Abstract
Proton exchange membrane fuel cells (PEMFCs) have received considerable interest due to their low operating temperature and high energy conversion rate. However, their practical implement suffers from significant performance challenge. In particular, proton exchange membrane (PEM) as the core component of PEMFCs, have shown a strong correlation between its properties (e.g., proton conductivity, dimensional stability) and the performance of fuel cells. Metal-organic frameworks (MOFs) as porous inorganic-organic hybrid materials have attracted extensive attention in gas storage, gas separation and reaction catalysis. Recently, the MOFs-modified PEMs have shown outstanding performance, which have great merit in commercial application. This manuscript presents an overview of the recent progress in the modification of PEMs with MOFs, with a special focus on the modification mechanism of MOFs on the properties of composite membranes. The characteristics of different types of MOFs in modified application were summarized.
Keywords: metal-organic framework, proton exchange membrane, modification, proton conductivity, fuel cells
Introduction
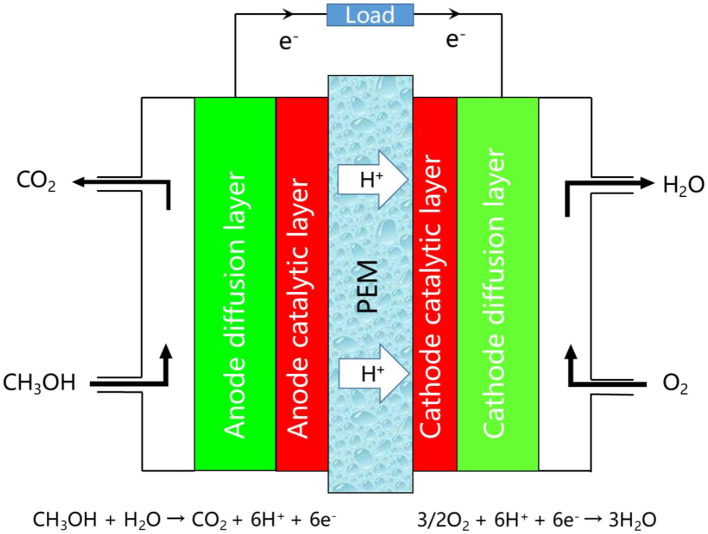
The shortage of fossil energy and the promotion of environmental protection concept have motivated the development of proton exchange membrane fuel cells (PEMFCs). PEMFCs were first developed by General Electric in the United States in the 1960s for the Gemini space program (Zhao et al., 2017; Teixeira et al., 2020). Compared with the other fuel cells (alkaline fuel cells, phosphoric acid fuel cells, molten carbonate fuel cells, etc.), PEMFCs possess several favorable features, including low operating temperature, fast start-up, high energy conversion rate, and environmentally friendliness (Moreno et al., 2015; Dekel, 2018; Pourrahmani et al., 2019; Zhang et al., 2019). PEMFCs are mainly composed of seal rings, collector plates and membrane electrode assemblies (MEAs) (Bose et al., 2014; Sanda et al., 2015; Li et al., 2020; Yang et al., 2020). MEAs convert the chemical energy of reactants into electrical energy, and directly determines the performance of the entire fuel cell system. Generally, MEAs consist of PEMs, catalytic layers and gas diffusion layers on both sides. Taking the direct methanol fuel cell (DEMFC) as an example, methanol reaches the anode catalytic layer through the gas diffusion layer, and the oxidation reaction takes place on the catalyst surface to generate electrons, protons and carbon dioxide (Jing et al., 2020; Junoh et al., 2020). Carbon dioxide is discharged through the anode outlet, and protons are transferred to the cathode catalyst layer through the PEM. At the same time, electrons are collected by the anode collector plates, and transferred to the cathode through the external circuit to form a current, which combines with protons and oxygens in the cathode catalyst layer to generate water (Rivera-Gavidia et al., 2020; Yan et al., 2020). The working principle of DEMFC is shown in Figure 1.
Figure 1.
Working principle of DEMFC.
In recent years, remarkable achievements have been made in the research of catalyst and water management (Lv et al., 2016; Deng et al., 2019a, 2020). Many researchers have prompted extensive investigated PEM due to it's the key component of MEAs (Yuan et al., 2020). As the carrier of proton transfer, the PEM transfers protons produced by the anode catalytic layer to the cathode catalytic layer and react with oxygen to generate water. At the same time, the PEM as a physical barrier separates the anode fuel from the cathode fuel to avoid direct contact between them. In addition, PEM have not conduct electrons, forcing electrons to conduct through external circuits to generate current (Han and Wu, 2018; Zhao et al., 2019). At present, the most widely used PEMs is the Nafion membrane of DuPont Company. However, due to the high price of Nafion membrane, scientists are actively seeking alternative materials to Nafion membrane, such as sulfonated poly (aryl ether)s (Hooshyari et al., 2020). The introduction of sulfonic acid group can obviously improve the conductivity of membranes. Nevertheless, these materials will appear serious swelling with the increase of sulfonation degree, which greatly reduces the dimensional stability, and aggravate the penetration of fuel. Moreover, these alternative materials and Nafion membrane have the disadvantage of relying excessively on moisture content to maintain stable performance. Currently, a wide range of strategies have been proposed including grafting, cross-link, polyelectrolyte modification and adding functional particles in the PEMs (Koros and Zhang, 2017; Zhang et al., 2019).
As a kind of inorganic-organic hybrid material with a certain crystal structure, MOFs are mainly used in gas storage, gas separation, magnetic materials and reaction catalysis (Makiura et al., 2010; Shah et al., 2013; Knebel et al., 2018; Luo et al., 2018; Deng et al., 2019b; Feng et al., 2019). In recent years, modification of PEMs with metal-organic frameworks (MOFs) has been intensively studied (Furukawa et al., 2014; Shiqiang et al., 2016; Luo et al., 2017; Shaari et al., 2018; Cai et al., 2019; Deng et al., 2019c; Wang et al., 2019). It is found that the open framework structure of MOFs improved the performance of the composite membrane by loading particles with special functions into the matrix as proton carriers (Pardo et al., 2011; Dybtsev et al., 2014; Díaz-Duran and Roncaroli, 2017; Liu et al., 2018). The large specific surface area of MOFs allows the composite membrane to promote the migration of protons while accommodating more bound water, which further improves the proton hopping conductivity. In addition, the small pore size of MOFs can hinder fuel and oxidant diffusion to improve selectivity, and most MOFs themselves have many proton hopping sites, which can elevate the conductivity (Matoga et al., 2015; Ono et al., 2017; Wu et al., 2017; Luo et al., 2019).
This review focus on the recent advances of a few MOFs modified PEMs, including UiO-series, MIL-series, ZIF-series and others. Especially, the effects of different series of MOFs materials on the electrical and mechanical properties of PEMs are extensively summarized. To conclude, the challenges and perspectives in this field are put forward. We believe this review can provide a progress report for the research of MOFs modified PEM, and provide some inductive assistance for researchers.
MOFs-Modified PEMs
Recently, MOFs with the high proton conductivity have received extensive attention. It was found that protons could be delivered through the coordination skeleton itself (Ohkoshi et al., 2010) or carriers (Bureekaew et al., 2009). However, the grain boundary structure of MOFs restricts the migration of proton conductors, resulting in insufficient conductivity. Furthermore, it is very difficult to directly process MOFs for fuel cells due to their special and diverse crystal structures (Taylor et al., 2015; Yang et al., 2015b; Pili et al., 2016; Van Goethem et al., 2018). Hybridization of MOFs with other polymers to form composite membranes is an effective strategy to solve this plight (Shekhah et al., 2011; Denny and Cohen, 2015; Kitao et al., 2017).
The PEMs modified by MOFs can be generally divided into two approaches. One of the approaches is to immerse the pores of MOFs with different proton carries, such as phytic@MIL, PIL@MIL, acids@MIL (Li et al., 2014a, 2016; Dong et al., 2017). Another approach is to modify the organic ligands of MOFs with functional groups (-SO3H, -NH2,−2COOH) to enhance the acidity and hydrophilicity. However, there are few reports on modified PEMs in different MOF categories. This section will summarize the performance improvement of PEMs with different categories of MOFs, include UiO-series, MIL-series, ZIF-series and other MOFs.
UiO-Series MOFs PEMs
UiO-series MOFs (UiO for University of Oslo) are composed of an inner Zr6O4(OH)4 core bounded to twelve terephthalate ligands (Cavka et al., 2008; Kandiah et al., 2010). They exhibited exceptional chemical and thermal stabilities which was benefiting from the highly oxyphilic nature of Zr(IV) atoms and their strong coordination bonds with carboxylate oxygen (Mukhopadhyay et al., 2019; Zeng et al., 2019). Among them, the unique spatial structure of UiO-66 endows with good stability for a wide class of solvents such as water, acidic conditions, high temperature and pressure. In addition, UiO-66 has attracted considerable interests in modifying PEMs due to their high working capacity, excellent thermal stability, and low-cost regenerability (Devautour-Vinot et al., 2012; Katz et al., 2013; Wu et al., 2013b; Liu et al., 2015). The conductivity of the PEMs by adding UiO-66 was increased by 10–50%, and the penetration of fuel was decreased significantly. Moreover, the different linkers of UiO-66 can be used to tailor the affinity with the polymer matrix, which was conducive to the preparation of composite PEMs by blending with polymer matrix (He et al., 2017). Table 1 lists some typical PEMs modified with UiO-series MOFs.
Table 1.
Properties of UiO-series MOF modified membranes.
| Compound | σ (S cm−1) | Ea (eV) | Conditions | References |
|---|---|---|---|---|
| Nafion/UiO-66 | 1.65 × 10−1 | n/a | 80oC, 95% RH | Donnadio et al., 2017 |
| Nafion/UiO-66-SO3H | 1.71 × 10−1 | n/a | 80oC, 95% RH | Donnadio et al., 2017 |
| Nafion/UiO-66-NH2 | 1.84 × 10−1 | n/a | 80oC, 95% RH | Rao et al., 2017b |
| Nafion/UiO-66-NH2+UiO-66-SO3H | 2.56 × 10−1 | n/a | 90oC, 95% RH | Rao et al., 2017b |
| CS/UiO-66-NH2+UiO-66-SO3H | 5.2 × 10−2 | 0.131 | 100oC, 98% RH | Dong et al., 2018 |
| SPEEK/GO@UiO-66-SO3H | 2.68 × 10−1 | 0.093 | 70oC, 95% RH | Sun et al., 2017a |
| Nafion/GO@UiO-66-NH2 | 3.03 × 10−1 | n/a | 90oC, 95% RH | Rao et al., 2017a |
| Nafion/PWA@UiO-66-NH2 | 9.2 × 10−2 | n/a | 25oC | Yang et al., 2019 |
Nafion membrane has become the most widely used PEMs by virtue of its high performance, and plays a significant role in the advancement of PEMFCs technology. The molecular structure of Nafion membrane is composed of hydrophobic poly tetra fluoroethylene main chain and perfluoroether branch chain with the hydrophilic sulfonic acid group at the end. This unique type of structure imparts Nafion membrane excellent chemical and thermal stability and favorable proton conductivity (Shao et al., 2007). However, the performance will be seriously reduced at low humidity, while sufficient humidification is necessary for superior performance. Compared with the pristine Nafion membrane, the proton conductivity of the composite membrane prepared by adding UiO-66 as filler into Nafion matrix was obviously improved by 30% under 95% RH (Donnadio et al., 2017). Research showed that the improvement of conductivity had no linear relationship with a load of MOFs fillers. The reason may be attributed to the excessive addition of low conductivity fillers that will offset the positive influence of fillers on the transport properties of ionomers. And the internal reunion of MOF fillers could cause resistance to conductivity (Khdhayyer et al., 2017; Lin et al., 2018). Consequently, the fillers should be added within a reasonable range, otherwise the adverse situation may occur.
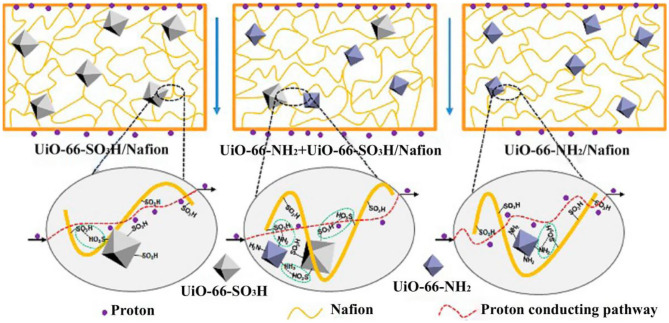
It was reported that UiO-66–SO3H possesses super protonic conductivity, numerous scholars have studied it as the main object of modified PEMs. Relative to the low proton conductivity of UiO-66 about 7 × 10−6 S cm−1, the proton conductivity of sulfonated UiO-66 has significantly increased by four orders of magnitude (Yang et al., 2011; Matthew et al., 2016; Patel et al., 2016). The sulfonic acid groups of UiO-66–SO3H are easily combined with water molecules, which could provide additional proton transfer pathways and more proton conduction sites. The proton conductivity of Nafion/UiO-66–SO3H was twice that of pristine Nafion membrane under the same conditions. The thermal stability of composite membrane was also slightly improved. Similar to the sulfonic acid groups, the amino groups can be used as proton carrier sites to improve proton conductivity (Ru et al., 2018). The composite membrane was synthesized by codoping of UiO−66-SO3H and UiO-66-NH2. It was found that the synergistic effect between UiO−66-SO3H and UiO-66-NH2 efficiently enhanced the proton conductivity of composite PEMs (Figure 2).
Figure 2.
Transport mechanism diagram of Nafion/UiO-66-NH2, Nafion/UiO-66-SO3H, and Nafion/UiO-66-NH2+UiO-66-SO3H. [Readapted with permission from Rao et al. (2017b) Copyright 2017, American Chemical Society].
Inspired by this, UiO-66-SO3H and UiO-66-NH2 were codoped in chitosan (CS) polymer to synthesize proton-conducting hybrid membranes (Dong et al., 2018). The novel dual-MOF-cofilled hybrid membranes could work in both hydrated and anhydrous conditions. Surprisingly, the maximum proton conductivity of the dual-MOF-cofilled hybrid membranes obtained 3.78 × 10−3 S cm−1 under anhydrous conditions at 120°C, which was roughly 29.1 times as the pure CS membrane. In addition, a PEMFC was constructed by using CS/UiO-66-SO3H+UiO-66-NH2 as a membrane, which gave an open-circuit voltage of 1.0 V, and power density of 10.6 mW cm−2. The experimental results showed that the cause may be: (1) the synergy between acid groups (–SO3H) and amino groups (-NH2) reduced the barrier of proton conduction and formed a hydrogen network, leading to an increase in proton conduction channels; (2) sulfonate functional groups of UiO-66-SO3H as proton donors increase the number of proton carriers and enhanced high-temperature resistance of dual-MOFs-cofilled hybrid membranes; (3) -NH2 groups of the embedded basic MOFs as proton acceptor provide proton hopping sites and build efficient acid-basic pairs between those adjacent MOFs (Yang et al., 2016).
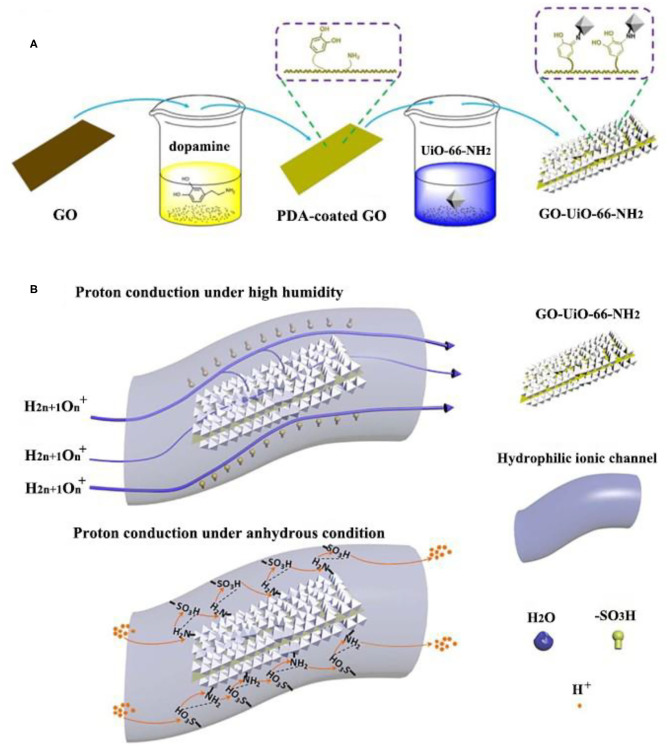
Graphene oxide (GO) has good proton conductivity due to high surface area and numerous oxygen-containing functional groups. Moreover, a large number of carboxyl groups of GO coordinate with Zr ions of UiO-66, which promote the uniform growth of UiO-66 on the surface of GO. GO@UiO-66-NH2 has been successfully prepared (Figure 3A) and incorporated to the Nafion matrix to fabricate Nafion/GO@UiO-66-NH2 mixed membrane (Rao et al., 2017a,b). Particularly, under 95% RH and anhydrous conditions, the highest proton conductivity of the composite membrane was 0.303 S cm−1 and 3.4 × 10−3 S cm−1 at 90°C, which were 1.57 times and 1.88 times higher than that of recast Nafion, respectively. The principle that GO@UiO-66-NH2 improves proton conduction can be clearly seen from Figure 3B. The reasons for improving proton conduction are speculated as follows: (1) massive of oxygen-containing functional groups in GO act as proton receptors; (2) excellent water affinity and high specific surface area of UiO-66-NH2 enhanced the water retention capacity of PEMs; (3) the continuous proton transport channels of UiO-66-NH2 was constructed by the tethering effect of GO surfaces and the good interaction between MOF particles; (4) the synergy between acid groups (–SO3H) of Nafion and basic groups (–NH2) of MOF provided extra proton hopping sites, and build efficient acid-basic pairs between those adjacent MOFs. In addition, the barrier effect of GO increased the tortuosity of transport channels and UiO-66-NH2 could trap methanol inside its pores, thus ameliorated the permeation of methanol.
Figure 3.
(A) Preparation of GO@UiO-66-NH2. (B) Schematic diagram of proton conductivity enhancements of GO@UiO-66-NH2. [Readapted with permission from Rao et al. (2017a) Copyright 2017, Elsevier].
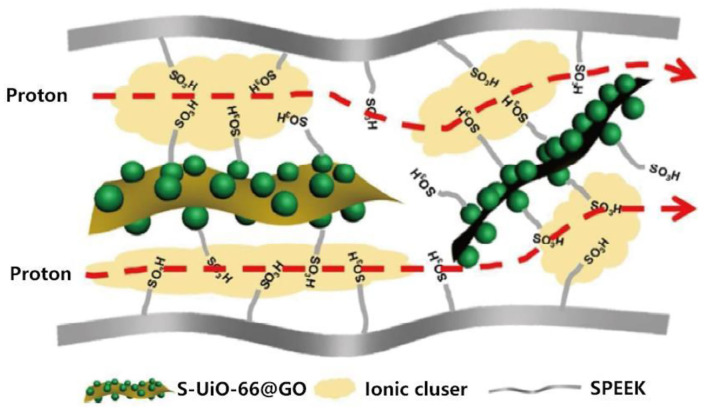
Sun et al. prepared GO@UiO-66-SO3H hybrid nanosheets via in situ surface growth method, and incorporated them into sulfonated polyether ether ketone (SPEEK) to synthesized SPEEK/GO@UiO-66-SO3H composite membranes (Yang et al., 2011; Sun et al., 2017a). Proton can be transported in the ionic nanochannels formed by the assembly of -SO3H groups of GO@UiO-66-SO3H together with -SO3H groups of SPEEK (as shown in Figure 4). The in-situ surface growth method effectively eliminated the agglomeration of UiO-66-SO3H, and improve proton conductivity of composite membranes. The effect of different functional groups (i.e., –SO3H, −2COOH, –NH2, and –Br) on proton conductivity of UiO-66 was investigated (Yang et al., 2015a). The results revealed that the proton conductivity of UiO-66-SO3H and UiO-66-2COOH were three orders of magnitude higher than pure UiO-66. Therefore, the –SO3H and −2COOH had great application potential in modifying the PEMs.
Figure 4.
Schematic illustration of the enhanced transport properties of the GO@UiO-66-SO3H. [Readapted with permission from Sun et al. (2017a) Copyright 2017, American Chemical Society].
Phosphotungstic acid has been coupled with modified UiO-66-NH2 and incorporated into the Nafion membrane by solution casting to improve the proton conductivity and ion-selectivity (Garibay and Cohen, 2010; Yang et al., 2019). The composite membrane exhibited a higher proton conductivity (9.2 × 10−2 S cm−1) and an ultra-high ion-selectivity (2.66 × 105 S min cm−3). In many cases, the performance of doping pure MOFs in PEMs was unsatisfactory. The effect of combining nanoparticles with MOFs was incredible to improve the performance composite membranes.
MIL-Series MOFs PEM
MIL-series MOFs (MIL for Materials of Institute Lavoisier) are synthesized by different transition metals and dicarboxylic ligands such as succinic acid and glutaric acid. Dicarboxylic ligands have been introduced many functional groups through appropriate chemical modifications. Compared with the traditional zeolite materials and graphite materials, MIL-series MOFs have the unique advantages of porosity, large specific surface area and unsaturated metal coordination sites. For instance, MIL-100 is a crystal with special topological structure synthesized by trivalent chromium and pyromellitic acid, that its pore diameter reaches 2.5–2.9 nm. MIL-101 with 3.0–3.4 nm of pore size and 5,900 m2/g of specific surface area is synthesized from trivalent chromium octahedral clusters and terephthalic acid. MIL-101 has two types of cage structure limited by 12 pentagonal faces for the smaller and by 16 faces for the larger. The proton conductivity of composite membranes can be obviously improved by adding MIL-series MOFs. The large number of coordinatively unsaturated metal sites (CUSs) of MIL-101 can provide abundant -OH group through hydrolysis. These -OH groups can form hydrogen-bond networks in the proton exchange membrane, which promote proton conduction through the Grotthuss mechanism. MIL-53 (Al) is connected with terephthalic acid group by aluminum as the connection point of metal organic framework, formed one-dimensional channels with a diameter of about 8.5 Å. Water molecules can be trapped by the hydrogen-bonding of carboxylic acid groups in these channels, which improve the conductivity of protons (Amdursky et al., 2016). In recent years, the research on the application of MIL-series MOFs has attracted wide attention from researchers.
Five types of MOFs were prepared (MIL-101, MIL-101-SO3H, H2SO4@MIL-101, H3PO4@MIL-101, CF3SO3H@MIL-101), and various hybrid membranes were synthesized via the solution casting method (Dong et al., 2017). The highest proton conductivity of pure CS membrane and hybrid membranes and other characteristic parameters are shown in Table 2. It is apparent that the incorporation of MIL-101 has increased proton conductivity by 2~3 times. The analysis indicated that the internal hydrogen-bond network interaction and the electrostatic effect can be contributed to the significant improvement of the proton conductivity (Hupp and Poeppelmeier, 2005). Furthermore, these non-volatile acids are more compatible with MOFs, which provide additional transport receptors and hopping sites while further enhancing the proton conductivity of the composite membranes.
Table 2.
The glass transition temperatures (Td), proton-conducting activation energy (Ea), and proton conductivity of hybrid membranes.
| Hybrid membranes | Td (°C) | Ea (eV) | σ (S cm−1) | References |
|---|---|---|---|---|
| CS | 217.7 | 0.189 | 0.030 | Dong et al., 2018 |
| CS/MIL-101 | 226.7 | 0.183 | 0.034 | Dong et al., 2017 |
| CS/S-MIL-101 | 234.3 | 0.174 | 0.064 | Dong et al., 2017 |
| CS/H2SO4@MIL-101 | 220.1 | 0.181 | 0.095 | Dong et al., 2017 |
| CS/H3PO4@MIL-101 | 226.8 | 0.175 | 0.083 | Dong et al., 2017 |
| CS/CF3SO3H@MIL-101 | 230.8 | 0.179 | 0.094 | Dong et al., 2017 |
Similar work has been done (Li et al., 2014b) to study the effect of sulfonic acid groups on the proton conductivity of composite membranes. They fabricated a new kind of hybrid membrane by incorporated sulfonated MIL-101(Cr) into the SPEEK matrix. The protons conductivity of SPEEK/sul-MIL-101(Cr) was increased up to 0.306 S cm −1, which was approximately doubled as the SPEEK. Similar to the previous reasons, sulfonic acid groups as proton transition centers increased the number of proton channels while forming hydrogen-bonded networks through the hydrolysis of CUSs. Phytic acid (myo-inositol hexaphosphonic acid) and phosphotungstic acid are conducive to the conduction of protons, but there are few reports on the research of phytic acid and phosphotungstic acid. Phytic acid has the content of phosphoric acid group next to phosphoric acid while also having good conductivity, chemical stability, and chelating ability. Incorporation of the phytic@MIL-101 into the Nafion membrane significantly enhanced the conductivity in low relative humidities (Li et al., 2014a). The proton conductivity of hybrid membranes was surprisingly up to 7.63 × 10−4 S cm−1 at 10% RH, which was 11 times higher than the Nafion membrane. Phosphotungstic acid (HPW) were formed by incorporated Na2WO4·2H2O and Na2HPO4 into the cavities of MIL-101(Cr), which avoided the leakage of HPW due to its high water solubility. The SPEEK/HPW@MIL-101 membranes prepared with HPW@MIL-101 in SPEEK exhibited good proton conductivity at low relative humidity (7.25 times higher than pristine SPEEK membrane; Zhang et al., 2017).
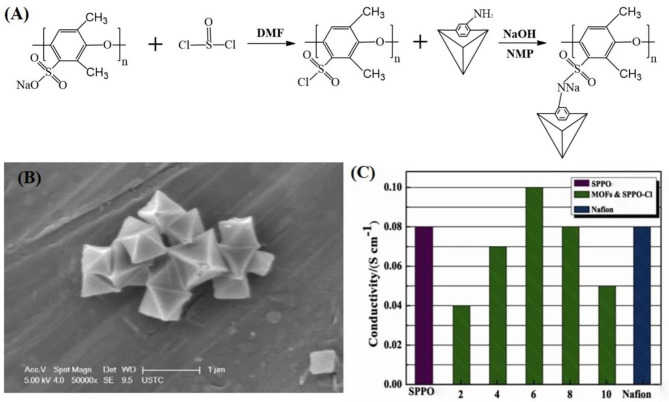
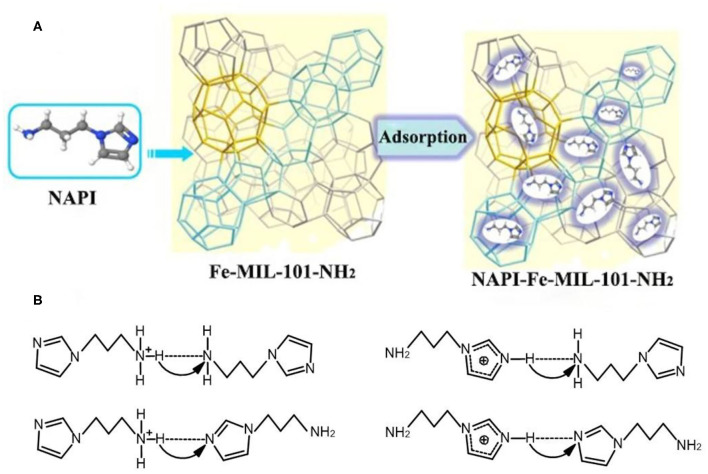
In addition to the above, amino functionalized MOFs is an effective strategy to improve conductivity of composite membranes (Anahidzade et al., 2018). A novel proton-conducting composite membrane was synthesized by amino-functionalized MIL-101 (Fe) particles with sulfonated poly (2, 6-dimethyl-1, 4-phenylene oxide) (SPPO) immersing in water and CHCl3 (Wu et al., 2013a). The procedure for preparing membranes with Fe-MIL-101-NH2-PPO–SO2Cl was shown in Figure 5A. Fe-MIL-101-NH2 particles were dispersed in matrix with almost no surface gap due to the chemical attachment between Fe-MIL-101-NH2 particles and SPPO matrix. The SEM showed that the Fe-MIL-101-NH2 crystals had an octagonal structure as presented in Figure 5B. Moreover, the additional protons were provided from the sulfonimide on a polymeric chain to further promote the proton conductivity. The proton conductivity of Fe-MIL-101-NH2-PPO–SO2Cl membrane reached to 0.10 S cm−1 at room temperature and 0.25 S cm−1 at 90°C, respectively, which was much higher than that of Nafion and pure SPPO membranes at the identical conditions (Figure 5C). On this basis, N-(3-aminopropyl)-imidazole (NAPI) was encapsulated in the frameworks of Fe-MIL-101-NH2, and mixed with SPPO to prepare composite PEM (Wu et al., 2014a). Liquid NAPI has much more proton acceptor/donor sites than imidazole, which ensured the continuous proton transfer by resided in the Fe-MIL-101-NH2 (Figure 6A). The schematic view of intermolecular proton transfer in NAPI as shown in Figure 6B. The composite membrane has maintained excellent proton conductivity and low methanol permeability at high temperature.
Figure 5.
(A) The synthetic procedure for membranes of Fe-MIL-101-NH2-PPO–SO2Cl. (B) SEM images of Fe-MIL-101-NH2 microcrystals. (C) Proton conductivities of the hybrid membranes at different MOF loadings at room temperature. [Readapted with permission from Wu et al. (2013a) Copyright 2013, Royal Society of Chemistry].
Figure 6.
Schematic illustration of (A) NAPI adsorption into Fe-MIL-101-NH2. (B) intermolecular proton transfer in NAPI. [Readapted with permission from Wu et al. (2014a) Copyright 2014, Elsevier].
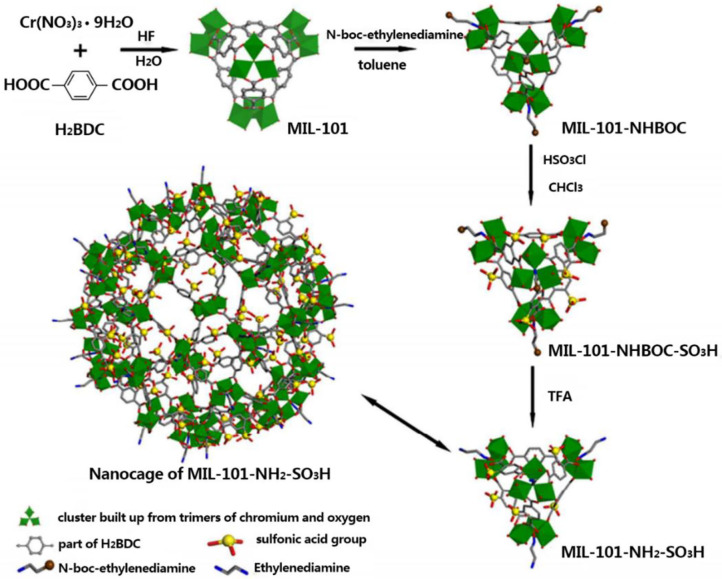
The sulfonic acid group could provide more proton conductive sites and transport pathways, and the amino group is the effective site for proton conduction. The combination of them greatly improve the conductivity of the composite membranes. A novel amino-sulfo-bifunctionalized MOFs named MIL-101-NH2-SO3H (MNS), was prepared and incorporated it into sulfonated poly (arylene ether ketone) containing naphthalene and fluorine moieties (SNF-PAEK) to form the MOFs-modified hybrid membranes (Ru et al., 2018). The schematic preparation and the nanostructure of MIL-101-NH2-SO3H are shown in Figure 7. The proton conductivity of these composite membranes was improved because MNS rearranges the microstructure of the membrane to accelerate the rate of proton migration.
Figure 7.
The schematic preparation and the nanostructure of MIL-101-NH2-SO3H. [Readapted with permission from Ru et al. (2018), Copyright 2018, American Chemical Society].
1-(1-Ethyl-3-imidazolio)propane-3-sulfonate (EIMS) has been developed as another material suitable for proton conduction. A hybrid polymer proton-conducting material, EIMS-HTFSA@MIL, was fabricated by impregnating EIMS and N,N-bis(trifluoromethanesulfonyl) amide (HTFSA) into MIL-101 via heterogeneous mixing and grinding method. The protonated –SO3H/HTFSA in EIMS-HTFSA@MIL acted as the proton source and unprotonated –/TFSA− matrix as the proton defect sites, enabling hydrogen ions hopping between the adjacent – groups for efficient transport (Sun et al., 2017c). Remarkably, the proton conductivity of EIMS-HTFSA@MIL reached 2 × 10−4 S cm−1 at 140°C and the pyrolysis temperature was up to 320°C. It was showed that it has unlimited potential for application in high-temperature fuel cells. Extending this work, Chen Hui and co-workers investigated the acid counter anion (R-) effect of PILs@MOF. They prepared various hybrid membranes, i.e., SA-EIMS@MIL-101, MSA-EIMS@MIL-101 and PTSA-EIMS@MIL-101 (SA = sulfate acid, MSA = methanesulfonate acid, PTSA = p-toluenesulfonate acid) by heterogeneously impregnating procedure (Chen et al., 2018). Compared to previous reported HTSFA, the R- has transfered protons more efficiently at low humidity due to their lower hydrophilicity. The van der Waals volumes of different acid counter anions are closely related to the proton conductivity of these hybrid membranes (Table 3). The steric hindrance inside the material became greater as the van der Waals volumes increased, which led to stronger blocking of hydrogen ions during transport, and made proton conductivity falling.
Table 3.
Proton conductivities at 50°C and 150°C and activation energies of membranes.
Kitagawa et al. reported the crucial influence of coordinated water or guest molecules on proton conduction in MOFs (Sadakiyo et al., 2009, 2012; Yamada et al., 2009). On the basis, two porous MOFs [CPO-27(Mg) and MIL-53(Al)] were incorporated into Nafion membrane for the first time to enhance the water retention ability performance of the membrane (Loiseau et al., 2004; Dietzel et al., 2008; Tsai et al., 2014). The unique one-dimensional pore structure of CPO-27 (Mg) provides an extra place for water storage. Moreover, CPO-27(Mg) and MIL-53(Al) have accommodated the condensed water in the micro-pore of skeleton and produced intense interaction with guest water molecules. Thus, the water uptake and the proton transport efficiencies of the composite membranes were greatly improved by 1.7 and 2.1 times compared with the recast Nafion membrane, respectively. These results showed that MOFs with excellent water retention ability can greatly improve the conductivity of composite membranes and had great potential for application in PEMFCs.
ZIF -Series MOFs PEM
Zeolite imidazolate frameworks (ZIFs), as a subclass of MOFs consist of a tetrahedral divalent metal cations CO2+ (or Zn2+) coordinated to four imidazolic acid rings with the metal–imidazolate–metal bond angles similar to the Si-O-Si angles of zeolites. The complete saturation of the metal provides an excellent thermal stability for ZIFs. In addition, the hydrophobic pores and surface structure of ZIFs are highly repulsion to water molecules, preventing the dissolution of the framework, giving ZIFs excellent chemical stability in alkaline aqueous solution, organic solvent and water. Several reports have revealed that the hybrid PEMs embedding ZIFs with a zeoltic topology possess superior stability and high proton conductivity (Park et al., 2006; Tan and Mahdi, 2016; Liu et al., 2020). The high microporosity and cavity volumes volume of ZIFs with a large number of proton carriers in the cavity enhances the conductivity of PEM. For instance, the unique structure of ZIF-8 with a large pores (11.6 Å in diameter) connected through small apertures (about 3.4 Å) have significantly reduced the penetration of methanol. The compatibility of ZIF-8 with the polymer matrix has a positive effect on the water management of composite membrane.
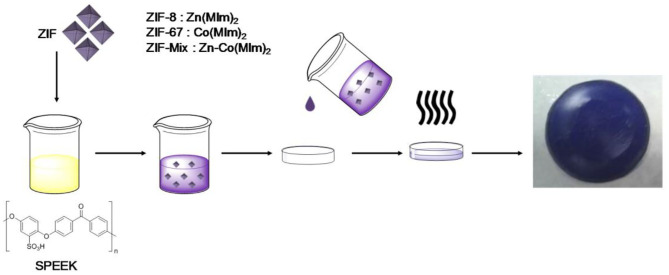
Based on the previous synthesis of PEI/ZIFs, Vicente Compañ and co-workers successfully prepared PBI@ZIF-8, PBI@ZIF-67, and PBI@ZIF-Mix (a Zn/Co bimetallic mixture) hybrid membranes (Shi et al., 2012; Vega et al., 2017; Escorihuela et al., 2018). Compared with the single ZIF doping, ZIF-mix doping is beneficial to improve the proton conductivity (Escorihuela et al., 2018). Moreover, Vicente Compañ et al. synthesized SPEEK@ZIF-8, SPEEK@ZIF-67, and SPEEK @ZIF-Mix hybrid membranes by similar methods (Figure 8; Barjola et al., 2018). The comparison between various membranes proton conductivity is shown in Table 4. The improvement of the conductivity can be attributed to the additional proton carriers provided from ZIFs.
Figure 8.
Schematic illustration of casting method for SPEEK@ZIFs membrane preparation. [Readapted with permission from Barjola et al. (2018), Copyright 2018, MDPI].
Table 4.
The proton conductivities of ZIF-series MOF modified membranes.
| Hybrid membranes | Additive | Conductivity (S cm−1) | Conditions | References |
|---|---|---|---|---|
| PBI@ZIF-8 | H3PO4 | 3.1 × 10−3 | 180oC, anhydrous | Escorihuela et al., 2018 |
| PBI@ZIF-67 | H3PO4 | 4.2 × 10−2 | 180oC, anhydrous | Escorihuela et al., 2018 |
| PBI@ZIF-Mix | H3PO4 | 9.2 × 10−2 | 180oC, anhydrous | Escorihuela et al., 2018 |
| SPEEK@ZIF-8 | – | 1.6 × 10−2 | 100oC | Barjola et al., 2018 |
| SPEEK@ZIF-67 | – | 1.5 × 10−2 | 100oC | Barjola et al., 2018 |
| SPEEK@ZIF-Mix | – | 2.9 × 10−2 | 100oC | Barjola et al., 2018 |
| PEI@ZIF-8 | TBA | 1.9 × 10−3 | 75oC, 95%RH | Vega et al., 2017 |
| PEI@ZIF-67 | TBA | 1.8 × 10−3 | 75oC, 95%RH | Vega et al., 2017 |
| PEI@ZIF-Mix | TBA | 3.0 × 10−3 | 75oC, 95%RH | Vega et al., 2017 |
| SPEEK@ZCN | CNTs | 5.0 × 10−2 | 120oC, 30%RH | Sun et al., 2017b |
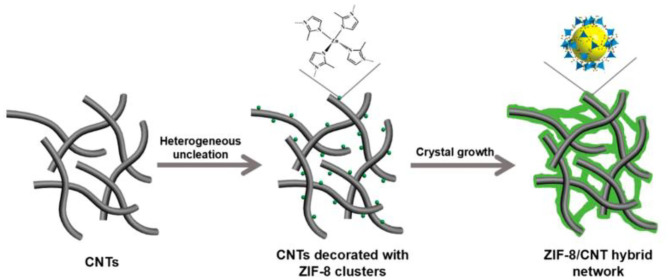
The—N-H from the ZIF-8 framework can provide proton, and reduce the obstacles in the proton conduction path through interact with GO rich in oxygen-containing groups (Zhang et al., 2013). Figure 9 shows that the proton conductivity of the ZIF-8@GO/Nafion hybrid membrane was improved 55 times higher than the Nafion membrane at 120°C and 40% RH (Yang et al., 2015b). A novel two-dimensional zeolite structure ZIF-8/CNT hybrid crosslinked networks (ZCN) was successfully synthesized as shown in Figure 10. The introduction of ZCN and SPEEK significantly improved the proton conductivity and inhibited methanol permeability (Sun et al., 2017b). The proton conductivity of SPEEK@ZCN composite membrane was reaching 0.05 S cm−1 at 120°C and 30% RH, which is one of the highest ZIFs modified PEM so far. In addition, it was found that the addition of two-dimensional fillers significantly improved the proton conductivity of the membrane (Chen et al., 2016; Jia et al., 2016).
Figure 9.
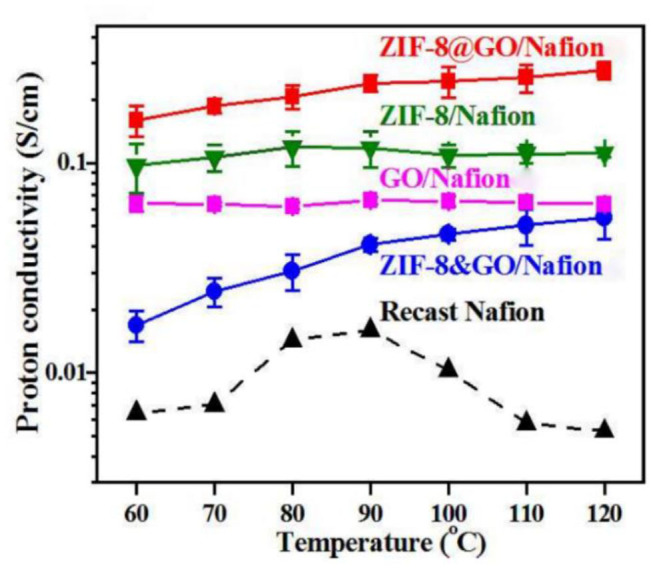
Pronton conductivity of ZIF-8@GO/Nafion and contrast membranes. [Readapted with permission from Yang et al. (2015b) Copyright 2015, Royal Society of Chemistry].
Figure 10.
Schematic illustration of in situ growth method for ZIF-8/CNT synthesized. [Readapted with permission from Sun et al. (2017b) Copyright 2017, American Chemical Society].
Poly(vinylphosphonic acid) (PVPA) has superior proton conductivity due to more phosphate groups and strong hydrogen bonding (Sen et al., 2016). However, the proton conductivity of PVPA decreases sharply at temperature above 100°C. The proton conductivity of PVPA can be improved by doping the ZIF-8 with high conductivity. The proton conductivity of ZIF-8@PVPA composite membrane has reached 3.2 × 10−3 S cm−1 at 140°C under anhydrous conditions, which was 3 times higher than that of pure PVPA membrane. In addition, the pyrolysis temperature of the composite membrane was increased to above 250°C. Poly(2-acrylamido-2-methyl-1-propanesulfonic acid) (PAMPS) is an acidic homopolymer with high proton conductivity. But it cannot be formed pristine membrane independently, which are greatly limited its application in fuel cells (Karlsson et al., 2002). The polymer composite membranes were prepared with poly(vinyl alcohol) (PVA), PAMPS and ZIF-8 in appropriate proportions, which overcome the shortcomings mentioned above (Erkartal et al., 2016).
Natural biomolecules can transport protons and ions through the synthesized transmembrane pores (Ordinario et al., 2014). Protein has been studied by many scholars as a typical proton conducting natural biomaterial (Nagle and Tristram-Nagle, 1983; Amdursky et al., 2016; Gopfrich et al., 2016). DNA@ZIF-8 membrane was prepared by incorporate single-strand DNA into the ZIF-8 membrane via a solid confinement conversion process (Guo et al., 2018). The composite membrane exhibited high proton conductivity of 0.17 S cm−1 at 75 °C, but low methanol permeability of 1.25 × 10−8 cm2 s−1 due to the smaller pore entrances size of ZIF-8. ZIF-8 has significant efficacy in solving ionic liquid leakage problems. Liu et al. fabricated an ionic liquids-polymer composite membrane with ZIF-8 fillers via the combination of the ionothermal and in situ crystallization method, which effectively inhibited the loss of ionic electrolyte (Liu et al., 2014).
Other Kinds of MOFs Modified PEM
In the previous sections, a series of typical MOFs and composite membranes with satisfactory performance were introduced. In addition, there are some other PEMs with satisfactory properties, but they have the disadvantages that the synthesis is tedious and expensive. Therefore, we will give a brief introduction to these PEMs in this section.
An oriented electrospun nanofiber proton-conducting membrane was synthesized by compose Zn2(C2O4)(C2N4H3)2(H2O)0.5(ZCCH) and SPPEK. The composite membranes exhibited excellent proton conductivity (8.2 × 10−2 S cm−1) under high temperature and anhydrous conditions, while the methanol permeability is only about 6% of Nafion-115 under the same conditions (Wu et al., 2014b). A hexaphosphate ester-based 3D MOF named JUC-200 and its composite membrane with poly(vinyl alcohol) (PVA) were prepared (Cai et al., 2017). The composite membrane exhibited excellent water tolerance and high conductivity in a solution of PH = 2.0.
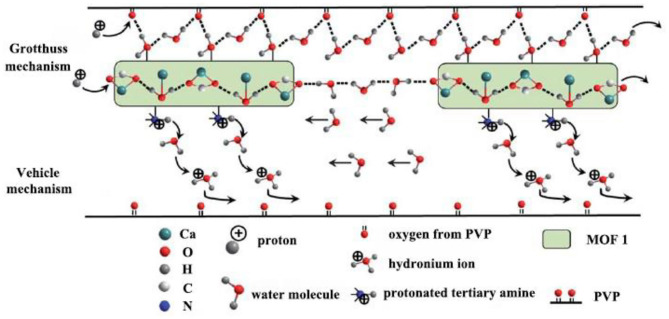
A chiral 2-D MOF named “MOF 1” was synthesized and embedded into PVP polymer matrix by spin-coating method to prepare composite membrane (Liang et al., 2013). From the Nyquist plot and Arrhenius-type plot, it was found that the proton conductivity of MOF 1 and composite membranes presented a downward trend with the decrease of relative humidity. However, the composite membrane has a significant increase in proton conductivity as the increase of MOF 1 content. The conductivity increased from 4.8 × 10−7 S cm−1 to 3.2 × 10−4 S cm−1 with the loading of MOF 1 submicrorods from 3% (MOF 1-PVP-3) to 50 % (MOF 1-PVP-50). It was obvious that the conductivity of MOF 1-PVP-50 was almost one thousand times higher than that of MOF 1-PVP-3. The increase in the conductivity of protons was not only attributed to the capacity of MOF particles to absorb water, but also related to the adsorption and humidification of water by PVP. Figure 11 presented the mechanism of proton transport in the MOF 1–PVP composite membrane.
Figure 11.
The possible mechanisms of proton transport in the MOF 1–PVP composite membrane. [Readapted with permission from Liang et al. (2013) Copyright 2013, Royal Society of Chemistry].
In recent years, a novel and effective method has been proposed to combine protic ionic liquids (PILs) with porous MOFs. PILs are composed of Brønsted acids and Brønsted bases (Santic et al., 2009; Horike et al., 2012), which as the most promising medium for proton conduction instead of water. PILs have attracted great attention due to their excellent thermal stability, high ionic conductive, low-volatile (Stoimenovski et al., 2010). Nevertheless, the high viscosity of PILs results in unsatisfactory conductivity. It can be seen that the mobility of charges is essentially related to the friction resistance produced by the viscosity of liquids from the Walden rule (Belieres and Angell, 2007). The porous structure of MOFs could disperse PILs sufficiently, which reduced viscosity and saving costs.
Conclusion
In this paper, we summarized recent advances in improving the performance of PEMs by doping MOFs. MOFs have large specific surface area which can accommodate a large number of water molecules and acidic groups, which providing sufficient receptors for proton transfer. And different structures can be designed according to the needs. However, the high manufacturing cost and unstable performance of purely MOF material crystalline membranes restrict the application in the fuel cells. Preparation of composite membranes by adding MOFs particles into polymers matrix is a simple method. A large amount of hydrogen bonds in MOFs can interact with polymers matrix to build a tighter hydrogen bond network and form additional proton transport channels. The MOFs-modified PEMs have shown outstanding fuel cell performance at elevated temperatures and anhydrous conditions, which has great merit in commercial application.
Different types of MOFs added to the PEMs substrate have different mechanisms for improving composite membrane performance. According to the research and analysis of several typical MOFs materials applied to PEM, it can be seen that UiO-series MOFs have a high-density spatial structure, which endows outstanding stability for water, acid conditions and other solvents. The stability of the composite membranes can be significantly improved by doping UiO-series MOFs to the matrix. MIL-series MOFs in PEMs contain a large number of CUSs, which can provide abundant hydroxyl groups through hydrolysis to promote the conduction of protons in composite membranes. ZIF-series MOFs have high selectivity and good chemical stability in alkaline aqueous solution and other solvents due to their unique hydrophobic pore and surface structure. PEMs containing ZIF-series MOFs can greatly reduce methanol permeability and improve selectivity while maintaining outstanding chemical and thermal stability. In addition, increasing the content of acid groups and mixing several MOFs have exhibited astonishing effect on the improvement of performance.
Many achievements have been made in the research of MOF/PEM composite membranes, which has greatly improved the performance of PEMFCs. However, there still have many challenges in developing MOF/PEM composite membranes:
select the appropriate MOFs and polymer matrix to improve the performance of composite membranes comprehensively;
identify the dispersing conditions to ensure that MOFs dispersed uniformly on the polymer matrix;
optimize the doping concentration of MOFs to obtain the best performance composite membranes;
These aspects directly affect the conductivity, methanol resistance, chemical and thermal stability of the composite membranes, and have an indispensable effect on the improvement of the performance.
More attempts are devoted to preparing high degree of functionalization, preferably both MOFs and new PEMs segments. Thus, more MOF/PEM composite membranes architectures must be designed as new strategies in the rational design of next-generation PEMs materials with high electrochemical PEMFCs performances over a long period of time. Vigorously promoting the development of MOF/PEM composite membranes toward environment-friendly and commercialization.
Author Contributions
QL: writing-original draft preparation and validation. PZ: funding acquisition, conceptualization, methodology, investigation, and data curation. ZL, DW, ZL, XP, and CL: writing-review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was supported by General Program of Civil Aviation Flight University of China (Grant No. J2020-111), National Key R&D Program of China (Grant No. 2018YFC0809500), the National Natural Science Foundation of China (Grant Nos. U1633203 and U1733126), and Sichuan Science and Technology Program (Grant No. 2018GZYZF0069).
References
- Amdursky N., Wang X., Meredith P., Bradley D. D., Stevens M. M. (2016). Long-range proton conduction across free-standing serum albumin mats. Adv. Mater. Weinheim 28, 2692–2698. 10.1002/adma.201505337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anahidzade N., Abdolmaleki A., Dinari M., Firouz Tadavani K., Zhiani M. (2018). Metal-organic framework anchored sulfonated poly(ether sulfone) as a high temperature proton exchange membrane for fuel cells. J. Memb. Sci 565, 281–292. 10.1016/j.memsci.2018.08.037 [DOI] [Google Scholar]
- Barjola A., Escorihuela J., Andrio A., Gimenez E., Compan V. (2018). Enhanced conductivity of composite membranes based on sulfonated poly(Ether Ether Ketone) (SPEEK) with zeolitic imidazolate frameworks (ZIFs). Nanomaterials (Basel) 8. 10.3390/nano8121042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belieres J.-P., Angell C. A. (2007). Protic ionic liquids preparation, characterization, and proton free energy level representation. J. Phys. Chem. B 111, 4926–4937. 10.1021/jp067589u [DOI] [PubMed] [Google Scholar]
- Bose A. B., Gopu S., Li W. (2014). Enhancement of proton exchange membrane fuel cells performance at elevated temperatures and lower humidities by incorporating immobilized phosphotungstic acid in electrodes. J. Power Sources 263, 217–222. 10.1016/j.jpowsour.2014.04.043 [DOI] [Google Scholar]
- Bureekaew S., Horike S., Higuchi M., Mizuno M., Kawamura T., Tanaka D., et al. (2009). One-dimensional imidazole aggregate in aluminium porous coordination polymers with high proton conductivity. Nat. Mater. 8, 831–836. 10.1038/nmat2526 [DOI] [PubMed] [Google Scholar]
- Cai K., Sun F., Liang X., Liu C., Zhao N., Zou X., et al. (2017). An acid-stable hexaphosphate ester based metal–organic framework and its polymer composite as proton exchange membrane. J. Mater. Chem. A 5, 12943–12950. 10.1039/C7TA00169J [DOI] [Google Scholar]
- Cai Y. Y., Yang Q., Zhu Z. Y., Sun Q. H., Zhu A. M., Zhang Q. G., et al. (2019). Achieving efficient proton conduction in a MOF-based proton exchange membrane through an encapsulation strategy. J. Memb. Sci 590:117277 10.1016/j.memsci.2019.117277 [DOI] [Google Scholar]
- Cavka J. H., Jakobsen S., Olsbye U., Guillou N., Lamberti C., Bordiga S., et al. (2008). A New zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc 130, 13850–13851. 10.1021/ja8057953 [DOI] [PubMed] [Google Scholar]
- Chen H., Han S.-Y., Liu R.-H., Chen T.-F., Bi K.-L., Liang J.-B., et al. (2018). High conductive, long-term durable, anhydrous proton conductive solid-state electrolyte based on a metal-organic framework impregnated with binary ionic liquids: Synthesis, characteristic and effect of anion. J. Power Sources 376, 168–176. 10.1016/j.jpowsour.2017.11.089 [DOI] [Google Scholar]
- Chen P., Hao L., Wu W., Li Y., Wang J. (2016). Polymer-inorganic hybrid proton conductive membranes: Effect of the interfacial transfer pathways. Electrochim. Acta 212, 426–439. 10.1016/j.electacta.2016.07.001 [DOI] [Google Scholar]
- Dekel D. R. (2018). Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 375, 158–169. 10.1016/j.jpowsour.2017.07.117 [DOI] [Google Scholar]
- Deng R., Han W., Yeung K. L. (2019a). Confined PFSA/MOF composite membranes in fuel cells for promoted water management and performance. Catalysis Today 331, 12–17. 10.1016/j.cattod.2018.05.016 [DOI] [Google Scholar]
- Deng Y., Chi B., Li J., Wang G., Zheng L., Shi X., et al. (2019b). Atomic Fe-Doped MOF-derived carbon polyhedrons with high active-center density and ultra-high performance toward PEM fuel cells. Adv. Energy Mater. 9 1–8. 10.1002/aenm.201802856 [DOI] [Google Scholar]
- Deng Y., Chi B., Tian X., Cui Z., Liu E., Jia Q., et al. (2019c). g-C3N4 promoted MOF derived hollow carbon nanopolyhedra doped with high density/fraction of single Fe atoms as an ultra-high performance non-precious catalyst towards acidic ORR and PEM fuel cells. J. Mater. Chem. A 7, 5020–5030. 10.1039/C8TA11785C [DOI] [Google Scholar]
- Deng Y., Tian X., Chi B., Wang Q., Ni W., Gao Y., et al. (2020). Hierarchically open-porous carbon networks enriched with exclusive Fe–Nx active sites as efficient oxygen reduction catalysts towards acidic H2–O2 PEM fuel cell and alkaline Zn–air battery. Chem. Eng. J.l 390, 1–32. 10.1016/j.cej.2020.124479 [DOI] [Google Scholar]
- Denny M. S., Jr., Cohen S. M. (2015). In situ modification of metal-organic frameworks in mixed-matrix membranes. Angew. Chem. Int. Ed Engl 54, 9029–9032. 10.1002/anie.201504077 [DOI] [PubMed] [Google Scholar]
- Devautour-Vinot S., Maurin G., Serre C., Horcajada P., Paula da Cunha D., Guillerm V., et al. (2012). Structure and dynamics of the functionalized MOF Type UiO-66(Zr): NMR and dielectric relaxation spectroscopies coupled with DFT calculations. Chem. Mater. 24, 2168–2177. 10.1021/cm300863c [DOI] [Google Scholar]
- Díaz-Duran A. K., Roncaroli F. (2017). MOF derived mesoporous nitrogen doped carbons with high activity towards oxygen reduction. Electrochim. Acta 251, 638–650. 10.1016/j.electacta.2017.08.055 [DOI] [Google Scholar]
- Dietzel P. D. C., Blom R., Fjellvåg H. (2008). Base-induced formation of two magnesium metal-organic framework compounds with a bifunctional tetratopic ligand. Eur. J. Inorg. Chem 2008, 3624–3632. 10.1002/ejic.200701284 [DOI] [Google Scholar]
- Dong X.-Y., Li J.-J., Han Z., Duan P.-G., Li L.-K., Zang S.-Q. (2017). Tuning the functional substituent group and guest of metal–organic frameworks in hybrid membranes for improved interface compatibility and proton conduction. J. Mater. Chem. A 5, 3464–3474. 10.1039/C6TA07761G [DOI] [Google Scholar]
- Dong X. Y., Wang J. H., Liu S. S., Han Z., Tang Q. J., Li F. F., et al. (2018). Synergy between isomorphous acid and basic metal-organic frameworks for anhydrous proton conduction of low-cost hybrid membranes at high temperatures. ACS Appl. Mater. Interfaces 10, 38209–38216. 10.1021/acsami.8b12846 [DOI] [PubMed] [Google Scholar]
- Donnadio A., Narducci R., Casciola M., Marmottini F., D'Amato R., Jazestani M., et al. (2017). Mixed membrane matrices based on nafion/UiO-66/SO3H-UiO-66 nano-MOFs: revealing the effect of crystal size, sulfonation, and filler loading on the mechanical and conductivity properties. ACS Appl. Mater. Interfaces 9, 42239–42246. 10.1021/acsami.7b14847 [DOI] [PubMed] [Google Scholar]
- Dybtsev D. N., Ponomareva V. G., Aliev S. B., Chupakhin A. P., Gallyamov M. R., Moroz N. K., et al. (2014). High proton conductivity and spectroscopic investigations of metal-organic framework materials impregnated by strong acids. ACS Appl. Mater. Interfaces 6, 5161–5167. 10.1021/am500438a [DOI] [PubMed] [Google Scholar]
- Erkartal M., Usta H., Citir M., Sen U. (2016). Proton conducting poly(vinyl alcohol) (PVA)/ poly(2-acrylamido-2-methylpropane sulfonic acid) (PAMPS)/ zeolitic imidazolate framework (ZIF) ternary composite membrane. J. Memb. Sci 499, 156–163. 10.1016/j.memsci.2015.10.032 [DOI] [Google Scholar]
- Escorihuela J., Sahuquillo O., Garcia-Bernabe A., Gimenez E., Compan V. (2018). Phosphoric acid doped polybenzimidazole (PBI)/zeolitic imidazolate framework composite membranes with significantly enhanced proton conductivity under low humidity conditions. Nanomaterials (Basel) 8:10. 10.3390/nano8100775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Yu S., Guo K., Li J., Li G. (2019). Water-mediated proton conduction for a highly stable strontium-organic framework from imidazole multi-carboxylate ligand. Polyhedron 169, 1–7. 10.1016/j.poly.2019.04.059 [DOI] [Google Scholar]
- Furukawa H., Gándara F., Zhang Y.-B., Jiang J., Queen W. L., Hudson M. R., et al. (2014). Water adsorption in porous metal–organic frameworks and related materials. J. Am. Chem. Soc 136, 4369–4381. 10.1021/ja500330a [DOI] [PubMed] [Google Scholar]
- Garibay S. J., Cohen S. M. (2010). Isoreticular synthesis and modification of frameworks with the UiO-66 topology. Chem Commun (Camb) 46, 7700–7702. 10.1039/c0cc02990d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopfrich K., Li C. Y., Mames I., Bhamidimarri S. P., Ricci M., Yoo J., et al. (2016). Ion Channels made from a single membrane-spanning DNA duplex. Nano Lett 16, 4665–4669. 10.1021/acs.nanolett.6b02039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Jiang Z., Ying W., Chen L., Liu Y., Wang X., et al. (2018). A DNA-threaded ZIF-8 membrane with high proton conductivity and low methanol permeability. Adv Mater 30(2). 10.1002/adma.201705155 [DOI] [PubMed] [Google Scholar]
- Han R., Wu P. (2018). composite proton-exchange membrane with highly improved proton conductivity prepared by in situ crystallization of porous organic cage. ACS Appl Mater Interfaces 10, 18351–18358. 10.1021/acsami.8b04311 [DOI] [PubMed] [Google Scholar]
- He T., Xu X., Ni B., Wang H., Long Y., Hu W., et al. (2017). Fast and scalable synthesis of uniform zirconium-, hafnium-based metal-organic framework nanocrystals. Nanoscale 9, 19209–19215. 10.1039/C7NR06274E [DOI] [PubMed] [Google Scholar]
- Hooshyari K., Moradi M., Salarizadeh P. (2020). Novel nanocomposite membranes based on PBI and doped-perovskite nanoparticles as a strategy for improving PEMFC performance at high temperatures. Intern. J. Energy Res. 44, 2617–2633. 10.1002/er.5001 [DOI] [Google Scholar]
- Horike S., Umeyama D., Inukai M., Itakura T., Kitagawa S. (2012). Coordination-network-based ionic plastic crystal for anhydrous proton conductivity. J. Am. Chem. Soc 134, 7612–7615. 10.1021/ja301875x [DOI] [PubMed] [Google Scholar]
- Hupp J. T., Poeppelmeier K. R. (2005). Chemistry. Better living through nanopore chemistry. Science 309, 2008–2009. 10.1126/science.1117808 [DOI] [PubMed] [Google Scholar]
- Jia W., Tang B., Wu P. (2016). Novel composite PEM with long-range ionic nanochannels induced by carbon nanotube/graphene oxide nanoribbon composites. ACS Appl. Mater. Interfaces 8, 28955–28963. 10.1021/acsami.6b07467 [DOI] [PubMed] [Google Scholar]
- Jing F., Sun R., Wang S., Sun H., Sun G. (2020). Effect of the anode structure on the stability of a direct methanol fuel cell. Energy Fuels 34, 3850–3857. 10.1021/acs.energyfuels.9b03923 [DOI] [Google Scholar]
- Junoh H., Jaafar J., Nordin N., Ismail A. F., Othman M. H. D., Rahman M. A., et al. (2020). Performance of polymer electrolyte membrane for direct methanol fuel cell application: perspective on morphological structure. Membranes (Basel) 10:34. 10.3390/membranes10030034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandiah M., Usseglio S., Svelle S., Olsbye U., Lillerud K. P., Tilset M. (2010). Post-synthetic modification of the metal–organic framework compound UiO-66. J. Mater. Chem. 20:9848 10.1039/c0jm02416c [DOI] [Google Scholar]
- Karlsson L. E., Jannasch P., WesslØn B. (2002). Preparation and solution properties of amphiphilic sulfonated acrylamide copolymers. Macromol. Chem. Phys. 203, 686–694. 10.1002/1521-3935(20020301)203:43.0.CO;2-C [DOI] [Google Scholar]
- Katz M. J., Brown Z. J., Colon Y. J., Siu P. W., Scheidt K. A., Snurr R. Q., et al. (2013). A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. (Camb) 49, 9449–9451. 10.1039/c3cc46105j [DOI] [PubMed] [Google Scholar]
- Khdhayyer M. R., Esposito E., Fuoco A., Monteleone M., Giorno L., Jansen J. C., et al. (2017). Mixed matrix membranes based on UiO-66 MOFs in the polymer of intrinsic microporosity PIM-1. Sep. Purificat. Technol. 173, 304–313. 10.1016/j.seppur.2016.09.036 [DOI] [Google Scholar]
- Kitao T., Zhang Y., Kitagawa S., Wang B., Uemura T. (2017). Hybridization of MOFs and polymers. Chem. Soc. Rev 46, 3108–3133. 10.1039/C7CS00041C [DOI] [PubMed] [Google Scholar]
- Knebel A., Zhou C., Huang A., Zhang J., Kustov L., Caro J. (2018). Smart metal-organic frameworks (MOFs): switching gas permeation through MOF membranes by external stimuli. Chem. Eng. Technol. 41, 224–234. 10.1002/ceat.201700635 [DOI] [Google Scholar]
- Koros W. J., Zhang C. (2017). Materials for next-generation molecularly selective synthetic membranes. Nat. Mater 16, 289–297. 10.1038/nmat4805 [DOI] [PubMed] [Google Scholar]
- Li Z., He G., Zhang B., Cao Y., Wu H., Jiang Z., et al. (2014a). Enhanced proton conductivity of Nafion hybrid membrane under different humidities by incorporating metal-organic frameworks with high phytic acid loading. ACS Appl. Mater. Interfaces 6, 9799–9807. 10.1021/am502236v [DOI] [PubMed] [Google Scholar]
- Li Z., He G., Zhao Y., Cao Y., Wu H., Li Y., et al. (2014b). Enhanced proton conductivity of proton exchange membranes by incorporating sulfonated metal-organic frameworks. J. Power Sources 262, 372–379. 10.1016/j.jpowsour.2014.03.123 [DOI] [Google Scholar]
- Li Z., Wang W., Chen Y., Xiong C., He G., Cao Y., et al. (2016). Constructing efficient ion nanochannels in alkaline anion exchange membranes by the in situ assembly of a poly(ionic liquid) in metal–organic frameworks. J. Mater. Chem. A 4, 2340–2348. 10.1039/C5TA10452A [DOI] [Google Scholar]
- Li Y., Li Z., Wang W., Sun J. (2020). Self-healing and highly elastic fluorine-free proton exchange membranes comprised of poly(vinyl alcohol) derivative and phytic acid for durable fuel cells. Sci. China Mater. 10.1007/s40843-020-1308-y. [Epub ahead of print]. [DOI] [Google Scholar]
- Liang X., Zhang F., Feng W., Zou X., Zhao C., Na H., et al. (2013). From metal–organic framework (MOF) to MOF–polymer composite membrane: enhancement of low-humidity proton conductivity. Chem. Sci. 4, 983–992. 10.1039/C2SC21927A [DOI] [Google Scholar]
- Lin K. A., Yang H., Hsu F. K. (2018). Zr-metal organic framework and derivatives for adsorptive and photocatalytic removal of acid dyes. Water Environ. Res. 90, 144–154. 10.2175/106143017X15054988926604 [DOI] [PubMed] [Google Scholar]
- Liu C., Zhang G., Zhao C., Li X., Li M., Na H. (2014). MOFs synthesized by the ionothermal method addressing the leaching problem of IL-polymer composite membranes. Chem. Commun. (Camb) 50, 14121–14124. 10.1039/C4CC05526H [DOI] [PubMed] [Google Scholar]
- Liu C., Zhao N., Zou X., Zhu G. (2018). Synthesis of an ultra-stable metal–organic framework for proton conduction. Cryst. Eng. Comm. 20, 3158–3161. 10.1039/C8CE00564H [DOI] [Google Scholar]
- Liu Q., Li Y., Zheng L., Shang J., Liu X., Yu R., et al. (2020). Sequential synthesis and active-site coordination principle of precious metal single-atom catalysts for oxygen reduction reaction and PEM fuel cells. Adv. Energy Mater. 10, 1–8. 10.1002/aenm.202000689 [DOI] [Google Scholar]
- Liu X., Demir N. K., Wu Z., Li K. (2015). Highly water-stable zirconium metal-organic framework UiO-66 membranes supported on alumina hollow fibers for desalination. J. Am. Chem. Soc. 137, 6999–7002. 10.1021/jacs.5b02276 [DOI] [PubMed] [Google Scholar]
- Loiseau T., Serre C., Huguenard C., Fink G., Taulelle F., Henry M., et al. (2004). A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chemistry 10, 1373–1382. 10.1002/chem.200305413 [DOI] [PubMed] [Google Scholar]
- Luo H. B., Ren Q., Wang P., Zhang J., Wang L., Ren X. M. (2019). High proton conductivity achieved by encapsulation of imidazole molecules into proton-conducting MOF-808. ACS Appl. Mater. Interfaces 11, 9164–9171. 10.1021/acsami.9b01075 [DOI] [PubMed] [Google Scholar]
- Luo H. B., Wang M., Liu S. X., Xue C., Tian Z. F., Zou Y., et al. (2017). Proton conductance of a superior water-stable metal-organic framework and its composite membrane with poly(vinylidene fluoride). Inorg. Chem 56, 4169–4175. 10.1021/acs.inorgchem.7b00122 [DOI] [PubMed] [Google Scholar]
- Luo H. B., Wang M., Zhang J., Tian Z. F., Zou Y., Ren X. M. (2018). Open-framework chalcogenide showing both intrinsic anhydrous and water-assisted high proton conductivity. ACS Appl. Mater. Interfaces 10, 2619–2627. 10.1021/acsami.7b17189 [DOI] [PubMed] [Google Scholar]
- Lv H., Wang S., Li J. K., Shao C. F., Zhou W., Shen X. J., et al. (2016). Self-assembled RuO2@IrOx core-shell nanocomposite as high efficient anode catalyst for PEM water electrolyzer. Appl. Surf. Sci 514, 145943–145975. 10.1016/j.apsusc.2020.145943 [DOI] [Google Scholar]
- Makiura R., Motoyama S., Umemura Y., Yamanaka H., Sakata O., Kitagawa H. (2010). Surface nano-architecture of a metal-organic framework. Nat. Mater 9, 565–571. 10.1038/nmat2769 [DOI] [PubMed] [Google Scholar]
- Matoga D., Oszajca M., Molenda M. (2015). Ground to conduct: mechanochemical synthesis of a metal-organic framework with high proton conductivity. Chem Commun (Camb) 51, 7637–7640. 10.1039/C5CC01789K [DOI] [PubMed] [Google Scholar]
- Matthew R., DeStefano T. I., Sergio J., Garibay J. T. H., Farha O. K. (2016). Room temperature synthesis of UiO-66 and the thermal modulation of densities of defect sites. Chem.Mater. 29, 1357–1361. 10.1021/acs.chemmater.6b05115 [DOI] [Google Scholar]
- Moreno N. G., Molina M. C., Gervasio D., Robles J. F. P. (2015). Approaches to polymer electrolyte membrane fuel cells (PEMFCs) and their cost. Renew. Sustainable Energy Rev. 52, 897–906. 10.1016/j.rser.2015.07.157 [DOI] [Google Scholar]
- Mukhopadhyay S., Debgupta J., Singh C., Sarkar R., Basu O., Das S. K. (2019). Designing UiO-66-based superprotonic conductor with the highest metal-organic framework based proton conductivity. ACS Appl. Mater. Interfaces 11, 13423–13432. 10.1021/acsami.9b01121 [DOI] [PubMed] [Google Scholar]
- Nagle J. F., Tristram-Nagle S. (1983). Hydrogen bonded chain mechanisms for proton conduction and proton pumping. The Journal of Membrane Biology 74(1), 1-14. 10.1007/BF01870590 [DOI] [PubMed] [Google Scholar]
- Ohkoshi S.-,I., Nakagawa K., Tomono K., Imoto K., Tsunobuchi Y., Tokoro H. (2010). High proton conductivity in prussian blue analogues and the interference effect by magnetic ordering. J. Am. Chem. Soc 132, 6620–6621. 10.1021/ja100385f [DOI] [PubMed] [Google Scholar]
- Ono K., Ishizaki M., Kanaizuka K., Togashi T., Yamada T., Kitagawa H., et al. (2017). Grain-boundary-free super-proton conduction of a solution-processed prussian-blue nanoparticle film. Angew. Chem. Int. Ed. Engl. 56, 5531–5535. 10.1002/anie.201701759 [DOI] [PubMed] [Google Scholar]
- Ordinario D. D., Phan L., Walkup W. G. t., Jocson J. M., Karshalev E., et al. (2014). Bulk protonic conductivity in a cephalopod structural protein. Nat. Chem 6, 596–602. 10.1038/nchem.1960 [DOI] [PubMed] [Google Scholar]
- Pardo E., Train C., Gontard G., Boubekeur K., Fabelo O., Liu H., et al. (2011). High proton conduction in a chiral ferromagnetic metal-organic quartz-like framework. J. Am. Chem. Soc 133, 15328–15331. 10.1021/ja206917z [DOI] [PubMed] [Google Scholar]
- Park K. S., Ni Z., Cote A. P., Choi J. Y., Huang R., Uribe-Romo F. J., et al. (2006). Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. U.S.A. 103, 10186–10191. 10.1073/pnas.0602439103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H. A., Mansor N., Gadipelli S., Brett D. J., Guo Z. (2016). Superacidity in Nafion/MOF hybrid membranes retains water at low humidity to enhance proton conduction for fuel cells. ACS Appl. Mater. Interfaces 8, 30687–30691. 10.1021/acsami.6b12240 [DOI] [PubMed] [Google Scholar]
- Pili S., Argent S. P., Morris C. G., Rought P., Garcia-Sakai V., Silverwood I. P., et al. (2016). Proton conduction in a phosphonate-based metal-organic framework mediated by intrinsic “free diffusion inside a sphere”. J. Am. Chem. Soc 138, 6352–6355. 10.1021/jacs.6b02194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourrahmani H., Moghimi M., Siavashi M. (2019). Thermal management in PEMFCs: The respective effects of porous media in the gas flow channel. Int. J. Hydrogen Energy 44, 3121–3137. 10.1016/j.ijhydene.2018.11.222 [DOI] [Google Scholar]
- Rao Z., Feng K., Tang B., Wu P. (2017a). Construction of well interconnected metal-organic framework structure for effectively promoting proton conductivity of proton exchange membrane. J. Memb. Sci. 533, 160–170. 10.1016/j.memsci.2017.03.031 [DOI] [Google Scholar]
- Rao Z., Tang B., Wu P. (2017b). Proton Conductivity of proton exchange membrane synergistically promoted by different functionalized metal-organic frameworks. ACS Appl. Mater. Interfaces 9, 22597–22603. 10.1021/acsami.7b05969 [DOI] [PubMed] [Google Scholar]
- Rivera-Gavidia L. M., Luis-Sunga M., Rodríguez J. L., Pastor E., García G. (2020). Methanol tolerant Pd-Based carbon supported catalysts as cathode materials for direct methanol fuel cells. Int. J. Hydrogen Energy. 10.1016/j.ijhydene.2020.01.167. [Epub ahead of print]. [DOI] [Google Scholar]
- Ru C., Li Z., Zhao C., Duan Y., Zhuang Z., Bu F., et al. (2018). Enhanced proton conductivity of sulfonated hybrid poly(arylene ether ketone) membranes by incorporating an amino-sulfo bifunctionalized metal-organic framework for direct methanol fuel cells. ACS Appl. Mater. Interfaces 10, 7963–7973. 10.1021/acsami.7b17299 [DOI] [PubMed] [Google Scholar]
- Sadakiyo M., Okawa H., Shigematsu A., Ohba M., Yamada T., Kitagawa H. (2012). Promotion of low-humidity proton conduction by controlling hydrophilicity in layered metal-organic frameworks. J. Am. Chem. Soc 134, 5472–5475. 10.1021/ja300122r [DOI] [PubMed] [Google Scholar]
- Sadakiyo M., Yamada T., Kitagawa H. (2009). Rational designs for highly proton-conductive metal–organic frameworks. J. Am. Chem. Soc 131, 9906–9907. 10.1021/ja9040016 [DOI] [PubMed] [Google Scholar]
- Sanda S., Biswas S., Konar S. (2015). Study of proton conductivity of a 2D flexible MOF and a 1D coordination polymer at higher temperature. Inorg. Chem 54, 1218–1222. 10.1021/ic502098h [DOI] [PubMed] [Google Scholar]
- Santic A., Wrobel W., Mutke M., Banhatti R. D., Funke K. (2009). Frequency-dependent fluidity and conductivity of an ionic liquid. Phys. Chem. Chem. Phys 11, 5930–5934. 10.1039/b904186a [DOI] [PubMed] [Google Scholar]
- Sen U., Erkartal M., Kung C. W., Ramani V., Hupp J. T., Farha O. K. (2016). Proton Conducting Self-Assembled Metal-Organic Framework/Polyelectrolyte Hollow Hybrid Nanostructures. ACS Appl. Mater. Interfaces 8, 23015–23021. 10.1021/acsami.6b05901 [DOI] [PubMed] [Google Scholar]
- Shaari N., Kamarudin S. K., Basri S., Shyuan L. K., Masdar M. S., Nordin D. (2018). Enhanced proton conductivity and methanol permeability reduction via sodium alginate electrolyte-sulfonated graphene oxide bio-membrane. Nanoscale Res. Lett 13:1. 10.1186/s11671-018-2493-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M. N., Gonzalez M. A., McCarthy M. C., Jeong H. K. (2013). An unconventional rapid synthesis of high performance metal-organic framework membranes. Langmuir 29, 7896–7902. 10.1021/la4014637 [DOI] [PubMed] [Google Scholar]
- Shao Y., Yin G., Wang Z., Gao Y. (2007). Proton exchange membrane fuel cell from low temperature to high temperature: material challenges. J. Power Sources 167, 235–242. 10.1016/j.jpowsour.2007.02.065 [DOI] [Google Scholar]
- Shekhah O., Liu J., Fischer R. A., Woll C. (2011). MOF thin films: existing and future applications. Chem. Soc. Rev 40, 1081–1106. 10.1039/c0cs00147c [DOI] [PubMed] [Google Scholar]
- Shi G. M., Yang T., Chung T. S. (2012). Polybenzimidazole (PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Memb. Sci 415–416, 577–586. 10.1016/j.memsci.2012.05.052 [DOI] [Google Scholar]
- Shiqiang Z., Lin L., Bharath B., Lee E. S. (2016). New nitrogen-doped graphene_MOF-modified catalyst for fuel cell systems. Ecs Trans. 72, 149–154. 10.1149/07208.0149ecst [DOI] [Google Scholar]
- Stoimenovski J., Izgorodina E. I., MacFarlane D. R. (2010). Ionicity and proton transfer in protic ionic liquids. Phys. Chem. Chem. Phys 12, 10341–10347. 10.1039/c0cp00239a [DOI] [PubMed] [Google Scholar]
- Sun H., Tang B., Wu P. (2017a). Rational design of S-UiO-66@GO hybrid nanosheets for proton exchange membranes with significantly enhanced transport performance. ACS Appl. Mater. Interfaces 9, 26077–26087. 10.1021/acsami.7b07651 [DOI] [PubMed] [Google Scholar]
- Sun H., Tang B., Wu P. (2017b). Two-dimensional zeolitic imidazolate framework/carbon nanotube hybrid networks modified proton exchange membranes for improving transport properties. ACS Appl. Mater. Interfaces 9, 35075–35085. 10.1021/acsami.7b13013 [DOI] [PubMed] [Google Scholar]
- Sun X. L., Deng W. H., Chen H., Han H. L., Taylor J. M., Wan C. Q., et al. (2017c). A metal-organic framework impregnated with a binary ionic liquid for safe proton conduction above 100 °C. Chemistry 23, 1248–1252. 10.1002/chem.201605215 [DOI] [PubMed] [Google Scholar]
- Tan J. C., Mahdi E. M. (2016). Dynamic molecular interactions between polyurethane and ZIF-8 in a polymer-MOF nanocomposite: microstructural, thermo-mechanical and viscoelastic effects. Polymer 97, 31–43. 10.1016/j.polymer.2016.05.012 [DOI] [Google Scholar]
- Taylor J. M., Dekura S., Ikeda R., Kitagawa H. (2015). Defect control to enhance proton conductivity in a metal–organic framework. Chem. Mater. 27, 2286–2289. 10.1021/acs.chemmater.5b00665 [DOI] [Google Scholar]
- Teixeira F. C., de S.á A. I., Teixeira A. P. S., Ortiz-Martínez V. M., Ortiz A., Ortiz I., et al. (2020). New modified Nafion-bisphosphonic acid composite membranes for enhanced proton conductivity and PEMFC performance. Int. J. Hydrogen Energy. 10.1016/j.ijhydene.2020.01.212. [Epub ahead of print]. [DOI] [Google Scholar]
- Tsai C.-H., Wang C.-C., Chang C.-Y., Lin C.-H., Chen-Yang Y. W. (2014). Enhancing performance of Nafion ® -based PEMFC by 1-D channel metal-organic frameworks as PEM filler. Int. J. Hydrogen Energy 39, 15696–15705. 10.1016/j.ijhydene.2014.07.134 [DOI] [Google Scholar]
- Van Goethem C., Verbeke R., Pfanmöller M., Koschine T., Dickmann M., Timpel-Lindner T., et al. (2018). The role of MOFs in Thin-Film Nanocomposite (TFN) membranes. J. Memb. Sci. 563, 938–948. 10.1016/j.memsci.2018.06.040 [DOI] [Google Scholar]
- Vega J., Andrio A., Lemus A. A., del Castillo L. F., Compañ V. (2017). Conductivity study of Zeolitic Imidazolate Frameworks, Tetrabutylammonium hydroxide doped with Zeolitic Imidazolate Frameworks, and mixed matrix membranes of Polyetherimide/Tetrabutylammonium hydroxide doped with Zeolitic Imidazolate Frameworks for proton conducting applications. Electrochim. Acta 258, 153–166. 10.1016/j.electacta.2017.10.095 [DOI] [Google Scholar]
- Wang J., Gong C., Wen S., Liu H., Qin C., Xiong C., et al. (2019). A facile approach of fabricating proton exchange membranes by incorporating polydopamine-functionalized carbon nanotubes into chitosan. Int. J. Hydrogen Energy 44, 6909–6918. 10.1016/j.ijhydene.2019.01.194 [DOI] [Google Scholar]
- Wu B., Liang G., Lin X., Wu L., Luo J., Xu T. (2014a). Immobilization of N-(3-aminopropyl)-imidazole through MOFs in proton conductive membrane for elevated temperature anhydrous applications. J. Memb. Sci 458, 86–95. 10.1016/j.memsci.2014.01.059 [DOI] [Google Scholar]
- Wu B., Lin X., Ge L., Wu L., Xu T. (2013a). A novel route for preparing highly proton conductive membrane materials with metal-organic frameworks. Chem. Commun. (Camb) 49, 143–145. 10.1039/C2CC37045J [DOI] [PubMed] [Google Scholar]
- Wu B., Pan J., Ge L., Wu L., Wang H., Xu T. (2014b). Oriented MOF-polymer composite nanofiber membranes for high proton conductivity at high temperature and anhydrous condition. Sci. Rep. 4:4334. 10.1038/srep04334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Chua Y. S., Krungleviciute V., Tyagi M., Chen P., Yildirim T., et al. (2013b). Unusual and highly tunable missing-linker defects in zirconium metal-organic framework UiO-66 and their important effects on gas adsorption. J. Am. Chem. Soc. 135, 10525–10532. 10.1021/ja404514r [DOI] [PubMed] [Google Scholar]
- Wu H., Yang F., Lv X.-L., Wang B., Zhang Y.-Z., Zhao M.-J., et al. (2017). A stable porphyrinic metal–organic framework pore-functionalized by high-density carboxylic groups for proton conduction. J. Mater. Chem. A 5, 14525–14529. 10.1039/C7TA03917D [DOI] [Google Scholar]
- Yamada T., Sadakiyo M., Kitagawa H. (2009). High proton conductivity of one-dimensional ferrous oxalate dihydrate. J. Am. Chem. Soc 131, 3144–3145. 10.1021/ja808681m [DOI] [PubMed] [Google Scholar]
- Yan X., Li H., Lin C., Chen J., Han A., Shen S., et al. (2020). An inorganic-framework proton exchange membrane for direct methanol fuel cells with increased energy density. Sustain. Energy Fuels 4, 772–778. 10.1039/C9SE00865A [DOI] [Google Scholar]
- Yang D., Bernales V., Islamoglu T., Farha O. K., Hupp J. T., Cramer C. J., et al. (2016). Tuning the surface chemistry of metal organic framework nodes: proton topology of the metal-oxide-like Zr6 nodes of UiO-66 and NU-1000. J. Am. Chem. Soc 138, 15189–15196. 10.1021/jacs.6b08273 [DOI] [PubMed] [Google Scholar]
- Yang F., Huang H., Wang X., Li F., Gong Y., Zhong C., et al. (2015a). Proton conductivities in functionalized UiO-66: tuned properties, thermogravimetry mass, and molecular simulation analyses. Cryst. Growth Des. 15, 5827–5833. 10.1021/acs.cgd.5b01190 [DOI] [Google Scholar]
- Yang L., Tang B., Wu P. (2015b). Metal–organic framework–graphene oxide composites: a facile method to highly improve the proton conductivity of PEMs operated under low humidity. J. Mater. Chem. A 3, 15838–15842. 10.1039/C5TA03507D [DOI] [Google Scholar]
- Yang Q., Wiersum A. D., Llewellyn P. L., Guillerm V., Serre C., Maurin G. (2011). Functionalizing porous zirconium terephthalate UiO-66(Zr) for natural gas upgrading: a computational exploration. Chem. Commun. (Camb) 47, 9603–9605. 10.1039/c1cc13543k [DOI] [PubMed] [Google Scholar]
- Yang X., Zhang G., Du L., Zhang J., Chiang F.-K., et al. (2020). PGM-Free Fe/N/C and ultralow loading Pt/C hybrid cathode catalysts with enhanced stability and activity in PEM fuel cells. ACS Appl. Mater. Interfaces 12, 13739–13749. 10.1021/acsami.9b18085 [DOI] [PubMed] [Google Scholar]
- Yang X. B., Zhao L., Goh K., Sui X. L., Meng L. H., Wang Z. B. (2019). Ultra-high ion selectivity of a modified nafion composite membrane for vanadium redox flow battery by incorporation of phosphotungstic acid coupled UiO-66-NH2. Chem. Select. 4, 4633–4641. 10.1002/slct.201900888 [DOI] [Google Scholar]
- Yuan W., Zhang X., Hou C., Zhang Y., Wang H., Liu X. (2020). Enhanced water management via the optimization of cathode microporous layer using 3D graphene frameworks for direct methanol fuel cell. J. Power Sources 451:227800 10.1016/j.jpowsour.2020.227800 [DOI] [Google Scholar]
- Zeng S., Lyu F., Sun L., Zhan Y., Ma F.-X., Lu J., et al. (2019). UiO-66-NO2 as an oxygen “pump” for enhancing oxygen reduction reaction performance. Chem. Mater. 31, 1646–1654. 10.1021/acs.chemmater.8b04934 [DOI] [Google Scholar]
- Zhang B., Cao Y., Li Z., Wu H., Yin Y., Cao L., et al. (2017). Proton exchange nanohybrid membranes with high phosphotungstic acid loading within metal-organic frameworks for PEMFC applications. Electrochim. Acta 240, 186–194. 10.1016/j.electacta.2017.04.087 [DOI] [Google Scholar]
- Zhang K., Lively R. P., Zhang C., Koros W. J., Chance R. R. (2013). Investigating the intrinsic ethanol/water separation capability of ZIF-8: an adsorption and diffusion study. J. Phys. Chem. C 117, 7214–7225. 10.1021/jp401548b [DOI] [Google Scholar]
- Zhang X., Liu Q., Xia L., Huang D., Fu X., Zhang R., et al. (2019). Poly(2,5-benzimidazole)/sulfonated sepiolite composite membranes with low phosphoric acid doping levels for PEMFC applications in a wide temperature range. J. Memb. Sci. 574, 282–298. 10.1016/j.memsci.2018.12.085 [DOI] [Google Scholar]
- Zhao Y., Li X., Wang S., Li W., Wang X., Chen S., et al. (2017). Proton exchange membranes prepared via atom transfer radical polymerization for proton exchange membrane fuel cell: Recent advances and perspectives. Int. J. Hydrogen Energy 42, 30013–30028. 10.1016/j.ijhydene.2017.08.167 [DOI] [Google Scholar]
- Zhao Y., Li X., Wang Z., Xie X., Qian W. (2019). Preparation of graft poly(Arylene Ether Sulfone)s-based copolymer with enhanced phase-separated morphology as proton exchange membranes via atom transfer radical polymerization. Polymers (Basel) 11:297. 10.3390/polym11081297 [DOI] [PMC free article] [PubMed] [Google Scholar]