Abstract
Background
Patients with lower extremity peripheral arterial disease (PAD) and sarcopenia are a population at risk requiring specific and targeted care. The aim of this review is to gather all relevant studies associating sarcopenia and PAD and to identify the underlying pathophysiological mechanisms as well as potential therapeutic strategies to improve skeletal muscle function.
Methods
A systematic review was carried out following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA).
Results
Data extraction allowed the evaluation of 140 publications; 87 met the inclusion criteria; of which 79 were included in the final review, reporting sufficient data for epidemiological and diagnostic criteria, mechanical analysis, and therapeutic approaches. Epidemiological analysis and diagnostic criteria were based on 18 studies following 2362 PAD patients [31.39% (SD 7.61) women], aged 72.42 (SD 2.84); sarcopenia was present in 34.63% (SD 12.86) of the patients. Mechanical and pathway analysis were based on five animal studies and 29 clinical reports, showing significantly altered muscle strength and function in 1352 PAD patients [26.49% (SD 17.32) women], aged 67.67 (SD 5.14) years; impaired muscle histology in 192 PAD patients (9.2% (SD 11.22) women), aged 64.3 (SD 0.99) years; +58.63% (SD 25.48) of oxidative stress in 69 PAD patients [16.96% (SD 8.10) women], aged 63.17 (SD 1.43) years; mitochondriopathy in 153 PAD patients [29.39% (SD 28.27) women], aged 63.50 (SD 1.83) years; +15.58% (SD 7.41) of inflammation in 900 PAD patients [40.77% (SD 3.71) women], aged 74.88 (SD 2.76) years; and altered signalling pathways in 51 PAD patients [34.45% (SD 32.23) women], aged 72.25 (SD 5.25) years. Therapeutic approaches analysis was based on seven animal studies and 21 clinical reports. In total, 884 patients followed an exercise therapy, and 18 received an angiogenesis treatment; 30.84% (SD 17.74) were women. Mean ages of patients studied were 66.85 (SD 3.96).
Conclusions
Sarcopenia and lower extremity PAD have musculoskeletal consequences that directly impair patients' quality of life and prognosis. Although PAD is primarily a vascular disease, all etiological factors of sarcopenia identified so far are present in PAD. Indeed, both sarcopenia and PAD are accompanied by oxidative stress, skeletal muscle mitochondrial impairments, inflammation, inhibition of specific pathways regulating muscle synthesis or protection (i.e. IGF‐1, RISK, and SAFE), and activation of molecules associated with muscle degradation. To date, besides revascularization, the best therapeutic strategy includes exercise, but approaches targeting the underlying mechanisms still deserve further studies.
Keywords: Sarcopenia, Peripheral arterial disease, Pathological pathways, Oxidative stress, Inflammation, Mitochondrial function, Exercise training
Introduction
Cardiovascular diseases are a major cause of death around the globe, with a prevalence gradually increasing as life expectancy rises. 1 Among them, lower extremity peripheral arterial disease (PAD) is defined by a reduction of or an obstruction to blood flow in the arteries, with symptoms ranging from intermittent claudication to critical limb ischemia (CLI), characterized by rest pain and/or ulcers. 2 , 3 , 4
Peripheral arterial disease is commonly accompanied by musculoskeletal abnormalities including generalized loss of skeletal muscle mass, strength, and physical performance—also called sarcopenia. 5 , 6 Both PAD and sarcopenia can run in parallel, many patients with PAD being also diagnosed with a sarcopenic condition (and probably even more are undiagnosed). In addition to worsen the loss of muscle mass and function in a vicious circle, sarcopenia further aggravates patients' quality of life and prognosis. 7 It is therefore essential to have a better understanding on how PAD and sarcopenia may impair skeletal muscle.
This systematic review resulted from the need to raise awareness on the importance of diagnosing sarcopenia in daily practice, and to better understand the pathological mechanisms underlying sarcopenia and PAD that might open the way towards new therapeutic advances. Therefore, the aim of this article is to gather all the relevant studies associating sarcopenia and PAD. To this end, the proposed systematic review will answer the following questions:
Epidemiological data: is sarcopenia a rare condition in patients with PAD?
Diagnostic criteria: how to diagnose sarcopenia and is sarcopenia a factor of poor prognosis for patients with PAD?
Mechanical analysis: how does sarcopenia affect skeletal muscle?
Therapeutic approaches: can we reverse the sarcopenic condition in patients with PAD?
Methods
Systematic review of the literature
A systematic review was performed following previously published Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 8
Eligibility criteria
Throughout the process of literature selection, clear inclusion and exclusion criteria were followed. Studies included were full text English or French publications without any chronological limit. All primary research studies reporting a link between sarcopenia and PAD were included. Studies not eligible for inclusion were reviews, letters, editorials, comments, book chapters, and studies focusing on other diseases. The main outcomes of interest were the presence of epidemiological data, mechanical data, and therapeutic data focusing on skeletal muscle aberrations and PAD. Studies that listed patient characteristics, assessment method of sarcopenia, duration of follow‐up, and prognostic outcome were considered to have epidemiological significance. Studies that listed mechanistic pathways leading to muscular defects in PAD were considered to have mechanical significance. Studies that listed therapies targeting skeletal muscle for PAD patients were considered to have therapeutic significant.
Information sources and search strategy
The PubMed database was analysed with a combined strategy using the subject heading terms (‘peripheral artery disease’ OR ‘peripheral arterial disease’ OR ‘critical limb ischemia’ OR ‘chronic limb‐threatening ischemia’) AND (‘sarcopenia’ OR ‘muscle atrophy’ OR ‘amyotrophy’ OR ‘muscle strength’ OR ‘muscle loss’ OR ‘muscle dysfunction’). All titles and abstracts collected from the search strategy were screened for relevance. When a relevant article was found, full text articles were retrieved. Studies that did not meet the inclusion criteria were excluded. The publications of the reference lists of included studies were searched and scanned for other potentially relevant studies. The full text of all potentially relevant articles was obtained and reviewed for eligibility.
Study records and data items
Data were extracted using a standardized form. This was done in duplicate to increase accuracy and to reduce measurement bias. Data extracted included study characteristics (year of publication, study design, population, and parameters determined) and particularly skeletal muscle characteristics (type of muscle, measurement method of muscle mass and strength) and main results.
Results
A flowchart showing study selection is given in Figure 1. Data extraction led to the evaluation of 140 publications, of which 87 met the inclusion criteria, and 79 were included in the final review. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 Of these, 18 gave sufficient data for epidemiological analysis and diagnostic criteria, 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 33 gave sufficient data for mechanistical analysis, 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 and 28 gave sufficient data for therapeutic approaches. 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87
FIGURE 1.

Flowchart of the systematic review.
1/Epidemiological data: Is sarcopenia a rare condition in patients with PAD?
Epidemiological analysis and diagnostic criteria were based on 17 studies following 2362 PAD patients; 31.39% (SD 7.61) were women. Mean age of patients studied was 72.42 (SD 2.84). Sarcopenia was present in 34.63% (SD 12.86) of the patients.
Sarcopenia is also described in conditions associated with PAD, such as metabolic syndrome. The metabolic syndrome—clustering of multiple metabolic abnormalities (i.e. diabetes mellitus, dyslipidemia, hypertension, or obesity)—is associated with an increased risk of cardiovascular morbidity and mortality and is highly prevalent in PAD patients. 9
Interestingly, in a rat model of PAD, the presence of diabetes mellitus worsened the ischemia–reperfusion‐induced skeletal muscle injury, as shown by a more severe decline in mitochondrial respiration and by enhanced levels of oxidative stress and apoptosis effectors in skeletal muscles. 10
In humans, patients living with both PAD and diabetes mellitus displayed musculoskeletal and biomechanical changes in the lower limbs. Indeed, Bartolo et al. recently showed a significant increase in the electromyogram muscle amplitude of PAD and diabetic patients, characteristic of less efficient lower muscle contractions and probable early fatigue. 11
Thus, skeletal muscle wasting is exacerbated in PAD patients presenting with a metabolic syndrome. Such patients represent a particularly fragile population that requires specific attention because of prominent atherosclerotic risk factors and poor outcomes. Moreover, the accumulation of skeletal muscle alterations might enhance the prevalence of sarcopenia—particularly hidden sarcopenia—in this fragile population. Indeed, it is important to note that a large proportion of these patients might be obese and thus, might be at risk of developing sarcopenic visceral and/or subcutaneous obesity. Such potential hidden condition is resulting in a higher risk of adverse outcomes. 12
2/Diagnostic criteria: How to diagnose sarcopenia and is sarcopenia a factor of poor prognosis for patients with PAD?
A/What is sarcopenia? Definition and diagnostic criteria
Sarcopenia is characterized by a decline in muscle mass and function with age or disease. It represents a significant burden for the patients, as it is associated with physical disability, increased hospitalization rates and mortality. 13 It is therefore essential for healthcare professionals to better recognize this condition. In this light and during the last decade, collaborative efforts were made all over the world, for example, Europe, America, and Asia in order to reach a consensus definition and diagnosis criteria for sarcopenia. To date, it appears accepted by the different working groups on sarcopenia that sarcopenia should be defined through three main criteria: (i) low muscle strength, (ii) low muscle quantity or quality, and (iii) low physical performance. Alone, Criterion 1 is considered the most reliable, and it should guide towards the diagnosis of sarcopenia. When combined, Criteria 1 and 2 account for the certainty of the diagnosis. Last, if all three criteria are met, sarcopenia is considered severe based on the link between low physical performance and poor prognosis.
Muscle strength assessment
According to the European Working Group on Sarcopenia in Older People (EWGSOP), low muscle strength can be assessed with measures of grip strength, with cut‐off points under 27 kg for men and 16 kg for women. The chair stand test can be used to measure impaired lower body strength (sarcopenia is suspected if the time required to complete five consecutive rises exceeds 15 s). Finally, isometric torque methods can be used to measure muscle extension/flexion power.
Muscle mass/quality assessment
Low muscle quantity can be assessed by measurements of appendicular skeletal muscle mass (ASM) or skeletal muscle mass index (ASM/height2) using bioelectrical impedance analysis (BIA) or dual X‐ray absorptiometry (DXA). The reference values for low ASM are under 20 kg for men and 15 kg for women and under 7.0 kg/m2 for men and 6.0 kg/m2 for women for low skeletal muscle mass index. Magnetic resonance imaging (MRI) or computed tomography (CT) can also be used to appreciate muscle quantity; however, appropriate cut‐offs values are not well defined for these measurements.
Physical performance assessment
Low physical performance can be predicted by low gait speed (≤0.8 m/s) or low score on the short physical performance battery test (≤8 point). 14
The data published show an important heterogeneity in the application of sarcopenic diagnostic criteria. Indeed, based on the articles in Tables 1 and 2, 12 articles are using a single‐measurement approach (measure of muscle mass alone in six articles, muscle strength alone in five articles and physical performance alone in one article); nine articles are using two points of measure (commonly muscle strength and physical performance), and no articles are using all three criteria (muscle strength, muscle mass, and muscle function). Where muscle mass is used, four articles focused on lumbar muscles, one on leg muscles, and three on individual psoas muscle; and values are either unindexed in five articles, indexed to tibial length square in one article, height square in one article, or even to the adjacent vertebral body in one article. These disparities make it difficult to aggregate and compare the resulting data. Further, it is unlikely that a single muscle might be used as a sentinel for sarcopenia diagnostic. These data support the need of further studies to reach a consensus allowing clear and valid diagnostic criteria for sarcopenia in PAD patients. 15
TABLE 1.
Association between sarcopenia and poor outcomes in PAD patients
| Reference | Patients population | Number of patients | Assessment method | Outcomes measured | Main results | ||
|---|---|---|---|---|---|---|---|
| Muscle strength | Muscle mass/quality | Physical performance | |||||
|
Shimazoe et al., 2019, Ann Vasc Surg |
CLI | 110 | ‐ | Skeletal muscle areas at the L3 level (CT) | Measures of basic aspects of activities related to self‐care and mobility | 3‐year overall survival; amputation‐free survival | Low activity of daily living was significantly associated with worse 3‐year overall survival and amputation‐free survival in patients with CLI and low muscle mass (defined as skeletal muscle area <114.0 cm2 for men and <89.8 cm2 for women). |
|
Taniguchi et al., 2019, Ann Vasc Dis |
CLI | 75 | ‐ | Cross‐sectional area of the psoas major muscles (CT) | ‐ | Limb salvage and overall survival | Low muscle mass (21.4 ± 3.8 kg/m2 in the sarcopenic group vs. 23.5 ± 3.1 kg/m2 in the non‐sarcopenic group) was associated with significantly lower limb salvage rates (73% vs. 100% at 2 years, P < 0.05) and overall survival rates (60% vs. 87% at 3 years, P < 0.05) |
|
Morisaki et al., 2019, Vascular |
CLI | 127 | ‐ | Low skeletal muscle mass index (CT) | Non‐ambulatory status | Overall survival | Low muscle mass (defined as skeletal muscle area <114.0 cm2 for men and <89.8 cm2 for women) was associated with significantly lower overall survival (89.7% in the CLI Frailty group vs. 60.5% in the CLI Non‐frailty group at 2 years after revascularization, P < 0.01) |
|
Reeve et al., 2018, J Vasc Surg |
Vascular disease (AAA, carotid stenosis, PAD) | 311 | Dominant hand grip strength | ‐ | ‐ N‐ | Comorbidity, cardiac risk | Low muscle strength (19.7 ± 6.5 kg in the frail vs. 36.8 ± 10.3 kg in the non‐frail patients) was associated with comorbidity (based on Charlson comorbidity index with 6.4 ± 2.2 points vs. 5.2 ± 2.2 points, P < 0.0001) and cardiac risk (based on revised cardiac risk index with 1.8 ± 0.8 vs. 1.5 ± 0.7, P < 0.018) |
|
Sugai et al., 2018, Circ J |
PAD | 327 | ‐ | Psoas muscle value (CT) | ‐ | Major adverse cardiovascular and limb events | Patients with major adverse cardiovascular and limb events had significantly lower mean psoas muscle value (41.0 ± 7.4 vs. 46.7 ± 5.7 Hounsfield unit, P < 0.001) than those without |
|
Matsubara et al., 2017, J Vasc Surg |
CLI | 114 | ‐ | Vertebral body at the L3 level (CT) | ‐ | Cardiovascular event‐free survival | Low muscle mass (defined as skeletal muscle area <114.0 cm2 for men and <89.8 cm2 for women) was associated with lower cardiovascular event‐free survival rates (43.1% for patients with sarcopenia vs. 91.2% without sarcopenia at 3 years, P < 0.01) |
|
Nyers et al., 2017, J Vasc Surg |
PAD | 188 | ‐ | Psoas‐L4 verterbal index (Cross‐sectional area of the bilateral psoas muscles and vertebral body at the L4 level) (CT) | ‐ | Amputation‐free survival | Muscle mass did not predict amputation‐free survival (with a psoas‐L4 vertebral index at 1.79 ± 0.55 for patients with 3 years amputation‐free survival vs. 1.78 ± 0.57 for patients without 3 years amputation‐free survival) |
|
Matsubara et al., 2015, J Vasc Surg |
CLI | 64 | ‐ | Vertebral body at the L3 level (CT) | ‐ | Overall survival | Low muscle mass (defined as skeletal muscle area <114.0 cm2 for men and <89.8 cm2 for women) was associated with lower survival rates (23.5% for patients with sarcopenia vs. 77.5% without sarcopenia at 5 years, P < 0.001) |
|
McDermott et al., 2012, J Am Coll Cardiol |
PAD | 434 | Knee extension/Isometric knee extension/Plantar flexion powerHand grip strength | Calf muscle density (CT) | ‐ | Comorbidities and mortality |
Lower calf muscle density was associated with higher cardiovascular disease mortality. Low plantar flexion strength, low baseline leg power and poor handgrip were associated with higher all‐cause mortality (using proportional hazards analyses) |
|
Singh et al., 2010, J Vasc Surg |
PAD | 410 | Knee extension/Isometric knee extension/Hip extension/Hip flexion power | ‐ | ‐ | Mortality | Low baseline strength for knee flexion/extension and hip extension were associated with higher all‐cause mortality in men. Poorer strength for knee flexion and hip extension were associated with higher cardiovascular mortality in men (using proportional hazards analyses) |
AAA, abdominal aortic aneurysm, CLI, critical limb ischemia; CT, computed tomography; F, female; M, male; PAD, peripheral artery disease.
TABLE 2.
Association between impaired muscle strength/function and PAD
| Reference | Patients population | Number of patients/controls | Assessment method | Main results | ||
|---|---|---|---|---|---|---|
| Muscle strength | Muscle mass/quality | Physical performance | ||||
|
Kakihana et al., 2017, J Vasc Surg |
PAD | 16/10 | ‐ | ‐ | 7‐m walkway embedded with a force plate test | PAD was associated with slower walk at self‐selected walking speed (88.32 ± 15.15 cm/s for PAD patients vs. 126.04 ± 16.31 cm/s for controls, P < 0.001) and at fast walking speed (119.90 ± 21.07 cm/s vs. 162.01 ± 21.47 cm/s for controls, P < 0.001); lower cadence at self‐selected walking speed (109.92 ± 12.17 step/min vs. 118.38 ± 7.28 steps/min, P < 0.001) and at fast walking speed (121.29 ± 11.39 steps/min vs. 135.11 ± 9.47 step/min, P < 0.001); and reduced peak hip flexor generation power at self‐selected walking speed (0.50 ± 0.18 W/kg vs. 1.00 ± 0.22 W/kg, P < 0.001) and at fast walking speed (0.78 ± 0.27 W/kg vs. 1.40 ± 0.39 W/kg, P < 0.001) |
|
Schieber et al., 2017, J Vasc Surg |
PAD | 94/16 | Maximal isometric plantar flexion contractions of 10 s | ‐ | ‐ | PAD patients exhibited strength deficits, with impaired peak torque values (69.1 ± 28.7 N.m for claudicating patients vs. 98.2 ± 27.6 N.m for controls, P < 0.01) |
|
Dziubek et al., 2015, Maturitas |
CLI | 85/50 | Force‐velocity parameters (peak torque, total work, average power) of the lower limb | ‐ | 6‐min walk test | PAD was associated with lower 6‐min walk distance (349.77 ± 65.08 m for PAD patients vs. 515.86 ± 96.39 for controls, P < 0.0001), lower mean walk speed (3.49 ± 0.65 km/h vs. 5.15 ± 0.96 km/h for controls, P < 0.01), and significantly lower values of force‐velocity parameters (including peak torque, total work and average power of the knee joint) compared with the control group (P < 0.005) |
|
Parmenter et al., 2013, J Vasc Surg |
PAD | 22/− | Maximum strength/endurance testing (hip extensors, hip abductors, quadriceps, hamstrings, plantar flexors, pectoral, upper back muscles) | ‐ | 6‐min walk test | Greater severity of PAD was associated with reduced bilateral hip extensor strength (r = 0.54, P = 0.007), whole body strength (r = 0.32, P = 0.05), shorter distance to first stop during the 6‐min walk test (r = 0.38, P = 0.05) and poorer single leg balance (r = 0.44, P = 0.03) (using univariate and stepwise multiple regression models) |
|
Câmara et al., 2012, Ann Vasc Surg |
PAD | 20/9 | Plantar flexion/dorsiflexion movements, knee extension/flexion movements | ‐ | Plantar flexion/dorsiflexion movements, knee extension/flexion movements |
PAD patients presented lower muscle strength in dorsiflexion (0.20 ± 0.10 N/m/kg for PAD patients vs. 0.29 ± 0.10 N/m/kg for controls, P < 0.01), plantar flexion (0.36 ± 0.20 N/m/kg vs. 0.53 ± 0.20 N/m/kg, P < 0.01) and knee flexion movements (0.50 ± 0.30 N/m/kg vs. 0.62 ± 0.10, P = 0.04). Also, PAD was associated with lower muscle endurance in dorsiflexion (8.0 ± 3.5 N/m/kg vs. 9.9 ± 6.6 N/m/kg, P = 0.01) and plantar flexion movements (20.0 ± 9.0 N/m/kg vs. 25.7 ± 10.7 N/m/kg, P = 0.02) |
|
Wurdeman et al., 2012, Gait Posture |
PAD | 30/32 | Joint moments and powers at early, mid and late stance (hip and knee and ankle joints) | ‐ | ‐ | PAD was associated with reduced peak hip power absorption in midstance (−0.788 ± 0.25 W/kg for PAD patients vs.−0.950 ± 0.27 W/kg for controls, P = 0.017), reduced peak knee power absorption in late stance (−0.729 ± 0.21 W/kg vs.−0.899 ± 0.33 W/kg for controls, P = 0.02), and reduced peak ankle power generation in late stance (2.677 ± 0.45 W/kg vs. 2.998 ± 0.60 W/kg, P = 0.021) |
|
Koutakis et al., 2010, J Vasc Surg |
PAD | 20/16 | Joint torques and powers at early, mid and late stance (hip, knee and ankle joints) | ‐ | Ambulation on a walkway |
PAD patients presented significantly reduced hip power generation in late stance (0.569 ± 0.18 W/kg for claudicating patients vs. 0.706 ± 0.24 W/kg for controls, P = 0.03), knee power absorption in late stance (−0.580 ± 0.25 W/kg vs. −0.882 ± 0.32 W/kg, P = 0.0015), and ankle power generation in late stance (2.178 ± 0.51 W/kg vs. 2.957 ± 0.69 W/kg, P = 0.0001) Also, PAD was associated with reduced gait velocity (1.09 ± 0.13 m/s for claudicating patients vs. 1.28 ± 0.13 m/s for controls, P = 0.0007) and stride length (1.27 ± 0.11 m vs. 1.47 ± 0.11 m for controls, P < 0.001) |
|
Koutakis et al., 2010, J Vasc Surg |
PAD | 20/10 | Joint torques and powers at early, mid and late stance (hip, knee and ankle joints) | ‐ | ‐ | PAD was associated with reduced knee power generation in early stance (0.26 ± 0.31 W/kg for claudicating patients vs. 0.62 ± 0.25 W/kg for controls, P < 0.05) and ankle power generation in late stance (2.05 ± 0.59 W/kg vs. 4.00 ± 0.88 W/kg for controls, P < 0.05) |
|
Herman et al., 2009, J Am Geriatr Soc |
PAD | 374/− |
Hip extension/flexion, knee extension/flexion strength Walking over a force platform |
‐ |
7‐m walking speed test 6‐min walk test Short physical performance battery |
In women with PAD, weaker baseline hip and knee flexion strength were associated with faster average annual decline in usual‐paced 4‐m walking velocity (P trend < 0.001 and P trend = 0.02 respectively) and in short physical performance battery test (P trend = 0.019 and P trend = 0.01, respectively) |
|
McDermott et al., 2008, J Am Geriatr Soc |
PAD | 424/271 |
Isometric knee extension/plantar flexion strength Handgrip strength Knee extension power |
‐ |
6‐min walk test 4‐m walking velocity test |
Lower arterial brachial index values were associated with lower plantar flexion strength (P trend = 0.04) and lower knee extension power (P trend < 0.001) |
|
Kuo et al., 2008, J Gerontol A Biol Sci Med Sci |
PAD | 206/1592 | Isokinetic dynamometer | ‐ | 20‐ft timed walk test | PAD associated with weak leg force, low gait speed and functional dependence (based on multiple logistic regression analyses) |
B/Sarcopenia in PAD: a factor of poor prognosis
Sarcopenia has been associated with multiple adverse events such as physical limitation, poor quality of life, and mortality and was shown to predict patients' prognosis and outcome following vascular procedures. 16 Similarly, recent data have revealed a link between sarcopenia, PAD and high comorbidity. In a 48‐month longitudinal study, a reduction in calf muscle density, lower limb extension/flexion power, and hand grip strength was shown to be associated with an increase in mortality in 434 PAD patients. 17 Interestingly, this conclusion was also reached when only one parameter of sarcopenia was measured (muscle quantity 18 or muscle strength 19 ). Indeed, in a retrospective study of 327 patients with PAD followed for up to 30 months, Sugai et al. found an independent link between low psoas muscle area and major adverse cardiovascular and limb events. Likewise, after a 9‐month follow‐up, Reeve et al. highlighted an association between lower grip strength and elevated comorbidity and cardiac risk in patients with vascular diseases, including PAD. Noteworthy, in a study realized on 410 patients with PAD followed for 60 months, poor leg extension/flexion power was reported to predict mortality in men but not in women. 20 This result might be explained by the larger proportion of men studied. Finally, a recent study following patients with PAD for 72 months showed no difference on overall survival between patients with high or low muscle mass, assessed through comparison of psoas‐L4 vertebral index. 21 Together, these results indicate that muscle strength rather than muscle mass is a poor prognosis factor in PAD. This might be attributable to the fact that muscle mass measurements are extremely susceptible to bias, supporting the need for homogenized technic used, muscle analysed, and cut‐off values of low muscle mass.
Sarcopenia was also linked with CLI and high mortality rates. Indeed, in 2015, and subsequently in 2017, Matsubara et al. reported that sarcopenia was associated with higher cardiovascular events and lower survival, in 64 and 114 patients suffering from CLI, respectively. 22 , 23 Recent papers confirmed this association in retrospective studies including patients with CLI, where low skeletal muscle mass was predictive of a worse overall survival 24 , 25 , 26 (Table 1).
3/Mechanistical analysis: how does sarcopenia affect skeletal muscle?
Mechanistical analysis was based on five animal studies [10; 43–44; 48; 58] and 29 clinical reports. This allowed the analysis of the following:
muscle strength and function, 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 based on 1352 PAD patients [26.49% (SD 17.32) women], aged 67.67 (SD 5.14) years;
muscle histology, 39 , 40 , 41 , 42 based on 192 PAD patients [9.2% (SD 11.22) women], aged 64.3 (SD 0.99) years;
oxidative stress, 43 , 44 , 45 , 46 , 47 based on 69 PAD patients [16.96% (SD 8.10) women], aged 63.17 (SD 1.43) years;
mitochondriopathy [43; 47–53], based on 153 PAD patients [29.39% (SD 28.27) women], aged 63.50 (SD 1.83) years;
inflammation, 54 , 55 , 56 based on 900 PAD patients [40.77% (SD 3.71) women], aged 74.88 (SD 2.76) years;
signalling pathways [10; 57–59], based on 51 PAD patients [34.45% (SD 32.23) women], aged 72.25 (SD 5.25) years.
Muscle dysfunction in PAD: the missing link to better understand the common mechanisms of sarcopenia and PAD pathophysiology
Patients with lower extremity PAD present various skeletal muscle defects, such as weak baseline strength, functional decline, and abnormal muscle histology. Although sarcopenia is currently defined by its clinical manifestations, all etiological factors identified so far—including excessive oxidative stress production, skeletal muscle mitochondrial impairments, high inflammation, and altered muscle kinetic process—are present in PAD and emphasizing skeletal muscle injuries (Figure 2).
FIGURE 2.
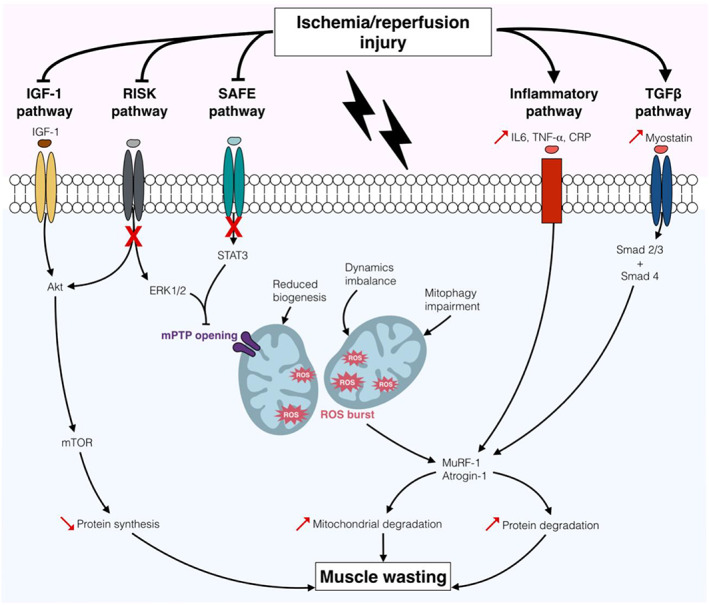
Major signalling pathways associated with sarcopenia and peripheral arterial disease (PAD). In the context of PAD, ischemia/reperfusion (I/R) injury induces a decrease in mitochondrial biogenesis, dynamics, and mitophagic activities, resulting in reactive oxygen species (ROS) burst and consecutive oxidative stress. Additionally, elevated levels of IL6, TNF‐α, and CRP are responsible for the activation of the inflammatory pathway. Ultimately, I/R‐induced oxidative stress and inflammation enhance the activity of the atrophy‐related ubiquitin ligases MuRF‐1 and atrogin‐1 and the degradation of mitochondria and proteins. I/R is also associated with defective stimulation of the muscle synthesis PI3K/Akt/mTOR pathway, notably via lower activity of the IGF‐1 and RISK pathways. Moreover, alteration of the protective pathways RISK and SAFE lead to persistent mitochondrial permeability transition pore (mPTP) opening, reduction in mitochondrial calcium retention capacity, and aggravation of mitochondrial dysfunction. Further, during I/R, myostatin overexpression results in enhanced activity of the muscle degradation pathway TGFβ.
A/Impaired muscle strength and function
Peripheral arterial disease pathophysiology is characterized by metabolic and structural myopathic changes in skeletal muscles, responsible for the decline in strength and function. 27 Lower extremity ischemia was shown to specifically impair proximal and distal leg muscles. Indeed, in a study of 424 patients with PAD, plantar flexion and knee extension strength was significantly lower when compared with age‐matched controls. 28 Similarly, analysis of strength and endurance of hip, knee, ankle, and plantar muscles of patients with and without PAD revealed an association between PAD and weaker leg muscles. 29 , 30 , 31 , 32 PAD physiopathology is thus characterized by a failure of specific muscles that are necessary for normal walking.
Accordingly, the loss of muscle strength appears to play a central role in the functional defects observed in patients. In the chronic stage of PAD, values of force‐velocity parameters of the lower limbs and 6‐min walk capacity were significantly lower compared with the control group. 33 Interestingly, the impairment in force and mobility was also seen in earlier stages of PAD, as shown by three independent studies. In the first study, 22 patients with PAD underwent 6‐min walking speed testing, maximum strength/endurance testing of lower limb muscles, as well as performance‐based testing of muscle function. Patients with PAD presented overall body disability, muscle weakness, and reduced physical performance. 34 The other two studies highlighted a gait impairment in patients with PAD, using a walkway embedded with a force plate. 35 , 36 Moreover, in a study of 374 patients with PAD, the correlation between poor strength/mobility and lower limb ischemia was found in women, but not men, probably because of their higher baseline strength. 37
It is important to note that in a total of 11 studies associating low muscle strength and/or function and/or quality and PAD, none used the term sarcopenia. Yet a sarcopenic diagnosis represents a turning point for patients, families, and clinicians. Indeed, beyond aggravating patients' prognosis, sarcopenia constitutes a great burden, as it participates to the isolation and dependence of patients with PAD 38 (Table 2).
B/Impaired muscle histology
The morphological and physiological consequences of the denervation–reinnervation process that occur in PAD were assessed in 26 patients with PAD based on gastrocnemius cross‐sectional area values and peak treadmill walking time. Overall, PAD was characterized by a general decline in type II muscle fibres number and size, responsible for the decay in general muscle strength. 39 Subsequent studies focusing on myofibre morphometrics of PAD gastrocnemius revealed significant changes in muscle quality, including lower diameter and density, rounder myofibres, and a fast‐to‐slow switch resulting in a predominance of type I fibres, as demonstrated by cross‐sectional area analysis. 40 , 41 , 42
Thus, the genesis of sarcopenia in PAD patients might be explained by the progressive loss of motoneurons included in type II motor units, which remains uncompensated despite reinnervation of muscle fibres by adaptive sprouting.
C/Oxidative stress
An imbalance between pro‐oxidant and antioxidant activities (also called oxidative stress) is responsible for reactive oxygen species (ROS) accumulation, mitochondrial respiratory chain dysfunctions, and oxidative damage of DNA. In PAD, ischemic lesions generate extensive oxidative stress. Accordingly, murine models of PAD displayed reduced antioxidant enzymes mRNA levels, increased ROS production, 43 increased oxidative stress, and impaired mitochondrial respiration, notably with reduced electron transport chain complexes I, III, and IV activities in ischemic muscles. 44
Similarly, human studies reported altered antioxidant enzyme activities, reduced electron transport chain complexes I, III, and IV activities and impaired mitochondrial respiration in patients with PAD. 45 PAD was also associated with significant oxidative stress and ROS production. 46 Interestingly, the extend of oxidative damage in PAD gastrocnemius was shown to be associated with advanced disease stage and lower myofibre cross‐sectional area. 47
Taken together, these data suggest the pathological implication of excessive oxidative stress in myofibres damage and PAD.
D/Mitochondriopathy
With disease and/or advancing age, mitochondrial dysfunctions accumulate, thus disrupting vital mitochondrial‐dependent activities. 47 Accordingly, studies in murine models of CLI revealed mitochondrial DNA damages in ischemic aged muscles, lower mitochondrial respiration, declined mitochondrial biogenesis, impaired calcium retention capacity and muscle atrophy, and muscle contractile deficits. 43 , 48
Human investigations regarding mitochondrial dysfunction in PAD showed an increase in skeletal muscle mitochondrial DNA injuries 49 and abnormal mitochondrial respiratory activity of ischemic muscles 50 compared with controls. Interestingly, Koutakis et al. investigated the potential role of the muscle specific intermediate filament desmin in PAD pathophysiology and linked abnormal desmin accumulation with low mitochondrial respiration. 51 Moreover, immunohistochemical analysis of muscle biopsies revealed accumulation of microtubule‐associated protein light chain 3 (LC3)—an autophagic marker—in the area depleted of mitochondria in PAD myofibres, thus suggesting an association between PAD and aberrant mitophagy process. 52 Last, a recent work has stressed a unique and severe mitochondriopathy touching patients with CLI. Indeed, besides the reduced mitochondrial oxidative capacity and respiratory activity also observed in patients with PAD, CLI was shown to be characterized by deficits in permeabilized myofibre mitochondrial function and decreased abundance of mitochondria‐associated mRNAs and proteins. 53
Overall, mitochondriopathy is thought to be a major contributor to sarcopenia and PAD, notably with significant disruption of mitochondrial biogenesis, dynamics, and mitophagy.
E/Inflammation
Systemic inflammation is considered an important pathophysiological mechanism in PAD and likely contributes to skeletal muscle wasting. Accordingly, several vascular inflammatory markers such as IL6, IL1 receptor antagonist, fibrinogen, and CRP were found elevated in PAD patients compared with controls subjects. 54 Further studies revealed an association between higher levels of these markers of inflammation and poorer 6‐min walk distance and overall performance (combining walking speed, balance, and chair rises exercises), 55 lower calf strength, and more adverse calf muscle characteristics. 56
It is therefore possible that sarcopenia in PAD patients finds its origin in altered inflammatory process, likely mediated by the dysregulation of multiple cytokine factors.
F/Impaired signalling pathways
IGF‐1 synthesis pathway
The IGF‐1/PI3K/Akt/mTOR signalling pathway is a key player in muscle growth, stimulating protein synthesis, and satellite cell proliferation in muscle, all together while simultaneously suppressing pathways responsible for protein degradation. Regarding the exact role played by this synthesis pathway in PAD, data are scarce. In 2004, Tuomisto et al. reported an up‐regulation of the anabolic factors IGF‐1 and IGF‐2 in atrophic and regenerating ischemic myocytes of patients with CLI. 57 The IGF system could promote skeletal muscle survival, regeneration and angiogenesis under chronic ischemia, notably via the VEGF pathway.
RISK and SAFE protective pathways
The RISK and SAFE pathways play essential roles in the reduction of ischemia/reperfusion (I/R) injuries, as they participate to muscle regeneration and mitochondrial integrity. Though this phenomenon is well documented in the field of cardiology, very little is known during PAD. In our rat model of PAD exposed to 3 h of ischemia followed by 2 h of reperfusion, the RISK and SAFE pathways were inefficiently activated, leading to mitochondrial dysfunctions, increased oxidative stress and apoptosis. 10
Impaired muscle degradation pathways
Several members of the TGFβ superfamily play a key role in protein degradation, among them, myostatin is well known for the extreme hyper muscularity of myostatin knock‐out mice and conversely, for the muscle atrophy of mice overexpressing myostatin. In mice models of PAD, silencing of myostatin led to gastrocnemius hypertrophy and improved running performance. 58
In humans, very little is known about the role of the TGFβ superfamily in PAD pathology. A recent research conducted by Ha et al. showed that TGFβ1 expression increased with advancing PAD severity. 59 Overall, myostatin is thought to be an important factor in the pathophysiology of sarcopenia and PAD.
Overall, evidence seem to indicate an imbalance between protein synthesis and degradation leading to reduced or impaired anabolic pathway and, in a broader sense, impaired muscle function and strength in PAD.
4/Therapeutic approaches: can we reverse the sarcopenic condition in patients with PAD?
Therapeutic analysis was based on seven animal studies [61–63; 83–86] and 21 clinical reports, Among the clinical reports, 19 were observational trial studies [64–68; 70–82; 87], and two were prospective studies [60; 69]. In total, 884 patients followed an exercise therapy, and 18 received an angiogenesis treatment; 30.84% (SD 17.74) were women. Mean age of patients studied was 66.85 (SD 3.96).
Treating sarcopenia in PAD
Lower limb revascularization surgery is the treatment of choice for patients suffering from CLI, enabling blood flow restoration and limb salvage, while reversing some sarcopenic features. Indeed, in a prospective study following 18 patients with CLI, surgical revascularization improved muscle strength, 6‐min walk distance, bodily pain and overall quality of life. 60 However, these muscular parameters are not currently tested in routine clinical practice, and therefore, we do not know whether ischemia‐related muscle impairment are normalized or whether sequela remain. Nevertheless, additional therapeutic approaches like exercise training or angiogenesis therapies seems useful to further sustain functional muscular improvement and to preserve patients' quality of life.
A/Exercise training
Molecular and cellular effects
In a mouse model of chronic CLI, moderate exercise consisting in a 3‐week treadmill training up to five times per week enhanced mitochondrial biogenesis and antioxidant enzymes mRNA levels, restored mitochondrial respiration and calcium retention capacity and reduced tissue damages, while generating low amount of oxidative stress. 61 On the other hand, Hain et al. showed that repeated cycles of electrical stimulation (mimicking exercise) resulted in increased NF‐κB activity and muscle fibre atrophy. 62 Further, 2‐week treadmill exercise was shown to have consequences on skeletal muscle mRNA expression in PAD models, notably by down‐regulating skeletal muscle regeneration markers. 63 These results might indicate that exercise intensity could be associated with adverse effects on skeletal muscle function and might also underscore the beneficial systemic effects of exercise.
In humans, studies show a significant increase of the inflammatory markers ICAM‐1, VCAM‐1, TNF‐α, and IL6 directly following one treadmill exercise. 64 , 65 However, a reduction in the inflammatory process was shown in PAD patients following either a 8‐week supervised training program 66 or a 12‐week homebased exercise training. 67 Last, 6 to 12 months of progressive resistance training was shown to increase type I and type II skeletal muscle fibre areas and capillary density. 68
Functional and vascular effects
In humans, the impact of exercise on PAD was assessed in prospective studies following training sessions for 4 weeks up to 12 months.
4‐week program
After a 4‐week rehabilitation program consisting in walking exercises, selective muscle strengthening, and sports, patients suffering from PAD showed significant improvement in walking distance. 69
8‐week program
Patients following 8 weeks of maximal strength training alone, 70 or combined with plantar flexion endurance training, 71 presented increased leg strength, force development, and walking performance.
12‐week program
A 12‐week homebased exercise training was shown to positively influence vascular function (microcirculation of the lower extremities measured by calf muscle haemoglobin oxygen saturation) and endurance (6‐min walk distance, peak walking time, and daily ambulatory activity) in PAD patients. 67
The effects of a 12‐week treadmill‐walking program on PAD were analysed in several independent studies. Notably Wang et al. demonstrated the importance of this training program in improving endurance (walking capacity) and strength (peak force, peak torques in plantar flexion) in patients with PAD. 72 The improvements in endurance (pain‐free walking distance, 6‐min walking distance, and peak exercise performance) were accompanied by a large decline in bilateral thigh lean mass 73 or by a significant increase in the number of denervated calf muscle fibres. 74 Here, regions remote to the ischemic lesion are mostly affected by a decline in muscle mass and muscle quality, possibly caused by sensory and motor nerve dysfunction during exercise. Further, this training program was shown to improve both muscular function (i.e. increased pain‐free walking distance) and endothelial function (i.e. increased flow‐mediated dilatation) in PAD patients. 75 Interestingly, a 12‐week treadmill walking exercise was found more effective than a 12‐week strength training program in term of exercise performance. 76
12‐week vs. 24‐week program
A supervised treadmill walking exercise program was shown to improve exercise performance and overall functional status after 12 weeks, with continued improvement after 24 weeks. 76 , 77
24‐week program
The effects of two training program were assessed in patients suffering from PAD. Improvements in 6‐min walk and treadmill walking performance, brachial artery flow‐mediated dilatation, and overall quality of life were observed in the group following supervised treadmill training; whereas climbing ability, treadmill performance, and quality of life were ameliorated in the group following resistance training, when compared with controls. 78
6‐month to 12‐month program
Two studies demonstrated the functional and vascular benefits of a 6‐month supervised walking exercise in PAD. Indeed, Andrew et al. observed improvements in peripheral circulation, walking economy, and cardiopulmonary function 79 ; while Schieber et al. observed improvements in walking distance, muscle strength, and gait biomechanics, as well as overall quality of life. 80 Moreover, in medium to longer term (i.e. 6 to 12 months follow‐up), progressive resistance training was shown to have beneficial effects on walking ability 81 and muscle strength. 82
In brief, exercise induces (i) molecular adaptations, notably with modifications of the transcript levels of mitochondrial biogenesis, antioxidant, oxidative phosphorylation enzymes, and reduction of the inflammatory process; (ii) cellular adaptations with enhanced mitochondrial plasticity, muscle fibre areas, and capillarization; and (iii) functional and vascular adaptations with enhanced functional status and improved microvascular circulation of the lower extremities in PAD (Table 3). Regular physical activity is highly recommended to prevent or reduce the onset of adverse effects occurring with PAD.
TABLE 3.
Effects of exercise on sarcopenia associated with PAD in experimental and clinical studies
| Reference | Population | Number studied (symptomatic/controls) | Exercise therapy | Outcomes measured | Main results | |||
|---|---|---|---|---|---|---|---|---|
| Exercise program | Duration | Muscle strength | Muscle mass/quality | Physical performance | ||||
|
Nagase et al., 2017, PLoS One |
Mice, PAD | 6/4 | Treadmill training | 2 weeks (twice a week) | ‐ | Quantitative analysis of mRNA levels | ‐ | Treadmill training significantly reduced the mRNA expression of skeletal muscle regeneration markers (P < 0.05) compared with the non‐exercised PAD group |
|
Lejay et al., 2017, Front Physiol |
Mice, CLI | 10/10 | Treadmill training | 3 weeks (5 times per week) | ‐ | Histological analysis | Functional score | Treadmill training reduced tissue damage (with a score of 1.9 for the exercised group vs. 4.0 for the non‐exercised group at day 30, P < 0.01), enhanced muscle function (with a score of 1.4 for the exercised group vs. 2.8 for the non‐exercised group at day 30, P < 0.01), stimulated mitochondrial biogenesis and anti‐oxidant defences |
|
Hain et al., 2011, Am J Physiol Regul Integr Comp Physiol |
Rats, PAD | Ns | Electrical stimulation causing repeated muscle contractions and mimicking exercise | 5 days | ‐ | Fibre cross‐sectional area | ‐ | Repeated cycles of muscle contraction decreased the mean fibre cross‐sectional area by 35% (1834 ± 219.9 μm2 in the exercised group vs. 2834 ± 132.5 μm2 in the non‐exercised group, P < 0.05) |
|
Schieber et al., 2019, J Vasc Surg |
Human, PAD | 47/− | Supervised walking exercise | 6 months (3 times per week) | Plantar flexor strength | ‐ | Walking distance, gait biomechanics | Supervised walking exercise improved muscle strength, walking distance and gait biomechanics |
|
Vun et al., 2016, J Vasc Surg |
Human, PAD | 36/− | Supervised treadmill exercise program | 12 weeks (twice a week) | ‐ | Whole‐body dual‐energy X‐ray absorptiometry |
Pain‐free walking distance 6‐min walking distance |
Supervised treadmill exercise improved pain‐free walking distance (213 ± 93 m after 12 weeks vs. 165 ± 78 m at baseline, P = 0.001) and 6‐min walk distance (421 ± 68 m after 12 weeks vs. 395 ± 78 m at baseline, P = 0.004) |
|
Gardner et al., 2014, J Am Heart Assoc |
Human, PAD | 60/− | Step‐monitored home walking to mild‐to‐moderate claudication pain | 12 weeks (3 times per week) | ‐ | ‐ |
6‐min walking distance Walking speed |
Home walking exercise improved 6‐min walk distance (372 ± 119 m after the 12‐week test vs. 328 ± 108 m at pre‐test, P < 0.001), peak walking time (490 ± 350 s vs. 380 ± 274 s at pre‐test, P < 0.001), and daily ambulatory activity notably with improvement in average cadence (11.8 ± 3.0 strides/min vs. 11.1 ± 2.7 strides/min, P < 0.01) |
|
Januszek et al., 2014, J Cardiol |
Human, PAD | 67/− | Supervised treadmill training | 12 weeks (3 times per week) | ‐ | ‐ | Maximal walking time | Treadmill training improved maximal walking time (+90%, P < 0.001) and flow‐mediated dilatation (+43%, P < 0.001) in PAD patients in comparison to baseline |
|
Pilz et al., 2014, Wien Klin Wochenschr |
Human, PAD | 42/− | Supervised exercise training on strength (couch pedal ergometer work on lower legs) and endurance (walk sessions) | 6 months (twice a week) |
Pushing power Pulling power Tip‐toe standing power |
‐ |
Pain‐free walking distance Walking‐speed |
Combined exercise program improved walking distance (568.9 ± 461.5 m after 6 months vs. 446.3 ± 276.6 m at baseline, P < 0.05), walking speed (4.39 ± 1.08 km/h vs. 4.17 ± 0.85 km/h at baseline, P < 0.05), pushing power (662.4 ± 530.4 J vs. 348.6 ± 270.3 J, P < 0.01), pulling power (96.4 ± 51.5 J vs. 58.7 ± 37.7 J, P < 0.0001), and tiptoe standing power (83.5 ± 48.6 repetitions vs. 49 ± 21.5 repetitions, P < 0.0001) |
| 52/− | 12 months (twice a week) | Combined exercise program further improved walking distance (647.8 ± 496.3 m after 12 months vs. 500.2 ± 427.9 m at baseline, P < 0.001), walking speed (4.53 ± 0.80 km/h vs. 4.03 ± 0.90 km/h at baseline, P < 0.0001), pushing power (637.8 ± 407.1 J vs. 337.2 ± 232.9 J, P < 0.001), pulling power (97.5 ± 59.8 J vs. 55.6 ± 38.8 J, P < 0.0001), and tiptoe standing power (84.9 ± 69.3 repetitions vs. 39.8 ± 15.3 repetitions, P < 0.0001) | ||||||
|
Parmenter et al., 2013, J Am Geriatr Soc |
Human, PAD | 7/− | High‐intensity progressive resistance training (weight lifting) | 6 months (3 times per week) | ‐ | ‐ | 6‐min walking distance | Progressive resistance training increased 6‐min walking distance (381.8 ± 151.6 m after 24 weeks of training vs. 321.9 ± 109.1 m at baseline, P = 0.02) |
|
Mosti et al., 2011, Scand J Med Sci Sports |
Human, PAD | 10/− | Leg press maximal strength training and plantar flexion endurance training | 8 weeks (3 times per week) |
Leg press maximal force Rate of force development |
‐ | Plantar flexion endurance | Exercise training improved muscle strength, notably with increased rates of force development (3675 ± 1315 N/s post‐test vs. 1943 ± 1027 N/s pre‐test, P < 0.01) and leg press maximal strength (152 ± 33 kg vs. 110 ± 24 kg, P < 0.01); but also walking distance (1203 ± 451 m vs. 1099 ± 463 m, P < 0.01) |
|
Cousin et al., 2011, Ann Phys Rehabil Med |
Human, PAD | 31/− | Walking sessions, selective muscle strengthening, general physical exercise | 4 weeks (5 days per week) |
Ankle plantar and dorsal flexors strength Concentric contractions at the angular velocity of 30°/s, 120°/s and 180°/s for muscle fatigue |
‐ | Walking distance on a treadmill <400 m | Rehabilitation program improved walking distance (977.4 ± 854.2 m upon completing the program vs. 282.4 ± 239.8 m at baseline, P < 0.0001) |
|
Saetre et al., Angiology, 2011 |
Human, PAD | 29/− | Supervised exercise training | 8 weeks (twice a week) | ‐ | Quantitative analysis of plasma inflammatory levels | Pain‐free walking distance, maximal walking distance | Exercise training reduced the plasma levels of E‐selectin (45.5 before training to 40.4 ng/ml after training, P = 0.013) and ICAM‐1 (342.0 to 298 ng/ml) in PAD patients. Both walking distance increased after exercise training (P < 0.01) |
|
Wang et al., 2010, Scand J Med Sci Sports |
Human, PAD | 10/− | Maximal strength training (dynamic leg press) | 8 weeks (3 times per week) |
Leg press force Rate of force development |
‐ | Walking economy test | Maximal strength training improved rates of force development (2901 ± 1848 N/s after the 8‐week training program vs. 1368 ± 893 N/s in the control period, P < 0.05), maximal strength (148 ± 33 kg vs. 114 ± 25 kg) and walking time to exhaustion (1095 ± 426 s vs. 1009 ± 448 s, P < 0.05) |
|
McDermott et al., 2009, JAMA |
Human, PAD | 156/− | Supervised treadmill walking training vs.resistance training | 24 weeks (3 times per week) | ‐ | ‐ | 6‐min walk performance, short physical performance battery, treadmill walking performance, walking impairment questionnaire, overall physical functioning score |
Supervised treadmill walking training improved 6‐min walk performance (by 35.9 m, P < 0.001), maximal treadmill walking time (by 3.44 min, P < 0.001) and overall quality of life (P = 0.02) compared to untrained controls. Resistance training increased maximal treadmill walking time (by 1.90 min, P = 0.009), stair climbing (P = 0.03) and overall quality of life (P = 0.04) |
|
Wang et al., 2006, Clin J Sport Med |
Human, PAD | 17/− | Supervised treadmill walking training | 12 weeks (3 times per week) | Calf‐muscle strength and endurance | ‐ | Walking capacity |
Supervised treadmill‐walking program improved peak torque at 30 degrees/s (175 ± 40 N/m post‐training vs. 159 ± 32 N/m at pre‐training, P < 0.01), mean peak force (358 ± 87 N vs. 314 ± 68 N, P < 0.001), and mean power (80 ± 26 W vs. 66 ± 19 W, P < 0.001). This training program also increased pain‐free walking time (382 ± 261 s vs. 137 ± 70 s, P < 0.001) and maximal walking time (696 ± 191 s vs. 314 ± 138 s, P < 0.001) |
|
Signorelli et al., 2003, Vasc Med |
Human, PAD | 20/20 | Treadmill test | 1 session | ‐ | Quantitative analysis of plasma inflammatory levels | ‐ | One treadmill exercise session increased plasma levels of ICAM‐1 (317 ± 4 at rest to 421 ± 10 ng/ml after exercise), VCAM‐1 (485 ± 14 to 576 ± 16), TNF‐α (14 ± 3 to 27 ± 5) and IL6 (12 ± 1 to 16 ± 2) in PAD patients |
|
McGuigan et al., 2001, J Gerontol A Biol Sci Med Sci |
Human, PAD | 11/− | Progressive resistance training | 6 months (3 times per week) |
Leg press strength Calf press strength |
Biopsies from gastrocnemius muscles | ‐ |
Progressive resistance training improved the 10‐repetition maximum loading leg (by 155%) and calf (by 126%) press strength in the trained subjects, at 24 weeks Training also increased type I (3442 ± 981 μm2 after training vs. 2695 ± 867 μm2 at pre‐training, P < 0.05) and type II muscle fibre area (4273 ± 1113 μm2 vs. 3421 ± 1148 μm2, P < 0.05) |
|
Brevetti et al., 2001, Clin Hemorheol Microcirc |
Human, PAD | 21/18 | Maximally tolerated treadmill exercise | 1 session | ‐ | Quantitative analysis of plasma inflammatory levels | ‐ | One treadmill exercise session increased plasma levels of ICAM‐1 (285 ± 15 at rest to 317 ± 16 ng/ml after exercise, P < 0.01) and VCAM‐1 (671 ± 45 to 751 ± 47 ng/ml, P < 0.05) in PAD patients, while no modifications were observed in controls |
|
Gardner et al., 2000, J Gerontol |
Human, PAD | 63/− | Supervised walking exercise | 6 months (3 times per week) | ‐ | ‐ | Walking economy | Exercise training improved walking economy by 10% (P < 0.05) compared with the untrained group |
|
Hiatt et al., 1996, J Appl Physiol |
Human, PAD | 26/− | Treadmill walking exercise | 12 weeks (3 times per week) | ‐ | Biopsies from gastrocnemius muscles | Peak exercise performance | Treadmill training was associated with improved exercise performance despite increased denervated fibres (7.6 ± 5.4 before exercise to 15.6 ± 7.5% after exercise, P < 0.05) |
|
Regensteiner et al., 1996, J Vasc Surg |
Human, PAD | 29/− | Supervised treadmill walking training | 12 weeks (3 h per week) or 24 weeks (3 h per week) | ‐ | ‐ | Functional status (questionnaires on walking ability, habitual physical activity level, and physical/social functioning, well‐being, overall health); monitored activity levels | Exercise training improved functional status and monitored activity level (P < 0.05) after 12 weeks and to a greater extend after 24 weeks |
|
Hiatt et al., 1994, Circulation |
Human, PAD | 29/− | Supervised treadmill walking training vs.strength training (resistive training of five muscle groups of each leg) | 12 weeks (3 h per week) or 24 weeks (3 h per week) | ‐ | ‐ | Peak exercise performance |
Patients in the 12 weeks treadmill training program had higher increase in peak walking time and higher improvement in peak oxygen consumption and onset of claudication pain compared with patients in the strength training program; with further improvements over 24 weeks of training |
CLI, critical limb ischemia; Ns, not specified; PAD, peripheral artery disease.
Angiogenesis
PAD and CLI are characterized by vascular dysfunction, reduced microvascular flow and altered angiogenesis process. On this basis, therapeutic angiogenesis represents a promising approach in the restoration of blood flow and treatment of ischemic lesions. In mouse models of CLI, angiogenic therapy consisting in bone marrow cells injections resulted in reduced limb necrosis and muscle impairment, enhanced gastrocnemius and quadriceps muscle mass, and blood flow regeneration, compared with untreated ischemic animals. 83 , 84 , 85 Interestingly, in a murine hindlimb ischemic model, injections of donepezil—an anti‐Alzheimer drug—upregulated angiomyogenesis factors (VEGF, HIF‐1α, and Akt) and reduced skeletal muscle atrophy. 86
Data in humans did not confirm the potential benefice of angiogenesis therapy in PAD, notably showing similar exercise performance and survival rates. 87
Concluding remarks and perspectives
With more than 200 million individuals affected worldwide, lower extremity PAD is a major issue for public healthcare. The morbidity and mortality rates are alarmingly high, especially in patients also presenting with sarcopenia. The mechanistic link between sarcopenia and PAD remains to be investigated but likely involves oxidative stress, mitochondrial dysfunction, inflammation and impaired muscle synthesis, and degradation pathways. Although difficult, the diagnosis of sarcopenia is crucial for PAD patients' care, as it determines prognosis, quality of life, and possible treatments. Indeed, targeting the muscular defects through exercise training could reverse the sarcopenic features observed in patients suffering from PAD and thus, ameliorate their quality of life and overall prognosis. Further, although therapeutic data are largely contradictory, complementary pharmacologic strategies focused on muscle mitochondrial dysfunction through oxidative stress, inflammation, and/or angiogenesis modulation should be further investigated in view of their potential usefulness as new innovative therapeutic approaches against sarcopenia (Figure 3).
FIGURE 3.
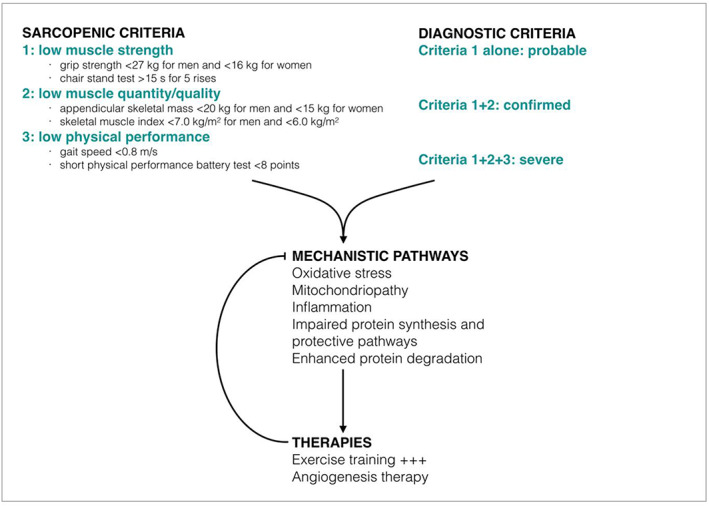
Sarcopenia and PAD: Diagnostic criteria, mechanistic pathways, and current therapies.
Ethical statement
The authors certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 88
Conflict of interest
None declared.
Pizzimenti M., Meyer A., Charles A. L., Giannini M., Chakfé N., Lejay A., and Geny B. (2020) Sarcopenia and peripheral arterial disease: a systematic review, Journal of Cachexia, Sarcopenia and Muscle, 11, 866–886. 10.1002/jcsm.12587
References
- 1. Mendis S, Puska P, Norrving B. Global atlas on cardiovascular disease prevention and control. Global atlas on cardiovascular disease prevention and control Published Online First: 2011https://www.cabdirect.org/cabdirect/abstract/20123402600 (accessed 10 Dec 2018).
- 2. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter‐society consensus for the management of peripheral arterial disease (TASC II). Eur J Vasc Endovasc Surg 2007;33:S1–S75. [DOI] [PubMed] [Google Scholar]
- 3. Aboyans V, Ricco JB, Bartelink MLE, Björck M, Brodmann M, Cohnert T, et al. Editor's Choice ‐ 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018;55:305–368. [DOI] [PubMed] [Google Scholar]
- 4. Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular guidelines on the management of chronic limb‐threatening ischemia. J Vasc Surg 2019;69:3S–125S.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDermott MM, Guralnik JM, Ferrucci L, Tian L, Pearce WH, Hoff F, et al. Physical activity, walking exercise, and calf skeletal muscle characteristics in patients with peripheral arterial disease. J Vasc Surg 2007;46:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019;393:2636–2646. [DOI] [PubMed] [Google Scholar]
- 7. Molnar AO, Eddeen AB, Ducharme R, Garg AX, Harel Z, McCallum MK, et al. Association of computed tomographic leg muscle characteristics with lower limb and cardiovascular events in patients with peripheral artery disease. J Am Heart Assoc 2018;7:e009943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moher D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med 2009;151:264. [DOI] [PubMed] [Google Scholar]
- 9. Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care 2001;24:1433–1437. [DOI] [PubMed] [Google Scholar]
- 10. Pottecher J, Adamopoulos C, Lejay A, Bouitbir J, Charles AL, Meyer A, et al. Diabetes worsens skeletal muscle mitochondrial function, oxidative stress, and apoptosis after lower‐limb ischemia‐reperfusion: implication of the RISK and SAFE pathways? Front Physiol 2018;9:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartolo E, Thorne CS, Gatt A, Formosa C. The influence of peripheral arterial disease on lower limb surface myoelectric signals in patients living with type II diabetes mellitus. Gait Posture 2019;73:228–232. [DOI] [PubMed] [Google Scholar]
- 12. Perna S, Spadaccini D, Rondanelli M. Sarcopenic obesity: time to target the phenotypes. J Cachexia Sarcopenia Muscle 2019;10:710–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baracos VE. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle 2017;8:527–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hale AL, Twomey K, Ewing JA, Langan EM III, Cull DL, Gray BH. Impact of sarcopenia on long‐term mortality following endovascular aneurysm repair. Vasc Med 2016;21:217–222. [DOI] [PubMed] [Google Scholar]
- 17. McDermott MM, Liu K, Tian L, Guralnik JM, Criqui MH, Liao Y, et al. Calf muscle characteristics, strength measures, and mortality in peripheral arterial disease: a longitudinal study. J Am Coll Cardiol 2012;59:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sugai T, Watanabe T, Otaki Y, Goto J, Watanabe K, Toshima T, et al. Decreased psoas muscle computed tomography value predicts poor outcome in peripheral artery disease. Circ J 2018;82:3069–3075. [DOI] [PubMed] [Google Scholar]
- 19. Reeve TE IV, Ur R, Craven TE, Kaan JH, Goldman MP, Edwards MS, et al. Grip strength measurement for frailty assessment in patients with vascular disease and associations with comorbidity, cardiac risk, and sarcopenia. J Vasc Surg 2018;67:1512–1520. [DOI] [PubMed] [Google Scholar]
- 20. Singh N, Liu K, Tian L, Criqui MH, Guralnik JM, Ferrucci L, et al. Leg strength predicts mortality in men but not in women with peripheral arterial disease. J Vasc Surg 2010;52:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nyers ES, Brothers TE. Perioperative psoas to lumbar vertebral index does not successfully predict amputation‐free survival after lower extremity revascularization. J Vasc Surg 2017;66:1820–1825. [DOI] [PubMed] [Google Scholar]
- 22. Matsubara Y, Matsumoto T, Aoyagi Y, Tanaka S, Okadome J, Morisaki K, et al. Sarcopenia is a prognostic factor for overall survival in patients with critical limb ischemia. J Vasc Surg 2015;61:945–950. [DOI] [PubMed] [Google Scholar]
- 23. Matsubara Y, Matsumoto T, Inoue K, Matsuda D, Yoshiga R, Yoshiya K, et al. Sarcopenia is a risk factor for cardiovascular events experienced by patients with critical limb ischemia. J Vasc Surg 2017;65:1390–1397. [DOI] [PubMed] [Google Scholar]
- 24. Morisaki K, Furuyama T, Matsubara Y, Inoue K, Kurose S, Yoshino S, et al. External validation of CLI Frailty Index and assessment of predictive value of modified CLI Frailty Index for patients with critical limb ischemia undergoing infrainguinal revascularization. Vascular 2019;1708538119836005, 10.1177/1708538119836005 [DOI] [PubMed] [Google Scholar]
- 25. Taniguchi R, Deguchi J, Hashimoto T, Sato O. Sarcopenia as a possible negative predictor of limb salvage in patients with chronic limb‐threatening ischemia. Ann Vasc Dis 2019;12:194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shimazoe H, Mii S, Koyanagi Y, Ishida M. Impact of low activity of daily living on the prognosis of patients with critical limb ischemia and sarcopenia. Ann Vasc Surg 2019;61:156–164. [DOI] [PubMed] [Google Scholar]
- 27. Myers SA, Applequist BC, Huisinga JM, Pipinos II, Johanning JM. Gait kinematics and kinetics are affected more by peripheral arterial disease than by age. J Rehabil Res Dev 2016;53:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McDermott MM, Tian L, Ferrucci L, Liu K, Guralnik JM, Liao Y, et al. Associations between lower extremity ischemia, upper and lower extremity strength, and functional impairment with peripheral arterial disease. J Am Geriatr Soc 2008;56:724–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schieber MN, Hasenkamp RM, Pipinos II, Johanning JM, Stergiou N, DeSpiegelaere HK, et al. Muscle strength and control characteristics are altered by peripheral artery disease. J Vasc Surg 2017;66:178–186.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Câmara LC, Ritti‐Dias RM, Menêses AL, Greve JMDA, Jacob Filho W, Santarém JM, et al. Isokinetic strength and endurance in proximal and distal muscles in patients with peripheral artery disease. Ann Vasc Surg 2012;26:1114–1119. [DOI] [PubMed] [Google Scholar]
- 31. Wurdeman SR, Koutakis P, Myers SA, Johanning JM, Pipinos II, Stergiou N. Patients with peripheral arterial disease exhibit reduced joint powers compared to velocity‐matched controls. Gait Posture 2012;36:506–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koutakis P, Johanning JM, Haynatzki GR, Myers SA, Stergiou N, Longo GM, et al. Abnormal joint powers before and after the onset of claudication symptoms. J Vasc Surg 2010;52:340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dziubek W, Bulińska K, Stefańska M, Woźniewski M, Kropielnicka K, Jasiński T, et al. Peripheral arterial disease decreases muscle torque and functional walking capacity in elderly. Maturitas 2015;81:480–486. [DOI] [PubMed] [Google Scholar]
- 34. Parmenter BJ, Raymond J, Dinnen PJ, Lusby RJ, Singh MAF. Preliminary evidence that low ankle‐brachial index is associated with reduced bilateral hip extensor strength and functional mobility in peripheral arterial disease. J Vasc Surg 2013;57:963–973.e1. [DOI] [PubMed] [Google Scholar]
- 35. Kakihana T, Ito O, Sekiguchi Y, Ito D, Goto H, Akamatsu D, et al. Hip flexor muscle dysfunction during walking at self‐selected and fast speed in patients with aortoiliac peripheral arterial disease. J Vasc Surg 2017;66:523–532. [DOI] [PubMed] [Google Scholar]
- 36. Koutakis P, Pipinos II, Myers SA, Stergiou N, Lynch TG, Johanning JM. Joint torques and powers are reduced during ambulation for both limbs in patients with unilateral claudication. J Vasc Surg 2010;51:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Herman SD, Liu K, Tian L, Guralnik JM, Ferrucci L, Criqui MH, et al. Baseline lower extremity strength and subsequent decline in functional performance at 6‐year follow‐up in persons with lower extremity peripheral arterial disease. J Am Geriatr Soc 2009;57:2246–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuo H‐K, Yu Y‐H. The relation of peripheral arterial disease to leg force, gait speed, and functional dependence among older adults. J Gerontol A Biol Sci Med Sci 2008;63:384–390. [DOI] [PubMed] [Google Scholar]
- 39. Regensteiner JG, Wolfel EE, Brass E, Carry MR, Ringel SP, Hargarten ME, et al. Chronic changes in skeletal muscle histology and function in peripheral arterial disease. Circulation 1993;87:413–421. [DOI] [PubMed] [Google Scholar]
- 40. Koutakis P, Myers SA, Cluff K, Ha DM, Haynatzki G, McComb RD, et al. Abnormal myofiber morphology and limb dysfunction in claudication. J Surg Res 2015;196:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. King S, Vanicek N, O'Brien TD. Dynamic muscle quality of the plantar flexors is impaired in claudicant patients with peripheral arterial disease and associated with poorer walking endurance. J Vasc Surg 2015;62:689–697. [DOI] [PubMed] [Google Scholar]
- 42. King SL, Vanicek N, O'Brien TD. Gastrocnemius muscle architecture and achilles tendon properties influence walking distance in claudicants with peripheral arterial disease. Muscle Nerve 2016;53:733–741. [DOI] [PubMed] [Google Scholar]
- 43. Lejay A, Choquet P, Thaveau F, Singh F, Schlagowski A, Charles AL, et al. A new murine model of sustainable and durable chronic critical limb ischemia fairly mimicking human pathology. Eur J Vasc Endovasc Surg 2015;49:205–212. [DOI] [PubMed] [Google Scholar]
- 44. Pipinos II, Swanson SA, Zhu Z, Nella AA, Weiss DJ, Gutti TL, et al. Chronically ischemic mouse skeletal muscle exhibits myopathy in association with mitochondrial dysfunction and oxidative damage. Am J Physiol Regul Integr Comp Physiol 2008;295:R290–R296.18480238 [Google Scholar]
- 45. Pipinos II, Judge AR, Zhu Z, Selsby JT, Swanson SA, Johanning JM, et al. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic Biol Med 2006;41:262–269. [DOI] [PubMed] [Google Scholar]
- 46. Hart CR, Layec G, Trinity JD, Kwon OS, Zhao J, Reese VR, et al. Increased skeletal muscle mitochondrial free radical production in peripheral arterial disease despite preserved mitochondrial respiratory capacity. Exp Physiol 2018;103:838–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weiss DJ, Casale GP, Koutakis P, Nella AA, Swanson SA, Zhu Z, et al. Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease. J Transl Med 2013;11:230–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schmidt CA, Ryan TE, Lin CT, Inigo MM, Green TD, Brault JJ, et al. Diminished force production and mitochondrial respiratory deficits are strain‐dependent myopathies of subacute limb ischemia. J Vasc Surg 2017;65:1504–1514.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bhat HK, Hiatt WR, Hoppel CL, Brass EP. Skeletal muscle mitochondrial DNA injury in patients with unilateral peripheral arterial disease. Circulation 1999;99:807–812. [DOI] [PubMed] [Google Scholar]
- 50. Pipinos II, Sharov VG, Shepard AD, Anagnostopoulos PV, Katsamouris A, Todor A, et al. Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J Vasc Surg 2003;38:827–832. [DOI] [PubMed] [Google Scholar]
- 51. Koutakis P, Miserlis D, Myers SA, Kim JKS, Zhu Z, Papoutsi E, et al. Abnormal accumulation of desmin in gastrocnemius myofibers of patients with peripheral artery disease: associations with altered myofiber morphology and density, mitochondrial dysfunction and impaired limb function. J Histochem Cytochem 2015;63:256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. White SH, McDermott MM, Sufit RL, Kosmac K, Bugg AW, Gonzalez‐Freire M, et al. Walking performance is positively correlated to calf muscle fiber size in peripheral artery disease subjects, but fibers show aberrant mitophagy: an observational study. J Transl Med 2016;14:284–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ryan TE, Yamaguchi DJ, Schmidt CA, Zeczycki TN, Shaikh SR, Brophy P, et al. Extensive skeletal muscle cell mitochondriopathy distinguishes critical limb ischemia patients from claudicants. JCI Insight 2018;3:https://www.ncbi.nlm.nih.gov/pubmed/30385731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McDermott MM, Guralnik JM, Corsi A, Albay M, Macchi C, Bandinelli S, et al. Patterns of inflammation associated with peripheral arterial disease: The InCHIANTI study. Am Heart J 2005;150:276–281. [DOI] [PubMed] [Google Scholar]
- 55. McDermott MM, Greenland P, Green D, Guralnik JM, Criqui MH, Liu K, et al. D‐dimer, inflammatory markers, and lower extremity functioning in patients with and without peripheral arterial disease. Circulation 2003;107:3191–3198. [DOI] [PubMed] [Google Scholar]
- 56. McDermott MM, Ferrucci L, Guralnik JM, Tian L, Green D, Liu K, et al. Elevated levels of inflammation, d‐dimer, and homocysteine are associated with adverse calf muscle characteristics and reduced calf strength in peripheral arterial disease. J Am Coll Cardiol 2007;50:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tuomisto TT, Rissanen TT, Vajanto I, Korkeela A, Rutanen J, Ylä‐Herttuala S. HIF‐VEGF‐VEGFR‐2, TNF‐alpha and IGF pathways are upregulated in critical human skeletal muscle ischemia as studied with DNA array. Atherosclerosis 2004;174:111–120. [DOI] [PubMed] [Google Scholar]
- 58. Sugo T, Terada M, Oikawa T, Miyata K, Nishimura S, Kenjo E, et al. Development of antibody‐siRNA conjugate targeted to cardiac and skeletal muscles. J Control Release 2016;237:1–13. [DOI] [PubMed] [Google Scholar]
- 59. Ha DM, Carpenter LC, Koutakis P, Swanson SA, Zhu Z, Hanna M, et al. Transforming growth factor‐beta 1 produced by vascular smooth muscle cells predicts fibrosis in the gastrocnemius of patients with peripheral artery disease. J Transl Med 2016;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Landry GJ, Esmonde NO, Lewis JR, Azarbal AF, Liem TK, Mitchell EL, et al. Objective measurement of lower extremity function and quality of life after surgical revascularization for critical lower extremity ischemia. J Vasc Surg 2014;60:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lejay A, Laverny G, Paradis S, Schlagowski AI, Charles AL, Singh F, et al. Moderate Exercise Allows for shorter Recovery Time in Critical Limb Ischemia. Front Physiol 2017;8:523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hain BA, Dodd SL, Judge AR. IκBα degradation is necessary for skeletal muscle atrophy associated with contractile claudication. Am J Physiol Regul Integr Comp Physiol 2011;300:R595–R604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nagase H, Yao S, Ikeda S. Acute and chronic effects of exercise on mRNA expression in the skeletal muscle of two mouse models of peripheral artery disease. PLoS ONE 2017;12:e0182456, 10.1371/journal.pone.0182456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brevetti G, De Caterina M, Martone VD, Ungaro B, Corrado F, Silvestro A, et al. Exercise increases soluble adhesion molecules ICAM‐1 and VCAM‐1 in patients with intermittent claudication. Clin Hemorheol Microcirc 2001;24:193–199. [PubMed] [Google Scholar]
- 65. Signorelli SS, Mazzarino MC, Pino LD, Malaponte G, Porto C, Pennisi G, et al. High circulating levels of cytokines (IL‐6 and TNFalpha), adhesion molecules (VCAM‐1 and ICAM‐1) and selectins in patients with peripheral arterial disease at rest and after a treadmill test. Vasc Med 2003;8:15–19. [DOI] [PubMed] [Google Scholar]
- 66. Saetre T, Enoksen E, Lyberg T, Stranden E, Jørgensen JJ, Sundhagen JO, et al. Supervised exercise training reduces plasma levels of the endothelial inflammatory markers E‐selectin and ICAM‐I in patients with peripheral arterial disease. Angiology 2011;62:301–305. [DOI] [PubMed] [Google Scholar]
- 67. Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step‐monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc 2014;3:e001107, 10.1161/JAHA.114.001107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McGuigan MR, Bronks R, Newton RU, Sharman MJ, Graham JC, Cody DV, et al. Resistance training in patients with peripheral arterial disease: effects on myosin isoforms, fiber type distribution, and capillary supply to skeletal muscle. J Gerontol A Biol Sci Med Sci 2001;56:B302–B310. [DOI] [PubMed] [Google Scholar]
- 69. Cousin A, Popielarz S, Wieczorek V, Tiffreau V, Mounier‐Vehier C, Thevenon A. Impact of a rehabilitation program on muscular strength and endurance in peripheral arterial occlusive disease patients. Ann Phys Rehabil Med 2011;54:429–442. [DOI] [PubMed] [Google Scholar]
- 70. Wang E, Helgerud J, Loe H, Indseth K, Kaehler N, Hoff J. Maximal strength training improves walking performance in peripheral arterial disease patients. Scand J Med Sci Sports 2010;20:764–770. [DOI] [PubMed] [Google Scholar]
- 71. Mosti MP, Wang E, Wiggen ØN, Helgerud J, Hoff J. Concurrent strength and endurance training improves physical capacity in patients with peripheral arterial disease. Scand J Med Sci Sports 2011;21:e308–e314. [DOI] [PubMed] [Google Scholar]
- 72. Wang J, Zhou S, Bronks R, Graham J, Myers S. Effects of supervised treadmill‐walking training on strength and endurance of the calf muscles of individuals with peripheral arterial disease. Clin J Sport Med 2006;16:397–400. [DOI] [PubMed] [Google Scholar]
- 73. Vun SV, Miller MD, Delaney CL, Allan RB, Spark JI. The effect of supervised exercise therapy for intermittent claudication on lower limb lean mass. J Vasc Surg 2016;64:1763–1769. [DOI] [PubMed] [Google Scholar]
- 74. Hiatt WR, Regensteiner JG, Wolfel EE, Carry MR, Brass EP. Effect of exercise training on skeletal muscle histology and metabolism in peripheral arterial disease. J Appl Physiol 1996;81:780–788. [DOI] [PubMed] [Google Scholar]
- 75. Januszek R, Mika P, Konik A, Petriczek T, Nowobilski R, Niżankowski R. Effect of treadmill training on endothelial function and walking abilities in patients with peripheral arterial disease. J Cardiol 2014;64:145–151. [DOI] [PubMed] [Google Scholar]
- 76. Hiatt WR, Wolfel EE, Meier RH, Regensteiner JG. Superiority of treadmill walking exercise versus strength training for patients with peripheral arterial disease. Implications for the mechanism of the training response. Circulation 1994;90:1866–1874. [DOI] [PubMed] [Google Scholar]
- 77. Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg 1996;23:104–115. [DOI] [PubMed] [Google Scholar]
- 78. McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 2009;301:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gardner AW, Katzel LI, Sorkin JD, Killewich LA, Ryan A, Flinn WR, et al. Improved functional outcomes following exercise rehabilitation in patients with intermittent claudication. J Gerontol A Biol Sci Med Sci 2000;55:M570–M577. [DOI] [PubMed] [Google Scholar]
- 80. Schieber, M.N. , Pipinos, I.I. , Johanning, J.M. , Casale, G.P. , Williams, M.A. , DeSpiegelaere, H.K. , Senderling, B. and Myers, S.A. Supervised walking exercise therapy improves gait biomechanics in patients with peripheral artery disease. J Vasc Surg Published Online First: 20 August 2019. doi:, 71, 575, 583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parmenter BJ, Raymond J, Dinnen P, Lusby RJ, Fiatarone Singh MA. High‐intensity progressive resistance training improves flat‐ground walking in older adults with symptomatic peripheral arterial disease. J Am Geriatr Soc 2013;61:1964–1970. [DOI] [PubMed] [Google Scholar]
- 82. Pilz M, Kandioler‐Honetz E, Wenkstetten‐Holub A, Doerrscheidt W, Mueller R, Kurz RW. Evaluation of 6‐ and 12‐month supervised exercise training on strength and endurance parameters in patients with peripheral arterial disease. Wien Klin Wochenschr 2014;126:383–389. [DOI] [PubMed] [Google Scholar]
- 83. Reis PEO, de Carvalho LP, Yasumura E, Silva FHD, Garcia BC, Beutel A, et al. Impact of angiogenic therapy in the treatment of critical lower limb ischemia in an animal model. Vasc Endovascular Surg 2014;48:207–216. [DOI] [PubMed] [Google Scholar]
- 84. Liu Q, Chen Z, Terry T, McNatt JM, Willerson JT, Zoldhelyi P. Intra‐arterial transplantation of adult bone marrow cells restores blood flow and regenerates skeletal muscle in ischemic limbs. Vasc Endovascular Surg 2009;43:433–443. [DOI] [PubMed] [Google Scholar]
- 85. Da Cunha FF, Martins L, Martin PKM, Stilhano RS, Gamero EJP, Han SW. Comparison of treatments of peripheral arterial disease with mesenchymal stromal cells and mesenchymal stromal cells modified with granulocyte and macrophage colony‐stimulating factor. Cytotherapy 2013;15:820–829. [DOI] [PubMed] [Google Scholar]
- 86. Noguchi T, Kakinuma Y, Arikawa M, Okazaki K, Hoshino E, Iiyama T, et al. Donepezil can improve ischemic muscle atrophy by activating angiomyogenic properties of satellite cells. Circ J 2014;78:2317–2324. [DOI] [PubMed] [Google Scholar]
- 87. Rajagopalan S, Trachtenberg J, Mohler E, Olin J, McBride S, Pak R, et al. Phase I study of direct administration of a replication deficient adenovirus vector containing the vascular endothelial growth factor cDNA (CI‐1023) to patients with claudication. Am J Cardiol 2002;90:512–516. [DOI] [PubMed] [Google Scholar]
- 88. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


