This post hoc analysis of data from a randomized clinical trial assesses whether high-dose erythropoietin given within 24 hours of birth through postmenstrual age of 32 completed weeks will decrease the need for blood transfusions in extremely low gestational age neonates.
Key Points
Question
Does high-dose erythropoietin given within 24 hours of birth to postmenstrual age of 32 weeks decrease the number of blood transfusions, cumulative volume of transfusions, and unique donor exposures in extremely preterm infants?
Findings
In this randomized phase 3 clinical trial that included 941 infants with gestational age of 24 weeks (0-7 days) to 27 weeks (6-7 days) at birth, erythropoietin given according to protocol significantly decreased the number of transfusions, the cumulative volume of transfusions, and the unique donor exposures.
Meaning
These findings suggest that high-dose erythropoietin decreases transfusion requirements in infants with a gestational age of 24 to 27 weeks and increases the number who remain transfusion free.
Abstract
Importance
Extremely preterm infants are among the populations receiving the highest levels of transfusions. Erythropoietin has not been recommended for premature infants because most studies have not demonstrated a decrease in donor exposure.
Objectives
To determine whether high-dose erythropoietin given within 24 hours of birth through postmenstrual age of 32 completed weeks will decrease the need for blood transfusions.
Design, Setting, and Participants
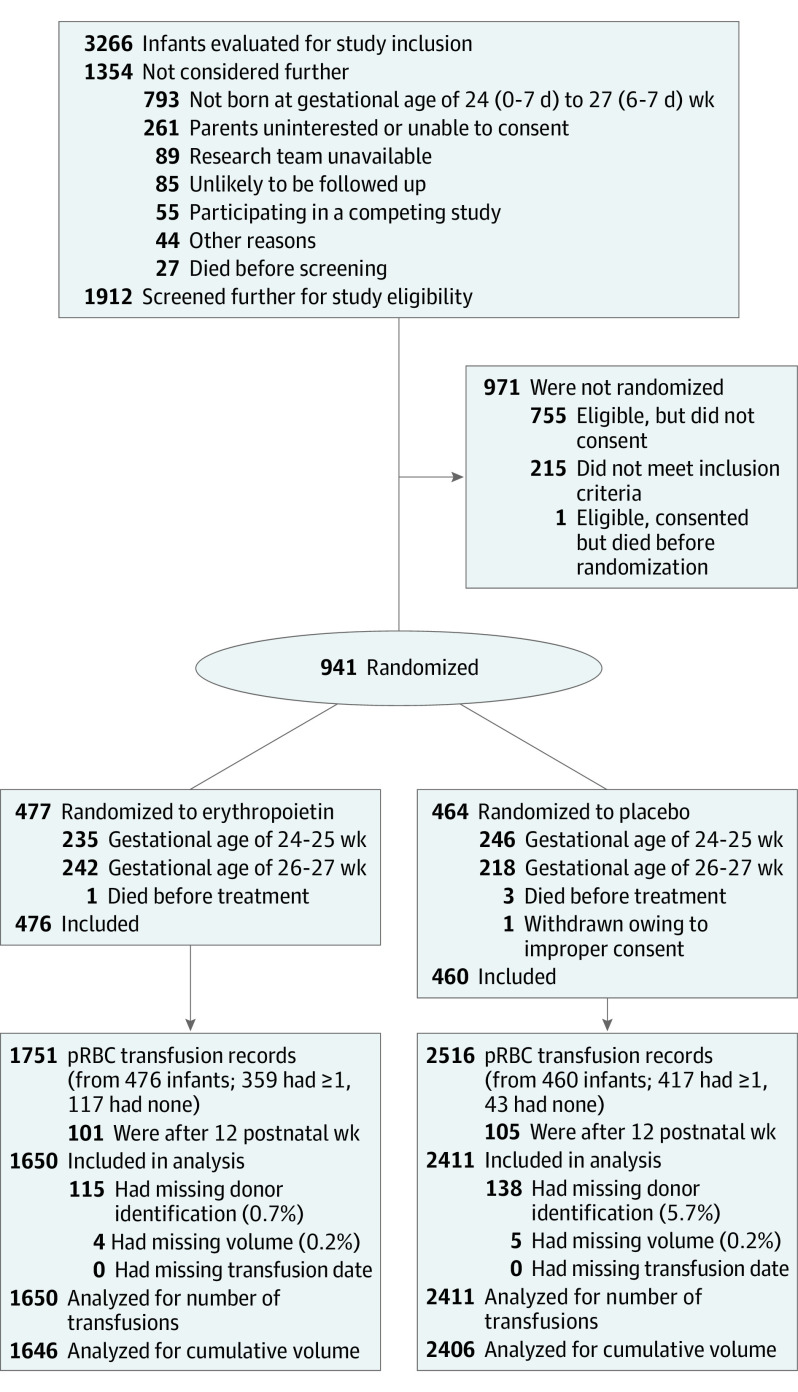
The Preterm Erythropoietin Neuroprotection Trial (PENUT) is a randomized, double-masked clinical trial with participants enrolled at 19 sites consisting of 30 neonatal intensive care units across the United States. Participants were born at a gestational age of 24 weeks (0-6 days) to 27 weeks (6-7 days). Exclusion criteria included conditions known to affect neurodevelopmental outcomes. Of 3266 patients screened, 2325 were excluded, and 941 were enrolled and randomized to erythropoietin (n = 477) or placebo (n = 464). Data were collected from December 12, 2013, to February 25, 2019, and analyzed from March 1 to June 15, 2019.
Interventions
In this post hoc analysis, erythropoietin, 1000 U/kg, or placebo was given every 48 hours for 6 doses, followed by 400 U/kg or sham injections 3 times a week through postmenstrual age of 32 weeks.
Main Outcomes and Measures
Need for transfusion, transfusion numbers and volume, number of donor exposures, and lowest daily hematocrit level are presented herein.
Results
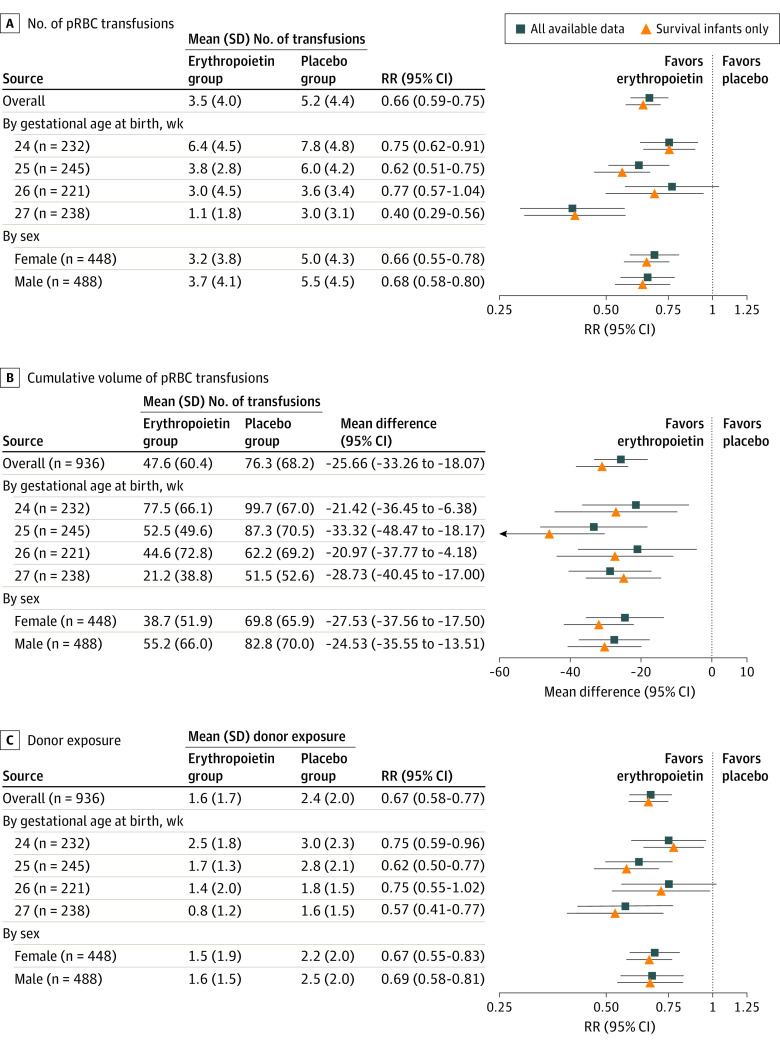
A total of 936 patients (488 male [52.1%]) were included in the analysis, with a mean (SD) gestational age of 25.6 (1.2) weeks and mean (SD) birth weight of 799 (189) g. Erythropoietin treatment (vs placebo) decreased the number of transfusions (unadjusted mean [SD], 3.5 [4.0] vs 5.2 [4.4]), with a relative rate (RR) of 0.66 (95% CI, 0.59-0.75); the cumulative transfused volume (mean [SD], 47.6 [60.4] vs 76.3 [68.2] mL), with a mean difference of −25.7 (95% CI, 18.1-33.3) mL; and donor exposure (mean [SD], 1.6 [1.7] vs 2.4 [2.0]), with an RR of 0.67 (95% CI, 0.58-0.77). Despite fewer transfusions, erythropoietin-treated infants tended to have higher hematocrit levels than placebo-treated infants, most noticeable at gestational week 33 in infants with a gestational age of 27 weeks (mean [SD] hematocrit level in erythropoietin-treated vs placebo-treated cohorts, 36.9% [5.5%] vs 30.4% [4.6%] (P < .001). Of 936 infants, 160 (17.1%) remained transfusion free at the end of 12 postnatal weeks, including 43 in the placebo group and 117 in the erythropoietin group (P < .001).
Conclusions and Relevance
These findings suggest that high-dose erythropoietin as used in the PENUT protocol was effective in reducing transfusion needs in this population of extremely preterm infants.
Trial Registration
ClinicalTrials.gov Identifier: NCT01378273
Introduction
Anemia is common in infants born at a gestational age of less than 28 weeks owing to a combination of phlebotomy losses, low circulating blood volume,1 a shortened life span for preterm red blood cells,2 growth-related blood volume expansion, anemia of prematurity, and variable use and adherence to transfusion guidelines.3,4 This clinical constellation results in multiple transfusions: historically, infants with a gestational age of less than 28 weeks receive a mean of 4 to 5 packed red blood cell (pRBC) transfusions during their initial hospitalization (range, 0 to >10).1,3,5,6
Erythropoietin administration has been investigated as a means to decrease transfusions and donor exposures in this patient population. Multiple doses (120-1400 U/kg/wk) and durations (2-10 wk or discharge) have been tested in preterm infants.7 Meta-analyses of both early and late use of erythropoietin in randomized clinical trials concluded that although the number and volume of transfusions are decreased by erythropoietin, treatment does not reliably decrease donor exposure and therefore was not recommended.8,9
The Preterm Erythropoietin Neuroprotection Trial (PENUT) is a randomized clinical trial designed to test the safety and efficacy of erythropoietin as a neuroprotective agent.10,11 This rigorously designed trial included a large cohort of well-characterized participants, permitting post hoc analyses to determine whether pRBC transfusions and donor exposures would be decreased in the erythropoietin-treated infants compared with placebo-treated control participants. We hypothesized that high-dose erythropoietin would increase the likelihood of being transfusion free and would decrease pRBC transfusion number, volume, and donor exposure. We now report the transfusion-related results of this trial.
Methods
PENUT is a randomized, placebo-controlled, double-masked clinical trial of erythropoietin neuroprotection in infants born at gestational ages of 24 weeks (0-7 days) to 27 weeks (6-7 days). The trial protocol is available in Supplement 1. PENUT enrolled and collected data on 941 infants at 19 sites consisting of 30 neonatal intensive care units across the United States from December 12, 2013, to February 25, 2019, 936 of whom received the first study drug dose and were considered for further analysis. Enrollment and initial treatment with the study drug occurred within 24 hours of birth after written informed consent was obtained from parents. Patients were excluded if they had known major life-threatening anomalies, known or suspected chromosomal anomalies, disseminated intravascular coagulopathy, polycythemia, hydrops fetalis, or a known congenital infection.10 PENUT was approved by the institutional review board at each site and followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Block randomization was used, and for multiple births, infants were randomized to the same treatment group. Randomization was stratified by site, gestational age (24-25 vs 26-27 weeks), and multiple gestation (twins, triplets, or more). The study was powered for the primary outcome of death or severe neurodevelopmental impairment measured at 2 years of age.10,11
Participants received erythropoietin, 1000 U/kg per dose, or placebo intravenously every 48 hours for 6 doses, followed by maintenance dosing of 400 U/kg per dose by subcutaneous injection or sham injections 3 times a week until postmenstrual age (PMA) of 32 weeks (6-7 days). PENUT is registered with the US Food and Drug Administration and ClinicalTrials.gov. All study investigators were blinded to treatment group except each site pharmacy and the investigator from the data coordinating center (B.A.C.), who generated the randomization tables.
Iron Supplementation
Iron supplementation guidelines for PENUT recommended, when enteral feedings were started, use of a standard iron-containing formula if breast milk was unavailable. When participants reached an enteral intake of 60 mL/kg/d and were at least 7 days of age, a starting enteral iron intake of 3 mg/kg/d was recommended. Iron intake should be increased to 6 mg/kg/d when infants achieved an enteral intake of 100 mL/kg/d.12 The serum ferritin level or the ratio of zinc protoporphyrin to heme (ZnPP:H)13 should be checked at 14 and 42 days, with iron dosing adjusted accordingly. If participants were not able to tolerate enteral feedings, they should receive maintenance iron supplementation parenterally (3 mg/kg/wk, adjusted based on iron indices).
Transfusion Guidelines
Because no consensus recommendations exist for transfusions in critically ill neonates, each site followed their own procedures for pRBC transfusion.14,15 Five sites did not use transfusion guidelines. The remaining sites used guidelines but differed in transfusion triggers, blood volume transfused per transfusion, and pRBC preservative solutions.
Data Collection
In addition to clinical characteristics and demographic information that was self-reported by the mother, pRBC transfusion number, cumulative blood volume, and number of unique donors were recorded for each participant. For those infants who did not receive transfusions, these values were recorded as zero. Transfusion records with missing data (transfusion volume or donor identification) were excluded from relevant analyses. The study also recorded clinically obtained hematocrit levels as available. When multiple hematocrit levels were recorded for a 24-hour period, we used the lowest value for this analysis.
Statistical Analysis
Data were analyzed from March 1 to June 15, 2019. Using a modified intention-to-treat approach, a total of 936 randomized infants who received the first dose of study treatment were included in the statistical analyses. Given enrollment of multiple births, we required that all analyses properly account for potential within-sibship correlation, so all statistical inferences used generalized estimating equations (GEE) with robust standard errors and an exchangeable working correlation structure.
Key aspects of transfusions were evaluated in this post hoc analysis, including the total number of pRBC transfusions, the cumulative transfusion volume, and donor exposure, defined as the number of unique donors. The analysis examined differences in these key outcomes between the 2 treatment groups through the first 12 weeks after birth. All 936 randomized infants were included in the analysis. To compare erythropoietin and control groups, we used GEE models based on Poisson regression for the number of transfusions and donor exposure and a GEE model based on linear regression for cumulative transfusion volume. All models were adjusted for gestational age at birth and recruitment site. In a sensitivity analysis, we included only the 823 infants who were alive by the end of the 2-year follow-up period. In addition, we performed subgroup evaluation by stratifying analyses based on gestational age at birth and sex. We used GEE regressions adjusted for recruitment site to generate subgroup-specific treatment comparisons. Statistical significance was set at 0.05 for each key outcome.
Exploratory analyses were conducted to evaluate treatment group differences in hematocrit levels, cumulative iron intake, serum ferritin levels, and ZnPP:H ratio. Because the length of erythropoietin treatment differed by gestational age (treatment was stopped at a PMA of 32 weeks), we stratified analyses of hematocrit levels over time based on gestational age group, and within each subgroup we used GEE by adjusting for site for inference on treatment group differences. For analysis of iron intake, cumulative enteral and intravenous iron intake for both treatment groups were compared using GEE regressions adjusted for gestational age at birth and recruitment site. Serum ferritin level and ZnPP:H ratio data were compared using GEE models based on log-linear regressions, adjusted for postnatal time of measurement, gestational age at birth, and recruitment site.
All statistical tests were 2 sided. Although we did not formally adjust for multiple testing in the exploratory analyses, we evaluated statistical significance at both the nominal 0.05 level and a conservative approximation to a Bonferroni correction when tests are presented for multiple follow-up periods. All statistical analyses were performed using the R statistical software package, version 3.5.1 (R Project for Statistical Computing).
Results
A total of 4061 transfusion records were evaluated for the 936 treated infants (488 male [52.1%] and 448 female [47.9%]; mean [SD] gestational age, 25.6 [1.2] weeks; and mean [SD] birth weight, 799 [189] g). A CONSORT diagram showing the screening, allocation, and transfusion data analyzed per group is shown in Figure 1. Demographic information for infants and their mothers is shown in the Table. The proportion of those who had any pRBC transfusion within the first 12 postnatal weeks was reported for each subgroup defined by the baseline characteristics. These proportions differed among subgroups stratified by maternal age (217 of 255 [85.1%] for <25 years vs 205 of 237 [86.5%] for 25-29 years vs 210 of 252 [83.3%] for 30-34 years vs 144 of 192 [75.0%] for ≥35 years; P = .03), risk of infection (565 of 689 [82.0%] vs 211 of 247 [85.4%]; P = .001), cesarean delivery (559 of 651 [85.9%] vs 217 of 285 [76.1%]; P < .001), delayed cord clamping (245 of 318 [77.0%] vs 317 of 362 [87.6%]; P = .001), sex (416 of 488 male [85.2%] vs 360 of 448 [80.4%]; P = .02), gestational age at birth (230 of 232 [99.1%] for 24 weeks vs 144 of 238 [60.5%] for 27 weeks; P < .001), birth weight less than the 10th percentile (133 of 147 [90.5%] vs 639 of 785 [81.4%]; P < .001), head circumference less than the 10th percentile (145 of 161 [90.1%] vs 612 of 752 [81.4%]; P < .001), Apgar score less than 5 at 5 minutes (182 of 189 [96.3%] vs 591 of 744 [79.4%]; P < .001), and early intracranial hemorrhage (174 of 197 [88.3%] vs 602 of 739 [81.5%]; P = .02).
Figure 1. CONSORT Diagram.
pRBC indicates packed red blood cell.
Table. Maternal and Neonatal Data at Baseline and pRBC Transfusion Within the First 12 Postnatal Weeks in 936 Infants.
| Characteristic | No. of infants | No. (%) with any pRBC transfusion in the first 12 weeks | P valuea |
|---|---|---|---|
| Randomization | |||
| Erythropoietin | 476 | 359 (75.4) | <.001 |
| Placebo | 460 | 417 (90.7) | |
| Maternal demographics | |||
| Age, y | .03 | ||
| <25 | 255 | 217 (85.1) | |
| 25-29 | 237 | 205 (86.5) | |
| 30-34 | 252 | 210 (83.3) | |
| ≥35 | 192 | 144 (75.0) | |
| Hispanic ethnicityb | .36 | ||
| Yes | 200 | 159 (79.5) | |
| No | 733 | 614 (83.8) | |
| Racec | .07 | ||
| White | 611 | 489 (80.0) | |
| Black | 240 | 219 (91.3) | |
| Educational attainmentd | .18 | ||
| ≤High school | 307 | 264 (86.0) | |
| Some college | 285 | 225 (78.9) | |
| ≥College degree | 229 | 186 (81.2) | |
| Neonatal data at enrollment | |||
| Complications | .34 | ||
| Maternal indications for delivery | |||
| Yes | 152 | 125 (82.2) | |
| No | 784 | 651 (83.0) | |
| Risk of infectione | .001 | ||
| Yes | 689 | 565 (82.0) | |
| No | 247 | 211 (85.4) | |
| Pregnancy-induced hypertension | .60 | ||
| Yes | 71 | 59 (83.1) | |
| No | 865 | 717 (82.9) | |
| Prenatal corticosteroid usef | .14 | ||
| Yes | 842 | 694 (82.4) | |
| No | 77 | 67 (87.0) | |
| Prenatal magnesium sulfate useg | .10 | ||
| Yes | 749 | 611 (81.6) | |
| No | 148 | 129 (87.2) | |
| Delivery complicationsh | .48 | ||
| Yes | 149 | 128 (85.9) | |
| No | 787 | 648 (82.3) | |
| Cesarean delivery | <.001 | ||
| Yes | 651 | 559 (85.9) | |
| No | 285 | 217 (76.1) | |
| Delayed cord clampingi | .001 | ||
| Yes | 318 | 245 (77.0) | |
| No | 362 | 317 (87.6) | |
| Sex | .02 | ||
| Male | 488 | 416 (85.2) | |
| Female | 448 | 360 (80.4) | |
| Gestational age at birth, wk | <.001 | ||
| 24 | 232 | 230 (99.1) | |
| 25 | 245 | 229 (93.5) | |
| 26 | 221 | 173 (78.3) | |
| 27 | 238 | 144 (60.5) | |
| Multiple gestation | .52 | ||
| Yes | 249 | 208 (83.5) | |
| No | 687 | 568 (82.7) | |
| Weight <10th percentilej | <.001 | ||
| Yes | 147 | 133 (90.5) | |
| No | 785 | 639 (81.4) | |
| Occipital frontal circumference <10th percentilek | <.001 | ||
| Yes | 161 | 145 (90.1) | |
| No | 752 | 612 (81.4) | |
| Apgar score at 5 min <5l | <.001 | ||
| Yes | 189 | 182 (96.3) | |
| No | 744 | 591 (79.4) | |
| Intracranial hemorrhage before first dose | .02 | ||
| Yes | 197 | 174 (88.3) | |
| No | 739 | 602 (81.5) | |
Abbreviation: pRBC, packed red blood cell.
P values were obtained from generalized estimating equation models evaluating the risk of having any transfusions among subgroups defined by the baseline characteristic, adjusting for assigned treatment, gestational weeks at birth, and site grouping.
Unknown or unreported for 3 participants.
Four individuals identified themselves with more than 1 race. Race was unknown or unreported for 81 participants.
Unknown or unreported for 115 participants.
Defined as presumed or clinically diagnosed chorioamnionitis.
Unknown for 17 participants.
Unknown for 39 participants.
Defined as the presence of 1 or more of the following: prolapsed cord, true knot, tear or rupture of cord, placental abruption, twin-twin transfusion, fetomaternal bleeding, ruptured uterus, or traumatic instrument delivery.
Unknown for 256 participants.
Missing for 4 participants.
Missing for 23 participants.
Missing for 3 participants.
Of 936 infants, 160 (17%) remained transfusion free at the end of 12 postnatal weeks. Of the 160 infants who were transfusion free, 43 (26.9%) were in the placebo group and 117 (73.1%) were in the erythropoietin group (P < .001). The number needed to treat to keep 1 child transfusion free was 7. Rates of transfusion within the first 12 weeks varied by gestational age and erythropoietin treatment. In infants with gestational age of 24 weeks, 112 (99.1%) in the erythropoietin group and 118 (99.2%) in the control group received transfusions. In infants with a gestational age of 25 weeks, 111 (91.7%) in the erythropoietin group and 118 (95.2%) in the placebo group received a transfusion. In infants with a gestational age of 26 weeks, 72 (69.9%) in the erythropoietin group and 101 (85.6%) in the placebo group received transfusions. In infants with a gestational age of 27 weeks, 64 (46.0%) in the erythropoietin group and 80 (80.8%) in the placebo group received transfusions. Cumulative transfusion volumes decreased as gestational age increased, with higher mean cumulative volumes in the placebo compared with the erythropoietin groups for all gestational ages (eFigure 1 in Supplement 2). For instance, at the end of week 12 (ie, day 84), the mean (SD) cumulative transfused volume in the erythropoietin group vs the placebo group was 77.5 (66.1) vs 99.7 (67.0) in infants with a gestational age of 24 weeks, 52.5 (49.6) vs 87.3 (70.5) in infants with a gestational age of 25 weeks, 44.6 (72.8) vs 62.2 (69.2) in infants with a gestational age of 26 weeks, and 21.2 (38.8) vs 51.5 (52.6) in infants with a gestational age of 27 weeks. More female infants remained transfusion free compared with male infants (88 of 448 [19.6%] vs 72 of 488 [14.8%]; treatment-adjusted P = .02).
Substantial variability in transfusion practice by site was observed. eFigure 2A in Supplement 2 shows the distribution of cumulative transfusion volume by site (range, 16-83 mL). Among the 5 site groupings with the highest median cumulative volume, the 25th percentile for transfusion volume ranged from 18 to 40 mL, whereas the 75th percentile ranged from 82 to 125 mL. eFigure 2B in Supplement 2 shows donor exposure by site. Donor exposure reflects both clinician transfusion practice and blood bank aliquoting practices.
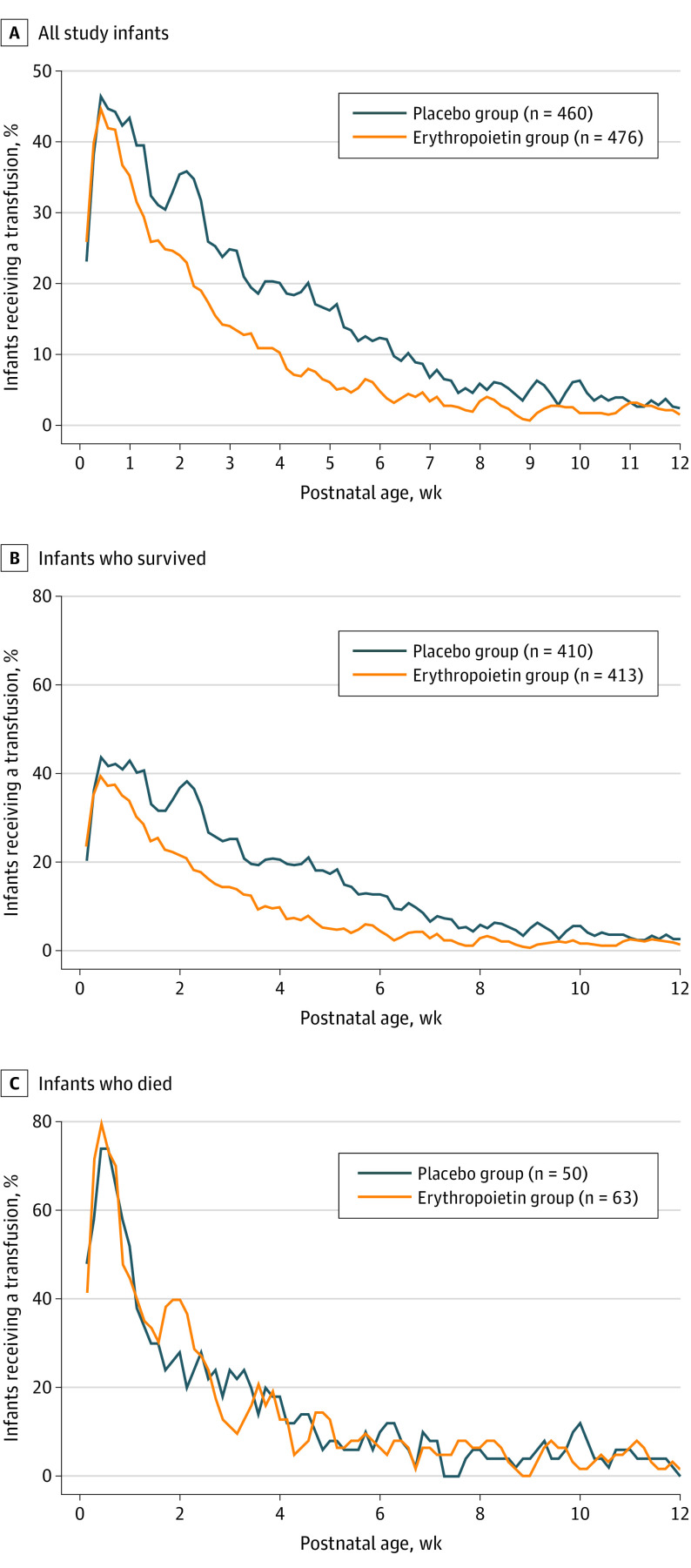
Most transfusions (3022 of 4061 [74.4%]) occurred during the first 4 weeks of life. Figure 2A shows the proportion of infants receiving transfusions for every 3-day window over time. By week 5, only 24 (5.0%) erythropoietin-treated infants received transfusions compared with 79 (17.2%) placebo-treated infants, and by week 8, 19 (4.0%) in erythropoietin-treated infants and 23 (5.0%) in placebo-treated infants received transfusions. There was a marked difference in transfusions for those infants who lived (Figure 2B) compared with those who died (Figure 2C), with 83 (73.5%) of those who died receiving transfusions in the first week of life compared with 325 survivors (39.5%). Among those who died, there was no difference in rates of transfusion by treatment group.
Figure 2. Transfusion Rate by Treatment Group and Survival Status.
Moving 3-day transfusion rates (ie, proportion of infants receiving a transfusion for every 3-day window) by treatment group were calculated for all infants (A [n = 936]), those who survived (B [n = 823]), and those who died (C [n = 113]).
Figure 3 compares the number of pRBC transfusions, cumulative volume, and unique donor exposures by treatment group. Each panel shows results for the PENUT cohort as a whole as well as by gestational age at birth and by sex. Comparing all participants across the treatment groups, the mean (SD) cumulative transfused volume in the erythropoietin group vs the placebo group was 47.6 (60.4) vs 76.3 (68.2) mL, with an adjusted difference of 25.7 mL (95% CI, 18.1-33.3 mL). The mean (SD) number of transfusions in the erythropoietin group vs the placebo group was 3.5 (4.0) vs 5.2 (4.4), with an adjusted relative rate (RR) of 0.66 (95% CI, 0.59-0.75). The mean (SD) donor exposure in the erythropoietin group vs the placebo group was 1.6 (1.7) vs 2.4 (2.0), with an RR of 0.67 (95% CI, 0.58-0.77). The overall difference results from both a higher fraction of participants in the erythropoietin group receiving no transfusions and a lower mean cumulative volume among those participants receiving transfusions. The mean (SD) volume among those in the erythropoietin group who received any transfusion was 63.1 (62.1) mL, whereas the mean (SD) volume among those in the placebo group receiving a transfusion was 84.2 (66.9) mL (adjusted difference, 21.7 mL; 95% CI, 13.0-30.4 mL). Because mortality affects transfusion exposures, results from sensitivity analysis using only infants who survived were also included. In survivors, erythropoietin significantly decreased the number, cumulative volume, and donor exposure in all groups. For instance, the mean (SD) cumulative transfused volume in the erythropoietin group vs the placebo group was 41.8 (52.2) vs 75.6 (65.3) mL, with an adjusted difference of 31.0 mL (95% CI, 23.6-38.4 mL) among survivors. When nonsurvivors were included, erythropoietin decreased the number, cumulative volume, and donor exposure in all but the cohort with a gestational age of 26 weeks.
Figure 3. Transfusion Exposures by Treatment Group.
The number of packed red blood cell (pRBC) transfusions (A [4061 records]), cumulative volume of pRBC transfusions (B [4052 records]), and donor exposure (C [3808 records]) were compared between treatment groups. Mean values were compared using generalized estimating equation models clustering on same-birth siblings, adjusted for gestational age and site. Relative rate (RR) of less than 1.00 favored the erythropoietin group.
The duration of study drug treatment varied by gestational week at birth, with infants born at a gestational age of 24 weeks (0-7 days) receiving 9 weeks of therapy and those born at a gestational age of 27 weeks (6-7 days) receiving 5 weeks of therapy. The difference between erythropoietin- and placebo-treated groups diminished after erythropoietin treatment was stopped at a PMA of 32 completed weeks. The magnitude and duration of treatment effect on hematocrit values is shown in Figure 4. Despite erythropoietin-treated infants receiving fewer transfusions, their lowest daily hematocrit level was significantly higher than that of placebo-treated infants. The difference in hematocrit level was the most noticeable at gestational week 33 (at which the last erythropoietin or placebo dose was given) in infants with a gestational age of 27 weeks: mean (SD) hematocrit level in the erythropoietin- vs placebo-treated cohort was 36.9% (5.5%) vs 30.4% (4.6%) (P < .001).
Figure 4. Lowest Daily Hematocrit Level Over Time by Gestational Age at Birth.
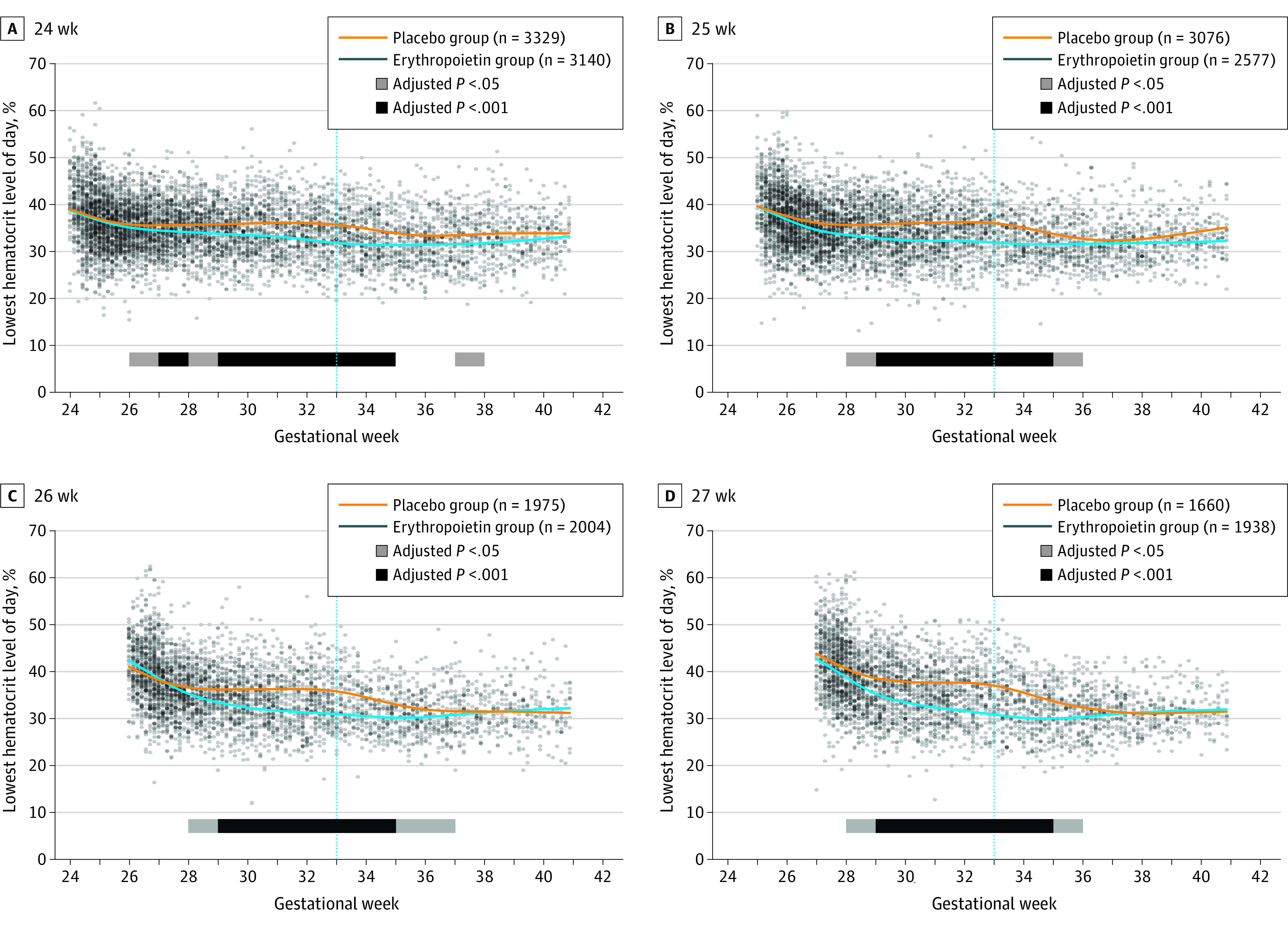
Mean weekly values by treatment group were compared using generalized estimating equation models clustering on same-birth siblings and adjusting for recruitment site. Significance was shown by P < .05 and P < .001 bars. The .001 level was chosen as an approximation to a conservative Bonferroni correction (.05 level divided by the total number of tests performed on the weekly data sets). Blue vertical line indicates week 33, at which the last erythropoietin or placebo dose was given.
Guidelines for iron intake recommended that supplementation start during the second week after birth, with adjustment based on assessment of either ferritin level or ZnPP:H ratio at day 14. Reflecting this adjustment, at 3 weeks of age, placebo-treated infants received a mean (SD) dosage of iron of 2.3 (2.7) mg/kg/d compared with 3.4 (3.0) mg/kg/d in the erythropoietin-treated group. By 6 weeks of age, the mean (SD) dosage increased to 3.3 (2.8) mg/kg/d and 4.8 (3.1) mg/kg/d, respectively (mean difference, 1.5 mg/kg/d). The cumulative intake of iron supplementation differed by treatment group, with the erythropoietin-treated cohort receiving significantly more iron than the placebo-treated cohort (eFigure 3 in Supplement 2). By the end of week 12, the mean (SD) cumulative enteral iron intake in the erythropoietin-treated cohort vs placebo-treated cohort was 275.1 (177.8) mg/kg vs 207.2 (143.7) mg/kg (P < .001). Despite the higher dose of iron in the erythropoietin-treated group, serum ferritin level and ZnPP:H ratio indicated that these infants were more likely to be iron deficient, with 192 (37.8%) ferritin values in erythropoietin-treated infants measuring below 76 ng/mL (to convert to μg/L, multiply by 1) compared with 53 (9.9%) ferritin measurements in the placebo-treated infants (eFigure 4 in Supplement 2). Similarly, ZnPP:H ratios were more likely to be greater than 115, consistent with iron deficiency.16,17
Discussion
Historically, in studies of preterm infants, erythropoietin has shown varied success in decreasing the number and volume of transfusions, likely owing to differences in targeted population, erythropoietin dosing, duration of treatment, and iron supplementation.9,18,19 A decrease in donor exposures has previously been reported in only 1 study20; however, we now show that, using the dosing schedule and duration of erythropoietin treatment chosen in PENUT, the risk of transfusion number, volume, and donor exposures can be significantly decreased in infants with gestational age less than 28 weeks. Although the timing and magnitude of the clinical response varies by gestational age (eFigure 1 in Supplement 2), with more mature infants responding more rapidly and effectively than those with earlier gestational ages, even the least mature infants benefitted (Figure 3). The differences by week of gestational age are likely owing to severity of illness and related need for phlebotomy, total blood volume available for phlebotomy, and response of hematopoietic organs during development (initially the liver, followed by the bone marrow). Despite these considerations, the treatment protocol used in PENUT was beneficial for all gestational ages, even resulting in a higher mean hematocrit level. Because most transfusions in preterm infants occur in the first weeks of life, starting early is necessary to maximize the benefit.
Because iron is incorporated into nascent red blood cells and erythropoietin stimulates erythropoiesis, it is critical to consider iron supplementation when using erythropoietin. In PENUT participants, use of iron supplements was greater in the erythropoietin-treated group, as expected. Guidelines for iron supplements were used in PENUT, with specific recommendations to adjust the iron supplementation based on either ferritin level or ZnPP:H ratio. As a result, the erythropoietin-treated infants received higher daily iron doses (a mean [SD] of 1.6 [0.2] mg/kg/d more than placebo-treated infants) with greater cumulative iron intake but less than the 6 to 9 mg/kg/d recommended when receiving erythropoietin for treatment of anemia and less than recommended by the PENUT guidelines.21,22,23,24 Erythropoietin-treated infants also received less iron in the form of transfusions. As a result, despite the higher dose of iron in the erythropoietin-treated group, serum ferritin levels and ZnPP:H ratios indicated that these infants were more likely to be iron deficient, with ferritin values more likely to be below 76 ng/mL (eFigure 4A in Supplement 2) and ZnPP:H ratios more likely to be greater than 115 (P < .001) (eFigure 4B in Supplement 2).16,17 Serum ferritin level may not be an ideal marker of iron status because it is an acute-phase reactant, and levels increase during inflammation. Therefore, if ferritin levels are low, they are a reliable marker of iron deficiency, but if normal or high, they may not reflect iron status accurately. The ZnPP:H ratio does not show similar changes in the face of inflammation; however, this test was used at fewer centers as an assessment of iron status.13 Because of these factors, the true incidence of iron deficiency may be higher in our study. Although neither serum ferritin levels nor ZnPP:H ratios are specific to brain iron content, it is well known that iron is first prioritized to erythropoiesis.25 Thus, when there is systemic (ferritin) or erythropoietic (ZnPP:H) evidence of iron depletion, the brain is likely already deficient. Levels below 40 ng/mL have been shown to compromise hippocampus-based recognition memory, and levels below 76 ng/mL have been associated with abnormalities in neonatal recognition memory,26 myelin-dependent speed of processing,27 and reflexes.28,29 Rigorous preclinical studies and longitudinal clinical studies have demonstrated that iron deficiency occurring during critical windows of brain development adversely affect neurodevelopment with irreversible effects despite later iron repletion.30,31,32 Specifically, after controlling for background factors, children and adolescents with iron deficiency relative to those with iron sufficiency show deficits in executive function, visuospatial cognition, mathematics, written expression, and motor functioning.33,34 We speculate that iron deficiency may have adversely affected neurodevelopmental outcomes in our study population.
Transfusions have been associated with several morbidities. In adults, transfusions are associated with increased incidence of multiorgan system failure and increased length of hospitalization and infection risk and are an independent factor associated with death.35,36 In addition to the rare transmission of blood-borne pathogens, transfusions have been associated with other morbidities, including transfusion-related acute lung injury,37,38 transfusion-related immune modulation,39 and transfusion-related cardiac overload.40,41 The association of transfusions with these problems in neonates has not been well studied because most extremely low gestational age newborns undergo transfusion, so comparing patients with and without transfusion is difficult; in preterm populations, the comparison is generally between liberal and restrictive transfusion guidelines.5,6 In preterm infants, pRBC transfusions have been implicated in the development of bronchopulmonary dysplasia,42 necrotizing enterocolitis,43,44,45,46 and retinopathy of prematurity. However, thus far a causative link between transfusions and these conditions has not been conclusively proven.47 These associations are complicated and influenced by many factors: infants with lower gestational age and lower birth weight are at higher risk of bronchopulmonary dysplasia, necrotizing enterocolitis, and retinopathy of prematurity but also at higher risk of transfusions and many complications of prematurity. Thus, it is difficult to separate these influences.
Limitations
Limitations of this study include the variability in transfusion practices by site, the missing information on 302 transfusions, and the absence of data on phlebotomy losses for each infant. Because missing data on transfusions were equally divided between the treatment groups, we believe our conclusions remain valid. The variability in site practice also contributed to the generalizability of our findings.
Conclusions
Given the known risks of pRBC transfusion, a common goal is to minimize this therapy. To this end, well known and safe interventions to reduce the need for pRBC transfusions should be used: implementation of transfusion guidelines, delayed cord clamping, use of cord blood for initial evaluations, decreasing blood draws, and use of microanalytic techniques. In addition, high-dose erythropoietin followed by maintenance dosing can be a safe and successful treatment strategy that results in fewer infants needing transfusions and an overall decrease in the number and volume of pRBC transfusions as well as donor exposures in treated infants. During erythropoietin treatment, it is important to pay careful attention to iron status because erythropoietin treatment in the absence of adequate iron supplementation can result in iron deficiency.
Trial Protocol
eFigure 1. Smoothed Average Cumulative Transfusion Volume by Treatment Group and Gestational Age
eFigure 2. Cumulative pRBC Transfusion Volume and Donor Exposure by Site Grouping
eFigure 3. Average Cumulative Iron Intake by Treatment Group
eFigure 4. Ferritin and ZnPP:H by Treatment Group
Data Sharing Statement
References
- 1.Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr. 2009;155(3):331-337.e1. doi: 10.1016/j.jpeds.2009.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuruvilla DJ, Widness JA, Nalbant D, et al. Estimation of adult and neonatal RBC lifespans in anemic neonates using RBCs labeled at several discrete biotin densities. Pediatr Res. 2017;81(6):905-910. doi: 10.1038/pr.2017.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henry E, Christensen RD, Sheffield MJ, et al. Why do four NICUs using identical RBC transfusion guidelines have different gestational age-adjusted RBC transfusion rates? J Perinatol. 2015;35(2):132-136. doi: 10.1038/jp.2014.171 [DOI] [PubMed] [Google Scholar]
- 4.Christensen RD, Carroll PD, Josephson CD. Evidence-based advances in transfusion practice in neonatal intensive care units. Neonatology. 2014;106(3):245-253. doi: 10.1159/000365135 [DOI] [PubMed] [Google Scholar]
- 5.Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115(6):1685-1691. doi: 10.1542/peds.2004-1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149(3):301-307. doi: 10.1016/j.jpeds.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 7.Juul S. Erythropoietin in anemia of prematurity. J Matern Fetal Neonatal Med. 2012;25(suppl 5):80-84. doi: 10.3109/14767058.2012.716987 [DOI] [PubMed] [Google Scholar]
- 8.Aher SM, Ohlsson A. Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2014;4(4):CD004868. doi: 10.1002/14651858.CD004868.pub4 [DOI] [PubMed] [Google Scholar]
- 9.Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2014;4(4):CD004863. doi: 10.1002/14651858.CD004863.pub4 [DOI] [PubMed] [Google Scholar]
- 10.Juul SE, Mayock DE, Comstock BA, Heagerty PJ. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern Health Neonatol Perinatol. 2015;1:27. doi: 10.1186/s40748-015-0028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juul SE, Comstock BA, Wadhawan R, et al. ; PENUT Trial Consortium . A randomized trial of erythropoietin for neuroprotection in preterm infants. N Engl J Med. 2020;382(3):233-243. doi: 10.1056/NEJMoa1907423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franz AR, Mihatsch WA, Sander S, Kron M, Pohlandt F. Prospective randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams. Pediatrics. 2000;106(4):700-706. doi: 10.1542/peds.106.4.700 [DOI] [PubMed] [Google Scholar]
- 13.German K, Vu PT, Grelli KN, Denton C, Lee G, Juul SE. Zinc protoporphyrin-to-heme ratio and ferritin as measures of iron sufficiency in the neonatal intensive care unit. J Pediatr. 2018;194:47-53. doi: 10.1016/j.jpeds.2017.10.041 [DOI] [PubMed] [Google Scholar]
- 14.Valentine SL, Bembea MM, Muszynski JA, et al. ; Pediatric Critical Care Transfusion and Anemia Expertise Initiative (TAXI); Pediatric Critical Care Blood Research Network (BloodNet); Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network . Consensus recommendations for RBC transfusion practice in critically ill children from the pediatric critical care transfusion and anemia expertise initiative. Pediatr Crit Care Med. 2018;19(9):884-898. doi: 10.1097/PCC.0000000000001613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacQueen BC, Baer VL, Scott DM, et al. Iron supplements for infants at risk for iron deficiency. Glob Pediatr Health. 2017;4:X17703836. doi: 10.1177/2333794X17703836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng CF, Zerzan JC, Johnson DB, Juul SE. Zinc protoporphyrin-to-heme ratios in high-risk and preterm infants. J Pediatr. 2012;161(1):81-87.e1. doi: 10.1016/j.jpeds.2011.12.048 [DOI] [PubMed] [Google Scholar]
- 17.Siddappa AM, Rao R, Long JD, Widness JA, Georgieff MK. The assessment of newborn iron stores at birth: a review of the literature and standards for ferritin concentrations. Neonatology. 2007;92(2):73-82. doi: 10.1159/000100805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aher SM, Ohlsson A. Late erythropoiesis-stimulating agents to prevent red blood cell transfusion in preterm or low birth weight infants. Cochrane Database Syst Rev. 2019;2:CD004868. doi: 10.1002/14651858.CD004868.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollak A, Hayde M, Hayn M, et al. Effect of intravenous iron supplementation on erythropoiesis in erythropoietin-treated premature infants. Pediatrics. 2001;107(1):78-85. doi: 10.1542/peds.107.1.78 [DOI] [PubMed] [Google Scholar]
- 20.Maier RF, Obladen M, Müller-Hansen I, et al. ; European Multicenter Erythropoietin Beta Study Group . Early treatment with erythropoietin beta ameliorates anemia and reduces transfusion requirements in infants with birth weights below 1000 g. J Pediatr. 2002;141(1):8-15. doi: 10.1067/mpd.2002.124309 [DOI] [PubMed] [Google Scholar]
- 21.Zamora TG, Guiang SF III, Widness JA, Georgieff MK. Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs. Pediatr Res. 2016;79(6):922-928. doi: 10.1038/pr.2016.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasper DC, Widness JA, Haiden N, et al. Characterization and differentiation of iron status in anemic very low birth weight infants using a diagnostic nomogram. Neonatology. 2009;95(2):164-171. doi: 10.1159/000153101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Widness JA, Serfass RE, Haiden N, Nelson SE, Lombard KA, Pollak A. Erythrocyte iron incorporation but not absorption is increased by intravenous iron administration in erythropoietin-treated premature infants. J Nutr. 2006;136(7):1868-1873. doi: 10.1093/jn/136.7.1868 [DOI] [PubMed] [Google Scholar]
- 24.Peters C, Georgieff MK, de Alarcon PA, et al. Effect of chronic erythropoietin administration on plasma iron in newborn lambs. Biol Neonate. 1996;70(4):218-228. doi: 10.1159/000244368 [DOI] [PubMed] [Google Scholar]
- 25.Rao R, Ennis K, Lubach GR, Lock EF, Georgieff MK, Coe CL. Metabolomic analysis of CSF indicates brain metabolic impairment precedes hematological indices of anemia in the iron-deficient infant monkey. Nutr Neurosci. 2018;21(1):40-48. doi: 10.1080/1028415X.2016.1217119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng F, Mai X, Zhan J, et al. Impact of fetal-neonatal iron deficiency on recognition memory at 2 months of age. J Pediatr. 2015;167(6):1226-1232. doi: 10.1016/j.jpeds.2015.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin SB, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J Pediatr. 2010;156(3):377-381. doi: 10.1016/j.jpeds.2009.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armony-Sivan R, Zhu B, Clark KM, et al. Iron deficiency (ID) at both birth and 9 months predicts right frontal EEG asymmetry in infancy. Dev Psychobiol. 2016;58(4):462-470. doi: 10.1002/dev.21388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armony-Sivan R, Eidelman AI, Lanir A, Sredni D, Yehuda S. Iron status and neurobehavioral development of premature infants. J Perinatol. 2004;24(12):757-762. doi: 10.1038/sj.jp.7211178 [DOI] [PubMed] [Google Scholar]
- 30.Congdon EL, Westerlund A, Algarin CR, et al. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J Pediatr. 2012;160(6):1027-1033. doi: 10.1016/j.jpeds.2011.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radlowski EC, Johnson RW. Perinatal iron deficiency and neurocognitive development. Front Hum Neurosci. 2013;7:585. doi: 10.3389/fnhum.2013.00585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lukowski AF, Koss M, Burden MJ, et al. Iron deficiency in infancy and neurocognitive functioning at 19 years: evidence of long-term deficits in executive function and recognition memory. Nutr Neurosci. 2010;13(2):54-70. doi: 10.1179/147683010X12611460763689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105(4):E51. doi: 10.1542/peds.105.4.e51 [DOI] [PubMed] [Google Scholar]
- 34.Shafir T, Angulo-Barroso R, Jing Y, Angelilli ML, Jacobson SW, Lozoff B. Iron deficiency and infant motor development. Early Hum Dev. 2008;84(7):479-485. doi: 10.1016/j.earlhumdev.2007.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hébert PC, Fergusson DA. Red blood cell transfusions in critically ill patients. JAMA. 2002;288(12):1525-1526. doi: 10.1001/jama.288.12.1525 [DOI] [PubMed] [Google Scholar]
- 36.Hébert PC, McDonald BJ, Tinmouth A. Clinical consequences of anemia and red cell transfusion in the critically ill. Crit Care Clin. 2004;20(2):225-235. doi: 10.1016/j.ccc.2003.12.006 [DOI] [PubMed] [Google Scholar]
- 37.Barrett NA, Kam PC. Transfusion-related acute lung injury: a literature review. Anaesthesia. 2006;61(8):777-785. doi: 10.1111/j.1365-2044.2006.04742.x [DOI] [PubMed] [Google Scholar]
- 38.Triulzi DJ. Transfusion-related acute lung injury: current concepts for the clinician. Anesth Analg. 2009;108(3):770-776. doi: 10.1213/ane.0b013e31819029b2 [DOI] [PubMed] [Google Scholar]
- 39.Stark MJ, Keir AK, Andersen CC. Does non-transferrin bound iron contribute to transfusion related immune-modulation in preterms? Arch Dis Child Fetal Neonatal Ed. 2013;98(5):F424-F429. doi: 10.1136/archdischild-2012-303353 [DOI] [PubMed] [Google Scholar]
- 40.Semple JW, Rebetz J, Kapur R. Transfusion-associated circulatory overload and transfusion-related acute lung injury. Blood. 2019;133(17):1840-1853. doi: 10.1182/blood-2018-10-860809 [DOI] [PubMed] [Google Scholar]
- 41.Simpson JD, Hopkins A, Amil A, Ross B, Enjeti AK. Transfusion-associated circulatory overload in ambulatory patients. Vox Sang. 2019;114(3):216-222. doi: 10.1111/vox.12753 [DOI] [PubMed] [Google Scholar]
- 42.Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Med Hypotheses. 2006;66(2):355-364. doi: 10.1016/j.mehy.2005.04.046 [DOI] [PubMed] [Google Scholar]
- 43.Mally P, Golombek SG, Mishra R, et al. Association of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates. Am J Perinatol. 2006;23(8):451-458. doi: 10.1055/s-2006-951300 [DOI] [PubMed] [Google Scholar]
- 44.Christensen RD, Lambert DK, Henry E, et al. Is “transfusion-associated necrotizing enterocolitis” an authentic pathogenic entity? Transfusion. 2010;50(5):1106-1112. doi: 10.1111/j.1537-2995.2009.02542.x [DOI] [PubMed] [Google Scholar]
- 45.Josephson CD, Wesolowski A, Bao G, et al. Do red cell transfusions increase the risk of necrotizing enterocolitis in premature infants? J Pediatr. 2010;157(6):972-978.e1, 3. doi: 10.1016/j.jpeds.2010.05.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teišerskas J, Bartašienė R, Tamelienė R. Associations between red blood cell transfusions and necrotizing enterocolitis in very low birth weight infants: ten-year data of a tertiary neonatal unit. Medicina (Kaunas). 2019;55(1):E16. doi: 10.3390/medicina55010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lust C, Vesoulis Z, Jackups R Jr, Liao S, Rao R, Mathur AM. Early red cell transfusion is associated with development of severe retinopathy of prematurity. J Perinatol. 2019;39(3):393-400. doi: 10.1038/s41372-018-0274-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. Smoothed Average Cumulative Transfusion Volume by Treatment Group and Gestational Age
eFigure 2. Cumulative pRBC Transfusion Volume and Donor Exposure by Site Grouping
eFigure 3. Average Cumulative Iron Intake by Treatment Group
eFigure 4. Ferritin and ZnPP:H by Treatment Group
Data Sharing Statement