Abstract
In Australia, disease registers for acute rheumatic fever (ARF) and rheumatic heart disease (RHD) were previously established to facilitate disease surveillance and control, yet little is known about the extent of case-ascertainment. We compared ARF/RHD case ascertainment based on Australian ARF/RHD register records with administrative hospital data from the Northern Territory (NT), South Australia (SA), Queensland (QLD) and Western Australia (WA) for cases 3–59 years of age. Agreement across data sources was compared for persons with an ARF episode or first-ever RHD diagnosis. ARF/RHD registers from the different jurisdictions were missing 26% of Indigenous hospitalised ARF/RHD cases overall (ranging 17–40% by jurisdiction) and 10% of non-Indigenous hospitalised ARF/RHD cases (3–28%). The proportion of hospitalised RHD cases (36%) was half the proportion of hospitalised ARF cases (70%) notified to the ARF/RHD registers. The registers were found to capture few RHD cases in metropolitan areas (SA Metro: 13%, QLD Metro: 35%, WA Metro: 14%). Indigenous status, older age, comorbidities, drug/alcohol abuse and disease severity were predictors of cases appearing in the hospital data only (p < 0.05); sex was not a determinant. This analysis confirms that there are biases associated with the epidemiological analysis of single sources of case ascertainment for ARF/RHD using Australian data.
Keywords: acute rheumatic fever, administrative data, epidemiology, linked data, disease register, rheumatic heart disease
1. Introduction
Rheumatic heart disease (RHD) is the most common cause of acquired heart disease in children globally [1]. It is a sequela of acute rheumatic fever (ARF), caused by an abnormal immunological response to a group A streptococcal (GAS) pharyngitis [2] or impetigo [3]. Spontaneous resolution of ARF symptoms occurs in most cases over weeks to months; however, 50–75% will progress to the chronic valvopathy of RHD [1]. Most will develop RHD as a result of recurrent episodes of ARF, however, valve damage can occur after a single episode [4]. In Australia, time to disease progression has been shown to vary. Some 35% of Indigenous cases progress to RHD within two years and 61% within ten years of their first ARF episode [5]. Although the incidence rates of ARF and RHD substantially decreased in high-income countries during the 20th century [6], there remains a significant burden of disease in disadvantaged minority populations, including Aboriginal and Torres Strait Islander (hereafter respectfully, Indigenous) populations in Australia who have some of the highest ARF/RHD rates globally [1,7].
Intramuscular benzathine penicillin G (BPG) is widely used as a secondary prophylaxis to prevent recurrences in ARF episodes and subsequent RHD [3,8]. Previous studies have demonstrated a decrease in the rates of ARF recurrences by 87% to 96% [9] and reductions in complications associated with RHD with the regular administration of BPG [1,10]. The World Health Organization (WHO) recommends that countries faced with high ARF/RHD rates have “adequate monitoring and surveillance, as an integrated component of national health systems responses” [2,11]; for an overview of the evolution of ARF/RHD registers globally, see [12].
In Australia, both ARF and RHD are notifiable conditions in the Northern Territory (NT), Western Australia (WA), South Australia (SA) and Queensland (QLD), with the timing of the introduction of notification varying in these jurisdictions. In the remaining states and territories, ARF and RHD are only partially or not notifiable. In addition, jurisdiction-based ARF/RHD registers were established independently and at different times across the NT (1997), WA (2009), QLD (2006), SA (2010) and New South Wales (NSW) (2015). This resulted in different operational definitions and underlying data structures [10,13]. In SA and NT, clinicians have the option to enter the data of patients diagnosed with ARF and RHD directly into the register, however, most opt to faxing and emailing the control programs [13]. In WA and QLD, clinicians do not have the ability to manually enter data into the registers, but complete a notification form instead [13]. This results in control program staff being heavily involved with manual data handling and case validation [13]. The Federal government has published two reports (2013 and 2017) summarizing data from the jurisdictional registers [14,15]. No integrated, national register exists.
According to the Australian guidelines for ARF and RHD diagnosis (a modification of the Jones and WHO criteria), both ARF and RHD are to be considered as differential diagnosis when evaluating Indigenous children and adolescents with cardiac symptoms, and hospitalisation is required for all ARF episodes as soon as possible after symptom onset [10]. RHD cases can be expected to be hospitalised for case management, especially for RHD-associated complications. Thus, in theory most cases identified through ARF/RHD register records should appear in hospital administrative data, and vice versa. In practice, gaps in case ascertainment on the ARF/RHD registers may persist and additional data is required to obtain more complete data for determining the ARF/RHD burden.
The END RHD in Australia: Study of Epidemiology (ERASE) Project aims to provide the first quasi-national epidemiological profile of ARF/RHD using linked data from multiple data sources. Linked administrative data allows for the follow-up of cases across health system contacts and facilities over time, avoiding overestimation of diagnoses and caseloads. The project works closely with the End RHD Centre for Research Excellence [16] which has developed a strategy to remove RHD as a public health problem in the country. ERASE has assembled a linked dataset covering five jurisdictions (NSW, NT, QLD, SA, WA) to facilitate ARF/RHD epidemiological research in Australia [17,18]. Pertinent to the current study, ARF and RHD case identification was based on probability-linked ARF/RHD register, hospital admission and death data.
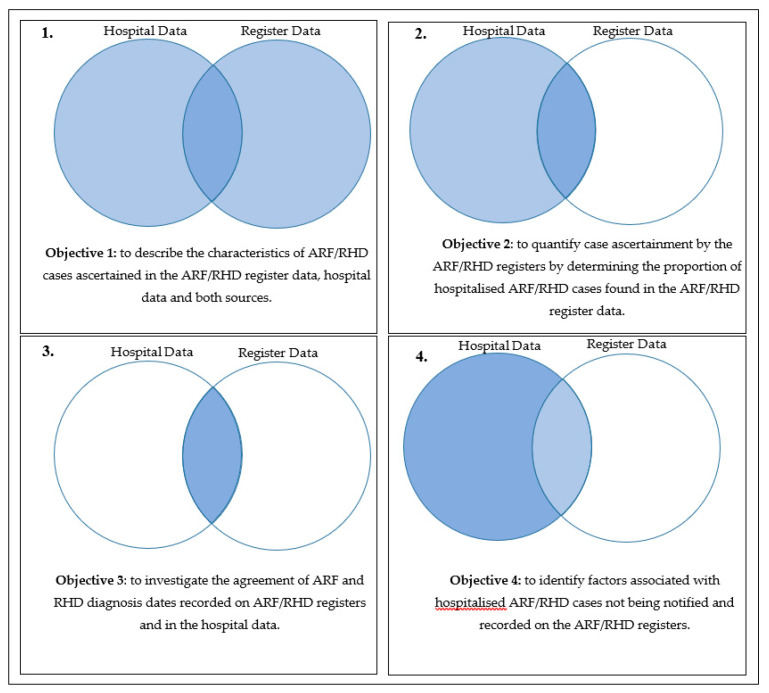
The overall aim of this study is to evaluate case ascertainment in the Australian ARF/RHD registers by comparing register records to administrative hospital data. The specific objectives were to:
-
(1)
describe the characteristics of ARF/RHD cases ascertained in the ARF/RHD register data, hospital data and both sources,
-
(2)
quantify case ascertainment by the ARF/RHD registers by determining the proportion of hospitalised ARF/RHD cases found in the ARF/RHD register data,
-
(3)
investigate the agreement of ARF and RHD diagnosis dates recorded on ARF/RHD registers and in the hospital data, and
-
(4)
identify factors associated with hospitalised ARF/RHD cases not being notified and recorded on the ARF/RHD registers.
2. Materials and Methods
2.1. Study Design and Data Sources
This is a validation study of case ascertainment based on ARF/RHD diagnoses recorded in two different case ascertainment systems, namely the register and administrative hospital records. The parent ERASE database identified ARF and RHD cases diagnosed in paediatric and adult populations in NT, SA, QLD, NSW and WA between July 2001 and June 2017. These five jurisdictions are home to 86% of the Australian Indigenous population (at 30 June 2016) [19]. Cases diagnosed outside their jurisdiction of residence were excluded, because we do not have access to cross-jurisdictionally linked data except for NT and SA. For the current study, ARF or RHD diagnoses had to be recorded in either data source (administrative hospital records or register) at least one calendar year following the establishment of a register in their jurisdiction of residence (NT from July 2001, SA from January 2013, QLD from January 2010 and WA from January 2011). Due to the NSW’s register’s recent inception, NSW data was not included. The study excluded foreign residents and patients with ARF/RHD diagnoses made before register establishment or outside an individual’s jurisdiction of residence. Administrative hospital data included records from public and private hospitals (NT and SA public hospitals only).
An ARF episode was defined as an episode recorded on an ARF/RHD register or as the principal diagnosis in an admissions record (International Classification of Diseases 10th Edition Australian Modification (ICD-10-AM) code: I00–I02) [14]. A unique episode was defined as an ARF record >90 days from the previous one and the earliest available diagnosis date was used to define episode onset [20,21].
A person was defined to be an RHD case identified on an ARF/RHD register from the earliest date they had an RHD severity assessment evaluated as “mild”, “moderate” or “severe” or had an RHD-related surgery or procedure recorded. Cases from the hospital data were defined from the earliest admission date where a predictive algorithm developed by the ERASE project assigned a predictive probability of being a valid RHD case. The prediction model considered ICD-10-AM codes I05–I09 as well as demographic and clinical variables [22,23]. RHD was defined to be “severe” if an individual had a hospital discharge diagnosis of heart failure, a procedure code indicating a valvular procedure or surgery (see Table A1 in the Appendix A) or was evaluated by a cardiologist as having severe RHD and documented on an ARF/RHD register accordingly.
The sample contains individuals 3–59 years of age at time of diagnosis. Individuals 60 years and over were excluded from the study, as this algorithm was not validated for this age group [17,18]. Individuals under the age of three were excluded to reduce the risk of misclassification of congenital heart disease as RHD.
Other variables of interest included baseline demographic (age, sex, vital status, jurisdiction of residence, Accessibility/Remoteness Index of Australia (ARIA) distinguishing between “very remote”, “remote”, “outer regional”, “inner regional” and “metropolitan” areas, socioeconomic status by population quintile (SES)), clinical (diagnosis (ARF or RHD), disease severity (mild/moderate or severe), ARF episode number (first-ever or recurrence), comorbidities and complications of RHD) and ARF/RHD register-related (last ARF/RHD diagnosis since register establishment) variables. The Socioeconomic Indexes for Areas (SEIFA) rank areas in Australia based on relative socio-economic advantage and disadvantage. For this study, Indigenous cases were allocated an Indigenous-specific socioeconomic index (Indigenous Relative Socioeconomic Outcomes index, IRSEO). The study used the most recent available recordings of SEIFA, IRSEO, ARIA, age and jurisdiction of residence for each individual. As comorbidity indices such as the Charlson Comorbidity Index [24] are not suitable for childhood conditions [25], individual comorbidities based on ICD-10-AM coded hospital admissions were investigated. The comorbidities included chronic obstructive pulmonary diseases, chronic kidney disease, other cardiovascular diseases, coronary heart disease, anticoagulant treatment, diabetes and mental health conditions. The complications considered for the analysis were heart failure, stroke, endocarditis and atrial fibrillation. Drugs/alcohol abuse and pregnancy (post-ARF/RHD diagnosis) were also investigated (see Table A2 in the Appendix A).
2.2. Statistical Methods
Figure 1 provides a visual overview of the cohorts for each sub-analysis. Univariate comparisons of demographic, clinical and ARF/RHD register-related variables were conducted to describe the characteristics of cases identified through the two different sources of ARF/RHD records. We identified statistical significance at the 0.05 level using a two-sided Chi-squared test. To describe case ascertainment on the registers, we calculated the percentage of ARF/RHD patients identified in hospital administrative data who also had an ARF/RHD record on a register. We also calculated the percentage of diagnosis dates recorded on an ARF/RHD register that were the equal or prior to hospital admission dates to determine the agreement of case ascertainment systems regarding the earliest reliable indication of an ARF/RHD diagnosis of each case. Inferential analyses were conducted using multivariate logistic regression models. The outcome variable identified the source of the record (1 = hospital only, 0 = register only or both register and hospital). Model selection was based on a priori inclusion of important covariates (SES, age, sex, disease severity, jurisdiction) and a backward stepwise regression methodology. The final model excluded complications due to its collinearity with disease severity. All analyses were performed using RStudio (V1.1.463) RStudio PBC, Boston, MA., USA), and Microsoft Excel (Microsoft Corporation, Redmond, CA, USA).
Figure 1.
Study objectives and sub-samples.
3. Results
3.1. Descriptive Analysis (Objective 1)
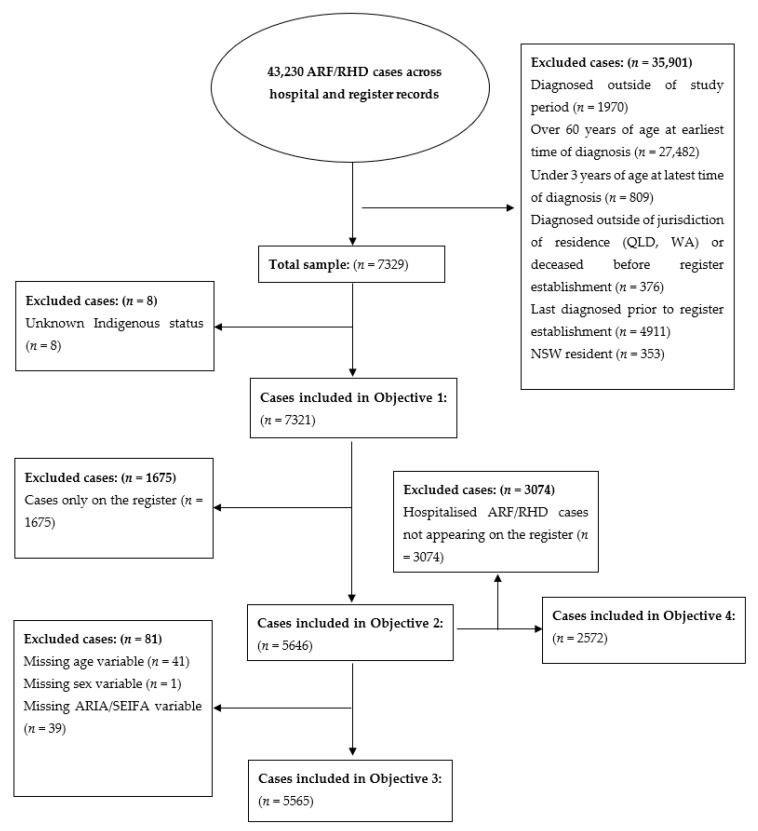
Of the 7321 cases in either source (Figure 2), 5824 were Indigenous (80%) (Table 1). Women comprised ~60% of cases for all data sources for both Indigenous and non-Indigenous patients. The cases only found in the hospital data had a higher percentage of individuals with ‘RHD only’ diagnoses, comorbidities, complications and a history of drugs/alcohol abuse compared with those only recorded on a register (all p < 0.001). Hospital cases were also older (p < 0.001), with >60% being 35 years or older among Indigenous patients and >60% being 45 years or older for non-Indigenous patients. This compares with 47% being 24 years or younger and 56% being 34 years or younger for Indigenous and non-Indigenous register only cases. Non-Indigenous cases had more than double the proportion of individuals appearing in the hospital data only (76%) compared with Indigenous cases (31%).
Figure 2.
Flowchart of sample selection.
Table 1.
Descriptive analysis of the study samples by Indigenous status and data sources.
| Variable | Indigenous (n = 5824) | Non–Indigenous (n = 1497) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital Only | Both | Register Only | Hospital Only | Both | Register Only | |||||||||
| Cases n | R% | n | R% | n | R% | p-Value | n | R% | n | R% | n | R% | p-Value | |
| Total | 1821 | 31% | 2484 | 43% | 1519 | 26% | 1135 | 76% | 206 | 14% | 156 | 10% | ||
| Most Recent Age (years) | <0.001 | <0.001 | ||||||||||||
| 3–14 | 107 | 12% | 535 | 59% | 261 | 29% | 35 | 38% | 32 | 35% | 24 | 26% | ||
| 15–24 | 159 | 12% | 720 | 54% | 457 | 34% | 50 | 36% | 46 | 33% | 42 | 30% | ||
| 25–34 | 294 | 25% | 548 | 46% | 344 | 29% | 90 | 67% | 23 | 17% | 22 | 16% | ||
| 35–44 | 441 | 46% | 319 | 33% | 209 | 22% | 149 | 78% | 28 | 15% | 15 | 8% | ||
| 45–54 | 424 | 54% | 225 | 28% | 141 | 18% | 262 | 83% | 36 | 11% | 18 | 6% | ||
| 55–75 | 393 | 62% | 137 | 22% | 107 | 17% | 511 | 87% | 41 | 7% | 35 | 6% | ||
| Sex | 0.153 | 0.725 | ||||||||||||
| Male | 653 | 30% | 944 | 43% | 592 | 27% | 443 | 75% | 80 | 14% | 66 | 11% | ||
| Female | 1168 | 32% | 1539 | 42% | 927 | 26% | 692 | 76% | 126 | 14% | 90 | 10% | ||
| Diagnosis | <0.001 | <0.001 | ||||||||||||
| ARF Only | 284 | 22% | 551 | 42% | 463 | 36% | 142 | 71% | 28 | 14% | 29 | 15% | ||
| RHD Only | 1457 | 47% | 856 | 27% | 809 | 26% | 985 | 81% | 119 | 10% | 108 | 9% | ||
| Both | 80 | 6% | 1077 | 77% | 247 | 18% | 8 | 9% | 59 | 69% | 19 | 22% | ||
| Disease Status | <0.001 | <0.001 | ||||||||||||
| Severe RHD | 765 | 40% | 911 | 48% | 226 | 12% | 740 | 80% | 133 | 14% | 48 | 5% | ||
| Non-Severe RHD | 772 | 29% | 1022 | 39% | 830 | 32% | 253 | 67% | 45 | 12% | 79 | 21% | ||
| ARF Only | 284 | 22% | 551 | 42% | 463 | 36% | 142 | 71% | 28 | 14% | 29 | 15% | ||
| ARF Recurrence | <0.001 | 0.122 | ||||||||||||
| Total | 365 | 14% | 1628 | 60% | 710 | 26% | 150 | 53% | 87 | 31% | 48 | 17% | ||
| No | 313 | 14% | 1222 | 55% | 668 | 30% | 145 | 53% | 81 | 30% | 48 | 18% | ||
| Yes | 51 | 10% | 406 | 81% | 42 | 8% | 5 | 45% | 6 | 55% | 0 | 0% | ||
| Vital Status | <0.001 | 0.056 | ||||||||||||
| Alive | 1403 | 27% | 2264 | 44% | 1483 | 29% | 1023 | 75% | 193 | 14% | 148 | 11% | ||
| Dead | 418 | 62% | 220 | 33% | 36 | 5% | 112 | 84% | 13 | 10% | 8 | 6% | ||
| State of Residence | <0.001 | <0.001 | ||||||||||||
| NT | 1207 | 36% | 1580 | 47% | 591 | 17% | 81 | 52% | 31 | 20% | 43 | 28% | ||
| SA | 54 | 47% | 33 | 28% | 29 | 25% | 135 | 91% | 8 | 5% | 6 | 4% | ||
| QLD | 380 | 24% | 585 | 38% | 593 | 38% | 705 | 73% | 163 | 17% | 101 | 10% | ||
| WA | 180 | 23% | 286 | 37% | 306 | 40% | 214 | 96% | <5 | 2% | 6 | 3% | ||
| Remoteness (ARIA) | <0.001 | <0.001 | ||||||||||||
| Inner regional | 164 | 54% | 91 | 30% | 50 | 16% | 867 | 82% | 136 | 13% | 53 | 5% | ||
| Outer Regional | 341 | 35% | 407 | 41% | 235 | 24% | 190 | 71% | 49 | 18% | 29 | 11% | ||
| Remote | 1303 | 31% | 1982 | 47% | 975 | 23% | 55 | 59% | 19 | 20% | 20 | 21% | ||
| Socioeconomic Status (SES) | <0.001 | <0.001 | ||||||||||||
| Quintile I & II | 245 | 42% | 202 | 34% | 141 | 24% | 362 | 87% | 31 | 7% | 25 | 6% | ||
| Quintile III | 282 | 35% | 343 | 43% | 176 | 22% | 248 | 78% | 49 | 15% | 21 | 7% | ||
| Quintile IV | 336 | 31% | 442 | 40% | 317 | 29% | 221 | 79% | 38 | 14% | 19 | 7% | ||
| Quintile V | 945 | 31% | 1493 | 49% | 626 | 20% | 283 | 70% | 86 | 21% | 36 | 9% | ||
| Last Interaction Since Register Establishment (years) | <0.001 | <0.001 | ||||||||||||
| Mean Time | 6.7 | 9.6 | 6.3 | 3.5 | 5.3 | 5.5 | ||||||||
| 0–2 | 238 | 40% | 113 | 19% | 247 | 41% | 295 | 84% | 34 | 10% | 21 | 6% | ||
| 2+ | 1583 | 30% | 2371 | 45% | 1272 | 24% | 840 | 73% | 172 | 15% | 135 | 12% | ||
| Comorbidity | ||||||||||||||
| Any | 1335 | 44% | 1268 | 42% | 415 | 14% | <0.001 | 873 | 84% | 128 | 12% | 38 | 4% | <0.001 |
| Complications | ||||||||||||||
| Any | 769 | 54% | 570 | 40% | 93 | 6% | <0.001 | 601 | 85% | 85 | 12% | 22 | 3% | <0.001 |
| Drug/Alcohol Abuse | ||||||||||||||
| Any Substance | 1240 | 43% | 1124 | 39% | 527 | 18% | <0.001 | 414 | 84% | 51 | 10% | 28 | 6% | <0.001 |
| Pregnancy | ||||||||||||||
| Females of Reproductive Age | 747 | 30% | 1095 | 43% | 679 | 27% | <0.001 | 250 | 70% | 64 | 18% | 45 | 13% | 0.004 |
| Pregnancy | 332 | 35% | 480 | 50% | 144 | 15% | <0.001 | 68 | 74% | 15 | 16% | 9 | 10% | 0.540 |
3.1.1. Indigenous Cases
The NT was the primary jurisdiction of residence for Indigenous cases (Table 1). Across all case ascertainment systems, Indigenous cases were predominantly from remote areas and from areas with the lowest SES (all p < 0.001). Cases from the two highest SES areas (Quintile I & II) were mostly found in the hospital data only. As social disadvantage increased, the proportion of individuals in the hospital data only appeared to decrease. There was little difference in the time of diagnosis relative to register establishment between cases only found in the hospital data and cases that were only register-recorded (40% versus 41% diagnosed within two years after register establishment, p < 0.001). 23% of Indigenous people in the hospital data also have a death record and accounted for 62% of all deceased Indigenous cases (Table 1). The majority of SA cases appeared in the hospital data only (47%) whereas the NT had the highest proportion of individuals appearing in both data sources (likely also related to the fact that NT has the largest number of cases overall). The characteristics of the cases in the hospital data only were similar when stratified by jurisdiction (see Table A3 in the Appendix A).
3.1.2. Non-Indigenous Cases
QLD was the primary jurisdiction of residence for non-Indigenous cases (Table 1). The cases appearing in the hospital data only were predominantly from the most socioeconomically advantaged areas, while cases appearing on both the register and hospital data or the register record only, predominantly resided in the most socioeconomically disadvantaged areas (p < 0.001). Cases residing remotely or in more disadvantaged areas had the lowest proportion of individuals in the hospital data only (Table 1).
3.2. Case Ascertainment on ARF/RHD Registers (Objective 2)
For both Indigenous and non-Indigenous cases, the proportion of hospitalised ARF/RHD cases that were also recorded on a register was lower for RHD cases (45% Indigenous, 13% non-Indigenous) compared with ARF cases (75% Indigenous, 33% non-Indigenous) (Table 2).
Table 2.
Count and percentage of hospital diagnosed ARF/RHD cases recorded on ARF/RHD registers by Indigenous status and jurisdiction.
| Indigenous Status | ARF | RHD | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Hospital Cases | Hospital Cases on the Register (%) | Total Hospital Cases |
Hospital Cases on the Register (%) | |||||
| Indigenous | ||||||||
| Jurisdiction | NT | 1133 | 874 | 77% | 2029 | 881 | 43% | |
| SA | 25 | 19 | 76% | 66 | 14 | 21% | ||
| QLD | 414 | 280 | 68% | 622 | 319 | 51% | ||
| WA | 226 | 172 | 76% | 283 | 131 | 46% | ||
| Total | 1798 | 1345 | 75% | 3000 | 1345 | 45% | ||
| Non-Indigenous | ||||||||
| Jurisdiction | NT | 30 | 13 | 43% | 87 | 19 | 22% | |
| SA | 8 | <5 | 12% | 135 | 7 | 5% | ||
| QLD | 167 | 59 | 35% | 721 | 116 | 16% | ||
| WA | 27 | <5 | 15% | 193 | <5 | 1% | ||
| Total | 232 | 77 | 33% | 1136 | 143 | 13% | ||
| Indigenous & non-Indigenous | 2030 | 1422 | 70% | 4136 | 1488 | 36% | ||
3.2.1. Indigenous Cases
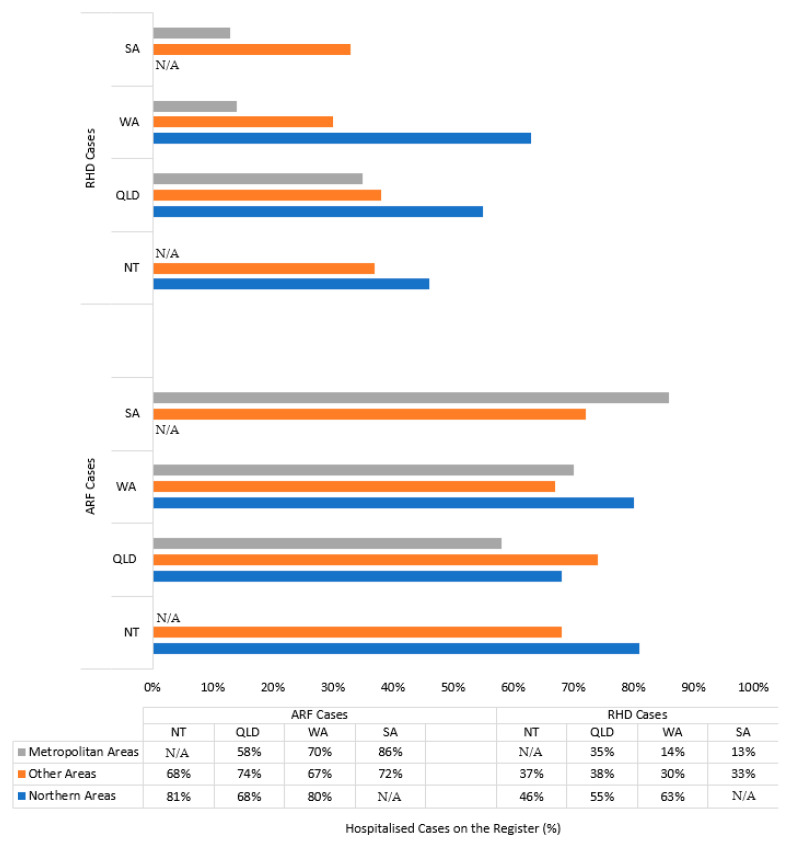
QLD had 68% of ARF cases appearing on the register, and SA had only 21% of Indigenous cases with RHD included on the register (Table 2). The Northern areas of Australia (NT Top End: 46%, QLD North: 55%, WA North: 63%) had a higher percentage value of RHD cases appearing on the register compared with the rest of the country (NT Central: 37%, QLD Other: 38%, WA Other: 30%, SA Other: 33%), and especially with metropolitan areas (QLD Metro: 35%, WA Metro: 14%, SA Metro: 13%) (Figure 3).
Figure 3.
Percentage of hospital diagnosed acute rheumatic fever (ARF) and rheumatic heart disease (RHD) cases recorded on ARF/RHD registers for Indigenous cases by jurisdiction and geography.
3.2.2. Non-Indigenous Cases
Non-Indigenous patients across all jurisdictions had less than 50% of hospitalised ARF cases and less than 25% of hospitalised RHD cases on the register (Table 2).
3.3. Agreement between Recorded Diagnosis Dates (Objective 3)
For both Indigenous and non-Indigenous cases, QLD and WA had >90% agreement between ARF episode dates recorded on their registers and hospital data (Table 3). In contrast, for Indigenous cases, NT and SA had <40% of ARF register episode dates on the registers agreeing with those found in hospital data and <15% for non-Indigenous episodes. The agreement in dates for RHD was higher for non-Indigenous cases compared with Indigenous cases (66% versus 60% across all jurisdictions).
Table 3.
Agreement between recorded diagnosis dates for ARF and RHD cases on both sources by Indigenous status, jurisdiction and Indigenous region.
| Indigenous Status | ARF | RHD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Episodes on Both Sources | Agreeing Date | Time Difference of Non-Agreeing Dates (Days) | Diagnosis on Both Sources | Agreeing Dates | Time Difference of Non-Agreeing Dates (Days) | ||||||||
| Indigenous | Total | n | % | Mean | Median | IQR | Total | n | % | Mean | Median | IQR | |
| Jurisdiction | NT | 979 | 396 | 40% | 6 | 3 | 5 | 881 | 548 | 62% | 347 | 8 | 324 |
| SA | 19 | 6 | 32% | 6 | 4 | 6 | 14 | 5 | 36% | 92 | 5 | 5 | |
| QLD | 279 | 268 | 96% | 15 | 14 | 22 | 319 | 163 | 51% | 331 | 56 | 445 | |
| WA | 186 | 174 | 94% | 3 | 2 | 2 | 131 | 93 | 71% | 258 | 59 | 262 | |
| Total | 1463 | 844 | 58% | 6 | 3 | 5 | 1345 | 809 | 60% | 332 | 16 | 348 | |
| Indigenous Regions (IREGs) | NT Top End | 733 | 306 | 42% | 5 | 2 | 4 | 666 | 405 | 61% | 298 | 6 | 187 |
| NT Central | 246 | 90 | 37% | 7 | 5 | 7 | 215 | 143 | 67% | 526 | 17 | 609 | |
| SA Other | 13 | <5 | 31% | 7 | 5 | 5 | 9 | <5 | 33% | 24 | 3 | 3 | |
| SA Metro | 6 | <5 | 33% | 6 | 2 | 5 | 5 | <5 | 40% | 227 | 7 | 331 | |
| QLD North | 244 | 235 | 96% | 15 | 14 | 18 | 273 | 133 | 49% | 317 | 49 | 334 | |
| QLD Other | 23 | 22 | 96% | 1 | 1 | 0 | 21 | 16 | 76% | 724 | 532 | 1262 | |
| QLD Metro | 12 | 11 | 92% | 27 | 27 | 0 | 25 | 14 | 56% | 336 | 439 | 531 | |
| WA North | 135 | 130 | 96% | 5 | 3 | 3 | 105 | 77 | 73% | 231 | 54 | 226 | |
| WA Other | 32 | 28 | 88% | 1 | 1 | 0 | 18 | 9 | 50% | 372 | 218 | 580 | |
| WA Metro | 19 | 16 | 84% | 2 | 2 | 1 | 8 | 7 | 88% | 1 | 1 | 0 | |
| Non-Indigenous | |||||||||||||
| Jurisdiction | NT | 13 | <5 | 15% | 7 | 5 | 7 | 19 | 13 | 68% | 254 | 74 | 350 |
| SA | <5 | 0 | 0% | 6 | 6 | 0 | 7 | <5 | 57% | 79 | 4 | 116 | |
| QLD | 58 | 56 | 97% | 14 | 14 | 12 | 116 | 76 | 66% | 239 | 6 | 141 | |
| WA | <5 | <5 | 100% | <5 | <5 | 100% | |||||||
| Total | 76 | 62 | 82% | 8 | 6 | 7 | 143 | 94 | 66% | 231 | 7 | 146 | |
Mean and median difference between dates recorded on the registers and hospital data were more variable among jurisdictions for RHD (mean: 79–347 days, median: 4–74 days) compared with ARF (mean: 3–15 days, median: 2–14 days). We also conducted a sensitivity analysis where we considered a 14-day ‘grace period’ for ARF onset and a 90-day ‘grace period’ for RHD onset. Any dates falling within these time windows across data sources were still considered to be in agreement. The agreement was greater than 89% for ARF and 77% for RHD (see Table A4 in the Appendix A).
3.4. Multivariate Inferential Analysis (Objective 4)
Non-Indigenous cases had 3.1 times higher odds of appearing in the hospital data only (95% CI 2.4–3.9, p < 0.001) after adjusting for age, sex, disease severity, vital status, jurisdiction, ARIA, SES, comorbidity, drugs/alcohol abuse and register establishment (Table 4). Older age, comorbidities, drugs/alcohol abuse and disease severity but not sex were determinants of register exclusion. As the age group of the cases increased the adjusted odds of appearing in the hospital data only increased considerably. After adjustment, as disease severity increased the odds of being in the hospital data only decreased. The adjusted odds ratios (AOR) were not statistically significant for ARIA, vital status, comorbidities and register establishment for non-Indigenous cases, and SES for Indigenous cases.
Table 4.
Multivariate inferential analysis of ARF and RHD cases only found in the hospital data.
| Variable | Total (n = 5565) | Indigenous (n = 4284) | Non-Indigenous (n = 1281) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | (CI) | AOR | (CI) | p Value | OR | (CI) | AOR | (CI) | p Value | OR | (CI) | AOR | (CI) | p Value | |
| Indigenous status | |||||||||||||||
| Non-Indigenous | 7.3 | (6.2–8.5) | 3.1 | (2.4–3.9) | <0.001 | ||||||||||
| Most Recent Age (years) | |||||||||||||||
| 15–24 | 1.1 | (0.85–1.4) | 1.2 | (0.89–1.5) | 0.267 | 1.1 | (0.84–1.4) | 1.2 | (0.86–1.5) | 0.347 | 1.0 | (0.53–1.9) | 1.2 | (0.55–2.5) | 0.691 |
| 25–34 | 2.7 | (2.1–3.3) | 3.8 | (2.9–5.1) | <0.001 | 2.7 | (2.1–3.4) | 3.5 | (2.5–4.8) | <0.001 | 3.6 | (1.8–7.0) | 7.6 | (3.3–18) | <0.001 |
| 35–44 | 6.8 | (5.4–8.5) | 10 | (7.4–14) | <0.001 | 6.9 | (5.4–8.9) | 9.7 | (7.0–14) | <0.001 | 4.8 | (2.6–9.2) | 9.1 | (4.0–22) | <0.001 |
| 45–54 | 11 | (8.4–13) | 14 | (11–20) | <0.001 | 9.4 | (7.3–12) | 14 | (10–20) | <0.001 | 6.8 | (3.7–12) | 16 | (7.0–38) | <0.001 |
| 55–75 | 21 | (16–26) | 23 | (17–32) | <0.001 | 14 | (11–19) | 22 | (15–32) | <0.001 | 12 | (6.7–21) | 31 | (14–72) | <0.001 |
| Sex | |||||||||||||||
| Male | 0.95 | (0.85–1.1) | 1.1 | (0.97–1.3) | 0.125 | 0.90 | (0.79–1.0) | 1.1 | (0.93–1.3) | 0.300 | 1.0 | (0.75–1.4) | 1.2 | (0.86–1.8) | 0.256 |
| Disease Status | |||||||||||||||
| Severe RHD | 1.9 | (1.7–2.2) | 0.18 | (0.14–0.23) | <0.001 | 1.6 | (1.4–1.9) | 0.20 | (0.15–0.26) | <0.001 | 1.1 | (0.68–1.7) | 0.08 | (0.04–0.17) | <0.001 |
| Mild/Moderate RHD | 1.3 | (1.1–1.5) | 0.45 | (0.37–0.56) | <0.001 | 1.5 | (1.2–1.7) | 0.49 | (0.39–0.61) | <0.001 | 1.1 | (0.64–1.8) | 0.22 | (0.11–0.44) | <0.001 |
| Vital Status | |||||||||||||||
| Dead | 2.4 | (2.0–2.8) | 1.8 | (1.5–2.3) | <0.001 | 3.1 | (2.6–3.7) | 1.8 | (1.5–2.3) | <0.001 | 1.7 | (0.97–3.2) | 1.2 | (0.62–2.4) | 0.619 |
| State of Residence | |||||||||||||||
| SA | 5.7 | (4.1–8.1) | 2.1 | (1.3–3.3) | 0.002 | 2.1 | (1.3–3.3) | 1.6 | (0.92–2.8) | 0.097 | 6.6 | (3.0–16) | 8.9 | (3.4–25) | <0.001 |
| QLD | 1.8 | (1.6–2.0) | 0.87 | (0.7–1.0) | 0.131 | 0.85 | (0.73–0.99) | 0.77 | (0.62–0.96) | 0.018 | 1.6 | (1.0–2.6) | 2.6 | (1.4–5.0) | 0.004 |
| WA | 1.7 | (1.4–2.0) | 1.1 | (0.87–1.4) | 0.421 | 0.83 | (0.68–1.0) | 0.81 | (0.62–1.1) | 0.110 | 21 | (8.1–73) | 30 | (9.7–118) | <0.001 |
| Last Diagnosis Since Register Establishment (years) | |||||||||||||||
| 2+ | 3.8 | (3.2–4.6) | 2.3 | (1.9–3.0) | <0.001 | 3.2 | (2.5–4.0) | 2.9 | (2.2–3.9) | <0.001 | 1.8 | (1.2–2.7) | 1.2 | (0.74–1.8) | 0.529 |
| Comorbidity | |||||||||||||||
| yes | 2.7 | (2.4–3.0) | 1.3 | (1.1–1.5) | 0.008 | 2.6 | (2.3–3.0) | 1.3 | (1.1–1.5) | 0.011 | 2.0 | (1.5–2.7) | 1.6 | (0.97–2.5) | 0.061 |
| Drug/Alcohol Abuse | |||||||||||||||
| yes | 1.7 | (1.5–1.9) | 1.3 | (1.1–1.5) | 0.001 | 2.6 | (2.3–3.0) | 1.3 | (1.1–1.5) | 0.004 | 1.8 | (1.3–2.6) | 1.6 | (1.1–2.4) | 0.029 |
| Remoteness (ARIA) | |||||||||||||||
| Inner Regional | 6.5 | (5.5–7.6) | 2.1 | (1.6–2.8) | <0.001 | 2.7 | (2.1–3.6) | 2.5 | (1.7–3.7) | <0.001 | 2.1 | (1.2–3.6) | 1.1 | (0.48–2.3) | 0.863 |
| Outer Regional | 1.7 | (1.5–2.0) | 1.0 | (0.9–1.3) | 0.736 | 1.3 | (1.1–1.5) | 1.1 | (0.85–1.4) | 0.515 | 1.3 | (0.71–2.4) | 0.82 | (0.38–1.7) | 0.600 |
| Socioeconomic Status | |||||||||||||||
| Quintile III | 0.41 | (0.33–0.52) | 0.71 | (0.53–0.95) | 0.022 | 0.68 | (0.53–0.87) | 1.1 | (0.83–1.5) | 0.465 | 0.42 | (0.26–0.68) | 0.44 | (0.25–0.76) | 0.003 |
| Quintile IV | 0.51 | (0.40–0.66) | 0.75 | (0.55–1.0) | 0.061 | 0.63 | (0.5–0.79) | 1.1 | (0.80–1.5) | 0.521 | 0.52 | (0.31–0.85) | 0.45 | (0.25–0.79) | 0.006 |
| Quintile V | 0.23 | (0.19–0.28) | 0.58 | (0.44–0.75) | <0.001 | 0.52 | (0.43–0.64) | 0.86 | (0.64–1.2) | 0.344 | 0.28 | (0.18–0.43) | 0.29 | (0.17–0.49) | <0.001 |
3.4.1. Indigenous Cases
Indigenous cases in the 55–75 age bracket had 22 times (95% CI 15–32, p < 0.001) higher odds of appearing in the hospital data only compared with individuals in the 3–14 age bracket. Cases who were last diagnosed more than two years after register establishment had 2.9 times the odds of not appearing on the register (95% CI 2.2–3.9, p < 0.001). When compared with cases from NT, cases residing in QLD had lower odds of being in the hospital data only (AOR: 0.77 95% CI: 0.62–0.96, p = 0.018). Those with comorbidities were 1.3 times more likely to appear in hospital records only (95% CI: 1.1–1.5, p = 0.011).
3.4.2. Non-Indigenous Cases
For non-Indigenous cases, those in the 55–75 age bracket had 31 times the odds of appearing in the hospital data only compared with those in the 3–14 age bracket (95% CI 14–72, p < 0.001). Social disadvantage increased the odds of inclusion in the register with the most disadvantaged areas being associated with the highest odds of inclusion (AOR: 0.29, 95% CI 0.17–0.49, p < 0.001). Compared with the NT, residence in SA, QLD or WA increased the odds of cases appearing in the hospital data only (varying AOR: 2.6–30, p < 0.05).
4. Discussion
Register-based ARF/RHD control programs are most useful when case ascertainment is high, and accurate, timely and complete information appears in the database [21,26,27]. This study presents the first quasi-national Australian analysis investigating case ascertainment of ARF and RHD cases through ARF/RHD registers and hospital records using linked administrative data. We found that no single source provides comprehensive case ascertainment by itself, with 31% and 26% of Indigenous and 76% and 10% of non-Indigenous cases missing from the register and hospital data respectively. Differences in patient characteristics captured through the two case ascertainment systems (particularly age, Indigenous status, disease severity and comorbidity profiles) highlight the potential for bias associated when only one data source is considered. Therefore, realistic epidemiological analyses and policy targets for ARF/RHD control cannot be developed using only hospital or register data, a limitation commonly faced in the previous literature. Analyses conducted using only hospital data will likely be biased towards older, comorbid cases with greater disease severity, residing in more metropolitan areas. If only register data is used, a bias towards a younger ARF/RHD population with lower disease severity and a lower socioeconomic profile would likely be incurred.
Twice as many ARF cases than RHD cases were found in both ARF/RHD register and hospital data. There was substantial variability between jurisdictions and regions in the reliability of case ascertainment through the ARF/RHD registers. The registers recorded proportionally more Indigenous cases from the Northern areas of Australia (NT Top End, QLD North, WA North) than non-Indigenous cases or individuals residing in other areas, especially metropolitan regions. The agreement between diagnosis dates across data sources also varied substantially by jurisdiction. For ARF, Queensland and WA performed substantially better than NT and SA.
We found that non-Indigenous status remains a significant predictor of being missed by the ARF/RHD registers of cases, after adjustment for age, sex, disease severity, vital status, jurisdiction, ARIA, SES, comorbidity, drugs/alcohol abuse and register establishment. Other significant determinants of exclusion from registers include older age, comorbidities, drugs/alcohol abuse and disease severity, but not sex.
Prior to this analysis, few studies have validated case ascertainment on ARF/RHD registers. Two Australian studies reported results on case ascertainment on the WA and NT ARF/RHD registers as incidental findings, rather than the primary study goal. In those small, regional studies, the WA and NT ARF/RHD registers were found to be incomplete (NT: 19% incomplete; Kimberley region of WA: 27% incomplete) [21,26] and containing errors due to the manual data entry process [13]. Studies in Fiji and New Zealand have also found gaps in case ascertainment on ARF/RHD registers [20,28].
The disparities between population groups and jurisdictions in case ascertainment observed in this study can be attributed to policy differences and operational considerations. All jurisdictions apart from SA, introduced ARF notification before the development of ARF/RHD disease registers, with rheumatic heart disease only becoming notifiable more recently. In SA, delayed notification requirements for ARF/RHD (in 2016 four years after the establishment of the ARF/RHD register) may have contributed to the lowest proportion of register notifications observed in this study. All jurisdictional registers mainly employ passive surveillance with the registration process relying on clinicians and health service providers being aware of ARF/RHD and their knowledge of having to complete notification forms [10]. Case ascertainment through the ARF/RHD register may be improved by more automated notification processes and standardised protocols for data entry; this would also reduce the labour-intensive need for manual data handling by the registers and the associated risks for data quality.
However, notification requirements can be complemented by other policies to increase case ascertainment. Outside of the NT, QLD had the highest proportion of hospitalised RHD cases recorded on its ARF/RHD register compared to the other jurisdictions despite RHD becoming notifiable outside of our research study period for this jurisdiction (2018). This may be related to comprehensive active case finding work in QLD that commenced in 2014 and identified 500 ARF/RHD cases previously unknown to the register [29], highlighting the importance of this approach to case ascertainment as part of control programs.
Operationally, the ARF/RHD registers face challenges such as limitations in resourcing with regard to infrastructure and staff. These operational constraints necessitate focusing on population groups and geographical areas where active case finding will likely identify a relatively large number of cases. It seems likely that such practical and clinically pertinent considerations have influenced the large representation of younger, Indigenous and Northern Australian cases recorded on ARF/RHD registers.
Patient transfers and low clinical awareness of ARF and RHD may have also lowered the case ascertainment by registers [10]. We found that metropolitan areas (generally better-resourced) had a relatively lower proportion of ARF/RHD patients on the registers compared with the rest of Australia (generally less-resourced). This suggests a need to increase clinical awareness and education of health service providers across Australia about ARF/RHD and notification requirements, including in metropolitan areas. The observed limitations in case ascertainment on the ARF/RHD registers and their likely causes point to the benefits of establishing a central, national ARF/RHD register based on automated notification and data management processes to achieve both more accurate epidemiological monitoring and more consistent real-time patient care including those across jurisdictional boundaries. Conversely, not all register recorded cases were found in the hospital data either. Despite guidelines recommending the hospitalisation of ARF patients [10], one-quarter (710 cases) of Indigenous ARF cases appeared in the register records only. As suggested by Artuso et al. [30], these cases were likely notified by primary health care services without hospitalisation due to a range of factors known to affect hospital utilisation by Indigenous patients including escort ineligibility/ availability, competing family priorities and mistrust of the health system.
For both case ascertainment methods, ARF/RHD register and hospital data, some cases may have been missed for various reasons. Sub-clinical ARF cases were likely under-represented due to a presumed high number of subclinical cases not seeking medical care, unavailability of primary health care data, underdiagnosis due to limited knowledge of ARF by clinicians and the clinical complexity associated with making an ARF diagnosis, including the lack of a definitive diagnostic test [10,31]. Miscoding in the hospital data (resulting in cases not being identified as ARF/RHD) and the limited study period may have also contributed to incomplete case ascertainment. Additionally, this study did not investigate case ascertainment for individuals last hospitalised for ARF/RHD before register establishment in their jurisdiction of residence, because the ARF/RHD registers do not generally engage in retrospective case finding. Current privacy limitations in cross-jurisdictional data linkage outside of SA and NT limited the tracking of patients across jurisdictions. It would be beneficial for a future study to investigate the characteristics of cases in primary health care services, as this data was not systematically available for this analysis. Small sample sizes, particularly among non-Indigenous cases, may have caused noise in the data and affected the power of the regression analysis. Furthermore, compared with cases appearing in the hospital data, our ability to describe the characteristics of the register-only cases was limited by the lack of recording of patient information relating to, for example, comorbidities, SES and remoteness. NSW was not included in our analysis, because its ARF/RHD register was only established at the end of 2016.
5. Conclusions
The NT, SA, QLD and WA ARF/RHD registers are part of the national Rheumatic Fever Strategy for control programs. Besides their surveillance function, an important goal of the register-based programs is to strengthen the prevention of ARF recurrences and minimise RHD progression by supporting the regular provision of secondary prophylaxis [13]. The effectiveness of the control programs is affected by incomplete case ascertainment. Pertinently, younger, Indigenous and Northern Australian cases are more comprehensively represented on ARF/RHD registers. However, differences in the characteristics of ARF/RHD cases across administrative hospital records and ARF/RHD registers demonstrate the need to utilise multiple sources when investigating the epidemiology of ARF/RHD in Australia to minimise systematic biases. Increased awareness of ARF/RHD in general and specifically of the notification requirements amongst clinicians would improve case ascertainment under the current operational systems. Furthermore, moving towards more integrated and automated systems, ideally implemented as a central, national ARF/RHD register, has the potential to improve communication and cooperation between the registers and health care services, minimise the workload of clinicians and the double handling of data and thus increase case ascertainment and data accuracy. This study demonstrates the need for sophisticated monitoring and surveillance systems in the global effort to reduce the burden of ARF and RHD.
Acknowledgments
The authors working on this paper have been supported by many collaborators across Australia, including Christopher Reid, Elizabeth Geelhoed, Daniel Williamson, Angelita Martini, Kalinda Griffiths, Jess de Dassell, Fadwa Al-Yaman, Anne Russell, Hideo Tohira, Pamela Bradshaw, Sara Noonan, Emma Haynes. The authors value the support/endorsement provided to the project by the following peak bodies representing the Aboriginal Community Controlled Health sector: Aboriginal Medical Services Alliance Northern Territory, Kimberley Aboriginal Medical Service (the health service serving the high-burden region of WA), Aboriginal Health Council of South Australia, and Aboriginal Health and Medical Research Council (NSW). We also received support from the Aboriginal divisions of Queensland and WA Health Departments. We are committed to providing feedback to these organisations ensuring that the findings are accessible and provide the evidence needed for policy that can reduce the burden of ARF and RHD in Australia. We acknowledge that figures and other statistics represent the loss of health and human life with profound impact and sadness for people, families, community and culture. We hope that the ‘numbers story’ emanating from this project can augment the ‘lived stories’ that reflect the voices of people with RHD and their families, thus jointly contributing to evidence to erase suffering from ARF and RHD in Australia. The authors also wish to thank the staff of the data linkage units of the State and Territory governments (WA, SA-NT, NSW, QLD) for linkage of the data. We thank the State and Territory Registries of Births, Deaths and Marriages, the State and Territory Coroners, and the National Coronial Information System for enabling Cause of Death Unit Record File data to be used for this project. Further, we thank the data custodians and data managers for the provision of the following data: Inpatient hospital data (five States and Territories) and RHD registers (five States and Territories).
Appendix A
Table A1.
ICD-AM-10 codes for procedures and surgeries related to RHD.
| Classification | ICD-10-AM Codes |
|---|---|
| RHD Procedure Codes | 3827001–3827003, 9622200, 3848808–3848811 |
| RHD Surgery Codes | 3845610, 3848300, 3848000, 3848100, 3848800, 3848801, 3848900, 3848901, 3845615, 3865304, 3848700, 3848501, 3848001, 3848101, 3847500, 3847700, 3848802, 3848803, 3848902, 3848500, 3845616, 3865305, 3845611, 3848002, 3848102, 3847501, 3847701, 3848804, 3848805, 3848903, 3845617, 3865306, 3845601, 3848806, 3848807, 3848904, 3848905, 3845618, 3865307, 3847502, 3847702 |
Table A2.
ICD-10-AM codes for comorbidities of ARF and RHD.
| Condition | ICD-10-AM Codes |
|---|---|
| Comorbiditie | |
| Chronic Obstructive Pulmonary Disease | J40 - J47 |
| Chronic Kidney Disease | E10.2, E11.2, E12.2, E13.2, E14.2, I15.0, I15.1, N39.1, N39.2, T82.4, Z94.0, Z99.2, I12, I13, N00 - N08, N11–N12, N14–N16, N18, N19, N25–N28, Q60–Q63, Z49 |
| Other Cardiovascular Diseases | I10 -I11, I15.2, I15.8, I15.9, I20 - I28, I30–I32, I34–I47, I49, I51, I52, I65-I89, I95–I99 |
| Coronary Heart Disease | I20–I25 |
| Anticoagulant | D62, D68.3, D68.4, D68.8 |
| Diabetes | E10–E14 |
| Mental Health Conditions | F202013F99 |
| Complications | |
| Stroke | I60–I64 |
| Heart Failure | I50 |
| Atrial Fibrillation | I48 |
| Endocarditis | I33 |
| Drug/Alcohol Abuse | |
| Alcohol Abuse | JZ50.2, Z71.4, Z72.1, F10, K70, E24.4, G31.2, G62.1, G72.1, I42.6, K29.2, K86.0, O35.4, T51.9 |
| Tobacco Smoking | Z71.6, Z72.0, F17, T65.2 |
| Drug abuse | Z71.5, F11 - F19, R78.1, R78.2, R78.3, R78.4, R78.5, T40, Y12, Z50.3, O35.5 |
| Pregnancy | |
| Pregnancy | O00 - O48, O60, O61-O77, O80-O82, O85-O92, O94, O9A, O98, O99, Z34, Z3A, F53, A34, E23, M83.0 |
Table A3.
Descriptive analysis of the study samples, stratified by jurisdiction.
| Variable | Indigenous (n = 1821) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NT | SA | QLD | WA | |||||||||
| n | Column Percent (C%) | Row Percent (R%) | n | C% | R% | n | C% | R% | n | C% | R% | |
| Total | 1207 | 100% | 36% | 54 | 100% | 47% | 380 | 100% | 24% | 180 | 100% | 23% |
| Most Recent Age (years) | ||||||||||||
| 3–14 | 39 | 3% | 9% | <5 | 4% | 9% | 49 | 13% | 16% | 17 | 9% | 12% |
| 15–24 | 78 | 6% | 11% | 5 | 9% | 24% | 50 | 13% | 13% | 26 | 14% | 13% |
| 25–34 | 189 | 16% | 27% | 12 | 22% | 55% | 58 | 15% | 19% | 35 | 19% | 20% |
| 35–44 | 341 | 28% | 55% | 9 | 17% | 64% | 55 | 14% | 26% | 36 | 20% | 28% |
| 45–54 | 291 | 24% | 59% | 8 | 15% | 57% | 94 | 25% | 47% | 31 | 17% | 37% |
| 55–75 | 268 | 22% | 65% | 18 | 33% | 78% | 72 | 19% | 50% | 35 | 19% | 61% |
| Sex | ||||||||||||
| Male | 427 | 35% | 33% | 20 | 37% | 50% | 154 | 41% | 25% | 52 | 29% | 19% |
| Female | 780 | 65% | 37% | 34 | 63% | 45% | 226 | 59% | 24% | 128 | 71% | 25% |
| Diagnosis | ||||||||||||
| ARF Only | 143 | 12% | 19% | 5 | 9% | 23% | 95 | 25% | 30% | 41 | 23% | 20% |
| RHD Only | 998 | 83% | 56% | 48 | 89% | 61% | 277 | 73% | 31% | 134 | 74% | 36% |
| Both | 66 | 5% | 8% | <5 | 2% | 7% | 8 | 2% | 2% | 5 | 3% | 3% |
| RHD Severity | ||||||||||||
| Severe RHD | 479 | 40% | 43% | 35 | 65% | 71% | 160 | 42% | 34% | 91 | 51% | 33% |
| Mild/ Moderate RHD | 585 | 48% | 39% | 14 | 26% | 31% | 125 | 33% | 16% | 48 | 27% | 16% |
| ARF Only | 143 | 12% | 19% | 5 | 9% | 23% | 95 | 25% | 30% | 41 | 23% | 20% |
| ARF Recurrence | ||||||||||||
| Total ARF Cases | 209 | 17% | 13% | 6 | 11% | 16% | 103 | 27% | 15% | 46 | 25% | 12% |
| No Recurrence | 170 | 81% | 14% | 5 | 83% | 14% | 99 | 96% | 17% | 39 | 85% | 12% |
| Recurrence | 39 | 19% | 12% | <5 | 17% | 100% | <5 | 4% | 5% | 7 | 15% | 9% |
| Indigenous (n = 1821) | ||||||||||||
| NT (n = 1207) | SA (n = 54) | QLD (n = 380) | WA (n = 180) | |||||||||
| Variable | n | C% | R% | n | C% | R% | n | C% | R% | n | C% | R% |
| Vital Status | ||||||||||||
| Alive | 882 | 73% | 31% | 49 | 91% | 42% | 317 | 83% | 22% | 155 | 86% | 21% |
| Dead | 325 | 27% | 62% | 5 | 9% | 71% | 63 | 17% | 66% | 25 | 14% | 56% |
| Remoteness (ARIA) | ||||||||||||
| Inner Regional | <5 | 0% | 100% | 24 | 44% | 73% | 75 | 20% | 49% | 62 | 34% | 53% |
| Outer Regional | 165 | 14% | 49% | 19 | 35% | 59% | 135 | 36% | 24% | 22 | 12% | 43% |
| Remote | 1028 | 85% | 36% | 9 | 17% | 39% | 170 | 45% | 21% | 96 | 53% | 16% |
| SES | ||||||||||||
| Quintile I & II | 124 | 10% | 48% | 18 | 33% | 95% | 59 | 16% | 34% | 44 | 24% | 32% |
| Quintile III | 111 | 9% | 51% | 7 | 13% | 54% | 137 | 36% | 27% | 27 | 15% | 51% |
| Quintile IV | 136 | 11% | 42% | 23 | 43% | 50% | 113 | 30% | 25% | 64 | 36% | 24% |
| Quintile V | 825 | 68% | 34% | <5 | 7% | 40% | 71 | 19% | 20% | 45 | 25% | 15% |
| Last Diagnosis Since Register Establishment (years) | ||||||||||||
| Mean Time | 8.3 | 2.1 | 4.0 | 2.7 | ||||||||
| 0–<2 | 93 | 8% | 74% | 19 | 35% | 59% | 69 | 18% | 24% | 57 | 32% | 37% |
| 2+ | 1114 | 92% | 34% | 35 | 65% | 42% | 311 | 82% | 24% | 123 | 68% | 20% |
| Comorbidity | ||||||||||||
| Any Comorbidity | 916 | 76% | 50% | 44 | 81% | 75% | 245 | 64% | 34% | 130 | 72% | 34% |
| Chronic Obstructive Pulmonary Diseases | 239 | 20% | 59% | 11 | 20% | 85% | 49 | 13% | 51% | 37 | 21% | 44% |
| Chronic Kidney Disease | 454 | 38% | 61% | 13 | 24% | 76% | 100 | 26% | 48% | 51 | 28% | 43% |
| Other Cardiovascular Diseases | 788 | 65% | 52% | 35 | 65% | 78% | 216 | 57% | 38% | 111 | 62% | 38% |
| Coronary Heart Diseases | 364 | 30% | 66% | 12 | 22% | 71% | 102 | 27% | 65% | 45 | 25% | 67% |
| Anticoagulant | 229 | 19% | 61% | 11 | 20% | 85% | 49 | 13% | 46% | 41 | 23% | 41% |
| Diabetes | 485 | 40% | 62% | 17 | 31% | 74% | 126 | 33% | 44% | 64 | 36% | 44% |
| Mental Health Conditions | 109 | 9% | 53% | 15 | 28% | 83% | 49 | 13% | 39% | 30 | 17% | 35% |
| Indigenous (n = 1,821) | ||||||||||||
| NT (n = 1,207) | SA (n = 54) | QLD (n = 380) | WA (n = 180) | |||||||||
| Variable | n | C% | R% | n | C% | R% | n | C% | R% | n | C% | R% |
| Complications | ||||||||||||
| Any Complication | 496 | 41% | 57% | 33 | 61% | 85% | 154 | 41% | 46% | 86 | 48% | 46% |
| Stroke | 50 | 4% | 58% | <5 | 6% | 100% | 17 | 4% | 49% | 8 | 4% | 35% |
| Heart Failure | 368 | 30% | 58% | 25 | 46% | 83% | 113 | 30% | 50% | 72 | 40% | 51% |
| Atrial Fibrillation | 278 | 23% | 56% | 19 | 35% | 83% | 94 | 25% | 43% | 55 | 31% | 47% |
| Endocarditis | 38 | 3% | 54% | <5 | 7% | 100% | 21 | 6% | 58% | 10 | 6% | 43% |
| Drug/Alcohol Abuse | ||||||||||||
| Any Substance | 834 | 69% | 49% | 40 | 74% | 74% | 244 | 64% | 34% | 122 | 68% | 30% |
| Alcohol Abuse | 463 | 38% | 54% | 23 | 43% | 72% | 107 | 28% | 38% | 56 | 31% | 28% |
| Drug Abuse | 56 | 5% | 47% | 8 | 15% | 73% | 33 | 9% | 38% | 24 | 13% | 36% |
| Tobacco Smoking | 756 | 63% | 49% | 36 | 67% | 72% | 232 | 61% | 34% | 116 | 64% | 30% |
| Pregnancy | ||||||||||||
| Females of Reproductive Age | 507 | 42% | 34% | 24 | 44% | 51% | 133 | 35% | 21% | 83 | 46% | 24% |
| Pregnancy | 234 | 46% | 39% | 10 | 42% | 67% | 57 | 43% | 25% | 31 | 37% | 27% |
Table A4.
Sensitivity analysis on the agreement between recorded diagnosis dates based on a 14-day grace period for ARF episodes and a 90-day grace period for RHD diagnosis by Indigenous status, jurisdiction and Indigenous regions.
| Indigenous Status | ARF | RHD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Episodes on Both Sources |
Agreeing Dates | Time Difference of Non-Agreeing Dates (Days) | Diagnosis on Both Sources |
Agreeing Dates | Time Difference of Non-Agreeing Dates (Days) | ||||||||
| Indigenous | Total | n | % | Mean | Median | IQR | Total | n | % | Mean | Median | IQR | |
| Jurisdiction | NT | 979 | 944 | 96% | 38 | 30 | 41 | 881 | 765 | 87% | 982 | 718 | 992 |
| SA | 19 | 17 | 89% | 20 | 20 | 2 | 14 | 12 | 86% | 400 | 400 | 269 | |
| QLD | 279 | 275 | 99% | 28 | 28 | 4 | 319 | 246 | 77% | 692 | 497 | 727 | |
| WA | 186 | 185 | 99% | 17 | 17 | 0 | 131 | 115 | 88% | 581 | 495 | 528 | |
| Total | 1463 | 1421 | 97% | 35 | 28 | 27 | 1345 | 1138 | 85% | 843 | 594 | 893 | |
| Indigenous Regions (IREGs) | NT Top End | 733 | 712 | 97% | 44 | 33 | 33 | 666 | 583 | 88% | 919 | 727 | 995 |
| NT Central | 246 | 232 | 94% | 27 | 17 | 8 | 215 | 182 | 85% | 1140 | 612 | 1031 | |
| SA Other | 13 | 12 | 92% | 22 | 22 | 0 | 9 | 8 | 89% | 131 | 131 | 0 | |
| SA Metro | 6 | 5 | 83% | 18 | 18 | 0 | 5 | <5 | 80% | 668 | 668 | 0 | |
| QLD North | 244 | 241 | 99% | 29 | 28 | 7 | 273 | 210 | 77% | 687 | 385 | 747 | |
| QLD Other | 23 | 23 | 100% | 21 | 18 | 86% | 1205 | 1267 | 642 | ||||
| QLD Metro | 12 | 11 | 92% | 27 | 27 | 0 | 25 | 18 | 72% | 523 | 530 | 118 | |
| WA North | 135 | 134 | 99% | 17 | 17 | 0 | 105 | 95 | 90% | 600 | 495 | 471 | |
| WA Other | 32 | 32 | 100% | 18 | 12 | 67% | 550 | 450 | 551 | ||||
| WA Metro | 19 | 19 | 100% | 8 | 8 | 100% | |||||||
| Non-Indigenous | |||||||||||||
| Jurisdiction | NT | 13 | 12 | 92% | 23 | 23 | 0 | 19 | 16 | 84% | 492 | 452 | 407 |
| SA | <5 | <5 | 100% | 7 | 6 | 86% | 232 | 232 | 0 | ||||
| QLD | 58 | 57 | 98% | 25 | 25 | 0 | 116 | 104 | 90% | 772 | 466 | 1190 | |
| WA | <5 | <5 | 100% | <5 | <5 | 100% | |||||||
| Total | 76 | 74 | 97% | 24 | 24 | 1 | 143 | 127 | 89% | 686 | 434 | 968 | |
Author Contributions
Conceptualization, T.A., J.M.K. and D.B.-S.; methodology, T.A., J.M.K. and D.B.-S.; software, T.A. and R.S.; formal analysis, T.A.; investigation, T.A., J.M.K. and D.B.-S.; resources, J.M.K.; data curation, J.M.K., R.S. and D.B.-S.; writing-original draft preparation, T.A.; writing-review and editing, T.A., J.M.K., R.S., K.D., M.A., V.W. and D.B.-S.; visualization, T.A.; supervision, J.M.K. and D.B.-S.; project administration, J.M.K.; funding acquisition, J.M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Health and Medical Research Council, 114652 and the Heart Foundation of Australia, 102043.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Carapetis J.R., Steer A.C., Mulholland E.K., Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 2.Remenyi B., Carapetis J., Wyber R., Taubert K., Mayosi B.M. Position statement of the World Heart Federation on the prevention and control of rheumatic heart disease. Nat. Rev. Cardiol. 2013;10:284–292. doi: 10.1038/nrcardio.2013.34. [DOI] [PubMed] [Google Scholar]
- 3.Carapetis J.R., Beaton A., Cunningham M.W., Guilherme L., Karthikeyan G., Mayosi B.M. Acute rheumatic fever and rheumatic heart disease. Nat. Rev. Dis. Primers. 2016;2:15084. doi: 10.1038/nrdp.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie B.J., Brewster D.R. Rheumatic fever in Aboriginal children. J. Paediatr. Child Health. 2002;38:223–225. doi: 10.1046/j.1440-1754.2002.00850.x. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence J.G., Carapetis J.R., Griffiths K., Edwards K., Condon J.R. Acute rheumatic fever and rheumatic heart disease: Incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation. 2013;128:492–501. doi: 10.1161/CIRCULATIONAHA.113.001477. [DOI] [PubMed] [Google Scholar]
- 6.Jackson S.J., Steer A.C., Campbell H. Systematic Review: Estimation of global burden of non-suppurative sequelae of upper respiratory tract infection: Rheumatic fever and post-streptococcal glomerulonephritis. Trop Med. Int. Health. 2011;16:2–11. doi: 10.1111/j.1365-3156.2010.02670.x. [DOI] [PubMed] [Google Scholar]
- 7.Marijon E., Mirabel M., Celermajer D.S., Jouven X. Rheumatic heart disease. Lancet. 2012;379:953–964. doi: 10.1016/S0140-6736(11)61171-9. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . A Review of the Technical Basis for the Control of Conditions Associated with Group A Streptococcal Infections [Internet] WHO; Geneva, Switzerland: 2005. [(accessed on 10 March 2019)]. Available online: https://www.who.int/maternal_child_adolescent/documents/fch_cah_05_08/en/ [Google Scholar]
- 9.Manyemba J., Mayosi B.M. Penicillin for secondary prevention of rheumatic fever. Cochrane Database Syst. Rev. 2002;3:CD002227. doi: 10.1002/14651858.CD002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RHDAustralia, National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand . The Australian Guideline for Prevention, Diagnosis and Management of Acute Rheumatic Fever and Rheumatic Heart Disease, [Internet] 2nd ed. RHDAustralia; Darwin, Australia: 2012. [(accessed on 10 March 2019)]. Available online: https://www.rhdaustralia.org.au/sites/default/files/resources/quick_reference_guides_0.pdf. [Google Scholar]
- 11.World Health Organization . Rheumatic Fever and Rheumatic Heart Disease: Report by the Director-General [Internet] WHO; Geneva, Switzerland: 2018. [(accessed on 1 April 2019)]. Available online: https://www.who.int/ncds/management/rheumatic-heart-disease-resolution/en/ [Google Scholar]
- 12.Wyber R., Kado J. Acute Rheumatic Fever and Rheumatic Heart Disease. Volume 12. Elsevier; Amsterdam, The Netherlands: 2020. Rheumatic Heart Disease Control Programs, Registers, and Access to Care; pp. 235–259. [Google Scholar]
- 13.Health Policy Analysis . Evaluation of the Commonwealth Rheumatic Fever Strategy: Final Report. Primary Healthcare Branch, Commonwealth Department of Health; Canberra, Australia: 2017. [Google Scholar]
- 14.Australian Consortium for Classification Development . International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Australian Modification. Independent Hospital Pricing Authority; Darlinghurst, Australia: 2017. [Google Scholar]
- 15.Australian Institute of Health and Welfare . Rheumatic Heart Disease and Acute Rheumatic Fever in Australia: 1996–2012 [Internet] AIHW; Canberra, Australia: 2013. [(accessed on 15 March 2019)]. Cat. no. CVD 60. Available online: https://www.aihw.gov.au/reports/heart-stroke-vascular-disease/rheumatic-heart-disease-and-acute-rheumatic-fever/contents/table-of-contents. [Google Scholar]
- 16.End Rheumatic Heart Disease Centre of Research Excellence . The Endgame Strategy. Telethon Kids Institute; Perth, Australalia: 2019. [(accessed on 17 October 2019)]. Available online: https://endrhd.telethonkids.org.au/our-research/ [Google Scholar]
- 17.Katzenellenbogen J.M., Bond-Smith D., Seth R.J., Dempsey K., Cannon J., Nedkoff L. The End Rheumatic Heart Disease in Australia Study of Epidemiology (ERASE) Project: Data sources, case ascertainment and cohort profile. Clin. Epidemiol. 2019;11:997–1010. doi: 10.2147/CLEP.S224621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katzenellenbogen J.M., Bond-Smith D., Seth R., Dempsey K., Cannon J., Stacey I. Contemporary incidence and prevalence of rheumatic fever and rheumatic heart disease in Australia using linked data: The case for policy change. J. Am. Heart Assoc. 2020 doi: 10.1161/JAHA.120.016851. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Australian Bureau of Statistics . Estimates of Aboriginal and Torres Strait Islander Australians, June 2016 [Internet] ABS; Canberra, Australia: 2018. [(accessed on 20 July 2020)]. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/mf/3238.0.55.001. [Google Scholar]
- 20.Moxon T.A., Reed P., Jelleyman T., Anderson P., Leversha A., Jackson C. Is a rheumatic fever register the best surveillance tool to evaluate rheumatic fever control in the Auckland region? N. Z. Med J. 2017;13:48–62. [PubMed] [Google Scholar]
- 21.Murdoch J., Davis S., Forrester J., Masuda L., Reeve C. Acute rheumatic fever and rheumatic heart disease in the Kimberley: Using hospitalisation data to find cases and describe trends. Aust. N. Z. J. Public Health. 2015;39:38–43. doi: 10.1111/1753-6405.12240. [DOI] [PubMed] [Google Scholar]
- 22.Bond-Smith D., Seth R., de Klerk N., Nedkoff L., Anderson M., Hung J. Development and evaluation of a prediction model for ascertaining rheumatic heart disease status in administrative data. Clin. Epidemiol. 2020;12:717–730. doi: 10.2147/CLEP.S241588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katzenellenbogen J.M., Nedkoff L., Cannon J., Kruger D., Pretty F., Carapetis J.R. Low positive predictive value of International Classification of Diseases, 10th Revision codes in relation to rheumatic heart disease: A challenge for global surveillance. Intern. Med. J. 2019;49:400–403. doi: 10.1111/imj.14221. [DOI] [PubMed] [Google Scholar]
- 24.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Torres-Espindola L.M., Demetrio-Rios J., Carmona-Aparicio L., Galvan-Diaz C., Perez-Garcia M., Chavez-Pacheco J.L. Comorbidity index as a predictor of mortality in pediatric patients with solid tumors. Front. Pediatr. 2019;7:48. doi: 10.3389/fped.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eissa S., Lee R., Binns P., Garstone G., McDonald M. Assessment of a register-based rheumatic heart disease secondary prevention program in an Australian Aboriginal community. Aust. N. Z. J. Public Health. 2005;29:521–525. doi: 10.1111/j.1467-842X.2005.tb00243.x. [DOI] [PubMed] [Google Scholar]
- 27.McDonald M., Brown A., Noonan S., Carapetis J.R. Preventing recurrent rheumatic fever: The role of register based programmes. Heart. 2005;91:1131–1133. doi: 10.1136/hrt.2004.057570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parks T., Kado J., Miller A.E., Ward B., Heenan R., Colquhoun S.M. Rheumatic heart disease-attributable mortality at ages 5-69 years in Fiji: A five-year, national, population-based record-linkage cohort study. PLoS Negl. Trop Dis. 2015;9:e0004033. doi: 10.1371/journal.pntd.0004033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambert S. Overview of Qld RHD Control Program and Statistics [Internet] RHDAustralia; Brisbane, Australia: 2016. [(accessed on 17 October 2019)]. Available online: https://www.rhdaustralia.org.au/resources/acute-rheumatic-fever-and-rheumatic-heart-disease. [Google Scholar]
- 30.Artuso S., Cargo M., Brown A., Daniel M. Factors influencing health care utilisation among Aboriginal cardiac patients in central Australia: A qualitative study. BMC Health Serv. Res. 2013;13:83. doi: 10.1186/1472-6963-13-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Australian Institute of Health Welfare . Acute Rheumatic Fever and Rheumatic Heart Disease in Australia. AIHW; Canberra, Australia: 2019. [Google Scholar]