Abstract
Background
A classic consequence of short‐term bed rest in older adults is the significant loss in skeletal muscle mass and muscle strength that underlies the accelerated physical performance deficits. Structured exercise programmes applied during acute hospitalization can prevent muscle function deterioration.
Methods
A single‐blind randomized clinical trial conducted in an acute care for elders unit in a tertiary public hospital in Navarre (Spain). Three hundred seventy hospitalized patients [56.5% female patients; mean age (standard deviation) 87.3 (4.9) years] were randomly allocated to an exercise intervention (n = 185) or a control (n = 185) group (usual care). The intervention consisted of a multicomponent exercise training programme performed during 5–7 consecutive days (2 sessions/day). The usual‐care group received habitual hospital care, which included physical rehabilitation when needed. The main endpoints were change in maximal dynamic strength (i.e. leg‐press, chest‐press, and knee extension exercises) and maximal isometric knee extensors and hip flexors strength from baseline to discharge. Changes in muscle power output at submaximal and maximal loads were also measured after the intervention.
Results
The physical exercise programme provided significant benefits over usual care. At discharge, the exercise group showed a mean increase of 19.6 kg [95% confidence interval (CI), 16.0, 23.2; P < 0.001] on the one‐repetition maximum (1RM) in the leg‐press exercise, 5.7 kg (95% CI, 4.7, 6.8; P < 0.001) on the 1RM in the chest‐press exercise, and 9.4 kg (95% CI, 7.3, 11.5; P < 0.001) on the 1RM in the knee extension exercise over usual‐care group. There were improvements in the intervention group also in the isometric maximal knee extension strength [14.8 Newtons (N); 95% CI, 11.2, 18.5 vs. −7.8 N; 95% CI, −11.0, −3.5 in the control group; P < 0.001] and the hip flexion strength (13.6 N; 95% CI, 10.7, 16.5 vs. −7.2 N; 95% CI, −10.1, −4.3; P < 0.001). Significant benefits were also observed in the exercise group for the muscle power output at submaximal loads (i.e. 30% 1RM, 45% 1RM, 60% 1RM, and 75% 1RM; all P < 0.001) over usual‐care group.
Conclusions
An individualized, multicomponent exercise training programme, with special emphasis on muscle power training, proved to be an effective therapy for improving muscle power output of lower limbs at submaximal loads and maximal muscle strength in older patients during acute hospitalization.
Keywords: Sarcopenia, Physical exercise, Hospitalized, Elderly
Introduction
Adequate hospital care for older adults with acute medical disorders is an important clinical concern in our ageing societies.1, 2, 3 In this regard, acute medical illnesses and subsequent hospitalization are major events in older people, leading to functional decline and frequently, long‐term disability.4, 5, 6 Loss of functional capacity associated with hospitalization increases the risk for higher resource use, caregiver burden, institutionalization, and death.7, 8, 9, 10 For these reasons, health care professionals and policy makers should prioritize the implementation of care procedures during hospitalization in older adults.
Complications and physical deterioration due to physical inactivity occur regardless of age. Low mobility during hospitalization is associated with a decline in activities of daily living and consequently, a rise in the rate of institutionalization and mortality.11 A classic consequence of short‐term bed rest in older adults is the significant loss in skeletal muscle mass that underlies the accelerated physical performance deficits.12 Previous studies have shown a rapid decline of >10% of total lean leg mass in healthy older adults after 7 to 10 days of in‐hospital inactivity13, 14, and lower muscle mass has been associated with a lower likelihood of survival after hospitalization in older patients.15 In addition, the ageing process causes increased protein degradation and lower protein synthesis16 and many neuromuscular changes17, 18, making older adults even more vulnerable to a negative impact of hospitalization on muscle strength and muscle mass.19
In this context, structured exercise and early rehabilitation programmes applied during acute hospitalization can prevent muscle function deterioration, abbreviate the periods of exacerbation of acute illness, and reduce the impact of subsequent health crises in hospitalized older adults.20, 21 Moreover, emerging evidence highlights that high‐velocity and low‐load resistance training (i.e. muscle power training) can improve muscle strength to a greater extent than traditional slow‐velocity resistance training.22, 23 A recent meta‐analysis of exercise training in older adults also found it was not associated with an increased risk of dropout because of health problems.24 However, studies focused on exercise interventions in hospitalized older adults are scarce. To the best of our knowledge, the effects of a multicomponent programme including muscle power training, balance, and gait‐retraining exercises on maximal muscle strength and muscle power characteristics in dynamic and isometric actions of lower and upper limbs have not been previously investigated in acutely hospitalized older adults.
The present study is in line with the long trajectory of research that has explored new possibilities to avoid dangers of prolonged bed rest.25 Thus, the main purpose of our study was to assess the effects of a multicomponent exercise training intervention on dynamic and isometric maximal muscle strength of lower and upper extremities and muscle power output in an acute care of the elderly (ACE) unit. We hypothesized that the aforementioned intervention would improve patients' muscle function during hospitalization over usual care.
Methods
Design
The study is a secondary analysis of a randomized controlled trial (RCT; NCT02300896)21, 26 conducted in the ACE unit of the Department of Geriatrics in a tertiary public hospital (Complejo Hospitalario de Navarra, Spain). This department has 35 allocated beds with a staff of eight geriatricians (distributed in the ACE unit, orthogeriatrics and outpatient consultations). Admissions to the ACE unit derive mainly from the Accident and Emergency Department, with heart failure, pulmonary, and infectious diseases being the main causes of admissions.
Acutely hospitalized patients who met the inclusion criteria were randomly assigned to the intervention or control (usual care) group within the first 48 h of admission. Usual care is offered to patients by the geriatricians of our department and consists of standard physiotherapy focused on walking exercises for restoring the functionality conditioned by potentially reversible pathologies. A formal exercise prescription was not provided at study entry, and patients were instructed to continue with the current activity practices along the duration of the study. The study followed the principles of the Declaration of Helsinki and was approved by the Complejo Hospitalario de Navarra Research Ethics Committee (Pyto 23/2014). All patients or their legal representatives provided written consent.
Participants and randomization
All of the patients admitted to the ACE unit were evaluated by geriatricians. We focused on a particularly vulnerable population but at the same time with a level of functional and cognitive capacity high enough to allow them to perform the physical exercise protocol. A trained research assistant conducted a screening interview to determine whether potentially eligible patients met the following inclusion criteria: age ≥ 75 years, Barthel index score ≥ 60 points, and able to ambulate (with/without assistance) and to communicate and collaborate with the research team. Exclusion criteria included expected length of stay < 6 days, very severe cognitive decline (i.e. Global Deterioration Scale score = 7), terminal illness, uncontrolled arrhythmias, acute pulmonary embolism and myocardial infarction, or extremity bone fracture in the past 3 months.
After the baseline assessment was performed, participants were randomly assigned following a 1:1 ratio, without restrictions. The simple randomization sequence was generated by a statistician not involve in the RCT using an online tool (www.randomizer.org) to allocate 185 patients in the exercise group (intervention group) and 185 patients in the usual‐care group (control group). In the randomization procedure used, the allocation probabilities were biassed during the process by the computer programme in order to try to maintain balance between treatment allocations. Assessment staff were blinded to the main study design and group allocation. It was not possible to blind the participants, and so they were explicitly informed and reminded not to discuss their randomization assignment with the assessment staff.
Intervention
The usual‐care group received habitual hospital care, which included physical rehabilitation when needed. For the intervention group, exercise training was programmed in two daily sessions (morning and evening) of 20 min duration during 5–7 consecutive days (including weekends) supervised by a qualified fitness specialist. Adherence to the exercise intervention programme was recorded in a daily register. A session was considered completed when ≥90% of the programmed exercises were successfully undertaken.
Each session was performed in a room equipped ad hoc in the ACE unit. Exercises were adapted from the ‘Vivifrail’ multicomponent physical exercise programme to prevent weakness and falls.27 The morning sessions included individualized progressive resistance, balance, and walking training exercises and were supervised by a physiotherapist (M.L.S.de.A) or a researcher (F.Z.F) with a PhD background in exercise physiology. The resistance exercises were tailored to the individual's functional capacity using variable resistance training machines (Matrix, Johnson Health Tech, Ibérica, S.L.; Torrejón de Ardoz, Spain and Exercycle S.L., BHGroup; Vitoria, Spain) aiming at two to three sets of 8 to 10 repetitions with a load equivalent to 30–60% of the one‐repetition maximum (1RM). Participants performed three exercises involving mainly lower limb muscles (squats rising from a chair, leg press, and bilateral knee extension) and one involving the upper body musculature (seated bench ‘chest’ press). They were instructed to perform the exercises at a high speed to optimize muscle power output, and care was taken to ensure proper exercise execution. Balance and gait‐retraining exercises gradually progressed in difficulty and included the following: semi‐tandem foot standing, line walking, stepping practice, walking with small obstacles, proprioceptive exercises on unstable surfaces (foam pads sequence), altering the base of support, and weight transfer from one leg to the other. The evening session consisted of functional unsupervised exercises using light loads (0.5–1.0 kg anklets and hand‐grip ball), such as knee extension/flexion, hip abduction, and daily walking in the corridor of the ACE unit with a duration based on the clinical physical exercise guide ‘Vivifrail’.27
Endpoints
As soon as the clinician in charge of the patient considered that their hemodynamic situation was acceptable, and the patient could collaborate, the following endpoints were assessed and the intervention was started. Endpoints were also measured on the day of discharge.
The endpoints were the change in dynamic and isometric maximal muscle strength and muscle power output during hospitalization (i.e. from admission to discharge).
Dynamic maximal muscle strength
Maximal dynamic strength was measured based on the results of a 1RM reached in bilateral leg‐press exercise, bench chest‐press, and knee extension exercises (Exercycle S.L., BHGroup, Vitoria, Spain). Four to five separate attempts were performed until the patient was not able to complete the concentric phase of the exercise. The last acceptable complete extension with the highest possible load was determined as the 1RM. The participants were instructed to perform each repetition as fast as possible during the 1RM assessment.
Isometric maximal muscle strength
Maximal isometric lower limb (right knee extensors and hip flexors) muscle strength was also measured using a manual dynamometer (MicroFET3, Hoogan Scientific, Salt Lake City, UT). Two maximal attempts were recorded, and the maximum reading was used for further analysis.
Muscle power output
The peak of power during the concentric actions was measured with the loads of 30, 45, 60, 75% of the 1RM, and 1RM in the leg‐press exercise. The muscle power output in the propulsive phase was recorded by connecting a velocity transducer to the weight plates (T‐Force System, Ergotech, Murcia, Spain).
Adverse events
Data related to length of hospital stay, falls during hospitalization, transfer after discharge, readmission rate, and mortality were also collected. Details of these endpoints have been published elsewhere. 21
Statistical analysis
All analyses were performed by the ‘intention‐to‐treat’ approach. After analysing missing data patients in both groups and comparing with the non‐missing data patients, a missing at random mechanism was assumed. Normality of data was checked graphically and through the Kolmogorov–Smirnov test. Between‐group comparisons of continuous variables were conducted using linear mixed models. Time was treated as a categorical variable. The models included group, time, and group by time interaction as fixed effects and participants as a random effect. For each group, data are expressed as change from baseline (admission) to discharge, determined by the time coefficients [95% confidence interval (CI)] of the model. The primary conclusions about effectiveness of exercise intervention were based on between‐group comparisons of change in dynamic maximal muscle strength from baseline (beginning of the intervention) to hospital discharge, as assessed with the leg‐press, bench chest‐press, and knee extension exercises. Comparisons between groups of secondary endpoints were also performed using the same statistical method. The effect size was calculated according to Cohen d, classified as small (0.20), medium (0.50), or large (>0.80) effect.28 All comparisons were two sided, with a significance level of 0.05. Statistical analysis was carried out using IBM‐SPSS v25 software (SPSS Inc., Chicago, IL).
Results
The study flow diagram is shown in Figure 1. No significant differences were found between groups at baseline for demographic and clinical characteristics for study endpoints (Table 1). Of the 370 patients included in the analyses, 209 were women (56.5%); mean age (standard deviation) was 87.3 (4.9) years (range 75–101 years); and 130 patients (35.1%) were nonagenarians. The median length of hospital stay was 8 days in both groups (interquartile range, 4 and 4 days, respectively). The mean number of intervention days for each patient was 5.3 ± 0.5 days, with most training days being consecutive (97%). The number of completed morning and evening sessions per patient averaged 5 ± 1 and 4 ± 1, respectively. Mean adherence to the intervention was 97% (95% CI, 95.7, 98.3) for the morning sessions (i.e. 806 successfully completed sessions of 841 total possible sessions) and 85% (95% CI, 79.7, 89.4) in the evening sessions (574 of 688); 73.9% of the participants performed leg‐press, 77.4% chest‐press, and 33.2% knee extension dynamic muscle strength measurements at both hospital admission and discharge. Considering the isometric maximal strength assessment, 78.1% and 93.9% of the patients completed knee extension and hip flexion endpoints, respectively. No adverse effects or falls associated with the prescribed exercises were recorded, and no patient had to interrupt the intervention or had their hospital stay modified because of it.
Figure 1.
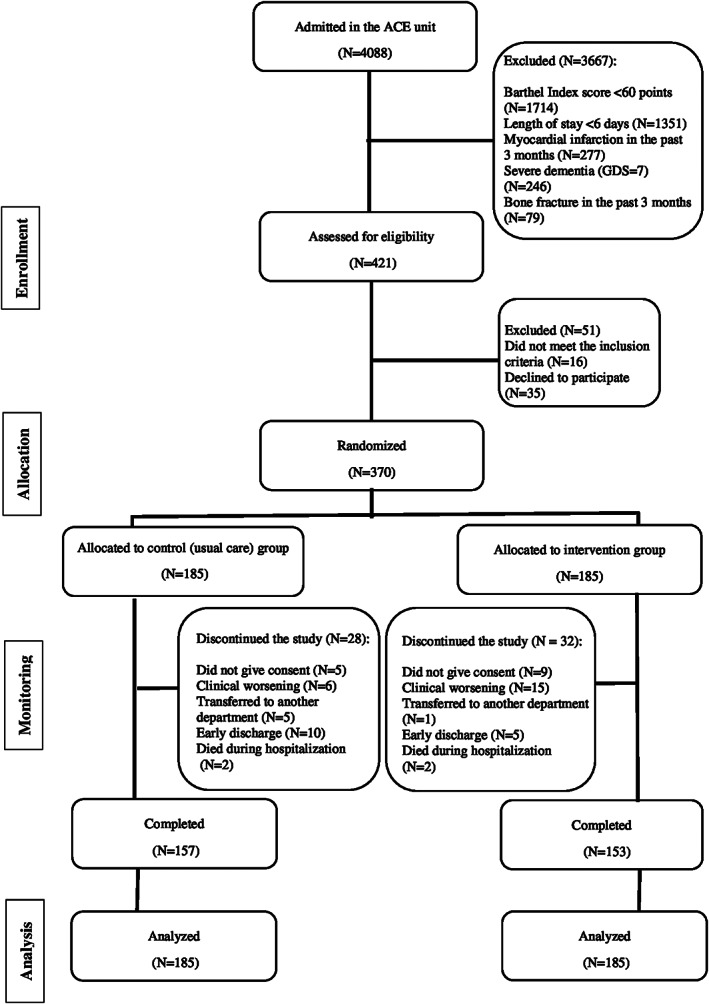
Study flow diagram.
Table 1.
Baseline characteristics of the participants
| Variable | Control group (n = 185) | Intervention group (n = 185) |
|---|---|---|
| Demographic data | ||
| Age (years) | 87.1 (5.2) | 87.6 (4.6) |
| Women (N, %) | 109 (59%) | 100 (54%) |
| Body mass index (kg/m2) | 26.9 (4.9) | 27.1 (4.4) |
| Clinical data | ||
| CIRS score (median, IQR) | 12 (5) | 13 (5) |
| MNA score (median, IQR) | 24 (4) | 24 (4) |
| MMSE (score) | 23 (4) | 22 (5) |
| Barthel index (score) | 83 (17) | 84 (17) |
| SPPB (points) | 4.7 (2.7) | 4.4 (2.5) |
| GVT (m/s) | 0.5 (0.2) | 0.5 (0.2) |
| Delirium (CAM, %) | 12% | 17% |
| Endpoint measures | ||
| Dynamic maximal muscle strength | ||
| 1RM leg press (kg) | 62 (31) | 57 (25) |
| 1RM chest press (kg) | 25 (12) | 24 (11) |
| 1RM knee extension (kg) | 41 (14) | 39 (13) |
| Isometric maximal muscle strength | ||
| 1RM knee extension (N) | 98 (37) | 97 (29) |
| 1RM hip flexion (N) | 90 (33) | 91 (27) |
| Peak of power at submaximal loads | ||
| 30% RM (W) | 59 (58) | 57 (41) |
| 45% RM (W) | 101 (79) | 81 (55) |
| 60% RM (W) | 102 (51) | 95 (56) |
| 75% RM (W) | 114 (55) | 107 (60) |
| Admission reason, N (%) | ||
| Cardiovascular | 67 (36) | 65 (35) |
| Infectious | 33 (18) | 33 (18) |
| Pulmonary | 20 (11) | 28 (15) |
| Gastrointestinal | 17 (9) | 20 (11) |
| Neurological | 9 (5) | 9 (5) |
| Other | 39 (21) | 30 (16) |
Data are mean (standard deviation) unless otherwise stated. No statistically significant differences were found between groups (all P > 0.05).
CAM, Confussion Assessment Method; CIRS, Cumulative Illness Rating Scale; EQ‐VAS, visual analogue scale of the EuroQol questionnaire (EQ‐5D); GDS, Yesavage Geriatric Depression Scale; GVT, Gait Velocity Test; IQR, interquartile range; MNA, Mini‐Nutritional Assessment; MMSE, Mini‐Mental State Examination; QoL, quality of life; SPPB, short physical performance battery; 1RM, one‐repetition maximum.
The primary analyses showed that the multicomponent programme seems to provide a significant benefit over the hospital usual care. Differences between the treatment groups revealed a significant intervention effect for all the endpoints assessed, except for the peak of power at 1RM in the leg‐press exercise (Figure 2). At discharge, the intervention group showed an increase of 19.6 kg (95% CI, 16.0, 23.2 kg; P < 0.001) on the 1RM in leg‐press exercise and 9.4 kg (95% CI, 7.3, 11.5 kg; P < 0.001) on the 1RM in the knee extension exercise over the usual‐care group. Furthermore, significant enhancements were observed in the physical exercise group at discharge on the maximal dynamic muscle strength in the bench chest‐press exercise of 4.0 kg (95% CI, 3.3, 4.7 kg) whereas no such trend was found in the control group (−1.8 kg; 95% CI, −2.6, −1.0 kg) (P < 0.001) (Figure 2). For the maximal isometric strength of lower limbs, the intervention group showed improvements at discharge in the knee extension of 22.1 N (95% CI, 16.9, 27.4 N; P < 0.001) and in the hip flexion of 20.8 N (95% CI, 16.7, 24.9 N; P < 0.001) (Supporting Information, Table S1).
Figure 2.
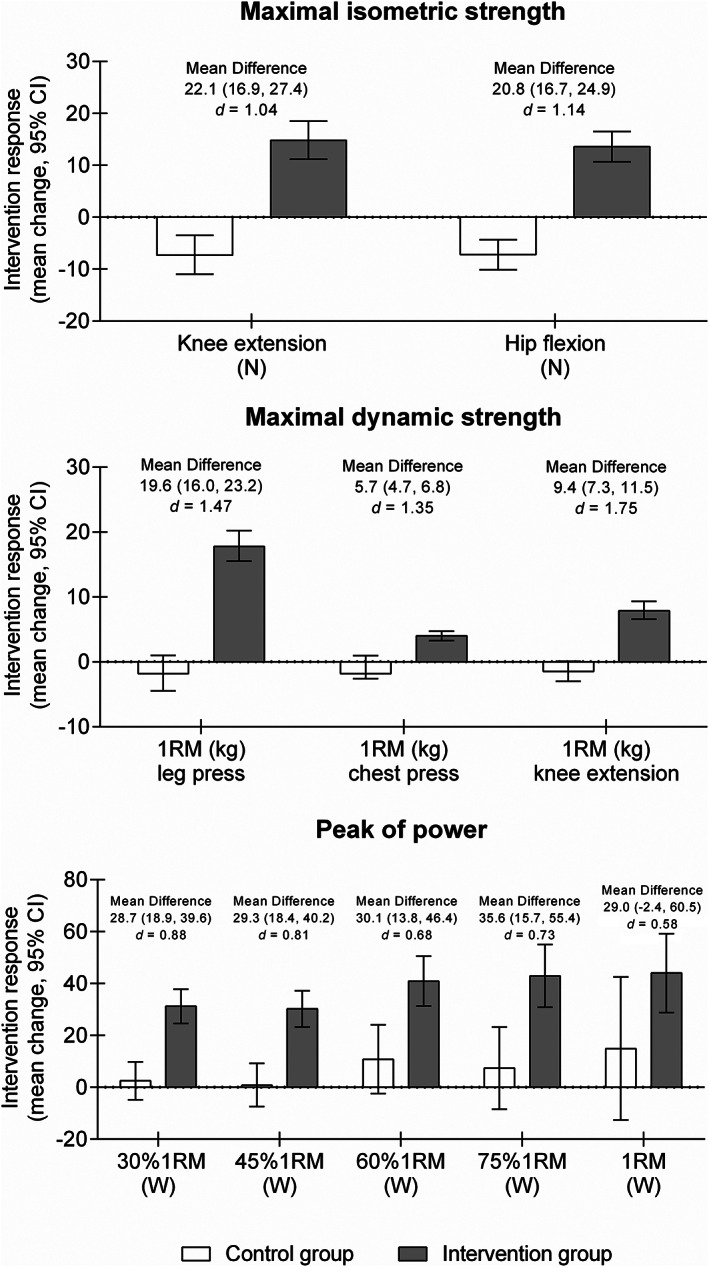
Changes in maximal muscle strength and muscle power for both groups. Mean difference corresponds to between‐group difference in each endpoint.
Considering the muscle power output, the physical exercise group showed enhancements in the peak of power at 30% 1RM of 28.7 W (95% CI, 18.9, 39.6 W; P < 0.001), at 45% 1RM of 29.3 W (95% CI, 18.4, 40.2 W; P < 0.001), at 60% 1RM of 30.1 W (95% CI, 13.8, 46.4 W; P < 0.001), and at 75% 1RM of 35.6 W (95% CI, 15.7, 55.4 W; P < 0.001) over the usual‐care group. No between‐group differences were observed in the peak of power at 1RM (P = 0.077) (Figure 2). The average power‐load curves of both groups in the concentric leg‐press actions are presented in Figure 3.
Figure 3.
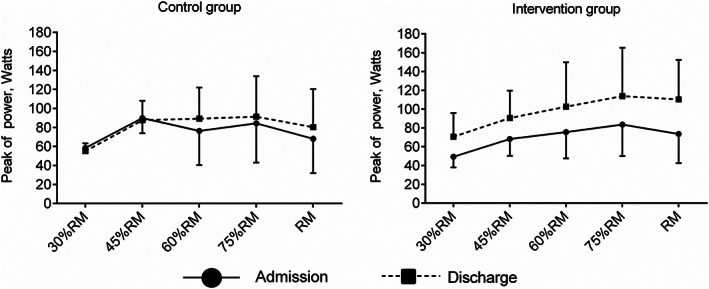
Muscle power values at submaximal and maximal loads by group.
Discussion
Low mobility during hospitalization leads to increased risk of morbidity, disability, and a decline in muscle function especially in older adults. Despite this, physical exercise interventions are rarely used in the rehabilitation or usual‐care programmes of hospitalized older medical patients. This study described enhancements obtained in the muscle power output of lower limbs in older adults admitted to an ACE unit after a median of 5 days of multicomponent exercise training. Additionally, improvements were also achieved in dynamic maximal strength measurements (i.e. leg‐press, chest‐press, and knee extension exercises) and isometric maximal strength outcomes (i.e. knee extension and hip flexion exercises) after the physical exercise programme compared with the control group. Therefore, our findings support that individualized physical exercise plays a crucial role in older medical patients during hospitalization.
Skeletal muscle is a complex tissue, important for locomotion, bone health, neuromuscular function, metabolism, as well as regulating the whole body's glucose homeostasis.29 Reduced lower limb muscle strength and loss of skeletal muscle mass (i.e. sarcopenia) have been associated with functional impairments and disability with ageing; attempts to counteract this process seem highly relevant.30 Our results show that an individualized physical exercise intervention, with special emphasis on progressive resistance training, seems to be an effective strategy to obtain improvements in maximal muscle strength of upper and lower limbs and to revert the loss of muscle strength often associated with hospitalization in older patients. This observation was consistent with other findings in which progressive resistance training has also been shown to be effective for increasing muscle strength, balance, and functional capacity in frail patients shortly after discharge due to acute medical illness31 or geriatric patients with multiple comorbidities.32
Muscle weakness and atrophy are probably the most functionally relevant and reversible parameters related to exercise in the older population.12 The exact mechanisms underlying the loss of muscle strength or power observed with ageing and development of muscle dysfunction is unknown; however, a decreased physical activity level, altered central and peripheral nervous system innervation, chronic low‐grade inflammation, infiltration of non‐contractile components within the muscle tissue, decline in protein synthesis, and anabolic hormones deficit have been identified as some of the contributing key factors.33
In this line, the significant enhancements obtained in lower limb muscle power after the exercise training programme over the usual‐care group have major implications for clinical practice: first, because skeletal muscle power decreases earlier and at a greater rate than muscle strength with advancing age23, 34, 35; second, because muscle power is a more discriminant predictor of physical functional performance in older adults23; and finally, because muscle power output plays a mediator role between functional endpoints in acutely hospitalized older adults.36 Recent evidence has suggested muscle power training as a cornerstone for managing functional status22 in older adults, and this type of exercise intervention has been demonstrated to be well tolerated, safe, and effective in this population.37, 38 Furthermore, improvements in muscle power are greater with resistance training interventions that emphasize high vs. low muscle contraction velocity.22 Accordingly, participants were encouraged to complete the concentric phase of each exercise as fast as possible during the exercise training programme, and consequently, significant gains were observed in the physical exercise group in all the muscle power measurements analysed at submaximal loads (i.e. 30, 45, 60, and 75% 1RM) during the 1RM leg‐press assessment compared with the control group. Moreover, the observation that the explosive muscle force capacity of the neuromuscular system remains trainable in older adults during hospitalization may have important implications for future early rehabilitation programmes, especially when considering the crucial role of muscle power output in maximal walking speed, postural balance, and other tasks of daily living. An advantage of this type of interventions (i.e. low‐load, high‐velocity muscle actions) is that the muscle force at which type II motor units are recruited (i.e. recruitment threshold) is markedly decreased in explosive‐type muscle actions,39 making it possible that type II motor units in such conditions may be recruited even using low to moderate loading intensities (i.e. 30–60% of 1RM). This is specially important because type II motor units are substantially lost due to its disuse across the ageing, which implies in severe loss in muscle power and mobility in older adults.17 Additionally, one of the characteristics of low‐load high‐velocity exercise programmes is that training is not performed close to the point of muscle failure, and thus, it may result in lower ratings of perceived exertion and reduced levels of delayed onset muscle soreness.
Our study has some limitations, including patients' difficulty to complete all the muscle strength and power measurements at admission and discharge. For example, only 23.8% of the patients in the control group and 31.9% of those in the intervention group were able to complete dynamic knee extension strength assessments, mainly due to the poor health condition presented at admission. Although patients were encouraged to complete the concentric phase of each repetition as fast as possible during the 1RM assessment, much of the muscle power data could not be recorded using the optical encoder because the contraction velocity was too slow to be captured by the measurement system. Notably, peak power data were recorded at 75% RM and 1RM in 58.9% and 31.0% of the older patients who completed the 1RM assessment, respectively. Additionally, the generalizability of our results is limited because of the inclusion of a selected population with relatively good functional capacity at preadmission (i.e. Barthel Index score ≥ 60 points), excluding those older adults with severe dementia, unstable hemodynamic condition, or who were unable to walk at admission, which increases the possibility of selection bias. Nevertheless, our RCT has many strengths. Thus far, research was carried out on a particularly vulnerable population of advanced age [overall mean 87.3 years; range 75–101 years, with 130 patients (35.1%) being nonagenarians] to develop a physical exercise intervention of a few days in acute settings. Also, patients with multiple comorbidities and mild dementia/cognitive impairment were included in the study, who are usually excluded from exercise studies. Regarding the multicomponent exercise training programme, a daily individualized adjustment of loads was performed specially in the power type resistance training protocol to optimize muscle performance adaptations and to avoid the muscle strength deterioration that is frequently associated with hospitalization in older adults.25 Physical exercise seems to be beneficial during hospitalization in this population21, but further research is needed to understand the mechanisms underlying the muscle strength improvements after such short intervention period (i.e. 5 ± 1 and 4 ± 1 morning and evening sessions) in acutely hospitalized older adults.40 Furthermore, the usefulness of innovative tools (i.e. inertial sensor units) for measuring muscle strength and peak of power during activities of daily living in clinical practice and the residual effect of exercise training on muscle performance at follow up (i.e. post‐discharge) should be explored in future trials.
Conclusions
An individualized, multicomponent exercise training programme, with special emphasis on progressive resistance training, proved to be an effective therapy for improving muscle power output of lower limbs at submaximal loads in very old patients during acute hospitalization. It was also shown to provide benefit in other skeletal muscle endpoints, such as dynamic and isometric maximal muscle strength of lower and upper extremities. These findings support the key role of physical exercise during hospitalization in older adults to minimize the hazards of prolonged bed rest, specifically muscle power and strength impairments. Therefore, further research to determine the effects and optimal dose of physical exercise in this particular population is warranted.
Conflict of interest
All authors have nothing to declare.
Supporting information
Table S1. Results of study endpoints by group
Acknowledgements
This study was funded by a Gobierno de Navarra project grant (Resolución 2186/2014) and acknowledged with the "Beca Ortiz de Landazuri" for the best research clinical project in 2014, as well as by a research grant PI17/01814 of the Ministerio de Economía, Industria y Competitivad (ISCIII, FEDER). We thank Fundación Miguel Servet (Navarrabiomed) for its support during the implementation of the trial, as well as Fundación Caja Navarra and Fundación la Caixa. R.R.‐V. is funded in part by a postdoctotal fellowship grant ID 420/2019 of the Public University of Navarre, Spain. Finally, we thank our patients and their families for their confidence in the research team.
The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle.41
Sáez de Asteasu M. L., Martínez‐Velilla N., Zambom‐Ferraresi F., Ramírez‐Vélez R., García‐Hermoso A., Cadore E. L., Casas‐Herrero Á., Galbete A., and Izquierdo M. (2020) Changes in muscle power after usual care or early structured exercise intervention in acutely hospitalized older adults, Journal of Cachexia, Sarcopenia and Muscle, 11, 997–1006. 10.1002/jcsm.12564.
Trial Registration: ClinicalTrials.gov Identifier: NCT02300896.
References
- 1. Gilbert T, Neuburger J, Kraindler J, Keeble E, Smith P, Ariti C, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rechel B, Grundy E, Robine JM, Cylus J, Mackenbach JP, Knai C, et al. Ageing in the European Union. Lancet 2013;381:1312–1322. [DOI] [PubMed] [Google Scholar]
- 3. Spillman BC, Lubitz J. The effect of longevity on spending for acute and long‐term care. N Engl J Med 2000;342:1409–1415. [DOI] [PubMed] [Google Scholar]
- 4. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA 2011;306:1782–1793. [DOI] [PubMed] [Google Scholar]
- 5. Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA 2004;292:2115–2124. [DOI] [PubMed] [Google Scholar]
- 6. Gill TM, Gahbauer EA, Han L, Allore HG. The role of intervening hospital admissions on trajectories of disability in the last year of life: prospective cohort study of older people. BMJ 2015;350:h2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Covinsky KE, Justice AC, Rosenthal GE, Palmer RM, Landefeld CS. Measuring prognosis and case mix in hospitalized elders. The importance of functional status. J Gen Intern Med 1997;12:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Covinsky KE, Wu AW, Landefeld CS, Connors AF Jr, Phillips RS, Tsevat J, et al. Health status versus quality of life in older patients: does the distinction matter? Am J Med 1999;106:435–440. [DOI] [PubMed] [Google Scholar]
- 9. Fortinsky RH, Covinsky KE, Palmer RM, Landefeld CS. Effects of functional status changes before and during hospitalization on nursing home admission of older adults. J Gerontol A Biol Sci Med Sci 1999;54:M521–M526. [DOI] [PubMed] [Google Scholar]
- 10. Inouye SK, Peduzzi PN, Robison JT, Hughes JS, Horwitz RI, Concato J. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA 1998;279:1187–1193. [DOI] [PubMed] [Google Scholar]
- 11. Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc 2004;52:1263–1270. [DOI] [PubMed] [Google Scholar]
- 12. Suetta C, Magnusson SP, Beyer N, Kjaer M. Effect of strength training on muscle function in elderly hospitalized patients. Scand J Med Sci Sports 2007;17:464–472. [DOI] [PubMed] [Google Scholar]
- 13. Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab 2012;302:E1113–E1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 2007;297:1772–1774. [DOI] [PubMed] [Google Scholar]
- 15. Verlaan S, Van Ancum JM, Pierik VD, Van Wijngaarden JP, Scheerman K, Meskers CG, et al. Muscle measures and nutritional status at hospital admission predict survival and independent living of older patients—the EMPOWER study. J Frailty Aging 2017;6:161–166. [DOI] [PubMed] [Google Scholar]
- 16. Cruz‐Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care 2010;13:1–7. [DOI] [PubMed] [Google Scholar]
- 17. Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports 2010;20:49–64. [DOI] [PubMed] [Google Scholar]
- 18. Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 2001;56:B209–B217. [DOI] [PubMed] [Google Scholar]
- 19. Van Ancum JM, Scheerman K, Jonkman NH, Smeenk HE, Kruizinga RC, Meskers CG, et al. Change in muscle strength and muscle mass in older hospitalized patients: a systematic review and meta‐analysis. Exp Gerontol 2017;92:34–41. [DOI] [PubMed] [Google Scholar]
- 20. de Morton NA, Keating JL, Jeffs K. The effect of exercise on outcomes for older acute medical inpatients compared with control or alternative treatments: a systematic review of randomized controlled trials. Clin Rehabil 2007;21:3–16. [DOI] [PubMed] [Google Scholar]
- 21. Martinez‐Velilla N, Casas‐Herrero A, Zambom‐Ferraresi F, de Asteasu ML, Lucia A, Galbete A, et al. Effect of exercise intervention on functional decline in very elderly patients during acute hospitalization: a randomized clinical trial. JAMA Intern Med 2019;179:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cadore EL, Izquierdo M. Muscle power training: a hallmark for muscle function retaining in frail clinical setting. J Am Med Dir Assoc 2018;19:190–192. [DOI] [PubMed] [Google Scholar]
- 23. Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 2012;40:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. García‐Hermoso A, Ramirez‐Vélez R, Sáez de Asteasu ML, Martínez‐Velilla N, Zambom‐Ferraresi F, Valenzuela PL, et al. Safety and effectiveness of long-term exercise interventions in older adults: a systematic review and meta-analysis of randomized controlled trials. Sports Med 2020. [Epub ahead of print] 10.1007/s40279-020-01259-y [DOI] [PubMed] [Google Scholar]
- 25. Asher RA. The dangers of going to bed. Br Med J 1947;2:967–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinez‐Velilla N, Casas‐Herrero A, Zambom‐Ferraresi F, Suárez N, Alonso‐Renedo J, Contín KC, et al. Functional and cognitive impairment prevention through early physical activity for geriatric hospitalized patients: study protocol for a randomized controlled trial. BMC Geriatr 2015;15:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Izquierdo M, Casas‐Herrero A, Zambom‐Ferraresi F, Martínez‐Velilla N, Alonso‐Bouzón C, Rodríguez‐Mañas L. Multicomponent physical exercise program VIVIFRAIL. 2017. Retrieved from http://www.vivifrail.com/resources/send/3‐documents/23‐e‐book‐interactive‐pdf.
- 28. Cohen J. Statistical power analysis for the behavioral sciences. L Erlbaum Associates 1988. [Google Scholar]
- 29. Suetta C, Maier AB. Is muscle failure a better term than sarcopenia? J Cachexia Sarcopenia Muscle 2019;10:1146–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tibaek S, Andersen CW, Pedersen SF, Rudolf KS. Does progressive resistance strength training as additional training have any measured effect on functional outcomes in older hospitalized patients? A single‐blinded randomized controlled trial. Clin Rehabil 2014;28:319–328. [DOI] [PubMed] [Google Scholar]
- 32. Scheerman K, Raaijmakers K, Otten RHJ, Meskers CGM, Maier AB. Effect of physical interventions on physical performance and physical activity in older patients during hospitalization: a systematic review. BMC Geriatr 2018;18:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM, Lang CH. Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am J Physiol Endocrinol Metab 2016;311:E594–E604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Izquierdo M, Ibanez J, Gorostiaga E, Anton A, Larrion JL, Haekkinen K. Maximal strength and power characteristics in isometric and dynamic actions of the upper and lower extremities in middle‐aged and older men. Acta Physiol Scand 1999;167:57–68. [DOI] [PubMed] [Google Scholar]
- 35. Izquierdo M, Aguado X, Gonzalez R, Lopez JL, Hakkinen K. Maximal and explosive force production capacity and balance performance in men of different ages. Eur J Appl Physiol Occup Physiol 1999;79:260–267. [DOI] [PubMed] [Google Scholar]
- 36. Saez de Asteasu ML, Martinez‐Velilla N, Zambom‐Ferraresi F, Casas‐Herrero A, Ramirez‐Velez R, Izquierdo M. Role of muscle power output as a mediator between gait variability and gait velocity in hospitalized older adults. Exp Gerontol 2019;124:110631. [DOI] [PubMed] [Google Scholar]
- 37. Cadore EL, Rodriguez‐Manas L, Sinclair A, Izquierdo M. Effects of different exercise interventions on risk of falls, gait ability, and balance in physically frail older adults: a systematic review. Rejuvenation Res 2013;16:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Izquierdo M, Hakkinen K, Ibanez J, Garrues M, Anton A, Zuniga A, et al. Effects of strength training on muscle power and serum hormones in middle‐aged and older men. J Appl Physiol (1985) 2001;90:1497–1507. [DOI] [PubMed] [Google Scholar]
- 39. Duchateau J, Enoka RM. Human motor unit recordings: origins and insight into the integrated motor system. Brain Res 2011;1409:42–61. [DOI] [PubMed] [Google Scholar]
- 40. Izquierdo M, Morley JE, Lucia A. Exercise in people over 85. BMJ. 2020. Feb 5;368:m402 10.1136/bmj.m402 [DOI] [PubMed] [Google Scholar]
- 41. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Results of study endpoints by group


