Abstract
Purpose
MiR-654-3p plays important roles in many types of malignant tumours. However, the biological function of miR-654-3p in non-small cell lung cancer (NSCLC) remains unknown. In this study, the role of miR-654-3p in NSCLC was investigated.
Methods
qRT-PCR was used to evaluate the level of miR-654-3p in NSCLC tissues and cell lines, while Cell Counting Kit-8, Annexin V/propidium iodide dual staining or TUNEL staining were used to investigate proliferation and apoptosis of NSCLC cells. Luciferase assays and Western blotting were performed to validate potential targets of miR-654-3p.
Results
MiR-654-3p levels were significantly decreased in NSCLC patients and cell lines and were significantly correlated with the tumour size and tumour node metastasis stage of NSCLC patients. In A549 cells, miR-654-3p overexpression significantly increased apoptosis and inhibited growth both in vivo and in vitro, while downregulation of miR-654-3p had the opposite effects. In addition, polo-like kinase 4 (PLK4) was shown to be a target gene of miR-654-3p that is negatively regulated by miR-654-3p in A549 cells. Furthermore, PLK4 was observed to be highly expressed in NSCLC tissues and cells, and PLK4 overexpression abolished the inhibitory effects of miR-654-3p overexpression on NSCLC cell proliferation. Finally, the animal experiment results further demonstrated that miR-654-3p inhibits tumour growth and regulates PLK4 expression.
Conclusion
Our results demonstrate that miR-654-3p functions as a growth-suppressing miRNA by targeting PLK4 in NSCLC.
Keywords: non-small cell lung cancer, miR-654-3p, polo-like kinase 4, apoptosis
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide, and non-small cell lung cancer (NSCLC) is the most common type of cancer affecting the lungs. It is estimated that approximately 85% of diagnosed lung cancers are NSCLC, including adenocarcinoma, squamous cell carcinoma, and many other histologies.1,2 The progression of NSCLC is a multistage process that is believed to include the deregulation of genes involved in cell cycle control, cell growth, apoptosis and cell migration. At present, an increasing number of studies have focused on investigating the roles of microRNAs (miRNAs) in the development of NSCLC. The results of these studies have shown that miRNAs play important roles in lung tumour development and progression, such as participation in the regulation of tumour cell proliferation and metastasis, cancer stem cell differentiation, and apoptosis.3–6 MiRNAs are a class of small non-coding genes that control the expression of their target mRNAs. In recent studies, many investigations have observed changes in the expression of many miRNAs, some of which showed significant correlations with the severity of NSCLC. Moreover, many differentially expressed miRNAs have been regarded as potential biomarkers for the diagnosis and prognosis NSCLC, as well as targets for treatment.7,8 MiR-654-3p, a recently identified miRNA, was first described publicly in 2010. However, its biological function was not disclosed until 2014.9 In papillary thyroid and ovarian cancers, the restoration of miR-654-3p has been reported to decrease cell proliferation and migration and induce the reprogramming of metastasis-related genes, suggesting a tumour suppressor role of this miRNA.10,11 Moreover, other published reports have confirmed that miR-654-3p may serve as a promising non-invasive biomarker to predict the efficacy of carbon ion radiotherapy for prostate cancer, suggesting that it has an important role in the development of malignant tumours.12 Nonetheless, the function and role of miR-654-3p in NSCLC has not yet been elucidated, although miR-654-3p expression has been reported to increase in NSCLC tissue.13 Therefore, in this study, we investigated miR-654-3p and its downstream biomolecular targets to gain a better understanding of the pathogenesis of NSCLC, the results of which may provide a promising therapeutic target for NSCLC.
Materials and Methods
Human Tissue Samples and Cell Culture
One hundred and seven (107) pairs of tumour and matched noncancerous tissue samples were collected from NSCLC patients (71 males and 59 females) who underwent surgical resection at the affiliated hospital of Southwest Medical University of China (Luzhou, China) from May 2017 to March 2019. The protocol (CE20160136) was approved by the Ethics Review Board of the affiliated hospital of Southwest Medical University and carried out in accordance with the Code of Ethics of the World Medical Association and the ethical guidelines of our institution. All patients were informed and provided written consent before the samples were collected. The inclusion criteria cover patients who had been diagnosed with NSCLC as the pathological type after surgical biopsy. Patients’ clinical medical records and signed informed consent forms were available. Exclusion criteria included NSCLC patients that had received radiotherapy, chemotherapy or immunotherapy, and patients that exhibited an unclear clinical stage of NSCLC or identified as non-pulmonary primary tumours. Also, NSCLC patients that exhibited other chronic lung diseases such as chronic obstructive pneumonia, chronic bronchitis, pulmonary interstitial diseases, pulmonary hypertension and tuberculosis, etc. were excluded. Detailed demographic information and clinical pathological parameters are presented in Table 1. Normal lung epithelial cells (BEAS-2B) and NSCLC cell lines (A549, H1299, SPC-A1 and H226) were obtained from the American Type Culture Collection (Manassas, VA, USA). All cells were cultured in RPMI-1640 medium supplemented with 10% foetal bovine serum, 50 U/mL penicillin, and 50 mg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
Table 1.
Relationship of MiR-654-3p with Pathological Features of NSCLC Patients
| Factors | Numbersa | MiR-654-3p Low | MiR-654-3p High | P value |
|---|---|---|---|---|
| Sex | 0.165 | |||
| Male | 51 | 27 | 24 | |
| Female | 56 | 38 | 18 | |
| Age (years) | 0.558 | |||
| <60 | 42 | 20 | 22 | |
| ≥60 | 65 | 35 | 30 | |
| Histological type | 0.691 | |||
| Squamous cell carcinoma | 45 | 28 | 17 | |
| Adenocarcinoma | 62 | 35 | 27 | |
| Differentiation | 0.167 | |||
| Well/Moderate | 43 | 19 | 24 | |
| Poor | 64 | 38 | 26 | |
| Tumour size | 0.007 | |||
| <5 cm | 58 | 22 | 36 | |
| ≥5 cm | 49 | 32 | 17 | |
| TNM stage | 0.002 | |||
| I+II | 39 | 15 | 24 | |
| III+IV | 68 | 48 | 20 |
Note: aThe patients (107) were divided into miR-654-3p high and low groups based on the median miR-654-3p level.
Abbreviation: TNM, tumour node metastases.
Cell Transfection
A549 cells were transfected with the LV-hsa-miR-654-3p-mimic vector (miR-654-3p-mimic) or the LV-hsa-miR-654-3p-inhibitor vector (miR-654-3p-inhibitor) (GenePharma, Shanghai, China), while the LV empty lentiviral construct (mimic or inhibitor control) served as a negative control. To generate a polo-like kinase 4 (PLK4) overexpression construct, the gene encoding human PLK4 was cloned to generate the plasmid pcDNA3.1-PLK4 (GenePharma) and then transferred into A549 cells according to the manufacturer’s instructions.
Quantitative Real-Time PCR
Total RNA was extracted from human tissues or cell lines using TRIzol reagent (Invitrogen, CA, USA) according to the manufacturer’s protocol. To assess the level of miR-654-3p, small RNAs were reverse transcribed to cDNA using a miRNA First-Strand cDNA Synthesis kit (Invitrogen) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) analysis of miR-654-3p expression was performed in triplicate with SYBR Green PCR Master Mix (Takara, Japan) according to the manufacturer’s instructions. U6 was used to normalize gene expression. Furthermore, to detect the mRNA level of PLK4, RNA was reverse transcribed to cDNA using oligo (dT) primers, and GAPDH was used to normalize gene expression. Data analysis was performed using the 2−ΔΔCt method.
MiRNA Target Prediction
The predicted target genes of miR-654-3p were identified using TargetScan (http://targetscan.org/) and miRDB (http://www.mirdb.org/miRDB/).
Luciferase Reporter Assay
Wild-type or mutant PLK4 3ʹ-UTR sequences were inserted into the vector pmir-GLO-promoter (Promega, Madison, USA). A549 cells were seeded in 24-well plates and transfected with 100 ng of the pmirGLO-PLK4-wild-type (WT), pmirGLO-PLK4-mutant (MT), miR-654-3p mimics, or normal control (NC, GenePharma, Shanghai, China) vectors by using Lipofectamine 2000 (Invitrogen, CA, USA). After 24 h, the relative luciferase activity was measured by using a Dual-Glo Luciferase Assay kit (Promega, USA).
Cell Proliferation Assay
A549 were grown to over 80% confluence and then digested with 0.25% trypsin into a single cell suspension. After counting, the cells were seeded in a 96-well plate at a density of 5×103 cells per well. The cells were cultured in RPMI-1640 medium supplemented with 10% foetal bovine serum, 50 U/mL penicillin, and 50 mg/mL streptomycin at 37°C in a humidified atmosphere containing 5% CO2. The plate was removed after 24, 48, and 72 h cultivation, and then 10 µL/well of Cell Counting Kit-8 solution was added (Sigma, St. Louis, MO, USA). Subsequently, the cells were incubated for an additional 4 hours, and the optical density (OD) of each well was measured at 450 nm by using a microplate reader (Thermo Scientific, MA, USA).
Apoptosis Assay
To assess apoptosis in vitro, 5×104 cells were seeded in each well of 24-well plate, and after 48 h, the cells were hydrated with PBS and then washed twice. Subsequently, staining was performed with Annexin V/propidium iodide (BD Biosciences, San Jose, CA, USA), and the cells were evaluated using a BD FACSCanto II (BD, Biosciences, USA) flow cytometer. To assess apoptosis in vivo, all specimens were fixed in 4% paraformaldehyde overnight at 4°C, permeabilized with 0.1% Triton X-100 and then incubated with TUNEL staining solution (Beyotime, Haimen, China) for 1 h according to the manufacturer’s protocol. TUNEL-positive cells were counted in five randomly selected fields of the slide under a microscope (Olympus).
Western Blotting Analysis
Radio immuno-precipitation assay buffer was used to prepare tissue or whole-cell lysates. Equal amounts of proteins were separated by 12% SDS-polyacrylamide gel electrophoresis and transferred to Polyvinylidene Fluoride membranes. Following blocking with 5% bovine serum albumin, the membranes were probed with anti-PLK4 and anti-GAPDH antibodies (Santa Cruz, CA, USA), which was followed by an incubation with a horseradish peroxidase-conjugated secondary antibody. GAPDH was used as a loading control.
Tumourigenicity Assay in vivo
Animals studies were approved by the Animal Care and Use Committee of the Southwest Medical University (SL20170874) and conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986 and associated guidelines. Twenty-four male BALB/c nude mice were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). To evaluate the effects of miR-654-3p on NSCLC growth in vivo, we prepared A549 cells transfected with the miR-654-3p mimic or miR-654-3p inhibitor and control cells, which were subcutaneously injected into the right subcutaneous anterior axillary of BALB/c nude mice (2×106 cells per mouse). The nude mice were randomly divided into the following three groups (n=8 each): the normal control group, the miR-654-3p inhibitor group and the mimic group. Tumour volume (V) was monitored every 4 days beginning at 12 days after the first injection and was calculated using the formula V = 0.5×L×W2, where “L” is tumour length and “W” is tumour width. The animals were sacrificed 32 days after injection, and the tumour tissues were removed.
Immunohistochemistry
Tissue sections were prepared from patients and animals and were deparaffinized and boiled in 10 mM citrate buffer (pH 6.0) for antigen retrieval. Endogenous peroxidase was blocked with 3% H2O2. Afterwards, all slides were blocked in serum, incubated with an anti-PLK4 antibody (Santa Cruz, CA, USA) at 4°C overnight and then incubated with an anti-rabbit secondary antibody. Finally, the slides were visualized with diaminobenzidine (Sigma). For immunohistochemical staining, five random fields were imaged under a microscope (Olympus) at 200× magnification.
Statistical Analysis
The data are presented as the means ± SD. Three replicates of each in vitro experiment were performed. One-way analysis of variance (ANOVA), least significant difference (LSD), Chi-square and Student’s t-test (SPSS 17.0, IBM, Inc., Chicago, IL, USA) were used for statistical analyses. The correlation analyses between miRNA expression with tumour size or PLK4 expression were performed using GraphPad Prism 8.0 version (GraphPad Software, San Diego, CA, USA).
Results
MiR-654-3p Expression in NSCLC Tissues and Cell Lines
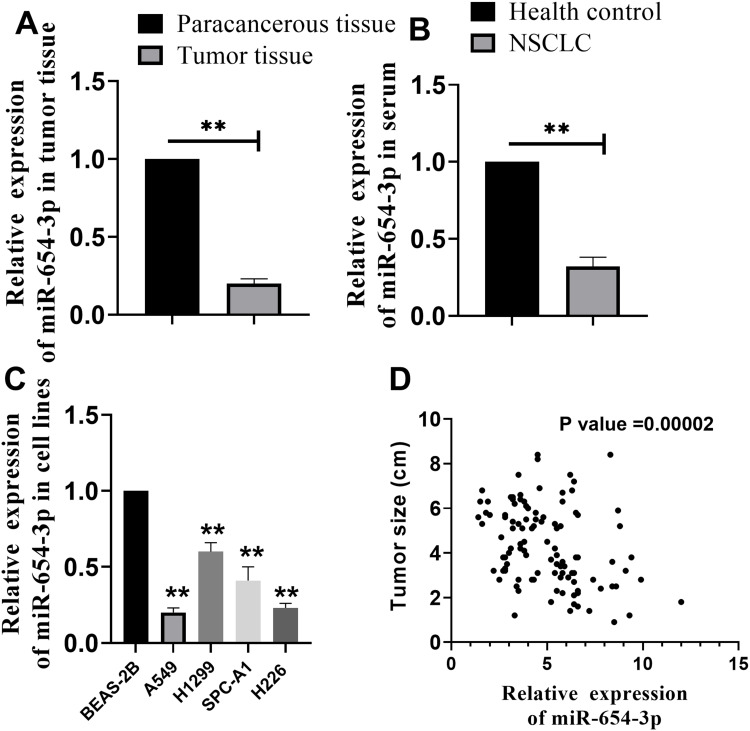
To investigate the important role of miR-654-3p in NSCLC, we assessed the expression pattern of miR-654-3p in NSCLC tissues collected from patients. As shown in Figure 1A, the level of miR-654-3p expression in the NSCLC tissues was lower than that observed in the paracancerous tissues (P<0.01). Furthermore, decreased miR-654-3p levels were also observed in the blood of NSCLC patients compared with that observed in the health controls (Figure 1B, P<0.01). Subsequently, we further investigated the relationship between miR-654-3p expression and tumour size in patients with NSCLC. To this end, we first divided the 107 patients into high and low expression groups based on the median miR-654-3p level. As shown in Table 1 and Figure 1D, miR-654-3p expression was negatively correlated with tumour size. Finally, we examined miR-654-3p levels in NSCLC cell lines. Compared with the control cells (normal lung epithelial cells, BEAS-2B), miR-654-3p expression was reduced in all NSCLC cell lines (Figure 1C, P<0.01), including A549, H1299, SPC-A1 and H226.
Figure 1.
MiR-654-3p expression in specimens from NSCLC patients. (A–B) MiR-654-3p expression in tumour tissue and blood from NSCLC patients was detected by qRT-PCR (N =107). (C) MiR-654-3p expression in normal lung epithelial cells (BEAS-2B) and NSCLC cell lines (A549, H1299, SPC-A1 and H226). (D) Correlation analysis of miR-654-3p and tumour size (N = 107; **P < 0.01 vs the paracancerous tissue, healthy control or BEAS-2B).
MiR-654-3p Inhibits the Proliferation of NSCLC Cells
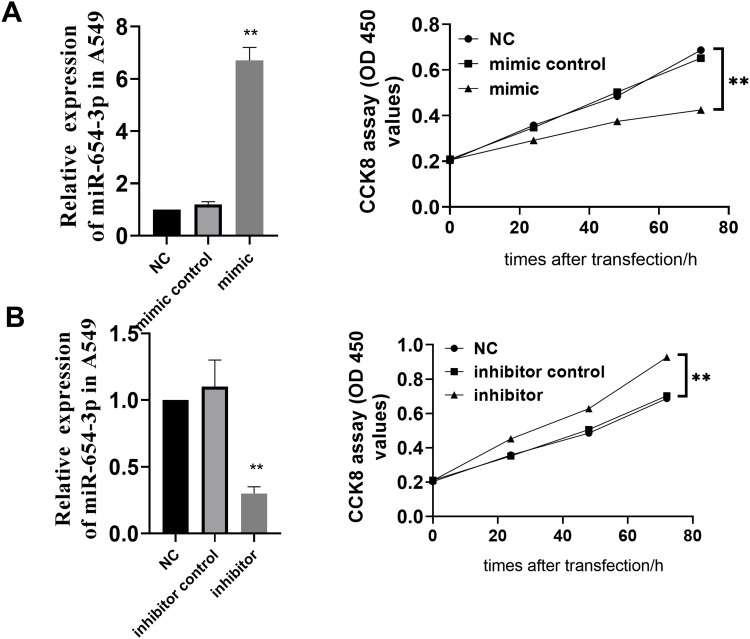
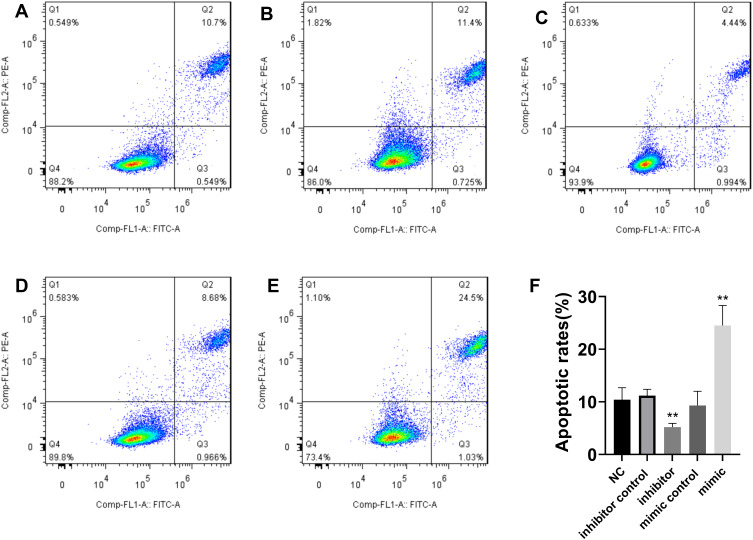
To study the effect of miR-654-3p on the proliferation of NSCLC cells, A549 cells were transfected with vectors LV-hsa-miR-654-3p-mimic (miR-654-3p mimic) and LV2-hsa-miR-654-3p-inhibitor (miR-654-3p inhibitor) to promote the overexpression and downregulation of miR-654-3p. As shown in Figure 2A and B, miR-654-3p levels were upregulated nearly 7-fold and decreased by approximately 70% in A549 cells transfected with the miR-654-3p mimic and miR-654-3p inhibitor, respectively (P<0.01). Overexpression of miR-654-3p significantly decreased the cell viability of A549 cells, while the downregulation of miR-654-3p markedly promoted A549 cell proliferation (P<0.01). The apoptotic assay results as presented in Figure 3 show that miR-654-3p overexpression significantly promoted A549 cells apoptosis, whereas miR-654-3p downregulation had the opposite effects (P<0.01).
Figure 2.
MiR-654-3p inhibits A549 cell proliferation. Cell viability was determined in A549 cells transfected with the LV2-hsa-miR-654-3p-mimic (A) or LV2- hsa-miR-654-3p-inhibitor vectors (B), and the levels of miR-654-3p were detected by qRT-PCR. The effect of miR-654-3p on cell viability was determined in A549 cells. The data are presented as the means ± SD of three measurements. **P< 0.01 vs the NC.
Figure 3.
MiR-654-3p promotes A549 cell apoptosis. A549 apoptotic death was determined by Annexin V/propidium iodide staining. (A): normal control (NC); (B): inhibitor control; (C): inhibitor; (D): mimic control; (E):mimic; (F): the data are presented as the means± SD of three measurements. **P < 0.01 vs the NC.
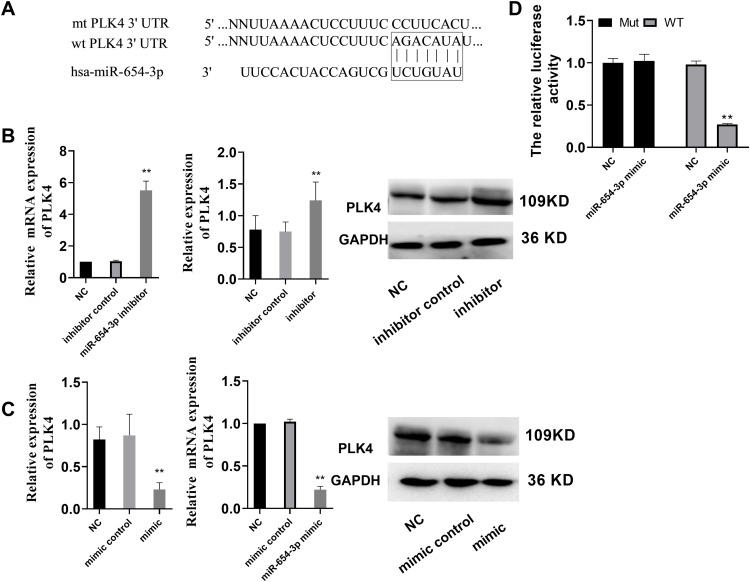
PLK4 is a Direct Target of MiR-654-3p
To better understand the molecular mechanism associated with the ability of miR-654-3p to promote cell death, we used the miRNA target analysis tools TargetScan and miRanda to predict putative target molecules of miR-654-3p. The results showed that PLK4 mRNA was a good match for miR-654-3p (Figure 4A). Therefore, PLK4 was selected for further investigation as a potential target of miR-654-3p in the present study. To validate PLK4 as a target of miR-654-3p, we performed a luciferase assay using luciferase expression plasmids containing either WT or MT PLK4. The results demonstrated that miR-654-3p inhibited luciferase activity in A549 cells with the PLK4-WT but not PLK4-MT construct (Figure 4D, P<0.01). Subsequently, we treated A549 cells with the miR-654-3p inhibitor or mimic and assessed PLK4 mRNA and protein levels by qRT-PCR and immunoblotting, respectively. As shown in Figure 4B and C, the miR-654-3p overexpression decreased PLK4 mRNA and protein levels in A549 cells, whereas miR-654-3p downregulation had the opposite effect (P<0.01).
Figure 4.
PLK4 is a direct target gene of miR-654-3p. (A) The predicted miR-654-3p-binding site in the 3ʹUTR of PLK4 mRNA and its mutated version. (B–C) PLK4 mRNA and protein expression levels were measured by qRT-PCR and Western blot analysis using GAPDH as the loading control after transfection of the miR-654-3p inhibitor or mimic in A549 cells, respectively. (D) Dual-luciferase reporter assay. The relative luciferase activity was assessed after cells were co-transfected with the miR-654-3p mimic. The data are presented as the means ± SD of three measurements. **P < 0.01 vs the NC.
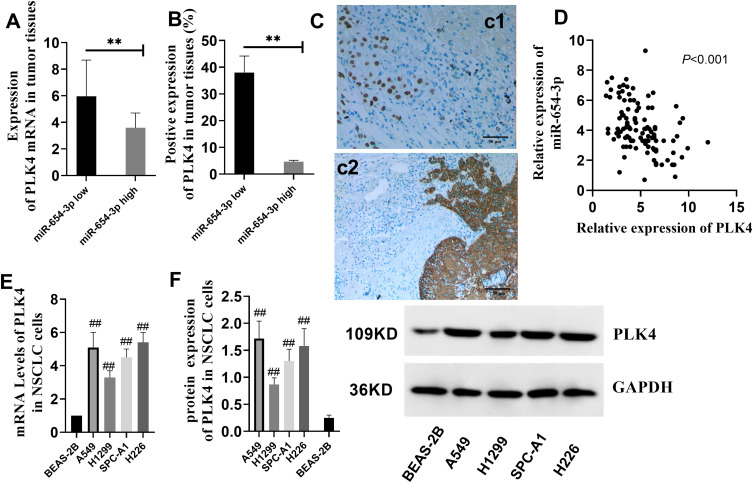
PLK4 Expression in NSCLC Patients
After confirming that PLK4 is a direct downstream target of miR-654-3p, we investigated the expression of PLK4 in NSCLC patients and cell lines. As shown in Figure 5A and B, PLK4 mRNA and protein levels in the miR-654-3p low group were significantly higher than those observed in the miR-654-3p high group (P<0.01). Furthermore, the level of miR-654-3p was negatively correlated with PLK4 expression (Figure 5D, P<0.01). In addition, PLK4 levels were also increased in the tumour tissues compared with those observed in the paracancerous tissues (Figure 5C, P<0.01). Consistent with these results, further analysis of PLK4 in NSCLC cells revealed that the PLK4 mRNA and protein levels were higher in A549, H1299, SPC-A1 and H226 cells than those observed in the BEAS-2B cells (Figure 5E and F, P<0.01).
Figure 5.
PLK4 expression is upregulated in NSCLC patients and cells. (A–B) The levels of PLK4 mRNA and protein expression in NSCLC patients were measured (**P< 0.01 vs the miR-654-3p low). (C) PLK4 expression in tumour tissue (c2) and adjacent tissue (c1). The pictures were captured with a final magnification (×200). (D) Correlation analysis of miR-654-3p and PLK4 (N=107). (E–F) PLK4 mRNA and protein expression in NSCLC cell lines. The data are presented as the means ± SD of three measurements. ##P < 0.01 vs the BEAS-2B.
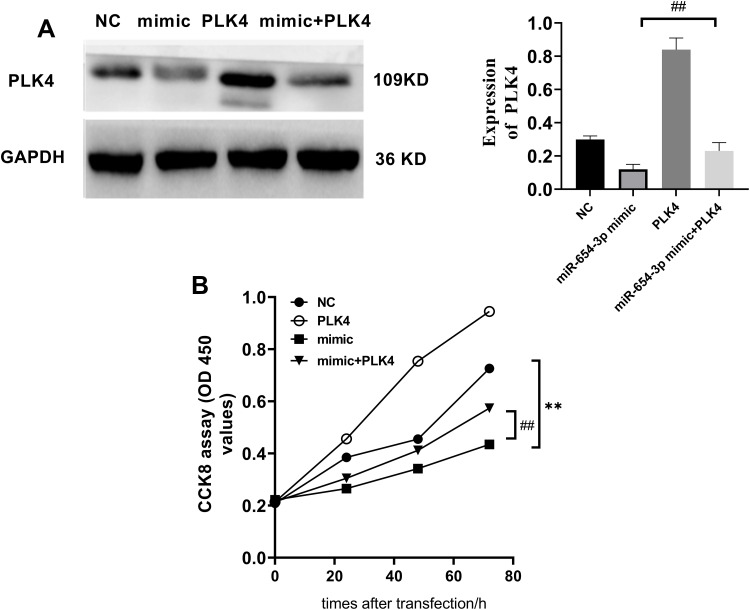
Targeting PLK4 is Required for the Biological Functions of MiR-654-3p in NSCLC Cells
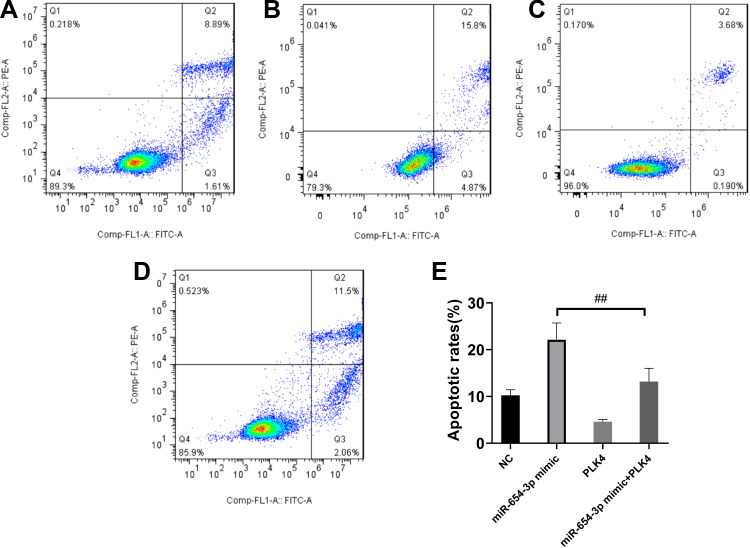
To determine whether miR-654-3p exerts its tumour-suppressing activity through the downregulation of PLK4, we overexpressed PLK4 in miR-654-3p mimic-transfected A549 cells (Figure 6A). As shown in Figure 6B, PLK4 overexpression decreased the miR-654-3p-induced inhibitory effects on A549 cell viability (P<0.01). Furthermore, the results presented in Figure 7 show that PLK4 overexpression in A549 cells inhibited miR-654-3p-induced apoptosis (P<0.01).
Figure 6.
PLK4 overexpression reverses the inhibitory effects of miR-654-3p on the proliferation of A549 cells. (A) The PLK4 expression vector significantly increased the expression of PLK4 in A549 cells treated with the miR-654-3p mimic. (B) The effect of miR-654-3p and PLK4 on cell viability was determined in A549 cells. The data are presented as the means ± SD of three measurements. **P < 0.01 vs the NC; ##P < 0.01 vs the miR-654-3p mimic.
Figure 7.
PLK4 overexpression reverses the effects of miR-654-3p on the apoptosis of A549 cells. A549 cell apoptosis was assessed by Annexin V/propidium iodide staining. (A): normal control (NC); (B): miR-654-3p mimic; (C): PLK4 vector; (D): miR-654-3p mimic+PLK4 vector, (E): the data are presented as the means ± SD of three measurements. ##P < 0.01 vs the miR-654-3p mimic.
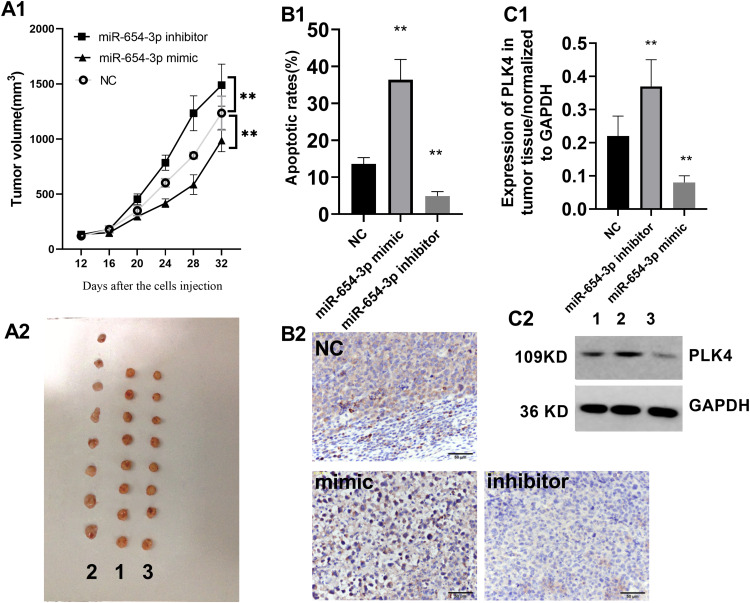
MiR-654-3p Represses A549 Cell-Mediated Tumourigenesis in vivo
Subsequently, we explored the function of miR-654-3p in the progression of NSCLC in vivo. We subcutaneously injected A549 cells in BALB/c nude mice to induce tumour formation. Twelve days after injection, the tumour volumes were measured every 4 days. As shown in Figure 8A, the tumour growth curves revealed a significantly higher growth rate in the miR-654-3p downregulated group and a lower growth rate in the miR-654-3p upregulated group compared with that observed in the control group (P<0.01). In addition, compared with the normal control (NC) group, the TUNEL staining intensities were decreased in the tumours from the miR-654-3p inhibitor group and increased in tumours from the miR-654-3p mimic group (Figure 8B, P<0.01). Finally, the miR-654-3p mimic and miR-654-3p inhibitor groups showed decreased and increased PLK4 levels in tumour tissues, respectively, compared with that observed in the NC group (Figure 8C, P<0.01).
Figure 8.
MiR-654-3p inhibits A549 xenograft tumourigenicity in vivo. (A1–A2) The tumour volume was recorded every 4 days starting at 12 days after the initial injection. 1: NC; 2: miR-654-3p inhibitor; 3: miR-654-3p mimic. (B1–B2) Apoptosis in tumour tissues was assessed by TUNEL staining, and the pictures were captured with a final magnification (×200); and (C1–C2) PLK4 expression in the implanted tumours was assessed by Western blotting. 1:NC; 2: miR-654-3p inhibitor; 3: miR-654-3p mimic. The data are presented as the means ± SD. N=8; **P < 0.01 vs the NC.
Discussion
Although miR-654-3p has been confirmed to be a tumour suppressor in thyroid and ovarian cancers, however, whether it suppresses the development of NSCLC remains unknown. In a previous investigation, a significant differential expression of miR-654-3p in NSCLC tissue was observed. However, they did not focus on biofunction of miR-654-3p in NSCLC development. The primary discovery of this study is the confirmation of the tumour suppressor activity of miR-654-3p in the development of NSCLC. Moreover, in another study, it has been shown that miR-654-3p was significantly altered after the application of carbon ion radiotherapy (CIRT), and the expression of miR-654-3p could be a promising non-invasive biomarker predicting the efficacy of CIRT. Differing with the study, we found the levels of miR-654-3p in the NSCLC serum were lower than those observed in the healthy control, suggesting miR-654-3p may have another medical value for NSCLC diagnosis. In the present study, we observed that miR-654-3p expression was downregulated in NSCLC tumour tissues compared with that observed in paracancerous tissues. Furthermore, miR-654-3p levels were also negatively correlated with tumour size and TNM stage. To better understand the biological role of miR-654-3p in NSCLC, we selected several NSCLC cell lines based on their characteristics [A549 from lung, H1266 from a metastatic site (lymph node), H226 from a metastatic site (pleural effusion), etc.] to assess the biological function of miR-654-3p. We observed that miR-654-3p expression was downregulated in NSCLC cell lines compared with that observed in normal lung epithelial cells (BEAS-2B). These findings were similar to those reported previously. For instance, Geraldo et al. reported that miR-654-3p levels decreased with long-term papillary thyroid carcinoma progression in mice and was inversely correlated with epithelial–mesenchymal transition.10 In another study, Formosa et al observed a severe and consistent downregulation of miRNAs, including miR-654-3p, in metastatic cell lines compared with that observed normal prostatic epithelial cells.9 These findings suggest that miR-654-3p should be further investigated as a tumour suppressor. To further elucidate its role in the development of NSCLC, we transfected A549 cells with miR-654-3p-mimic or miR-654-3p-inhibitor vectors and investigated the proliferative ability and growth of both cell lines in vitro and in vivo. We observed that miR-654-3p overexpression significantly inhibited A549 cell growth and promoted apoptosis both in vivo and in vitro. Our data also indicated that miR-654-3p downregulation in NSCLC may contribute to the progression of NSCLC.
An additional discovery of this study is the identification of a new target gene of miR-654-3p. In a previous study, Gu et al proposed that Frizzled-10 (FZD10) may be an important downstream target gene of miR-654-3p due to its important roles in osteoporosis.14 FZD10 is well known to promote the development of tumours as demonstrated by the requirement for FZD10 to sustain cancer cell proliferation and downregulates gene expression in many types of tumours.15–17 However, no additional details regarding the targets of miR-654-3p have been reported. To gain a better understanding of the anticancer mechanism of miR-654-3p, we used several online tools to predict potential molecular targets of miR-654-3p in NSCLC. However, every single online tool provided hundreds of potential genes targeted by miR-654-3p. Therefore, we focused on the gene predicted by all online tools simultaneously, with high expression in the lung tissue (checked by https://www.proteinatlas.org/ENSG00000142731-PLK4) and with potential diagnostic value in NSCLC.18 As a result, PLK4 was selected as a target gene of miR-654-3p for further study. PLKs are regulatory serine/threonine kinases of the cell cycle involved in mitotic entry and exit, spindle formation, cytokinesis, and meiosis.19 PLK4 is a PLK member that has been confirmed to be a regulator of centriole duplication.19,20 Under physiological conditions, human PLK4 shows increased expression in tissues with high levels of proliferation, such as embryonic tissues and testis.21 Moreover, the aberrant overexpression of PLK4 has been observed in several cancers.22–24 In recent studies, PLK4 expression was shown to be significantly higher in malignant versus normal lungs and conferred an unfavourable survival rate, while PLK4 inhibition produces polyploidy and apoptotic death of lung cancers.25,26 All of these results support our hypothesis that PLK4 may be a downstream target of miR-654-3p. In our study, we observed that PLK4 expression was upregulated in NSCLC tissues compared with the adjacent tissue and was negatively correlated with miR-654-3p in NSCLC patients. Subsequent analysis verified that miR-654-3p robustly suppresses PLK4 mRNA and protein expression in A549 cells with wild-type but not the mutant PLK4, demonstrating that PLK4 is the direct target of miR-654-3p. Furthermore, PLK4 overexpression in A549 cells decreased the miR-654-3p-induced inhibitory effects on cell viability, suggesting that PLK4 downregulation is at least partially responsible for the miR-654-3p-mediated increase in apoptosis in NSCLC. Our research has some limitations. First, we did not detect blood PLK4 levels and analyse its relationship with miR-654-3p in NSCLC patients. Second, peripheral blood miRs have potential value for tumour diagnosis. Although we evaluated blood miR-654-3p levels, we did not observe a correlation between blood miR-654-3p levels and tumour parameters (eg, tumour size and TNM stage). Thus, in future studies, additional clinical samples are required to reanalyse the diagnostic value of miR-654-3p in NSCLC.
Conclusion
In summary, in this study, we demonstrated that miR-654-3p inhibits the proliferation and tumourigenesis of NSCLC cells in vitro and in vivo by directly targeting PLK4, suggesting that miR-654-3p may serve as a potential indicator and therapeutic target for the progression of NSCLC.
Acknowledgment
This work was financially supported by the Sichuan University Education Foundation (TSCI002).
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi: 10.1016/S0025-6196(11)60735-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho JH. Immunotherapy for non-small cell lung cancer: current status and future obstacles. Immune Netw. 2017;17(6):378–391. doi: 10.4110/in.2017.17.6.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C, Yin Y, Liu X, Xi X, Xue W, Qu Y. Non-small cell lung cancer associated microRNA expression signature: integrated bioinformatics analysis, validation and clinical significance. Oncotarget. 2017;8(15):24564–24578. doi: 10.18632/oncotarget.15596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun DM, Tang BF, Li ZX, et al. MiR-29c reduces the cisplatin resistance of non-small cell lung cancer cells by negatively regulating the PI3K/Akt pathway. Sci Rep. 2018;8(1):8007. doi: 10.1038/s41598-018-26381-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue X, Fei X, Hou W, Zhang Y, Liu L, Hu R. miR-342-3p suppresses cell proliferation and migration by targeting AGR2 in non-small cell lung cancer. Cancer Lett. 2018;412:170–178. doi: 10.1016/j.canlet.2017.10.024 [DOI] [PubMed] [Google Scholar]
- 6.Yang F, Wei K, Qin Z, et al. MiR-598 suppresses invasion and migration by negative regulation of derlin-1 and epithelial-mesenchymal transition in non-small cell lung cancer. Cell Physiol Biochem. 2018;47(1):245–256. doi: 10.1159/000489803 [DOI] [PubMed] [Google Scholar]
- 7.Mizuno K, Mataki H, Seki N, Kumamoto T, Kamikawaji K, Inoue H. MicroRNAs in non-small cell lung cancer and idiopathic pulmonary fibrosis. J Hum Genet. 2017;62(1):57–65. doi: 10.1038/jhg.2016.98 [DOI] [PubMed] [Google Scholar]
- 8.Florczuk M, Szpechcinski A, Chorostowska-Wynimko J. miRNAs as biomarkers and therapeutic targets in non-small cell lung cancer: current perspectives. Target Oncol. 2017;12(2):179–200. doi: 10.1007/s11523-017-0478-5 [DOI] [PubMed] [Google Scholar]
- 9.Formosa A, Markert EK, Lena AM, et al. MicroRNAs, miR-154, miR-299-5p, miR-376a, miR-376c, miR-377, miR-381, miR-487b, miR-485-3p, miR-495 and miR-654-3p, mapped to the 14q32.31 locus, regulate proliferation, apoptosis, migration and invasion in metastatic prostate cancer cells. Oncogene. 2014;33(44):5173–5182. doi: 10.1038/onc.2013.451 [DOI] [PubMed] [Google Scholar]
- 10.Geraldo MV, Nakaya HI, Kimura ET. Down-regulation of 14q32-encoded miRNAs and tumor suppressor role for miR-654-3p in papillary thyroid cancer. Oncotarget. 2017;8(6):9597–9607. doi: 10.18632/oncotarget.14162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S, Zhang X, Fu X, Li W, Xing S, Yang Y. Identification of common differentially-expressed miRNAs in ovarian cancer cells and their exosomes compared with normal ovarian surface epithelial cell cells. Oncol Lett. 2018;16(2):2391–2401. doi: 10.3892/ol.2018.8954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Q, Li P, Weng M, et al. Nano-Vesicles are a potential tool to monitor therapeutic efficacy of carbon ion radiotherapy in prostate cancer. J Biomed Nanotechnol. 2018;14(1):168–178. doi: 10.1166/jbn.2018.2503 [DOI] [PubMed] [Google Scholar]
- 13.Xu C, Zheng Y, Lian D, Ye S, Yang J, Zeng Z. Analysis of microRNA expression profile identifies novel biomarkers for non-small cell lung cancer. Tumori. 2015;101(1):104–110. doi: 10.5301/tj.5000224 [DOI] [PubMed] [Google Scholar]
- 14.Gu H, Wu L, Chen H, et al. Identification of differentially expressed microRNAs in the bone marrow of osteoporosis patients. Am J Transl Res. 2019;11(5):2940–2954. [PMC free article] [PubMed] [Google Scholar]
- 15.Scavo MP, Depalo N, Rizzi F, et al. FZD10 Carried by Exosomes Sustains Cancer Cell Proliferation. Cells. 2019;8(8):pii: E777. doi: 10.3390/cells8080777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong C, Qu S, Lv XB, et al. BRMS1L suppresses breast cancer metastasis by inducing epigenetic silence of FZD10. Nat Commun. 2014;5:5406. doi: 10.1038/ncomms6406 [DOI] [PubMed] [Google Scholar]
- 17.Nagayama S, Yamada E, Kohno Y, et al. Inverse correlation of the up-regulation of FZD10 expression and the activation of beta-catenin in synchronous colorectal tumors. Cancer Sci. 2009;100(3):405–412. doi: 10.1111/j.1349-7006.2008.01052.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Q, Fan G, Dong Y. Polo-like kinase 4 correlates with greater tumor size, lymph node metastasis and confers poor survival in non-small cell lung cancer. J Clin Lab Anal. 2019;e23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y, Wang X. PLK4: a promising target for cancer therapy. J Cancer Res Clin Oncol. 2019;145(10):2413–2422. doi: 10.1007/s00432-019-02994-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, et al. AK/PLK4 is required for centriole duplication and flagella development. Curr Biol. 2005;15(24):2199–2207. doi: 10.1016/j.cub.2005.11.042 [DOI] [PubMed] [Google Scholar]
- 21.Karn T, Holtrich U, Wolf G, Hock B, Strebhardt K, Rubsamenwaigmann H. Human SAK related to the PLK/polo family of cell cycle kinases shows high mRNA expression in testis. Oncol Rep. 1997;4:505–510. doi: 10.3892/or.4.3.505 [DOI] [PubMed] [Google Scholar]
- 22.Tian X, Zhou D, Chen L, et al. Polo-like kinase 4 mediates epithelial-mesenchymal transition in neuroblastoma via PI3K/Akt signaling pathway. Cell Death Dis. 2018;9(2):54. doi: 10.1038/s41419-017-0088-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Dai K, Wang C, et al. Expression of polo-like kinase 4(PLK4) in breast cancer and its response to taxane-based neoadjuvant chemotherapy. J Cancer. 2016;7(9):1125–1132. doi: 10.7150/jca.14307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dementyeva E, Kryukov F, Kubiczkova L, et al. Clinical implication of centrosome amplification and expression of centrosomal functional genes in multiple myeloma. J Transl Med. 2013;11(1):77. doi: 10.1186/1479-5876-11-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawakami M, Mustachio LM, Zheng L, et al. Polo-like kinase 4 inhibition produces polyploidy and apoptotic death of lung cancers. Proc Natl Acad Sci U S A. 2018;115(8):1913–1918. doi: 10.1073/pnas.1719760115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinmura K, Kato H, Kawanishi Y, et al. POLQ overexpression is associated with an increased somatic mutation load and PLK4 Overexpression in lung adenocarcinoma. Cancers. 2019;11(5):pii: E722. doi: 10.3390/cancers11050722 [DOI] [PMC free article] [PubMed] [Google Scholar]