Abstract
Abnormal reward responsiveness and rumination each are associated with elevated inflammation and mood symptoms. Ruminating on positive and negative affect, or dampening positive affect, may amplify, or buffer, the associations of reward hyper/hyposensitivity with inflammation and mood symptoms. Young adults (N=109) with high or moderate reward sensitivity completed reward responsiveness and ruminative style measures at the initial visit of a longitudinal study of mood symptoms, a blood draw to assess inflammatory biomarkers, and mood symptom measures at the study visits before and after the day of the blood draw. The interaction between high reward responsiveness and rumination on positive affect was associated with higher levels of an inflammatory composite measure and hypomanic symptoms. The interaction between lower reward responsiveness and high dampening of positive affect was associated with higher levels of the inflammatory composite measure and depressive symptoms. Lower reward responsiveness also interacted with low rumination on positive affect to predict increases in depressive symptoms and higher levels of the inflammatory composite. Thus, levels of reward responsiveness and ruminative response styles may synergistically influence the development of inflammatory phenotypes and both hypomanic and depressive mood symptoms.
Keywords: depression, hypomania, inflammation, rumination, reward responsiveness
Introduction
Although they show some differential patterns of vulnerability, unipolar depression (UD) and bipolar spectrum disorders (BSDs) share notable overlap in their risk factors and biological correlates (Cuellar, Johnson, & Winters, 2005; Johnson & Kizer, 2002). Thus, there is value in developing integrated transdiagnostic models that incorporate common risk factors for both ends of the mood spectrum. For example, both aberrant reward responsiveness and rumination are well-established risk factors for UDs and BSDs (e.g., Alloy, Olino, Freed, & Nusslock, 2016; Gruber, Eidelman, Johnson, Smith, & Harvey, 2011; Johnson, McKenzie, & McMurrich, 2008; Nolen-Hoeksema & Morrow, 1991). Inasmuch as inflammation is gaining support as part of the pathophysiology of mood disorders (Brietzke et al., 2012; Dowlati et al., 2010; Kim, Jung, Myint, Kim, & Park, 2007), and abnormal reward responsiveness and rumination both have been independently linked to heightened inflammation (Nusslock & Miller, 2016; Zoccola, Figueroa, Rabideau, & Woody, 2014), research that investigates the interplay between rumination, reward responsiveness, and inflammation in the development of mood symptomatology is warranted. Given initial evidence that rumination amplifies the effect of some risk factors (e.g. anxiety) on inflammation in ways that increase risk for depressive symptoms (Moriarity et al., 2018), this study investigated whether the interaction of trait ruminative response styles and trait reward responsiveness is associated with future inflammatory biomarkers and changes in depressive and hypomanic/manic (referred to throughout as (hypo)manic) symptoms.
Aberrant Reward Responsiveness is a Risk Factor for Mood Psychopathology
The behavioral approach system (BAS)/reward hypersensitivity theory of BSDs (e.g., Alloy, Nusslock, & Boland, 2015; Alloy et al., 2016; Johnson, Edge, Holmes, & Carver, 2012) posits that individuals hypersensitive to reward are at risk for (hypo)manic symptoms through excessive reward activation states triggered by rewards, goal-striving, goal-attainment, and, in the case of irritability, goal-blockage or frustration (Carver, 2004; Harmon-Jones, 2003). Likewise, reward hyperresponsive individuals are vulnerable to depressive symptoms when their reward systems become excessively deactivated in response to irreconcilable failures or losses. In this model, reward responsiveness refers to the strength of reward processing and approach motivation, mechanisms that regulate reward pursuit. There is strong support for the BAS/reward hypersensitivity theory with respect to (hypo)manic symptoms and episodes (Alloy et al., 2012; Boland et al., 2016). However, evidence suggests that reward hypersensitivity may have an indirect effect on depressive symptoms (e.g., Boland et al., 2016), highlighting the importance of investigating potential moderators and mediators of the association between reward responsiveness and mood symptoms.
Reward models of UD more frequently describe low reward responsiveness as a risk factor (e.g., Henriques & Davidson, 2000; Morgan, Olino, Mcmakin, Ryan, & Forbes, 2013). Reduced reward processing as measured by electroencephalographic event related potentials has been shown to predict prospective depression onset in adolescent girls (Bress, Foti, Kotov, Klein, & Hajcak, 2013). Further, a recent coordinate-based meta-analysis of fMRI reward studies found that, relative to healthy controls, UD individuals consistently show less striatal activation in response to reward (Ng, Alloy, & Smith, in press), and less striatal activation when anticipating rewards predicted increases in depressive symptoms at a two-year follow-up (Morgan et al., 2013). In summary, whereas high reward responsiveness may provide risk for (hypo)mania and bipolar depression (Alloy et al., 2012; 2016), low reward responsiveness may provide risk for UD (Henriques & Davidson, 2000; Morgan et al., 2013).
Rumination Confers Risk for Mood Symptoms
Rumination, the tendency to passively focus on one’s mood and its causes and consequences, is a well-established risk factor for depressive symptoms and episodes. Rumination on negative affect has been demonstrated to maintain or worsen depressive symptoms (e.g., Nolen-Hoeksema, 1991) and predict onset of depressive episodes (e.g., Spasojevic & Alloy, 2001). There also are several studies demonstrating that rumination on negative affect is elevated in euthymic, depressive, and (hypo)manic phases of BSDs compared to controls (e.g., Gruber et al., 2011; Johnson et al., 2008) and is associated with (hypo)manic symptoms (Knowles, Tai, Christensen, & Bentall, 2005).
In addition to rumination on negative thoughts and emotions, rumination on positive affect is associated with mood symptoms as well. Ruminative styles that amplify positive emotions have been associated with (hypo)manic symptoms in undergraduates (Feldman, Joorman, & Johnson, 2008). Conversely, ruminative styles that lessen positive moods via responding to positive affect with thoughts about what could go wrong (referred to as dampening of positive affect) has predicted current and future depressive symptoms (Feldman et al., 2008; Raes, Smets, Nelis, & Schoofs, 2012). Thus, rumination on both negative and positive affect can be considered a transdiagnostic risk factor for mood symptoms.
Reward Responsiveness and Inflammation
Both reward hyper- and hyposensitivity have been associated with elevated levels of inflammatory biomarkers (Eisenberger, Moieni, Inagaki, Muscatell, & Irwin, 2017; Felger & Miller, 2012; Miller, Maletic, & Raison, 2009; Nusslock & Miller, 2016), potentially via moderating levels of physiological arousal in the context of reward-salient stimuli. This is supported by research associating reward hypersensitivity with heightened negative affect in response to stressors (e.g. anger and anxiety; Carver, 2004; Harmon-Jones, 2003; Hundt et al., 2013), which, in turn, has been associated with large stress-evoked changes in inflammatory biomarkers (Carroll et al., 2011; Moons, Eisenberger, & Taylor, 2010). Additionally, higher levels of an inflammatory composite variable have been associated with larger orbitofrontal cortex responses to reward anticipation among reward hypersensitive individuals (Chat et al., 2019). Further, administration of inflammatory challenges has been associated with heightened neural sensitivity to both negative and positive social feedback (Eisenberger et al., 2017).
Both hyper- and hypo-reward responsiveness also are associated with behaviors that can increase inflammation. Individuals with both high and low reward responsiveness are more likely to engage in substance use and have high-fat diets (Alloy et al., 2009; Loxton & Tipman, 2017; Volkow, Wang, Fowler, & Telang, 2008), which promote inflammation. Additionally, both reward hyper- and hyposensitivity are associated with increased occurrence of goal failures and losses (e.g., Boland et al., 2016; Liu & Alloy, 2010), which can trigger stress-related changes in inflammation. Relatedly, elevated goal-striving tendencies are associated with elevated inflammatory biomarkers (Miller & Wrosch, 2007). Further, low reward responsiveness is associated with lack of goal pursuit, which may preclude successes (e.g., relationships, employment), resulting in stress that may, in turn, exacerbate inflammation. In conclusion, there is evidence that both extremes of reward responsiveness might confer risk for inflammation via increasing intensity and/or frequency of stressors and inflammation-enhancing behaviors.
Rumination and Inflammation
The perseverative cognition hypothesis (Brosschot, Gerin, & Thayer, 2006) posits that perseverative cognitive styles, including rumination, amplify physiological reactions to both physical and psychological stressors. This prolongs stress-related activation, ultimately contributing to a shift in baselines for physiological regulation, which is believed to confer risk for adverse outcomes (McEwen & Stellar, 1993; Slavich & Irwin, 2014). This theory is supported by findings that rumination is associated with greater increases in C-reactive protein (CRP), and slower recovery, following a stressor compared to a distraction condition (Zoccola et al., 2014). Although the majority of research has tested the relationship between rumination on negative affect and inflammation, there is evidence that individuals experiencing stress, but not controls, who engage in neutral, reflective perseverative cognitions about their stressors also are at risk for heightened inflammation (Segerstrom, Schipper, & Greenberg, 2008). Thus, rumination about a stressful experience, regardless of the valence of affect, might amplify risk for increases in inflammation.
Theory: The Interaction Between Reward Responsiveness and Rumination
Rumination and reward responsiveness might interact to synergistically increase risk for inflammation and mood symptoms. Reward hyperresponsive individuals might experience increased physiological arousal during goal pursuit and reward anticipation/attainment as well as increased salience of reward-related failures, which might be amplified by rumination on positive or negative affect, respectively. Conversely, reward hyporesponsiveness might be associated with lower goal pursuit that would otherwise lead to success and decrease stress, which also could be amplified by rumination on negative affect, or protected against by rumination on positive affect via mitigating negative arousal and stress associated with a lack of goal attainment. Further, dampening of positive affect might buffer the risk conferred by reward hyperresponsiveness by decreasing physiological arousal during positive mood states post-goal attainment. Conversely, dampening of positive affect may reduce the perceived benefit of positive events, thus decreasing the frequency of goal pursuit that might reduce stress, exacerbating the risk associated with reward hyposensitivity. Moreover, as both extremes of reward responsiveness are associated with increased frequency of goal failures and losses (Boland et al., 2016; Liu & Alloy, 2010), the tendency to ruminate on negative affect might extend the duration of physiological arousal to these negative events. Thus, there is value in extending previous findings to examine whether the interaction of reward responsiveness and ruminative response styles is associated with inflammation and changes in mood symptoms.
The Present Study
This study examined whether the interaction of reward responsiveness and ruminative response styles, both known independent risk factors for mood symptoms and inflammation, are associated with inflammatory biomarkers and changes in mood symptoms. We had three hypotheses for symptoms and two for inflammation. Regarding symptoms, we predicted that: response styles associated with high positive affect (i.e., both high levels of rumination on positive affect and low tendency to dampen positive affect) would amplify the positive association between higher reward responsiveness and (hypo)manic symptoms (Hypothesis S-1); response styles associated with low positive affect (i.e., both low levels of rumination on positive affect, as well as the tendency to dampen positive affect) also would amplify the positive association between lower reward responsiveness and depressive symptoms (Hypothesis S-2); and brooding on negative affect would amplify the positive association between lower reward responsiveness and depressive symptoms (Hypothesis S-3). Regarding inflammation, we predicted that cognitive styles associated with high positive affect (i.e., high rumination on positive affect, as well as low tendency to dampen positive affect) would amplify the positive association between high reward responsiveness and inflammation and, similarly, that cognitive styles associated with low positive affect (i.e., both low levels of rumination on positive affect, as well as the tendency to dampen positive affect) would amplify the positive association between low reward responsiveness and inflammation (Hypothesis I-1). We also hypothesized that high brooding on negative affect would amplify the positive association of low reward responsiveness and inflammation (Hypothesis I-2).
Method
Participants and Procedures
Participants were drawn from the Teen Emotion and Motivation (TEAM) Project (Alloy et al., 2012), an ongoing prospective, longitudinal study that aims to identify various risk factors related to the onset and course of BSDs. Participants were selected via a two-phase screening procedure from the greater Philadelphia area (Alloy et al., 2012). In Phase I, 9,991 students aged 14–19 were recruited from 13 Philadelphia public high schools and two local universities. They completed two self-report trait measures of reward sensitivity: the Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) Scales (Carver & White, 1994) and Sensitivity to Punishment/Sensitivity to Reward Questionnaire (Torrubia, Ávila, Moltó, & Caseras, 2001) at the screener (see Figure 1 for a complete study timeline). Students who scored in the top 15th percentile on both the BAS-Total (BAS-T) of the BIS/BAS and the Sensitivity to Reward Scale (SR) of the SPSRQ composed the high BAS (high reward sensitivity) group, and those who scored between the 40th and 60th percentiles on both of these measures composed the moderate BAS (moderate reward sensitivity) group. Participants with low BAS sensitivity were excluded because low BAS sensitivity is associated with vulnerability to unipolar depression (see Alloy et al., 2016; Nusslock & Alloy, 2017 for reviews) and a major aim of Project TEAM was to examine vulnerability to first onset of BSDs. The screening sample was representative of adolescents ages 14–19 in the Philadelphia area on race, sex, and age (Alloy et al., 2012).
Figure 1.
Study timeline
A random subset of high BAS and moderate BAS participants were invited to a Phase II screening and were administered an expanded Schedule for Affective Disorders and Schizophrenia—Lifetime diagnostic interview (exp-SADS-L; Alloy et al., 2008; Endicott & Spitzer, 1978) by interviewers blind to participants’ BAS risk group status. The exp-SADS-L interview was expanded to allow for generation of both DSM-IV-TR (American Psychiatric Association, 2000) and Research Diagnostic Criteria (RDC; Endicott & Spitzer, 1978) diagnoses. In Phase II, parents provided written consent and adolescents provided written assent for those under the age of 18, and participants 18 or older completed their own written consent. Participants were excluded from the final Project TEAM sample if they met DSM-IV-TR and/or RDC criteria for a lifetime history of any psychotic disorder or were not fluent in English.
This study included a subset of 109 participants (72 high BAS, 37 moderate BAS, 32 with a history of BSD, 52% female, 57% White, 43% non-white, mean age at blood draw = 21.5, SD = 2.1 years) who attended an optional study session to complete a blood draw as part of an exploratory aim added several years after the start of the larger study (see Figure 1 for study timeline).
As part of the first visit after screening, participants completed trait measures of ruminative response styles and reward sensitivity. They also completed mood symptom measures, the 7 Up 7 Down questionnaire (7U7D; Youngstrom, Murray, Johnson, & Findling, 2013) and the Beck Depression Inventory (BDI; Beck, Brown, & Steer, 1996), approximately every 6 months after the first session. For this study, symptom measures were taken from the closest timepoint prior to, and after, the blood draw. The 7U7D assesses symptoms of both (hypo)mania and depression, but it does not include some important symptoms of depression (e.g., anhedonia). To compliment this measure, analyses also included the BDI, which is a more thorough measure of depressive symptomology. Unfortunately, there was no more comprehensive measure of (hypo)manic symptoms in this sample. To reduce participant burden, some measures, including the 7U7D, were completed online before the study visit, while others were completed at the study visit (e.g., the BDI). It was not uncommon for participants to complete their online questionnaires but miss or reschedule their in-person study session, resulting in differences in time between assessments across symptom measures. Eighty-five participants had pre- and post-blood draw 7U7D data and 81 had pre- and post-blood draw BDI data (see Figure 1 for study timeline). The variability between time points was due in part to the measurement timing described above as well as that the optional blood draw was collected at a different session than the regular study visits. The 109 participants who completed blood draws did not differ from the 665 recruited for the main study on measures of ruminative response styles, reward sensitivity, or race (all p’s > .05); however, there was a higher proportion of males in this subsample than in the full sample, χ2(1)=7.42, p = .006.
Measures
Exp-SADS-L
The exp-SADS-L (Alloy et al., 2008; 2012), an expanded version of the SADS-L (Endicott & Spitzer, 1978), is a semi-structured diagnostic interview used to assess lifetime and current Axis I psychiatric disorders during Phase II screening. It was designed to generate both DSM-IV-TR and RDC diagnoses, to increase items and improve the probes in the mood disorder sections, and to diagnose and evaluate eating disorders, attention-deficit/hyperactivity disorder, acute stress disorder, medical history, family history, and organic rule-out conditions (Alloy et al., 2012). Before administering the exp-SADS-L, post-doctoral fellows, doctoral students, and research assistants in clinical psychology received approximately 200 hours of extensive training. The exp-SADS-L has demonstrated high inter-rater reliability, with k ≥ .95 for major depressive episodes based on 80 interviews (Alloy et al., 2000) and k ≥ .96 for BSDs based on 105 interviews (Alloy et al., 2008).
BIS/BAS Scales
The BIS/BAS Scales (Carver & White, 1994) are a widely used self-report questionnaire that assesses individual differences in sensitivity to threats and rewards. Participants responded to 20 questions on a 4-point Likert scale ranging from strongly disagree to strongly agree. The scales consist of one BIS subscale, and three BAS subscales: Reward Responsiveness, Drive, and Fun-Seeking. The five Reward Responsiveness subscale items (e.g., “When I get something I want, I feel excited and energized”) measure positive responses to reward stimuli. The four Drive subscale items (e.g., “When I want something, I usually go all-out to get it”) evaluate vigor and persistence in reward pursuit. The four Fun-Seeking subscale items (e.g., “I will often do things for no other reason than that they might be fun”) assess willingness to approach rewards and novel stimuli on impulse. Internal consistencies (α’s = .66-.76) and retest reliabilities (r’s = .59-.69) for the subscales are satisfactory (Carver & White, 1994). Summing all the BAS items results in the BAS-T score, which was used to select the high and moderate BAS participants in Phase I and demonstrated good internal consistency in Project TEAM (α = .80). Inasmuch as the Reward Responsiveness subscale assesses the arousal felt following reward attainment, the mechanism through which we theorize reward sensitivity affects inflammation, only the Reward Responsiveness subscale was used in these analyses.
SPSRQ
The SPSRQ (Torrubia et al., 2001) is composed of two subscales, sensitivity to reward (SR) and sensitivity to punishment (SP) with 24 “yes” or “no” questions in each. Both subscales have good internal consistency and retest reliability (Torrubia et al., 2001). Research also supports the construct validity of both the BIS/BAS and SPSRQ measures. In the current study, the SR and SP scales demonstrated good internal consistency with α’s = .76 and .84, respectively, and the SR subscale was correlated with the BAS-T (r = .40, p < .01) in the Phase I sample. Although the SR subscale was used to help select participants for the overall Project TEAM sample in Phase I, it was not used in the current study’s analyses.
Rumination on Positive Affect Scale
The Rumination on Positive Affect Scale (RPAS; Feldman et al., 2008) is a 17-item self-report questionnaire used to measure the propensity to respond to positive affective states with responses that diminish positive affect (Dampening subscale) and enhance positive affect through recurrent thoughts about one’s positive affective experience (Emotion-Focused subscale) and positive qualities (Self-Focused subscale) as traits. The Emotion-Focused and Self-Focused subscales correlate with self-esteem and vulnerability to hypomania (Feldman et al., 2008), whereas the Dampening subscale correlates with a history of depression (Johnson et al., 2008). In the current study, all three subscales demonstrated good internal consistency (α’s ranged from .76 to .83). In an effort to reduce the number of analyses and type I error, only the Dampening and Self-Focused subscales (retest reliability r = .62 for both]), but not the Emotion-Focused subscale, were used in analyses. This is because the Self-Focused subscale has been shown to be positively correlated with (hypo)manic and negatively correlated with depressive symptoms, whereas the Emotion-Focused subscale was not found to be correlated with depressive symptoms (Feldman et al., 2008).
Ruminative Responses Scale
The Brooding subscale of the Ruminative Responses Scale (RRS; Treynor, Gonzalez, & Nolen-Hoeksema, 2003) was used to assess brooding rumination in response to negative affect as a trait. The subscale has five items, which were scored on 4-point Likert scales (1 = almost never, 4 = almost always). It has been found to predict depressive symptoms longitudinally and have good retest reliability (r’s = .60-.62, Treynor et al., 2003). In the present study, the Brooding subscale also demonstrated good internal consistency (α = .78).
Beck Depression Inventory (BDI)
The BDI (Beck et al., 1996) is a 21-item self-report scale that assesses affective, motivational, cognitive, and somatic symptoms of depression. Items are scored from 0 to 3, with higher numbers indicating greater symptom severity. The BDI has demonstrated good internal consistency, retest reliability, and concurrent validity with clinical depression ratings in both clinical (r = .72) and nonclinical (r = .60) samples (Beck et al., 1996). The BDI demonstrated high internal consistency in this sample (α = .90).
7 Up 7 Down Inventory (7U7D)
The 7U7D (Youngstrom et al., 2013) is a 14-item (scored 0–3) brief version of the General Behavior Inventory (Depue, Krauss, Spoont, & Arbisi, 1989). The depression and (hypo)mania subscales each have seven items; both subscales have demonstrated good internal consistency, convergent and discriminant validity, and discrimination between diagnostic groups (Youngstrom et al., 2013). Both the depression and (hypo)mania scales had high internal consistency in this sample (α’s = .93 and .90, respectively).
Immune Assays
Plasma levels of C-reactive protein (CRP), interleukin (IL)-6, IL-8, IL-10, and tumor necrosis factor alpha (TNF-α) were measured. These inflammatory biomarkers were chosen because they previously have been associated with (hypo)manic and depressive symptoms. Blood was drawn into an EDTA-treated Vacutainer by antecubital venipuncture and stored in an ultracold freezer at −80°C until the day of assay. Time of day of the blood draw and participants’ body mass index (BMI) were recorded from directly measured height and weight. CRP was measured with a high-sensitivity immunoturbidimetric assay on a Roche/Hitachi cobas c502 analyzer. Average intra- and inter-assay coefficients of variation were 2.5% and 5.6%, respectively. This assay’s lower limit of detection is 0.2 mg/L. The cytokines IL-6, IL-8, IL-10, and TNF-α were measured in duplicate by electrochemiluminescence on a SECTOR Imager 2400A (MesoScale Discovery) with a Human Pro-Inflammatory 4-Plex Ultra-Sensitive assay (MesoScale Discovery), following instructions provided by the manufacturer (Fu, Zhu, & Van Eyk, 2010). The kit’s lower limits of detection range from 0.10 pg/mL (IL-8) to 0.80 pg/mL (IL-10). Across runs, the average intra-assay coefficients of variation were 3.79% (IL-6), 2.24% (IL-8), 4.13% (IL-10), and 3.33% (TNF-α).
Data Analysis Plan
All descriptive statistics, correlations, and analyses were conducted in SPSS (v23; IBM Corp, 2016). All moderation analyses were conducted using Model 1 in the Process Macro (Hayes, 2013). Bivariate correlations between demographic and physical characteristics and primary study variables were calculated.
Two sets of primary hypothesis-testing analyses were conducted. First, moderation analyses tested our three hypotheses for mood symptoms. Specifically, we tested whether reward responsiveness interacted with rumination on positive affect or dampening of positive affect to predict (hypo)manic symptoms (Hypothesis S-1); whether reward responsiveness interacted with rumination on positive affect or dampening of positive affect to predict depressive symptoms (Hypothesis S-2); and whether reward responsiveness interacted with brooding on negative affect to predict higher depressive symptoms (Hypothesis S-3). These analyses controlled for gender, race, and age at measurement due to the established associations between these characteristics and mood symptoms (Twenge & Nolen-Hoeksema, 2002), as well as medications related to mood symptoms, therapy at the time of the assessment, time in study, and symptoms at the previous study visit to estimate change in symptoms. Second, to examine our inflammatory hypotheses we tested if the interaction between reward responsiveness and rumination on positive affect or dampening of positive affect was associated with inflammation (Hypothesis I-1) and if the interaction between reward responsiveness and brooding on negative affect was associated with inflammation (Hypothesis I-2). Our inflammatory outcome was a composite of z-standardized and log (100 × value) transformed CRP, IL-6, IL-8, and TNF-α. Models controlled for variables associated with inflammatory biomarkers, specifically gender and race (Alanna et al., 2011), age at blood draw (Mills, Scott, Wray, Cohen-Woods, & Baune, 2013), BMI at blood draw (Howren, Lamkin, & Suls, 2009), birth control use (Kalo-Klein & Witkins, 1989), anti-inflammatory medications, and time of day of the blood draw (Dominguez-Rodriguez, Abreu-Gonzalex, & Kaski, 2009). An inflammation composite score (ICS) was computed both as a proxy measure of systemic inflammation and to reduce issues of multiple comparisons, as has been done previously (Miller, Brody, Yu, & Chen, 2014; Nusslock et al., 2019). Significant results for the ICS were probed for each specific inflammatory biomarker to examine whether certain biomarkers might be driving the effects. The anti-inflammatory cytokine IL-10 was not analyzed, due to its primarily anti-inflammatory properties. All significant interaction terms were probed for Johnson-Neyman significance regions to determine at what levels of the moderator there were significant effects of reward responsivity.
CRP values > 10 mg/L may indicate an acute infection, and thus, six participants with CRP > 10 mg/L were excluded from the analyses (Bell et al., 2017; de Ferranti, Gauvreau, Ludwih, Newburger, & Rifai, 2006). Raw CRP, IL-6, IL-8, and TNF-α values were significantly skewed, and thus, were log-transformed for follow-up analyses (log(100 × value)).
Results
Preliminary Analyses
Descriptive statistics and bivariate correlations for the primary study variables, including the means and standard deviations of untransformed inflammatory biomarkers, are summarized in Supplemental Table 1. Independent samples t-tests were used to investigate potential differences in primary study variables as a function of gender, race, and BAS-group status. Females reported higher levels of brooding and had higher concentrations of the ICS, log CRP, and log IL-6 (p’s = .018, .042, <.001, and = .003, respectively). Non-white participants reported higher trait brooding than white participants (p = .017). Participants in the high BAS group had higher levels of trait self-focused positive rumination (p = .040), trait brooding (p = .004), trait reward responsiveness (p < .001), pre-blood draw 7U7D mania and depression scores (p’s < .001, =.001, respectively), and log IL-6 (p = .014). Bivariate correlations between continuous covariates and primary dependent variables are in Supplementary Table 2.
Reward responsiveness and rumination interact to predict mood symptoms
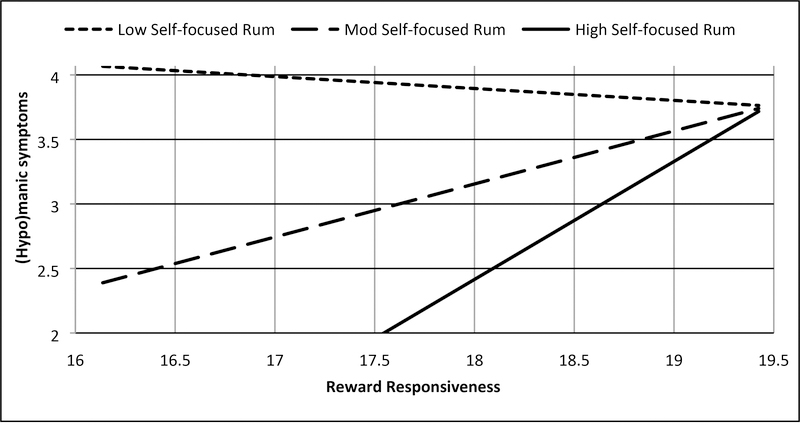
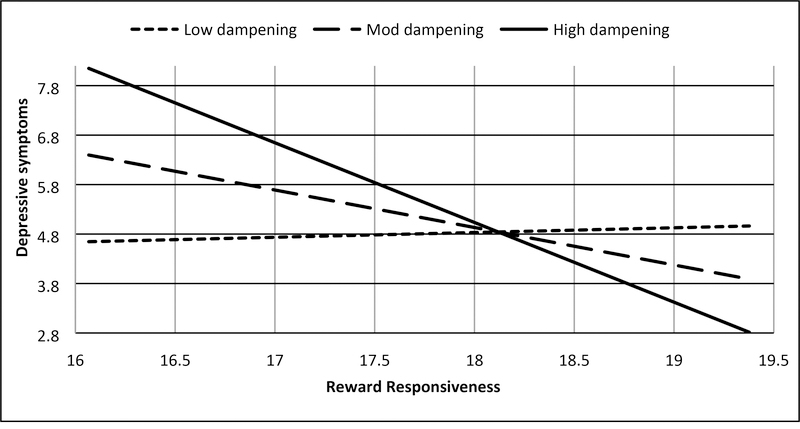
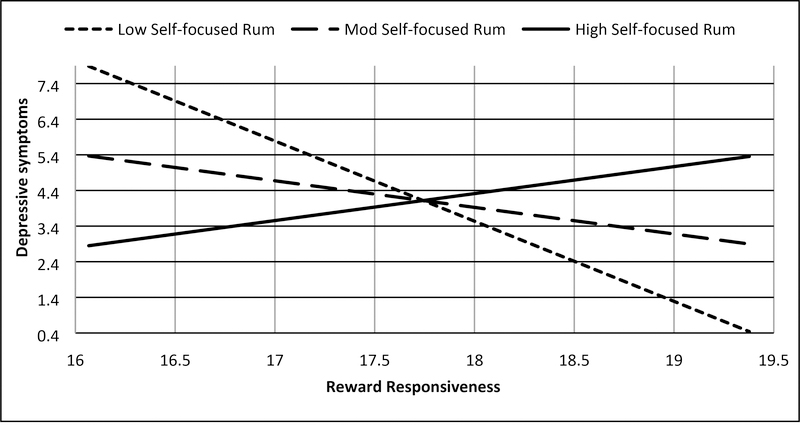
Two significant interactions emerged consistent with Hypotheses S-1 and S-2. Consistent with Hypothesis S-1, trait reward responsiveness interacted with trait self-focused rumination on positive affect to predict (hypo)manic symptoms (7U; b = .187, SE = .089, t = 2.101, p = .040, 95% CI: .009, .365; Figure 2a, Supplemental Table 3), such that higher reward responsiveness combined with higher self-focused rumination on positive affect to predict higher (hypo)manic symptoms (Johnson-Neyman significance region: above 63rd percentile of rumination; b = .530, SE = .265, t = 1.998, p = .050, 95% CI: .000, 1.061). As predicted in Hypothesis S-2, trait reward responsiveness interacted with trait dampening of positive affect to predict depressive symptoms (BDI; b = −.157, SE = .073, t = −2.139, p = .036, 95% CI: −.303, −.010; Figure 3a, Supplemental Table 4), such that lower reward responsiveness interacted with heightened dampening of positive affect to predict higher depressive symptoms (Johnson-Neyman significance region: above 56th percentile of dampening; b = −.769, SE = .385, t = −2.000, p = .050, 95% CI: −1.539, .000). Also consistent with Hypothesis S-2, trait reward responsiveness interacted with trait self-focused rumination on positive affect to predict depressive symptoms (BDI; b = .523, SE = .132, t = 3.958, p < .001, 95% CI: .259, .787; Figure 3b, Supplemental Table 3), such that lower reward responsiveness interacted with lower rumination on positive affect to predict higher depressive symptoms (Johnson-Neyman significance region; below 60th percentile of rumination; b = −.741, SE = .371, t = −2.000, p = .050, 95% CI: −1.482, .000). There was a second Johnson-Neyman significance region finding that high rumination on positive affect provided a buffering effect of the risk low reward responsiveness conferred for depressive symptoms (Johnson-Neyman significance region; above 83rd percentile of rumination; b = 1.219, SE = .610, t = 2.000, p = .050, 95% CI: .000, 2.439). We did not find that the interaction between reward responsiveness and brooding on negative affect predicted depressive symptoms (Hypothesis S-3, Supplemental Table 5) or that the interaction between reward responsiveness and dampening of positive affect predicted (hypo)manic symptoms (Hypothesis S-1, Supplemental Table 4, both p’s > .05).
Figure 2a.
BAS Reward responsiveness and self-focused rumination on positive affect interact to predict (hypo)mania symptoms (7U7D)
Note: Low = −1 SD from mean, Mod = mean, High = +1 SD from mean, range of reward responsiveness = 14–20, range of (hypo)manic symptoms = 2–9. Values represented were truncated to focus on simple slopes.
Figure 3a.
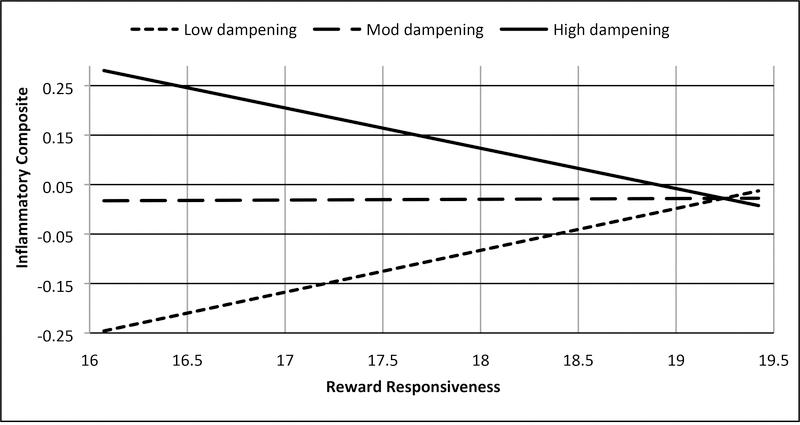
BAS Reward responsiveness and dampening of positive affect interact to predict depressive symptoms (BDI)
Note: Low = −1 SD from mean, Mod = mean, High = +1 SD from mean, Range of reward responsiveness = 14–20, range of depressive symptoms = 0–32. Values represented were truncated to focus on simple slopes.
Figure 3b.
BAS Reward responsiveness and self-focused rumination on positive affect interact to predict depressive symptoms (BDI)
Note: Rum = rumination, Low = −1 SD from mean, Mod = mean, High = +1 SD from mean, range of reward responsiveness = 14–20, range of depressive symptoms = 0–32. Values represented were truncated to focus on simple slopes
Reward responsiveness and rumination are synergistically associated with biomarkers
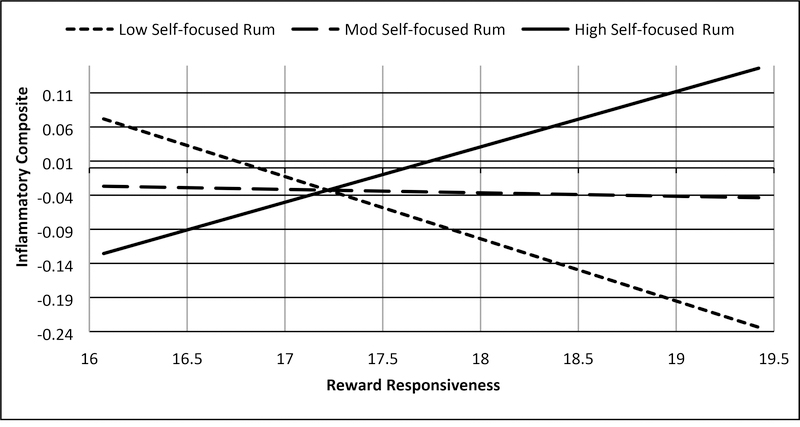
Two interactions testing Hypotheses I-1 were significant in these analyses. First, consistent with support for Hypothesis S-1 predicting (hypo)manic symptoms, the interaction between trait reward responsiveness and trait self-focused rumination on positive affect was associated with the ICS (b = .032, SE = .014, t = 2.312, p = .023, 95% CI: .004, .059; Figure 2b, Supplemental Table 3), such that higher reward responsiveness combined with higher self-focused rumination on positive affect was associated with greater inflammation (Johnson-Neyman significance region: above 95th percentile of rumination; b = .134, SE = .067, t = 1.987, p = .050, 95% CI: .000, .278) and that low reward responsiveness combined with low self-focused rumination to predict greater inflammation (Johnson-Neyman significance region; below 6th percentile of rumination; b = −.152, SE = .076, t = −1.987, p = .050, 95% CI: −.304, .000). Second, consistent with Hypothesis S-2 predicting depressive symptoms, the interaction between trait reward responsiveness and trait dampening of positive affect was associated with the ICS (b = −.015, SE = .006, t = −2.308, p = .023, 95% CI: −.028, −.002; Figure 3c, Supplemental Table 4), such that lower reward responsiveness combined with higher dampening of positive affect was associated with higher inflammation (Johnson-Neyman significance region; above 94th percentile of dampening; b = −.144, SE = .073, t = −1.987, p = .050, 95% CI: −.289, .000). The interaction between reward responsiveness and brooding on negative affect was not associated with the ICS (Hypothesis I-2, Supplemental Table 5).
Figure 2b.
BAS Reward responsiveness and self-focused rumination on positive affect interact to be associated with inflammatory composite
Note: Rum = rumination, Low = −1 SD from mean, Mod = mean, High = +1 SD from mean, range of reward responsiveness = 14–20, range of inflammatory composite = −2.00 – 1.94. Values represented were truncated to focus on simple slopes
Figure 3c.
BAS Reward responsiveness and dampening of positive affect interact to be associated with inflammatory composite
Note: Low = −1 SD from mean, Mod = mean, High = +1 SD from mean, range of reward responsiveness = 14–20, range of inflammatory composite = −2.00 – 1.94. Values represented were truncated to focus on simple slopes.
Follow-up analyses probing the results with the composite suggest that they might be driven by individual biomarkers. Consistent with the interaction between reward responsiveness and self-focused rumination above, higher trait reward responsiveness and higher trait self-focused rumination on positive affect was associated with more IL-8 (b = .019, SE = .008, t = 2.532, p = .013, 95% CI: .004, .034). Also, consistent with the interaction between reward responsiveness and dampening above, lower trait reward responsiveness combined with higher trait dampening of positive affect was associated with higher CRP (b = −.012, SE = .005, t = − 2.338, p = .022, 95% CI: −.023, −.002).
Discussion
The literature on inflammation and mood symptoms has expanded rapidly over the past decade; however, there is a dearth of integrated models of inflammation and mood symptom risk, particularly for (hypo)manic symptoms. Congruent with the perspective that mood symptoms exist along a continuum, the current study utilized a transdiagnostic approach and found that the interaction of reward responsiveness and ruminative response styles was associated with inflammatory markers and predicted changes in both depressive and (hypo)manic symptoms.
Consistent with two hypotheses, higher reward responsiveness interacted with rumination on positive affect to predict greater (hypo)manic symptoms, and lower reward responsiveness interacted with both high dampening of positive affect and lower rumination on positive affect to predict higher depressive symptoms. Importantly, probing of the interaction between reward responsiveness and dampening of positive affect predicting depressive symptoms suggested that low levels of dampening might also be a protective factor. These results lend support for the role of higher and lower reward responsiveness in differentially increasing risk for (hypo)manic and depressive symptoms, respectively (Alloy et al., 2016), and indicate that the tendency to amplify (via rumination) or dampen positive affect enhances these associations. Individuals with high reward responsiveness are expected to experience positive affective arousal during reward anticipation and following reward receipt, and if they also amplify this positive affect through rumination, this combination may be especially likely to lead to greater (hypo)manic symptoms. Likewise, individuals with blunted reward responsiveness who also tend to not savor, or even dampen, positive affective experiences may be particularly vulnerable to depressive symptoms.
However, one of our hypotheses, specifically that lower reward responsiveness would interact with brooding on negative affect to predict increased depressive symptoms (Hypothesis S-3) was not supported. Further, part of Hypothesis S-1, namely that dampening positive affect would buffer the risk conferred by higher reward responsiveness for (hypo)manic symptoms also was not supported. It is possible that failure to attain goals is less salient in individuals with low reward responsiveness, who then are less likely to have negative emotions to brood on. Additionally, heightened reward responsiveness might not increase risk for (hypo)manic symptoms via increased positive affect, rendering the tendency to dampen positive emotions irrelevant. Finally, the two significant models that predicted depressive symptoms only did so with the BDI, not the 7U7D depression scale. This might be the result of the BDI more thoroughly measuring depressive symptomatology than the 7U7D, which doesn’t include all symptoms of depression, resulting in these measures reflecting different depressive constructs. In particular, the 7U7D does not include assessment of anhedonia, which among depressive symptoms, has been most strongly associated with low reward responsiveness (Nusslock & Alloy, 2017).
Further, our results suggest that the interaction between reward responsiveness and ruminative response styles that promote or dampen positive affect is associated with higher inflammation, indexed by an inflammatory composite, providing additional support for the ability of rumination to amplify the effect of other psychological risk factors on inflammation (Moriarity et al., 2018). Importantly, the interactions associated with inflammation exhibited the same pattern as two of the interactions predicting mood symptoms. Specifically, consistent with Hypotheses I-1 both 1) higher reward responsiveness and rumination on positive affect, and 2) lower reward responsiveness and dampening of positive affect were associated with greater inflammation. Follow-up analyses suggested that these results might have been driven by IL-8 and CRP, respectively. Indeed, consistent with an expansion of the perseverative cognition hypothesis (Brosschot et al., 2006; Zoccola et al., 2014), rumination may amplify the intensity and duration of physiological arousal associated with psychological states of goal-pursuit/achievement or goal absence/failure associated with higher and lower reward responsiveness, respectively. Although these results support our hypotheses, it is important to note that, unlike the models predicting symptoms, inflammation only was measured at one timepoint, precluding truly predictive models.
Unexpectedly, there were no significant models with brooding on negative affect associated with inflammation, contrary to prior studies (Moriarity et al., 2018; Zoccola et al., 2014). This could be due to the decision to use an ICS, whereas prior studies tested specific biomarkers. Although this analytic strategy helped avoid issues of multiple comparisons, there is evidence that specific biomarkers have differential relationships with discrete psychological variables (Fried et al., 2018). Thus, it is possible that brooding on negative affect might have moderated the association between reward responsiveness and individual biomarkers.
This study has several methodological strengths. It has a longitudinal design, allowing for temporal precedence of the predictors relative to the outcomes. Additionally, mood symptoms were measured twice, allowing for tests of how the predictors were associated with change in symptomatology. It also included participants selected for and shown to be at high-risk for the development of a BSD (Alloy et al., 2012), elevating the likelihood of observing (hypo)manic symptoms and increasing this study’s clinical relevance.
However, these results should be considered in light of the following limitations. First, there was considerable variability in the time between the assessments and different average times to follow-up for the BDI and 7U7D. As a result, participants had different lengths of time for both predictors and outcomes to change, which could influence the relationships observed in this study. Future research should attempt to replicate these results with different time lags to evaluate if these associations are robust to differences in time-to-follow-up. Additionally, future tests of this model ideally would have less variability in time-to-follow-up to allow a more concrete understanding of the temporal contexts in which these associations are evident. However, this concern is somewhat mitigated by the relatively high stability of the predictor measures; thus, it is unlikely that they changed considerably over time. Unfortunately, because the blood draws were introduced as an optional addition to Project TEAM, there were no repeated inflammatory biomarker measures, precluding the ability to examine change. Also, although the use of an inflammatory composite variable as a measure of systemic inflammation has been used before (Miller et al., 2014; Nusslock et al., 2019) and reduced potential concerns about multiple comparisons, it is a relatively new technique that has not been psychometrically evaluated. Further, it is possible that some interactions might have been associated with individual biomarkers, but not the composite. Also, it is possible that inflammation influences reward responsiveness, but only having pre-blood draw measures of reward responsiveness precluded investigation of bidirectional relationships. Additionally, the original TEAM sample was selected for high vs. moderate levels of reward sensitivity, which might limit the generalizability of these results. Thus, future research should test these hypotheses in a sample that includes the full spectrum of reward responsiveness. Finally, we did not find any specific tests of the discriminant validity between these measures of reward sensitivity and affective rumination and mood symptoms in the extant literature, which must be considered when interpreting the results.
Theoretically, inflammation could mediate the relationship between reward responsiveness and mood symptoms, conditional on rumination. Unfortunately, this dataset was underpowered for testing moderated mediation analyses and only had one timepoint of immune measures, precluding the ability to control for initial levels of inflammation. Future research using a more appropriate dataset should test such indirect effects. Another important future direction for this research would be to evaluate how positive and negative rumination, in the context of positive and negative life events (which were not measured in the current study), may interact with reward sensitivity to predict inflammation and mood symptoms.
Conclusion
Both reward responsiveness and rumination are important risk factors for unipolar and bipolar mood symptoms (Johnson et al., 2008; Nolen-Hoeksema & Morrow, 1991; Nusslock & Alloy, 2017). This study is the first to demonstrate that they act synergistically to increase risk for depressive and (hypo)manic symptoms. Their interaction also is associated with inflammation, which may account for some of the associations between these risk factors and mood symptoms. This study supports the integration of current models of mood disorder etiology involving reward sensitivity and ruminative response styles. It also highlights how these risk factors might influence inflammation in a way that increases risk for later mood symptoms.
Supplementary Material
Highlights.
Examined reward and ruminative style predictors of mood symptoms and inflammation
High reward responsivity & positive rumination interact to predict (hypo)mania
High reward responsivity & positive rumination interact to predict inflammation
Low reward responsivity & positive affect dampening interact to predict depression
Low reward responsivity & positive dampening interact to predict inflammation
Acknowledgments
This research was supported by National Institute of Mental Health Grants MH077908 and MH102310 to Lauren B. Alloy and MH100117 to Robin Nusslock.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alanna M, Zhao L, Ahmed Y, Stoyanova N, Hooper WC, Gibbons G, … Vaccarino V Association between depression and inflammation-differences by race and sex: the META-Health study. Psychosomatic Medicine, 73(6), 462–468. 10.1097/PSY.0b013e318222379c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Hogan ME, Whitehouse WG, Rose DT, Robinson MS, … Lapkin JB (2000). The Temple-Wisconsin cognitive vulnerability to depression project: Lifetime history of Axis I psychopathology in individuals at high and low cognitive risk for depression. Journal of Abnormal Psychology, 109(3), 403–418. 10.1037//0021-843X.109.3.403 [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, … Hogan ME (2008). Behavioral approach system and behavioral inhibition system sensitivities and bipolar spectrum disorders : prospective prediction of bipolar mood episodes. Bipolar Disorders, 10, 310–322. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Wagner CA, Whitehouse WG, Abramson LY, Hogan ME, … Harmon-jones E (2009). Bipolar spectrum - substance use co-occurrence: Behavioral approach system ( BAS ) sensitivity and impulsiveness as shared personality vulnerabilities. Journal of Personality and Social Psychology, 97(3), 549–565. 10.1037/a0016061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner C. a, Liu RT, Grant D. a, … Abramson LY (2012). High Behavioral Approach System (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: a prospective behavioral high-risk design. Journal of Abnormal Psychology, 121(2), 339–351. 10.1037/a0025877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Nusslock R, & Boland EM (2015). The development and course of bipolar spectrum disorders: An integrated reward and circadian rhythm dysregulation model. Annual Review of Clinical Psychology, 11, 213–250. 10.1146/annurev-clinpsy-032814-112902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Olino T, Freed RD, & Nusslock R (2016). Role of reward sensitivity and processing in major depressive and bipolar spectrum disorders. Behavior Therapy, 47(5), 600–621. https://doi.org/10.1016Zj.beth.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Urosevic S, Abramson LY, Jager-Hyman S, Nusslock R, Whitehouse WG, & Hogan M (2012). Progression along the bipolar spectrum: A longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. Journal of Abnormal Psychology, 121(1), 16–27. 10.1037/a0023973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association, A. P. (2000). Diagnostic and statistical manual of mental disorders: DSM-IV-TR (4th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Beck AT, Brown G, & Steer R (1996). Beck Depression Inventory, 2nd Edition. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Bell JA, Kivimaki M, Bullmore ET, Steptoe A, Bullmore E, Vértes PE, … Carvalho LA (2017). Repeated exposure to systemic inflammation and risk of new depressive symptoms among older adults. Translational Psychiatry, 7(8), e1208 10.1038/tp.2017.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland EM, Stange JP, LaBelle DR, Shapero BG, Weiss RB, Abramson LY, & Alloy LB (2016). Affective disruption from social rhythm and behavioral approach system (BAS) sensitivities: A test of the integration of the social zeitgeber and BAS theories of bipolar disorder. Clinical Psychological Science, 4(3), 418–432. https://doi.org/10.n77/2167702615603368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, & Hajcak G (2013). Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology, 50, 74–81. 10.1111/j.1469-8986.2012.01485.x [DOI] [PubMed] [Google Scholar]
- Brietzke E, Mansur RB, Soczynska JK, Kapczinski F, Bressan RA, & McIntyre RS Towards a multifactorial approach for prediction of bipolar disorder in at risk populations. Journal of Affective Disorders, 740(1), 82–91. 10.1016/jjad.2012.02.016 [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, & Thayer JF (2006). The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research, 60(2), 113–124. 10.1016/jjpsychores.2005.06.074 [DOI] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, & Marsland AL (2011). Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain, Behavior, and Immunity, 25(2), 232–238. https://doi.org/10.1016Zj.bbi.2010.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS (2004). Negative affects deriving from the behavioral approach system. Emotion, 4(1), 3–22. 10.1037/1528-3542AL3 [DOI] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology, 67(2), 319–333. 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- Cuellar AK, Johnson SL, & Winters R (2005). Distinctions between bipolar and unipolar depression. Clinical Psychology Review, 25, 307–339. https://doi.Org/10.1016/j.cpr.2004.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ferranti SD, Gauvreau K, Ludwih DS, Newburger JW, & Rifai N (2006). Inflammation and changes in metabolic syndrome abnormalities in US adolescents: Findings from the 1988–1994 and 1999–2000 National health and nutrition examination surveys. Clinical Chemistry, 52(7), 1325–1330. [DOI] [PubMed] [Google Scholar]
- Depue RA, & Iacono WG (1989). Neurobehavioral aspects of affective disorders. Annual Review of Psychology, 40, 457–492. 10.1146/annurev.ps.40.020189.002325 [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss S, Spoont MR, & Arbisi P (1989). General Behavior Inventory identification of unipolar and bipolar affective conditions in a nonclinical university population. Journal of Abnormal Psychology, 98(2), 117–126. [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A, Abreu-Gonzalex P, & Kaski JC (2009). Inflammatory systemic biomarkers in setting acute coronary syndromes - effects of the diurnal variation. Current Drug Targets, 10(10), 1001–1008. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, & Lanctôt KL (2010). A meta-analysis of cytokines in major depression. Biological Psychiatry, 67(5), 446–457. https://doi.org/10.1016Zj.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Moieni M, Inagaki TK, Muscatell KA, & Irwin MR (2017). In sickness and in health: The co-regulation of inflammation and social behavior. Neuropsychopharmacology, 42(1), 242–253. 10.1038/npp.2016.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, & Spitzer R (1978). A diagnostic interview The schedule for affective disorders and schizophrenia. Archives of General Psychiatry, 35(7), 837–844. [DOI] [PubMed] [Google Scholar]
- Feldman GC, Joorman J, & Johnson SL (2008). Responses to positive affect: A self-report measure of rumination and dampening. Cognitive Therapy and Research, 32(4), 507–528. 10.1109/TMI.2012.2196707.Separate [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, & Miller AH (2012). Cytokine effects on the basal ganglia and dopamine function: The subcortical source of inflammatory malaise. Frontiers in Neuroendocrinology, 33(3), 315–327. 10.1016/j.yfrne.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI, von Stockert S, Haslbeck JMB, Lamers F, Schoevers RA, & Penninx BWJH (2018). Using network analysis to examine links between individual depressive symptoms, inflammatory markers, and covariates, 2–22. [DOI] [PubMed] [Google Scholar]
- Fu Q, Zhu J, & Van Eyk J (2010). Comparison of multiplex immunoassay platforms. Clinical Chemistry, 56(2), 314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Eidelman P, Johnson SL, Smith B, & Harvey AG (2011). Hooked on a feeling: Rumination about positive and negative emotion in inter-episode bipolar disorder. Journal of Abnormal Psychology, 120(4), 956–961. 10.1037/a0023667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E (2003). Anger and the behavioral approach system. Personality and Individual Differences, 35(5), 995–1005. 10.1016/S0191-8869(02)00313-6 [DOI] [Google Scholar]
- Hayes A (2013). Introduction to mediation, moderation, and conditional process analysis : a regression-based approach. New York: Guilford Press. [Google Scholar]
- Henriques JB, & Davidson RJ (2000). Decreased responsiveness to reward in depression. Cognition and Emotion, 14(5), 711–724. 10.1080/02699930050117684 [DOI] [Google Scholar]
- Howren MB, Lamkin DM, & Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine, 71(2), 171–186. 10.1097/PSY.0b013e3181907c1b [DOI] [PubMed] [Google Scholar]
- Hundt NE, Brown LH, Kimbrel NA, Walsh MA, Nelson-Gray R, & Kwapil TR (2013). Reinforcement sensitivity theory predicts positive and negative affect in daily life. Personality and Individual Differences, 54(3), 350–354. https://doi.Org/10.1016/j.paid.2012.09.021 [Google Scholar]
- IBM Corp. (2016). IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp. [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, & Carver CS (2012). The behavioral activation system and mania. Annual Review of Clinical Psychology, 8, 243–267. 10.1146/annurev-clinpsy-032511-143148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, & Kizer A (2002). Bipolar and unipolar depression: A comparison of clinical phenomenology and psychosocial predictors In Gotlib IH & Hammen CL (Eds.), Handbook of depression (pp. 141–165). New York, NY: Guillford Press. [Google Scholar]
- Johnson SL, McKenzie G, & McMurrich S (2008). Ruminative responses to negative and positive affect among students diagnosed with bipolar disorder and major depressive disorder. Cognitive Therapy and Research, 32(5), 702–713. 10.1007/s10608-007-9158-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalo-Klein A, & Witkins SS (1989). Candida albicans: cellular immune system interactions during different stages of the menstrual cycle. American Journal of Obstetrics & Gynecology, 161(5), 1132–1136. [DOI] [PubMed] [Google Scholar]
- Kim YK, Jung HG, Myint AM, Kim H, & Park SH (2007). Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. Journal of Affective Disorders, 104(1–3), 91–95. 10.1016/jjad.2007.02.018 [DOI] [PubMed] [Google Scholar]
- Knowles R, Tai S, Christensen I, & Bentall R (2005). Coping with depression and vulnerability to mania: A factor analytic study of the Nolen-Hoeksema (1991) Response Styles Questionnaire. British Journal of Clinical Psychology, 44(1), 99–112. 10.1348/014466504X20062 [DOI] [PubMed] [Google Scholar]
- Liu RT, & Alloy LB (2010). Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clinical Psychology Review, 30(5), 582–593. 10.1016/jxpr.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loxton NJ, & Tipman RJ (2017). Reward sensitivity and food addiction in women. Appetite, 115, 28–35. https://doi.org/10.1016Zj.appet.2016.10.022 [DOI] [PubMed] [Google Scholar]
- McEwen B, & Stellar E (1993). Stress and the individual: mechanisms leading to disease. Arch Intern Med, 153, 2093–2101. [PubMed] [Google Scholar]
- Miller AH, Maletic V, & Raison CL (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry, 65(9), 732–741. 10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Brody GH, Yu T, & Chen E (2014). A family-oriented psychosocial intervention reduces inflammation in low-SES African American youth. Proceedings of the National Academy of Sciences, 111(31), 11287–11292. 10.1073/pnas.1406578111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, & Wrosch C (2007). You’ve gotta know when to fold ‘em adolescence. Psychological Science, 18(9), 773–777. [DOI] [PubMed] [Google Scholar]
- Mills NT, Scott JG, Wray NR, Cohen-Woods S, & Baune BT (2013). Research review: The role of cytokines in depression in adolescents: A systematic review. Journal of Child Psychology and Psychiatry and Allied Disciplines, 54(8), 816–835. 10.1111/jcpp.12080 [DOI] [PubMed] [Google Scholar]
- Moons WG, Eisenberger NI, & Taylor SE (2010). Anger and fear responses to stress have different biological profiles. Brain, Behavior, and Immunity, 24(2), 215–219. https://doi.Org/10.1016/j.bbi.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Morgan JK, Olino TM, Mcmakin DL, Ryan ND, & Forbes EE (2013). Neural response to reward as a predictor of rise in depressive symptoms in adolescence. Neurobiol Dis, 52, 66–74. https://doi.org/10.1016Zj.nbd.2012.03.039.Neural [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity DP, McArthur BA, Ellman LM, Coe CL, Abramson LY, & Alloy LB (2018). Immunocognitive model of depression secondary to anxiety in adolescents. Journal of Youth and Adolescence, 47(12), 2625–2636. 10.1007/s10964-018-0905-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TH, Alloy LB, & Smith DV (2018). Meta-analysis of Reward Processing in Major Depressive Disorder: Distinct Abnormalities within the Reward Circuit? BioRxiv, 332981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (1991). Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology, 100(4), 569–582. 10.1037/0021-843X.100A569 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, & Morrow J (1991). A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta Earthquake. Journal of Personality and Social Psychology, 61(1), 115–121. 10.1037/0022-3514.61.1.115 [DOI] [PubMed] [Google Scholar]
- Nusslock R, & Alloy LB (2017). Reward processing and mood-related symptoms: An RDoC and translational neuroscience perspective. Journal of Affective Disorders, 216(2017), 3–16. 10.1016/jjad.2017.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Brody GH, Armstrong CC, Carroll AL, Sweet LH, Yu T, … Miller GE (2019). Higher peripheral inflammatory signaling associated with lower resting-state functional brain connectivity in emotion regulation and central executive networks. Biological Psychiatry, 1–10. 10.1016/j.biopsych.2019.03.968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Miller GE (2016). Early-life adversity and physical and emotional health across the lifespan: A neuroimmune network hypothesis. Biological Psychiatry, 50(1), 23–32. 10.1016/_j.biopsych.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes F, Smets J, Nelis S, & Schoofs H (2012). Dampening of positive affect prospectively predicts depressive symptoms in non-clinical samples. Cognition and Emotion, 26(1), 75–82. 10.1080/02699931.2011.555474 [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Schipper LJ, & Greenberg RN (2008). Caregiving, repetitive thought, and immune response to vaccination in older adults. Brain Behavior and Immunity, 22(5), 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Irwin MR (2014). From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojevic J, & Alloy LB (2001). Rumination as a common mechanism relating depressive risk factors to depression. Emotion, 1(1), 25–37. 10.1037//1528-3542.1.1.25 [DOI] [PubMed] [Google Scholar]
- Torrubia R, Ávila C, Molto J, & Caseras X (2001). The Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Personality and Individual Differences, 31(6), 837–862. 10.1016/S0191-8869(00)00183-5 [DOI] [Google Scholar]
- Treynor W, Gonzalez R, & Nolen-Hoeksema S (2003). Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research, 27(3), 247–259. 10.1023/A:1023910315561 [DOI] [Google Scholar]
- Twenge JM, & Nolen-hoeksema S (2002). Age, gender, race, socioeconomic status, and birth cohort differences on the Children’ s Depression Inventory: A meta-analysis. Journal of Abnormal Psychology, 777(4), 578–588. https://doi.Org/10.1037//0021-843X.111.4.578 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, & Telang F (2008). Overlapping neuronal circuits in addiction and obesity : evidence of systems pathology. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1507), 3191–3200. 10.1098/rstb.2008.0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstrom EA, Murray G, Johnson SL, & Findling RL (2013). The 7 Up 7 Down Inventory: A 14-item measure of manic and depressive tendencies carved from the General Behavior Inventory. Psychological Assessment, 25(4), 1377–1383. 10.1037/a0033975.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccola PM, Figueroa WS, Rabideau EM, & Woody A (2014). Differential effects of post-stressor rumination and distraction on C-reactive protein in healthy women. Health Psychology, 33(12), 1606–1609. 10.1037/hea0000019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.