Abstract
Background
Frailty is a geriatric syndrome characterized by increased susceptibility to adverse health outcomes. One major determinant thereof is the gradual weakening of the musculoskeletal system and the associated osteosarcopenia. To improve our understanding of the underlying pathophysiology and, more importantly, to test potential interventions aimed at counteracting frailty, suitable animal models are needed.
Methods
To evaluate the relevance of prematurely aged PolgA(D257A/D257A) mice as a model for frailty and osteosarcopenia, we quantified the clinical mouse frailty index in PolgA(D257A/D257A) and wild‐type littermates (PolgA(+/+), WT) with age and concertedly assessed the quantity and quality of bone and muscle tissue. Lastly, the anabolic responsiveness of skeletal muscle, muscle progenitors, and bone was assessed.
Results
PolgA(D257A/D257A) accumulated health deficits at a higher rate compared with WT, resulting in a higher frailty index at 40 and 46 weeks of age (+166%, +278%, P < 0.0001), respectively, with no differences between genotypes at 34 weeks. Concomitantly, PolgA(D257A/D257A) displayed progressive musculoskeletal deterioration such as reduced bone and muscle mass as well as impaired functionality thereof. In addition to lower muscle weights (−14%, P < 0.05, −23%, P < 0.0001) and fibre area (−20%, P < 0.05, −22%, P < 0.0001) at 40 and 46 weeks, respectively, PolgA(D257A/D257A) showed impairments in grip strength and concentric muscle forces (P < 0.05). PolgA(D257A/D257A) mutation altered the acute response to various anabolic stimuli in skeletal muscle and muscle progenitors. While PolgA(D257A/D257A) muscles were hypersensitive to eccentric contractions as well as leucine administration, shown by larger downstream signalling response of the mechanistic target of rapamycin complex 1, myogenic progenitors cultured in vitro showed severe anabolic resistance to leucine and robust impairments in cell proliferation. Longitudinal micro‐computed tomography analysis of the sixth caudal vertebrae showed that PolgA(D257A/D257A) had lower bone morphometric parameters (e.g. bone volume fraction, trabecular, and cortical thickness, P < 0.05) as well as reduced remodelling activities (e.g. bone formation and resorption rate, P < 0.05) compared with WT. When subjected to 4 weeks of cyclic loading, young but not aged PolgA(D257A/D257A) caudal vertebrae showed load‐induced bone adaptation, suggesting reduced mechanosensitivity with age.
Conclusions
PolgA(D257A/D257A) mutation leads to hallmarks of age‐related frailty and osteosarcopenia and provides a powerful model to better understand the relationship between frailty and the aging musculoskeletal system.
Keywords: Aging, Osteopenia, Sarcopenia, Osteosarcopenia, mTORC1, in vivo micro‐CT
Introduction
Although there is no universally accepted definition of frailty, 1 it is considered as an age‐related syndrome characterized by the decline of multiple physiological functions, leading to the accumulation of health deficits and thus a higher vulnerability to adverse health outcomes such as morbidity and mortality. 2 One of the most prominent components of frailty is the progressive weakening of the musculoskeletal system, 3 , 4 , 5 leading to common age‐related diseases such as osteopenia and sarcopenia. There is growing evidence that both diseases often co‐exist in frail older individuals (also termed osteosarcopenia 6 , 7 ), thereby further increasing the risk for negative outcomes such as falls and fractures. 8 , 9 Although several anabolic interventions such as dietary protein supplementation and mechanical stimulation are known to promote muscle and bone formation in young individuals, the molecular insights behind osteopenia and sarcopenia in the elderly population are lacking.
In the field of muscle physiology, studies in humans and rodents have shown that aged muscles are less responsive to well‐known anabolic stimuli such as amino acids 10 , 11 , 12 and muscle contractions 13 ; this phenomenon, termed ‘anabolic resistance’, likely results from reduced protein synthesis due to diminished intracellular signalling through the mechanistic target of rapamycin complex 1 (mTORC1) pathway. 14 , 15 , 16 Next to impairments in intramuscular mTORC1 signalling, age‐related sarcopenia has been associated with a decrease in number 17 , 18 and proliferation capacity 19 , 20 of myogenic progenitors or satellite cells. These are instrumental not only for the maintenance of muscle fibres, but also for the adaptive responses to exercise and regeneration upon injury. 21 In the field of bone physiology, evidence pointing towards altered mechanosensitivity with age has also been shown in humans 22 and in mice. 23 , 24 , 25 , 26 However, this effect might be site specific as studies using a tibia‐loading model showed a reduced response of trabecular 23 , 24 and cortical 25 , 26 bone formation with age, while bone adaptation in response to loading of the caudal vertebrae was maintained with age. 27
Therefore, whether and how age‐related changes in the responsiveness to anabolic stimuli occur remains unclear. A better understanding of the pathophysiology of osteosarcopenia will help to identify interventions to strengthen the musculoskeletal system, which ultimately will be beneficial for the prevention and/or treatment of frailty.
In order to address this, tools such as the frailty index (FI) have been established to quantify the accumulation of age‐related health deficits (e.g. loss of hearing, tremoring, comorbid diseases) in humans 28 and, more recently, also in mice. 29 Indeed, the striking similarities between key features of the FI scores in humans and in mice 30 have highlighted the potential of rodent frailty models to not only improve our understanding of frailty but also serve as a tool to test responses to interventions designed to modify (or even prevent) frailty. 31 , 32 , 33 In this study, we aimed to evaluate the PolgA(D257A/D257A) mouse (referred to as PolgA), which exhibits an accelerated aging phenotype due to elevated mitochondrial DNA point mutations and systemic mitochondrial dysfunction, 34 , 35 as a model of frailty and osteosarcopenia. While these mice are known to develop multiple signs of aging (e.g. hair loss, greying, hearing loss) earlier (around 40 weeks of age) than their wild‐type littermates (PolgA( +/+), referred to as WT), the frailty phenotype has to the best of our knowledge not yet been assessed in these mice. Furthermore, although several studies have reported lower muscle weights in PolgA mice compared with their WT littermates, 34 , 36 , 37 little is known about their muscle quality and functionality. To address this, forelimb grip strength and concentric muscle forces were measured in vivo and ex vivo, respectively, in addition to the evaluation of hind limb muscle masses. Furthermore, the response to acute anabolic stimuli such as eccentric contractions (ECCs) and leucine administration were assessed. With respect to the bone phenotype, only two studies have reported reduced femoral bone density using X‐ray densitometry. 34 , 35 Although this technique is still the gold standard to assess bone mineral density (i.e. bone quantity) clinically in humans, it does not provide insight regarding the quality of bone tissue. Therefore, bone phenotyping in pre‐clinical studies is commonly performed using high‐resolution micro‐computed tomography (micro‐CT) as it allows additional standardized evaluation of the three‐dimensional (3D) bone microarchitecture. 38 Furthermore, using in vivo micro‐CT, dynamic bone remodelling activities can be tracked longitudinally providing information both on bone formation and bone resorption. 25 , 39 These markers are important for better understanding of age‐related changes in bone microarchitecture due to osteopenia. Coupled with longitudinal measurements of FI, we aimed to monitor individual mice during the process of aging, allowing us to capture the onset of osteosarcopenia and to track other signs of aging. Lastly, by longitudinally monitoring bone adaptation in response to a long‐term mechanical loading intervention, we aimed to investigate whether PolgA bones show altered mechanosensitivity with age.
Materials and methods
Study design
The study consisted of three parts. For Parts 1 and 2, female mice were aged up to 46 weeks and divided into four groups. Three of the groups were used to cross‐sectionally compare the musculoskeletal and frailty phenotype of PolgA and WT mice at 34, 40, and 46 weeks, respectively (in vivo and ex vivo, Part 1). The fourth group was longitudinally monitored between the ages of 20 and 40 weeks to investigate the changes in bone microarchitecture and frailty over time (in vivo, Part 2). Lastly, the effects of various anabolic interventions on bone and muscle tissue were assessed (in vivo, ex vivo, in vitro). The number of mice and sample sizes in various animal studies are provided directly in the figure legends and in the Supporting Information, Table S1. The sample sizes for in vivo experiments were selected based on previous experiments, and power analysis (power set at 0.80) was used to determine the sample size required for ex vivo experiments. All mouse experiments described in the present study were carried out in strict accordance with the recommendations and regulations in the Animal Welfare Ordinance (TSchV 455.1) of the Swiss Federal Food Safety and Veterinary Office, and results are reported following the principles of the ARRIVE guidelines (www.nc3rs.org.uk/arrive-guidelines).
Animals
All animal procedures were approved by the local authorities (licence numbers 262/2016 and ZH255‐16, Verterinäramt des Kantons Zürich, Zurich, Switzerland). A colony of the mouse strain expressing an exonuclease‐deficient version of the mitochondrial DNA polymerase γ (PolgAD257A, B6.129S7(Cg)‐Polgtm1Prol/J, JAX stock 017341, The Jackson Laboratory) was bred and maintained at the ETH Phenomics Center (12:12 h light–dark cycle, maintenance feed and water ad libitum, three to five animals per cage). The mice were bred and genotyped as described in the Supporting Information. In order to confirm that the premature aging phenotypes were associated with mitochondrial dysfunction, the activity of complex IV enzyme (COX IV) in m. gastrocnemius (GAS) was measured (Supporting Information). Compared with WT, PolgA muscles had lower COX IV activity both at 40 and 46 weeks (−19% and −24%, P < 0.0001, respectively), thus confirming that the mice used in this study had the same phenotype as those previously reported. 36 , 37 , 40
Quantification of frailty index
As recommended in the recently established toolbox for the longitudinal assessment of health span in aging mice, 41 frailty was quantified using the Mouse Clinical FI, 29 which includes the assessment of 31 non‐invasive clinical items. For 29 of these items, mice were given a score of 0 if not present, 0.5 if there was a mild deficit, and 1 for a severe deficit. The final two items were weight and body surface temperature, which were scored based on the number of standard deviations from a reference mean in young adult mice as previously described. 29 To compare rates of deficit accumulation between PolgA and WT, the natural log of the FI was plotted against age. The slope of this line provides an estimate of the rate of deficit accumulation, as shown in previous studies. 29 , 42 , 43
Forelimb grip strength
Forelimb grip strength was measured using a force tension apparatus (Grip Strength Meter, 47200, Ugo Basile) at 40 and 46 weeks of age. Once mice gripped the stationary bar with their forepaws, they were pulled horizontally at the base of their tail until they let go of the bar. The process was repeated five times to determine the average peak grip force value (gram‐force) used for analysis. All measurements were performed by the same experienced user.
Muscle harvesting and force measurements
PolgA and WT GAS, TA, and m. soleus (SOL) were excised under anaesthesia (5% isoflurane/oxygen) and snap frozen in liquid nitrogen. Furthermore, m. extensor digitorum longus (EDL) from both legs were dissected and maintained in 4°C Krebs–Henseleit buffer supplemented with 1 × MEM amino acid mixture (Invitrogen) and 25 mM glucose. For assessment of ex vivo force production, the EDL of the right leg was attached to the lever arms of an Aurora system (Aurora Scientific) and submerged in continuously gassed Krebs–Henseleit buffer maintained at 37°C. After 5 minutes (min) of temperature acclimation, muscle length was adjusted until a single stimulus pulse elicited maximum force during a twitch (Lo) under isometric conditions. After 5 min rest, a force frequency protocol was initiated by subsequently providing a pulse train (lasting 250 ms) of 1‐30‐50‐80‐150‐250 and 300 Hz with 1 min rest between every intensity.
Eccentric contraction protocol
Five min after the force frequency protocol, EDL from the right legs were subjected to an eccentric training protocol according to O'Neil et al. 44 consisting of 60 contractions in a 22 min time window. In this study, the pulse train was changed to 200 Hz (compared with 100 Hz in O'Neil et al.), because pilot studies suggested that 100 Hz was not sufficient to induce maximal eccentric force in older muscle. After the contractions, the muscle was maintained in 37°C Krebs–Henseleit buffer + 1×MEM amino acid mixture and 25 mM glucose before snap freezing 1 h after the last contraction. The control EDL from the contralateral leg was kept in the same 37°C Krebs–Henseleit buffer during the complete ECC period.
Leucine administration
Mice that were 46 weeks old were fasted for 5 h in the beginning of their light cycle, after which saline (0.9% NaCl, CTL) or leucine (0.4 g/kg, LEU) was administered via oral gavage. L‐Leucine (Sigma‐Aldrich) was dissolved in a stock solution of 40 g/L, heated (40–50°C), and acidified as described previously. 45 Thirty min later, hind limb muscles were weighted and snap frozen for further analysis.
Leucine stimulation in vitro
Primary muscle progenitor cells (MPs) were isolated from muscle tissue as described in the Supporting Information. A total of 200 000 cells were seeded in six‐well plates. Myogenic differentiation medium containing low‐glucose Dulbecco's modified Eagle medium, 2% horse serum (Invitrogen), and 1% P/S was added 24 h later. Fully differentiated MPs were treated 72 h later according to one of the following conditions: (1) STV, 1 h Dulbecco's modified Eagle medium w/o amino acids (Biomol GmbH) + 10% dialyzed fetal bovine serum (Invitrogen) + 1% P/S; (2) LEU 0.8, 1 h STV + 1 h 0.8 mM L‐Leucine (Sigma‐Aldrich); and (3) LEU 5, 1 h STV + 1 h 5 mM L‐Leucine. Experiments were performed in biological triplicate and technical duplicate. 5‐Ethynyl‐2´‐deoxyuridine (EdU) analysis was performed according to manufacturer's protocol (Thermo Fischer Scientific) after a 4 h pulse with 1 μg/mL EdU. For proliferation analysis, 20 000 cells were plated on 12‐well plates, and three plates were counted per time point over 5 days.
Immunoblotting
Details have been described previously. 46 Briefly, bone tissue (20–25 mg) was first pulverized with mortar and pestle and subsequently homogenized with a tissue homogenizer (Omni THq). Muscle tissue (10–15 mg) was homogenized directly with a tissue homogenizer. Lysis was performed in ice‐cold lysis buffer (for specifications, see Table S2). Homogenates were centrifuged at 10 000 g for 10 min at 4°C. Supernatant was collected and protein concentration was measured using the DC protein assay kit. About 10–25 μg of total protein was loaded in a 15‐well pre‐casted gradient gel (Bio‐Rad, 456‐8086). After electrophoresis, a picture of the gel was taken under ultraviolet light to determine protein loading using strain‐free technology. Proteins were transferred via semidry transfer onto a polyvinylidene difluoride membrane (Bio‐Rad, 170‐4156) and subsequently blocked for 1 h at room temperature with 5% milk in tris‐buffered saline–Tween. Membranes were incubated overnight at 4°C with primary antibodies (listed in Table S2). The appropriate secondary antibodies for anti‐rabbit and anti‐mouse IgG horseradish peroxidase‐linked antibodies (Table S2) were used for chemiluminescent detection of proteins. Membranes were scanned with a chemidoc imaging system (Bio‐Rad) and quantified using Image Lab software (Bio‐Rad).
Micro‐computed tomography imaging and analysis
For femoral analysis, the right femora were harvested, placed in 70% ethanol, and scanned with micro‐CT (micro‐CT40, Scanco Medical AG, isotropic nominal resolution: 10 μm; 55 kVp, 145 μA, 200 ms integration time). A 3D constrained Gaussian filter (sigma 1.2, support 1) was applied to the image data, after which images were segmented by a global thresholding procedure. 47 Standard bone microstructural parameters were calculated in trabecular (BV/TV, Tb. Th, Tb. N, Tb. Sp), cortical (Ct.Ar/Tt.Ar, Ct.BV, Ct.MV, Ct.Ar, Tt.Ar, Ct. Th, Ps. Pm, Ec.Pm), and whole (apparent volume density (AVD) and length) bone using automatically selected masks for these regions. 48
For analysis of the sixth caudal vertebra (CV6), in vivo micro‐CT (vivaCT 40, Scanco Medical AG, isotropic nominal resolution: 10.5 μm; 55 kVp, 145 μA, 350 ms integration time, 500 projections per 180°) was performed every 2 weeks between 20 and 40 weeks of age. Animals were anaesthetised with isoflurane (induction/maintenance: 5%/1–2% isoflurane/oxygen). Micro‐CT data were processed, and standard bone microstructural parameters were calculated in trabecular, cortical, and whole bone by using automatically selected masks for these regions as described previously. 49
To calculate dynamic morphometric parameters, micro‐CT images from consecutive time points were registered onto one another. The voxels present only at the initial time point were considered resorbed, whereas voxels present only at the later time point were considered formed. Voxels that were present at both time points were considered as quiescent bone. By overlaying the images, morphometrical analysis of bone formation and resorption sites within the trabecular region allowed calculations of bone formation rate (BFR), bone resorption rate (BRR), mineral apposition rate (MAR), mineral resorption rate (MRR), mineralizing surface (MS), and eroded surface (ES). 39
Analysis of bone turnover markers
Immediately following euthanasia, blood samples from 40‐week‐old PolgA and WT mice were obtained by cardiac puncture. Serum was separated by centrifugation and stored at −80°C until further analysis. Markers for bone formation and resorption were measured in technical duplicates using enzyme‐linked immunosorbent assay kits for N‐terminal propeptide of type I procollagen (PINP, AC‐33F1, Immunodiagnostics) and for C‐terminal cross‐linked telopeptides of type I collagen (CTX‐I, RatLaps, AC‐06F1, Immunodiagnostics) according to manufacturer's instructions.
Cyclic mechanical loading of the sixth caudal vertebra
Sixth caudal vertebra were subjected to a cyclic loading regime, which has previously been shown to have anabolic effects in 15‐week‐old female C57BL/6J mice. 50 Briefly, stainless steel pins (Fine Science Tools) were inserted into the fifth and seventh caudal vertebrae of 35‐week‐old and 12‐week‐old female mice. Three weeks after surgery, the mice received either sham (0N control) or 8N cyclic (10 Hz) loading for 5 min, three times per week over 4 weeks. Weekly in vivo micro‐CT images (vivaCT 40 or vivaCT80, Scanco Medical AG) were acquired and analysed as described above.
Statistical analysis
Data are reported as mean and standard error of the mean (±SEM), unless otherwise stated. All analyses, with the exception of the longitudinal micro‐CT data, were performed using GraphPad Prism (8.0.0). Unpaired or paired Student's t‐test, Mann–Whitney test, one‐way or two‐way analysis of variance with post‐hoc multiple comparison testing were performed as indicated in figure legends. For the analysis of the longitudinal micro‐CT data, linear mixed‐effects modelling was used with Tukey's post‐hoc multiple comparison testing corrected with Bonferroni criteria (SPSS 24.0.0.0). Fixed effects were allocated to the age and genotype. Random effects were allocated to the animal to account for the natural differences in bone morphometry in different mice. Significance was set at α < 0.05 in all experiments.
Results
With age, PolgA mice become frailer and display signs of co‐existing osteopenia and sarcopenia.
To characterize the frailty and the musculoskeletal phenotypes of the PolgA model, female PolgA(D257A/D257A) (referred to as PolgA) and WT littermates (PolgA(+/+)) were aged in parallel and sacrificed at 34, 40, and 46 weeks, respectively. Using the clinical mouse FI as a tool to quantify the accumulation of health deficits, 29 we observed that older (40 and 46 weeks), but not younger (34 weeks), PolgA mice had higher FI scores compared with WT (Figure 1A). This divergence between genotypes at later time points was also observed when FI was assessed longitudinally in individual mice at 34, 38, and 40 weeks, respectively. Specifically, between 34 and 40 weeks, the mean FI in PolgA mice increased from 0.05 ± 0.02 to 0.15 ± 0.02 (mean ± standard deviation, P < 0.0001), whereas the increase in WT from 0.04 ± 0.03 to 0.06 ± 0.04 was less pronounced (P < 0.05, Figure 1B). Thus, compared with WT, PolgA mice had 73% (P < 0.05) and 128% (P < 0.001) higher FI at 38 and 40 weeks, respectively. Furthermore, the slope of the natural logarithm of the FI vs. age curve, which has been shown to correspond to the rate of deficit accumulation in humans, 43 was 0.025 in PolgA mice and 0.012 in WT, suggesting that PolgA mice became frailer faster than WT (P < 0.05, Figure 1C).
FIGURE 1.
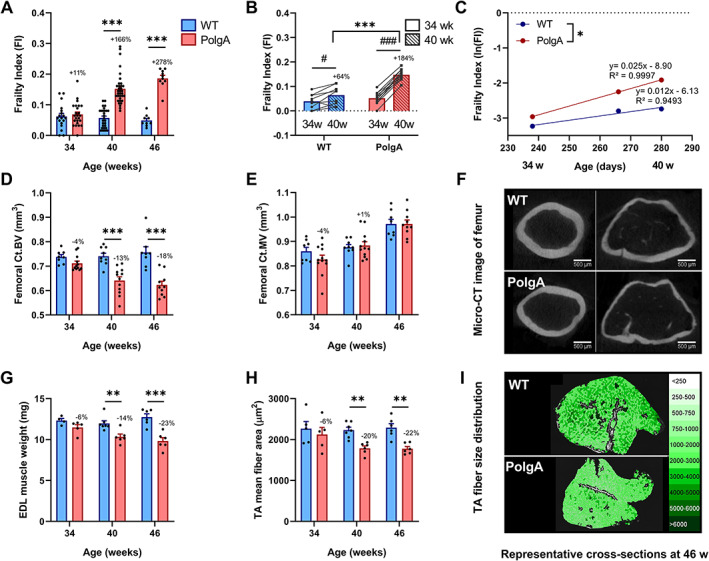
Comparison of the frailty and musculoskeletal phenotypes at different ages. (A) Frailty index (FI) assessed in PolgA and wild type (WT) at 34, 40, and 46 weeks (n = 9–35/group). (B) Longitudinal monitoring of FI in individual mice (n = 9–12/group) at 34 (solid bars) and 40 weeks (striped bars). (C) The natural logarithm FI was plotted as a function of age. The slope of the regression lines through these data, which represent the rate of deficit accumulation, was higher in PolgA mice. (D–I) PolgA mice displayed lower bone and muscle mass and cross‐sectional area compared with WT. (D) Femoral cortical bone volume (Ct.BV) and (E) cortical marrow volume (Ct.MV) (n = 8–12/group). (G) Muscle weight and (H) fibre area (n = 5–7/group). Representative cross sections of (C) femoral bone and (F) m. tibialis anterior (TA) muscle of PolgA and WT mice at 46 weeks. Data represent mean ± SEM; * P < 0.05, ** P < 0.01, *** P < 0.0001. WT (blue bars) vs. PolgA (red bars) determined by unpaired t‐test, corrected for multiple comparisons by Bonferroni; for (B), *** P < 0.0001 WT vs. PolgA and #P < 0.05, ###P < 0.0001 over time determined by two‐way analysis of variance.
Concomitant to the increase in FI with age, older PolgA mice displayed deteriorations of the musculoskeletal system. Specifically, micro‐CT analysis of the femora showed that the cortical bone volume (Ct.BV) was similar between genotypes at 34 weeks of age (P > 0.05), but then diverged over time such that PolgA had lower Ct.BV at 40 and 46 weeks, respectively (P < 0.0001, Figure 1D). In line with the decline in Ct.BV, the cortical area (Ct.Ar) and thickness (Ct. Th) were lower in PolgA femora compared with WT at 40 (−13% and −11%, P < 0.0001) and 46 weeks (−17% and −15%, P < 0.0001), respectively (Table S3). The cortical marrow volume (Ct.MV) increased both in WT and PolgA with age with no differences between genotypes at any of the ages (P > 0.05, Figure 1E). The total cross‐sectional area within the periosteal envelope (Tt.Ar) was similar between genotypes at 34 weeks of age, but significantly lower in PolgA compared with WT at 40 and 46 weeks (−5% and −7%, P < 0.01), respectively (Table S3). Hence, while PolgA and WT showed similar endocortical expansion with age, PolgA showed reduced periosteal expansion at 40 and 46 weeks, respectively. Furthermore, PolgA had slightly but significantly shorter femora compared with WT at 40 and 46 weeks (−2% and −1%, P < 0.001, Table S3), respectively. Regarding the muscle tissue, the weights of EDL and fibre cross‐sectional area of m. tibialis anterior (TA) were similar between genotypes at 34 weeks (P > 0.05), but lower in PolgA mice at 40 (−14% and −20%, P < 0.01) and 46 weeks (−23% P < 0.0001, −22% P < 0.01), respectively (P < 0.05, Figure 1G–I). Similarly, lower weights of TA and m. gastrocnemius (GAS) in PolgA mice as compared with WT were observed at 40 (−20%, P < 0.0001 and −23%, P < 0.0001) and 46 weeks (−22%, P < 0.0001 and −16%, P < 0.05), respectively.
Decreased forelimb grip strength and concentric force in PolgA mice
Considering that PolgA mice showed clear reductions in muscle mass and cross‐sectional area at 40 and 46 weeks, we aimed to comprehensively characterize the muscular functionality at these time points. At 40 weeks, in vivo assessment of the forelimb grip strength, a widely used method to assess muscle function in rodents, showed that PolgA mice had lower grip strength relative to body weight (P < 0.05, Figure 2A). Furthermore, when the EDL was subjected to a force frequency protocol, PolgA showed a tendency towards decreased absolute force at 250 and 300 Hz (P < 0.10), while relative force was not affected (Figure 2B,C). At 46 weeks, the grip strength was 11% lower in PolgA mice compared with WT, but did not reach significance (Figure 2D). However, the differences in forces were more exacerbated with a lower absolute and relative force at 80 to 300 Hz (absolute) and 250 to 300 Hz (relative) in the PolgA (P < 0.05, Figure 2E,F).
FIGURE 2.
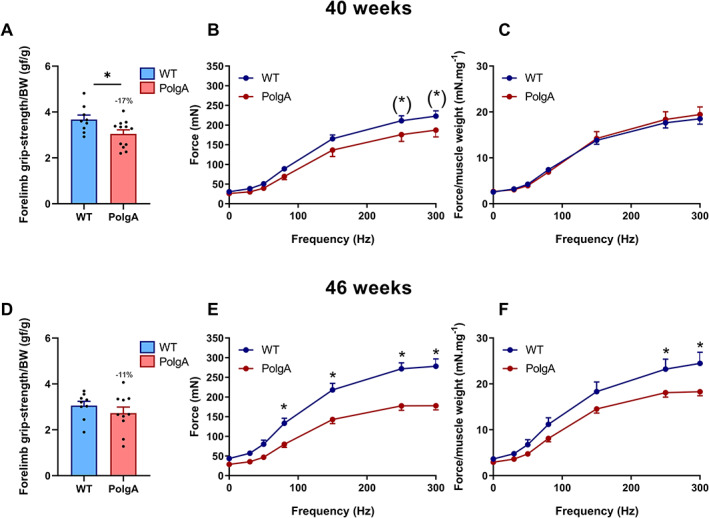
Muscle phenotyping at 40 (upper row, A–C) and 46 weeks (lower row, D–F). (A,D) forelimb grip strength (n = 9–12/group). (B,E) force frequency at 1, 30, 50, 80, 150, 250, and 300 Hz. (C,F) force frequency relative to EDL muscle weight (n = 6–8/group). Data represent mean ± SEM; (*)P < 0.10, * P < 0.05 by Student's t‐tests (A–D) and two‐way ANOVA (E–H).
PolgA mutation induces higher basal and eccentric contraction evoked mechanistic target of rapamycin complex 1 and mechanotransduction signalling.
Because PolgA muscles were atrophied and weaker than those of their WT littermates of the same chronological age, we used an isolated ex vivo model to assess the acute response to eccentric contractions (ECC), which have been shown to effectively activate mTORC1, the main regulator of skeletal muscle protein synthesis. 51 , 52 Data of muscle forces evoked by ECC are presented in Table 1. PolgA had similar average force during the 60 ECC, but tended to have a lower peak force during the first set (Table 1).
TABLE 1.
Forces eccentric muscle contractions at 40 and 46 weeks
| Average peak force (mV) | 40 weeks | 46 weeks | ||
|---|---|---|---|---|
| WT | PolgA | WT | PolgA | |
| All contractions | 149.9 ± 9.7 | 142.8 ± 15.7 | 141.6 ± 9.6 | 137.3 ± 8.8 |
| 1st set | 193.3 ± 12.4 | 158.8 ± 17.5 | 188.5 ± 6.6 | 164.6 ± 8.6(*) |
| 10th set | 108.8 ± 8.0 | 107.5 ± 11.2 | 98.6 ± 8.2 | 111.8 ± 5.8 |
WT, wild type.
(*) P < 0.10 WT vs. PolgA at 40 and 46 weeks, respectively determined by unpaired t‐test, corrected for multiple comparisons by Bonferroni.
At 40 weeks, ECC evoked increased phosphorylation of the downstream mTORC1 kinase ribosomal protein S6 kinase 1 (S6K1) at Thr389 compared with the contralateral control leg in both WT (+147 ± 50%, P < 0.05) and PolgA (406 ± 161%, P < 0.05), but the increase was more pronounced in PolgA, resulting in a higher pS6K1 upon ECC in the PolgA (∆ECC‐CTL, P < 0.05) (Figure 3A). The direct downstream kinase of pS6K1, S6 ribosomal protein (RPS6) showed similar increases in phosphorylation at Ser235/236 upon ECC in WT and PolgA (64 ± 24% and 352 ± 146%, P < 0.05), whereas there was only a trend towards higher activation in PolgA compared with WT (∆ECC‐CTL, P = 0.07) (Figure 3B). At 46 weeks, downstream mTORC1 targets showed similar effects with a trend towards hyper‐phosphorylated pS6K1 and pRPS6 after ECC contractions in the PolgA compared with WT (P = 0.10, Figure 3D,E). Moreover, basal pRPS6 was higher in PolgA (1257 ± 427%, P < 0.05) compared with WT (Figure 3E), suggesting both basal and contraction‐induced hyperactivation of mTORC1 in PolgA mutated muscle.
FIGURE 3.
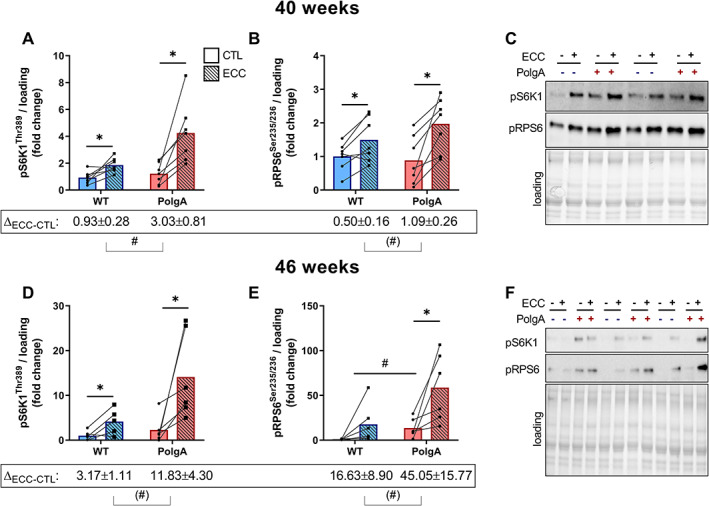
Downstream mechanistic target of rapamycin complex 1 (mTORC1) signalling upon eccentric contraction (ECC) in wild type (WT) and PolgA EDL. Downstream mTORC1 targets pS6K1 and pRPS6 increased upon ECC both at 40 (A,B) and 46 weeks (D,E). (C,F) representative blots. Data represent mean ± SEM, n = 6–7/group, within genotypes: * P < 0.05, ** P < 0.001 ECC (squares/striped bars) vs. CTL (circles/solid bars) by paired Student's t‐test, between genotypes: (#)P < 0.10, #P < 0.05 by unpaired Student's t‐test ∆ECC‐CTL and basal values.
One of the proposed pathways for high‐load contractions to regulate mTORC1 is via activation of the stress responsive mitogen‐activated protein kinase (MAPK) pathway. 53 To investigate whether the hyperactive mTORC1 signalling in the PolgA muscle was related to increased MAPK response, we measured activation of (stress‐activated protein kinase/Jun‐amino‐terminal Kinase) pSAPK/JNK upon ECC. At 40 weeks, the stress‐responsive pSAPK/JNK at Thr138/Tyr185 after ECC increased by 113 ± 35.8% (P < 0.05) in WT and by 156 ± 26% (P < 0.001) in PolgA (Figure 4A), with a tendency towards hyperactivation in PolgA compared with WT (∆ECC‐CTL, P = 0.09). At 46 weeks, the increase in pSAPK/JNK upon ECC was more pronounced in the PolgA (2410 ± 1035%, P < 0.05) than in the WT (1410 ± 179%, P < 0.05), resulting in a higher phosphorylation in PolgA muscle after ECC (∆ECC‐CTL, p < 0.05) and a tendency towards higher basal pSAPK/JNK (P = 0.07, Figure 4D). Interestingly, another arm of the stress‐responsive MAPK pathway, p42‐44 MAPK at Thr202/Tyr204, was not affected by ECC; however, basal p44/42 signalling was higher in PolgA muscle compared with WT at 46 weeks (Figure 4B,D). These data show that hyperactive mTORC1 signalling mirrors increased basal and contraction‐induced MAPK signalling in PolgA mutated muscle.
FIGURE 4.
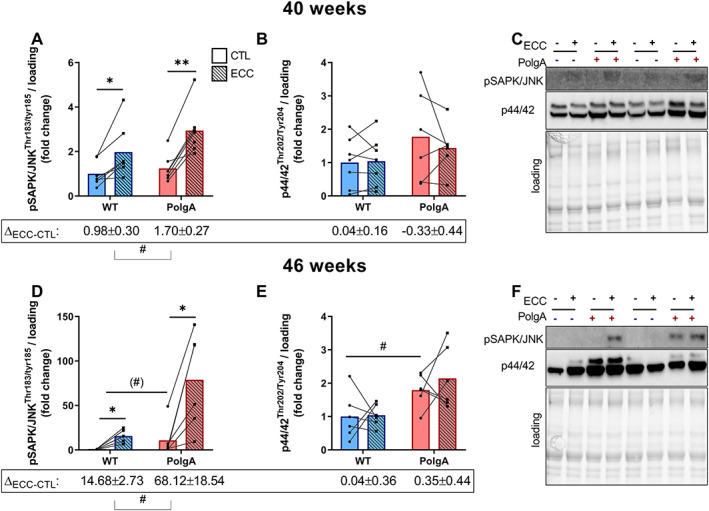
Downstream mitogen‐activated protein kinase (MAPK) signalling upon eccentric contraction (ECC) in wild type (WT) and PolgA EDL. pSAPK/JNK, but not p44/42 MAPK ERK1/2 increased upon ECC both at 40 (A,B) and 46 weeks (D,E). (C,F) representative blots. Data represent mean ± SEM, n = 6–7/group, within genotypes: * P < 0.05, ** P < 0.001 ECC (squares/striped bars) vs. CTL (circles/solid bars) by paired Student's t‐test, between genotypes: (#)P < 0.10, #P < 0.05 by unpaired Student's t‐test ∆ECC‐CTL and basal values.
PolgA whole muscles are hypersensitive to leucine in vivo, while their primary myotubes are resistant to leucine in vitro
To examine whether other anabolic stimuli also hyperactivate mTORC1 signalling in PolgA muscle, we administered a submaximal (0.4 g/kg) dose of leucine via gavage to 46‐week‐old WT and PolgA littermates. Leucine induced an increase in pRPS6 in both WT (P < 0.05) and PolgA (P < 0.0001) TA, whereas pS6K1 was also increased in PolgA (P < 0.001). The increase was 3.3 ± 0.8 and 11.4 ± 5.3 fold higher in PolgA for both pRPS6 and pS6K1, respectively (P < 0.001, Figure 5A–C). These data demonstrate that the amino acid leucine increases downstream mTORC1 signalling to a greater extent in PolgA mutated compared with WT muscle.
FIGURE 5.
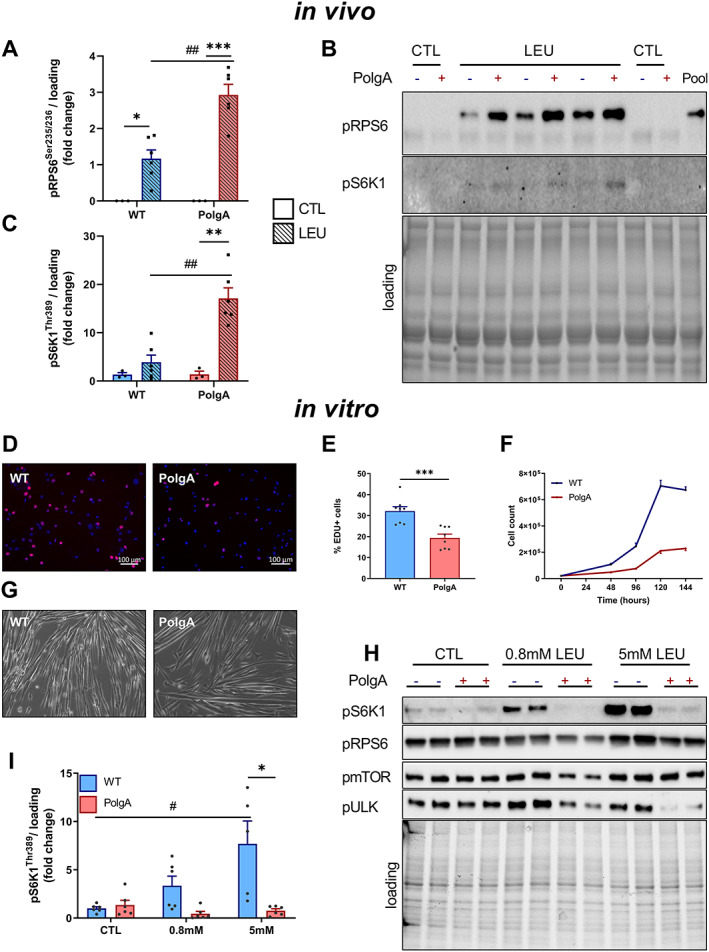
Downstream mechanistic target of rapamycin complex 1 (mTORC1) signalling after leucine administration in vivo and in vitro. (A–C). pRPS6 and pS6K1 signalling 30 min after submaximal dose of leucine gavage in vivo in 46‐week‐old wild type (WT) and PolgA. Data represent mean ± SEM, n = 3–6/group, within genotypes: * P < 0.05, ** P < 0.001, *** P < 0.0001 LEU (squares/striped bars) vs. CTL (circles/solid bars), between genotypes: (#)P < 0.10, #P < 0.05, ##P < 0.001 determined by two‐way analysis of variance. (C) Representative blots. (D–I) Proliferation and downstream mTORC1 signalling in primary myotubes from WT and PolgA muscle. (D,E) Cell proliferation was analysed by EdU labelling and (F) cell count. (G) Brightfield of two‐dimensional differentiated myotubes. (H) Representative blots of dose‐response leucine experiment in fully differentiated WT and PolgA myoblasts. (I) Quantification of pS6K1 from three independent experiments. Data represent mean ± SEM, * P < 0.05 WT (blue line/bars) vs. PolgA (red line/bars), #P < 0.05 over time determined by two‐way analysis of variance.
In vivo, leucine sensing towards mTORC1 might be altered by the presence of other amino acids or growth factors. To rule out such interference, we cultured MPs from WT and PolgA hind limb muscle to verify proliferation capacity and leucine sensing without the availability of other amino acids. Strikingly, both EdU incorporation and proliferation (Figure 5D–F) were reduced in PolgA derived MPs. Furthermore, after differentiation, which was unaffected by PolgA mutation (Figure 5G), we subjected myotubes to varying doses of leucine after 60 min of starvation. In contrast to our in vivo data, PolgA myotubes showed a strong anabolic resistance to leucine, as indicated by lower pS6K1 at 0.8 and 5 mM leucine, respectively (Figure 5H,I). These data show that PolgA muscle progenitors have reduced cell proliferation capacity in vitro, potentially due to lower sensitivity to leucine.
Reduced bone quantity and quality in PolgA mice
As the results of the cross‐sectional experiments described above suggest that PolgA mice display clear signs of co‐existing osteopenia and sarcopenia from 40 weeks onwards, we aimed to longitudinally track individual mice during the transition from young to frail status in order to gain a better understanding of when exactly and to what extent bone loss (osteopenia) occurs in individual mice. Therefore, we used an in vivo micro‐CT approach to monitor the dynamic changes in the bone microarchitecture of CV6 in individual mice between the ages of 20 and 40 weeks (Figure 6). The comparison between genotypes showed that at 20 weeks, PolgA and WT had similar trabecular morphometric parameters. Initially, bone volume fraction (BV/TV) and trabecular thickness (Tb. Th) increased in both genotypes, but then started to diverge such that BV/TV and Tb. Th were higher in WT mice from 30 weeks onwards (P < 0.05, Figure 6A,B). Furthermore, from 30 weeks onwards, Tb. Th continuously increased in WT (+5%, P < 0.0001), whereas it did not change in PolgA (−1.3%, P > 0.5, Figure 6B). No significant differences between genotypes were detected for the trabecular number (Tb. N) and separation (Tb. Sp, Figure 6C,D). Nevertheless, Tb. N in WT decreased between 30 and 40 weeks (−2.5%, p < 0.05), whereas Tb. N did not change over time in PolgA mice (−0.3%, p > 0.05, Figure 6C). The reduction in Tb. N together with the increase in Tb. Sp in WT explain the slight decrease in BV/TV in WT mice from 36 weeks onwards. A significant interaction between age and genotype, indicating that the time course developed differently between genotypes, was found for Tb. Th, Tb. N, and Tb. Sp.
FIGURE 6.
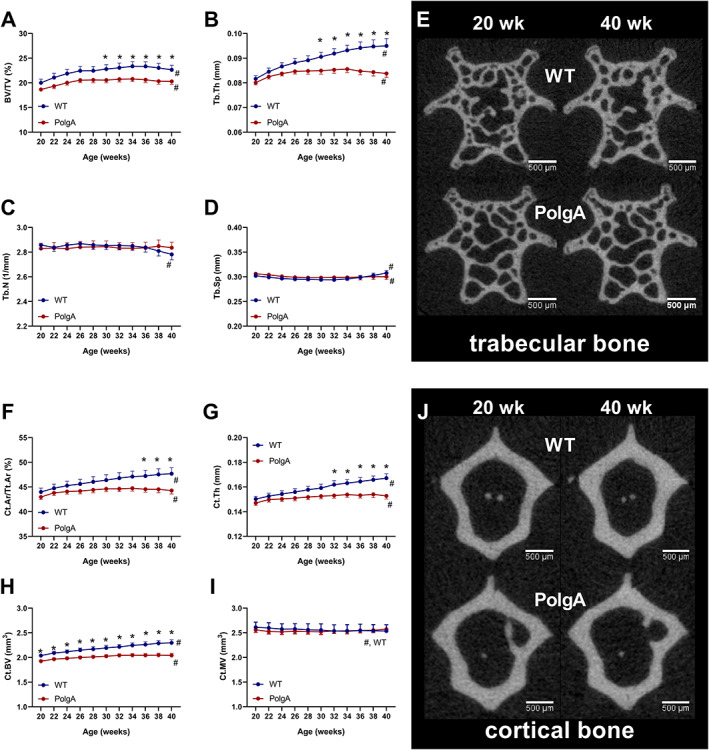
Longitudinal monitoring of trabecular and cortical bone morphometric parameters over 20 weeks: (A) bone volume fraction (BV/TV), (B) trabecular thickness (Tb. Th), (C) trabecular number (Tb. N), (D) trabecular separation (Tb. Sp), (F) cortical area fraction (Ct.Ar/Tt.Ar), (G) cortical thickness (Ct. Th), (H) cortical bone volume (Ct.BV), and (I) cortical marrow volume (Ct.MV). (E,J) Bone microstructure (cross sections) of representative (median) wild type (WT) (top row) and PolgA (bottom row) mice at 20 (left) and 40 (right) weeks of age. In WT mice, thickening of trabeculae and cortex can be observed, while in PolgA mice, little change can be seen between time points. Data represent mean ± SEM; n = 9 WT and n = 12 PolgA, * P < 0.05 WT (blue line) vs. PolgA (red line); #P < 0.05 over time determined by linear mixed model and Tukey's post‐hoc.
Similar results were obtained when the cortical bone morphometric parameters were analysed. At 20 weeks, there were no differences between genotypes in cortical area fraction (Ct.Ar/Tt.Ar), Ct. Th, and Ct.MV (Figure 6F,G,I). Ct.BV was already lower (−6%, P < 0.05) in PolgA compared with WT at 20 weeks (Figure 6H) with this difference becoming more accentuated up to 40 weeks (−11%, P < 0.001). Ct. Th increased over time, but then diverged such that Ct. Th was higher than that of PolgA mice from 32 weeks onwards (P < 0.05). This resulted in a higher Ct.Ar/Tt.Ar in PolgA mice from 36 weeks onwards (P < 0.05). No differences in Ct.MV were detected between genotypes. A significant interaction between age and genotype was found for Ct.Ar/Tt.Ar, Ct. Th, Ct.BV, and Ct.MV. The thickening of the trabecular and cortical structure was also visually apparent from the micro‐CT images and can be appreciated when the same cross‐section is observed at 20 and 40 weeks (Figure 6E,J).
Reduced bone remodelling in PolgA mice
In addition to monitoring changes in bone morphometry over time, we used in vivo micro‐CT to quantify dynamic bone formation and resorption parameters in PolgA and WT caudal vertebrae, respectively (Figure 7). On average, PolgA mice had lower BFR (−23.2%, P < 0.05) and BRR (−28.8%, P < 0.05) compared with WT (Figure 7A,B). BFR and BRR changed over time in both genotypes (P < 0.001); however, the time course did not develop differently between genotypes (interaction effect, P > 0.05). Hence, the net remodelling rate (BFR‐BRR) was not different between genotypes. The MAR and MRR, which represent the thickness of formation and resorption packages, were lower (−8.9%, P < 0.05 and −20.1%, P < 0.001) in PolgA compared with WT (Figure 7C,D). MAR did not change over time and showed no significant interaction between age and genotype (Figure 6C). On the other hand, there was a significant interaction effect of age and genotype on MRR (P < 0.05). Over time, MRR increased in WT, while MRR remained constant in PolgA (Figure 7D). The mineralized surface (MS), which represents the surface of formation sites, was lower in PolgA (−11.8%, P < 0.0001), whereas the ES was similar between genotypes (P > 0.05) (Figure 7E,F). MS and ES changed over time in both genotypes; however, the time course did not develop differently between genotypes (interaction effect, P > 0.05). In line with lower BFR and BRR observed by micro‐CT, bone turnover markers for formation (N‐terminal propeptide of type I procollagen and resorption; C‐terminal telopeptides of type I collagen, CTX‐I) were lower in serum from PolgA (−56% and −49%, P < 0.05) compared with WT at 40 weeks of age (Figure 7H,I). Overall, these results suggest that PolgA mice have reduced bone turnover compared with WT.
FIGURE 7.
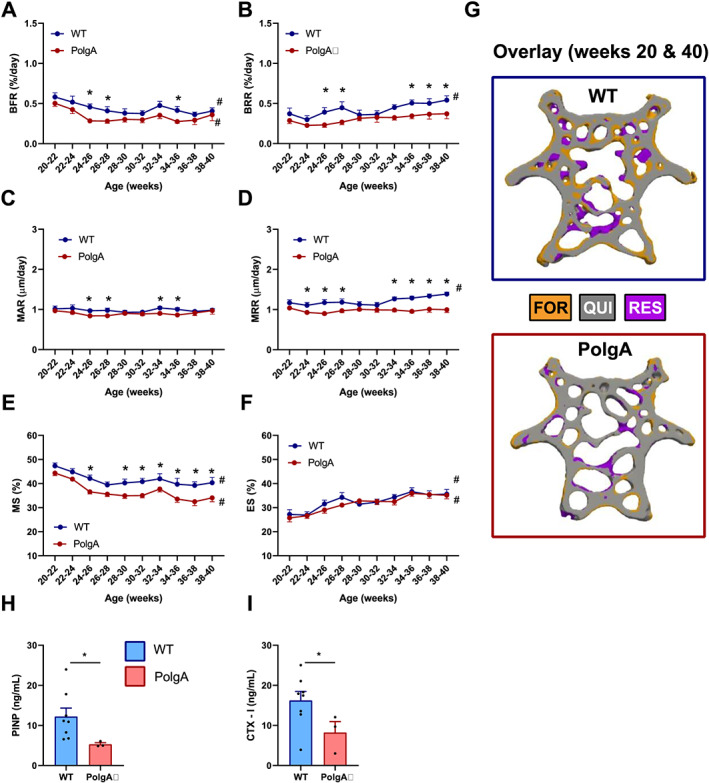
Longitudinal monitoring of dynamic bone morphometry parameters over 20 weeks: (A) bone formation rate (BFR), (B) bone resorption rate (BRR), (C) mineral apposition rate (MAR), (D)mineral resorption rate (MRR), (E)mineralizing surface (MS) and (F) eroding surface (ES). Data represent mean ± SEM; n = 9 wild type (WT) and n = 12 PolgA; * P < 0.05 WT (blue line) vs. PolgA (red line); #P < 0.05 over time determined by linear mixed model and Tukey's post‐hoc. (G) Overlay of thresholded micro‐computed tomography images from 20 and 40 weeks showing sites of formation (orange), quiescence (grey), and resorption (blue) in representative WT and PolgA mouse. Serum bone turnover markers for (H) formation (PINP) and (I) resorption (CTX‐1) at 40 weeks of age. Data represent mean ± SEM; n = 8 WT and n = 3 PolgA; * P < 0.05 WT (blue bar) vs. PolgA (red bar) determined by Mann–Whitney test.
To assess whether the osteopenic phenotype in PolgA mice could be linked to alterations in mTORC1 signalling, we assessed basal mTORC1 and MAPK signalling in caudal vertebrae at 40 weeks of age. Overall, the expression of downstream mTORC1 and MAPK was lower in vertebrae compared with muscle tissue. Therefore, the bone immunoblots were overexposed in order to observe a signal. Compared with WT, PolgA mice tended to have lower pS6K1 at Thr389 (−69%, P ≤ 0.1) while no significant difference was observed for the direct downstream kinase thereof (RPS6) at Ser235/236 (P > 0.05) (Figure 8A,B). Basal pSAPK/JNK at Thr183/Tyr185 was lower (−60%, P < 0.001) in PolgA compared with WT, while no difference was observed in p44/42 at Thr202/Tyr204 (P > 0.05) (Figure 8C,D).
FIGURE 8.
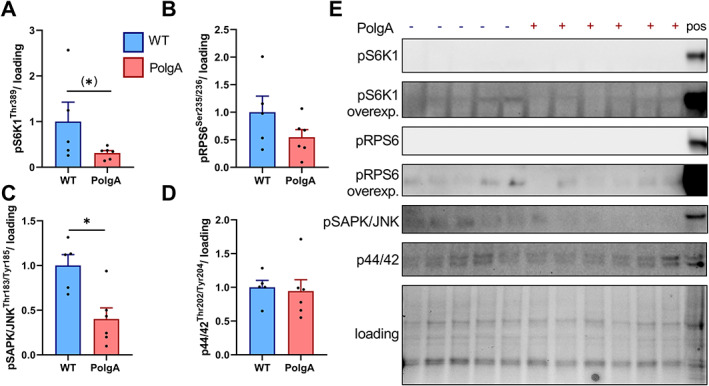
Basal mechanistic target of rapamycin complex 1 (mTORC1) and mitogen‐activated protein kinase (MAPK) signalling in wild type (WT) and PolgA caudal vertebrae at 40 weeks. PolgA mice showed a tendency towards reduced downstream mTORC1 targets pS6K1 (A) and pRPS6 (B). Downstream MAPK target pSAPK/JNK (C), but not p44/42 MAPK (D) was lower in PolgA compared with WT. (E) Representative blots with positive control (muscle tissue). Overall signal was low in bone lysates, so pS6K1 and pRPS6 are overexposed. Data represent mean ± SEM, n = 5–6/genotype, (*)P ≤ 0.10, * P < 0.05 PolgA (red bars) vs. WT (blue) by unpaired Student's t‐test.
Reduced mechanosensitivity of PolgA caudal vertebrae with age
To investigate whether prematurely aged PolgA bones are mechanosensitive, CV6 were cyclically loaded three times per week over 4 weeks using a previously developed loading device. 50 Figure 9 shows the absolute values of trabecular bone morphometric parameters at the end of the mechanical loading intervention in young (Figure 9A,B) and aged (Figure 9C,D) mice. At young age, loading elicited an anabolic response both in WT and PolgA mice such that BV/TV and Tb. Th were higher (P < 0.0001) in the loaded compared with the sham‐loaded controls (Figure 9A,B). Strikingly, the anabolic effect was maintained with age in WT (P < 0.05), whereas PolgA mice did not respond to the mechanical loading intervention, with no differences detected between loaded and non‐loaded controls (Figure 9C,D).
FIGURE 9.
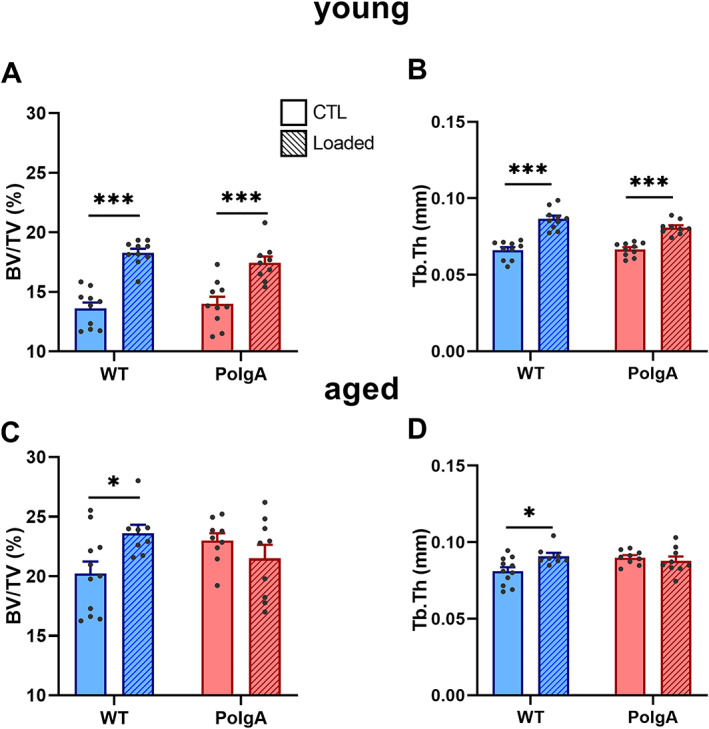
Effect of cyclic mechanical loading on trabecular bone morphometric parameters in PolgA and wild‐type (WT) mice at young (top) and old age (bottom): (A,C) bone volume fraction (BV/TV) and (B,D) trabecular thickness (Tb. Th) at the end of the loading intervention. Data represent mean ± SEM, n = 8–11/group; * P < 0.05, *** P < 0.0001 CTL (circles/solid bars) vs. loading (squares/striped bars).
Discussion
Due to the accumulation of mitochondrial DNA mutations with age, PolgA mice are known to develop multiple signs of aging (alopecia, greying, hearing loss, kyphosis, reduced bone, and muscle mass) early in life. 34 , 35 , 87 Here, we used the clinical mouse FI, 29 which was recently reverse translated from the FI in humans, 28 to quantify the accumulation of these health deficits over time in individual mice. Furthermore, as the weakening of the musculoskeletal system is a major component of age‐related frailty, we used a combination of in vivo, ex vivo, and in vitro techniques to comprehensively characterize the musculoskeletal phenotype of this model. Lastly, the effects of various anabolic interventions on the musculoskeletal system of PolgA mice were assessed.
This study demonstrated that in comparison with their chronologically aged WT littermates, PolgA mice developed many hallmarks of aging over time, which collectively resulted in PolgA mice becoming frailer with age. Of the 31 items included in the FI scoring, changes in the integument category were the most striking. Specifically, PolgA mice showed a loss in fur colour (from black to grey/brown) and a decline in the coat condition (ruffled fur, ungroomed appearance). Furthermore, they showed signs of enlarged abdomen and kyphosis. At 40 weeks of age (280 days), PolgA mice had mean frailty scores of 0.15 ± 0.02 (mean ± standard deviation), whereas WT mice had 0.06 ± 0.04. A previous study using male C57BL/6J mice reported mean FI values of 0.05 ± 0.05 in young (30–299 days), 0.21 ± 0.04 in middle‐aged (300–599 days), and 0.29 ± 0.07 in older mice (above 600 days). 30 Comparing these values to the ones observed in our study, the (female) WT mice (at 40 weeks <299 days) fall in the category of young mice, whereas the PolgA mice fall into the category of ‘middle‐aged’ mice. Although some studies have reported higher FI scores in female mice compared with male mice, 29 , 54 others showed no differences in frailty between sexes 55 , 56 ; furthermore, it seems that the rate of deficit accumulation is similar between sexes. 54 Nevertheless, future studies in which PolgA mice are aged for a longer period of time (>300 days) would be necessary to investigate the degree of frailty at later stages in life. Our ethical permit for breeding was constrained to a maximum of 46 weeks as previous studies reported median lifespans of PolgA mice ranging from ~59 weeks (416 days), 34 48 weeks (336 days), 35 down to only 36 weeks (250 days). 37 The discrepancies between studies regarding the lifespan are also apparent for the age at which symptoms of aging first appeared (ranging from 6 37 to 9 months 34 , 35 ). As not only homozygous (PolgA(D257A/D257A)) but also heterozygous (PolgA( D257A/+)) mice progressively accumulate mitochondrial DNA point mutations with age, we suspect that the differences between studies resulted from differences in breeding schemes. Because all the mice used in our study were bred according to a standardized breeding scheme, thereby displaying only a single generation of mutation burden, the onset of the accelerated aging phenotype may have been delayed in the present work as compared with other studies. Nevertheless, we observed that PolgA mice became frailer faster than WT, as shown by plotting the natural logarithm of the FI as a function of age and fitting the resulting relationship with a linear function. To our knowledge, we are the first to report the frailty phenotyping in the PolgA mouse model.
In line with increased FI with age, the musculoskeletal phenotype clearly diverged over time between genotypes, with PolgA mice developing multiple signs of osteosarcopenia with age. In agreement with previous reports showing lower muscle weights 34 , 36 , 37 and reduced femoral bone mineral density assessed by X‐ray densitometry, 34 , 35 phenotyping of the femora and hind limb muscles revealed lower bone and muscle mass as well as cross‐sectional area in PolgA mice compared with their WT littermates at 40 and 46 weeks of age. These reductions were not observed at 34 weeks, thus indicating a progressive weakening of the musculoskeletal system in the PolgA mice. As the clinical diagnosis of sarcopenia is not only characterized by reduced muscle mass but also by reduced muscle function and quality, we assessed the forelimb grip strength, muscle force, and intramuscular signalling upon anabolic stimuli. In agreement with clinically diagnosed sarcopenia, PolgA displayed lowered muscle strength, indicated by reduced grip strength and lower concentric contraction force at different intensities.
One mechanism commonly thought of as a contributor to sarcopenia is the age‐related lowered sensitivity to anabolic stimuli. This phenomenon, known as anabolic resistance, has been associated with the reduced acute activation of mTORC1, 13 , 14 , 15 the central protein complex that integrates external stimuli to regulate cell growth. 57 , 58 , 59 , 60 Note that the interpretation of the data described in previous studies, which showed increased anabolic resistance upon heavy‐load contractions, might be confounded by the fact that the same relative load (% 1 repetition max) was used in old vs. young humans. 13 The lower absolute load in the aged subjects might have caused lower mechanical stress on the muscle, as demonstrated by lower MAPK signalling, 61 and thus a dampened increase in mTORC1 signalling. By using the protocol described by O'Neil et al., 44 we could overcome this confounding factor by controlling the load applied to the muscles. Interestingly, although the average force produced during the 60 contractions was similar between WT and PolgA, both MAPK and downstream mTORC1 signalling were remarkably elevated in PolgA mutated muscle. Notably, leucine also activated mTORC1 to a greater extent in 46‐week‐old PolgA muscle compared with their chronologically aged WT littermates. This is intriguing as ECCs and leucine activate mTORC1 signalling via independent mechanisms. 62 Potentially, increased contraction‐induced mechanical signalling is responsible for increased mTORC1 activity in response to contractions, but further research is needed to fully elucidate the mechanisms behind mTORC1 hyperactivity in PolgA muscle. Next to stimulus‐induced mTORC1 activity, we also found hyperactive basal mTORC1 in PolgA at 46 weeks of age. In fact, basal mTORC1 hyperactivity has previously also been shown in other models of progeria, 63 aging, 64 and progeria‐like syndromes (i.e. laminopathies). 65 , 66 Furthermore, chronically increased mTORC1 activity has been shown to be sufficient to cause progressive muscle atrophy, fibre damage, fibre death, and muscle weakness in humans and in mice. 67 Therefore, treatment with mTORC1 inhibitors has been proposed as a potential therapeutic approach to rescue elevated mTOR signalling in these models. 65 , 66 , 67
In vivo, the systemic availability of amino acids/nutrients and differences in the local niche might have interfered with hyperactive mTORC1 signalling in PolgA. To rule this out, we used an in vitro approach to study the activation of mTORC1 in MPs that were stimulated with identical concentrations of leucine, without the availability of other amino acids. We were able to demonstrate that PolgA primary MPs had substantial defects in proliferative capacity and were completely insensitive to leucine with respect to mTORC1 anabolic signalling. Thus, it seems that mitochondrial dysfunction per se is sufficient to induce impairments in muscle stem cell mTORC1 signalling, regardless of exposure to the local niche. The differences in downstream mTORC1 signalling upon anabolic signals between whole muscle and their respective stem cells are puzzling, but can potentially be induced by the higher accumulation of mitochondrial damage under proliferative in vitro conditions when compared with post‐mitotic differentiated muscle tissue that develops apoptosis at a later stage. 34 Nonetheless, these data warrant caution in the translation of MP‐derived experimental data to the in vivo muscle setting and suggest that the accumulation of mitochondrial damage is tissue specific and can profoundly change the mTORC1 response to leucine.
With respect to the evaluation of the bone phenotype, this study is, to the best of our knowledge, the first to use standardized micro‐CT approaches to comprehensively characterize the bone phenotype of the PolgA mouse model. 38 , 39 , 48 Albeit still considered the clinical gold standard for assessing bone mineral density in humans, X‐ray densitometry, which was previously used to evaluate the PolgA bone phenotype, 34 , 35 is confounded by the size and positioning of the bone and furthermore does not provide any information on the bone microarchitecture. 68 , 69 Nevertheless, the results of this study did recapitulate the clear reductions measured in femoral bone mineral density in PolgA mice with age. 34 , 35 Note that the accuracy of the aforementioned phenotyping of the femora, both in the previous as well as in the current study, is limited by the cross‐sectional study design. This type of study design cannot account for the intrinsic initial variability between animals at baseline, 70 , 71 thereby making it impossible to determine the amount of bone lost in individual mice. We therefore used an established in vivo micro‐CT approach to monitor the spatio‐temporal changes in bone microarchitecture in the caudal vertebrae of individual mice during the transition from healthy to frail state. Multiple bone morphometric parameters diverged over time, from being similar at 20 weeks to lower in PolgA mice with age. Interestingly though, this difference did not arise due to significant bone loss in PolgA mice, but rather in the failure to achieve normal peak bone mass. We and others have previously reported the absence of age‐related bone loss in caudal vertebrae. 72 , 73 Hence, mouse caudal vertebrae may not be optimal for investigating age‐related bone loss. As the bone morphometric parameters in the femora declined with age in PolgA mice, this study suggests that age‐related changes in bone microarchitecture differ depending on the skeletal site that is analysed. Indeed, a more severe age‐related deterioration of bone microarchitecture in long bones compared with lumbar vertebrae has previously been reported. 71 , 72 , 74 In a cross‐sectional study performed by Glatt et al., the cross‐sectional area of the lumbar and caudal vertebrae of female C57BL/6 mice increased by 25% between the age of 2 and 20 months, while the femoral cross‐sectional area declined by 3%. 71 Furthermore, using in vivo micro‐CT, we and others have previously shown differences in age‐related changes in the lumbar and caudal vertebrae as compared with the tibiae of C57BL/6 mice. 73 , 74 Both studies showed continuous declines in cortical bone of the tibiae, while no changes or even increases were observed in the lumbar and caudal vertebrae, respectively. 73 , 74 We therefore assume that the osteopenic phenotype in PolgA mice is stronger in long bones compared with vertebrae. Hence, monitoring the long bones of PolgA mice by in vivo micro‐CT may be beneficial in future studies. Beyond enhanced phenotyping, the current study capitalized on the ability of in vivo micro‐CT to provide information on the dynamic coupling between bone resorption and formation. Compared with WT littermates, PolgA had lower bone remodelling activities as shown by reduced bone formation and resorption rates, with no differences in the net remodelling rate. These results were confirmed by lower bone turnover markers measured in PolgA serum compared with WT. Similarly, senile osteoporosis in humans is characterized by low bone turnover, as opposed to the high bone turnover rates (higher resorption activities) observed during postmenopausal osteoporosis. Despite the advantages of in vivo micro‐CT, the anaesthesia, cumulative radiation, and stress associated with repeated CT measurements could have an effect on the bone morphometry and the well‐being of the animals. 75 , 76 , 77 , 78 , 79 However, as effects of radiation are of greater concern in young, growing mice, and furthermore have been shown to be independent of surgical treatments (e.g. ovariectomy), 75 we suspect that any effects of radiation would be similar in WT and PolgA mice. Nevertheless, we compared the bone remodelling activities of PolgA and WT mice used in this study (subjected to 11 in vivo measurements) with those of corresponding age‐matched controls that received only two in vivo scans at 38 and 40 weeks, respectively, and saw no statistical differences between the groups. Furthermore, we did not observe any negative effects of any of these variables on the well‐being of the animals.
Finally, after having established that PolgA mutation altered the acute sensitivity to anabolic stimuli in muscles and their progenitors, we investigated the responsiveness of PolgA bones to a previously established 4 week mechanical loading regime. 49 Interestingly, prematurely aged PolgA mice were not responsive to cyclic loading of the caudal vertebrae over 4 weeks, whereas loading had beneficial effects in WT mice. Although there are controversial reports on the maintenance of bone mechanosensitivity with age in mice, 23 , 24 , 25 , 26 these results conflicted with our previous observation that caudal vertebrae remain mechanosensitive to cyclic mechanical loading with age. 27 Interestingly, in that study, 82‐week‐old C57BL/6J mice showed a greater anabolic response compared with the 52‐week‐old mice; hence, it is possible that even older PolgA mice would respond differently than the ones used in this study. Furthermore, the load in this study was not adjusted to account for differences in the initial bone volume fraction between PolgA and WT mice. An individualized loading approach, which ensures that all bones are subjected to the same mechanical strain, could yield differences in the mechanosensitivity of the PolgA and WT mice. Nevertheless, adapting the loading for the lower bone mass in the PolgA would decrease the stimulus, and therefore, we do not expect a positive response at this even lower stimulus. Nonetheless, further research is therefore needed to elucidate the mechanosensitivity of PolgA bones. Of further notice was that BV/TV and Tb. Th declined over time in the aged sham‐loaded WT mice, whereas loading was able to reduce this bone loss. As our in vivo micro‐CT monitoring of the caudal vertebrae did not show any bone loss up to 40 weeks of age (Figures 6 and 7), we suspect that the bone loss occurring in the loaded WT mice was due to the surgical insertion of the pins required for loading. Interestingly, this decline in trabecular bone was not observed in the PolgA mice, thus explaining the high BV/TV and Tb. Th in the sham‐loaded PolgA mice at endpoint. We have previously observed similar bone loss in 52‐week‐old mice with loading being able to reduce this bone loss. 27 Similar to our current study though, the 15‐week‐old and 82‐week‐old mice did not show any bone loss. It is possible that the prematurely aged PolgA mice behave similarly to the 82‐week‐old mice of that study. Nevertheless, the fact that PolgA bones lose mechano‐responsiveness to loading with age while PolgA muscles show increased acute response to mechanical stimuli is intriguing. Along the same lines were the contrasting results in terms of basal mTORC1 and MAPK signalling, where basal downstream mTORC1 and MAPK signalling was almost absent in PolgA mutated bone, while it was hyperactive in PolgA muscle. Tissue‐specific differences in mTORC1 signalling have been shown previously. 80 Genetic reduction of mTOR expression had beneficial effects on neurological and muscle function (e.g. rotarod, grip strength) in aging mice, whereas declines in bone volume and immune function were accelerated. 80 Moreover, the pharmacological inhibition of mTORC1 signalling has been shown to impair osteogenic 81 , 82 and osteoclast 83 differentiation in vitro as well as induce trabecular bone loss in vivo, 84 while rapamycin treatment reduced muscle fibre loss in aging mice. 67 Hence, although treatments with mTOR inhibitors could be beneficial for long‐term muscle health, this might not be the case for bone health. Future studies are required to better understand discrepancies and potential interactions in mTORC1 signalling between muscle and bone and whether such differences can be linked to altered mechanosensitivity with age.
There are a number of limitations to our study that should be mentioned. First, the acute response to anabolic stimuli in the muscle tissue cannot be directly compared with the long‐term loading regime (over 4 weeks) to which the bone tissue was subjected. Physiological resistance training protocols leading to skeletal muscle hypertrophy in mice are difficult to achieve. The protocols, which are available such as voluntary resistance running 85 and high‐intensity interval running, 86 might be cumbersome due to a reduced exercise capacity in PolgA. 87 Nevertheless, both voluntary 87 and forced 37 long‐term endurance exercise regimes have been shown to improve the progeroid phenotype and locomotor behaviour in PolgA mice. Hence, future studies should investigate the potential of longer term resistance exercise training in alleviation of mTORC1 hyperactivity in (mouse models of) progeria. To be noted though is that the above‐mentioned exercise regimes were applied already at young (10–12 weeks) up to old age, thereby making it unknown whether they would have the same effect in older PolgA mice already displaying signs of aging. In this respect, future studies subjecting PolgA caudal vertebrae to cyclic loading regimes throuoghout life (i.e. from young until old age) would be interesting to assess whether the reduction in bone mechanosensitivity with age could be prevented.
A further limitation of our study was that the evaluation of the bone remodelling activities was mainly based on time‐lapsed micro‐CT analysis, whereas two‐dimensional (2D) histomorphometry using fluorescent labels would allow for the assessment of bone formation and mineralization at an even higher resolution. 88 However, owing to the lack of an appropriate marker, bone resorption can only be estimated from the number of osteoclasts. In this regard, the non‐destructive nature of time‐lapsed micro‐CT imaging provides a major advantage as both bone formation and bone resorption can be assessed longitudinally in individual mice, 25 , 39 thereby reducing the number of animals needed for experiments. Nevertheless, we and others have previously shown high correlations between dynamic histomorphometric parameters quantified by micro‐CT and conventional histomorphometry, respectively. 25 , 39 , 89 A further advantage of micro‐CT, however, is that bone histomorphometric parameters can be assessed in the entire 3D compartment rather than in a limited number of 2D histological sections. This does not only reduce labor‐intensive processing of the samples but also reduces interobserver and intraobserver errors associated with the analysis thereof. 90 , 91 The final determining advantage for using in vivo micro‐CT in this particular study though was the long‐term period of 20 weeks over which bone remodelling activities were assessed. Due to the resorption of fluorochrome labels, 2D histomorphometry requires very short intervals (2–3 days) between marker injections 92 and hence is not well suited for long‐term studies.
In conclusion, we show that PolgA mice develop multiple hallmarks of aging, such as reduced bone remodelling and muscle mass early in life, which collectively can be quantified using the mouse FI. We further demonstrate acute mTORC1 hyperactivity in PolgA muscle upon anabolic signals, which is related to diminished satellite cell proliferation. By mimicking many aspects of osteosarcopenia, the PolgA mouse provides a powerful model that facilitates our understanding of the relationship between muscles and bones and also between the aging musculoskeletal system and frailty.
Funding
This manuscript is based upon work supported by the Swiss National Science Foundation (SNF IZCNZ0‐174586), the European Cooperation in Science and Technology (COST Action BM1402: MouseAGE), and the European Research Council (ERC Advanced MechAGE ERC‐2016‐ADG‐741883).
Author contributions
A. C. S. and G. D. H. designed the experiments, collected the data, and wrote the manuscript. G. A. K. designed the experiments, collected the data, and reviewed the manuscript. M. E. collected the data and reviewed the manuscript. E. S. W. provided support establishing methods and reviewed the manuscript. R. M. and K. D. B. designed the experiments and wrote the manuscript.
Conflict of interests
None declared.
Supporting information
Table S1. Animal experiments: Overview, setup and analysis details.
Table S2. Reagents and antibodies used for immunoblotting.
Table S3. Cortical bone morphometry of femora.
Acknowledgements
We gratefully acknowledge Dr Ilaria Bellantuono for her guidance and inputs for the quantification of the clinical mouse FI. We acknowledge Susanne Friedrich for performing the force‐grip assessments. The authors certify that they comply with the ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. 95
Scheuren A. C., D'Hulst G., Kuhn G. A., Masschelein E., Wehrle E., De Bock K., and Müller R. (2020) Hallmarks of frailty and osteosarcopenia in prematurely aged PolgA(D257A/D257A) mice, Journal of Cachexia, Sarcopenia and Muscle, 11, 1121–1140. 10.1002/jcsm.12588
References
- 1. Rodríguez‐Mañas L, Gonzalez‐Colaço Harmand M, Carcaillon L, Scuteri A, Sinclair A, Pelaez M, et al. Searching for an operational definition of frailty: a delphi method based consensus statement. The Frailty Operative Definition‐Consensus Conference Project. J Gerontol A Biol Sci Med Sci 2013;68:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Whitson HE, Duan‐Porter W, Schmader KE, Morey MC, Cohen HJ, Colon‐Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A 2016;71:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McGuigan FE, Bartosch P, Åkesson KE. Musculoskeletal health and frailty. Best Pract Res Clin Rheumatol 2017;31:145–159. [DOI] [PubMed] [Google Scholar]
- 4. Milte R, Crotty M. Musculoskeletal health, frailty and functional decline. Best Pract Res Clin Rheumatol 2014;28:395–410. [DOI] [PubMed] [Google Scholar]
- 5. Frisoli A, Chaves PH, Ingham SJM, Fried LP. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community‐dwelling older women: results from the Women's Health and Aging Study (WHAS) II. Bone 2011;48:952–957. [DOI] [PubMed] [Google Scholar]
- 6. Hassan E, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician 2017;46:849–853. [PubMed] [Google Scholar]
- 7. Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int 2017;28:2781–2790. [DOI] [PubMed] [Google Scholar]
- 8. Yu R, Leung J, Woo J. Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) Study. J Am Med Dir Assoc 2014;15:551–558. [DOI] [PubMed] [Google Scholar]
- 9. Huo YR, Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Gunawardene P, et al. Comprehensive nutritional status in sarco‐osteoporotic older fallers. J Nutr Health Aging 2015;19:474–480. [DOI] [PubMed] [Google Scholar]
- 10. van Dijk M, Nagel J, Dijk FJ, Salles J, Verlaan S, Walrand S, et al. Sarcopenia in older mice is characterized by a decreased anabolic response to a protein meal. Arch Gerontol Geriatr 2017;69:134–143. [DOI] [PubMed] [Google Scholar]
- 11. Chalil S, Pierre N, Bakker AD, Manders RJ, Pletsers A, Francaux M, et al. Aging related ER stress is not responsible for anabolic resistance in mouse skeletal muscle. Biochem Biophys Res Commun 2015;468:702–707. [DOI] [PubMed] [Google Scholar]
- 12. Katsanos CS, Kobayashi H, Sheffield‐Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 2005;82:1065–1073. [DOI] [PubMed] [Google Scholar]
- 13. Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, et al. Aging impairs contraction‐induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 2011;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Francaux M, Demeulder B, Naslain D, Fortin R, Lutz O, Caty G, et al. Aging reduces the activation of the mTORC1 pathway after resistance exercise and protein intake in human skeletal muscle: potential role of REDD1 and impaired anabolic sensitivity. Nutrients 2016;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422–424. [DOI] [PubMed] [Google Scholar]
- 16. Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: interventions to counteract the 'anabolic resistance' of ageing. Nutr Metab (Lond) 2011;8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nederveen JP, Joanisse S, Snijders T, Ivankovic V, Baker SK, Phillips SM, et al. Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia Sarcopenia Muscle 2016;7:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HHCM, van Loon LJC. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 2007;292:E151–E157. [DOI] [PubMed] [Google Scholar]
- 19. Barberi L, Scicchitano BM, De Rossi M, Bigot A, Duguez S, Wielgosik A, et al. Age‐dependent alteration in muscle regeneration: the critical role of tissue niche. Biogerontology 2013;14:273–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parker MH. The altered fate of aging satellite cells is determined by signaling and epigenetic changes. Front Genet 2015;6:59–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 2013;93:23–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stattin K, Michaëlsson K, Larsson SC, Wolk A, Byberg L. Leisure‐time physical activity and risk of fracture: a cohort study of 66,940 men and women. J Bone Miner Res 2017;32:1599–1606. [DOI] [PubMed] [Google Scholar]
- 23. Lynch ME, Main RP, Xu Q, Schmicker TL, Schaffler MB, Wright TM, et al. Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging. Bone 2011;49:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Willie BM, Birkhold AI, Razi H, Thiele T, Aido M, Kruck B, et al. Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female C57Bl/6 mice coincides with a reduction in deformation to load. Bone 2013;55:335–346. [DOI] [PubMed] [Google Scholar]
- 25. Birkhold AI, Razi H, Duda GN, Weinkamer R, Checa S, Willie BM. Mineralizing surface is the main target of mechanical stimulation independent of age: 3D dynamic in vivo morphometry. Bone 2014;66:15–25. [DOI] [PubMed] [Google Scholar]
- 26. Holguin N, Brodt MD, Sanchez ME, Silva MJ. Aging diminishes lamellar and woven bone formation induced by tibial compression in adult C57BL/6. Bone 2014;65:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lambers FM, Kuhn G, Weigt C, Koch KM, Schulte FA, Müller R. Bone adaptation to cyclic loading in murine caudal vertebrae is maintained with age and directly correlated to the local micromechanical environment. J Biomech 2015;48:1179–1187. [DOI] [PubMed] [Google Scholar]
- 28. Jones DM, Song XW, Rockwood K. Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc 2004;52:1929–1933. [DOI] [PubMed] [Google Scholar]
- 29. Whitehead JC, Hildebrand BA, Sun M, Rockwood MR, Rose RA, Rockwood K, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci 2014;69:621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rockwood K, Blodgett JM, Theou O, Sun MH, Feridooni HA, Mitnitski A, et al. A frailty index based on deficit accumulation quantifies mortality risk in humans and in mice. Sci Rep 2017;7:43068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Banga S, Heinze‐Milne SD, Howlett SE. Rodent models of frailty and their application in preclinical research. Mech Ageing Dev 2019;179:1–10. [DOI] [PubMed] [Google Scholar]
- 32. Trendelenburg AU, Scheuren AC, Potter P, Müller R, Bellantuono I. Geroprotectors: a role in the treatment of frailty. Mech Ageing Dev 2019;180:11–20. [DOI] [PubMed] [Google Scholar]
- 33. Bellantuono RDI, Ehninger D, Fernandes A, Howlett SE, Müller R, Potter P, et al. Find drugs that delay many diseases of old age. Nature 2018;554:293–295. [Google Scholar]
- 34. Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005;309:481–484. [DOI] [PubMed] [Google Scholar]
- 35. Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004;429:417–423. [DOI] [PubMed] [Google Scholar]
- 36. Kolesar JE, Safdar A, Abadi A, MacNeil LG, Crane JD, Tarnopolsky MA, et al. Defects in mitochondrial DNA replication and oxidative damage in muscle of mtDNA mutator mice. Free Radic Biol Med 2014;75:241–251. [DOI] [PubMed] [Google Scholar]
- 37. Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, et al. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. P Natl Acad Sci USA 2011;108:4135–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro‐computed tomography. J Bone Miner Res 2010;25:1468–1486. [DOI] [PubMed] [Google Scholar]
- 39. Schulte FA, Lambers FM, Kuhn G, Müller R. In vivo micro‐computed tomography allows direct three‐dimensional quantification of both bone formation and bone resorption parameters using time‐lapsed imaging. Bone 2011;48:433–442. [DOI] [PubMed] [Google Scholar]
- 40. Dai Y, Kiselak T, Clark J, Clore E, Zheng K, Cheng A, et al. Behavioral and metabolic characterization of heterozygous and homozygous POLG mutator mice. Mitochondrion 2013;13:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bellantuono I, de Cabo R, Ehninger D, Di Germanio C, Lawrie A, Miller J, et al. A toolbox for the longitudinal assessment of healthspan in aging mice. Nat Protoc 2020;15:540–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yorke A, Kane AE, Friesen CLH, Howlett SE, O'Blenes S. Development of a rat clinical frailty index. J Gerontol A Biol Sci Med Sci 2017;72: 897−+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mitnitski A, Rockwood K. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci 2007;62:722–727. [DOI] [PubMed] [Google Scholar]
- 44. O'Neil TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3‐kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol‐London 2009;587:3691–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tseng H‐C, Lee C‐Y, Weng W‐L, Shiah IM. Solubilities of amino acids in water at various pH values under 298.15K. Fluid Phase Equilibria 2009;285:90–95. [Google Scholar]
- 46. D'Hulst G, Jamart C, Van Thienen R, Hespel P, Francaux M, Deldicque L. Effect of acute environmental hypoxia on protein metabolism in human skeletal muscle. Acta Physiol (Oxf) 2013;208:251–264. [DOI] [PubMed] [Google Scholar]
- 47. Rüegsegger P, Koller B, Müller R. A microtomographic system for the nondestructive evaluation of bone architecture. Calcif Tissue Int 1996;58:24–29. [DOI] [PubMed] [Google Scholar]
- 48. Kohler T, Stauber M, Donahue LR, Muller R. Automated compartmental analysis for high‐throughput skeletal phenotyping in femora of genetic mouse models. Bone 2007;41:659–667. [DOI] [PubMed] [Google Scholar]
- 49. Lambers FM, Schulte FA, Kuhn G, Webster DJ, Müller R. Mouse tail vertebrae adapt to cyclic mechanical loading by increasing bone formation rate and decreasing bone resorption rate as shown by time‐lapsed in vivo imaging of dynamic bone morphometry. Bone 2011;49:1340–1350. [DOI] [PubMed] [Google Scholar]
- 50. Webster DJ, Morley PL, van Lenthe GH, Muller R. A novel in vivo mouse model for mechanically stimulated bone adaptation—a combined experimental and computational validation study. Comput Methods Biomech Biomed Engin 2008;11:435–441. [DOI] [PubMed] [Google Scholar]
- 51. Jacobs BL, You JS, Frey JW, Goodman CA, Gundermann DM, Hornberger TA. Eccentric contractions increase the phosphorylation of tuberous sclerosis complex‐2 (TSC2) and alter the targeting of TSC2 and the mechanistic target of rapamycin to the lysosome. J Physiol 2013;591:4611–4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ogasawara R, Jensen TE, Goodman CA, Hornberger TA. Resistance exercise‐induced hypertrophy: a potential role for rapamycin‐insensitive mTOR. Exerc Sport Sci Rev 2019;47:188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rahnert JA, Burkholder TJ. High‐frequency electrical stimulation reveals a p38‐mTOR signaling module correlated with force‐time integral. J Exp Biol 2013;216:2619–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kane AE, Shin S, Wong AA, Fertan E, Faustova NS, Howlett SE, et al. Sex differences in healthspan predict lifespan in the 3xTg‐AD mouse model of Alzheimer's disease. Front Aging Neurosci 2018;10:172. 10.3389/fnagi.2018.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Parks RJ, Fares E, MacDonald JK, Ernst MC, Sinal CJ, Rockwood K, et al. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci 2012;67:217–227. [DOI] [PubMed] [Google Scholar]
- 56. Kane AE, Hilmer SN, Boyer D, Gavin K, Nines D, Howlett SE, et al. Impact of longevity interventions on a validated mouse clinical frailty index. J Gerontol A Biol Sci Med Sci 2015;71:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012;149:274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yoon M‐S. mTOR as a key regulator in maintaining skeletal muscle mass. Front Physiol 2017;8:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 2013;280:4294–4314. [DOI] [PubMed] [Google Scholar]
- 60. Hornberger TA, Sukhija KB, Chien S. Regulation of mTOR by mechanically induced signaling events in skeletal muscle. Cell Cycle 2006;5:1391–1396. [DOI] [PubMed] [Google Scholar]
- 61. Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen‐activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol 2003;547:977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Drummond MJ, Rasmussen BB. Leucine‐enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr Opin Clin Nutr 2008;11:222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Takayama K, Kawakami Y, Lavasani M, Mu X, Cummins JH, Yurube T, et al. mTOR signaling plays a critical role in the defects observed in muscle‐derived stem/progenitor cells isolated from a murine model of accelerated aging. J Orthop Res 2017;35:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Joseph GA, Wang SX, Jacobs CE, Zhou W, Kimble GC, Tse HW, et al. Partial inhibition of mTORC1 in aged rats counteracts the decline in muscle mass and reverses molecular signaling associated with sarcopenia. Mol Cell Biol 2019;39:e00141–e00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ramos FJ, Chen SC, Garelick MG, Dai D‐F, Liao C‐Y, Schreiber KH, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C‐deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med 2012;4: 144ra103‐144ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kawakami Y, Hambright WS, Takayama K, Mu X, Lu A, Cummins JH, et al. Rapamycin rescues age‐related changes in muscle‐derived stem/progenitor cells from progeroid mice. Mol Ther Methods Clin Dev 2019;14:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tang H, Inoki K, Brooks SV, Okazawa H, Lee M, Wang J, et al. mTORC1 underlies age‐related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell 2019;0:e12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gasser JA. Assessing bone quantity by pQCT. Bone 1995;17:S145–S154. [DOI] [PubMed] [Google Scholar]
- 69. Rosen HN, Tollin S, Balena R, Middlebrooks VL, Beamer WG, Donohue LR, et al. Differentiating between orchiectomized rats and controls using measurements of trabecular bone density: a comparison among DXA, Histomorphometry, and peripheral quantitative computerized tomography. Calcif Tissue Int 1995;57:35–39. [DOI] [PubMed] [Google Scholar]
- 70. Silva MJ, Brodt MD, Lynch MA, Stephens AL, Wood DJ, Civitelli R. Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle‐aged mice. PLoS ONE 2012;7:e34980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age‐related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res 2007;22:1197–1207. [DOI] [PubMed] [Google Scholar]
- 72. Willinghamm MD, Brodt MD, Lee KL, Stephens AL, Ye J, Silva MJ. Age‐related changes in bone structure and strength in female and male BALB/c mice. Calcif Tissue Int 2010;86:470–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lambers F. Functional bone imaging in an in vivo mouse model of bone adaptation, aging and disease. 2011.
- 74. Buie HR, Moore CP, Boyd SK. Postpubertal architectural developmental patterns differ between the L3 vertebra and proximal tibia in three inbred strains of mice. J Bone Miner Res 2008;23:2048–2059. [DOI] [PubMed] [Google Scholar]
- 75. Klinck RJ, Campbell GM, Boyd SK. Radiation effects on bone architecture in mice and rats resulting from in vivo micro‐computed tomography scanning. Med Eng Phys 2008;30:888–895. [DOI] [PubMed] [Google Scholar]
- 76. Laperre K, Depypere M, van Gastel N, Torrekens S, Moermans K, Bogaerts R, et al. Development of micro‐CT protocols for in vivo follow‐up of mouse bone architecture without major radiation side effects. Bone 2011;49:613–622. [DOI] [PubMed] [Google Scholar]
- 77. Hildebrandt IJ, Su H, Weber WA. Anesthesia and other considerations for in vivo imaging of small animals. ILAR J 2008;49:17–26. [DOI] [PubMed] [Google Scholar]
- 78. Hohlbaum K, Bert B, Dietze S, Palme R, Fink H, Thöne‐Reineke C. Severity classification of repeated isoflurane anesthesia in C57BL/6JRj mice—assessing the degree of distress. PLoS ONE 2017;12:e0179588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tremoleda JL, Khalil M, Gompels LL, Wylezinska‐Arridge M, Vincent T, Gsell W. Imaging technologies for preclinical models of bone and joint disorders. EJNMMI Res 2011;1:11–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wu JJ, Liu J, Chen Edmund B, Wang Jennifer J, Cao L, Narayan N, et al. Increased mammalian lifespan and a segmental and tissue‐specific slowing of aging after genetic reduction of mTOR expression. Cell Rep 2013;4:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Singha UK, Jiang Y, Yu S, Luo M, Lu Y, Zhang J, et al. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3‐E1 cells and primary mouse bone marrow stromal cells. J Cell Biochem 2008;103:434–446. [DOI] [PubMed] [Google Scholar]
- 82. Chen J, Tu X, Esen E, Joeng KS, Lin C, Arbeit JM, et al. WNT7B promotes bone formation in part through mTORC1. PLoS Genet 2014;10:e1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dai Q, Xie F, Han Y, Ma X, Zhou S, Jiang L, et al. Inactivation of regulatory‐associated protein of mTOR (raptor)/mammalian target of rapamycin complex 1 (mTORC1) signaling in osteoclasts increases bone mass by inhibiting osteoclast differentiation in mice. J Biol Chem 2017;292:196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, et al. Matrix IGF‐1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med 2012;18:1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. D'Hulst G, Palmer AS, Masschelein E, Bar‐Nur O, De Bock K. Voluntary resistance running as a model to induce mTOR activation in mouse skeletal muscle. Front Physiol 2019;10:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Goh Q, Song T, Petrany MJ, Cramer AA, Sun C, Sadayappan S, et al. Myonuclear accretion is a determinant of exercise‐induced remodeling in skeletal muscle. Elife 2019;8:e44876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ross JM, Coppotelli G, Branca RM, Kim KM, Lehtio J, Sinclair DA, et al. Voluntary exercise normalizes the proteomic landscape in muscle and brain and improves the phenotype of progeroid mice. Aging Cell 2019;18:e13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 1987;2:595–610. [DOI] [PubMed] [Google Scholar]
- 89. Oz U, Ruellas AC, Westgate PM, Cevidanes LH, Huja SS. Novel application and validation of in vivo micro‐CT to study bone modelling in 3D. Orthod Craniofac Res 2019;22:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Compston JE, Vedi S, Stellon AJ. Interobserver variation in bone histomorphometry. Bone 1986;7:161–161. [DOI] [PubMed] [Google Scholar]
- 91. Wright CDP, Vedi S, Garrahan NJ, Stanton M, Duffy SW, Compston JE. Combined interobserver and intermethod variation in bone histomorphometry. Bone 1992;13:205–208. [DOI] [PubMed] [Google Scholar]
- 92. Erben RG, Glosmann M. Histomorphometry in rodents. Methods Mol Biol 1914;2019:411–435. [DOI] [PubMed] [Google Scholar]
- 93. Gorski T, Mathes S, Krutzfeldt J. Uncoupling protein 1 expression in adipocytes derived from skeletal muscle fibro/adipogenic progenitors is under genetic and hormonal control. J Cachexia Sarcopenia Muscle 2018;9:384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mayeuf‐Louchart A, Hardy D, Thorel Q, Roux P, Gueniot L, Briand D, et al. MuscleJ: a high‐content analysis method to study skeletal muscle with a new Fiji tool. Skelet Muscle 2018;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Animal experiments: Overview, setup and analysis details.
Table S2. Reagents and antibodies used for immunoblotting.
Table S3. Cortical bone morphometry of femora.


