Most Zika virus (ZIKV) vaccine research has focused on the E or prM-E proteins and the induction of high levels of neutralizing antibodies. However, these ZIKV neutralizing antibodies cross-react with other flaviviruses, which may aggravate the disease via an antibody-dependent enhancement (ADE) mechanism. ZIKV NS1 protein may be an alternative antigen for vaccine development, since antibodies to NS1 do not bind to the virion, thereby eliminating the risk of ADE. Here, we show that recombinant VSV and DNA vaccines expressing NS1, alone, confer partial protection against ZIKV infection in both immunocompetent and immunodeficient mice, highlighting the value of NS1 as a potential vaccine candidate.
KEYWORDS: NS1, Zika virus, vaccine
ABSTRACT
The nonstructural protein 1 (NS1) of several flaviviruses, including West Nile, dengue, and yellow fever viruses, is capable of inducing variable degrees of protection against flavivirus infection in animal models. However, the immunogenicity of NS1 protein of Zika virus (ZIKV) is less understood. Here, we determined the efficacy of ZIKV NS1-based vaccine candidates using two delivery platforms, methyltransferase-defective recombinant vesicular stomatitis virus (mtdVSV) and a DNA vaccine. We first show that expression of ZIKV NS1 could be significantly enhanced by optimizing the signal peptide. A single dose of mtdVSV-NS1-based vaccine or two doses of DNA vaccine induced high levels of NS1-specfic antibody and T cell immune responses but provided only partial protection against ZIKV viremia in BALB/c mice. In Ifnar1−/− mice, neither NS1-based vaccine provided protection against a lethal high dose (105 PFU) ZIKV challenge, but mtdVSV-NS1-based vaccine prevented deaths from a low dose (103 PFU) challenge, though they experienced viremia and body weight loss. We conclude that ZIKV NS1 alone conferred substantial, but not complete, protection against ZIKV infection. Nevertheless, these results highlight the value of ZIKV NS1 for vaccine development.
IMPORTANCE Most Zika virus (ZIKV) vaccine research has focused on the E or prM-E proteins and the induction of high levels of neutralizing antibodies. However, these ZIKV neutralizing antibodies cross-react with other flaviviruses, which may aggravate the disease via an antibody-dependent enhancement (ADE) mechanism. ZIKV NS1 protein may be an alternative antigen for vaccine development, since antibodies to NS1 do not bind to the virion, thereby eliminating the risk of ADE. Here, we show that recombinant VSV and DNA vaccines expressing NS1, alone, confer partial protection against ZIKV infection in both immunocompetent and immunodeficient mice, highlighting the value of NS1 as a potential vaccine candidate.
INTRODUCTION
The recent outbreaks of Zika virus (ZIKV) have been associated with severe diseases, including microcephaly, Guillain-Barré syndrome and other neurological disorders, highlighting the urgent need to develop a safe and efficacious vaccine (1, 2). ZIKV belongs to the family Flaviviridae which includes other important human pathogens, dengue virus (DENV), West Nile virus (WNV), yellow fever virus (YFV), and Japanese encephalitis virus (JEV). Similar to other flaviviruses, the ZIKV genome encodes a polyprotein precursor, which is posttranslationally cleaved into three structural proteins (capsid, C; premembrane, prM; and envelope, E) and seven nonstructural (NS) proteins (3, 4). Soon after the initial ZIKV outbreaks occurred in South America, several recombinant vaccine candidates were developed and tested in small animal models and/or nonhuman primates (5–7). These DNA, mRNA, subunit, and recombinant vectored vaccine candidates presented the E or prM-E proteins, the targets for neutralizing antibodies. The levels of immune protection provided by these vaccines have correlated with the neutralizing antibody titer. However, recent studies have shown that some ZIKV antibodies cross-react with the four serotypes of dengue virus, aggravating their infection via an antibody-dependent enhancement (ADE) mechanism (8–10). Therefore, there is a need to explore the possibility of using other ZIKV proteins, such as nonstructural protein 1 (NS1), as a vaccine candidate.
The flavivirus NS1 protein is a multifunctional protein that is essential in viral replication, immune evasion, and pathogenesis (11–14). Within infected cells, the NS1 protein is glycosylated and has three forms: an intracellular monomer, a membrane-bound homodimer, and a secreted homohexamer. The intracellular NS1 participates in viral replication, whereas the membrane-bound homodimer and the secreted extracellular NS1 play an important role in immune evasion and viral pathogenesis (13–16). In addition, NS1 protein interacts with the viral prM and E proteins and is critical for virus maturation (17). The overall crystal structure of ZIKV NS1 is similar to those of other flaviviruses, organizing into three distinct domains: an N-terminal β-roll, an epitope-rich wing domain, and a C-terminal β-ladder. A flexible intertwined loop is present in the wing domain, allowing NS1 to associate with membranes during replication and immature virions during particle morphogenesis and to facilitate the interactions that form the hexameric lipoprotein complex (15).
Vaccination with the NS1 protein of several flaviviruses, including WNV, YFV, JEV, and DEN, can provide protection against infection with the corresponding flavivirus in animal models even without inducing detectable neutralizing antibodies (18–24). However, passive administration of monoclonal antibodies (MAbs) against NS1 can protect mice against the corresponding flavivirus infection (22). Thus, NS1 could be an alternative antigen for vaccine development. The primary advantage of using NS1 alone as an immunogen is that NS1 antibodies do not mediate ADE of other flavivirus infections (22). Indeed, the potential of using NS1 protein as a ZIKV vaccine candidate has been reported. Brault et al. showed that intramuscular immunization of immunocompetent CD-1/ICR mice with the modified vaccinia Ankara (MVA) vector expressing NS1 (MVA-ZIKV-NS1) vaccine candidate provided 100% protection against a lethal intracerebral dose of ZIKV (strain MR766) (25). However, the MVA-ZIKV-NS1-immunized mice were challenged with ZIKV via the intracerebral route, which is not a natural transmission model for ZIKV infection (25). We and others have recently shown that coexpression of NS1 with prM-E protein synergistically induced ZIKV-specific antibody and T cell immune responses, inhibiting ZIKV infection and highlighting the value of incorporating NS1 with prM-E in a vaccine (26). In addition, passive immunization of Stat2−/− mice with ZIKV NS1 MAbs or NS1 antisera was capable of protecting against ZIKV challenge, although mice exhibited some weight loss and ZIKV clinical signs (27, 28). More recently, it was shown that ZIKV NS1 contains T cell epitopes which are critical for protection against ZIKV infection in a DNA vaccine experiment (29). However, three immunizations with 50 or 100 μg of DNA failed to induce protection against ZIKV infection in Ifnar1−/− mice, which lack the interferon alpha and beta receptor and, therefore, the type I interferon response (29).
We have now systematically determined the efficacy of NS1 as a vaccine immunogen for protection against ZIKV infection in both immunocompetent and immunodeficient mice using two vaccine delivery platforms, an attenuated vesicular stomatitis virus (VSV) vector and pCI plasmid DNA vaccine. We chose VSV as a vaccine vector because it is able to produce high protein levels (26, 30). Furthermore, the first VSV-based vaccine was recently approved by the U.S. Food and Drug Administration (FDA), one that protects against Ebola virus. We first optimized the expression of ZIKV NS1 protein by fusing different signal peptides to the N terminus of the NS1 protein. BALB/c mice immunized with a single dose of recombinant VSV expressing NS1 or two doses of DNA vaccine expressing NS1 triggered high levels of NS1-specific antibodies, a T cell immune response and partial protection against ZIKV viremia. Neither vaccine protected Ifnar1−/− mice against a 105 PFU ZIKV challenge, but they survived from a 103 PFU challenge, although not without weight loss and viremia. Thus, we concluded that the vaccination of the ZIKV NS1 protein provides substantial but not complete protection against ZIKV infection and that its protection capability is dependent on the challenge dose.
RESULTS
Recovery of recombinant methyltransferase-defective VSVs expressing ZIKV NS1.
The goal of our study is to determine the efficacy of immunization with ZIKV NS1 in protection from ZIKV infection. For this purpose, we used an attenuated VSV as a vector to deliver ZIKV NS1 protein, since the VSV system has been shown to express high levels of foreign proteins (31). Since VSV is virulent for rodents, we first developed an attenuate VSV vector. Previously, we found that a point mutation (D1762A) in the mRNA cap methyltransferase catalytic site of the VSV large (L) polymerase protein abolished both guanine N-7 and ribose 2′-O methylation (32). The resultant recombinant virus carrying D1762A (rVSV-D1762A) was avirulent in BALB/c mice (33). Thus, we introduced the D1762A mutation into a plasmid encoding the VSV antigenome pVSV-GxxL, resulting in the construction of pVSV-D1762A-GxxL.
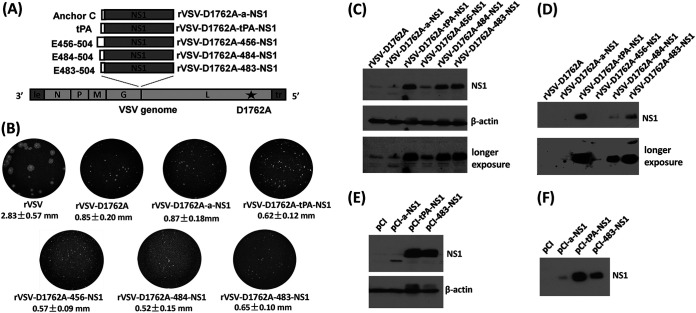
We inserted the ZIKV NS1 gene into the gene junction between the VSV G and L genes of the full-length genomic cDNA, pVSV-D1762A-GxxL, and recovered five recombinant VSVs (rVSV) expressing ZIKV-NS1 differing only in their signal peptide sequence (Fig. 1A). The first recombinant virus, rVSV-D1762A-a-NS1, includes the anchor C signal peptide fused to the N terminus of the NS1 protein. The anchor C sequence is essential for expression of the ZIKV prM-E or E protein (34). Recombinant rVSV-D1762A-tPA-NS1 contains the signal sequence of human tissue plasminogen activator (tPA) fused to the NS1 N terminus. The tPA signal peptide is commonly used to optimize the expression of type 1 glycoproteins and was used in a DNA vaccine expressing the NS1 of dengue type 2 virus (35). We also constructed three recombinant viruses containing the C-terminal transmembrane domain of the ZIKV E protein, which functions as a natural signal peptide for the NS1 protein and might enhance NS1 protein expression (36, 37). Previously, a similar strategy was successfully used for the expression of DENV and WNV NS1 protein (37). Based on the prediction by SignalP 4.1 Server (http://www.cbs.dtu.dk/services/SignalP), this signal peptide includes amino acid residues 456 to 504 at the C terminus of the ZIKV E protein (E456-504). Since the exact length of this signal sequence is uncertain, we constructed three recombinant viruses with different lengths of this signal sequence in the NS1 protein. These recombinant viruses were named rVSV-D1762A-456-NS1, rVSV-D1762A-484-NS1, and rVSV-D1762A-483-NS1 and contained amino acid residues from 456 to 504, 484 to 504, and 483 to 504, respectively, at the C-terminal end of the ZIKV E protein as the signal peptide (Fig. 1A).
FIG 1.
Recovery of mtdVSVs expressing ZIKV NS1. (A) Strategy to construct recombinant mtdVSV expressing ZIKV NS1. (Top panel) Different signal sequences were used for expression of NS1 protein. C-terminal anchor of ZIKV-C (anchor C), human tissue plasminogen activator (tPA), ZIKV-E 456-504 (E456), ZIKV-E 483-504 (E483), and ZIKV-E 484-504 (E484) were fused to the full-length NS1 gene (352 amino acids) of ZIKV Cambodian strain by PCR. The resultant DNA fragments (a-NS1, tPA-NS1, 456-NS1, 483-NS1, and 484-NS1) were digested by XhoI and SmaI and inserted into the same sites at the gene junction between G and L in the VSV genome. (Bottom panel) Organization of nonsegmented negative-sense VSV genome. Le, VSV leader sequence; N, nucleocapsid gene; P, phosphoprotein gene; M, matrix protein gene; G, glycoprotein gene; L, large polymerase gene; Tr, VSV trailer sequence. A single point mutation (D1762A) in the SAM binding site in the L protein was introduced into the VSV backbone. (B) Plaque morphology of mtdVSVs expressing ZIKV NS1. Recombinant VSVs were recovered from infectious cDNA clones. The plaque morphology of each recombinant virus is shown. All plaques were developed after 36 h of incubation. The diameters of a total of 10 plaques were measured for each recombinant virus. (C to F) Expression of ZIKV NS1 protein in cell lysate and cell culture medium. BSRT7 cells were infected with each mtdVSV expressing ZIKV NS1 at an MOI of 3.0. (C) At 12 h postinfection, the cell lysate was harvested. (D) At 24 h postinfection, the cell culture supernatants were harvested. To express NS1 by plasmids, HEK293T cells were transfected with pCI, pCI-a-NS1, pCI-tPA-NS1, or pCI-483-NS1 using Lipofectamine 2000. At 72 h posttransfection, the cell lysate (E) and cell culture supernatants (F) were harvested. All of the samples (15 μl of cell lysate and 20 μl of culture medium each sample) were analyzed by Western blotting using ZIKV NS1-specific antibody.
After successful recovery, all recombinant viruses were plaque purified. All recombinant viruses contain the desired insertions as shown by sequencing of the VSV genome. No additional mutation was found in the genome except for the D1762A substitution in the L gene, as designed. The parental rVSV formed large plaques at 36 h postinoculation with average diameter of 2.83 ± 0.57 mm (mean ± the standard deviation) (Fig. 1B). As expected, all other recombinant viruses formed significantly smaller plaques due to the D1762 attenuating mutation they share (Fig. 1B).
The rVSV-D1762A-a-NS1 had a plaque size of 0.87 ± 0.18 mm, which was similar to the backbone virus rVSV-D1762A (0.85 ± 0.20 mm). Recombinant rVSV-D1762A-tPA-NS1, rVSV-D1762A-456-NS1, rVSV-D1762A-483-NS1, and rVSV-D1762A-484-NS1 formed even smaller plaques with average sizes of 0.62 ± 0.12 mm, 0.57 ± 0.09 mm, 0.65 ± 0.10 mm, and 0.52 ± 0.15 mm, respectively (Fig. 1B). The addition of the NS1 gene did not affect the plaque size, but the recombinant viruses with the natural leader sequences (C-terminal of ZIKV E) in NS1 appear to have a slightly decreased plaque size (Fig. 1B). These results suggest that methyltransferase-defective rVSVs (mtdVSVs) expressing NS1 had dramatic defects in replication and/or cell-to-cell spread.
High-level expression of ZIKV NS1 protein by the mtdVSV vector.
We assessed the expression of the ZIKV NS1 protein in BSRT7 cells infected by these mtdVSVs expressing NS1. The molecular weight of ZIKV NS1 protein ranges from 40 to 43 kDa due to the differences in its posttranslational modification (15). By Western blotting, the 42-kDa NS1 protein was detected in the lysates from all cell cultures infected with the mtdVSV vectors carrying the NS1 gene, except for rVSV-D1762A-a-NS1, and cells infected with rVSV-D1762A-456-NS1 only expressed a small amount of NS1 (Fig. 1C). We also analyzed the NS1 protein in cell culture supernatants. A strong NS1 protein band was detected in cell culture medium from rVSV-D1762A-tPA-NS1 and rVSV-D1762A-483-NS1-infected cells, even without the need for concentrating the NS1 protein. However, the amount of NS1 protein secreted from rVSV-D1762A-484-NS1-infected cells was much lower than rVSV-D1762A-483-NS1 (Fig. 1D) despite the fact that the signal peptide of NS1 in rVSV-D1762A-484-NS1 contains only one additional amino acid. Taken together, these results show that ZIKV NS1 protein is highly expressed and secreted by cells infected with rVSV-D1762A-tPA-NS1 and rVSV-D1762A-483-NS1. With the help of the added signal peptide, the NS1 protein was secreted from infected cells at levels that were easily detectable by Western blotting.
Expression of ZIKV proteins by the DNA vector.
We also constructed several plasmids to use as DNA vaccines to deliver the ZIKV NS1 antigen. Since we observed high expression of NS1 containing the tPA and E483 signal sequences in the VSV vector, the tPA-NS1 and E483-NS1 genes were cloned into the pCI mammalian expression vector to generate pCI-tPA-NS1 and pCI-483-NS1, respectively. We also constructed pCI-a-NS1 to use as a control. These recombinant plasmids were transfected into HEK293T cells to assess their expression of ZIKV NS1. Similar to the mtdVSVs expressing NS1, the NS1 protein was present in lysates from cells transfected with pCI-tPA-NS1 or pCI-483-NS1. NS1 protein was detectable from pCI-a-NS1 but at a much lower level than the other two plasmids (Fig. 1E). NS1 protein was also detectable in the cell culture medium from pCI-tPA-NS1 and pCI-483-NS1 (Fig. 1F).
A single dose of mtdVSV-NS1 vaccines or two doses of NS1-DNA vaccines induce a high level of NS1 antibody in BALB/c mice.
We sought to determine whether the mtdVSV-NS1 and NS1-DNA vaccines were immunogenic in BALB/c mice. Mice were inoculated intranasally with a single dose of mtdVSV-based vaccines (106 PFU/mouse). For the DNA vaccines, BALB/c mice were intramuscularly injected with 50 μg of plasmid and boosted with the same dose 2 weeks later. Recombinant VSV expressing prM-E (rVSV-G1670A-prM-E) was used as a positive control, as we previously showed that rVSV-G1670A-prM-E induced an immune response to the E protein that included a high level of antibody and provided complete protection against ZIKV-induced viremia in mice (26).
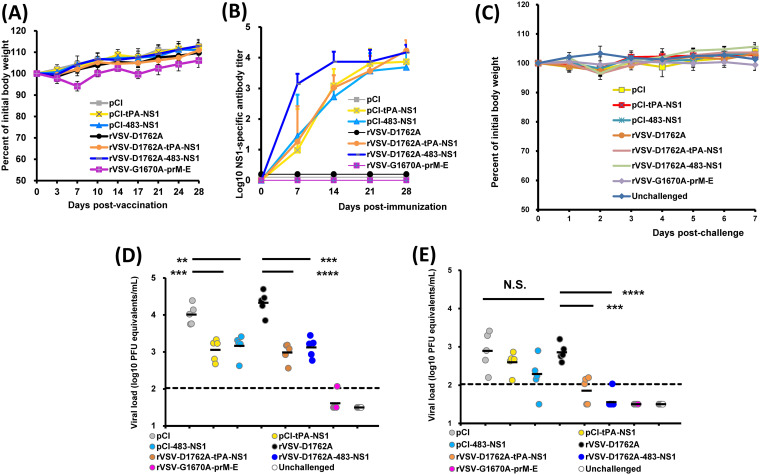
The vector, rVSV-G1670A-prM-E, carries the G1670A attenuating mutation that abolishes the G-N-7 but not the 2′-O methylation and retains low virulence in mice. Consistent with our previous observation, rVSV-G1670A-prM-E-immunized mice had no clinical signs but showed mild body weight loss at day 7 postinoculation but quickly recovered (Fig. 2A). In contrast, mice inoculated with rVSV-D1762A-tPA-NS1 and rVSV-D1762A-483-NS1 had no body weight loss or any VSV-associated clinical signs during the entire experimental period (Fig. 2A), suggesting that these two recombinant VSVs were completely attenuated in BALB/c mice. As expected, no body weight losses were observed in the DNA vaccine groups (Fig. 2A).
FIG 2.
A single dose of either mtdVSV-NS1 or two doses of DNA vaccination induced a high level of NS1-specific antibody response and provided partial protection against ZIKV infection in BALB/c mice. BALB/c mice were intranasally inoculated with DMEM or 106 PFU of each recombinant virus. For DNA vaccination, mice were vaccinated intramuscularly with 50 μg of plasmid DNA and were boosted with the same dose 2 weeks later. At week 4, mice were intraperitoneally administered 1.8 mg of anti-IFNAR1 blocking antibody and 24 h later challenged intravenously with 106 PFU of ZIKV Cambodian strain. (A) Dynamic of mouse body weight changes after vaccination. Data are averages for five mice ± the standard deviation. (B) Kinetics of ZIKV NS1-specific antibody induced by mtdVSV-NS1 vaccines and DNA vaccines. Recombinant rVSV-G1670A-prM-E was used as a control. Serum samples were collected weekly and analyzed by ELISA for NS1-specific serum IgG antibody. The data are expressed as the GMT of five mice ± the standard deviation. (C) Dynamic of body weight of changes after challenge with ZIKV. Mice were challenged with 106 PFU of ZIKV Cambodian strain. After challenge, the body weight for each mouse was measured each day until day 7 postchallenge. Data are averages for five mice ± the standard deviation. (D) Viremia in mice on day 3 after challenge with ZIKV. After challenge, blood samples were collected at day 3, and the amount of ZIKV RNA was quantitated by real-time RT-PCR and calculated to log10 PFU eq/ml. (E) Viremia in mice on day 7 after challenge with ZIKV. Viremia was expressed as log10 PFU eq/ml. Data in panels D and E are the GMT of five mice (black bars) and were analyzed by using Student t test and compared to the empty vector rVSV-D1762A group or the pCI group (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
The NS1-specific antibody response was monitored weekly by enzyme-linked immunosorbent assay (ELISA) (Fig. 2B). At week 1 postimmunization, three of five mice in the rVSV-D1762A-tPA-NS1 group had detectable NS1 antibody, whereas all the mice in rVSV-D1762A-483-NS1 group developed a high level of NS1 antibody. From weeks 2 to 4 postvaccination, all mice in these two groups developed and maintained high levels of NS1-specific antibody. This result shows that rVSV-D1762A-483-NS1 had an earlier NS1-specific antibody response than did rVSV-D1762A-tPA-NS1. For the DNA vaccines, two and three mice vaccinated with pCI-tPA-NS1 and pCI-483-NS1, respectively, developed detectable NS1 antibody at week 1 postvaccination. All the mice in these two groups developed NS1 antibody at week 2. After booster immunization, the NS1 antibody titer further increased but had a slightly lower antibody titer compared to mtdVSV-based NS1 vaccine at week 4 (day 28 postimmunization). As expected, rVSV-G1670A-prM-E induced a high level of ZIKV E-specific but not NS1-specific antibody during the 4 weeks of vaccination period. These results demonstrate that a single dose of mtdVSV-based NS1 vaccine or two doses of DNA vaccines triggered a high level of NS1-specific antibody response. These results also suggest that the mtdVSV-based NS1 vaccine is more immunogenic than the DNA vaccine.
A single dose of mtdVSV-NS1 based vaccines or two doses of DNA vaccines provide partial protection against ZIKV-induced viremia in BALB/c mice.
Next, we examined whether NS1-based vaccine candidates can provide protection against ZIKV-induced viremia in BALB/c mice. The VSV-NS1-immunized mice were challenged intraperitoneally with 106 PFU of the ZIKV Cambodian strain (FSS13025) at week 4 postimmunization. In a previous study by Richner et al. (38), BALB/c mice were shown to be more susceptible to ZIKV infection after receiving passively transferred anti-IFNAR1 antibody, based on the observation that mice with anti-IFNAR1 antibody treatment showed significantly higher viremia and more body weight loss after challenge with a mouse-adapted ZIKV strain (Dakar 41519). Therefore, all mice were intraperitoneally injected with 2 mg of the IFNAR1 antibody at 24 h prior to the ZIKV challenge. Mouse body weight was monitored daily for 7 days after ZIKV challenge. No significant body weight losses or ZIKV-associated clinical sign were observed in any group, including the unvaccinated but challenged controls (Fig. 2C), which was consistent with many other observations that ZIKV infection does not cause illness in immunocompetent mice. Blood samples were collected from each mouse at days 3 and 7 postchallenge to measure the viremia. As expected, the unvaccinated challenged groups (pCI and rVSV-D1762A) developed a high level of ZIKV-induced viremia, reaching 104.01 and 104.33 PFU eq/ml on average at day 3 (Fig. 2D) postchallenge, and viremia declined at day 7 (Fig. 2E). This result was consistent with the previous observations that ZIKV only causes transient viremia in BALB/c mice (39, 40). As the positive control, mice vaccinated with rVSV-G1670A-prM-E were completely protected from viremia at days 3 and 7, a finding consistent with our previous study (26). The mice vaccinated with the DNA vaccines or mtd-VSV-NS1 based vaccines had a significantly lower viremia at day 3 compared to the controls, pCI and rVSV-D1762A backbone (Fig. 2D). However, viremia (103.00 to 103.16 PFU eq/ml) was detected in the DNA vaccine or mtdVSV-NS1 based vaccine groups, ∼1 log lower than the controls but remained 1 log higher than the detection limit (Fig. 2D). At day 7, viremia had been cleared in the mtdVSV-NS1 vaccine groups, whereas 102.29 to 102.60 PFU eq/ml were still detectable in the DNA vaccine groups (Fig. 2E). Collectively, these results showed that a single dose of mtdVSV-NS1 vaccine or two doses of DNA vaccine expressing NS1 provided partial protection in BALB/c mice against ZIKV-induced viremia. In addition, the protection efficacy of the mtdVSV-based NS1 vaccine was higher than that of the DNA vaccine.
T helper cell responses to NS1 induced by the mtdVSV or DNA vaccines.
T helper cells are required for antibody induction and cytotoxic T cell development that protect against virus infection. Previously, we found that the NS1 protein modulated the T cell immune response when coexpressed with prM-E protein from VSV vector (26). Specifically, we found that mice immunized with prM-E-NS1 had higher IL-5, IL-10, and IL-17 T cell responses than mice immunized with prM-E, suggesting that coexpression of NS1 enhanced Th2 and Th17 responses (26). However, whether NS1 alone can trigger a T cell immune response was not clear. To address this question, an identical animal immunization experiment was performed except that rVSV-G1670A-prM-E was not included in the study. At week 4 postimmunization, mice were euthanized, spleen T cells were isolated and restimulated with NS1 antigen, and the proliferation of T helper cells that express specific cytokines was determined by flow cytometry after intracellular staining with anticytokines. Population of each kind of cell was gated by different cytokine signals. An example of gating strategy was depicted in Fig. 3.
FIG 3.
Representative gating strategy for flow cytometry analysis of antigen-specific cytokines producing CD4+ T helper cells. Spleen cells were restimulated in vitro with the antigen for 5 days. Cells were subjected to extracellular staining with lineage-specific antibodies (i.e., anti-CD3 and anti-CD4 [BioLegend, San Diego, CA]) and, after fixation, to intracellular staining with cytokine-specific antibodies (BioLegend). For flow cytometry analysis, the cells were first gated on singlets (not shown), and then live cells were selected (R1) based on forward (FSC) and side (SSC) scatters. Live cells were then analyzed for expression of CD4 versus CD3, and the double-positive population was identified as CD4+ T cells (CD3+ CD4+). The frequency of cells producing a given cytokine among CD3+ CD4+ cells was determined by analyzing the number of cytokine-positive cells (R2).
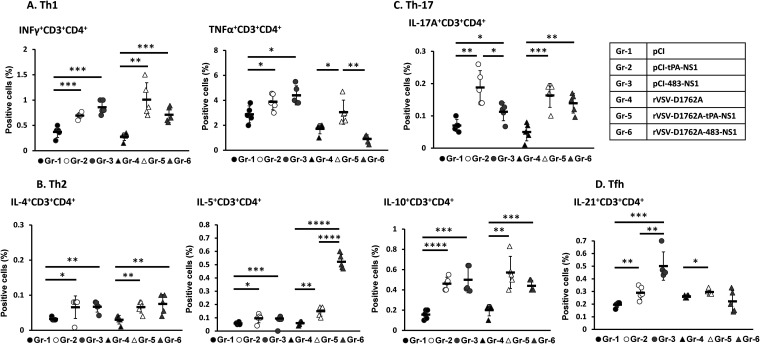
Th1 cells, which produce cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), play an important role in protection against intracellular pathogens such as viruses. The CD4+ IFN-γ+ response in spleen cells isolated from mice immunized with pCI-tPA-NS1 and pCI-483-NS1 was significantly higher than the pCI control group (Fig. 4A). Similarly, the CD4+ IFN-γ+ response in rVSV-D1762A-tPA-NS1 and rVSV-D1762A-483-NS1 was significantly higher than in the rVSV-D1762A control (Fig. 4A). These results demonstrate that the NS1-based vaccines activated ZIKV NS1-specific IFN-γ-producing T helper cells (CD4+ IFN-γ+). Similarly, TNF-α-producing T helper cells (CD4+ TNF-α+) were significantly higher than pCI vector control in pCI-tPA-NS1- and pCI-483-NS1-immunized mice (Fig. 4A). Interestingly, TNF-α-producing T helper cells (CD4+ TNF-α+) were detected in the group immunized with rVSV-D1762A-tPA-NS1 but not in the group immunized with rVSV-D1762A-483-NS1 (Fig. 4A).
FIG 4.
mtdVSV-NS1 and DNA vaccine expressing NS1 induces ZIKV-specific T helper cell responses. Six-week-old BALB/c mice (five mice per group) were immunized with each vaccine candidate and euthanized at week 4 postimmunization. The spleen from each mouse was isolated and homogenized, and a cell suspension was prepared, split into three wells (triplicate per mouse), and cultured in 96-well plates in the presence of 20 μg/ml of ZIKV NS1 protein for 5 days. (A) Proliferation of ZIKV-specific Th1 cells (IFN-γ+ CD3+ CD4+ and TNF-α+ CD3+ CD4+). (B) Proliferation of ZIKV-specific Th2 cells (IL-4+ CD3+ CD4+, IL-5+ CD3+ CD4+, and IL-10+ CD3+ CD4+). (C) Proliferation of ZIKV-specific Th17 cells (IL-17A+ CD3+ CD4+). (D) Proliferation of ZIKV-specific and Tfh cells (IL-21+ CD3+ CD4+). Cells were determined by flow cytometry after extra- and intracellular staining with the CD3/CD4 antibody and corresponding anticytokines. Data are expressed as mean of percent positive cells (n = 5 mice) ± the standard deviation. An asterisk (*) indicates the statistical difference with empty vector pCI or rVSV-D1762A group determined using a Student t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
Th2 cells, which produce cytokines such as interleukin-4 (IL-4), IL-5, and IL-10, play important roles in antibody responses. Both mtdVSV NS1 and DNA vaccine induced significantly higher-level proliferation of CD4+ IL-4+, CD4+ IL-5+, and CD4+ IL-10+ T cells than did their controls (P < 0.05) (Fig. 4B), demonstrating that NS1-based vaccine triggers a Th2 response. We also measured IL-21, the signature product of follicular T helper cells (Tfh), and IL-17A, the product of Th17 cells that facilitate antibody production and affinity maturation. Both mtdVSV NS1 and DNA vaccines induced significantly higher CD4+ IL-17A+ T cell (Th17) responses than did their controls (P < 0.05) (Fig. 4C). Both DNA vaccines also induced a significantly higher Tfh response (CD4+ IL-21+) than did the pCI control (P < 0.05) (Fig. 4D). Recombinant rVSV-D1762A-tPA-NS1 induced a higher CD4+ IL-21+ response (P < 0.05), whereas rVSV-D1762A-483-NS1 had no significant difference from rVSV-D1762A (P > 0.05) (Fig. 4D). These results demonstrated that both mtdVSV and DNA vaccine expressing ZIKV NS1 were capable of triggering ZIKV NS1-specific T helper cell responses to support both cell-mediated immunity and antibody responses against ZIKV.
Both mtdVSV-NS1 vaccine and DNA vaccine provide partial protection against a high dose of ZIKV challenge in Ifnar1–/– mice.
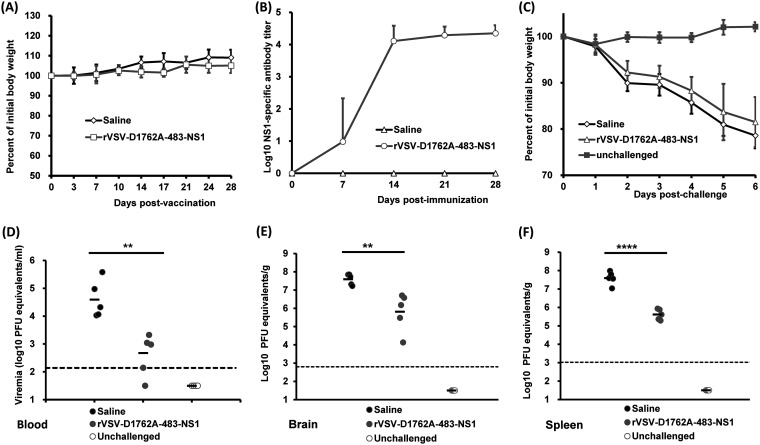
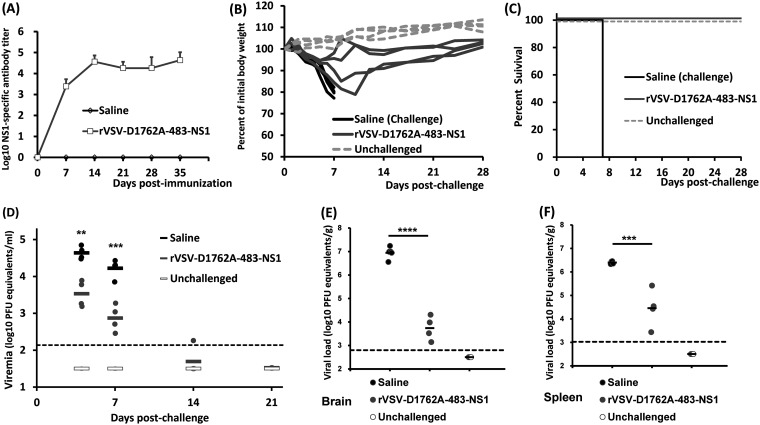
Finally, we determined the ability of mtdVSV NS1 and DNA vaccines to protect Ifnar1−/− mice from lethal ZIKV challenge. The Ifnar1−/− mice have their INFAR (IFN-α receptor) knocked out and, as a result, lack the type I IFN response, the innate response that limits viral infection. For that reason, Ifnar1−/− mice are highly permissive for both ZIKV and VSV infection (41–44). We chose rVSV-D1762A-483-NS1 in this experiment because it induced higher NS1 antibody than rVSV-D1762A-tPA-NS1 in BALB/c mice. Briefly, Ifnar1−/− mice were intramuscularly immunized with 103 PFU of rVSV-D1762A-483-NS1. Two weeks later, Ifnar1−/− mice were boosted with 105 PFU of rVSV-D1762A-483-NS1 in order to induce high NS1 antibody. At week 2 after the booster vaccination, mice were challenged with 105 PFU of ZIKV Cambodian strain. Mice inoculated with rVSV-D1762A-483-NS1 had no body weight loss (Fig. 5A) or any abnormal reaction during the 4-week immunization period, suggesting that the mtdVSV-based NS1 vaccine was completely attenuated in Ifnar1−/− mice. Two mice immunized with rVSV-D1762A-483-NS1 developed NS1-specific antibody by 1 week postimmunization, and all the mice in this group developed high levels of NS1 antibody by 2 weeks postimmunization despite the fact that a relatively low dose (103 PFU) was used for the initial vaccination (Fig. 5B). After the booster vaccination, NS1 antibody did not have a significant increase at weeks 3 and 4 (Fig. 5B). At week 4 postimmunization, mice from the unvaccinated group (saline) and rVSV-D1762A-483-NS1 group were challenged by ZIKV at a dose of 105 PFU per mouse. We chose this dose, as we knew that prM-E- or prM-E-NS1-based vaccine candidates provided complete protection in Ifnar1−/− mice. After ZIKV challenge, both rVSV-D1762A-483-NS1 and saline groups developed ZIKV-associated clinical signs and had significant body weight loss (Fig. 5C). However, two of five mice immunized with rVSV-D1762A-483-NS1 showed less body weight loss, and these two mice and one other mouse in this group survived at day 7 postchallenge despite the fact that they exhibited obvious clinical signs. In contrast, all the mice in the saline group were dead at day 6 postchallenge. Next, we measured ZIKV-induced viremia in these mice at day 3 postchallenge. The mice immunized by rVSV-D1762A-483-NS1 had a significantly lower (P < 0.01, t test) ZIKV viral load in the blood compared to the saline group (unimmunized and challenged control group) (Fig. 5D). After euthanasia at day 7 postchallenge, brain and spleen tissues were collected for detection of ZIKV viral load. Similar to the viral load in the blood, significantly less viral load was detected in both brain (P < 0.01) and spleen (P < 0.0001) tissues of mice immunized by rVSV-D1762A-483-NS1 than in the control group (Fig. 5E and F). However, it should be noted that ZIKV was still detectable in blood, brain, and spleen after ZIKV challenge despite a high level of NS1 antibody induced by immunization with rVSV-D1762A-483-NS1. Thus, we conclude that mtdVSV-NS1 vaccine provided partial protection against a lethal ZIKV challenge in Ifnar1−/− mice. These results also suggest that the protection efficacy of NS1 alone was lower than that of prM-E or prM-E-NS1, given the fact that VSV-based prM-E or prM-E-NS1 vaccine candidates were capable of providing complete protection against 105 PFU of ZIKV challenge (26).
FIG 5.
mtdVSV-NS1 vaccine provided partial protection against lethal ZIKV challenge in Ifnar1−/− mice. Ifnar1−/− mice were immunized intramuscularly with rVSV-D1762A-483-NS1 at a dose of 1 × 103 PFU/mouse and boosted with 1 × 105 PFU/mouse after 2 weeks. At week 4 postimmunization, mice were intraperitoneally challenged with 105 PFU of ZIKV Cambodian strain. (A) Dynamics of mouse body weight change after vaccination with rVSV-D1762A-483-NS1. Mouse body weight was measured every 3 or 4 days, and the results are shown as the mean of five mice ± the standard deviation. (B) Kinetics of ZIKV NS1-specific antibody induced by rVSV-D1762A-483-NS1. Serum samples were collected weekly and analyzed by ELISA for NS1-specific serum IgG Ab. The data are expressed as the GMT of five mice plus the standard deviation. (C) Body weight changes after ZIKV challenge. After ZIKV challenge, the body weight for each mouse was measured daily. The average body weights of five mice ± the standard deviations are shown. (D) Viremia in Ifnar1−/− mice after challenge with ZIKV. Viremia at day 3 postchallenge was measured by real-time RT-PCR and calculated to PFU equivalent RNA/ml. (E) ZIKV titer in brain tissues. At day 7 postchallenge, Ifnar1−/− mice were terminated; brain and spleen tissues were harvested and analyzed by real-time RT-PCR and calculated to the PFU equivalent RNA/g tissue. (F) ZIKV titer in spleen tissues. Data for each mouse are shown and are expressed as the GMT of five mice ± the standard deviations, determined using a Student t test, and compared to the saline group (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
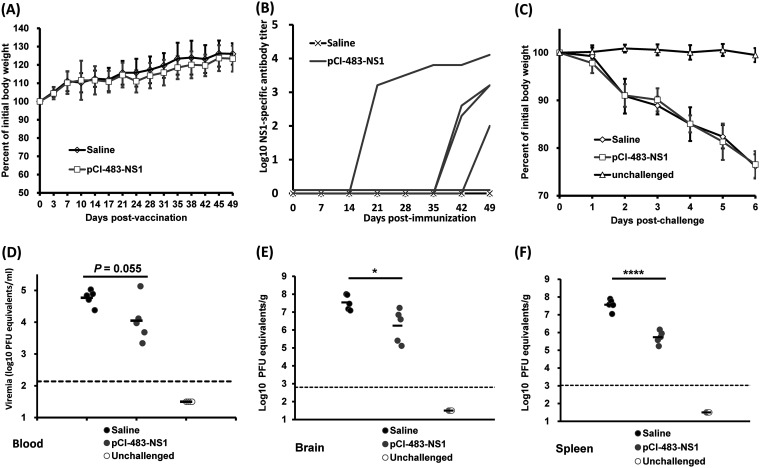
Similarly, we assessed the immunogenicity of the DNA vaccine expressing NS1 in Ifnar1−/− mice. Briefly, Ifnar1−/− mice were intramuscularly injected with 50 μg of pCI-483-NS1. No body weight loss (Fig. 6A) or any abnormal reaction was observed following the DNA vaccination. No NS1 antibody was detected in the first 2 weeks (Fig. 6B). Thus, mice were boosted with the same amount of DNA vaccine at week 2. At week 3 (week 1 after the booster vaccination), only one mouse in the pCI-483-NS1 group developed NS1-specific antibody (Fig. 6B). In this case, a third vaccination was performed 5 weeks after the first inoculation. By week 7 postvaccination, 4 of 5 mice in this group had developed a high level of NS1-specific antibody (Fig. 6B). At week 7, mice were challenged with 105 PFU of the ZIKV Cambodian strain. All mice in the DNA vaccine group and saline group developed severe ZIKV-associated clinical signs and significant body weight loss compared to unchallenged control (Fig. 6C). In addition, no significant difference in body weight loss was observed between the DNA vaccine group and the challenged control group (P > 0.05). All the mice in the saline group died at day 6 postchallenge. One of five mice immunized with pCI-483-NS1 survived at day 7 but was euthanized due to the severity of the disease. The pCI-483-NS1 group had a lower viremia level (P = 0.055, t test) than the saline group at day 3 postchallenge (Fig. 6D). However, it had higher viremia compared to the rVSV-D1762A-483-NS1 group (compare Fig. 6D to Fig. 5D). Despite the disease severity, the viral load in the brain and spleen of the pCI-483-NS1 group was significantly lower than the saline control (Fig. 6E and F). These results show that the DNA vaccine expressing NS1 provides only partial protection against a lethal challenge of ZIKV in Ifnar1−/− mice. These results also suggest that the efficacy of the DNA vaccine was lower than mtdVSV-based NS1 vaccine.
FIG 6.
DNA vaccine expressing NS1 provided partial protection against lethal ZIKV challenge in Ifnar1−/− mice. Ifnar1−/− mice were intramuscularly immunized with 50 μg of pCI-483-NS1 and were boosted with the same dose at weeks 2 and 5. At week 7, mice were intraperitoneally challenged with 105 PFU of ZIKV Cambodian strain. (A) Dynamics of mouse body weight change after vaccination with DNA vaccine. Mouse body weight was measured every 3 or 4 days, and the results are shown as the means of five mice ± standard deviations. (B) Kinetics of ZIKV NS1-specific antibody induced by DNA vaccine. Serum samples were collected weekly and analyzed by ELISA for NS1-specific serum IgG antibody. The data are expressed as the GMT of five mice plus the standard deviations. (C) Body weight changes after ZIKV challenge. After ZIKV challenge, the body weight for each mouse was measured daily. The average body weights of five mice ± the standard deviations are shown. (D) Viremia in Ifnar1−/− mice after challenge with ZIKV. Viremia at day 3 postchallenge was measured by real-time RT-PCR and calculated to PFU equivalent RNA/ml. (E) ZIKV titer in brain tissues. At day 7 postchallenge, Ifnar1−/− mice were terminated; brain and spleen tissues were harvested and analyzed by real-time RT-PCR and calculated to PFU equivalent RNA/g tissue. (F) ZIKV titer in spleen tissues. Data for each mouse are shown and are expressed as the GMT of five mice ± standard deviations, analyzed using a Student t test, and compared to the saline group (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
The mtdVSV-NS1 vaccine protects Ifnar1–/– mice from a lower dose ZIKV challenge.
Since Ifnar1−/− mice are highly sensitive to ZIKV infection, it is possible that the mtdVSV-NS1 vaccine be more effective against a lower challenge dose. To test this possibility, Ifnar1−/− mice were immunized intramuscularly with rVSV-D1762A-483-NS1 or saline at a dose of 105 PFU and boosted with same dose 2 weeks later. As shown in Fig. 7A, the vaccinated mice developed a high level of NS1-specific antibody at week 1 that increased by week 2 and remained stable through week 5. At that time, mice were challenged with 103 PFU of ZIKV. All mice in the saline group developed ZIKV clinical signs (ruffled fur, hindlimb paralysis to total paralysis), had significant body weight losses, and died within 7 days after ZIKV challenge (Fig. 7B). Two of four mice vaccinated with rVSV-D1762A-483-NS1 had moderate body weight loss and ruffled fur, and one of these two mice also had partial hindlimb paralysis. However, these mice had recovered by day 8, and all began to gain weight between days 8 and 10 (Fig. 7B). The other two mice in the rVSV-D1762A-483-NS1 group had mild body weight loss without the other symptoms and recovered quickly (Fig. 7B). ZIKV-induced viremia was detectable in rVSV-D1762A-483-NS1 group at day 4 or 7 postchallenge, although it was significantly lower than the saline group (Fig. 7D). Importantly, mice vaccinated with rVSV-D1762A-483-NS1 all survived the 28-day follow-up after ZIKV challenge (Fig. 7C), at which point they were terminated. ZIKV RNA was still detectable in brain (Fig. 7E) and spleen (Fig. 7F) in the rVSV-D1762A-483-NS1 group. Thus, these results demonstrate that the protection efficacy of the mtdVSV-NS1 vaccine was improved at the lower challenge dose. However, the protection was not complete since the mice experienced weight loss, mild clinical signs, and viremia.
FIG 7.
mtdVSV-NS1 vaccine provides an improved protection against a lower dose of ZIKV challenge in Ifnar1−/− mice. Ifnar1−/− mice were immunized intramuscularly with rVSV-D1762A-483-NS1 at a dose of 1 × 105 PFU/mouse and were boosted with the same dose after 2 weeks. At week 4 postimmunization, mice were intraperitoneally challenged with 103 PFU of ZIKV Cambodian strain. (A) Kinetics of ZIKV NS1-specific antibody induced by rVSV-D1762A-483-NS1. Serum samples were collected weekly and analyzed by ELISA for NS1-specific serum IgG Ab. The data are expressed as the GMT of five mice ± the standard deviations. (B) Body weight change of each mouse after ZIKV challenge. After ZIKV challenge, the body weight for each mouse was measured daily. Each line is the representative of an individual animal. (C) Survival curve of Ifnar1−/− mice after ZIKV challenge. All four mice in the rVSV-D1762A-483-NS1 group survived until day 28 postchallenge, whereas all the mice in the saline group died at day 7 after ZIKV challenge. (D) Viremia in Ifnar1−/− mice after challenge with ZIKV. Viremia at days 4, 7, 14, and 21 postchallenge was measured by real-time RT-PCR and calculated to PFU equivalent RNA/ml. (E) ZIKV titer in brain tissues. At day 28 postchallenge, Ifnar1−/− mice were terminated; brain and spleen tissues were harvested and analyzed by real-time RT-PCR and calculated to PFU equivalent RNA/g tissue. (F) ZIKV titer in spleen tissues. Data are expressed as the GMT of five mice ± the standard deviations, analyzed using a Student t test, and compared to the saline group (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
DISCUSSION
In this study, we optimized ZIKV NS1 protein expression and secretion by fusing different signal peptides to NS1. We found that both the tPA and E483 signal peptides significantly increased NS1 expression within cells and its secretion. We assessed two vaccine platforms, an attenuated VSV vector and a DNA vaccine, expressing NS1 protein for their efficacy against ZIKV infection in BALB/c and Ifnar1−/− mice. We found that both NS1-based vaccine platforms provided partial protection against lethal ZIKV infection and viremia in BALB/c mice challenged with 106 PFU ZIKV and in Ifnar1−/− mice challenged with 105 PFU ZIKV, despite both vaccine platforms triggering a high level of NS1-specific antibody and T cell immune response. At a lower ZIKV challenge dose, 103 PFU ZIKV, the protection efficacy of NS1-based vaccine was remarkably improved in Ifnar1−/− mice. However, it was still unable to provide complete protection against ZIKV infection. These results demonstrated that vaccination with NS1 alone is capable of inducing substantial protection against ZIKV infection, but the efficacy is less than prM-E or prM-E-NS1.
The ability of the NS1 proteins of several other flaviviruses to provide protection against their respective viruses has previously been studied. Immunization with purified NS1, recombinant NS1, a DNA vaccine expressing NS1, or passively administered MAbs against NS1, provided substantial protection against lethal infections by WNV, JEV, DENV, and YFV (20, 22, 45–47). In some flaviviruses, the protective efficacy of NS1 was comparable to that of prM-E. For example, mice immunized with the plasmid-expressed JEV NS1 provided 90% protection after a lethal JEV challenge, whereas plasmid-expressed prM-E only provided 70% protection (46). For DENV, it was shown that immune responses elicited by vaccination with DENV2 NS1 could protect against a lethal challenge with a high dose of DENV2 (48). In addition, vaccination with DENV1, DENV3, or DENV4 NS1 provided substantial cross protection against a heterologous DENV2 lethal challenge (48). In that study, 6- to 8-week-old Ifnar−/− C57BL/6 mice were immunized with NS1 from DENV1, -2, -3, and -4, and those immunizations provided 75, 100, 60, and 60% protection, respectively, against the challenge of DENV2 derived from strain PL046 (48).
A recent study showed that intramuscular vaccination with a single dose of MVA-ZIKV-NS1 in CD-1/ICR mice provided complete protection (including weight loss, clinical signs, mortality, and virus burden in the brain) against 105 PFU of a neurovirulent African strain of ZIKV (MR766) via an intracerebral challenge (25). In contrast to that study, we found that NS1 alone only provides partial protection against ZIKV challenge in BALB/c and Ifnar1−/− mice using two vaccine platforms, mtdVSV-based NS1 vaccine and DNA vaccine expressing NS1 protein. Although ZIKV did not cause significant illness in immunocompetent mice (such as BALB/c mice), they developed viremia upon challenge with ZIKV. We showed that both mtdVSV-NS1 and DNA vaccines significantly reduced levels of viremia upon challenge of vaccinated mice compared to the unvaccinated control groups (rVSV-D1762A and pCI) by day 3 postchallenge. However, this protection was not complete, since mice in the NS1-based vaccine groups had an ∼1 log PFU eq of ZIKV above the detection limit. As a positive control, a prM-E-based vaccine construct provided complete protection against viremia, and no ZIKV RNA was detected in blood samples during the entire experiment. Our results were consistent with a recent study which showed that BALB/c mice immunized intradermally with three times of DNA vaccine (pVAX-tpaNS1, 50 or 100 μg) provided partial protection against viremia at day 1 after ZIKV challenge (29).
Ifnar1−/− mice lack the interferon type I receptor (IFNAR). Therefore, they lack the signaling responses to type I interferons. This defect makes the Ifnar1−/− mouse a highly sensitive model for evaluating the safety and efficacy of ZIKV vaccine candidates (49, 50). Previously, it was shown that prM-E-based DNA, mRNA, recombinant viral vectored ZIKV vaccine candidates, and inactivated vaccine and live attenuated ZIKV vaccine candidates provided complete protection against ZIKV challenge in Ifnar1−/− mice (reviewed in references 5 and 7). In the present study, we used an mtdVSV (rVSV-D1762A), which is completely defective in both G-N-7 and ribose 2′-O methylation, as the vector to deliver ZIKV NS1. During the study, none of the mice vaccinated with 105 PFU of mtdVSV-NS1 displayed any weight loss or any symptoms characteristic of wild-type VSV infection. However, 50 PFU of wild-type VSV is lethal to Ifnar1−/− mice (44). This difference indicates that the rVSV-D1762A vector is completely attenuated and safe for Ifnar1−/− mice. However, we found that Ifnar1−/− mice immunized with mtdVSV-NS1 and DNA vaccines experienced significant ZIKV-associated clinical signs and body weight loss after a higher dose (105 PFU) ZIKV challenge, despite the presence of high levels of NS1 antibody present in the immunized mice. In addition, significant levels of ZIKV were detected in the blood, brain, and spleen of immunized animals following challenge. At a lower dose of ZIKV challenge (103 PFU), the protection efficacy of mtdVSV-NS1 was improved, since all mice survived and regained body weight. However, this protection was not complete because mice still experienced mild ZIKV-associated clinical signs, viremia, and brain and spleen infection. These data showed that NS1-based vaccine candidates were insufficient to provide complete protection against ZIKV infection in Ifnar1−/− mice. Similarly, Grubor-Bauk et al. recently showed that Ifnar1−/− mice immunized intradermally three times with 50 μg of DNA vaccine (pVAX-tpaNS1) failed to protect against a lethal challenge dose of 103 CCID50 (50% cell culture infectious dose) of the MR766 strain of ZIKV (29).
It appears that the protection efficacy of NS1 reported in our study and Grubor-Bauk study is lower than that in the Brault study (25). Several experimental differences, including the animal model, ZIKV strain, and challenge route and dose, may have contributed to the differences in outcome. We challenged BALB/c and Ifnar1−/− mice with the ZIKV Cambodian strain via the intraperitoneal route at doses of 106 and 105/103 PFU, respectively. However, Brault et al. used an outbred mouse model CD-1/ICR challenged with an African strain of ZIKV (MR766) via an intracerebral route at a dose of 105 PFU (25). For challenge experiments in BALB/c mice, we used anti-IFNAR receptor antibody to render mice more susceptible to ZIKV infection prior to challenge. Initially, we chose a challenge dose of 106 PFU (for BALB/c) or 105 PFU (for Ifnar1−/−) of ZIKV because our previous study showed that mtdVSV expressing prM-E or prM-E-NS1 and DNA vaccine expressing prM-E conferred complete protection against the ZIKV Cambodian strain at this dose in these mouse strains (26). Clearly, the protective efficacy of NS1 alone is lower than that of prM-E or prM-E-NS1 under our experimental conditions.
Although we showed that NS1 alone induces only partial protection under our experimental conditions, there are many reasons for including NS1 as a ZIKV vaccine antigen. Currently, most research on ZIKV subunit vaccines has focused on prM and E proteins, which relies on triggering high levels of neutralizing antibody (5, 7). However, several studies have shown that neutralizing antibody can cross-react with other species of flaviviruses, facilitating ZIKV infection through the antibody-dependent enhancement (ADE) mechanism (8–10). Since NS1 is not a component of the ZIKV virion, antibodies against NS1 protein will not directly neutralize ZIKV. However, because antibodies to NS1 do not bind to the virion, there is no risk of disease enhancement. Recently, Bailey et al. identified four monoclonal antibodies (MAbs) from a ZIKV-infected patient that target the NS1 protein (27). As expected, these MAbs are nonneutralizing and can engage FcγR without inducing ADE of infection in vitro. Passive immunization of Stat2−/− mice with one of MAbs (AA12) conferred protective efficacy against lethal challenges of African and Asian lineage ZIKV strains, although the mice still experienced weight loss and clinical signs (27). In a follow-up study, Bailey et al. detected a high level of NS1-specific antibody in mice vaccinated with a DNA vaccine plasmid expressing NS1 (pCAGGS-NS1) together with two doses of adjuvanted NS1 protein (28). Passive transfer of these immune sera was able to significantly protect (60 to 80% survival rate) STAT2 knockout mice against lethal challenge by MR766 ZIKV strain at a dose of 158 PFU per mouse (28). These results demonstrated that NS1 antibody provided substantial but incomplete protection against ZIKV infection. Since both NS1 and prM-E can provide protection against ZIKV infection, coexpression of NS1 and prM-E may provide synergetic effects. In fact, we have previously shown that expression of prM-E-NS1, more than prM-E, triggered a stronger cellular immunity that is critical for virus clearance (26). In addition, other researchers showed that coexpression of NS1 with prM-E had a significantly higher immunogenicity than prM-E when they were delivered by an adenovirus vector (51). One approach is to combine NS1 with neutralizing epitope-deleted prM-E in one vaccine construct which may not only enhance immunogenicity but also avoid ADE.
It has been reported that NS1 of flaviviruses induces protection through different mechanisms. MAbs against WNV NS1 protected against WNV infection through C1q-independent and Fcγ receptor-dependent or -independent pathways (22). Similarly, it was shown that ZIKV NS1 MAb-induced protection is Fc dependent, based on the observation that a mutated antibody unable to activate Fc effector functions failed to provide protection in vivo (27). For both mtdVSV and DNA vaccines, we detected a high level of NS1 antibody and NS1-specific T cell activation in mice. Similar results were observed when mice were immunized with MVA-ZIKV-NS1 (25). Recently, Grubor-Bauk et al. found that the C-terminal β-ladder domain of ZIKV NS1 protein contains novel NS1-specific cytotoxic CD8+ T lymphocyte and T helper cell epitopes, which are critical for conferring protection against ZIKV infection in a DNA vaccination experiment (29). Therefore, it is likely that both Fc-receptor-meditated mechanisms and T-cell-mediated mechanisms collectively contribute to the protection conferred by NS1-based vaccines.
In this study, we found that the expression and immunogenicity of NS1 was influenced by different signal peptides. We compared signal peptides from tPA, anchor C, and different lengths of the transmembrane domain at the C terminus of the ZIKV E protein. We found that NS1 expression was the highest in cell culture when tPA or E483-504 signal peptide was used. However, in BALB/c mice, the rise of NS1-specific antibody in rVSV-D1762A-tPA-NS1 was significantly delayed compared to rVSV-D1762A-483-NS1, which triggered high antibody levels as early as 7 days after vaccination. The higher or faster immune response in the rVSV-D1762A-483-NS1 group might have been due to E483-504 being the natural signal peptide close to the cleavage site between E and NS1 protein, which subsequently produces more NS1 protein in its optimal conformation. We also found that NS1 delivered by the mtdVSV vector was more efficacious than that from the DNA vaccine vector. A single vaccination with mtdVSV-based vaccine was sufficient to induce a high level of NS1 antibody, whereas the DNA vaccine required two or three vaccinations to induce a similar level of NS1 antibody. In addition, the protective efficacy of the mtdVSV-based vaccine against ZIKV infection was higher than the DNA vaccine. Given the recent successful application of VSV-based Ebola virus vaccine in humans (52, 53) and the official approval of VSV-based Ebola virus vaccine by the FDA, it is anticipated that more VSV-based vaccines for human trials will be forthcoming.
In summary, we have developed and optimized mtdVSV-based vaccines and DNA vaccines expressing the ZIKV NS1 protein and showed that NS1 alone was capable of triggering high humoral and cellular immune responses and providing substantial but incomplete protection against ZIKV infection in both immunocompetent and immunodeficient mice.
MATERIALS AND METHODS
Cell lines, viruses, and plasmid construction.
BHK-21 cells (ATCC no. CCL-10), Vero cells (ATCC no. CCL-81), and 293T cells (ATCC no. CRL-3216) were purchased from the American Type Culture Collection (Manassas, VA). BSRT7 cells, which stably express T7 RNA polymerase, are clones of BHK-21 cells. All cell lines were grown in Dulbecco modified Eagle medium (DMEM; Life Technologies) supplemented with 10% FBS. ZIKV Cambodian strain (FSS13025) was provided by the World Reference Center for Emerging Viruses and Arboviruses (University of Texas Medical Branch), propagated in Vero cells, and titrated using a standard plaque assay (54). Plasmids encoding VSV N (pN), P (pP), and L (pL) genes, and an infectious cDNA clone of the viral genome, pVSV1(+), were generous gifts from Gail Wertz (55). Plasmid pVSV1(+) GxxL, which contains SmaI and XhoI at the G and L gene junction, was kindly provided by Sean Whelan. To further attenuate the VSV vector, we took advantage of a point mutation, D1762A, in the large (L) polymerase protein, which rendered a recombinant virus that is completely defective in mRNA cap G-N-7 and ribose 2′-O methylation (32, 33). Using site-directed mutagenesis, the D1762A mutation was introduced into the pVSV1(+) GxxL plasmid. The full-length nonstructural protein 1 (NS1) was amplified from an infectious cDNA clone of ZIKV Cambodian strain by high-fidelity PCR. The upper stream primers used for PCR contain different signal sequences, including C-terminal anchor of ZIKV-C (anchor C), human tissue plasminogen activator (tPA), or amino acids from 456 to 504 (E456), 483 to 504 (E483), and 484 to 504 (E484) at the C terminus of ZIKV E protein. These DNA fragments were digested with SmaI and XhoI and cloned into pVSV(+)GxxL at the same sites. The resulting plasmids were designated pVSV(+)-D1762A-a-NS1, pVSV(+)-D1762A-tPA-NS1, pVSV(+)-D1762A-456-NS1, pVSV(+)-D1762A-484-NS1, and pVSV(+)-D1762A-483-NS1. To prepare DNA vaccine plasmids, the NS1 genes of ZIKV Cambodian strain, together with the different signal sequences, were cloned into a pCI vector (Promega) which resulted in the construction of pCI-a-NS1, pCI-tPA-NS1, and pCI-483-NS1. All constructs were confirmed by sequencing.
Recovery of rVSV expressing ZIKV antigens.
Recovery of recombinant VSV (rVSV) from the infectious clone was carried out as described previously (55). Briefly, rVSV was recovered by cotransfection of the plasmid encoding VSV genome and support plasmids encoding VSV nucleocapsid complex (pN, pP, and pL) into BSRT7 cells infected with a recombinant vaccinia virus (vTF7-3) expressing T7 RNA polymerase (kindly provided by Bernard Moss). At 96 h posttransfection, cell culture fluids were collected and filtered through a 0.2-μm filter, and the recombinant virus was further amplified in BSRT7 cells. Subsequently, the viruses were plaque purified as described previously (32). Individual plaques were isolated, and seed stocks were amplified in BSRT7 cells. The viral titer was determined by a plaque assay performed in Vero cells.
RT-PCR.
Viral RNA was extracted from recombinant VSVs by using an RNeasy minikit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. ZIKV genes were amplified by using a one-step reverse transcription-PCR (RT-PCR) kit (Qiagen) with primers annealing to the VSV G gene at position 4524 (5′-CGAGTTGGTATTTATCTTTGC-3′) and the L gene at position 4831 (5′-GTACGTCATGCGCTCATCG-3′) (numbering refers to the complete VSV Indiana genome sequence). The amplified products were analyzed on 1% agarose gel electrophoresis and confirmed by DNA sequencing.
Analysis of the expression of ZIKV NS1 by rVSV or DNA vaccine plasmids by Western blotting.
Confluent BSRT7 cells were infected with each rVSV expressing ZIKV NS1 or rVSV-D1762A at a multiplicity of infection (MOI) of 3.0. For the pCI plasmid, HEK293T cells were transfected with 2 μg of pCI, pCI-a-NS1, pCI-tPA-NS1, or pCI-483-NS1 using Lipofectamine 2000. At the indicated times, cell culture medium was harvested and clarified at 3,000 rpm for 5 min. In the meantime, cells were harvested in lysis buffer containing 5% β-mercaptoethanol, 0.01% NP-40, and 2% sodium dodecyl sulfate (SDS). Proteins were separated by 10% SDS-PAGE and transferred to a Hybond enhanced chemiluminescence nitrocellulose membrane (Amersham) in a Mini Trans-Blot electrophoretic transfer cell (Bio-Rad). The blot was probed with rabbit anti-ZIKV NS1 antiserum (Alpha Diagnostic International, Inc., San Antonio, TX) at a dilution of 1:1,500, followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (Santa Cruz) at a dilution of 1:5,000. The blot was developed with SuperSignal West Pico chemiluminescent substrate (Thermo Scientific) and exposed to Kodak BioMax MR film.
Purification of ZIKV.
Two confluent T150 flasks of Vero cells were infected with the ZIKV Cambodian strain at an MOI of 0.01 in a volume of 2 ml of DMEM. After 1 h of absorption, 20 ml of DMEM (supplemented with 5% fetal bovine serum) was added, and infected cells were incubated at 37°C for 72 h. When an extensive cytopathic effect was observed, cell culture fluids were clarified by centrifugation at 3,000 × g for 30 min. Viruses were concentrated through a 40% (wt/vol) sucrose cushion by centrifugation at 30,000 × g for 2 h at 4°C in a Ty50.2 rotor (Beckman). The pellet was resuspended in 0.3 ml of NTE buffer (100 mM NaCl, 10 mM Tris, 1 mM EDTA [pH 7.4]). The viral titer was determined by a plaque assay.
Animal experiments.
All mice were housed within ULAR facilities of The Ohio State University under approved Institutional Laboratory Animal Care and Use Committee (IACUC) guidelines. Each inoculation group was separately housed in rodent cages under biosafety level 2 (BSL-2) conditions.
Experiment 1: determining whether mtdVSVs and DNA plasmids expressing NS1 are immunogenic in BALB/c mice.
Thirty-five 6-week-old specific-pathogen-free female BALB/c mice (Charles River Laboratories, Wilmington, MA) were randomly divided into seven groups (five mice per group). Mice in group 1 were intramuscularly inoculated with an empty pCI vector (with no insertion). Mice in groups 2 and 3 were inoculated with pCI-tPA-NS1 and pCI-483-NS1, respectively. Mice in groups 4 to 6 were intranasally inoculated with rVSV-D1762A (with no insertion), rVSV-D1762A-tPA-NS1, and rVSV-D1762A-483-NS1. Mice in group 7 were immunized intranasally with rVSV-G1670A-prM-E. For DNA vaccination, mice were immunized intramuscularly with 50 μg of plasmids and boosted with the same dose 2 weeks later. For VSV-based vaccination, each mouse was inoculated intranasally at a dose of 1 × 106 PFU in a volume of 50 μl. After inoculation, the animals were evaluated twice every day for mortality and the presence of any symptoms of VSV infection. The body weight of each mouse was monitored twice a week. Blood samples were collected from each mouse weekly by bleeding the facial vein, and serum was isolated for antibody detection. At week 4 postinoculation, all mice were euthanized.
Experiment 2: determining whether mtdVSV and DNA vaccines can protect BALB/c mice against viremia.
Forty 6-week-old specific-pathogen-free female BALB/c mice (Charles River Laboratories) were randomly divided into eight groups (five mice per group). Mice in groups 1 to 7 were immunized as described for experiment 1. Mice in group 8 were immunized with saline as an unimmunized and unchallenged control. At week 4 postimmunization, mice in groups 1 to 6 were intraperitoneally challenged with ZIKV Cambodian strain at a dose of 1 × 106 PFU per mouse. At 24 h prior to ZIKV challenge, mice were intraperitoneally administered 2 mg of anti-IFNAR1 (Leinco Technologies, Fenton, MO) blocking antibody. After challenge, the animals were evaluated twice daily for mortality and the presence of any symptoms of ZIKV infection. The body weight for each mouse was monitored daily. At days 3 and 7, blood was collected from each mouse for measuring ZIKV RNA by real-time RT-PCR. At day 7 postchallenge, all mice from each group were euthanized.
Experiment 3: determining whether mtdVSV and DNA vaccines can trigger ZIKV-specific T cell response in BALB/c mice.
Thirty-five 6-week-old SPF female BALB/c mice (Charles River Laboratories) were randomly divided into seven groups (five mice per group) and immunized with mtdVSV or DNA vaccines as described for experiment 1. At week 4 postinoculation, all mice were euthanized, and spleens were isolated from each mouse for ZIKV-specific T cell response analysis.
Experiment 4: determining whether mtdVSV vaccine can protect Ifnar1–/– mice against a high dose of ZIKV challenge.
Fifteen 8-week-old female Ifnar1−/− mice (Jackson Laboratories) were randomly divided into three groups (five per group). Mice in groups 1 to 3 were immunized intramuscularly with saline (unvaccinated, unchallenged control), saline (unvaccinated, challenged control), and rVSV-D1762A-483-NS1 at a dose of 103 PFU per mouse in a volume of 100 μl. Then, 2 weeks later, mice in groups 1 and 2 were inoculated intramuscularly with the same amount of saline, while mice in group 3 were boosted intramuscularly with rVSV-D1762A-483-NS1 at a dose of 105 PFU per mouse to maximize the immune response. After immunization, mice were evaluated every 3 days for body weight. The safety of the mtdVSV-NS1 vaccine candidate was evaluated twice a day. Blood samples were collected weekly from each mouse for detection of NS1-specific antibody. At week 4 postimmunization, mice in groups 2 and 3 were intraperitoneally challenged with ZIKV Cambodian strain at a dose of 1 × 105 PFU per mouse. After being challenged, the animals were evaluated twice every day for mortality and the presence of any clinical sign of ZIKV infection. The body weight for each mouse was monitored daily. Blood was collected at days 3 and 7 for the detection of viremia. At day 7 postchallenge, all mice from each group were euthanized, and the brain and spleen from each mouse were collected for virus quantification.
Experiment 5: determining whether DNA vaccine can protect Ifnar1–/– mice against a high dose of ZIKV challenge.
The experimental design here was similar to that of experiment 3 described above. Fifteen 6-week-old female Ifnar1−/− mice (Jackson Laboratories) were randomly divided into three groups (five per group). Mice in groups 1 to 3 were immunized intramuscularly with saline (unvaccinated unchallenged control), saline (unvaccinated challenged control), and 50 μg of pCI-483-NS1 plasmid in a volume of 100 μl. At weeks 2 and 5 postvaccination, mice in groups 1 and 2 were inoculated intramuscularly with the same amount of saline, while mice in group 3 were boosted intramuscularly with the same dose of pCI-483-NS1. After immunization, blood samples were collected weekly from each mouse for detection of antibody. At week 7 postimmunization, mice in groups 2 and 3 were intraperitoneally challenged with ZIKV Cambodian strain at a dose of 1 × 105 PFU per mouse. After being challenged, the animals were evaluated twice every day for mortality and the presence of any clinical sign of ZIKV infection. The body weight of each mouse was monitored daily. Blood was collected at days 3 and 7 for the detection of viremia. At day 7 postchallenge, all mice from each group were euthanized, and the brain and spleen from each mouse were collected for virus quantification.
Experiment 6: determining whether mtdVSV vaccine can protect Ifnar1–/– mice against a lower dose of ZIKV challenge.
Twelve 6 to 8-week-old male Ifnar1−/− mice (Jackson Laboratories) were randomly divided into three groups (four per group). Mice in groups 1 to 3 were immunized intramuscularly with saline (unvaccinated, unchallenged control), saline (unvaccinated, challenged control), and rVSV-D1762A-483-NS1 at a dose of 105 PFU per mouse in a volume of 100 μl. Then, 2 weeks later, mice in groups 1 and 2 were inoculated intramuscularly with the same amount of saline, while mice in group 3 were boosted intramuscularly with same dose of rVSV-D1762A-483-NS1. Blood samples were collected weekly from each mouse for detection of NS1-specific antibody. At week 5 postimmunization, mice in groups 2 and 3 were intraperitoneally challenged with ZIKV Cambodian strain at a dose of 1 × 103 PFU per mouse. After being challenged, the animals were evaluated twice every day for mortality and the presence of any clinical sign of ZIKV infection. The body weight for each mouse was monitored daily. Blood was collected on days 4, 7, 14, and 21 for the detection of viremia. At day 28 postchallenge, all mice from each group were euthanized, and the brain and spleen from each mouse were collected for virus quantification.
Detection of ZIKV NS1-specific antibody by ELISA.
Ninety-six-well plates were first coated with 50 μl of highly purified NS1 protein (MyBioSource, Inc., San Diego, CA; 4 μg/ml, in 50 mM Na2CO3 buffer [pH 9.6]) per well at 4°C overnight and then blocked with bovine serum albumin (1% [wt/vol] in phosphate-buffered saline [PBS] at 100 μl/well) at 37°C for 2 h. Subsequently, individual serum samples were tested for ZIKV NS1-specific Ab on antigen-coated plates. Briefly, serum samples were 2-fold serially diluted and added to NS1 protein-coated wells. After incubation at room temperature for 2 h, the plates were washed five times with PBS-Tween (0.05%), followed by incubation in 50 μl of goat anti-mouse IgG HRP-conjugated secondary antibodies (Sigma) at a dilution of 1:2,000 for 1 h. The plates were washed, developed with 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB), and stopped by the addition of 100 μl of H2SO4 (2 mol/liter); the optical density at 450 nm then was determined by using a BioTek microplate reader. Endpoint titers were determined as the reciprocal of the highest dilution that had an absorbance value 2.1-fold greater than the background level (DMEM control). Antibody titers were calculated using the geometric mean titers (GMT).
Analysis of ZIKV-specific T cell responses.
Spleen cells from animal experiment 3 were aseptically removed from mice 28 days after immunization and minced by pressing them through a cell strainer. Red blood cells were removed by incubation in 0.84% ammonium chloride and, following a series of washes in RPMI 1640, resuspended in RPMI 1640 supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate, 10 mM HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum. The cell concentrations were adjusted to 3 × 106 cells/ml, and 100 μl was added to each well of a 96-well microtiter plate. Cells were cultured either alone or in the presence of 20 μg/ml of ZIKV NS1 protein, which might restimulate the proliferation of NS1-specific T cells, for 5 days at 37°C in a 5% CO2 atmosphere. Culture supernatants were collected from each well and frozen at –80°C until analysis of secreted cytokines using Bio-Plex Pro Mouse Cytokine Standard 23-Plex, group I (Bio-Rad Laboratories, Inc., Hercules, CA) according to the manufacturer’s instructions. The frequencies of NS1 antigen-specific Th1 cells (IFN-α+ CD4+ CD3+ or TNF-β+ CD4+ CD3+), Th2 cells (IL-4+ CD4+ CD3+, IL-5+ CD4+ CD3+, or IL-10+ CD4+ CD3+), Th17 cells (IL-17A+ CD4+ CD3+), and Tfh cells (IL-21+ CD4+ CD3+) were determined by intracellular staining with the corresponding anti-cytokine antibodies (Table 1) after additional incubation in the presence of phorbol myristate acetate and ionomycin. The cells were then analyzed with the aid of an Attune flow cytometer. Population of each kind of cells was gated by different cytokine signals (for example, Th1 cells determined by Th1-IFN-α or Th1-TNF-β cells, which were selected by IFN-α/CD4/CD3 triple positive or TNF-β/CD4/CD3 triple positive, respectively). Data are expressed as the mean percent positive cells from the population of all T helper cell (CD3+ CD4+) ± 1 standard deviation, and statistical differences are indicated in the figures by asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001, and ****, P < 0.0001).
TABLE 1.
Antibodies used for ZIKV NS1-specific T cell response
| Ab | Fluorochrome | Clone | Concn (mg/ml) | Catalog no. | Company |
|---|---|---|---|---|---|
| Anti-CD3 | Alexa Fluor 700 | 17A2 | 0.5 | 100216 | BioLegend |
| Anti-CD4 | Alexa Fluor 750 | GK1.5 | 0.2 | 100460 | BioLegend |
| Anti-IFN-γ | Alexa Fluor 488 | XMG1.2 | 0.5 | 505813 | BioLegend |
| Anti-TNF-α | PerCP Cy5.5 | MP6-XT22 | 0.2 | 506322 | BioLegend |
| Anti-IL-5 | PE | TRFK5 | 0.2 | 504304 | BioLegend |
| Anti-IL-21 | Alexa Fluor 647 | IC594R | 0.2 | 1506741 | R&D Systems |
| Anti-IL-10 | PE-Cy7 | JES5-16E3 | 0.2 | 505026 | BioLegend |
| Anti-IL-17A | Brilliant Violet 650 | TC11-18H10.1 | 0.5 | 506929 | BioLegend |
| Anti-IL-4 | Brilliant Violet 605 | 11B11 | 0.2 | 504125 | BioLegend |
Measurement of viral burden.
At the indicated time points after ZIKV challenge, blood was collected, and organs were recovered. Organs were weighed and homogenized using a bead-beater apparatus (MagNA Lyser; Roche). The total RNA was extracted from tissue samples and blood by using TRIzol Reagent (Life Technologies, Carlsbad, CA). Reverse transcription (RT) was conducted using a primer (5′-CTCGTCTCTTCTTCTCCTTCCTAGCATTGA-3′) targeting the E gene of ZIKV and the Superscript III transcriptase kit (Invitrogen, Carlsbad, CA). The RT products were then used to perform real-time PCR using primers specifically targeting the capsid gene of ZIKV (forward, 5′-CATCAGGATGGTCTTGGCGATTCTAGC-3′; reverse, 5′-CTCGTCTCTTCTTCTCCTTCCTAGCATTGA-3′) in a StepOne real-time PCR system (Applied Biosystems). A standard curve was generated using a serial dilution of ZIKV RNA extracted from known PFU titer of infectious virus. The amplification cycles used were as follows: 2 min at 50°C, 10 min at 95°C, and 40 cycles of 15 s at 95°C and 1 min at 60°C. The threshold for detection of fluorescence above the background was set within the exponential phase of the amplification curves. For each assay, 10-fold dilutions of standard viral RNA were generated, and negative-control samples and double-distilled water (ddH2O) were included in each assay. Viral burden is expressed on a log10 scale as viral PFU equivalents per gram or per milliliter.
Quantitative and statistical analyses.
Quantitative analysis was performed either by densitometric scanning of autoradiographs or by using a phosphorimager (Typhoon; GE Healthcare) and ImageQuant TL software (GE Healthcare, Piscataway, NJ). Statistical analysis was performed by one-way multiple comparisons using SPSS 8.0 statistical analysis software (SPSS, Inc., Chicago, IL). A P value of ≤0.05 was considered statistically significant.
ACKNOWLEDGMENTS
This study was supported by internal funds from The Ohio State University (J.L. and P.N.B.) and Nationwide Children’s Hospital (M.E.P.) and grants from the National Institutes of Health (NIH; P01 AI112524 to M.E.P. and J.L. and R01 AI090060 to J.L.). Work in S.-L.L.’s lab was supported by NIH grants R01 AI112381, R01 AI150473, and R21 AI109464. Work in P.N.B.'s lab was supported by NIH grant R01 DK101323.
REFERENCES
- 1.Brasil P, Pereira JP, Moreira ME, Ribeiro Nogueira RM, Damasceno L, Wakimoto M, Rabello RS, Valderramos SG, Halai U-A, Salles TS, Zin AA, Horovitz D, Daltro P, Boechat M, Raja Gabaglia C, Carvalho de Sequeira P, Pilotto JH, Medialdea-Carrera R, Cotrim da Cunha D, Abreu de Carvalho LM, Pone M, Machado Siqueira A, Calvet GA, Rodrigues Baião AE, Neves ES, Nassar de Carvalho PR, Hasue RH, Marschik PB, Einspieler C, Janzen C, Cherry JD, Bispo de Filippis AM, Nielsen-Saines K. 2016. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med 375:2321–2334. doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao-Lormeau V-M, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial A-L, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra J-C, Despres P, Fournier E, Mallet H-P, Musso D, Fontanet A, Neil J, Ghawché F. 2016. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers TJ, Hahn CS, Galler R, Rice CM. 1990. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol 44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 4.Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, Shi J, Lok SM. 2016. Structure of the thermally stable Zika virus. Nature 533:425–428. doi: 10.1038/nature17994. [DOI] [PubMed] [Google Scholar]
- 5.Diamond MS, Ledgerwood JE, Pierson TC. 2019. Zika virus vaccine development: progress in the face of new challenges. Annu Rev Med 70:121–135. doi: 10.1146/annurev-med-040717-051127. [DOI] [PubMed] [Google Scholar]
- 6.Richner JM, Diamond MS. 2018. Zika virus vaccines: immune response, current status, and future challenges. Curr Opin Immunol 53:130–136. doi: 10.1016/j.coi.2018.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shan C, Xie X, Shi PY. 2018. Zika virus vaccine: progress and challenges. Cell Host Microbe 24:12–17. doi: 10.1016/j.chom.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, Nachbagauer R, Foster GA, Krysztof D, Tortorella D, Stramer SL, Garcia-Sastre A, Krammer F, Lim JK. 2017. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 356:175–180. doi: 10.1126/science.aal4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton G. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, Foglierini M, Pedotti M, Simonelli L, Dowall S, Atkinson B, Percivalle E, Simmons CP, Varani L, Blum J, Baldanti F, Cameroni E, Hewson R, Harris E, Lanzavecchia A, Sallusto F, Corti D. 2016. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 11.Muller DA, Young PR. 2013. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis, and application as a diagnostic biomarker. Antiviral Res 98:192–208. doi: 10.1016/j.antiviral.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Watterson D, Modhiran N, Young PR. 2016. The many faces of the flavivirus NS1 protein offer a multitude of options for inhibitor design. Antiviral Res 130:7–18. doi: 10.1016/j.antiviral.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Glasner DR, Puerta-Guardo H, Beatty PR, Harris E. 2018. The good, the bad, and the shocking: the multiple roles of dengue virus nonstructural protein 1 in protection and pathogenesis. Annu Rev Virol 5:227–253. doi: 10.1146/annurev-virology-101416-041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puerta-Guardo H, Glasner DR, Espinosa DA, Biering SB, Patana M, Ratnasiri K, Wang C, Beatty PR, Harris E. 2019. Flavivirus NS1 triggers tissue-specific vascular endothelial dysfunction reflecting disease tropism. Cell Rep 26:1598–1613. doi: 10.1016/j.celrep.2019.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown WC, Akey DL, Konwerski JR, Tarrasch JT, Skiniotis G, Kuhn RJ, Smith JL. 2016. Extended surface for membrane association in Zika virus NS1 structure. Nat Struct Mol Biol 23:865–867. doi: 10.1038/nsmb.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutsche I, Coulibaly F, Voss JE, Salmon J, d’Alayer J, Ermonval M, Larquet E, Charneau P, Krey T, Mégret F, Guittet E, Rey FA, Flamand M. 2011. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc Natl Acad Sci U S A 108:8003–8008. doi: 10.1073/pnas.1017338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scaturro P, Cortese M, Chatel-Chaix L, Fischl W, Bartenschlager R. 2015. Dengue virus nonstructural protein 1 modulates infectious particle production via interaction with the structural proteins. PLoS Pathog 11:e1005277. doi: 10.1371/journal.ppat.1005277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa SM, Freire MS, Alves AM. 2006. DNA vaccine against the nonstructural 1 protein (NS1) of dengue 2 virus. Vaccine 24:4562–4564. doi: 10.1016/j.vaccine.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Wu SF, Liao CL, Lin YL, Yeh CT, Chen LK, Huang YF, Chou HY, Huang JL, Shaio MF, Sytwu HK. 2003. Evaluation of protective efficacy and immune mechanisms of using a nonstructural protein NS1 in DNA vaccine against dengue 2 virus in mice. Vaccine 21:3919–3929. doi: 10.1016/s0264-410x(03)00310-4. [DOI] [PubMed] [Google Scholar]
- 20.Hall RA, Brand TN, Lobigs M, Sangster MY, Howard MJ, Mackenzie JS. 1996. Protective immune responses to the E and NS1 proteins of Murray Valley encephalitis virus in hybrids of flavivirus-resistant mice. J Gen Virol 77:1287–1294. doi: 10.1099/0022-1317-77-6-1287. [DOI] [PubMed] [Google Scholar]
- 21.Whiteman MC, Li L, Wicker JA, Kinney RM, Huang C, Beasley DW, Chung KM, Diamond MS, Solomon T, Barrett AD. 2010. Development and characterization of non-glycosylated E and NS1 mutant viruses as a potential candidate vaccine for West Nile virus. Vaccine 28:1075–1083. doi: 10.1016/j.vaccine.2009.10.112. [DOI] [PubMed] [Google Scholar]
- 22.Chung KM, Nybakken GE, Thompson BS, Engle MJ, Marri A, Fremont DH, Diamond MS. 2006. Antibodies against West Nile Virus nonstructural protein NS1 prevent lethal infection through Fc gamma receptor-dependent and -independent mechanisms. J Virol 80:1340–1351. doi: 10.1128/JVI.80.3.1340-1351.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edeling MA, Diamond MS, Fremont DH. 2014. Structural basis of flavivirus NS1 assembly and antibody recognition. Proc Natl Acad Sci U S A 111:4285–4290. doi: 10.1073/pnas.1322036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hertz T, Beatty PR, MacMillen Z, Killingbeck SS, Wang C, Harris E. 2017. Antibody epitopes identified in critical regions of dengue virus nonstructural 1 protein in mouse vaccination and natural human infections. J Immunol 198:4025–4035. doi: 10.4049/jimmunol.1700029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brault AC, Domi A, McDonald EM, Talmi-Frank D, McCurley N, Basu R, Robinson HL, Hellerstein M, Duggal NK, Bowen RA, Guirakhoo F. 2017. A Zika vaccine targeting NS1 protein protects immunocompetent adult mice in a lethal challenge model. Sci Rep 7:14769. doi: 10.1038/s41598-017-15039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li A, Yu J, Lu M, Ma Y, Attia Z, Shan C, Xue M, Liang X, Craig K, Makadiya N, He JJ, Jennings R, Shi PY, Peeples ME, Liu SL, Boyaka PN, Li J. 2018. A Zika virus vaccine expressing premembrane-envelope-NS1 polyprotein. Nat Commun 9:3067. doi: 10.1038/s41467-018-05276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey MJ, Duehr J, Dulin H, Broecker F, Brown JA, Arumemi FO, Bermudez Gonzalez MC, Leyva-Grado VH, Evans MJ, Simon V, Lim JK, Krammer F, Hai R, Palese P, Tan GS. 2018. Human antibodies targeting Zika virus NS1 provide protection against disease in a mouse model. Nat Commun 9:4560. doi: 10.1038/s41467-018-07008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey MJ, Broecker F, Duehr J, Arumemi F, Krammer F, Palese P, Tan GS. 2019. Antibodies elicited by an NS1-based vaccine protect mice against Zika virus. mBio 10:e02861-18. doi: 10.1128/mBio.02861-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grubor-Bauk B, Wijesundara DK, Masavuli M, Abbink P, Peterson RL, Prow NA, Larocca RA, Mekonnen ZA, Shrestha A, Eyre NS, Beard MR, Gummow J, Carr J, Robertson SA, Hayball JD, Barouch DH, Gowans EJ. 2019. NS1 DNA vaccination protects against Zika infection through T cell-mediated immunity in immunocompetent mice. Sci Adv 5:eaax2388. doi: 10.1126/sciadv.aax2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bukreyev A, Skiadopoulos MH, Murphy BR, Collins PL. 2006. Nonsegmented negative-strand viruses as vaccine vectors. J Virol 80:10293–10306. doi: 10.1128/JVI.00919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whelan SP, Barr JN, Wertz GW. 2004. Transcription and replication of nonsegmented negative-strand RNA viruses. Curr Top Microbiol Immunol 283:61–119. doi: 10.1007/978-3-662-06099-5_3. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Fontaine-Rodriguez EC, Whelan SP. 2005. Amino acid residues within conserved domain VI of the vesicular stomatitis virus large polymerase protein essential for mRNA cap methyltransferase activity. J Virol 79:13373–13384. doi: 10.1128/JVI.79.21.13373-13384.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma Y, Wei Y, Zhang X, Zhang Y, Cai H, Zhu Y, Shilo K, Oglesbee M, Krakowka S, Whelan SP, Li J. 2014. mRNA cap methylation influences pathogenesis of vesicular stomatitis virus in vivo. J Virol 88:2913–2926. doi: 10.1128/JVI.03420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rana J, Slon Campos JL, Leccese G, Francolini M, Bestagno M, Poggianella M, Burrone OR. 2018. Role of capsid anchor in the morphogenesis of Zika virus. J Virol 92:e01174-18. doi: 10.1128/JVI.01174-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa SM, Paes MV, Barreto DF, Pinhao AT, Barth OM, Queiroz JL, Armoa GR, Freire MS, Alves AM. 2006. Protection against dengue type 2 virus induced in mice immunized with a DNA plasmid encoding the nonstructural 1 (NS1) gene fused to the tissue plasminogen activator signal sequence. Vaccine 24:195–205. doi: 10.1016/j.vaccine.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Z, Chan JF, Tee KM, Choi GK, Lau SK, Woo PC, Tse H, Yuen KY. 2016. Comparative genomic analysis of pre-epidemic and epidemic Zika virus strains for virological factors potentially associated with the rapidly expanding epidemic. Emerg Microbes Infect 5:e22. doi: 10.1038/emi.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Youn S, Cho H, Fremont DH, Diamond MS. 2010. A short N-terminal peptide motif on flavivirus nonstructural protein NS1 modulates cellular targeting and immune recognition. J Virol 84:9516–9532. doi: 10.1128/JVI.00775-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, Ciaramella G, Diamond MS. 2017. Modified mRNA vaccines protect against Zika virus infection. Cell 168:1114–1125. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, Gordon DN, Gallagher JR, Chen X, Todd JP, Tsybovsky Y, Harris A, Huang YS, Higgs S, Vanlandingham DL, Andersen H, Lewis MG, De La Barrera R, Eckels KH, Jarman RG, Nason MC, Barouch DH, Roederer M, Kong WP, Mascola JR, Pierson TC, Graham BS. 2016. Rapid development of a DNA vaccine for Zika virus. Science 354:237–240. doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larocca RA, Abbink P, Peron JPS, Zanotto PMDA, Iampietro MJ, Badamchi-Zadeh A, Boyd M, Ng’ang’a D, Kirilova M, Nityanandam R, Mercado NB, Li Z, Moseley ET, Bricault CA, Borducchi EN, Giglio PB, Jetton D, Neubauer G, Nkolola JP, Maxfield LF, De La Barrera RA, Jarman RG, Eckels KH, Michael NL, Thomas SJ, Barouch DH. 2016. Vaccine protection against Zika virus from Brazil. Nature 536:474–478. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. 2016. A mouse model of Zika virus pathogenesis. Cell Host Microbe 19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. 2016. Characterization of a novel murine model to study Zika virus. Am J Trop Med Hyg 94:1362–1369. doi: 10.4269/ajtmh.16-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinhoff U, Muller U, Schertler A, Hengartner H, Aguet M, Zinkernagel RM. 1995. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J Virol 69:2153–2158. doi: 10.1128/JVI.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Broek MF, Muller U, Huang S, Zinkernagel RM, Aguet M. 1995. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev 148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 45.Costa SM, Azevedo AS, Paes MV, Sarges FS, Freire MS, Alves AM. 2007. DNA vaccines against dengue virus based on the ns1 gene: the influence of different signal sequences on the protein expression and its correlation to the immune response elicited in mice. Virology 358:413–423. doi: 10.1016/j.virol.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 46.Lin YL, Chen LK, Liao CL, Yeh CT, Ma SH, Chen JL, Huang YL, Chen SS, Chiang HY. 1998. DNA immunization with Japanese encephalitis virus nonstructural protein NS1 elicits protective immunity in mice. J Virol 72:191–200. doi: 10.1128/JVI.72.1.191-200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlesinger JJ, Brandriss MW, Walsh EE. 1987. Protection of mice against dengue 2 virus encephalitis by immunization with the dengue 2 virus nonstructural glycoprotein NS1. J Gen Virol 68:853–857. doi: 10.1099/0022-1317-68-3-853. [DOI] [PubMed] [Google Scholar]
- 48.Beatty PR, Puerta-Guardo H, Killingbeck SS, Glasner DR, Hopkins K, Harris E. 2015. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 7:304ra141. doi: 10.1126/scitranslmed.aaa3787. [DOI] [PubMed] [Google Scholar]
- 49.Morrison TE, Diamond MS. 2017. Animal models of Zika virus infection, pathogenesis, and immunity. J Virol 91:e00009-17. doi: 10.1128/JVI.00009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miner JJ, Diamond MS. 2017. Zika virus pathogenesis and tissue tropism. Cell Host Microbe 21:134–142. doi: 10.1016/j.chom.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Qu L, Ye X, Yi C, Zheng X, Hao M, Su W, Yao Z, Chen P, Zhang S, Feng Y, Wang Q, Yan Q, Li P, Li H, Li F, Pan W, Niu X, Xu R, Feng L, Chen L. 2018. Incorporation of NS1 and prM/M are important to confer effective protection of adenovirus-vectored Zika virus vaccine carrying E protein. NPJ Vaccines 3:29. doi: 10.1038/s41541-018-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suder E, Furuyama W, Feldmann H, Marzi A, de Wit E. 2018. The vesicular stomatitis virus-based Ebola virus vaccine: from concept to clinical trials. Hum Vaccin Immunother 14:2107–2113. doi: 10.1080/21645515.2018.1473698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, Carroll MW, Doumbia M, Draguez B, Duraffour S, Enwere G, Grais R, Gunther S, Hossmann S, Kondé MK, Kone S, Kuisma E, Levine MM, Mandal S, Norheim G, Riveros X, Soumah A, Trelle S, Vicari AS, Watson CH, Kéïta S, Kieny MP, Røttingen J-A. 2015. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 386:857–866. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 54.Shan C, Xie X, Muruato AE, Rossi SL, Roundy CM, Azar SR, Yang Y, Tesh RB, Bourne N, Barrett AD, Vasilakis N, Weaver SC, Shi PY. 2016. An infectious cDNA clone of Zika virus to study viral virulence, mosquito transmission, and antiviral inhibitors. Cell Host Microbe 19:891–900. doi: 10.1016/j.chom.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whelan SP, Ball LA, Barr JN, Wertz GT. 1995. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci U S A 92:8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]