Abstract
This study describes critical factors affecting germination of somatic embryos and plantlet regeneration in Pinus massoniana. Somatic embryos from the same embryogenic line 27 of P. massoniana were used as test materials. The supplementation of activated charcoal (AC) in the medium was essential for the germination of mature somatic embryos, while the addition of excessive AC to the medium was prohibitive for somatic embryo germination. The highest germination rate was found on the medium containing 10 g/l AC, and the addition of 5 g/l AC to the medium was optimal to the growth of germinating somatic embryos. Thidiazuron (TDZ) was linearly related to the number of sprouting axillary buds. However, the growth of sprouting buds was retarded when > 4 µmol/l TDZ was added into culture medium. Exogenous plant growth regulators added to the medium significantly improved the root regeneration capacity of shoots. The highest root regeneration rate was observed under the treatment of 1.2 µmol/l ɑ-naphthaleneacetic acid (NAA) plus 2 µmol/l paclobutrazol (PBZ), reaching 96.3%. One year after the field transfer, the growth performance of plant height, caliper, and survival rate for rooted shoots was significantly better than that of plantlets directly developed via somatic embryogenesis. The presented results provide useful instruction for the establishment of plantlets originating from somatic embryos, and would be able to make a great contribution to the clonal forestry of P. massoniana.
Keywords: Clonal forestry, Indirect somatic embryogenesis, Masson pine, Paclobutrazol, Thidiazuron
Introduction
Masson pine (Pinus massoniana), a native species of southern China, is one of the most important tree species in subtropical regions, and is cultivated for the production of timber and natural resin (Ding et al. 2006). To date, seedlings propagated by seeds are the main planting materials for the establishment of P. massoniana plantations, resulting in the low productivity of forests due to the great genetic variations among trees (Zhu et al. 2010). To enhance stand productivity, and promote the industrial development of P. massoniana, clonal forestry is urgent, with the aim of genetic gains with cloning.
Concerning the clonal propagation of P. massoniana, the utility of cutting propagation has been indicated (Shen 2018). However, donor plant age is negatively related to the root regeneration capacities of P. massoniana cuttings (Ji et al. 1996), as Ragonezi et al. reported factors influencing adventitious root regeneration in conifers (2010); hence, this method is limited to the propagation of selected genotypes from adult P. massoniana trees. The protocol of plantlet regeneration from the initiation of axillary buds/shoots via an organogenesis approach has been widely developed in plants (Sul and Korban 1994; Nunes et al. 2018). For P. massoniana, the approach has also been studied for many years (Huang and Wei 1994), and numerous researchers have reported that the protocol for micropropagation using juvenile or mature materials in P. massoniana has been established (Li et al. 2009; Zhu et al. 2010; Yin et al. 2013; Yao and Wang 2016; Wang et al. 2019; Wang and Yao 2017), while the high cost of the process caused difficulties in mass production on a commercial scale. Propagation via somatic embryogenesis has many advantages. The in vitro propagation of elite trees could be very effective when it is combined with cryopreservation of embryonal cells (Klimaszewska et al. 2016). This method was able to provide unlimited somatic embryos for the production of plantlets during the selection of elite genotypes in a field test. Furthermore, the utilization of artificial seeds (Aquea et al. 2008) and genetic transformation using single cells initiated from somatic embryos (Walters et al. 2010) confirmed the superiority of somatic embryogenesis.
For conifers, many bottlenecks cause difficulties in plantlet regeneration via somatic embryogenesis, such as a low initiation rate, poor maturity of somatic embryos and difficult development of the root system (Klimaszewska et al. 2016). Among Pinus species, results that showed plantlet regeneration were published for at least 16 species (Klimaszewska et al. 2007; Montalbán et al. 2010; Pullman et al. 2015), e.g., P. strobus, P. radiata, and P. taeda. However, it is not clear whether regenerated plantlets were successfully transferred to the field for all of these Pinus species. In P. massoniana, previously published protocols made great progress on the initiation, proliferation, and maturation of somatic embryos (Yang et al. 2011), while the efficiency of germination and plantlet regeneration was quite low. To date, the results of the field transfer of plantlets have not been reported for this species. Further research, especially on the establishment of regenerated plantlets via somatic embryogenesis, is needed.
Recently, we established an efficient protocol for the micropropagation of P. massoniana using the shoots formed from zygotic embryos and performed the field transfer of regenerated plants. Our observed results showed that the growth performance of micropropagated plantlets was significantly better than that of seed seedlings. It is well known that root mass directly affects plant growth, and hence, the natural environment for plants with robust roots is much easier to adapt than that for plants with poor roots, and consequently, plants with robust roots grow better than those with poor roots (Aderson et al. 1992). P. massoniana is a tree species with developed tap roots but sparse lateral roots, which restrains the growth of plantlets after transplantation (Ding et al. 2006). It is possible that the variation in root properties leads to the increased growth adaptability of plants due to the conversion from a tap root system to adventitious root system following the induction of in vitro root regeneration.
Concerning the difficulty in root formation as well as the potential inefficiency of plantlet regeneration and field transfer via somatic embryogenesis, this study aims to develop a protocol for the establishment of plantlets formed from somatic embryos through the improvement of the root system. Considering the success in regenerating P. radiata plants through a combined pathway of somatic embryogenesis and organogenesis (Montalbán et al. 2011), immature cones collected from open-pollinated trees in a P. massoniana seed orchard were first used as donors for the origination of somatic embryogenesis. Then, the effects of factors affecting plantlet regeneration, including activated charcoal (AC), the impacts of radicle cutting, and exogenous plant growth regulators on somatic embryo germination, shoot multiplication/elongation, and adventitious root induction, were investigated using mature somatic embryo explants in the present study. The growth performance of regenerated plants through traditional somatic embryogenesis as well as the methodology described in this study were both assessed during the first year of field transfer.
Materials and methods
Plant material
Immature cones of Pinus massoniana Lamb were collected from open-pollinated trees in a seed orchard established by Paiyang-Shan Forest Farm in Nanning, China (latitude: 22°07′6″N, longitude: 107°03′40″E, elevation: 620 m). From 2013 to 2016, fifty cones were collected weekly during the month of June. The cones were put in paper bags and stored at 4 ℃ for a maximum of one week. Intact cones were washed with running water for an hour and sterilized in H2O2 10% (v/v) plus five drops of Tween 80 for 20 min. The cones were rinsed 3 ~ 4 times with sterile distilled water and then split and immature seeds extracted in aseptic conditions. Following the methodology, Yang et al. (2011) described, seed coats were removed, megagametophytes were aseptically excised out, and horizontally placed onto LP media (Aitken-Christie et al. 1988) with plant growth regulators and amino acids. Finally, fast-growing and vigorous embryogenic cell lines were obtained, and mature somatic embryos from the most stable and productive cell line 27 were used for further experimentation in this study.
Germination of somatic embryos
After the establishment of the induction (Fig. 1a, d), proliferation (Fig. 1b, e) and maturation (Fig. 1c, f) from embryogenic cells were sequentially achieved, mature somatic embryos (Fig. 1g) without any pre-germination treatment were directly placed on half-strength modified Murashige-Skoog (MS) (Murashige and Skoog 1962) medium (MMS medium) (Yao and Wang 2016) containing 0–12 g/l AC in 2017 for germination of 30–40 days (Fig. 1h) with 80 µmol/m2s light intensity at 25 ± 0.5 °C until the formation of main leaves (Fig. 1i). Medium lacking AC was used as the control treatment. In this experiment, 3 replicates and 15 embryos per replication were performed for each AC treatment. A total of 315 well-developed embryos (15 somatic embryos × 3 replicates × 7 AC treatments) were tested. In addition, 110 mature embryos were directly transferred to half-strength MMS medium containing 10 g/l AC for germination culture of 200–230 days until the establishment of plantlets (Fig. 1j, referred to as somaplants in this study). During the culture, mature embryos were transferred monthly to fresh medium containing the same components.
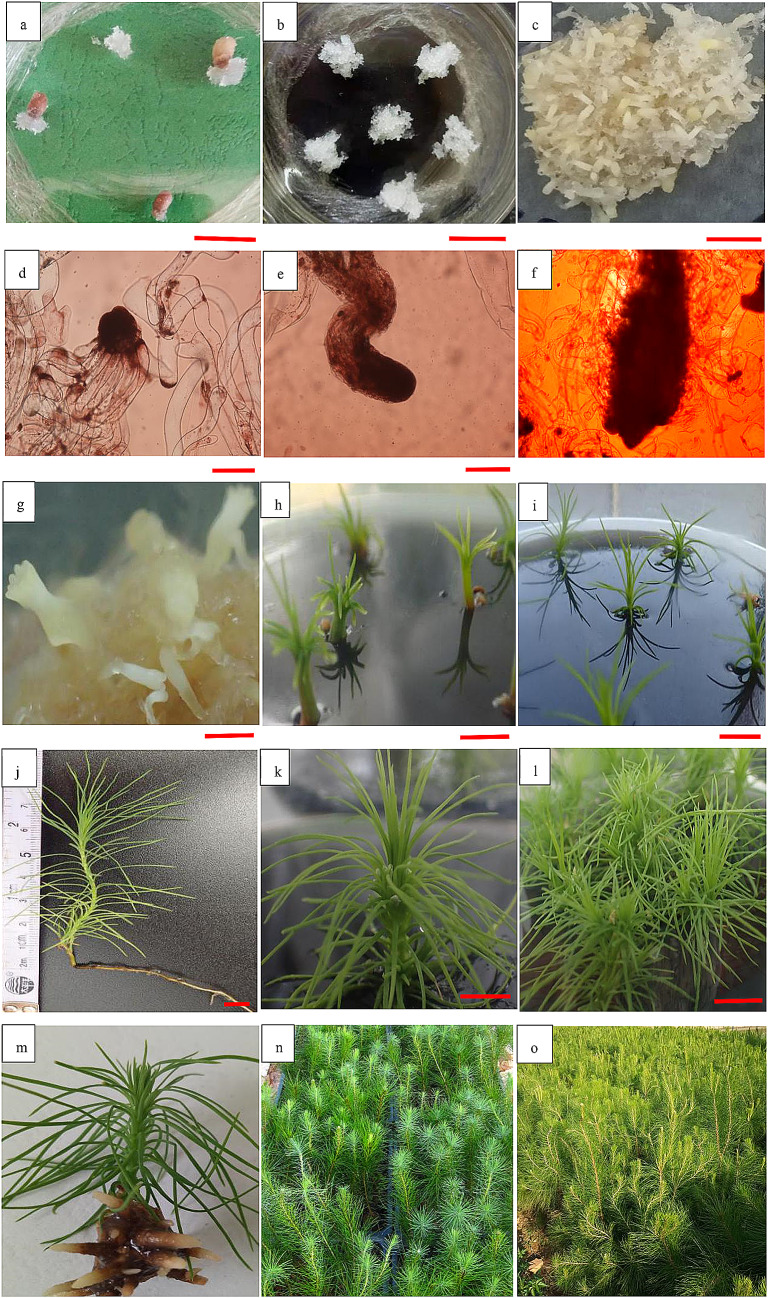
Fig. 1.
Establishment of plantlets originating from somatic embryos of embryogenic line 27 in Pinus massoniana. a–l Somatic embryogenesis: induction of embryogenic callus (a), proliferation (b), maturation (c, g), germination (h), shoot tips collected from elongated epicotyls of germinating somatic embryos (i), somatic plant with tap root system (j), shoot tips with axillary buds induced (k), and clustered shoots (l). d–f Microscopic observation on embryogenic structures: induced embryogenic cell (d), proliferative somatic embryo (e), mature somatic embryo (f). m–o Regenerated plantlets via adventitious root induction: in vitro rooted shoots (m), regenerated plantlets three months after transplanting in the nursery (n), and growth performance of plantlets transplanted into the soil for one year (o). Scale bars: 200 μm (d, e), 500 μm (c, f, h, k), 2 mm (g), 1 cm (a, b, i, j, l, m), 10 cm (n), 20 cm (o)
In vitro plantlet regeneration
The 1st stage
To promote the development of germinating somatic embryos (GSE), epicotyls from GSE following radicle cutting were transferred into MMS medium containing 0–10 g/l AC. After the in vitro culture for 40 days, shoots were developed and the shoot length was aseptically determined. GSE without radicle cutting were regarded as the control. The culture conditions were the same as those described in the germination experiment. A total of 288 epicotyls (8 epicotyls × 3 replicates × 6 AC treatments × 2 radicle cutting treatments) were sampled at this stage.
The 2nd stage
To reinvigorate the shoots originating from GSE, 30–50 mm long shoots tips were excised from elongated epicotyls and cultured on MMS medium with the addition of 0–8 µmol/l thidiazuron (TDZ) for multiplication culture (Fig. 1k). After 35 days of TDZ treatment, sprouting axillary buds ≥ 5 mm were counted for the calculation of total bud number per explant, and then shoots with clustered buds (Fig. 1l) were transferred to MMS medium supplemented with 5 g/l AC and no plant growth regulators. Fifty days after transfer, buds were elongated, and buds ≥ 30 mm were used to calculate the number of effective buds per explant. At this stage, the light intensity was reduced to 40 µmol/m2s, and the temperature was unchanged in contrast to the culture conditions used in the 1st stage. A total of 225 shoots (15 shoot tips × 3 replicates × 5 TDZ treatments) were tested.
The 3rd stage
Buds ≥ 30 mm were referred to as shoots, which were excised in 20–30 mm length and transferred on half-strength MMS medium containing 2 µmol/l paclobutrazol (PBZ) and/or 1.2 µmol/l ɑ-naphthaleneacetic acid (NAA) to induce adventitious root regeneration. Medium with no plant growth regulators was used as the control. The culture conditions were the same as those described in the 2nd stage. Approximately, 50–60 days after the induction of in vitro root regeneration, rooted shoots (Fig. 1m) with roots ≥ 20 mm were counted. The root regeneration rate is expressed as the percentage of rooted shoots among total shoots cultured on the root regeneration medium. Here, a total of 450 shoots (50 shoots × 3 replicates × 3 plant growth regulator treatments) were tested.
Acclimatization and field transfer
For acclimatization (Fig. 1n, o), in vitro regenerated plantlets and somaplants were removed from the root regeneration medium to become acclimatized with a progressive decrease in relative humidity according to the method we previously published (Wang and Yao 2017). In Mar. 2018, 120 acclimatized plantlets and 30 somaplants approximately 10 cm high were transplanted to the field under a plant spacing of 2 m × 3 m (plants × rows). Following the field transfer, the growth of height and caliper as well as the survival rate of transplanted plants from both in vitro regenerated plantlets and somaplants were measured monthly until March 2019. The plant age was determined in accordance with the time of field transfer in this study.
Data analyses
All percentage data were transformed (before the calculation of statistics and statistical comparison) using arcsine square root transformation (Compton et al. 1994) to normalize the error distribution prior to variance analysis. A linear relationship between the number of total buds and exogenous TDZ concentrations was analysed using linear regression analysis; the number of total buds was considered the dependent variable, and the TDZ concentration was considered the predictor variable. Regression analyses were carried out using the general linear model (GLM) procedure in the SAS statistical package (SAS Institute Inc., North Tustin, USA). Statistical analyses used were a factorial ANOVA (analysis of variance, AC concentration, radicle cutting treatment, the number of buds, TDZ concentration, exogenous plant growth regulator types, plant age or seedling sources as factors), Duncan’s test (significant statistical difference among AC concentration, TDZ concentration, exogenous plant growth regulators types or plant age) and t tests (significant statistical difference between radicle cutting and no cutting, the number of total, and effective buds or seedling sources within plant age or measurement time). Variance analyses were performed using the SPSS 19.0 software package (SPSS Inc., Chicago, IL, USA).
Results
Effects of AC on somatic embryo germination
Mature embryos from the same cell line were cultured on AC or AC-free medium. To investigate somatic embryo germination, the germination rate was calculated as the percentage of germinating somatic embryos among 15 somatic embryos in 3 replicates. Mature embryos from AC-free medium were 0% germinating, and they grew into browning and hygrophanous after culturing on the medium. Concerning AC supplementation, the germination rate of somatic embryos was significantly increased by the addition of AC to the medium, and the highest germination rate and the best growth performance of germinating somatic embryos were observed at the 10 g/l AC treatment in our present study (Table 1). The high AC concentration (12 g/l) applied in the medium led to the reduction in the germination rate as well as growth effects of germinating somatic embryos in comparison to the 10 g/l AC treatment.
Table 1.
Germination and growth of mature somatic embryos from embryogenic line 27 under activated charcoal (AC) treatment in Pinus massoniana
| AC/g/l | Germination rate/% | Growth of mature somatic embryos |
|---|---|---|
| 0 | 0f | Serious browning and hygrophanous |
| 2 | 4.9 ± 1.2e | Turned green, but stubby, and no differentiation |
| 4 | 45.9 ± 3.2d | Differentiated into epicotyls and hypocotyls, but not developed root system |
| 6 | 55.7 ± 2.4c | Formed new and slender shoots and short roots |
| 8 | 84.4 ± 4.7b | Formed healthy shoots and robust roots |
| 10 | 97.0 ± 3.6a | Formed healthy shoots and robust roots |
| 12 | 84.2 ± 5.7b | Formed robust roots, but new shoots were yellow and slim |
The germination rate is calculated as the percentage of germinating somatic embryos among 15 somatic embryos in 3 replicates. Meansstandard errors (SE) within a column followed by the same small letter are not significantly different according to Duncan’s multiple range tests (Duncan’s tests) at P ≤ 0.05
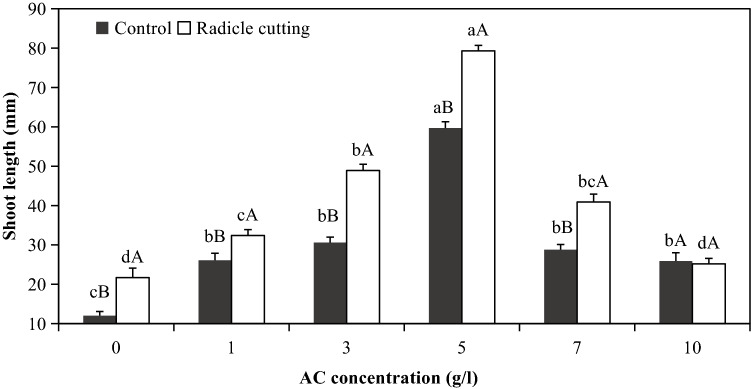
Effects of AC and radicle cutting on shoot elongation
Irrespective of radicle cutting influences, AC promotes the elongation of shoots in germinating somatic embryos, while the promotive effects of AC on shoot elongation were decreased by the increase in AC concentration if it was higher than 5 g/l; hence, the best growth responses of shoots were investigated under the 5 g/l AC treatment (Fig. 2). Under the treatment of 10 g/l AC, there was no difference between radicle cutting and the control (no radicle cutting); however, in the range of 0–7 g/l AC, radicle cutting enhanced shoot elongation when compared with the control within the same AC concentration range (Fig. 2).
Fig. 2.
Elongation of shoots from germinating somatic embryos of embryogenic line 27 under AC and/or radicle cutting treatment in Pinus massoniana. Small letters indicate the differences among AC treatments within the control or radicle cutting treatment (Duncan’s tests, P ≤ 0.05), and capital letters indicate the differences between the control and radicle cutting treatment within the AC treatment (t test, P ≤ 0.05). Bars in the figure mean standard errors (SE), and the same as the following
Effects of TDZ on shoot multiplication
Shoots excised from elongated somatic embryos were cultured on the medium containing 0–8 µmol/l TDZ. After culturing for 35 days, we found that there was a remarkable linear relationship between the TDZ concentration and the number of total sprouting axillary buds (R2 = 0.98, Fig. 3). For the application of 8 µmol/l TDZ in the medium, the number of total buds was the highest, reaching 9.4 per explant. Following the transfer of shoots with buds on TDZ-free plus 5 g/l AC medium for 50 days, significant differences in the number of effective (vigorous, fast-growing and healthy) buds derived from different TDZ treatments were found (Fig. 3). With the increase in the TDZ concentration, the number of effective buds was first enhanced and then decreased. A peak in the number of effective buds was observed under the treatment of 4 µmol/l TDZ. Additionally, there was no difference between the number of total and effective buds under the 0–4 µmol/l TDZ treatment.
Fig. 3.
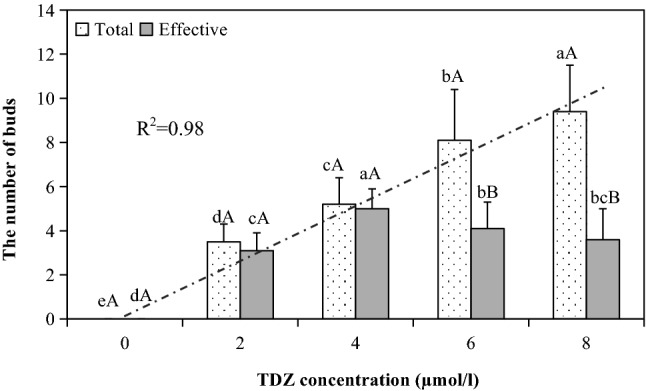
Multiplication of shoots originating from somatic embryos of embryogenic line 27 under thidiazuron (TDZ) treatment in Pinus massoniana. Sprouting axillary buds ≥ 5 mm were counted for the calculation of total bud number per explant, and elongated buds ≥ 30 mm were used to calculate the number of effective buds per explant. Small letters indicate the differences among TDZ treatments for the number of total buds or effective buds per explant (Duncan’s tests, P ≤ 0.05), and capital letters indicate the differences between the number of total and effective buds within a TDZ treatment (t test, P ≤ 0.05)
Effects of exogenous plant growth regulators on root regeneration capacity
It was clear that the addition of exogenous plant growth regulators in the root regeneration medium was necessary (Fig. 4). Shoots cultured on the plant growth regulator-free medium were 0% rooted. However, for shoots exposed to NAA and/or PBZ medium, the root regeneration rate was significantly increased, ranging from 55.3 to 96.3%. When compared with the medium with only NAA, the combinations of NAA and PBZ in the medium led to a significant enhancement of the root regeneration by74.1% (Fig. 4).
Fig. 4.
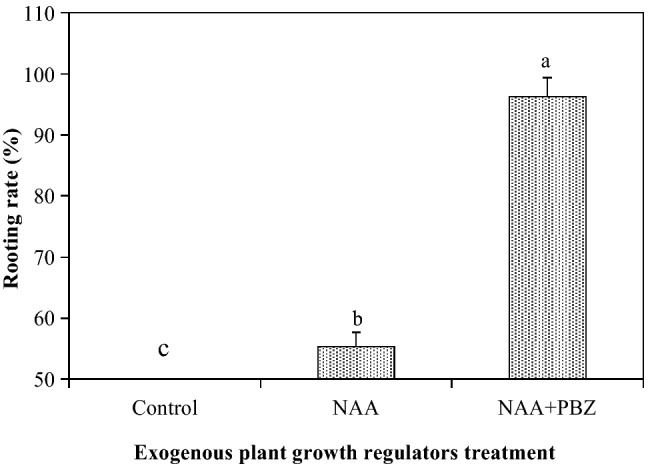
Root regeneration responses of in vitro cultured shoots originating from somatic embryos of embryogenic line 27 to exogenous 1.2 µmol/l ɑ-naphthaleneacetic acid (NAA) and/or 2 µmol/l paclobutrazol (PBZ) added to the root regeneration medium in Pinus massoniana. Small letters indicate the differences among exogenous plant growth regulator treatments according to Duncan’s tests at P ≤ 0.05
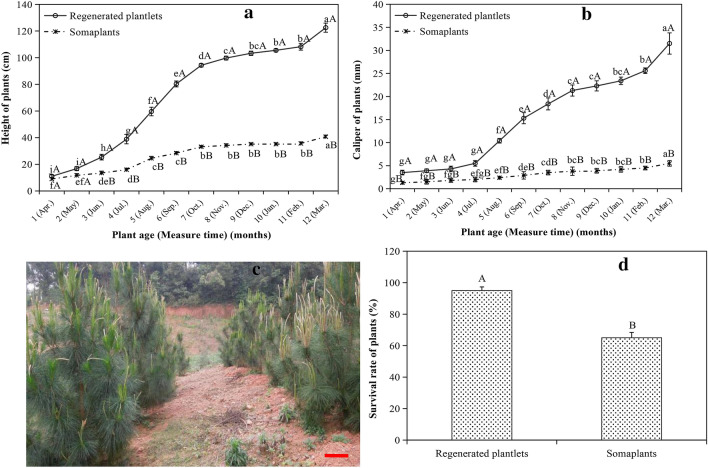
Growth performance after field transfer
Although gradual increases in plant height and caliper were determined with the development of plant age for the plants from the two sources, plantlets in vitro regenerated with the methodology described in this study grew faster than the somaplants during the one-year field transfer (Fig. 5a, b), and young stands were efficiently established using the regenerated plantlets in one-year planting (Fig. 5c). For the growth performance of plant height, the height of regenerated plantlets was higher than that of somaplants from the start of the 3-month field transfer (Fig. 5a). Concerning the growth tendency, the height growth of somaplants was gentle, while the growth of regenerated plants was sharp. After transplanting in the field for 3–7 or 12 months (from Jun. to Oct., or Mar.), the plant height was increased monthly in regenerated plantlets. For somaplants, a monthly increase in plant height was only observed 5-month (Aug.) after field transfer (Fig. 5a). However, for caliper growth performance, the caliper of regenerated plants was significantly higher than that of somaplants during field transfer (Fig. 5b). In addition, a significantly higher survival rate of plants was observed in regenerated plantlets in comparison to that of somaplants after one-year field transfer (Fig. 5d).
Fig. 5.
Growth performance of somaplants (somatic plantlets directly via somatic embryogenesis) and in vitro regenerated plantlets via organogenesis originating from somatic embryos of embryogenic line 27 after one year of planting in a Pinus massoniana field. a Changes in plant height with the plant age. b Changes in plant caliper with the plant age. c In vitro regenerated plantlets originating from somatic embryos after planting for one year in the field. d Changes in survival rate of plants one year after transplanting of both regenerated plantlets and somaplants. Scale bar: 15 cm. In figures a, b and d, small letters indicate the differences among plant ages for somaplants or regenerated plantlets (Duncan’s tests, P ≤ 0.05), and capital letters indicate the differences between somaplants and regenerated plantlets within plant age (t test, P ≤ 0.05)
Discussion
Previous studies reported that AC affected the availability of different metallic species when it was applied in the culture medium (De Diego et al. 2008; Thomas 2008; Ragonezi et al. 2010). For Pinus species, AC plays an important role in micropropagation due to its ability to absorb phenolics and residual plant growth regulators (Van Winkle and Pullman 2003). Pan and van Staden (1998) declared that AC was able to promote or inhibit in vitro growth of plants, whereas its promotive or inhibitory effects depended on the tree species and materials used. The promotive impact of AC on P. massoniana micropropagation has been confirmed in our previous studies (Yao and Wang 2016; Wang and Yao 2017). In the present study, in comparison to the AC-free medium, the medium with AC addition was beneficial to the germination of mature somatic embryos and the shoot elongation of germinating somatic embryos. However, reduced increments in both germination rate and shoot length were also observed to result from the supplementation of a high concentration of AC in the medium. These results indicate that the presence of AC in the medium used for germination and shoot elongation is essential in P. massoniana in vitro culture, while to obtain the best culture responses to AC application, the amount of AC applied in the medium needs to be optimized for the material type (e.g., 10 g/l AC for somatic embryo germination and 5 g/l AC for shoot elongation). Concerning shoot elongation, despite better performance of shoot growth observed under the conditions of radicle cutting and AC addition than that under the no radicle cutting condition in a range of 0–7 g/l AC, the shoot elongation can be mainly attributed to AC promotive effects, as significantly longer shoots were cultured on AC medium than on AC-free medium. To simplify the procedure of P. massoniana micropropagation, combinations of AC and plant growth regulators were used for shoot multiplication and elongation in our previous experiment; however, poor responses of in vitro culture to the combinations were found (Wang and Yao 2017). This result showed that AC remarkably influenced the availability of exogenous plant growth regulators in P. massoniana, implying that the promotive effect of AC was possibly related to its capacity to absorb residual plant growth regulators in the present study.
Plant growth regulators are vital for shoot and root growth in the in vitro culture of plants (Huang et al. 2007; Ragonezi et al. 2010; Marhavý et al. 2011, 2013; Mauriat et al. 2014; McAdam et al. 2016). TDZ, which is often substituted for phenylurea as a plant growth regulator with cytokinin-like activity, is widely used in various plant culture systems (Huetteman and Preece 1993; Kanyand et al. 1994; Siddique and Anis 2007). The gradually increased addition of TDZ to the medium of shoot multiplication contributed to an increasing number of sprouting axillary buds in this experiment. However, the number of effective buds (elongated to ≥ 30 mm on TDZ-free and 5 g/l AC medium) was not enhanced with the supplementation of the TDZ concentration in the medium, and a peak of the effective bud number occurred in the medium containing 4 µmol/l TDZ. We suggest that the use of 4 µmol/l TDZ in medium could be optimal for the multiplication of shoots originating from somatic embryos in P. massoniana irrespective of the aid of AC in absorbing residual plant growth regulators.
Regarding the role of plant growth regulators in adventitious root regeneration, our results demonstrated that NAA or NAA + PBZ significantly improved the root regeneration capacity of shoots in vitro cultured P. massoniana. NAA, an auxin analogue, has been extensively used as an effective stimulator of root formation in plants (Li et al. 2009; Ragonezi et al. 2010; Zhu et al. 2010; Yao and Wang 2016; Wang and Yao 2017; Shiji and Siril 2018). In P. massoniana, the promotive effect of NAA on root regeneration in vitro has been confirmed in our previous studies (Wang and Yao 2017), while the root regeneration protocol is not well developed for the mass propagation of selected genotypes in this species. Physiological age is closely related to the root regeneration capacity of P. massoniana, and hence, the root regeneration rate of shoots originating from juvenile materials, including seedlings, is often high according to our previously published results (Wang et al. 2019). To obtain rooted shoots with robust root systems, in general root growth (root regeneration rate) and root formation (root length) need to be simultaneously considered. Here, only shoots with roots ≥ 20 mm can only be counted as rooted shoots. In this study, the low root regeneration rate of 55.3% observed from the NAA medium indicated that NAA was not effective to the root formation in P. massoniana. However, the combined application of NAA and PBZ in the root regeneration medium obviously enhanced the percentage of rooted shoots with a well-developed root system, suggesting the promotive effects of PBZ on root formation. PBZ, an inhibitor of gibberellin (GA) biosynthesis, is extensively used as a promotive regulator of root development in plants (Watson 1996, 2004; Ragonezi et al. 2010; Salari et al. 2017; Muhammad et al. 2018). There is a remarkable crosstalk between GA and indole acetic acid (IAA, a core plant hormone promoting plant growth) (Huang et al. 2007), and it is mostly reported that GA promotes the growth of plants (Hardtke 2003); hence, GA is usually required for the formation of adventitious roots by IAA modulating its response (Fu and Harberd 2003). In the present study, the observed influences of PBZ on root development indicated the possibly negative roles of GA in regulating root formation. Further research on the relationships between GA and root regeneration capacity during the development of adventitious roots is needed in P. massoniana. Pinus species are the most widely distributed conifers in the world, whereas most of them are recalcitrant to adventitious root regeneration. More positive responses of plant regeneration to exogenous PBZ in culture medium can be expected.
To evaluate the adaptability of rooted shoots with well-developed root systems to the natural environment, plantlets regenerated in vitro with the methodology described in the present study and plantlets directly originating from somatic embryogenesis (somaplants) were both transferred to the field at a similar plant height (approximately 10 cm). For one year after the field transfer, the variations in plant height and caliper were investigated monthly for both plant sources (i.e., regenerated plantlets and somaplants). Our results showed that regenerated plantlets grew significantly faster than those from somaplants. Clearly, root mass influences the growth of aboveground parts in plants. P. massoniana is a species with a taproot system that has a poor root system in seeded seedlings (Ding et al. 2006). Previous studies clarified that root cutting can effectively improve root mass and cause an increase in seedling growth and the acceleration of seedling establishment, resulting in the wide use of the technology in the cultivation of P. massoniana seedlings (Wan 2016; Lin 2019). Here, the regenerated plantlets developed a robust adventitious root system via radicle cutting, suggesting that a critical reason for the difficulty of somaplants adapting to harsh conditions could be their poor root system. This finding would be able to provide useful instruction for the improvement and application of somatic embryogenesis in practice.
Conclusion
This study explored critical factors affecting in vitro plantlet regeneration originating from somatic embryos and assessed growth performance following field transfer. The application of AC in the medium promoted germination and growth of mature somatic embryos under optimizing AC concentration conditions, and the promotive effect of AC on growth could be strengthened by combining radicle cutting of germinating somatic embryos. TDZ was essential for shoot multiplication, while the use of high concentrations of TDZ in the medium is a retardant to shoot growth. The application of exogenous NAA plus PBZ in culture medium significantly enhanced the root regeneration capacity of shoots. In comparison to somaplants, the growth performance of in vitro plantlets regenerated with the methodology described in this study was remarkably improved one year after field transfer. A young stand was first established using plantlets originating from somatic embryos of P. massoniana.
Acknowledgements
We thank the local Paiyangshan Forest Farm for their valuable fieldwork and collection of plant materials. We thank the anonymous reviewers for their valuable comments. This project was supported by the Science and Technology Plan of Guangxi (nos. AD17195078, 2017GXNSFAA198037, 2018GXNSFDA281020, and AA17204087-1), and the National Natural Science Foundation of China grant (nos. 31960311, and 31360178).
Author contributions
The research topic and framework was defined, and the whole study was supervised by RY. YW performed the tissue culture work and the interpretation of data. Evaluations on plantlet regeneration and field growth were carried out by YW. RY wrote and revised the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that there was no conflict of interest.
Footnotes
Ruiling Yao and Yin Wang contributed equally to this work.
Contributor Information
Ruiling Yao, Email: jullyudi@163.com.
Yin Wang, Email: yinvvang@163.com.
References
- Aderson AB, Frampton LJ, McKeand SE. Tissue-culture shoot and root system effects on field performance of loblolly pine. Can J Forest Res. 1992;22:56–61. [Google Scholar]
- Aitken-Christie J, Singh AP, Davies H. Multiplication of meristematic tissue: a new tissue culture system for radiata pine. In: Hanover JW, Keathley DE, editors. Genetic manipulation of woody plants. New York: Plenum; 1988. pp. 413–432. [Google Scholar]
- Aquea F, Poupin MJ, Matus JT, Gebauer M, Medina C, Arce-Johnson P. Synthetic seed production from somatic embryos of Pinus radiata. Biotechnol Lett. 2008;30:1847–1852. doi: 10.1007/s10529-008-9754-x. [DOI] [PubMed] [Google Scholar]
- De Diego N, Montalbán IA, Fernández E, Moncaleán P. In vitro regeneration of Pinus pinaster adult trees. Can J Forest Res. 2008;38:2607–2615. [Google Scholar]
- Ding GJ, Zhou ZC, Wang ZR. Cultivation and utilization of pulpwood stand for Pinus massoniana. Beijing: Forestry Publisher of China; 2006. [Google Scholar]
- Fu X, Harberd NP. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature. 2003;421:740–743. doi: 10.1038/nature01387. [DOI] [PubMed] [Google Scholar]
- Hardtke CS. Gibberellin signaling: GRASs growing roots. Curr Biol. 2003;13:366–367. doi: 10.1016/s0960-9822(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Huang JQ, Wei ZM. Tissue and protoplast culture of Pinus species. Chin Bull Bot. 1994;11:34–42. [Google Scholar]
- Huang Y, Ji KS, Zhai JR. Relationship between rooting ability and endogenous phytohormone changes in successive continuous generation cuttings of Buxussinica var. parvifolia, an endangered woody species in China. Forest Stud China. 2007;9:189–197. [Google Scholar]
- Huetteman CA, Preece JE. Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tiss Org. 1993;33:105–119. [Google Scholar]
- Ji KS, Wang ZR, Chen TH, Wang MX. Cyclophysis and effect of rejuvenation with continued cuttage in Pinus massoniana cutting propagation. J Zhejiang Forest Coll. 1996;16:341–345. [Google Scholar]
- Kanyand N, Dessai AP, Prakash SC. Thidiazuron promotes high frequency regeneration of peanut (Arachis hypogea) plants in vitro. Plant Cell Rep. 1994;14:1–5. doi: 10.1007/BF00233288. [DOI] [PubMed] [Google Scholar]
- Klimaszewska K, Hargreaves C, Lelu-Walter M, Trontin J. Advances in conifer somatic embryogenesis since year 2000. In: Germanà MA, Lambardi M, editors. In vitro embryogenesis in higher plants methods in molecular biology. New York: Springer Science and Business Media; 2016. pp. 131–166. [DOI] [PubMed] [Google Scholar]
- Klimaszewska K, Trontin JF, Becwar MR, Devillard C, Park YS, Lelu-Walter MA. Recent progress in somatic embryogenesis of four Pinus spp. Tree For Sci Biotechnol. 2007;1:11–25. [Google Scholar]
- Li XY, Lv CQ, Huang BL, Wu QM, Zhang MH. Adventitious roots' induction of Pinus massoniana shoots in test tubes and anatomical observation. J Northwest Forest Coll. 2009;24:80–84. [Google Scholar]
- Lin Y. Effects of root cutting on the Pinus massoniana seedlings and afforestation. Fujian Forest. 2019;7:46–48. [Google Scholar]
- Marhavý P, Bielach A, Abas L, Abuzeineh A, Duclercq J, Tanaka H, Pařezová M, Petrášek J, Friml J, Kleine-Vehn J, et al. Cytokinin modulates endocytic trafficking of PIN1 auxin efflux carrier to control plant organogenesis. Dev Cell. 2011;21:796–804. doi: 10.1016/j.devcel.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Marhavý P, Vanstraelen M, De Rybel B, Zhaojun D, Bennett MJ, Beeckman T, Benkova E. Auxin reflux between the endodermis and pericycle promotes lateral root initiation. EMBO J. 2013;32:149–158. doi: 10.1038/emboj.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriat M, Petterle A, Bellini C, Moritz T. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. Plant J. 2014;78:372–384. doi: 10.1111/tpj.12478. [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ, Ross JJ. Shoot-derived abscisic acid promotes root growth. Plant Cell Environ. 2016;39:652–659. doi: 10.1111/pce.12669. [DOI] [PubMed] [Google Scholar]
- Montalbán IA, De Diego N, Aguirre Igartua E, Setién A, Moncaleán P. A combined pathway of somatic embryogenesis and organogenesis to regenerate radiata pine plants. Plant Biotechnol Rep. 2011;5:177–186. [Google Scholar]
- Montalbán IA, De Diego N, Moncaleán P. Bottlenecks in Pinus radiata somatic embryogenesis: improving maturation and germination. Trees. 2010;24:1061–1071. [Google Scholar]
- Muhammad K, Su W, Irshad A, Meng X, Cui W, Zhang X, Mou S, Aaqil K, Han Q. Application of paclobutrazol affect maize grain yield by regulating root morphological and physiological characteristics under a semi-arid region. Sci Rep. 2018 doi: 10.1038/s41598-018-23166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nunes S, Sousa D, Pereira VT, Correia S, Marum L, Santos C, Dias MC. Efficient protocol for in vitro mass micropropagation of slash pine. Vitro Cell Dev Biol-Plant. 2018;54:175–183. [Google Scholar]
- Pan JJ, van Staden J. The use of activated charcoal in in vitro culture—a review. Plant Growth Regul. 1998;26:155–163. [Google Scholar]
- Pullman GS, Zeng X, Copeland-Kamp B, Crockett J, Lucrezi J, May SW, Bucalo K. Conifer somatic embryogenesis: improvements by supplementation of medium with oxidation–reduction agents. Tree Physiol. 2015;35:209–224. doi: 10.1093/treephys/tpu117. [DOI] [PubMed] [Google Scholar]
- Ragonezi C, Klimaszewska K, Castro MR, Lima M, de Oliveira P, Zavattieri MA. Adventitious rooting of conifers: influence of physical and chemical factors. Trees. 2010;24:975–992. [Google Scholar]
- Salari H, Baninasab B, Akbari M, Rohani AM. Effect of paclobutrazol on adventitious root formation of IBA-treated cuttings of ‘Zard’ and ‘Dakal’ Olive (Olea europaea L.) Cultivars. Asian J Appl Sci. 2017;5:692–699. [Google Scholar]
- Shen XH. Technology innovation-afforestation of Pinus species with cuttings in Guangdong. Forest Technol Comm. 2018;61:63–64. [Google Scholar]
- Shiji PC, Siril EA. An improved micropropagation and ex vitro rooting of a commercially important crop Henna (Lawsonia inermis L.) Physiol Mol Biol Pla. 2018;24:1273–1278. doi: 10.1007/s12298-018-0600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique I, Anis M. In vitro shoot multiplication and plantlet regeneration from nodal explants of Cassia angustifolia-a medicinal plant. Acta Physiol Plant. 2007;29:333–338. [Google Scholar]
- Sul I, Korban SS. Effect of different cytokinins on axillary shoot proliferation and elongation of several genotypes of Sequoia sempervirens. Vitro Cell Dev Biol-Plant. 1994;30:131–135. [Google Scholar]
- Thomas TD. The role of activated charcoal in plant tissue culture. Biotechnol Adv. 2008;26:618–631. doi: 10.1016/j.biotechadv.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Van Winkle SC, Pullman GS. The combined impact of pH and activated carbon on the elemental composition of a liquid conifer embryogenic tissue initiation medium. Plant Cell Rep. 2003;22:303–311. doi: 10.1007/s00299-003-0686-6. [DOI] [PubMed] [Google Scholar]
- Walters C, Find JI, Grace LJ. Somatic embryogenesis and genetic transformation in Pinus radiata. In: Mohan SJ, Gupta PK, editors. Protocol for somatic embryogenesis in woody plants. Dordrecht: Springer; 2010. pp. 11–24. [Google Scholar]
- Wan XD. Root cutting and its effect in seedling culture of Pinus massoniana. South China Agric. 2016;10(35–36):52. [Google Scholar]
- Wang Y, Yao RL. Plantlet regeneration of adult Pinus massoniana Lamb. trees using explants collected in March and thidiazuron in culture medium. J Forest Res. 2017;28:1169–1175. [Google Scholar]
- Wang Y, Yao RL, Li HJ, Zhang Y. In vitro sterilized culture of nodal segments based on explants physiological rejuvenation in Pinus massoniana. Plant Physiol J. 2019;55:1375–1384. [Google Scholar]
- Watson G. Effect of transplanting and paclobutrazol on root growth of ‘Green Column’ black maple and ‘Summit’ green ash. J Environ Hortic. 2004;22:209–212. [Google Scholar]
- Watson GW. Tree root system enhancement with paclobutrazol. J Arbor. 1996;22:211–217. [Google Scholar]
- Yang MH, Zhang DL, Li ZH, Jin XC, Ding GJ. Somatic embryogenesis with immature embryos of masson pine (Pinus massoniana Lamb.) Plant Physiol J. 2011;47:904–912. [Google Scholar]
- Yao RL, Wang Y. An effective protocol for regenerating mature Pinus massoniana L. trees by tissue culture. Res J Biotechnol. 2016;11:75–80. [Google Scholar]
- Yin SL, Zhang DL, Yang MH, Li ZH, Wang Q, Shi K. Effects of explant collecting time and storage duration on callus induction of Pinus massoniana. Guangxi Forest Sci. 2013;42:8–13. [Google Scholar]
- Zhu LH, Wu XQ, Qu HY, Ji J, Ye JR. Micropropagation of Pinus massoniana and mycorrhiza formation in vitro. Plant Cell Tiss Org. 2010;102:121–128. [Google Scholar]