This study presents the isolation of the very first freshwater cyanophage, PA-SR01, that infects Pseudanabaena, and fills an important knowledge gap on freshwater cyanophages as well as cyanophages infecting Pseudanabaena.
KEYWORDS: cyanophages, freshwater, full genome, isolation, Pseudanabaena
ABSTRACT
Cyanobacteria are the major primary producers in both freshwater and marine environments. However, the majority of freshwater cyanophages remain unknown due to the limited number of cyanophage isolates. In this study, we present a novel lytic freshwater cyanophage, PA-SR01, which was isolated from the Singapore Serangoon Reservoir. To our knowledge, this is the first isolate of a cyanophage that has been found to infect the cyanobacterium Pseudanabaena. PA-SR01 has a narrow host range, a short latent period, and is chloroform sensitive. Distinct from the majority of cyanophage isolates, PA-SR01 has a tailless morphology. It is a double-stranded DNA virus with a 137,012-bp genome. Functional annotation for the predicted open reading frames (ORFs) of the PA-SR01 genome identified genes with putative functions related to DNA metabolism, structural proteins, lysis, host-derived metabolic genes, and DNA packaging. Out of 166 predicted ORFs, only 17 ORFs have homology with genes with known function. Phylogenetic analysis of the major capsid protein and terminase large subunit further suggests that phage PA-SR01 is evolutionary distinct from known cyanophages. Metagenomics sequence recruitment onto the PA-SR01 genome indicates that PA-SR01 represents a new evolutionary lineage of phage which shares considerable genetic similarities with phage sequences in aquatic environments and could play key ecological roles.
IMPORTANCE This study presents the isolation of the very first freshwater cyanophage, PA-SR01, that infects Pseudanabaena, and fills an important knowledge gap on freshwater cyanophages as well as cyanophages infecting Pseudanabaena.
INTRODUCTION
Cyanobacteria play important roles in primary production and trophic interactions. They are the dominant autotrophs in most aquatic environments, such as freshwater and marine environments (1). Viruses infecting cyanobacteria are referred to as cyanophages and can play major roles in the dynamics, genetic diversity, and structure of cyanobacterial communities (2–4). Compared to marine cyanophages, which have been widely studied (5), there are very limited studies on freshwater cyanophages (6, 7).
To better understand the biological interactions and evolutionary relationships between cyanophages and their host, cyanophage whole-genome sequences could provide a solid platform to elucidate such relationships (8–12). At the same time, as metagenomics becomes a more prevalent approach to monitoring environmental cyanophage diversity, genomic sequences of cultured cyanophages are needed for more precise annotation of the viral metagenome. Most viral sequences in metagenomic databases cannot be allocated putative functions and there are still many viral contigs of unknown identity in metagenomes (13, 14). This further strengthens the case for the need to acquire more genomic sequences of new cyanophage isolates, especially in the case of freshwater cyanophages.
Based on their morphological differences, cyanophages are generally categorized within three families, the Myoviridae, Podoviridae, and Siphoviridae, which belong to the order Caudovirales. Myoviridae have long contractile tails, Podoviridae have short noncontratile tails, while Siphoviridae have long flexible tails (15). Only one tailless cyanophage has been isolated to date (16).
PA-SR01 infects and lyses freshwater Pseudanabaena strain KCZY-C8. Despite reports of several cases of Pseudanabaena presence in cyanobacterial blooms (17–19), there have been no Pseudanabaena-infecting phages isolated to date to the best of our knowledge. PA-SR01 is the first cyanophage infecting and lysing Pseudanabaena. To explore the biological properties and ecological roles of PA-SR01, we first studied the morphology and infection process, followed by sequencing the PA-SR01 genome and performing functional gene annotation. To further understand its environmental presence, recruitment of metagenomics reads onto the PA-SR01 genome was performed and revealed its prevalence in aquatic systems around the globe.
RESULTS AND DISCUSSION
Physical properties of phage PA-SR01.
The transmission electron microscopy images of PA-SR01 phage (Fig. 1) showed numerous virus particles with similar size and morphology. Unlike most isolated tailed cyanophages, the cross sections of the viral particles appeared hexagonal, without any tails attached, indicating that the virus had an icosahedral symmetry and was tailless. The average diameter of viral particles ranged from 88 to 95 nm (mean ± SD = 91 ± 3 nm). Similar tailless freshwater cyanophages have also been identified in Lake Donghu, China (16).
FIG 1.

Transmission electron micrographs showing morphological features of PA-SR01. Micrographs show empty capsid (A) and original phage particles (B).
Host specificity.
PA-SR01 lysed only Pseudanabaena strain KCZY-C8 and not the additional 2 Pseudanabaena strains and 17 cyanobacterial species (Table 1). Based on this result, we concluded that PA-SR01 is strain specific rather than species specific, and has a narrow host range. To understand whether PA-SR01 infectivity is correlated with geographical location, more Pseudanabaena strains will need to be isolated from other tropical water bodies.
TABLE 1.
List of cyanobacteria used for host range test
| Genus | Strain | Origin | Susceptibility |
|---|---|---|---|
| Cylindrospermopsis | CS505 | Lakes in tropical Queensland | − |
| Cylindrospermopsis | CS511 | Lakes in tropical Queensland | − |
| Cylindrospermopsis | Cyl UPR | Upper Peirce Reservoir | − |
| Cylindrospermopsis | CS509 | Lakes in tropical Queensland | − |
| Cylindrospermopsis | Cy2.2 | Serangoon Reservoir | − |
| Cylindrospermopsis | Cy3.4 | Serangoon Reservoir | − |
| Limnothrix | MRS2 | Marina Reservoir | − |
| Microcystis | I21 | Serangoon Reservoir | − |
| Microcystis | B | Kranji Reservoir | − |
| Microcystis | I1 | Serangoon Reservoir | − |
| Microcystis | I31 | Serangoon Reservoir | − |
| Microcystis | M1 | Marina Reservoir | − |
| Microcystis | K18 | Kranji Reservoir | − |
| Pseudanabaena | KCZY-C8 | Serangoon Reservoir | + |
| Pseudanabaena | MRS2 | Marina Reservoir | − |
| Pseudanabaena | M13A | Marina Reservoir | − |
| Synechococcus | IA | Serangoon Reservoir | − |
| Synechococcus | Cip1 | Serangoon Reservoir | − |
| Synechococcus | Cip6 | Serangoon Reservoir | − |
| Synechococcus | R4S1 | Serangoon Reservoir | − |
| Synechococcus | Cip21 | Serangoon Reservoir | − |
Chloroform sensitivity.
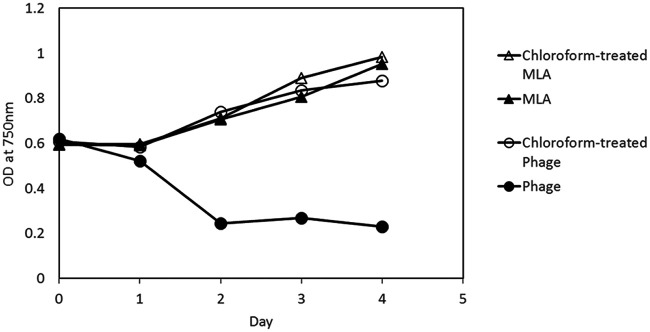
The infectivity of PA-SR01 is chloroform sensitive (Fig. 2). Chloroform treatment makes phage lose its infectivity and there is no observable effect on host cell growth with or without addition of chloroform-treated MLA medium. Chloroform sensitivity serves as a first indication of a viral lipid component (20). Chloroform can dissolve lipids that may be structural components of infection mechanisms in lipid-containing phage (21). However, chloroform sensitivity alone does not prove the presence of lipid in viral particles. Further studies are needed to confirm the presence of lipids in PA-SR01. This figure also shows that the latent period of PA-SR01 is approximately 1 day, which is relatively short compared to other freshwater cyanophages such as S-LBS1 (latent period of 4 days), PaV-LD (latent period of 2 days), and S-EIV1 (latent period of 2 days) (9, 10, 12).
FIG 2.
Effect of chloroform on the infectivity of PA-SR01. OD at 750 nm is shown for Pseudanabaena cultures grown in untreated (open triangle) or chloroform-treated MLA medium (black triangle), or else inoculated with chloroform-treated (black circles) or untreated viruses (open circles).
Genomic overview.
The 137,012-bp genome of PA-SR01 is circularly permutated (Fig. 3) with a GC content of 39.5%. One hundred sixty-six ORFs were predicted, though most ORFs in PA-SR01 did not have homologous genes of known function. In total, 47 ORFs had significant similarity to other sequences, and more than 70% of the ORFs could not be annotated to any homologs. Only 11 ORFs were similar to phage sequences and only 17 were similar to genes of known function (BLASTp; E value cutoff = 10−5). Three clustered tRNA genes, tRNAMet, tRNAAsp, and tRNAGly, were identified (Table 2).
FIG 3.

Genomic map of PA-SR01. Circles from outermost to innermost correspond to (i) predicted ORFs (BLASTp, nr database, E value of <0.00001) on forward strand and (ii) reverse strand; (iii) GC content plotted with green representing G+C content and purple representing A+T content. Only ORFs of >100 bp are shown and are colored as follows: black, hypothetical protein; gray, no homolog; pink, DNA packaging; blue, structural protein; crimson, host-derived metabolic gene; brown, DNA metabolism; purple, lysis.
TABLE 2.
tRNAs predicted with tRNAscan-SE2
| tRNA number | tRNA start | tRNA end | tRNA type | Anticodon |
|---|---|---|---|---|
| 1 | 58579 | 58502 | Met | CAT |
| 2 | 58497 | 58427 | Asp | ATC |
| 3 | 58356 | 58284 | Gly | TCC |
Genome annotation of PA-SR01 ORFs identified putative genes with functions associated with structural proteins, DNA metabolism, DNA packaging, and lysis (Table 3). HHpred was used to ascribe function to additional ORFs (Table 4). This resulted in the identification of genes encoding putative functions associated with DNA-binding domains (ORF3), Mu-like prophage I protein (ORF24), major capsid protein (ORF32), and PD-(D/E)XK endonuclease (ORF139). In total, 21 ORFs showing homology to genes of known function were obtained.
TABLE 3.
Predicted ORFs of cyanophage PA-SR01 with similarity to genes of known function
| ORF | GenBank ID | Strand | % identity | E value | Putative protein encoded | Organism |
|---|---|---|---|---|---|---|
| 12 | WP_097778008.1 | + | 29 | 1.00E−55 | Phage terminase large subunit | Faecalibacterium prausnitzii |
| 29 | RTL07602.1 | − | 38 | 8.40E−72 | DEAD/DEAH box helicase | Candidatus Dependentiae bacterium |
| 41 | ADP97718.1 | + | 21 | 3.40E−26 | Phage tail tape measure protein, family | Marinobacter adhaerens HP15 |
| 55 | QBQ77753.1 | − | 35 | 8.80E−26 | Putative group I intron endonuclease | Escherichia phage vB_EcoM_WFbE185 |
| 66 | YP_009100781.1 | - | 35 | 3.70E−23 | Homing endonuclease | Shigella phage Shf125875 |
| 68 | KKP95496.1 | + | 30 | 1.00E−11 | Crossover junction endodeoxyribonuclease RuvC | Candidate division TM6 bacterium |
| 77 | WP_138275383.1 | + | 33 | 6.00E−09 | Crossover junction endodeoxyribonuclease RuvC | Candidatus Rhodoluna limnophila |
| 81 | WP_050045131.1 | + | 90 | 2.00E−218 | IS200/IS605 family element transposase accessory protein TnpB | Tolypothrix bouteillei |
| 83 | WP_070058383.1 | + | 51 | 7.00E−16 | Septal ring lytic transglycosylase RlpA family protein | Marinobacter sp. X15-166B |
| 90 | WP_131120822.1 | − | 48 | 7.10E−19 | KilA-N domain-containing protein | Westiellopsis prolifica |
| 98 | OBQ26706.1 | − | 39 | 1.10E−20 | Appr-1-p processing protein | Aphanizomenon flos-aquae LD13 |
| 103 | QBQ73194.1 | − | 68 | 5.70E−92 | FAD-dependent thymidylate synthase | Nodularia phage vB_NspS-kac65v151 |
| 107 | BBI90448.1 | − | 34 | 6.00E−112 | Ribonucleotide-diphosphate reductase subunit beta | Tenacibaculum phage PTm1 |
| 108 | WP_018298810.1 | − | 49 | 3.20E−198 | Ribonucleoside-diphosphate reductase subunit alpha | Fangia hongkongensis |
| 114 | PSB68310.1 | − | 35 | 6.00E−99 | DNA-directed DNA polymerase | Filamentous cyanobacterium CCP1 |
| 116 | WP_119501154.1 | − | 30 | 3.00E−08 | Deoxynucleotide monophosphate kinase | Alteromonas sp. RKMC-009 |
| 125 | YP_009042791.1 | − | 48 | 1.50E−21 | Recombination protein | Anabaena phage A-4L |
TABLE 4.
ORFs with distant homology to PA-SR01 identified using HHpred analysis
| ORF | Pfam IDa | Strand | E value | Putative protein encoded |
|---|---|---|---|---|
| 3 | PF10544.9 | + | 5.2E−16 | T5orf172 domain |
| 24 | PF10123.9 | + | 4.5E−18 | Mu-like prophage I protein |
| 32 | PF03864.15 | + | 1.5E−30 | Phage major capsid protein E |
| 139 | PF11645.8 | − | 0.0000018 | PD-(D/E)XK endonuclease |
Pfam ID data can be found at https://pfam.xfam.org.
Seven rho-independent terminators were predicted by Findterm (Table 5); 6 are downstream of ORFs with unknown function (ORF30, ORF33, ORF61, ORF62, ORF73, and ORF102) and one is downstream of a gene predicted to encode a Kila-N domain-containing protein (ORF90).
TABLE 5.
PA-SR01 rho-independent terminators predicted by Findterm
| Terminator | Start | End | Length (bp) | Strand | Energy (kCal) | Upstream ORF | Distance to ORF |
|---|---|---|---|---|---|---|---|
| Term1 | 26973 | 26924 | 50 | + | −18.8 | 30 | 21 |
| Term2 | 30131 | 30088 | 44 | + | −17.3 | 33 | 139 |
| Term3 | 61015 | 60972 | 44 | + | −21.5 | 61 | 2 |
| Term4 | 61988 | 61943 | 46 | + | −22.7 | 62 | 104 |
| Term5 | 71819 | 71770 | 50 | + | −20.9 | 73 | 89 |
| Term6 | 86765 | 86805 | 41 | − | −16.9 | 90 | 67 |
| Term7 | 95947 | 95996 | 50 | − | −18 | 102 | 87 |
PA-SR01 is morphologically distinct from known Caudovirales, and this is also reflected in the genes shared between them. Out of 166 ORFs predicted, merely 6 ORFs were homologous to genes from known Caudovirales. This suggested that PA-SR01 did not belong to the order Caudovirales, which was further supported by the observed tailless morphology of PA-SR01.
Structural genes.
ORF41 is the only ORF in PA-SR01 encoding tail tape measure protein (TMP), which is a tail-associated protein. Tail tape measure protein of tailed phages determines the tail length and enables DNA transition into the host cell during infection (22). Despite the name suggesting its widespread presence in tail phage genomes, the tail tape measure protein-encoding gene has, nevertheless, also been observed to be present in tailless phages (10). This indicates that tail tape measuring protein is not unique to tailed phages. Very few sequences encoding known phage structural proteins were found in the PA-SR01 genome other than the major capsid protein (ORF32). This further supports the structural distinction of PA-SR01 from other known phages. With SDS-PAGE analysis, 4 structural proteins of about 16, 28, 47, and 99 kDa (Fig. 4) were resolved. ORF32, encoding the major capsid protein, can be matched to the 47-kDa band. ORF46, ORF9, and ORF101, encoding hypothetical proteins, can be matched to the 99-kDa, 28-kDa, and 16-kDa bands, respectively. However, 4 structural proteins do not represent the full picture, as 13 structural proteins have been identified in phage with a similar genomic size (10, 23). To obtain a more thorough understanding of structural proteins in PA-SR01, a mass spectrometer approach is needed.
FIG 4.

SDS-PAGE for structural proteins of PA-SR01.
Host-derived genes.
Host-derived metabolic genes are commonly present in cyanophages and play important roles in interactions between cyanophage and their host (24). For example, a survey of 33 cyanophages revealed that psbA was found in 88% of the cyanophage genomes and 50% of the cyanophages contained both psbA and psbD genes (25). Besides photosynthetic genes, other host-derived genes have also been found that are responsible for phycobilisome degradation, carbon metabolism, phosphate uptake, and nucleotide biosynthesis (11, 26–28). The only host-derived metabolic gene identified in PA-SR01 genome is ribonucleotide-diphosphate reductase (RNR) (24). This suggests that PA-SR01 is evolutionary distinct from known cyanophages and could have its own special metabolic genes that require further study.
In PA-SR01, ORF107 and ORF108 were found homologous to ribonucleotide-diphosphate reductase (RNR) subunit alpha and beta, respectively. The RNR gene product can reduce ribonucleotide diphosphate to deoxy-ribonucleotide diphosphate, which is a precursor of DNA (26). Cyanophage can thus make use of RNRs to degrade host DNA to provide building blocks for synthesizing genomes of phage progeny. RNR genes are considered essential for the rapid replication found in lytic phage (29), and this could be a contributing factor to the short latent period of PA-SR01.
Nucleotide metabolism.
Besides RNR, there were several genes identified that are involved in nucleotide metabolism. The PA-SR01 genome encodes a homolog (ORF103) of FAD-dependent thymidylate synthase (ThyX) that produces thymidylate (dTMP) de novo from dUMP (30). The importance of ThyX in phage genome replication has been demonstrated in double-stranded DNA virus (31). In the PA-SR01 genome, another gene possibly involved in nucleotide metabolism is ORF116, encoding a homolog of deoxy-nucleotide monophosphate kinase which may phosphorylate dGMP, dTMP, and 5-hydroxymethyl-dCMP to be used in producing new viral DNA genomes (32). Both ThyX and deoxy-nucleotide monophosphate kinase might contribute to phage genome replication in PA-SR01. dTMP produced by ThyX could be phosphorylated to dTDP, which could be further phosphorylated by nucleoside-diphosphate kinase (NDPK) to form dTTP, a monomer that can be utilized by DNA polymerase (ORF114) to generate long-chain DNA molecules. However, no homologs of NDPK were found in the PA-SR01 genome, and thus further studies are needed to better understand the detailed nucleotide biosynthesis strategy of PA-SR01.
Insertion element.
PA-SR01 has one ORF showing extremely high similarity to the ORF from cyanobacterium Tolypothrix bouteillei. ORF81 has 90% amino acid sequence similarity to IS200/IS605 family element transposase accessory protein. Such a high sequence similarity is rare in phage genome and suggests that this ORF originated from recent horizontal gene transfer. Similar insertion sequences (IS) have been observed in other phage genomes (11, 33) and their functions remain unknown. IS elements are rare in phage genomes and are considered disadvantageous for bacteriophage propagation as they could disrupt the efficiency of phage genome organization (33). This also supports the hypothesis that ORF81 came from recent horizontal gene transfer, as it is less likely for a phage genome with IS elements to propagate and pass on its gene over many generations compared to phage without IS elements.
Lysis-associated genes.
The lysozyme homolog is commonly found in cyanophage and is believed to be the functional gene for cell lysis (9, 11, 12). However, no homologs of lysozyme can be found in the PA-SR01 genome; instead, ORF83 encodes a putative septal ring lytic transglycosylase RlpA family protein. Lytic transglycosylases represent a major class of enzymes capable of lysing bacterial cell walls with the same substrate specificity as lysozyme. Across different families of lytic transglycosylases, family 4 has been shown to be involved with bacteriophage-induced lysis (34). Rare lipoprotein A (RlpA) was found to be a new lytic transglycosylase with strong preference for naked glycan strands (35). ORF83, encoding a homolog of the RlpA family, could be the key gene responsible for cell lysis. This suggests that PA-SR01 adopts a different lysis strategy from known cyanophages and that PA-SR01 is likely to be evolutionary distinct.
PA-SR01, a new evolutionary lineage of cyanophage.
PA-SR01 represents a new evolutionary lineage of cyanophage based on its genomic content. There is a lack of structural gene similarity between the PA-SR01 genome and other phage genomes, with the exception of the major capsid protein (ORF32) and tail-tape measuring protein (ORF41). This is further supported by the morphological features of PA-SR01. To our knowledge, PA-SR01 is only the second tailless cyanophage discovered and a vast majority of cultured cyanophages belong to the order Caudovirales. Besides structural distinction, PA-SR01 adopts a different lysis strategy from other cyanophages, based on the fact that lytic transglycosylase instead of lysozyme is found in the PA-SR01 genome.
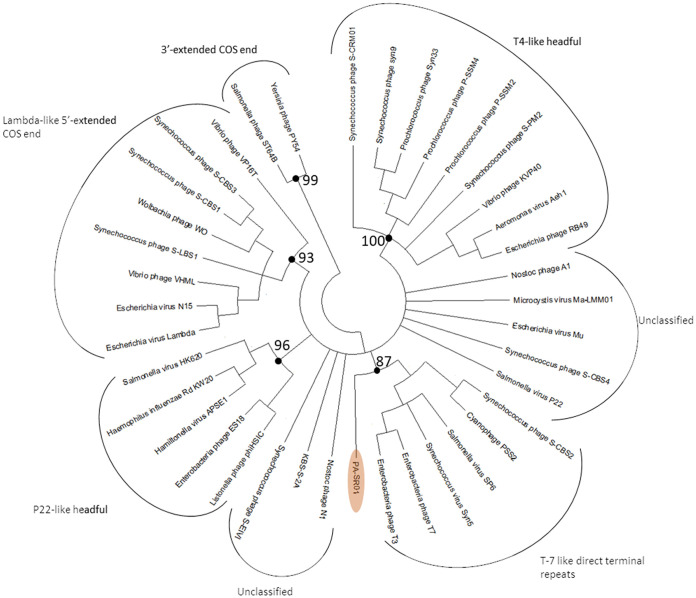
Phylogenetic analysis of the terminase large subunit (terL) and major capsid protein shows that PA-SR01 is evolutionary distinct from other cyanophage isolates. Although PA-SR01 terL is related to T7-like phages, it does not fall within the group of T7-like phages (Fig. 5). Furthermore, the amino acid sequence percentage identity shared between PA-SR01 terL and S-CBS2 terL is merely 26%. The BLASTP result of PA-SR01 terL showed much greater similarity to noncyanophage terL sequences, indicating an evolutionary divergence of terL in PA-SR01.
FIG 5.
Maximum likelihood amino acid tree of the viral terminase large subunit (terL). Bootstrap values are indicated (100 replicates).
Maximum likelihood amino acid tree of the major capsid protein provides further evidence that PA-SR01 is evolutionarily distinct from other cyanophage isolates (Fig. 6). A majority of the phages fall within the three main groups, Myoviridae, Siphoviridae, and Podoviridae, respectively. However, PA-SR01 does not fall within any of the clades and represents an independent branch, providing further support of the evolutionary divergence of PA-SR01 from other phages.
FIG 6.
Maximum likelihood amino acid tree of the viral major capsid protein. Bootstrap values are indicated (100 replicates).
PA-SR01 sequence similarities in the environment.
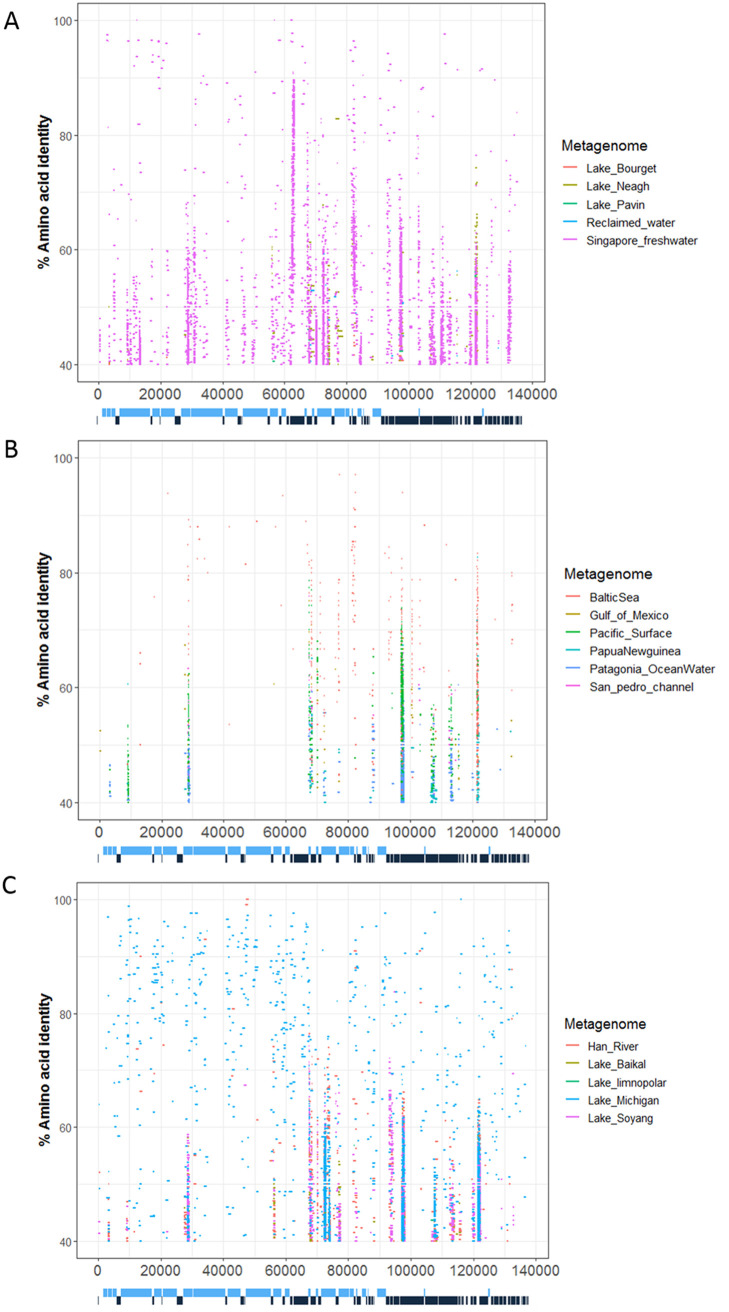
The widespread occurrence of viral sequences similar to PA-SR01 in the environment is shown by the recruitment of metagenomics reads onto the translated PA-SR01 genome. Both marine and freshwater environments were investigated in this analysis (Fig. 7A to C), and 146 ORFs were mapped with at least with one freshwater metagenome and 106 ORFs were mapped with multiple freshwater metagenomes. Twenty-six ORFs were extensively mapped to freshwater metagenomes. Fifty-eight ORFs were mapped to at least one marine metagenome and twenty-two ORFs were mapped across several marine metagenomes. This indicates that PA-SR01-like phages are much more prominent in freshwater. Seven ORFs (ORF12, ORF29, ORF64, ORF103, ORF114, ORF121, and ORF134) were extensively mapped with marine metagenomes and they were all extensively mapped with freshwater metagenomes as well. This suggests that phages adopting similar packaging strategies (e.g., ORF12 encoding a terminase large subunit) and similar DNA metabolism (e.g., ORF29 encoding a DEAD/DEAH box helicase, ORF114 encoding a DNA-directed DNA polymerase, and ORF103 encoding an FAD-dependent thymidylate synthase) are widespread in aquatic environments.
FIG 7.
Prevalence of viral sequences similar to PA-SR01 in environmental viral metagenomic data. Fragment recruitment of reads from environmental viral metagenomic data onto the genome of PA-SR01. Each horizontal line represents a read recruited from one of the following publicly available metagenomics data sets: (A) freshwater viral metagenome: Lake Pavin, Lake Bourget, Lake Neagh, reclaimed water virus, and Singapore urban freshwater; (B) marine viral metagenomes: Baltic Sea, Papua New Guinea, Patagonia, Gulf of Mexico, San Pedro Channel, and Pacific Ocean surface; (C) freshwater viral metagenome: Lake Baikal, Lake Limonopolar, Lake Michigan, Lake Soyang, and Han River.
The FAD-dependent thymidylate synthase ThyX (ORF103) and a hypothetical protein (ORF134) have the most recruited sequences across both marine and freshwater metagenomes. ThyX is a key gene in double-stranded phage genome replication, suggesting that phages with similar DNA replication strategy are widespread in aquatic environments and ThyX is an important part of phage DNA replication for both marine and freshwater phages. There are also a large number of recruited reads to IS200/IS605 family element transposase accessory protein TnpB (ORF81), located around 80 kbp. In contrast to the marine environment, 5 out of 10 freshwater metagenomes were recruited onto ORF81. As mentioned previously, TnpB was considered disadvantageous for bacteriophage and is not widely present in known cultured phage (11, 33). The recruited reads from multiple metagenomes onto ORF81 suggests that TnpB might not be detrimental for phage propagation or there could be extensive horizontal gene transfer of TnpB gene from host bacteria to phage.
It is clear in Fig. 7 that we observed many more recruited reads from freshwater viral metagenomes than marine. The majority of ORFs across the genome of PA-SR01 have recruited sequences in the metagenome from urban freshwaters in Singapore. This was expected, since PA-SR01 was isolated from a water body in Singapore and it is likely that viral sequences similar to PA-SR01 are widely distributed locally. Surprisingly, metagenomes from Lake Michigan produced a comparable amount of recruited reads on the PA-SR01 genome. This strongly suggests the widespread presence of viral sequences similar to PA-SR01 around the globe.
Further evidence for the widespread presence of viral sequences similar to PA-SR01 in the environment is provided by the relative abundance of different cyanophages in the Lake Michigan metagenome (Fig. 8). It is clear that viral sequences similar to PA-SR01 are prevalent in the Lake Michigan metagenome. Although there are several other phages having higher normalized recruited reads than PA-SR01, they are of comparable amount. Furthermore, the number of recruited reads of PA-SR01 is apparently much higher than the majority of other known cyanophages examined in this analysis. Admittedly, the occurrence of PA-SR01 relative to other cyanophages is overestimated due to the fact that only the blast hit with highest E value was recruited. For example, in the list of selected cyanophages there are several P-HM1-like phages, e.g., P-RSM4, P-SSM2, P-TIM68 and Syn1. Significant sequence similarity and core genes are shared among those phages, but only one phage genome would recruit each read, causing the dilution of read numbers assigned to each P-HM1-like cyanophage. Nonetheless, the data indicate that viral sequences similar to PA-SR01 phages are relatively abundant in freshwater environments.
FIG 8.
Relative abundance of viral sequences similar to PA-SR01 relative to other cyanophages in Lake Michigan. The number of reads was normalized to the number of genome length of each phage as well as the metagenome database size.
The relative abundance of viral sequences similar to PA-SR01 in the Pacific Ocean surface water (Fig. 9) is much lower than that in Lake Michigan and is among the least abundant, suggesting that viral sequences similar to PA-SR01 are more prevalent in freshwater environments. Since PA-SR01 was isolated from freshwater, it is more likely to have genes specific to freshwater environments.
FIG 9.
Relative abundance of viral sequences similar to PA-SR01 relative to other cyanophages in the Pacific Ocean surface. The number of reads was normalized to the number of genome length of each phage as well as the metagenome database size.
In conclusion, this study describes the characteristics and genome of PA-SR01, a rare tailless cyanophage with a uniquely different set of genes from other known cyanophages. PA-SR01 infects a tropical isolate of Pseudanabaena sp. and represents a new evolutionary lineage of cyanophage. Comparative metagenomics data indicate the global prevalence of PA-SR-01-like phages in both freshwater and marine environments. PA-SR01 and related viruses are likely to play major roles in controlling and shaping Pseudanabaena populations. Given the large number of genes without homologies in PA-SR01, more work is needed to characterize the phage-host interactions and ecological roles of PA-SR01.
MATERIALS AND METHODS
Host cells.
The Pseudanabaena strain KCZY-C8 was isolated in February 2019 from a tropical eutrophic fresh water body (Singapore Serangoon Reservoir) at 1°23′26.2"N 103°54'58.7"E 15 cm below the surface water. The strain was isolated by micropipetting from a surface water sample into sterile MLA medium (36) at 25°C. Identification of the strain was determined to the level of genus following the morphological characteristics (cell shape, dimension, and organization within trichome) reported in Bergey’s Manual of Systematics of Archaea and Bacteria (37) and other studies (38–40). Detailed traits of KCZY-C8 can be found in Fig. 10. We also used partial bacterial 16S rRNA sequence to verify the strain identity (Table 6). The culture was then incubated and maintained in batch culture at 25°C under low radiance (20 μmol photons m−2s−1) with a 12-h/12-h light/dark cycle.
FIG 10.
(A) Light microscope image of host strain KCZY-C8. (B) SEM image of host strain KCZY-C8. Both images show that most of the trichomes of KCZY-C8 are in agreement with the morphological characteristics of Pseudanabaena reported by Bergey’s Manual of Systematic Archaea and Bacteria (37), as well as other studies (38–40), as follows: cell division in one plane and intercellular breakage of trichome (filament); trichomes are straight; trichomes comprised of 3 barrel-shaped cells (usually 3 to 10 cells per trichome); cell length wider than diameter (diameter = 1μm, length = 3 μm); cell walls are constricted at the junction between adjacent cells.
TABLE 6.
BLASTN result of host 16S Sequence against NCBI RefSeq database
| Strain | Accession no. | % identity | Host 16S sequence |
|---|---|---|---|
| Pseudanabaena sp. ABRG5-3 | NZ_AP017560.1 | 93.81 | ATCTGNCGTGTCTCAGTCCAGTGTGACTGGTCATCCTCTCAGACCAGTTACCGATCGTCGCCATGGTGTGCCTTTACCACTCCATCTAGCTAATCGGACGCAAGCTCATCTACAGATGATAAATCTTTCACCCGAAGGCATATCCGGTATTAGCAGTCGTTTCCAACTGTTGTCCCGAGTCTGTAGGTAGATTCTTACGCGTTACTCACCCGTAA |
| Pseudanabaena biceps PCC7429 | NZ_ALWB01000102.1 | 91.827 | |
| Pseudanabaena sp. UWO310 | NZ_SELU01000117.1 | 91.827 | |
| Pseudanabaena sp. Roaring Creek | NZ_LIRE01000034.1 | 91.827 | |
| Pseudanabaena sp. BC1403 | NZ_PDDM01000058.1 | 91.346 | |
| Pseudanabaena SR411 | NZ_NDHW01000108.1 | 91.346 |
Cyanophage isolation.
Cyanophage PA-SR01 was isolated from viral concentrates collected from surface water as described above. Briefly, 450 ml of water was filtered through 0.2 μm (Nuclepore) pore size filters. The virus-sized particles in the filtrate were concentrated 100- to 200-fold with a 100-kDa molecular weight (MW) cutoff in ultrafiltration centrifugal tubes (Amicon Ultra-15 centrifugal filter units; Millipore). Viral concentrate was stored at 4°C in dark before any further action. Viral concentrate was serially diluted up to 107 times. PA-SR01 was isolated by adding the aliquots to an exponentially growing culture of Pseudanabaena strain KCZY-C8 in a 24-well microtiter plate and incubating at 25°C under low radiance (20 μmol photons m−2s−1) with 12-h/12-h light/dark cycle for 14 days. Culture lysis was determined by a substantial decrease in optical density at 750 nm (OD750) compared with control cultures (41). A clonal viral isolate was obtained by three rounds of extinction dilution (42) in 96-well microtiter plates with exponentially growing Pseudanabaena strain KCZY-C8.
Amplification and purification of PA-SR01.
The cyanophage was amplified by adding 1% (vol/vol) of the virus isolate to 30-ml cultures of Pseudanabaena strain KCZY-C8. Both phage-added and control culture were incubated until lysis took place in the culture flasks with phage. The lysates were centrifuged at 15,000 × g for 5 min to remove cellular debris. The supernatant containing the majority of viral particles was filtered through a 0.22-μm syringe filter (Minisart syringe filter, Satorius) to remove cellular debris. These purified viral particles were used for subsequent infection experiments.
Transmission electron microscopy.
Thirty milliliters of PA-SR01 lysate was centrifuged at 15,000 × g for 5 min followed by filtering through a 0.22-μm syringe filter to remove the cellular debris. The filtered lysate was centrifuged at 5,000 × g with a 100-kDa MW cutoff in ultrafiltration centrifugal tubes (Amicon Ultra-15 centrifugal filter units; Millipore) to increase the phage particle concentration. For staining, 20 μl of gadolinium triacetate (1% wt/wt) was adsorbed to the surface of copper grids at room temperature for 1 min. Excess liquid was blotted from the side of the copper grids with clean filter paper. The grids were viewed and photographed on a JEOL JEM-2100F field emission gun transmission electron microscope at the National University of Singapore Faculty of Chemical and Biomolecular Engineering.
Host range.
PA-SR01 infectivity was tested against local freshwater isolates of cyanobacteria strains, as well as cyanobacteria obtained from the Commonwealth Scientific and Industrial Research Organisation (CSIRO) culture collection. PA-SR01 phage lysate (1 ml) was added to cultures of exponentially growing cyanobacteria as listed in Table 1. Growth of cyanobacteria cultures without PA-SR01 addition was also monitored to serve as a control. Infectivity was determined by a reduction in OD reading compared to control.
Chloroform sensitivity.
Chloroform sensitivity of the cyanophage was tested. Filtered lysate (1 ml) was mixed with 1 ml of chloroform followed by shaking manually for 10 min. Chloroform removal was carried out by centrifugation at 4,100 × g for 5 min at room temperature. The aqueous phase was transferred to a 1.5 ml microcentrifuge tube and incubated for 6 h at room temperature to remove any remaining chloroform. One milliliter of chloroform was added to treat 1 ml of MLA medium to serve as the control. Chloroform-treated MLA, nontreated MLA, and treated and nontreated virus particles were added to exponentially growing Pseudanabaena strain KCZY-C8 cultures and the OD was measured over 6 days.
DNA extraction, purification, and sequencing.
Pseudanabaena strain KCZY-C8 was grown in 300 ml of MLA medium at 25°C under low irradiance (20 μmol photons m−2s−1) with a 12-h/12-h light/dark cycle until lysis. The lysates were centrifuged at 15,000 × g for 5 min to remove cellular debris. The supernatant containing the majority of viral particles was filtered through a 0.22-μm syringe filter (Minisart syringe filter, Satorius) to remove cellular debris. In order to remove free nucleic acid, the lysate was treated with DNase I. The treated lysate was concentrated with a 100-kDa MW cutoff ultrafiltration centrifugal tube (Amicon Ultra-15 centrifugal filter units; Millipore) at 5,000 × g to a final volume of 1 ml. QIAamp DNA minikit was used to extract viral DNA with 20 μl of RNase A added in the first step to remove RNA. The cyanophage genome was sequenced using an Illumina High throughput sequencer, with a 150-bp paired-end library constructed using a New England BioLab Next Ultra DNA library prep kit.
Genome assembly.
The sequencing data were trimmed using BBDuk (version 35.43) to remove adaptors and Phix reads. Reads were de novo assembled into contigs by MetaSPAdes genome assembler (3.12.0) (43).
Genome annotation.
The open reading frames (ORFs) were predicted using GeneMarkS (44) and Prodigal (45); where the prediction differed, the longer of the two was kept. Homology searching was performed with BLASTp against NCBI nonredundant (nr) database (accessed in October 2019). Sequences with E values of <10−5 were considered to be homologs. HHpred against protein data bank (PDB) and Pfam database were used to predict more distant homologs (46). The genome was analyzed for tRNA genes with tRNAscan-SE 2.0 (47) and for Rho-independent terminators using Findterm (48), with the energy threshold set to −16 kCal. A genomic map was generated with CGview (49).
SDS-PAGE analysis for structural protein.
Purified PA-SR01 was diluted in SDS buffer (5:1, vol/vol) and heated at 95°C for 5 min. The sample was then resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) using a Mini-PROTEAN Tetra Cell (Bio-Rad Laboratories). The Mini-PROTEAN TGX stain-free precast gel was run in an SDS running buffer (pH 8.3) at 120 V for 1.5 h using a PageRuler unstained protein ladder (Thermo Fisher) for size calibration.
Phylogenetic analysis.
The large terminase subunit (terL) and major capsid protein were compared phylogenetically with those from other cyanophages and bacteriophages (Table 7) using Mega-X software (version 10.1.6). ClustalX was used to align the inferred amino acid sequences with default parameters. Based on the multiple sequence alignment, the Jones-Taylor-Thornton (JTT) model was selected and a maximum likelihood tree was constructed with 100 bootstrap replicates.
TABLE 7.
Accession numbers of genes used in phylogenetic analysis
| Accession no. | Gene | Organism |
|---|---|---|
| ADZ31560.1 | Terminase large subunit | Planktothrix phage PaV-LD |
| AHV82220.1 | Terminase large subunit | Synechococcus phage S-EIVl |
| YP_004508468.1 | Terminase large subunit | Synechococcus phage S-CRM01 |
| YP_717790.1 | Terminase large subunit | Synechococcus phage syn9 |
| YP_214662.1 | Terminase large subunit | Prochlorococcus phage P-SSM4 |
| YP_214360.1 | Terminase large subunit | Prochlorococcus phage P-SSM2 |
| CAF34164.1 | Terminase large subunit | Synechococcus phage S-PM2 |
| NP_899601.1 | Terminase large subunit | Vibrio phage KVP40 |
| AAQ17878.1 | Terminase large subunit | Aeromonas virus Aeh1 |
| NP_891724.1 | Terminase large subunit | Escherichia phage RB49 |
| AND75577.1 | Terminase large subunit | Nostoc phage A1 |
| Q9T1W6.1 | Terminase large subunit | Escherichia virus Mu |
| YP_063734.1 | Terminase large subunit | Salmonella virus P22 |
| NP_853601 | Terminase large subunit | Salmonella virus SP6 |
| P03694 | Terminase large subunit | Enterobacteria phage T7 |
| P10310 | Terminase large subunit | Enterobacteria phage T3 |
| YP_224236 | Terminase large subunit | Listonella phage phiHSIC |
| YP_224140 | Terminase large subunit | Enterobacteria phage ES18 |
| NP_050979 | Terminase large subunit | Hamiltonella virus APSE1 |
| P44184 | Terminase large subunit | Haemophilus influenzae phage KW20 |
| NP_112076 | Terminase large subunit | Salmonella virus HK620 |
| AAA96534 | Terminase large subunit | Escherichia virus lambda |
| NP_046897 | Terminase large subunit | Escherichia virus N15 |
| NP_758915 | Terminase large subunit | Vibrio phage VHML |
| BAA89621 | Terminase large subunit | Wolbachia phage WO |
| AAQ96470 | Terminase large subunit | Vibrio phage VP16T |
| NP_700375 | Terminase large subunit | Salmonella phage ST64B |
| NP_892047 | Terminase large subunit | Yersinia phage PY54 |
| YP_004323723.1 | Terminase large subunit | Prochlorococcus phage Syn33 |
| BAF36209.1 | Terminase large subunit | Microcystis virus Ma-LMM01 |
| AND75488.1 | Terminase large subunit | Nostoc phage N1 |
| ADP06606.1 | Terminase large subunit | Synechococcus phage S-CBS1 |
| ADF42459.1 | Terminase large subunit | Synechococcus phage S-CBS3 |
| ADF42432.1 | Terminase large subunit | Synechococcus phage S-CBS2 |
| AEX55977.1 | Terminase large subunit | Synechococcus phage S-CBS4 |
| ACT65564.1 | Terminase large subunit | Cyanophage PSS2 |
| AGH57666.1 | Terminase large subunit | Cyanophage KBS-S-2A |
| ABP87967.1 | Terminase large subunit | Synechococcus virus Syn5 |
| ATS93174.1 | Terminase large subunit | Synechococcus phage S-LBS1 |
| YP_009173820.1 | Terminase large subunit | Synechococcus virus P60 |
| ABC46474.1 | Major capsid protein | Cyanophage AN-15 |
| ABI33181.1 | Major capsid protein | Phormidium virus WMP4 |
| YP_001285797.1 | Major capsid protein | Phormidium virus WMP3 |
| YP_009042803.1 | Major capsid protein | Anabaena phage A-4L |
| AND75579.1 | Major capsid protein | Nostoc phage A1 |
| ADZ31580.1 | Major capsid protein | Planktothrix phage PaV-LD |
| YP_008766991.1 | Major capsid protein | Cyanophage PP |
| YP_009217771.1 | Major capsid protein | Microcystis phage MaMV-DC |
| AND75491.1 | Major capsid protein | Nostoc phage N1 |
| AOO10060.1 | Major capsid protein | Synechococcus phage S-RIM2 |
| ATS93177.1 | Major capsid protein | Synechococcus phage S-LBS1 |
| NP_680487.1 | Major capsid protein | Lactobacillus phage A2 |
| ATW59308.1 | Major capsid protein | Aphanizomenon phage vB_AphaS-CL131 |
| YP_214206.1 | Major capsid protein | Prochlorococcus virus PSSP7 |
| ABP87946.1 | Major capsid protein | Synechococcus virus Syn5 |
| YP_009173806.1 | Major capsid protein | Synechococcus virus P60 |
| NP_041998.1 | Major capsid protein | Escherichia phage T7 |
| ABA46378 | Major capsid protein | Bacillus phage Cherry |
| YP_338188.1 | Major capsid protein | Bacillus phage Gamma |
| YP_003347636.1 | Major capsid protein | Klebsiella phage KP34 |
| YP_717802.1 | Major capsid protein | Synechococcus phage syn9 |
| YP_214367.1 | Major capsid protein | Prochlorococcus phage P-SSM2 |
| NP_891732.1 | Major capsid protein | Escherichia phage RB49 |
| NP_861877.1 | Major capsid protein | Escherichia phage RB69 |
| NP_049787.1 | Major capsid protein | Escherichia virus T4 |
| YP_003097339.1 | Major capsid protein | Synechococcus phage S-RSM4 |
Recruitment of reads to metagenomics.
The presence of viral sequences similar to PA-SR01 in aquatic environments was investigated by recruiting viral metagenomics data onto the genome of PA-SR01 (50). In total, 88 gigabytes of freshwater metagenome data and 173 gigabytes of marine metagenome data were used (Table 8). Briefly, metagenomic data were first made into a BLAST nucleotide database and queried with the predicted protein sequence of PA-SR01 using tBLASTn (E value of ≤10−5), which performed a six-frame translation of the subject nucleotide sequence into protein sequence (51). Metagenomics nucleotide reads with a blast hit to PA-SR01 were then extracted from each metagenome and used as query to blast (BLASTx, E value of ≤10−5, max_target_seqs = 1) against a viral protein database containing predicted proteins of PA-SR01 phage and another 2,536 bacteriophage genomes from the NCBI Reference Sequence Database (RefSeq; accessed on Jan 2020). If the best hit was related to PA-SR01 instead of the other phages, it was recruited as viral sequences similar to PA-SR01 and mapped onto the genome of PA-SR01, based on percentage identity of amino acid sequence using ggplot2 (52).
TABLE 8.
SRA accession numbers of metagenomics data used for metagenome mapping
| Source | Database size (Gb) | Accession no. | Marine or freshwater |
|---|---|---|---|
| Pacific Ocean surface | 76.10 | ERR3256930 | Marine |
| 76.10 | ERR3256932 | Marine | |
| 76.10 | ERR3256934 | Marine | |
| 76.10 | ERR3256936 | Marine | |
| 76.10 | ERR3256953 | Marine | |
| 76.10 | ERR3256955 | Marine | |
| 76.10 | ERR3256957 | Marine | |
| 76.10 | ERR3256959 | Marine | |
| 76.10 | ERR3256964 | Marine | |
| 76.10 | ERR3256973 | Marine | |
| 76.10 | ERR3256977 | Marine | |
| 76.10 | ERR3256975 | Marine | |
| Baltic Sea | 51.10 | SRR7254009 | Marine |
| 51.10 | SRR7253988 | Marine | |
| 51.10 | SRR7253990 | Marine | |
| 51.10 | SRR7253989 | Marine | |
| 51.10 | SRR7254008 | Marine | |
| 51.10 | SRR7254007 | Marine | |
| 51.10 | SRR7254010 | Marine | |
| Singapore urban water | 40.00 | SRR5995660–SRR5995697 | Freshwater |
| Lake Michigan | 23.40 | SRR1915829 | Freshwater |
| 23.40 | SRR1915851 | Freshwater | |
| 23.40 | SRR1974489 | Freshwater | |
| 23.40 | SRR1974496–SRR1974508 | Freshwater | |
| 23.40 | SRR1974510–SRR1974511 | Freshwater | |
| 23.40 | SRR1974513 | Freshwater | |
| 23.40 | SRR1974515 | Freshwater | |
| 23.40 | SRR1296481 | Freshwater | |
| 23.40 | SRR1302020 | Freshwater | |
| 23.40 | SRR1301999 | Freshwater | |
| 23.40 | SRR1974488 | Freshwater | |
| 23.40 | SRR1974490 | Freshwater | |
| 23.40 | SRR1974491 | Freshwater | |
| 23.40 | SRR1974493–SRR1974495 | Freshwater | |
| 23.40 | SRR1974509 | Freshwater | |
| 23.40 | SRR1974512 | Freshwater | |
| 23.40 | SRR1974514 | Freshwater | |
| 23.40 | SRR1974516–SRR1974517 | Freshwater | |
| 23.40 | SRR1302010 | Freshwater | |
| San Pedro channel | 20.60 | SRR10600460 | Marine |
| 20.60 | SRR10600461 | Marine | |
| Patagonia | 15.80 | SRR5145173–SRR5145178 | Marine |
| Lake Soyang | 13.90 | ERS2758845 | Freshwater |
| 13.90 | ERS2759121 | Freshwater | |
| 13.90 | ERS2759122 | Freshwater | |
| Gulf of Mexico | 9.40 | SRR11048275 | Marine |
| Han River | 7.50 | ERS1546404 | Freshwater |
| 7.50 | ERS1546406 | Freshwater | |
| Papua New Guinea | 5.30 | SRR5644412–SRR5644431 | Marine |
| Lake Neagh | 1.30 | SRR2147000 | Freshwater |
| Lake Baikal | 0.87 | SRR5936590 | Freshwater |
| Lake Limnopolar | 0.39 | SRR1658897–SRR1658894 | Freshwater |
| Reclaimed water virus | 0.38 | SRR014585–SRR014589 | Freshwater |
| Lake Bourget | 0.33 | ERR019478 | Freshwater |
| Lake Pavin | 0.37 | ERR019477 | Freshwater |
The BLAST hits number to PA-SR01 was normalized by dividing by the total number of predicted ORFs and the size of the metagenome (in gigabytes), which provides a normalized measure to compare recruitments across metagenomes of different size. Similar recruitment analysis was also performed for other phage genomes (Table 9).
TABLE 9.
List of cyanophages selected for metagenomics recruitment analysis
| Cyanophage | Accession no. |
|---|---|
| Synechococcus phage ACG-2014b | NC_027130 |
| Synechococcus phage S-CAM1 | NC_020837 |
| Synechococcus phage S-CBP1 | NC_025456 |
| Synechococcus phage S-CBS1 | NC_016164 |
| Synechococcus phage S-CRM01 | NC_015569 |
| Synechococcus phage S-PM2 | NC_006820 |
| Synechococcus phage S-RIP1 | NC_020867 |
| Synechococcus phage S-SKS1 | NC_020851 |
| Synechococcus phage S-SM1 | NC_015282 |
| Synechococcus phage S-WAM1 | NC_031944 |
| Synechococcus phage syn9 | NC_008296 |
| Synechococcus virus P60 | NC_003390 |
| Prochlorococcus phage MED4-184 | NC_020847 |
| Prochlorococcus phage P-GSP1 | NC_020878 |
| Prochlorococcus phage P-HM1 | NC_015280 |
| Prochlorococcus phage P-RSM4 | NC_015283 |
| Prochlorococcus phage P-SSM2 | NC_006883 |
| Prochlorococcus phage P-SSP10 | NC_020835 |
| Prochlorococcus phage P-SSP3 | NC_020874 |
| Prochlorococcus phage P-TIM68 | NC_028955 |
| Prochlorococcus phage Syn1 | NC_015288 |
| Prochlorococcus virus PSSP7 | NC_006882 |
| Microcystis phage MaMV-DC | NC_029002 |
| Microcystis virus Ma-LMM01 | NC_008562 |
| Planktothrix phage PaV-LD | NC_016564 |
| Synechococcus phage S-EIV1 | KJ410740.1 |
Data availability.
The whole-genome sequence of the phage is available in GenBank under accession number MT234670.
ACKNOWLEDGMENTS
This research was supported by the Singapore National Research Foundation, Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) program.
We are grateful to PUB, Singapore's national water agency, for providing logistical field support in this study. We thank the YUNG Lab from the National University of Singapore Department of Chemical and Biomolecular Engineering for their support on the TEM imaging. We also thank our colleagues Goh Kwan Chien and Sim Zhiyang for providing the host cyanobacteria Pseudanabaena strain KCZY-C8.
We declare no conflicts of interest.
REFERENCES
- 1.Saad A, Atia A. 2014. Review on freshwater blue-green algae (cyanobacteria): occurrence, classification and toxicology. Biosci, Biotechnol Res Asia 11:1319–1325. doi: 10.13005/bbra/1522. [DOI] [Google Scholar]
- 2.Sullivan MB, Waterbury JB, Chisholm SW. 2003. Cyanophages infecting the oceanic cyanobacterium Prochlorococcus. Nature 424:1047–1051. doi: 10.1038/nature01929. [DOI] [PubMed] [Google Scholar]
- 3.Fuhrman JA. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541–548. doi: 10.1038/21119. [DOI] [PubMed] [Google Scholar]
- 4.Suttle CA. 2000. The ecology of cyanobacteria: their diversity in time and space. Kluwer Academic Publishers, Dordrecht, The Netherlands. [Google Scholar]
- 5.Suttle CA. 2005. Viruses in the sea. Nature 437:356–361. doi: 10.1038/nature04160. [DOI] [PubMed] [Google Scholar]
- 6.Middelboe M, Jacquet S, Weinbauer M. 2008. Viruses in freshwater ecosystems: an introduction to the exploration of viruses in new aquatic habitats. Freshwater Biology 53:1069–1075. doi: 10.1111/j.1365-2427.2008.02014.x. [DOI] [Google Scholar]
- 7.Xia H, Li T, Deng F, Hu Z. 2013. Freshwater cyanophages. Virol Sin 28:253–259. doi: 10.1007/s12250-013-3370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreher TW, Brown N, Bozarth CS, Schwartz AD, Riscoe E, Thrash C, Bennett SE, Tzeng SC, Maier CS. 2011. A freshwater cyanophage whose genome indicates close relationships to photosynthetic marine cyanomyophages. Environ Microbiol 13:1858–1874. doi: 10.1111/j.1462-2920.2011.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chenard C, Chan AM, Vincent WF, Suttle CA. 2015. Polar freshwater cyanophage S-EIV1 represents a new widespread evolutionary lineage of phages. ISME J 9:2046–2058. doi: 10.1038/ismej.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao EB, Gui JF, Zhang QY. 2012. A novel cyanophage with a cyanobacterial nonbleaching protein A gene in the genome. J Virol 86:236–245. doi: 10.1128/JVI.06282-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida T, Nagasaki K, Takashima Y, Shirai Y, Tomaru Y, Takao Y, Sakamoto S, Hiroishi S, Ogata H. 2008. Ma-LMM01 infecting toxic Microcystis aeruginosa illuminates diverse cyanophage genome strategies. J Bacteriol 190:1762–1772. doi: 10.1128/JB.01534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong KX, Suttle CA, Baudoux AC, Derelle E, Colombet J, Cho A, Caleta J, Six C, Jacquet S. 2018. A new freshwater cyanosiphovirus harboring integrase. Front Microbiol 9:2204. doi: 10.3389/fmicb.2018.02204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu X, Tay QXM, Te SH, Saeidi N, Goh SG, Kushmaro A, Thompson JR, Gin KY. 2018. Geospatial distribution of viromes in tropical freshwater ecosystems. Water Res 137:220–232. doi: 10.1016/j.watres.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coutinho FH, Silveira CB, Gregoracci GB, Thompson CC, Edwards RA, Brussaard CPD, Dutilh BE, Thompson FL. 2017. Marine viruses discovered via metagenomics shed light on viral strategies throughout the oceans. Nat Commun 8:15955. doi: 10.1038/ncomms15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safferman RS, Cannon RE, Desjardins PR, Gromov BV, Haselkorn R, Sherman LA, Shilo M. 1983. Classification and nomenclature of viruses of cyanobacteria. Intervirology 19:61–66. doi: 10.1159/000149339. [DOI] [PubMed] [Google Scholar]
- 16.Gao EB, Yuan XP, Li R, Zhang QY. 2009. Isolation of a novel cyanophage infectious to the filamentous cyanobacterium Planktothrix agardhii (Cyanophyceae) from Lake Donghu, China. Aquat Microb Ecol 54:163–170. doi: 10.3354/ame01266. [DOI] [Google Scholar]
- 17.Stal LJ, Albertano P, Bergman B, Bröckel KV, Gallon JR, Hayes PK, Sivonen K, Walsby AE. 2003. BASIC: Baltic Sea cyanobacteria. An investigation of the structure and dynamics of water blooms of cyanobacteria in the Baltic Sea—responses to a changing environment. Continental Shelf Res 23:1695–1714. doi: 10.1016/j.csr.2003.06.001. [DOI] [Google Scholar]
- 18.Kim SG, Rhee SK, Ahn CY, Ko SR, Choi GG, Bae JW, Park YH, Oh HM. 2006. Determination of cyanobacterial diversity during algal blooms in Daechung Reservoir, Korea, on the basis of cpcBA intergenic spacer region analysis. Appl Environ Microbiol 72:3252–3258. doi: 10.1128/AEM.72.5.3252-3258.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willame R, Boutte C, Grubisic S, Wilmotte A, Komárek J, Hoffmann L. 2006. Morphological and molecular characterization of planktonic cyanobacteria from Belgium and Luxembourg. J Phycology 42:1312–1332. doi: 10.1111/j.1529-8817.2006.00284.x. [DOI] [Google Scholar]
- 20.Atanasova NS, Sencilo A, Pietilä MK, Roine E, Oksanen HM, Bamford DH. 2015. Comparison of lipid-containing bacterial and archaeal viruses. Adv Virus Res 92:1–61. doi: 10.1016/bs.aivir.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Espejo RT, Canelo ES. 1968. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology 34:738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- 22.Mahony J, Alqarni M, Stockdale S, Spinelli S, Feyereisen M, Cambillau C, Sinderen DV. 2016. Functional and structural dissection of the tape measure protein of lactococcal phage TP901–1. Sci Rep 6:36667. doi: 10.1038/srep36667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coloma SE, Dienstbier A, Bamford DH, Sivonen K, Roine E, Hiltunen T. 2017. Newly isolated Nodularia phage influences cyanobacterial community dynamics. Environ Microbiol 19:273–286. doi: 10.1111/1462-2920.13601. [DOI] [PubMed] [Google Scholar]
- 24.Gao EB, Huang Y, Ning D. 2016. Metabolic genes within cyanophage genomes: implications for diversity and evolution. Genes (Basel) 7:80. doi: 10.3390/genes7100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan MB, Lindell D, Lee JA, Thompson LR, Bielawski JP, Chisholm SW. 2006. Prevalence and evolution of core photosystem II genes in marine cyanobacterial viruses and their hosts. PLoS Biol 4:e234. doi: 10.1371/journal.pbio.0040234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gleason FK, Olszewski NE. 2002. Isolation of the gene for the B12-dependent ribonucleotide reductase from Anabaena sp. strain PCC 7120 and expression in Escherichia coli. J Bacteriol 184:6544–6550. doi: 10.1128/jb.184.23.6544-6550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson LR, Zeng Q, Kelly L, Huang KH, Singer AU, Stubbe J, Chisholm SW. 2011. Phage auxiliary metabolic genes and the redirection of cyanobacterial host carbon metabolism. Proc Natl Acad Sci U S A 108:E757–64. doi: 10.1073/pnas.1102164108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martiny AC, Huang Y, Li W. 2009. Occurrence of phosphate acquisition genes in Prochlorococcus cells from different ocean regions. Environ Microbiol 11:1340–1347. doi: 10.1111/j.1462-2920.2009.01860.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen F, Lu J. 2002. Genomic sequence and evolution of marine cyanophage P60: a new insight on lytic and lysogenic phages. Appl Environ Microbiol 68:2589–2594. doi: 10.1128/aem.68.5.2589-2594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Myllykallio H, Lipowski G, Leduc D, Filee J, Forterre P, Liebl U. 2002. An alternative flavin-dependent mechanism for thymidylate synthesis. Science 297:105–107. doi: 10.1126/science.1072113. [DOI] [PubMed] [Google Scholar]
- 31.Graziani S, Xia Y, Gurnon JR, Van Etten JL, Leduc D, Skouloubris S, Myllykallio H, Liebl U. 2004. Functional analysis of FAD-dependent thymidylate synthase ThyX from Paramecium bursaria chlorella virus-1. J Biol Chem 279:54340–54347. doi: 10.1074/jbc.M409121200. [DOI] [PubMed] [Google Scholar]
- 32.Duckworth DH, Bessman MJ. 1967. The enzymology of virus-infected bacteria. X. A biochemical-genetic study of the deoxynucleotide kinase induced by wild type and amber mutants of phage T4. J Biol Chem 242:2877–2885. [PubMed] [Google Scholar]
- 33.Sakaguchi Y, Hayashi T, Kurokawa K, Nakayama K, Oshima K, Fujinaga Y, Ohnishi M, Ohtsubo E, Hattori M, Oguma K. 2005. The genome sequence of Clostridium botulinum type C neurotoxin-converting phage and the molecular mechanisms of unstable lysogeny. Proc Natl Acad Sci U S A 102:17472–17477. doi: 10.1073/pnas.0505503102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scheurwater E, Reid CW, Clarke AJ. 2008. Lytic transglycosylases: bacterial space-making autolysins. Int J Biochem Cell Biol 40:586–591. doi: 10.1016/j.biocel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Jorgenson MA, Chen Y, Yahashiri A, Popham DL, Weiss DS. 2014. The bacterial septal ring protein RlpA is a lytic transglycosylase that contributes to rod shape and daughter cell separation in Pseudomonas aeruginosa. Mol Microbiol 93:113–128. doi: 10.1111/mmi.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolch CJS, Blackburn SI. 1996. Isolation and purification of Australian isolates of the toxic cyanobacterium Microcystis aeruginosa Kütz. J Appl Phycol 8:5–13. doi: 10.1007/BF02186215. [DOI] [Google Scholar]
- 37.Whitman WB. 2015. Bergeys manual of systematics of archaea and bacteria. John Wiley & Sons, Inc, Hoboken, New Jersey. [Google Scholar]
- 38.Tuji A, Niiyama Y. 2018. Two new Pseudanabaena (Cyanobacteria, Synechococcales) species from Japan, Pseudanabaena cinerea and Pseudanabaena yagii, which produce 2-methylisoborneol. Phycological Res 66:291–299. doi: 10.1111/pre.12327. [DOI] [Google Scholar]
- 39.Komárek J, Kaštovský J, Mareš J, Johansen JR. 2014. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera), using a polyphasic approach. Prelisa 86:295–335. [Google Scholar]
- 40.Gao J, Zhu J, Wang M, Dong W. 2018. Dominance and growth factors of Pseudanabaena sp. in drinking water source reservoirs, southern China. Sustainability 10:3936. doi: 10.3390/su10113936. [DOI] [Google Scholar]
- 41.Yeo BH, Gin K-H. 2013. Cyanophages infecting Anabaena circinalis and Anabaena cylindrica in a tropical reservoir. Bacteriophage 3:e25571. doi: 10.4161/bact.25571. [DOI] [Google Scholar]
- 42.Nagasaki K, Yamaguchi M. 1997. Isolation of a virus infectious to the harmful bloom causing microalga Heterosigma akashiwo (Raphidophyceae). Aquat Microb Ecol 13:135–140. doi: 10.3354/ame013135. [DOI] [Google Scholar]
- 43.Nurk S, Meleshko D, Korobeynikov A, Pevzner PA. 2017. metaSPAdes: a new versatile metagenomic assembler. Genome Res 27:824–834. doi: 10.1101/gr.213959.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Besemer J, Lomsadze A, Borodovsky M. 2001. GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res 29:2607–2618. doi: 10.1093/nar/29.12.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soding J, Biegert A, Lupas AN. 2005. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res 33:W244–8. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lowe TM, Chan PP. 2016. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res 44:W54–7. doi: 10.1093/nar/gkw413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solovyev V, Salamov A. 2011. In Li RW. (ed), Metagenomics and its applications in agriculture, biomedicine and environmental studies Nova Science Publishers, Hauppauge, NY. [Google Scholar]
- 49.Grant JR, Stothard P. 2008. The CGView Server: a comparative genomics tool for circular genomes. Nucleic Acids Res 36:W181–4. doi: 10.1093/nar/gkn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y, Temperton B, Thrash JC, Schwalbach MS, Vergin KL, Landry ZC, Ellisman M, Deerinck T, Sullivan MB, Giovannoni SJ. 2013. Abundant SAR11 viruses in the ocean. Nature 494:357–360. doi: 10.1038/nature11921. [DOI] [PubMed] [Google Scholar]
- 51.Wheeler D, Bhagwat M. 2007. In Bergman NH. (ed), Comparative genomics, vol 1 and 2. Springer Publishing Company Inc, Berlin, Germany. [Google Scholar]
- 52.Wickham H. 2009. ggplot2: elegant graphics for data analysis. Springer Publishing Company, Inc, Berlin, Germany. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The whole-genome sequence of the phage is available in GenBank under accession number MT234670.