Abstract
Malaria during pregnancy results in intrauterine growth restriction, fetal anemia, and infant mortality. Women are more susceptible to malaria during pregnancy due to malaria‐induced inflammation and the sequestration of infected red blood cells in the placenta, which bind to the chondroitin sulfate portion of syndecan‐1 on the syncytiotrophoblast and in the intervillous space. Syndecan‐1 is a dimeric proteoglycan with an extracellular ectodomain that is cleaved from the transmembrane domain (referred to as “shedding”) by matrix metalloproteinases (MMPs), likely the secreted MMP‐9. The ectodomain includes four binding sites for chondroitin sulfate, which are proximal to the transmembrane domain, and six distal binding sites primarily for heparan sulfate. This “shedding” of syndecan‐1 is inhibited by the presence of the heparan sulfate chains, which can be removed by heparanase. The intervillous space contains fibrin strands and syndecan‐1 ectodomains free of heparan sulfate. The following is proposed as the sequence of events that leads to and is primarily responsible for sequestration in the intervillous space of the placenta. Inflammation associated with malaria triggers increased heparanase activity that degrades the heparan sulfate on the membrane‐bound syndecan‐1. Inflammation also upregulates MMP‐9 and the removal of heparan sulfate gives MMP‐9 access to cleave syndecan‐1, thereby releasing dimeric syndecan‐1 ectodomains with at least four chondroitin sulfate chains attached. These multivalent ectodomains bind infected RBCs together leading to their aggregation and entrapment in intervillous fibrin. This mechanism suggests possible new targets for anti‐placental malaria drugs such as the inhibition of MMP‐9. Doxycycline is an antimalarial drug which inhibits MMP‐9.
Keywords: fibrin, heparanase, malaria, metalloproteinase, placenta, sequestration, syndecan‐1
Abbreviations
- CSA
chondroitin sulfate A
- CSC
chondroitin sulfate C
- CSPG
chondroitin sulfate proteoglycan
- DBL
Duffy binding like
- kDa
kilodaltons
- MMP
matrix metalloproteinase
- MT‐MMPs
membrane‐type matrix metalloproteinase
- PfEMP1
Plasmodium falciparum erythrocyte membrane protein 1
- RBC
red blood cell
1. INTRODUCTION
Pregnant women are more susceptible to malaria and more severely affected (Beaudet et al., 2014; Huynh, Cottrell, Cot, & Briand, 2015; Kourtis, Read, & Jamieson, 2014; Menendez, 2006; Moore et al., 2017). This is particularly true for pregnant women infected with Plasmodium falciparum due to inflammation and cell sequestration in the placenta (referred to as placental malaria). P. falciparum causes the most severe disease and is the most deadly among several species of the unicellular protozoan genus Plasmodium (White et al., 2014). The next most important species is Plasmodium vivax, which causes less placental infection and no observed placental inflammation (Carter & Mendis, 2002; Chaikitgosiyakul et al., 2014; Mayor et al., 2012; McGready et al., 2004).
The virulence of P. falciparum is largely due to two features. First, P. falciparum parasites can infect all red blood cells (RBCs) whereas P. vivax infects only reticulocytes and relatively young erythrocytes (Douglas et al., 2012). Second, RBCs infected by certain stages (trophozoites and schizonts) of P. falciparum can adhere to the endothelial walls of blood vessels, other RBCs, and platelets leading to extensive sequestration in the microvasculature (Franke‐Fayard, Fonager, Braks, Khan, & Janse, 2010; Haldar, Murphy, Milner, & Taylor, 2007; Rowe, Claessens, Corrigan, & Arman, 2009; Udeinya, Miller, McGregor, & Jensen, 1983). This allows these parasites to evade the spleen where the infected RBCs could be removed from circulation and destroyed. Adhesion and sequestration occurs with P. vivax (Costa et al., 2011) but to a lesser extent than P. falciparum. In areas where P. falciparum malaria is endemic, the most sensitive population is children who have not yet developed immunity to malaria. The next most sensitive population is pregnant women, particularly primigravida and secundigravida, who may have immunity to common malaria (to be referred to as nonplacental malaria or peripheral malaria) but not to the pronounced sequestration of infected RBCs that occur in placental malaria.
1.1. The asexual P. falciparum life cycle and inflammation
Humans get malaria when they receive a bite from an infected female mosquito of the genus Anopheles (Michalakis & Renaud, 2009). Parasites infect the salivary glands of the mosquito and the malarial sporozoites are carried into the host blood in the saliva from the mosquito (Beier, 1998). Sporozoites infect hepatocytes in the liver where, over a period of 8–10 days, they develop into schizonts that each can release about a thousand merozoites into the blood. Merozoites rapidly invade RBCs where the parasites feed on hemoglobin which is plentiful in infected red cells. Free heme is generated and detoxified in the parasite's digestive vacuole by converting it into inert dark brown crystals referred to as malaria pigment or hemozoin. Merozoites develop into the ring stage which then go on to develop into trophozoites and then schizonts. Infected RBCs containing schizonts then burst and release more merozoites into the blood (Cowman & Crabb, 2006). RBCs infected with trophozoites and schizonts are the ones capable of adhering to the endothelium and thus the form of the parasite most commonly observed in a peripheral blood smear is the ring form which infects RBCs that do not adhere to the endothelium.
When the schizonts burst to release new merozoites, they also release hemozoin, cytokines, and other toxic factors. The hemozoin and cytokines are pro‐inflammatory and attract leukocytes to the sites of sequestration (Ordi et al., 1998; Pichyangkul, Saengkrai, & Webster, 1994; Prato, Giribaldi, Polimeni, Gallo, & Arese, 2005; Sherry et al., 1995). The resulting inflammation is responsible for the characteristic symptoms of severe malaria such as fever, chills, and impaired consciousness (Clark, Budd, Alleva, & Cowden, 2006; Cowman & Crabb, 2006; Rasti, Wahlgren, & Chen, 2004; Vogetseder, Ospelt, Reindl, Schober, & Schmutzhard, 2004).
1.2. Mechanism of adhesion and sequestration in nonplacental malaria
The P. falciparum parasites generate protein receptors, referred to as P. falciparum erythrocyte membrane protein 1 (PfEMP1) receptors, that become embedded in the plasma membrane of the RBC (Gamain, Gratepanche, Miller, & Baruch, 2002; Rowe et al., 2009). These receptors form clusters on the cell surface, which are referred to as knobs (Duffy & Fried, 1999; Udeinya et al., 1983). Different forms of the PfEMP1 receptors can bind to a variety of targets (including endothelial surface molecules such as CD36, ICAM1, thrombospondin, VCAM‐1, E‐selectin, P‐selectin, and CD31; Rowe et al., 2009) but each infected RBC commonly binds to one type of target receptor. In general, sequestration in the microvasculature results from the binding of the cysteine‐rich interdomain region 1 of PfEMP1 receptors to the CD36 glycoprotein receptors on the surface of endothelium, RBCs, infected RBCs, and platelets (Gamain et al., 2002; Rowe et al., 2009). The binding selectivity of the PfEMP1 receptor can be switched at each new asexual cycle allowing the parasite to adapt to new conditions.
The sequestration leads to microcirculatory obstruction which causes impaired tissue perfusion leading to local hypoxia (Clark et al., 2006). Infected RBCs are better at adhering to the vascular endothelium of certain tissues including the bone marrow (Ekvall, 2003; Haldar & Mohandas, 2009; Wickramasinghe & Abdalla, 2000; Wickramasinghe, Phillips, Looareesuwan, Warrell, & Hughes, 1987). This adhesion in the bone marrow impacts RBC production which contributes to anemia (Franke‐Fayard et al., 2010; Haldar & Mohandas, 2009; Lamikanra et al., 2007; Obaldia et al., 2018; Smalley, Abdalla, & Brown, 1980). With successive malarial infections, the host becomes able to generate antibodies that block the binding of the PfEMP1 receptors to the CD36 glycoprotein receptors (Doolan, Dobaño, & Baird, 2009). In endemic areas, most adults have acquired this immunity.
2. MALARIA IN PREGNANCY
During pregnancy, women in endemic areas become more susceptible to malaria infection and are more severely affected. This sensitivity is due to the generation of infected RBCs with PfEMP1 receptors with active Duffy binding‐like (DBL)γ domains (referred to as VAR2CSA receptors) that can bind to the chondroitin sulfate A (CSA) portion of chondroitin sulfate A proteoglycans which is the target receptor in the placenta. These infected RBCs with VAR2CSA receptors start out as a small portion of the total infected RBCS but are selected for and develop rapidly (Achur, Valiyaveettil, Alkhalil, Ockenhouse, & Gowda, 2000; Brabin et al., 2004; Salanti et al., 2004; Srivastava et al., 2010; Ezebialu et al., 2012; Ouédraogo et al., 2012; Kalilani‐Phiri et al., 2013; Pehrson et al., 2016; Sharma & Shukla, 2017). Women in endemic areas likely have immunity to the common form of malaria caused by infected RBCs that bind to CD36 receptors but primigravidae do not have immunity to VAR2CSA‐binding infected RBCs that bind in the placenta and cause placental malaria. Infected pregnant women eventually form antibodies that block the binding of infected RBCs to chondroitin sulfate A proteoglycans and infections during subsequent pregnancies are not as severe (Desai et al., 2007; Duffy & Fried, 2003; Ezebialu et al., 2012; McLean, Ataide, Simpson, Beeson, & Fowkes, 2015; Moore et al., 2017; Rogerson, 2017; Rogerson, Hviid, Duffy, Leke, & Taylor, 2007; Sharma & Shukla, 2017; Uneke, 2007a, 2007b).
The prevalence of malaria in pregnancy depends on the stage of gestation. For example, in one study, the prevalence of peripheral malaria for primigravidae in Kenya peaked at 85.7% between gestation Weeks 13 and 16 and then gradually decreased to 42.9% between gestation Weeks 33 and 36 (Brabin, 1983). The pattern for multigravidae was similar but lower with a peak of 51.7% between gestation Weeks 13 and 16 and 23.5% between Weeks 33 and 36. In an area of low transmission (the Thailand–Myanmar border), a total of 16.4% (8221) of 50,060 pregnant women had falciparum and/or vivax malaria (6.3% with falciparum malaria only, 7.2% with vivax malaria only and 3.0% with both; Moore et al., 2017). In this study, the peak rates of malaria were observed at gestation Weeks 6 and 5 for P. falciparum and P. vivax, respectively.
3. CHARACTERISTICS OF PLACENTAL MALARIA
Infected RBCs with VAR2CSA receptors are found attached to the apical lining of the syncytiotrophoblast but even more notably in the intervillous space, sometimes together with monocytes and macrophages with ingested pigment (Beeson, Amin, Kanjala, & Rogerson, 2002; Brabin, 1983; Chaikitgosiyakul et al., 2014; de Moraes, Tadokoro, Gómez‐Conde, Olivieri, & Penha‐Gonçalves, 2013; Duffy & Fried, 2001a, 2001b; Fried & Duffy, 1996; Imamura, Sugiyama, Cuevas, Makunde, & Nakamura, 2002; Ismail et al., 2000; Muthusamy et al., 2004; Walter, Garin, & Blot, 1982). This congestion leads to decreased uterine artery blood flow (Brabin & Johnson, 2005; Dorman et al., 2002; Griffin et al., 2012; Umbers, Aitken, & Rogerson, 2011). Patients with placental malaria may or may not also have an apparent peripheral blood infection. In one study, among 109 patients with acute placental malarial, peripheral blood malaria was apparent by microscopy in 47% of cases (Ismail et al., 2000). In another study, 254 (70%) of 365 women with peripheral malaria also had parasitized term placentas resulting in a significant (p < 0.001) association between peripheral malaria and placental malaria (Ezebialu et al., 2012).
3.1. Prevalence of placental malaria
As would be expected, placental malaria is more common in areas where malaria is endemic (Fehintola et al., 2017). In sub‐Saharan Africa, placental malaria is almost entirely due to P. falciparum. Across 21 studies conducted in nine sub‐Saharan African countries in which evidence of malaria was not a criterion for enrollment (studies were reported between 1925 and 2017), the percentage of 10,032 term pregnancies that were infected with placental malaria was 29.9% (Adebami, Owa, Oyedeji, Oyelami, & Omoniyi‐Esan, 2007; Anagnos, Lanoie, Palmieri, Ziefer, & Connor, 1986; Bassey, Nyengidiki, & John, 2015; Brabin, 1983; Bulmer, Rasheed, Francis, Morrison, & Greenwood, 1993; Ezebialu et al., 2012; Fehintola et al., 2017; Kalilani‐Phiri et al., 2013; Matteelli et al., 1994; Menendez et al., 2000). In areas of low transmission, the rate of placental malaria is lower. Among 400 pregnant women in a study conducted in Panama (Clark, 1915), there were 19 with placental malaria (4.8%).
3.2. Relationship between placental malaria and stage of gestation
In most studies, the definitive diagnosis of placental malaria has been based on the histological examination of the term placenta and, in general, there is little information about the extent of placental malaria early in gestation (Cohee et al., 2016; Valea et al., 2012). Reports of the stages of pregnancy that can be affected include women who are earlier than 4 months of pregnancy (Cottrell, Mary, Barro, & Cot, 2007), earlier than 20 weeks of gestation (Griffin et al., 2012), and 10 weeks of gestation and greater (Valea et al., 2012). In another study, 46 of 75 (48%) women had parasitized placentas following peripheral malaria detected during the second trimester and 10 of 75 (13%) had parasitized placentas following peripheral malaria detected during the third trimester (Kalilani‐Phiri et al., 2013).
Other studies, though, have used methods to detect the presence of placental infected RBCs in peripheral blood prior to delivery. For example, isolates of infected RBCs collected from peripheral blood can be evaluated for transcripts of the var2csa gene by polymerase chain reaction (PCR; Snounou et al., 1993; Mockenhaupt, Ulmen, von Gaertner, Bedu‐Addo, & Bienzle, 2002). In a study in Benin, pregnant women were enrolled during the first or second trimester. At enrollment, the median gestational age was 15.9 weeks and the interquartile range was 13.7–17.0 weeks. Peripheral blood was collected periodically resulting in 50 samples collected prior to delivery and 41 samples at delivery (Doritchamou et al., 2012). For analysis, the samples were placed into three categories based on when they were collected—prior to gestation week 13, after gestation week 13 but before delivery, and at delivery. A variety of approaches including the evaluation of var2csa gene transcripts using PCR was used to show that some of the women from all stages had infected RBCs in peripheral blood with the placental phenotype (i.e., binding to chondroitin sulfate A) and that the affinity of the infected RBCs for chondroitin sulfate was similar for the three stages of gestation evaluated.
3.3. Stages of infection in the placenta
The stages of infection within the placenta have been identified histologically and one system of classification originally developed by Bulmer, Rasheed, Francis, et al. (1993), Bulmer, Rasheed, Morrison, Francis, and Greenwood (1993), and subsequently followed by Adebami et al. (2007) and Ezebialu et al. (2012), is shown below:
Active infection: Parasites and pigments in maternal RBCs in the intervillous spaces but no pigment or cells within fibrin.
Active‐on‐past infection: Parasites and pigment in maternal RBCs and pigment and/or cells within fibrin.
Past infection: Parasites not present and pigment and/or cells confined to fibrin.
In Bulmer, Rasheed, Francis, et al. (1993), 121 placentas from women in The Gambia were examined and 9.9% had active infections, 16.5% had active‐on‐past infections, and 29.8% showed evidence of past infections. In Ezebialu et al. (2012), 365 placentas from women in Nigeria were examined, 6.3% showed active infection, 53.7% showed active‐on‐past infections, and 9.6% showed past infection. Note: in Bulmer, Rasheed, Francis, et al. (1993), fibrin is described as both intervillous fibrin and perivillous fibrin although detailed definitions are not provided. The terminology and definitions to be used below are as follows. The term intervillous fibrin will refer to the phenomenon wherein fibrin strands are observed across the intervillous space of the placenta and are not limited to the space close to the villi. Dense deposits of fibrin that occur on the surface of the villi will be referred to as perivillous fibrinoids (after Frank et al. (1994) and Kaufmann, Huppertz, and Frank (1996)).
3.4. Other factors
In addition to sequestration in the placenta, the severity of the disease and the outcome of the pregnancy in women with malaria are adversely impacted by the following factors:
Maternal anemia (Brabin, 1983; Matteelli et al., 1994; Guyatt & Snow, 2001; Uneke, 2007a, 2007b; Mokuolu et al., 2009; Prentice et al., 2010; Verhoef et al., 2010; Kalilani‐Phiri et al., 2013; Bassey et al., 2015; Maternal and Child Survival Program (MCSP), President's Malaria Initiative (PMI), and Centers for Disease Control (CDC), 2015; Fehintola et al., 2017);
Pre‐eclampsia (Brabin & Johnson, 2005), and,
Intervillous infiltration of mononuclear inflammatory cells and macrophages (Carmona‐Fonseca, Arango, & Maestre, 2013; Imamura et al., 2002; Ismail et al., 2000; Maeno et al., 1993; Muehlenbachs et al., 2010; Ordi et al., 1998; Sharma & Shukla, 2017; Uneke, 2007a) that are associated with the malaria‐induced release of chemokines and cytokines in the placenta (Fried et al., 1998; Suguitan et al., 2003).
4. IMPACT OF MALARIA ON THE OFFSPRING
The potential impact of malaria on the offspring is reviewed in Menendez et al. (2000), Dorman et al. (2002), Uneke (2007a), (2007b), and De Beaudrap et al. (2013). One common effect is intrauterine growth restriction and low birth weight (Adebami et al., 2007; Bassey et al., 2015; Brabin et al., 2004; Chandrasiri et al., 2014; Cottrell et al., 2007; de Moraes et al., 2013; Gaccioli & Lager, 2016; Griffin et al., 2012; Huynh et al., 2015; Matteelli, Caligaris, Castelli, & Carosi, 1997; Mokuolu et al., 2009; Moore et al., 2017; Rasti et al., 2004; Rijken et al., 2012; Umbers et al., 2011). In a study in Burkina Faso (Valea et al., 2012), women first infected in the first trimester were more likely to have babies with low birth weight (incidence 13/31 = 42%) than women first infected in the second and third semesters. In a study of 832 infants in Uganda (De Beaudrap et al., 2016), postnatal growth was most affected if the mother had had malaria during the last 12 weeks of pregnancy. Malaria in pregnancy is also associated with prenatal, perinatal, and postnatal mortality (McGready et al., 2012; Schantz‐Dunn & Nour, 2009) and it has been estimated that 75,000–200,000 infant deaths each year are attributable to malaria in pregnancy (Steketee, Nahlen, Parise, & Menendez, 2001).
Other potential adverse effects on the offspring are fetal anemia (Brabin et al., 2004; le Cessie et al., 2002; Moore et al., 2017; Uneke, 2007b), postnatal anemia (Accrombessi et al., 2015), preterm birth (De Beaudrap et al., 2016; Dorman et al., 2002; Menendez et al., 2000; Schantz‐Dunn & Nour, 2009), and transplacental transmission of malaria/congenital malaria (Bergström et al., 1993; De Beaudrap et al., 2016).
5. ROLE OF CHONDROITIN SULFATE PROTEOGLYCANS IN PLACENTAL MALARIA
5.1. Glycosaminoglycans and proteoglycans in humans
The endothelial glycocalyx consists of a network of membrane‐bound proteoglycans and glycoproteins which cover the endothelium on the luminal side (Pries, Secomb, & Gaehtgens, 2000; Reitsma, Slaaf, Vink, van Zandvoort, & oude Egbrink, 2007). Proteoglycans consist of core proteins with attached glycosaminoglycans (GAGs). GAGs are unbranched chains of repeating disaccharide units and, with the exceptions of heparin and hyaluronic acid, are attached to core proteins. Proteoglycans can have from 1 to more than 100 GAG side chains, each attached to a serine side chain on the core protein via a specific tetrasaccharide link (Alberts et al., 2015). Also, the length of the GAG side chains varies. For most proteoglycans, the attached GAGs consist of chains of chondroitin sulfate and/or heparan sulfate (Alexopoulou, Multhaupt, & Couchman, 2007; Deepa et al., 2004; Elenius & Jalkanen, 1994; Kokenyesi & Bernfield, 1994). The sulfation of chondroitin and heparan occurs by chondroitin sulfate sulfotransferases and heparan sulfate transferases, respectively. Sulfate groups can be removed from some GAGs by extracellular endosulfatases (Morimoto‐Tomita, Uchimura, Werb, Hemmerich, & Rosen, 2002; Yang et al., 2007).
Chondroitin sulfate is actually the name of a family of GAGs. The disaccharide repeating units of chondroitin sulfate can consist of d‐glucuronic acid and N‐acetylgalactosamine with N‐acetylgalactosamine being sulfated in the 4‐carbon position (referred to as chondroitin‐4‐sulfate or chondroitin sulfate A [CSA]) or the 6‐carbon position (referred to as chondroitin‐6‐sulfate or chondroitin sulfate C [CSC]). In some cases, both chondroitin sulfate A disaccharides and chondroitin sulfate C disaccharides can be included within the same chain. Alternatively, the disaccharide can consist of l‐iduronic acid (instead of glucuronic acid) and galactosamine (instead of N‐acetylgalactosamine) with the 4‐carbon position of the galactosamine being sulfated (referred to as chondroitin sulfate B or dermatan sulfate). Chondroitin sulfate proteoglycans (CSPGs) are cell surface and extracellular matrix components.
5.2. Evidence that the ligand for placental malaria is chondroitin sulfate A
Various lines of evidence suggest that infected RBCs with VAR2CSA receptors bind specifically to the chondroitin sulfate A portion of chondroitin sulfate A proteoglycans on the surface of the syncytiotrophoblast and in the intervillous space of the placenta. There is also extensive evidence that these infected RBCs with VAR2CSA receptors that bind to chondroitin sulfate A are responsible for the sequestration of cells in the placenta. First, Rogerson, Chaiyaroj, Ng, Reeder, and Brown (1995) showed that RBCs infected with certain strains of P. falciparum can bind to chondroitin sulfate A attached to a plastic Petri dish. Fried and Duffy (1996) showed that:
Infected RBCs from term infected placentas would bind to chondroitin sulfate A attached to a plastic Petri dish but not to other extracellular matrix proteins or to other known IRBC receptors, whereas infected RBCs from nonpregnant donors did not bind to chondroitin sulfate A.
Infected RBCs from infected placentas would also bind within the intervillous space in sections from uninfected term placentas and this binding was inhibited by chondroitin sulfate A.
Peripheral infected RBCs from nonpregnant women bound to CD36 attached to a plastic Petri dish but not to chondroitin sulfate A. Peripheral infected RBCs from pregnant women could bind to either CD36 or chondroitin sulfate A.
Gysin, Pouvelle, Fievet, Scherf, and Lépolard (1999) showed that soluble chondroitin sulfate A can break apart the clusters of cells involved in placental malaria.
These findings were confirmed and extended in multiple subsequent studies (Achur et al., 2000; Beeson, Chai, Rogerson, Lawson, & Brown, 1998; Cooke, Rogerson, Brown, & Coppel, 1996; Duffy & Fried, 1999; Fried & Duffy, 1998; Fried, Lauder, & Duffy, 2000; Pouvelle, Fusaï, Lépolard, & Gysin, 1998). More recently, it was shown in an ex vivo model of perfused placental tissue that soluble chondroitin sulfate A and specific antibodies directed against VAR2CSA inhibited the binding of infected RBCs (Pehrson et al., 2016). Also, it was found that soluble chondroitin sulfate A inhibited the binding of the recombinant VAR2CSA receptor to both the syncytiotrophoblast and the intervillous mesh in tissue sections of term placentas (Ayres Pereira et al., 2016). The same authors reported that an antibody to chondroitin sulfate (CS‐56), which binds to both chondroitin sulfate A and chondroitin sulfate C, bound in placental tissue sections to both the syncytiotrophoblast and the intervillous mesh with a pattern similar to that of VAR2CSA as well as the truncated active portion of recombinant VAR2CSA previously reported on by Clausen et al. (2012) (referred to in Ayres Pereira et al. (2016), as rVAR2).
5.3. Characterization of the VAR2CSA receptor for chondroitin sulfate A
Clausen et al., 2012 studied the properties of different fragments of the PfEMP1 receptor VAR2CSA including the binding to isolated placental CSPG samples. They identified a minimal chondroitin sulfate A binding region (ID1‐DBL2Xb) with a molecular weight (MW) of 62 kDa that retained high affinity binding (K D = 21.8 nM) to a CSPG, decorin, which is a small proteoglycan (MW ~40 kDa) with a single chondroitin sulfate chain. Larger VAR2CSA fragments which contained this 62 kDa segment had somewhat greater affinity including FV2 (the full‐length VAR2CSA protein without the N‐terminal segment) for which the K D was 5.2 nM.
5.4. The size of the binding site for VAR2CSA on chondroitin sulfate A
Several studies have contributed to the characterization of the binding site(s) on the chondroitin sulfate A ligand. Both Beeson et al. (1998) and Pouvelle et al. (1998) prepared chondroitin sulfate A chains of various lengths and studied their effects on the binding of infected RBCs to chondroitin sulfate A attached to plastic Petri dishes. Beeson et al. observed that 90 μg/mL of an oligosaccharide with seven chondroitin sulfate A disaccharide units reduced infected RBC binding to 50% of control whereas 90 μg/mL of an oligosaccharide with eight chondroitin sulfate A disaccharide units reduced infected RBC binding to 20% of control. The same concentration of an oligosaccharide with seven chondroitin sulfate C disaccharide units did not inhibit infected RBC adhesion. Pouvelle et al. observed that 100 μg/mL of a chain of approximately 4 kDa (~9 chondroitin sulfate A disaccharide units) was needed to inhibit the binding of infected RBCs to chondroitin sulfate A by 50% and a chain of approximately 9 kDa (~19 disaccharide units) was needed to inhibit the binding by the same amount as the full VAR2CSA molecule.
Alkhalil, Achur, Valiyaveettil, Ockenhouse, and Gowda (2000) measured the adhesion of chondroitin sulfate A‐adherent infected RBCs to purified CSPGs from human placental intervillous space. They prepared oligosaccharides of chondroitin sulfate A of various sizes and with varying extent of sulfation and measured the ability of these oligosaccharides to inhibit the adhesion of the infected RBCs to chondroitin sulfate A. They reported that the maximal inhibition of infected RBC binding (90–93% inhibition) was seen with oligosaccharides consisting of six chondroitin disaccharide units and about 30–38% of maximal 4‐sulfate content. Oligosaccharides consisting of six chondroitin sulphate C disaccharide units and 89–98% of maximal sulfate content caused a lesser (32–36%) inhibition of infected RBC binding. Oligosaccharides consisting of six chondroitin sulfate C disaccharide units had no binding inhibitory activity. Chai, Beeson, and Lawson (2002) observed that the minimum chain length for effective inhibition of infected RBC binding consisted of six chondroitin disaccharide units with four or five being chondroitin sulfate A and one or two being unsulfated.
Thus, it seems likely that the binding site for VAR2CSA on placental chondroitin sulfate A consists of between 6 and 19 chondroitin disaccharide units.
5.5. Placental proteoglycans
5.5.1. Distribution and characterization of chondroitin sulfate and heparan sulfate in the term human placenta
Achur et al. (2000) studied CSPGs and heparan sulfate from the uninfected term human placenta and the binding of infected RBCs to these CSPGs. The CSPGs that they identified were described as two major low‐sulfate CSPGs from the intervillous space, minor amounts of two different CSPGs associated with cells, and major amounts of dermatan sulfate‐like CSPG from the fibrous tissue. In the more common CSPG from the intervillous space, about one‐half of the N‐acetylgalactosamine groups in the GAG chains were sulfated in the 4‐position. In the less common CSPG from the intervillous space, about 1 in 10 of the N‐acetylgalactosamine groups in the GAG chains were sulfated in the 4‐position. There were also two primary CSPGs identified as being cell associated and these were also not fully sulfated with about one‐half of the N‐acetylgalactosamine groups being sulfated.
Achur et al., 2000 detected no heparan sulfate in the material from the intervillous space, whereas approximately 60% of the GAGs from the cell‐associated proteoglycans were degraded by heparinase III, indicating a large content of heparan sulfate.
Achur et al. (2000) also observed high affinity binding of infected RBCs to both the intervillous space‐derived CSPG and cell‐associated CSPG with greater affinity observed for the intervillous space‐derived CSPG. For both types of CSPGs, the binding was inhibited by soluble chondroitin sulfate A and prevented by pretreating the CSPGs with chondroitinase ABC which degrades chondroitin sulfate A, chondroitin sulfate B, and chondroitin sulfate C.
Beaudet et al. (2014) washed excess blood from three whole human placentas using chilled phosphate buffered saline and then dissected them into three regions—the cotyledons, the chorionic plate, and umbilical cord. The GAGs of each of the three regions were characterized. In the cotyledons (which presumably included much of the contents of the intervillous space), the GAGs included not only chondroitin sulfate A and unsulfated disaccharides but also chondroitin sulfate 6 and heparan sulfate. Among the three placentas, the ratio of chondroitin sulfate to heparan sulfate in the cotyledons ranged from 3 to 5. Achur et al. (2000) had not detected the presence of chondroitin sulfate C (C6S) in placental CSPG. Beaudet et al. had the following explanation: “The observation of 6S disaccharide and other minor disaccharides in the current study may be attributable to the inclusion of GAGs from the fibrous scaffold of the lobes and/or the increased sensitivity of the ultraperformance liquid chromatography‐mass spectrometric method used.”
Beaudet et al. also evaluated the binding of the GAG preparations from the three regions to the 62‐kDa chondroitin sulfate A binding fragment from VAR2CSA (ID1‐DBL2Xb) studied by Clausen et al. (2012). The affinity for the binding to the chondroitin sulfate A‐binding region (ID1‐DBL2Xb) by chondroitin sulfate isolated from the cotyledons was only slight less (K D = 37 nM) than that obtained for the binding to the chondroitin sulfate A‐binding region (ID1‐DBL2Xb) by the CSPG decorin (K D = 21.8 nM).
5.5.2. Syndecan‐1
One of the proteoglycans of the endothelial glycocalyx of the human syncytiotrophoblast is syndecan‐1 (Jokimaa et al., 1998; Szabo et al., 2013). Syndecans are proteoglycan components of both the cell surface and the extracellular matrix. Syndecan‐1 exists as a dimer and each monomeric polypeptide has 311 amino acid residues, a molecular weight of 33 kDa and includes a short cytoplasmic domain of 34 amino acid residues, a single transmembrane domain of ~42 amino acid residues, and an extracellular ectodomain of ~235 amino acid residues (Elenius & Jalkanen, 1994; Kokenyesi & Bernfield, 1994; Manon‐Jensen, Itoh, & Couchman, 2010; Saunders, Jalkanen, O'Farrel, & Bernfield, 1989). Each syndecan‐1 monomer has five serine–glycine sites that serve as the attachment points for glycosylation. There are two sites on the end of the ectodomain proximal to the transmembrane domain that bind only chondroitin sulfate chains and three sites at the distal end of the ectodomain that primarily bind heparan sulfate but can also bind short chondroitin sulfate chains (Kokenyesi & Bernfield, 1994; Manon‐Jensen et al., 2010; Saunders et al., 1989). Depending on the source, the MW of syndecan‐1 monomers including the GAG chains range from 100 kDa to greater than 300 kDa (Ramani, Pruett, Thompson, DeLucas, & Sanderson, 2012).
Syndecan 1 is strongly expressed on the apical surface of syncytiotrophoblast cells (Jokimaa et al., 1998). The extracellular ectodomain can be cleaved from the transmembrane portion by matrix metalloproteinases (MMPs, discussed below) and become soluble (Bernfield et al., 1999). During gestation, the concentrations of syndecan‐1 (presumably ectodomain) in the serum from peripheral blood increased steadily at each time point (nonpregnant and gestational Weeks 10, 20, 30, and 38) through gestational Week 30 and, at gestational Week 38, there was a 159‐fold increase compared to nonpregnant controls (Hofmann‐Kiefer et al., 2013). In contrast, the serum concentrations of heparan sulfate remained fairly constant during gestation and, at gestational Week 38, represented only approximately 0.05% of the level of syndecan‐1.
Two findings suggest that the binding site for the VAR2CSA receptors on infected RBCs in the placenta is syndecan‐1 with chondroitin sulfate A chains (Ayres Pereira et al., 2016). First, syndecan‐1 was one of two CSPGs from placental extracts that bound to rVAR2 (the minimal active portion of recombinant VAR2CSA). Second, in a proximity ligation assay using tissue from third trimester placentas that had been perfused with RBCs infected with VAR2CSA‐expressing parasites, it was demonstrated that an antibody to syndecan‐1 colocalized with antibodies to VAR2CSA and rVAR2, binding to both the syncytiotrophoblast and the intervillous mesh.
Despite the extensive knowledge about the importance of syndecan‐1 and chondroitin sulfate in the sequestration of infected RBCs in the placenta, the precise mechanism for this accumulation of infected RBCs in placental malaria is not known.
5.5.3. Metalloproteinases
The extracellular ectodomain of syndecan‐1 and other proteoglycans can be enzymatically cleaved close to the transmembrane domain by MMPs generating soluble proteoglycan ectodomains (referred to as “shedding”; Bernfield et al., 1999). MMPs are a family of zinc‐dependent endopeptidases that primarily act outside the cell and are involved in the breakdown of the extracellular matrix. MMPs are either secreted and soluble (Lichtenthaler et al., 2018), embedded in the plasma membrane (i.e., transmembrane) or outside the cell but anchored to a glycosylphosphatidylinositol group on the cell surface (Manon‐Jensen et al., 2010). The latter two types are referred to as membrane‐type MMPs (MT‐MMPs) and are primarily involved in pericellular degradation of the extracellular matrix whereas soluble MMPs are not constrained to the pericellular space (Itoh et al., 2001). Syndecan shedding by MMPs can be induced by several inflammatory factors associated with pathological conditions such as infection (Hayashida, Bartlett, Chen, & Park, 2010; Manon‐Jensen et al., 2010; Nam & Park, 2012; Park, Pier, Hinkes, & Bernfield, 2001; Teng, Aquino, & Park, 2012). The soluble syndecan‐1 ectodomain can compete with the intact membrane‐bound form for binding to extracellular ligands (Manon‐Jensen et al., 2010).
The MMPs that are both expressed in the syncytiotrophoblast (Chen & Khalil, 2017; Hiden et al., 2012; Kaitu'u‐Lino et al., 2012; Kaitu'u‐Lino, Palmer, Tuohey, Ye, & Tong, 2012; Majali‐Martinez et al., 2016; Sundrani, Chavan‐Gautam, Pisal, Mehendale, & Joshi, 2012) and capable of shedding syndecan‐1 (Brule et al., 2006; Endo et al., 2003; Fears & Woods, 2006; Hayashida et al., 2010; Li, Park, Wilson, & Parks, 2002; Purushothaman et al., 2010; Purushothaman, Chen, Yang, & Sanderson, 2008; Roper, Williamson, & Bass, 2012; Stepp, Pal‐Ghosh, Tadvalkar, & Pajoohesh‐Ganji, 2015; Vuoriluoto, Högnäs, Meller, Lehti, & Ivaska, 2011) are the secreted gelatinase MMP‐9 as well as MMP‐14 (MT1‐MMP) and MMP‐16 (MT3‐MMP) which are both transmembrane MMPs (Manon‐Jensen et al., 2010; Shofuda, Yasumitsu, & Nishihashi, 1997). The polypeptide structure of MMP‐9 includes three fibronectin II‐like repeats (Manon‐Jensen et al., 2010; Uhlin‐Hansen, 2013).
5.5.4. Heparanase and MMP‐9
Using myeloma cells obtained from bone marrow aspirates (Yang et al., 2007) and multiple human cell lines (Ramani et al., 2012), it was demonstrated that the presence of heparan sulfate chains on syndecan‐1 inhibited the shedding of the syndecan‐1 ectodomain so a reduction in the heparan sulfate present on syndecan‐1 leads to increased shedding. Heparan sulfate chains on syndecan‐1 are degraded to oligosaccharides by heparanase which acts both at the cell surface and within the extracellular matrix and is upregulated by inflammation (Hulett et al., 1999; Ramani et al., 2012; Vlodavsky et al., 1999). The expression of heparanase in myeloma cell also led to the upregulation of MMP‐9, which sheds syndecan‐1 (Purushothaman et al., 2008). Thus, inflammation can induce a chain of events that leads to the increased shedding of the syndecan‐1 ectodomain.
MMP‐9 appears to have a role in inflammation and placental malaria. When human monocytes were incubated in vitro with P. falciparum trophozoite‐parasitized RBCs and hemozoin, the monocytes phagocytosed the infected RBCs and hemozoin, resulting in increased MMP‐9 activity and increased TNF‐α production (Prato et al., 2005). The secretion of MMP‐9 from cultured human microvascular endothelial cells was induced by exposing these cells in vitro to trophozoite‐parasitized RBCs (D'Alessandro, Basilico, & Prato, 2013). Other studies have also shown the possible pro‐inflammatory role of MMP‐9 in nonplacental malaria and some have suggested the potential use of MMP‐9 inhibitors as drugs for nonplacental malaria, particularly for complicated malaria (Dietmann et al., 2008; Polimeni & Prato, 2014; Prato & Giribaldi, 2011; Van den Steen et al., 2006). In a study in Ghana which compared 54 pregnant women with the minor T allele for an MMP‐9 promoter to 248 pregnant women with the more common CC genotype, the percent of women with peripheral parasites, placental parasites, and placental hemozoin was lower for the women with the T allele, suggesting a possible role for MMP‐9 in placental malaria (Apoorv et al., 2015).
6. ROLE OF FIBRIN IN THE ACCUMULATION OF INFECTED RBCS IN THE INTERVILLOUS SPACE
Intervillous fibrin was observed by light microscopy in the placentas of 20–29% of uninfected women in the United States who delivered early between gestational Weeks 23 and 27 (N ≥ 84 at each weekly gestational age; total N = 947; overall rate of intervillous fibrin was 24%; Hecht et al., 2008). In some extreme cases, fibrin can fill the intervillous space (Fuke et al., 1994; Waters & Ashikaga, 2006) and this observation has been associated with abortion (Gaither & Sampson, 1968).
It must be that intervillous fibrin is polymerized to be visible by light microscopy. The precursor to fibrin, fibrinogen, is a soluble glycoprotein (MW ~340 kDa) that is found in human plasma at concentrations normally in the range of 150–400 mg/dL (Asselta, Duga, & Tenchini, 2006; Cieśla, Adamczyk, Barbasz, & Wasilewska, 2013; Marucco, Fenoglio, Turci, & Fubini, 2013). Fibrinogen can bind to integrin αIIbβ3 on the surface of platelets that have been appropriately activated (Du et al., 1993). Fibrinogen and fibrin also bind to fibronectin (Makogonenko, Tsurupa, Ingham, & Medved, 2002) and fibrin to MMP‐9 (Makowski and Ramsby, 1998). Thrombin is an enzyme that binds to the surface of circulating platelets and can convert fibrinogen to insoluble fibrin strands. This machinery is used for hemostasis which is normally prevented by factors released from the endothelial cells of intact blood vessels. However, the normal process of coagulation is disrupted by malaria (Angchaisuksiri, 2014; Vogetseder et al., 2004).
The initiation of the sequestration in the intervillous space in malaria has been described as free parasites becoming enmeshed in fibrin strands (Anagnos et al., 1986; Duffy & Fried, 2001a, p. 73). Extensive intervillous mesh‐like structures were detected by fibrin antibody in term placentas from women with malaria (Imamura et al., 2002). Muthusamy et al. (2004) describe fibrous, filamentous materials in the intervillous space of infected placentas. When infected RBCs from infected placentas were incubated with uninfected term placentas, the cells bound only in the periphery of the intervillous space (Fried & Duffy, 1996) indicating a difference been infected and uninfected placentas. However, in a subsequent study using placental‐type infected RBCs (i.e., expressing VAR2CSA), intervillous mesh was observed in the placentas from healthy women although the mesh was not as dense as seen with infected placentas (Ayres Pereira et al., 2016).
Fibrin can also contribute to the formation of perivillous or intravillous fibrinoids or perivillous fibrinoid plaques which occur on the surface of or within the placental villi of healthy women (Frank et al., 1994; Hargitai, Marton, & Cox, 2004; Kaufmann et al., 1996; Rampersad, Cervar‐Zivkovic, & Nelson, 2011). These fibrinoid deposits are a feature of placental malaria and, as mentioned above, when containing hemozoin or cells without parasites, are considered evidence of previous malaria infection (Bulmer, Rasheed, Francis, et al., 1993; Bulmer, Rasheed, Morrison, et al., 1993; Mostafa et al., 2015). When excessive, these perivillous fibrinoid deposits have been associated with a marked upregulation of platelet tissue factor that initiates thrombin formation from prothrombin. This upregulation of platelet tissue factor was suggested to be responsible for the formation of these malaria‐related fibrinoid deposits as a type of clotting (Imamura et al., 2002).
7. REVIEW OF KEY POINTS
7.1. In pregnant women with placental malaria
Placental malaria is caused by the sequestration of infected RBCs in the placenta and is associated with marked inflammation due partly at least to the release by infected RBCs of cytokines and hemozoin.
The binding of infected RBCs to the syncytiotrophoblast and in the intervillous space is caused by the binding of the PfEMP‐1 receptor VAR2CSA on the surface of infected RBCs to the chondroitin sulfate portion of a CSPG, likely syndecan‐1.
7.2. In various in vitro and in vivo human models
Syndecan‐1 is a transmembrane proteoglycan with an extracellular ectodomain and is found on the luminal surface of the syncytiotrophoblast.
The syndecan‐1 ectodomain is shed by MMP(s) and serum concentrations of syndecan‐1 ectodomain progressively increase during gestation.
Syndecan‐1 in the intervillous space must be the syndecan‐1 ectodomain as intact syndecan‐1 is a membrane protein.
Both the cell‐associated and intervillous proteoglycans have substantial chondroitin sulfate but there is substantial heparan sulfate only in the cell‐associated fraction.
The lack of heparan sulfate on the proteoglycans in the intervillous space could imply that the syndecan‐1 is not shed until the heparan sulfate on the syndecan‐1 ectodomain has been removed, presumably by heparanase.
Heparan sulfate on syndecan‐1 inhibits the shedding of the syndecan‐1 ectodomain, perhaps by forming a canopy that blocks the access of secreted MMPs to the base of the ectodomain.
Heparanase degrades the heparan sulfate on syndecan‐1 and is upregulated by inflammation.
Heparanase expression also leads to the upregulation of MMP‐9.
Inflammation is known to increase syndecan shedding and the upregulation of heparanase and MMP‐9 could be the primary mechanism.
8. PROPOSED MECHANISM FOR SEQUESTRATION IN THE INTERVILLOUS SPACE
The endothelial glycocalyx is complex. Nevertheless, it is proposed that the following cascade of events plays a significant role in the genesis of the sequestration of infected RBCs in the intervillous space in placental malaria (see Figures 1 and 2):
Since syndecan‐1 (see Figure 2a) appears not be shed until heparan sulfate has been removed, it is likely that syndecan‐1 is shed by a soluble MMP (i.e., MMP‐9) that would not have access to the ectodomain cleavage site until the canopy of heparan sulfate has been removed.
Inflammation associated with malaria triggers increased heparanase activity that degrades the heparan sulfate on the cell surface syndecan‐1.
- The enhanced expression of heparanase then does both of the following:
- It stimulates the formation of MMP‐9.
- It removes the heparan sulfate barrier (Figure 2b) which enables the following:
-
iInfected RBCs have access to and bind to the chondroitin sulfate chains on the intact membranous syndecan‐1 on the surface of the syncytiotrophoblast.
-
iiSoluble MMP‐9 has access to the cleavage site at the base of the syndecan‐1 ectodomain.
-
i
These actions lead to the shedding of syndecan‐1 ectodomain with its chondroitin sulfate chains intact but with its heparan sulfate chains largely removed (Figure 2c).
The dimeric syndecan‐1 ectodomain then is left with at least four chondroitin sulfate chains making it able to bind to infected RBCs in the intervillous space and, since it is effectively multivalent, to link infected RBCs together causing them to aggregate (Figure 2d). It may be that P. falciparum exploits syndecan‐1 shedding to enhance virulence as was reported for Pseudomonas aeruginosa (Park et al., 2001).
The presence of fibronectin II‐like repeats in MMP‐9, the binding of fibrinogen and fibrin to fibronectin, and the resultant binding of fibrin to MMP‐9 could allow fibronectin and MMP‐9 to contribute to the formation of the intervillous mesh.
The similar incidences of intervillous fibrin between gestational Weeks 23 and 27 in a study or pregnant women in the United States (24%) and the estimate provided here for the prevalence of placental malaria in sub‐Saharan Africa (30%), a high transmission area, could be more than a coincidence. It seems possible that the fibrin that occurs spontaneously in the placentas of some women provides the initial lattice for the trapping of aggregated infected RBCs (that may also have platelets and uninfected RBCs bound to them) and leads to placental malaria. Thus, the presence of intervillous fibrin mesh could predispose women to placental malaria.
The finding of pigment or cells (without parasites) within perivillous fibrinoids on the surface of the syncytiotrophoblast in cases of past placental malaria could indicate that these fibrinoids are the product of the intervillous mesh after it has collapsed.
Figure 1.
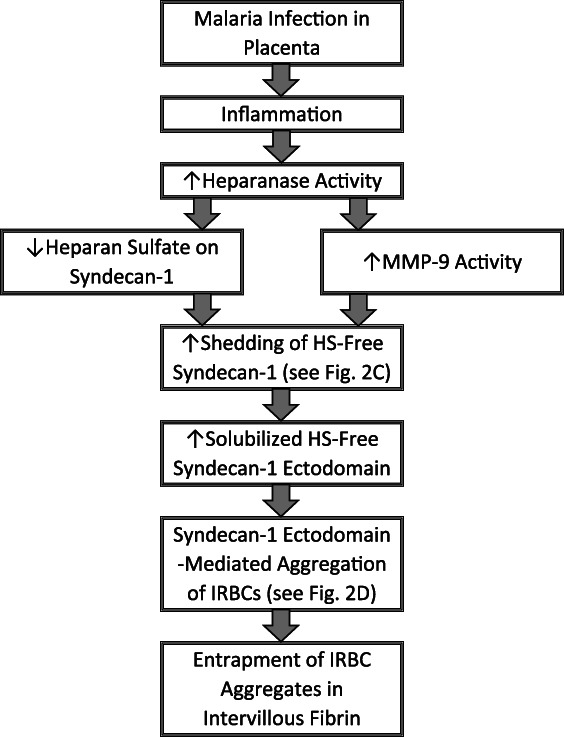
Flowchart of the proposed primary mechanism for placental malaria. Shown here are the steps outlined in Section 8. HS = heparan sulfate; infected RBCs = infected red blood cells; MMP‐9 = matrix metalloproteinase 9
Figure 2.
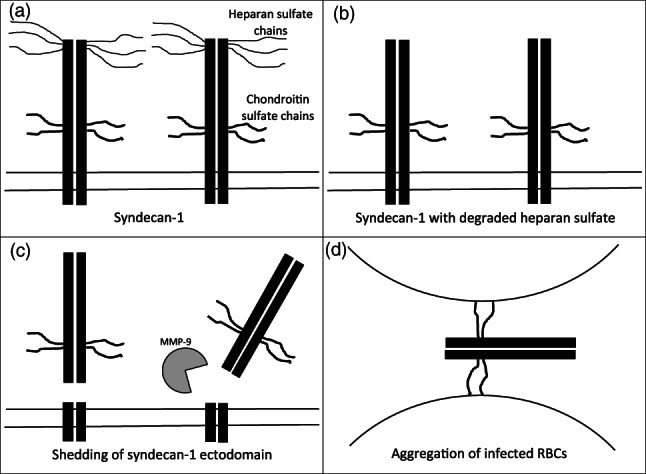
Schematic representation of the proposed sequential changes in syndecan‐1 in placental malaria. (a) A depiction of intact syndecan‐1 embedded in the plasma membrane of the syncytiotrophoblast showing the heparan sulfate chains on the ectodomain distal from the cell surface and the chondroitin sulfate chains on the ectodomain proximal to the cell surface. (b) Syndecan‐1 after the heparan sulfate chains have been removed by heparanase. (c) The removal of the heparan sulfate chains has cleared the way for MMP‐9 to access the ectodomain cleavage site near the surface of the syncytiotrophoblast. (d) The cleaved syndecan‐1 ectodomain with chondroitin sulfate chains has been released, allowing it to bind to multiple infected RBCs. The depictions of syndecan‐1 shown here were influenced by related models shown in Elenius and Jalkanen (1994), Bernfield et al. (1999), Pries et al. (2000), Manon‐Jensen et al. (2010), Roper et al. (2012), and Itoh et al. (2015). HS = heparan sulfate; infected RBCs = infected red blood cells; MMP‐9 = matrix metalloproteinase 9
9. POSSIBLE IMPLICATIONS FOR THE TREATMENT OF PLACENTAL MALARIA
If intervillous fibrin before infection is a prerequisite or contributing factor for placental malaria, then treatments that eliminate intervillous fibrin could be preventative for placental malaria. Also, agents that inhibit heparanase or the shedding of syndecan‐1 ectodomain (in particular, MMP‐9) could be effective against placental malaria.
Doxycycline is a tetracycline antibiotic that has been used to treat and prevent malaria (Cross, Ling, Day, McGready, & Paris, 2016; Gaillard, Madamet, & Pradines, 2015; Tan et al., 2011; WHO, 2015) and has been recommended for intermittent preventive treatment of P. falciparum malaria in pregnant women (Gaillard, Boxberger, Madamet, & Pradines, 2018). Doxycycline is also an inhibitor of MMP‐9 (Dursun, Kim, Solomon, & Pflugfelder, 2001; Sochor et al., 2009) and can be used for treatment of conditions related to its MMP‐9 inhibitory activity, for example, chronic wounds (Stechmiller, Cowan, & Schultz, 2010) and acute lung injury (Doroszko et al., 2010; Fujita et al., 2007; Zhang, Gong, Liu, Guo, & Ge, 2014). Doxycycline can also inhibit the pro‐inflammatory effects of IL‐17 (Obradović et al., 2016). Tetracyclines share a linear fused tetracyclic nucleus and some tetracyclines (including the original tetracycline, referred to as tetracycline) not including doxycycline have been shown to cause dental staining in human neonates when administered during the second or third trimesters (Cohlan, 1977; Kline, Blattner, & Lunin, 1964; Toaff & Ravid, 1966). Initially contraindicated for use in pregnancy because of a possible tetracycline class effect, recent reviews have found little evidence to suggest an adverse effect of doxycycline on pregnancy (Cross et al., 2016; Gaillard et al., 2015) and doxycycline use during the first and second trimesters to combat various infections other than malaria has had positive effects on pregnancy outcome (Gaillard et al., 2018; Kazy, Puho, & Czeizel, 2007). Doxycycline may have beneficial effects on placental malaria due to both its antibiotic effects and its activity as an MMP‐9 inhibitor and thus may be particularly useful against placental malaria.
ACKNOWLEDGMENTS
The author wishes to acknowledge the Medicines for Malaria Venture for enabling this article to be open access.
Clark RL. Genesis of placental sequestration in malaria and possible targets for drugs for placental malaria. Birth Defects Research. 2019;111:569–583. 10.1002/bdr2.1496
REFERENCES
- Accrombessi, M. , Ouédraogo, S. , Agbota, G. C. , Gonzalez, R. , Massougbodji, A. , Menéndez, C. , & Cot, M. (2015). Malaria in pregnancy is a predictor of infant haemoglobin concentrations during the first year of life in Benin, West Africa. PLoS One, 10(6), e0129510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achur, R. N. , Valiyaveettil, M. , Alkhalil, A. , Ockenhouse, C. F. , & Gowda, D. C. (2000). Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum‐infected erythrocytes to the placenta. The Journal of Biological Chemistry, 275(51), 40344–40356. [DOI] [PubMed] [Google Scholar]
- Adebami, O. J. , Owa, J. A. , Oyedeji, G. A. , Oyelami, O. A. , & Omoniyi‐Esan, G. O. (2007). Association between placental and cord blood malaria infection and fetal malnutrition in an area of malaria holoendemicity. The American Journal of Tropical Medicine and Hygiene, 77, 169–200. [PubMed] [Google Scholar]
- Alberts, B. , Johnson, A. , Lewis, J. , Morgan, D. , Raff, M. , Roberts, K. , & Walter, P. (2015). Molecular biology of the cell (pp. 1057–1061). New York, NY: Garland Science. [Google Scholar]
- Alexopoulou, A. N. , Multhaupt, H. A. , & Couchman, J. R. (2007). Syndecans in wound healing, inflammation and vascular biology. The International Journal of Biochemistry & Cell Biology, 39(3), 505–528. [DOI] [PubMed] [Google Scholar]
- Alkhalil, A. , Achur, R. N. , Valiyaveettil, M. , Ockenhouse, C. F. , & Gowda, D. C. (2000). Structural requirements for the adherence of Plasmodium falciparum‐infected erythrocytes to chondroitin sulfate proteoglycans of human placenta. The Journal of Biological Chemistry, 275(51), 40357–40364. [DOI] [PubMed] [Google Scholar]
- Anagnos, D. , Lanoie, L. O. , Palmieri, J. R. , Ziefer, A. , & Connor, D. H. (1986). Effects of placental malaria on mothers and neonates from Zaire. Zeitschrift für Parasitenkunde, 72(1), 57–64. [DOI] [PubMed] [Google Scholar]
- Angchaisuksiri, P. (2014). Coagulopathy in malaria. Thrombosis Research, 133(1), 5–9. [DOI] [PubMed] [Google Scholar]
- Apoorv, T. S. , Babu, P. P. , Meese, S. , Gai, P. P. , Bedu‐Addo, G. , & Mockenhaupt, F. P. (2015). Matrix metalloproteinase‐9 polymorphism 1562 C > T (rs3918242) associated with protection against placental malaria. The American Journal of Tropical Medicine and Hygiene, 93(1), 186–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselta, R. , Duga, S. , & Tenchini, M. L. (2006). The molecular basis of quantitative fibrinogen disorders. Journal of Thrombosis and Haemostasis, 4(10), 2115–2129. [DOI] [PubMed] [Google Scholar]
- Ayres Pereira, M. , Mandel Clausen, T. , Pehrson, C. , Mao, Y. , Resende, M. , Daugaard, M. , … Salanti, A. (2016). Placental sequestration of Plasmodium falciparum malaria parasites is mediated by the interaction between VAR2CSA and chondroitin sulfate A on syndecan‐1. PLoS Pathogens, 12(8), e1005831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassey, G. , Nyengidiki, T. K. , & John, C. T. (2015). Prevalence of placenta plasmodium parasitemia and pregnancy outcome in asymptomatic patients at delivery in a university teaching hospital in Nigeria. Nigerian Journal of Clinical Practice, 18(1), 27–32. [DOI] [PubMed] [Google Scholar]
- Beaudet, J. M. , Mansur, L. , Joo, E. J. , Kamhi, E. , Yang, B. , Clausen, T. M. , … Linhardt, R. J. (2014). Characterization of human placental glycosaminoglycans and regional binding to VAR2CSA in malaria infected erythrocytes. Glycoconjugate Journal, 31(2), 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson, J. G. , Amin, N. , Kanjala, M. , & Rogerson, S. J. (2002). Selective accumulation of mature asexual stages of Plasmodium falciparum‐infected erythrocytes in the placenta. Infection and Immunity, 70(10), 5412–5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeson, J. G. , Chai, W. , Rogerson, S. J. , Lawson, A. M. , & Brown, G. V. (1998). Inhibition of binding of malaria‐infected erythrocytes by a tetradecasaccharide fraction from chondroitin sulfate A. Infection and Immunity, 66(7), 3397–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier, J. C. (1998). Malaria parasite development in mosquitoes. Annual Review of Entomology, 43, 519–543. [DOI] [PubMed] [Google Scholar]
- Bergström, S. , Fernandes, A. , Schwalbach, J. , Perez, O. , & Miyar, R. (1993). Materno‐fetal transmission of pregnancy malaria: An immunoparasitological study on 202 parturients in Maputo. Gynecologic and Obstetric Investigation, 35(2), 103–107. [DOI] [PubMed] [Google Scholar]
- Bernfield, M. , Gotte, M. , Park, P. W. , Reizes, O. , Fitzgerald, M. L. , Lincecum, J. , & Zako, M. (1999). Functions of cell surface heparan sulfate proteoglycans. Annual Review of Biochemistry, 68, 729–777. [DOI] [PubMed] [Google Scholar]
- Brabin, B. J. (1983). An analysis of malaria in pregnancy in Africa. Bulletin of the World Health Organization, 61(6), 1005–1016. [PMC free article] [PubMed] [Google Scholar]
- Brabin, B. J. , & Johnson, P. M. (2005). Placental malaria and pre‐eclampsia through the looking glass backwards? Journal of Reproductive Immunology, 65(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Brabin, B. J. , Romagosa, C. , Abdelgalil, S. , Menéndez, C. , Verhoeff, F. H. , McGready, R. , … Ordi, J. (2004). The sick placenta‐the role of malaria. Placenta, 25(5), 359–378. [DOI] [PubMed] [Google Scholar]
- Brule, S. , Charnaux, N. , Sutton, A. , Ledoux, D. , Chaigneau, T. , Saffar, L. , & Gattegno, L. (2006). The shedding of syndecan‐4 and syndecan‐1 from HeLa cells and human primary macrophages is accelerated by SDF‐1/CXCL12 and mediated by the matrix metalloproteinase‐9. Glycobiology, 16, 488–501. [DOI] [PubMed] [Google Scholar]
- Bulmer, J. N. , Rasheed, F. N. , Francis, N. , Morrison, L. , & Greenwood, B. M. (1993). Placental malaria. I. Pathological classification. Histopathology, 22, 211–218. [DOI] [PubMed] [Google Scholar]
- Bulmer, J. N. , Rasheed, F. N. , Morrison, L. , Francis, N. , & Greenwood, B. M. (1993). Placental malaria. II. A semi‐quantitative investigation of the pathological features. Histopathology, 22(3), 219–225. [DOI] [PubMed] [Google Scholar]
- Carmona‐Fonseca, J. , Arango, E. , & Maestre, A. (2013). Placental malaria in Colombia: Histopathologic findings in Plasmodium vivax and P. falciparum infections. The American Journal of Tropical Medicine and Hygiene, 88(6), 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, R. , & Mendis, K. N. (2002). Evolutionary and historical aspects of the burden of malaria. Clinical Microbiology Reviews, 15(4), 564–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, W. , Beeson, J. G. , & Lawson, A. M. (2002). The structural motif in chondroitin sulfate for adhesion of Plasmodium falciparum‐infected erythrocytes comprises disaccharide units of 4‐O‐sulfated and non‐sulfated N‐acetylgalactosamine linked to glucuronic acid. The Journal of Biological Chemistry, 277(25), 22438–22446. [DOI] [PubMed] [Google Scholar]
- Chaikitgosiyakul, S. , Rijken, M. J. , Muehlenbachs, A. , Lee, S. J. , Chaisri, U. , Viriyavejakul, P. , … McGready, R. (2014). A morphometric and histological study of placental malaria shows significant changes to villous architecture in both Plasmodium falciparum and Plasmodium vivax infection. Malaria Journal, 13, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasiri, U. P. , Chua, C. L. , Umbers, A. J. , Chaluluka, E. , Glazier, J. D. , Rogerson, S. J. , & Boeuf, P. (2014). Insight into the pathogenesis of fetal growth restriction in placental malaria: Decreased placental glucose transporter isoform 1 expression. The Journal of Infectious Diseases, 209(10), 1663–1667. [DOI] [PubMed] [Google Scholar]
- Chen, J. , & Khalil, R. A. (2017). Matrix metalloproteinases in normal pregnancy and preeclampsia. Progress in Molecular Biology and Translational Science, 148, 87–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieśla, M. , Adamczyk, Z. , Barbasz, J. , & Wasilewska, M. (2013). Mechanisms of fibrinogen adsorption at solid substrates at lower pH. Langmuir, 29(23), 7005–7016. [DOI] [PubMed] [Google Scholar]
- Clark, H. C. (1915). The diagnostic value of the placental blood film in aestivo‐autumnal malaria. Journal of Experimental Medicine, 22, 427–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, I. A. , Budd, A. C. , Alleva, L. M. , & Cowden, W. B. (2006). Human malarial disease: A consequence of inflammatory cytokine release. Malaria Journal, 5, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen, T. M. , Christoffersen, S. , Dahlbäck, M. , Langkilde, A. E. , Jensen, K. E. , Resende, M. , … Salanti, A. (2012). Structural and functional insight into how the Plasmodium falciparum VAR2CSA protein mediates binding to chondroitin sulfate A in placental malaria. The Journal of Biological Chemistry, 287(28), 23332–23345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohee, L. M. , Kalilani‐Phiri, L. , Mawindo, P. , Joshi, S. , Adams, M. , Kenefic, L. , … Laufer, M. K. (2016). Parasite dynamics in the peripheral blood and the placenta during pregnancy‐associated malaria infection. Malaria Journal, 15(1), 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohlan, S. Q. (1977). Tetracycline staining of teeth. Teratology, 15(1), 127–129. [DOI] [PubMed] [Google Scholar]
- Cooke, B. M. , Rogerson, S. J. , Brown, G. V. , & Coppel, R. L. (1996). Adhesion of malaria‐infected red blood cells to chondroitin sulfate A under flow conditions. Blood, 88(10), 4040–4044. [PubMed] [Google Scholar]
- Costa, F. T. , Lopes, S. C. , Ferrer, M. , Leite, J. A. , Martin‐Jaular, L. , Bernabeu, M. , … del Portillo, H. (2011). On cytoadhesion of Plasmodium vivax: Raison d'être? Memórias do Instituto Oswaldo Cruz, 106(Suppl. 1), 79–84. [DOI] [PubMed] [Google Scholar]
- Cottrell, G. , Mary, J. Y. , Barro, D. , & Cot, M. (2007). The importance of the period of malarial infection during pregnancy on birth weight in tropical Africa. The American Journal of Tropical Medicine and Hygiene, 76(5), 849–854. [PubMed] [Google Scholar]
- Cowman, A. F. , & Crabb, B. S. (2006). Invasion of red blood cells by malaria parasites. Cell, 124(4), 755–766. [DOI] [PubMed] [Google Scholar]
- Cross, R. , Ling, C. , Day, N. P. , McGready, R. , & Paris, D. H. (2016). Revisiting doxycycline in pregnancy and early childhood—Time to rebuild its reputation? Expert Opinion on Drug Safety, 15(3), 367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessandro, S. , Basilico, N. , & Prato, M. (2013). Effects of Plasmodium falciparum‐infected erythrocytes on matrix metalloproteinase‐9 regulation in human microvascular endothelial cells. Asian Pacific Journal of Tropical Medicine, 6, 195–199. [DOI] [PubMed] [Google Scholar]
- De Beaudrap, P. , Turyakira, E. , Nabasumba, C. , Tumwebaze, B. , Piola, P. , Boum Ii, Y. , & McGready, R. (2016). Timing of malaria in pregnancy and impact on infant growth and morbidity: A cohort study in Uganda. Malaria Journal, 15, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaudrap, P. , Turyakira, E. , White, L. J. , Nabasumba, C. , Tumwebaze, B. , Muehlenbachs, A. , … Piola, P. (2013). Impact of malaria during pregnancy on pregnancy outcomes in a Ugandan prospective cohort with intensive malaria screening and prompt treatment. Malaria Journal, 12, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Moraes, L. V. , Tadokoro, C. E. , Gómez‐Conde, I. , Olivieri, D. N. , & Penha‐Gonçalves, C. (2013). Intravital placenta imaging reveals microcirculatory dynamics impact on sequestration and phagocytosis of Plasmodium‐infected erythrocytes. PLoS Pathogens, 9(1), e1003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa, S. S. , Yamada, S. , Zako, M. , Goldberger, O. , & Sugahara, K. (2004). Chondroitin sulfate chains on syndecan‐1 and syndecan‐4 from normal murine mammary gland epithelial cells are structurally and functionally distinct and cooperate with heparan sulfate chains to bind growth factors. A novel function to control binding of midkine, pleiotrophin, and basic fibroblast growth factor. The Journal of Biological Chemistry, 279(36), 37368–37376. [DOI] [PubMed] [Google Scholar]
- Desai, M. , ter Kuile, F. O. , Nosten, F. , McGready, R. , Asamoa, K. , Brabin, B. , & Newman, R. D. (2007). Epidemiology and burden of malaria in pregnancy. The Lancet Infectious Diseases, 7(2), 93–104. [DOI] [PubMed] [Google Scholar]
- Dietmann, A. , Helbok, R. , Lackner, P. , Issifou, S. , Lell, B. , Matsiegui, P. B. , … Kremsner, P. G. (2008). Matrix metalloproteinases and their tissue inhibitors (TIMPs) in Plasmodium falciparum malaria: Serum levels of TIMP‐1 are associated with disease severity. The Journal of Infectious Diseases, 197(11), 1614–1620. [DOI] [PubMed] [Google Scholar]
- Doolan, D. L. , Dobaño, C. , & Baird, J. K. (2009). Acquired immunity to malaria. Clinical Microbiology Reviews, 22(1), 13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doritchamou, J. , Bertin, G. , Moussiliou, A. , Bigey, P. , Viwami, F. , Ezinmegnon, S. , … Tuikue Ndam, N. (2012). First‐trimester Plasmodium falciparum infections display a typical "placental" phenotype. The Journal of Infectious Diseases, 206(12), 1911–1919. [DOI] [PubMed] [Google Scholar]
- Dorman, E. K. , Shulman, C. E. , Kingdom, J. , Bulmer, J. N. , Mwendwa, J. , Peshu, N. , & Marsh, K. (2002). Impaired uteroplacental blood flow in pregnancies complicated by falciparum malaria. Ultrasound in Obstetrics & Gynecology, 19(2), 165–170. [DOI] [PubMed] [Google Scholar]
- Doroszko, A. , Hurst, T. S. , Polewicz, D. , Sawicka, J. , Fert‐Bober, J. , Johnson, D. H. , & Sawicki, G. (2010). Effects of MMP‐9 inhibition by doxycycline on proteome of lungs in high tidal volume mechanical ventilation‐induced acute lung injury. Proteome Science, 8, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas, N. M. , Anstey, N. M. , Buffet, P. A. , Poespoprodjo, J. R. , Yeo, T. W. , White, N. J. , & Price, R. N. (2012). The anaemia of Plasmodium vivax malaria. Malaria Journal, 11, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, X. , Gu, M. , Weisel, J. W. , Nagaswami, C. , Bennett, J. S. , Bowditch, R. , & Ginsberg, M. H. (1993). Long range propagation of conformational changes in integrin alpha IIb beta 3. The Journal of Biological Chemistry, 268(31), 23087–23092. [PubMed] [Google Scholar]
- Duffy, P. E. , & Fried, M. (1999). Malaria during pregnancy: Parasites, antibodies and chondroitin sulphate A. Biochemical Society Transactions, 27(4), 478–482. [DOI] [PubMed] [Google Scholar]
- Duffy, P. E. , & Fried, M. (2001a). Malaria in pregnancy: Deadly parasite, susceptible host (Vol. 73, 1st ed., p. 104). Oxfordshire, UK: Taylor & Francis. [Google Scholar]
- Duffy, P. E. , & Fried, M. (2001b). Biomedicine. Turncoat antibodies. Science, 293(5537), 2009–2010. [DOI] [PubMed] [Google Scholar]
- Duffy, P. E. , & Fried, M. (2003). Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infection and Immunity, 71(11), 6620–6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun, D. , Kim, M. C. , Solomon, A. , & Pflugfelder, S. C. (2001). Treatment of recalcitrant recurrent corneal erosions with inhibitors of matrix metalloproteinase‐9, doxycycline and corticosteroids. American Journal of Ophthalmology, 132(1), 8–13. [DOI] [PubMed] [Google Scholar]
- Ekvall, H. (2003). Malaria and anemia. Current Opinion in Hematology, 10(2), 108–114. [DOI] [PubMed] [Google Scholar]
- Elenius, K. , & Jalkanen, M. (1994). Function of the syndecans—A family of cell surface proteoglycans. Journal of Cell Science, 107(Pt. 11), 2975–2982. [DOI] [PubMed] [Google Scholar]
- Endo, K. , Takino, T. , Miyamori, H. , Kinsen, H. , Yoshizaki, T. , Furukawa, M. , & Sato, H. (2003). Cleavage of syndecan‐1 by membrane type matrix metalloproteinase‐1 stimulates cell migration. The Journal of Biological Chemistry, 278(42), 40764–40770. [DOI] [PubMed] [Google Scholar]
- Ezebialu, I. U. , Eke, A. C. , Ezeagwuna, D. A. , Nwachukwu, C. E. , Ifediata, F. , & Ezebialu, C. U. (2012). Prevalence, pattern, and determinants of placental malaria in a population of southeastern Nigerian parturients. International Journal of Infectious Diseases, 16(12), e860–e865. [DOI] [PubMed] [Google Scholar]
- Fears, C. Y. , & Woods, A. (2006). The role of syndecans in disease and wound healing. Matrix Biology, 25, 443–456. [DOI] [PubMed] [Google Scholar]
- Fehintola, A. O. , Fehintola, F. O. , Loto, O. M. , Fasubaa, O. B. , Bakare, B. , & Ogundele, O. (2017). Pregnancy and fetal outcome of placental malaria parasitemia in Ile‐Ife, Nigeria. Tropical Journal of Obstetrics and Gynaecology, 33(3), 310–316. [Google Scholar]
- Frank, H. G. , Malekzadeh, F. , Kertschanska, S. , Crescimanno, C. , Castellucci, M. , Lang, I. , … Kaufmann, P. (1994). Immunohistochemistry of two different types of placental fibrinoid. Acta Anatomica (Basel), 150(1), 55–68. [DOI] [PubMed] [Google Scholar]
- Franke‐Fayard, B. , Fonager, J. , Braks, A. , Khan, S. M. , & Janse, C. J. (2010). Sequestration and tissue accumulation of human malaria parasites: Can we learn anything from rodent models of malaria? PLoS Pathogens, 6(9), e1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried, M. , & Duffy, P. E. (1996). Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science, 272(5267), 1502–1504. [DOI] [PubMed] [Google Scholar]
- Fried, M. , & Duffy, P. E. (1998). Maternal malaria and parasite adhesion. Journal of Molecular Medicine (Berlin), 76(3–4), 162–171. [DOI] [PubMed] [Google Scholar]
- Fried, M. , Muga, R. O. , Misore, A. O. , & Duffy, P. E. (1998). Malaria elicits type 1 cytokines in the human placenta: IFN‐gamma and TNF‐alpha associated with pregnancy outcomes. J Immunol, 160, 2523–2530. [PubMed] [Google Scholar]
- Fried, M. , Lauder, R. M. , & Duffy, P. E. (2000). Plasmodium falciparum: Adhesion of placental isolates modulated by the sulfation characteristics of the glycosaminoglycan receptor. Experimental Parasitology, 95(1), 75–78. [DOI] [PubMed] [Google Scholar]
- Fujita, M. , Harada, E. , Ikegame, S. , Ye, Q. , Ouchi, H. , Inoshima, I. , & Nakanishi, Y. (2007). Doxycycline attenuated lung injury by its biological effect apart from its antimicrobial function. Pulmonary Pharmacology & Therapeutics, 20(6), 669–675. [DOI] [PubMed] [Google Scholar]
- Fuke, Y. , Aono, T. , Imai, S. , Suehara, N. , Fujita, T. , & Nakayama, M. (1994). Clinical significance and treatment of massive intervillous fibrin deposition associated with recurrent fetal growth retardation. Gynecologic and Obstetric Investigation, 38(1), 5–9. [DOI] [PubMed] [Google Scholar]
- Gaccioli, F. , & Lager, S. (2016). Placental nutrient transport and intrauterine growth restriction. Frontiers in Physiology, 7, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard, T. , Boxberger, M. , Madamet, M. , & Pradines, B. (2018). Has doxycycline, in combination with anti‐malarial drugs, a role to play in intermittent preventive treatment of Plasmodium falciparum malaria infection in pregnant women in Africa? Malaria Journal, 17(1), 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard, T. , Madamet, M. , & Pradines, B. (2015). Tetracyclines in malaria. Malaria Journal, 14, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither, D. B. , & Sampson, C. C. (1968). Intervillous fibrin deposition associated with spontaneous abortion. Analysis of 100 cases. Journal of the National Medical Association, 60(6), 497–499. [PMC free article] [PubMed] [Google Scholar]
- Gamain, B. , Gratepanche, S. , Miller, L. H. , & Baruch, D. I. (2002). Molecular basis for the dichotomy in Plasmodium falciparum adhesion to CD36 and chondroitin sulfate A. Proceedings of the National Academy of Sciences of the United States of America, 99(15), 10020–10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin, J. B. , Lokomba, V. , Landis, S. H. , Thorp, J. M., Jr. , Herring, A. H. , Tshefu, A. K. , … Meshnick, S. R. (2012). Plasmodium falciparum parasitaemia in the first half of pregnancy, uterine and umbilical artery blood flow, and foetal growth: A longitudinal Doppler ultrasound study. Malaria Journal, 11, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt, H. L. , & Snow, R. W. (2001). The epidemiology and burden of Plasmodium falciparum‐related anemia among pregnant women in sub‐Saharan Africa. The American Journal of Tropical Medicine and Hygiene, 64(Suppl. 1–2), 36–44. [DOI] [PubMed] [Google Scholar]
- Gysin, J. , Pouvelle, B. , Fievet, N. , Scherf, A. , & Lépolard, C. (1999). Ex vivo desequestration of Plasmodium falciparum‐infected erythrocytes from human placenta by chondroitin sulfate A. Infection and Immunity, 67(12), 6596–6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar, K. , & Mohandas, N. (2009). Malaria, erythrocytic infection, and anemia. Hematology. American Society of Hematology. Education Program, 2009, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar, K. , Murphy, S. C. , Milner, D. A. , & Taylor, T. E. (2007). Malaria: Mechanisms of erythrocytic infection and pathological correlates of severe disease. Annual Review of Pathology, 2, 217–249. [DOI] [PubMed] [Google Scholar]
- Hargitai, B. , Marton, T. , & Cox, P. M. (2004). Best practice no 178. Examination of the human placenta. Journal of Clinical Pathology, 57(8), 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida, K. , Bartlett, A. H. , Chen, Y. , & Park, P. W. (2010). Molecular and cellular mechanisms of ectodomain shedding. The Anatomical Record, 293, 925–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht, J. L. , Allred, E. N. , Kliman, H. J. , Zambrano, E. , Doss, B. J. , Husain, A. , … ELGAN Study Investigators . (2008). Histological characteristics of singleton placentas delivered before the 28th week of gestation. Pathology, 40(4), 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiden, U. , Lassance, L. , Tabrizi, N. G. , Miedl, H. , Tam‐Amersdorfer, C. , Cetin, I. , … Desoye, G. (2012). Fetal insulin and IGF‐II contribute to gestational diabetes mellitus (GDM)‐associated up‐regulation of membrane‐type matrix metalloproteinase 1 (MT1‐MMP) in the human feto‐placental endothelium. The Journal of Clinical Endocrinology and Metabolism, 97(10), 3613–3621. [DOI] [PubMed] [Google Scholar]
- Hofmann‐Kiefer, K. F. , Knabl, J. , Martinoff, N. , Schiessl, B. , Conzen, P. , Rehm, M. , … Chappell, D. (2013). Increased serum concentrations of circulating glycocalyx components in HELLP syndrome compared to healthy pregnancy: An observational study. Reproductive Sciences, 20(3), 318–325. [DOI] [PubMed] [Google Scholar]
- Hulett, M. D. , Freeman, C. , Hamdorf, B. J. , Baker, R. T. , Harris, M. J. , & Parish, C. R. (1999). Cloning of mammalian heparanase, an important enzyme in tumor invasion and metastasis. Nature Medicine, 5(7), 803–809. [DOI] [PubMed] [Google Scholar]
- Huynh, B.‐T. , Cottrell, G. , Cot, M. , & Briand, V. (2015). Burden of malaria in early pregnancy: A neglected problem? Clinical Infectious Diseases, 60, 598–604. [DOI] [PubMed] [Google Scholar]
- Imamura, T. , Sugiyama, T. , Cuevas, L. E. , Makunde, R. , & Nakamura, S. (2002). Expression of tissue factor, the clotting initiator, on macrophages in Plasmodium falciparum‐infected placentas. Journal of Infectious Diseases, 186(3), 436–440. [DOI] [PubMed] [Google Scholar]
- Ismail, M. R. , Ordi, J. , Menendez, C. , Ventura, P. J. , Aponte, J. J. , Kahigwa, E. , … Alonso, P. L. (2000). Placental pathology in malaria: A histological, immunohistochemical, and quantitative study. Human Pathology, 31(1), 85–93. [DOI] [PubMed] [Google Scholar]
- Itoh, Y. , Takamura, A. , Ito, N. , Maru, Y. , Sato, H. , Suenaga, N. , … Seiki, M. (2001). Homophilic complex formation of MT1‐MMP facilitates proMMP‐2 activation on the cell surface and promotes tumor cell invasion. The EMBO Journal, 20, 4782–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, Y. (2015). Membrane‐type matrix metalloproteinases: Their functions and regulations. Matrix Biology, 44‐46, 207–223. [DOI] [PubMed] [Google Scholar]
- Jokimaa, V. , Inki, P. , Kujari, H. , Hirvonen, O. , Ekholm, E. , & Anttila, L. (1998). Expression of syndecan‐1 in human placenta and decidua. Placenta, 19(2–3), 157–163. [DOI] [PubMed] [Google Scholar]
- Kaitu'u‐Lino, T. J. , Palmer, K. , Tuohey, L. , Ye, L. , & Tong, S. (2012). MMP‐15 is upregulated in preeclampsia, but does not cleave endoglin to produce soluble endoglin. PLoS One, 7(6), e39864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitu'u‐Lino, T. J. , Palmer, K. R. , Whitehead, C. L. , Williams, E. , Lappas, M. , & Tong, S. (2012). MMP‐14 is expressed in preeclamptic placentas and mediates release of soluble endoglin. The American Journal of Pathology, 180(3), 888–894. [DOI] [PubMed] [Google Scholar]
- Kalilani‐Phiri, L. , Thesing, P. C. , Nyirenda, O. M. , Mawindo, P. , Madanitsa, M. , Membe, G. , … Laufer, M. K. (2013). Timing of malaria infection during pregnancy has characteristic maternal, infant and placental outcomes. PLoS One, 8(9), e74643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann, P. , Huppertz, B. , & Frank, H. G. (1996). The fibrinoids of the human placenta: Origin, composition and functional relevance. Annals of Anatomy, 178(6), 485–501. [DOI] [PubMed] [Google Scholar]
- Kazy, Z. , Puho, E. H. , & Czeizel, A. E. (2007). Effects of doxycycline treatment during pregnancy for birth outcomes. Reproductive Toxicology, 24, 279–280. [DOI] [PubMed] [Google Scholar]
- Kline, A. H. , Blattner, R. I. , & Lunin, M. (1964). Transplacental effect of tetracyclines on teeth. Journal of the American Medical Association, 188, 178–180. [DOI] [PubMed] [Google Scholar]
- Kokenyesi, R. , & Bernfield, M. (1994). Core protein structure and sequence determine the site and presence of heparan sulfate and chondroitin sulfate on syndecan‐1. The Journal of Biological Chemistry, 269, 12304–12309. [PubMed] [Google Scholar]
- Kourtis, A. P. , Read, J. S. , & Jamieson, D. J. (2014). Pregnancy and infection. The New England Journal of Medicine, 370(23), 2211–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamikanra, A. A. , Brown, D. , Potocnik, A. , Casals‐Pascual, C. , Langhorne, J. , & Roberts, D. J. (2007). Malarial anemia: Of mice and men. Blood, 110(1), 18–28. [DOI] [PubMed] [Google Scholar]
- le Cessie, S. , Verhoeff, F. H. , Mengistie, G. , Kazembe, P. , Broadhead, R. , & Brabin, B. J. (2002). Changes in haemoglobin levels in infants in Malawi: Effect of low birth weight and fetal anaemia. Archives of Disease in Childhood. Fetal and Neonatal Edition, 86(3), F182–F187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Park, P. W. , Wilson, C. L. , & Parks, W. C. (2002). Matrilysin shedding of syndecan‐1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell, 111(5), 635–646. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler, S. F. , Lemberg, M. K. , & Fluhrer, R. (2018). Proteolytic ectodomain shedding of membrane proteins in mammals‐hardware, concepts, and recent developments. The EMBO Journal, 37(15), e99456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno, Y. , Steketee, R. W. , Nagatake, T. , Tegoshi, T. , Desowitz, R. S. , Wirima, J. J. , & Aikawa, M. (1993). Immunoglobulin complex deposits in Plasmodium falciparum‐infected placentas from Malawi and Papua New Guinea. The American Journal of Tropical Medicine and Hygiene, 49, 574–580. [DOI] [PubMed] [Google Scholar]
- Majali‐Martinez, A. , Hiden, U. , Ghaffari‐Tabrizi‐Wizsy, N. , U1, L. , Desoye, G. , & Dieber‐Rotheneder, M. (2016). Placental membrane‐type metalloproteinases (MT‐MMPs): Key players in pregnancy. Cell Adhesion & Migration, 10(1–2), 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makogonenko, E. , Tsurupa, G. , Ingham, K. , & Medved, L. (2002). Interaction of fibrin(ogen) with fibronectin: Further characterization and localization of the fibronectin‐binding site. Biochemistry, 41(25), 7907–7913. [DOI] [PubMed] [Google Scholar]
- Makowski, G. S. , & Ramsby, M. L. (1998). Binding of matrix metalloproteinase 9 to fibrin is mediated by amorphous calcium‐phosphate. Inflammation, 22(6), 599–617. [DOI] [PubMed] [Google Scholar]
- Manon‐Jensen, T. , Itoh, Y. , & Couchman, J. R. (2010). Proteoglycans in health and disease: The multiple roles of syndecan shedding. The FEBS Journal, 277(19), 3876–3889. [DOI] [PubMed] [Google Scholar]
- Marucco, A. , Fenoglio, I. , Turci, F. , & Fubini, B. (2013). Interaction of fibrinogen and albumin with titanium dioxide nanoparticles of different crystalline phases. Journal of Physics: Conference Series, 429, 012014. [Google Scholar]
- Maternal and Child Survival Program (MCSP), President's Malaria Initiative (PMI), and Centers for Disease Control (CDC) . (2015). Controlling maternal anemia and malaria: ensuring pregnant women receive effective interventions to prevent malaria and anemia: What program managers and policymakers should know. https://www.mcsprogram.org/resource/controlling-maternal-anemia-and-malaria-ensuring-pregnant-women-receive-effective-interventions-to-prevent-malaria-and-anemia-what-program-managers-and-policymakers-should-know/.
- Matteelli, A. , Caligaris, S. , Castelli, F. , & Carosi, G. (1997). The placenta and malaria. Annals of Tropical Medicine and Parasitology, 91(7), 803–810. [DOI] [PubMed] [Google Scholar]
- Matteelli, A. , Donato, F. , Shein, A. , Muchi, J. A. , Leopardi, O. , Astori, L. , & Carosi, G. (1994). Malaria and anaemia in pregnant women in urban Zanzibar, Tanzania. Annals of Tropical Medicine and Parasitology, 88(5), 475–483. [DOI] [PubMed] [Google Scholar]
- Mayor, A. , Bardají, A. , Felger, I. , King, C. L. , Cisteró, P. , Dobaño, C. , … Rogerson, S. (2012). Placental infection with Plasmodium vivax: A histopathological and molecular study. The Journal of Infectious Diseases, 206, 1904–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGready, R. , Davison, B. B. , Stepniewska, K. , Cho, T. , Shee, H. , Brockman, A. , … Nosten, F. (2004). The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. The American Journal of Tropical Medicine and Hygiene, 70, 398–407. [PubMed] [Google Scholar]
- McGready, R. , Lee, S. J. , Wiladphaingern, J. , Ashley, E. A. , Rijken, M. J. , Boel, M. , … Nosten, F. H. (2012). Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: A population‐based study. The Lancet Infectious Diseases, 12(5), 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean, A. R. , Ataide, R. , Simpson, J. A. , Beeson, J. G. , & Fowkes, F. J. (2015). Malaria and immunity during pregnancy and postpartum: A tale of two species. Parasitology, 142(8), 999–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez, C. (2006). Malaria during pregnancy. Current Molecular Medicine, 6(2), 269–273. [DOI] [PubMed] [Google Scholar]
- Menendez, C. , Ordi, J. , Ismail, M. R. , Ventura, P. J. , Aponte, J. J. , Kahigwa, E. , … Alonso, P. L. (2000). The impact of placental malaria on gestational age and birth weight. The Journal of Infectious Diseases, 181(5), 1740–1745. [DOI] [PubMed] [Google Scholar]
- Michalakis, Y. , & Renaud, F. (2009). Malaria: Evolution in vector control. Nature, 462, 298–300. [DOI] [PubMed] [Google Scholar]
- Mockenhaupt, F. P. , Ulmen, U. , von Gaertner, C. , Bedu‐Addo, G. , & Bienzle, U. (2002). Diagnosis of placental malaria. Journal of Clinical Microbiology, 40(1), 306–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokuolu, O. A. , Falade, C. O. , Orogade, A. A. , Okafor, H. U. , Adedoyin, O. T. , Oguonu, T. A. , … Callahan, M. V. (2009). Malaria at parturition in Nigeria: Current status and delivery outcome. Infectious Diseases in Obstetrics and Gynecology, 2009, 1, 473971–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, K. A. , Simpson, J. A. , Wiladphaingern, J. , Min, A. M. , Pimanpanarak, M. , Paw, M. K. , … McGready, R. (2017). Influence of the number and timing of malaria episodes during pregnancy on prematurity and small‐for‐gestational‐age in an area of low transmission. BMC Medicine, 15(1), 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto‐Tomita, M. , Uchimura, K. , Werb, Z. , Hemmerich, S. , & Rosen, S. D. (2002). Cloning and characterization of two extracellular heparin‐degrading endosulfatases in mice and humans. The Journal of Biological Chemistry, 277, 49175–49185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa, A. G. , Bilal, N. E. , Abass, A. E. , Elhassan, E. M. , Mohmmed, A. A. , & Adam, I. (2015). Coagulation and fibrinolysis indicators and placental malaria infection in an area characterized by unstable malaria transmission in Central Sudan. Malaria Research and Treatment, 2015, 369237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenbachs, A. , Fried, M. , McGready, R. , Harrington, W. , Mutabingwa, T. K. , Nosten, F. , & Duffy, P. E. (2010). A novel histological grading scheme for placental malaria applied in areas of high and low malaria transmission. The Journal of Infectious Diseases, 202(10), 1608–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusamy, A. , Achur, R. N. , Bhavanandan, V. P. , Fouda, G. G. , Taylor, D. W. , & Gowda, D. C. (2004). Plasmodium falciparum‐infected erythrocytes adhere both in the intervillous space and on the villous surface of human placenta by binding to the low‐sulfated chondroitin sulfate proteoglycan receptor. The American Journal of Pathology, 164(6), 2013–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, E. J. , & Park, P. W. (2012). Shedding of cell membrane‐bound proteoglycans. Methods in Molecular Biology, 836, 291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaldia, N. I. I. I. , Meibalan, E. , Sa, J. M. , Ma, S. , Clark, M. A. , Mejia, P. , … Marti, M. (2018). Bone marrow is a major parasite reservoir in Plasmodium vivax infection. MBio, 9(3), e00625–e00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradović, H. , Krstić, J. , Kukolj, T. , Trivanović, D. , Đorđević, I. O. , Mojsilović, S. , … Santibañez, J. F. (2016). Doxycycline inhibits IL‐17‐stimulated MMP‐9 expression by downregulating ERK1/2 activation: Implications in myogenic differentiation. Mediators of Inflammation, 2016, 2939658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordi, J. , Ismail, M. R. , Ventura, P. J. , Kahigwa, E. , Hirt, R. , Cardesa, A. , … Menendez, C. (1998). Massive chronic intervillositis of the placenta associated with malaria infection. The American Journal of Surgical Pathology, 22, 1006–1011. [DOI] [PubMed] [Google Scholar]
- Ouédraogo, A. , Tiono, A. B. , Diarra, A. , Bougouma, E. C. , Nébié, I. , Konaté, A. T. , & Sirima, S. B. (2012). Transplacental transmission of Plasmodium falciparum in a highly malaria endemic area of Burkina Faso. J Trop Med., 2012, 109705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, P. W. , Pier, G. B. , Hinkes, M. T. , & Bernfield, M. (2001). Exploitation of syndecan‐1 shedding by Pseudomonas aeruginosa enhances virulence. Nature, 411, 98–102. [DOI] [PubMed] [Google Scholar]
- Pehrson, C. , Mathiesen, L. , Heno, K. K. , Salanti, A. , Resende, M. , Dzikowski, R. , … Nielsen, M. A. (2016). Adhesion of Plasmodium falciparum infected erythrocytes in ex vivo perfused placental tissue: A novel model of placental malaria. Malaria Journal, 15(1), 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichyangkul, S. , Saengkrai, P. , & Webster, H. K. (1994). Plasmodium falciparum pigment induces monocytes to release high levels of tumor necrosis factoralpha and interleukin‐1 beta. The American Journal of Tropical Medicine and Hygiene, 51, 430–435. [PubMed] [Google Scholar]
- Polimeni, M. , & Prato, M. (2014). Host matrix metalloproteinases in cerebral malaria: New kids on the block against blood‐brain barrier integrity? Fluids Barriers CNS., 11(1), 1 10.1186/2045-8118-11-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouvelle, B. , Fusaï, T. , Lépolard, C. , & Gysin, J. (1998). Biological and biochemical characteristics of cytoadhesion of Plasmodium falciparum‐infected erythrocytes to chondroitin‐4‐sulfate. Infection and Immunity, 66(10), 4950–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prato, M. , & Giribaldi, G. (2011). Matrix Metalloproteinase‐9 and Haemozoin: Wedding rings for human host and Plasmodium falciparum parasite in complicated malaria. Journal of Tropical Medicine, 2011, 628435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prato, M. , Giribaldi, G. , Polimeni, M. , Gallo, V. , & Arese, P. (2005). Phagocytosis of hemozoin enhances matrix metalloproteinase‐9 activity and TNF‐alpha production in human monocytes: Role of matrix metalloproteinases in the pathogenesis of falciparum malaria. Journal of Immunology, 175, 6436–6442. [DOI] [PubMed] [Google Scholar]
- Prentice, A. M. , Cox, S. E. , & Nweneka, C. V. (2010). Asymptomatic malaria in the etiology of iron deficiency anemia: a nutritionist's viewpoint. The American Journal of Clinical Nutrition, 92(6), 1283–1284. [DOI] [PubMed] [Google Scholar]
- Pries, A. R. , Secomb, T. W. , & Gaehtgens, P. (2000). The endothelial surface layer. Pflügers Archiv, 440(5), 653–666. [DOI] [PubMed] [Google Scholar]
- Purushothaman, A. , Chen, L. , Yang, Y. , & Sanderson, R. D. (2008). Heparanase stimulation of protease expression implicates it as a master regulator of the aggressive tumor phenotype in myeloma. The Journal of Biological Chemistry, 283(47), 32628–32636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman, A. , Uyama, T. , Kobayashi, F. , Yamada, S. , Sugahara, K. , Rapraeger, A. C. , & Sanderson, R. D. (2010). Heparanase‐enhanced shedding of syndecan‐1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood, 115, 2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani, V. C. , Pruett, P. S. , Thompson, C. A. , DeLucas, L. D. , & Sanderson, R. D. (2012). Heparan sulfate chains of syndecan‐1 regulate ectodomain shedding. The Journal of Biological Chemistry, 287(13), 9952–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersad, R. , Cervar‐Zivkovic, M. , & Nelson, D. M. (2011). Development and anatomy of the human placenta In Kay H., Nelson D. M., & Wang Y. (Eds.), The placenta: From development to disease (pp. 19–26). West Sussex, England: Wiley‐Blackwell. [Google Scholar]
- Rasti, N. , Wahlgren, M. , & Chen, Q. (2004). Molecular aspects of malaria pathogenesis. FEMS Immunology and Medical Microbiology, 41(1), 9–26. [DOI] [PubMed] [Google Scholar]
- Reitsma, S. , Slaaf, D. W. , Vink, H. , van Zandvoort, M. A. , & Oude Egbrink, M. G. (2007). The endothelial glycocalyx: Composition, functions, and visualization. Pflügers Archiv, 454(3), 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijken, M. J. , Papageorghiou, A. T. , Thiptharakun, S. , Kiricharoen, S. , Dwell, S. L. , Wiladphaingern, J. , … McGready, R. (2012). Ultrasound evidence of early fetal growth restriction after maternal malaria infection. PLoS One, 7(2), e31411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson, S. J. (2017). Management of malaria in pregnancy. The Indian Journal of Medical Research, 146(3), 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson, S. J. , Chaiyaroj, S. C. , Ng, K. , Reeder, J. C. , & Brown, G. V. (1995). Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum‐infected erythrocytes. The Journal of Experimental Medicine, 182(1), 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson, S. J. , Hviid, L. , Duffy, P. E. , Leke, R. F. , & Taylor, D. W. (2007). Malaria in pregnancy: Pathogenesis and immunity. The Lancet Infectious Diseases, 7(2), 105–117. [DOI] [PubMed] [Google Scholar]
- Roper, J. A. , Williamson, R. C. , & Bass, M. D. (2012). Syndecan and integrin interactomes: Large complexes in small spaces. Current Opinion in Structural Biology, 22(5), 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe, J. A. , Claessens, A. , Corrigan, R. A. , & Arman, M. (2009). Adhesion of Plasmodium falciparum‐infected erythrocytes to human cells: Molecular mechanisms and therapeutic implications. Expert Reviews in Molecular Medicine, 11, e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanti, A. , Dahlbäck, M. , Turner, L. , Nielsen, M. A. , Barfod, L. , Magistrado, P. , … Theander, T. G. (2004). Evidence for the involvement of VAR2CSA in pregnancy‐associated malaria. The Journal of Experimental Medicine, 200(9), 1197–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, S. , Jalkanen, M. , O'Farrel, S. , & Bernfield, M. (1989). Molecular cloning of syndecan, an integral membrane proteoglycan. The Journal of Cell Biology, 108, 1547–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz‐Dunn, J. , & Nour, N. M. (2009). Malaria and pregnancy: A global health perspective. Reviews in Obstetrics and Gynecology, 2(3), 186–192. [PMC free article] [PubMed] [Google Scholar]
- Sharma, L. , & Shukla, G. (2017). Placental malaria: A new insight into the pathophysiology. Frontiers of Medicine (Lausanne), 4, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry, B. A. , Alava, G. , Tracey, K. J. , Martiney, J. , Cerami, A. , & Slater, A. F. (1995). Malaria‐specific metabolite hemozoin mediates the release of several potent endogenous pyrogens (TNF, MIP‐1 alpha, and MIP‐1 beta) in vitro, and altered thermoregulation in vivo. Journal of Inflammation, 45, 85–96. [PubMed] [Google Scholar]
- Shofuda, K. , Yasumitsu, H. , & Nishihashi, A. (1997). Expression of three membrane type matrix metalloproteinases (MT‐MMPs) in rat vascular smooth muscle cells and characterization of MT3‐MMPs with and without transmembrane domain. The Journal of Biological Chemistry, 272(15), 9749–9754. [DOI] [PubMed] [Google Scholar]
- Smalley, M. E. , Abdalla, S. , & Brown, J. (1980). The distribution of Plasmodium falciparum in the peripheral blood and bone marrow of Gambian children. Transactions of the Royal Society of Tropical Medicine and Hygiene, 75(1), 103–105. [DOI] [PubMed] [Google Scholar]
- Snounou, G. , Viriyakosol, S. , Zhu, X. P. , Jarra, W. , Pinheiro, L. , do Rosario, V. E. , … Brown, K. N. (1993). High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Molecular and Biochemical Parasitology, 61(2), 315–320. [DOI] [PubMed] [Google Scholar]
- Sochor, M. , Richter, S. , Schmidt, A. , Hempel, S. , Hopt, U. T. , & Keck, T. (2009). Inhibition of matrix metal‐loproteinase‐9 with doxycycline reduces pan‐creatitis‐associated lung injury. Digestion, 80, 65–73. [DOI] [PubMed] [Google Scholar]
- Srivastava, A. , Gangnard, S. , Round, A. , Dechavanne, S. , Juillerat, A. , Raynal, B. , … Gamain, B. (2010). Full‐length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high‐affinity binding to CSA. Proceedings of the National Academy of Sciences of the United States of America, 107(11), 4884–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechmiller, J. , Cowan, L. , & Schultz, G. (2010). The role of doxycycline as a matrix metalloproteinase inhibitor for the treatment of chronic wounds. Biological Research for Nursing, 11(4), 336–344. [DOI] [PubMed] [Google Scholar]
- Steketee, R. W. , Nahlen, B. L. , Parise, M. E. , & Menendez, C. (2001). The burden of malaria in pregnancy in malaria‐endemic areas. The American Journal of Tropical Medicine and Hygiene, 64(Suppl. 1–2), 28–35. [DOI] [PubMed] [Google Scholar]
- Stepp, M. A. , Pal‐Ghosh, S. , Tadvalkar, G. , & Pajoohesh‐Ganji, A. (2015). Syndecan‐1 and its expanding list of contacts. Advances in Wound Care (New Rochelle), 4(4), 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suguitan, A. L., Jr. , Leke, R. G. , Fouda, G. , Zhou, A. , Thuita, L. , Metenou, S. , … Taylor, D. W. (2003). Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. The Journal of Infectious Diseases., 188, 1074–1082. [DOI] [PubMed] [Google Scholar]
- Sundrani, D. P. , Chavan‐Gautam, P. M. , Pisal, H. R. , Mehendale, S. S. , & Joshi, S. R. (2012). Matrix metalloproteinase‐1 and ‐9 in human placenta during spontaneous vaginal delivery and caesarean sectioning in preterm pregnancy. PLoS One, 7(1), e29855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo, S. , Xu, Y. , Romero, R. , Fule, T. , Karaszi, K. , Bhatti, G. , … Than, N. G. (2013). Changes of placental syndecan‐1 expression in preeclampsia and HELLP syndrome. Virchows Archiv: An International Journal of Pathology, 463(3), 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, K. R. , Magill, A. J. , Parise, M. E. , Arguin, P. M. , & Centers for Disease Control and Prevention . (2011). Doxycycline for malaria chemoprophylaxis and treatment: Report from the CDC expert meeting on malaria chemoprophylaxis. The American Journal of Tropical Medicine and Hygiene, 84(4), 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng, Y. H. , Aquino, R. S. , & Park, P. W. (2012). Molecular functions of syndecan‐1 in disease. Matrix Biology, 31, 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toaff, R. , & Ravid, R. (1966). Tetracyclines and the teeth. Lancet, 2(7457), 281–282. [DOI] [PubMed] [Google Scholar]
- Udeinya, I. J. , Miller, L. H. , McGregor, I. A. , & Jensen, J. B. (1983). Plasmodium falciparum strain‐specific antibody blocks binding of infected erythrocytes to amelanotic melanoma cells. Nature, 303, 429–431. [DOI] [PubMed] [Google Scholar]
- Uhlin‐Hansen L. 2013. Heterodimers of proteoglycans and matrix metalloproteinase 9. The 20th Proteoglycan Forum. March 9, 2013, Tokyo Medical and Dental University. http://www.glycoforum.gr.jp/square/20thPGF/pdf/pgf09.pdf
- Umbers, A. J. , Aitken, E. H. , & Rogerson, S. J. (2011). Malaria in pregnancy: Small babies, big problem. Trends in Parasitology, 27(4), 168–175. [DOI] [PubMed] [Google Scholar]
- Uneke, C. J. (2007a). Impact of placental Plasmodium falciparum malaria on pregnancy and perinatal outcome in sub‐Saharan Africa I: Introduction to placental malaria. The Yale Journal of Biology and Medicine, 80(2), 39–50. [PMC free article] [PubMed] [Google Scholar]
- Uneke, C. J. (2007b). Impact of placental Plasmodium falciparum malaria on pregnancy and perinatal outcome in sub‐Saharan Africa: II: Effects of placental malaria on perinatal outcome; malaria and HIV. The Yale Journal of Biology and Medicine, 80(3), 95–103. [PMC free article] [PubMed] [Google Scholar]
- Valea, I. , Tinto, H. , Drabo, M. K. , Huybregts, L. , Sorgho, H. , Ouedraogo, J. B. , … FSP/MISAME Study Group . (2012). An analysis of timing and frequency of malaria infection during pregnancy in relation to the risk of low birth weight, anaemia and perinatal mortality in Burkina Faso. Malaria Journal, 11, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Steen, P. E. , Van Aelst, I. , Starckx, S. , Maskos, K. , Opdenakker, G. , & Pagenstecher, A. (2006). Matrix metalloproteinases, tissue inhibitors of MMPs and TACE in experimental cerebral malaria. Laboratory Investigation, 86(9), 873–888. [DOI] [PubMed] [Google Scholar]
- Verhoef, H. (2010). Asymptomatic malaria in the etiology of iron deficiency anemia: a malariologist's viewpoint. The American Journal of Clinical Nutrition, 92(6), 1285–1286. [DOI] [PubMed] [Google Scholar]
- Vlodavsky, I. , Friedmann, Y. , Elkin, M. , Aingorn, H. , Atzmon, R. , Ishai‐Michaeli, R. , … Pecker, I. (1999). Mammalian heparanase: Gene cloning, expression and function in tumor progression and metastasis. Nature Medicine, 5(7), 793–802. [DOI] [PubMed] [Google Scholar]
- Vogetseder, A. , Ospelt, C. , Reindl, M. , Schober, M. , & Schmutzhard, E. (2004). Time course of coagulation parameters, cytokines and adhesion molecules in Plasmodium falciparum malaria. Tropical Medicine and International Health, 9(7), 767–773. [DOI] [PubMed] [Google Scholar]
- Vuoriluoto, K. , Högnäs, G. , Meller, P. , Lehti, K. , & Ivaska, J. (2011). Syndecan‐1 and ‐4 differentially regulate oncogenic K‐ras dependent cell invasion into collagen through α2β1 integrin and MT1‐MMP. Matrix Biology, 30(3), 207–217. [DOI] [PubMed] [Google Scholar]
- Walter, P. R. , Garin, Y. , & Blot, P. (1982). Placental pathologic changes in malaria—A histologic and ultrastructural study. The American Journal of Pathology, 109, 330–342. [PMC free article] [PubMed] [Google Scholar]
- Waters, B. L. , & Ashikaga, T. (2006). Significance of perivillous fibrin/oid deposition in uterine evacuation specimens. The American Journal of Surgical Pathology, 30(6), 760–765. [DOI] [PubMed] [Google Scholar]
- White, N. J. , Pukrittayakamee, S. , Hien, T. T. , Faiz, M. A. , Mokuolu, O. A. , & Dondorp, A. M. (2014). Malaria. Lancet, 383(9918), 723–735. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe, S. N. , & Abdalla, S. H. (2000). Blood and bone marrow changes in malaria. Baillière's Best Practice & Research. Clinical Haematology, 13(2), 277–299. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe, S. N. , Phillips, R. E. , Looareesuwan, S. , Warrell, D. A. , & Hughes, M. (1987). The bone marrow in human cerebral malaria: Parasite sequestration within sinusoids. British Journal of Haematology, 66(3), 295–306. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2015). Guidelines for the treatment of malaria (3rd ed., p. 83). Geneva, Switzerland: World Health Organization. [Google Scholar]
- Yang, Y. , MacLeod, V. , Dai, Y. , Khotskaya‐Sample, Y. , Shriver, Z. , Venkataraman, G. , … Sanderson, R. D. (2007). The syndecan‐1 heparan sulfate proteoglycan is a viable target for myeloma therapy. Blood, 110(6), 2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Macleod, V. , Miao, H. Q. , Theus, A. , Zhan, F. , Shaughnessy, J. D., Jr. , … Sanderson, R. D. (2007). Heparanase enhances syndecan‐1 shedding: A novel mechanism for stimulation of tumor growth and metastasis. The Journal of Biological Chemistry, 282(18), 13326–13333. [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Gong, W. , Liu, H. , Guo, Z. , & Ge, S. (2014). Inhibition of matrix metalloproteinase‐9 with low‐dose doxycycline reduces acute lung injury induced by cardiopulmonary bypass. International Journal of Clinical and Experimental Medicine, 7(12), 4975–4982. [PMC free article] [PubMed] [Google Scholar]


