Abstract
A dentin biomodification strategy with selective proanthocyanidin (PAC)–enriched extracts reinforces dentin and dentin-resin interfaces. Enrichment of the extracts according to the degree of polymerization allows exploration of bioactive principles of PACs and structure-activity relationships. This study investigated the sustained dentin matrix biomodification and dentin-resin bioadhesion of 2 fractions consisting exclusively of B-type PAC dimers with or without a single galloyl motif (specifically, DIMERG and DIMERNG) and their precursor material, enriched grape seed extract (e-GSE; Vitis vinifera). The biomodification potential was determined by long-term evaluation of the apparent modulus of elasticity and collagen solubility (hydroxyproline release). Chemical characterization of the dentin matrix was performed by attenuated total reflectance–Fourier-transform infrared spectroscopy. The bioadhesive properties were assessed by a microtensile bond strength test at different time points, and macro-hybrid layers were produced to verify the degree of conversion of the adhesive resin. Fractions consisting of DIMERG, DIMERNG, and their precursor, e-GSE, increased the modulus of elasticity at all time points and reduced collagen degradation. Specimens treated with DIMERNG remained stable throughout 12 mo of storage, whereas a significant drop in the modulus of elasticity was observed for the DIMERG and e-GSE groups at 6 mo. The fractions and precursor did not affect the degree of resin conversion at the hybrid layer. Changes in infrared resonances corresponding to collagen cross-links in the dentin matrix occurred for all treatments. Higher bond strength was observed for dentin treated with e-GSE as compared with DIMERG and DIMERNG; all biointerfaces remained stable after 12 mo. Nongalloylated PACs mediate stable dentin biomodification, which includes protective activity against collagen degradation and reinforcement of the anchoring dentin matrix. Collectively, PACs with a higher degree of oligomerization offer a robust bioadhesion between the hydrophilic dentin matrix and the hydrophobic adhesive.
Keywords: extracellular matrix, biomodification, bioadhesion, biostability, grape seed extract, type I collagen
Introduction
Adhesion protocols in dentistry rely on the formation of micromechanical retention of a dental methacrylate resin infiltrate to enamel and dentin. In the case of dentin, effective infiltration of resin monomers within the dentin extracellular matrix (ECM), biostability of the underlying tissue, and low permeability of the polymerized resin system are key for the success of adhesive restorations (Navarra et al. 2012; Matuda et al. 2016; Silva Sousa et al. 2016; Cadenaro et al. 2019).
Bioinspired approaches to address pitfalls associated with unstable dentin-resin interfaces target the reinforcement of the dentin ECM and dentin-resin adhesion through biomodification by plant-derived proanthocyanidins (PACs; Bedran-Russo et al. 2008; Bedran-Russo et al. 2012; Bedran-Russo et al. 2014). Multiscale enhancement of the mechanical properties, biodegradability of dentin, and extended durability of dentin-resin adhesive interfaces were reported for PAC extracts, specifically from Vitis vinifera (Vv) grape seeds (Leme et al. 2015; Leme-Kraus et al. 2017; Aydin et al. 2019). Preliminary studies have indicated that the dentin biomodification potency is influenced by a rich structural diversity of PACs, such as the degree of polymerization (DP; Vidal, Leme, et al. 2014), galloylation (Vidal, Aguiar, et al. 2014), and the stereochemistry or positioning of the hydroxyl groups (Dong et al. 2013; Aydin et al. 2019). Recently, 18-mo analysis found that sources of galloylated PACs exhibited higher initial dentin biomodification; however, these effects were not as sustainable (Aydin et al. 2019).
The development of phytochemical separation protocols enables studies of isolated oligomeric PACs from crude extracts and their enrichment into fractions that contain PACs with a desired DP range. The chosen workflow involved the preparation of isolates from Vv with a combination of phytochemical separation methods, such as 2-phase solvent system partitioning and fast centrifugal partitioning chromatography, fraction profiling by HPLC and UHPLC (high- and ultrahigh-performance liquid chromatography, respectively), and structural analysis by NMR (nuclear magnetic resonance). The outcomes show that dimeric PACs from Vv can induce a 5.7-fold increase in the modulus of elasticity of the dentin matrix (Phansalkar et al. 2015). The phytochemical profiles are important fingerprints of the oligomeric PACs and can be used as parameters for the evaluation of their potential for dentin biomodification. Furthermore, it is essential to investigate the separation method and enrichment of the bioactives applied for dentin biomodification for the development of standardized materials, which are a prerequisite for biological and clinical reproducibility.
The primary goal of this study is to conduct an in-depth analysis of PAC structural variations, particularly the role of the galloylation of PACs with an identical DP, targeting their interactivity with the dentin ECM, the stability of the biomodified tissue, and their bioadhesive properties. Therefore, we investigated the effect of 2 exclusively dimeric fractions of B-type PACs—1 galloylated (DIMERG) and 1 nongalloylated (DIMERNG), obtained from highly bioactive Vv (grape seed) extract—on the sustained biomodification potential of the dentin ECM and the strength and stability of dentin–hydrophobic resin biointerfaces.
Materials and Methods
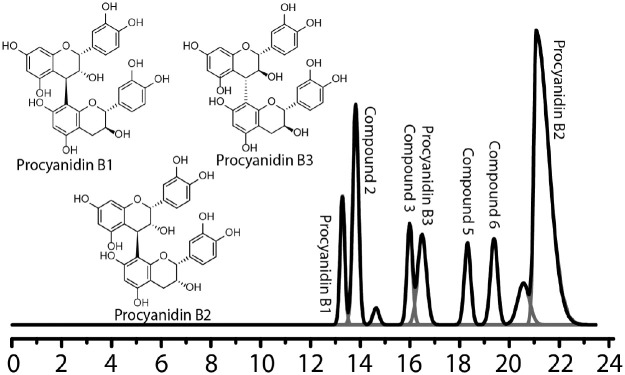
Preparation of dimeric fractions followed a fractionation method previously published (Phansalkar et al. 2015). Briefly, 2 dimeric fractions from an oligomeric enriched grape seed extract (e-GSE) from the crude extract of Vv seeds (MegaNatural Gold Grape Seed Extract, No. 206112508-01/122112505-01; Polyphenolics) were separated by fast centrifugal partitioning chromatography. HPLC-ultraviolet phytochemical profiles confirmed that fraction DIMERG consisted of monogalloylated dimers and that fraction DIMERNG consisted of nongalloylated dimers. The structures of major dimers present in both fractions are shown in Figure 1.
Figure 1.
Deconvoluted HPLC-UV chromatogram of fraction 4 generated in OriginPro. Peaks are labeled with the constituent dimers (Phansalkar et al. 2019). Structures of major dimeric PACs present in fractions DIMERG and DIMERNG. HPLC-UV, high-performance liquid chromatography–ultraviolet; PAC, proanthocyanidin.
Studies of the Dentin Matrix
Mechanical Properties and Biostability
The bulk apparent modulus of elasticity of the dentin matrix was assessed by a 3-point bending test with a maximum deformation of 3% (EZ Graph; Shimadzu), as described previously (Bedran-Russo et al. 2008). Briefly, extracted human third molars (Institutional Review Board protocol No. 2011-0312) were sectioned (Isomet 1000; Buehler) into dentin specimens (0.5 × 1.7 × 6.0 mm, thickness × width × length) and demineralized (10% phosphoric acid for 5 h). DIMERG, DIMERNG, and e-GSE were assessed at 6.5% w/v (pH 7.2) in 20mM Hepes. Each specimen (n = 15 per group) was immersed in 100 µL of the solution for 1 h under agitation at 25 °C. The apparent modulus of elasticity was assessed immediately after treatment and at 6- and 12-mo storage in simulated body fluid (SBF; 5mM Hepes, 2.5mM CaCl2, 0.05mM ZnCl2, 0.3mM NaN3) at 37 °C. Data were statistically analyzed by 2-way analysis of variance (ANOVA) and Games-Howell test (α = 0.05).
Collagen solubilization by endogenous proteases was estimated by the amount of solubilized hydroxyproline (HYP) in the storage media (SBF; Leme-Kraus et al. 2017). The detailed method is described in the Appendix. Briefly, SBF collected every 2 wk (n = 15) was pooled into 2 time points: 0 to 6 mo and 7 to 12 mo. Aliquots were hydrolyzed and mixed with 0.056M chloramine T reagent (25 min), and 1M Ehrlich’s reagent was used to develop color (60 °C for 40 min). Absorbance was measured at a 550-nm wavelength (Spectramax Plus; Molecular Devices). Reference values for HYP concentrations (0.5, 1, 2, 3, 4, and 5 µg/mL; Appendix Fig. 1) were used to produce a standard curve. Specimens were prepared in duplicate and analyzed with 2-way ANOVA and Tukey’s post hoc test (α = 0.05).
Chemical Characterization of the Biomodified Dentin
Chemical characterization of the biomodified dentin matrix was performed by Fourier-transform infrared spectroscopy (FTIR; Nicolet 6700, Thermo Fisher Scientific), equipped with an attenuated total reflectance (ATR) apparatus. Spectra were collected between 4,500 cm−1 to 600 cm−1 with a mean 100 scans per sample and at a resolution of 4.0 cm−1 (n = 3). Data were recorded and analyzed with OMNIC Spectra Software (Thermo Fisher Scientific).
The areas under the resonance at 1,630 cm−1 (amide I), 1,550 cm−1 (amide II), 1,240 cm−1 (amide III), and 1,450 cm−1 (CH2 scissoring) were obtained following a 2-point baseline correction, normalization, and peak area integration. Structural variations in the collagen triple helix were investigated by the ratio between amide III and CH2 scissoring (A1240/A1450) and the degree of cross-linking by the ratio between amide II and CH2 scissoring (A1550/A1450; Gordon et al. 1974; Sylvester et al. 1989; Madhan et al. 2005; Liu, Dusevich, and Wang 2014; Liu, Yao, et al. 2014; Liu et al. 2015; Du et al. 2018). Comparisons among DIMERG, DIMERNG, e-GSE, and control for each ratio were analyzed by 1-way ANOVA and Tukey’s post hoc test (α = 0.05).
Studies of Dentin-Resin Interface
Dentin-Resin Bioadhesion
The dentin-resin bioadhesive properties of DIMERG and DIMERNG were compared with their precursor, e-GSE, through a standard microtensile bond strength (TBS) test with an experimental hydrophobic adhesive resin (H0; Silva Sousa et al. 2016; Leme-Kraus et al. 2017). Adhesive composition and detailed method description are available in the Appendix. Briefly, occlusal dentin surfaces (n = 7 per group) were etched (35% phosphoric acid solution), and primer was applied for 1 min (DIMERG, DIMERNG, and e-GSE prepared at 15% w/v) and rinsed. H0 adhesive was then applied and light cured for 40 s (Optilux 501; Kerr Dental), followed by incremental placement of resin composite buildup. The same protocol was followed for the control group, except there was no primer. After 24 h at 37 °C in SBF, interfaces were serially sectioned, and dentin-resin specimens were tested in tensile at 24 h and 6 and 12 mo (microtensile tester; Bisco). Data were statistically analyzed with 2-way ANOVA and Tukey’s post hoc tests (α = 0.05). Pearson’s correlation tests with a 1-tailed significance level of 0.05 were performed to investigate correlations between TBS and dentin apparent modulus of elasticity and between TBS and HYP release. Representative fractured specimens (Appendix Fig. 2) were observed under scanning electron microscopy (Hitachi Ltd.).
Adhesive Resin Degree of Conversion in a Macro-Hybrid Layer Model
Macro-hybrid layers (Chiaraputt et al. 2008; Matuda et al. 2016) were prepared, and the degree of conversion (Cadenaro et al. 2009; Malacarne-Zanon et al. 2009; Parthasarathy et al. 2012) of the adhesive system at the hybrid layer was assessed, as described in the Appendix. Briefly, dentin specimens were demineralized and treated for 1 h with biomodification primers (DIMERG, DIMERNG, and e-GSE) and control (Hepes). Each specimen (n = 5 per group) was dehydrated in ascending ethanol/water concentrations and infiltrated with H0 resin (Matuda et al. 2016). Specimens were light cured (60 s) on the top and bottom surfaces (Optilux 501) and polished with SiC paper grits 600, 800, and 1,200 with a water-free silicon (Silicon Oil; Aldrich). Infrared spectra of the uncured and cured macrohybrid specimens were obtained with a FTIR-ATR (Nicolet 6700; Thermo Fisher Scientific), and the degree of conversion was calculated with the following formula: degree of conversion (%) = [1 – (R cured / R uncured)] × 100, where R is the ratio between the aliphatic C=C and aromatic C=C (constant) peaks, respectively at 1,638 cm−1 and 1,608 cm−1 of the cured and uncured resin. Data were statistically analyzed by 1-way ANOVA and Tukey’s post hoc (α = 0.05).
Results
Studies of Dentin Matrix
Mechanical Properties and Biodegradation
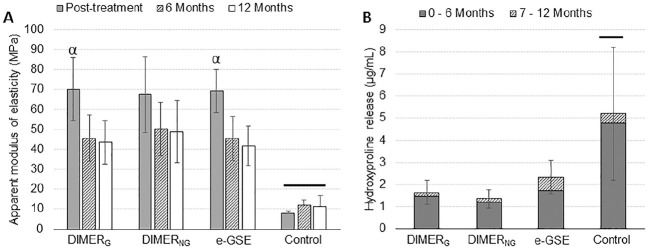
Figure 2A shows the results of the apparent modulus of elasticity. There were significant interactions between the studied factors (time and dentin treatment, P < 0.001). Statistically significant differences were found among treatments, with a higher modulus of elasticity for the e-GSE, DIMERG, and DIMERNG groups when compared with control at all time points (P < 0.001). A significant decrease in modulus of elasticity occurred at 6 mo for dentin treated with DIMERG (P < 0.001) and e-GSE (P < 0.001). The DIMERNG group remained stable after 6 mo in SBF (P = 0.056). There were no significant differences in the modulus of elasticity of specimens treated with DIMERG (P = 0.0894), DIMERNG (P = 0.973), and e-GSE (P = 0.630) at 6 and 12 mo.
Figure 2.
Studies of dentin matrix’s apparent modulus of elasticity (E) and total hydroxyproline released in the media. (A) The apparent modulus of elasticity of groups DIMERG, DIMERNG, and e-GSE immediately after treatment and after 6 and 12 mo of storage. (B) Cumulative hydroxyproline release after 12 mo of storage. Symbol (α) is used to show significant differences between posttreatment and 6 mo for DIMERG (P < 0.001) and e-GSE (P < 0.001); bars depict differences in apparent modulus of elasticity and hydroxyproline release between control (Hepes) and DIMERG, DIMERNG, and e-GSE (P < 0.001). Error bars depict standard deviation. e-GSE, enriched oligomeric grape seed extract.
Results of cumulative HYP release over the 12-mo aging period (Fig. 2B) showed significantly lower HYP (1.7- to 2.6-fold lower) released from dentin matrices treated with e-GSE, DIMERG, and DIMERNG when compared with the control group (P < 0.001). There were no significant differences among e-GSE, DIMERG, and DIMERNG (P > 0.05).
Chemical Characterization of the Biomodified Dentin
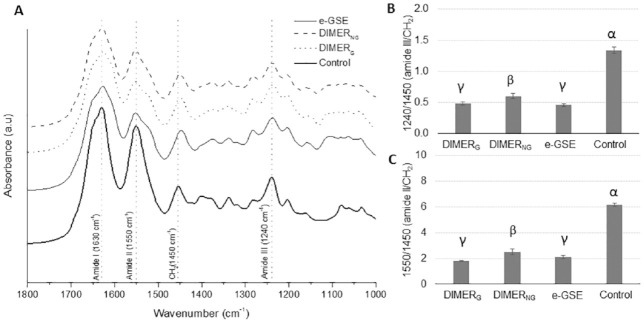
The FTIR spectra of the dentin matrix biomodified with DIMERG, DIMERNG, e-GSE, and control are shown in Figure 3A. All spectra showed resonances that are characteristic of type I collagen, such as those of the stretching vibrations of the C=O groups associated with amide I (~1,630 cm−1), the presence of mixed C-N stretch and in-plane bend of N-H vibrations and CH2 bending as amide II (~1,550 cm−1), N-H bending and C-N stretching vibrations with contributions from the C=O in-plane bending, the C-Cα stretching vibration for amide III (~1,240 cm−1), and scissoring mode vibrations of CH2 bonds (~1,450 cm−1). The lowest A1240/A1450 ratio was observed for DIMERG and e-GSE treatment, followed by DIMERNG treatment, when compared with control (P < 0.001; Fig. 3B). A significant decrease in the A1550/A1450 ratio (Fig. 3C) was observed in the DIMERG, DIMERNG, and e-GSE groups when compared with the control group (P < 0.001). The bands in ~1,630 cm−1 and ~1,552 cm−1 for amide I and amide II, respectively, showed a slight shift for the DIMERG and e-GSE groups (~1,625 to 1,627 cm−1). The ~1,240-cm−1 band (amide III) did not shift among groups, although the intensity of this band was lower for PAC-treated groups as compared with control.
Figure 3.
FTIR chemical characterization dentin collagen. (A) FTIR spectra of collagen and collagen biomodified by DIMERG, DIMERNG, e-GSE, where peaks assigned to collagen are depicted by the dashed lines. Ratios calculated from the areas under the peak assigned to (B) amide III over CH2 scissoring (1,240/1,450 cm−1) and (C) amide II over CH2 (1,550/1,450 cm−1). Symbols (α, β, γ) depict statistically significant differences between groups (P < 0.05). Error bars depict standard deviation. e-GSE, enriched oligomeric grape seed extract; FTIR, Fourier-transform infrared spectroscopy.
Dentin-Resin Interface
Studies of Dentin-Resin Bioadhesion
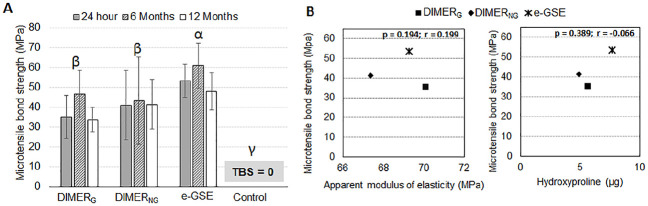
The results of TBS are depicted in Figure 4A. No interactions were observed between the factors time and dentin treatment (P = 0.737). The e-GSE group exhibited significantly higher bond strength than both DIMERG (P = 0.001) and DIMERNG (P = 0.015) groups. No statistically significant difference was observed between the DIMERG and DIMERNG groups (P = 0.670). Aging in artificial saliva up to 12 mo did not affect the TBS of the experimental groups (P = 0.378), while no specimens remained bonded in the control group (TBS = 0). No significant correlation was observed between TBS and the apparent modulus of elasticity (P = 0.194, r = 0.199) or between TBS and HYP release (P = 0.389, r = −0.066), as shown in Figure 4B. Subtle differences in fracture pattern among groups were compatible with the bioadhesive performance (Appendix Fig. 2).
Figure 4.
Bioadhesive properties of the dentin-resin interface and correlation studies. (A) TBS of the experimental groups. All priming solutions resulted in stable adhesion after 24-h, 6-mo, and 12-mo aging in SBF (P > 0.05). Solid bar depicts statistically significant difference between groups e-GSE, DIMERG, and DIMERNG (P < 0.05). (B) Graphs showing that there was no statistically significant correlation between TBS and apparent modulus of elasticity (P = 0.194) or TBS and hydroxyproline release (P = 0.389). Error bars depict standard deviation. e-GSE, enriched oligomeric grape seed extract; SBF, simulated body fluid; TBS, microtensile bond strength.
Adhesive Resin Degree of Conversion in a Macro-Hybrid Layer Model
The degrees of conversion of the adhesive resin in the macro-hybrid layers were as follows: 72.2% ± 4.2% for DIMERG, 73.8% ± 4.2% for DIMERNG, and 72.9% ± 1.6% for e-GSE treatment. The degree of conversion of the experimental resin within the macrohybrid layer was in the range of 74.3% ± 5.0%. There were no statistically significant differences in the resin degree of conversion among control, DIMERG, DIMERNG, and e-GSE (P = 0.715).
Discussion
The study revealed key PAC properties that contribute to a strong and stable bioadhesion between resin-based materials and the sustained interaction with the dentin matrix. The dimeric fractions increased the dentin matrix’s modulus of elasticity and prevented biodegradation in the same manner as their precursor material, e-GSE. Long-term evaluation of the biomodified dentin matrix’s biomechanics revealed that biomodification with galloylated PACs is less durable, targeting the roles of the galloyl moieties in terms of both reactivity and stability of the biomodified dentin matrix. The studies also revealed that dentin matrices treated with e-GSE, composed of PACs with DP up to 7, show stronger adhesion as compared with treatment with the dimeric fractions DIMERG and DIMERNG. The FTIR-based chemical profiling of the biomodified dentin matrix revealed changes in peak intensities mediated by PAC interactions with type I collagen. There was no correlation between dentin-resin bioadhesion and the outcomes of dentin ECM biomechanics and biostability (Fig. 4B), leading to the conclusion that dimeric fractions and their precursor, e-GSE, exert different mechanisms of biomodification associated with improvement of the dentin-resin biointerface.
Dentin biomodification with DIMERG, DIMERNG, and e-GSE increased the apparent modulus of elasticity of the dentin matrix (Fig. 2A) up to 7-fold. However, in both e-GSE and DIMERG, an initial drop in the modulus of elasticity of the dentin matrix was observed at the 6-mo time point, with modulus of elasticity stabilization for the remaining time. While the galloylated PAC fraction elicited strong immediate bioactivity, it did not sustain the dentin biomodification as compared with nongalloylated PACs (DIMERNG). The high biomodification potency of galloylated compounds was first reported for monomeric galloylated compounds (EGCG), where a direct correlation was observed between potency and the number of phenolic hydroxyl (–OH) groups (Vidal, Leme, et al. 2014). However, in subsequent studies, the monomeric nongalloylated counterpart (EGC) did not enhance the mechanical properties, thus limiting any further relationships. The present findings show that the phenolic –OH groups of the galloyl motif increase the compound’s reactivity and hydrophilicity (Iglesias et al. 2010; Vidal, Leme, et al. 2014; Aydin et al. 2019); however, the sustainability of the bioactivity was found to be diminished, which is plausible considering that the galloyl motif is prone to hydrolysis of the ester bond linkage (Lee et al. 2010; Krook and Hagerman 2012).
While higher release of HYP was observed for the e-GSE group from 7 to 12 mo, there were no significant differences in the cumulative HYP release among DIMERG, DIMERNG, and e-GSE over 12 mo. All treatments resulted in statistically lower HYP release when compared with the control group (Fig. 2B). Both dimeric fractions were able to reduce collagen degradation, meaning that smaller molecular weight compounds are effective in preventing collagen degradation (Vidal, Leme, et al. 2014). All biomodification agents induced cross-links within the different levels of hierarchy of the dentin matrix, likely reinforcing and hiding specific binding sites of endogenous proteases in the type I collagen (Vidal, Leme, et al. 2014). Additionally, PACs with lower molecular weight could aid by improving the wettability and infiltration of the biomodification solution within the dentin ECM (Jenkins et al. 2013). Regardless of the treatment, higher amounts of HYP were found in the storage solution during the first 6 mo of storage, likely due to a higher initial activity of endogenous proteases.
PAC primers did not induce secondary structural changes to type I collagen, as depicted by lack of shifts in ~1,240 cm−1 (amide III). However, lower intensity was observed for all PAC-modified groups (ratio A1240/A1450), which can be attributed to spectral traces of treatment reagents (Liu, Dusevich, and Wang 2014). e-GSE, DIMERNG, and DIMERG induced cross-linking in the dentin matrix, as evidenced by the decrease in the ratio between the amide II and CH2 scissoring bands (A1550/A1450), resulting in a reduction in –NH2 and consequently the band amide II (Silva Junior et al. 2015; Oliveira et al. 2019). Specific for the dimeric fractions, the A1550/A1450 ratio was significantly different from each other probably due to the higher reactivity of the galloylated compounds present in DIMERG when compared with DIMERNG.
All PAC primers promoted robust bioadhesion (Fig. 4A). Notably, the control group had no adhesion with the same hydrophobic adhesive resin (H0). The amphiphilic nature of PACs is due to their hydrophilic polar hydroxyl groups, paired with the hydrophobic aromatic rings (Iglesias et al. 2010) to promote the adhesion between the hydrophobic resin (H0) and the dentin ECM (Leme-Kraus et al. 2017). Moreover, the adhesion mediated by the priming solutions DIMERG, DIMERNG, and e-GSE remained stable after 6 and 12 mo. Priming of the dentin matrix with a PAC-rich solution can overcome problems leading to long-term degradation of the dentin-resin interface (Leme-Kraus et al. 2017). Comparisons among the biomodification primers revealed that priming solutions consisting of DIMERG and DIMERNG did not accomplish the same adhesion strength as the precursor e-GSE. Gravimetrically, the fraction DIMERG accounted for 2.6% w/w of the e-GSE, whereas DIMERNG accounted for 6.4% w/w of the e-GSE (Phansalkar et al. 2015). The intermediary order oligomers present in e-GSE likely contributed to the bioadhesive properties (Leme-Kraus et al. 2017), as both the reducing and chelating capacities increase with the increased DP of PACs (Iglesias et al. 2010).
To exclude any possible influence of PACs bound to the dentin matrix on the polymerization of the adhesive system, a macromodel of the hybrid layer was used to determine the degree of conversion of the adhesive resin. The results showed that the application of a PAC primer before the infiltration of the dentin matrix by the adhesive system did not impair the polymerization of the adhesive resin. An earlier study found a low degree of conversion of a commercially available adhesive system containing galloylated monomers (EGCG; Du et al. 2012). In the present study, PACs were not incorporated into the adhesive resin blend; instead, their use as rinse-out primer enabled unbound PACs to be removed. This approach can reinforce and stabilize the anchoring ECM and underlying dentin (Leme et al. 2015) and minimize the risk of secondary caries adjacent to the restoration margins (Kim et al. 2017).
In conclusion, while the use of galloylated PACs for dentin biomodification results in high initial activity, the stability of the biomodified matrix is diminished as compared with nongalloylated PACs, likely due to hydrolysis of the ester bond linkage of 3-O-galloylated motif. All PAC-rich biomodification agents induced collagen cross-links in the dentin ECM. However, between the dimeric fractions, higher reactivity was depicted by the galloylated fraction, DIMERG. Galloylation did not affect the stability of dentin-resin adhesion of dimeric PACs. Higher-order (higher DP) oligomeric PACs, as in e-GSE, promoted stronger dentin-resin adhesion. Finally, the lack of correlation between enhanced dentin biomechanics concurrent with lower biodegradability and the poorer bioadhesive performance by PAC dimers indicated the diverse mechanisms of interactions with the dentin matrix and the importance in defining PACs–dentin structure activity relationships.
Author Contributions
A.A. Leme-Kraus, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; R.S. Phansalkar, M.C. dos Reis, B. Aydin, Y. Alania, contributed to data acquisition and analysis, critically revised the manuscript; A.B.S. Sousa, contributed to data acquisition, critically revised the manuscript; J. McAlpine, S.N. Chen, contributed to data analysis, critically revised the manuscript; G.F. Pauli, contributed to conception and data interpretation, critically revised the manuscript; A.K. Bedran-Russo, contributed to conception, design, data analysis and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034519892959 for Dimeric Proanthocyanidins on the Stability of Dentin and Adhesive Biointerfaces by A.A. Leme-Kraus, R.S. Phansalkar, M.C. dos Reis, B. Aydin, A.B.S. Sousa, Y. Alania, J. McAlpine, S.N. Chen, G.F. Pauli and A.K. Bedran-Russo in Journal of Dental Research
Acknowledgments
The authors thank Bisco Inc. for providing the experimental resin formulations.
Footnotes
A supplemental appendix to this article is available online.
This study was supported by National Institutes of Health / National Institute of Dental and Craniofacial Research research grant DE021040.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: A.K. Bedran-Russo  https://orcid.org/0000-0002-3670-9519
https://orcid.org/0000-0002-3670-9519
References
- Aydin B, Leme-Kraus AA, Vidal CMP, Aguiar TR, Phansalkar RS, Nam JW, McAlpine JB, Chen SN, Pauli GF, Bedran-Russo AK. 2019. Evidence to the role of interflavan linkages and galloylation of proanthocyanidins at sustaining long-term dentin biomodification. Dent Mater. 35(2):328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedran-Russo AK, Castellan CS, Shinohara MS, Hassan L, Antunes A. 2012. Characterization of biomodified dentin matrices for potential preventive and reparative therapies. Acta Biomater. 7(4):1735–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedran-Russo AK, Pashley DH, Agee K, Drummond JL, Miescke KJ. 2008. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J Biomed Mater Res B Appl Biomater. 86(2):330–334. [DOI] [PubMed] [Google Scholar]
- Bedran-Russo AK, Pauli GF, Chen SN, McAlpine J, Castellan CS, Phansalkar RS, Aguiar TR, Vidal CM, Napotilano JG, Nam JW, et al. 2014. Dentin biomodification: strategies, renewable resources and clinical applications. Dent Mater. 30(1):62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenaro M, Breschi L, Rueggeberg FA, Suchko M, Grodin E, Agee K, Di Lenarda R, Tay FR, Pashley DH. 2009. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dent Mater. 25(5):621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenaro M, Maravic T, Comba A, Mazzoni A, Fanfoni L, Hilton T, Ferracane J, Breschi L. 2019. The role of polymerization in adhesive dentistry. Dent Mater. 35(1):e1–e22. [DOI] [PubMed] [Google Scholar]
- Chiaraputt S, Mai S, Huffman BP, Kapur R, Agee KA, Yiu CK, Chan DC, Harnirattisai C, Arola DD, Rueggeberg FA, et al. 2008. Changes in resin-infiltrated dentin stiffness after water storage. J Dent Res. 87(7):655–660. [DOI] [PubMed] [Google Scholar]
- Dong XQ, Zou B, Zhang Y, Ge ZZ, Du J, Li CM. 2013. Preparation of a-type proanthocyanidin dimers from peanut skins and persimmon pulp and comparison of the antioxidant activity of a-type and b-type dimers. Fitoterapia. 91:128–139. [DOI] [PubMed] [Google Scholar]
- Du T, Niu X, Li Z, Li P, Feng Q, Fan Y. 2018. Crosslinking induces high mineralization of apatite minerals on collagen fibers. Int J Biol Macromol. 113:450–457. [DOI] [PubMed] [Google Scholar]
- Du X, Huang X, Huang C, Wang Y, Zhang Y. 2012. Epigallocatechin-3-gallate (EGCG) enhances the therapeutic activity of a dental adhesive. J Dent. 40(6):485–492. [DOI] [PubMed] [Google Scholar]
- Gordon PL, Huang C, Lord RC, Yannas IV. 1974. The far-infrared spectrum of collagen. Macromolecules. 7(6):954–956. [DOI] [PubMed] [Google Scholar]
- Iglesias J, Pazos M, Lois S, Medina I. 2010. Contribution of galloylation and polymerization to the antioxidant activity of polyphenols in fish lipid systems. J Agric Food Chem. 58(12):7423–7431. [DOI] [PubMed] [Google Scholar]
- Jenkins CL, Meredith HJ, Wilker JJ. 2013. Molecular weight effects upon the adhesive bonding of a mussel mimetic polymer. ACS Appl Mater Interfaces. 5(11):5091–5096. [DOI] [PubMed] [Google Scholar]
- Kim GE, Leme-Kraus AA, Phansalkar R, Viana G, Wu C, Chen SN, Pauli GF, Bedran-Russo A. 2017. Effect of bioactive primers on bacterial-induced secondary caries at the tooth-resin interface. Oper Dent. 42(2):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook MA, Hagerman AE. 2012. Stability of polyphenols epigallocatechin gallate and pentagalloyl glucose in a simulated digestive system. Food Res Int. 49(1):112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RJ, Lee VS, Tzen JT, Lee MR. 2010. Study of the release of gallic acid from (-)-epigallocatechin gallate in old oolong tea by mass spectrometry. Rapid Commun Mass Spectrom. 24(7):851–858. [DOI] [PubMed] [Google Scholar]
- Leme AA, Vidal CM, Hassan LS, Bedran-Russo AK. 2015. Potential role of surface wettability on the long-term stability of dentin bonds after surface biomodification. J Biomech. 48(10):2067–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leme-Kraus AA, Aydin B, Vidal CM, Phansalkar RM, Nam JW, McAlpine J, Pauli GF, Chen S, Bedran-Russo AK. 2017. Biostability of the proanthocyanidins-dentin complex and adhesion studies. J Dent Res. 96(4):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bai X, Li S, Liu Y, Keightley A, Wang Y. 2015. Molecular weight and galloylation affect grape seed extract constituents’ ability to cross-link dentin collagen in clinically relevant time. Dent Mater. 31(7):814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Dusevich V, Wang Y. 2014. Addition of grape seed extract renders phosphoric acid a collagen-stabilizing etchant. J Dent Res. 93(8):821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yao X, Liu YW, Wang Y. 2014. A Fourier transform infrared spectroscopy analysis of carious dentin from transparent zone to normal zone. Caries Res. 48(4):320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhan B, Subramanian V, Rao JR, Nair BU, Ramasami T. 2005. Stabilization of collagen using plant polyphenol: role of catechin. Int J Biol Macromol. 37(1–2):47–53. [DOI] [PubMed] [Google Scholar]
- Malacarne-Zanon J, Pashley DH, Agee KA, Foulger S, Alves MC, Breschi L, Cadenaro M, Garcia FP, Carrilho MR. 2009. Effects of ethanol addition on the water sorption/solubility and percent conversion of comonomers in model dental adhesives. Dent Mater. 25(10):1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuda LS, Marchi GM, Aguiar TR, Leme AA, Ambrosano GM, Bedran-Russo AK. 2016. Dental adhesives and strategies for displacement of water/solvents from collagen fibrils. Dent Mater. 32(6):723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra CO, Breschi L, Turco G, Diolosa M, Fontanive L, Manzoli L, Di Lenarda R, Cadenaro M. 2012. Degree of conversion of two-step etch-and-rinse adhesives: in situ micro-Raman analysis. J Dent. 40(9):711–717. [DOI] [PubMed] [Google Scholar]
- Oliveira PN, Montembault A, Sudre G, Alcouffe P, Marcon L, Gehan H, Lux F, Albespy K, Centis V, Campos D, et al. 2019. Self-crosslinked fibrous collagen/chitosan blends: processing, properties evaluation and monitoring of degradation by bi-fluorescence imaging. Int J Biol Macromol. 131:353–367. [DOI] [PubMed] [Google Scholar]
- Parthasarathy R, Misra A, Park J, Ye Q, Spencer P. 2012. Diffusion coefficients of water and leachables in methacrylate-based crosslinked polymers using absorption experiments. J Mater Sci Mater Med. 23(5):1157–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phansalkar RS, Nam JW, Chen SN, McAlpine JB, Napolitano JG, Leme A, Vidal CMP, Aguiar T, Bedran-Russo AK, Pauli GF. 2015. A galloylated dimeric proanthocyanidin from grape seed exhibits dentin biomodification potential. Fitoterapia. 101:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phansalkar RS, Nam JW, Leme-Kraus AA, Gan LS, Zhou B, McAlpine JB, Chen SN, Bedran-Russo AK, Pauli GF. 2019. Proanthocyanidin dimers and trimers from Vitis vinifera provide diverse structural motifs for the evaluation of dentin biomodification. J Nat Prod. 82(9):2387–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Junior ZS, Botta SB, Ana PA, Franca CM, Fernandes KP, Mesquita-Ferrari RA, Deana A, Bussadori SK. 2015. Effect of papain-based gel on type I collagen—spectroscopy applied for microstructural analysis. Sci Rep. 5:11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Sousa AB, Vidal CMP, Leme-Kraus AA, Pires-de-Souza FCP, Bedran-Russo AK. 2016. Experimental primers containing synthetic and natural compounds reduce enzymatic activity at the dentin-adhesive interface under cyclic loading. Dent Mater. 32(10):1248–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester MF, Yannas IV, Salzman EW, Forbes MJ. 1989. Collagen banded fibril structure and the collagen-platelet reaction. Thromb Res. 55(1):135–148. [DOI] [PubMed] [Google Scholar]
- Vidal CM, Aguiar TR, Phansalkar R, McAlpine JB, Napolitano JG, Chen SN, Araujo LS, Pauli GF, Bedran-Russo A. 2014. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 10(7):3288–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal CM, Leme AA, Aguiar TR, Phansalkar R, Nam JW, Bisson J, McAlpine JB, Chen SN, Pauli GF, Bedran-Russo A. 2014. Mimicking the hierarchical functions of dentin collagen cross-links with plant derived phenols and phenolic acids. Langmuir. 30(49):14887–14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034519892959 for Dimeric Proanthocyanidins on the Stability of Dentin and Adhesive Biointerfaces by A.A. Leme-Kraus, R.S. Phansalkar, M.C. dos Reis, B. Aydin, A.B.S. Sousa, Y. Alania, J. McAlpine, S.N. Chen, G.F. Pauli and A.K. Bedran-Russo in Journal of Dental Research