Abstract

Pulmonary arterial hypertension is a rare and devastating disease characterized by an abnormal chronic increase in pulmonary arterial pressure above 20 mmHg at rest, with a poor prognosis if not treated. Currently, there is not a single fully effective therapy, even though a dozen of drugs have been developed in the last decades. Pulmonary arterial hypertension is a multifactorial disease, meaning that several molecular mechanisms are implicated in its pathology. The main molecular pathways regulating the pulmonary vasomotor tone—endothelin, nitric oxide, and prostacyclin—are the most biologically and therapeutically explored to date. However, drugs targeting these pathways have already found their limitations. In the last years, translational research and clinical trials have made a strong effort in suggesting and testing novel therapeutic strategies for this disease. These approaches involve targeting the main molecular pathways with novel drugs, drug repurposing for novel targets, and also using combinatorial therapies. In this review, we summarize current strategies and drugs targeting the endothelin, nitric oxide, and prostacyclin pathways, as well as, the emerging new drugs proposed to cope with vascular remodelling, metabolic switch, perivascular inflammation, epigenetic modifications, estrogen deregulation, serotonin, and other neurohumoral mechanisms characteristic of this disease. Nowadays, pulmonary arterial hypertension remains an incurable disease; however, the incoming new knowledge makes us believe that new promising therapies are coming to the clinical arena soon.
Keywords: pulmonary arterial hypertension, pharmacotherapy, drug targets, emerging therapies
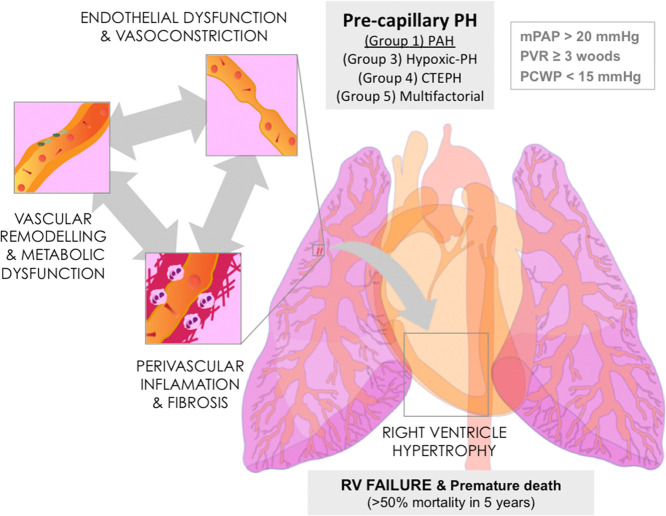
Pulmonary arterial hypertension (PAH) is an aggressive rare condition with a poor prognosis—its prevalence ranges from 15 to 60 cases per million in Europe—and a high morbidity and mortality rate, more than 40% over 5 years after diagnosis.1−4 According to the last consensus reached at the Sixth World Symposium on Pulmonary Hypertension in 2018,5 PAH, also called precapillary pulmonary hypertension (PH), is defined by an abnormal chronic increase in the mean pulmonary arterial pressure (mPAP) ≥ 20 mmHg, together with a pulmonary capillary wedge pressure (PCWP) ≤ 15 mmHg and a pulmonary vascular resistance (PVR) ≥ 3 Woods units at rest.5 Such a chronic increase in mPAP and PVR originates by functional and structural changes in the small pulmonary arterioles, endothelial damage, media thickening, and inflammation, causing an aberrant vascular remodelling together with an increase in vascular tone6 (Figure 1). Anomalous production of different endothelial vasoactive mediators, for instance nitric oxide (NO), prostacyclin, or endothelin (ET-1) are common characteristics of PAH7,8 (Figure 2). As a consequence, chronic pressure overload progressively affects the right ventricle (RV), inducing in a first instance a compensatory RV hypertrophy, and eventually RV dysfunction followed by failure and premature death within 5 years if not treated.9,10
Figure 1.
Hallmarks of PAH. The initial endothelial dysfunction compromising vascular dilation and permeability is followed by perivascular inflammation, fibrosis, metabolic dysfunction, and vascular remodelling, all contributing to the rise in mPAP, RV hypertrophy, failure, and premature death: mPAP, mean pulmonary arterial pressure; RV, right ventricle; PH, pulmonary hypertension; PAH, pulmonary arterial hypertension; CTEPH, chronic thromboembolic PH; PVR, pulmonary vascular resistance; PCWP, pulmonary capillary wedge pressure. Individual illustrations were kindly provided by Dr Carlos Galan-Arriola.
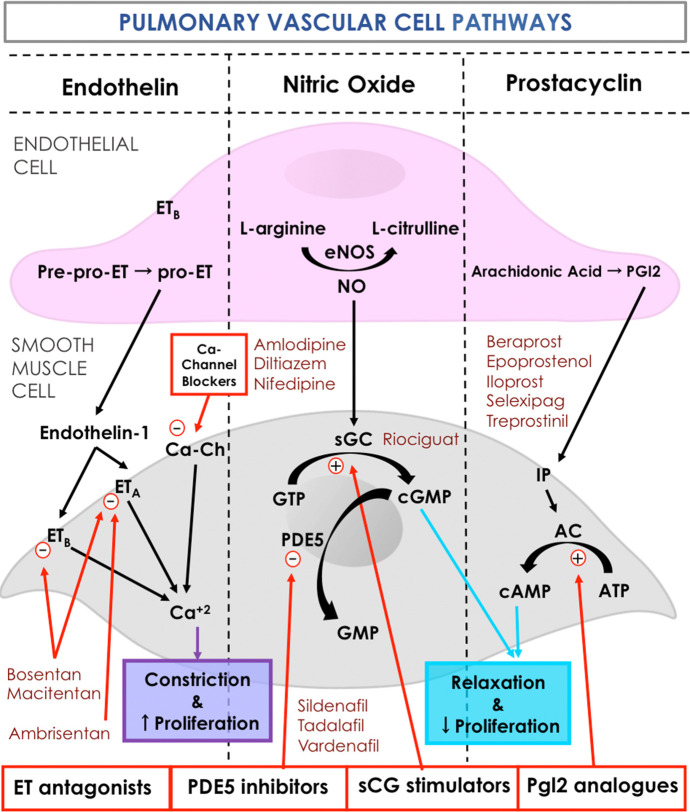
Figure 2.
Current targets and therapies in PAH. ET, indicates endothelin; ETA, endothelin receptor type A; ETB, endothelin receptor type B; PGI2, prostaglandin I2; IP, PGI2 receptor; NO, nitric oxide; PDE5, phosphodiesterase type 5; sGC, soluble guanylate cyclase; GMP, guanosine triphosphate; cGMP, cyclic guanosine triphosphate; cAMP, cyclic adenosine monophosphate; ATP, adenosine triphosphate; AC, adenyl cyclase; Ca-Ch, calcium channel. Individual illustrations were kindly provided by Dr. Carlos Galan-Arriola.
On the basis of the latest classification, PH is classified into five groups. Among them, PAH is classified within group 1 (Figure 1). At the same time, group 1 PAH can be considered either idiopathic, heritable, associated with other diseases such as congenital heart disease, connective tissue disease, portal hypertension, or associated with exposure to certain toxins and drugs and even related to HIV infection. In addition, depending on the symptoms, pathophysiology, response to drug treatment, and clinical situation, PAH is classified into four different clinical categories–from mild to severe–according to the World Health Organization (WHO).2,5 According to the guidelines for its management, the diagnosis of PH requires clinical suspicion based on symptoms–shortness of breath, fatigue, weakness, angina and syncope–and physical examination–electrocardiogram, chest radiograph, lung computed tomography scan, pulmonary function and 6 min walking distance test. Additionally, confirmation of hemodynamic values is essential for the diagnosis and for a better description of its severity. This is normally done by right heart catheterization and vasoreactivity, echocardiography and cardiac magnetic resonance.2
Several drugs have been developed in the last decades giving a considerable improvement in the life quality and expectancy of PAH patients. Current drugs are able to improve physical exercise tolerance—the 6 min walking distance test—and pulmonary circulation hemodynamics up to some point, aiming to reduce vascular tone and remodelling.2 However, these therapies normally focus on controlling symptoms and signs, which can extend and improve the life of patients, but the disease still remains incurable. The most common current therapeutic strategies seek to restore the appropriate balance between vasoconstriction and vasodilation, and also to have an antiproliferative effect, which mitigates the anomalous proliferation of pulmonary endothelial and smooth muscle cells (SMCs).11 Although there is currently no fully effective treatment for PAH, novel strategies are continuously emerging to cope with other pathological signs such as metabolic switch, inflammation, or epigenetics among others. This review aims to bring together current available treatments, and some of the new potential targets and emerging therapies under investigation to fight PAH.
Current Drug Targets and Treatments
The exact mechanisms that trigger the onset of PAH still remain undefined. Nonetheless, it is well established that endothelial dysfunction is one of the hallmarks of the disease (Figure 1). Endothelial cells (ECs) play an important role in regulating vascular tone, vascular remodelling and inflammation through the expression of vasoactive mediators. The three main pathways regulating the pulmonary vasomotor tone are presented in Figure 2: ET-1, NO, and prostacyclin pathways. EC dysfunction leads to increased vascular tone by reducing the production of vasodilators (prostacyclin and NO), while increasing the production of ET-1, a potent vasoconstrictor. At the same time, it stimulates perivascular inflammation and SMC proliferation in pulmonary arteries through a cascade of molecular and cross-cellular responses.6
Currently, specific FAD-approved PAH therapies target these three pathways and, depending on their activity, are classified into four distinct classes: endothelin-receptor antagonists (ERAs), phosphodiesterase type 5 (PDE5) inhibitors, soluble guanylate cyclase (sGC) stimulators, or prostacyclin analogues. ET-1-mediated vasoconstriction can be inhibited by selective or nonselective ET-1 receptor antagonists. The NO pathway can be modulated by the use of PDE5 inhibitors, as well as stimulators of the sGC, both increasing cyclic guanosine monophosphate (cGMP) levels, and therefore stimulating vasodilation,12 or even by exogenous NO therapy.6 The prostacyclin pathway can be enhanced by the administration of prostanoid compounds, which act as powerful vasodilators or nonprostanoid IP receptor agonists.12 Additionally, certain patients with acute vasoreactivity are recommended to receive Ca2+ channel blockers. Therapies, both current and emerging, blend with distinct targets in SMCs in the pulmonary arteries (Figure 2).
Endothelin Receptor Antagonist
The discovery of increased ET-1 levels, a potent vasoconstrictor, among PAH patients was the initial reason to consider endothelin receptor antagonists as a potential therapy.13 Endothelins encompass three similar 21-amino acid peptides (ET-1, ET-2, and ET-3) derived from a 212 amino acid precursor that undergoes successive proteolytical cleavages by furin-type proprotein convertase14 and endothelin converting enzymes (ECE1 and ECE2).15 These active 21-amino acid peptides can be stored or secreted in response to a stimulus. ET peptides act on two individual membrane receptors, ETA and ETB16,17—widely expressed across the body and first described in the lungs—with ETA having equal affinity to isoforms ET-1 and ET-2, and ETB presenting affinity to all three isoforms.18 ET-1 is the most abundant ET and is a potent vasoconstrictor as it binds to smooth muscular ETA and ETB receptors, especially during PAH (Figure 2). On the other side, stimulation of the endothelial ETB receptor releases vasodilators such as NO and prostacyclin (prostaglandin I2) causing vasodilation, but also possibly contributing to cell proliferation. In addition, preclinical research on ERAs has demonstrated numerous benefits ranging from improved hemodynamics, right ventricular hypertrophy, and survival to a reduction in vascular remodelling, fibrosis, and even improved endothelial function of pulmonary vessels.19
Altogether, the vasodilatory effect of ERAs has been considered a relevant way to fight PAH. In this context, bosentan, a mixed ETA/ETB antagonist was the first FDA-approved drug in WHO functional class II–IV PAH patients.20 More recently ETA-selective antagonists such as ambrisentan and macitentan have been also approved for patients at class II–III. Ambrisentan is a highly selective ETA antagonist, which is characterized by a long half-life that allows for a single daily dose and with less liver toxicity than bosentan. Macitentan is a lipophilic, dual ETA and ETB antagonist, which presents better tissue penetration and prolonged binding to the receptors in pulmonary artery SMCs, reducing vascular resistance, decreasing the mortality rate during treatment, and improving the results of the 6 min walking distance test.19,21 Currently, clinical trials to assess combination therapy with PDE-5 inhibitors are ongoing.22
Prostanoids
Prostanoids are arachidonic acid-derived eicosanoids that act as vasoactive mediators by binding and activating G protein-coupled receptors, which fall into three functional categories: relaxant receptors (DP, EP2, EP4, and IP), contractile receptors (EP1, FP, and TP), and the inhibitory receptor EP3. Prostacyclin I2 (PGI2), is an endogenous prostanoid with vasodilation, antiproliferative, and antithrombotic properties. PGI2 is released mainly by ECs in pulmonary arteries and binds to its receptor, IP, leading to SMC relaxation and vasodilation via adenylate cyclase-mediated increase in cyclic adenosine monophosphate (cAMP) levels (Figure 2). Intracellular prostacyclin has also been shown to activate the nuclear receptor peroxisome proliferator-activated receptors (PPAR)23 to inhibit SMC proliferation by cAMP-mediated inhibition of extracellular signal-regulated kinase 1/2 (Erk1/2) signaling.24 It is also known to avoid thrombocyte aggregation by inhibiting platelet activation. Production of prostacyclin has been reported to be downregulated in PAH patients.25,26 Therefore, several therapeutic strategies are based on administration of prostacyclin analogues to reestablish prostacyclin signaling and thereby counteract the deleterious vasoconstrictive effect of ET-1 activity.27
Stable prostanoid analogues are available as drugs, such as epoprostenol, a highly efficient drug with undesirable effects due to its short half-life and its requirement for continuous intravenous infusion; treprostinil with greater stability and longer half-life so it can be administered by subcutaneous, inhaled, intravenous, or oral routes; beraprost (oral administration but with moderate efficacy); and iloprost, which can be inhaled or applied intravenously with a similar efficiency to intravenous epoprostenol but longer half-life (30–45 min).28 The limitations of therapies based on prostacyclin analogues, particularly their short half-life, has led to the development of nonprostanoid prostacyclin receptor agonists, such as selexipag.29,30 Its oral administration causes rapid absorption and hydrolization to its more active metabolite, ACT-333679, by carboxylesterase in the liver. Although both selexipag and ACT-333679 present a high degree of affinity to the IP receptor, the latter is 37 times more potent in activating IP receptors. This is an important advantage in relation to nonselective prostanoids, which are known to have a shared affinity for a variety of prostanoid receptors, including contractile receptors.31,32 Promising results have been obtained with selexipag, as it considerably decreases the risk of death, hospitalization, long-term oxygen therapy, and lung transplantation.22 In addition, retrospective studies have shown that a combination of selexipag with other established PAH therapies stabilizes exercise capacity, right ventricular function, and life quality, while also reducing arterial pressure and symptoms.29,30
Phosphodiesterase Type 5 Inhibitors
The endothelial dysfunction characteristic of PAH affects NO, which is the main physiological regulator of vasodilation. In healthy conditions, NO is synthesized in the vascular endothelium by the nitric oxide synthase (eNOS) (Figure 2). Once synthesized, its diffusion toward SMC activates the sGC that converts GTP into cyclic GMP, thus activating the intracellular cascade leading to vasodilation via protein kinase G and Rho GTPases among others. The lack of NO during PAH favors the increase in vascular tone, therefore maintaining an adequate intracellular cGMP concentration was an obvious therapeutic target to explore.33 In addition to its vasodilatory effect, cGMP has a role on SMC proliferation, with increased cGMP levels having been demonstrated to inhibit proliferation in cultured cells.33 Among the regulators of this pathway, PDE-5 is an enzyme highly present in pulmonary arteries, specifically in SMCs. PDE-5 hydrolyzes cGMP into GMP, thus regulating the vasodilation induced by NO. PDE-5, which is overexpressed in lungs and RVs of PAH patients,34 has been demonstrated to play an important role in PAH. Inhibition of PDE-5 results in an intracellular rise of cGMP levels and therefore has been proven to enhance vasodilation, reduce proliferation, and improve RV function.33,35
Presently, three selective PDE-5 inhibitors, sildenafil, tadalafil, and vardenafil, have been approved for the treatment of PAH. Administration of these inhibitors results in a decrease of mPAP and PVR, and a better functional test. Additionally, tadalafil has been proven to delay disease progression.35 In a recent metanalysis including 36 clinical trials, 19 of them on PAH, involving around 3000 patients with all PH groups, PDE-5 inhibitors were more likely to improve the patients WHO functional class, increase distance of walking 48 m further in the 6 min walking distance tests, and decrease mortality rates compared to placebo.36 While there is no clear benefit of PDE-5 inhibitors in other forms of PH, and a recent trial even found a deleterious effect in group 2 PH,37 the metanalysis mentioned above concluded that this drug family has clear beneficial effects in group 1 PAH. Sildenafil, tadalafil, and vardenafil are all efficacious in this clinical setting, and clinicians should consider the side-effect profile for each individual when choosing which PDE5 inhibitor to prescribe.36
Soluble Guanylate Cyclase Stimulator
Still within the same cellular NO pathway, other strategies have been demonstrated useful for treating PAH. Riociguat is a stimulator of sGC, therefore improving cGMP synthesis even in the absence of NO (Figure 2). Apart from directly stimulating sGC, riociguat also stabilizes its bond with NO, which increases considerably the consequent production of cGMP. The result of this combination is vasodilation and thereby reduction on PVR. Additionally, riociguat induces antiaggregation and antiproliferation as a result of increasing the concentration of cGMP and reacting synergistically with NO. Unlike previous PDE-5 inhibitors, riociguat is independent of endogenous NO. In addition, riociguat showed a superior effect when compared with sildenafil as it not only reduces RV hypertrophy and vascular remodelling but also exhibits an antifibrotic, anti-inflammatory, and antiproliferative effect, improving RV function and pulmonary hemodynamics in animal models of PAH.10,38,39
Nowadays in the clinics, riociguat is the only drug approved both for PAH (group 1 PH), and for patients suffering chronic thromboembolic pulmonary hypertension (group 4 PH).35 Since a proportion of patients under PDE-5 inhibitors do not reach the goals, selected patients with PAH may benefit from switching to riociguat. However, this strategy needs to be further investigated since there are still a few large, randomized and controlled trials comparing drugs alone or in combination.40
Calcium Channel Blockers
An acute increase of intracellular Ca2+ occurs in SMCs as the last step on which all vasoconstrictive signals converge (Figure 2). Calcium channel blockers (CCBs) were therefore initially introduced as systemic antihypertensive drugs because of their ability to reduce intracellular Ca2+, thus avoiding vasoconstriction. However, evidence on their use in PAH suggests several limitations. In fact, generalized use of these drugs are avoided and only recommended in PAH patients with a positive acute vasoreactivity test and that do not present low cardiac output states or elevated right atrial pressure, as CCBs can lead to hemodynamic compromise.41 In that context, the three main classes of CCBs–such as nifedipine, diltiazem, or amlodipine–differ in terms of their chemical composition and display distinct effects determined by biophysical and conformation-dependent synergy with the L-type Ca2+ channel. CCBs constrain inflow of calcium into SMCs, resulting in vasodilation.6 However, according to current standards, this treatment is applied only if the PAH patient exhibits an appropriate systemic blood pressure (>90 mmHg), belonging to functional class I–III before the initiation of therapy.42 Although CCBs can only be used in a limited number of PAH patients and its benefits are still under consideration, certain groups of PAH patients receive some benefit, and therefore, it might be worthwhile to further explore its use as adjunctive therapy.
Emerging Therapies and Targets
Undoubtedly, the research community has achieved immense progress in tackling PAH over the past decade. Novel aspects of PAH are known to be at the origin of the disease: from the initial endothelial damage, followed by perivascular inflammation, together with a sustained cellular stress, all being responsible for the vascular remodelling. All these environmental changes induce a cellular and metabolic switch that enables pulmonary vascular cells to acquire a cancer-like phenotype, characterized both by a pro-proliferative and an antiapoptotic status that pushes vascular cells to proliferate massively, and thus having an important role on the reduction of the lumen size, flow increase, and consequent rise of PAP. Indeed, the cancer theory of PAH has been in place for several years offering an opportunity to exploit therapeutic strategies used currently in cancer: either avoiding proliferative pathways, regulating mitochondrial metabolism, reducing oxidative stress and DNA instability, deregulating epigenetics, or avoiding excessive inflammatory response, among others.43 Herein, we summarize these cellular and molecular deregulations that are becoming more relevant as new targets for potential therapeutic avenues against PAH and their most relevant drugs (Table 1).
Table 1. Selected Emerging Therapeutic Agents in PAHa.
Promising novel pathways are presented in the table together with dysfunctional consequences and possible therapeutic manipulation according to erroneous functions. ACE, angiotensin-converting enzyme; ALK1, activin receptor-like kinase 1; AT-2, angiotensin 2, BMPR2, bone morphogenetic protein receptor type 2; CDCs, cardiosphere-derived cells; DHEA, dehydroepiandrosterone; ECM, extracellular matrix; EGF, epidermal growth factor; EPCs, endothelial progenitor cells; FGF, fibroblast growth factor; HDAC, histone deacetylase; IL, interleukin; mTOR, mammalian target of rapamycin; MIF, macrophage migration-inhibitory factor; PARP, poly-ADP ribose polymerase; PDGF, platelet-derived growth factor; PDK, pyruvate dehydrogenase kinase; ROS, reactive oxygen species; RAAS, renin-angiotensin-aldosterone system; SSRIs, selective serotonin reuptake inhibitor; TXA2, thromboxane A2; VEGF, vascular endothelial growth factor. Individual illustrations were kindly provided by Dr Carlos Galan-Arriola.
Mitochondrial Metabolism and Oxidative Stress
Mitochondria regulate many environmental and molecular cues and as oxygen sensors can alter the production of mitochondria-derived ROS and cause oxidative stress. Because the lungs’ main function is the distribution of oxygen, the pulmonary vasculature and in particular ECs that are at the front line, are very sensitive to the slightest changes in oxygen levels.44 Accordingly, higher ROS production and mitochondrial dysfunction is observed in patients with PAH.45 Since mitochondrial metabolic alterations have been mostly studied in the oncology field, the knowledge acquired in the treatment of these metabolic alterations is now being applied to PAH. Here we will approach several drugs—elamipretide, dichloroacetate, bardoxolone and allopurinol—that are being investigated as promising PAH therapies.
Elamipretide, a novel mitochondrial-targeted drug, is a cell-permeable tetrapeptide that can selectively bind to cardiolipin. Cardiolipin is the main phospholipid of the inner mitochondria membrane and has recently been described to play central roles in mitochondrial metabolism, biogenesis, dynamics, and mitophagy.46 Cardiolipin has been shown to interact with cytochrome C,47,48 which is indispensable for the OXPHOS system and also acts as a radical scavenger. By binding cardiolipin, elamipretide inhibits the formation of the cardiolipin-cytochrome C complex and its underlying peroxidase activity,49 and consequently, reduces ROS production.50 This peptide is also of interest in PAH since it has been demonstrated to attenuate transverse aortic constriction-induced PAH in mice.51 In addition, it is the first in its class to enter clinical trials and has been shown to improve mitochondrial function in the failing heart.52
Pulmonary vascular cells also undergo metabolic changes during PAH, with a shift from oxidative phosphorylation to glycolysis and lactate production, a phenomenon known as the Warburg Effect. Although aerobic glycolysis is less efficient in the production of energy, it leads to increased generation of metabolites that benefit cell proliferation, and SMC hyperproliferation is a key process in the development of PAH. In this context, dichloroacetate (DCA) has been found useful in patients suffering idiopathic PAH. DCA inhibits mitochondrial pyruvate dehydrogenase kinase (PDK), therefore reversing this metabolic switch and re-establishing glucose oxidation.53,54 In the rat model, DCA treatment reverted monocrotaline-induced PAH and RV remodelling, while also preventing the formation of neointimal lesions.53,55,56
The nuclear factor erythroid 2-related factor 2 (Nrf2) is a key regulator of cellular responses to oxidative damage. Under conditions of reduced oxidative stress, Nrf2 associates to Kelch-like ECH-associated protein 1 (Keap1) that acts as a E3 ubiquitin ligase substrate adaptor and targets NRF2 for rapid proteasomal degradation.57−59 However, Keap1 also acts as a redox sensor and upon increased oxidative stress frees Nrf2, allowing it to translocate to the nucleus and activate transcription of downstream antioxidant gene targets.60,61 During recent years, bardoxolone, a small electrophile compound that is in advanced stages of clinical trials (NCT03068130 and NCT0203697), has been found to act as a positive modulator of Nrf2 function by releasing it from the inhibitory Nrf2-Keap1 complex.
Another example of therapies that are currently under investigation against the metabolic dysfunction in PAH3,62 is allopurinol, a drug that has been vastly used to inhibit uric acid and that is being repurposed for PAH to reduce oxidative stress. By inhibiting xanthine oxidase, allopurinol limits the generation of both ROS and uric acid. Finally, preclinical data also support the use of insulin resistant drugs such as rosiglitazone, metformin, and glucagon-like peptide 1 (GLP-1) receptor agonists as potential therapeutic strategies for PAH.63
Perivascular Inflammation
Perivascular infiltration of inflammatory cells (macrophages, B and T lymphocytes and dendritic cells) has been described in pulmonary vessels of PAH patients,64−66 as well as the presence of autoantibodies, autoimmune disorders (e.g., HIV), and increased levels of many cytokines (IL-1β, IL-6, IL-18, TNFα, and MCP-1).67−70 Altogether, these several pieces of evidence highlight the importance of an altered immune response in PAH.
In this context, the inflammasome, a key regulator of innate immune response, has received special attention. Inflammasomes are a family of multiprotein complexes—present mostly in macrophages but also ECs, neutrophils, and dendritic cells—that activate in response to pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) generated by the host cell. Examples of DAMPs include the production of ROS, release of oxidized mitochondrial DNA, mitochondrial stress, and changes in intracellular calcium levels. Activation of the inflammasome leads to increased expression of pro-inflammatory IL-1β and IL-18, which then promotes the release of several secondary cytokines, including IL-2, IL-6, and IL-12.71 Several approaches aimed at modulating inflammasome activity at distinct levels of the signaling cascade have been employed. Examples of such targets are IL-1, IL-6.
Repurposing of anakinra, a recombinant IL-1 receptor antagonist used for rheumatoid arthritis, inhibits both IL-1α and IL-1β function and has been shown to be beneficial for PAH patients.72 Tocilizumab, an IL-6R-blocking monoclonal antibody, also used for rheumatoid arthritis,73 is currently in clinical trials for its repurposing for PAH. In 2015, a selective NLRP3 inhibitor, MCC950,74 was described and has revealed promising results in several animal models of cardiovascular and lung disease75−78 but remains to be tested in humans.
Since autoantibodies have a high prevalence in PAH, one other therapeutic strategy that has been used is the administration of rituximab, an anti-CD20 monoclonal antibody, that selectively targets B lymphocytes inducing its lysis. When administered in rat models of SU5416-induced PAH, rituximab led to decreased PASMC proliferation, lower mPAP and decreased RV remodelling.79 Although few studies support the efficacy of immunosuppressants in humans,80 some have recently shown the concomitant use of immunosuppressant together with PAH-specific therapy as a bridge therapy in severe systemic lupus erythematosus associated PAH (cyclophosphamide and corticosteroids).81
DNA Damage Accumulation and Mutations
As mentioned above, the primary mechanisms that trigger the onset of PAH still remain undefined. However, it is known that oxidative stress and inflammation are a hallmark in the development and progression of PAH and that these factors pose a threat to DNA integrity. Accordingly, high levels of DNA damage are observed in PAH patients and may potentiate the hyperproliferative and apoptosis-resistant phenotype of these vascular cells.82−84 In this regard, anticancer drugs have been assessed as a potential new treatment. For instance, two clinically available inhibitors of poly-ADP-ribose polymerase (PARP), olaparib and veliparib, promote cell death by preventing the repair of DNA damage. Inhibition of PARP significantly mitigated PAH in several animal models of PAH63,80 and therefore, olaparib is currently in clinical trials for repurposing for PAH patients (NCT03782818).63 However, these drugs normally exhibit strong side effects and further clinical trials will bring more evidence about risk/benefit balance in patients.
On the other side, deregulation of the transforming growth factor-β/bone morphogenetic protein (TGFβ/BMP) signaling pathway, which controls cell proliferation, may be due to pathogenic mutations. This is the case of the bone morphogenetic protein receptor type II (BMPR2) signaling impairment, which is present in 80% of patients with a family history of PAH and 25% of idiopathic PAH.85 BMPR2 is a serine/threonine receptor kinase that receives signals from BMPs and induces several cellular functions such as osteogenesis, cell growth, and cell differentiation. Although BMPR2 is expressed in a wide number of cells types, it is especially highly expressed in the pulmonary vascular endothelium. Loss of BMPR2 promotes endothelial-to-mesenchymal transition and favors endothelial dysfunction.40,86 Regulation of the TGFβ/BMP signaling pathway is very complex and includes interaction with Smad complexes, a family of transcription factors that regulates the expression of several genes related to the mentioned cellular functions controlled by this pathway. Through loss of antiproliferative Smad1/5 signaling, pulmonary artery SMCs with BMPR2 mutations become hyperproliferative and resistant to the growth-suppressive effects of bone morphogenetic proteins.87 A few therapeutic strategies have been developed to reverse impairment of BMPR2 function, such as gene therapy via intravenous adenoviral BMPR2 gene delivery, which proved to be efficient in murine models of PAH.88 Other strategies such as chloroquine, which is supposed to inhibit BMPR2 lysosomal degradation exerted a positive effect in rats;89 BMP9 administration in heterozygous BMPR2 knockout mice also had a beneficial effect;90 ataluren, that renders ribosomes less sensitive to stop-codons, has been used to restore full-length BMPR2 protein thus reestablishing the pathway.91 Lastly and very promising, are drugs that regulate the TGF-β/BMP pathway, such as sotatercep. Sotatercep is a recombinant human ActRIIA IgG1 Fc fusion protein that acts as a TGFβ ligand trap and thereby inhibits TGFβ signaling and has been found useful in inhibiting cell proliferation. Indeed, very recently sotatercept was shown to have a potent effect in avoiding vascular remodelling, by inhibiting Smad2/3-mediated cell proliferation and by enhancing apoptosis of pulmonary vascular cells in animal models,92 and is currently enrolled in phase 2 clinical trials (NCT03738150 and NCT03496207). Altogether, there is significant evidence to consider these regulators of the TGF-β/BMP pathway as potential therapies for human PAH in the near future.
Epigenetic Modifications
Nowadays, there is a rising interest in epigenetics as epigenetic modifications on cellular DNA or histones that affect gene expression are reversible and do not change the gene sequence.43 Clearly, it may allow finding new therapeutic targets in many different diseases. Even though the role of histone modification is still poorly understood in PAH, histone deacetylase (HDAC) inhibitors have been used in PAH due to their favorable effects in inflammation and cancer.93 While research results have been mixed, studies performed on rat models produced positive outcomes in pulmonary vascular remodelling.94 This new, innovative strategy needs further assessment with more selective HDAC inhibitors or genetic manipulations.95 Vorinostat, is one of the most representative HDAC inhibitors; however, it is a general inhibitor that acts on class I, II, and IV of HDACs. A selective inhibition of HDAC-6 by tubastatin A has been suggested to be more specific and to have less undesirable effects.96 It is also interesting to bring attention to Bromodomain-containing protein 4 (BRD4) inhibitors, such as apabetalone. BRD4 is a member of the bromodomain and extra terminal domain family (BET) that recruits transcriptional regulatory complexes to acetylated chromatin97 and that is upregulated in PASMCs isolated from PAH patients.98 By binding to BRD4, apabetalone has been shown to reverse vascular remodelling in animal models by repressing pathways that contribute to the hyperproliferative, apoptosis-resistant and proinflammatory phenotype of ECs and PASMCs.98 In addition, the next promising epigenetic targets will also be miRNAs as they are involved in the pathogenesis of PAH, even though the exact side effects are still unknown.63 In this regard, the miR-143/145 family has received special attention since it seems useful in controlling PPAR expression.99
Growth Factors and Vascular Remodelling
As mentioned before, there is high resemblance between tumor growth and the aberrant vascular cell proliferation characteristic of PAH: both cancer and pulmonary vascular cells share a metabolic switch toward a glycolytic pathway—the so-called Warburg effect—that increases proliferation and inhibits apoptosis of the SMCs, fibroblasts, and ECs, driving thus toward an aberrant vascular remodelling.62 In this field, research on several growth factors, including platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF), has demonstrated these growth factors to be related not only to pathological proliferation but also to the migration of pulmonary vascular cells during PAH.100 PDGFR is heavily upregulated in small pulmonary arteries and induces proliferation. This has opened an opportunity for research on drug repurposing of the oncology therapeutic arsenal. This is the case for the tyrosine kinase receptor inhibitor—including PDGFR—imatinib, which leads to extensive reversal of pulmonary arterial remodelling.9 Although very promising results were initially obtained from several clinical trials, long-term treatment with imatinib led to a significant incidence of severe subdural hematoma.101 Although contradictory, it is also worth mentioning that some cancer patients receiving dasatinib, another tyrosine kinase inhibitor, have developed PAH as a rare side effect. All the accumulated evidence has led the classification of the association of dasatinib with PAH as defined by the Sixth World Symposium on Pulmonary Hypertension 2018, which in turn has introduced new doubts on the use of this drug family.5
Other oncological drugs tested in PAH include elastase inhibitors such as elafin. Elafin is an endogenous human protein that plays a direct role in tumor suppression. Additionally, elafin has been found, on several animal models, to be an effective strategy in the treatment of inflammatory vascular, systemic, and pulmonary diseases.102 Regarding PAH, it has been found to induce apoptosis of SMCs by promoting the interaction between BMPR2-Caveolin-1, which enhances this signaling pathway, and thus prevents and reverses vascular remodelling in experimental models of PAH.63,103 These findings have encouraged initiation of clinical trials of elafin for PAH (NCT03522935).
Serotonergic System
An unexpected secondary effect found with the use of appetite suppressive drugs, that operate as serotonin transporter substrates and serotonergic agonists (e.g., aminorex, benfluorex, and dexfenfluramine), was the sudden occurrence of PAH.104 This event pointed researchers toward the involvement of the serotonergic system in PAH pathogenesis. Serotonin is a pulmonary vasoconstrictor and leads to SMC proliferation. These serotonin-driven processes are mediated by serotonin receptors 5-HT and serotonin transporters (SERT), respectively.105,106 In response to serotonin stimulation, SMCs show increased expression of SERT and increased cell proliferation, with more serotonin secretion by pulmonary ECs, reduced platelet storage of serotonin, and increased peripheral blood serotonin levels.107 Additionally, it is also known that serotonin induces 5-HT1B-dependent ROS production, Nrf-2 dysfunction, and Rho-kinase activation in SMC that contribute to vascular injury.108 All this evidence pointed out the potential use of either 5-HT receptor antagonists, SERT inhibitors, or even inhibitors of serotonin synthesis by the tryptophan hydroxylase 1 (TPH1), which has been studied in animal models and may prove to be a valid therapeutic strategy against PAH.104 Nevertheless, to date clinical trials have been disappointing in this strategy, as was the case with the 5-HT2A/2B receptor antagonist terguride, which despite its promising results in animal models109,110 showed no benefit in a phase 2 clinical trial. Similarly, the use of fluoxetine, a selective serotonin reuptake inhibitor, was not associated with any improvement in PAH patients (NCT03638908),110 despite positive results in murine preclinical models.111 However, there is still hope for new strategies targeting serotonin signaling. Rodatristat, an inhibitor of TPH1, seems to be a promising emerging therapy in this regard, and the future development of new 5-HT1B receptor antagonists might also be beneficial.104
Neurohumoral Modulation
Increased neurohumoral activation during PAH supports the idea of using aldosterone antagonists (spironolactone or eplerenone), angiotensin II receptor blockers (losartan), β-blockers (bisoprolol, nebivolol, or carvedilol) and other relevant strategies such as the vasoactive intestinal peptide (VIP).3 However, the use of these strategies still generates uncertainty due to their unclear pathological or compensatory role and safety. Even though these drugs are generally well tolerated, severe adverse effects occur in some patients due to their systemic effect, such as bradycardia, hypotension, hypoxaemia, worsening of right ventricular function, and death.
The renin–angiotensin–aldosterone system plays a major role not only in regulating blood pressure but also in the regulation of inflammation, proliferation, and fibrosis in pulmonary diseases. Despite some promising evidence of the anti-inflammatory properties of inhibiting the angiotensin converting enzyme (ACE)/angiotensin (Ang) II/AT1 receptor (R) axis in lung diseases, ACE inhibitors have not been shown to be beneficial in PAH patients.112 Nevertheless, the stimulation of the angiotensin-converting enzyme 2 (ACE2)/Ang (1–7)/Mas receptor pathway, which counteracts the ACE/AngII/AT1R pathway by catalyzing the hydrolysis of AngII into the vasodilator angiotensin-(1–7), has been demonstrated in murine models to be a potential strategy to treat PAH. On the other hand, the aldosterone antagonist, eplerenone, has demonstrated a good reduction of PVR in preclinical models, together with other improvements in proliferation and extracellular matrix deposition.113 More research is needed to clarify the potential use of this pathway as a target in PAH.
Since their discovery, β-blockers have revolutionized the field of cardiovascular therapies, being widely used for left ventricle failure management, systemic hypertension, arrhythmia, and other noncardiovascular diseases.114 However, current guidelines do not recommend their use in PAH patients to avoid the negative inotropic and chronotropic effects that will result in systemic hypotension and a decreased exercise capacity.115,116 Nevertheless, some β-blockers have been shown to improve RV function. While results in PAH patients with bisoprolol (a selective β1-blocker) suggest a deteriorated cardiac function driven by a decrease in heart rate, cardiac index, and 6 min walking test,117 others β-blockers are showing better outcomes. For instance, nebivolol (a third generation β1-blocker with β2/β3-agonist activity that confers a NO-dependent vasodilation effect)114 has been found to be promising compared to other β-blockers since it improves endothelial dysfunction, vascular remodelling, and RV function in animal models.118 Additionally, carvedilol (a β1/2-blocker with vasodilation properties due to its ability to block α1-adenergic and to release NO)114 has been recently associated with an improvement in patients suffering PAH, without any evidence of decrease exercise capacity, RV functional deterioration and well tolerated systemic effects.119 In the same line, mirabegron (not a β-blocker but a β3-agonist),114 known to produce vasodilation and prevention of ventricular remodelling in different conditions, has been demonstrated to reduce PVR and improved RV performance in a porcine model that resembles certain aspects of postcapillary PH.120 The same drug is currently in phase 2 clinical trial [NCT02775539] as a potential treatment for PH secondary to chronic heart failure.121 Further research on mirabegron is needed to clarify whether or not this drug might also be a potential treatment for PAH.
In addition, new clinical studies suggest that administration of VIP, an inhibitor of SMC proliferation and pulmonary vasodilation that is downregulated in pulmonary arteries of PAH patients, yields positive results in PAH.122 Further research to fully understand VIP’s positive effects in PAH is being performed (NCT03315507).123 All these strategies are therefore still being investigated in order to assess the optimal dose and time of treatment.3,107
Estrogen Signaling
PAH is observed more frequently in women than in men, and men suffering PAH have an increased level of estrogen.124 Estrogen is a hormone found in every important organ such as lung and heart in both genders. Women that metabolize estrogen to 16-estrogens, suffer from PAH more often than women that metabolize estrogen to 2- or 4-estrogens. The metabolism of estrogen drives penetrance in men, although not to the same extent as in women. In clinical trials, inhibiting estrogen signaling as a therapeutic strategy has displayed a positive effect on RV hypertrophy in animal models.125 Reducing estrogen levels with drugs such as anastrozole, which inhibit the conversion from androgen to estrogens, or blocking the estrogen receptor with drugs such as fulvestrant or tamoxifen may be new promising therapeutic targets. In addition, dehydroepiandrosterone (DHEA), a steroid hormone which modulates endothelial function, has anti-inflammatory effects, promotes pulmonary vasodilation, normalizes the apoptosis/proliferation balance, and improves RV function by reducing oxidative stress is currently being investigated in clinical trials and represents a promising therapy.3
Stem Cells
Cardiac and vascular stem cells have been suggested before to be potential new therapies for cardiovascular regeneration.126,127 Some investigations in the field of PAH have also shown potentially positive results of stem cell-based therapy with endothelial progenitor cells (EPCs)—able to partially rescue endothelial function—and cardiosphere-derived cells (CDCs), which have demonstrated to ameliorate both vascular and ventricular remodelling and no adverse effects were reported.127−133 In addition to these cell types, some other cell types have been explored in stem cell therapy animal models of PAH—few of them directly targeting the RV for RV failure prevention–with modest but promising results.134
Regarding the use of EPCs, the phase 1 PH and Angiogenic Cell Therapy (PHACeT) trial (NCT00469027) demonstrated that the delivery of EPCs overexpressing eNOS was well-tolerated hemodynamically in patients with PAH.128 However, this trial showed very modest improvement on hemodynamic variables. Further trials on EPCs (NCT00257413 and NCT00641836), based on previous experimental data suggesting that transplantation of autologous EPCs attenuates monocrotaline-induced PH in rats,129 also showed that this therapy might have beneficial effects on exercise capacity and pulmonary hemodynamics in patients with idiopathic PAH.130 In this line, other studies also demonstrated that idiopathic PAH patients had reduced the numbers of EPCs with a clear correlation with invasive hemodynamic parameters. In addition, the same study found increased levels of circulating EPCs in patients treated with sildenafil.131 More recently, a trial on children with PAH caused by congenital heart disease, found that EPCs may become an effective treatment and a protective factor in children with higher mPAP values.133 However, further research is required in order to understand the full mechanism and confirm the beneficial results of this therapy.126,127
CDCs are cardiac progenitor cells with potent anti-inflammatory and immunomodulatory properties126 that have been demonstrated to attenuate hemodynamic changes, perivascular inflammation, and pulmonary arteriolar remodelling in animal models. In addition, an improvement of RV systolic function and a decrease of RV hypertrophy were found either as a consequence of a reduction in occlusive arteriopathy or even as an additive effect directly over the RV.134 Currently, the ALPHA (Allogeneic CDCs for PH therapy; NCT03145298) phase 1 clinical trial is investigating the safety and feasibility of intravenous administration of CDCs to be further studied as an adjunctive therapy in PAH patients in which inflammation and immune dysfunction are key pathophysiologic drivers. However, studies performed in a large animal model of overloaded RV dysfunction tested the feasibility and effects of CDC therapy on the RV failure secondary to chronic overload and found modest effects.135 Nevertheless, a reduction in RV fibrosis and a protection against arrhythmia was found after intraepicardial administration.135 This could represent promising results targeting the transition from RV hypertrophy to RV failure that needs to be deeply explored.
Although some results are promising, more research is needed to clarify whether stem cells could represent a potential therapy on PAH.
Future Directions
Even though in the last 20 years several new drugs have been suggested, developed, and approved for use against PAH, a fully efficient treatment for this disease has still not emerged.137 Current drug therapies aim both to slow down the processes responsible for the occlusion of pulmonary arteries, such as vasoconstriction and vascular remodelling, which are the main reasons of the increased blood pressure. However, fully effective drugs still do not exist since the ones currently in place are only partially able to attenuate symptoms and signs, but none of these diverse therapeutic strategies have been able to cure or stop the disease.
PAH is a multifactorial disease and no single pathway exhibits a dominant pathogenic role, therefore there is no separate class of drugs with unvarying capability to treat all the pathways and all affected patients. Medical advances over the past decade have enabled a better understanding and a significant improvement in therapeutic strategies as we have described herein. Apart from the main vascular pathways involved in vasoconstriction and vascular remodelling, other targets emerged from the basic knowledge to the clinics. A new set of incoming drugs is targeting metabolic dysfunction, perivascular inflammation, excessive cell proliferation, fibrosis, neurohumoral imbalance, genetic damages, and epigenetic modifications, among others. All of these are important pieces of the big puzzle of PAH. It is unlikely that only one of those potential therapies will be the cure for the disease. In addition, new drugs still have to display an additional benefit to the current optimized treatments.137 Nevertheless, in case emerging therapies come to the clinical arena, a combination of new and currently used drugs might be able to multitarget the disease if strategically used from early stages.
In this regard, the use of monotherapy has been significantly reduced because of its lower capacity to improve symptoms and life expectancy, and is being replaced by the combination of two or even three of the available drugs.136 Hundreds of clinical trials are currently ongoing and most of them are focused on new drugs being applied to old families within a multitherapy approach.110,136,137 However, single therapy is still used in early stage patients with low cardiovascular risk. It is also worth noting that most data regarding the use of combination therapy is obtained from sequential additions to the continuing single data therapy rather than from the inception of such therapeutic treatment, which does not help to achieve solid conclusions.
Although the field is progressing quickly in the last years, there is still room for improvements in many other aspects. First of all, animal models need to better resemble all aspects of the disease not only in small but also larger animals. In addition, standardization of late stage preclinical multicenter-trials with comparison of current and emerging therapies for testing reproducibility138 might help to increase the chances of success. Furthermore, a deep focus into the different steps of the disease might also be important to adapt therapeutic approaches with clearly different targets. In early stages preserving endothelium might be the main focus, in intermediate stages stopping vascular remodelling and perivascular inflammation could be key, while in more advanced stages the focus lies in preservation of RV function. In this regard, further basic research on the endothelial-to-mesenchymal transition within the pulmonary vasculature or on the fatal progression from RV hypertrophy to failure, that is, the main mortality predictor of PAH, will be invaluable. Additionally, more knowledge coming from multiomic approaches and noninvasive and radio-ligand imaging techniques will probably help us to understand in the near future how and when these changes occur, therefore helping to better adapt the administration of incoming therapies to the right moment. Finally, the knowledge acquired from other diseases that share some common characteristics such as metabolic disorders, mitochondrial disease, cardiac toxicity, inflammatory diseases, or aberrant cell growth will be continuously helping the research community in suggesting drug repurposing and assessing new potential therapies, which will eventually open new unexplored avenues and hopes.
Conclusions
Despite recent pharmacological advancements, PAH still remains an incurable disease. Future basic research in this field will improve our knowledge and understanding about the molecular basis behind PAH and will probably shed light on new pathways to further explore. In addition, thanks to translational research, a considerable number of potential therapies are continuously emerging and some are now ready to be tested. Finally, forthcoming clinical studies will clarify whether these emerging innovative strategies are safe and effective to treat PAH patients. Notwithstanding, substantial collaborative research from multiple perspectives is still needed before a final cure for PAH is reached.
Acknowledgments
The authors would like to acknowledge Dr. Carlos Galan-Arriola for the invaluable help with drawings illustrating the figures, and Dr. Borja Ibáñez for his constant support as head of the Translational Laboratory for Cardiovascular Imaging and Therapy at CNIC. E.O. is a recipient of funds from Programa de Atracción de Talento (2017-T1/BMD-5185) of Comunidad de Madrid. The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia, Innovación y Universidades (MCNU), and the Pro CNIC Foundation, and is a Severo Ochoa Center of Excellence (SEV-2015-0505).
Glossary
Abbreviations
- AC
adenyl cyclase
- ATP
adenosine triphosphate
- ACE
angiotensin-converting enzyme
- ALK1
activin receptor-like kinase 1
- ATII
angiotensin II
- BRD4
bromodomain-containing protein 4
- BMPR2
bone morphogenetic protein receptor type 2
- CDCs
cardiosphere-derived cells
- Ca-Ch
calcium channel.
- cGM
cyclic guanosine triphosphate
- cAMP
cyclic adenosine monophosphate
- DHEA
dehydroepiandrosteron
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EPCs
endothelial progenitor cells
- ET
endothelin
- ERAs
ET receptor antagonists
- ETA
endothelin receptor type A
- ETB
endothelin receptor type B
- FGF
fibroblast growth factor
- FDA
Food and Drug Administration.
- GMP
guanosine triphosphate
- HDAC
histone deacetylase
- IL
interleukin
- IP
PGI2 receptor
- mTOR
mammalian target of rapamycin
- MIF
macrophage migration-inhibitory factor
- mPAP
mean pulmonary arterial pressure
- NO
nitric oxide
- PAH
pulmonary arterial hypertension
- PCWP
pulmonary capillary wedge pressure
- PH
pulmonary hypertension
- PVR
pulmonary vascular resistance
- RV
right ventricle
- SMC
smooth muscle cells
- PDE5
phosphodiesterase type 5
- PARP
poly-ADP ribose polymerase
- PGI2
prostaglandin I2
- PDGF
platelet-derived growth factor
- PDK
pyruvate dehydrogenase kinase
- ROS
reactive oxygen species
- RAAS
renin-angiotensin-aldosterone system
- SSRIs
selective serotonin reuptake inhibitor
- sGC
soluble guanylate cyclase
- TXA2
thromboxane A2
- VEGF
vascular endothelial growth factor
- WHO
World Health Organization
The authors declare no competing financial interest.
References
- Farber H. W.; Miller D. P.; Poms A. D.; Badesch D. B.; Frost A. E.; Muros-Le Rouzic E.; Romero A. J.; Benton W. W.; Elliott C. G.; McGoon M. D.; Benza R. L. (2015) Five-Year outcomes of patients enrolled in the REVEAL Registry. Chest 148 (4), 1043. 10.1378/chest.15-0300. [DOI] [PubMed] [Google Scholar]
- Galie N.; Humbert M.; Vachiery J.-L.; Gibbs S.; Lang I.; Torbicki A.; Simonneau G.; Peacock A.; Vonk Noordegraaf A.; Beghetti M.; Ghofrani A.; Gomez Sanchez M. A.; Hansmann G.; Klepetko W.; Lancellotti P.; Matucci M.; McDonagh T.; Pierard L. A.; Trindade P. T.; Zompatori M.; Hoeper M. (2016) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 37 (1), 67. 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- Prins K. W.; Thenappan T. (2016) World Health Organization Group I Pulmonary Hypertension: Epidemiology and Pathophysiology.. Cardiol Clin 34 (3), 363–74. 10.1016/j.ccl.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijeratne D. T.; Lajkosz K.; Brogly S. B.; Lougheed M. D.; Jiang L.; Housin A.; Barber D.; Johnson A.; Doliszny K. M.; Archer S. L. (2018) Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension: A Population-Based Cohort Study in Ontario, Canada.. Circ Cardiovasc Qual Outcomes 11 (2), e003973 10.1161/CIRCOUTCOMES.117.003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneau G.; Montani D.; Celermajer D. S.; Denton C. P.; Gatzoulis M. A.; Krowka M.; Williams P. G.; Souza R. (2019) Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 53 (1), 1801913. 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell N. W.; Adnot S.; Archer S. L.; Dupuis J.; Jones P. L.; MacLean M. R.; McMurtry I. F.; Stenmark K. R.; Thistlethwaite P. A.; Weissmann N.; Yuan J. X.; Weir E. K. (2009) Cellular and molecular basis of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 54 (1 Suppl), S20–31. 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs W.; van de Veerdonk M. C.; Trip P.; de Man F.; Heymans M. W.; Marcus J. T.; Kawut S. M.; Bogaard H.-J.; Boonstra A.; Vonk Noordegraaf A. (2014) The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest 145 (6), 1230. 10.1378/chest.13-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawut S. M.; Al-Naamani N.; Agerstrand C.; Berman Rosenzweig E.; Rowan C.; Barst R. J.; Bergmann S.; Horn E. M. (2009) Determinants of right ventricular ejection fraction in pulmonary arterial hypertension. Chest 135 (3), 752. 10.1378/chest.08-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermuly R. T.; Dony E.; Ghofrani H. A.; Pullamsetti S.; Savai R.; Roth M.; Sydykov A.; Lai Y. J.; Weissmann N.; Seeger W.; Grimminger F. (2005) Reversal of experimental pulmonary hypertension by PDGF inhibition. J. Clin. Invest. 115 (10), 2811–21. 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbicki A. (2016) Definition of pulmonary hypertension challenged?. Nat. Rev. Cardiol. 13 (5), 250. 10.1038/nrcardio.2016.44. [DOI] [PubMed] [Google Scholar]
- Humbert M.; Sitbon O.; Simonneau G. (2004) Treatment of pulmonary arterial hypertension. N. Engl. J. Med. 351 (14), 1425. 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- O’Callaghan D. S.; Savale L.; Montani D.; Jaïs X.; Sitbon O.; Simonneau G.; Humbert M. (2011) Treatment of pulmonary arterial hypertension with targeted therapies. Nat. Rev. Cardiol. 8 (9), 526. 10.1038/nrcardio.2011.104. [DOI] [PubMed] [Google Scholar]
- Channick R. N.; Sitbon O.; Barst R. J.; Manes A.; Rubin L. J. (2004) Endothelin receptor antagonists in pulmonary arterial hypertension. J. Am. Coll. Cardiol. 43 (12 Suppl S), 62S–67S. 10.1016/j.jacc.2004.02.042. [DOI] [PubMed] [Google Scholar]
- Denault J. B.; Claing A.; D’Orleans-Juste P.; Sawamura T.; Kido T.; Masaki T.; Leduc R. (1995) Processing of proendothelin-1 by human furin convertase. FEBS Lett. 362 (3), 276–80. 10.1016/0014-5793(95)00249-9. [DOI] [PubMed] [Google Scholar]
- Takahashi M.; Matsushita Y.; Iijima Y.; Tanzawa K. (1993) Purification and characterization of endothelin-converting enzyme from rat lung. J. Biol. Chem. 268 (28), 21394–8. [PubMed] [Google Scholar]
- Arai H.; Hori S.; Aramori I.; Ohkubo H.; Nakanishi S. (1990) Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348 (6303), 730–2. 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Sakurai T.; Yanagisawa M.; Takuwat Y.; Miyazakit H.; Kimura S.; Goto K.; Masaki T. (1990) Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 348 (6303), 732–5. 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Houde M.; Desbiens L.; D’Orleans-Juste P. (2016) Endothelin-1: Biosynthesis, Signaling and Vasoreactivity. Adv. Pharmacol. 77, 143–75. 10.1016/bs.apha.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Dupuis J.; Hoeper M. M. (2008) Endothelin receptor antagonists in pulmonary arterial hypertension. Eur. Respir. J. 31 (2), 407–15. 10.1183/09031936.00078207. [DOI] [PubMed] [Google Scholar]
- Sahni S.; Ojrzanowski M.; Majewski S.; Talwar A. (2016) Pulmonary arterial hypertension: a current review of pharmacological management. Pneumonol. Alergol. Pol. 84 (1), 47. 10.5603/PiAP.a2015.0084. [DOI] [PubMed] [Google Scholar]
- Tynan T.; Hird K.; Hannon T.; Gabbay E. (2019) Pulmonary arterial hypertension outcomes upon endothelin-1 receptor antagonist switch to macitentan. J. Int. Med. Res. 47 (5), 2177–2186. 10.1177/0300060519840130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale M.; Ferraretti A.; Monaco I.; Grazioli D.; Di Biase M.; Brunetti N. D. (2018) Endothelin-receptor antagonists in the management of pulmonary arterial hypertension: where do we stand?. Vasc. Health Risk Manage. 14, 253–264. 10.2147/VHRM.S133921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katusic Z. S.; Santhanam A. V.; He T. (2012) Vascular effects of prostacyclin: does activation of PPARdelta play a role?. Trends Pharmacol. Sci. 33 (10), 559–64. 10.1016/j.tips.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. C.; Cindrova-Davies T.; Skepper J. N.; Sellers L. A. (2004) Prostacyclin induces apoptosis of vascular smooth muscle cells by a cAMP-mediated inhibition of extracellular signal-regulated kinase activity and can counteract the mitogenic activity of endothelin-1 or basic fibroblast growth factor. Circ. Res. 94 (6), 759–67. 10.1161/01.RES.0000121568.40692.97. [DOI] [PubMed] [Google Scholar]
- Christman B. W.; McPherson C. D.; Newman J. H.; King G. A.; Bernard G. R.; Groves B. M.; Loyd J. E. (1992) An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N. Engl. J. Med. 327 (2), 70–5. 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- Tuder R. M.; Cool C. D.; Geraci M. W.; Wang J.; Abman S. H.; Wright L.; Badesch D.; Voelkel N. F. (1999) Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 159 (6), 1925–32. 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- Pluchart H.; Khouri C.; Blaise S.; Roustit M.; Cracowski J. L. (2017) Targeting the Prostacyclin Pathway: Beyond Pulmonary Arterial Hypertension. Trends Pharmacol. Sci. 38 (6), 512–523. 10.1016/j.tips.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Chen Y. F.; Jowett S.; Barton P.; Malottki K.; Hyde C.; Gibbs J. S. R.; Pepke-Zaba J.; Fry-Smith A.; Roberts J.; Moore D. (2009) Clinical and cost-effectiveness of epoprostenol, iloprost, bosentan, sitaxentan and sildenafil for pulmonary arterial hypertension within their licensed indications: a systematic review and economic evaluation. Health Technol. Assess. 13, 1–320. 10.3310/hta13490. [DOI] [PubMed] [Google Scholar]
- Berlier C.; Schwarz E. I.; Saxer S.; Lichtblau M.; Ulrich S. (2019) Real-Life Experience with Selexipag as an Add-On Therapy to Oral Combination Therapy in Patients with Pulmonary Arterial or Distal Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Analysis. Lung 197 (3), 353–360. 10.1007/s00408-019-00222-7. [DOI] [PubMed] [Google Scholar]
- Hardin E. A.; Chin K. M. (2016) Selexipag in the treatment of pulmonary arterial hypertension: design, development, and therapy. Drug Des., Dev. Ther. 10, 3747–3754. 10.2147/DDDT.S103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y. M.; Jones R. L.; Chan K. M.; Stock A. I.; Ho J. K. (1994) Potent contractile actions of prostanoid EP3-receptor agonists on human isolated pulmonary artery. Br. J. Pharmacol. 113 (2), 369–74. 10.1111/j.1476-5381.1994.tb16997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch L.; de Montpreville V.; Brink C.; Norel X. (2001) Prostanoid EP(1)- and TP-receptors involved in the contraction of human pulmonary veins. Br. J. Pharmacol. 134 (8), 1671–8. 10.1038/sj.bjp.0704423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins M. R.; Wharton J.; Grimminger F.; Ghofrani H. A. (2008) Phosphodiesterase inhibitors for the treatment of pulmonary hypertension. Eur. Respir. J. 32 (1), 198–209. 10.1183/09031936.00124007. [DOI] [PubMed] [Google Scholar]
- Nagendran J.; Archer S. L.; Soliman D.; Gurtu V.; Moudgil R.; Haromy A.; St Aubin C.; Webster L.; Rebeyka I. M.; Ross D. B.; Light P. E.; Dyck J. R.; Michelakis E. D. (2007) Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 116 (3), 238. 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- Watanabe H. (2018) Treatment Selection in Pulmonary Arterial Hypertension: Phosphodiesterase Type 5 Inhibitors versus Soluble Guanylate Cyclase Stimulator. Eur. Cardiol 13 (1), 35. 10.15420/ecr.2017:22:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes H.; Brown Z.; Burns A.; Williams T. (2019) Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database Syst. Rev. 1, CD012621. 10.1002/14651858.CD012621.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo J.; Yotti R.; Garcia-Orta R.; Sanchez-Fernandez P. L.; Castano M.; Segovia-Cubero J.; Escribano-Subias P.; San Roman J. A.; Borras X.; Alonso-Gomez A.; Botas J.; Crespo-Leiro M. G.; Velasco S.; Bayes-Genis A.; Lopez A.; Munoz-Aguilera R.; de Teresa E.; Gonzalez-Juanatey J. R.; Evangelista A.; Mombiela T.; Gonzalez-Mansilla A.; Elizaga J.; Martin-Moreiras J.; Gonzalez-Santos J. M.; Moreno-Escobar E.; Fernandez-Aviles F.; et al. (2018) Sildenafil for Improving Outcomes after, V. C. i., Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur. Heart J. 39 (15), 1255–1264. 10.1093/eurheartj/ehx700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghofrani H.-A.; Galie N.; Grimminger F.; Grunig E.; Humbert M.; Jing Z.-C.; Keogh A. M.; Langleben D.; Kilama M. O.; Fritsch A.; Neuser D.; Rubin L. J. (2013) Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 369 (4), 330–40. 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- Toxvig A. K.; Wehland M.; Grimm D.; Infanger M.; Kruger M. (2019) A focus on riociguat in the treatment of pulmonary arterial hypertension. Basic Clin. Pharmacol. Toxicol. 125 (3), 202–214. 10.1111/bcpt.13272. [DOI] [PubMed] [Google Scholar]
- Hopper R. K.; Moonen J. R.; Diebold I.; Cao A.; Rhodes C. J.; Tojais N. F.; Hennigs J. K.; Gu M.; Wang L.; Rabinovitch M. (2016) In Pulmonary Arterial Hypertension, Reduced BMPR2 Promotes Endothelial-to-Mesenchymal Transition via HMGA1 and Its Target Slug. Circulation 133 (18), 1783–94. 10.1161/CIRCULATIONAHA.115.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medarov B. I.; Judson M. A. (2015) The role of calcium channel blockers for the treatment of pulmonary arterial hypertension: How much do we actually know and how could they be positioned today?. Respir Med. 109 (5), 557–64. 10.1016/j.rmed.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Fan Z.; Chen Y.; Liu H. (2015) Calcium channel blockers for pulmonary arterial hypertension. Cochrane Database Syst. Rev. (9), CD010066. 10.1002/14651858.CD010066.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucherat O.; Vitry G.; Trinh I.; Paulin R.; Provencher S.; Bonnet S. (2017) The cancer theory of pulmonary arterial hypertension. Pulm. Circ. 7 (2), 285. 10.1177/2045893217701438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshikawa Y.; Ono S.; Suzuki S.; Tanita T.; Chida M.; Song C.; Noda M.; Tabata T.; Voelkel N. F.; Fujimura S. (2001) Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J. Appl. Physiol. 90 (4), 1299. 10.1152/jappl.2001.90.4.1299. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh N.; Patel R. S.; Eapen D. J.; Veledar E.; Al Kassem H.; Manocha P.; Khayata M.; Zafari A. M.; Sperling L.; Jones D. P.; Quyyumi A. A. (2014) Oxidative stress is associated with increased pulmonary artery systolic pressure in humans. Hypertension 63 (6), 1270–5. 10.1161/HYPERTENSIONAHA.113.02360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies G.; Paradies V.; Ruggiero F. M.; Petrosillo G. (2019) Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells 8 (7), 728. 10.3390/cells8070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinibaldi F.; Howes B. D.; Piro M. C.; Polticelli F.; Bombelli C.; Ferri T.; Coletta M.; Smulevich G.; Santucci R. (2010) Extended cardiolipin anchorage to cytochrome c: a model for protein-mitochondrial membrane binding. JBIC, J. Biol. Inorg. Chem. 15 (5), 689–700. 10.1007/s00775-010-0636-z. [DOI] [PubMed] [Google Scholar]
- Tuominen E. K.; Wallace C. J.; Kinnunen P. K. (2002) Phospholipid-cytochrome c interaction: evidence for the extended lipid anchorage. J. Biol. Chem. 277 (11), 8822–6. 10.1074/jbc.M200056200. [DOI] [PubMed] [Google Scholar]
- Kagan V. E.; Tyurin V. A.; Jiang J.; Tyurina Y. Y.; Ritov V. B.; Amoscato A. A.; Osipov A. N.; Belikova N. A.; Kapralov A. A.; Kini V.; Vlasova I. I.; Zhao Q.; Zou M.; Di P.; Svistunenko D. A.; Kurnikov I. V.; Borisenko G. G. (2005) Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat. Chem. Biol. 1 (4), 223–32. 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- Szeto H. H. (2014) First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br. J. Pharmacol. 171 (8), 2029–50. 10.1111/bph.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. I.; Huang T. H.; Sung P. H.; Chen Y. L.; Chua S.; Chai H. Y.; Chung S. Y.; Liu C. F.; Sun C. K.; Chang H. W.; Zhen Y. Y.; Lee F. Y.; Yip H. K. (2016) Administration of antioxidant peptide SS-31 attenuates transverse aortic constriction-induced pulmonary arterial hypertension in mice. Acta Pharmacol. Sin. 37 (5), 589–603. 10.1038/aps.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield K. C.; Sparagna G. C.; Chau S.; Phillips E. K.; Ambardekar A. V.; Aftab M.; Mitchell M. B.; Sucharov C. C.; Miyamoto S. D.; Stauffer B. L. (2019) Elamipretide Improves Mitochondrial Function in the Failing Human Heart. JACC Basic Transl Sci. 4 (2), 147. 10.1016/j.jacbts.2018.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B.; Yan J.; Shen Y.; Liu Y.; Ma Z. (2014) Dichloroacetate prevents but not reverses the formation of neointimal lesions in a rat model of severe pulmonary arterial hypertension. Mol. Med. Rep. 10 (4), 2144–52. 10.3892/mmr.2014.2432. [DOI] [PubMed] [Google Scholar]
- Michelakis E. D.; Gurtu V.; Webster L.; Barnes G.; Watson G.; Howard L.; Cupitt J.; Paterson I.; Thompson R. B.; Chow K.; O’Regan D. P.; Zhao L.; Wharton J.; Kiely D. G.; Kinnaird A.; Boukouris A. E.; White C.; Nagendran J.; Freed D. H.; Wort S. J.; Gibbs J. S. R.; Wilkins M. R. (2017) Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci. Transl. Med. 9 (413), eaao4583. 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- McMurtry M. S.; Bonnet S.; Wu X.; Dyck J. R.; Haromy A.; Hashimoto K.; Michelakis E. D. (2004) Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ. Res. 95 (8), 830–40. 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- Sun X. Q.; Zhang R.; Zhang H. D.; Yuan P.; Wang X. J.; Zhao Q. H.; Wang L.; Jiang R.; Jan Bogaard H.; Jing Z. C. (2016) Reversal of right ventricular remodeling by dichloroacetate is related to inhibition of mitochondria-dependent apoptosis. Hypertens. Res. 39 (5), 302–11. 10.1038/hr.2015.153. [DOI] [PubMed] [Google Scholar]
- Cullinan S. B.; Gordan J. D.; Jin J.; Harper J. W.; Diehl J. A. (2004) The Keap1-BTB protein is an adaptor that bridges Nrf2 to a Cul3-based E3 ligase: oxidative stress sensing by a Cul3-Keap1 ligase. Mol. Cell. Biol. 24 (19), 8477–86. 10.1128/MCB.24.19.8477-8486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A.; Kang M. I.; Okawa H.; Ohtsuji M.; Zenke Y.; Chiba T.; Igarashi K.; Yamamoto M. (2004) Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 24 (16), 7130–9. 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. D.; Lo S. C.; Cross J. V.; Templeton D. J.; Hannink M. (2004) Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell. Biol. 24 (24), 10941–53. 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A.; Rojo A. I.; Wells G.; Hayes J. D.; Cousin S. P.; Rumsey W. L.; Attucks O. C.; Franklin S.; Levonen A. L.; Kensler T. W.; Dinkova-Kostova A. T. (2019) Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discovery 18 (4), 295–317. 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- Smith R. E.; Tran K.; Smith C. C.; McDonald M.; Shejwalkar P.; Hara K. (2016) The Role of the Nrf2/ARE Antioxidant System in Preventing Cardiovascular Diseases. Diseases 4 (4), 34. 10.3390/diseases4040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. Y.; Rubin L. J. (2017) Metabolic dysfunction in pulmonary hypertension: from basic science to clinical practice. Eur. Respir Rev. 26 (146), 170094. 10.1183/16000617.0094-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins M. R.; Aman J.; Harbaum L.; Ulrich A.; Wharton J.; Rhodes C. J. (2018) Recent advances in pulmonary arterial hypertension. F1000Research 7, 1128. 10.12688/f1000research.14984.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frid M. G.; Brunetti J. A.; Burke D. L.; Carpenter T. C.; Davie N. J.; Reeves J. T.; Roedersheimer M. T.; van Rooijen N.; Stenmark K. R. (2006) Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am. J. Pathol. 168 (2), 659–69. 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek M. J.; Mouchaers K. T.; Niessen H. M.; Hadi A. M.; Kupreishvili K.; Boonstra A.; Voskuyl A. E.; Belien J. A.; Smit E. F.; Dijkmans B. C.; Vonk-Noordegraaf A.; Grunberg K. (2010) Characteristics of interstitial fibrosis and inflammatory cell infiltration in right ventricles of systemic sclerosis-associated pulmonary arterial hypertension. Int. J. Rheumatol. 2010, 1. 10.1155/2010/604615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuder R. M.; Groves B.; Badesch D. B.; Voelkel N. F. (1994) Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am. J. Pathol. 144 (2), 275–85. [PMC free article] [PubMed] [Google Scholar]
- Soon E.; Holmes A. M.; Treacy C. M.; Doughty N. J.; Southgate L.; Machado R. D.; Trembath R. C.; Jennings S.; Barker L.; Nicklin P.; Walker C.; Budd D. C.; Pepke-Zaba J.; Morrell N. W. (2010) Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 122 (9), 920–7. 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- Voelkel N. F.; Tuder R. M.; Bridges J.; Arend W. P. (1994) Interleukin-1 receptor antagonist treatment reduces pulmonary hypertension generated in rats by monocrotaline. Am. J. Respir. Cell Mol. Biol. 11 (6), 664–75. 10.1165/ajrcmb.11.6.7946395. [DOI] [PubMed] [Google Scholar]
- Morisawa D.; Hirotani S.; Oboshi M.; Nishimura K.; Sawada H.; Eguchi A.; Okuhara Y.; Iwasaku T.; Naito Y.; Mano T.; Okamura H.; Masuyama T. (2016) Interleukin-18 disruption suppresses hypoxia-induced pulmonary artery hypertension in mice. Int. J. Cardiol. 202, 522–4. 10.1016/j.ijcard.2015.09.118. [DOI] [PubMed] [Google Scholar]
- Parpaleix A.; Amsellem V.; Houssaini A.; Abid S.; Breau M.; Marcos E.; Sawaki D.; Delcroix M.; Quarck R.; Maillard A.; Couillin I.; Ryffel B.; Adnot S. (2016) Role of interleukin-1 receptor 1/MyD88 signalling in the development and progression of pulmonary hypertension. Eur. Respir. J. 48 (2), 470–83. 10.1183/13993003.01448-2015. [DOI] [PubMed] [Google Scholar]
- Cahill C. M.; Rogers J. T. (2008) Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J. Biol. Chem. 283 (38), 25900–12. 10.1074/jbc.M707692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trankle C. R.; Canada J. M.; Kadariya D.; Markley R.; De Chazal H. M.; Pinson J.; Fox A.; Van Tassell B. W.; Abbate A.; Grinnan D. (2019) IL-1 Blockade Reduces Inflammation in Pulmonary Arterial Hypertension and Right Ventricular Failure: A Single-Arm, Open-Label, Phase IB/II Pilot Study. Am. J. Respir. Crit. Care Med. 199 (3), 381–384. 10.1164/rccm.201809-1631LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazici Y.; Curtis J. R.; Ince A.; Baraf H.; Malamet R. L.; Teng L. L.; Kavanaugh A. (2012) Efficacy of tocilizumab in patients with moderate to severe active rheumatoid arthritis and a previous inadequate response to disease-modifying antirheumatic drugs: the ROSE study. Ann. Rheum. Dis. 71 (2), 198–205. 10.1136/ard.2010.148700. [DOI] [PubMed] [Google Scholar]
- Coll R. C.; Robertson A. A.; Chae J. J.; Higgins S. C.; Munoz-Planillo R.; Inserra M. C.; Vetter I.; Dungan L. S.; Monks B. G.; Stutz A.; Croker D. E.; Butler M. S.; Haneklaus M.; Sutton C. E.; Nunez G.; Latz E.; Kastner D. L.; Mills K. H.; Masters S. L.; Schroder K.; Cooper M. A.; O’Neill L. A. (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21 (3), 248–55. 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavillard L. E.; Canadas-Lozano D.; Alcocer-Gomez E.; Marin-Aguilar F.; Pereira S.; Robertson A. A. B.; Muntane J.; Ryffel B.; Cooper M. A.; Quiles J. L.; Bullon P.; Ruiz-Cabello J.; Cordero M. D. (2017) NLRP3-inflammasome inhibition prevents high fat and high sugar diets-induced heart damage through autophagy induction. Oncotarget 8 (59), 99740–99756. 10.18632/oncotarget.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primiano M. J.; Lefker B. A.; Bowman M. R.; Bree A. G.; Hubeau C.; Bonin P. D.; Mangan M.; Dower K.; Monks B. G.; Cushing L.; Wang S.; Guzova J.; Jiao A.; Lin L. L.; Latz E.; Hepworth D.; Hall J. P. (2016) Efficacy and Pharmacology of the NLRP3 Inflammasome Inhibitor CP-456,773 (CRID3) in Murine Models of Dermal and Pulmonary Inflammation. J. Immunol. 197 (6), 2421–33. 10.4049/jimmunol.1600035. [DOI] [PubMed] [Google Scholar]
- van der Heijden T.; Kritikou E.; Venema W.; van Duijn J.; van Santbrink P. J.; Slutter B.; Foks A. C.; Bot I.; Kuiper J. (2017) NLRP3 Inflammasome Inhibition by MCC950 Reduces Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice-Brief Report. Arterioscler., Thromb., Vasc. Biol. 37 (8), 1457–1461. 10.1161/ATVBAHA.117.309575. [DOI] [PubMed] [Google Scholar]
- van Hout G. P.; Bosch L.; Ellenbroek G. H.; de Haan J. J.; van Solinge W. W.; Cooper M. A.; Arslan F.; de Jager S. C.; Robertson A. A.; Pasterkamp G.; Hoefer I. E. (2016) The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur. Heart J. 38 (11), 828–836. [DOI] [PubMed] [Google Scholar]
- Mizuno S.; Farkas L.; Al Husseini A.; Farkas D.; Gomez-Arroyo J.; Kraskauskas D.; Nicolls M. R.; Cool C. D.; Bogaard H. J.; Voelkel N. F. (2012) Severe pulmonary arterial hypertension induced by SU5416 and ovalbumin immunization. Am. J. Respir. Cell Mol. Biol. 47 (5), 679–87. 10.1165/rcmb.2012-0077OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloche J.; Pflieger A.; Vaillancourt M.; Paulin R.; Potus F.; Zervopoulos S.; Graydon C.; Courboulin A.; Breuils-Bonnet S.; Tremblay E.; Couture C.; Michelakis E. D.; Provencher S.; Bonnet S. (2014) Role for DNA damage signaling in pulmonary arterial hypertension. Circulation 129 (7), 786–97. 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- Sanges S.; Savale L.; Lamblin N.; Remy-Jardin M.; Humbert M.; Sobanski V. (2019) Efficacy of immunosuppressants with bridge vasodilator therapy in severe lupus erythematosus-associated pulmonary arterial hypertension. ESC Heart Fail 6 (6), 1322–1325. 10.1002/ehf2.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldred M. A.; Comhair S. A.; Varella-Garcia M.; Asosingh K.; Xu W.; Noon G. P.; Thistlethwaite P. A.; Tuder R. M.; Erzurum S. C.; Geraci M. W.; Coldren C. D. (2010) Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 182 (9), 1153–60. 10.1164/rccm.201003-0491OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici C.; Drake K. M.; Rigelsky C. M.; McNelly L. N.; Meade S. L.; Comhair S. A.; Erzurum S. C.; Aldred M. A. (2015) Increased Mutagen Sensitivity and DNA Damage in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 192 (2), 219–28. 10.1164/rccm.201411-2128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M. E.; Halley G. R.; Golpon H. A.; Voelkel N. F.; Tuder R. M. (2001) Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ. Res. 88 (1), E2–E11. 10.1161/01.RES.88.1.e2. [DOI] [PubMed] [Google Scholar]
- Fessel J. P.; Loyd J. E.; Austin E. D. (2011) The Genetics of Pulmonary Arterial Hypertension in the Post-BMPR2 Era. Pulm. Circ. 1 (3), 305. 10.4103/2045-8932.87293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Duffhues G.; Garcia de Vinuesa A.; van de Pol V.; Geerts M. E.; de Vries M. R.; Janson S. G.; van Dam H.; Lindeman J. H.; Goumans M. J.; Ten Dijke P. (2019) Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J. Pathol. 247 (3), 333–346. 10.1002/path.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frump A.; Prewitt A.; de Caestecker M. P. (2018) BMPR2 mutations and endothelial dysfunction in pulmonary arterial hypertension (2017 Grover Conference Series). Pulm. Circ. 8 (2), 2045894018765840 10.1177/2045894018765840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds A. M.; Holmes M. D.; Danilov S. M.; Reynolds P. N. (2012) Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur. Respir. J. 39 (2), 329–43. 10.1183/09031936.00187310. [DOI] [PubMed] [Google Scholar]
- Morrell N. W.; Aldred M. A.; Chung W. K.; Elliott C. G.; Nichols W. C.; Soubrier F.; Trembath R. C.; Loyd J. E. (2019) Genetics and genomics of pulmonary arterial hypertension. Eur. Respir. J. 53 (1), 1801899. 10.1183/13993003.01899-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L.; Mallet C.; Mazerbourg S.; Feige J. J.; Bailly S. (2007) Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) in endothelial cells. Blood 109 (5), 1953–61. 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- Roy B.; Friesen W. J.; Tomizawa Y.; Leszyk J. D.; Zhuo J.; Johnson B.; Dakka J.; Trotta C. R.; Xue X.; Mutyam V.; Keeling K. M.; Mobley J. A.; Rowe S. M.; Bedwell D. M.; Welch E. M.; Jacobson A. (2016) Ataluren stimulates ribosomal selection of near-cognate tRNAs to promote nonsense suppression. Proc. Natl. Acad. Sci. U. S. A. 113 (44), 12508–12513. 10.1073/pnas.1605336113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung L. M.; Yang P.; Joshi S.; Augur Z. M.; Kim S. S. J.; Bocobo G. A.; Dinter T.; Troncone L.; Chen P. S.; McNeil M. E.; Southwood M.; Poli de Frias S.; Knopf J.; Rosas I. O.; Sako D.; Pearsall R. S.; Quisel J. D.; Li G.; Kumar R.; Yu P. B. (2020) ACTRIIA-Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension. Sci. Transl. Med. 12 (543), eaaz5660. 10.1126/scitranslmed.aaz5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullamsetti S. S.; Schermuly R.; Ghofrani A.; Weissmann N.; Grimminger F.; Seeger W. (2014) Novel and emerging therapies for pulmonary hypertension. Am. J. Respir. Crit. Care Med. 189 (4), 394–400. 10.1164/rccm.201308-1543PP. [DOI] [PubMed] [Google Scholar]
- Zhao L.; Chen C.-N.; Hajji N.; Oliver E.; Cotroneo E.; Wharton J.; Wang D.; Li M.; McKinsey T. A.; Stenmark K. R.; Wilkins M. R. (2012) Histone Deacetylation Inhibition in Pulmonary Hypertension Therapeutic Potential of Valproic Acid and Suberoylanilide Hydroxamic Acid. Circulation 126 (4), 455. 10.1161/CIRCULATIONAHA.112.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavasin M. A.; Stenmark K. R.; McKinsey T. A. (2015) Emerging roles for histone deacetylases in pulmonary hypertension and right ventricular remodeling (2013 Grover Conference series). Pulm. Circ. 5 (1), 63–72. 10.1086/679700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucherat O.; Chabot S.; Paulin R.; Trinh I.; Bourgeois A.; Potus F.; Lampron M. C.; Lambert C.; Breuils-Bonnet S.; Nadeau V.; Paradis R.; Goncharova E. A.; Provencher S.; Bonnet S. (2017) HDAC6: A Novel Histone Deacetylase Implicated in Pulmonary Arterial Hypertension. Sci. Rep. 7 (1), 4546. 10.1038/s41598-017-04874-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.; Vakoc C. R. (2014) The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol. Cell 54 (5), 728–36. 10.1016/j.molcel.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Feen D. E.; Kurakula K.; Tremblay E.; Boucherat O.; Bossers G. P. L.; Szulcek R.; Bourgeois A.; Lampron M. C.; Habbout K.; Martineau S.; Paulin R.; Kulikowski E.; Jahagirdar R.; Schalij I.; Bogaard H. J.; Bartelds B.; Provencher S.; Berger R. M. F.; Bonnet S.; Goumans M. J. (2019) Multicenter Preclinical Validation of BET Inhibition for the Treatment of Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 200 (7), 910–920. 10.1164/rccm.201812-2275OC. [DOI] [PubMed] [Google Scholar]
- Rothman A. M. K.; Arnold N. D.; Pickworth J. A.; Iremonger J.; Ciuclan L.; Allen R. M. H.; Guth-Gundel S.; Southwood M.; Morrell N. W.; Thomas M.; Francis S. E.; Rowlands D. J.; Lawrie A. (2016) MicroRNA-140–5p and SMURF1 regulate pulmonary arterial hypertension. J. Clin. Invest. 126 (7), 2495. 10.1172/JCI83361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun P. M.; Mouthon L.; Barbera J. A.; Eddahibi S.; Flores S. C.; Grimminger F.; Jones P. L.; Maitland M. L.; Michelakis E. D.; Morrell N. W.; Newman J. H.; Rabinovitch M.; Schermuly R.; Stenmark K. R.; Voelkel N. F.; Yuan J. X.; Humbert M. (2009) Inflammation, growth factors, and pulmonary vascular remodeling. J. Am. Coll. Cardiol. 54 (1 Suppl), S10–9. 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Speich R.; Treder U.; Domenighetti G.; Huber L. C.; Ulrich S. (2014) Weaning from intravenous prostanoids and normalization of hemodynamics by long-term imatinib therapy in severe idiopathic pulmonary arterial hypertension. Int. J. Clin. Pharm. 36 (2), 256–60. 10.1007/s11096-013-9881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L.; Wiedow O. (2011) Therapeutic potential of human elafin. Biochem. Soc. Trans. 39 (5), 1450–4. 10.1042/BST0391450. [DOI] [PubMed] [Google Scholar]
- Nickel N. P.; Spiekerkoetter E.; Gu M.; Li C. G.; Li H.; Kaschwich M.; Diebold I.; Hennigs J. K.; Kim K. Y.; Miyagawa K.; Wang L.; Cao A.; Sa S.; Jiang X.; Stockstill R. W.; Nicolls M. R.; Zamanian R. T.; Bland R. D.; Rabinovitch M. (2015) Elafin Reverses Pulmonary Hypertension via Caveolin-1-Dependent Bone Morphogenetic Protein Signaling. Am. J. Respir. Crit. Care Med. 191 (11), 1273–86. 10.1164/rccm.201412-2291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean M. M. R. (2018) The serotonin hypothesis in pulmonary hypertension revisited: targets for novel therapies (2017 Grover Conference Series). Pulm. Circ. 8 (2), 204589401875912 10.1177/2045894018759125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean M. R.; Sweeney G.; Baird M.; McCulloch K. M.; Houslay M.; Morecroft I. (1996) 5-Hydroxytryptamine receptors mediating vasoconstriction in pulmonary arteries from control and pulmonary hypertensive rats. Br. J. Pharmacol. 119 (5), 917–30. 10.1111/j.1476-5381.1996.tb15760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahibi S.; Fabre V.; Boni C.; Martres M. P.; Raffestin B.; Hamon M.; Adnot S. (1999) Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells. Relationship with the mitogenic action of serotonin. Circ. Res. 84 (3), 329–36. 10.1161/01.RES.84.3.329. [DOI] [PubMed] [Google Scholar]
- Humbert M.; Lau Edmund M. T.; Montani D.; Jaïs X.; Sitbon O.; Simonneau G. (2014) Advances in Therapeutic Interventions for Patients With Pulmonary Arterial Hypertension. Circulation 130 (24), 2189. 10.1161/CIRCULATIONAHA.114.006974. [DOI] [PubMed] [Google Scholar]
- Hood K. Y.; Mair K. M.; Harvey A. P.; Montezano A. C.; Touyz R. M.; MacLean M. R. (2017) Serotonin Signaling Through the 5-HT1B Receptor and NADPH Oxidase 1 in Pulmonary Arterial Hypertension. Arterioscler., Thromb., Vasc. Biol. 37 (7), 1361–1370. 10.1161/ATVBAHA.116.308929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrascu R.; Kulcke C.; Konigshoff M.; Kouri F.; Yang X.; Morrell N.; Ghofrani H. A.; Weissmann N.; Reiter R.; Seeger W.; Grimminger F.; Eickelberg O.; Schermuly R. T.; Pullamsetti S. S. (2011) Terguride ameliorates monocrotaline-induced pulmonary hypertension in rats. Eur. Respir. J. 37 (5), 1104–18. 10.1183/09031936.00126010. [DOI] [PubMed] [Google Scholar]
- Sitbon O.; Gomberg-Maitland M.; Granton J.; Lewis M. I.; Mathai S. C.; Rainisio M.; Stockbridge N. L.; Wilkins M. R.; Zamanian R. T.; Rubin L. J. (2019) Clinical trial design and new therapies for pulmonary arterial hypertension. Eur. Respir. J. 53 (1), 1801908. 10.1183/13993003.01908-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai F. G.; Zhang X. H.; Wang H. L. (2009) Fluoxetine protects against monocrotaline-induced pulmonary arterial hypertension: potential roles of induction of apoptosis and upregulation of Kv1.5 channels in rats. Clin. Exp. Pharmacol. Physiol. 36 (8), 850–6. 10.1111/j.1440-1681.2009.05168.x. [DOI] [PubMed] [Google Scholar]
- Tan W. S. D.; Liao W.; Zhou S.; Mei D.; Wong W. F. (2018) Targeting the renin-angiotensin system as novel therapeutic strategy for pulmonary diseases. Curr. Opin. Pharmacol. 40, 9–17. 10.1016/j.coph.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Boehm M.; Arnold N.; Braithwaite A.; Pickworth J.; Lu C.; Novoyatleva T.; Kiely D. G.; Grimminger F.; Ghofrani H. A.; Weissmann N.; Seeger W.; Lawrie A.; Schermuly R. T.; Kojonazarov B. (2018) Eplerenone attenuates pathological pulmonary vascular rather than right ventricular remodeling in pulmonary arterial hypertension. BMC Pulm. Med. 18 (1), 41. 10.1186/s12890-018-0604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver E.; Mayor F. Jr.; D’Ocon P. (2019) Beta-blockers: Historical Perspective and Mechanisms of Action. Rev. Esp. Cardiol. 72 (10), 853–862. 10.1016/j.recesp.2019.02.023. [DOI] [PubMed] [Google Scholar]
- Provencher S.; Herve P.; Jais X.; Lebrec D.; Humbert M.; Simonneau G.; Sitbon O. (2006) Deleterious effects of beta-blockers on exercise capacity and hemodynamics in patients with portopulmonary hypertension. Gastroenterology 130 (1), 120–6. 10.1053/j.gastro.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Watson G.; Oliver E.; Zhao L.; Wilkins M. R. (2013) Pulmonary hypertension: old targets revisited (statins, PPARs, beta-blockers). Handb. Exp. Pharmacol. 218, 531–48. 10.1007/978-3-662-45805-1_21. [DOI] [PubMed] [Google Scholar]
- van Campen J. S.; de Boer K.; van de Veerdonk M. C.; van der Bruggen C. E.; Allaart C. P.; Raijmakers P. G.; Heymans M. W.; Marcus J. T.; Harms H. J.; Handoko M. L.; de Man F. S.; Vonk Noordegraaf A.; Bogaard H. J. (2016) Bisoprolol in idiopathic pulmonary arterial hypertension: an explorative study. Eur. Respir. J. 48 (3), 787–96. 10.1183/13993003.00090-2016. [DOI] [PubMed] [Google Scholar]
- Perros F.; Ranchoux B.; Izikki M.; Bentebbal S.; Happé C.; Antigny F.; Jourdon P.; Dorfmüller P.; Lecerf F.; Fadel E.; Simonneau G.; Humbert M.; Bogaard H. J.; Eddahibi S. (2015) Nebivolol for Improving Endothelial Dysfunction, Pulmonary Vascular Remodeling, and Right Heart Function in Pulmonary Hypertension. J. Am. Coll. Cardiol. 65 (7), 668. 10.1016/j.jacc.2014.11.050. [DOI] [PubMed] [Google Scholar]
- Farha S.; Saygin D.; Park M. M.; Cheong H. I.; Asosingh K.; Comhair S. A.; Stephens O. R.; Roach E. C.; Sharp J.; Highland K. B.; DiFilippo F. P.; Neumann D. R.; Tang W. H. W.; Erzurum S. C. (2017) Pulmonary arterial hypertension treatment with carvedilol for heart failure: a randomized controlled trial. JCI Insight 2 (16), 95240 10.1172/jci.insight.95240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alvarez A.; Pereda D.; Garcia-Lunar I.; Sanz-Rosa D.; Fernandez-Jimenez R.; Garcia-Prieto J.; Nuno-Ayala M.; Sierra F.; Santiago E.; Sandoval E.; Campelos P.; Aguero J.; Pizarro G.; Peinado V. I.; Fernandez-Friera L.; Garcia-Ruiz J. M.; Barbera J. A.; Castella M.; Sabate M.; Fuster V.; Ibanez B. (2016) Beta-3 adrenergic agonists reduce pulmonary vascular resistance and improve right ventricular performance in a porcine model of chronic pulmonary hypertension. Basic Res. Cardiol. 111 (4), 49. 10.1007/s00395-016-0567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lunar I.; Blanco I.; Fernandez-Friera L.; Prat-Gonzalez S.; Jorda P.; Sanchez J.; Pereda D.; Pujadas S.; Rivas M.; Sole-Gonzalez E.; Vazquez J.; Blazquez Z.; Garcia-Picart J.; Caravaca P.; Escalera N.; Garcia-Pavia P.; Delgado J.; Segovia-Cubero J.; Fuster V.; Roig E.; Barbera J. A.; Ibanez B.; Garcia-Alvarez A. (2020) Design of the beta3-Adrenergic Agonist Treatment in Chronic Pulmonary Hypertension Secondary to Heart Failure Trial. JACC Basic Transl Sci. 5 (4), 317–327. 10.1016/j.jacbts.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov V.; Mosgoeller W.; Ziesche R.; Raderer M.; Stiebellehner L.; Vonbank K.; Funk G. C.; Hamilton G.; Novotny C.; Burian B.; Block L. H. (2003) Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. J. Clin. Invest. 111 (9), 1339–46. 10.1172/JCI17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiè N.; Ghofrani A.-H. (2013) New horizons in pulmonary arterial hypertension therapies. European Respiratory Review 22 (130), 503. 10.1183/09059180.00006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh M. E.; Hemnes A. R. (2010) Pulmonary hypertension in women. Expert Rev. Cardiovasc. Ther. 8 (11), 1549–58. 10.1586/erc.10.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L.; Cogan J. D.; Hedges L. K.; Nunley B.; Hamid R.; Austin E. D. (2018) The Y Chromosome Regulates BMPR2 Expression via SRY: A Possible Reason “Why” Fewer Males Develop Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 198 (12), 1581. 10.1164/rccm.201802-0308LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Colon E.; Oliver E. (2018) Cardiac Stem Cells: A Plethora of Potential Therapies for Myocardial Regeneration Within Reach. Stem Cell Genetics for Biomedical Research 135–171. 10.1007/978-3-319-90695-9_7. [DOI] [Google Scholar]
- Lavoie J. R.; Stewart D. J. (2012) Genetically modified endothelial progenitor cells in the therapy of cardiovascular disease and pulmonary hypertension. Curr. Vasc. Pharmacol. 10 (3), 289. 10.2174/157016112799959413. [DOI] [PubMed] [Google Scholar]
- Granton J.; Langleben D.; Kutryk M. B.; Camack N.; Galipeau J.; Courtman D. W.; Stewart D. J. (2015) Endothelial NO-Synthase Gene-Enhanced Progenitor Cell Therapy for Pulmonary Arterial Hypertension: The PHACeT Trial. Circ. Res. 117 (7), 645–54. 10.1161/CIRCRESAHA.114.305951. [DOI] [PubMed] [Google Scholar]
- Huang W.-C.; Ke M.-W.; Cheng C.-C.; Chiou S.-H.; Wann S.-R.; Shu C.-W.; Chiou K.-R.; Tseng C.-J.; Pan H.-W.; Mar G.-Y.; Liu C.-P. (2016) Therapeutic Benefits of Induced Pluripotent Stem Cells in Monocrotaline-Induced Pulmonary Arterial Hypertension. PLoS One 11 (2), e0142476. 10.1371/journal.pone.0142476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. X.; Zhang F. R.; Shang Y. P.; Zhu J. H.; Xie X. D.; Tao Q. M.; Zhu J. H.; Chen J. Z. (2007) Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial.. J. Am. Coll. Cardiol. 49 (14), 1566–1571. 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Diller G. P.; van Eijl S.; Okonko D. O.; Howard L. S.; Ali O.; Thum T.; Wort S. J.; Bédard E.; Gibbs J. S.; Bauersachs J.; Hobbs A. J.; Wilkins M. R.; Gatzoulis M. A.; Wharton J. (2008) Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation 117 (23), 3020–3030. 10.1161/CIRCULATIONAHA.108.769646. [DOI] [PubMed] [Google Scholar]
- Sun H. X.; Li G. J.; Du Z. H.; Bing Z.; Ji Z. X.; Luo G.; Pan S. L. (2019) The relationship between endothelial progenitor cells and pulmonary arterial hypertension in children with congenital heart disease. BMC Pediatr. 19 (1), 502. 10.1186/s12887-019-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton R. C.; Fournier M.; Xu X.; Marban E.; Lewis M. I. (2017) Therapeutic benefits of intravenous cardiosphere-derived cell therapy in rats with pulmonary hypertension. PLoS One 12 (8), e0183557 10.1371/journal.pone.0183557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loisel F.; Provost B.; Haddad F.; Guihaire J.; Amsallem M.; Vrtovec B.; Fadel E.; Uzan G.; Mercier O. (2018) Stem cell therapy targeting the right ventricle in pulmonary arterial hypertension: is it a potential avenue of therapy?. Pulm. Circ. 8 (2), 2045893218755979 10.1177/2045893218755979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert V.; Gouadon E.; Capderou A.; Le Bret E.; Ly M.; Dinanian S.; Renaud J. F.; Pucéat M.; Rücker-Martin C. (2015) Right ventricular failure secondary to chronic overload in congenital heart diseases: benefits of cell therapy using human embryonic stem cell-derived cardiac progenitors. J. Thorac. Cardiovasc. Surg. 149 (3), 708–15e1. 10.1016/j.jtcvs.2014.11.033. [DOI] [PubMed] [Google Scholar]
- Hoeper M. M. (2016) Combination or monotherapy for pulmonary arterial hypertension?. Lancet Respir. Med. 4 (4), 247. 10.1016/S2213-2600(16)00058-8. [DOI] [PubMed] [Google Scholar]
- Prior D. L.; Adams H.; Williams T. J. (2016) Update on pharmacotherapy for pulmonary hypertension. Med. J. Aust. 205 (6), 271. 10.5694/mja16.00468. [DOI] [PubMed] [Google Scholar]
- Rossello X.; Rodriguez-Sinovas A.; Vilahur G.; Crisostomo V.; Jorge I.; Zaragoza C.; Zamorano J. L.; Bermejo J.; Ordonez A.; Bosca L.; Vazquez J.; Badimon L.; Sanchez-Margallo F. M.; Fernandez-Aviles F.; Garcia-Dorado D.; Ibanez B. (2019) CIBER-CLAP (CIBERCV Cardioprotection Large Animal Platform): A multicenter preclinical network for testing reproducibility in cardiovascular interventions. Sci. Rep. 9 (1), 20290. 10.1038/s41598-019-56613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]