This randomized clinical trial evaluates the health effects of using oral sustained-release morphine to treat moderate to very severe breathlessness in patients with advanced chronic obstructive pulmonary disease.
Key Points
Question
Does regular, low-dose, oral sustained-release morphine improve disease-specific health status or cause respiratory adverse effects in patients with moderate to very severe chronic breathlessness due to advanced chronic obstructive pulmonary disease?
Findings
In this randomized clinical trial of 111 patients with chronic obstructive pulmonary disease, morphine significantly improved Chronic Obstructive Pulmonary Disease Assessment Test scores. No clinically relevant respiratory adverse effects occurred during 4 weeks of treatment.
Meaning
Use of regular, low-dose, oral sustained-release morphine for 4 weeks may have a positive effect on Chronic Obstructive Pulmonary Disease Assessment Test scores in patients with moderate to severe breathlessness without causing respiratory adverse effects, confirming its current role in palliative treatment for chronic breathlessness.
Abstract
Importance
Morphine is used as palliative treatment of chronic breathlessness in patients with advanced chronic obstructive pulmonary disease (COPD). Evidence on respiratory adverse effects and health status is scarce and conflicting.
Objective
To assess the effects of regular, low-dose, oral sustained-release morphine on disease-specific health status (COPD Assessment Test; CAT), respiratory outcomes, and breathlessness in patients with COPD.
Interventions
Participants were randomly assigned to 10 mg of regular, oral sustained-release morphine or placebo twice daily for 4 weeks, with the possibility to increase to 3 times daily after 1 or 2 weeks.
Design, Setting, and Participants
The Morphine for Treatment of Dyspnea in Patients With COPD (MORDYC) study was a randomized, double-blind, and placebo-controlled study of a 4-week intervention. Patients were enrolled between November 1, 2016, and January 24, 2019. Participants were recruited in a pulmonary rehabilitation center and 2 general hospitals after completion of a pulmonary rehabilitation program. Outpatients with COPD and moderate to very severe chronic breathlessness (modified Medical Research Council [mMRC] breathlessness grades 2-4) despite optimal pharmacological and nonpharmacological treatment were included. A total of 1380 patients were screened, 916 were ineligible, and 340 declined to participate.
Main Outcomes and Measures
Primary outcomes were CAT score (higher scores represent worse health status) and arterial partial pressure of carbon dioxide (Paco2). Secondary outcome was breathlessness in the previous 24 hours (numeric rating scale). Data were analyzed by intention to treat. Subgroup analyses in participants with mMRC grades 3 to 4 were performed.
Results
A total of 111 of 124 included participants were analyzed (mean [SD] age, 65.4 [8.0] years; 60 men [54%]). Difference in CAT score was 2.18 points lower in the morphine group (95% CI, –4.14 to –0.22 points; P = .03). Difference in Paco2 was 1.19 mm Hg higher in the morphine group (95% CI, –2.70 to 5.07 mm Hg; P = .55). Breathlessness remained unchanged. Worst breathlessness improved in participants with mMRC grades 3 to 4 (1.33 points lower in the morphine group; 95% CI, –2.50 to –0.16 points; P = .03). Five participants of 54 in the morphine group (9%) and 1 participant of 57 in the placebo group (2%) withdrew because of adverse effects. No morphine-related hospital admissions or deaths occurred.
Conclusions and Relevance
In this randomized clinical trial, regular, low-dose, oral sustained-release morphine for 4 weeks improved disease-specific health status in patients with COPD without affecting Paco2 or causing serious adverse effects. The worst breathlessness improved in participants with mMRC grades 3 to 4. A larger randomized clinical trial with longer follow-up in patients with mMRC grades 3 to 4 is warranted.
Trial Registration
ClinicalTrials.gov Identifier: NCT02429050
Introduction
Chronic breathlessness is one of the most frequently reported symptoms of patients with advanced chronic obstructive pulmonary disease (COPD).1,2 The underlying pathophysiology is complex, and it has a considerable effect on prognosis and health status (defined as the effect of health on the ability to perform and derive fulfillment from activities of daily life, including health-related quality of life and functional status3).4,5 Breathlessness management is an important treatment goal.6 Previous authors proposed palliative pharmacological treatment with low-dose opioids for patients with refractory breathlessness despite optimal pharmacological and nonpharmacological treatment.7 This recommendation has been included in international and national guidelines.8,9,10 Evidence for this recommendation is still limited. Two meta-analyses reported small improvements in breathlessness after opioid treatment in patients with different life-limiting illnesses.11,12 Analyses in patients with COPD treated for at least 4 days showed an improvement of 5 to 12 points on a 0 to 100 visual analog scale.11,12 No effect on health status or functional performance could be shown because only a few studies included small populations. A recent study by Currow et al13 prescribing regular, low-dose, oral sustained-release morphine for 1 week to patients with chronic breathlessness due to several conditions also showed no change in health status and suggested morphine will only reduce breathlessness in patients with severe chronic breathlessness.
Moreover, physicians remain reluctant to prescribe opioids for breathlessness in COPD for fear of respiratory depression.14,15 A recent systematic review found no evidence for respiratory adverse effects after treatment with low-dose opioids for chronic breathlessness.16 However, most studies were small, and only a few measured arterial blood gases. To our knowledge, no large randomized clinical trials (RCTs) adequately powered to measure the effect of opioids on respiratory outcomes have yet been conducted.
Therefore, the primary aims of the Morphine for Treatment of Dyspnea in Patients With COPD (MORDYC) study17 were to assess (1) whether and to what extent regular, low-dose, oral sustained-release morphine improves disease-specific health status in patients with moderate to very severe chronic breathlessness due to advanced COPD and (2) whether and to what extent regular, low-dose, oral sustained-release morphine leads to respiratory adverse effects. Secondary aims were to assess the effect of regular, low-dose, oral sustained-release morphine on functional performance and breathlessness.
Methods
Study Design
The MORDYC study is a randomized, double-blind, placebo-controlled, parallel-arm intervention study.17 Participants were treated with regular, low-dose, oral sustained-release morphine or placebo for 4 weeks. The study protocol (Supplement 1) was approved by the medical ethics committee of Maastricht University Medical Center (METC152002). All participants provided written informed consent. Results are reported according to the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Participants
Adult patients with a confirmed diagnosis of COPD based on the Global Initiative for Obstructive Lung Disease (postbronchodilator forced expiratory volume in 1 second per forced vital capacity ratio <0.70)6 were recruited from CIRO (a center of expertise for chronic organ failure in Horn, the Netherlands); Zuyderland Hospital in Heerlen, the Netherlands; and VieCuri Medical Center in Venlo, the Netherlands. Inclusion criteria were modified Medical Research Council (mMRC) breathlessness grades 2, 3, or 418 despite optimal pharmacological and nonpharmacological treatment, including having completed a pulmonary rehabilitation program.6 See eMethods 1 in Supplement 2 for details.
Randomization and Blinding
Randomization was performed by a web-based random number generator using minimization and stratification for age (<55 years; 55-65 years; 65-75 years; or >75 years) and mMRC grade.19 Participants and investigators were blinded.
Study Procedures
Participants received 10 mg of regular, oral sustained-release morphine or placebo twice daily. The dose could be adjusted to 3 times daily after 1 or 2 weeks in nonresponders (<1 point improvement in severity of mean breathlessness on a 0 to 10 numeric rating scale [NRS] compared with baseline20,21). All participants received a prescription for macrogol (13.8 g) once daily and metoclopramide (10 mg) 3 times daily, both as needed.
After baseline, participants were contacted by phone after 2 days and 3 weeks. Home visits took place after 1 and 2 weeks (Figure 1).
Figure 1. Study Design.
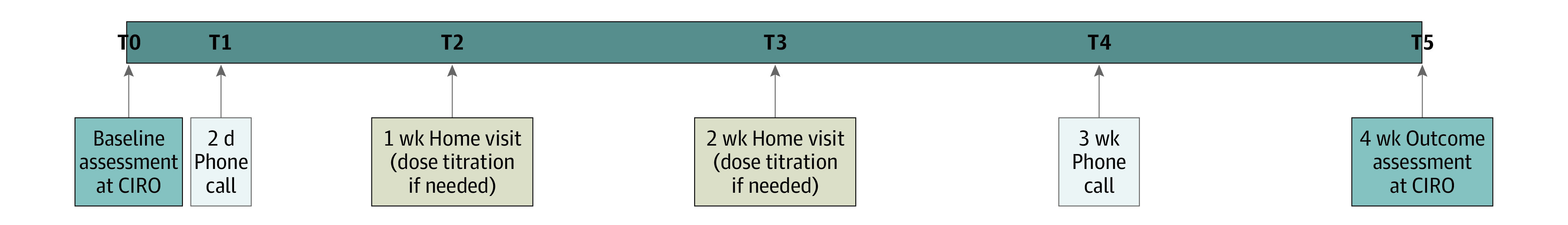
T indicates time.
Outcomes
At baseline, demographic and clinical characteristics were collected from the patient file or based on self-report. Disease-specific health status was determined using the COPD Assessment Test22,23 (CAT) (higher scores represent worse health status, minimal clinical important difference [MCID] 2.0-3.0 points24) at time T0, T2, T3, and T5. Arterial partial pressure of carbon dioxide (Paco2) was assessed in arterial blood at T0 and T5. A priori, the project group defined a change of 7.5 mm Hg as clinically relevant.17
Secondary outcomes included functional performance, respiratory outcomes, and severity of breathlessness. Functional performance consisted of functional exercise performance (6-minute walk test [6MWT]25), general mobility (Timed Up and Go [TUG] test26), and care dependency (Care Dependency Scale [CDS]27,28). The 6MWT and CDS were assessed at T0 and T5, and the TUG test was performed at T0, T2, T3, and T5.
Secondary respiratory outcomes included (1) partial arterial pressure of oxygen (Pao2), arterial oxygen saturation (Sao2), percentage of time that the overnight pulse oxygen saturation (SpO2) was below 90%, mean overnight SpO2, and lung function at T0 and T5; and (2) respiratory rate (RR), transcutaneous carbon dioxide pressure (PtcCO2), and transcutaneous SpO2 at T0, T2, T3, and T5.
Severity of mean and worst breathlessness in the previous 24 hours was self-reported at T0 to T5 on a 0 to 10 NRS,29 with 0 being not breathless at all and 10 being the worst imaginable breathlessness.
Morphine-related adverse effects, medication use, and incidence of acute COPD exacerbations or hospitalizations were discussed during T0 to T5 (eMethods 2 in Supplement 2).
Sample Size
To detect a mean (SD) change in CAT of 3.8 (6.1) points (at the time of study design, the estimated MCID22,30), 54 participants per group were needed (significance level 5%, power 90%). Furthermore, 10 participants per group were needed to detect a mean (SD) change in Paco2 of 7.5 (5.331) mm Hg . Considering a dropout rate of 13%,32 62 participants per group needed to be included.
Statistical Analysis
Continuous data were described as mean (SD) or median (interquartile range). Categorical data were shown as number (percentage).
Given the longitudinal nature of the data, mean change between the morphine and placebo group was assessed, including time by group interaction. For Paco2, Pao2, overnight oximetry, lung function, 6MWT, and CDS, a linear regression model was developed. For CAT, RR, PtcCO2, SpO2, TUG, and NRS, a linear mixed-effects model was developed. Different covariance structures were considered (eTable 1 in Supplement 2), and the best-fitting model was selected using χ2 tests. Mean difference (95% CI) between groups was presented. Post hoc subgroup analyses were performed in the original study population with mMRC grades 3 to 4 at baseline. Furthermore, post hoc analyses of the CAT on item level were performed.
Analyses were performed according to intention to treat but excluding participants who withdrew between randomization and exposure to the intervention.33 For the analyses, SPSS version 25.0 (IBM Corp) was used. A 2-sided level of significance was set at P ≤ .05. Data were analyzed from September 11, 2019, to May 11, 2020.
Results
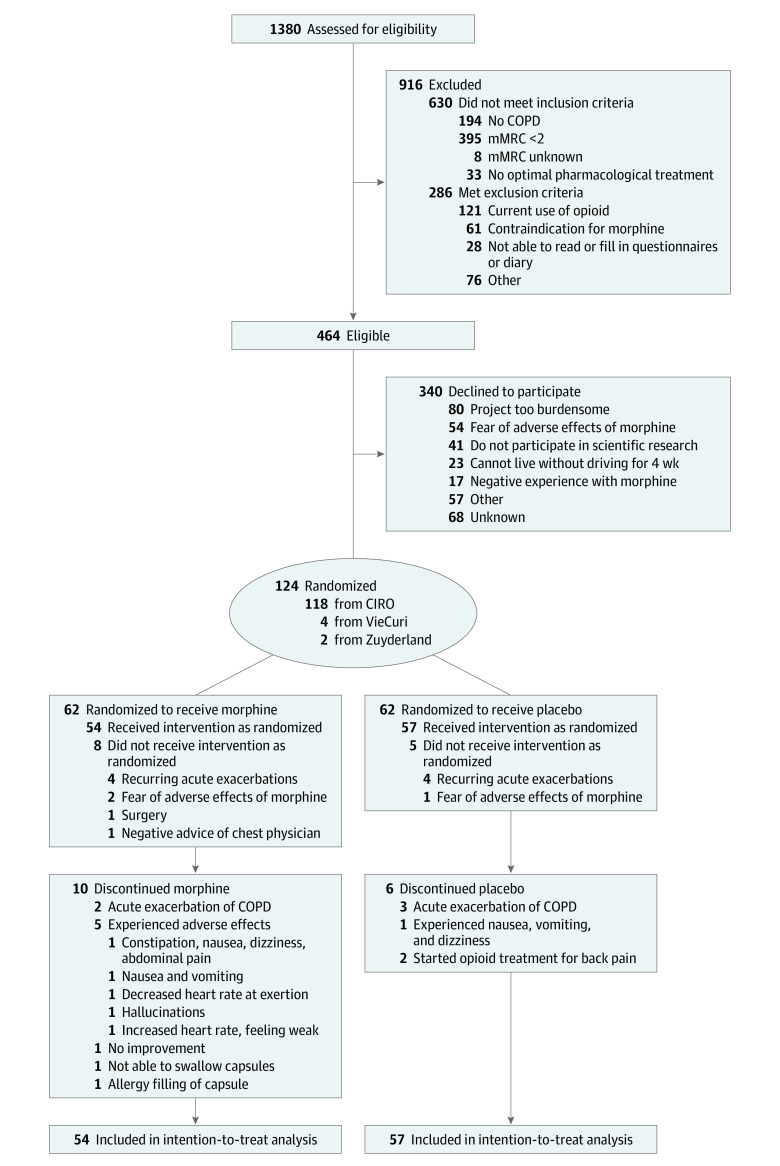
Between November 1, 2016, and January 24, 2019, 1380 patients were screened, 464 were eligible, and 124 participants were randomized (response rate, 27%) (Figure 2). Between randomization and baseline assessment, 13 participants withdrew. The remaining 111 participants had a mean (SD) age of 65.4 (8.0) years, and 60 were men (54%) (Table 1). Participants who enrolled did not differ from those who declined to participate regarding age or sex, but participants who enrolled experienced more severe breathlessness (41 of 124 participants [33%] had mMRC grade 3 and 11 [9%] had mMRC grade 4 vs 59 of 340 nonparticipants [17%] who had mMRC grade 3 and 25 [7%] who had mMRC grade 4; P = .009). The proportion of participants completing the treatment was 81% (n = 44 of 54) in the morphine group and 89% (n = 51 of 57) in the placebo group.
Figure 2. Flowchart of the Morphine for Treatment of Dyspnea in Patients With COPD (MORDYC) Study.
COPD indicates chronic obstructive pulmonary disease; mMRC, modified Medical Research Council.
Table 1. Baseline Characteristics of Total Study Population and Subgroup of Participants With mMRC Grades 3-4.
| Variable | No. (%)a | |||
|---|---|---|---|---|
| Total study population (n = 111) | Subgroup with mMRC grades 3-4 (n = 49) | |||
| Morphine (n = 54) | Placebo (n = 57) | Morphine (n = 23) | Placebo (n = 26) | |
| Demographics | ||||
| Age, mean (SD), y | 65.0 (8.0) | 65.7 (8.0) | 66.6 (8.1) | 64.5 (9.0) |
| Male | 28 (52) | 32 (56) | 12 (52) | 12 (46) |
| BMI, mean (SD), kg/m2 | 27.6 (6.6) | 27.2 (5.3) | 27.5 (6.1) | 26.1 (6.2) |
| Marital status | ||||
| Single | 9 (17) | 13 (23) | 2 (9) | 5 (19) |
| Married/cohabitation | 42 (78) | 44 (77) | 19 (83) | 21 (81) |
| In a relationship but living apart | 3 (6) | 0 | 2 (9) | 0 |
| Medical characteristics | ||||
| Current smoking | 7 (13) | 7 (12) | 3 (13) | 4 (15) |
| Pack-years, median (IQR) | 40 (29.8-51.3) | 40 (30-50) | 40 (30-50) | 40 (59-69.3) |
| CCI, median (IQR), pointsb | 1.5 (1-2) | 1 (1-3) | 1 (1-2) | 1 (1-3) |
| Prior myocardial infarction | 7 (13) | 4 (7) | 4 (17) | 1 (4) |
| Congestive heart failure | 2 (4) | 6 (11) | 2 (9) | 4 (15) |
| Peripheral vascular disease | 6 (11) | 6 (11) | 2 (9) | 1 (4) |
| History of cerebrovascular disease | 4 (7) | 4 (7) | 0 | 3 (12) |
| Rheumatologic disease | 1 (2) | 3 (5) | 0 | 2 (8) |
| Peptic ulcer disease | 0 | 1 (2) | 0 | 1 (4) |
| Mild liver disease | 1 (2) | 2 (4) | 0 | 2 (8) |
| Diabetes | 9 (17) | 7 (12) | 6 (26) | 1 (4) |
| Moderate to severe renal disease | 2 (4) | 1 (2) | 0 | 1 (4) |
| Diabetes with chronic complications | 1 (2) | 0 | 1 (4) | 0 |
| Cancer without metastases, leukemia or lymphoma | 5 (9) | 9 (16) | 2 (9) | 4 (15) |
| Exacerbations <12 mo, median (IQR) | 2 (1-4) | 2 (0-3.5) | 3 (2-4) | 4 (1-4) |
| Hospital admissions <12 mo, median (IQR) | 0 (0-1) | 0 (0-1) | 1 (0-1) | 0 (0-1) |
| Treatment used | ||||
| LAMA | 52 (96)c | 57 (100) | 23 (100) | 26 (100) |
| LABA | 53 (98)c | 57 (100) | 23 (100) | 26 (100) |
| ICS | 43 (80) | 45 (79) | 18 (78) | 21 (81) |
| LTOT | 22 (41) | 25 (44) | 10 (43) | 13 (50) |
| NIV | 12 (22) | 12 (21) | 3 (13) | 5 (19) |
| Pulmonary function, median (IQR) | ||||
| FEV1, L | 0.99 (0.71-1.31) | 0.95 (0.64-1.25) | 0.87 (0.66-1.02) | 0.88 (0.58-1.18) |
| FEV1, % predicted | 38 (29-53) | 34 (25-49) | 30 (24-42) | 35 (23-51) |
| FVC, L | 2.88 (2.39-3.74) | 2.92 (2.32-3.66) | 2.72 (2.03-3.66) | 2.71 (2.22-3.39) |
| FEV1/FVC | 0.32 (0.27-0.41) | 0.31 (0.25-0.42) | 0.29 (0.27-0.34) | 0.32 (0.26-0.44) |
| IC/TLC, mean (SD) | 0.31 (0.09)d | 0.31 (0.10)e | 0.29 (0.07)d | 0.30 (0.11)d |
| ITGV, mean (SD), L | 4.79 (1.26)d | 5.08 (1.53)f | 5.02 (1.34)d | 5.06 (1.85) |
| Clinical characteristics | ||||
| mMRC grade at T0 | ||||
| 2 | 31 (57) | 31 (54) | 0 | 0 |
| 3 | 20 (37) | 19 (33) | 20 (87) | 19 (73) |
| 4 | 3 (6) | 7 (12) | 3 (13) | 7 (27) |
| CAT score, mean (SD) | 22.8 (6.3) | 21.4 (7.4) | 23.2 (5.8) | 24.0 (6.5) |
| 6MWT, mean (SD), m | 354 (85)d | 343 (114)d | 332 (84)d | 285 (114)d |
| Paco2, median (IQR), mm Hg | 40.6 (37.6-44.5) | 41.4 (36.8-45.9) | 42.9 (35.3-45.9) | 39.9 (36.7-47.9) |
| Sao2, median (IQR), % | 93.1 (91.4-94.5) | 93.8 (89.9-95.3) | 94.2 (92.5-95.1) | 93.1 (89.2-95.5) |
Abbreviations: BMI, body mass index; CAT, COPD Assessment Test; CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IC/TLC, inspiratory capacity to total lung capacity ratio; ICS, inhaled corticosteroids; ITGV, intrathoracic gas volume; IQR, interquartile range; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTOT, long-term oxygen therapy; mMRC, modified Medical Research Council; 6MWT, 6-minute walk test; NIV, noninvasive positive pressure ventilation; Paco2, arterial partial pressure of carbon dioxide; Sao2, arterial oxygen saturation.
Values are written as No. (%) unless otherwise indicated.
None of the participants had dementia, hemiplegia, moderate or severe liver disease, metastatic solid tumor, or acquired immunodeficiency syndrome.
One patient only used LABA therapy, and 1 patient had LAMA-LABA therapy prescribed but stopped using this on their own initiative.
Test not performed in 1 participant.
Test not performed in 3 participants.
Test not performed in 4 participants.
Health Status
The difference in CAT score between the treatment groups was –2.18 points (95% CI, –4.14 to –0.22 points; P = .03; Table 2) favoring morphine. When examining the CAT score on item level, the difference between the groups was significant for walking the stairs or hill (–0.43 points; 95% CI, –0.80 to –0.07 points; P = .02; Table 3).
Table 2. Mean Difference in Outcomes for Total Study Population and Subgroup of Participants With mMRC Grades 3 to 4.
| Variable | Morphine vs placebo | |||
|---|---|---|---|---|
| Total study population (n = 111) | Subgroup with mMRC grades 3-4 (n = 49) | |||
| Mean difference (95% CI) | P value | Mean difference (95% CI) | P value | |
| Primary outcomes | ||||
| CAT score | −2.18 (−4.14 to −0.22) | .03 | −1.17 (−4.17 to 1.84) | .44 |
| Paco2, mm Hg | 1.19 (−2.70 to 5.07) | .55 | 1.84 (−4.95 to 8.64) | .59 |
| Secondary outcomes | ||||
| Pao2, mm Hg | −3.79 (−9.70 to 2.12) | .21 | −5.92 (−15.73 to 3.90) | .23 |
| Sao2, % | −1.09 (−2.93 to 0.75) | .24 | −1.72 (−5.02 to 1.58) | .30 |
| Respiratory rate | −1.46 (−2.84 to −0.09) | .04 | −0.73 (−2.79 to 1.34) | .49 |
| PtcCO2, mm Hg | 1.39 (−0.65 to 3.42) | .18 | 1.02 (−1.78 to 3.82) | .47 |
| SpO2, % | −0.33 (−0.95 to 0.29) | .29 | −0.09 (−1.09 to 0.91) | .86 |
| % Time overnight SpO2 below 90% | −0.04 (−19.61 to 19.52)a | >.99 | 10.84 (−19.64 to 41.32)b | .48 |
| Overnight SpO2, % | 0.16 (−1.48 to 1.81)a | .84 | −0.03 (−2.90 to 2.85)b | .99 |
| FEV1, L | −0.02 (−0.29 to 0.26) | .91 | 0.04 (−0.34 to 0.42) | .83 |
| FEV1, % predicted | −0.27 (−9.92 to 9.38) | .96 | 2.57 (−12.07 to 17.22) | .73 |
| FVC, L | −0.15 (−0.68 to 0.38) | .57 | −0.17 (−0.96 to 0.63) | .68 |
| FEV1/FVC | 0.02 (−0.05 to 0.08) | .60 | 0.04 (−0.06 to 0.14) | .42 |
| IC/TLC | 0.02 (−0.04 to 0.07)c | .58 | 0.03 (−0.06 to 0.11)d | .56 |
| IC, L | −0.03 (−0.49 to 0.42)e | .88 | −0.07 (−0.74 to 0.61)b | .85 |
| ITGV, L | −0.42 (−1.27 to 0.44)e | .34 | −0.65 (−2.17 to 0.88)b | .40 |
| Functional exercise performance (6MWT) | −5.07 (−61.38 to 51.20)b | .86 | 1.49 (−87.47 to 90.46)b | .97 |
| General mobility (TUG) | −0.04 (−0.54 to 0.47)d | .89 | 0.00 (−0.87 to 0.87)b | .99 |
| Care dependency (CDS) | −0.33 (−3.34 to 2.69) | .83 | −1.56 (−6.65 to 3.52) | .54 |
| Breathlessness previous 24 h (NRS) | ||||
| Mean | −0.60 (−1.55 to 0.35) | .21 | −1.31 (−2.80 to 0.17) | .08 |
| Worst | −0.56 (−1.41 to 0.28) | .19 | −1.33 (−2.50 to −0.16) | .03 |
Abbreviations: CAT, COPD Assessment Test; CDS, Care Dependence Scale; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IC, inspiratory capacity; IC/TLC, inspiratory capacity to total lung capacity ratio; ITGV, intrathoracic gas volume; mMRC, modified Medical Research Council; 6MWT, 6-minute walk test; NRS, numeric rating scale; Paco2, arterial partial pressure of carbon dioxide; Pao2, partial arterial pressure of oxygen; PtcCO2, transcutaneous carbon dioxide pressure; Sao2, arterial oxygen saturation; SpO2, pulse oxygen saturation; TUG, Timed Up and Go Test.
Test not performed in 3 participants.
Test not performed in 1 participant.
Test not performed in 5 participants.
Test not performed in 2 participants.
Test not performed in 4 participants.
Table 3. Mean Difference in CAT Item Scores for Total Study Population and Subgroup of Participants With mMRC Grades 3 to 4.
| Score item | Morphine vs placebo | |||
|---|---|---|---|---|
| Total study population (n = 111) | Subgroup with mMRC grades 3-4 (n = 49) | |||
| Mean difference (95% CI) | P value | Mean difference (95% CI) | P value | |
| Total score | −2.18 (−4.14 to −0.22) | .03 | −1.17 (−4.17 to 1.84) | .44 |
| Coughing | −0.31 (−0.70 to 0.08) | .12 | −0.12 (−0.74 to 0.49) | .70 |
| Phlegm | −0.12 (−0.49 to 0.25) | .52 | −0.13 (−0.64 to 0.38) | .61 |
| Chest tightness | −0.06 (−0.55 to 0.44) | .83 | 0.59 (−0.09 to 1.27) | .09 |
| Walking stairs or hill | −0.43 (−0.80 to −0.07) | .02 | −0.45 (−0.96 to 0.05) | .08 |
| Activities at home | −0.11 (−0.58 to 0.35) | .63 | −0.33 (−1.04 to 0.37) | .35 |
| Confidence leaving home | −0.31 (−0.86 to 0.25) | .28 | 0.14 (−0.79 to 1.08) | .76 |
| Sleeping | −0.16 (−0.78 to 0.45) | .60 | −0.30 (−1.34 to 0.75) | .57 |
| Energy | −0.45 (−1.07 to 0.16) | .15 | −0.22 (−1.22 to 0.78) | .66 |
Abbreviations: CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; mMRC, modified Medical Research Council.
In the subgroup with mMRC grades 3 to 4, the difference between the treatment groups was not significant (–1.17 points; 95% CI, –4.17 to 1.84 points; P = .44; Table 2), as were the scores on item level (Table 3). Results for the CAT scores per assessment are shown in eTable 2 in Supplement 2.
Respiratory Outcomes
Change in Paco2 did not differ significantly or clinically between the treatment groups (1.19 mm Hg; 95% CI, –2.70 to 5.07 mm Hg; P = .55; Table 2). The subgroup with mMRC grades 3 to 4 also showed no significant or clinically relevant difference in Paco2 (1.84 mm Hg; 95% CI, –4.95 to 8.64 mm Hg; P = .59; Table 2).
The difference in RR between the treatment groups was significant, favoring morphine (–1.46; 95% CI, –2.84 to –0.09; P = .04; Table 2). In the subgroup with mMRC grades 3 to 4, no difference in RR was seen (–0.73; 95% CI, –2.79 to 1.34; P = .49; Table 2). Differences in Pao2, Sao2, PtcCO2, SpO2, overnight SpO2, the amount of time SpO2 was below 90% during the night, and all lung function parameters were not significant (Table 2).
Functional Performance
No difference in distance walked in the 6MWT was observed between the treatment groups in the total study population (–5.07 m; 95% CI, –61.38 to 51.20 m; P = .86) and in the subgroup with mMRC grades 3 to 4 (1.49 m; 95% CI, –87.47 to 90.46 m; P = .97). The TUG time and CDS scores also did not differ significantly (Table 2).
Breathlessness
There was no significant or clinically relevant change in mean or worst breathlessness in the previous 24 hours between the treatment groups (Table 2). Within the morphine group, 21 of 44 participants (48%) responded to the treatment (improvement of 1.0 point on NRS mean breathlessness); within the placebo group, 18 of 51 participants (35%) responded (P = .25). In the subgroup with mMRC grades 3 to 4, the difference in mean breathlessness between the treatment groups was not significant (–1.31; 95% CI, –2.80 to 0.17; P = .08; Table 2). Change in worst breathlessness in the previous 24 hours differed between the treatment groups (–1.33; 95% CI, –2.50 to –0.16; P = .03; Table 2). Results of NRS scores for each assessment are shown in eTable 2 in Supplement 2.
Intervention and Adverse Effects
In 68 of 106 participants (64%) who were still in the study at time T2, treatment dose was increased to 3 times daily at T2 or T3: 27 of 51 (53%) in the morphine group and 41 of 55 (75%) in the placebo group (P = .02). In 1 participant (2%) in the morphine group and 3 participants (5%) in the placebo group, treatment dose was increased at T2 but decreased at T3 again because of adverse effects. The final mean (SD) number of capsules in the morphine group was 2.55 (0.50) capsules and in the placebo group was 2.73 (0.45) capsules (P = .07); 24 participants of 44 (55%) in the morphine group and 37 participants of 51 (73%) in the placebo group used 3 capsules per day at T5 (P = .07; eTable 3 in Supplement 2).
Self-reported compliance was 67%, with a median number of forgotten capsules of 2 (interquartile range, 1-5). Both the proportion of noncompliant participants and the number of forgotten capsules were equal between the treatment groups (P = .51 and P = .44, respectively). Reasons for not taking study medication included forgetting to take the medication (n = 28; 25%), feeling the medication was not helping (n = 1; 1%), and experiencing adverse effects (n = 6; 5%).
A total of 53 of 111 (48%) participants guessed correctly whether they received morphine or placebo (20 [37%] in the morphine group and 33 [58%] in the placebo group). A total of 20 of 111 (18%) had no idea what intervention they received (12 [22%] in the morphine group and 8 [14%] in the placebo group).
The number of participants experiencing 1 or more adverse effects of interest (nausea, vomiting and retching, drowsiness, constipation, and sleeplessness) did not differ between the morphine group and placebo group (43 of 53 [81%] vs 40 of 57 [70%], respectively; P = .18). Change in constipation NRS scores between baseline and T5 between the treatment groups was significant (1.53 points; 95% CI, 0.44 to 2.62 points; P = .006; eTable 4 in Supplement 2). Detailed results of participants experiencing adverse effects and change in NRS scores are shown in eTables 4 and 5 in Supplement 2. Other spontaneously reported adverse effects did not differ between the treatment groups.
Eighteen of 111 participants (16%) experienced a moderate to severe COPD exacerbation (a worsening of symptoms treated with antibiotics and/or corticosteroids6): 7 (13%) in the morphine group and 11 (19%) in the placebo group (P = .37). Three hospital admissions (all for COPD exacerbation) occurred, with 1 of 54 (2%) in the morphine group and 2 of 57 (4%) in the placebo group (P = .59). No morphine-related deaths occurred.
Discussion
In patients with moderate to very severe chronic breathlessness due to COPD, disease-specific health status improved after administering regular, low-dose, oral sustained-release morphine. These effects were obtained without any change in respiratory outcomes or functional performance. Regular, low-dose, oral sustained-release morphine for 4 weeks was well tolerated, with only mild opioid-related adverse effects.
To our knowledge, this is the first study powered to detect a change in respiratory outcomes of morphine treatment. Our study clearly illustrates that fear of respiratory depression or other respiratory adverse effects cannot be substantiated. Respiratory rate decreased without a change in Paco2 or Pao2, indicating no clinically relevant differences in alveolar ventilation. Low-dose morphine treatment, therefore, seems to be safe even in this group of patients with moderate to very severe COPD. These results suggest that fear of respiratory depression, mentioned by physicians,14,15 might be unfounded and are in accordance with our previous meta-analysis.16
Our results show a significant and clinically relevant improvement in CAT after morphine treatment. This improvement did not reach the predetermined MCID of the CAT as originally used for the sample size calculation.30 However, this MCID was reassessed by Smid et al in 201724 and is now defined as a change of 2.0 to 3.0 points. Therefore, we conclude that the reported differences in CAT are on the lower bound of clinical relevance for this population.
Previous reviews on opioid treatment showed an effect on breathlessness but not on health status.11,12 Otherwise, Currow et al13 recently published an RCT in which 284 patients with chronic breathlessness due to several conditions were treated with regular, low-dose, oral sustained-release morphine. No effect on mean or worst breathlessness in the previous 24 hours, current breathlessness, health status, or functional capacity was shown after 1 week of treatment. Where our total study population also did not show an effect on breathlessness, our subgroup with mMRC grades 3 to 4 showed an improvement on worst breathlessness of 1.33 points at 4 weeks. Moreover, the effect on mean breathlessness was 1.31 points at 4 weeks, which did not reach the level of significance, possibly due to a lack of power.
Interestingly, the morphine group reported CAT improvement on walking the stairs or hill compared with the placebo group. We cannot exclude that this improvement in daily life activities masks the expected effect on breathlessness. Indeed, palliative treatment may allow patients to be more active in daily living.34 Patients will be able to do more before reaching the same level of breathlessness. Previous studies exploring the effect of breathlessness treatment (morphine, supplemental oxygen) on exercise capacity in laboratory settings have shown similar results.35 However, this suggested improvement in daily functioning was not reflected in an objectifiable change of functional performance as assessed in this study.
Morphine treatment was well tolerated by the participants of this study. The mean difference in NRS scores between the morphine and placebo group was only significant for constipation, which was consistent with other studies.11,13 The complaints resolved after symptom treatment or early study termination. In this group of patients with COPD, there were no hospital admissions for or deaths due to morphine-related adverse effects. A large observational study on patients with COPD who are oxygen dependent also showed no association between low-dose opioids and increased hospital admissions or deaths.36
Strengths and Limitations
Our study has several strengths and limitations. First, to our knowledge, this study is the first double-blind, placebo-controlled, parallel-arm RCT with a 4-week morphine treatment for chronic breathlessness in patients with COPD. Second, to our knowledge, this is the first RCT including CAT and Paco2 as primary outcomes. Moreover, a thorough assessment of adverse respiratory effects by means of 14 outcomes was performed. Third, all participants completed a comprehensive pulmonary rehabilitation program ensuring treatment was both pharmacologically and nonpharmacologically optimized.
Some limitations need to be recognized. The main limitation is the large number of patients who were unwilling to participate, contributing to insufficient inclusion of our original target population. Where we expected a response rate of 50%, only 27% of eligible patients gave informed consent. As a result, we had to expand the inclusion criteria to participants with mMRC grade 2. Currow et al13 experienced a delayed inclusion as well, also leading to the expansion of the inclusion criteria to mMRC grade 2. As concluded by Johnson et al,19 patients with less severe chronic breathlessness are less likely to benefit from opioid treatment. In future studies, only patients with mMRC grades 3 to 4 should be included. Second, the predefined sample size was not reached for the CAT. The prior MCID of 3.8 was estimated by an anchor-based method and was the best estimate for our patient population at the time of study design.30 The MCID was re-estimated by Smid et al24 by combining all known MCIDs from anchor-based and distribution-based estimations, making this MCID of 2.0 to 3.0 more accurate. Therefore, we are confident that our findings are clinically relevant. Third, functional performance was assessed by a standard battery of tests. At least, it can be questioned whether these forms of exercise testing are appropriate in this stage of the disease to assess daily functional performance. Direct assessment of low-grade daily life activities probably would be more appropriate.35 Physical activity is heterogeneous, and physical activity patterns fluctuate.37 Combining activity monitoring with, for example, Ecological Momentary Assessment can give more insight into the fluctuation of breathlessness and its effect on physical activity and quality of life over the day.38 Fourth, the occurrence of adverse effects and the fact that participants were not blinded to laxative use might have compromised blinding. The occurrence of adverse effects was equal between treatment arms, but intensity of constipation was significantly different. However, because only 48% of participants guessed their treatment (morphine or placebo) correctly, we assume blinding was only minimally compromised. In addition, although this study was one of the first with a trial duration of more than 1 week, the long-term effects of morphine and possible adverse effects remain unknown.
Conclusions
In conclusion, this study has shown that regular, low-dose, oral sustained-release morphine for 4 weeks may have a positive effect on disease-specific health status in patients with moderate to very severe breathlessness. Also, regular, low-dose, oral sustained-release morphine does not appear to lead to respiratory adverse effects. However, our results should be confirmed in a future RCT only including patients with severe to very severe chronic breathlessness and optimized pharmacological and nonpharmacological treatment of their COPD. Given the low response rate, a multicenter approach should be considered. Furthermore, to confirm and substantiate the results on CAT score, the study should include a measure of daily physical activity. Finally, to assess long-term effects and safety, more than 4 weeks of follow-up are needed.
Trial Protocol
eMethods 1. Participant eligibility criteria and recruitment
eMethods 2. Description of outcome measures
eTable 1. Covariance structures
eTable 2. Mean difference in CAT score and breathlessness scores per assessment for total study population and subgroup participants with mMRC grade 3-4
eTable 3. Dose increase during the study for total study population
eTable 4. Participants experiencing adverse effects
eTable 5. Numeric rating scores for adverse effects
eReferences
Data Sharing Statement
References
- 1.Janssen DJ, Spruit MA, Uszko-Lencer NH, Schols JM, Wouters EF. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med. 2011;14(6):735-743. doi: 10.1089/jpm.2010.0479 [DOI] [PubMed] [Google Scholar]
- 2.Agusti A, Calverley PMA, Celli B, et al. ; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators . Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11(1):122. doi: 10.1186/1465-9921-11-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis JR, Patrick DL. The assessment of health status among patients with COPD. Eur Respir J Suppl. 2003;41(41):36s-45s. doi: 10.1183/09031936.03.00078102 [DOI] [PubMed] [Google Scholar]
- 4.Johnson MJ, Yorke J, Hansen-Flaschen J, et al. . Towards an expert consensus to delineate a clinical syndrome of chronic breathlessness. Eur Respir J. 2017;49(5):1602277. doi: 10.1183/13993003.02277-2016 [DOI] [PubMed] [Google Scholar]
- 5.Celli BR, Cote CG, Marin JM, et al. . The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005-1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 6.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2020 report. Accessed December 18, 2019. https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf
- 7.Rocker G, Horton R, Currow D, Goodridge D, Young J, Booth S. Palliation of dyspnoea in advanced COPD: revisiting a role for opioids. Thorax. 2009;64(10):910-915. doi: 10.1136/thx.2009.116699 [DOI] [PubMed] [Google Scholar]
- 8.Lanken PN, Terry PB, Delisser HM, et al. ; ATS End-of-Life Care Task Force . An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177(8):912-927. doi: 10.1164/rccm.200605-587ST [DOI] [PubMed] [Google Scholar]
- 9.Mahler DA, Selecky PA, Harrod CG, et al. . American College of Chest Physicians consensus statement on the management of dyspnea in patients with advanced lung or heart disease. Chest. 2010;137(3):674-691. doi: 10.1378/chest.09-1543 [DOI] [PubMed] [Google Scholar]
- 10.Baas AAF, Zylicz Z, Hesselmann GM Pallialine richtlijn dyspnoe en hoesten 2.0 Accessed March 13, 2015. http://pallialine.nl/dyspnoe.
- 11.Barnes H, McDonald J, Smallwood N, Manser R. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev. 2016;3(3):CD011008. doi: 10.1002/14651858.CD011008.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekström M, Nilsson F, Abernethy AA, Currow DC. Effects of opioids on breathlessness and exercise capacity in chronic obstructive pulmonary disease: a systematic review. Ann Am Thorac Soc. 2015;12(7):1079-1092. doi: 10.1513/AnnalsATS.201501-034OC [DOI] [PubMed] [Google Scholar]
- 13.Currow D, Louw S, McCloud P, et al. ; Australian National Palliative Care Clinical Studies Collaborative (PaCCSC) . Regular, sustained-release morphine for chronic breathlessness: a multicentre, double-blind, randomised, placebo-controlled trial. Thorax. 2020;75(1):50-56. doi: 10.1136/thoraxjnl-2019-213681 [DOI] [PubMed] [Google Scholar]
- 14.Janssen DJ, de Hosson SM, bij de Vaate E, Mooren KJ, Baas AA. Attitudes toward opioids for refractory dyspnea in COPD among Dutch chest physicians. Chron Respir Dis. 2015;12(2):85-92. doi: 10.1177/1479972315571926 [DOI] [PubMed] [Google Scholar]
- 15.Young J, Donahue M, Farquhar M, Simpson C, Rocker G. Using opioids to treat dyspnea in advanced COPD: attitudes and experiences of family physicians and respiratory therapists. Can Fam Physician. 2012;58(7):e401-e407. [PMC free article] [PubMed] [Google Scholar]
- 16.Verberkt CA, van den Beuken-van Everdingen MHJ, Schols JMGA, et al. . Respiratory adverse effects of opioids for breathlessness: a systematic review and meta-analysis. Eur Respir J. 2017;50(5):1701153. doi: 10.1183/13993003.01153-2017 [DOI] [PubMed] [Google Scholar]
- 17.Verberkt CA, van den Beuken-van Everdingen MH, Franssen FM, et al. . A randomized controlled trial on the benefits and respiratory adverse effects of morphine for refractory dyspnea in patients with COPD: protocol of the MORDYC study. Contemp Clin Trials. 2016;47:228-234. doi: 10.1016/j.cct.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 18.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581-586. doi: 10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson MJ, Bland JM, Oxberry SG, Abernethy AP, Currow DC. Opioids for chronic refractory breathlessness: patient predictors of beneficial response. Eur Respir J. 2013;42(3):758-766. doi: 10.1183/09031936.00139812 [DOI] [PubMed] [Google Scholar]
- 20.Johnson MJ, Bland JM, Oxberry SG, Abernethy AP, Currow DC. Clinically important differences in the intensity of chronic refractory breathlessness. J Pain Symptom Manage. 2013;46(6):957-963. doi: 10.1016/j.jpainsymman.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 21.Currow DC, McDonald C, Oaten S, et al. . Once-daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study. J Pain Symptom Manage. 2011;42(3):388-399. doi: 10.1016/j.jpainsymman.2010.11.021 [DOI] [PubMed] [Google Scholar]
- 22.Dodd JW, Hogg L, Nolan J, et al. . The COPD assessment test (CAT): response to pulmonary rehabilitation: a multicentre, prospective study. Thorax. 2011;66(5):425-429. doi: 10.1136/thx.2010.156372 [DOI] [PubMed] [Google Scholar]
- 23.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 24.Smid DE, Franssen FM, Houben-Wilke S, et al. . Responsiveness and MCID estimates for CAT, CCQ, and HADS in patients with COPD undergoing pulmonary rehabilitation: a prospective analysis. J Am Med Dir Assoc. 2017;18(1):53-58. doi: 10.1016/j.jamda.2016.08.002 [DOI] [PubMed] [Google Scholar]
- 25.Holland AE, Spruit MA, Troosters T, et al. . An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446. doi: 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 26.Podsiadlo D, Richardson S. The Timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142-148. doi: 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 27.Dijkstra A, Tiesinga LJ, Goossen WT, Dassen TW. Further psychometric testing of the Dutch Care Dependency Scale on two different patient groups. Int J Nurs Pract. 2002;8(6):305-314. doi: 10.1046/j.1440-172X.2002.00384.x [DOI] [PubMed] [Google Scholar]
- 28.Dijkstra A, Brown L, Havens B, et al. . An international psychometric testing of the care dependency scale. J Adv Nurs. 2000;31(4):944-952. doi: 10.1046/j.1365-2648.2000.01354.x [DOI] [PubMed] [Google Scholar]
- 29.Dorman S, Byrne A, Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? a systematic review. Palliat Med. 2007;21(3):177-191. doi: 10.1177/0269216307076398 [DOI] [PubMed] [Google Scholar]
- 30.Kon SS, Canavan JL, Jones SE, et al. . Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med. 2014;2(3):195-203. doi: 10.1016/S2213-2600(14)70001-3 [DOI] [PubMed] [Google Scholar]
- 31.Spruit MA, Pennings HJ, Janssen PP, et al. . Extra-pulmonary features in COPD patients entering rehabilitation after stratification for MRC dyspnea grade. Respir Med. 2007;101(12):2454-2463. doi: 10.1016/j.rmed.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 32.Poole PJ, Veale AG, Black PN. The effect of sustained-release morphine on breathlessness and quality of life in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(6 pt 1):1877-1880. doi: 10.1164/ajrccm.157.6.9711061 [DOI] [PubMed] [Google Scholar]
- 33.Kochovska S, Huang C, Johnson MJ, et al. . Intention-to-treat analyses for randomized controlled trials in hospice/palliative care: the case for analyses to be of people exposed to the intervention. J Pain Symptom Manage. 2020;59(3):637-645. doi: 10.1016/j.jpainsymman.2019.10.026 [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. WHO definition of palliative care. Accessed August 30, 2018. https://www.who.int/cancer/palliative/definition/en/
- 35.Ekström M. Why treatment efficacy on breathlessness in laboratory but not daily life trials? the importance of standardized exertion. Curr Opin Support Palliat Care. 2019;13(3):179-183. doi: 10.1097/SPC.0000000000000444 [DOI] [PubMed] [Google Scholar]
- 36.Ekström MP, Bornefalk-Hermansson A, Abernethy AP, Currow DC. Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ. 2014;348:g445. doi: 10.1136/bmj.g445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mesquita R, Spina G, Pitta F, et al. . Physical activity patterns and clusters in 1001 patients with COPD. Chron Respir Dis. 2017;14(3):256-269. doi: 10.1177/1479972316687207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janssen DJA, Johnson MJ. Palliative treatment of chronic breathlessness syndrome: the need for P5 medicine. Thorax. 2020;75(1):2-3. doi: 10.1136/thoraxjnl-2019-214008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods 1. Participant eligibility criteria and recruitment
eMethods 2. Description of outcome measures
eTable 1. Covariance structures
eTable 2. Mean difference in CAT score and breathlessness scores per assessment for total study population and subgroup participants with mMRC grade 3-4
eTable 3. Dose increase during the study for total study population
eTable 4. Participants experiencing adverse effects
eTable 5. Numeric rating scores for adverse effects
eReferences
Data Sharing Statement