Abstract
Mitochondria and peroxisomes are ubiquitous subcellular organelles that are highly dynamic and possess a high degree of plasticity. These organelles proliferate through division of pre-existing organelles. Studies on yeast, mammalian cells, and unicellular algae have led to a surprising finding that mitochondria and peroxisomes share the components of their division machineries. At the heart of the mitochondrial and peroxisomal division machineries is a GTPase dynamin-like protein, Dnm1/Drp1, which forms a contractile ring around the neck of the dividing organelles. During division, Dnm1/Drp1 functions as a motor protein and constricts the membrane. This mechanochemical work is achieved by utilizing energy from GTP hydrolysis. Over the last two decades, studies have focused on the structure and assembly of Dnm1/Drp1 molecules around the neck. However, the regulation of GTP during the division of mitochondrion and peroxisome is not well understood. Here, we review the current understanding of Dnm1/Drp1-mediated divisions of mitochondria and peroxisomes, exploring the mechanisms of GTP regulation during the Dnm1/Drp1 function, and provide new perspectives on their potential contribution to mitochondrial and peroxisomal biogenesis.
Keywords: mitochondrial division, peroxisomal division, dynamin-related protein Dnm1/Drp1, nucleoside-diphosphate kinase, local GTP generation
1. Introduction
Mitochondria and peroxisomes play vital roles in cellular metabolism. Double-membrane-bounded mitochondria contain their own DNA derived from an endosymbiotic ancestor and are the powerhouse of eukaryotic cells, playing roles in the regulation of the cellular redox state, Ca2+ homeostasis, and apoptosis [1,2,3]. Single-membrane-bounded peroxisomes were originally defined as a carrier of flavin-oxidases, producing H2O2 and catalase [4]. Subsequently, peroxisomes were found to exert important metabolic functions in lipid homeostasis [5]. Mitochondria and peroxisomes are metabolically linked organelles and share matrix enzyme activities, including fatty-acid β-oxidation enzymes [6]. Since mitochondria and peroxisomes share functions, their morphogenesis and number need to be coordinated. Importantly, the number of both organelles is maintained by the division of pre-existing organelles using the same division machinery [7,8]. A range of pathological conditions, including cancer, aging, neurodegeneration and metabolic diseases, are associated with disorders in mitochondrial and peroxisomal division [9,10,11]. Thereby, the divisions of mitochondria and peroxisomes have been studied extensively over the last two decades with various model systems including yeast, mammals, and algae. There are two important findings in this field. (1) The identification of dynamin-like protein Dnm1/Drp1 (Dnm1 in yeast and algae; Drp1, DRP1, or DLP1 in mammals), which catalyzes the membrane fission of mitochondria [12,13,14] and peroxisomes [7] in yeast and mammalian cells as a model system. Dnm1/Drp1 is a dynamin family member as well as a classical dynamin involved in the endocytosis [15]. Similar to classical dynamin, Dnm1/Drp1 contains a GTPase domain (G-domain) and forms a helical polymer to constrict the membrane tubules upon binding to and hydrolyzing GTP [16,17]. (2) The identification of an electron-dense “ring” around the neck of the dividing organelles. This electron-dense ring was first identified around the neck of dividing mitochondrion in a unicellular alga, Cyanidioschyzon merolae [18,19]. It was also observed later around the neck of a dividing peroxisome [20]. A similar electron-dense neck has also been observed around the constriction site of mitochondrion in yeast [16] and mammalian cells [21]. These findings raise a view that mitochondria and peroxisomes divide via the constriction of the ring-shaped division machinery composed of Dnm1/Drp1 [22,23]. Research in this field has previously focused on the receptor-mediated recruitment of the Dnm1/Drp1-based division machinery to membrane fission sites [24]. In addition to exploring of the function of Dnm1/Drp1 receptors, a recent study on C. merolae demonstrated that the energy source of Dnm1/Drp1, GTP is spatio-temporally regulated during the divisions of mitochondrion and peroxisome, raising an idea that the energy source for the organelle division machinery is locally generated [25]. In this review, we briefly compile the current knowledge about membrane remodeling of mitochondria and peroxisomes in yeast, algae, and mammalian cells. We also address molecular mechanisms underlying the energetic regulation of Dnm1/Drp1-based machineries, in regards to mitochondrial and peroxisomal division.
2. Mitochondrial Dynamics
2.1. In Yeast and Mammals
In yeast and mammalian cells, membrane fission and fusion are responsible for maintaining the morphology and number of mitochondria [26,27,28]. The core component of the mitochondrial fission machinery is Dnm1/Drp1 that was first identified as Dnm1 by yeast mutagenesis [12,13,14]. Dnm1 is a dynamin-related GTPase, which is recruited to the mitochondrial outer membrane (MOM), where it self-assembles via GTP binding followed by membrane constriction (Figure 1A). Loss of Dnm1 results in long interconnected mitochondrial networks [13,14]. Receptors with transmembrane domains are involved in recruitment of Dnm1 to the MOM. Genetics studies in yeast identified the receptor proteins of Dnm1, namely mitochondrial fission 1 protein (Fis1) [29], mitochondrial division protein 1 (Mdv1) [30], and CCR4-associated factor 4 (Caf4) [31]. In mammals, a Dnm1 ortholog, Drp1 [32], and its receptor protein, Fis1, have been identified [21]. However, it has been reported that Fis1 is dispensable for Drp1-mediated mitochondrial fission in mammalian cells [33,34] and important under a specific physiological condition, such as stress-induced mitophagy [35]. More recent studies have shown that Fis1 negatively regulates mitochondrial fusion [36]. As such, Fis1 may exert functions other than in the recruitment of Drp1 in mammalian cells. Mdv1 and Caf4 are not conserved in mammals [37]. Instead, recruitment of Drp1 likely depends on other receptors, such as mitochondrial fission factor (Mff) [33], which was originally identified by the small interfering RNAscreening of the cultured Drosophila melanogaster cells [38], and mitochondrial dynamics proteins of 49 kDa (MiD49) and 51 kDa/mitochondrial elongation factor 1 (MiD51/MIEF1) conserved in vertebrates [39,40]. In addition, the mitochondrial distribution and morphology protein 36 (Mdm36), and a cortical protein, nuclear migration protein 1 (Num1), are also known to regulate mitochondrial fission and distribution in yeast [41,42]. Mammalian orthologs of Mdm36 and Num1 have not yet been identified.
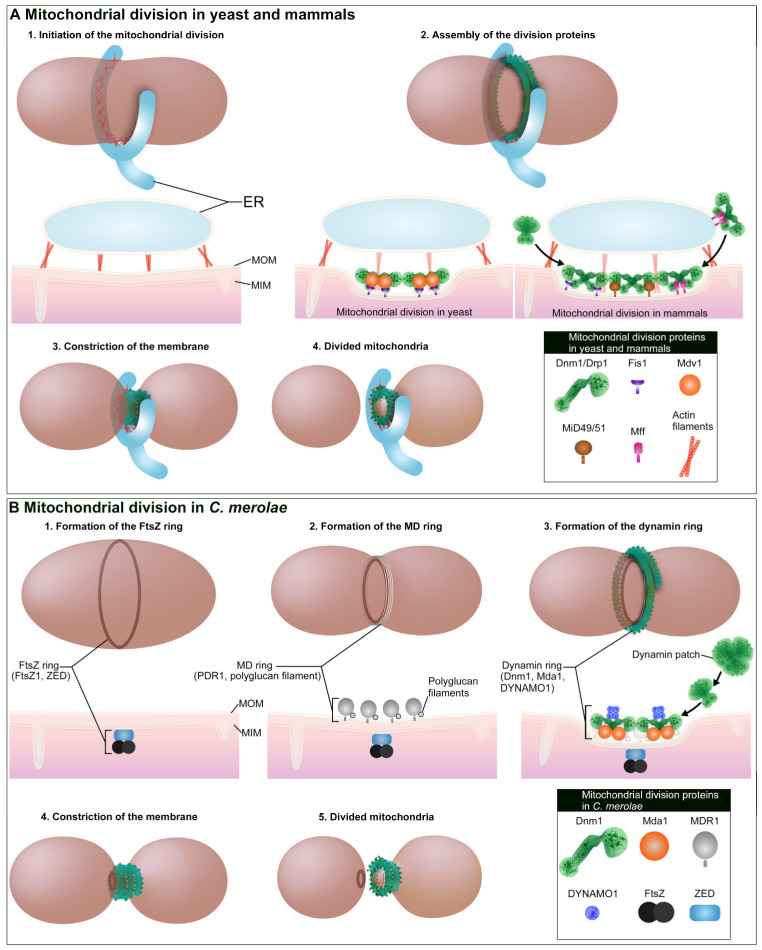
Figure 1.
Mitochondria are divided by Dnm1/Drp1-based membrane fission machinery. Schematic images represent dividing mitochondria and their cross sections at the division plane. (A), Mitochondrial division in yeast and mammals is initiated at the endoplasmic reticulum (ER)-mitochondrion contact site. At the contact site, ER tubules encircle the mitochondria and polymerized actin is formed. Receptor proteins Fis1 and Mdv1/Caf4 are involved in the recruitment of Dnm1 to mitochondrial outer membrane (MOM) in yeast, while Fis1, Mff, and MiD49/51 are involved in the recruitment of Drp1 in mammals. In mammals, the ER–mitochondrion contact site also participates in the recruitment of Drp1. Polymerized Dnm1/Drp1 constricts and pinches off mitochondrial division site. (B) In C. merolae, a mitochondrion is divided by mitochondrion-dividing (MD) machinery (FtsZ ring, MD ring and dynamin ring). The first event of the mitochondrial division is formation of the FtsZ ring. ZED is important for the FtsZ ring formation on the matrix side of MIM followed by the formation of MD ring. MDR1 is involved in the formation of MD ring. Dynamin ring is composed of Dnm1, and Mdv1/Caf4 ortholog Mda1 is involved in the recruitment of Dnm1. Dnm1 is likely recruited from cytosolic dynamin patches. During the constriction of MD machinery, DYNAMO1 generates GTP and supports the GTPase activity of Dnm1.
Mitochondrial fusion is also regulated by dynamin-related proteins. The first identified mitochondrial fusion gene is fuzzy onions (fzo1) in D. melanogaster [43]. Fzo1 is a transmembrane protein with its GTPase domain that is exposed to the cytoplasm. A molecular genetic study using yeast found that fzo1 mutation causes a fragmented mitochondrial phenotype as a result of blocking the fusion of MOM [44]. Fzo1 and Dnm1 double-mutations alter this fragmented phenotype to wild-type mitochondrial morphology, indicating that the balance between fission and fusion plays an important role in the morphology of mitochondria [14]. In yeast, the fusion of mitochondrial inner membrane (MIM) is regulated by a dynamin-related GTPase, Mgm1 [45]. In mammalian cells, the fusion of MOM is regulated by Fzo1 orthologs, namely mitofusins 1 and 2 (Mfn1 and Mfn2) [46], and MIM is regulated by the Mgm1 ortholog optic atrophy-1 (OPA-1) [47,48].
In the initiation of mitochondrial dynamics, the endoplasmic reticulum (ER) plays an important role. During mitochondrial division, ER tubules encircle and constrict mitochondrial tubules prior to the recruitment of Dnm1/Drp1 to the mitochondria [49]. At the ER-marked mitochondrial division site, an ER-associated formin, INF2, facilitates the polymerization of actin to generate small patches of the actin–myosin II cytoskeleton [50,51,52]. Since Mff functions via its affinity to membrane curvature and recruits Drp1 in mammalian cells, it is proposed that the ER tubules and actin cytoskeleton trigger a mechanical force that recruits Mff and Drp1 [53]. Interestingly, the Drp1 oligomers, Mff and Fis1, are located on the ER membrane as puncta, and these fission components are transferred to mitochondrial division site upon the ER–mitochondria contact site [54]. Therefore, the ER most likely serves as both an initiator of mitochondrial division and a platform of mitochondrial fission machinery. Similar to mitochondrial division, mitochondrial fusion also occurs at the ER-mitochondria contact site [55]. It is consistent with the fusion protein of MOM, Mfn2, which is involved in mitochondrial–ER tethering [56].
In addition to the receptor proteins and the ER-actin cytoskeleton, a mitochondria-specific polyanionic phospholipid cardiolipin also regulates mitochondrial dynamics. Cardiolipin is primarily localized at the MIM, but it is also found at the MOM [57] where Drp1 is found. Cardiolipin directly binds to an insert B region of Drp1 and promotes its oligomerization followed by stimulation of its GTPase activity, the so-called assembly-stimulated GTPase activity [58,59]. Overexpression of an insert B mutant of Drp1 with a reduced binding affinity to cardiolipin-containing membranes does not rescue the defect of mitochondrial fission in Drp1-knockout cells [60]. Cardiolipin has been also shown to interact with mitochondrial fusion proteins, such as yeast Mgm1 and human Opa1, and stimulate their GTPase activity, although these dynamin-like proteins do not contain PH domain [61,62]. Thereby, cardiolipin is important for both the fission and fusion of mitochondria.
Mutations in the mitochondrial division genes are associated with human disease [9,63]. The first reported patient was a newborn female patient with microcephaly, abnormal brain development, optic atrophy and hypoplasia, persistent lactic acidemia, and a mildly elevated plasma concentration of very-long-chain fatty acids. The patient had a dominant negative heterozygous mutation at G395D in the DRP1 gene, manifested as a severe fission defect of mitochondria and died one month after birth [64]. Patients with mutations in the Mff gene are associated with early-onset Leigh-like basal ganglia disease [65]. In cells derived from these patients, fission defect in the mitochondria and impaired DRP1 recruitment are observed. Several clinical diseases have also been shown to be associated with mutations in mitochondrial fusion genes. Mfn2 is mutated in patients with Charcot–Marie–Tooth type 2A [66], while mutations of the OPA1 gene are associated with dominant optic atrophy [67,68].
2.2. In C. merolae
Unlike yeast and mammalian cells which contain numerous mitochondria and peroxisomes, the unicellular red algae C. merolae contains a single mitochondrion and peroxisome per cell [19]. The division of these organelles is highly synchronized by the cycles of light/dark stimulation [20,69], allowing for both snapshots of sequential events of individual mitochondrial and peroxisomal division and the visualization of the entire picture of the division machinery. Furthermore, during synchronization, division machineries can be isolated in bulk; this allows the components of the division machinery to be identified by mass spectrometry and 100% sequenced for their genomic information [70,71]. In C. merolae, mitochondria only divide and do not fuse, unlike the mitochondria in yeast and mammalian cells. Consistent with this membrane remodeling, C. merolae does not contain any mitochondrial fusion genes, such as Mfn and OPA-1 [70]. The division machinery of the mitochondrion, called mitochondrion-dividing (MD) machinery, consists of three types of ring-shaped structures: the mitochondrion-dividing (MD) ring, the dynamin ring, and the FtsZ ring [23] (Figure 1B). Using transmission electron microscopy, the MD ring was identified as an electron-dense ring-like structure wrapped around the neck of dividing mitochondria [18]. There are two types of MD rings: an outer MD ring, which forms at the cytoplasmic side of the MOM, and an inner MD ring, which forms within the matrix beneath the MIM. The outer MD ring is composed of a bundle of polyglucan nanofilaments (~5 nm in width) and glycosyltransferase MITOCHONDRION-DIVIDING RING1 (MDR1) that regulates the synthesis of the filaments [72]. Molecular details of the inner MD ring are not yet clear. The dynamin ring is composed of Dnm1 and formed around the MD ring [73]. In the cytoplasm, Dnm1-positive signals are observed as 10–20 cytoplasmic puncta (dynamin patches), and Dnm1 is likely to be recruited from the dynamin patches to the mitochondrial division site [73]. The dynamin patches do not contain GTP; thus, Dnm1 is probably in a GTP-unbound form before recruitment [74]. The Dnm1 receptor proteins encoded in C. merolae are mitochondrial division apparatus 1 (Mda1) and Fis1 [70,71]. Mda1 is a WD40 repeat protein homologous to yeast Mdv1 and Caf4. Mda1 localizes to the mitochondrial division site prior to Dnm1 recruitment and its stable homo-oligomer is a core structure of the MD machinery [75]. Fis1 is assumed to be an adaptor between Dnm1 and Mda1, but its function in C. merolae remains unclear. The nucleoside diphosphate kinase (NDPK) protein DYNAMO1 was recently identified as an interacting partner of Dnm1 [25]. NDPK domain catalyzes the GTP generating reaction by transferring γ-phosphate from ATP [76]. DYNAMO1 is essential for the recruitment of Dnm1 and the constriction of Dnm1-dependent mitochondrial division by facilitating G-domain activity (both GTP binding and hydrolysis) of Dnm1 and providing GTP to Dnm1, respectively. The FtsZ ring is composed of an alphaproteobacterial-type filamenting temperature sensitive mutant Z1 (FtsZ1), a remnant of bacterial cell division apparatus [77,78]. FtsZ ring formation depends on the interaction between FtsZ1 and a bacterial ZapA-like protein, ZED [79]. An exact role of FtsZ ring in mitochondrial division is not fully understood, but is thought to play an important role in the constriction of MIM and the positioning of the MD machinery [73].
The molecular mechanisms of the initiation of mitochondrial division are not yet well understood in C. merolae. In yeast and mammals, the ER and actin–myosin cytoskeleton are involved in the initiation of mitochondrial division, as discussed in the previous section. In C. merolae, the ER extends towards the mitochondrial division site [80]. However, since C. merolae lacks an actin–myosin system [70,71,81], it remains unclear whether the ER is involved in the initiation of mitochondrial division. Instead of the ER and actin–myosin-mediated initiation of the mitochondrial division, the FtsZ ring is thought to play a pivotal role in the positioning of the MD machinery [73]. Light/dark cycle-induced synchronization captures the sequential event of mitochondrial division and demonstrates that the FtsZ ring forms on the matrix side of MIM followed by the recruitment of Mda1 and Dnm1 at the same site on MOM [73,75]. The exact molecular mechanism of the FtsZ ring function is not yet understood, particularly how the FtsZ ring engages in the crosstalk with the division proteins over the MIM and MOM, and it is not known what signal induces FtsZ ring formation. Understanding of these issues would be an exciting topic for the investigation in future studies.
3. Peroxisomal Dynamics
3.1. In Yeast and Mammals
Peroxisomal proliferation by “growth and division” is a widely accepted dogma in yeast and mammals [82,83]. This process involves the synthesis of peroxisomal proteins on cytosolic free-polyribosomes and their post-translational transportation into the peroxisome matrix and membrane, followed by peroxisomal division. Peroxisomal membrane protein 11 (PEX11) is the first protein to be identified that plays an important role in peroxisomal division (Figure 2A). PEX11 was originally identified as Pmp27p using yeast mutagenesis [84]. The disruption of this gene in yeast caused a significant reduction in peroxisome abundance, while its overexpression yielded the opposite result [84,85,86]. In mammals, the PEX11 isoform, PEX11β, mediates membrane growth by remodeling, deforming, and elongating the peroxisomal membrane prior to fission [87,88]. In addition to its membrane-shaping function, PEX11β is also involved in the recruitment of division factors. Peroxisome division in mammals is regulated by Drp1 [7,89], Fis1 [8,90], and Mff [38,91]. Pex11β interacts with Mff in a Drp1-dependent manner, suggesting that Mff plays a key role in the fission of the peroxisomal membrane in a concerted manner with Pex11β and Drp1 [91]. A functional complex comprising Pex11β, Mff, and Drp1 promotes the Mff-mediated fission during peroxisomal division [91,92]. The involvement of PEX11 in the regulation of peroxisomal number is conserved between yeast and mammals, although the manner of Dnm1/Drp1 action is different in yeast. In the yeast Saccharomyces cerevisiae, dynamin-like protein vacuolar protein sorting 1 (Vps1) is involved in peroxisomal division [93], while Dnm1, Fis1, and Mdv1 are required for division when cells are under the peroxisomal growth condition in the presence of oleate [94]. On the other hand, a study using the yeast Hansenula polymorpha showed that Dnm1, but not Vps1, plays a crucial role in peroxisomal division [95]. In H. polymorpha, PEX11 directly binds to Dnm1 and functions as a GTPase-activating protein (GAP) [96]. Therefore, Dnm1 function may be dispensable in a subset of cell types or environment in yeast.
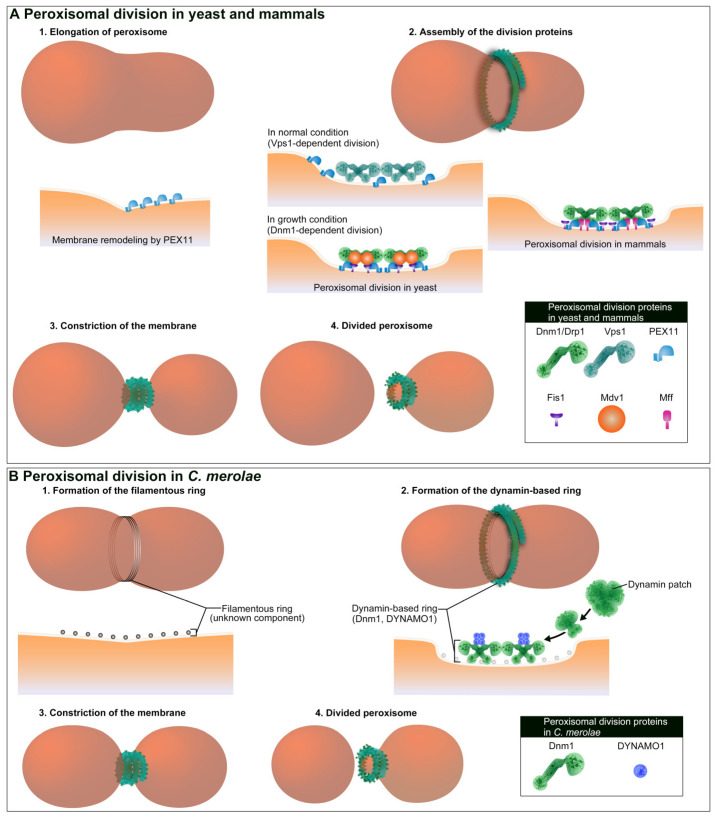
Figure 2.
Peroxisomes are divided by Dnm1/Drp1-based membrane fission machinery. (A) Peroxisomal division in yeast and mammals are initiated by PEX11. In yeast S. cerevisiae, Vps1 and PEX11 are involved in the peroxisomal division in normal conditions. In growth condition, Dnm1, PEX11, Fis1, and Mdv1 are involved in the division. In mammals, Drp1, PEX11β, Fis1, and Mff are involved in the division. (B) In C. merolae, a peroxisome is divided by peroxisomal-dividing (POD) machinery (filamentous ring and dynamin-based ring). The filamentous ring is thought to be formed first at the division site followed by formation of the dynamin-based ring. Dnm1 is likely recruited from cytosolic dynamin patches. DYNAMO1 regulates both recruitment of Dnm1 and GTP-dependent constriction of dynamin-based ring.
As described in the previous section, the ER plays an important role in the initiation of mitochondrial division and the recruitment of Dnm1/Drp1, and also plays a pivotal role in peroxisomal proliferation. In yeast and mammalian cells, peroxisomal membrane peroxins 3 and 16 (Pex3 and Pex16) have been observed emerging from ER [97,98,99,100,101,102]. Thereby, Pex-containing pre-peroxisomal vesicle is proposed to play an important role in the biogenesis of peroxisomes followed by Dnm1/Drp1-mediated membrane fission [103]. The mechanism by which Pex-containing pre-peroxisomal vesicles participate in peroxisomal biogenesis is not yet clearly understood. However, it has been proposed that the vesicles fuse with each other or with pre-existing peroxisome to generate a larger matured peroxisome [104,105]. A recent study suggested that Pex3 is targeted to mitochondria, and Pex3-containing vesicles bud off from MOM [106]. This study also suggested that Pex3-containing vesicles fuse to Pex16-containing vesicles from the ER for peroxisome assembly. Thus, mitochondria may also be involved in the proliferation of peroxisomes, in addition to the ER. The identification of other factors involved in the regulation of the fusion of Pex-containing vesicles is another interesting topic for future studies.
As for mitochondrial division proteins, mutations in peroxisomal division proteins are also responsible for human diseases. The first reported mutation was a Chinese hamster ovary (CHO) cell mutant, ZP121, which was impaired in DRP1 with one-point temperature-sensitive and dominant negative mutation at G363D in the middle region [89]. In terms of the manifestation of peroxisomal dysmorphogenesis in humans, only three patients have been identified with a different defect in two proteins involved in the division of peroxisomes. The first patient was a severely affected female patient with a dominant negative heterozygous mutation at G395D in DLP1, who manifested a severe fission defect of both peroxisomes and mitochondria and died one month after birth [64]. The second patient with a dysfunctional DRP1 harboring a G362D mutation was more recently reported [107]. A patient with defective peroxisomal division due to a homozygous nonsense mutation in the PEX11β gene was reported as the 14th complementation group of the peroxisome biogenesis disorders [108,109].
3.2. In C. merolae
In a unicellular red algae C. merolae, the division of a peroxisome occurs during M phase and always takes place after mitochondrial division. The peroxisome morphology is plastic, as in mammalian cells, and is a result of the physical interaction of peroxisomes with mitochondria during mitosis [110]. Morphological changes take place in peroxisomes during their physical interaction with mitochondria, including an increase in their volume before peroxisomal division. The post-translational import of catalase is linked to an increase in the volume of a peroxisome [110]. Thus, a peroxisome in C. merolae proliferates via its growth and division like in yeast and mammalian cells. As such, the interaction of peroxisome with the mitochondrion may play an important role in its the morphological plasticity. The division machinery of the peroxisome, called the peroxisomal-dividing (POD) machinery, consists of two types of ring-shaped structures, namely a filamentous ring and a dynamin-based (DB) ring. Both are formed at the cytoplasmic side of peroxisomal membrane constriction site [20] (Figure 2B). The POD machinery does not contain a FtsZ ring or an electron-dense ring in the matrix side. The filamentous ring is composed of a bundle of 4-nm wide filaments, which is similar to the structure of the MD ring, but its components remain unknown. The DB ring contains Dnm1 [20] and DYNAMO1 [25], thus sharing components with the dynamin ring of the MD machinery. The DB ring is formed from a single spot on the POD machinery, called the dynamin-based ring organizing center (DOC), which functions as the nucleation site of Dnm1 [74]. GTP binding is likely to play an essential role in the assembly of Dnm1. Similar to the dynamin ring in the MD machinery, Dnm1 is recruited from the dynamin patches to the division site of the peroxisome [20,74]. DYNAMO1 provides GTP to Dnm1 on the DB ring, which is critical for generating the constriction force of the POD machinery, as well as the constriction of the MD machinery. Unlike mitochondrial division, DYNAMO1 is not involved in recruiting Dnm1 to the membrane fission site during peroxisomal division. Future studies will elucidate how C. merolae regulates Dnm1 recruitment during the peroxisomal division. C. merolae encodes Pex11β and thus Pex11β-Dnm1 interaction may be sufficient for the recruitment of Dnm1 to the peroxisomal division site.
4. Regulation of GTP during Dnm1/Drp1 Function
Dnm1/Drp1 is a core component in the division machineries of the mitochondria and peroxisomes in yeast, mammals, and C. merolae [11,22,23]. The key regulatory mechanisms of the Dnm1/Drp1-based division machinery include the step of recruitment of Dnm1/Drp1 to the membrane, the receptor-mediated protein recruitment, and the mobilization of GTP. We are certain that various Dnm1/Drp1 receptor proteins described in the previous sections are required for Dnm1/Drp1 recruitment. However, their regulation of GTP remains unclear. GTP is the only energy source used for Dnm1/Drp1 recruitment and membrane constriction, and GTP binding is important in the recruitment of Dnm1/Drp1. In yeast, the binding of GTP to the G-domain alters the structure of Dnm1 by exposing the insert B region involved in the membrane interaction [111]. After GTP binding, Dnm1 is able to form a highly ordered helix-shaped structure [16]. Mdv1 is known to preferentially interact with GTP-bound Dnm1 [112]. In mammals, GTP binding alters the conformation of Drp1 and allows for the interaction between Drp1 and Mid49 [113], followed by polymerization with Mff on the mitochondrial membrane [114]. Moreover, GTP and GDP influence the binding kinetics between Drp1 and actin filaments [115]. During the constriction, the Dnm1/Drp1-helical polymer is thought to demonstrate ratchet motion [17] similar to classical dynamin, which requires continuous consumption of GTP until the diameter of the membrane reaches its limit, at ~4 nm, for spontaneous membrane fission [116,117]. Although GTP binding is important for the recruitment of Dnm1/Drp1, its affinity to GTP is weak. That is, the Km of basal G-domain is at least 1 mM [118]. Dnm1/Drp1 has a relatively high rate of GTP hydrolysis on the membrane [16,118,119], at least 5000-fold higher than that of small GTPases [120]. Indeed, a study using yeast Dnm1 reported that significant constriction of lipid tubules mediated by the Dnm1-helical polymer requires extraordinarily high levels of GTP concentration (~1 mM) [17], consistent with the idea that the allosteric enhancer of G-domain is required to elevate the affinity of Dnm1 to GTP [118]. Several earlier studies have showed that Dnm1/Drp1 binding proteins, such as Mdv1 and Mff, enhance G-domain function [121,122]. In addition to these Dnm1/Drp1 binding proteins present on the MOM, cytoplasmic proteins also participate in the enhancement of the G-domain function. A study in mammalian cells found that mitochondrial fragmentation is induced by cytoplasmic cyclin C, which is released from cell nucleus in response to oxidative stress [123]. Cyclin C directly binds to Drp1 and increases the affinity of Drp1 to GTP. As GTP binding to Drp1 is required for the interaction of Drp1 with MiD49 [113], enhancing the affinity of Drp1 to GTP may play an important role in the recruitment of Drp1 to MOM from the cytoplasm. In C. merolae, the ortholog of Drp1, Dnm1, is likely recruited from the dynamin patches in the cytoplasm [73]. The Dnm1 in the dynamin patches is likely a GTP-unbound form [74]. The GTP-unbound form of Dnm1 is thought to be altered to GTP-bound form during the recruitment in C. merolae. Consistent with this, a recent study found that the NDPK protein DYNAMO1 facilitates the G-domain function and regulates the recruitment of Dnm1 to the division site of mitochondrion [25]. This indicates that GTP is needed during Drp1 recruitment and that DYNAMO1 functions as an enhancer of the G-domain function of Dnm1. The enzyme activity of NDPK is not required for the recruitment, but it is essential during the membrane constriction. During the membrane constriction in a mitochondrion and a peroxisome, DYNAMO1 localizes to the MD and POD machineries and locally generates GTP from ATP. Abolishing this activity results in stalling of the constriction of a mitochondrion and a peroxisome. Thus, DYNAMO1 is an essential GTP regulator for the Dnm1-based division machinery of a mitochondrion and a peroxisome. It is not known whether NDPK orthologs, if any, are important in Dnm1/Drp1 function in yeast and mammalian cells, while NDPK function is reported to be required during mitochondrial fusion. One of the NDPK isoforms in mammals, non-metastatic cells 4 (NME4), has been suggested to provide GTP for the OPA-1 function during MIM fusion [124,125]. Recently, another isoform NME3, was found to regulate the function of Mfn1 and Mfn2 during MOM fusion. However, in this case, GTP generation activity was not required for Mfn1 and Mfn2 functions [126]. Thus, mitochondrial fusion in mammals seems to be regulated by the NDPK protein. Fission and fusion occur in rapid succession at the same region on mitochondria [127]. Moreover, these two opposing membrane remodeling processes frequently occur at the ER–mitochondrial contact sites [49,55,128], where Drp1 and Mfn1 are colocalized [129]. Therefore, NDPK protein may also be accessible to Drp1, in addition to Mfn1. In future studies, the issue as to whether local GTP generation during mitochondrial fission is conserved in yeast and mammals will need to be addressed.
5. Molecular Mechanisms Underlying Local GTP Generation around the Organelle Division Machinery
Local GTP generation on dynamin family proteins has been reported in both classical dynamin and dynamin-related proteins. In D. melanogaster, a mutation of the NDPK gene, called abnormal wing disc (Awd), is identified as an enhancer of the shibire mutant phenotype, which has a defect in dynamin-dependent synaptic vesicle endocytosis [130]. In mammals, NDPK proteins are important for both clathrin-mediated and clathrin-independent endocytosis [131,132], during which the NDPK isoforms, NME1 and NME2, bind to classical dynamin and generate GTP locally on the endocytic sites. Thus, NDPK function is essential for classical dynamin. For the mitochondrial dynamics, another isoform, NME4, produces GTP on OPA-1 during MIM fusion [124,125]. For the Dnm1-dependent division of a mitochondrion and a peroxisome, the NDPK ortholog DYNAMO1 generates GTP on Dnm1 in C. merolae [25]. Local GTP generation by the NDPK protein is a conserved phenomenon among dynamin family proteins, although molecular mechanisms of GTP generation have a discrepancy between the two proposed models: one is that GTP is channeled within the complex of dynamin family proteins and NDPK [133,134], and the other is that GTP concentration is enriched locally around the membrane fission site [25].
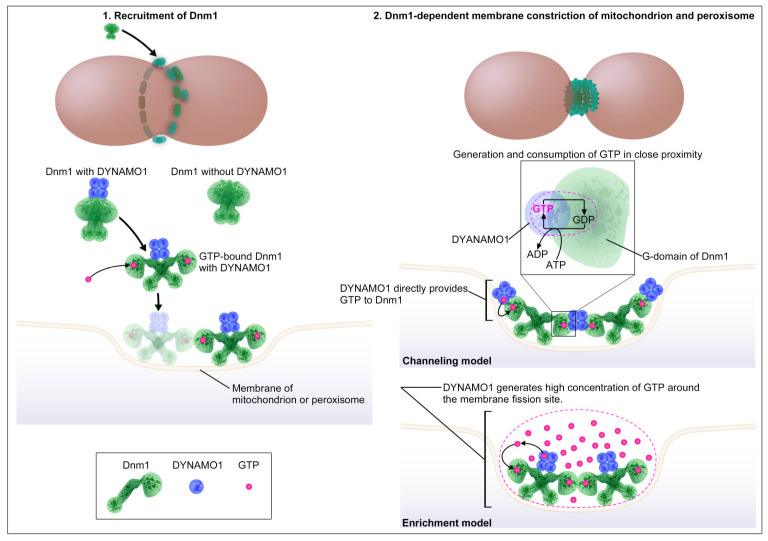
In the channeling model (Figure 3), GTP generation within the dynamin family protein-NDPK protein complex maximizes the efficiency of GTP delivery to the G-domain of Dnm1. Maximizing enzyme kinetics usually takes place in a spatial proximity within the complex of multifunctional enzymes to be separated from the diffusion equilibrium, a process known as “channeling” [135]. A well-known example of this channeling is the glycolysis reaction between glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the phosphoglycerate kinase (PGK) complex [136], and it is also proposed for the NDPK reaction [133]. The caveat of the channeling model is the unbalanced kinetics between the generation and hydrolysis of GTP. The turnover of NDPK is Kcat = 600/s [137], which suggests >500–2000-fold higher enzyme kinetics than the GTPase activity of the membrane-bound classical dynamin or Dnm1 orthologs in yeast, algae, and mammals [118,119,138]. Thus, the catalytic domains of the NDPK protein and the G-domain of Dnm1 need to be in close proximity to secure a high GTP concentration ratio and to promote GTP hydrolysis without leakage of the nucleotides. However, the GTP-holding pocket in the G-domain faces each other between the adjusting helical turns in a helical polymer of Dnm1/Drp1, as well as in classical dynamin [17,116]. In this case, the interaction between the NDPK domain and the G-domain would compete with the formation of the G-domain dimer. Structural studies of the NDPK protein-dynamin family protein complex are required for further analysis and discussion of the channeling model.
Figure 3.
DYNAMO1 locally generates GTP for the GTPase activity of Dnm1 during the division of mitochondrion and peroxisome in C. merolae. During the recruitment of Dnm1 to mitochondrial membrane, DYNAMO1 binds to Dnm1 and promotes the recruitment by enhancing the G-domain function of Dnm1. During the constriction of Dnm1-based membrane fission machinery, DYNAMO1 is thought to provide GTP locally to Dnm1 by mechanisms called a channeling model or an enrichment model. In the channeling model, DYNAMO1 provides GTP in close proximity to G-domain of Dnm1. In the enrichment model, DYNAMO1 elevates local GTP concentration around the membrane fission site.
The enrichment model (Figure 3) reconciles the fact that the dynamin family proteins, including Dnm1/Drp1, have a low affinity to GTP and a high rate of GTP-hydrolysis, which cannot be supported by the physiological levels of GTP. As mentioned above, the enzyme kinetics of NDPK is much higher than that of the GTPase activity of dynamin family proteins. Thus, a balance between GTP generation and consumption may not exist around NDPK-enriched membrane fission sites. In cells, NDPK forms a tetramer or a hexamer [139]. The functional membrane fission ring contains ~100 molecules of Drp1 during mitochondrial fission in mammalian cells, based on the quantification of endogenous GFP-tagged proteins [140], which are ~200 nm in diameter [53]. An accurate model for the number of G-domain dimers and the number of helix turns is required in the future studies. Given the parameters of enzyme kinetics between NDPK and Dnm1 or classical dynamin, excess of GTP may be generated around the membrane fission sites to increase the concentration of local GTP. However, the diffusion coefficient of nucleotides is ~360 µm2/s [141], and it is uncertain whether GTP can be locally enriched by overcoming the diffusion kinetics. This could be understood by carefully considering the coupling between the generation/consumption and diffusion of GTP. One way to locally enrich GTP is to manipulate the diffusion coefficient, as seen during the diffusion of calcium ions, whose diffusion coefficient is slowed down ~10 times as a result of molecular crowding or the restriction of movement by the cytoskeleton and cellular organelles [142,143]. Another mechanism by which local enrichment can be achieved is liquid-phase separation. Recently, the liquid-phase separation of cycling GMP-AMP synthase (cGAS) that converts GTP and ATP to cAMP is found in mammalian cells [144]. In this study, the enrichment of ATP or GTP within the cGAS-DNA liquid droplets is demonstrated. Thus, even small molecules, such as ATP and GTP, could be phase separated. However, there is only little information on the buffering elements that can slow down the diffusion coefficient of GTP, or on intrinsically disordered proteins that help in the phase separation of GTP around membrane fission sites. For example, transmission electron microscopy images of platinum replicas of an unroofed cell show that the mitochondria are surrounded with a dense cytoskeletal network in mammalian cells [52]. During clathrin-mediated endocytosis, early stage endocytic proteins have been shown to form phase separated droplets on the endocytic sites [145]. Future studies would need to examine a mobile fraction of GTP around the division sites of mitochondrion and peroxisome.
6. Conclusions and Perspectives
In this review, we summarize the most recent studies regarding GTP regulation during the Dnm1/Drp1-dependent membrane fission of mitochondria and peroxisomes. In addition to the importance of receptor-mediated Dnm1/Drp1 recruitment during the division of mitochondria and peroxisomes, the emerging studies have highlighted the importance of the replenishment of GTP during membrane fission. We also discussed the issues regarding the molecular mechanism underlying how local GTP generation is generated by NDPK protein on the division machineries of mitochondria and peroxisomes, as exampled by GTP channeling and GTP enrichment models. To elucidate these two models, various approaches need to be developed in future studies. To characterize the GTP channeling model, the structure of the interface between dynamin family protein and NDPK protein on the membrane needs to be elucidated. For the GTP enrichment model, the visualization of GTP concentration is vital. Recently, a circularly permutated YFP (cpYFP)-based GTP sensor was developed [146]. GTP imaging using the cpYFP could open another door in the field of NDPK protein and dynamin protein research. Currently, the working model of these proteins remains unknown. Several questions remain to be answered, such as when does local GTP generation start? How many molecules of NDPK protein are required per membrane fission machinery? What is the geometry of the NDPK proteins in the membrane fission machinery? To address these issues, the structure of the membrane fission machinery needs to be visualized using in situ cryo-electron tomography and subtomogram averaging techniques. Although these represent considerable challenges, the results will provide a new direction of research towards the membrane fission events conserved across eukaryotic cells.
Author Contributions
Conceptualization, Y.I. and Y.F.; writing—original draft preparation, Y.I.; writing—review and editing, Y.F. and K.I.; supervision, Y.I. and Y.F.; project administration, Y.I. and Y.F.; funding acquisition, Y.I. and Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Japan Society for the Promotion of Science Postdoctoral Research Fellowship for Research Abroad and in part by grants from the Japan Society for the Promotion of Science Fellowships grant number 14J04556. Ministry of Education, Culture, Sports, Science, and Technology of Japan, Grants-in-Aid for Scientific Research grant numbers JP24247038, JP25112518, JP25116717, JP26116007, JP15K14511, JP15K21743, and JP17H03675. Takeda Science Foundation. Naito Foundation. (Japan Foundation for Applied Enzymology and Novartis Foundation (Japan) for the Promotion of Science.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ernster L., Schatz G. Mitochondria: A Historical Review. J. Cell Biol. 1981;91:227s–255s. doi: 10.1083/jcb.91.3.227s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McBride H.M., Neuspiel M., Wasiak S. Mitochondria: More than Just a Powerhouse. Curr. Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 3.Yun J., Finkel T. Mitohormesis. Cell Metab. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Duve C., Baudhuin P. Peroxisomes (Microbodies and Related Particles) Physiol. Rev. 1966;46:323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- 5.van den Bosch H., Schutgens R.B.H., Wanders R.J.A., Tager J.M. Biochemistry of Peroxisomes. Annu. Rev. Biochem. 1992;61:157–197. doi: 10.1146/annurev.bi.61.070192.001105. [DOI] [PubMed] [Google Scholar]
- 6.Schrader M., Yoon Y. Mitochondria and Peroxisomes: Are the ‘Big Brother’and the ‘Little Sister’Closer than Assumed? Bioessays. 2007;29:1105–1114. doi: 10.1002/bies.20659. [DOI] [PubMed] [Google Scholar]
- 7.Koch A., Thiemann M., Grabenbauer M., Yoon Y., McNiven M.A., Schrader M. Dynamin-like Protein 1 Is Involved in Peroxisomal Fission. J. Biol. Chem. 2003;278:8597–8605. doi: 10.1074/jbc.M211761200. [DOI] [PubMed] [Google Scholar]
- 8.Koch A., Yoon Y., Bonekamp N.A., McNiven M.A., Schrader M. A Role for Fis1 in Both Mitochondrial and Peroxisomal Fission in Mammalian Cells. Mol. Biol. Cell. 2005;16:5077–5086. doi: 10.1091/mbc.e05-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itoh K., Nakamura K., Iijima M., Sesaki H. Mitochondrial Dynamics in Neurodegeneration. Trends Cell Biol. 2013;23:64–71. doi: 10.1016/j.tcb.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong S.-B., Hall A.R., Hausenloy D.J. Mitochondrial Dynamics in Cardiovascular Health and Disease. Antioxid. Redox Signal. 2013;19:400–414. doi: 10.1089/ars.2012.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrader M., Castro I., Fahimi H.D., Islinger M. Molecular Machines Involved in Peroxisome Biogenesis and Maintenance. Springer Science and Business Media LLC; Berlin/Heidelberg, Germany: 2014. Peroxisome Morphology in Pathologies; pp. 125–151. [Google Scholar]
- 12.Otsuga D., Keegan B.R., Brisch E., Thatcher J.W., Hermann G.J., Bleazard W., Shaw J.M. The Dynamin-Related GTPase, Dnm1p, Controls Mitochondrial Morphology in Yeast. J. Cell Biol. 1998;143:333–349. doi: 10.1083/jcb.143.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleazard W., McCaffery J.M., King E.J., Bale S., Mozdy A., Tieu Q., Nunnari J., Shaw J.M. The Dynamin-Related GTPase Dnm1 Regulates Mitochondrial Fission in Yeast. Nat. Cell Biol. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sesaki H., Jensen R.E. Division versus Fusion: Dnm1p and Fzo1p Antagonistically Regulate Mitochondrial Shape. J. Cell Biol. 1999;147:699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Praefcke G.J.K., McMahon H.T. The Dynamin Superfamily: Universal Membrane Tubulation and Fission Molecules? Nat. Rev. Mol. Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 16.Ingerman E., Perkins E.M., Marino M., Mears J.A., McCaffery J.M., Hinshaw J.E., Nunnari J. Dnm1 Forms Spirals That Are Structurally Tailored to Fit Mitochondria. J. Cell Biol. 2005;170:1021–1027. doi: 10.1083/jcb.200506078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mears J.A., Lackner L.L., Fang S., Ingerman E., Nunnari J., Hinshaw J.E. Conformational Changes in Dnm1 Support a Contractile Mechanism for Mitochondrial Fission. Nat. Struct. Mol. Biol. 2011;18:20–26. doi: 10.1038/nsmb.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroiwa T., Suzuki K., Itou R., Toda K., O’keefe T.C., Kuroiwa H. Mitochondria-Dividing Ring: Ultrastructual Basis for the Mechanism of Mitochondrial Division in Cyanidioschyzon Merolae. Protoplasma. 1995;186:12–23. doi: 10.1007/BF01276930. [DOI] [Google Scholar]
- 19.Miyagishima S., Itoh R., Toda K., Kuroiwa H., Kuroiwa T. Real-Time Analyses of Chloroplast and Mitochondrial Division and Differences in the Behavior of Their Dividing Rings during Contraction. Planta. 1999;207:343–353. doi: 10.1007/s004250050491. [DOI] [Google Scholar]
- 20.Imoto Y., Kuroiwa H., Yoshida Y., Ohnuma M., Fujiwara T., Yoshida M., Nishida K., Yagisawa F., Hirooka S., Miyagishima S. Single-Membrane–Bounded Peroxisome Division Revealed by Isolation of Dynamin-Based Machinery. Proc. Natl. Acad. Sci. USA. 2013;110:9583–9588. doi: 10.1073/pnas.1303483110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon Y., Krueger E.W., Oswald B.J., McNiven M.A. The Mitochondrial Protein HFis1 Regulates Mitochondrial Fission in Mammalian Cells through an Interaction with the Dynamin-Like Protein DLP1. Mol. Cell. Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osteryoung K.W., Nunnari J. The Division of Endosymbiotic Organelles. Science. 2003;302:1698–1704. doi: 10.1126/science.1082192. [DOI] [PubMed] [Google Scholar]
- 23.Kuroiwa T., Misumi O., Nishida K., Yagisawa F., Yoshida Y., Fujiwara T., Kuroiwa H. Vesicle, Mitochondrial, and Plastid Division Machineries with Emphasis on Dynamin and Electron-Dense Rings. Int. Rev. Cell Mol. Biol. 2008;271:97–152. doi: 10.1016/S1937-6448(08)01203-3. [DOI] [PubMed] [Google Scholar]
- 24.Bui H.T., Shaw J.M. Dynamin Assembly Strategies and Adaptor Proteins in Mitochondrial Fission. Curr. Biol. 2013;23:R891–R899. doi: 10.1016/j.cub.2013.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imoto Y., Abe Y., Honsho M., Okumoto K., Ohnuma M., Kuroiwa H., Kuroiwa T., Fujiki Y. Onsite GTP Fuelling via DYNAMO1 Drives Division of Mitochondria and Peroxisomes. Nat. Commun. 2018;9:1–12. doi: 10.1038/s41467-018-07009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto K., Shaw J.M. Mitochondrial Morphology and Dynamics in Yeast and Multicellular Eukaryotes. Annu. Rev. Genet. 2005;39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- 27.Friedman J.R., Nunnari J. Mitochondrial Form and Function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murata D., Arai K., Iijima M., Sesaki H. Mitochondrial Division, Fusion and Degradation. J. Biochem. (Tokyo) 2020;167:233–241. doi: 10.1093/jb/mvz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mozdy A.D., McCaffery J.M., Shaw J.M. Dnm1p GTPase-Mediated Mitochondrial Fission Is a Multi-Step Process Requiring the Novel Integral Membrane Component Fis1p. J. Cell Biol. 2000;151:367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tieu Q., Nunnari J. Mdv1p Is a WD Repeat Protein That Interacts with the Dynamin-Related GTPase, Dnm1p, to Trigger Mitochondrial Division. J. Cell Biol. 2000;151:353–366. doi: 10.1083/jcb.151.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffin E.E., Graumann J., Chan D.C. The WD40 Protein Caf4p Is a Component of the Mitochondrial Fission Machinery and Recruits Dnm1p to Mitochondria. J. Cell Biol. 2005;170:237–248. doi: 10.1083/jcb.200503148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smirnova E., Griparic L., Shurland D.-L., van Der Bliek A.M. Dynamin-Related Protein Drp1 Is Required for Mitochondrial Division in Mammalian Cells. Mol. Biol. Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otera H., Wang C., Cleland M.M., Setoguchi K., Yokota S., Youle R.J., Mihara K. Mff Is an Essential Factor for Mitochondrial Recruitment of Drp1 during Mitochondrial Fission in Mammalian Cells. J. Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Losón O.C., Song Z., Chen H., Chan D.C. Fis1, Mff, MiD49, and MiD51 Mediate Drp1 Recruitment in Mitochondrial Fission. Mol. Biol. Cell. 2013;24:659–667. doi: 10.1091/mbc.e12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Q., Yamano K., Head B.P., Kawajiri S., Cheung J.T., Wang C., Cho J.-H., Hattori N., Youle R.J., van der Bliek A.M. Mutations in Fis1 Disrupt Orderly Disposal of Defective Mitochondria. Mol. Biol. Cell. 2014;25:145–159. doi: 10.1091/mbc.e13-09-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu R., Jin S.-B., Lendahl U., Nistér M., Zhao J. Human Fis1 Regulates Mitochondrial Dynamics through Inhibition of the Fusion Machinery. EMBO J. 2019;38 doi: 10.15252/embj.201899748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy M., Reddy P.H., Iijima M., Sesaki H. Mitochondrial Division and Fusion in Metabolism. Curr. Opin. Cell Biol. 2015;33:111–118. doi: 10.1016/j.ceb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandre-Babbe S., van der Bliek A.M. The Novel Tail-Anchored Membrane Protein Mff Controls Mitochondrial and Peroxisomal Fission in Mammalian Cells. Mol. Biol. Cell. 2008;19:2402–2412. doi: 10.1091/mbc.e07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer C.S., Osellame L.D., Laine D., Koutsopoulos O.S., Frazier A.E., Ryan M.T. MiD49 and MiD51, New Components of the Mitochondrial Fission Machinery. EMBO Rep. 2011;12:565–573. doi: 10.1038/embor.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao J., Liu T., Jin S., Wang X., Qu M., Uhlén P., Tomilin N., Shupliakov O., Lendahl U., Nistér M. Human MIEF1 Recruits Drp1 to Mitochondrial Outer Membranes and Promotes Mitochondrial Fusion Rather than Fission. EMBO J. 2011;30:2762–2778. doi: 10.1038/emboj.2011.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerveny K.L., Studer S.L., Jensen R.E., Sesaki H. Yeast Mitochondrial Division and Distribution Require the Cortical Num1 Protein. Dev. Cell. 2007;12:363–375. doi: 10.1016/j.devcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 42.Hammermeister M., Schödel K., Westermann B. Mdm36 Is a Mitochondrial Fission-Promoting Protein in Saccharomyces Cerevisiae. Mol. Biol. Cell. 2010;21:2443–2452. doi: 10.1091/mbc.e10-02-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hales K.G., Fuller M.T. Developmentally Regulated Mitochondrial Fusion Mediated by a Conserved, Novel, Predicted GTPase. Cell. 1997;90:121–129. doi: 10.1016/S0092-8674(00)80319-0. [DOI] [PubMed] [Google Scholar]
- 44.Hermann G.J., Thatcher J.W., Mills J.P., Hales K.G., Fuller M.T., Nunnari J., Shaw J.M. Mitochondrial Fusion in Yeast Requires the Transmembrane GTPase Fzo1p. J. Cell Biol. 1998;143:359–373. doi: 10.1083/jcb.143.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong E.D., Wagner J.A., Gorsich S.W., McCaffery J.M., Shaw J.M., Nunnari J. The Dynamin-Related GTPase, Mgm1p, Is an Intermembrane Space Protein Required for Maintenance of Fusion Competent Mitochondria. J. Cell Biol. 2000;151:341–352. doi: 10.1083/jcb.151.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santel A., Fuller M.T. Control of Mitochondrial Morphology by a Human Mitofusin. J. Cell Sci. 2001;114:867–874. doi: 10.1242/jcs.114.5.867. [DOI] [PubMed] [Google Scholar]
- 47.Olichon A., Baricault L., Gas N., Guillou E., Valette A., Belenguer P., Lenaers G. Loss of OPA1 Perturbates the Mitochondrial Inner Membrane Structure and Integrity, Leading to Cytochrome c Release and Apoptosis. J. Biol. Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 48.Griparic L., Head B., van der Bliek A.M. Mitochondrial Function and Biogenesis. Springer; Berlin/Heidelberg, Germany: 2004. Mitochondrial Fission and Fusion Machineries; pp. 227–249. [Google Scholar]
- 49.Friedman J.R., Lackner L.L., West M., DiBenedetto J.R., Nunnari J., Voeltz G.K. ER Tubules Mark Sites of Mitochondrial Division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Korobova F., Ramabhadran V., Higgs H.N. An Actin-Dependent Step in Mitochondrial Fission Mediated by the ER-Associated Formin INF2. Science. 2013;339:464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korobova F., Gauvin T.J., Higgs H.N. A Role for Myosin II in Mammalian Mitochondrial Fission. Curr. Biol. 2014;24:409–414. doi: 10.1016/j.cub.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang C., Svitkina T.M. Ultrastructure and Dynamics of the Actin−myosin II Cytoskeleton during Mitochondrial Fission. Nat. Cell Biol. 2019;21:603–613. doi: 10.1038/s41556-019-0313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji W., Hatch A.L., Merrill R.A., Strack S., Higgs H.N. Actin Filaments Target the Oligomeric Maturation of the Dynamin GTPase Drp1 to Mitochondrial Fission Sites. Elife. 2015;4:e11553. doi: 10.7554/eLife.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji W.-K., Chakrabarti R., Fan X., Schoenfeld L., Strack S., Higgs H.N. Receptor-Mediated Drp1 Oligomerization on Endoplasmic Reticulum. J. Cell Biol. 2017;216:4123–4139. doi: 10.1083/jcb.201610057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo Y., Li D., Zhang S., Yang Y., Liu J.-J., Wang X., Liu C., Milkie D.E., Moore R.P., Tulu U.S., et al. Visualizing Intracellular Organelle and Cytoskeletal Interactions at Nanoscale Resolution on Millisecond Timescales. Cell. 2018;175:1430–1442. doi: 10.1016/j.cell.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 56.De Brito O.M., Scorrano L. Mitofusin 2 Tethers Endoplasmic Reticulum to Mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 57.Ardail D., Privat J.P., Egret-Charlier M., Levrat C., Lerme F., Louisot P. Mitochondrial Contact Sites. Lipid Composition and Dynamics. J. Biol. Chem. 1990;265:18797–18802. [PubMed] [Google Scholar]
- 58.Bustillo-Zabalbeitia I., Montessuit S., Raemy E., Basañez G., Terrones O., Martinou J.-C. Specific Interaction with Cardiolipin Triggers Functional Activation of Dynamin-Related Protein 1. PLoS ONE. 2014;9:e102738. doi: 10.1371/journal.pone.0102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Macdonald P.J., Stepanyants N., Mehrotra N., Mears J.A., Qi X., Sesaki H., Ramachandran R. A Dimeric Equilibrium Intermediate Nucleates Drp1 Reassembly on Mitochondrial Membranes for Fission. Mol. Biol. Cell. 2014;25:1905–1915. doi: 10.1091/mbc.e14-02-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stepanyants N., Macdonald P.J., Francy C.A., Mears J.A., Qi X., Ramachandran R. Cardiolipin’s Propensity for Phase Transition and Its Reorganization by Dynamin-Related Protein 1 Form a Basis for Mitochondrial Membrane Fission. Mol. Biol. Cell. 2015;26:3104–3116. doi: 10.1091/mbc.E15-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeVay R.M., Dominguez-Ramirez L., Lackner L.L., Hoppins S., Stahlberg H., Nunnari J. Coassembly of Mgm1 Isoforms Requires Cardiolipin and Mediates Mitochondrial Inner Membrane Fusion. J. Cell Biol. 2009;186:793–803. doi: 10.1083/jcb.200906098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ban T., Heymann J.A.W., Song Z., Hinshaw J.E., Chan D.C. OPA1 Disease Alleles Causing Dominant Optic Atrophy Have Defects in Cardiolipin-Stimulated GTP Hydrolysis and Membrane Tubulation. Hum. Mol. Genet. 2010;19:2113–2122. doi: 10.1093/hmg/ddq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burté F., Carelli V., Chinnery P.F., Yu-Wai-Man P. Disturbed Mitochondrial Dynamics and Neurodegenerative Disorders. Nat. Rev. Neurol. 2015;11:11–24. doi: 10.1038/nrneurol.2014.228. [DOI] [PubMed] [Google Scholar]
- 64.Waterham H.R., Koster J., van Roermund C.W.T., Mooyer P.A.W., Wanders R.J.A., Leonard J.V. A Lethal Defect of Mitochondrial and Peroxisomal Fission. N. Engl. J. Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 65.Koch J., Feichtinger R.G., Freisinger P., Pies M., Schrödl F., Iuso A., Sperl W., Mayr J.A., Prokisch H., Haack T.B. Disturbed Mitochondrial and Peroxisomal Dynamics Due to Loss of MFF Causes Leigh-like Encephalopathy, Optic Atrophy and Peripheral Neuropathy. J. Med. Genet. 2016;53:270–278. doi: 10.1136/jmedgenet-2015-103500. [DOI] [PubMed] [Google Scholar]
- 66.Züchner S., Mersiyanova I.V., Muglia M., Bissar-Tadmouri N., Rochelle J., Dadali E.L., Zappia M., Nelis E., Patitucci A., Senderek J., et al. Mutations in the Mitochondrial GTPase Mitofusin 2 Cause Charcot-Marie-Tooth Neuropathy Type 2A. Nat. Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- 67.Alexander C., Votruba M., Pesch U.E.A., Thiselton D.L., Mayer S., Moore A., Rodriguez M., Kellner U., Leo-Kottler B., Auburger G., et al. OPA1, Encoding a Dynamin-Related GTPase, Is Mutated in Autosomal Dominant Optic Atrophy Linked to Chromosome 3q28. Nat. Genet. 2000;26:211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 68.Delettre C., Lenaers G., Griffoin J.-M., Gigarel N., Lorenzo C., Belenguer P., Pelloquin L., Grosgeorge J., Turc-Carel C., Perret E., et al. Nuclear Gene OPA1, Encoding a Mitochondrial Dynamin-Related Protein, Is Mutated in Dominant Optic Atrophy. Nat. Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki K., Ehara T., Osafune T., Kuroiwa H., Kawano S., Kuroiwa T. Behavior of Mitochondria, Chloroplasts and Their Nuclei during the Mitotic Cycle in the Ultramicroalga Cyanidioschyzon Merolae. Eur. J. Cell Biol. 1994;63:280–288. [PubMed] [Google Scholar]
- 70.Matsuzaki M., Misumi O., Shin-i T., Maruyama S., Takahara M., Miyagishima S., Mori T., Nishida K., Yagisawa F., Nishida K., et al. Genome Sequence of the Ultrasmall Unicellular Red Alga Cyanidioschyzon Merolae 10D. Nature. 2004;428:653–657. doi: 10.1038/nature02398. [DOI] [PubMed] [Google Scholar]
- 71.Nozaki H., Takano H., Misumi O., Terasawa K., Matsuzaki M., Maruyama S., Nishida K., Yagisawa F., Yoshida Y., Fujiwara T., et al. A 100%-Complete Sequence Reveals Unusually Simple Genomic Features in the Hot-Spring Red Alga Cyanidioschyzon Merolae. BMC Biol. 2007;5:28. doi: 10.1186/1741-7007-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshida Y., Kuroiwa H., Shimada T., Yoshida M., Ohnuma M., Fujiwara T., Imoto Y., Yagisawa F., Nishida K., Hirooka S., et al. Glycosyltransferase MDR1 Assembles a Dividing Ring for Mitochondrial Proliferation Comprising Polyglucan Nanofilaments. Proc. Natl. Acad. Sci. USA. 2017;114:13284–13289. doi: 10.1073/pnas.1715008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nishida K., Takahara M., Miyagishima S., Kuroiwa H., Matsuzaki M., Kuroiwa T. Dynamic Recruitment of Dynamin for Final Mitochondrial Severance in a Primitive Red Alga. Proc. Natl. Acad. Sci. USA. 2003;100:2146–2151. doi: 10.1073/pnas.0436886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imoto Y., Abe Y., Okumoto K., Honsho M., Kuroiwa H., Kuroiwa T., Fujiki Y. Defining the Dynamin-Based Ring Organizing Center on the Peroxisome-Dividing Machinery Isolated from Cyanidioschyzon Merolae. J. Cell Sci. 2017;130:853–867. doi: 10.1242/jcs.199182. [DOI] [PubMed] [Google Scholar]
- 75.Nishida K., Yagisawa F., Kuroiwa H., Yoshida Y., Kuroiwa T. WD40 Protein Mda1 Is Purified with Dnm1 and Forms a Dividing Ring for Mitochondria before Dnm1 in Cyanidioschyzon Merolae. Proc. Natl. Acad. Sci. USA. 2007;104:4736–4741. doi: 10.1073/pnas.0609364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Norman A.W., Wedding R.T., Black M.K. Detection of Phosphohistidine in Nucleoside Diphosphokinase Isolated from Jerusalem Artichoke Mitochondria. Biochem. Biophys. Res. Commun. 1965;20:703–709. doi: 10.1016/0006-291X(65)90073-2. [DOI] [PubMed] [Google Scholar]
- 77.Takahara M., Takahashi H., Matsunaga S., Miyagishima S., Takano H., Sakai A., Kawano S., Kuroiwa T. A Putative Mitochondrial FtsZ Gene Is Present in the Unicellular Primitive Red Alga Cyanidioschyzon Merolae. Mol. Gen. Genet. MGG. 2000;264:452–460. doi: 10.1007/s004380000307. [DOI] [PubMed] [Google Scholar]
- 78.Takahara M., Kuroiwa H., Miyagishima S., Mori T., Kuroiwa T. Localization of the Mitochondrial FtsZ Protein in a Dividing Mitochondrion. Cytologia (Tokyo) 2001;66:421–425. doi: 10.1508/cytologia.66.421. [DOI] [Google Scholar]
- 79.Yoshida Y., Kuroiwa H., Hirooka S., Fujiwara T., Ohnuma M., Yoshida M., Misumi O., Kawano S., Kuroiwa T. The Bacterial ZapA-like Protein ZED Is Required for Mitochondrial Division. Curr. Biol. 2009;19:1491–1497. doi: 10.1016/j.cub.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 80.Yagisawa F., Fujiwara T., Kuroiwa H., Nishida K., Imoto Y., Kuroiwa T. Mitotic Inheritance of Endoplasmic Reticulum in the Primitive Red Alga Cyanidioschyzon Merolae. Protoplasma. 2012;249:1129–1135. doi: 10.1007/s00709-011-0359-1. [DOI] [PubMed] [Google Scholar]
- 81.Kuroiwa T. The Primitive Red Algae Cyanidium Caldarium and Cyanidioschyzon Merolae as Model System for Investigating the Dividing Apparatus of Mitochondria and Plastids. BioEssays. 1998;20:344–354. doi: 10.1002/(SICI)1521-1878(199804)20:4<344::AID-BIES11>3.0.CO;2-2. [DOI] [Google Scholar]
- 82.Lazarow P.B., Fujiki Y. Biogenesis of Peroxisomes. Annu. Rev. Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- 83.Thoms S., Erdmann R. Dynamin-Related Proteins and Pex11 Proteins in Peroxisome Division and Proliferation. FEBS J. 2005;272:5169–5181. doi: 10.1111/j.1742-4658.2005.04939.x. [DOI] [PubMed] [Google Scholar]
- 84.Erdmann R., Blobel G. Giant Peroxisomes in Oleic Acid-Induced Saccharomyces Cerevisiae Lacking the Peroxisomal Membrane Protein Pmp27p. J. Cell Biol. 1995;128:509–523. doi: 10.1083/jcb.128.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marshall P.A., Krimkevich Y.I., Lark R.H., Dyer J.M., Veenhuis M., Goodman J.M. Pmp27 Promotes Peroxisomal Proliferation. J. Cell Biol. 1995;129:345–355. doi: 10.1083/jcb.129.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sakai Y., Marshall P.A., Saiganji A., Takabe K., Saiki H., Kato N., Goodman J.M. The Candida Boidinii Peroxisomal Membrane Protein Pmp30 Has a Role in Peroxisomal Proliferation and Is Functionally Homologous to Pmp27 from Saccharomyces Cerevisiae. J. Bacteriol. 1995;177:6773–6781. doi: 10.1128/JB.177.23.6773-6781.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schrader M., Reuber B.E., Morrell J.C., Jimenez-Sanchez G., Obie C., Stroh T.A., Valle D., Schroer T.A., Gould S.J. Expression of PEX11β Mediates Peroxisome Proliferation in the Absence of Extracellular Stimuli. J. Biol. Chem. 1998;273:29607–29614. doi: 10.1074/jbc.273.45.29607. [DOI] [PubMed] [Google Scholar]
- 88.Li X., Gould S.J. PEX11 Promotes Peroxisome Division Independently of Peroxisome Metabolism. J. Cell Biol. 2002;156:643–651. doi: 10.1083/jcb.200112028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tanaka A., Kobayashi S., Fujiki Y. Peroxisome Division Is Impaired in a CHO Cell Mutant with an Inactivating Point-Mutation in Dynamin-like Protein 1 Gene. Exp. Cell Res. 2006;312:1671–1684. doi: 10.1016/j.yexcr.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 90.Kobayashi S., Tanaka A., Fujiki Y. Fis1, DLP1, and Pex11p Coordinately Regulate Peroxisome Morphogenesis. Exp. Cell Res. 2007;313:1675–1686. doi: 10.1016/j.yexcr.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 91.Itoyama A., Michiyuki S., Honsho M., Yamamoto T., Moser A., Yoshida Y., Fujiki Y. Mff Functions with Pex11pβ and DLP1 in Peroxisomal Fission. Biol. Open. 2013;2:998–1006. doi: 10.1242/bio.20135298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoshida Y., Niwa H., Honsho M., Itoyama A., Fujiki Y. Pex11mediates Peroxisomal Proliferation by Promoting Deformation of the Lipid Membrane. Biol. Open. 2015;4:710–721. doi: 10.1242/bio.201410801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoepfner D., van den Berg M., Philippsen P., Tabak H.F., Hettema E.H. A Role for Vps1p, Actin, and the Myo2p Motor in Peroxisome Abundance and Inheritance in Saccharomyces Cerevisiae. J. Cell Biol. 2001;155:979–990. doi: 10.1083/jcb.200107028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuravi K., Nagotu S., Krikken A.M., Sjollema K., Deckers M., Erdmann R., Veenhuis M., Klei I.J. van der. Dynamin-Related Proteins Vps1p and Dnm1p Control Peroxisome Abundance in Saccharomyces Cerevisiae. J. Cell Sci. 2006;119:3994–4001. doi: 10.1242/jcs.03166. [DOI] [PubMed] [Google Scholar]
- 95.Nagotu S., Saraya R., Otzen M., Veenhuis M., van der Klei I.J. Peroxisome Proliferation in Hansenula Polymorpha Requires Dnm1p Which Mediates Fission but Not de Novo Formation. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2008;1783:760–769. doi: 10.1016/j.bbamcr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 96.Williams C., Opalinski L., Landgraf C., Costello J., Schrader M., Krikken A.M., Knoops K., Kram A.M., Volkmer R., van der Klei I.J. The Membrane Remodeling Protein Pex11p Activates the GTPase Dnm1p during Peroxisomal Fission. Proc. Natl. Acad. Sci. USA. 2015;112:6377–6382. doi: 10.1073/pnas.1418736112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Titorenko V.I., Rachubinski R.A. The Life Cycle of the Peroxisome. Nat. Rev. Mol. Cell Biol. 2001;2:357–368. doi: 10.1038/35073063. [DOI] [PubMed] [Google Scholar]
- 98.Hoepfner D., Schildknegt D., Braakman I., Philippsen P., Tabak H.F. Contribution of the Endoplasmic Reticulum to Peroxisome Formation. Cell. 2005;122:85–95. doi: 10.1016/j.cell.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 99.Kragt A., Voorn-Brouwer T., van den Berg M., Distel B. Endoplasmic Reticulum-Directed Pex3p Routes to Peroxisomes and Restores Peroxisome Formation in a Saccharomyces Cerevisiae Pex3Δ Strain. J. Biol. Chem. 2005;280:34350–34357. doi: 10.1074/jbc.M505432200. [DOI] [PubMed] [Google Scholar]
- 100.Tam Y.Y.C., Fagarasanu A., Fagarasanu M., Rachubinski R.A. Pex3p Initiates the Formation of a Preperoxisomal Compartment from a Subdomain of the Endoplasmic Reticulum in Saccharomyces Cerevisiae. J. Biol. Chem. 2005;280:34933–34939. doi: 10.1074/jbc.M506208200. [DOI] [PubMed] [Google Scholar]
- 101.Kim P.K., Mullen R.T., Schumann U., Lippincott-Schwartz J. The Origin and Maintenance of Mammalian Peroxisomes Involves a de Novo PEX16-Dependent Pathway from the ER. J. Cell Biol. 2006;173:521–532. doi: 10.1083/jcb.200601036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Motley A.M., Hettema E.H. Yeast Peroxisomes Multiply by Growth and Division. J. Cell Biol. 2007;178:399–410. doi: 10.1083/jcb.200702167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schrader M., Fahimi H.D. Peroxisomes and Oxidative Stress. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2006;1763:1755–1766. doi: 10.1016/j.bbamcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 104.Boukh-Viner T., Guo T., Alexandrian A., Cerracchio A., Gregg C., Haile S., Kyskan R., Milijevic S., Oren D., Solomon J., et al. Dynamic Ergosterol- and Ceramide-Rich Domains in the Peroxisomal Membrane Serve as an Organizing Platform for Peroxisome Fusion. J. Cell Biol. 2005;168:761–773. doi: 10.1083/jcb.200409045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van der Zand A., Gent J., Braakman I., Tabak H.F. Biochemically Distinct Vesicles from the Endoplasmic Reticulum Fuse to Form Peroxisomes. Cell. 2012;149:397–409. doi: 10.1016/j.cell.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 106.Sugiura A., Mattie S., Prudent J., McBride H.M. Newly Born Peroxisomes Are a Hybrid of Mitochondrial and ER-Derived Pre-Peroxisomes. Nature. 2017;542:251–254. doi: 10.1038/nature21375. [DOI] [PubMed] [Google Scholar]
- 107.Vanstone J.R., Smith A.M., McBride S., Naas T., Holcik M., Antoun G., Harper M.-E., Michaud J., Sell E., Chakraborty P., et al. DNM1L- Related Mitochondrial Fission Defect Presenting as Refractory Epilepsy. Eur. J. Hum. Genet. 2016;24:1084–1088. doi: 10.1038/ejhg.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ebberink M.S., Koster J., Visser G., van Spronsen F., Stolte-Dijkstra I., Smit G.P.A., Fock J.M., Kemp S., Wanders R.J.A., Waterham H.R. A Novel Defect of Peroxisome Division Due to a Homozygous Non-Sense Mutation in the PEX11β Gene. J. Med. Genet. 2012;49:307–313. doi: 10.1136/jmedgenet-2012-100778. [DOI] [PubMed] [Google Scholar]
- 109.Thoms S., Gärtner J. First PEX11β Patient Extends Spectrum of Peroxisomal Biogenesis Disorder Phenotypes. J. Med. Genet. 2012;49:314–316. doi: 10.1136/jmedgenet-2012-100899. [DOI] [PubMed] [Google Scholar]
- 110.Miyagishima S., Itoh R., Toda K., Kuroiwa H., Nishimura M., Kuroiwa T. Microbody Proliferation and Segregation Cycle in the Single-Microbody Alga Cyanidioschyzon Merolae. Planta. 1999;208:326–336. doi: 10.1007/s004250050566. [DOI] [Google Scholar]
- 111.Bui H.T., Karren M.A., Bhar D., Shaw J.M. A Novel Motif in the Yeast Mitochondrial Dynamin Dnm1 Is Essential for Adaptor Binding and Membrane Recruitment. J. Cell Biol. 2012;199:613–622. doi: 10.1083/jcb.201207079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Naylor K., Ingerman E., Okreglak V., Marino M., Hinshaw J.E., Nunnari J. Mdv1 Interacts with Assembled Dnm1 to Promote Mitochondrial Division. J. Biol. Chem. 2006;281:2177–2183. doi: 10.1074/jbc.M507943200. [DOI] [PubMed] [Google Scholar]
- 113.Kalia R., Wang R.Y.-R., Yusuf A., Thomas P.V., Agard D.A., Shaw J.M., Frost A. Structural Basis of Mitochondrial Receptor Binding and Constriction by DRP1. Nature. 2018;558:401–405. doi: 10.1038/s41586-018-0211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Helle S.C.J., Feng Q., Aebersold M.J., Hirt L., Grüter R.R., Vahid A., Sirianni A., Mostowy S., Snedeker J.G., Šarić A., et al. Mechanical Force Induces Mitochondrial Fission. eLife. 2017;6:e30292. doi: 10.7554/eLife.30292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hatch A.L., Ji W.-K., Merrill R.A., Strack S., Higgs H.N. Actin Filaments as Dynamic Reservoirs for Drp1 Recruitment. Mol. Biol. Cell. 2016;27:3109–3121. doi: 10.1091/mbc.e16-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chappie J.S., Mears J.A., Fang S., Leonard M., Schmid S.L., Milligan R.A., Hinshaw J.E., Dyda F. A Pseudoatomic Model of the Dynamin Polymer Identifies a Hydrolysis-Dependent Powerstroke. Cell. 2011;147:209–222. doi: 10.1016/j.cell.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Antonny B., Burd C., Camilli P.D., Chen E., Daumke O., Faelber K., Ford M., Frolov V.A., Frost A., Hinshaw J.E., et al. Membrane Fission by Dynamin: What We Know and What We Need to Know. EMBO J. 2016;35:2270–2284. doi: 10.15252/embj.201694613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Macdonald P.J., Francy C.A., Stepanyants N., Lehman L., Baglio A., Mears J.A., Qi X., Ramachandran R. Distinct Splice Variants of Dynamin-Related Protein 1 Differentially Utilize Mitochondrial Fission Factor as an Effector of Cooperative GTPase Activity. J. Biol. Chem. 2016;291:493–507. doi: 10.1074/jbc.M115.680181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bohuszewicz O., Low H.H. Structure of a Mitochondrial Fission Dynamin in the Closed Conformation. Nat. Struct. Mol. Biol. 2018;25:722–731. doi: 10.1038/s41594-018-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bourne H.R., Sanders D.A., McCormick F. The GTPase Superfamily: Conserved Structure and Molecular Mechanism. Nature. 1991;349:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- 121.Lackner L.L., Horner J.S., Nunnari J. Mechanistic Analysis of a Dynamin Effector. Science. 2009;325:874–877. doi: 10.1126/science.1176921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koirala S., Guo Q., Kalia R., Bui H.T., Eckert D.M., Frost A., Shaw J.M. Interchangeable Adaptors Regulate Mitochondrial Dynamin Assembly for Membrane Scission. Proc. Natl. Acad. Sci. USA. 2013;110:E1342–E1351. doi: 10.1073/pnas.1300855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ganesan V., Willis S.D., Chang K.-T., Beluch S., Cooper K.F., Strich R. Cyclin C Directly Stimulates Drp1 GTP Affinity to Mediate Stress-Induced Mitochondrial Hyperfission. Mol. Biol. Cell. 2018;30:302–311. doi: 10.1091/mbc.E18-07-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tokarska-Schlattner M., Boissan M., Munier A., Borot C., Mailleau C., Speer O., Schlattner U., Lacombe M.-L. The Nucleoside Diphosphate Kinase D (NM23-H4) Binds the Inner Mitochondrial Membrane with High Affinity to Cardiolipin and Couples Nucleotide Transfer with Respiration. J. Biol. Chem. 2008;283:26198–26207. doi: 10.1074/jbc.M803132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schlattner U., Tokarska-Schlattner M., Ramirez S., Tyurina Y.Y., Amoscato A.A., Mohammadyani D., Huang Z., Jiang J., Yanamala N., Seffouh A., et al. Dual Function of Mitochondrial Nm23-H4 Protein in Phosphotransfer and Intermembrane Lipid Transfer A CARDIOLIPIN-DEPENDENT SWITCH. J. Biol. Chem. 2013;288:111–121. doi: 10.1074/jbc.M112.408633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen C.-W., Wang H.-L., Huang C.-W., Huang C.-Y., Lim W.K., Tu I.-C., Koorapati A., Hsieh S.-T., Kan H.-W., Tzeng S.-R., et al. Two Separate Functions of NME3 Critical for Cell Survival Underlie a Neurodegenerative Disorder. Proc. Natl. Acad. Sci. USA. 2019;116:566–574. doi: 10.1073/pnas.1818629116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu X., Weaver D., Shirihai O., Hajnóczky G. Mitochondrial ‘Kiss-and-Run’: Interplay between Mitochondrial Motility and Fusion–Fission Dynamics. EMBO J. 2009;28:3074–3089. doi: 10.1038/emboj.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lewis S.C., Uchiyama L.F., Nunnari J. ER-Mitochondria Contacts Couple MtDNA Synthesis with Mitochondrial Division in Human Cells. Science. 2016;353:aaf5549. doi: 10.1126/science.aaf5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abrisch R.G., Gumbin S.C., Wisniewski B.T., Lackner L.L., Voeltz G.K. Fission and Fusion Machineries Converge at ER Contact Sites to Regulate Mitochondrial Morphology. J. Cell Biol. 2020;219:e201911122. doi: 10.1083/jcb.201911122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krishnan K.S., Rikhy R., Rao S., Shivalkar M., Mosko M., Narayanan R., Etter P., Estes P.S., Ramaswami M. Nucleoside Diphosphate Kinase, a Source of GTP, Is Required for Dynamin-Dependent Synaptic Vesicle Recycling. Neuron. 2001;30:197–210. doi: 10.1016/S0896-6273(01)00273-2. [DOI] [PubMed] [Google Scholar]
- 131.Dammai V., Adryan B., Lavenburg K.R., Hsu T. Drosophila Awd, the Homolog of Human Nm23, Regulates FGF Receptor Levels and Functions Synergistically with Shi/Dynamin during Tracheal Development. Genes Dev. 2003;17:2812–2824. doi: 10.1101/gad.1096903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boissan M., Montagnac G., Shen Q., Griparic L., Guitton J., Romao M., Sauvonnet N., Lagache T., Lascu I., Raposo G., et al. Nucleoside Diphosphate Kinases Fuel Dynamin Superfamily Proteins with GTP for Membrane Remodeling. Science. 2014;344:1510–1515. doi: 10.1126/science.1253768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zala D., Schlattner U., Desvignes T., Bobe J., Roux A., Chavrier P., Boissan M. The Advantage of Channeling Nucleotides for Very Processive Functions. F1000Research. 2017;6:724. doi: 10.12688/f1000research.11561.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Boissan M., Schlattner U., Lacombe M.-L. The NDPK/NME Superfamily: State of the Art. Lab. Invest. 2018;98:164–174. doi: 10.1038/labinvest.2017.137. [DOI] [PubMed] [Google Scholar]
- 135.Ovádi J., Sreret P.A. Macromolecular Compartmentation and Channeling. In: Walter H., Brooks D.E., Srere P.A., editors. International Review of Cytology. Volume 192. Microcompartmentation and Phase Separation in Cytoplasm; Academic Press; Cambridge, MA, USA: 1999. pp. 255–280. [DOI] [PubMed] [Google Scholar]
- 136.Weber J.P., Bernhard S.A. Transfer of 1,3-Diphosphoglycerate between Glyceraldehyde 3-Phosphate Dehydrogenase and 3-Phosphoglycerate Kinase via an Enzyme-Substrate-Enzyme Complex. Biochemistry. 1982;21:4189–4194. doi: 10.1021/bi00260a042. [DOI] [PubMed] [Google Scholar]
- 137.Lascu I., Schaertl S., Wang C., Sarger C., Giartosio A., Briand G., Lacombe M.-L., Konrad M. A Point Mutation of Human Nucleoside Diphosphate Kinase A Found in Aggressive Neuroblastoma Affects Protein Folding. J. Biol. Chem. 1997;272:15599–15602. doi: 10.1074/jbc.272.25.15599. [DOI] [PubMed] [Google Scholar]
- 138.Warnock D.E., Hinshaw J.E., Schmid S.L. Dynamin Self-Assembly Stimulates Its GTPase Activity. J. Biol. Chem. 1996;271:22310–22314. doi: 10.1074/jbc.271.37.22310. [DOI] [PubMed] [Google Scholar]
- 139.Kim Y.-I., Park S., Jeoung D.-I., Lee H. Point Mutations Affecting the Oligomeric Structure of Nm23-H1 Abrogates Its Inhibitory Activity on Colonization and Invasion of Prostate Cancer Cells. Biochem. Biophys. Res. Commun. 2003;307:281–289. doi: 10.1016/S0006-291X(03)01195-1. [DOI] [PubMed] [Google Scholar]
- 140.Michalska B.M., Kwapiszewska K., Szczepanowska J., Kalwarczyk T., Patalas-Krawczyk P., Szczepański K., Hołyst R., Duszyński J., Szymański J. Insight into the Fission Mechanism by Quantitative Characterization of Drp1 Protein Distribution in the Living Cell. Sci. Rep. 2018;8:1–15. doi: 10.1038/s41598-018-26578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hubley M.J., Locke B.R., Moerland T.S. The Effects of Temperature, PH, and Magnesium on the Diffusion Coefficient of ATP in Solutions of Physiological Ionic Strength. Biochim. Biophys. Acta BBA—Gen. Subj. 1996;1291:115–121. doi: 10.1016/0304-4165(96)00053-0. [DOI] [PubMed] [Google Scholar]
- 142.Biess A., Korkotian E., Holcman D. Barriers to Diffusion in Dendrites and Estimation of Calcium Spread Following Synaptic Inputs. PLoS Comput. Biol. 2011;7:e1002182. doi: 10.1371/journal.pcbi.1002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guerrier C., Holcman D. The First 100 Nm Inside the Pre-Synaptic Terminal Where Calcium Diffusion Triggers Vesicular Release. Front. Synaptic Neurosci. 2018;10:23. doi: 10.3389/fnsyn.2018.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Du M., Chen Z.J. DNA-Induced Liquid Phase Condensation of CGAS Activates Innate Immune Signaling. Science. 2018;361:704–709. doi: 10.1126/science.aat1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bergeron-Sandoval L.-P., Heris H.K., Hendricks A.G., Ehrlicher A.J., François P., Pappu R.V., Michnick S.W. Endocytosis Caused by Liquid-Liquid Phase Separation of Proteins. bioRxiv. 2017:145664. [Google Scholar]
- 146.Bianchi-Smiraglia A., Rana M.S., Foley C.E., Paul L.M., Lipchick B.C., Moparthy S., Moparthy K., Fink E.E., Bagati A., Hurley E., et al. Internally Ratiometric Fluorescent Sensors for Evaluation of Intracellular GTP Levels and Distribution. Nat. Methods. 2017;14:1003–1009. doi: 10.1038/nmeth.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]