breaking of free radical chain by donation of a hydrogen atom (Loganayaki et al. 2013). In the present study, the ability of EPS-K2 to reduce the Fe3+/ferricyanide complex to the ferrous form (Fe2+) was determined and compared with that of the synthetic antioxidant BHA (Fig. 9b). Results showed that EPS-K2 is less sensitive to the ferric reducing assay, which is possibly, attributed to the functional groups of polysaccharide that are less reactive with the Fe3+/ferricyanide complex. At a concentration of 10 g/l, the reducing power of EPS-K2 was four times less than BHA (Fig. 9b).
The ferrous ion-chelating ability is considered as one of the important antioxidant properties. It was monitored by measuring iron ferrozine complex at 562 nm. EDTA, the versatile chelating agent, was used as positive control. Results reported in Fig. 9c showed that the metal chelating activity increased with increasing the concentrations of EPS-K2 which exhibited 31.1% of Fe2+ chelating effect at concentration of 10 g/l. Arun et al. (2017) reported higher value of Fe2+ chelating ability (49%) for the EPS produced by Halolactibacillus miurensis.
Oxidative DNA damage protective activity of EPS-K2 was investigated using a free radical-induced plasmid DNA breaks system in vitro. The attack of the hydroxyl radical (OH•) generated from the Fenton reaction results in the cleavage of one of the phosphodiester chains of the supercoiled DNA and generates three forms, including supercoiled, open circular and linear form (Hajji et al. 2019). As shown in lane 2 (Fig. 9d), the incubation of the plasmid DNA with Fenton’s reagent generated a complete degradation of DNA, indicating that OH· produced multiple double-strand DNA breaks. Interestingly, the addition of EPS-K2 at 5, 7.5 and 10 g/l (lane 3, 4 and 5, respectively) resulted in a partial retention of supercoiled DNA and produced open circular form and linear form of DNA (Fig. 9d). On the one hand, the generation of circular form of DNA is the result of single-strand breaks and on the other hand, the generation of linear form of DNA is the outcome of double-strand breaks (Burrows and Muller 1998). Therefore, it can be assumed that EPS-K2 prevented OH• induced DNA breaks and mitigated the oxidative stress, indicating its antioxidant effect. According to the results depicted in Fig. 9, EPS-K2 possessed great ability of providing hydrogen atom or electron and, therefore, quenching reactive oxygen species. This action could prevent the free radicals-mediated oxidation of DNA through directly scavenging OH• and, therefore, protecting the supercoiled plasmid DNA (Hajji et al. 2019).
Conclusion
In the current study, a hetero-exopolysaccharide EPS-K2 from Halomonas smyrnensis K2 was produced and chemically described to determine its structure. EPS-K2 has a relative molecular weight of 396 kDa. It was mainly composed of mannose, glucose, galactose (with the following molar percent: 66:20:14). EPS-K2 showed good thermostability with a degrading temperature around 260 °C. In vitro biological activity tests revealed that EPS-K2 exhibited strong anti-biofilm activity and could significantly quench free radicals, suggesting that it could be a natural source of antioxidant additives for nutraceuticals and functional food. Our findings establish a valuable basis to develop the potential applications of EPS-K2 in the different food, cosmetics and medical fields.
Compliance with ethical standards
Conflict of interest
We declare that we have no conflict of interest.
Footnotes
Ichrak Joulak and Ilaria Finore contributed equally to this work.
References
- Abid Y, Casillo A, Gharsallah H, Joulak I, Lanzetta R, Corsaro MM, Attia H, Azabou S. Production and structural characterization of exopolysaccharides from newly isolated probiotic lactic acid bacteria. Int J Biol Macromol. 2018;108:719–728. doi: 10.1016/j.ijbiomac.2017.10.155. [DOI] [PubMed] [Google Scholar]
- Arias S, del Moral A, Ferrer MR, Tallon R, Quesada E, Bejar V. Mauran, an exopolysaccharide produced by the halophilic bacterium Halomonas maura, with a novel composition and interesting properties for biotechnology. Extremophiles. 2003;7(4):319–326. doi: 10.1007/s00792-003-0325-8. [DOI] [PubMed] [Google Scholar]
- Arun J, Selvakumar S, Sathishkumar R, Moovendhan M, Ananthan G, Maruthiah T, Palavesam A. In vitro antioxidant activities of an exopolysaccharide from a salt pan bacterium Halolactibacillus miurensis. Carbohydr Polym. 2017;155:400–406. doi: 10.1016/j.carbpol.2016.08.085. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Borrachero MV, Payá J, Bonilla M, Monzo J. The use of thermogravimetric analysis technique for the characterization of construction materials: the gypsum case. J Therm Anal Calorim. 2018;91(2):503–509. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bremer PJ, Geesey GG. An evaluation of biofilm development utilizingnon-destructive attenuated total reflectance Fourier transform infrared spectroscopy. Biofouling. 1991;3:89–100. [Google Scholar]
- Burrows CJ, Muller JG. Oxidative nucleobase modifications leading to strand scission. Chem Rev. 1998;98:1109–1151. doi: 10.1021/cr960421s. [DOI] [PubMed] [Google Scholar]
- Caruso C, Rizzo C, Mangano S, Poli A, Di Donato P, Nicolaus B, Finore I, Di Marco G, Michaud L, Lo Giudice A. Isolation, characterization and optimization of EPSs produced by a cold-adapted Marinobacter isolate from Antarctic seawater. Antarct Sci. 2019;31(2):69–79. [Google Scholar]
- Casillo A, Ståhle J, Parrilli E, Sannino F, Mitchell DE, Pieretti G, Gibson MI, Marino G, Lanzetta R, Parrilli M, Widmalm G, Tutino ML, Corsaro MM. Structural characterization of an all-aminosugar-containing capsular polysaccharide from Colwellia psychrerythraea 34H. Anton Leeuw Int J G. 2017;110:1377–1387. doi: 10.1007/s10482-017-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casillo A, Lanzetta R, Parrilli M, Corsaro MM. Exopolysaccharides from marine and marine extremophilic bacteria: structures, properties, ecological roles and applications. Mar Drugs. 2018;16:69–103. doi: 10.3390/md16020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury AR, Saluja P, Prasad GS. Pullulan production by an osmotolerant Aureobasidium pullulans RBF-4A3 isolated from flowers of Caesulia axillaris. Carbohydr Polym. 2011;83:1547–1552. [Google Scholar]
- Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 1990;38:674–677. [Google Scholar]
- Deshmukh S, Kanekar P, Bhadekar R. Production and characterization of exopolysaccharide from marine moderately halophilic bacterium Halomonas symrnensis SVD III. Int J Pharm Pharm Sci. 2017;9:146–152. [Google Scholar]
- Di Donato P, Taurisano V, Tommonaro G, Pasquale V, Silván Jiménez JM, de Pascual ST, Poli A, Nicolaus B. Biological properties of polyphenols extracts from agro industry’s wastes. Waste Biomass Valori. 2017;9:1567–1578. [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- Erginer M, Coskunkan B, Morova T, Rende D, Bucak S, Baysal N, Ozisik R, Eroglu MS, Agirbasli M, Öner ET. Sulfated Halomonas levan as a heparin-mimetic bioactive glycan. New Biotechnol. 2016;33:S151–S152. doi: 10.1016/j.carbpol.2016.04.092. [DOI] [PubMed] [Google Scholar]
- Erkorkmaz BA, Toksoy Öner E. Development of a cost-effective microbial levan production process by Halomonas cultures. New Biotechnol. 2016;33:S114. [Google Scholar]
- Finore I, Poli A, Di Donato P, Lama L, Trincone A, Fagnano M, Mori M, Nicolaus B, Tramice A. The hemicellulose extract from Cynara cardunculus: a source of value-added biomolecules produced by xylanolytic thermozymes. Green Chem. 2016;18:2460–2472. [Google Scholar]
- Finore I, Lama L, Di Donato P, Romano I, Tramice A, Leone L, Nicolaus B, Poli A. Parageobacillus thermantarcticus, an antarctic cell factory: from crop residue valorization by green chemistry to astrobiology studies. Diversity. 2019;11(8):128–151. [Google Scholar]
- Freitas F, Torres CAV, Reis MAM. Engineering aspects of microbial exopolysaccharide production. Bioresour Technol. 2017;245:1674–1683. doi: 10.1016/j.biortech.2017.05.092. [DOI] [PubMed] [Google Scholar]
- Hajji M, Hamdi M, Sellimi S, Ksouda G, Laouer H, Li S, Nasri M. Structural characterization, antioxidant and antibacterial activities of a novel polysaccharide from Periploca laevigata root barks. Carbohydr Polym. 2019;206:380–388. doi: 10.1016/j.carbpol.2018.11.020. [DOI] [PubMed] [Google Scholar]
- Insulkar P, Kerkar S, Lele SS. Purification and structural-functional characterization of an exopolysaccharide from Bacillus licheniformis PASS26 with in vitro antitumor and wound healing activities. Int J Biol Macromol. 2018;120:1441–1450. doi: 10.1016/j.ijbiomac.2018.09.147. [DOI] [PubMed] [Google Scholar]
- Jiang P, Li J, Han F, Duan G, Lu X, Gu Y, Yu W. Antibiofilm activity of an exopolysaccharide from marine bacterium Vibrio sp. QY101. PLoS On. 2011;6:1–11. doi: 10.1371/journal.pone.0018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joulak I, Finore I, Nicolaus B, Leone L, Schiano Moriello A, Attia H, Poli A, Azabou S. Evaluation of the production of exopolysaccharides by newly isolated Halomonas strains from Tunisian hypersaline environments. Int J Biol Macromol. 2019;138:658–666. doi: 10.1016/j.ijbiomac.2019.07.128. [DOI] [PubMed] [Google Scholar]
- Kim Y, Oh S, Kim SH. Released exopolysaccharide (r-EPS) produced from probiotic bacteria reduce biofilm formation of enterohemorrhagic Escherichia coli O157:H7. Biochem Biophys Res Commun. 2009;379:324–329. doi: 10.1016/j.bbrc.2008.12.053. [DOI] [PubMed] [Google Scholar]
- Lama L, Tramice A, Finore I, Anzelmo G, Calandrelli V, Pagnotta E, Tommonaro G, Poli A, Di Donato P, Nicolaus B, Fagnano M, Mori M, Impagliazzo A, Trincone A. Degradative actions of microbial xylanolytic activities on hemicelluloses from rhizome of Arundo donax. AMB Express. 2014;4:55–64. doi: 10.1186/s13568-014-0055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Kim HR, Kim J, Jang YS. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J Agric Food Chem. 2002;50:6490–6496. doi: 10.1021/jf020388c. [DOI] [PubMed] [Google Scholar]
- Li P, Liu Z, Xu R. Chemical characterisation of the released polysaccharide from the cyanobacterium Aphanothece halophytica GR02. J Appl Phycol. 2001;13(1):71–77. [Google Scholar]
- Llamas I, Amjres H, Mata JA, Quesada E, Béjar V. The potential biotechnological applications of the exopolysaccharide produced by the halophilic bacterium Halomonas almeriensis. Molecules. 2012;17(6):7103–7120. doi: 10.3390/molecules17067103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganayaki N, Siddhuraju P, Manian S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J Food Sci Technol. 2013;50:687–695. doi: 10.1007/s13197-011-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cánovas MJ, Quesada E, Llamas I, Béjar V. Halomonas ventosae sp. nov., a moderately halophilic, denitrifying, exopolysaccharide-producing bacterium. Int J Syst Evol Microbiol. 2004;54:733–737. doi: 10.1099/ijs.0.02942-0. [DOI] [PubMed] [Google Scholar]
- Mata JA, Béjar V, Bressollier P, Tallon R, Urdaci MC, Quesada E, Llamas I. Characterization of exopolysaccharides produced by three moderately halophilic bacteria belonging to the family Alteromonadaceae. J Appl Microbiol. 2008;105:521–528. doi: 10.1111/j.1365-2672.2008.03789.x. [DOI] [PubMed] [Google Scholar]
- Nicolaus B, Kambourova M, Öner ET. Exopolysaccharides from extremophiles: from fundamentals to biotechnology. Environ Technol. 2010;31:1145–1158. doi: 10.1080/09593330903552094. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. JPN J Nutr Diet. 1986;44:307–315. [Google Scholar]
- Poli A, Esposito E, Orlando P, Lama L, Giordano A, de Appolonia F, Nicolaus B, Gambacorta A (2007) Halomonas alkaliantarctica sp. nov., isolated from saline lake Cape Russell in Antarctica, an alkalophilic moderately halophilic, exopolysaccharide-producing bacterium. Syst Appl Microbiol 30(1):31-8 [DOI] [PubMed]
- Poli A, Kazak H, Gürleyendağ B, Tommonaro G, Pieretti G, Öner ET, Nicolaus B. High level synthesis of levan by a novel Halomonas species growing on defined media. Carbohydr Polym. 2009;78:651–657. [Google Scholar]
- Poli A, Finore I, Romano I, Gioiello A, Lama L, Nicolaus B. Microbial diversity in extreme marine habitats and their biomolecules. Microorganisms. 2017;5:25–55. doi: 10.3390/microorganisms5020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli A, Di Donato P, Finore I, Leone L, Nicolaus B (2019) Sources, biosynthesis, properties, structures and applications of halophilic exopolysaccharides. In: Özlem Ateş Duru (Ed) Microbial exopolysaccharides: current research and developments, Caister Academic Press, pp 25–56
- Radchenkova N, Boyadzhieva I, Atanasova N, Poli A, Finore I, Di Donato P, Nicolaus B, Panchev I, Kuncheva M, Kambourova M. Extracellular polymer substance synthesized by a halophilic bacterium Chromohalobacter canadensis 28. Appl Microbiol Biotechnol. 2018;102(11):4937–4949. doi: 10.1007/s00253-018-8901-0. [DOI] [PubMed] [Google Scholar]
- Rani RP, Anandharaj M, Sabhapathy P, Ravindran AD. Physiochemical and biological characterization of novel exopolysaccharide produced by Bacillus tequilensis FR9 isolated from chicken. Int J Biol Macromo. 2017;96:1–10. doi: 10.1016/j.ijbiomac.2016.11.122. [DOI] [PubMed] [Google Scholar]
- Rendueles O, Kaplan JB, Chigo JM. Anti-biofilm polysaccharides. Environ Microbiol. 2014;15:334–346. doi: 10.1111/j.1462-2920.2012.02810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ruiz C, Srivastava GK, Carranza D, Mata JA, Llamas I, Santamaría M, Quesada E, Molina IJ. An exopolysaccharide produced by the novel halophilic bacterium Halomonas stenophila strain B100 selectively induces apoptosis in human T leukaemia cells. Appl Microbiol Biotechnol. 2011;89(2):345–355. doi: 10.1007/s00253-010-2886-7. [DOI] [PubMed] [Google Scholar]
- Sam S, Kucukasik F, Yenigun O, Nicolaus B, Oner ET, Yukselen MA. Flocculating performances of exopolysaccharides produced by a halophilic bacterial strain cultivated on agro-industrial waste. Bioresour Technol. 2011;102:1788–1794. doi: 10.1016/j.biortech.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Sarilmiser HK, Öner ET. Investigation of anti-cancer activity of linear and aldehyde activated levan from Halomonas smyrnensis AAD6T. Biochem Eng J. 2014;92:28–34. [Google Scholar]
- Sarilmiser HK, Ates O, Ozdemir G, Arga KY, Oner ET. Effective stimulating factors for microbial levan production by Halomonas smyrnensis AAD6T. J Biosci Bioeng. 2015;119:455–463. doi: 10.1016/j.jbiosc.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Shang N, Xu R, Li P. Structure characterization of an exopolysaccharide produced by Bifidobacterium animalis RH. Carbohydr Polym. 2013;91(1):28–134. doi: 10.1016/j.carbpol.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Singh RP, Shukla MK, Mishra A, Kumari P, Reddy CRK, Jha B. Isolation and characterization of exopolysaccharides from seaweed associated bacteria Bacillus licheniformis. Carbohydr Polym. 2011;84:1019–1026. [Google Scholar]
- Sogutcu E, Öner ET, Arga KY. Identification of exopolysaccharide biosynthesis mechanism of Halomonas smyrnensis AAD6T. New Biotechnol. 2012;29:S145. [Google Scholar]
- Southgate DAT. Determination of food carbohydrates. London: Applied Science Publishers LTD; 1976. [Google Scholar]
- Spanò A, Laganà P, Visalli G, Maugeri TL, Gugliandolo C. In Vitro Antibiofilm activity of an exopolysaccharide from the marine thermophilic Bacillus licheniformis T14. Curr Microbiol. 2016;72:518–528. doi: 10.1007/s00284-015-0981-9. [DOI] [PubMed] [Google Scholar]
- Sran KS, Sundharam SS, Krishnamurthi S, Roy Choudhury A. Production, characterization and bio-emulsifying activity of a novel thermostable exopolysaccharide produced by a marine strain of Rhodobacter johrii CDR-SL 7Cii. Int J Biol Macromol. 2019;127:240–249. doi: 10.1016/j.ijbiomac.2019.01.045. [DOI] [PubMed] [Google Scholar]
- Tohme S, Hacıosmanoğlu GG, Eroğlu MS, Kasavi C, Genç S, Can ZS, Toksoy Oner E. Halomonas smyrnensis as a cell factory for co-production of PHB and levan. Int J Biol Macromol. 2018;118:1238–1246. doi: 10.1016/j.ijbiomac.2018.06.197. [DOI] [PubMed] [Google Scholar]
- Vidhyalakshmi R, Valli NC, Narendra Kumar G, Sunkar S. Bacillus circulans exopolysaccharide: production, characterization and bioactivities. Int J Biol Macromol. 2016;87:405–414. doi: 10.1016/j.ijbiomac.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Salem DR, Sani RK. Extremophilic exopolysaccharides: a review and new perspectives on engineering strategies and applications. Carbohydr Polym. 2019;205:8–26. doi: 10.1016/j.carbpol.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Wittschier N, Lengsfeld C, Vorthems S, Stratmann U, Ernst JF, Verspohl EJ, Hensel A. Large molecules as anti-adhesive compounds against pathogens. J Pharm Pharmacol. 2007;59:777–786. doi: 10.1211/jpp.59.6.0004. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang D, Deng J, Yang J, Shi C, Zhou F, Shi Z. Activity and structural characteristics of peach gum exudates. Int J Polym Sci Article ID. 2018 doi: 10.1155/2018/4593735. [DOI] [Google Scholar]
- Yu L, Xu S, Deng C, Li H, Yang Q, Xu Z, Chen J. Preparation and partial structural characterization of the exopolysaccharide from Bacillus mucilaginosus SM-01. Carbohydr Polym. 2016;146:217–223. doi: 10.1016/j.carbpol.2016.03.038. [DOI] [PubMed] [Google Scholar]