Abstract
Background:
The Hirao reaction discovered ca. 35 years ago is an important P–C coupling protocol between dialkyl phosphites and aryl halides in the presence of Pd(PPh3)4 as the catalyst and a base to provide aryl phosphonates. Then, the reaction was extended to other P-reagents, such as secondary phosphine oxides and H-phosphinates and to other aryl and hetaryl derivatives to afford also phosphinic esters and tertiary phosphine oxides. Instead of the Pd(PPh3)4 catalyst, Pd(OAc)2 and Ni-salts were also applied as catalyst precursors together with a number of mono- and bidentate P-ligands.
Objective:
In our review, we undertook to summarize the target reaction with a special stress on the developments attained in the last 6 years, hence this paper is an update of our earlier reviews in a similar topic.
Conclusions:
“Greener” syntheses aimed at utilizing phase transfer catalytic and microwave-assisted approaches, even under “P-ligand-free. or even solvent-free conditions are the up-to date versions of the classical Hirao reaction. The mechanism of the reaction is also in the focus these days.
Keywords: Hirao reaction, P-C coupling, Pd-catalyst, Phosphonates, Phosphinates, Phosphine oxides, Green synthesis
1. THE TRADITIONAL HIRAO REACTION
The Hirao reaction that is, a P-C coupling to furnish phosphonates or phosphine oxides, was reviewed 7 years ago by us [1, 2]. The purpose of this survey was to give an update on the newer developments. Of course, the most important precedents have also been summarized.
1.1. Palladium(0)-catalyzed Hirao Reactions
Hirao et al. described the first P-C coupling reaction between vinyl- or aryl halides and dialkyl phosphites in the presence of tetrakis(triphenylphosphine)palladium as the catalyst, applying organic bases in toluene as the medium, or without the use of any solvent (Scheme 1) [3-5].
Scheme 1.
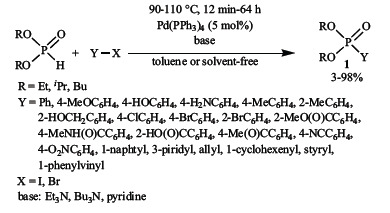
Then, the Pd(PPh3)4-catalyzed Hirao reaction was extended to a number of cases. Beside dialkyl phosphites [6-22], H-phosphinates
[23-28] and secondary phosphine oxides [29-31] were also reacted with aryl- and vinyl halides, or sulfonates [31-36]. The most com-monly used bases were triethylamine and N, N-diisopropyl-ethylamine, but N-methyl morpholine was also used [24, 34]. The solvent may be toluene and THF [35], or dipolar aprotic solvents, e.g. acetonitrile [34, 37], DMF [33, 38] or DMSO [38]. The P-C coupling reaction of optically active H-phosphinates and alkylarylphosphine oxides proceeded with the complete retention of configuration [39-43].
1.2. P-C Coupling in the Presence of Metal Salts and Ligands
1.2.1. The use of Pd(II) Sources and P-ligands
The use of palladium-precursors (e.g. Pd(OAc)2, PdCl2 or even Pd(dba)2) in combination with mono- and bidentate P-ligands is more user-friendly than applying the sensitive and rather expensive Pd(PPh3)4. In this case, the active catalyst is formed in situ by reduction of the Pd(II) to Pd(0).
The generally accepted catalytic cycle of the Hirao reaction [44] is similar to the mechanism of the well-known Pd-catalyzed C-C couplings [45], as it follows the classic three steps (Scheme 2): the oxidative addition of the aryl (or vinyl) halide to the Pd0Ln to form an “Ar-PdIILn-X” complex (A). The next step is the ligand exchange, when the Y2P(O)H reagent enters the catalytic cycle and replaces the X- anion in the Pd(II) complex. Finally, the reductive elimination from species B leads to the desired product (2), while the active Pd(0) catalyst is regenerated.
Scheme 2.
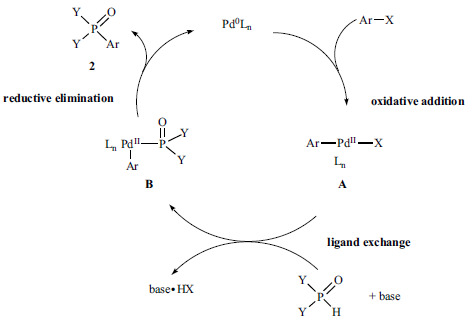
Investigation of the reaction mechanism revealed that, the trivalent tautomeric form (Y2POH) of the Y2P(O)H reagent is involved in the change of ligands step [46, 47]. It should also be noted that the presence of PPh3 and added anions may make the ligand substitution and the whole catalytic cycle more complex [48].
Arylphosphonates (3), important building blocks of biologically active compounds [51, 55, 56], flame retardants [54] or catalyst ligands [66, 67] may be easily synthetized by the Hirao reaction of dialkyl phosphites with aryl halides or triflates (Scheme 3, Table 1).
Scheme 3.
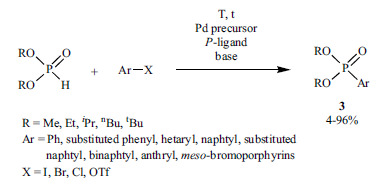
Table 1. The synthesis of arylphosphonates using Pd(II) precursors and P-ligands.
| Pd-precursor | P-Ligand | Base | Solvent | Reaction Conditions | Ref. |
|---|---|---|---|---|---|
| Pd(OAc)2 | PPh3 | Et3N, Cy2MeN, iPr2EtN | EtOH | reflux, 16-68 h | [49-56] |
| Cs2CO3 | toluene | 110 °C, 18 h | [57] | ||
| Pd(OAc)2 | PPh3, dppf, dppb, dppp, BINAP | Et3N, iPr2NEt | THF, MeCN, DMF, DMSO, 1,4-dioxane, toluene |
60-110 °C, 1.5-72 h | [44, 58-66] |
| Pd(OAc)2, Pd(dba)2 | dppb | iPr2NEt, Et3N | DMSO, toluene | 25-100 °C, 12-72 h | [66-70] |
| Pd(dppf)Cl2 | iPr2NEt, Et3N | MeCN, toluene | 82-90 °C, 15-24 h | [71,72] | |
Several arylphosphonates were prepared in the presence of Pd(OAc)2 and the simplest P-ligand, PPh3 [49-57]. The addition of tetra-n-butylammoniumchloride, -bromide or -acetate as anionic additives could enhance the coupling reactions, when Pd(OAc)2 was used as the catalyst precursor and PPh3 as the ligand [44]. KOAc and NaOAc were also applied as ionic additives in the Hirao reaction [58-62]. In the presence of Pd(OAc)2 or Pd(dba)2 as the Pd precursor, dppf [58-65], dppb [58, 66-70], dppp [58] and BINAP [58] could also be used as ligands. Pd(dppf)2Cl2 was also a suitable catalyst to promote the coupling reaction of dialkyl phosphites [71, 72].
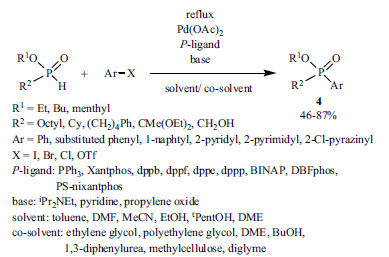
Using H-phosphinates as the reactant, both bromoarenes and less reactive chloroarenes were suitable substrates in the cross-coupling (Scheme 4) [47, 48, 73-75]. The reactions were performed using Pd(OAc)2 as the catalyst precursor and different ligands (PPh3, Xantphos, dppb, dppf, dppe, dppp, BINAP, DBFphos, PS-nixantphos), along with bases (iPr2NEt, pyridine, propylene oxide). The best results were obtained applying Xantphos [47, 48, 73], dppb [74] or dppf [75] as the P-ligand, and N,N-diisopro-pylethylamine as the base. Employing a solvent/co-solvent system was beneficial, as it may support the tautomerization of the P-reagent, and thus the ligand-exchange step of the catalytic cycle. The Hirao reaction of (R)-menthyl(hydroxymethyl)-H-phosphinate (R1 = menthyl, R2 = CH2OH) took place with the retention of the configuration at P [73].
Scheme 4.
An important and widely investigated Pd-catalyzed Hirao reaction is the coupling of aromatic species and secondary phosphine oxides, as valuable tertiary phosphines that can be applied as ligands in transition metal complexes can be formed by the reduction of the resulting phosphine oxides. It became clear that the Pd(OAc)2 - bidendate ligand (dppp or dppb) system has a high tolerance for functional groups (Scheme 5) [76-81]. During the preparation of various P-containing ligands, the phosphorylation of 2-bromobenzaldehyde [76], 2-(2-bromophenoxy)tetrahydro- 2H-pyran [77] and 2’-iodo-6,6’-dimethoxy-N,N-dimethyl-[1,1’-biphenyl]-2-amine [78] with diarylphosphine oxides took place in moderate to good yields (37-92%). The selective substitution of the iodide group of 3-bromoiodobenzene could be achieved in rather a good yield (65%) [79]. The cross-coupling of sterically hindered dibromo-3-spirobis(indene) [80] and a dinoflate bis carbazole derivative [81] with diphenylphosphine oxide took also place with high selectivity.
Scheme 5.
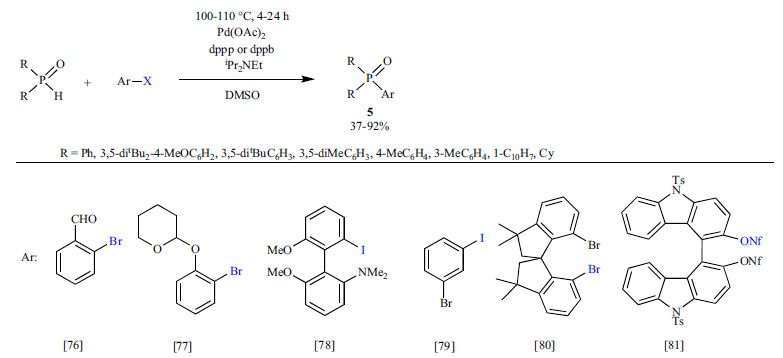
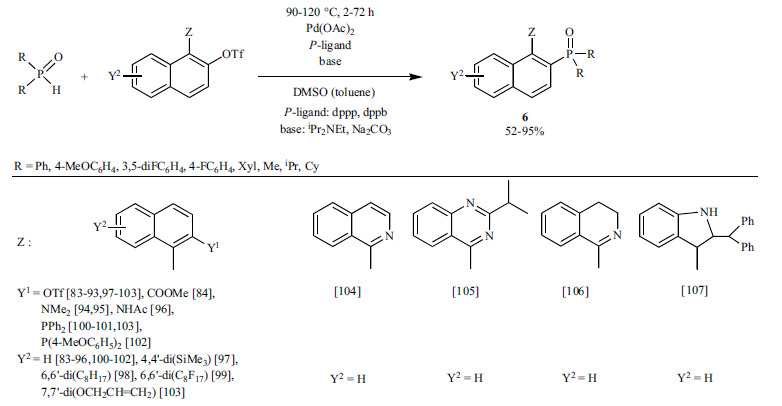
BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) can be considered as the most powerful, and one of the most commonly used bidentate chiral ligands in asymmetric catalysis [82]. In the synthesis of BINAP derivatives (6), the reaction of axial chiral triflates and diaryl or dialkyl phosphine oxides is most often carried out applying Pd(OAc)2 as the Pd precursor and iPr2NEt [83-102, 104-107] or Na2CO3 [103] as the base in DMSO (Scheme 6). Both the (R) and (S) binaphyl structures could be functionalized in different ways. In the case of binaphthyl bistriflates, the selective phosphorylation of only one triflate unit was possible [83-93]. Hence, the bis-phosphonylation could not be realized, and a “bypass” procedure had to be developed for the preparation of bisphosphines: after the mono-phosphonylation, the resulting phosphine oxide was reduced with a silane and then the second cross-coupling took place [100-103]. Heterocyclic analogs of BINAP as potential catalysts of new enantioselective approaches, such as biaryl isoquinolines (called Quinazolinaps) [104, 105], 1-aryl-3,4-dihydroisoquinolines (DHIQs) [106] and a new class of naphthyl-indole heterobiaryl skeletons [107] were also developed.
Scheme 6.
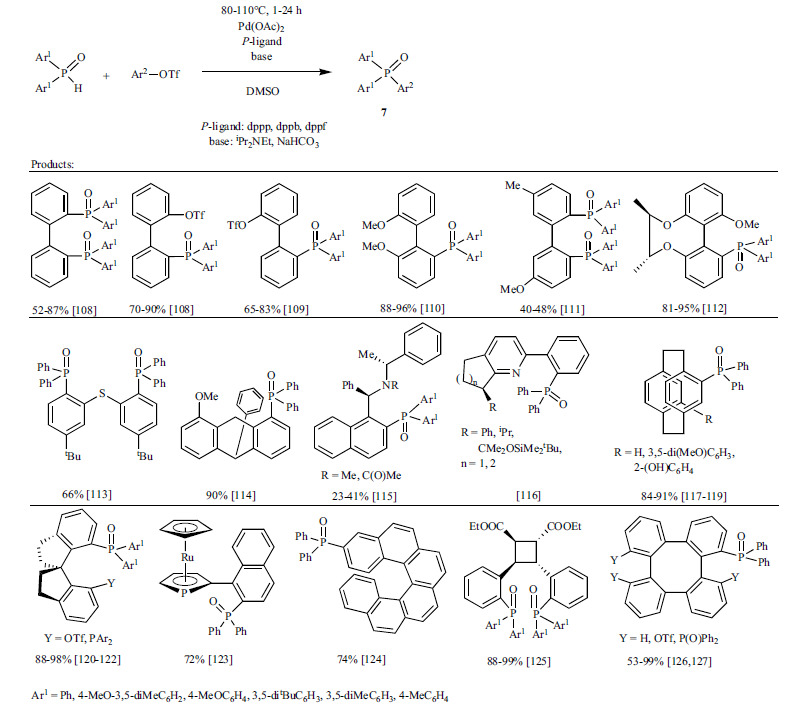
Synthesis of the representatives of other, even more complex aromatic tertiary phosphine oxides (7) is shown in Scheme 7 [108-127]. The coupling reaction of sterically hindered substrates means a real challenge and usually requires long reaction times. Despite the difficulties, selective mono- [108, 120-122], double [108, 111, 113, 125-127] and quadruple [125-127] phosphorylations could be achieved.
Scheme 7.
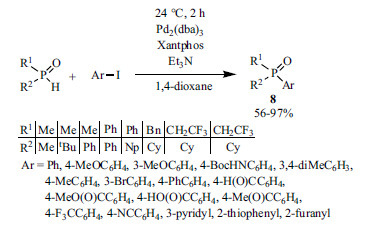
Beside Pd(OAc)2, tris(dibenzylideneacetone)dipalladium(0) is also a suitable catalyst for P-C coupling reactions to afford tertiary phosphine oxides. A general method was developed for the reaction of aryl iodides and dialkyl-, alkylaryl- or diarylphosphine oxides in the presence of Pd2(dba)3 and Xantphos using triethylamine as the base (Scheme 8) [128].
Scheme 8.
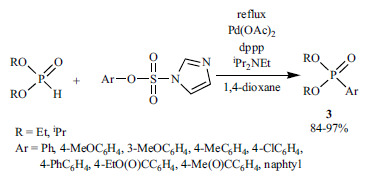
(2-Bromophenyl)diphenylphosphine oxide (9) could be synthetized in a yield of 65% from 2-bromoiodobenzene by applying Pd2(dba)3 as the catalyst, and dppp as the P-ligand in toluene (Scheme 9) [129].
Scheme 9.
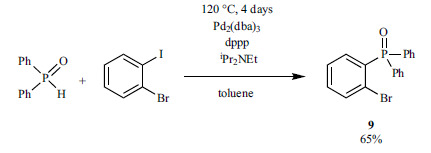
A series of intermediates for P-ligands were synthesized by the reaction of aryl triflates and diaryl phosphine oxides using Pd2(dba)3 (Scheme 10) [130-136]. The selective transformation of the triflate group of 2-bromophenyl trifluoromethanesulfonate [130], 2-bromo-4-methoxyphenyl trifluoromethanesulphonate [131] and 1-bromonaphthalen-2-yl trifluoromethanesulfonates [132-134] could be achieved in the presence of a dppp ligand and Hünig-base in toluene. (1-(Naphtho [2,3-b]furan-9-yl)naphthalen-2-yl)diaryl-phosphine oxides were obtained by similar cross-coupling reactions employing dppb as the ligand and DMSO as the solvent [135]. A double P-C coupling of 5,5'-diamino-(1,1’-biphenyl)-2,2'-diyl bis(trifluoromethanesulfonate) with diphenylphosphine oxide via N-Boc-protection and acidic cleavage resulted in the corresponding product in a 20% overall yield [136].
Scheme 10.
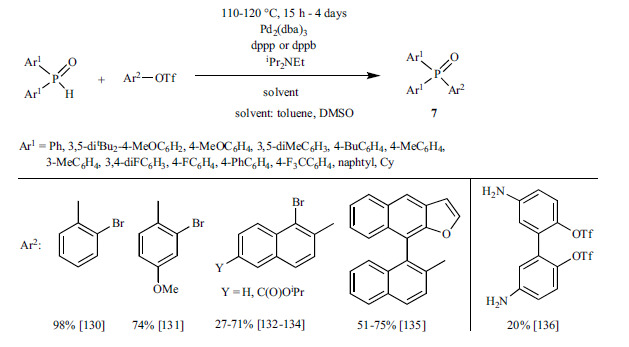
Finally, methods are summarized that apply other substrates other than aryl halides or triflates. Fu et al. reported a convenient method for the coupling reaction of aryl tosylates or mesylates and dialkyl phosphites or ethyl phenyl-H-phosphinate. The best results were obtained using Pd(OAc)2 as the catalyst with CM-Phos as the ligand and DIPEA as the base (Scheme 11) [137].
Scheme 11.
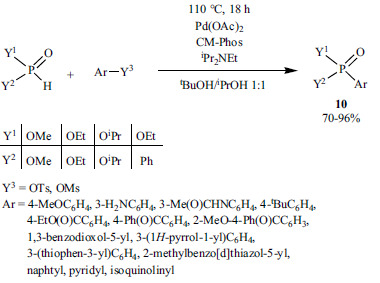
Chinese researchers described the Pd-catalyzed reactions of dialkyl phosphites and aryl imidazolyl sulfonates (Scheme 12) [138]. The reaction has a high tolerance of functional groups, although it cannot be regarded as an atom-efficient approach.
Scheme 12.
Arylboronic acids were reacted with diphenylphosphine oxide, ethyl phenyl-H-phosphinate or diethyl phosphite. The best results were obtained applying the Pd(OAc)2-dppb system as the catalyst (Scheme 13) [139].
Scheme 13.
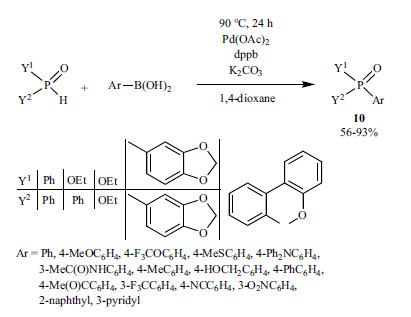
The desulfitative cross-coupling reaction of sodium benzenesulfinate and dialkyl phosphites was performed in the presence of silver carbonate as the oxidant, and tetrabutyl-ammonium chloride (TBAC) as an additive (Scheme 14) [140]. The role of Ag2CO3 has not been clarified.
Scheme 14.
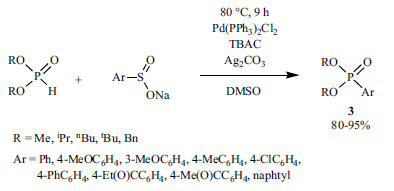
1.2.2. Nickel(II)-catalyzed Hirao Reactions
Beside the widely-used Pd catalysts, the cheaper Ni-precursors in combination with P- and N-ligands may also be used. However, this field is much less investigated. There are a few examples utilizing a Ni(II) precursor - ligand system. During the arylation of dimethyl phosphite and diphenylphosphine oxide, NiCl2 was combined with P-ligands (PPh3 or dppp) (Scheme 15) [141, 142].
Scheme 15.
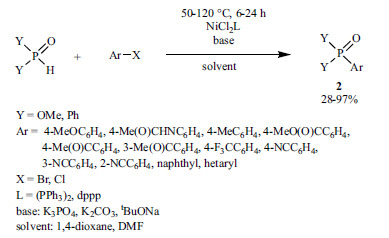
The reaction of aryl boronic acids and >P(O)H reagents was performed in the presence of Ni(II) salts and N-ligands (Scheme 16) [143].
Scheme 16.
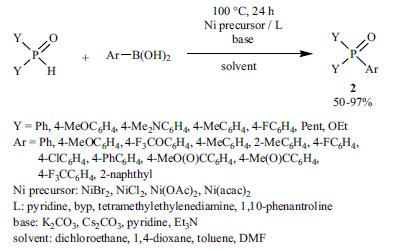
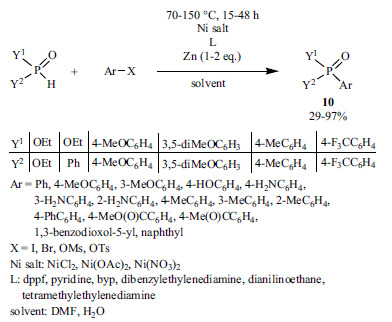
The coupling of aryl halides, mesylates and tosylates with >P(O)H species using Ni(II)-precursors and dppp or N-ligands and zinc dust led to the formation of triarylphosphine oxides, aryl-phosphonates and aryl-phosphinates (Scheme 17) [144, 145]. According to the authors, the zinc powder was required for the reduction of Ni(II) to Ni(0).
Scheme 17.
1.2.3. Environmental-friendly Approaches
Over the past few years, “green” techniques including phase transfer catalysis (PTC) and microwave (MW) irradiation came into fashion.
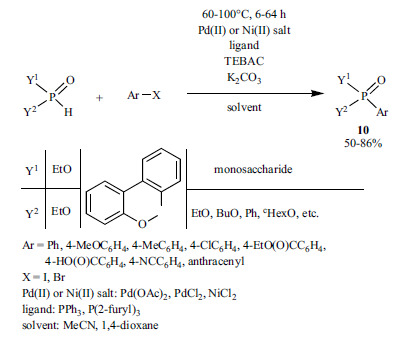
The PTC-promoted Hirao reaction employing triethylbenzyl-ammonium chloride (TEBAC) was developed by Beletskaya et al. (Scheme 18) [146-149]. The reaction of diethyl phosphite and bromoarenes performed in the presence of Pd(OAc)2 gave better results using tris(2-furyl)phosphine as the P-ligand, as compared to the case applying triphenylphosphine [146, 147]. 6-Aryl-6H-dibenzo[c,e][1,2]oxaphosphorine 2-oxides were prepared by both Pd- and Ni-catalyzed PTC-assisted coupling reactions [148]. It is noteworthy that the solid-liquid phase coupling of “phosphorylated” monosaccharides could also be performed under PTC conditions [149].
Scheme 18.
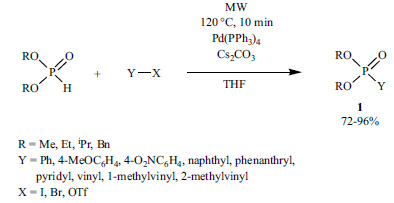
The use of the MW technique in organic chemistry has spread. In this way, the yields and selectivity could be improved, the needless excess of reagents could be eliminated, and the catalytic-systems could be simplified [150]. In 1997, a Pd(PPh3)2Cl2-catalyzed P-C coupling reaction performed in a Teflon autoclave placed in a kitchen MW oven was reported [151]. Ten years later, Stawinski et al. developed a general MW-assisted method for the P-C coupling of dialkyl phosphites and substituted aryl, hetaryl and vinyl reagents in the presence of Pd(PPh3)4 as the catalyst, Cs2CO3 as the base, and THF as the solvent using a dedicated MW oven (Scheme 19) [152].
Scheme 19.
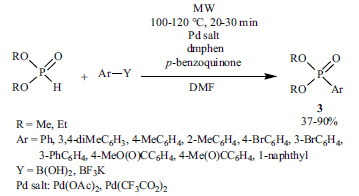
Later on, the reaction of a wide range of substrates was carried out under MW irradiation. For example, the coupling of aryl boronic acids or aryltrifluoroborates and dialkyl phosphites was performed employing Pd salts as the catalyst precursor and dmphen as the ligand (Scheme 20) [153].
Scheme 20.
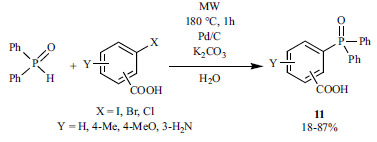
Water-soluble tertiary phosphine oxides were prepared by the Pd/C catalyzed coupling reaction of halobenzoic acids and diphenylphosphine oxide in water (Scheme 21) [154].
Scheme 21.
2. NOVEL DEVELOPMENTS ON THE HIRAO REACTION
Extensions and novel advances on the Hirao reaction attained in the last four years are presented in this chapter.
2.1. Extensions of the Pd-catalyzed Hirao Reaction
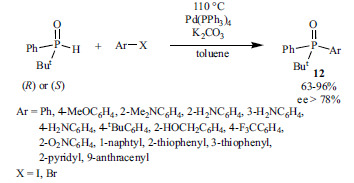
Optically active P(V) derivatives, the precursors of P(III) ligands are of importance. Beside resolution [155], enantioselective syntheses may also lead to optically active phosphine oxides [156-158]. Transition-metal-catalyzed cross-coupling reactions may also be suitable for the preparation of optically active phosphine oxides. The Pd(PPh3)4-catalyzed coupling of aryl halides and enantio-merically pure tert-butylphenylphosphine oxide carried out in the presence of K2CO3 as the base and toluene as the solvent afforded enantiomerically enriched (ee>78%) tertiary phosphine oxides in yields of 63-96% (Scheme 22) [159].
Scheme 22.
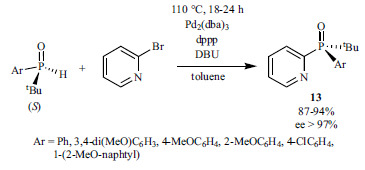
(S)-Tert-butyl-aryl phosphine oxides were coupled with 2-bromopyridine using Pd2(dba)3 as the catalyst, dppp as the ligand, and DBU as the base to give products 13 with excellent selectivity (ee>97%) (Scheme 23) [160]. The retention of the P-atom was proved by single-crystal X-ray analysis.
Scheme 23.
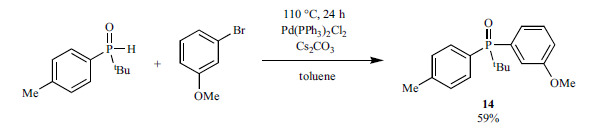
Stankevič et al. prepared the m-anisyl-t-butyl-p-tolylphosphine oxide (14) by the Pd(PPh3)2Cl2-catalyzed reaction of t-butyl-p-tolylphosphine oxide and 3-bromoanizole (Scheme 24) [161].
Scheme 24.
The palladium-catalyzed Hirao reaction was extended to the phosphorylation of aryl amides (Scheme 25) [162]. Substrates containing acyclic and cyclic N-activating groups were reacted with dialkyl phosphites. Mechanistic studies suggested that the course of the reaction is similar to that of the original Hirao reaction of aryl halides, and the key step of the catalytic cycle is the insertion of the metal into the C-N bond.
Scheme 25.
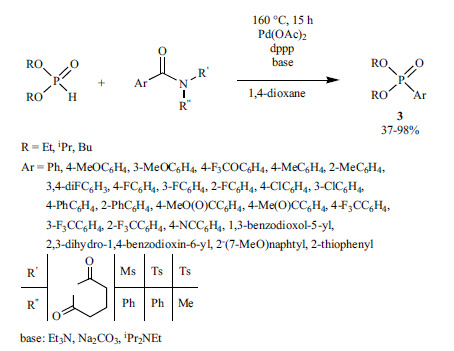
A method for the arylation of P-stereogenic secondary phosphine oxides was developed to provide optically active tertiary phosphine oxides (15) (Scheme 26) [163]. The P-C coupling of ortho-substituted aryl iodides carried out applying Pd(CF3CO2)2 as the precursor for Pd(0) and a chiral ligand (L*) furnished the target tertiary phosphine oxides (15) in moderate to good enantio-selectivities (ee: 19-83%).
Scheme 26.
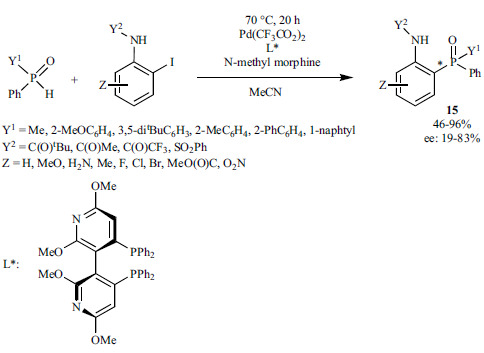
Chinese researchers reported a protocol for the P-C coupling of heteroaryl boronic acids and dialkyl phosphites in the presence of PdCl2 and PPh3, and in the absence of any base (Scheme 27) [164]. At the same time, Ag2O was needed as an additive, whose role has not been clarified.
Scheme 27.
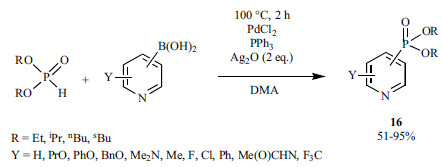
Arylphosphonates (3) were prepared via C-Si bond cleavage by the reaction of arylsilanes and dialkyl phosphites performed in the presence of Pd(PPh3)Cl2 as the catalyst (Scheme 28) [165]. Potassium fluoride is necessary to activate the arylsilane before its entering the catalytic cycle. Ag2CO3 was assumed as a reoxidant at the end of the catalytic cycle, however, this has not been proved.
Scheme 28.
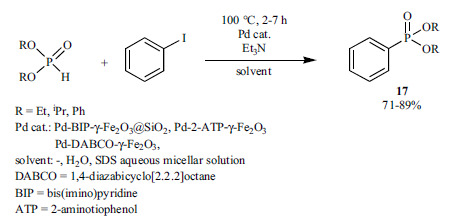
New magnetically recoverable heterogeneous palladium catalysts were developed to make the Hirao reaction environ-mentally more capable (Scheme 29) [166-168]. At first, the Pd complex of an “NNN” princer ligand (BIP) supported on nanomagnetic γ-Fe2O3@SiO2 was prepared, which proved to be a good catalyst in the cross-coupling reaction of diethyl phosphite and iodobenzene under solvent-free conditions [166]. The Pd complex of 2-aminothiophenol (Pd-2-ATP-γ-Fe2O3) [167] and DABCO (Pd-DABCO-γ-Fe2O3) [168] could be used as a catalyst in pure water, or in sodium dodecyl sulfate (SDS) aqueous micellar solution. The heterogeneous catalysts could be reused in five or six consecutive cycles, without any significant loss in their catalytic activity.
Scheme 29.
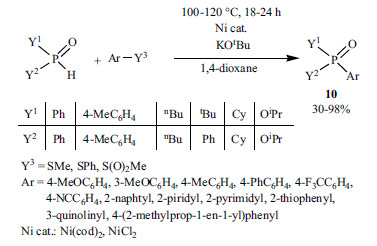
2.2. Nickel-catalyzed P-C Coupling Reactions
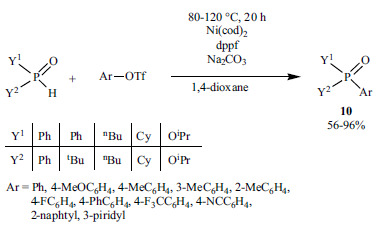
In the last years, the Ni-catalyzed P-C bond formation was also developed. Han and his co-workers elaborated the P-arylation of different >P(O)H compounds with aryl triflates in the presence of Ni(cod)2 as the Ni(0) precursor and dppf as the P-ligand (Scheme 30) [169].
Scheme 30.
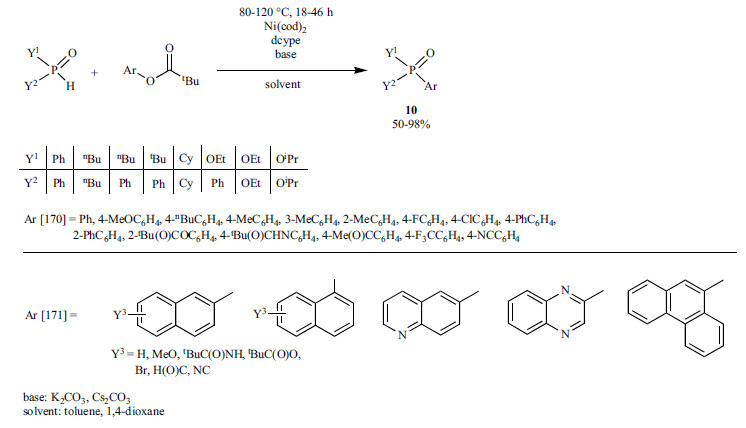
The same research group also used aryl pivalates as C-O bond activated substrates for coupling reactions with >P(O)H reagents (Scheme 31) [170, 171]. Application of the Ni(cod)2/dcype catalyst system led to the corresponding P=O derivatives (10) in good yields (50-98%). Beside phenol esters, benzylic and allylic esters could also be phosphorylated.
Scheme 31.
The combination of Ni- and photoredox catalysis allowed the cross-coupling of “C-O-S” containing aryl- and vinyl compounds (tosylates, sulfonates and sulfamates) with >P(O)H reagents under mild conditions (Scheme 32) [172]. In this case, Ni(cod)2 applied together with N-ligands, eg. 1,10-phenanthroline or dtbbyp, served as the catalyst.
Scheme 32.

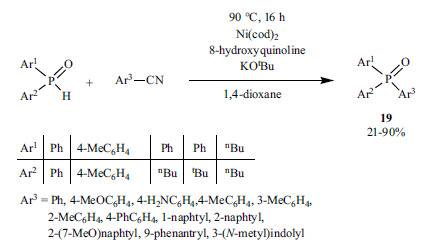
The use of Ni(cod)2 as the Ni-source, and 8-hydroxyquinoline as the N-ligand allowed the synthesis of tertiary phosphine oxides
(19) from aryl nitriles and secondary phosphine oxides (Scheme 33) [173].
Scheme 33.
The coupling of amides with dialkyl phosphites described in subchapter 2.1, Scheme 25 was also accomplished using Ni(dppp)Cl2 as the catalyst (Scheme 34) [162].
Scheme 34.
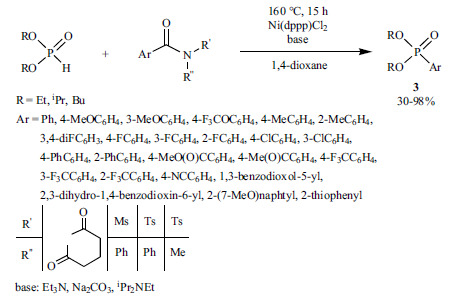
An example for the application of Ni(OAc)2 together with a tiophene-based diphosphine ligand (dcypt) using the esters of aromatic carboxylic acids as the substrate is shown in Scheme 35 [174]. Utilizing NaF as an additive increased the efficiency of the coupling, although its exact role is unknown.
Scheme 35.
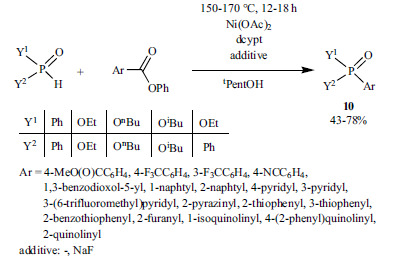
According to a recent study, the Ni-catalyzed P-C coupling reaction of aryl bromides and dialkyl phosphites was performed under electrochemical conditions to provide the corresponding aryl phosphonates (3) in moderate to good yields (15-91%) (Scheme 36) [175].
Scheme 36.
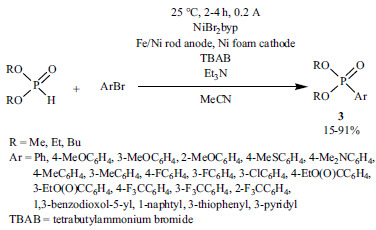
2.3. Copper-catalyzed P-C Couplings
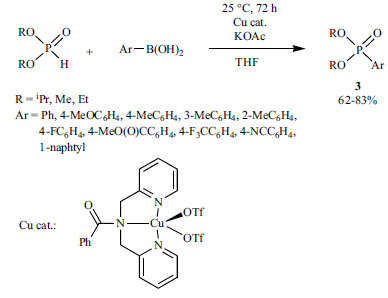
The application of Cu as the catalyst is by far less investigated, thus it is a more challenging field. Cu-catalyzed methods for C-P bond formation were summarized in a recent review [176]. Since then, just a few methods have been described for the coupling of >P(O)H reagents and aryl halides using Cu(I) or Cu(II) precursors and N-ligands [177-182]. A novel method involves the reaction of aryl boronic acids and dialkyl phosphites performed in the presence of a Cu(II) complex, (Benz-bpa)Cu(CF3SO3)2 as the catalyst, and KOAc as an additive (Scheme 37) [183].
Scheme 37.
The enantioselective Cu-catalyzed P-C coupling reactions represent a new trend (Scheme 38) [184]. Optically active tertiary phosphine oxides (20) could be prepared from racemic secondary phosphine oxides and diaryliodonium tetrafluoroborate salts in the presence of Cu(II) triflate as the metal source, (S,S)-PhPyBox as the chiral N-ligand, and K2HPO4 as the base in acetonitrile-water mixture, in mostly high enantioselectivities (ee: 50-98%). The role of water has not been mentioned.
Scheme 38.

2.4. Other Methods
Special catalytic systems have also been developed. Visible light photoredox catalysis was combined with an Au catalyst to promote the reaction of aryldiazonium salts with H-phosphinates and H-phosphonates (Scheme 39) [185]. In this way, the reactions could be carried out at room temperature, without the addition of any base. However, the synthesis of the starting diazonium salts from commercially available anilines means an extra step.
Scheme 39.
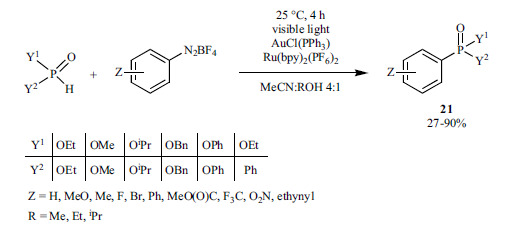
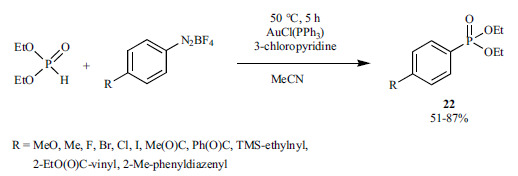
A similar Au(I)-assisted redox catalytic coupling using diethyl phosphite and aryldiazonium salts was also developed (Scheme 40) [186]. In this case, 3-chloropyridine served as the base.
Scheme 40.
3. HIRAO REACTIONS WITHOUT THE ADDITION OF USUAL P-LIGANDS
Keglevich and his co-workers found that the Hirao reaction of bromoarenes with dialkyl phosphites, H-phosphinates or secondary phosphine oxides may take place without the addition of usual P-ligands, but using the >P(O)H reactant in excess under MW conditions (Scheme 41) [187, 188]. It was found that both electron-donating and electron-withdrawing substituents of the aromatic ring decrease the reactivity dictating harsher reaction conditions (175-200°C).
Scheme 41.

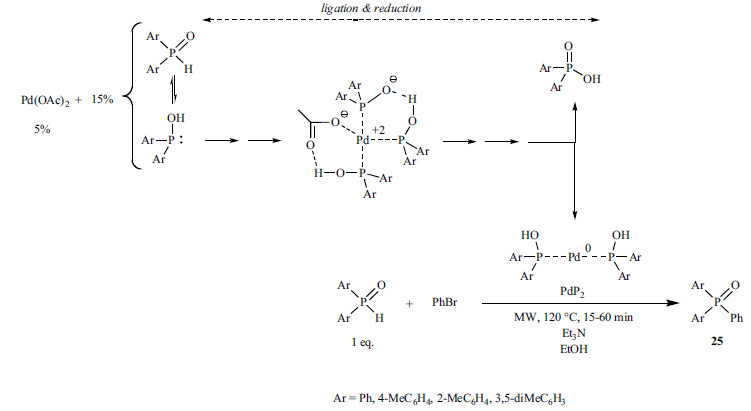
Our assumption was that in the Pd(OAc)2-catalyzed “P-ligand-free” Hirao reactions the trivalent tautomeric form of the excess of the >P(O)H reactant may serve as the P-ligand. To justify this, the reaction of bromobenzene with diethyl phosphite and diphenyl-phosphine oxide was investigated in detail (Scheme 42) [189].
Scheme 42.

Experiments showed that the use of only 1 equivalent of diethyl phosphite or diphenylphosphine oxide was not enough, as the yields were only 54% (24a) and 54% (24b), respectively. In the presence of 10% of Pd(OAc)2, the optimal amount of the >P(O)H reagent was 1.3 equivalents, leading to yields of 74% (24a) and 84% (24b), respectively. Theoretical calculations were in accord with the preparative results, and suggested that the >P(O)H reagents have a triple role in the cross-coupling process: 1 equivalent of it serves as the reactant, 10% of the >P(O)H species ensures the reduction of Pd(II) to Pd(0), while 20% of the reagent provides the P-ligand of the Pd-complexes of type (HO)Y2P…Pd…PY2(OH).
The formation of the active catalyst (PdP2) in the Hirao reaction of bromobenzene and diaryl phosphine oxides was studied in detail (Scheme 43). Quantum chemical calculations revealed that the activity of the PdP2 catalyst has a greater impact on the P-C coupling, than the intrinsic reactivity of the Ar2P(O)H compound. Hence, the catalyst containing (2-MeC6H4)POH as the ligand is more efficient, than that with Ph2POH ligand [190].
Scheme 43.
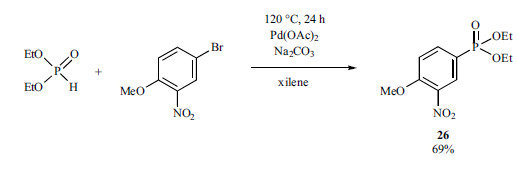
Later on, Hirao et al. have also studied a “P-ligand-free” P-C coupling reaction. Diethyl (4-methoxy-3-nitrophenyl)phosphonate (26) was synthetized from 4-bromo-1-methoxy-2-nitrobenzene applying only Pd(OAc)2 as the catalyst, and Na2CO3 as the base in xylene as the medium (Scheme 44) [191].
Scheme 44.
Recently, 2- and 4-phosphonated 13α-estrones (27 and 28) have been prepared by MW-assisted P-C coupling reactions [192]. Although the best results (yields of 66-93%) were obtained in the presence of Pd(PPh3)4, Pd(OAc)2 together with the excess of the Y2P(O)H reagent could also be used as the catalyst (to afford yields of 58-77%) (Scheme 45). The products proved to be potent estron-based OATP2B1 inhibitors.
Scheme 45.
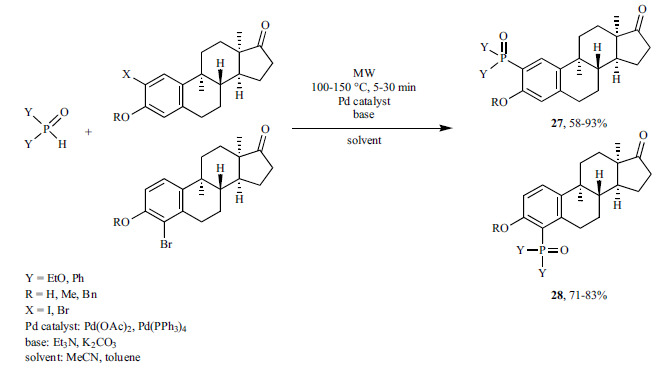
A “ligand-free” MW-promoted desulfitative coupling of arylsulfinate salts with dialkyl phosphites was performed using PdCl2 as the catalyst under MW irradiation (Scheme 46) [193]. Ag2CO3 was assumed to be involved in the oxidation of the arylsulfinate substrate, but this has not been proved.
Scheme 46.
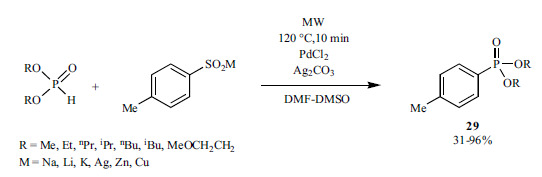
The Keglevich group also developed a Ni-catalyzed “P-ligand-free” P-C coupling process (Scheme 47) [194]. During the arylation of the >P(O)H compounds, NiCl2 was applied as the catalyst and triethylamine or potassium carbonate as the base under MW irradiation.
Scheme 47.
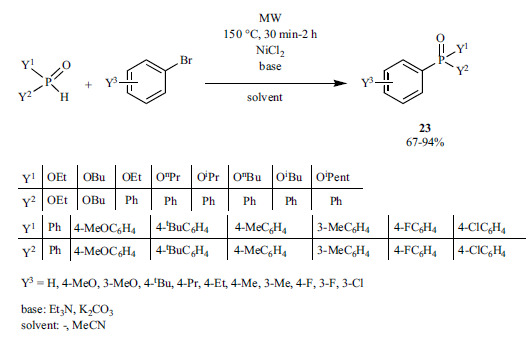
The coupling of aryl sulfides, sulfoxides or sulfones with >P(O)H reagents was carried out in the presence of Ni(cod)2 without the use of conventional ligands (Scheme 48) [195]. The reaction of diphenylphosphine oxide and thioanisol could be catalyzed by NiCl2.
Scheme 48.
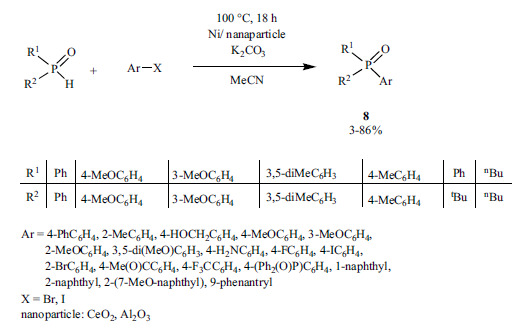
A heterogeneous Ni-catalyst supported by CeO2 or Al2O3 was utilized in the P-C coupling of aryl halides and secondary phosphine oxides without any added ligand (Scheme 49) [196]. However, the catalyst used in the reaction of 1-bromonaphthalene and diphenylphosphine oxide, and separated from the mixture by filtration practically lost its activity in the second load.
Scheme 49.
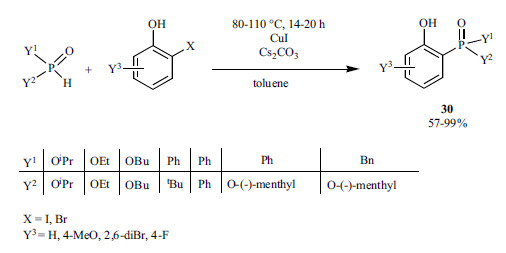
Chinese researchers published the Cu-catalyzed “ligand-free” coupling reaction of >P(O)H compounds and aryl bromides or iodides to give the 2-phosphorylated phenolic derivatives (28) (Scheme 50) [197]. The reaction of (-)-menthyl phenylphosphinate and (-)-menthyl benzylphosphinate occurred with the retention of configuration at the P-atom. According to the plausible mechanism, the oxidative addition may take place via a “Cu-phenolate” intermediate, although the mechanism was not proved.
Scheme 50.
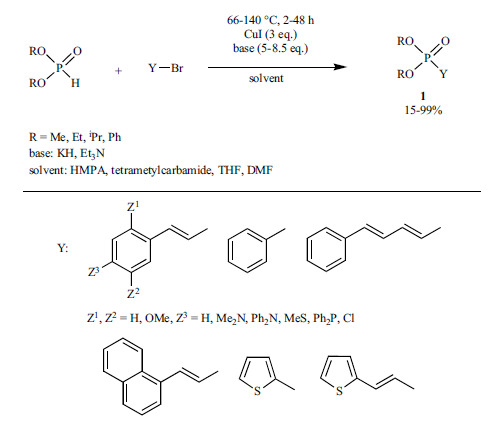
The reaction of vinyl or aryl halides with dialkyl phosphites was studied by applying excess of Cu(I)-iodide and the base (Scheme 51) [198]. The best results (yields of 78-99%) could be obtained in the presence of KH/HMPA system, but the results with triethylamine as the base and THF as the solvent also deserve attention providing product 1 in yields of 24-76%.
Scheme 51.
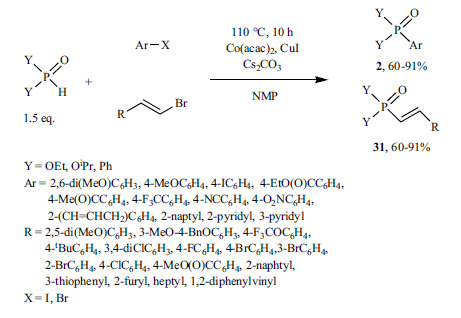
A Co-catalyzed and Cu-assisted P-C coupling of aryl/vinyl bromides and dialkyl phosphites or diphenylphosphine oxide was also described in the absence of any added ligand (Scheme 52) [199]. The corresponding products (2 and 31) were obtained in good yields (60-91%). The authors suggested that Co(I) formed from Co(II) by reduction with acac in the first step of the catalytic cycle may be the active form. At the same time, the transmetallation step is supported by Cu(I). It is noteworthy that the >P(O)H reactant was used in excess, so the participation of the >POH form cannot be excluded in the process.
Scheme 52.
Regarding the Ni-, Cu- or even the co-catalyzed “ligand-free” coupling protocols, it should be emphasized that these reactions may be of a complex nature, therefore further investigations are necessary to understand the mechanism of each system.
Nowadays, metal-free catalysis is of great importance. For this purpose, the P-C coupling of diaryl phosphine oxides with iodo-, or bromobenzoic acids was performed in the absence of any catalyst in water as the solvent under MW conditions (Scheme 53) [200]. Unfortunately, this method is limited only to the reaction of halobenzoic acids, but may be regarded as the “greenest” accomplishment that has been so far elaborated.
Scheme 53.
CONCLUSION
These days, the Hirao reaction is an important synthetic tool to provide phosphonates, phosphinates and tertiary phosphine oxides as useful intermediates. Toluene, DMSO, acetonitrile, 1,4-dioxane, THF, DMF, EtOH (or other alcohols) are the typical solvents used in the Hirao reaction. The selection of the solvent should also depend on the catalyst/catalyst precursor. Pd(PPh3)4 is, in most cases, applied together with toluene, while with Pd(OAc)2 as the catalyst precursor, DMSO is the most often used solvent. The best medium for the Ni-catalyzed P-C couplings is 1,4-dioxane, and sometimes acetonitrile. One can see that mainly aprotic solvents are applied, but protic solvents may also emerge. Both organic and ionic bases may be used in the Hirao reaction. Regarding the Pd-catalysts, triethylamine and diisopropylethylamine are the most often used tertiary amines, but the use of pyridine, DBU and N-methylmorpholine was also described. In certain cases, K2CO3, Cs2CO3 and Na2CO3 played the role of the deprotonating agent. In the Ni-catalyzed cases, all bases mentioned were used. The originally applied Pd(PPh3)4 catalyst may be replaced by Pd(OAc)2 used together with mono- and bidentate P-ligands. The best protocol is when the >P(O)H reagent serves not only as the reactant, but, via its tautomeric form (>P-OH), also as the P-ligand. In this “green” approach, the >P(O)H species have to be measured in a suitable excess (in three equivalents to the catalyst precursor), and MW-assistance is needed. The optimum temperature may be in the range of 120-150 °C. Ethanol and acetonitrile may be the best solvents, but in special cases, there is no need for any solvent. Triethylamine seems to be the best base. Other metals, such as Ni and Cu may also be used as catalysts. The environmentally-friendly variations of the P-C coupling reactions offer newer possibilities. Evaluation of the mechanism of the Hirao reaction has had a positive impact on finding the optimum conditions. The reactivity of the Hirao reaction is, of course, influenced by the substituent in the aromatic ring. Both the electron-donating and the electron-withdrawing groups decrease the reactivity. On the other hand, the P-C coupling reactions remained chemoselective also in the presence of different substituents, like halogeno-, ethoxycarbonyl- and acetyl groups.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was sponsored by the National Research Development and Innovation Fund (K119202). Réka Henyecz was supported through the New National Excellence Program of the Ministry of Human Capacities (ÚNKP-18-3-I-BME-118).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Jablonkai E., Keglevich G. P-C Bond formation by coupling reactions utilizing >P(O)H species as the reagents. Curr. Org. Chem. 2014;11:429–453. [Google Scholar]
- 2.Jablonkai E., Keglevich G. Advances and new variations of the hirao reaction. Org. Prep. Proced. Int. 2014;46:281–316. [Google Scholar]
- 3.Hirao T., Masunaga T., Ohshiro Y., Agawa T. Stereoselective synthesis of vinylphosphonate. Tetrahedron Lett. 1980;21:3595–3598. [Google Scholar]
- 4.Hirao T., Masunaga T., Yamada N., Ohshiro Y., Agawa T. Palladium-catalyzed new carbon-phosphorus bond formation. Bull. Chem. Soc. Jpn. 1982;55:909–913. [Google Scholar]
- 5.Hirao T., Masunaga T., Ohshiro Y., Agawa T. A novel synthesis of dialkyl arenephosphonates. Synthesis. 1981;1981(1):56–57. [Google Scholar]
- 6.Bulot J.J., Aboujaoude E.E., Collignon N. Preparation d’aminophenyl-, nitrophenyl, pyridyl-, et quinolylphosphonates sous photostimulation ou assistance metallique; Acces aux acides aminophosphoniques correspondants. Phosphorus Sulfur Silicon Relat. Elem. 1984;21:197–204. [Google Scholar]
- 7.Kazankova M.A., Trostyanskaya I.G., Lutsenko S.V., Beletskaya I.P. Nickel- and palladium-catalyzed cross-coupling as a route to 1- and 2-aikoxy- or dialkylaminovinylphosphonates. Tetrahedron Lett. 1999;40:569–572. [Google Scholar]
- 8.Machnitzki P., Nickel T., Stelzer O., Landgrafe C. Consecutive Pd-catalyzed p-c coupling reactions and nucleophilic phosphanylation - X-ray structure of Ph2P-C6H4-m-PO3Na2 · 5.5 H2O ·iPrOH. Eur. J. Inorg. Chem. 1998;1998:1029–1034. [Google Scholar]
- 9.Kant M., Bischoff S., Siefken R., Gründemann E., Köckritz A. Synthesis and characterization of 4- and 4,49-phosphorylated 2,29-bis(diphenylphosphanyl)-1,19-binaphthyls. Eur. J. Org. Chem. 2001;2001:477–481. [Google Scholar]
- 10.Zhong P., Xiong Z.X., Huang X. A facile regio- and stereocontrolled synthesis of (E)-vinylphosphonates VIA cross coupling of (E)-vinyl iodides with dialkyl phosphites. Synth. Commun. 2000;30:273–278. [Google Scholar]
- 11.Kim Y-C., Brown S.G., Harden T.K., Boyer J.L., Dubyak G., King B.F., Burnstock G., Jacobson K.A. Structure−activity relationships of pyridoxal phosphate derivatives as potent and selective antagonists of P2X1 receptors. J. Med. Chem. 2001;44:340–349. doi: 10.1021/jm9904203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi Y., William A., Tokoro Y. Sharpless asymmetric dihydroxylation of trans-propenylphosphonate by using a modified AD-mix-α and the synthesis of fosfomycin. J. Org. Chem. 2001;66:7903–7906. doi: 10.1021/jo010701u. [DOI] [PubMed] [Google Scholar]
- 13.Muthukumaran K., Loewe R.S., Ambroise A., Tamaru S-I., Li Q., Mathur G., Bocian D.F., Misra V., Lindsey J.S. Porphyrins bearing arylphosphonic acid tethers for attachmentto oxide surfaces. J. Org. Chem. 2004;69:1444–1452. doi: 10.1021/jo034945l. [DOI] [PubMed] [Google Scholar]
- 14.Ziessel R.F., Charbonnière L.J., Mameri S., Camerel F. Bridging of bipyridine units by phenylphosphine links: Linear and cyclic oligomers and some acid derivatives. J. Org. Chem. 2005;70:9835–9840. doi: 10.1021/jo051586g. [DOI] [PubMed] [Google Scholar]
- 15.Řehoř I., Kubiček V., Kotek J., Hermann P., Lukeš I., Száková J., Elst L.V., Muller R.N., Peters J.A. 1H NMR relaxivity of aqueous suspensions of titanium dioxide nanoparticles coated with a gadolinium(III) chelate of a DOTA-monoamide with a phenylphosphonate pendant arm. J. Mater. Chem. 2009;19:1494–1500. [Google Scholar]
- 16.Ghalib M., Niaz B., Jones P.G., Heinicke J.W. σ2-P Ligands: convenient syntheses of N-methyl-1,3-benzazaphospholes. Tetrahedron Lett. 2012;53:5012–5014. [Google Scholar]
- 17.Li L., Li A., Song L., Wang Z-H., Zhou X-H., Yang T., Huang W. Synthesis, structure and properties of a tetranuclear europium(III) complex based on 9,9-dimethylfluorene-2,7-diphosphonic acid. J. Mol. Struct. 2014;1067:37–42. [Google Scholar]
- 18.Bennett J.A., Hope E.G., Singh K., Stuart A.M. Synthesis and coordination chemistry of fluorinated phosphonic acids. J. Fluor. Chem. 2009;130:615–620. [Google Scholar]
- 19.Maffei M., Buono G. A two step synthesis of 2-oxo-2-vinyl 1,3,2-dioxaphospholanes and -dioxaphosphorinanes. Tetrahedron. 2003;59:8821–8825. [Google Scholar]
- 20.Johansson T., Stawinski J. Synthesis of dinucleoside pyridylphosphonates involving palladium(0)-catalysed phosphorus-carbon bond formation as a key step. Chem. Commun. (Camb.) 2001:2564–2565. [Google Scholar]
- 21.Parrish J., Tong L., Wang M., Chen X., Lansdon E.B., Cannizzaro C., Zheng X., Desai M.C., Xu L. Synthesis and biological evaluation of phosphonate analogues of nevirapine. Bioorg. Med. Chem. Lett. 2013;23:1493–1497. doi: 10.1016/j.bmcl.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 22.Maeda K., Miyagawa T., Furuko A., Onouchi H., Yashima E. Dual memory of enantiomeric helices in poly(phenylacetylene)s induced by a single enantiomer through helix inversion and dual storage of the enantiomeric helicity memories. Macromolecules. 2015;48:4281–4293. [Google Scholar]
- 23.Schuman M., Lopez X., Karplus M., Gouverneur V. Synthesis of a novel diarylphosphinic acid: A distorted ground state mimic and transition state analogue for amide hydrolysis. Tetrahedron. 2001;57:10299–10307. [Google Scholar]
- 24.Luke G.P., Shakespeare W.C. A simple and efficent preparation of (arylphosphinyl)-methylphosphonates. Synth. Commun. 2002;32:2951–2957. [Google Scholar]
- 25.Cristau H-J., Hervé A., Loiseau F., Virieux D. Synthesis of new arylhydroxymethylphosphinic acids and derivatives. Synthesis. 2003;14:2216–2220. [Google Scholar]
- 26.Walton J.W., Carr R., Evans N.H. Funk, A.M.; Kenwright, A.M.; Parker, D.; Yufit, D.S.; Botta, M.; De Pinto, S.; Wong K.-L. Isostructural series of nine-coordinate chiral lanthanide complexes based on triazacyclononane. Inorg. Chem. 2012;51:8042–8056. doi: 10.1021/ic300147p. [DOI] [PubMed] [Google Scholar]
- 27.Németh G., Greff Z., Sipos A., Varga Z., Székely R., Sebestyén M., Jászay Z., Béni Sz., Nemes Z., Pirat J-L., Volle J-N., Virieux D., Gyuris Á., Kelemenics K., Áy É., Minarovits J., Szathmary S., Kéri G., Őrfi L. Synthesis and evaluation of phosphorus containing, specific CDK9/CycT1 inhibitors. J. Med. Chem. 2014;57:3939–3965. doi: 10.1021/jm401742r. [DOI] [PubMed] [Google Scholar]
- 28.Fourgeaud P., Volle J-N., Vors J-P., Békro Y-A., Pirat J-A., Virieux D. 5-H-1,2-oxaphosphole 2-oxides, key building blocks for diversity oriented chemical libraries. Tetrahedron. 2016;72:7912–7925. [Google Scholar]
- 29.Rankic D.A., Parvez M., Keay B.A. 3,3′-Substituted BINAP derivatives containing C-bound substituents: Applications in asymmetric hydrogenation reactions. Tetrahedron. 2012;23:754–763. [Google Scholar]
- 30.Trost B.M., Radinov R. On the effect of a cation binding site in an asymmetric ligand for a catalyzed nucleophilic substitution reaction. J. Am. Chem. Soc. 1997;119:5962–5963. [Google Scholar]
- 31.Hall R.G., Riebli P. Preparation of new phosphine oxide synthons: synthesis of an analogue of muscarinic antagonists. Synlett. 1999;10:1633–1635. [Google Scholar]
- 32.Lu X., Zhu J. Palladium-catalyzed reaction of aryl polyflouroalkanesulfonates with O,O-dialkyl phosphonates. Synthesis. 1987;1987:726–727. [Google Scholar]
- 33.Holt D.A., Erb J.M. Palladium-catalyzed phosphorylation of alkenyl triflates. Tetrahedron Lett. 1989;30:5393–5396. [Google Scholar]
- 34.Petrakis K.S., Nagabhushan T.L. Palladium-catalyzed substitutions of triflates derived from tyrosine-containing peptides and simpler hydroxyarenes forming 4-(diethoxyphosphinyl)phenylalanines and diethyl arylphosphonates. J. Am. Chem. Soc. 1987;109:2831–2833. [Google Scholar]
- 35.Kobayashi Y., William A.D. Palladium- and nickel-catalyzed coupling reactions of α-bromoalkenylphosphonates with arylboronic acids and lithium alkenylborates. Adv. Synth. Catal. 2004;346:1749–1757. [Google Scholar]
- 36.Norris M.R., Concepcion J.J., Glasson C.R.K., Fang Z., Lapides A.M., Ashford D.L., Templeton J.L., Meyer T.J. Synthesis of phosphonic acid derivatized bipyridine ligands and their ruthenium complexes. Inorg. Chem. 2013;52:12492–12501. doi: 10.1021/ic4014976. [DOI] [PubMed] [Google Scholar]
- 37.Chauhan S.S., Varshney A., Verma B., Pennington M.W. Efficient synthesis of protected L-phosphonophenylalanine (Ppa) derivatives suitable for solid phase peptide synthesis. Tetrahedron Lett. 2007;48:4051–4054. doi: 10.1007/978-0-387-73657-0_88. [DOI] [PubMed] [Google Scholar]
- 38.Defacqz N., de Buerger B., Touillaux R., Cordi A., Marchand-Brynaert J. Direct phosphonylation of mono- and dihalogenoanilines. Synthesis. 1999;1999(8):1368–1372. [Google Scholar]
- 39.Xu Y., Li Z., Xia J., Guo H., Huang Y. Palladium-catalysed synthesis of unsymmetrical alkyl aryl-phenylphosphinates. Synthesis. 1983;1983:377–378. [Google Scholar]
- 40.Xu Y., Zhang J. Palladium-catalysed synthesis of functionalised alkyl alkylarylphosphinates. Synthesis. 1984;1984:778–780. [Google Scholar]
- 41.Xu Y., Li Z., Xia J., Guo H., Huang Y. Palladium-catalysed synthesis of alkylarylphenylphosphine oxides. Synthesis. 1984;1984:781–782. [Google Scholar]
- 42.Zhang J., Xu Y., Huang G., Guo H. Palladium-catalyzed synthesis of chiral, nonracemic isopropyl arylmethyphosphinates. Tetrahedron Lett. 1988;29:1955–1958. [Google Scholar]
- 43.Xu Y., Wei H., Zhang J., Huang G. An Efficent synthesis of chiral, nonracemic isopropyl alkenylmethyphosphinates via palladium route. Tetrahedron Lett. 1989;30:949–952. [Google Scholar]
- 44.Kalek M., Stawinski J. Pd(0)-catalyzed phosphorus-carbon bond formation. Mechanistic and synthetic studies on the role of the palladium sources and anionic additives. Organometallics. 2007;26:5840–5847. [Google Scholar]
- 45.Amatore C., Jutand A. Anionic Pd(0) and Pd(II) intermediates in palladium-catalyzed heck and cross-coupling reactions. Acc. Chem. Res. 2000;33:314–321. doi: 10.1021/ar980063a. [DOI] [PubMed] [Google Scholar]
- 46.Kalek M., Stawinski J. Palladium-catalyzed C-P bond formation: Mechanistic studies on the ligand substitution and the reductive elimination. An intramolecular catalysis by the Acetate group in PdII complexes. Organometallics. 2008;27:5876–5888. [Google Scholar]
- 47.Deal E.L., Petit C., Montchamp J-L. Palladium-catalyzed cross-coupling of h-phosphinate esters with chloroarenes. Org. Lett. 2011;13:3270–3273. doi: 10.1021/ol201222n. [DOI] [PubMed] [Google Scholar]
- 48.Berger O., Petit C., Deal E.L., Montchamp J-L. Phosphorus-carbon bond formation: Palladium-catalyzed cross-coupling of H-phosphinates and other P(O)H-containing compounds. Adv. Synth. Catal. 2013;355:1361–1373. [Google Scholar]
- 49.Bessmertnykh A., Douaihy C.M., Guilard R. Direct synthesis of amino-substituted aromatic phosphonates via palladium-catalyzed coupling of aromatic mono- and dibromides with diethyl phosphite. Chem. Lett. 2009;38:738–739. [Google Scholar]
- 50.Gooßen L.J., Dezfuli M.K. Practical protocol for the palladium-catalyzed synthesis of arylphosphonates from bromoarenes and diethyl phosphite. Synlett. 2005:445–448. [Google Scholar]
- 51.Chen X., Kopecky D.J., Mihalic J., Jeffries S., Min X., Heath J., Deignan J., Lai S., Fu Z., Guimaraes C., Shen S., Li S., Johnstone S., Thibault S., Xu H., Cardozo M., Shen W., Walker N., Kayser F., Wang Z. Structure-guided design, synthesis, and evaluation of guanine-derived inhibitors of the eIF4E mRNA−cap interaction. J. Med. Chem. 2012;55:3837–3851. doi: 10.1021/jm300037x. [DOI] [PubMed] [Google Scholar]
- 52.Bonnaventure I., Charette A.B. Probing the importance of the hemilabile site of bis(phosphine) monoxide ligands in the copper-catalyzed addition of diethylzinc to N-phosphinoylimines: Discovery of new effective chiral ligands. J. Org. Chem. 2008;73:6330–6340. doi: 10.1021/jo800969x. [DOI] [PubMed] [Google Scholar]
- 53.Contrella N.D., Sampson J.R., Jordan R.F. Copolymerization of ethylene and methyl acrylate by cationic palladium catalysts that contain phosphine-diethyl phosphonate ancillary ligands. Organometallics. 2014;33:3546–3555. [Google Scholar]
- 54.Benin V., Durganalab S., Morgan A.B. Synthesis and flame retardant testing of new boronated and phosphonated aromatic compounds. J. Mater. Chem. 2012;22:1180–1190. [Google Scholar]
- 55.Reisinger B., Kuzmanovic N., Lçffler P., Merkl R., Kçnig B., Sterner R. Exploiting protein symmetry to design light-controllable enzyme inhibitors. Angew. Chem. Int. Ed. 2014;53:595–598. doi: 10.1002/anie.201307207. [DOI] [PubMed] [Google Scholar]
- 56.Enakieva Y.Y., Bessmertnykh A.G., Gorbunova Y.G., Stern C., Rousselin Y., Tsivadze A.Y., Guilard R. Synthesis of meso-polyphosphorylporphyrins and example of self-assembling. Org. Lett. 2009;11:3842–3845. doi: 10.1021/ol901421e. [DOI] [PubMed] [Google Scholar]
- 57.Cummings S.P., Savchenko J., Fanwick P.E., Kharlamova A., Ren T. Diruthenium alkynyl compounds with phosphonate capping groups. Organometallics. 2013;32:1129–1132. [Google Scholar]
- 58.Kalek M., Jezowska M., Stawinski J. Preparation of arylphosphonates by palladium(0)-catalyzed cross-coupling in the presence of acetate additives: Synthetic and mechanistic studies. Adv. Synth. Catal. 2009;351:3207–3216. [Google Scholar]
- 59.Chen B., Ding J., Wang L., Jinga X., Wanga F. A solution-processable phosphonate functionalized deep-blue fluorescent emitter for efficient single-layer small molecule organic light-emitting diodes. Chem. Commun. (Camb.) 2012;48:8970–8972. doi: 10.1039/c2cc34712a. [DOI] [PubMed] [Google Scholar]
- 60.Mulhern K.R., Orchard A., Watson D.F., Detty M.R. Influence of surface-attachment functionality on the aggregation, persistence, and electron-transfer reactivity of chalcogenorhodamine dyes on TiO2. Langmuir. 2012;28:7071–7082. doi: 10.1021/la300668k. [DOI] [PubMed] [Google Scholar]
- 61.Rechmann R., Sarfraz A., Götzinger A.C., Dirksen E., Müller T.J.J., Erbe A. Surface functionalization of oxide-covered zinc and iron with phosphonated phenylethynyl phenothiazine. Langmuir. 2015;31:7306–7316. doi: 10.1021/acs.langmuir.5b01370. [DOI] [PubMed] [Google Scholar]
- 62.Lilley M., Mambwe B., Jackson R.F.W., Muimo R. 4-Phosphothiophen-2-yl alanine: A new 5-membered analogue of phosphotyrosine. Chem. Commun. (Camb.) 2014;50:9343–9345. doi: 10.1039/c4cc03393k. [DOI] [PubMed] [Google Scholar]
- 63.Bazaga-García M., Colodrero R.M.P., Papadaki M., Garczarek P., Zoń J., Olivera-Pastor P., Losilla E.R., León-Reina L., Aranda M.A.G., Choquesillo-Lazarte D., Demadis K.D., Cabeza A. Guest molecule-responsive functional calcium phosphonate frameworks for tuned proton conductivity. J. Am. Chem. Soc. 2014;136:5731–5739. doi: 10.1021/ja500356z. [DOI] [PubMed] [Google Scholar]
- 64.Belabassi Y., Alzghari S., Montchamp J-L. Revisiting the hirao cross-coupling: improved synthesis of aryl and heteroaryl phosphonates. J. Organomet. Chem. 2008;693:3171–3178. doi: 10.1016/j.jorganchem.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lemeune A., Mitrofanov A.Y., Rousselin Y., Stern C., Guilard R., Enakieva Y.Y., Gorbunova Y.G., Nefedov S.E. Supramolecular architectures based on phosphonic acid diesters. Phosphorus. Sulfur. 2015;190:831–836. [Google Scholar]
- 66.Nandi M., Jin J. RajanBabu, T.V. Synergistic effects of hemilabile coordination and counterions in homogeneous catalysis: new tunable monophosphine ligands for hydrovinylation reactions. J. Am. Chem. Soc. 1999;121:9899–9900. [Google Scholar]
- 67.Yan Y-Y. RajanBabu, T.V. Highly flexible synthetic routes to functionalized phospholanes from carbohydrates. J. Org. Chem. 2000;65:900–906. [Google Scholar]
- 68.Hiney R.M., Higham L.J., Müller-Bunz H., Gilheany D.G. Taming a functional group: Creating air-stable, chiral primary phosphanes. Angew. Chem. 2006;118:7406–7409. doi: 10.1002/anie.200602143. [DOI] [PubMed] [Google Scholar]
- 69.Davies L.H., Stewart B., Harrington R.W., Clegg W., Higham L.J. Air-stable, highly fluorescent primary phosphanes. Angew. Chem. Int. Ed. 2012;51:4921–4924. doi: 10.1002/anie.201108416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davies L.H., Wallis J.F., Harrington R.W., Waddell P.G., Higham L.J. Air-stable fluorescent primary phosphine complexes of molybdenum and tungsten. J. Coord. Chem. 2016;69:2069–2080. [Google Scholar]
- 71.Nikishkin N.I., Huskens J., Assenmacher J., Wilden A., Modolob G., Verboom W. Palladium-catalyzed cross-coupling of various phosphorus pronucleophiles with chloropyrazines: Synthesis of novel Am(III)-selective extractants. Org. Biomol. Chem. 2012;10:5443–5451. doi: 10.1039/c2ob25787d. [DOI] [PubMed] [Google Scholar]
- 72.Brewster T.P., Konezny S.J., Sheehan S.W., Martini L.A., Schmuttenmaer C.A., Batista V.S., Crabtree R.H. Hydroxamate anchors for improved photoconversion in dye-sensitized solar cells. Inorg. Chem. 2013;52:6752–6764. doi: 10.1021/ic4010856. [DOI] [PubMed] [Google Scholar]
- 73.Berger O., Montchamp J-L. General synthesis of P-stereogenic compounds: the menthyl phosphinate approach. Org. Biomol. Chem. 2016;14:7552–7562. doi: 10.1039/c6ob01413e. [DOI] [PubMed] [Google Scholar]
- 74.Nakano K., Oyama H., Nishimura Y., Nakasako S., Nozaki K. λ5-phospha[7]helicenes: synthesis, properties, and columnar aggregation with one-way chirality. Angew. Chem. Int. Ed. 2012;51:695–699. doi: 10.1002/anie.201106157. [DOI] [PubMed] [Google Scholar]
- 75.Gavara L., Petit C., Montchamp J-L. DBU-promoted alkylation of alkyl phosphinates and H-phosphonates. Tetrahedron Lett. 2012;53:5000–5003. [Google Scholar]
- 76.Nakano H., Suzuki Y., Kabuto C., Fujita R., Hongo H. Chiral phosphinooxathiane ligands for catalytic asymmetric Diels-Alder reaction. J. Org. Chem. 2002;67:5011–5014. doi: 10.1021/jo0201474. [DOI] [PubMed] [Google Scholar]
- 77.Nishimura T., Maeda Y., Hayashi T. Chiral diene-phosphine tridentate ligands for rhodium-catalyzed asymmetric cycloisomerization of 1,6-enynes. Org. Lett. 2011;13:3674–3677. doi: 10.1021/ol2013236. [DOI] [PubMed] [Google Scholar]
- 78.Zhou Z., Zhang Y., Xia W., Chen H., Liang H., He X., Yu S., Cao R., Qiu L. Palladium-catalyzed suzuki-miyaura coupling reactions of boronic acid derivatives with aryl chlorides. Asian J. Org. Chem. 2016;5:1260–1268. [Google Scholar]
- 79.Kitagaki S., Nakamura K., Kawabata C., Ishikawa A., Takenaga N., Yoshida K. Planar chiral [2.2]paracyclophane-based phosphine-phenols: use in enantioselective [3 + 2] annulations of allenoates and N-tosylimines. Org. Biomol. Chem. 2018;16:1770–1778. doi: 10.1039/c8ob00248g. [DOI] [PubMed] [Google Scholar]
- 80.Sun W., Gu H., Lin X. Synthesis and application of hexamethyl-1,1′-spirobiindane-based phosphine-oxazoline ligands in ni-catalyzed asymmetric arylation of cyclic aldimines. J. Org. Chem. 2018;83:4034–4043. doi: 10.1021/acs.joc.8b00422. [DOI] [PubMed] [Google Scholar]
- 81.Botman P.N.M., Fraanje J., Goubitz K., Peschar R., Verhoeven J.W., van Maarseveen J.H., Hiemstra H. Synthesis, properties and applications of bicap: a new family of carbazole-based diphosphine ligands. Adv. Synth. Catal. 2004;346:743–754. [Google Scholar]
- 82.Berthod M., Mignani G., Woodward G., Lemaire M. modified BINAP: the how and the why. Chem. Rev. 2005;105:1801–1836. doi: 10.1021/cr040652w. [DOI] [PubMed] [Google Scholar]
- 83.Kurz L., Lee G., Morgans D., Jr, Waldyke M.J., Ward T. Stereospecific functionalization of (R)-(-)-1,1′-bi-2-naphtol triflate. Tetrahedron Lett. 1990;31:6321–6324. [Google Scholar]
- 84.Uozumi Y., Suzuki N., Ogiwara A., Hayashi T. Preparation of optically active binaphthylmonophosphines (MOP’s) containing various functional groups. Tetrahedron. 1994;50:4293–4302. [Google Scholar]
- 85.Cai D., Payack J.F., Bender D.R., Hughes D.L., Verhoeven T.R., Reider P.J. Synthesis of chiral 2,2′-bis(diphenylphosphino)-1,l’-binaphthyl (BINAP) via a novel nickel-catalyzed phosphine insertion. J. Org. Chem. 1994;59:7180–7181. [Google Scholar]
- 86.Xu J-X., Chen M-Y., Zheng Z-J., Cao J. J.; Xu, Z.; Cui, Y.-M.; Xu, L.-W. Platinum-catalyzed multicomponent alcoholysis/hydrosilylation and bis-hydrosilylation of alkynes with dihydrosilanes. ChemCatChem. 2017;9:3111–3116. [Google Scholar]
- 87.Chen J-X., Daeuble J.F., Stryker J.M. Phosphine effects in the copper(I) hydride-catalyzed hydrogenation of ketones and regioselective 1,2-reduction of α,β-unsaturated ketones and aldehydes. hydrogenation of decalin and steroidal ketones and enones. Tetrahedron. 2000;56:2789–2798. [Google Scholar]
- 88.Uozumi Y., Tanahashi A., Lee S-Y., Hayashi T. Synthesis of optically active 2-(diary1phosphino)-1,l’-binaphthyls, efficient chiral monodentate phosphine ligands. J. Org. Chem. 1993;58:1945–1948. [Google Scholar]
- 89.Cho S., Shibasaki M. Synthesis and evaluation of a new chiral ligand: 2-Diphenylarsino-2′-diphenylphosphino-1,1′-binaphthyl (BINAPAs). Tetrahedron Lett. 1998;39:1773–1776. [Google Scholar]
- 90.Shi M., Li C-Q. Catalytic, asymmetric aza-Baylis-Hillman reaction of N-sulfonated imines with 2-cyclohexen-1-one and 2-cyclopenten-1-one in the presence of a chiral phosphine Lewis base. Tetrahedron. 2005;16:1385–1391. [Google Scholar]
- 91.Hamada T., Chieffi A., Åhman J., Buchwald S.L. An improved catalyst for the asymmetric arylation of ketone enolates. J. Am. Chem. Soc. 2002;124:1261–1268. doi: 10.1021/ja011122+. [DOI] [PubMed] [Google Scholar]
- 92.Wei B., Chen C., You C., Lv H., Zhang X. Efficient synthesis of (S,R)-Bn-Yanphos and Rh/(S,R)-Bn-Yanphos catalyzed asymmetric hydroformylation of vinyl heteroarenes. Org. Chem. Front. 2017;4:288–291. [Google Scholar]
- 93.Duclos M-C., Singjunla Y., Petit C., Favre-Réguillon A., Jeanneau E., Popowycz F., Métay E., Lemaire M. Synthesis and evaluation of P-chirogenic monodentate binaphthyl phosphines. Tetrahedron Lett. 2012;53:5984–5986. [Google Scholar]
- 94.Ding K., Wang Y., Yun H., Liu J., Wu Y., Terada M., Okubo Y., Mikami K. Highly efficient and practical optical resolution of 2-amino-2′-hydroxy-1,1′-binaphthyl by molecular complexation with n-benzylcinchonidium chloride: A direct transformation to binaphthyl amino phosphine. Chemistry. 1999;5:1734–1737. [Google Scholar]
- 95.Vyskočil Š., Smřcina M., Hanuš V., Polášek M., Kočovský P. Derivatives of 2-Amino-2′-diphenylphosphino-1,1′-binaphthyl (MAP) and their application in asymmetric palladium(0)-catalyzed allylic substitution. J. Org. Chem. 1998;63:7738–7748. [Google Scholar]
- 96.Anstiss C., Karuso P., Richardson M., Liu L. Synthesis of new BINAP-based aminophosphines and their 31P-NMR spectroscopy. Molecules. 2013;18:2788–2802. doi: 10.3390/molecules18032788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ngo H.L., Lin W. Development of 4,4′-substituted-XylBINAP ligands for highly enantioselective hydrogenation of ketones. J. Org. Chem. 2005;70:1177–1187. doi: 10.1021/jo048333s. [DOI] [PubMed] [Google Scholar]
- 98.Han J-W., Hayashi T. Enhanced catalytic activity in asymmetric hydrosilylation of 1,3-dienes with a soluble palladium catalyst. Tetrahedron. 2002;13:325–331. [Google Scholar]
- 99.Maillard D., Bayardon J., Kurichiparambil J.D., Nguefack-Fournier C., Sinou D. Chiral perfluorous analogues of MOP. Synthesis and applications in catalysis. Tetrahedron. 2002;13:1449–1456. [Google Scholar]
- 100.Gladiali S., Pulacchini S., Fabbri D., Manassero M., Sansoni M. 2-Diphenylphosphino-2′-diphenylphosphinyl-1,1′-binaphthalene (BINAPO), an axially chiral heterobidentate ligand for enantioselective catalysis. Tetrahedron. 1998;9:391–395. [Google Scholar]
- 101.Gladiali S., Taras R., Ceder R.M., Rocamora M., Muller G., Solans X., Font-Bardia M. Asymmetric allylic alkylation catalyzed by Pd(II)-complexes with (S)-BINPO, a hemilabile axially chiral P,O-heterodonor inducer. Tetrahedron. 2004;15:1477–1485. [Google Scholar]
- 102.Bayardon J., Cavazzini M., Maillard D., Pozzi G., Quicib S., Sinou D. Chiral fluorous phosphorus ligands based on the binaphthyl skeleton: synthesis and applications in asymmetric catalysis. Tetrahedron. 2003;14:2215–2224. [Google Scholar]
- 103.Yuan W-C., Cun L-F., Gong L-Z., Mi A-Q., Jiang Y-Z. Preparation of chiral 7,7′-disubstituted BINAPs for Rh-catalyzed 1,4-addition of arylboronic acids. Tetrahedron Lett. 2005;46:509–512. [Google Scholar]
- 104.Alcock N.W., Brown J.M., Hulmes D.I. Synthesis and resolution of l-(2-diphenylphosphino-l-naphthyl)isoquinoline; a P-N chelating ligand for asymmetric catalysis. Tetrahedron. 1993;4:743–756. [Google Scholar]
- 105.Maxwell A.C., Franc C., Pouchain L., Müller-Bunza H., Guiry P.J. Electronically varied quinazolinaps for asymmetric catalysis. Org. Biomol. Chem. 2008;6:3848–3853. doi: 10.1039/b810936b. [DOI] [PubMed] [Google Scholar]
- 106.Rosell J.M., Staniland S., Turner N.J., Clayden J. Substituent effects on axial chirality in 1-aryl-3,4-dihydroisoquinolines: controlling the rate of bond rotation. Tetrahedron. 2016;72:5172–5177. [Google Scholar]
- 107.Zhang H-H., Wang C-S., Li C., Mei G-J., Li Y., Shi F. Design and enantioselective construction of axially chiral naphthyl-indole skeletons. Angew. Chem. Int. Ed. 2016;55:1–7. doi: 10.1002/anie.201608150. [DOI] [PubMed] [Google Scholar]
- 108.Mikami K., Aikawa K., Korenaga T. General synthetic route to chiral flexible biphenylphosphine ligands: The use of a chiral additive enables the preparation and observation of metal complexes incorporating the enantiopure form. Org. Lett. 2001;3:243–245. doi: 10.1021/ol0068896. [DOI] [PubMed] [Google Scholar]
- 109.Tian F., Yao D., Zhang Y.J., Zhang W. Phosphine-oxazoline ligands with an axial-unfixed biphenyl backbone: the effects of the substituent at oxazoline ring and P phenyl ring on Pd-catalyzed asymmetric allylic alkylation. Tetrahedron. 2009;65:9609–9615. [Google Scholar]
- 110.Xia W., Li Y., Zhou Z., Chen H., Liang H., Yu S., He X., Zhang Y., Pang J., Zhou Z., Qiu L. Synthesis of chiral-bridged atropisomeric monophosphine ligands with tunable dihedral angles and their applications in asymmetric suzuki-miyaura coupling reactions. Adv. Synth. Catal. 2017;359:1656–1662. [Google Scholar]
- 111.Liang Y., Wang Z., Ding K. Generation of self-supported noyori-type catalysts using achiral bridged-biphep for heterogeneous asymmetric hydrogenation of ketones. Adv. Synth. Catal. 2006;348:1533–1538. [Google Scholar]
- 112.Wang S., Li J., Miao T., Wu W., Li Q., Zhuang Y., Zhou Z., Qiu L. Highly efficient synthesis of a class of novel chiral-bridged atropisomeric monophosphine ligands via simple desymmetrization and their applications in asymmetric suzuki-miyaura coupling reaction. Org. Lett. 2012;14:1966–1969. doi: 10.1021/ol300721p. [DOI] [PubMed] [Google Scholar]
- 113.Morohashi N., Akahira Y., Tanaka S., Nishiyama K., Kajiwara T., Hattori T. Synthesis of a sulfur-bridged diphosphine ligand and its unique complexation properties toward palladium(II) ion. Chem. Lett. 2008;37:418–419. [Google Scholar]
- 114.Leung F.K-C., Ishiwari F., Shoji Y., Nishikawa T., Takeda R., Nagata Y., Suginome M., Uozumi Y., Yamada Y.M.A. Fukushima. T. Synthesis and catalytic applications of a triptycene-based monophosphine ligand for palladium-mediated organic transformations. ACS Omega. 2017;2:1930–1937. doi: 10.1021/acsomega.7b00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y., Li X., Ding K. Synthesis of a new type of chiral amino phosphine ligands for asymmetric catalysis. Tetrahedron. 2002;13:1291–1297. [Google Scholar]
- 116.Ito K., Kashiwagi R., Iwasaki K., Katsuki T. Asymmetric allylic alkylation using a palladium complex of chiral 2-(phosphinoaryl)pyridine ligands. Synlett. 1999:1563–1566. [Google Scholar]
- 117.Zhang T-Z., Dai L-X., Hou X-L. Synthesis of planar chiral [2.2]paracyclophane monophosphine ligands and their application in the umpolung allylation of aldehydes. Tetrahedron. 2007;18:251–259. [Google Scholar]
- 118.Kitagaki S., Ohta Y., Tomonaga S., Takahashi R., Mukai C. Synthesis of planar chiral pseudo-ortho-substituted aryl[2.2]paracyclophanes by stepwise successive palladium-catalyzed coupling reactions. Tetrahedron. 2011;22:986–991. [Google Scholar]
- 119.Takenaga N., Adachi S., Furusawa A., Nakamura K., Suzuki N., Ohta Y., Komizu M., Mukai C., Kitagaki S. Planar chiral [2.2]paracyclophane-based phosphine-phenol catalysts: Application to the aza-Morita-Baylis-Hillman reaction of N-sulfonated imines with various vinyl ketones. Tetrahedron. 2016;72:6892–6897. [Google Scholar]
- 120.Xie J-H., Wang L-X., Fu Y., Zhu S-F., Fang B-M., Duan H-F., Zhou Q-L. Synthesis of spiro diphosphine ligands and their application in asymmetric hydrogenation of ketones. J. Am. Chem. Soc. 2003;125:4404–4405. doi: 10.1021/ja029907i. [DOI] [PubMed] [Google Scholar]
- 121.Xie J-H., Duan H-F., Fan B-M., Cheng X., Wang L-X., Zhou Q-L. Application of SDP ligands for Pd-catalyzed allylic alkylation. Adv. Synth. Catal. 2004;346:625–632. [Google Scholar]
- 122.Li S., Zhu S-F., Zhang C-M., Song S., Zhou Q-L. Iridium-catalyzed enantioselective hydrogenation of α,β-unsaturated carboxylic acids. J. Am. Chem. Soc. 2008;130:8584–8585. doi: 10.1021/ja802399v. [DOI] [PubMed] [Google Scholar]
- 123.Carmichael D., Ricard L., Seeboth N. Planar-to-Axial Chirality Relay in Phospharuthenocenes. A rotationally hindered 2-(2′-diphenylphosphinonaphth-1′-yl)phospharuthenocene. Organometallics. 2007;26:2964–2970. [Google Scholar]
- 124.Teplý F., Stará I.G., Starý I., Kollárovič A., Šaman D., Vyskočil Š., Fiedler P. Synthesis of 3-hexahelicenol and its transformation to 3-hexahelicenylamines, diphenylphosphine, methyl carboxylate, and dimethylthiocarbamate. J. Org. Chem. 2003;68:5193–5197. doi: 10.1021/jo034369t. [DOI] [PubMed] [Google Scholar]
- 125.Zhao D., Ding K. A new type of C2-symmetric bisphospine ligands with a cyclobutane backbone: practical synthesis and application. Org. Lett. 2003;5:1349–1351. doi: 10.1021/ol034299c. [DOI] [PubMed] [Google Scholar]
- 126.Wen J-F., Hong W., Yuan K., Mak T.C.W., Wong H.N.C. Synthesis, resolution, and applications of 1,16-dihydroxytetraphenylene as a novel building block in molecular recognition and assembly. J. Org. Chem. 2003;68:8918–8931. doi: 10.1021/jo0302408. [DOI] [PubMed] [Google Scholar]
- 127.Peng H-Y., Lam C-K., Mak T.C.W., Cai Z., Ma W-T., Li Y-X., Wong H.N.C. Chiral rodlike platinum complexes, double helical chains, and potential asymmetric hydrogenation ligand based on “linear” building blocks: 1,8,9,16-tetrahydroxytetraphenylene and 1,8,9,16-tetrakis(diphenylphosphino)tetraphenylene. J. Am. Chem. Soc. 2005;127:9603–9611. doi: 10.1021/ja051013l. [DOI] [PubMed] [Google Scholar]
- 128.Bloomfield A.J., Herzon S.B. Room temperature, palladium-mediated p-arylation of secondary phosphine oxides. Org. Lett. 2012;14:4370–4373. doi: 10.1021/ol301831k. [DOI] [PubMed] [Google Scholar]
- 129.Zhang H., Hu R-B., Zhang X-Y., Li S-X., Yang S-D. Palladium-catalyzed R2(O)P directed C(sp2)-H acetoxylation. Chem. Commun. (Camb.) 2014;50:4686–4689. doi: 10.1039/c4cc01238k. [DOI] [PubMed] [Google Scholar]
- 130.Matsumura K., Shimizu H., Saito T., Kumobayashi H. Synthesis and application of chiral phospholane ligands bearing a sterically and electrically adjustable moiety. Adv. Synth. Catal. 2003;345:180–184. [Google Scholar]
- 131.Wang C., Yang G., Zhuang J., Zhang W. From tropos to atropos: 5,5′-bridged 2,2′-bis(diphenylphosphino)biphenyls as chiral ligands for highly enantioselective palladium-catalyzed hydrogenation of α-phthalimide ketones. Tetrahedron Lett. 2010;51:2044–2047. [Google Scholar]
- 132.Zhou Y., Zhang X., Liang H., Cao Z., Zhao X., He Y., Wang S., Pang J., Zhou Z., Ke Z., Qiu L. Enantioselective synthesis of axially chiral biaryl monophosphine oxides via direct asymmetric suzuki coupling and dft investigations of the enantioselectivity. ACS Catal. 2014;4:1390–1397. [Google Scholar]
- 133.Feng J., Li B., He Y., Gu Z. Enantioselective synthesis of atropisomeric vinyl arene compounds by palladium catalysis: A carbene strategy. Angew. Chem. Int. Ed. 2016;55:2186–2190. doi: 10.1002/anie.201509571. [DOI] [PubMed] [Google Scholar]
- 134.Li B., Zhang M., Huanga X., Gu Z. Synthesis of 2,3-dihydro-1h-phosphindole-1-oxides via the t-buli-mediated rearrangement of vinylbromide and phosphine oxide. Org. Chem. Front. 2017;4:1854–1857. [Google Scholar]
- 135.Zhou Z., Liang H., Xia W., Chen H., Zhang Y., He X., Yu S., Cao R., Qiu L. Synthesis of a class of binaphthyl monophosphine ligands with a naphthofuran skeleton and their applications in Suzuki-Miyaura coupling reactions. New J. Chem. 2018;42:5967–5971. [Google Scholar]
- 136.Storch G., Siebert M., Rominger F., Trapp O. 5,5′-Diamino-BIPHEP ligands bearing small selector units for non-covalent binding of chiral analytes in solution. Chem. Commun. (Camb.) 2015;51:15665–15668. doi: 10.1039/c5cc06306j. [DOI] [PubMed] [Google Scholar]
- 137.Fu W.C., So C.M., Kwong F.Y. Palladium-catalyzed phosphorylation of aryl mesylates and tosylates. Org. Lett. 2015;17:5906–5909. doi: 10.1021/acs.orglett.5b03104. [DOI] [PubMed] [Google Scholar]
- 138.Luo Y., Wu J. Synthesis of arylphosphonates via palladium-catalyzed coupling reactions of aryl imidazolylsulfonates with h-phosphonate diesters. Organometallics. 2009;28:6823–6826. [Google Scholar]
- 139.Fu T., Qiao H., Peng Z., Hu G., Wu X., Gao Y., Zhao Y. Palladium-catalyzed air-based oxidative coupling of arylboronic acids with H-phosphine oxides leading to aryl phosphine oxides. Org. Biomol. Chem. 2014;12:2895–2902. doi: 10.1039/c3ob42470g. [DOI] [PubMed] [Google Scholar]
- 140.Miao T., Wang L. Palladium-catalyzed desulfitative cross-coupling reaction of sodium arylsulfinates with H-phosphonate diesters. Adv. Synth. Catal. 2014;356:967–971. [Google Scholar]
- 141.Zhao Y-L., Wu G-J., Li Y., Gao L-X., Han F-S. [NiCl2(dppp)]-Catalyzed cross-coupling of aryl halides with dialkyl phosphite, diphenylphosphine oxide, and diphenylphosphine. Chemistry. 2012;18:9622–9627. doi: 10.1002/chem.201103723. [DOI] [PubMed] [Google Scholar]
- 142.Zhang H-Y., Sun M., Ma Y-N., Tian Q-P., Yang S-D. Nickel-catalyzed C-P cross-coupling of diphenylphosphine oxide with aryl chlorides. Org. Biomol. Chem. 2012;10:9627–9633. doi: 10.1039/c2ob26874d. [DOI] [PubMed] [Google Scholar]
- 143.Hu G., Chen W., Fu T., Peng Z., Qiao H., Gao Y., Zhao Y. Nickel-catalyzed C-P cross-coupling of arylboronic acids with P(O)H compounds. Org. Lett. 2013;15:5362–5365. doi: 10.1021/ol402672e. [DOI] [PubMed] [Google Scholar]
- 144.Shen C., Yang G., Zhang W. Nickel-catalyzed C-P coupling of aryl mesylates and tosylates with H(O)PR1R2. Org. Biomol. Chem. 2012;10:3500–3505. doi: 10.1039/c2ob25225b. [DOI] [PubMed] [Google Scholar]
- 145.Zhang X., Liu H., Hu X., Tang G., Zhu J., Zhao Y. Ni(II)/Zn catalyzed reductive coupling of aryl halides with diphenylphosphine oxide in water. Org. Lett. 2011;13:3478–3481. doi: 10.1021/ol201141m. [DOI] [PubMed] [Google Scholar]
- 146.Novikova Z.S., Demik N.N., Agarkov A.Yu., Beletskaya I.P. Palladium-catalyzed arylation of diethyl hydrogen phosphite. Russ. J. Org. Chem. 1995;31:129. [Google Scholar]
- 147.Kabachnik M.M., Solntseva M.D., Izmer V.V., Novikova Z.S., Beletskaya I.P. Palladium-catalyzed phase-transfer arylation of dialkyl phosphonates. Russ. J. Org. Chem. 1998;34:93–97. [Google Scholar]
- 148.Beletskaya I.P., Neganova E.G., Veits Yu.A. Arylation of 6H-dibenzo[c,e][1,2λ5]oxaphosphinine 6-oxide. Russ. J. Org. Chem. 2004;40:1782–1786. [Google Scholar]
- 149.Beletskaya I.P., Karlstedt N.B., Nifant’ev E.E., Khodarev D.V., Kukhareva T.S., Nikolaev A.V., Ross A.J. Palladium-catalyzed P-arylation of hydrophosphoryl derivatives of protected monosaccharides. Russ. J. Org. Chem. 2006;42:1780–1785. [Google Scholar]
- 150.Keglevich G. Milestones in Microwave Chemistry. Switzerland: Springer; 2016. [Google Scholar]
- 151.Villemin D., Jaffrès P-A., Siméon F. Phosphorus Sulfur Silicon Relat. Elem. 1997;130:59. [Google Scholar]
- 152.Kalek M., Stawinski J. Efficient synthesis of mono- and diarylphosphinic acids: a microwave-assisted palladium-catalyzed cross-coupling of aryl halides with phosphinate. Tetrahedron. 2009;65:10406–10412. [Google Scholar]
- 153.Andaloussi M., Lindh J., Sävmarker J., Sjöberg P.J.R., Larhed M. Microwave-promoted palladium(II)-catalyzed C-P bond formation by using arylboronic acids or aryltrifluoroborates. Chemistry. 2009;15:13069–13074. doi: 10.1002/chem.200901473. [DOI] [PubMed] [Google Scholar]
- 154.Rummelt S.M., Ranocchiari M., van Bokhoven J.A. Synthesis of water-soluble phosphine oxides by Pd/C-catalyzed P-C coupling in water. Org. Lett. 2012;14:2188–2190. doi: 10.1021/ol300582y. [DOI] [PubMed] [Google Scholar]
- 155.Bagi P., Ujj V., Czugler M., Fogassy E., Keglevich G. Resolution of P-stereogenic P-heterocycles via the formation of diastereomeric molecular and coordination complexes (a review). Dalton Trans. 2016;45:1823–1842. doi: 10.1039/c5dt02999f. [DOI] [PubMed] [Google Scholar]
- 156.Dutartre M., Bayardon J., Jugé S. Applications and stereoselective syntheses of P-chirogenic phosphorus compounds. Chem. Soc. Rev. 2016;45:5771–5794. doi: 10.1039/c6cs00031b. [DOI] [PubMed] [Google Scholar]
- 157.Chen T., Han L-B. Optically active H-phosphinates and their stereospecific transformations into optically active P-stereogenic organophosphoryl compounds. Synlett. 2015;26:1153–1163. [Google Scholar]
- 158.Kolodiazhnyi O.I. Recent advances in asymmetric synthesis of P-stereogenic phosphorus compounds. Top. Curr. Chem. 2015;360:161–236. doi: 10.1007/128_2014_564. [DOI] [PubMed] [Google Scholar]
- 159.Chrzanowski J., Krasowska D., Urbaniak M., Sieroń L., Pokora-Sobczak P., Demchuk O.M., Drabowicz J. Synthesis of enantioenriched aryl‐tert‐butylphenylphosphine oxides via cross-coupling reactions of tert‐butylphenylphosphine oxide with aryl halides. Eur. J. Org. Chem. 2018;33:4614–4627. [Google Scholar]
- 160.Han Z.S., Wu H., Xu Y., Zhang Y., Qu B., Li Z., Caldwell D.R., Fandrick K.R., Zhang L., Roschangar F., Song J.J., Senanayake C.H. General and stereoselective method for the synthesis of sterically congested and structurally diverse p-stereogenic secondary phosphine oxides. Org. Lett. 2017;19:1796–1799. doi: 10.1021/acs.orglett.7b00568. [DOI] [PubMed] [Google Scholar]
- 161.Stankevič M., Pisklak J., Włodarczyk K. Aryl group - a leaving group in arylphosphine oxides. Tetrahedron. 2016;72:810–824. [Google Scholar]
- 162.Liu C., Szostak M. Decarbonylative phosphorylation of amides by palladium and nickel catalysis: The hirao cross-coupling of amide derivatives. Angew. Chem. Int. Ed. 2017;56:12718–12722. doi: 10.1002/anie.201707102. [DOI] [PubMed] [Google Scholar]
- 163.Zhang Y., He H., Wanga Q., Cai Q. Asymmetric synthesis of chiral P-stereogenic triaryl phosphine oxides via Pd-catalyzed kinetic arylation of diaryl phosphine oxides. Tetrahedron Lett. 2016;57:5308–5311. [Google Scholar]
- 164.Geng Z., Zhang Y., Zheng L., Li J., Zou D., Wu Y., Wu Y. Pd-catalyzed C-P Coupling of Heteroaryl Boronic Acid with H-Phosphonate Diester. Tetrahedron Lett. 2016;57:3063–3066. [Google Scholar]
- 165.Luo H., Liu H., Chen X., Wang K., Luo X., Wang K. Ar-P bond construction by the Pd-catalyzed oxidative cross-coupling of arylsilanes with H-phosphonates via C-Si bond cleavage. Chem. Commun. (Camb.) 2017;53:956–958. doi: 10.1039/c6cc08408g. [DOI] [PubMed] [Google Scholar]
- 166.Sobhani S., Vahidi Z., Zeraatkar Z., Khodadadi S. A Pd complex of a NNN pincer ligand supported on γ-Fe2O3@SiO2 as the first magnetically recoverable heterogeneous catalyst for C-P bond forming reactions. RSC Advances. 2015;5:36552–36559. [Google Scholar]
- 167.Sobhani S., Vahidi Z. P-arylation of aryl halides by an environmentally compatible method. Can. J. Chem. 2017;95:1280–1284. [Google Scholar]
- 168.Sobhani S., Zeraatkar Z. A new magnetically recoverable heterogeneous palladium catalyst for phosphonation reactions in aqueous micellar solution. Appl. Organomet. Chem. 2016;30:12–19. [Google Scholar]
- 169.Yang J., Xiao J., Chen T., Han L-B. Nickel-catalyzed phosphorylation of aryl triflates with P(O)-H compounds. J. Organomet. Chem. 2016;820:120–124. [Google Scholar]
- 170.Yang J., Xiao J., Chen T., Han L-B. Nickel-Catalyzed Phosphorylation of Phenol Derivatives via C-O/P-H Cross Coupling. J. Org. Chem. 2016;81:3911–3916. doi: 10.1021/acs.joc.6b00289. [DOI] [PubMed] [Google Scholar]
- 171.Yang J., Chen T., Han L-B. C-P Bond-forming reactions via C-O/P-H cross coupling catalyzed by nickel. J. Am. Chem. Soc. 2015;137:1782–1785. doi: 10.1021/ja512498u. [DOI] [PubMed] [Google Scholar]
- 172.Liao L-L., Gui Y-Y., Zhang X-B., Shen G., Liu H-D., Zhou W-J., Li J., Yu D-G. Phosphorylation of alkenyl and aryl C−O bonds via photoredox/nickel dual catalysis. Org. Lett. 2017;19:3735–3738. doi: 10.1021/acs.orglett.7b01561. [DOI] [PubMed] [Google Scholar]
- 173.Zhang J-S., Chen T., Yanga J., Han L-B. Nickel-catalyzed P-C bond formation via P-H/C-CN cross couplings. Chem. Commun. (Camb.) 2015;51:7540–7542. doi: 10.1039/c5cc01182e. [DOI] [PubMed] [Google Scholar]
- 174.Isshiki R., Muto K., Yamaguchi J. Decarbonylative C−P bond formation using aromatic esters and organophosphorus compounds. Org. Lett. 2018;20:1150–1153. doi: 10.1021/acs.orglett.8b00080. [DOI] [PubMed] [Google Scholar]
- 175.Sengmany S., Ollivier A., Gall E.L., Léonel E. A mild electroassisted synthesis of (hetero)arylphosphonates. Org. Biomol. Chem. 2018;16:4495–4500. doi: 10.1039/c8ob00500a. [DOI] [PubMed] [Google Scholar]
- 176.Zhang H., Zhang X-Y., Dong D-Q., Wang Z-L. Copper-catalyzed cross-coupling reactions for C-P bonds formation. RSC Advances. 2015;5:52824–52831. [Google Scholar]
- 177.Gelman D., Jiang L., Buchwald S.L. Copper-catalyzed C-P bond construction via direct coupling of secondary phosphines and phosphites with aryl and vinyl halides. Org. Lett. 2003;5:2315–2318. doi: 10.1021/ol0346640. [DOI] [PubMed] [Google Scholar]
- 178.Huang C., Tang X., Fu H., Jiang Y. Zhao. Y. Proline/pipecolinic acid-promoted copper-catalyzed P-arylation. J. Org. Chem. 2006;71:5020–5022. doi: 10.1021/jo060492j. [DOI] [PubMed] [Google Scholar]
- 179.Rao H., Jin Y., Fu H., Jiang Y., Zhao Y. A Versatile and efficient ligand for copper-catalyzed formation of C-N, C-O, and P-C bonds: Pyrrolidine-2-phosphonic acid phenyl monoester. Chemistry. 2006;12:3636–3646. doi: 10.1002/chem.200501473. [DOI] [PubMed] [Google Scholar]
- 180.Jiang D.S., Jiang Q., Fu H., Jiang Y.Y., Zhao Y.F. Efficient copper-catalyzed coupling of 2-haloacetanilides with phosphine oxides and phosphites under mild conditions. Synthesis. 2008;21:3473–3477. [Google Scholar]
- 181.Karlstedt N.B., Beletskaya I.P. Copper-catalyzed cross-coupling of diethyl phosphonate with aryl iodides. Russ. J. Org. Chem. 2011;47:1011–1014. [Google Scholar]
- 182.Stankevič M., Włodarczyk A. Efficient copper(I)-catalyzed coupling of secondary phosphine oxides with aryl halides. Tetrahedron. 2013;69:73–81. [Google Scholar]
- 183.Wan H., Zhao Y., Wang Q., Zhang Y., Li Y. The Cu-catalyzed C-P coupling of phosphonate esters with arylboronic acids. Russ. J. Gen. Chem. 2016;86:150–153. [Google Scholar]
- 184.Beaud R., Phipps R.J., Gaunt M.J. Enantioselective Cu-catalyzed arylation of secondary phosphine oxides with diaryliodonium salts toward the synthesis of P-chiral phosphines. J. Am. Chem. Soc. 2016;138:13183–13186. doi: 10.1021/jacs.6b09334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.He Y., Wuab H., Toste F.D. A dual catalytic strategy for carbon-phosphorus cross-coupling via gold and photoredox catalysis. Chem. Sci. (Camb.) 2015;6:1194–1198. doi: 10.1039/c4sc03092c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Peng H., Cai R., Xu C., Chen H., Shi X. Nucleophile promoted gold redox catalysis with diazonium salts: C-Br, C-S and C-P bond formation through catalytic Sandmeyer coupling. Chem. Sci. (Camb.) 2016;7:6190–6196. doi: 10.1039/c6sc01742h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jablonkai E., Keglevich G. P-ligand-free, microwave-assisted variation of the Hirao reaction under solvent-free conditions; the P-C coupling reaction of >P(O)H species and bromoarenes. Tetrahedron Lett. 2013;54:4185–4188. [Google Scholar]
- 188.Keglevich G., Jablonkai E., Balázs L.B.A. “green” variation of the Hirao reaction: the P-C coupling of diethyl phosphite, alkyl phenyl-H-phosphinates and secondary phosphine oxides with bromoarenes using a P-ligand-free Pd(OAc)2 catalyst under microwave and solvent-free conditions. RSC Advances. 2014;4:22808–22816. [Google Scholar]
- 189.Keglevich G., Henyecz R., Mucsi Z., Kiss N.Z. The palladium acetate-catalyzed microwave-assisted hirao reaction without an added phosphorus ligand as a “green” protocol: A quantum chemical study on the mechanism. Adv. Synth. Catal. 2017;359:4322–4331. doi: 10.1002/adsc.201700895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Henyecz R., Mucsi Z., Keglevich G. Palladium-catalyzed microwave-assisted Hirao reaction utilizing the excess of the diarylphosphine oxide reagent as the P-ligand; a study on the activity and formation of the “PdP2” catalyst. Pure Appl. Chem. 2019;91:121–134. [Google Scholar]
- 191.Amaya T., Abe Y., Inada Y., Hirao T. Synthesis of self-doped conducting polyaniline bearing phosphonic acid. Tetrahedron Lett. 2014;55:3976–3978. [Google Scholar]
- 192.Jójárt R., Pécsy S., Keglevich G., Szécsi M., Rigó R., Özvegy-Laczka C., Kecskeméti G., Mernyák E. Pd-Catalyzed microwave-assisted synthesis of phosphonated 13α-estrones as potential OATP2B1, 17β-HSD1 and/or STS inhibitors. Beilstein J. Org. Chem. 2018;14:2838–2845. doi: 10.3762/bjoc.14.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li J., Bi X., Wang H., Xiao J. Palladium-catalyzed desulfitative C-P coupling of arylsulfinate metal salts and H-phosphonates. RSC Advances. 2014;4:19214–19217. [Google Scholar]
- 194.Jablonkai E., Balázs L.B., Keglevich G. A P-ligand-free nickel-catalyzed variation of the hirao reaction under microwave conditions. Curr. Org. Chem. 2015;19:197–202. [Google Scholar]
- 195.Yang J., Xiao J., Chen T., Yin S-F., Han L-B. Efficient nickel-catalyzed phosphinylation of C-S bonds forming C-P bonds. Chem. Commun. (Camb.) 2016;52:12233–12236. doi: 10.1039/c6cc06048j. [DOI] [PubMed] [Google Scholar]
- 196.Łastawiecka E., Flis A., Stankevič M. Greluk, M.; Słowik, G.; Gac, W. P-Arylation of secondary phosphine oxides catalyzed by nickel-supported nanoparticles. Org. Chem. Front. 2018;5:2079–2085. [Google Scholar]
- 197.Xiong B., Li M., Liu Y., Zhou Y., Zhao C., Goto M., Yin S-F., Han L-B. Stereoselective Synthesis of Phosphoryl-Substituted Phenols. Adv. Synth. Catal. 2014;356:781–794. [Google Scholar]
- 198.Ogawa T., Usuki N., Ono N. A new synthesis of ð-electron conjugated phosphonates and phosphonic bis(diethylamides) and their SHG activities. J. Chem. Soc., Perkin Trans. 1. 1998:2953–2958. [Google Scholar]
- 199.Ghosh T., Maity P., Kundu D., Ranu B.C. Cobalt catalyzed, copper assisted C(sp2)-P cross coupling. New J. Chem. 2016;40:9556–9564. [Google Scholar]
- 200.Jablonkai E., Keglevich G. Catalyst-free P-C coupling reactions of halobenzoic acids and secondary phosphine oxides under microwave irradiation in water. Tetrahedron Lett. 2015;56:1638–1640. [Google Scholar]


