Abstract
Recent advances in the application of environmentally benign acid catalysts in organic synthesis are reviewed. The work includes three main parts; (i) description of environmentally benign acid catalysts, (ii) synthesis with heterogeneous and (iii) homogeneous catalysts. The first part provides a brief overview of acid catalysts, both solid acids (metal oxides, zeolites, clays, ion-exchange resins, metal-organic framework based catalysts) and those that are soluble in green solvents (water, alcohols) and at the same time could be regenerated after reactions (metal triflates, heteropoly acids, acidic organocatalysts etc.). The synthesis sections review a broad array of the most common and practical reactions such as Friedel-Crafts and related reactions (acylation, alkylations, hydroxyalkylations, halogenations, nitrations etc.), multicomponent reactions, rearrange-ments and ring transformations (cyclizations, ring opening). Both the heterogeneous and homogeneous catalytic synthesis parts include an overview of asymmetric acid catalysis with chiral Lewis and Brønsted acids. Although a broad array of catalytic processes are discussed, emphasis is placed on applications with commercially available catalysts as well as those of sustainable nature; thus individual examples are critically reviewed regarding their contribution to sustainable synthesis.
Keywords: Solid acid, heterogeneous catalysis, metal oxides, clay, zeolite, metal-organic-frameworks, heteropoly acids, ion-exchange resins, sulfated metal oxides
1. INTRODUCTION
Acidity-basicity is one of the oldest concepts in the history of chemistry beginning with Lavoisier and Davy over two centuries ago [1]. While the first general description was developed by Arrhenius [2], it was later refined and replaced by the Brønsted-Lowry [3] and Lewis [4] concepts. Accordingly, acid catalysis has extensive history that covers a broad range of applications such as electrophilic addition, esterification, ester hydrolysis, aromatic electrophilic substitution or rearrangement reactions [5]. Con-ventional mineral acids, such as HCl, H2SO4 or HNO3 were the traditional catalysts of choice for these transformations. Despite their usefulness, these acids are highly corrosive; many of them release gases that pose safety and health hazards. In addition, most of them are impossible to reuse. In short, their application is undesirable under the Green Chemistry principles [6]. Due to the importance of acid catalysis, significant efforts were made to address this problem. The two major ways to replace these harmful acids are the application of solid acids via heterogeneous catalytic reactions and the use of homogeneous (soluble) acid catalysts that do not undergo hydrolysis under aqueous conditions and can be recycled. The latter acids are soluble in water and several organic solvents thus acting as homogeneous catalysts. Both groups of catalysts are now frequently used in a broad variety of organic reactions, and are extensively reviewed [7].
In this account we will survey the applications of these catalysts in environmentally benign processes. Although we provide major sources of earlier reviews and books, the emphasis will be placed on recent advances, thus most of the original work reviewed in this article will cover the past decade between 2008 and 2018. Even with this limitation, due to the large number of papers published in this period and the page limitations of this work, the focus will be on providing a broad scope of reactions with representative examples.
2. ENVIRONMENTALLY BENIGN ACID CATALYSTS
Environmentally benign acid catalysts form two major groups depending on the type of catalysis they perform. Solid acids are not soluble in water or organic solvents and the reactions occur on the surface of these materials thus forming heterogeneous catalytic systems. In contrast, there are acids that, although solid under normal conditions, readily dissolve in water and many organic solvents thus performing homogeneous catalysis. In this part we summarize the most frequently used catalyst in both groups (Fig. 1).
Fig. (1).
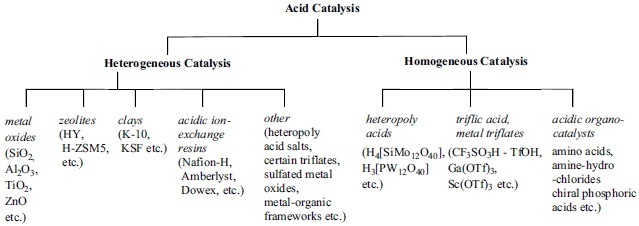
Classification of green acid catalysts.
It is worth noting that there is a limited overlap between the two major groups. There are several acids, mainly inorganic acids and salts, such as heteropoly acids or metal triflates that are not soluble in certain organic solvents, thus with the appropriate selection of the solvent they can be used as either heterogeneous or homo-geneous catalysts. Their water solubility and stability under aqueous conditions still aid their recycling during work up pro-cedures.
2.1. Heterogeneous Catalysis - Solid Acids
Solid acid catalysts have been extensively used in organic synthesis as well as in the pharmaceutical industry [8]. Their advantages include their practical utility (durability, easy work-up by filtration), their potential recyclability, their low cost and overall their great green potential. These catalysts are of many types depending on the composition and the structure they possess. The aforementioned assets combined with various other specific properties attracted vast interest toward their applications. Due to the polar nature of these materials, most are excellent microwave absorbers thus can act as a catalyst as well as an internal heating medium for a broad array of transformations [9-11]. The main types of solid acid-catalysts; namely metal oxides, zeolites, clays, acidic ion exchange resins, heteropoly acid salts and metal organic frameworks and sulfated metal oxides; will be briefly described in this section.
The first large group of solid acids is based on metal oxides [12]. Although their acidity is relatively limited, due to their broad availability and low cost they represent a versatile group of weak acid catalysts for many transformations [13]. They include the common silica or acidic alumina catalysts. When metal oxides are treated with sulfuric acid they form a special group of catalysts named sulfated metal oxides [14]. The reaction with sulfuric acid appears to enhance the Lewis acid character and significantly strengthen acidity. Some of these materials, such as sulfated zirconia are commonly used Lewis acid catalysts in organic synthesis [15].
Probably the most utilized solid acid catalysts originate from natural aluminosilicates, zeolites and clays, although the success of the natural materials inspired extensive efforts in the preparation of semi-synthetic and synthetic alternatives.
Clays are aluminosilicates constituted of multiple layers of polyhedrons. Tetrahedral silicone oxides and octahedral hydrous alumina are their common building blocks [16]. Clays have demonstrated remarkable catalytic activity due to their porous structure that provides a unique environment in which molecules can interact in specific ways allowing reactions to take place. In other words, they act as a micro-reactor capable of initiating reactions on account of their inherent properties such as adsorption-, swelling- and ion-exchange capacities. Various types of clays exist; depending on their properties, they are suitable for catalyzing a broad scope of transformations [17]. Clays can be classified into two categories: the negatively charged clays that welcome balancing cations and the positively charged clays containing anions in their interlayer space [18]. The second class of clays is often synthesized while the first one is prepared from natural sources, most commonly montmorillonites. The natural clays can be used directly in their original form or after undergoing chemical treatments that enhance an existing characteristic or specifically introduce an extrinsic feature. An acidic activation method is often employed to enhance their acidic properties. To do so, several mineral acid solutions can be applied including phosphoric acid, sulfuric acid or nitric acid [19]. The resulting materials exhibit an increase in acidity and the number of active sites and surface area. The widespread montmorillonites are cationic clays, member of the smectite group [20], possess remarkable absorption capacity, high surface area as well as interesting acidic properties which altogether contribute to their success as catalysts in various reactions [21]. Montmorillonites K-10 and KSF, both acid treated montmoril-lonites, are the most widely used acidic clays in organic synthesis [22].
Zeolites are also aluminosilicates, however, in contrast to the layered structure of clays these highly porous, crystalline materials exhibiting a well-defined channel and cage-based structure [23] and less commonly a lamellar structure [24]. The molecular dimensions of their channels and cages contribute greatly to the catalytic potential of zeolitic materials [25]. Similarly to clays, the catalytic properties of zeolites are due in part to the exchangeable ions and water molecules trapped in their structure as well as their com-monly high adsorption capacity [26]. It is important to mention that zeolites also offer the possibility to introduce specific active sites and to modulate the existing electronic features giving rise, for example, to zeolites with enhanced acidic properties. In catalysis, the most commonly used acidic zeolites are zeolites X and Y, employed in catalytic cracking, and zeolite ZSM-5, a fundamental catalysts in the petroleum industry [27]. In general, although zeolites are strong acids at high temperatures, their acidity is relatively modest at the common temperature range of organic synthesis [27]. Efforts to prepare zeolites with stronger acid sites by functionalizing them with organic moieties led to promising results; nonetheless, this issue still remains a challenge [26].
Acidic ion exchange resins are versatile materials used as catalysts for many organic transformations as well [28]. Their framework makes possible the synthesis of hybrid materials with tunable acidity. Depending upon their pore size, chemical structure and formulation, they are useful in a number of transformations. Ionic exchange resins exist as either cationic or anionic forms. The so-called acidic ionic exchange resins are typically of cationic type. Amberlyst and Nafion are two common sulfonic acid based-resins employed in numerous catalytic processes [29]. While the Amberlyst type is based on a polystyrene-sulfonic acid motif, Nafion possesses a perfluorinated backbone with sulfonic acid entities.
The high electron withdrawing character of fluorine in Nafion significantly enhances its acidity to the superacidic level. Amberlyts are able to catalyze the conversion of acetals and carbonyls to alcohols, the conversion of acids to esters, among others. Nafion has been successfully employed as solid-acid catalyst for several reaction types such as oxidation reactions, protection and deprotection of alcohols and carbonyl containing compounds, as well as rearrangements [30]. It is worth mentioning that their BET surface area, however, is rather low (~9 m2/g) and they often suffer from a poor porosity. To address these issues and fully take advantage of the strong acidity of Nafion-H a Nafion-silica nanocomposite material was developed, in which the small Nafion-H particles are trapped in a porous silica network significantly enhancing both the BET surface and available active centers [31].
Metal-organic frameworks (MOF) [32] constitute a highly porous class of materials possessing an important internal surface area; thus they have found applications as gas storage media, and as absorbents but they also appear to be excellent catalysts. They have the particularity to be composed of organic linkers and inorganic metal ions [33]. Unlike zeolites that have a restricted number of structures, the hybrid nature of MOFs allows multiple combinations of both components yielding practically infinite structural possibilities. Various synthetic methods exist to prepare MOFs. The self-assembly approach is likely the most common: two solutions containing the organic and inorganic components, respectively, are mixed, often under solvothermal conditions that result in a crystalline framework [34]. Clusters of metal-carboxylates are common secondary building blocks acting as nodes in the MOF architecture. They come together to form a network linked by organic ligands such as terephtalic acid (Fig. 2). By selecting a particular ligands/metal cluster combination, the pore size as well as the flexibility of the material can be controlled and specific functionalizations can also be introduced. These easily tunable solid catalysts have therefore the potential to favor certain reactive intermediates and promote specific pathways, thus control the reactivity and selectivity of an organic reaction [35]. Moreover, the incorporation of chiral linkers extends the applications of the MOF to asymmetric catalysis [36].
Fig. (2).
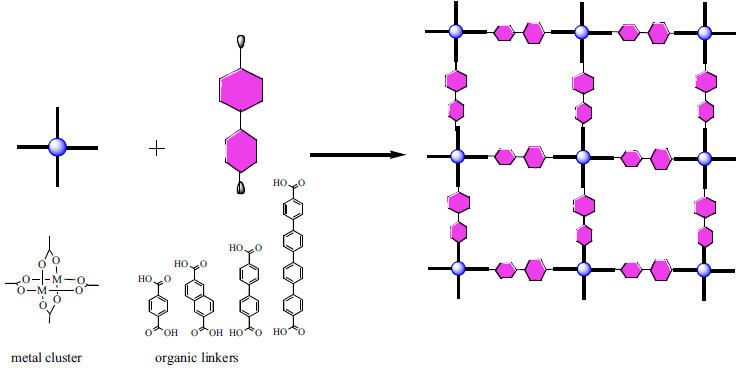
Representation of the synthesis of a metal organic framework with examples of metal cluster/organic linkers [37].
Several composite materials are also often used solid acid catalysts. There are three major groups of catalysts that are particularly important in this area; the (i) carbon-, (ii) ionic liquid- and (iii) carbon silica composite based acids.
The first group is based on sulfonated carbon-based materials that were observed to be efficient and highly active solid Brønsted acid catalyst [38]. These catalysts resemble to graphite sheets that, after sulfonation, contain a high density of sulfonic acid groups. In fact the acid density of these materials surpasses that of Nafion-H by a factor of five. These materials are commonly prepared from susatinable sources, such as natural carbohydrates, e.g. starch, cellulose etc. by partial cabonization and sulfonation [39]. They are used in a broad variety of applications for example metal-doped alternatives were applied in the Suzuki coupling and oxidation [40].
The ionic liquid-base composites in which ionic liquids are immobilized on solid surfaces, e.g. sulfonated silica also attracted significant attention [41]. These materials, such as ionic liquid coated sulfonated carbon-silica composites were found to be novel solid acid catalyst for aqueous phase organic synthesis [42].
Sulfonated carbon-silica composites are also important catalytic materials [43]. These catalysts have been applied in a variety of transformations, such as the Hantzsch cyclization [44], multi-component synthesis of heterocycles [45], the Michael addition of indoles to α,β-unsaturated ketones [46], chemoselective protection of aldehydes, and C, N, O-acylations [47].
Another new emerging class of solid acid catalysts is the nanoscale version of the polymer-supported acid catalysts (PSAs). The so called “nanoacid” corresponds to polymer-based nanoparticles functionalized with sulfonic acid groups [48]. A synthetic method reported the use of emulsion polymerization technique in presence of a reactive surfactant to prepare such material [48]. The co-monomers, styrene and divinylbenzene, employed were crosslinked under specific conditions in order to form a backbone of nanoparticles that remains heterogeneous in solution. The surfactant, octylbenzene sulfonic acid, possessing a benzenesulfonate moiety was then reacted with the particles depositing the sulfonic acid groups at their surface. The resulting material showed good performance in catalyzing the formation of urethane from isocyanate and alcohol. While nanoacid catalysts offer a greater surface area than PSAs and therefore possess a high potential to perform acid-catalyzed organic reactions, their success remain to be demonstrated as very few examples are available in the literature. It is interesting to note that the term nanoacid has been used for other materials, such as heteropolyoxometallate-based materials that possess nanometer size as well as strong acidic properties [49].
The last solid acid catalyst type discussed in this section is heteropoly acid salts, fundamentally different from the other types described previously. Heteropoly acids are made of hydrogen, oxygen, heteroatoms, and metals often including tungsten or molybdenum. In other words, they can be considered special clusters of transition metal oxides [12]. Heteropoly acids are commonly soluble in water and many organic solvents. Their corresponding salts, obtained by replacing some or most of the hydrogens by large cations such as cesium, however, exhibit low solubility in both aqueous and organic media [50]. The resulting heteropoly acid salts have the potential to be recycled; they exhibit unique superacidic and multielectronic properties in addition to an important surface area when nanocrystallites are abundant within their structure. As a result they are interesting catalysts especially for acid or redox catalyzed reactions [51]. Their chemical composition can also be easily modified to adjust the desired redox and acid-base properties. The salts are often classified by the structure of the heteropolyanion that constitutes the fundamental unit called the primary structure. A secondary structure is generated by the connection of these heteropoly anions by hydrogen bonding. HPAs have been successfully used to promote alcohol dehydration, alkylation, esterification, polymerization and oxidation among other reactions [52, 53]. Despite their high acid strength however, some HPAs have a rather low surface area [54], which certainly is a drawback in heterogeneous applications.
The above short summary indicates that although the traditional solid acids, such as zeolites, clays or metal oxides (neat or sulfated) still dominate the field of solid acids, several new classes of these catalysts such as metal-organic frameworks, composite- materials and nanoacids have been developed to provide further variety and application possibilities for the traditional reactions and also allow the extension of the already broad scope applications.
2.2. Homogeneous Catalysis – Stable Soluble Acids
In its over two hundred years of history, homogeneous acid catalysis was successfully applied in a variety of transformations such as esterification, hydrolysis, aromatic electrophilic substitution and electrophilic addition, and many others. The traditional mineral acids, such as HCl or H2SO4 served as efficient catalysts for these reactions in the past. However, these acids are commonly highly corrosive, and many discharge poisonous fumes that represent safety and health hazards. The same is true for the conventional Lewis acids, such as aluminum chloride and bromide, or other metal-halogen compounds (TiCl4, SnCl4 etc.). These acids are all moisture sensitive, and decompose to metal oxides and hydrogen halides upon contact with water, whether it is during a reaction or a work-up procedure. In addition to the safety and health concerns this undesirable feature will also exclude the recycling of these catalysts and produce a significant amount of waste. Accordingly, significant effort has been made to replace these acids with greener alternatives both in industrial and laboratory applications. There are two major desired characteristics; (i) the acid should be stable under aqueous conditions and should not hydrolyze to allow recovery and recycling and (ii) it should have low vapor pressure in order to maintain a stable acid or catalyst concentration during a reaction and avoid toxic fumes. There have been several attempts on applying water soluble acids that do not hydrolyze under aqueous conditions and therefore are recyclable. The most typical examples are heteropoly acids [55] such as the commercially available H3[PMo12O40], H4[SiMo12O40], H4[SiW12O40], or H3[PW12O40]. These Keggin-type HPAs are soluble in water as well as in several organic solvents, and readily promote the typical Brønsted acid catalyzed reactions. However, due to their moisture insensitivity they can be regenerated from the reaction mixture upon removal of the solvent. The practical usefulness of HPAs generated extensive efforts in their synthesis and a large number of polyoxometallates have been prepared and applied for various purposes [56]. Another important group is that of trifluoromethanesulfonic acid (triflic acid) and its triflates [57], common salts of various metals, such as copper, gallium, lanthanides etc. and triflic acid that function as Lewis acids. In contrast to the traditional Lewis acids (such as AlCl3), these acids resist aqueous hydrolysis and can be recovered from such solutions. According to their properties, metal triflates are well-known for forming complexes with organic ligands, thus opening a way for a broad range of applications including asymmetric catalysis [58]. However, due to their considerable cost, their widespread applications, particularly at the industrial scale, are not yet established.
The third large group of potentially sustainable acid catalysts is the acidic organocatalysts. These materials are organic compounds that possess considerable acidity and thus are capable of promoting acid-catalyzed reactions. Most of them, although not soluble in aqueous medium, are water tolerant and would remain stable during aqueous workup conditions. The traditional examples are alkyl or aryl sulfonic acids, such as para-toluenesulfonic acid (pTSA), that catalyze a variety of transformations [59]. Another such example, camphor sulfonic acid (CSA) extends the application of these acids to asymmetric synthesis [60]. Although, amino acids also possess weak inherent acidity and are often used in organocatalysis, the focus of the applications is not in acid-catalyzed reactions unless they are used as chiral ligands.
A new class of acidic organocatalysts, chiral phosphoric acids, was first reported in 2004, involving asymmetric organocatalytic Mannich reactions catalyzed by 3,3’-disubstituted (BINOL)-derived phosphoric acids [61]. These pioneering studies initiated extensive interest on the application of these unique compounds. Since their introduction, a large variety of transformations were carried out by chiral phosphoric acids, including Kabachnik-Fields, Pictet-Spengler, Strecker, Friedel-Crafts or Biginelli reactions, just to name a few [62].
The field of green soluble acid catalysts has been dominated by triflates and heteopoly acids on the past two decades. More recently, acidic organocatalyst were introduced and found exceptionally active and selective in a number of applications, mostly in the field of asymmetric catalysis. Although organo-catalysts are inherently green, their industrial applications are delayed by several factors. Among these, the often high cost (e.g. BINOL-based phosphoric acids), nearly stoichiometric amount required, and the difficult recyclability are the most detrimental issues that need to be addressed in future developments.
3. SYNTHETIC APPLICATIONS IN HETEROGENEOUS SYSTEMS
3.1. Friedel-Crafts and Related Reactions
The Friedel-Crafts reactions are one of the most fundamental transformations in organic chemistry. These processes are based on electrophilic aromatic substitutions and include acylation, alkylations, halogenation, nitration, sulfonation etc. Due to their practical importance the field has been the subject of numerous reviews and books, including Olah’s extensive series [63]. Despite the nearly one and a half century old history, Friedel-Crafts reactions are widely used in organic synthesis to create carbon-carbon bonds and therefore prepare useful substituted aromatic compounds [64]. The catalysts that were conventionally employed to perform such reactions are traditional Lewis-acids such as AlCl3 or BF3. They are used in stoichiometric quantities and consequently generate a considerable amount of toxic waste. In addition, the regioselectivity when using these catalysts is often rather poor [65]. Solid acid catalysts offer many advantages over the traditional Lewis acid catalysts in a typical Friedel-Crafts reaction. Unlike conventional Lewis-acids, they have been shown to efficiently catalyze these reactions with good recyclability and interesting selectivity.
Acylation. The role of common zeolites and their metal-doped counterparts was studied for the acylation of anisole with propanoic acid under solvent-free conditions (Scheme 1). The catalysts tested were ZSM-5 and BEA zeolites as well as their nickel, silver or iron-loaded derivatives [66]. Independently of the catalyst used, the para- and ortho-products were predominant. The modified catalysts exhibited lower conversion compared to the original zeolites. This was explained by the replacement of Brønsted acid sites with Lewis acid sites that appeared to be detrimental for the reaction. The most efficient catalyst was found to be the pristine ZSM-5 zeolite yielding about 70% conversion and 80% selectivity. The same acylation could be carried out by MOF-based catalysts with high phosphotungstic acid loading [67].
Scheme 1.

Yadav and Kamble developed a new solid superacidic mesoporous UDCat-5 catalyst for the similar acylation of toluene with propionic anhydride [68]. The reaction occurred at 180°C, with relatively moderate yield (68%), however, with exclusive regioselectivity. The reaction was carried out under solvent-free condition.
Sulfated zirconia was found to be an efficient catalyst for the acylation of ferrocene with acyl chlorides, including long chain compounds such as heptanoyl chloride (Scheme 2). The reaction was carried out in dichloroethane under reflux conditions. The catalyst could be recycled in the reaction; five consecutive reactions resulted in no significant drop in activity and yields, in fact some activation (yield 88%) occurred during the subsequent reactions.
Scheme 2.

Alkylations. The microwave-assisted K-10-catalyzed alkylation and alkylation/electrophilic annulation of indoles were accomplished by using alcohols and diols as green alkylation reagents (Scheme 3) [69]. The alkylation with tert-butyl alcohol occurred with moderate to high yields with high selectivity toward the C-3 position of indole. The solid acid appeared to show little sensitivity to the water that is eliminated during the reaction. Using 2,5-hexanediol the reaction occurred in a three steps sequence; alkylation, annulation and dehydrogenative aromatization to form 1,4-dimethylcarbazoles in good to excellent yields.
Scheme 3.
In a relevant extension, Cirujano et al. described a solvent-free Friedel-Crafts alkylation of indoles with alcohols (Scheme 4), avoiding the use of the often required organic solvents [70]. In particular, the performance of ionic liquids and solid acid catalysts (metal organic frameworks and zeolites) were compared in terms of activity, selectivity and reusability. The major conclusion of the study was that the ionic liquids provide the best activity but the microporous frameworks provided better recyclability and better selectivity. Given the importance of lowering the environmental impact, the microporous solids are of great interests. They also showed improved selectivity towards small mono-alkylated products. HY zeolites were found to be the most attractive option in terms of yield, cost and recyclability for this reaction.
Scheme 4.

Acidic cation-exchange resins were also employed to catalyze Friedel-Crafts-type reactions [71]. Thanks to their inherent Brønsted acidity, they are capable of catalyzing the formation of carbocations or other electrophiles essential for the occurrence of an aromatic electrophilic substitution. The commercially available and low-cost sulfonic acid resin D072 was used for the hydroarylation of styrene-derivatives and electron-rich arenes (Scheme 5). Under the optimized conditions the yields were close to quantitative with selectivity in favor of the main isomer reaching 100% for some reagents or as low as 60% for some others. The activity of the selected resin was also investigated in a fixed bed flow reactor in order to evaluate its potential use in large-scale applications. The activity was maintained and the selectivity was improved compared to the batch conditions. While dichloroethane is not a green solvent, 100% atom economy, the green alkylating agent and the recyclable catalyst substantially compensate this disadvantage.
Scheme 5.

The well-known, perfluoroalkyl-sulfonic acid resin Nafion-H served as a catalyst in the preparation of triarylmethanes from benzaldehydes and arenes under microwave irradiation and solvent-free conditions (Scheme 6) [72]. The combination of the solid acid catalyst and the activation method proved to be effective to produce high yields in short reaction times. Different regioselectivities were obtained depending on the activation methods: microwave irradiation favored the formation of ortho-ortho products while conventional heating mostly yielded para-para products. The obvious advantages of the method lie mainly in the nature of the catalyst that is environmentally friendly and can be filtered off for an easier product separation, as well as in the use of a green alkylating agent, that only produces water as byproduct. However, the recyclability of the catalyst was not investigated.
Scheme 6.
A similar process has been developed for the synthesis of bis(indolyl)methanes via the alkylation of indoles with aldehydes (Scheme 7). According to the significantly enhanced reactivity of indoles, the inexpensive and abundant catalyst SiO2 could be used under solvent-free conditions [73]. The reaction showed a broad substrate scope using a variety of aromatic aldehydes all providing high yields. Although the highest yields were obtained with silica as a catalyst, the reaction was not catalyst specific. The similarly abundant and inexpensive acidic Al2O3 was also found to be an effective catalyst for the reaction, however, resulting in slightly lower yields.
Scheme 7.

Due to its significant acidity and high surface area, K-10 montmorillonite is a widely used catalyst for various transfor-mations including the Friedel-Crafts reaction. As shown in Scheme 8, K-10 efficiently catalyzed the alkylation of indoles and pyrrole with nitroalkenes under solvent-free conditions at moderate temperature (60°C) providing high yields [74]. In addition to the low cost and environmentally benign nature of the catalyst, it appeared recyclable during three consecutive cycles.
Scheme 8.
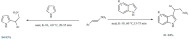
Several works have exploited the catalytic potential of non-conventional biomass, such as metal hyper-accumulator plants, in combination with the same common porous support, mon-tmorillonite K-10 [75]. Grison et al. demonstrated the clear advantages of heterogeneous catalysts over their homogeneous counterparts [76]. The heterogeneous plant-based Lewis acids afforded high yields and good recyclability with consistent yields for up to four consecutive cycles. Two reactions were studied: the acylation of anisole with acetic anhydride and the alkylation of aromatics with alkyl halides (Scheme 9). The combination of Zn hyper-accumulative plants supported on K-10 was demonstrated to be the most efficient catalyst providing yields ranging from 50% to 100% depending on the reactants. In addition, the results obtained with these ecocatalysts were considerably superior to the results obtained with the commercial Lewis-Acid ZnCl2.
Scheme 9.

Numerous examples have shown the performance of metal organic frameworks in Friedel-Crafts reaction as well. They often are functionalized in order to improve their performance. Urea-containing MOF have recently attracted attention for the alkylation of indoles with nitroalkenes. Urea-based compounds are known for their capacity to participate in hydrogen-bonding and therefore act as organocatalysts. In traditional homogeneous systems, they undergo self-quenching and consequently require high catalyst loading. Functionalizing MOFs with urea moiety yielded interesting results. Luo et al. synthesized a MOF from V-shaped dicarboxylate ligands and dicopper units [77]. The obtained simple 2D structure was believed to ease the access of the substrates while stabilizing the urea moieties, previously attached to the building blocks. The resulting heterogeneous catalyst was highly active in the Friedel-Crafts alkylation of indoles with nitrostyrene (Scheme 10); it was tolerant towards various substituents and exhibited good recyclability. Another similar study reported the alkylation of indoles with various nitroalkenes catalyzed by a zinc-based MOF containing ureylenic acid as the hydrogen-bond-donor [78]. The authors also observed a significant increase in activity upon heterogenization of the urea organocatalyst. When employed as catalysts for Friedel-Crafts reactions, the desired products were obtained in high yields in toluene at 60°C.
Scheme 10.
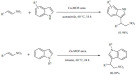
The synthesis of a large number of organofluorine indole and pyrrole derivatives was achieved by a K-10-catalyzed aromatic electrophilic hydroxyalkylation (Scheme 11) [79]. Several of the synthesized products showed good to excellent activity in the inhibition of the fibril formation of Alzheimer’s disease’s amyloid beta peptide [80].
Scheme 11.
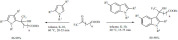
Halogenations. The chlorination of aromatic compounds was performed using various types of zeolites. One of them, the proton-exchanged zeolite X, catalyzed the chlorination of toluene at ambient temperature in different solvents using tert-butyl hypo-chlorite (Scheme 12) [81]. The solvent effect appeared to be an important factor for the selectivity as well as the conversion. The best results in terms of both yield and selectivity were obtained in ether, probably due to its polarity that is believed to stabilize the oxonium intermediate and aid the selectivity. Tetrachloromethane also provided high yields; however, both solvents cause many health and environmental issues.
Scheme 12.

Mohan et al. reported a regioselective bromination reaction using the widely-used montmorillonite K-10 clay in presence of N-bromosuccinimide (Scheme 13) [82]. Various aralkyl ketones were converted to α-monobrominated aralkyl ketones in methanol at mild temperature (65°C) with excellent yields and short reaction times. In addition to the simplicity and the efficiency of the method, the reusability of the catalyst is another benefit of the method surpassing other existing procedures that suffer from disadvantages such as the use of toxic brominating agents, long reaction times or tedious workup.
Scheme 13.

Nitration: Several works have reported successful nitration of aromatics over zeolites in attempts to substitute the concentrated acidic solution mixture (nitric and sulfuric acids) that is tradi-tionally employed for this reaction type. For instance, the nitration of phenol over the acidic zeolite Hβ in ethanol resulted in a combined yield of 86% with a para/ortho ratio approaching 1 (Scheme 14, a) [69]. Although the regioselectivity could be imp-roved by using hexane instead of ethanol, selecting this solvent is not in favor of the environmental impact. In addition, the method doesn’t completely eliminate the use of acidic solutions since a dilute solution of nitric acid was still used as the nitrating agent. Another work employed similar zeolites in dichloroethane under reflux for 48 h (Scheme 14, b) [69]. It was observed that the best results were obtained when zeolites had large pores to optimize the reaction rate and restrictive channel structure to better control the regioselectivity. While the combined yield was lower than for the first method, the selectivity was higher. This method used isopropyl nitrate, another nitrating agent.
Scheme 14.

Kumar et al. reported the nitration of aromatics with silica supported perchloric acid or bisulfate under solvent-free micro-wave-driven conditions (Scheme 15) [83]. The green nitrating agent NaNO2 in combination with the supported acidic catalyst yielded excellent results in short reaction times. Perchloric acid was preferred over bisulfate due to its low nucleophilicity and higher acidity. The catalyst was easily prepared and could be reused for four consecutive cycles.
Scheme 15.

3.2. Condensations
Condensation reactions, an acid or base-catalyzed sequence of an addition and an elimination reactions, are versatile and constitute a broad scope of transformations that enable the synthesis of a wide range of products [84].
The K-10–catalyzed microwave-assisted condensation of substituted aryl trifluoromethyl ketones and different primary amines yielded the corresponding trifluoromethyl imines (Scheme 16) in good to excellent yields. Although most of the reaction conditions qualify as green, high temperature (175°C) and somewhat long reaction times (45-60 min) were required even with microwave activation to counter the low reactivity of the trifluoromethyl ketones. Despite the relatively harsh conditions the K-10/microwave protocol was still significantly greener than a similar, conventionally heated process that was catalyzed by PTSA; for some reactants as long as 7 days of heating at 175°C was needed to achieve 70-80% yields. The K-10 catalyst could be reused without any loss of activity [85]. The method could be successfully extended for the synthesis of chiral amines by carrying out the condensation with enantiomeric methyl-benzyl amines and hydrogenate the imines to secondary amines and finally remove the benzyl group by hydrogenolysis [86].
Scheme 16.
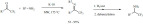
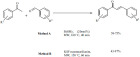
Chalcone derivatives were synthesized via the aldol con-densation of acetophenones and benzaldehydes. The process was catalyzed by boric acid and was carried out under microwave-assisted solvent-free conditions (Method A) [87]. Due to the low solubility of boric acid, the reaction occurred in a rather hetero-geneous system and the products were obtained in medium to good yields. The chalcones were obtained as the E stereoisomer with almost exclusive selectivity and were isolated in a pure form after a simple extraction of the product mixture (Scheme 17, Method A). In a very similar approach (Method B) KSF montmorillonite was used as a catalyst [88]. The products were obtained in medium to good yields. Electron withdrawing substituents appeared to negatively affect the yield. (Scheme 17, Method B).
Scheme 17.
Acidic ion exchange resins, such as Amberlite IR 120H, were also found to be excellent catalysts for the condensation reaction of aryl aldehydes and hydroxylamines (Scheme 18) [89]. The solvent-free reactions provided high yields in short reaction times. The ion exchange resin could be readily recycled with only a negligible drop in activity even after the fourth consecutive run. The reaction appeared to be surprisingly substrate tolerant; a broad group of benzaldehydes was subjected to the experimental conditions without significant changes in yield.
Scheme 18.

3.3. Multicomponent Reactions
The concept of multicomponent reactions is very simple; a cascade of reactions occurs in a specific order in one pot by simply adding the reactants to the reaction mixture at the beginning of the reaction producing a well-defined product selectively. This way, large compounds of complex structure can be prepared without the need of several purification steps of intermediates thus saving time, energy and effort, while also avoiding the formation of a potentially large amount of chemical waste. Therefore, multicomponent reactions are particularly attractive for green synthesis [90]. Given their attractive features these reactions have a long history dating back to the classic Hantzsch or Biginelli reactions from the late 1800s. Newer applications such as the Ugi reaction reinvigorated this field in the late 1950s.
The traditional Hantzsch synthesis was successfully modified to an even greener heterogeneous catalytic approach by using a simple physical mixture of Pd/C and K-10 montmorillonite. The use of this catalyst mixture is a typical example of bifunctional catalysis. The solid acid K-10 efficiently catalyzed the condensation and cyclization of aldehyde, ethyl acetoacetate and ammonium acetate to result in typical Hantzsch esters. The successive dehydrogenative aromatization of the intermediate was catalyzed by the noble metal component to yield pyridine derivatives in moderate to excellent yields (Scheme 19) [91].
Scheme 19.

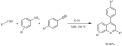
A similar, K-10-catalyzed microwave-assisted approach was developed for the synthesis of quinolones by a three component reaction using anilines, aldehydes and terminal aryl alkynes (Scheme 20) [92]. The reaction pathway is of domino nature; the first step is the formation of an imine between the aldehyde and the aniline, which is immediately followed by the intermolecular addition of the triple bond to the imine. This intermediate then undergoes cyclization. Although the above steps are catalyzed by the strong acid centers of K-10, the last step, the oxidative aromatization, is due to the mild oxidative properties of this catalyst that is common to other montmorillonites as well.
Scheme 20.
The preparation of substituted pyrroles was achieved by Jeong et al. using a four-component reaction [93]. The authors reacted a β-ketoester, an amine, an aldehyde and nitromethane in the presence of catalytic amount of p-toluenesulfonic acid doped polystyrene (PTSA-PS) (Scheme 21). The microwave irradiation resulted in high yields in short reaction times as compared to conventional heating. The catalyst was reused in seven consecutive reactions with only a negligible loss in activity.
Scheme 21.

3.4. Couplings
The traditional coupling reactions, such as the Heck, Suzuki or Negishi couplings are typical metal-catalyzed reactions [94]. Since their original discoveries the field has enjoyed considerable success [95] resulting in the Nobel Prize for the three scientists [96]. Thus acid-catalyzed approaches are relatively rare and occur through a different mechanism.
An environmentally benign metal-free, K-10 montmorillonite catalyzed domino-style approach was developed for the direct synthesis of biaryls by solid phase diazotization of anilines and successive nucleophilic attack by aromatic hydrocarbons that led to C-C bond formation (Scheme 22) [97]. The reaction provided excellent yields using a broad range of substituted anilines and a variety of aromatic nucleophiles. The catalyst was found to be recyclable five times without a notable loss of activity.
Scheme 22.

3.5. Rearrangements
Rearrangements are useful transformations to obtain compounds that are difficult or tedious to obtain in a direct route. Solid acids such as zeolites played an important role in early rearrangements in the chemical, particularly in the petrochemical industry, serving as catalysts for alkene isomerization or cracking reactions.
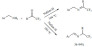
The superacidic Nafion-H and Nafion SAC-13 were found to be effective solid acid catalysts for the synthesis of trifluoromethyl ketimines from benzylamines and trifluoromethylated ketones (Scheme 23) [98]. The reaction occurs via a condensation and a subsequent benzylimine-benzaldimine rearrangement via a facile 1,3-hydrogen shift. The reaction can be carried out under a wide range of reaction conditions such as microwave irradiation and flow system. It appeared that with the appropriate selection of the temperature one could direct the reaction to form the simple Schiff-base (164°C) or the rearranged benzaldimine (185°C) as major products. As the major goal of this work was to synthesize aldminines via the rearrangement, the initial step toward the formation of the benzylimine was not investigated separately and yields were not determined.
Scheme 23.
3.6. Ring Transformations
Ring transformations include cyclization as well as ring-opening reactions, both are important synthetic tools [99].
Cyclizations: The above described Pd/C/K-10 bifunctional catalyst was applied in the microwave-assisted synthesis of pyrazoles from chalcones and hydrazines. The concept was also similar to those mentioned above, K-10 catalyzed the condensation and subsequent cyclization, and the Pd promoted the last dehydrogenation/aromatization step. A broad variety of hydrazines and chalcones were applied to assess the scope of the method. The products were obtained in good to excellent yields (Scheme 24) [100].
Scheme 24.

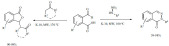
Phthalaldehydic acid (2-carboxybenzaldehyde) served as starting materials for the synthesis of valuable products. Its K-10 montmorillonite-catalyzed reaction with methylaryl and cyclic ketones yielded isobenzofuran-1(3H)-ones in excellent yields (90-98%) via condensation and successive lactonization reactions (Scheme 25) [101]. A similar reaction using aryl-hydrazines led to the formation of phthalazinones by forming a hydrazone intermediate that immediately underwent cyclization (Scheme 25) [102]. The application of microwave irradiation substantially increased the reaction rates in both reactions and the products formed in short reaction times (1-40 min).
Scheme 25.
The synthesis of a broad variety of N-acyl-indoles and sulfonyl-pyrroles was completed by the cyclization of 2,5-dimethoxy-tetrahydrofuran with amides [103] or sulfonamides and amines [104] by microwave-assisted K-10 catalysis (Scheme 26). The product N-sulfonyl-pyrroles were found to be effective (IC50=32-135 nM) inhibitors of the FBPase enzyme, and thus could be considered as drug candidates for type 2 diabetes [105].
Scheme 26.
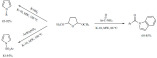
The synthesis of benzimidazoles, benzodiazepines, quinoxali-nones was achieved by a solvent-free microwave-assisted K-10 montmorillonite-catalyzed method. A variety of substituted o-phenylenediamines readily reacted with ketones, aldehydes and bifunctional reagents to form the products (Scheme 27) [106]. The bicyclic condensed heterocycles were obtained in moderate to excellent yields (53-99%) and excellent selectivities in short reaction times (mostly 1-10 min).
Scheme 27.
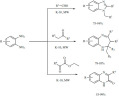
The synthesis of β-carboline-core based drug candidates was achieved by a Pd/K-10-catalyzed microwave-assisted Pictet-Spengler cyclization of substituted tryptamines with various aromatic aldehydes and glyoxals (Scheme 28) [107]. The reaction proceeded by the acid (K-10)-catalyzed formation of an imine between the tryptamine and the carbonyl compounds and the subsequent cyclization to the tetrahydro intermediate. The additional Pd content of the catalyst completed the process by catalyzing the oxidative dehydrogenation/aromatization step. The compounds were found to be excellent inhibitors of amyloid-beta oligomer formation and butyrylcholinesterase activity, and also showed moderate anti-fibril properties [108].
Scheme 28.
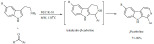
A similar approach was applied for the K-10-catalyzed microwave-assisted synthesis of quinolines from anilines and cinnamaldehydes (Scheme 29) [109]. Reactions were completed in a matter of minutes and although mostly excellent yields were obtained, substrates with potential electron pair donor substituents, such as methoxy, only resulted in moderate yields, likely due to a complexation with the Lewis acid centers of the catalyst.
Scheme 29.

The microwave-assisted solid phase diazotization of o-phenylenediamines with K-10 montmorillonite appeared to be a novel method for the synthesis of 1,2,3-benzotriazoles (Scheme 30) [110]. The method was based on a sequence of reactions that was initiated by the acid centers of K-10, namely the production of nitrous acid that reacted with the amino group of the phenylene-diamine. Once the nitroso intermediate formed it underwent an immediate ring closure with the other NH2 group due to their close proximity forming the benzotriazoles. The reaction showed broad applicability providing high yields for all substrates used.
Scheme 30.
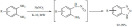
The K-10-catalyzed microwave-assisted 5-exo-dig cyclo-condensation of alk-3-yn-1-ones with hydrazines led to the successful synthesis of diversely substituted pyrazoles (Scheme 31) [111]. The combined application of the clay-based solid acid and microwave irradiation allowed the elimination of organic solvents during the heating and the products were obtained in excellent yields. Due to the different reactivity of the carbonyl group and the triple bond the reaction occurred with exclusive selectivity.
Scheme 31.

Several K-10-catalyzed cyclization reactions were assessed regarding their energy efficiency comparing microwave-assisted and conventionally heated examples. Six reactions were compared using the normalized energy consumption. It was found that the microwave activated ones were more energy efficient than the traditional counterparts in five of the six reactions. However, the one example when the traditional heating was more energy efficient indicates that every reaction should be individually assessed for energy efficiency, and the green label should not be automatically applied for microwave-assisted synthesis [112].
Cu triflate catalyst was successfully applied for the synthesis of fused chromenopyridines. The reaction has been performed under green conditions, using air as oxidant in a solvent-free system [113]. Due to the favorable redox properties of Cu, the Lewis acid catalyzed the Michael-addition as well as the final oxidation of the intermediate to the product (Scheme 32). Although the intermediate underwent aromatization even in a catalyst free system, the yield and reaction rate improved significantly when the catalyst was present providing excellent yields.
Scheme 32.

The solvent-free Pechmann condensation was applied for the preparation of coumarins via an acylation-cyclization sequence of phenols with methyl acetoacetate using zirconium(IV) phospho-tungstate (ZrPW) as a solid acid catalyst [114]. The expected products were obtained in short reaction times, albeit in moderate yields (Scheme 33). The catalyst appeared to be recyclable, although it required an acid recovery treatment to restore its catalytic activity. A similar study applied Envirocat EPZ-10 as a catalyst for this reaction [115]. Although the conditions were about the same, the new catalyst appeared to make a significant difference and resulted in good yields.
Scheme 33.

The successive ring opening-cyclization of epoxychalcones in the presence of montmorillonite KSF under microwave-assisted solvent-free conditions resulted in the formation of flavonols (Scheme 34) [116]. A broad scope of different aromatic substituents was tolerated and the formation of the products occurred in excellent yields in very short reaction times.
Scheme 34.

Aziridines are versatile synthetic building blocks that are used in highly regio- and stereoselective ring-opening reactions. K-10 montmorillonite has been applied as a catalyst for the synthesis of aziridines in a simple, ambient temperature approach using imines and ethyl diazoacetate as starting materials (Scheme 35) [117]. The reaction resulted in the formation of cis-aziridines. The solid, easily recyclable catalyst, the short, room temperature reactions, the high atom economy, the excellent yields (90%) and exclusive selectivity all point toward an excellent example of sustainable synthesis.
Scheme 35.

A microwave-assisted aminolysis of epoxides was catalyzed by BiCl3 on SiO2, an efficient heterogeneous catalyst. The reaction readily provided β-amino alcohols in high yields under solvent free conditions [118]. The catalyst could be recycled for five times without loss in activity. The microwave activation enhanced the yields even under shorter reaction times compared to conventional heating. A broad variety of epoxides could be successfully applied with negligible substituent effect (Scheme 36).
Scheme 36.

Acids have been proven to serve as effective catalysts for the ring-opening reaction of lactones as well. As acidic conditions are also likely to catalyze the reverse reaction, i.e. lactone formation, the ring opening often is only successful if an immediate subsequent reaction is involved further transforming the primary product hydroxy-acids. In a recent study, Zhu et al. showed that metal triflates can be efficient in the ring-opening-hydrogenolysis of lactones to produce the corresponding carboxylic acids (Scheme 37) [119]. While several different metal triflates were effective in the reaction, tungsten was found to be the most active. The metal cation acts as a Lewis-acid in both the ring-opening polymerization as well as the depolymerization forming an unsaturated acid that is then reduced by a conventional Pd/C catalyst. A broad range of different sized lactones could be transformed under relatively mild conditions and in mostly high yields.
Scheme 37.

Gowda and Chakraborty used the environmentally benign Lewis acid FeCl3 as catalyst for the ring-opening polymerization of lactones (Scheme 38) [120]. The reactions gave full conversion to the polymer and good molecular weights of the polymers were obtained. The molecular weight distribution was shown to be superior to that obtained with other more complex iron catalysts.
Scheme 38.

Similar reactivity was obtained for the ring-opening polymerization of β-butyrolactone and lactide using a zinc-amine complex [121]. Miller et al. demonstrated that the Sb2O5 is effective in catalyzing the ring-opening polymerization/poly-condensation reaction between lactones and aromatic hydroxyacids [122]. The products were obtained in high yields and good molecular weights.
3.7. Asymmetric Synthetic Applications
The most common way to design heterogeneous catalytic applications is to immobilize either a chiral modifier on the surface of a solid catalyst or a chiral catalyst (e.g. metal complex) on the surface of a catalyst support.
The group of Freire and Pires showed that the asymmetric epoxidation of styrenes was possible under heterogeneous conditions using a manganese (III) salen complex immobilized on functionalized porous clay heterostructures (PCHs) [123]. The thus prepared catalyst enabled to obtain the desired epoxides in high enantiomeric excess but only in low yields (Scheme 39). While the method is attractive due the use of a heterogeneous catalyst and the high enantioselectivities obtained, the use of dichloromethane as solvent and the non-recyclability of the catalyst limit the performance with regard to green chemistry metrics.
Scheme 39.

Lin et al. used a chiral metal-organic framework (CMOF) containing Mn-salen unit as linker for the asymmetric epoxidation of cyclic alkenes [124]. By generating isoreticular MOFs with different cavity size the authors observed that the epoxidation rate was clearly dependent on the channel dimension. While the catalyst could be recycled for up to 4 times without loss of activity and enantiomeric excess, the reaction suffered from the need of dichloromethane as solvent and 2-(tert-butyl-sulfonyl)iodosyl-benzene as oxidant (Scheme 40).
Scheme 40.

Similar results were obtained by Huang et al. using a Jacobsen’s type catalyst bound to zirconium poly(styrene-phenylvinylphosphonate)-phosphate (ZPS-PVPA) and mCPBA/ NMO as an oxidizer [125]. The reactions proceeded with high yields and enantioselectivities and the catalyst was reused for up to seven times without notable loss of activity and enantioselectivity. The authors showed that the reaction performed well on a large scale using the same procedure giving identical yields and enantioselectivities. Dichloromethane, used as the solvent for the reaction, however, led to an increase in the environmental impact of the reaction.
Using a CMOF containing a Ni-salen moiety as catalyst the insertion reaction of carbon dioxide into propylene oxide was possible [126]. The reaction used racemic propylene oxide as starting material and did not require the use of a solvent (Scheme 41). Although the enantioselectivities obtained were moderate and the conversion was low, this method represents an interesting new procedure for a chiral resolution reaction.
Scheme 41.

The sequential one-pot asymmetric epoxidation-ring-opening reaction of alkenes was shown to be effectively catalyzed by a multivariate MOF [127]. The MOFs used contained salen units with different metal ions as active centers. A variety of nucleophiles was used in the ring opening and the products were generally obtained in high yields and regio- as well as enantioselectivities (Scheme 42). As described previously, 2-(tert-butyl- sulfonyl)iodo-sylbenzene was used as the oxidant and dichloromethane as solvent which significantly increases the environmental impact of the reaction.
Scheme 42.
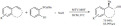
Jiang and co-workers showed that a porphyrin-salen based MOF can be used as a catalyst in the asymmetric cyanosilylation of aldehydes (Scheme 43) [128]. The reaction provided the desired products with moderate to high yields and enantioselectivities. The easy product separation and recyclability of the catalyst are negatively counterbalanced by the use of stochiometric amounts of PPh3O as an activating agent and the use of dichloromethane as a solvent.
Scheme 43.

Cui et al. used a chiral porous organic framework (POF) functionalized with Rh as Lewis acid catalyst for the asymmetric conjugate addition of arylboronic acids to unsaturated ketones [129]. A variety of enones and arylboronic acids were coupled in mostly high yields and enantioselectivities (Scheme 44). The catalyst was easily recovered by centrifugation and could be reused for five consecutive runs without significant loss in activity and enantioselectivity. The environmental impact of the reaction is increased by the fact that 50 mol% KOH have to be added and the reaction needs to be run in a dioxan-water mixture. The authors also demonstrated that the chiral POFs can be used as a separating agent in chromatography.
Scheme 44.

3.8. Oxidation of Alkenes
The oxidation of alkenes is traditionally performed by using organic peroxides or inorganic metal oxides in high oxidation states, both systems producing a large amount of low-value byproducts. To overcome this issue the use of solid acids in combination with environmentally friendly oxidizing agents such as hydrogen peroxide or oxygen has been explored. Xia et al. used 30% H2O2 and a solid phosphotungstic acid catalyst for the epoxidation of cyclohexene (Scheme 45) [130]. The catalyst was prepared by simple exchange reaction with an anion-exchange resin and performed the desired reaction in high yields and selectivities. The catalyst could be recycled for up to five times without losing activity and selectivity. The authors also demonstrated that the scope could be extended for other substrates but with decreased yields and selectivities.
Scheme 45.

In a very similar approach the same group used a polystyrene-bound peroxophosphotungstic acid as a catalyst for the epoxidation of various dienes with H2O2 [131]. The best results were obtained with dicyclopentadiene where the monoepoxide was obtained in nearly quantitative yield. The catalyst could be recycled for up to eight times without losing activity.
The solid acid KSF montmorillonite successfully catalyzed the aqueous phase oxidative cleavage of styrenes to benzaldehyde with air, in a process that can be viewed as an environmentally benign alternative to ozonolysis (Scheme 46) [132]. Although the reaction showed high selectivity and conversion in the oxidation of styrene, the cleavage of substituted derivatives occurred in lower yields and selectivities. The Fe-content of the catalysts was proposed as a major contributor to the mode of action, although the acid centers played a role in the cleavage of the intermediates.
Scheme 46.

3.9. Miscellaneous Reactions
It appears that acidic montmorillonites possess properties that allow them to serve as mild oxidation catalysts as well. K-10 montmorillonite successfully catalyzed the partial oxidation of benzylamines to benzaldehydes and the immediate successive condensation of the product benzaldehydes with the excess amine to form benzylidene-benzylamines. Using a mixture of benzyl-amines and anilines mixed products, e.g. benzylidene-anilines were also obtained in excellent yields (Scheme 47) [133, 134].
Scheme 47.
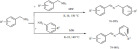
A tailored catalyst, methanesulfonic acid deposited on Al2O3, was applied in the esterification of carboxylic acids in a solvent-free, microwave-assisted process [135]. The scope of the reaction was broad; a variety of aromatic and aliphatic acids could be esterified with common alcohols. The process provided high yields in short reactions (Scheme 48) and could be extended to the esterification of α-aminoacids.
Scheme 48.

Solid acids have also been applied in the degradation and transformation of natural saccharides using a variety of these materials [136]. For example a patented method proposed a highly effective polymerization of building blocks derived from unlimited resources such as glycosides, employing solid superacid catalysts [137]. The method consists of reacting the starting materials with sulfonated metal oxides, such as sulfonated iron oxide or zirconia sulfate, by a melt polymerization or a solution polymerization process. For instance, D-glucose was polymerized without solvent in an electric furnace using an equivalent amount of sulfated zirconia at a temperature corresponding to the melting point of the saccharide for 24h. The method allowed the production of multiple polymers of saccharides in one simple step making the procedure relatively environmentally friendly compared to the wasteful multi-steps traditional methods.
The above subchapter gives an overview of the many application possibilities of solid acids in synthetic processes. A wide variety of approaches are described. Essentially, solid acids can be applied and thus can replace harmful mineral acids in every process that has been attempted by traditional acid catalysis. It is widely believed that these materials are the future of acid catalysis and accordingly the development of the field is rapid and extensive. In addition to the common solid acids, such as clays, zeolites and acidic ion-exchange resins, several new classes of materials were introduced to the field and helped extending applications and will have a great impact on upcoming developments of new and potentially greener processes.
4. SYNTHETIC APPLICATIONS IN HOMOGENEOUS SYSTEMS
4.1. Friedel-Crafts and Related Reactions
The major goal in contemporary, sustainable homogeneous Friedel-Crafts chemistry is to replace the traditional catalysts, such as mineral acids and moisture sensitive Lewis acids with water stable recyclable acids.
Acylation: Metal triflates are one of the most popular green acid catalysts in homogeneous catalytic applications. Anisole derivatives were acylated with acetic anhydride using Sc(OTf3) and a dendritic terpyridine ligand as the catalyst (Scheme 49) [138]. The appli-cation of microwave heating ensured short reaction time when derivatives with strong electron withdrawing groups were used. The catalyst, while soluble in the reaction medium, was also found recyclable, which is an important green feature and a significant step forward from traditional methods that use aluminum chloride.
Scheme 49.

Bi(OTf)3 was described as an efficient catalyst for the microwave-assisted solvent-free Friedel-Crafts acylation of benzene derivatives with benzoic anhydride [139]. The benzoylation readily occurred with a wide variety of aromatics in short reaction times (Scheme 50). The catalyst was found recyclable and only a negligible activity loss was observed after five consecutive reactions. It was noted that although conventional heating also produced the products it required significantly longer reaction times.
Scheme 50.

Alkylation: An efficient triflic acid catalyzed one-pot synthesis of N-substituted pyrroles, indoles, and carbazoles was achieved using a successive cyclization/annelation approach [140]. The reaction was carried out applying aryl sulfonamides and 2,5-dimethoxy-tetrahydrofuran as a starting materials (Scheme 51). Although the first step to form pyrroles is a simple cyclization, the subsequent steps to form indoles and carbazoles occurred via the aromatic alkylation and aromatization. The reactant/triflic acid ratio successfully controlled the formation of the desired product, thus indoles or carbazoles could be prepared without isolating any intermediate product. Despite the use of dichloromethane the rest of the system appears to be green involving a recyclable acid, the one pot-five step reaction (for carbazoles) and the room temperature reactions.
Scheme 51.
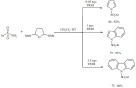
Triflic acid was also found to be an effective catalyst for the preparation of unnatural organofluorine amino acid esters [141]. The highly stereoselective aminoalkylation of indoles and pyrroles was carried out by using chiral 3,3,3-trifluoro-pyruvate-α-methylbenzyl imines. The reaction sequence was completed by a subsequent Pd-catalyzed hydrogenolysis to remove the methyl-benzyl group provided the products in high yields and excellent enantioselectivities. The product α-trifluoromethyl-α-(heteroaryl)-glycine esters were isolated in high yields and excellent enantioselectivity (Scheme 52).
Scheme 52.
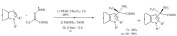
Halogenation: The bromination of aromatic compounds, alkenes and alkynes was achieved using ammonium vanadate as a catalyst using tetrabutylammonium bromide as a bromine source (Scheme 53) [142]. Hydrogen peroxide was applied as an auxiliary oxidant to produce Br2 from the reagent. The process gave a broad variety of products in good yields under mild conditions, e.g. at room temperature. When alkenes and alkynes used the reaction resulted in mixtures. Using alkenes, 1,2-dibromo and hydroxy-bromo products formed, while in the case of alkynes, the reaction stopped at the dibromoalkene phase resulting in the formation of E/Z isomers with high selectivity for the Z-product.
Scheme 53.
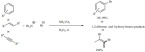
Nitration: Heravi et al. described the nitration of aromatics with vanadium substituted phosphomolibdic acid in acetic anhydride as a solvent using nitric acid as the nitrating agent (Scheme 54) [143]. Although the yields were mostly moderate the catalyst could be reused without significant loss in its activity.
Scheme 54.

Bismuth (III) triflate was also found to be an excellent catalyst for the above reaction using toluene as a model substrate [144]. Using dichloroethane as solvent and HNO3 as the nitrating agent the catalyst resulted in complete conversion of the starting material, essentially yielding 100% product. The catalyst was reused in the reaction five times and no activity loss was observed during the subsequent runs. The same reaction could be carried out using N2O5 as a nitrating agent [145].
4.2. Multicomponent Reactions
Reported methods about multi-component sustainable syntheses catalyzed by homogeneous acid catalysts are scarce in the literature. A few acid driven-organic reactions in homogeneous systems are described in this part. Most methods use heteropolyacids, acidic organocatalysts and metal triflates; some other methods use unique homogeneous acid catalysts and constitute isolated examples. It must be noted that although they possess relatively good environ-mental-friendliness, their green potential is not always ideal. The limited number of existing methods, together with the yet-to-be improved sustainability of the presently selected works, illustrates the need for more research in this area.
While ionic liquids are often used as reaction media, their specific properties also allow them to promote different reactions depending on their composition. As a result they have the ability to serve as catalysts as well. For instance, acidic functions can be introduced within the structure of the salt, providing acidic properties. A novel ionic liquid with Brønsted acidic properties was reported by Zare et al. and applied as a catalyst for the three-component synthesis of napthol derivatives [146]. The ionic liquid 1,3-disulfonic acid imidazolium hydrogen sulfate (DsimHSO4) successfully promoted the condensation of β-naphthol with arylaldehydes and alkyl carbamates or amides with high yields in short reaction times (Scheme 55). Despite the controversial eco-friendliness of ILs due mainly to their long and potentially wasteful synthesis, in this study, the IL was used in catalytic amount and was recyclable; consequently it limits the amount of waste generated. In addition, the proposed method has a significantly lower environ-mental impact than other methods often suffering from toxic catalysts in high loading, harsh conditions or long reaction times.
Scheme 55.
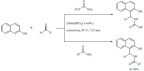
Biologically active tetrahydropyridine derivatives were synthesized in presence of a similar ionic liquid ([Py-SO3H]HSO4 - sulfonic acid pyridinium hydrogen sulfate) serving as an eco-friendly catalyst (Scheme 56) [147]. The one-pot three-component condensation of various aromatic aldehydes, aromatic amines and ethyl acetoacetate was carried out without solvent at 100°C to reach yields above 80%. The IL played the role of both Brønsted and Lewis acid to assist the formation of the multiple intermediates. Not only did the reaction proceed better without solvent but the method also proved to be significantly more efficient that previously reported systems using organic solvents and/or toxic catalysts. After extracting the reaction mixture, the IL was used for five consecutive cycles with no noticeable loss of activity.
Scheme 56.

Polymers are tunable structures that can easily be functionalized. Attaching acidic functions to a polymeric chain forms an interesting material that can potentially be used as a catalyst. A simple and green protocol was reported for the three-component synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones (Scheme 57) employing the water-soluble and reusable polyvinylsulfonic acid as a Brønsted acid catalyst [148]. This soluble solid-state analogue of sulfuric acid offers many advantages: it has a higher acid density than sulfuric acid, it can be used in aqueous media and has the potential to be recycled, it is cost-effective and easy to handle. As a result, the acid-catalyzed cyclocondensation of aldehydes, ethyl acetoacetate and urea or thiourea was conducted in water at 90°C affording excellent yields. The products were extracted from the mixture and the catalyst could be recycled after simple evaporation of water. The results obtained were comparable and even superior to most conventional metal-based procedures both in terms of yield and reaction rate.
Scheme 57.
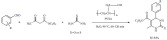
Indium triflate is another metal-triflate with catalytic activity [149]. The four component reaction of aldehydes, isocyanides, azidotrimethylsilane, and aliphatic alcohols was reported using indium triflate as a mild Lewis-acid catalyst (Scheme 58). The reaction starts with the Lewis-acid driven formation of carbo-xonium ion intermediate from the aldehyde and the aliphatic alcohol that also serves as a solvent. Interestingly, an optimum catalyst loading was reported for this reaction with yields ranging from moderate to good depending in part on the nucleophilicity of the isocyanide. The reaction shows some green features; it required low catalyst loading of a stable Lewis acid. However, the catalyst was not recyclable and afforded only moderate yields for some substrates. As another drawback of the method; excess amount of some of the reactants was necessary to observe conversion.
Scheme 58.

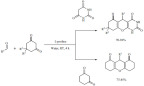
A few organocatalyst-driven multi component syntheses were reported under mild conditions in aqueous medium making them interesting methods from the green chemistry perspective. For instance, L-proline is a well-known organocatalyst that has successfully catalyzed multiple organic transformations. Myrboth et al. described the asymmetric multi-component synthesis of chromeno [2,3-d]pyrimidine-triones and xanthene dione derivatives using L-proline as a catalyst [150]. Aromatic aldehydes, dimethyl-cyclohexane-1,3-dione/cyclohexane-1,3-dione and barbituric acid were reacted at room temperature in water to afford chromeno [2,3-d]pyrimidine-triones in good to excellent yields and moderate to high enantioselectivities (Scheme 59). The method was equally successful when using two equivalents of cyclohexane-1,3-dione in place of barbituric acid to form xanthene dione derivatives. The reaction was mostly assisted by the nucleophilic properties of the amino group of L-proline although its acidic properties contributed. The yields seemed not to be affected by the presence of electron withdrawing or donating substituents. The very mild reaction conditions and the non-toxic nature of the catalyst certainly contribute to reduce the environmental impact of the method despite the relatively high catalyst loading (15%).
Scheme 59.
The functionalization of organocatalysts with Brønsted acid groups creates acidic organocatalysts suitable to perform con-ventional Brønsted acid-catalyzed reactions. A guanidinium-based sulfonic acid functionalized organocatalyst was developed and proved its effectiveness to replace the traditional liquid mineral acids for the multi-component synthesis of a variety of quinoxaline-, pyrazine- and urazole-derivatives in aqueous media (Scheme 60) [151]. The reactions proceeded smoothly in less than two hours at room temperature or reflux depending on the substrate type. The method also presented the benefits of heterogeneous systems because the product precipitated and was filtered out while the catalyst was extracted from water with ethyl acetate and reused for other cycles with consistent activity.
Scheme 60.
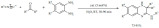
A similar method was developed by Wang et al. for the synthesis of the polycyclic benzoazulen-1-ones [152]. Although the authors called this procedure “catalyst-free” one should not overlook the fact that the solvent acetic acid indeed acted as a mild strength acid catalyst. The microwave activation and the small amount of mild acid represented sufficient conditions to achieve high yields in short times. The substrate scope of the reaction was broad, showing little substituent effect (Scheme 61).
Scheme 61.

A solvent free A3-coupling was developed for the synthesis of quinolines (Scheme 62) [153]. Microwave heating was used to activate the system and FeCl3 was used as a catalyst. The reaction provided high yields for most substrates, although starting materials with strong electron-withdrawing substituents lowered activity and decreased yields. The environmentally benign catalyst, the high reaction rates and relatively short times are the main green advantages of the process.
Scheme 62.

Imidazo [1,2-a]pyridines were synthesized by the multi-component Groebke-Bienaymé-Blackburn reaction of 2-amino-pyridines, aromatic aldehydes and isonitriles [154]. With the application of microwave activation, solvent-free conditions and Yb(OTf)3 catalyst the products were obtained in excellent yields in short, only 5 min long, reactions (Scheme 63). The sustainable conditions, the nearly quantitative yield ensured that the environmental impact of this method was significantly lower than that of available alternatives.
Scheme 63.

A heteropolyacid-catalyzed multicomponent condensation of an aryl aldehyde, acetyl chloride, acetonitrile, and enolizable ketone for the preparation of β-acetamido ketones (Scheme 64) was described by Tayabee and Tizabi [155]. Several heteropolyacids were screened to compare their relative activity. The vanadium-substituted Keggin-type H5PW10V2O40 achieved the highest yields. Interestingly, despite its lower Brønsted acidity it showed better performance than the traditional Keggin-type heteropolyacid H3PW12O40. In fact, the high catalytic activity is believed to be not only due to the acidity but also to the electron acceptor property of vanadium. Although, the high yields and short reaction times suggest an environmentally friendly protocol, there are a few disadvantages. The reflux conditions, the use of acetonitrile as solvent, as well as the required preparation of the metal-substituted heteropolyacid all increase the overall environmental impact of the protocol.
Scheme 64.

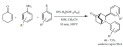
The heteropoly acid-catalyzed microwave assisted three component aza Diels-Alder cyclization of cyclohexenone, substi-tuted anilines and benzaldehydes to prepare azabicyclo[2.2.2]octan-5-ones (Scheme 65) was described by Borkin et al. [156]. The multistep reaction occurred in a one pot fashion. In the first step a Schiff base formed in situ, which underwent an intermolecular cyclization with the cyclohexenone. Despite the short reaction times the yields were moderate to good and the products formed with excellent diastereoselectivity. The compounds were also tested in preliminary cholinesterase and amyloid β fibrillogenesis inhibition assays to probe them as potential anti Alzheimer’s disease agents.
Scheme 65.
4.3. Ring Transformations
The formation of substituted quinolines was described using zinc chloride as the Lewis acid catalyst for the cyclization of ketones with imines (Scheme 66) [157]. The reaction can also be performed as one-pot sequence without prior formation of the starting imines. The quinolines were obtained in moderate yields. Acetic acid was used as the solvent for the reaction allowing the formation of quinolines under environmentally friendly conditions.
Scheme 66.
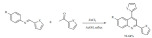
Zhao et al. described a green way for the synthesis of substituted 3,2’-tetrahydrofuryl spiroxoindoles through cyclization of 3-allyl-3-hydroxy-2-oxoindoles (Scheme 67) using methanesul-fonic acid (MSA) as catalyst [158]. The reaction did not require any metal and water was used as the solvent. The authors showed that performing the reaction on a gram-scale was easily possible.
Scheme 67.
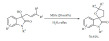
The group of Gooßen found that silver triflate was an effective Lewis-acid catalyst for the synthesis of γ-lactones from unsaturated fatty acids (Scheme 68) [159]. The silver served as the catalyst for the migration of the double bond into proper position, as well as a catalyst for the lactone formation. Even though chlorobenzene was used as the solvent, the advantage of the reaction is in the remarkably high yield compared to previous known examples and given that several double bond migration steps have to be achieved before the cyclization can occur. Oligomerization was found to be the major side reaction, however, the formation of δ-lactones was not observed.
Scheme 68.

The synthesis of benzobis-azoles from an aromatic orthoester and diamino diols or dithiols using a Lewis acid catalyst (Scheme 69) was described by Jeffries-EL et al. [160]. Y(OTf)3 was used as environmentally benign catalyst and the desired products where obtained in high yields. The benzobisazoles formed served as monomers for the generation of conjugated polymers for use in organic LED. The drawback from the environmental point is that a pyridine/THF mixture had to be used as solvent for the reaction.
Scheme 69.

Uozumi and co-workers showed that camphorsulfonic acid (CSA) can be used as efficient catalyst for the synthesis of 4(3H)-quinazolinones [161]. The reaction used a water-ethyl lactate mixture as green solvent and was able to produce the desired compounds from a large variety of 2-aminobenzamides and 1,3-diketones (Scheme 70). The diketones used can be both cyclic and acyclic and they undergo a C-C bond cleavage during the reaction.
Scheme 70.

The solvent- and metal-free synthesis of quinolines using a Brønsted acid was demonstrated by Bao et al. [162]. N-alkylamines were reacted with alkenes or alkynes and a catalytic amount of triflic acid to afford the formation of the quinolines in high yields (Scheme 71). The reaction did not require any solvent and used oxygen as a green oxidant.
Scheme 71.

The group of Jirgenson developed a Lewis acid-catalyzed protocol for the formation of vinyloxazolines and vinyloxazines [163, 164]. Using bis(imidates) as starting materials the formation of the desired products was possible using a catalytic amount of FeCl3 or AlCl3 (Scheme 72). The products were obtained in high yields and short reaction times. While the use of iron chloride is still acceptable within green chemistry, the use of aluminium chloride and dichloromethane should be avoided. The same group could also show that bis(trichloroacetimidate) can serve as starting material for the reaction [165].
Scheme 72.

Cossy et al. demonstrated that iron and indium salts can serve as effective Lewis acid catalysts for the cyclization of tert-butyl carbonates to form cyclic carbonates [166]. Both five- and six-membered cyclic carbonates can be formed using this method. While iron proved to be effective for substrates containing only carbon and hydrogen, indium was shown to be superior when heteroatoms are present in the starting material (Scheme 73). The syn diastereomer was always the major product obtained, albeit the selectivity was moderate in certain cases. Another drawback of the method was the use of the not-green solvent acetonitrile.
Scheme 73.

A scandium triflate catalyzed [3+3] annulation to form tetrahydro-β-carbolines and tetrahydroisoquinolines was developed by Wang et al. [167]. The transformation used benzylic alcohols and aziridines as substrates to give the desired products in moderate yields (Scheme 74). The [3+3] process applied allows for greater product diversity than previously established methods. While scandium triflate can be considered a green Lewis acid, the use of 1,2-dichloroethane as solvent counterbalances the overall environmental friendliness of the procedure.
Scheme 74.
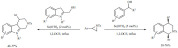
A new strategy for the en-yne-cyclizations was developed by Zhang and co-workers [168]. Instead of the traditional π-activation they chose to use a Brønsted acid to perform σ-activation of alkyne enones to trigger the cyclization and yield the desired 5-membered ring products (Scheme 75). Depending on the starting material used, both saturated and unsaturated ring systems were obtained in moderate to high yields. While the initial cyclization yielded a mixture of syn and anti-isomers; a base-catalyzed isomerization led to the selective formation of the anti-isomer. Depending on the substrate, 1,2-dichloroethane or nitromethane had to be used as the solvent which increased the environmental impact of the reaction.
Scheme 75.
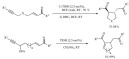
Lithium bromide was applied as Lewis acid catalyst for the ring expansion of epoxides with α-mercaptocarboxylic acids to form 1,4-oxathian-2-ones (Scheme 76) [169]. The reaction proceeded under solvent-free conditions at room temperature resulting in the products in high yields. The epoxide opening was achieved with high regioselectivity and the catalyst was recovered after the reaction and reused without any loss of efficiency.
Scheme 76.

The ring-opening reaction of epoxides with ammonium thiocyanate was efficiently catalyzed by a PEG-supported sulfonic acid [170]. The water soluble polymer combines the best features of homogeneous catalysis with the easy separation of a heterogeneous catalyst. The reaction was carried out under mild conditions in water as the solvent and formed the β-hydroxy thiocyanates in excellent yields (Scheme 77). High regioselectivities were obtained during the opening of the epoxide.
Scheme 77.

Nazari et al. described that a similar reaction of epoxides can also be catalyzed by imidazole-1-yl-acetic acid [171]. Ammonium thiocyanate and sodium azide was used as reagents for the ring opening reaction (Scheme 78). The catalyst used was recycled after the reaction and reused for consecutive reactions without significant loss of activity. The products were obtained with high regioselectivity in most cases.
Scheme 78.
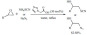
The ring opening of epoxides with aromatic amines (Scheme 79) was described by Halimehjani et al. using boric acid and glycerol as a green catalytic system in water as the solvent [172]. The reactions proceeded with high yields and high regioselectivities under mild conditions. Glycerol acts as chelating agent for the boric acid thus increasing its acidity.
Scheme 79.
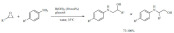
The catalytic etherification of glycidol with different alcohols (Scheme 80) was described by Capacchione et al. [173]. Using a very low loading of Al(OTf)3 as the catalyst the monoalkyl glycerol ethers were obtained in mostly high yields. The etherification happens preferentially at the terminal position, although the ratio between terminal and internal alkylation was not very high in many cases. The alcohol used for alkylation additionally served as the solvent, no other solvent was needed for the reaction.
Scheme 80.

Majee and co-workers developed a method for the ring-opening of aziridines with various nucleophiles (Scheme 81) using an imidazole-based zwitterionic organocatalyst [174]. The catalyst acts as hydrogen donor to activate the aziridine towards the attack of the nucleophile. No solvent was needed for the reaction of carbon-, oxygen- and nitrogen-nucleophiles and the products were obtained in high yields and high regioselectivities with concern to the position of ring opening.
Scheme 81.
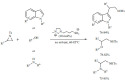
Iron chloride was used as the Lewis acid catalyst in the ring-opening polymerization of cyclic lactones by Chakraborty and Gowda [175]. The authors showed that ε-caprolactone, δ-valerolactone and β-butyrolactone can be polymerized effectively (Scheme 82) to form polymers with a narrow molecular weight distribution. Through the addition of alcohols and the variation of the monomer-catalyst ratio the polymer properties can easily be tuned.
Scheme 82.

The ring-opening alternating co-polymerization of cyclic anhydrides and epoxides (Scheme 83) was described using a Lewis acid-base pair as catalytic system under solvent-free conditions [176]. The combination of an organozinc compound with a N-heterocyclic organobase catalyzed the polymerization in most cases with high conversions, extremely high degree of alternation and a narrow molecular weight distribution.
Scheme 83.

The transformation of furfuryl alcohol to alkyl levulinates also readily occurred in methanol with microwave activation [177]. Several metal salts, including CrCl3, ZnSO4, AlCl3, or Al2(SO4)3 were tested as catalysts. Al2(SO4)3 showed the best performance, likely due to its somewhat elevated Brønsted acidity. The high dielectric constant of the solvent, probably contributed to the efficiency of the alcoholysis of furfuryl alcohol via its strong microwave absorption ability that ensured that in a 5 min reaction at 150°C, the reaction occurred (Scheme 84) in 80% yield and nearly exclusive selectivity. During mechanistic investigations of the reaction mechanism key intermediates (methoxymethylfuran (MMF) and 4,5,5-trimethoxypentan-2-one (TMP)) were isolated. The catalyst was reused for six consecutive cycles with negligible loss in activity. Thus despite using DCM as solvent in the work up the method is rapid, efficient and partially environmentally benign.
Scheme 84.

4.4. Asymmetric Synthetic Applications
Catalysis by chiral phosphoric acids: Huang et al. showed that the catalytic asymmetric synthesis of chiral 1,3-butadienyl-2-carbinols was possible by using a chiral phosphoric acid as a catalyst [178]. The reaction between aldehydes and homoallenyl-boronates (Scheme 85) did not require the use of a metal catalyst and furnished the products in excellent yields and enantio-selectivities. The reaction was performed on a gram scale and the products were of interest due to their synthetic utility as shown by the authors through the synthesis of a NCSi-gb analogue using their methodology.
Scheme 85.
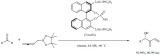
The generation of chiral quaternary centers was achieved by List et al. Chiral phosphoric acids enantioselectively catalyzed the α-amination of α-branched ketones (Scheme 86), a transformation that cannot easily be achieved by traditional amine-catalysis [179]. High yields and good enantioselectivities were obtained for the amination in the α-position of several different cyclic α-branched ketones with diazocarboxylates.
Scheme 86.
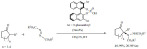
A solvent free variation of the above reaction was published by Toste et al. using a different chiral phosphoric acid [180]. Proper mixing was achieved by dissolving the starting materials in CH2Cl2. After evaporating the solvent, the residue was heated at 45°C for 40-60 h to obtain the α-aminated products in high yield and high enantioselectivities.
Chiral phosphoric acids were also applied for the Friedel-Crafts addition of benzoxazinones with substituted pyrroles (Scheme 87) [181]. The products were formed in high yields and enantio-selectivities generating a quaternary stereocenter. The presence of the CF3-group seems to be essential as it contributes to hydrogen binding in the activation mode of the reaction.
Scheme 87.
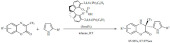
The asymmetric Friedel-Crafts addition of acetals to naphthols (Scheme 88) was described by Da et al. [182]. Using a chiral phosphoric acid as catalyst and acetic acid as a co-catalyst the addition products were obtained in moderate yields and enantioselectivities. Even though the reaction was performed in dichloroethane as a solvent, the major benefit of the applied system was that the reaction could be performed under mild conditions.
Scheme 88.
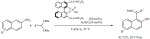
The asymmetric transfer hydrogenation of a range of imines could also be catalyzed by chiral phosphoric acids. The enantioselective hydrogenation of different substituted quinolines (Scheme 89) was shown by Rueping and his coworkers using only 0.5mol% of a chiral phosphoric acid as a catalyst [183]. Both a traditional batch reactor and a continuous-flow system were able to perform the reaction with the latter significantly improving the reaction yield (67 vs. 97%). More and Bhanage showed that the sustainable solvent diethyl carbonate could be used for the same reaction [184]. Similar high yields and enantiomeric excesses were obtained using a Hantzsch ester as the hydrogen donor.
Scheme 89.
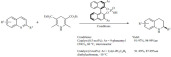
A binaphthol-derived phosphoric acid in combination with a cinchona alkaloid derived amine was an effective catalytic system for the enantioselective epoxidation of α,β-unsaturated aldehydes (Scheme 90) yielding the products in moderate to high yield and high enantioselectivities [185]. Although the reaction was per-formed in THF, a not-green solvent, the system benefits from the use of hydrogen peroxide as an oxidant and the fact that the aldehyde could be used as a substrate, which is often not possible using other protocols due to the oxidation of the carbonyl group.
Scheme 90.
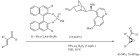
The enantioselective epoxidation of enynes was performed by using scandium triflate as the Lewis acid in presence of a chiral N,N’-dioxide as ligand [186]. The selective epoxidation of the alkene (Scheme 91) was achieved in excellent yields and enantioselectivities using hydrogen peroxide as the oxidant. A low catalyst loading of 0.5% was applied and the reaction could also easily be performed on a gram scale. The high selectivity towards the alkene epoxidation overcomes the drawbacks associated with the synthesis of the ligand and the use of THF as a solvent.
Scheme 91.
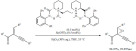
Using ZnCl2 as a Lewis acid in combination with a prolineamide as chiral catalyst the asymmetric aldol reactions of aromatic aldehydes with ketones (Scheme 92) was described in high yields, syn:anti ratios and enantiomeric excess [187]. In addition to the use of an environmentally benign catalyst the system also benefited from the use of a 1:1 water-ethanol mixture as a solvent system.
Scheme 92.
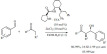
Penhoat et al. showed that this reaction can also be performed using unmodified proline and ZnCl2 as Lewis acid [188]. The study had a smaller substrate scope and DMSO had to be used as a co-solvent making this approach less appealing from a sustainability point of view than the before mentioned ZnCl2-prolineamide system.
An environmentally friendly variation of the Mukaiyama aldol reaction was developed by Kobayashi and his co-workers [189]. It was shown that a large variety of Lewis acids can be used for the reaction of aldehydes with silyl enol ethers (Scheme 93) in combination with a chiral bipyridine ligand. The environmentally friendly metal ions iron and bismuth showed especially good results using an aqueous solvent system. The desired aldol products were obtained in high yields and excellent syn:anti selectivities as well as enantiomeric excess.
Scheme 93.
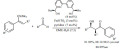
The asymmetric Michael addition using a catalyst made of Yb(OTf)3 as Lewis acid and L-proline as a chiral ligand (Scheme 94) afforded the corresponding addition products with yields up to 94% and high enantioselectivities under environmentally friendly conditions [190]. The reaction was performed in water as a solvent and the aqueous phase, containing the catalyst, was recycled several times without appreciable loss of catalytic activity.
Scheme 94.

An asymmetric version of the Strecker reaction using a chiral Brønsted acid was described by Barbero et al. [191]. The use of substituted o-benzenedisulfonimides as a chiral catalyst allowed the formation of the α-aminonitrile from acetophenones, anilines and TMSCN in high yields and enantiomeric excess (Scheme 95). The reaction was performed under solvent-free conditions and the catalyst was recycled three times without decrease in yield or enantioselectivity.
Scheme 95.
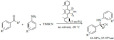
Using a chiral Mg(OTf)2-BOX complex as a Lewis acid catalyst the formal [3+2] cycloaddition to form chiral γ-lactams (Scheme 96) was possible in mostly high yields and enantio-selectivities [192]. While the use of the imine was necessary for the success of the reaction, both stereoisomers of the imine could be used as starting material in the reaction, a separation was not necessary. A drawback from the green point of view is the need for diethylether as solvent in the reaction.
Scheme 96.
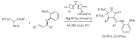
4.5. Miscellaneous Reactions
The combined application of acidic organocatalysis and heterogeneous catalytic hydrogenations allowed to solve a long standing problem the hydrogenation of indoles under aqueous conditions [193]. The typical conditions for indole hydrogenation are somewhat harsh and often result in indole over-hydrogenation. The reaction was carried out at ambient temperature in water as a solvent by using pTSA as an acidic organocatalyst that protonated indole and made it soluble in the aqueous phase. The protonation also destabilized the aromatic system helping the subsequent hydrogenation step (Scheme 97) to produce the products in high yield.
Scheme 97.
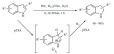
The N-formylation of amines (Scheme 98) was achieved in a ZnCl2 initiated Lewis acid-catalyzed process [194]. The reactions proceeded without any solvent and the products were obtained in moderate to high yields. Both aromatic and aliphatic amines were successfully used as substrates for the reaction.
Scheme 98.

While ZnCl2 was also used as the catalyst for the amide formation between fatty acids and long-chain amines (Scheme 99), Sugi and co-workers showed that FeCl3 performed slightly better in this transformation [195]. Using 20 mol% of the catalyst, the desired amides were obtained in mainly high yields. The use of the not-green solvent mesitylene was needed to remove water during the reaction to ensure complete conversion of the starting materials.
Scheme 99.

Another amidation reaction, using alcohols and nitriles (Scheme 64) was described in a Ca(II) triflate-catalyzed Ritter reaction (Scheme 100) [196]. The solvent-free microwave-assisted reaction resulted in high yields in short reaction times for a variety of alkyl and aryl substituted alcohols and nitriles. The reaction required the presence of a phase transfer agent (Bu4NPF6) which was thought to be necessary to activate the hydroxyl group.
Scheme 100.

Gu et al. showed that aqueous solutions of gluconic acid acted as a promoting medium for various reactions [197]. Amongst others, the Michael addition of indoles with various α,β-unsaturated compounds (Scheme 101) was performed under milder conditions than previously reported. The reaction medium not only allowed to obtain the products in moderate to excellent yields, but also eased the separation of the product to a simple extraction. The reaction medium could then be reused for several runs without decrease in reaction yield.
Scheme 101.

Simple amine hydrochlorides were demonstrated to be efficient, mild acid catalysts. Pyridium hydrochloride was found to be the catalyst of choice for the syntheses of tungsten tert-butylimido and adamantylimido alkylidene complexes, which are potential alkene methathesis catalysts [198].
The conversion of biomass to produce chemicals is becoming more-and-more attractive due to the need of identifying new non-petroleum based feedstock. While the advantages of using such biodegradable and abundant starting materials are obvious, the methods to transform them often used corrosive mineral acids. Research to develop new protocols offering lower environmental impact is in the forefront of biomass related research.
The cyclization of sorbitol to isosorbide (Scheme 102) was successfully developed in a microwave-assisted transformation in ionic liquids [199]. The best performing IL was N,N,N-trimethyl-N-propylammonium-bis(trifluoromethylsulfonyl)amide ([TMPA][NTf2]). Under optimized conditions the product yield was above 60% when the reaction was performed at 180°C for 10 min with a catalytic amount of p-toluenesulfonic acid. The IL likely improved the nucleophilicity of the substrate. The IL could be recycled for up to five times with only a negligible drop in yields. Although the reaction conditions are mostly green, the recovery process required the use of dichloromethane and thus contributed to the waste generation.
Scheme 102.

In similar processes the microwave-assisted dehydration of sugars by niobium and zirconium phosphates [200] and mesoporous SBA-15 catalysts [201] resulted in the formation of 5-hydroxy-methyl-furfural.
Based on the above surveyed results, the applications of water stable solid acids, although substantial in the reviewed period, probably are less extensive than those with solid acids. The well-known examples, such as heteropoly acids and metal triflates still dominate this field. Most of the new developments occurred in the area of organocatalysis especially in asymmetric organocatalysis. The introduction of the BINOL-based chiral phosphoric acids significantly extended the application possibilities and aided the expansion of this field that was led by chirally modified Lewis acids for an extended period. The future developments must address, however, the significant challenges these new catalysts face, in order to extend their applications, potentially, to the industrial level.
CONCLUSIONS
Recent advances in the application of environmentally benign acid catalysts have been reviewed. Due to the importance and magnitude of the contribution of the acid-catalyzed reactions to the development of sustainable synthetic methods, the topic remains at the forefront of synthesis research. The focus of new developments is finding novel applications for traditional reactions that occur under obsolete and unsustainable conditions. While the directions during the nearly 150 years of acid catalysis substantially changed over the past decades, the applications of acid catalysts will remain an important tool in organic synthesis development. Hence, the continuous development of green acid catalytic processes will likely be one of the major driving forces in the growth of sustainable synthesis development for the preparation of pharmaceuticals, fine chemicals and materials.
ACKNOWLEDGEMENTS
Support of our work by the University of Massachusetts Boston is gratefully acknowledged.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Bell R.P. The use of the terms “acid” and “base”. Q. Rev. Chem. Soc. 1947;1:113–125. [Google Scholar]
- 2.Bell R.P. The Proton in Chemistry. 2nd ed. London: Chapman and Hall; 1973. p. 4. [Google Scholar]
- 3.a Brönsted J.N. Einige Bemerkungen über den Begriff der Säuren und Basen. Recl. Trav. Chim. Pays Bas. 1923;42:718–728. [Google Scholar]; b Lowry T.M. The uniqueness of hydrogen. J. Soc. Chem. Ind. 1923;42:43–47. [Google Scholar]
- 4.Lewis G.N. Valence and the Structure of Atoms and Molecules. Vol. 14. The Chemical Catalog Company, Inc.; 1923. (American Chemical Society Monograph Series). [Google Scholar]
- 5.Yamamoto H., Ishihara K. Acid Catalysis in Modern Organic Synthesis. Weinheim: Wiley-VCH; 2008. [Google Scholar]
- 6.Török B., Dransfield T. Green Chemistry: An inclusive approach. Oxford: Elsevier; 2018. [Google Scholar]
- 7.Molnár Á. Acids and Acid Catalysis – Homogeneous. In: Horvath I.T., editor. Encyclopedia of Catalysis. Vol. 1. New York: Wiley; 2003. pp. 40–86. [Google Scholar]; b Bag S., Dasgupta S., Török B. Microwave-assisted heterogeneous catalysis: An environmentally benign tool for contemporary organic synthesis. Curr. Org. Synth. 2011;8:237–261. [Google Scholar]
- 8.a Clark J.H. Solid acids for green chemistry. Acc. Chem. Res. 2002;35:791–797. doi: 10.1021/ar010072a. [DOI] [PubMed] [Google Scholar]; Gates B.C. Catalysis by Solid Acids. In: Horvath I.T., editor. Encyclopedia of Catalysis. Vol. 2. New York: Wiley; 2003. pp. 104–142. [Google Scholar]; c Gupta P., Mahajan A. Green chemistry approaches as sustainable alternatives to conventional strategies in the pharmaceutical industry. RSC Advances. 2015;5:26686–26705. [Google Scholar]; d Gupta P., Paul S. Solid acids: Green alternatives for acid catalysis. Catal. Today. 2014;236:153–170. [Google Scholar]
- 9.Kokel A., Schäfer C., Török B. Application of microwave-assisted hetero-geneous catalysis in sustainable synthesis design. Green Chem. 2017;19:3729–3751. [Google Scholar]
- 10.Cho H., Schäfer C., Török B. Microwave-assisted solid acid catalysis. In: Horikoshi S., Serpone N., editors. Microwaves in Catalysis – Fundamental Research and Scale-up Technology. Wiley; 2015. pp. 193–213. [Google Scholar]
- 11.Daştan A., Kulkarni A., Török B. Environmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approaches. Green Chem. 2012;14:17–37. [Google Scholar]
- 12.a Wachs I.E., Briand L.E., Jehng J-M. Molecular structure and reactivity of the group V metal oxides. Catal. Today. 2000;57:323–330. [Google Scholar]; b Ushikubo T. Recent topics of research and development of catalysis by niobium and tantalum oxides. Catal. Today. 2000;57:331–338. [Google Scholar]; Hutchings G.J., Bartley J.K., Rhodes C., Taylor S.H., Wells R.P.K., Willock D.J. Metal Oxides. In: Horvath I.T., editor. Encyclopedia of Catalysis. Vol. 4. New York: Wiley; 2003. pp. 602–694. [Google Scholar]
- 13.Gawande M.B., Pandey R.K., Jayaram R.V. Role of mixed metal oxides in catalysis science—versatile applications in organic synthesis. Catal. Sci. Technol. 2012;2:1113–1125. [Google Scholar]
- 14.Venkatesh K.R., Hu J., Dogan C., Tierney J.W., Wender I. Sulfated metal oxides and related solid acids: Comparison of protonic acid strengths. Energy Fuels. 1995;9:888–893. [Google Scholar]
- 15.Arata K. Organic syntheses catalyzed by superacidic metal oxides: Sulfated zirconia and related compounds. Green Chem. 2009;11:1719–1728. [Google Scholar]
- 16.Vaccari A. Clays and catalysis: a promising future. Appl. Clay Sci. 1999;14:161–198. [Google Scholar]
- 17.Balogh M., Laszlo P. Organic Chemistry Using Clays. Berlin: Springer-Verlag; 1993. [Google Scholar]; b Nikalje M.D., Phukan P., Sudalai A. Recent advances in clay-catalyzed organic transformations. Org. Prep. Proced. Int., 2000, 32, 1-40; (d) Varma, R.S. Clay and clay-supported reagents in organic synthesis. Tetrahedron. 2002;58:1235–1255. [Google Scholar]
- 18.Dasgupta S., Török B. Application of clay catalysts in organic synthesis. A review. Org. Prep. Proced. Int. 2008;40:1–65. [Google Scholar]
- 19.Cseri T., Békássy S., Figueras F., Cseke E., de Menorval L-C., Dutartre R. Characterization of clay-based K catalysts and their application in Friedel-Crafts alkylation of aromatics. Appl. Catal. A Gen. 1995;132:141–155. [Google Scholar]
- 20.Nagendrappa G. Organic synthesis using clay and clay-supported catalysts. Appl. Clay Sci. 2011;53:106–138. [Google Scholar]
- 21.Ghadiri M., Chrzanowski W., Rohanizadeh R. Biomedical applications of cationic clay minerals. RSC Advances. 2015;5:29467–29481. [Google Scholar]
- 22.Kumar B.S., Dhakshinamoorthy A., Pitchumani K. K10 montmorillonite clays as environmentally benign catalysts for organic reactions. Catal. Sci. Technol. 2014;4:2378–2396. [Google Scholar]
- 23.Janssens B., Catry P., Claessens R., Baron G., Jacobs P.A. In: Progress in Zeolite and Microporous Materials, Studies in Surface Science and Catalysis, Chon, H.; Ihm, S.-K. Uh Y.S., editor. Vol. 105. Amsterdam: Elsevier; 1997. p. 1211. [Google Scholar]; Csicsery S.M., Kiricsi I. Shape-Selective Catalysis. In: Horvath I.T., editor. Encyclopedia of Catalysis. Vol. 6. New York: Wiley; 2003. pp. 307–338. [Google Scholar]
- 24.Tsapatsis M., Fan W. A new, yet familiar, lamellar zeolite. ChemCatChem. 2010;2:246–248. [Google Scholar]
- 25.Paillaud J., Harbuzaru B., Patarin J., Bats N. Extra-large-pore zeolites with two-dimensional channels formed by 14 and 12 rings. Science. 2004;304:990–992. doi: 10.1126/science.1098242. [DOI] [PubMed] [Google Scholar]
- 26.Busca G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017;254:3–16. [Google Scholar]
- 27.Corma A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003;216:298–312. [Google Scholar]
- 28.a Olah G.A., Iyer P.S., Prakash G.K.S. Perfluorinated resinsulphonic acid (Nafion-H) catalysis in synthesis. Synthesis. 1986:513–531. [Google Scholar]; b Prakash G.K.S., Olah G.A. Acid-Base Catalysis. Tanabe, K.; Hattori, H.; Yamaguchi, T.; Tanaka T. Eds.; Kodansha: Tokyo; 1989. p. 59. [Google Scholar]; c Molnár Á. Nafion-silica nanocomposites: A new generation of water-tolerant solid acids of high efficiency. Curr. Org. Chem. 2008;12:159–181. [Google Scholar]
- 29.Xu Y., Gu W., Gin D. Heterogeneous catalysis using a nanostructured solid acid resin based on lyotropic liquid crystals. J. Am. Chem. Soc. 2004;126:1616–1617. doi: 10.1021/ja038501i. [DOI] [PubMed] [Google Scholar]
- 30.Kidwai M., Chauhan R., Bhatnagar S. Nafion-H (R): A versatile catalyst for organic synthesis. Curr. Org. Chem. 2015;19:72–98. [Google Scholar]
- 31.a Harmer M.A., Farneth W.E., Sun Q. High surface area nafion resin/silica nanocomposites: A new class of solid acid catalyst. J. Am. Chem. Soc. 1996;118:7708–7715. [Google Scholar]; b Török B., Kiricsi I., Molnár Á., Olah G.A. Acidity and catalytic activity of nafion-H/silica nanocomposite catalyst and a comparison with silica-supported perfluorinated acids. J. Catal. 2000;193:132–138. [Google Scholar]
- 32.a Li H., Eddaoudi M., O’Keeffe M., Yaghi O.M. Design and synthesis of an exceptionally stable and highly porous metal–organic framework. Nature. 1999;402:276–279. [Google Scholar]; b Eddaoudi M., Kim J., Rosi N., Vodak D., Wachter J., O’Keeffe M., Yaghi O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science. 2002;295:469–472. doi: 10.1126/science.1067208. [DOI] [PubMed] [Google Scholar]
- 33.Zhou H., Kitagawa S. Metal-organic frameworks (MOFs). Chem. Soc. Rev. 2014;43:5415–5418. doi: 10.1039/c4cs90059f. [DOI] [PubMed] [Google Scholar]
- 34.Liang W., D’Alessandro D.M. Microwave-assisted solvothermal synthesis of zirconium oxide based metal-organic frameworks. Chem. Commun. (Camb.) 2013;49:3706–3708. doi: 10.1039/c3cc40368h. [DOI] [PubMed] [Google Scholar]
- 35.Corma A., Garcia H., Llabres i Xamena F.X. Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 2010;110:4606–4655. doi: 10.1021/cr9003924. [DOI] [PubMed] [Google Scholar]
- 36.Yoon M., Srirambalaji R., Kim K. Homochiral metal-organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 2012;112:1196–1231. doi: 10.1021/cr2003147. [DOI] [PubMed] [Google Scholar]
- 37.Liu J., Lukose B., Shekhah O., Arslan H.K., Weidler P., Gliemann H., Braese S., Grosjean S., Godt A., Feng X., Muellen K., Magdau I., Heine T., Woell C. A novel series of isoreticular metal organic frameworks: realizing metastable structures by liquid phase epitaxy. Sci. Rep. 2012;2:921–936. doi: 10.1038/srep00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hara M., Yoshida T., Takagaki A., Takata T., Kondo J.N., Domen K., Hayashi S.A. A carbon material as a strong protonic acid. Angew. Chem. Int. Ed. 2004;43:2955–2958. doi: 10.1002/anie.200453947. [DOI] [PubMed] [Google Scholar]
- 39.Suganuma S., Nakajima K., Kitano M., Yamaguchi D., Kato H., Hayashi S., Hara M. Synthesis and acid catalysis of cellulose derived carbon-based solid acid. Solid State Sci. 2010;12:1029–1034. [Google Scholar]
- 40.Jamwal N., Sodhi R.K., Gupta P., Paul S. Nano Pd(0) supported on cellulose: A highly efficient and recyclable heterogeneous catalyst for the Suzuki coupling and aerobic oxidation of benzyl alcohols under liquid phase catalysis. Int. J. Biol. Macromol. 2011;49:930–935. doi: 10.1016/j.ijbiomac.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 41.a Chrobok A., Baj S., Pudło W., Jarzebski A. Supported hydrogensulfate ionic liquid catalysis in Baeyer–Villiger reaction. Appl. Catal. A Gen. 2009;366:22–28. [Google Scholar]; b Sugimura R., Qiao K., Tomida D., Yokoyama C. Immobilization of acidic ionic liquids by copolymerization with styrene and their catalytic use for acetal formation. Catal. Commun. 2007;8:770–772. [Google Scholar]; c Amarasekara A.S., Owereh O.S. Synthesis of a sulfonic acid functionalized acidic ionic liquid modified silica catalyst and applications in the hydrolysis of cellulose. Catal. Commun. 2010;11:1072–1075. [Google Scholar]
- 42.Gupta P., Kour M., Paul S., Clark J.H. Ionic liquid coated sulfonated carbon/silica composites: novel heterogeneous catalysts for organic syntheses in water. RSC Advances. 2014;4:7461–7470. [Google Scholar]
- 43.a Nakajima K., Okamura M., Kondo J.N., Domen K., Tatsumi T., Hayashi S., Hara M. Amorphous carbon bearing sulfonic acid groups in mesoporous silica as a selective catalyst. Chem. Mater. 2009;21:186–193. [Google Scholar]; b De Vyver S.V. Peng, L.; Geboers, J.; Schepers, H.; Clippel, F. de.; Gommes, C.J.; Goderis, B.; Jacobs, P.A.; Sels, B.F. Sulfonated silica/carbon nanocomposites as novel catalysts for hydrolysis of cellulose to glucose. Green Chem. 2010;12:1560–1563. [Google Scholar]
- 44.Gupta P., Paul S. Sulfonated carbon/silica composites: highly efficient heterogeneous catalysts for the one-pot synthesis of hantzsch 1,4-dihydropyridines, 2,4,5-trisubstituted imidazoles and 2-arylbenzimidazoles. Curr. Catal. 2014;3:53–64. [Google Scholar]
- 45.Gupta P., Kumar V., Paul S. Silica functionalized sulfonic acid catalyzed one-pot synthesis of 4,5,8a-triarylhex-ahydropyrimido[4,5-d]pyrimidine-2,7(1H,3H)-diones under liquid phase catalysis. J. Braz. Chem. Soc. 2010;21:349–354. [Google Scholar]
- 46.Gupta P., Paul S. Sulfonated carbon/silica composite functionalized Lewis acids for one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles, 3,4-dihydropyrimidin-2(1H)-ones and for Michael addition of indole to α,β-unsaturated ketones. J. Mol. Catal. Chem. 2012;352:75–80. [Google Scholar]
- 47.Gupta P., Paul S. Amorphous carbon-silica composites bearing sulfonic acid as solid acid catalysts for the chemoselective protection of aldehydes as 1,1-diacetates and for N-, O- and S-acylations. Green Chem. 2011;13:2365–2372. [Google Scholar]
- 48.Chen Z., Zhong W., Tang D., Zhang G. Preparation of organic nanoacid catalyst for urethane formation. Chin. J. Chem. Phys. 2017;30:339–342. [Google Scholar]
- 49.Liu T., Imber B., Diemann E., Liu G., Cokleski K., Li H., Chen Z., Müller A. Deprotonations and charges of well-defined {Mo72Fe30} nanoacids simply stepwise tuned by pH allow control/ variation of related self-assembly processes. J. Am. Chem. Soc. 2006;128:15914–15920. doi: 10.1021/ja066133n. [DOI] [PubMed] [Google Scholar]
- 50.Kozhevnikov I.V. Catalysis by Polyoxometalates. Chichester: Wiley; 2002. [Google Scholar]; b Kozhevnikov I.V. Friedel–Crafts acylation and related reactions catalysed by heteropoly acids. Appl. Catal. A Gen. 2003;256:3–18. [Google Scholar]; Misono M. Heteropoly Acids. In: Horvath I.T., editor. Encyclopedia of Catalysis. Vol. 3. New York: Wiley; 2003. pp. 433–447. [Google Scholar]
- 51.Contreras Coronel N., da Silva M.J. Lacunar keggin heteropolyacid salts: soluble, solid and solid-supported catalysts. J. Cluster Sci. 2018;29:195–205. [Google Scholar]
- 52.Heravi M.M., Sadjadi S., Oskooie H.A., Shoar R.H., Bamoharram F.F. Heteropolyacids as heterogeneous and recyclable catalysts for the synthesis of benzimidazoles. Catal. Commun. 2008;9:504–507. [Google Scholar]
- 53.Micek-Ilnicka A. The role of water in the catalysis on solid heteropolyacids. J. Mol. Catal. Chem. 2009;308:1–14. [Google Scholar]
- 54.Sathicq A.G., Romanelli G.P., Palermo V., Vazquez P.G., Thomas H.J. Heterocyclic amine salts of Keggin heteropolyacids used as catalyst for the selective oxidation of sulfides to sulfoxides. Tetrahedron Lett. 2008;49:1441–1444. [Google Scholar]
- 55.Pope M.T. Heteropoly and Isopoly Oxometalates. Springer; 1983. [Google Scholar]
- 56.a Vila-Nadal L., Cronin L. Design and synthesis of polyoxometalate-framework materials from cluster precursors. Nat. Rev. Mater. 2017;2:17054. [Google Scholar]; b Gumerova N.I., Rompel A. Synthesis, structures and applications of electron-rich polyoxometalates. Nat. Rev. Chem. 2018;2:0112. [Google Scholar]
- 57.Kobayashi S. Rare earth metal trifluoromethanesulfonates as water-tolerant lewis acid catalysts in organic synthesis. Synlett, 1994, 689-701; (b) Kobayashi, S.; Sugiura, M.; Kitagawa, H.; Lam, W.W.-L. Rare-earth metal triflates in organic synthesis. Chem. Rev. 2002;102:2227–2302. doi: 10.1021/cr010289i. [DOI] [PubMed] [Google Scholar]
- 58.Manabe K., Kobayashi S. Catalytic asymmetric carbon–carbon bond‐forming reactions in aqueous media. Chem. Eur. J., 2002, 8, 4094-4101; (b) Shen, K.; Liu, X.; Lin, L.; Feng, X. Recent progress in enantioselective synthesis of C3-functionalized oxindoles: Rare earth metals take action. Chem. Sci. (Camb.) 2012;3:327–334. doi: 10.1002/1521-3765(20020916)8:18<4094::AID-CHEM4094>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 59.Mishra A.K., Biswas S. Brønsted acid catalyzed functionalization of aromatic alcohols through nucleophilic substitution of hydroxyl group. J. Org. Chem., 2016, 81, 2355-2363; (b) Bovonsombat, P.; Ali, R.; Khan, C.; Leykajarakul, J.; Pla-on, K.; Aphimanchindakul, S.; Pungcharoenpong, N.; Timsuea, N.; Arunrat, A.; Punpongjareorn, N. Facile p-toluenesulfonic acid-promoted para-selective monobromination and chlorination of phenol and analogues. Tetrahedron. 2010;66:6928–6935. [Google Scholar]
- 60.a Goldberg S.I., Miller N.C. Asymmetric selection during dehydration of achiral alcohols in the presence of (+)-camphorsulfonic acid. J. Chem. Soc. Chem. Commun. 1969:1409–1410. [Google Scholar]; b Liu C., Lu Y. Primary amine/(+)-CSA salt-promoted organocatalytic conjugate addition of nitro esters to enones. Org. Lett. 2010;12:2278–2281. doi: 10.1021/ol1006407. [DOI] [PubMed] [Google Scholar]
- 61.a Akiyama T., Itoh J., Yokota K., Fuchibe K. Enantioselective Mannich-type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. 2004;43:1566–1568. doi: 10.1002/anie.200353240. [DOI] [PubMed] [Google Scholar]; b Uraguchi D., Terada M. Chiral Brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 2004;126:5356–5357. doi: 10.1021/ja0491533. [DOI] [PubMed] [Google Scholar]
- 62.a Cheng X., Goddard R., Buth G., List B. Direct catalytic asymmetric three-component Kabachnik-Fields reaction. Angew. Chem. Int. Ed. 2008;47:5079–5081. doi: 10.1002/anie.200801173. [DOI] [PubMed] [Google Scholar]; b Seayad J., Seayad A.M., List B. Catalytic asymmetric Pictet-Spengler reaction. J. Am. Chem. Soc. 2006;128:1086–1087. doi: 10.1021/ja057444l. [DOI] [PubMed] [Google Scholar]; c Terada M., Sorimachi K. Enantioselective Friedel-Crafts reaction of electron-rich alkenes catalyzed by chiral Brønsted acid. J. Am. Chem. Soc. 2007;129:292–293. doi: 10.1021/ja0678166. [DOI] [PubMed] [Google Scholar]; d Chen X-H., Xu X-Y., Liu H., Cun L-F., Gong L-Z. Highly enantioselective organocatalytic Biginelli reaction. J. Am. Chem. Soc. 2006;128:14802–14803. doi: 10.1021/ja065267y. [DOI] [PubMed] [Google Scholar]
- 63.Olah G.A. Friedel-Crafts and Related Reactions. New York: Wiley; 1964. [Google Scholar]; Olah G.A. Friedel-Crafts Chemistry. New York: Wiley-Interscience; 1973. [Google Scholar]
- 64.Dasgupta S., Török B. Environmentally benign contemporary friedel-crafts chemistry by solid acids. Curr. Org. Synth. 2008;5:321–342. [Google Scholar]
- 65.El-Hiti G.A., Smith K., Hegazy A.S. Catalytic, green and regioselective friedel-crafts acylation of simple aromatics and heterocycles over zeolites. Curr. Org. Chem. 2015;19:585–598. [Google Scholar]
- 66.Bernardon C., Ben Osman M., Laugel G., Louis B., Pale P. Acidity versus metal-induced Lewis acidity in zeolites for Friedel-Crafts acylation. Compt. Rend. Chim. 2017;20:20–29. [Google Scholar]
- 67.Khder A.R.S., Hassan H.M.A., El-Shall M.S. Metal-organic frameworks with high tungstophosphoric acid loading as heterogeneous acid catalysts. Appl. Catal. A Gen. 2014;487:110–118. [Google Scholar]
- 68.Yadav G.D. Kamble, S.B. Atom efficient Friedel-Crafts acylation of toluene with propionic anhydride over solid mesoporous superacid UDCaT-5. Appl. Catal. A Gen. 2012;433-434:265–274. [Google Scholar]
- 69.Kulkarni A., Quang P., Török B. Microwave-assisted solid acid-catalyzed Friedel-Crafts alkylation and electrohylic annulation of indoles using alcohols as alkylating agents. Synthesis. 2009:4010–4014. [Google Scholar]
- 70.Cirujano F.G., Stalpaert M., De Vos D.E. Ionic liquids vs. microporous solids as reusable reaction media for the catalytic C-H functionalization of indoles with alcohols. Green Chem. 2018;20:2481–2485. [Google Scholar]
- 71.Wen J., Qi H., Kong X., Chen L., Yan X. Hydroarylation of styrenes with electron-rich arenes over acidic ion-exchange resins. Synth. Commun. 2014;44:1893–1903. [Google Scholar]
- 72.Prakash S.G.K., Fogassy G., Olah G.A. Microwave-assisted nafion-h catalyzed friedel-crafts type reaction of aromatic aldehydes with arenes: Synthesis of triarylmethanes. Catal. Lett. 2010;138:155–159. [Google Scholar]
- 73.Zhang D.W., Zhang Y.M., Zhang Y.L., Zhao T.Q., Liu H.W., Gan Y.M., Gu Q. Efficient solvent-free synthesis of bis(indolyl)methanes on SiO2 solid support under microwave irradiation. Chem. Pap. 2015;69:470–478. [Google Scholar]
- 74.An L., Zhang L., Zou J., Zhang G. Montmorillonite K10: Catalyst for friedel-crafts alkylation of indoles and pyrrole with nitroalkenes under solventless conditions. Synth. Commun. 2010;40:1978–1984. [Google Scholar]
- 75.Hechelski M., Ghinet A., Louvel B., Dufrenoy P., Rigo B., Daich A., Waterlot C. From conventional lewis acids to heterogeneous montmorillonite K10: Eco-friendly plant-based catalysts used as green lewis acids. ChemSusChem. 2018;11:1249–1277. doi: 10.1002/cssc.201702435. [DOI] [PubMed] [Google Scholar]
- 76.Losfeld G., Escande V., de La Blache P.V., L’Huillier L., Grison C. Design and performance of supported Lewis acid catalysts derived from metal contaminated biomass for Friedel-Crafts alkylation and acylation. Catal. Today. 2012;189:111–116. [Google Scholar]
- 77.Zhu C., Mao Q., Li D., Li C., Zhou Y., Wu X., Luo Y., Li Y. A readily available urea based MOF that act as a highly active heterogeneous catalyst for Friedel-Crafts reaction of indoles and nitrostryenes. Catal. Commun. 2018;104:123–127. [Google Scholar]
- 78.Rao P.C., Mandal S. Friedel-Crafts alkylation of indoles with nitroalkenes through hydrogen-bond-donating metal-organic framework. ChemCatChem. 2017;9:1172–1176. [Google Scholar]
- 79.Abid M., Török B. Synthesis of N-heteroaryl-trifluoromethyl-hydroxyl alkanoic acid esters by highly efficient solid acid catalyzed hydroxyalkylation of indoles and pyrroles with activated trifluoromethyl ketones. Adv. Synth. Catal. 2005;347:1797–1803. [Google Scholar]
- 80.a Török M., Abid M., Mhadgut S.C., Török B. Organofluorine inhibitors of amyloid fibrillogenesis. Biochemistry. 2006;45:5377–5383. doi: 10.1021/bi0601104. [DOI] [PubMed] [Google Scholar]; b Török B., Sood A., Bag S., Kulkarni A., Borkin D., Lawler E., Dasgupta S., Landge S.M., Abid M., Zhou W., Foster M., LeVine H., III, Török M. Structure-activity relationship of organofluorine inhibitors of amyloid-beta self-assembly. ChemMedChem. 2012;7:910–919. doi: 10.1002/cmdc.201100569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith K., El-Hiti G.A. Use of zeolites for greener and more para-selective electrophilic aromatic substitution reactions. Green Chem. 2011;11:1579–1608. [Google Scholar]
- 82.Mohan R.B., Reddy N.C.G. Regioselective α-bromination of aralkyl ketones using N-bromosuccinimide in the presence of montmorillonite K-10 clay: a simple and efficient method. Synth. Commun. 2013;43:2603–2614. [Google Scholar]
- 83.Kumar M.S., Sriram Y.H., Venkateswarlu M., Rajanna K.C., Sudhakar M.S., Venkana P., Saiprakash P.K. Silica-supported perchloric acid and potassium bisulfate as reusable green catalysts for nitration of aromatics under solvent-free microwave conditions. Synth. Commun. 2017;48:59–67. [Google Scholar]
- 84.Mackie R.K., Smith D.M., Aitken R.A. Guidebook to Organic Synthesis. Harlow: Pearson; 1999. p. 69. [Google Scholar]
- 85.Abid M., Savolainen M., Landge S., Hu J., Prakash G.K.S., Olah G.A., Török B. Synthesis of trifluoromethyl-imines by solid acid/superacid catalyzed microwave assisted approach. J. Fluor. Chem. 2007;128:587–594. doi: 10.1016/j.jfluchem.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dasgupta S., Morzhina E., Schäfer C., Mhadgut S.C., Prakash G.K.S., Török B. Synthesis of chiral trifluoromethyl benzylamines by heterogeneous catalytic reductive amination. Top. Catal. 2016;59:1207–1213. [Google Scholar]
- 87.Brun E., Safer A., Carreaux F., Bourahla K., L’helgoua’ch J.M., Bazureau J.P., Villalgordo J.M. Microwave-assisted condensation reactions of acetophenone derivatives and activated methylene compounds with aldehydes catalyzed by boric acid under solvent-free conditions. Molecules. 2015;20:11617–11631. doi: 10.3390/molecules200611617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rocchi D., González J.F., Menéndez J.C. Montmorillonite clay-promoted, solvent-free cross-aldol condensations under focused microwave irradiation. Molecules. 2014;19:7317–7326. doi: 10.3390/molecules19067317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Varghese A., Nizam A., Kulkarni R., George L. Amberlite IR‐120H: An improved reusable solid phase catalyst for the synthesis of nitriles under solvent free microwave irradiation. Eur. J. Chem. 2012;3:247–251. [Google Scholar]
- 90.Biggs-Houck J.E., Younai A., Shaw J.T. Recent advances in multicomponent reactions for diversity oriented synthesis. Curr. Opin. Chem. Biol. 2010;14:371–382. doi: 10.1016/j.cbpa.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 91.De Paolis O., Baffoe J., Landge S.M., Török B. Multicomponent domino cyclization-oxidative aromatization on a bifunctional Pd/C/K-10 catalyst: An environmentally benign approach toward the synthesis of pyridines. Synthesis. 2008:3423–3428. [Google Scholar]
- 92.Kulkarni A., Török B. Microwave-assisted multicomponent domino cyclization-aromatization: An efficient approach for the synthesis of substituted quinolines. Green Chem. 2010;12:875–878. [Google Scholar]
- 93.Shinde V.V., Lee S.D., Jeong Y.S., Jeong Y.T. p-Toluenesulfonic acid doped polystyrene (PS-PTSA): solvent-free microwave assisted cross-coupling-cyclization–oxidation to build one-pot diversely functionalized pyrrole from aldehyde, amine, active methylene, and nitroalkane. Tetrahedron Lett. 2015;56:859–865. [Google Scholar]
- 94.Heck R.F. Acylation, methylation, and carboxyalkylation of olefins by Group VIII metal derivatives. J. Am. Chem. Soc. 1968;90:5518–5526. [Google Scholar]
- 95.Molnár Á. Palladium-Catalyzed Coupling Reactions: Practical Aspects and Future Developments. Weinheim: Wiley-VCH; 2013. [Google Scholar]
- 96. https://www.nobelprize.org/nobel_prizes/chemistry/laureates/2010/ (accessed on 09/28/2018).
- 97.Pandey G., Török B. K-10 montmorillonite-catalyzed solid phase diazotizations: environmentally benign coupling of diazonium salts with aromatic hydrocarbons to biaryls. Green Chem. 2017;19:2515–2519. [Google Scholar]
- 98.Prakash G.K.S., Glinton K.E., Panja C., Gurung L., Battamack P.T., Török B., Mathew T., Olah G.A. Thermocontrolled benzylimine–benzaldimine rearrangement over Nafion-H catalysts for efficient entry into α-trifluoromethylbenzylamines. Tetrahedron Lett. 2012;53:607–611. [Google Scholar]
- 99.Mackie R.K. Smith, D.M., Aitken, R.A. Guidebook to Organic Synthesis. Harlow: Pearson; 1999. p. 120. [Google Scholar]
- 100.Landge S.M., Schmidt A., Outerbridge V., Török B. Synthesis of pyrazoles by a one-pot tandem cyclization-dehydrogenation approach on Pd/C/K-10 catalyst. Synlett. 2007:1600–1604. [Google Scholar]
- 101.Landge S.M., Berryman M., Török B. Microwave-assisted solid acid-catalyzed one-pot synthesis of isobenzofuran-1(3H)-ones. Tetrahedron Lett. 2008;49:4505–4508. [Google Scholar]
- 102.Outerbridge V.M., Landge S.M., Tamaki H., Török B. Microwave-promoted solid-acid-catalyzed one pot synthesis of phthalazinones. Synthesis. 2009;2009:1801–1806. [Google Scholar]
- 103.Abid M., De Paolis O., Török B. A novel one-pot synthesis of N-acylindoles from primary aromatic amides. Synlett. 2008;2008:410–413. [Google Scholar]
- 104.Abid M., Landge S.M., Török B. An efficient and rapid synthesis of N-pyrroles by microwave-assisted solid acid catalysis. Org. Prep. Proced. Int. 2006;38:495–500. [Google Scholar]
- 105.Rudnitskaya A., Borkin D.A., Huynh K., Török B., Stieglitz K. Rational design, synthesis and potency of N-substituted indoles, pyrroles and triarylpyrazoles as potential fructose 1,6-biphosphatase inhibitors. ChemMedChem. 2010;5:384–389. doi: 10.1002/cmdc.200900493. [DOI] [PubMed] [Google Scholar]
- 106.Landge S.M., Török B. Synthesis of condensed benzo[N,N]-heterocycles by microwave-assisted solid acid catalysis. Catal. Lett. 2008;122:338–343. [Google Scholar]
- 107.Kulkarni A., Abid M., Török B., Huang X. A direct synthesis of β-carbolines via a three-step one-pot domino approach with a bifunctional Pd/C/K-10 catalyst. Tetrahedron Lett. 2009;50:1791–1794. [Google Scholar]
- 108.Horton W., Sood A., Peerannawar S., Kugyela N., Kulkarni A., Tulsan R., Tran C.D., Soule J., LeVine H., III, Török B., Török M. Synthesis and application of β-carbolines as novel multi-functional anti- Alzheimer’s disease agents. Bioorg. Med. Chem. Lett. 2017;27:232–236. doi: 10.1016/j.bmcl.2016.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.De Paolis O., Teixeira L., Török B. Synthesis of quinolines by a solid acid-catalyzed microwave-assisted domino cyclization-aromatization approach. Tetrahedron Lett. 2009;50:2939–2942. [Google Scholar]
- 110.Kokel A., Török B. Microwave-assisted solid phase diazotation: a method for the environmentally benign synthesis of benzotriazoles. Green Chem. 2017;19:5390–5395. [Google Scholar]
- 111.Borkin D.A., Puscau M., Carlson A., Solan A., Wheeler K.A., Török B., Dembinski R. Synthesis of diversely 1,3,5-trisubstituted pyrazoles via 5-exo-dig cyclization. Org. Biomol. Chem. 2012;10:4505–4508. doi: 10.1039/c2ob25580d. [DOI] [PubMed] [Google Scholar]
- 112.Cho H., Török F., Török B. Energy efficiency of heterogeneous catalytic microwave-assisted organic reactions. Green Chem. 2014;16:3623–3634. [Google Scholar]
- 113.Yadav A., Biswas S., Mobin S.M., Samanta S. Efficient Cu(OTf)2-catalyzed and microwave-assisted rapid synthesis of 3,4-fused chromenopyridinones under neat conditions. Tetrahedron Lett. 2017;58:3634–3639. [Google Scholar]
- 114.Ghodke S., Chudasama U. Solvent free synthesis of coumarins using environment friendly solid acid catalysts. Appl. Catal. A Gen. 2013;453:219–226. [Google Scholar]
- 115.Chavan O.S., Shioorkar M.G., Jadhav S.A., Sakhare M.A., Pawar Y.M., Shivaji B., Chavan S.B., Baseer M.A. Envirocat EPZ-10: An efficient catalyst for synthesis of coumarins by Pechmann reactin under solvent free microwave irradiation method. Heterocyclic Lett. 2017;7:377–380. [Google Scholar]
- 116.Babu M., Pitchumani K., Ramesh P. An expeditious synthesis of flavonols promoted by montmorillonite KSF clay and assisted by microwave irradiation under solvent-free conditions. Helv. Chim. Acta. 2013;96:1269–1272. [Google Scholar]
- 117.Borkin D.A., Carlson A., Török B. K-10-catalyzed highly diastereoselective synthesis of aziridines. Synlett. 2010:745–748. [Google Scholar]
- 118.Mohsenzadeh F., Aghapoor K., Darabi H.R., Jalali M.R., Halvagar M.R. Greener aminolysis of epoxides on BiCl3/SiO2. Compt. Rend. Chim. 2016;19:978–985. [Google Scholar]
- 119.Zhu R., Jiang J-L., Li X-L., Deng J., Fu Y. A comprehensive study on metal triflate-promoted hydrogenolysis of lactones to carboxylic acids: From synthetic and mechanistic perspectives. ACS Catal. 2017;7:7520–7528. [Google Scholar]
- 120.Gowda R.R., Chakraborty D. Environmentally benign process for bulk ring opening polymerization of lactones using iron and ruthenium chloride catalysts. J. Mol. Catal. Chem. 2009;301:84–92. [Google Scholar]
- 121.Kernbichl S., Reiter M., Bucalon D.H., Altmann P.J., Kronast A., Rieger B. Synthesis of lewis acidic, aromatic aminotroponiminate zinc complexes for the ring-opening polymerization of cyclic esters. Inorg. Chem. 2018;57:9931–9940. doi: 10.1021/acs.inorgchem.8b01060. [DOI] [PubMed] [Google Scholar]
- 122.Nguyen H.T.H., Short G.N., Qi P., Miller S.A. Copolymerization of lactones and bioaromatics via concurrent ring-opening polymerization/ polycondensation. Green Chem. 2017;19:1877–1889. [Google Scholar]
- 123.Kuźniarska-Biernacka I., Pereira C., Carvalho A.P., Pires J., Freire C. Epoxidation of olefins catalyzed by manganese(III) salen complexes grafted to porous heterostructured clays. Appl. Clay Sci. 2011;53:195–203. [Google Scholar]
- 124.Song F., Wang C., Falkowski J.M., Ma L., Lin W. Isoreticular chiral metal-organic frameworks for asymmetric alkene epoxidation: tuning catalytic activity by controlling framework catenation and varying open channel sizes. J. Am. Chem. Soc. 2010;132:15930–15938. doi: 10.1021/ja1069773. [DOI] [PubMed] [Google Scholar]
- 125.Huang J., Ding W., Cai J. Heterogeneous Jacobsen’s catalyst on alkoxyl‐modified zirconium poly (styrene‐phenylvinylphospho-nate)‐phosphate (ZPS‐PVPA) for asymmetric epoxidation. Appl. Organomet. Chem. 2017;31:e3861 [Google Scholar]
- 126.Ren Y., Cheng X., Yang S., Qi C., Jiang H., Mao Q. A chiral mixed metal–organic framework based on a Ni(saldpen) metalloligand: synthesis, characterization and catalytic performances. Dalton Trans. 2013;42:9930–9937. doi: 10.1039/c3dt50664a. [DOI] [PubMed] [Google Scholar]
- 127.Xia Q., Li Z., Tan C., Liu Y., Gong W., Cui Y. Y. Multivariate metal−organic frameworks as multifunctional heterogeneous asymmetric catalysts for sequential reactions. J. Am. Chem. Soc. 2017;139:8259–8266. doi: 10.1021/jacs.7b03113. [DOI] [PubMed] [Google Scholar]
- 128.Li J., Ren Y., Qi C., Jiang H. The first porphyrin–salen based chiral metal–organic framework for asymmetric cyanosilylation of aldehydes. Chem. Commun. (Camb.) 2017;53:8223–8226. doi: 10.1039/c7cc03499g. [DOI] [PubMed] [Google Scholar]
- 129.Dong J., Liu Y., Cui Y. Chiral porous organic frameworks for asymmetric heterogeneous catalysis and gas chromatographic separation. Chem. Commun. (Camb.) 2014;50:14949–14952. doi: 10.1039/c4cc07648f. [DOI] [PubMed] [Google Scholar]
- 130.Peng C., Lu X-H., Ma X-T., Shen Y. Wei, C.-C.; He, J.; Zhou, D.; Xia, Q.-H. Highly efficient epoxidation of cyclohexene with aqueous H2O2 over powdered anion-resin supported solid catalysts. J. Mol. Catal. Chem. 2016;423:393–399. [Google Scholar]
- 131.Shen Y., Lu X-H., Wei C-C., Ma X-T., Peng C., He J., Zhou D., Xia Q-H. Highly selective mono-epoxidation of dicyclopentadiene with aqueous H2O2 over heterogeneous peroxo-phosphotungstic catalysts. Mol. Catal. 2017;433:185–192. [Google Scholar]
- 132.Schäfer C., Ellstrom C.J., Török B. Heterogeneous catalytic aqueous phase oxidative cleavage of styrenes to benzaldehydes: An environmentally benign alternative to ozonolysis. Top. Catal. 2018;61:643–651. [Google Scholar]
- 133.Landge S.M., Atanassova V., Thimmaiah M., Török B. Microwave-assisted oxidative coupling of amines to imines on solid acid catalysts. Tetrahedron Lett. 2007;48:5161–5164. [Google Scholar]
- 134.Atanassova V., Ganno K., Kulkarni A., Landge S.M., Curtis S., Foster M., Török B. Mechanistic study on the oxidative coupling of amines to imines on K-10 montmorillonite. Appl. Clay Sci. 2011;53:220–226. [Google Scholar]
- 135.Fabian L., Gómez M., Kuran J.A.C., Moltrasio G., Moglioni A. Efficient microwave-assisted esterification reaction employing methanesulfonic acid supported on alumina as catalyst. Synth. Commun. 2014;44:2386–2392. [Google Scholar]
- 136.a Gallo J.M.R., Trapp M.A. The chemical conversion of biomass-derived saccharides: An overview. J. Braz. Chem. Soc. 2017;28:1586–1607. [Google Scholar]; b De S., Duttab S., Saha B. Critical design of heterogeneous catalysts for biomass valorization: current thrust and emerging prospects. Catal. Sci. Technol. 2016;6:7364–7385. [Google Scholar]; c Chatterjee C., Pong F., Sen A. Chemical conversion pathways for carbohydrates. Green Chem. 2015;17:40–71. [Google Scholar]; d Pagan-Torres Y.J., Gallo J.M.R., Wang D., Pham H.N., Libera J.A., Marshall C.L., Elam J.W., Datye A.K., Dumesic J.A. Synthesis of highly ordered hydrothermally stable mesoporous niobia catalysts by atomic layer deposition. ACS Catal. 2011;1:1234–1245. [Google Scholar]
- 137.Nishimura S.C. Paris. France: European Patent Office. European Patent No EP 1 123 939 B1. 2004. [Google Scholar]
- 138.Perrier A., Keller M., Caminade A., Majoral J., Ouali A. Efficient and recyclable rare earth-based catalysts for Friedel-Crafts acylations under microwave heating: dendrimers show the way. Green Chem. 2013;15:2075–2080. [Google Scholar]
- 139.Tran P.H., Nguyen H.T., Hansen P.E., Le T.N. Greener Friedel-Crafts acylation using microwave-enhanced reactivity of bismuth triflate in the Friedel-Crafts benzoylation of aromatic compounds with benzoic anhydride. Chem. Select. 2017;2:571–575. [Google Scholar]
- 140.Abid M., Teixeira L., Török B. Triflic acid controlled successive annelation of aromatic sulfonamides: An efficient one-pot synthesis of N-sulfonyl pyrroles, indoles and carbazoles. Tetrahedron Lett. 2007;48:4047–4050. doi: 10.1016/j.tetlet.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abid M., Teixeira L., Török B. Triflic acid-catalyzed highly stereoselective friedel-crafts aminoalkylation of indoles and pyrroles. Org. Lett. 2008;10:933–935. doi: 10.1021/ol703095d. [DOI] [PubMed] [Google Scholar]
- 142.Mendoza F., Ruíz-Guerrero R., Hernández-Fuentes C., Molina P., Norzagaray-Campos M., Reguera E. On the bromination of aromatics, alkenes and alkynes using alkylammonium bromide: Towards the mimic of bromoperoxidases reactivity. Tetrahedron Lett. 2016;57:5644–5648. [Google Scholar]
- 143.Heravi M.M., Bakhtiari K., Benmorad T., Bamoharram F.F., Oskooie H., Tehrani M.H. Nitration of aromatic compounds catalyzed by divanadium substituted molybdophosphoric acid H5[PMo10V2O40]. Monatsh. Chem. 2007;138:449–452. [Google Scholar]
- 144.Zhu W., Liu Y., Niu G., Wang Q., Li J., Zhu X., Li Y., Xue H., Yang G. First application of bismuth triflate as an efficient, non-transition metallic and reusable catalyst for aromatic nitration. Catal. Commun. 2012;29:145–148. [Google Scholar]
- 145.Qian H., Wang Y., Liu D., Lv C. Bismuth triflate catalyzed mononitration of aromatic compounds with N2O5. Lett. Org. Chem. 2014;11:509–512. [Google Scholar]
- 146.Zare A., Yousofi T., Moosavi-Zare A.R. Ionic liquid 1,3-disulfonic acid imidazolium hydrogen sulfate: a novel and highly efficient catalyst for the preparation of 1-carbamatoalkyl-2-naphthols and 1-amidoalkyl-2-naphthols. RSC Advances. 2012;2:7988–7991. [Google Scholar]
- 147.Mohammadi S., Abbasi M. Design of ionic liquid sulfonic acid pyridinium hydrogen sulfate as an efficient, eco-friendly, and reusable catalyst for one-pot synthesis of highly functionalized tetrahydropyridines. Res. Chem. Intermed. 2015;41:8877–8890. [Google Scholar]
- 148.Rahmatpour A. Polyvinylsulfonic acid: An efficient, water-soluble and reusable Brønsted acid catalyst for the three-component synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones in water and ethanol. Catal. Lett. 2012;142:1505–1511. [Google Scholar]
- 149.Yanai H., Sakiyama T., Oguchi T., Taguchi T. Four component reaction of aldehydes, isocyanides, Me3SiN3, and aliphatic alcohols catalyzed by indium triflate. Tetrahedron Lett. 2012;53:3161–3164. [Google Scholar]
- 150.Bhattacharjee D., Sutradhar D., Chandra A.K., Myrboh B. L-proline as an efficient asymmetric induction catalyst in the synthesis of chromeno[2,3-d]pyrimidine-triones, xanthenes in water. Tetrahedron. 2017;73:3497–3504. [Google Scholar]
- 151.Seyyedhamzeh M., Shaabani S., Sangachin M.H., Shaabani A. Guanidinium-based sulfonic acid as a new Bronsted acid organocatalyst in organic synthesis in water. Res. Chem. Intermed. 2016;42:2845–2855. [Google Scholar]
- 152.Wang S., Cheng C., Wu F., Jiang B., Shi F., Tu S., Rajale T., Li G. Microwave-assisted multi-component reaction in water leading to highly regioselective formation of benzo[f]azulen-1-ones. Tetrahedron. 2011;67:4485–4493. doi: 10.1016/j.tet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Naidoo S., Jeena V. A green, solvent-free one-pot synthesis of disubstituted quinolines via A3-coupling using 1mol % FeCl3. Heterocycles. 2016;92:1655–1664. [Google Scholar]
- 154.Ansari A.J., Sharma S., Pathare R.S., Gopal K., Sawant D.M., Pardasani R.T. Solvent–free multicomponent synthesis of biologically–active fused–imidazo heterocycles catalyzed by reusable Yb(OTf)3 under microwave irradiation. Chem. Select. 2016;1:1016–1021. [Google Scholar]
- 155.Tayebee R., Tizabi S. One-pot four-component dakin-west synthesis of beta-acetamido ketones catalyzed by a vanadium-substituted heteropolyacid. Chin. J. Catal. 2012;33:923–932. [Google Scholar]
- 156.Borkin D., Morzhina E., Datta S., Rudnitskaya A., Sood A., Török M., Török B. Heteropoly acid-catalyzed microwave-assisted three-component aza-diels-alder cyclizations: diastereoselective synthesis of potential drug candidates for alzheimer’s disease. Org. Biomol. Chem. 2011;9:1394–1401. doi: 10.1039/c0ob00638f. [DOI] [PubMed] [Google Scholar]
- 157.Ishak C.Y., Wahbi H.I., Mohamed M.E. Synthesis and characterization of some new 6-substituted-2, 4-di (hetar-2-yl) quinolines via micheal addition - ring closure reaction of schiff base n-(hetar-2- yl) methylene aniline with hetarylketones. Int. J. Pharm. Phytopharm. Res. 2013;2:431–435. [Google Scholar]
- 158.Zhang J-H., Wang R-B., Li D-F., Zhoa L-M. Green method to preparing oxindole-fused spirotetrahydrofuran scaffolds through methanesulfonic acid-catalyzed cyclization reactions of 3‐allyl-3-hydroxy-2-oxindole in water. ACS Omega. 2017;2:7022–7028. doi: 10.1021/acsomega.7b01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gooßen L.J., Ohlmann D.M., Dierker M. Silver triflate-catalysed synthesis of γ-lactones from fatty acids. Green Chem. 2010;12:197–200. [Google Scholar]
- 160.Mike J.F., Intemann J.J., Cai M., Xiao T., Shinar R., Shinar J., Jeffries-EL M. Efficient synthesis of benzobisazole terpolymers containing thiophene and fluorene. Polym. Chem. 2011;2:2299–2305. [Google Scholar]
- 161.Shen G., Zhou H., Du P., Liu S., Zou K., Uozumi Y. Brønsted acid-catalyzed selective C–C bond cleavage of 1,3-diketones: A facile synthesis of 4(3H)-quinazolinones in aqueous ethyl lactate. RSC Advances. 2015;5:85646–85651. [Google Scholar]
- 162.Ahmed W., Zhang S., Yu X., Yamamoto Y., Bao M. Brønsted acid-catalyzed metal- and solvent-free quinoline synthesis from N-alkyl anilines and alkynes or alkenes. Green Chem. 2018;20:261–265. [Google Scholar]
- 163.Grigorjeva L., Jirgensons A. Lewis acid catalyzed intramolecular allylic substitution of bis(trichloroacetimidates): A versatile approach to racemic unsaturated amino acids. Eur. J. Org. Chem. 2011;13:2421–2425. [Google Scholar]
- 164.Klimovica K., Grigorjeva L., Maleckis A., Popelis J., Jirgensons A. C-quaternary vinylglycinols by metal-catalyzed cyclization of allylic bistrichloroacetimidates. Synlett. 2011;19:2849–2851. [Google Scholar]
- 165.Grigorjeva L., Maleckis A., Klimovica K., Skvorcova M., Ivdra N., Leitis G., Jirgensons A. Novel synthesis of 2-trichloromethyl-4-vinyloxazoline and its derivatization by ring cleavage reactions. Chem. Heterocycl. Compd. 2012;48:919–924. [Google Scholar]
- 166.Cornil J., Gonnard L., Guérinot A., Reymond S., Cossy J. Lewis acid catalyzed synthesis of cyclic carbonates, precursors of 1,2- and 1,3-diols. Eur. J. Org. Chem. 2014;23:4958–4962. [Google Scholar]
- 167.Wang S., Chai Z., Zhou S., Wang S., Zhu X., Wei Y. A novel lewis acid catalyzed [3 + 3]-annulation strategy for the syntheses of tetrahydro-β-carbolines and tetrahydroisoquinolines. Org. Lett. 2013;15:2628–2631. doi: 10.1021/ol4008525. [DOI] [PubMed] [Google Scholar]
- 168.Yu Z., Liu L., Zhang J. Triflic acid-catalyzed enynes cyclization: A new strategy beyond electrophilic π-activation. Chemistry. 2016;22:8488–8492. doi: 10.1002/chem.201601599. [DOI] [PubMed] [Google Scholar]
- 169.Singh A.K., Rai A. Yadav. L.D.S. LiBr catalyzed solvent-free ring expansion of epoxides to 1,4-oxathian-2-ones with a-mercaptocarboxylic acids. Tetrahedron Lett. 2011;52:3614–3617. [Google Scholar]
- 170.Kiasat A.R., Mehrjadi M.F. PEG-SO3H as eco-friendly polymeric catalyst for regioselective ring opening of epoxides using thiocyanate anion in water: An efficient route to synthesis of b-hydroxy thiocyanate. Catal. Commun. 2008;9:1497–1500. [Google Scholar]
- 171.Nazari S. Iravani, N.; Ahmady, A.Z.; Vafaee-Nezhad, M.; Keshavarz, M. Imidazol-1-yl-acetic acid as a green simple bifunctional organocatalyst for the regioselective conversion of epoxides to 1,2-azido alcohols and -hydroxythiocyanates. Curr. Organocatal. 2014;1:7–12. [Google Scholar]
- 172.Halimehjani A.Z., Gholami H., Saidi M.R. Boric acid/glycerol as an efficient catalyst for regioselective epoxide ring opening by aromatic amines in water. Green Chem. Lett. Rev. 2012;5:1–5. [Google Scholar]
- 173.Cucciniello R., Ricciardi M., Vitiello R., Di Serio M., Proto A., Capacchione C. Synthesis of monoalkyl glyceryl ethers by ring opening of glycidol with alcohols in the presence of lewis acids. ChemSusChem. 2016;9:3272–3275. doi: 10.1002/cssc.201600989. [DOI] [PubMed] [Google Scholar]
- 174.Ghosal N.C., Santra S., Das S., Hajra A., Zyryanov G.V., Majee A. Organocatalysis by an aprotic imidazolium zwitterion: regioselective ring-opening of aziridines and applicable to gram scale synthesis. Green Chem. 2016;18:565–574. [Google Scholar]
- 175.Gowda R.R., Chakraborty D. Environmentally benign process for bulk ring opening polymerization of lactones using iron and ruthenium chloride catalysts. J. Mol. Catal. Chem. 2009;301:84–92. [Google Scholar]
- 176.Ji H-Y., Wang B., Pan L., Li S-Y. Lewis pairs for ring-opening alternating copolymerization of cyclic anhydrides and epoxides. Green Chem. 2018;20:641–648. [Google Scholar]
- 177.Huang Y., Yang T., Zhou M., Pan H., Fu Y. Microwave-assisted alcoholysis of furfural alcohol into alkyl levulinates catalyzed by metal salts. Green Chem. 2016;18:1516–1523. [Google Scholar]
- 178.Huang Y., Yang X. Lv, Z.;Cai, C.; Kai, C.; Pei, Y.; Feng, Y. Asymmetric synthesis of 1,3-butadienyl-2-carbinols by the homoallenylboration of aldehydes with a chiral phosphoric acid catalyst. Angew. Chem. Int. Ed. 2015;54:7299–7302. doi: 10.1002/anie.201501832. [DOI] [PubMed] [Google Scholar]
- 179.Shevchenko G.A., Pupo G., List B. Catalytic asymmetric α-amination of α-branched ketones via enol catalysis. Synlett. 2015;26:1413–1416. [Google Scholar]
- 180.Yang X., Toste F.D. Direct asymmetric amination of α‐branched cyclic ketones catalyzed by a chiral phosphoric acid. J. Am. Chem. Soc. 2015;137:3205–3208. doi: 10.1021/jacs.5b00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lou H., Wang Y., Jin E., Lin X. Organocatalytic asymmetric synthesis of dihydrobenzoxazinones bearing trifluoromethylated quaternary stereocenters. J. Org. Chem. 2016;81:2019–2026. doi: 10.1021/acs.joc.5b02848. [DOI] [PubMed] [Google Scholar]
- 182.Qin L., Wang P., Zhang Y., Ren Z., Zhang X., Da C-S. Direct asymmetric friedel–crafts reaction of naphthols with acetals catalyzed by chiral brønsted acids. Synlett. 2016;27:571–574. [Google Scholar]
- 183.Rueping M., Bootwicha T., Sugiono E. Continuous-flow catalytic asymmetric hydrogenations: Reaction optimization using FTIR inline analysis. Beilstein J. Org. Chem. 2012;8:300–307. doi: 10.3762/bjoc.8.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.More G.V., Bhanage B.M. Chiral phosphoric acid catalyzed asymmetric transfer hydrogenation of quinolines in a sustainable solvent. Tetrahedron. 2015;26:1174–1179. [Google Scholar]
- 185.Lifchits O., Reisinger C.M., List B. Catalytic asymmetric epoxidation of α-branched enals. J. Am. Chem. Soc. 2010;132:10227–10229. doi: 10.1021/ja1037935. [DOI] [PubMed] [Google Scholar]
- 186.Zhang H., Yao Q., Lin L., Xu C., Liu X., Feng X. Catalytic asymmetric epoxidation of electron-deficient enynes promoted by chiral N,N′-dioxide-scandium(III) complex. Adv. Synth. Catal. 2017;359:3454–3459. [Google Scholar]
- 187.Chen G., Fu X., Li C., Wu C., Miao Q. Highly efficient direct a larger-scale aldol reactions catalyzed by a flexible prolinamide based-metal Lewis acid bifunctional catalyst in the presence of water. J. Organomet. Chem. 2012;702:19–26. [Google Scholar]
- 188.Penhoat M., Barbry D., Rolando C. Direct asymmetric aldol reaction co-catalyzed by L-proline and group 12 elements Lewis acids in the presence of water. Tetrahedron Lett. 2011;52:159–162. [Google Scholar]
- 189.Kitanosono T., Ollevier T., Kobayash S. Iron- and bismuth-catalyzed asymmetric mukaiyama aldol reactions in aqueous media. Chem. Asian J. 2013;8:3051–3062. doi: 10.1002/asia.201301149. [DOI] [PubMed] [Google Scholar]
- 190.Aplander K., Ding R., Krasavin M., Lindström U.M., Wennerberg J. Asymmetric lewis acid catalysis in water: α-amino acids as effective ligands in aqueous biphasic catalytic michael additions. Eur. J. Org. Chem. 2009;6:810–821. [Google Scholar]
- 191.Barbero M., Cadamuro S., Dughera S., Torregrossa R. Chiral derivatives of 1,2-benzenedisulfonimide as efficient Brønsted acid catalysts in the Strecker reaction. Org. Biomol. Chem. 2014;12:3902–3911. doi: 10.1039/c4ob00552j. [DOI] [PubMed] [Google Scholar]
- 192.Espinosa M., Blay G., Cardona L., Munoz M.C. Pedro, J.R. Catalytic asymmetric formal [3+2] cycloaddition of 2-isocyanatomalonate esters and unsaturated imines: Synthesis of highly substituted chiral γ-lactams. Chemistry. 2017;23:14707–14711. doi: 10.1002/chem.201702777. [DOI] [PubMed] [Google Scholar]
- 193.Kulkarni A., Zhou W., Török B. Heterogeneous catalytic hydrogenation of unprotected indoles in water: A green solution to a long-standing challenge. Org. Lett. 2011;13:5124–5127. doi: 10.1021/ol2019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shekhar A.C., Kumar A.R., Sathaiah G., Paul V.L., Sridhar M., Rao P.S. Facile N-formylation of amines using Lewis acids as novel catalysts. Tetrahedron Lett. 2009;50:7099–7101. [Google Scholar]
- 195.Terada Y., Ieda N., Komura K., Sugi Y. Multivalent metal salts as versatile catalysts for the amidation of long-chain aliphatic acids with aliphatic amines. Synthesis. 2008;15:2318–2320. [Google Scholar]
- 196.Yaragorla S., Singh G., Saini P.L., Reddy M.K. Microwave assisted, Ca(II)-catalyzed Ritter reaction for the green synthesis of amides. Tetrahedron Lett. 2014;55:4657–4660. [Google Scholar]
- 197.Zhou B., Yang J., Li M., Gu Y. Gluconic acid aqueous solution as a sustainable and recyclable promoting medium for organic reactions. Green Chem. 2011;13:2204–2211. [Google Scholar]
- 198.Jeong H., Axtell J.C., Török B., Schrock R.R., Muller P. Syntheses of tungsten tert-butylimido and adamantylimido alkylidene complexes employing pyridinium chloride as the acid. Organometallics. 2012;31:6522–6525. [Google Scholar]
- 199.Kamimura A., Murata K., Tanaka Y., Okagawa T., Matsumoto H., Kaiso K., Yoshimoto M. Rapid conversion of sorbitol to isosorbide in hydrophobic ionic liquids under microwave irradiation. ChemSusChem. 2014;7:3257–3259. doi: 10.1002/cssc.201402655. [DOI] [PubMed] [Google Scholar]
- 200.Antonetti C., Melloni M., Licursi D. Fulignati, S.; Ribechini, E.; Rivas, S.; Parajó, J.C.; Cavani, F.; Raspolli Galletti, A.M. Microwave-assisted dehydration of fructose and inulin to HMF catalyzed by niobium and zirconium phosphate catalysts. Appl. Catal. B. 2017;206:364–377. [Google Scholar]
- 201.Bhanja P., Modak A., Chatterjee S., Bhaumik A. Bifunctionalized mesoporous SBA-15: A new heterogeneous catalyst for the facile synthesis of 5-hydroxymethylfurfural. ACS Sustain. Chem.& Eng. 2017;5:2763–2773. [Google Scholar]


