Version Changes
Revised. Amendments from Version 1
The manuscript has been updated in response to the reviewer’s helpful and insightful comments. The most important changes are that the figures have been redesigned and the emphasis on the head-orientation study reduced. The MR images have been updated to use a consistent set of slices, Figures 3 & 4 have been merged into a single figure, and the average within-subject CoV has been added. Figure 1 (the number of spokes) and Figure 6 (colour scheme) have been updated for clarity. We hope that these new figures are clearer and more intuitive than the previous figures. The language used to refer to the head orientation study has been clarified to refer to results as “highly statistically significant” rather than “strong”. A reviewer provided a plausible explanation for the negative values of ihMTR in CSF, namely the use of Fermi pulses in the preparation module, and this limitation has been discussed. A table with the mean ihMTR and inverse ihMTR values has been added. The discussion has been expanded to better set the context of the paper within existing literature, with better comparisons between our results and previous papers. We think the resulting paper is much improved and thank the reviewers again for their valued input.
Abstract
Background: Inhomogeneous Magnetization Transfer (ihMT) is an emerging, uniquely myelin-specific magnetic resonance imaging (MRI) contrast. Current ihMT acquisitions utilise fast Gradient Echo sequences which are among the most acoustically noisy MRI sequences, reducing patient comfort during acquisition. We sought to address this by modifying a near silent MRI sequence to include ihMT contrast.
Methods: A Magnetization Transfer preparation module was incorporated into a radial Zero Echo-Time sequence. Repeatability of the ihMT ratio and inverse ihMT ratio were assessed in a cohort of healthy subjects. We also investigated how head orientation affects ihMT across subjects, as a previous study in a single subject suggests this as a potential confound.
Results: We demonstrated that ihMT ratios comparable to existing, acoustically loud, implementations could be obtained with the silent sequence. We observed a small but significant effect of head orientation on inverse ihMTR.
Conclusions: Silent ihMT imaging is a comparable alternative to conventional, noisy, alternatives. For all future ihMT studies we recommend careful positioning of the subject within the scanner.
Keywords: ihMT, Silent MRI, Myelin, ZTE, RUFIS
Introduction
Myelin is a critical part of a healthy nervous system and hence visualising it in vivo is of great use to clinicians. Fortuitously, myelin displays multiple physical properties that give rise to contrast in Magnetic Resonance (MR) images. Tissue containing myelinated axons has lower longitudinal and transverse relaxation times 1, 2, lower susceptibility 3, increased Magnetization Transfer (MT) effects 4, and reduced diffusion 5, compared to non-myelinated tissue. However, while all of these MR parameters are sensitive to myelination, they are not specific, as other biological processes demonstrate the same effects 6. An additional MR-relevant property of myelin is that it is semi-crystalline in nature, being formed of closely packed proteins and lipids 7. This regular structure can maintain dipolar order, and recent work has shown that this can be exploited to produce inhomogeneous Magnetization Transfer (ihMT) contrast 8– 10. Although other substances and tissues such as muscle can exhibit ihMT 11, 12, it is possible to tune the acquisition parameters specifically to the properties of myelin 13. This, along with the fact that there are no other candidate substances that can exhibit ihMT within the central nervous system, suggests that ihMT has the potential to produce genuinely myelin-specific contrast 8.
Previous work has shown that within a single subject the ihMT effect exhibits a dependence on the orientation of axons to the magnetic field 10, 14, 15. This is attributable to the preferential alignment of myelin sheaths with the axons, leading to a non-uniform distribution of orientations of the lipids and proteins in the sheath 16, 17. As the orientation of WM to the magnetic field will also depend on the orientation of the head, this positioning of the patient within the scanner may influence ihMT metrics. To our knowledge, this effect of bulk orientation across subjects has not been directly investigated.
Recent ihMT imaging methods utilise fast gradient-echo sequences to acquire full-brain images in a reasonable time frame 18, 19. Such sequences are among the loudest MR sequences as they utilise a short Repetition Time (TR) and require rapid switching of high amplitude field gradients 20. Acoustic noise is a leading cause of discomfort for subjects during an MR examination 21, 22, and is of particular concern in paediatric and fetal MRI 23– 26.
It is possible to make 3D gradient echo acquisitions almost silent by swapping from the standard Cartesian to a radial zero echo-time (ZTE) acquisition scheme 27– 29, but due to the fixed (near zero) echo-time and RF amplifier limits it can be difficult to achieve strong tissue contrasts in such sequences 30, 31. In the current work we incorporate an MT preparation module into a radial ZTE sequence without compromising the acoustic noise level and show that myelin-weighted contrast can be achieved at the expense of only a small increase in scan time. The primary aim was to measure the repeatability of semi-quantitative ihMT and inverse ihMT ratios 14. As a secondary aim we hence investigated the effect of head orientation across subjects on the ihMT effect.
Methods
MR sequence
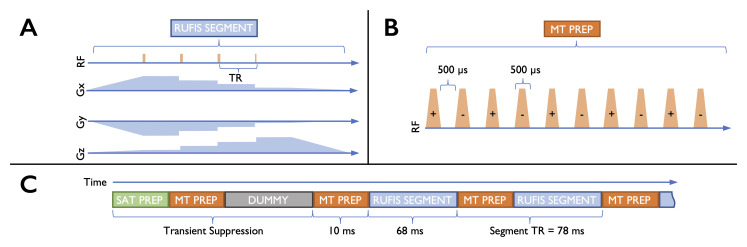
The Rotating Ultra-Fast Imaging Sequence (RUFIS) was originally introduced to image flowing liquids 27. It is essentially a gradient echo sequence, where each TR consists of a single RF pulse followed by a readout. The principal difference, illustrated in Figure 1, is that the readout gradient is held constant during the TR, including during the excitation pulse, and hence each readout consists of a ‘spoke’ that starts in the center of k-space and moves towards one edge. This is in contrast to a standard Cartesian sequence where the imaging gradients acquire a line from one side of k-space to the other.
Figure 1.
A - Sequence diagram for a RUFIS segment. The gradients are first ramped to a constant amplitude, and short hard RF pulses are used to avoid slab profile effects from the constant gradient magnitude. For clarity, only four spokes are illustrated in the segment, our acquisition had 32. B - The MT preparation block consists of multiple saturation pulses. To generate the ihMT effect the sign of the saturation frequency is alternated, for standard MT preparation all pulses would have the same sign. C - The overall sequence consists of RUFIS segments separated by MT preparation blocks. At the start of each image a saturation module nulls all signal, and dummy segments where no data is acquired are played until the steady-state magnetization is reached.
The innovations introduced in order to be robust to flow effects have numerous serendipitous effects, chiefly massively reduced acoustic noise levels compared to conventional MR imaging schemes 29. As shown in Figure 1A, the imaging gradients have constant magnitude and only their orientation is varied during the sequence in order to appropriately sample k-space. At the end of each TR the gradient direction is changed by a small step on each gradient channel. As there are no rapid or large gradient changes, which are the major source of acoustic noise, RUFIS acquisitions are extremely quiet.
Because Radio Frequency (RF) excitation occurs in the presence of the imaging gradients in RUFIS, high bandwidth excitation pulses are required to mitigate slab profile effects. In practice, very short hard pulses (on the order of 10µs) are required, which limits the range of available flip-angles to a few degrees due to RF amplifier and transmit coil limitations 30. This restricts a naïve RUFIS implementation to Proton-Density (PD) weighting 31. To circumvent this limitation, the sequence can be segmented, with preparation pulses played in between segments; this approach has previously been utilised for T2 32 and diffusion prepared RUFIS imaging 33.
MT is a common technique for generating contrast in tissues that have high fractions of non-aqueous hydrogen protons 4, 34. Such tissues include White Matter (WM) in the brain, and so MT has seen wide application in WM diseases 35, 36. The most common acquisition method is a Cartesian gradient echo sequence with an off-resonance saturation pulse added to every TR 37. It is not feasible to play a saturation pulse in every TR within RUFIS due to the constant presence of the imaging gradient. If a saturation pulse was played while this gradient was present, the effective frequency offset of the pulse would differ across the field of view. Instead, we added a train of saturation pulses, shown in Figure 1B as a preparation module before each segment.
The approach of prepared segmented MT imaging has already been demonstrated with a Cartesian readout 38, and can be tuned to produce an increased sensitivity to myelin by varying the width and spacing of the saturation pulses 12. Cartesian readouts can choose their k-space view order to preferentially weight the center of k−space to the MT effect. Because the center of k-space is sampled in every repetition in RUFIS, such view re-ordering is not possible. T1 recovery during a segment is hence a significant issue in RUFIS, as it will dilute any weighting from a preparation module. We therefore used a short segment length to minimise T1 recovery. This results in playing the preparation pulses more frequently, which lengthens scan time. However, MT preparation requires very little dead time compared to either T1 or T2 preparation, and so the overall increase in scan time is minimal.
To minimise any contamination from transient signals at the start of scanning or when switching between different MT preparation schemes, we adopted two complementary measures. The first was the addition of a saturation module played once at the start of each volume. This consisted of a single adiabatic 90 degree pulse and spoiler to effectively null all longitudinal magnetization. Following this module, 48 dummy segments were played, where the no data was acquired, to allow the signal in brain parenchyma to approach a steady-state. The 48 dummy segments lasted for 3.3 seconds, which is longer than the approximate T1 of parenchyma (around 1s) at 3T. The full sequence schematic is shown in Figure 1C.
Imaging study
We recruited 6 male and 6 female subjects (age range 25 to 54 years) through local advertisement within our research establishment (King’s College London). Subjects gave written informed consent in accordance with ethics approved by the King’s College London REC (approval number 04/Q0706/72), and standard MRI exclusion criteria were applied. Each subject had two imaging sessions, spaced approximately a week apart, in a 3 Tesla scanner using a 12-channel head coil (Discovery MR750, GE Healthcare). Each session consisted of two ihMT scans, for a total of four ihMT scans per subject. In addition to the ihMT data, to provide an anatomical reference, in the first imaging session a standard T1-weighted image was acquired using the ADNI-GO protocol 39. No special instructions were given to subjects as to their head positions in order to acquire a representative sample of orientations with respect to the main magnetic field.
The ihMT scans consisted of five images (see below). All volumes were acquired with the following parameters: 22cm field of view, 1.5mm isotropic voxel size, readout bandwidth ±25kHz, TR 1.764ms, spokes-per-segment 32. We used a 2° hard pulse for excitation. This was lengthened from the manufacturer default of 8µs to 24µs to lower the B1 amplitude and hence minimise any saturation of the bound pool from the excitation pulses. A train of 10 Fermi saturation pulses was played between each segment with pulse-width of 500µs and a 500µs gap in between. The pulses had a root-mean-square B1 of 8.75µT and an offset frequency of 7kHz. This corresponds to a root-mean-square B1 of 6.2µT over the course of the preparation module. The total RUFIS segment time and preparation time (including ramps and switching time) were 68.6ms and 10.8ms respectively. We did not add an explicit spoiling gradient after the pulse train, instead relying on the initial gradient ramp of the acquisition segment to spoil spuriously generated transverse magnetization from the MT pulses.
To isolate the ihMT contrast images acquired under both single-sided and dual-sided irradiation are required. The five volumes were acquired with the following scheme saturation scheme: +/-, -/+, none, +, -, where + or - refers to the sign of the saturation offset frequency. We refer to the volumes with only positive or negative saturation frequency as MT-weighted and those with dual-sided saturation as enhanced MT (eMT) weighted. Scan time per ihMT volume was 65 seconds, and 5 minutes 41 seconds for all five volumes. The acoustic noise was measured with an MR-compatible microphone (Casella CEL-63X, IDEAL Industries) located in the scanner bore.
Previous work has shown that ihMT ratio (ihMTR) is sensitive to confounds from B1 (RF transmit inhomogeneity) and T1-weighting, but this can be potentially mitigated through the use of an inverse ihMTR 14, and such inverse metrics have also been used to compensate for T1 effects in Chemical Exchange Saturation Transfer experiments 40. To calculate this we additionally acquired a volume with T1-weighting. Because it is difficult to achieve high flip-angles with the short block pulses in RUFIS 30, we opted to replace the ihMT preparation train with a single on-resonance 10ms 25° pulse. The on-resonance preparation pulse generates a large amount of unwanted transverse magnetization compared to the off-resonance MT pulses, and so we added a 10 cycles-per-voxel spoiler gradient which increased the preparation module time to 11.3ms. The spoiler gradient ramp time was lengthened to reduce acoustic noise to the level of the rest of the RUFIS sequence.
The RUFIS images were reconstructed using the manufacturer’s proprietary Orchestra toolbox (GE Healthcare). The reconstruction consisted of nearest-neighbour gridding on a twice-oversampled grid 41, k-space center filling 42, 43, and Total Generalised Variation (TGV) regularization 33, 44. The TGV regularisation parameter was set to λ = 0.01 with a maximum of 64 iterations.
Analysis
We first motion corrected the MT-weighted images with mcFLIRT 45. The MTR, eMTR, ihMTR, inverse ihMTR and MT-asymmetry were calculated using an open-source C++ program added to QUIT 46. MT-asymmetry is a measure of whether the absorption rate differs for positive or negative irradiation frequencies 47. The parameters were defined to match 14:
where S +, S −, S +/− and S −/+ refer to the signal from the saturation schemes defined above, and S T1 is the signal from the T1-prepared image.
We calculated an affine transform from the eMTR image to each subject’s standard T1-weighted image using ANTs 48. The eMTR image was selected because the contrast is broadly similar to the T1-weighted. We then constructed a study template from all subject’s T1-weighted images, and non-linearly registered the resulting image to the MNI atlas 49, 50. Analysis then proceeded in two complementary directions: first, for illustrative purposes, we resampled each subject’s MT metrics in MNI space, and second, for a quantitative region of interest (ROI) analysis we resampled the JHU WM atlas into the native space of each scanning session. To minimise the number of resampling operations all transforms between the MNI space and the MT-weighted native space were concatenated before application. Ten bilateral white matter tract ROIs were selected 51.
Mean average value within the ROI was calculated for each ROI at each of the 4 scans (2 repeats at 2 sessions) for MTR, eMTR, ihMTR and inverse ihMTR. Intra-class correlation coefficients (ICC) were calculated over the 4 measurements using the regularised mixed-effects method of 52. Specifically, two-way random ICC(2,1) values and 95% confidence intervals (bootstrap percentile method; 1000 resamples) were extracted from a random effects model with random effects of subject and scan. Regularisation of variance components was achieved via a weakly informative gamma prior (shape parameter 2, rate parameter 0.5) 52. Calculations were performed in R version 3.6.2 using the blme package version 1.0.4 53. ICC values were classified as poor if they were less than 0.5, moderate if between 0.5 and 0.75, good between 0.75 and 0.9, and excellent above 0.9 54.
Finally, we investigated how head orientation affects ihMTR. As a proxy for how each subject’s head was aligned with the main magnetic field, we calculated the angle between the Z-direction (head-foot axis) of the MT scan space and the MNI atlas. This was found by first concatenating the three affine transforms (MT- to T1-weighted, T1-weighted to study template, and study template to atlas), applying the concatenated transform to the vector Z = (0, 0, 1) and then calculating the dot product between the result and Z.
We only examined the effect of orientation on ihMTRinv, as this is expected to be robust against B1- and T1-effects 14, which may also have a spatial or angular dependence 55. To probe the impact of head orientation in the presence of potential between-participant differences, we calculated linear mixed effects models using blme as above. These models included mean ihMTRinv values from all 10 ROIs, main effects and interactions of head angle & ROI and both a random and fixed effect for each participant, with subject allowed to interact with ROI. Of prime interest was the average linear effect of head angle within-subject and whether this varied over the ROIs. Statistical significance was tested with ANOVA adjusted by the Kenwood-Roger procedure, with p<0.05 considered significant.
Results
Images
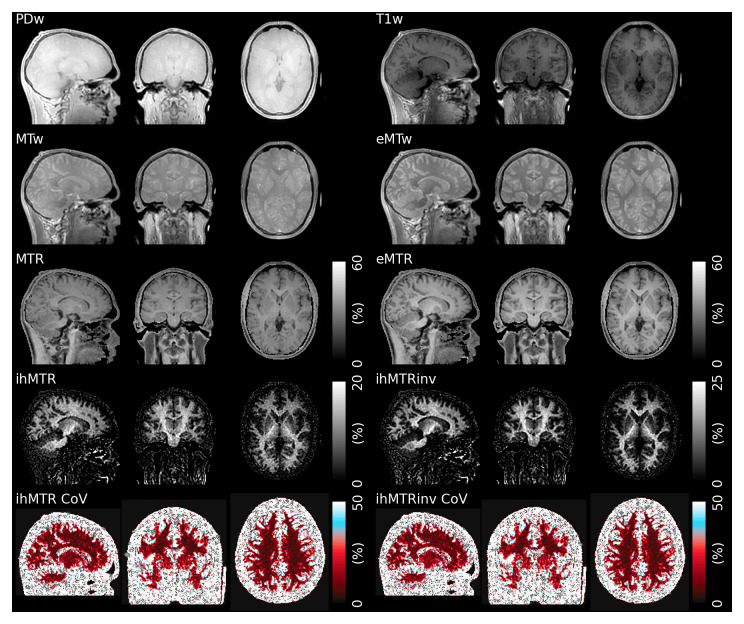
Figure 2 shows the acquired raw images and calculated MT ratios from a single subject. The acoustic noise was measured as 72 dB, compared to a 69 dB background level, which is similar to our previous work where comparable Cartesian sequences were approximately 30 dB louder 30.
Figure 2. Example raw weighted images (PD, T1, MT and eMT), ratio images (MTR, eMTR, ihMTR and ihMTRinv) and within-subject CoV for one subject.
The inverse ihMTR shows a subtly improved contrast between white and grey matter compared to ihMTR, particularly in the cerebellum and putamen (note the different color scale).
The eMT-weighted image in particular demonstrates good grey matter (GM)/WM contrast, while the MTR image exhibits little GM/WM contrast. The ihMTR, which is the difference between eMTR and MTR (with a scaling factor of two), hence also shows very good GM/WM contrast. It is very close to zero outside the brain, in contrast to both MTR and eMTR which are high in tissues outside the brain. The inverse ihMTR exhibits improved GM/WM contrast compared to ihMTR in the deep GM structures, such as the putamen, the cerebellum and reduced B1 inhomogeneity effects throughout WM.
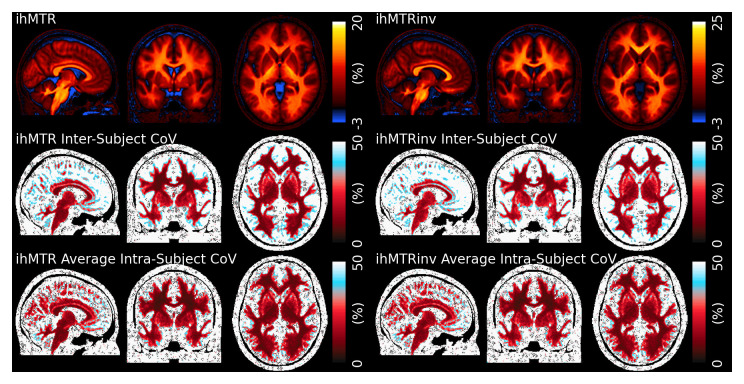
Figure 3 shows the mean, between-subject and average within-subject Coefficient of Variation (CoV) for ihMTR and inverse ihMTR in MNI space, while Table 1 summarises the average ihMTR and inverse ihMTR for the ROIs we examined. The ihMTR and inverse ihMTR values were, respectively, about 12% and 15% in WM, with values in tracts oriented parallel to the main magnetic field slightly higher as expected 15. Values were lower in GM, and we observed a small negative ihMTR in cerebral spinal fluid (CSF) and the eyeballs.
Figure 3. Top - ihMTR and ihMTRinv values averaged across all scans in MNI space.
An asymmetric color scale has been used to highlight the small negative of ihMTR in cerebral spinal fluid. Heightened values can be observed in WM tracts oritented parallel to B0. Middle – Between-subject CoV calculated across all scans and subjects. Bottom – Average within-subject CoV. The within-subject CoV is lower than the between-subject in the cortex.
Table 1. The mean and standard deviation of ihMTR and inverse ihMTR in the 10 selected ROIs, calculated across all subjects.
| ROI | ihMTR
Mean (%) |
ihMTR SD
(%) |
Inverse ihMTR
Mean (%) |
Inverse ihMTR SD
(%) |
|---|---|---|---|---|
| Genu of CC | 10.81 | 0.16 | 15.20 | 0.38 |
| Body of CC | 11.79 | 0.17 | 14.70 | 0.44 |
| Splenium of CC | 12.52 | 0.18 | 15.78 | 0.52 |
|
Corticospinal
tract |
15.18 | 0.08 | 18.52 | 0.58 |
|
Cerebral
peduncle |
13.89 | 0.17 | 15.14 | 0.48 |
| Internal Capsule | 12.67 | 0.22 | 15.47 | 0.53 |
| Corona Radiata | 12.67 | 0.16 | 16.54 | 0.40 |
|
Thalamic
Radiation |
12.17 | 0.19 | 15.67 | 0.48 |
|
Cingl. Cingulate
Gyrus |
10.82 | 0.15 | 13.30 | 0.40 |
|
Cingl.
Hippocampus |
10.49 | 0.41 | 10.49 | 0.76 |
The between-subject CoV was approximately 10% in WM, but approaches 50% in GM and reached over 100% in CSF. The average within-subject CoV was lower, at around 8% in WM and 30% in GM. The average value of inverse ihMTR is higher, at approximately 20%. The contrast in parenchyma is broadly similar to ihMTR, but there are subtle differences in deep GM, frontal WM and the brain stem and values in CSF are close to zero instead of negative. Both the between-subject and average within-subject CoV in WM is slightly higher than for ihMTR.
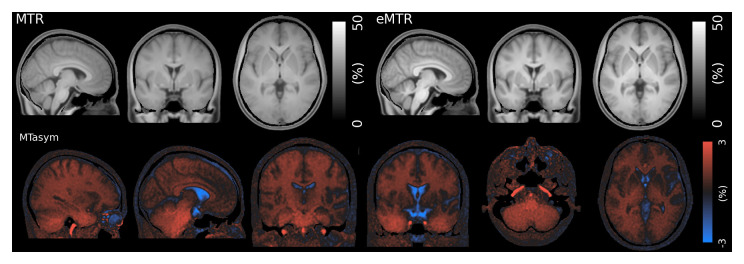
Figure 4 shows the mean MTR, eMTR and MT-asymmetry. The MTR image shows only limited contrast between WM and GM despite the high levels of saturation power, while the eMTR image shows the expected improved contrast. In contrast to ihMTR, non-zero MTR and eMTR can be observed in tissues outside the brain. We found a small consistently positive value of MT asymmetry in cerebral WM, which was increased in cerebellar WM and the major ascending arteries. MT asymmetry was negative in CSF and the eyeballs.
Figure 4. Mean MTR, eMTR and MT-Asymmetry in atlas space.
Elevated levels of MT asymmetry can be observed in the ascending arteries and cerebellum.
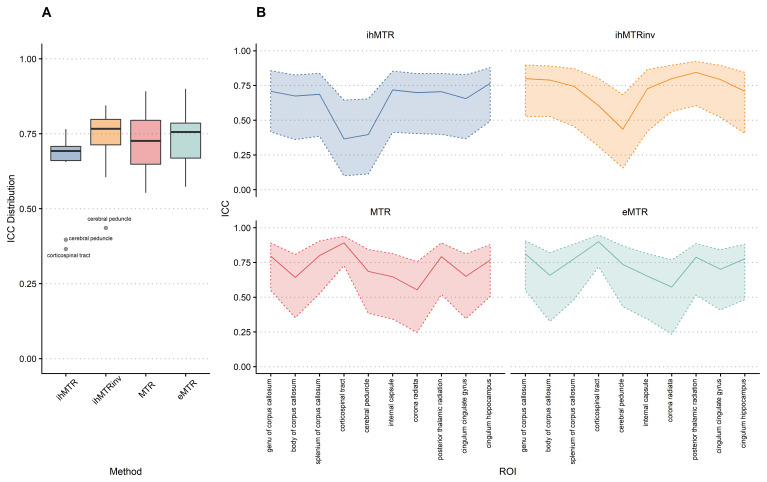
Reliability
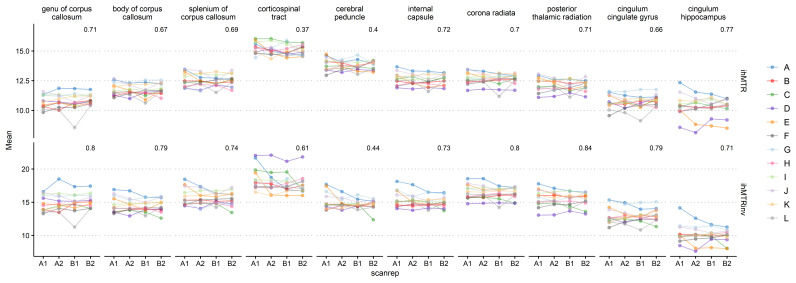
Figure 5 shows the obtained ICC values in the atlas ROIs. ICC values were moderate or good for all measures, except for the cerebral peduncles in both ihMTR and inverse ihMTR and corticospinal tract in ihMTR only, where the ICC values were poor. ICC values were slightly higher for inverse ihMTR compared to ihMTR. The cerebral peduncle and corticospinal tract ROIs commonly gave results atypical of the remaining ROIs. Figure 6 shows the mean values of ihMTR and inverse ihMTR for all ROIs across all four scans. Most ROIs show good reliability and repeatability 56, but there are several obvious outliers, for instance subject D in the corticospinal tract for inverse ihMTR, and subjects D and E for ihMTR in the cingulum hippocampus.
Figure 5.
A - Intra-class correlation coefficient (ICC) distribution (Tukey Boxplot). B - ICC (solid line) and 95% bootstrap confidence intervals (dashed lines and shaded area), displaying a profile over the regions of interest (ROIs).
Figure 6. Subject-level variability in ihMTR and inverse ihMTR for the 10 atlas regions of interest.
Head orientation
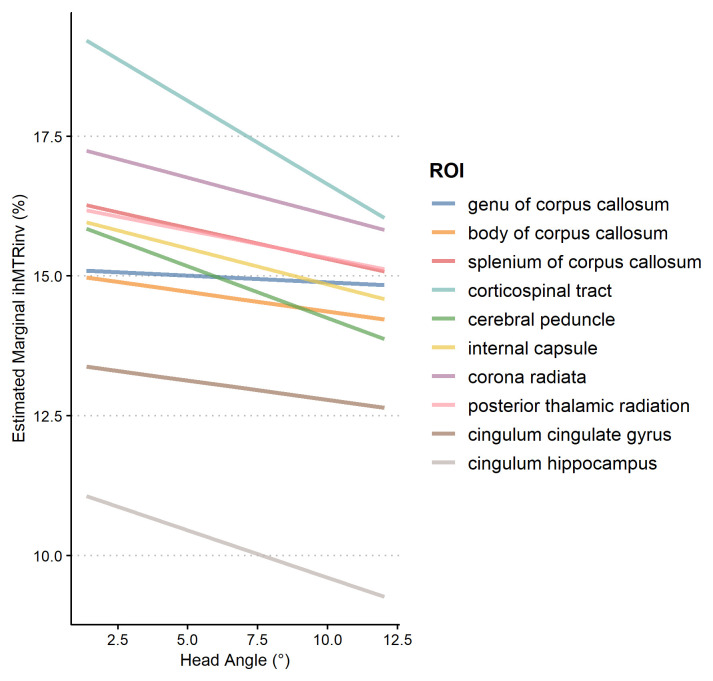
The observed median head orientation angle was 7° (lower quartile 3.2°, upper quartile 10.3°). Subject D showed particularly elevated values around 25° (excluding subject D the maximum observed angle was 12°). Closer examination revealed that subject D had a fairly small head and in both sessions was scanned with their head tilted back within the coil (data not shown). Our analysis revealed a significant main effect of head angle ( F = 27.7, p = 2.50 × 10 −7) on ihMTRinv, such that with increased rotation angle inverse ihMTR values were lower, illustrated in Figure 7. There was no significant interaction between angle and ROI ( F = 1.47, p = 0.15) indicating effects were relatively homogeneous over the ROIs. Because subject D (angle = 25°) could be interpreted as an outlier, we repeated the analysis with subject D excluded and results were comparable (main effect of angle: F = 37.24, p = 3.00 × 10 −9; angle ×ROI interaction: F = 1.32, p = 0.23).
Figure 7. Estimated effects of participant head angle× region of interest (ROI) for inverse ihMTR.
As the angle of the head increases, the value of inverse ihMTR tends to decrease.
Discussion
We have demonstrated full-brain 3D myelin-weighted ihMT images acquired with a silent and fast imaging sequence. The MT preparation module increases scan-time by a minimal amount and does not compromise the silent nature of RUFIS acquisition. ihMT has shown potential for assessing myelination in multiple sclerosis 57, 58, and the use of a silent sequence will extend this potential to noise intolerant patient cohorts, for instance non-sedated infants.
The single-sided saturation MT-weighted and MTR images showed fairly flat contrast between WM and GM. This is to be expected, as the 7 kHz frequency offset chosen is optimal for the ihMT effect, whereas a smaller offset, for example 2 kHz, would likely generate larger MT contrast. Although the eMTR image exhibits good WM/GM contrast, it also shows significant signal in other tissues outside the brain, such as muscle, cartilage and blood.
Combining the eMT and MT-weighted images into an ihMT ratio increases the specificity of the sequence to myelin, as evidenced by an ihMTR close to zero outside the brain. Our ihMTR values, at around 12% in WM, are similar to previous literature using a comparable preparation module with a Cartesian readout 13, 38, but lower than recent papers using a low-duty cycle preparation module 13, 18. Our protocol was adapted from that presented in 38, which had comparable levels of power deposition during the preparation module, and the principal difference is our acquisition module acquires a larger number (32) of center-out readout spokes with a low flip-angle instead of a small number of Cartesian readouts with a higher flip-angle.
To mitigate against T1 recovery during the readout segment, which repeatedly samples the center of k-space, we minimised the number of acquired spokes to 32. The resulting segment time of less than 70 ms is much shorter than typical T1 times in parenchyma (approximately 1 s at 3T). Increasing the number of spokes per segment would lead to a reduction in scan-time, at a cost of increased T1 recovery and potentially reduced ihMTR.
A particular drawback of radial ZTE sequences compared to Cartesian is constrained SNR. We observed some residual T1-weighted contrast in the PD-weighted reference image. Reducing the excitation flip-angle below 2° to further reduce the T1-weighting would incur a linear reduction in SNR (from the small flip-angle approximation sin α ≈ α), which would likely yield unacceptable image quality. We used Total Generalized Variation regularization in our reconstruction to primarily to improve image quality, whereas for Cartesian sequences such methods are generally used with parallel imaging to speed up acquisitions 18, 38, 44. Although non-cartesian parallel imaging methods exist 59, to our knowledge none has been specifically tailored to 3D radial acquisitions. Despite this limitation, we acquired 1.5 mm isotropic maps in 6 minutes, which is competitive with a recent cartesian ihMT acquisition with an MP-RAGE type readout which acquired 2.4 mm isotropic maps in the same time 18.
We observed a small negative ihMTR in CSF and the vitreous humour of the eyeball. The likely cause of this is a small, unwanted, difference in direct saturation effects between our single-sided and dual-sided irradiation preparation modules. Changing the shape of the preparation pulses to one with better controlled sidebands, for example Hann or Gaussian, would likely remove this effect 38. Use of the inverse ihMTR appeared to mitigate T1 and B1+ effects and led to improved contrast between WM and GM, and more consistent contrast within WM, for instance the internal capsule and genu of the corpus callosum have different ihMTR values but similar inverse ihMTR values. To fully determine whether the inverse ihMTR reduced B1+ contributions would require the acquisition of additional B1+ maps, which was beyond the scope of the current work.
We observed a large MT asymmetry in the carotid arteries and an elevated value in the cerebellum compared to the cerebrum. Blood is known to exhibit an MT effect, due to a high concentration of protein 60– 62, and this is also known to be asymmetric 63. PET and Dynamic Contrast Enhanced MRI measurements indicate that the cerebellum has increased vascularity and cerebral blood volume compared to the cerebrum 64, 65. Hence this result appears to be consistent with previous literature.
The CoV maps in Figure 3 showed high values in cortical GM, indicating poor reliability of ihMTR metrics in the cortex. This is partly to be expected due to the very small absolute values of ihMTR in both GM. However, the average within-subject CoV was lower than the between-subject CoV, indicating that partial volume effects and registration quality affected the between-subject figure. These issues are not unique to ihMTR but affect all quantitative MRI measures 66, 67.
The repeatability of both the ihMTR and inverse ihMTR, as defined by the ICC scores, fell on the boundary of the moderate (0.5-0.75) and good (0.75-0.9) categories with the exception of the cerebral peduncles and corticospinal tract which had notably worse scores. The ICC scores of inverse ihMTR were slightly superior to ihMTR. Our ICC values are lower than a repeatability study of a steady-state Cartesian ihMT study 51. However, the values are not directly comparable as that study used a 2.4×2.4×3.2 mm voxel size compared to our 1.5 mm isotropic voxel size, with a similar overall scan time. Our lower ICC values can hence at least be partly attributed to the smaller voxel size and correspondingly lower SNR.
As shown in Figure 6 most subjects had consistent measures across all four scans but others, notably subject A, showed high variability across sessions. This variation can at least in part be attributed to the orientation of the head with the main magnetic field. We found that the observed ihMTR decreased in all ROIs as the observed rotation angle of the head increased. Our method for quantifying the rotation angle is imperfect, as it cannot distinguish positive and negative rotations and the choice of the MNI template as “zero” is arbitrary. We also did not control for the average angle of each ROI, variations in tract orientation within an ROI, the effect of hemisphere, or the effect of inter-volume motion during the ihMT scan. Despite these limitations, we showed a small but highly statistically significant effect of angle on inverse ihMTR, and so conclude that head orientation is a potential confound in ihMT studies. Recent work has incorporated prospective motion correction into a cartesian ihMT sequence 18, and such approaches could be of benefit in this radial implementation to minimise both inter- and intra-volume motion artefacts in problematic patient cohorts (e.g. infants).
Conclusion
We have demonstrated that MT-weighting can generate significant additional GM/WM contrast in silent ZTE images with minimal extension of scan time. We have shown that the derived semi-quantitative MT ratios have good repeatability, and that the inverse ihMTR has advantages over the ihMTR. However, the ihMT effect depends on the orientation of the subject’s head within the bore, and hence we recommend that careful attention is paid to participant’s positioning in future work.
Data availability
Underlying data
Figshare: Silent Myelin-Weighted MR Imaging, https://doi.org/10.6084/m9.figshare.12090645 68.
This project contains the following data:
Atlas images in MNI space format
ROI summary statistics in Comma Separated Value format
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Funding Statement
TCW received funding from the Wellcome/EPSRC Centre for Medical Engineering (Award Number WT 203148/Z/16/Z). ND, AJL, EL & SCRW thank the NIHR Maudsley Biomedical Research Centre at South London Maudsley Foundation Trust and King's College London for their support and funding this study. EL also thanks GE Healthcare for joint funding of his PhD studentship.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 3 approved, 1 approved with reservations]
References
- 1. Bock NA, Hashim E, Janik R, et al. : Optimizing T 1-weighted imaging of cortical myelin content at 3.0 T. Neuroimage. 2013;65:1–12. 10.1016/j.neuroimage.2012.09.051 [DOI] [PubMed] [Google Scholar]
- 2. Liu H, Rubino C, Dvorak AV, et al. : Myelin Water Atlas: A Template for Myelin Distribution in the Brain. J Neuroimaging. 2019;29(6):699–706. 10.1111/jon.12657 [DOI] [PubMed] [Google Scholar]
- 3. Lee J, Shmueli K, Kang BT, et al. : The contribution of myelin to magnetic susceptibility-weighted contrasts in high-field MRI of the brain. Neuroimage. 2012;59(4):3967–3975. 10.1016/j.neuroimage.2011.10.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolff SD, Balaban RS: Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med. 1989;10(1):135–144. 10.1002/mrm.1910100113 [DOI] [PubMed] [Google Scholar]
- 5. De Santis S, Drakesmith M, Bells S, et al. : Why diffusion tensor MRI does well only some of the time: variance and covariance of white matter tissue microstructure attributes in the living human brain. Neuroimage. 2014;89(100):35–44. 10.1016/j.neuroimage.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wood TC, Simmons C, Hurley SA, et al. : Whole-brain ex-vivo quantitative MRI of the cuprizone mouse model. PeerJ. 2016;4:e2632. 10.7717/peerj.2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boullerne AI: The history of myelin. Exp Neurol. 2016;283(Pt B):431–445. 10.1016/j.expneurol.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Varma G, Duhamel G, de Bazelaire C, et al. : Magnetization transfer from inhomogeneously broadened lines: A potential marker for myelin. Magn Reson Med. 2015;73(2):614–622. 10.1002/mrm.25174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Girard OM, Prevost VH, Varma G, et al. : Magnetization transfer from inhomogeneously broadened lines (ihMT): Experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn Reson Med. 2015;73(6):2111–2121. 10.1002/mrm.25330 [DOI] [PubMed] [Google Scholar]
- 10. Manning AP, Chang KL, MacKay AL, et al. : The physical mechanism of “inhomogeneous” magnetization transfer MRI. J Magn Reson. 2017;274(Supplement C):125–136. 10.1016/j.jmr.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 11. Swanson SD, Malyarenko DI, Fabiilli ML, et al. : Molecular, dynamic, and structural origin of inhomogeneous magnetization transfer in lipid membranes. Magn Reson Med. 2017;77(3):1318–1328. 10.1002/mrm.26210 [DOI] [PubMed] [Google Scholar]
- 12. Duhamel G, Prevost VH, Cayre M, et al. : Validating the sensitivity of inhomogeneous magnetization transfer (ihMT) MRI to myelin with fluorescence microscopy. Neuroimage. 2019;199:289–303. 10.1016/j.neuroimage.2019.05.061 [DOI] [PubMed] [Google Scholar]
- 13. Varma G, Girard OM, Mchinda S, et al. : Low duty-cycle pulsed irradiation reduces magnetization transfer and increases the inhomogeneous magnetization transfer effect. J Magn Reson. 2018;296:60–71. 10.1016/j.jmr.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 14. Geeraert BL, Lebel RM, Mah AC, et al. : A comparison of inhomogeneous magnetization transfer, myelin volume fraction, and diffusion tensor imaging measures in healthy children. Neuroimage. 2018;182:343–350. 10.1016/j.neuroimage.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 15. Ercan E, Varma G, Mädler B, et al. : Microstructural correlates of 3D steady-state inhomogeneous magnetization transfer (ihMT) in the human brain white matter assessed by myelin water imaging and diffusion tensor imaging. Magn Reson Med. 2018;80(6):2402–2414. 10.1002/mrm.27211 [DOI] [PubMed] [Google Scholar]
- 16. Pampel A, Müller DK, Anwander A, et al. : Orientation dependence of magnetization transfer parameters in human white matter. NeuroImage. 2015;114:136–146. 10.1016/j.neuroimage.2015.03.068 [DOI] [PubMed] [Google Scholar]
- 17. Girard OM, Prevost VH, Mchinda M, et al. : 472 - Anisotropy of inhomogeneous Magnetization Transfer (ihMT) in White Matter.2017; ISMRM 25th Annual Meeting. Reference Source [Google Scholar]
- 18. Varma G, Munsch F, Burns B, et al. : Three-dimensional inhomogeneous magnetization transfer with rapid gradient-echo (3D ihMTRAGE) imaging. Magn Reson Med. 2020. 10.1002/mrm.28324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malik SJ, Teixeira RPAG, West DJ, et al. : Steady-state imaging with inhomogeneous magnetization transfer contrast using multiband radiofrequency pulses. Magn Reson Med. 2020;83(3):935–949. 10.1002/mrm.27984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin C, Li H, Li X, et al. : Temporary Hearing Threshold Shift in Healthy Volunteers with Hearing Protection Caused by Acoustic Noise Exposure during 3-T Multisequence MR Neuroimaging. Radiology. 2018;286(2):602–608. 10.1148/radiol.2017161622 [DOI] [PubMed] [Google Scholar]
- 21. McNulty JP, McNulty S: Acoustic noise in magnetic resonance imaging: An ongoing issue. Radiography. 2009;15(4):320–326. 10.1016/j.radi.2009.01.001 [DOI] [Google Scholar]
- 22. van Minde D, Klaming L, Weda H: Pinpointing Moments of High Anxiety During an MRI Examination. Int J Behav Med. 2014;21(3):487–95. 10.1007/s12529-013-9339-5 [DOI] [PubMed] [Google Scholar]
- 23. Hughes EJ, Winchman T, Padormo F, et al. : A dedicated neonatal brain imaging system. Magn Reson Med. 2017;78(2):794–804. 10.1002/mrm.26462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raschle N, Zuk J, Ortiz-Mantilla S, et al. : Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann N Y Acad Sci. 2012;1252(1):43–50. 10.1111/j.1749-6632.2012.06457.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hutter J, Price AN, Cordero-Grande L, et al. : Quiet echo planar imaging for functional and diffusion MRI. Magn Reson Med. 2018;79(3):1447–1459. 10.1002/mrm.26810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edwards AD, Arthurs OJ: Paediatric MRI under sedation: is it necessary? What is the evidence for the alternatives? Pediatr Radiol. 2011;41(11):1353–1364. 10.1007/s00247-011-2147-7 [DOI] [PubMed] [Google Scholar]
- 27. Madio DP, Lowe IJ: Ultra-fast imaging using low flip angles and FIDs. Magn Reson Med. 1995;34(4):525–529. 10.1002/mrm.1910340407 [DOI] [PubMed] [Google Scholar]
- 28. Weiger M, Pruessmann KP: MRI with Zero Echo Time.In Robin K. Harris, editor, Encyclopedia of Magnetic Resonance.John Wiley & Sons, Ltd Chichester, UK.2012. 10.5167/uzh-73614 [DOI] [Google Scholar]
- 29. Alibek S, Vogel M, Sun W, et al. : Acoustic noise reduction in MRI using Silent Scan: an initial experience. Diagn Interv Radiol. 2014;20(4):360–363. 10.5152/dir.2014.13458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ljungberg E, Wood T, Solana AB, et al. : Silent T 1 mapping using the variable flip angle method with B 1 correction. Magn Reson Med. 2020;84(2):813–824. 10.1002/mrm.28178 [DOI] [PubMed] [Google Scholar]
- 31. Wiesinger F, Sacolick LI, Menini A, et al. : Zero TE MR bone imaging in the head. Magn Reson Med. 2016;75(1):107–114. 10.1002/mrm.25545 [DOI] [PubMed] [Google Scholar]
- 32. Solana AB, Menini A, Sacolick LI, et al. : Quiet and distortion-free, whole brain BOLD fMRI using T 2-prepared RUFIS. Magn Reson Med. 2016;75(4):1402–1412. 10.1002/mrm.25658 [DOI] [PubMed] [Google Scholar]
- 33. Yuan J, Hu Y, Menini A, et al. : Near-silent distortionless DWI using magnetization-prepared RUFIS. Magn Reson Med. 2020;84(1):170–181. 10.1002/mrm.28106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Henkelman RM, Stanisz GJ, Graham SJ: Magnetization transfer in MRI: a review. NMR Biomed. 2001;14(2):57–64. 10.1002/nbm.683 [DOI] [PubMed] [Google Scholar]
- 35. Tozer D, Ramani A, Barker GJ, et al. : Quantitative magnetization transfer mapping of bound protons in multiple sclerosis. Magn Reson Med. 2003;50(1):83–91. 10.1002/mrm.10514 [DOI] [PubMed] [Google Scholar]
- 36. Dortch RD, Moore J, Li K, et al. : Quantitative magnetization transfer imaging of human brain at 7 T. Neuroimage. 2013;64:640–649. 10.1016/j.neuroimage.2012.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Portnoy S, Stanisz GJ: Modeling pulsed magnetization transfer. Magn Reson Med. 2007;58(1):144–155. 10.1002/mrm.21244 [DOI] [PubMed] [Google Scholar]
- 38. Mchinda S, Varma G, Prevost VH, et al. : Whole brain inhomogeneous magnetization transfer (ihMT) imaging: Sensitivity enhancement within a steady-state gradient echo sequence. Magn Reson Med. 2018;79(5):2607–2619. 10.1002/mrm.26907 [DOI] [PubMed] [Google Scholar]
- 39. Leung KK, Malone IM, Ourselin S, et al. : Effects of changing from non-accelerated to accelerated MRI for follow-up in brain atrophy measurement. Neuroimage. 2015;107:46–53. 10.1016/j.neuroimage.2014.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zaiss M, Xu J, Goerke S, et al. : Inverse Z-spectrum analysis for spillover-, MT-, and T 1 -corrected steady-state pulsed CEST-MRI--application to pH-weighted MRI of acute stroke. NMR Biomed. 2014;27(3):240–252. 10.1002/nbm.3054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oesterle C, Markl M, Strecker R, et al. : Spiral reconstruction by regridding to a large rectilinear matrix: a practical solution for routine systems. J Magn Reson Imaging. 1999;10(1):84–92. [DOI] [PubMed] [Google Scholar]
- 42. Wu Y, Dai G, Ackerman JL, et al. : Water- and fat-suppressed proton projection MRI (WASPI) of rat femur bone. Magn Reson Med. 2007;57(3):554–567. 10.1002/mrm.21174 [DOI] [PubMed] [Google Scholar]
- 43. Froidevaux R, Weiger M, Brunner DO, et al. : Filling the dead-time gap in zero echo time MRI: Principles compared. Magn Reson Med. 2018;79(4):2036–2045. 10.1002/mrm.26875 [DOI] [PubMed] [Google Scholar]
- 44. Knoll F, Bredies K, Pock T, et al. : Second order total generalized variation (TGV) for MRI. Magn Reson Med. 2011;65(2):480–491. 10.1002/mrm.22595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jenkinson M, Bannister P, Brady M, et al. : Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- 46. Wood TC: QUIT: QUantitative Imaging Tools. J Open Source Softw. 2018;3(26):656 10.21105/joss.00656 [DOI] [Google Scholar]
- 47. Hua J, Jones CK, Blakeley J, et al. : Quantitative description of the asymmetry in magnetization transfer effects around the water resonance in the human brain. Magn Reson Med. 2007;58(4):786–793. 10.1002/mrm.21387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Avants BB, Tustison NJ, Song G, et al. : A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Avants BB, Yushkevich P, Pluta J, et al. : The optimal template effect in hippocampus studies of diseased populations. Neuroimage. 2010;49(3):2457–2466. 10.1016/j.neuroimage.2009.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tustison NJ, Avants BB, Cook PA, et al. : N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310–1320. 10.1109/TMI.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang L, Chen T, Tian H, et al. : Reproducibility of inhomogeneous magnetization transfer (ihMT): A test-retest, multi-site study. Magn Reson Imaging. 2019;57:243–249. 10.1016/j.mri.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 52. Chen G, Taylor PA, Haller SP, et al. : Intraclass correlation: Improved modeling approaches and applications for neuroimaging. Hum Brain Mapp. 2018;39(3):1187–1206. 10.1002/hbm.23909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chung Y, Rabe-Hesketh S, Dorie V, et al. : A nondegenerate penalized likelihood estimator for variance parameters in multilevel models. Psychometrika. 2013;78(4):685–709. 10.1007/s11336-013-9328-2 [DOI] [PubMed] [Google Scholar]
- 54. Koo TK, Li MY: A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155–163. 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schyboll F, Jaekel U, Weber B, et al. : The impact of fibre orientation on T 1-relaxation and apparent tissue water content in white matter. MAGMA. 2018;31(4):501–510. 10.1007/s10334-018-0678-8 [DOI] [PubMed] [Google Scholar]
- 56. Vavasour IM, Clark CM, Li DK, et al. : Reproducibility and reliability of MR measurements in white matter: clinical implications. Neuroimage. 2006;32(2):637–642. 10.1016/j.neuroimage.2006.03.036 [DOI] [PubMed] [Google Scholar]
- 57. Van Obberghen E, Mchinda S, le Troter A, et al. : Evaluation of the Sensitivity of Inhomogeneous Magnetization Transfer (ihMT) MRI for Multiple Sclerosis. AJNR Am J Neuroradiol. 2018;39(4):634–641. 10.3174/ajnr.A5563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhang L, Wen B, Chen T, et al. : A comparison study of inhomogeneous magnetization transfer (ihMT) and magnetization transfer (MT) in multiple sclerosis based on whole brain acquisition at 3.0 T. Magn Reson Imaging. 2020;70:43–49. 10.1016/j.mri.2020.03.010 [DOI] [PubMed] [Google Scholar]
- 59. Luo T, Noll DC, Fessler JA, et al. : A GRAPPA algorithm for arbitrary 2D/3D non-Cartesian sampling trajectories with rapid calibration. Magn Reson Med. 2019;82(3):1101–1112. 10.1002/mrm.27801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Morrison C, Henkelman RM: A model for magnetization transfer in tissues. Magn Reson Med. 1995;33(4):475–482. 10.1002/mrm.1910330404 [DOI] [PubMed] [Google Scholar]
- 61. Spees WM, Yablonskiy DA, Oswood MC, et al. : Water proton MR properties of human blood at 1.5 Tesla: magnetic susceptibility, T 1, T 2, T* 2, and non-Lorentzian signal behavior. Magn Reson Med. 2001;45(4):533–542. 10.1002/mrm.1072 [DOI] [PubMed] [Google Scholar]
- 62. Kim T, Hendrich K, Kim SG: Functional MRI with magnetization transfer effects: Determination of BOLD and arterial blood volume changes. Magn Reson Med. 2008;60(6):1518–1523. 10.1002/mrm.21766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Song AW, Wolff SD, Balaban RS, et al. : The effect of off-resonance radiofrequency pulse saturation on fMRI contrast. NMR Biomed. 1997;10(4–5):208–215. [DOI] [PubMed] [Google Scholar]
- 64. Turkheimer FE, Edison P, Pavese N, et al. : Reference and Target Region Modeling of [11C]-(R)-PK11195 Brain Studies. J Nucl Med. 2007;48(1):158–67. [PubMed] [Google Scholar]
- 65. Østergaard L, Weisskoff RM, Chesler DA, et al. : High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36(5):715–725. 10.1002/mrm.1910360510 [DOI] [PubMed] [Google Scholar]
- 66. Piredda GF, Hilbert T, Granziera C, et al. : Quantitative brain relaxation atlases for personalized detection and characterization of brain pathology. Magn Reson Med. 2020;83(1):337–351. 10.1002/mrm.27927 [DOI] [PubMed] [Google Scholar]
- 67. Bonnier G, Fischi-Gomez E, Roche A, et al. : Personalized pathology maps to quantify diffuse and focal brain damage. Neuroimage Clin. 2019;21:101607. 10.1016/j.nicl.2018.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tobias W: Silent Myelin-Weighted MR Imaging. figshare.Dataset.2020. 10.6084/m9.figshare.12090645.v1 [DOI] [Google Scholar]