Abstract
Atopic dermatitis (AD) is an eczematous, pruritic skin disorder with extensive barrier dysfunction and elevated interleukin (IL)-4 and IL-13 signatures. The barrier dysfunction correlates with the downregulation of barrier-related molecules such as filaggrin (FLG), loricrin (LOR), and involucrin (IVL). IL-4 and IL-13 potently inhibit the expression of these molecules by activating signal transducer and activator of transcription (STAT)6 and STAT3. In addition to IL-4 and IL-13, IL-22 and IL-17A are probably involved in the barrier dysfunction by inhibiting the expression of these barrier-related molecules. In contrast, natural or medicinal ligands for aryl hydrocarbon receptor (AHR) are potent upregulators of FLG, LOR, and IVL expression. As IL-4, IL-13, IL-22, and IL-17A are all capable of inducing oxidative stress, antioxidative AHR agonists such as coal tar, glyteer, and tapinarof exert particular therapeutic efficacy for AD. These antioxidative AHR ligands are known to activate an antioxidative transcription factor, nuclear factor E2-related factor 2 (NRF2). This article focuses on the mechanisms by which FLG, LOR, and IVL expression is regulated by IL-4, IL-13, IL-22, and IL-17A. The author also summarizes how AHR and NRF2 dual activators exert their beneficial effects in the treatment of AD.
Keywords: atopic dermatitis, skin barrier, filaggrin, filaggrin-2, loricrin, involucrin, IL-4, IL-13, IL-17A, IL-22
1. Introduction
The human epidermis is composed of stratified layers, differentiating from the basal layer on the basement membrane, towards spinous and granular layers, and finally to the outermost cornified layer [1,2,3,4,5,6]. Keratinocytes are the major constituent of epidermal cells. In this stratification process, the keratinocytes undergo well-orchestrated differentiation to optimize the skin barrier to survive the dry, harsh terrestrial conditions. Basal layer keratinocytes, expressing keratin 5 (K5) and K14, divide and move up to the spinous layer. Spinous layer keratinocytes then switch their keratin profile to K1 and K10. Keratins connect to the desmosomes, which are the cell–cell adhesion structures specific for stratified epithelium [1,2,3,4,5,6]. In the granular layer, keratinocytes commit to synthesize keratohyalin granules, which are primarily composed of profilaggrin and loricrin (LOR) [1,2,3,4,5,6]. This process coincides with the decreases of K1 and K10, increase of calcium and activation of protein kinase C [2]. In normal orthokeratotic conditions, cells are denucleated when the uppermost keratinocytes in the granular layer turn into cornified cells (corneocytes) in the cornified layer. Profilaggrin is processed to filaggrin (FLG) repeats and the cleaved N-terminus of profilaggrin translocates into the nucleus and may trigger the denucleation process [7,8,9]. The cornified cells provide a specialized cell membrane called the cornified envelope. The cornified envelope is composed of various cytoskeletal and barrier-related molecules, including K1, K10, desmosomal proteins (envoplakin and periplakin), LOR, FLG, filaggrin-2 (FLG2), and involucrin (IVL), which are crosslinked by transglutaminase 1 and partly by transglutaminases 3 and 5 [1,2,3,4,5,6]. Granular layer keratinocytes also produce lamellar granules, which are rich in polar lipids, glycosphingolipids, free sterols, and phospholipids. The lamellar granule lipids are released and integrated into the intercellular space lipids containing sterols, fatty acids and ceramides in the cornified layer. Some ceramides with ultralong omega-hydroxyl chains are covalently bound to the outside of the cornified envelope via scaffolds of IVL, envoplakin and periplakin [1,2,3,4,5,6]. This thin lipid layer covering the cornified envelope is called the corneocyte-bound lipid envelope [6,10]. These three structures, cornified envelope, corneocyte-bound lipid envelope, and intercellular space lipids, are key players in maintaining appropriate barrier function [1,2,3,4,5,6].
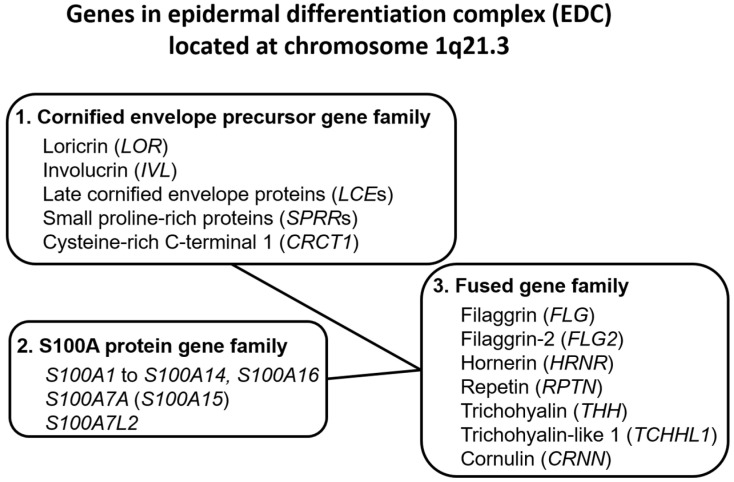
Upon terminal differentiation, keratinocytes synthesize a number of barrier-related molecules, which are sequentially incorporated into the above-mentioned three cardinal barrier structures. Notably, many barrier-related molecules expressed in the granular layer are genetically mapped to the chromosome 1q21.3 locus, which is called the epidermal differentiation complex (EDC) [5,11]. FLG, FLG2, LOR, and IVL genes are all located in the EDC [5,11]. The EDC also includes a series of genes encoding S100A proteins, such as S100A7 [5,12,13]. Most S100A proteins exert antimicrobial and proinflammatory effects [5,12]. LOR, IVL, late cornified envelope protein genes (LCEs), and small proline-rich protein genes (SPRRs) are classified into the “cornified envelope precursor gene family” [5]. FLG, FLG2, and hornerin (HRNR) genes are classified into the “fused gene family” evolved from the “S100A protein gene family” and the “cornified envelope precursor gene family” [5] (Figure 1).
Figure 1.
Genes encoding the epidermal differentiation complex.
The expression of EDC genes is not stable, but is actively modulated by external stimuli, including ultraviolet irradiation and photoproducts [14,15], dioxins, and other oxidative pollutants [4,16], bioproducts of commensal or symbiotic microorganisms such as Malassezia and Staphylococcus epidermidis [17,18,19], cosmetics [20], and various phytochemicals [21,22,23,24,25]. These chemical stimulants activate the xenobiotic chemical sensor aryl hydrocarbon receptor (AHR), upregulate the expression of barrier-related proteins, and accelerate the terminal differentiation of keratinocytes [4,14,16,18,26]. Consistent with this, long-lasting activation of AHR by dioxins induces the exaggerated terminal differentiation of keratinocytes and sebocytes, leading to the development of chloracne [27,28,29]. In particular, the exaggerated AHR activation converts sebocyte differentiation from sebaceous cell differentiation to keratinocytic differentiation, which results in the loss of sebocytes and keratinous cyst formation [29,30]. This is probably the major cause of chloracne. Although the mechanisms of accelerated keratinization are not fully understood, intracellular levels of reactive oxygen species (ROS) are one of the major modulators [4,31,32,33,34,35,36,37,38]. The intracellular oxidative stress level is fine-tuned by the oxidative stress-prone AHR system [4,31,39] and the antioxidative nuclear factor E2-related factor 2 (NRF2) system [32,33,34,35,36,37,38,40,41,42,43].
The expression of EDC genes is also actively modulated by internal stimuli such as cytokines. The gene expression of FLG, FLG2, LOR, IVL, and S100A7 is differentially affected in atopic dermatitis (AD) and psoriasis [44,45]. The dysregulated expression of FLG, FLG2, LOR, and IVL is known to be normalized by specific biologic treatments, for example, blockade of interleukin-4 (IL-4) and IL-13 in AD [44] or blockade of IL-17A in psoriasis [45]. The levels of IL-22 are elevated in both AD [46,47] and psoriasis [48,49]. IL-22 is also known to modulate the gene expression of these barrier-related molecules [50,51,52]. As the expression of IVL, LOR, FLG, FLG2, and other EDC genes is differentially altered by these pathogenic cytokines, the expression of these molecules is commonly used as reliable markers for evaluating the therapeutic efficacy of relevant biologics [44,45]. The purpose of this article is to review the current evidence on the regulatory activities of IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2 against IVL, LOR, FLG, and FLG2 gene expression with special reference to AD.
2. Roles of IVL, LOR, FLG, and FLG2 in Epidermal Barrier Formation
IVL has high structural homology with LOR in the glutamine- and lysine-rich amino- and carboxy-terminal domains [5]. IVL is expressed in the upper spinous layer, but mainly in the granular layers, and is involved in the initial step of cornified envelope formation. Cornified envelope formation starts from desmosomes where IVL is crosslinked with envoplakin, periplakin, and keratin filaments by transglutaminase 1 [5,6]. This protein complex also becomes the scaffold for the corneocyte-bound lipid envelope [6,10].
LOR is the most abundant component of the cornified envelope [1,3,5]. It is very hydrophobic, insoluble, and is easily polymerized via disulfide crosslinking in ambient air, making it suitable as a protein that reinforces the cornified envelope [1,5]. LOR is expressed in the granular layer and is crosslinked to IVL, envoplakin, and periplakin scaffolds by transglutaminase 1 [1,5].
Profilaggrin consists of a conserved small N-terminal domain, 10–12 FLG repeats and a C-terminal domain [5]. Profilaggrin to FLG processing requires several proteases, such as profilaggrin endopeptidase 1, matriptase 1, and channel-activating protease 1. FLG is involved in aggregating the K1 and K10 filaments into higher-molecular-weight parallel structures that facilitate the incorporation of K1 and K10 into the cornified envelope and contribute to the thin granular keratinocyte shape [1,5,53]. FLG peptides are simultaneously degraded by caspase 14 and calpain 1 into free hydrophilic amino acids, which maintain the intracellular water content [1,5,6]. Ichthyosis vulgaris is caused by the loss-of-function mutation of FLG [54]. Loss-of-function mutations of FLG have been demonstrated in a subpopulation of patients with AD, at rates ranging from 10% to 50% depending on the ethnicity [55,56]. Therefore, AD is a significant comorbidity with ichthyosis vulgaris [57,58].
FLG2 contains two distinct repeat domains, A and B. The A domain presents high homology with hornerin repeats and the B domain is homologous to FLG [5,59]. FLG2 is also expressed in the keratohyalin granules in the granular layer [5,59]. The expression of FLG and FLG2 is downregulated in skin treated with 5% or 10% lactic acid, with such downregulation often used to define sensitive skin [60]. The expression of FLG and FLG2 has also been reported to be downregulated by tape stripping [61]. In a three-dimensional reconstituted human epidermis model, FLG2 downregulation was found to induce parakeratosis, compact stratum corneum, increased pH, and reduced amounts of free amino acids with reduced proteolytic processing of corneodesmosin, hornerin, and filaggrin in parallel with reduced amounts of caspase-14 [62]. In another report, the expression of FLG2 was described as being colocalized with corneodesmosin in the cornified cells [63]. The absence of FLG2 induces the marked reduction of corneodesmosin expression [63]. Thus, FLG2 exerts a specialized function different from FLG.
Under physiological conditions, IVL is detected from the uppermost spinous layer to granular layer keratinocytes (early-phase epidermal terminal differentiation), but the expression of LOR, FLG, and FLG2 is more confined to granular cell layer keratinocytes (late-phase epidermal terminal differentiation) [5,64,65,66].
3. Upregulation of IVL, LOR, and FLG by AHR Activation
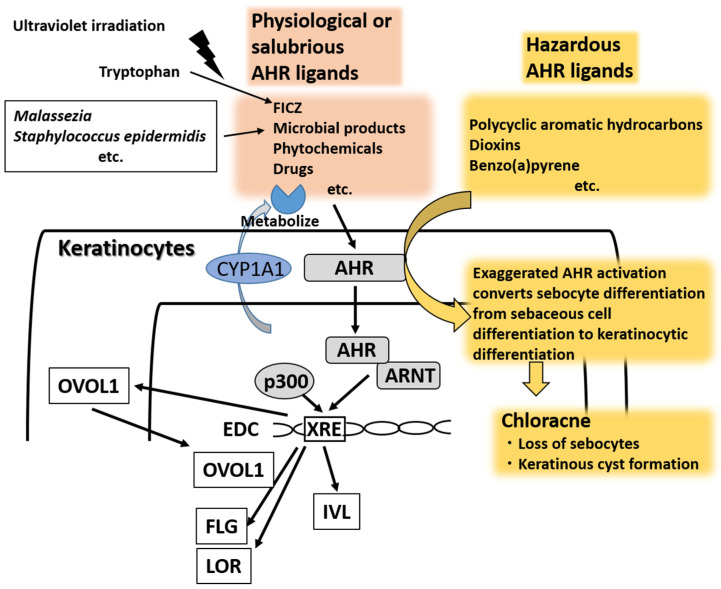
As a chemical sensor, AHR is one of the major transcription factors for EDC genes in keratinocytes. It was originally called dioxin receptor because environmental pollutants such as polycyclic aromatic hydrocarbons and dioxins bind to it with high affinity and generate ROS production [27,28,29,67,68,69]. In the absence of ligands, AHR resides in the cytoplasm, where it forms a protein complex with heat shock protein 90 (HSP90), hepatitis B virus X-associated protein 2 (XAP-2, also known as AIP or Ara9), p23, and c-Src protein kinase [70,71,72]. After ligand binding, AHR dissociates from the cytoplasmic complex and a nuclear translocation site of AHR is exposed. Then, AHR is translocated into the nucleus where it dimerizes with AHR-nuclear translocator (ARNT), binds DNA responsive elements called xenobiotic responsive elements (XREs), and upregulates the transcription of target genes, such as phase I metabolizing enzyme cytochrome P450 (CYP) members (i.e., CYP1A1, CYP1A2, and CYP1B1) [73,74] (Figure 2).
Figure 2.
There are many physiological or salubrious aryl hydrocarbon receptor (AHR) ligands such as tryptophan photoproduct 6-formylindolo [3,2-b] carbazole (FICZ), microbial products from Malassezia and Staphylococcus epidermidis, phytochemicals and drugs. AHR activated by ligands translocates into the nucleus and is heterodimerized with AHR-nuclear translocator (ARNT). The ligand–AHR–ARNT complex binds XRE regions with p300 cofactor and upregulates the transcription of target genes and associated protein expression, including for CYP1A1, OVOL1, filaggrin (FLG), loricrin (LOR), and involucrin (IVL). Cytoplasmic OVOL1 translocates into the nucleus and contributes to the upregulation of FLG and LOR, but not that of IVL. The effects of physiological or salubrious AHR ligands are transient because they are rapidly metabolized or degraded by CYP1A1. In contrast, hazardous AHR ligands such as polycyclic aromatic hydrocarbons, dioxins and benzo(a)pyrene are stable and long-lasting in the body because they are not easily metabolized by CYP1A1. The exaggerated AHR activation converts sebocyte differentiation from sebaceous cell differentiation to keratinocytic differentiation. This results in chloracne characterized by the loss of sebocytes and keratinous cyst formation. FICZ, 6-formylindolo [3,2-b] carbazole; XRE, xenobiotic responsive element.
Loertscher et al. were the first to demonstrate that exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces the premature or accelerated terminal differentiation of epidermal keratinocytes with upregulated expression of IVL, LOR, and FLG in three-dimensional skin equivalent models and in vivo models [65,75]. Their findings were later confirmed by Sutter’s group [76]. In monolayer culture, the effects of AHR activation are apparent in human keratinocytes under high-calcium or high-cell-density conditions, in which keratinocytes undergo more differentiation than proliferation [77,78]. In addition, AHR signaling is preferentially activated in differentiated rather than in proliferating keratinocytes [79]. Reciprocally, growth-promoting conditions involving treatment with epidermal growth factor or transforming growth factor α attenuate the TCDD-AHR/ARNT-mediated CYP1A1 and FLG expression [16,78]. The recruitment of nuclear factor p300 plays an essential role in the AHR/ARNT-mediated transcription of target genes [78]. Epidermal growth factor receptor signaling also requires p300 for its proper activity. The competitive usage of p300 by epidermal growth factor receptor activation leads to repression of the recruitment of p300 to the AHR/ARNT transcriptional complex, and eventually suppresses the AHR/ARNT activity to induce transcription of the target gene CYP1A1 [78] (Figure 2).
Activation of the AHR/ARNT system by TCDD is known to enhance the expression of IVL, LOR, FLG, and FLG2 genes, as well as many other EDC genes, such as hornerin, repetin, small proline-rich protein 2A, late cornified envelope protein 3A, and S100A7 [4,16,65]. The coordinated upregulation of EDC gene products by AHR/ARNT activation highlights the essential involvement of the AHR/ARNT system in epidermal terminal differentiation and skin barrier function. Moreover, AHR antagonists, GNF351 and CH223191, have been shown to inhibit FLG and IVL expression [79]. In addition, TCDD-induced AHR/ARNT activation increases the expression of 75% of the genes required for de novo ceramide biosynthesis, leading to the overproduction of ceramides 1–7 and 9 without affecting the levels of cholesterol and free fatty acids [4]. Moreover, the increased production of the cornified envelope by TCDD is blocked in the presence of antioxidative agents, indicating the important role of oxidative stress in the TCDD-AHR/ARNT-induced acceleration of epidermal terminal differentiation [4]. TCDD-induced ROS production is AHR- and CYP1A1-dependent because TCDD-induced ROS production was shown to be inhibited in AHR-silenced or CYP1A1-silenced cells [80].
Human skin is rich in tryptophan [81,82]. Ultraviolet irradiation generates tryptophan photoproducts such as 6-formylindolo [3,2-b] carbazole (FICZ), which is a high-affinity ligand for AHR [83]. This explains why ultraviolet irradiation upregulates the expression of CYP1A1, a target gene downstream of AHR/ARNT signaling [84,85,86]. FICZ upregulates the FLG expression in an AHR-dependent manner [15,26].
AHR is a promiscuous receptor and is activated by many other ligands such as bioproducts of commensal or symbiotic microorganisms (Malassezia and Staphylococcus epidermidis) [17,19,87], cosmetics [20], various phytochemicals [21,22,23,24,25], and drugs [88,89]. These AHR ligands are all known to upregulate the expression of FLG [17,20,22,23,24,25], IVL [17,22], LOR [22,23,24], and transglutaminase 1 [17]. Notably, AHR upregulates the FLG and LOR expression via another transcription factor, OVO-like 1 (OVOL1) [21,22,26,90,91], but OVOL1 is relatively dispensable for AHR-induced IVL upregulation [22] (Figure 2). OVOL1 promotes the differentiation of epidermal keratinocytes by repressing c-Myc-mediated cell proliferation [92,93,94]. Interestingly, the genes encoding FLG, OVOL1, and IL-13 have been reported as the top three genes conferring susceptibility to AD [95].
Under physiological conditions, endogenous AHR ligands, such as tryptophan photoproducts [15,26,83] and microbial bioproducts [17,19,87], may upregulate the expression of EDC genes via AHR activation and maintain the healthy epidermal barrier. In line with this, the expression of FLG, LOR, and IVL is downregulated in keratinocytes with AHR knockdown or in the presence of AHR antagonists during the terminal differentiation of keratinocytes [79]. However, the effects of endogenous AHR ligands are transient because these ligands are rapidly degraded by AHR-induced CYP1A1 [83,96]. In contrast, TCDD and other dioxins are likely to induce long-lasting and exaggerated activation of AHR because these agents are chemically stable and resistant to degradation via CYP1A1 [27,28]. The high-level activation of AHR accelerates the keratinization process of sebocytes and keratinoctytes, leading to chloracne [27,28,29,30,97].
4. Decreased Expression of IVL, LOR, and FLG in AD
AD is a common and heterogeneous eczematous skin disorder characterized by Th2-deviated skin inflammation, barrier disruption, and chronic pruritus [98,99,100]. Frequent relapse with intense pruritus deteriorates the quality of life and decreases the treatment satisfaction of afflicted patients [101,102,103,104,105]. Skin barrier dysfunction is associated with the reduced production of terminal differentiation molecules such as FLG [10,31,106,107,108,109].
The expression of INV and LOR was also found to be decreased in the lesional and nonlesional skin of AD [31,110]. Abnormal skin barrier integrity also causes the increased colonization of microbes such as Staphylococcus aureus, which further exacerbates Th2-deviated skin inflammation [111,112].
Although the strongest genetic risk factors for AD are loss-of-function mutations in the FLG gene [95], FLG mutations were not found in all AD patients, were less common in Southern Europeans with AD [113] and were even absent in patients with AD from some African countries [114], suggesting that FLG mutations only partly explain FLG protein downregulation in AD. Moreover, FLG mutation was shown not to be related to the development of AD in patients from a subtropical island in Japan [115].
Notably, IL-4 and IL-13 are known to decrease the FLG [20,25,26,31,110,116], LOR [110], and IVL [31,110] expression in vitro. Therefore, a Th2-polarized inflammatory milieu in AD may be more influential in the downregulation of FLG expression than loss-of-function mutation of the FLG gene [91,106,117]. The pathogenic importance of IL-4 and IL-13 has recently been reinforced by the excellent treatment response of patients with AD to the anti-IL-4 receptor α (IL-4Rα, IL4R) antibody dupilumab, which inhibits both IL-4 and IL-13 signals [44,118]. More recently, large-scale transcriptomic analysis revealed the specific and dominant role of IL-13 in the lesional skin of AD because IL-4 expression was nearly undetectable [119]. Consistent with this notion, the anti-IL-13 antibody tralokinumab was shown to successfully improve AD [120].
In addition to FLG, the expression of LOR, IVL, and FLG2 is downregulated or occurs prematurely in the lesional and nonlesional skin of AD compared with their expression in the normal skin of healthy individuals [26,31,110,121,122,123,124]. In line with these reports, topical steroids significantly improve clinical inflammatory signs and normalize transepidermal water loss in lesional AD skin with the upregulation of FLG and LOR expression [125]. These improvements are associated with downregulation of the Th2 (IL-13 and IL-31) signature [125]. Similar results have been obtained in dupilumab treatment. The expression of FLG and LOR is decreased in the lesional skin compared with that in the nonlesional skin in AD [44]. Dupilumab, but not placebo, restores the downregulation of FLG and LOR [44]. It is also known that the expression of FLG, FLG2, and LOR is downregulated in the patch test site of paraphenylenediamine in patients with allergic contact dermatitis [126].
5. Downregulation of IVL, LOR, and FLG by IL-4/IL-13
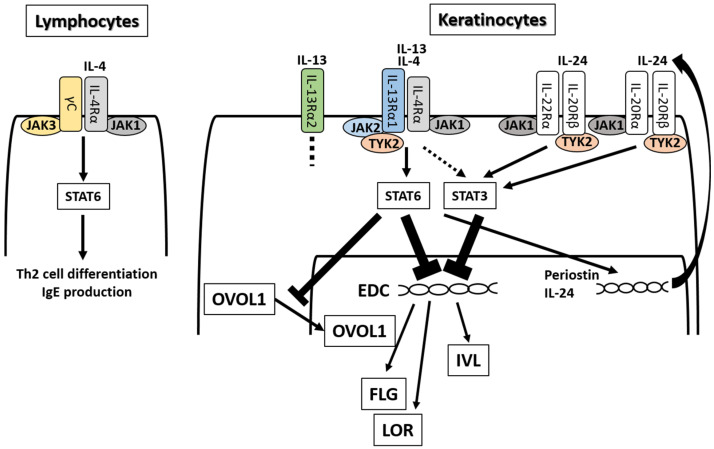
As described above, IL-4Rα signaling by IL-4 and IL-13 is known to reduce the expression of EDC molecules, including IVL [31,110,127,128], LOR [31,127,128], FLG [20,25,26,31,110,116,122,127,128], FLG2 [122,127,128], and hornerin [31,122] in keratinocytes (Figure 3). IL-4 has two heterodimeric receptors (IL-4Rα/γc and IL-4Rα/IL-13Rα1) [129]. IL-13 also has two receptors (IL-4Rα/IL-13Rα1 and IL-13Rα2) [129]. IL-4Rα/γc signaling preferentially occurs in hematopoietic cells, while IL-4Rα/IL-13Rα1 signaling predominantly occurs in nonhematopoietic ones [129,130]. In lymphocytes, IL-4, but not IL-13, induces Th2 cell differentiation and IgE production via IL-4Rα/γc heterodimer [131] (Figure 3). The importance of IL-4Rα/IL-13Rα1 in nonhematopoietic cells coincides with the fact that IL-13, but not IL-4, is preferentially expressed in the lesional skin of AD [44,119]. IL-13Rα2 is a decoy receptor for IL-13 and inhibits IL-13 signaling by trapping functional IL-13 [129,132,133].
Figure 3.
IL-4 and IL-13 have partly shared receptor systems. IL-4 binds IL-4Rα/γC in lymphocytes and other hematopoietic cells, activates the JAK1/JAK3-STAT6 pathway, and induces Th2 differentiation and IgE production. IL-4 and IL-13 share IL-4Rα/IL-13Rα1 in keratinocytes, activate the JAK1/JAK2/TYK2-STAT6 and -STAT3 pathway, and inhibit the expression of EDC molecules such as FLG, LOR, and IVL. IL-4 and IL-13 also inhibit the cytoplasmic-to-nuclear translocation of OVOL1 and downregulate the expression of FLG and LOR. IL-4/IL-13-mediated STAT6 activation upregulates periostin and subsequently enhances IL-24 production. IL-24 binds to IL-20Rβ/IL-22Rα or IL-20Rβ/IL-20Rα, activates the JAK1/TYK2-STAT3 pathway, and inhibits the expression of FLG.
After ligation by IL-4, IL-4Rα/γc heterodimer activates Janus kinase 1 (JAK1) and JAK3 and induces the activation (phosphorylation) of signal transducer and activator of transcription (STAT)6 [129,134,135]. IL-4 and IL-13 bind IL-4Rα/IL-13Rα1, activate JAK1, JAK2, and tyrosine kinase 2 (TYK2), and induce the phosphorylation of STAT6 [129,130,136,137]. Although evidence for the STAT6 activation by IL-4 and IL-13 has been consistently found, several groups suggest the possibility that IL-4 and IL-13 activate other STAT family members including STAT1 [128], STAT3 [127,128], and STAT5 [138].
It is still controversial whether IL-4/IL-13-mediated STAT6 activation is fully responsible for the downregulation of EDC molecules [127]. In Stat6 transgenic mice, the cutaneous expression of Lor and Ivl was shown to be significantly decreased compared with that in control wild-type mice [110]. In contrast, the permeability barrier function was found to be upregulated in Stat6-deficient mice with significantly increased Lor expression [139]. Moreover, the Flg and Ivl expression tended to be upregulated in Stat6-deficient mice, but this did not reach statistical significance [139].
Amano et al. highlighted a significant role of the IL-4/IL-13-mediated activation of STAT3, but not STAT6, in the downregulation of FLG and LOR using keratinocytes treated with specific small interfering RNA (siRNA) for STAT3 or STAT6 [127]. IL-4/IL-13-mediated downregulation of FLG and LOR was shown to be canceled in keratinocytes treated with STAT3 siRNA, but not in those with STAT6 siRNA [127]. Notably, IL-4/IL-13-mediated upregulation of CCL26 and CXCL6 expression was canceled in keratinocytes treated with STAT6 siRNA, but not in those with STAT3 siRNA [127]. These results indicate that the IL-4/IL-13-mediated activation of STAT3 transmits signals different from those by the IL-4/IL-13-mediated activation of STAT6, and that IL-4/IL-13-mediated STAT3 activation is probably responsible for the downregulation of EDC molecules in keratinocytes [127]. As both IL-4Rα/γc and IL-4Rα/IL-13Rα1 require the JAK family for their activation, different kinds of JAK inhibitors potently restore the IL-4/IL-13-mediated downregulation of EDC molecules (IVL, LOR, FLG, and FLG2) and improve skin barrier function in vitro and in vivo [127,128].
The IL-13-mediated activation of STAT3 may be caused by another cytokine pathway (Figure 3). IL-4- and IL-13-mediated activation of STAT6 could feasibly upregulate IL-24 production [140,141]. IL-13-induced STAT6 activation upregulates the production of periostin, which promotes allergic inflammation and fibrosis in keratinocytes [142,143]. Periostin stimulates keratinocytes to produce IL-24 [144,145]. IL-24 has two heterodimeric receptors, IL-22Rα/IL-20Rβ and IL-20Rα/IL-20Rβ [146]. After ligation with IL-24, both receptors activate JAK1/TYK2 and STAT3, and downregulate FLG expression [144,145] (Figure 3).
6. Medicinal Use of AHR/NRF2 Dual Activators for AD
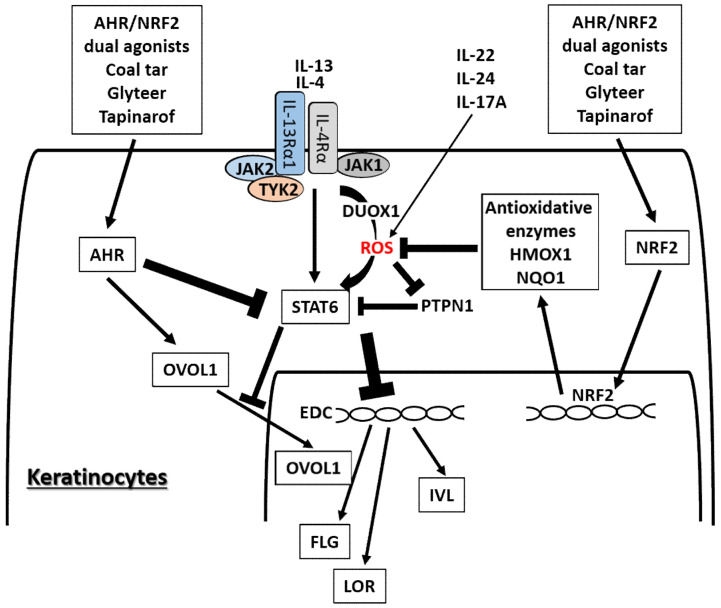
Apart from long-lasting and hazardous AHR ligands, many phytochemical AHR ligands play potentially salubrious roles for skin barrier function by upregulating FLG, LOR, and IVL [21,22,23,24,25]. Some AHR ligands are potent NRF2 activators and are expected to be valuable for medicinal use for eczematous skin diseases [21,147] where oxidative stress is upregulated [148,149]. Antioxidative AHR agonists such as coal tar [31], soybean tar glyteer [25,26], and tapinarof [150,151,152] have been widely used or undergone clinical trials for the treatment of inflammatory skin diseases including AD (Figure 4). Coal tar has been integrated into topical skin treatments for more than 2000 years [31]. Glyteer is a delipidated soybean tar licensed in 1924 in Japan and is still covered under the Japanese national medical insurance system as an ointment, in which it is mixed with dexamethasone [25,153]. Tapinarof {5-[(E)-2-phenylethenyl]-2-[propan-2-yl] benzene-1,3-diol, WBI-1001, GSK2894512 or benvitimod} is a naturally derived (but now fully synthetic) hydroxylated stilbene produced by bacterial symbionts of entomopathogenic nematodes [150,152,154,155]. Coal tar, glyteer, and tapinarof could feasibly upregulate the EDC molecules, including FLG, IVL, and hornerin, via AHR activation and exert their antioxidative function via NRF2 activation [25,31,152]. Recent clinical trials of topical tapinarof have proved its efficacy for AD compared with placebo control [150,151,156].
Figure 4.
Medicinal AHR/NRF2 dual agonists can restore the IL-4/IL-13-STAT6-induced downregulation of FLG, LOR, and IVL expression. Coal tar and glyteer activate AHR and inhibit the STAT6 effects partially by increasing the entry of OVOL1 into the nucleus. IL-4 and IL-13 activate dual oxidase protein 1 (DUOX1), generate reactive oxygen species (ROS) production, and promote STAT6 phosphorylation in keratinocytes. On the other hand, the activation or phosphorylation of STAT6 by IL-4 and IL-13 is negatively regulated by protein-tyrosine phosphatase, nonreceptor-type 1 (PTPN1) because PTPN1 dephosphorylates the phosphorylated STAT6. ROS induced by IL-4 and IL-13 inhibit PTPN1 activity and subsequently enhance STAT6 phosphorylation. AHR/NRF2 dual agonists also activate NRF2 and upregulate the antioxidative enzymes such as NAD(P)H quinone oxidoreductase 1 (NQO1) and heme oxygenase 1 (HMOX1), which neutralize the IL-4/IL-13-induced ROS and downregulate STAT6 phosphorylation by revitalizing the PTPN1 activity. IL-22, IL-24, and IL-17A also induce ROS production.
AHR and NRF2 dual activators, coal tar and glyteer, have been shown to restore the IL-4/IL-13-mediated downregulation of IVL, LOR, and FLG [25,31]. IL-4 and IL-13 activate dual oxidase protein 1 (DUOX1), generate ROS production and promote STAT6 phosphorylation in keratinocytes [157] (Figure 4). On the other hand, the activation or phosphorylation of STAT6 by IL-4 and IL-13 is negatively regulated by protein-tyrosine phosphatase, nonreceptor-type 1 (PTPN1) because PTPN1 dephosphorylates the phosphorylated STAT6 [157,158,159]. Oxidative stress induced by IL-4 and IL-13 inhibits PTPN1 activity and subsequently enhances STAT6 phosphorylation [157,158,159]. Coal tar activates NRF2 and upregulates antioxidative enzymes such as NAD(P)H quinone oxidoreductase 1 (NQO1), which neutralize the IL-4/IL-13-induced ROS and downregulate STAT6 phosphorylation by revitalizing the PTPN1 activity [31]. The antioxidative AHR agonist glyteer inhibits the IL-4-induced downregulation of FLG, and activates NRF2 and downstream antioxidative enzymes such as NQO1 [24,25]. NRF2 activation also upregulates various other antioxidative enzymes such as heme oxygenase 1 (HMOX1) [40,42,43,143,160] and glutathione peroxidase 2 (GPX2) [161]. As oxidative stress is also capable of enhancing STAT3 activation, it is possible that antioxidative agents may inhibit the STAT3 pathway [162,163].
In parallel with this, other NRF2-activating phytochemicals exhibit similar inhibitory action on IL-4/IL-13-mediated FLG downregulation [20,24], IL-13-mediated periostin upregulation [143] and imiquimod-induced STAT3 activation [164,165] in keratinocytes. IL-4 also stimulates dendritic cells via STAT6 activation to produce CCL17 and CCL22, which are potent chemokines for recruiting Th2 cells [166]. In addition, IL-4 stimulates dendritic cells to upregulate the expression of receptors for IL-31, which is the major pruritogenic cytokine in AD [167]. In addition, antioxidative glyteer inhibits the IL-4/STAT6-mediated expression of CCL17 and CCL22 expression as well as upregulation of the IL-31 receptor [166,167].
OVOL1 is an important transcription factor for epidermal terminal differentiation. It resides in the cytoplasm in a steady-state condition, but activated OVOL1 translocates into the nucleus and regulates downstream gene expression [26,90,92]. OVOL1 suppresses c-Myc expression and inhibits keratinocyte proliferation [93,94], but it conversely promotes epidermal differentiation and upregulates the expression of FLG and LOR [22,26,90]. AHR activation upregulates the expression of OVOL1, induces its cytoplasmic-to-nuclear translocation, and increases the expression of FLG and LOR [22,26,90,92]. IL-4 does not affect or rather enhances OVOL1 expression, but it inhibits the cytoplasmic-to-nuclear translocation of OVOL1, which correlates with the downregulation of FLG expression [26,90]. AHR activation restores the IL-4-mediated inhibition of OVOL1 nuclear translocation and recovers the IL-4-induced FLG downregulation [26,90]. Interestingly, AHR activation also upregulates IVL expression, but its regulation is OVOL1-independent [22]. In addition, IL-4 and IL-13 themselves increase the mRNA and protein expression of AHR in B cells and keratinocytes [168,169]. This suggests a mutually compensatory (or seesaw) regulation between Th2 and AHR signaling. Keratinocytes are a rich source of pro-Th2 cytokines such as IL-33 [98]. AHR-mediated OVOL1 activation is also functional in inhibiting IL-33 production in keratinocytes [170].
7. Downregulation of IVL, LOR, and FLG by IL-22
Increased IL-17A and IL-22 signatures are also shown in AD [44,46,47,99,125,171,172,173]. However, the pathogenic roles of Th17 and Th22 cell infiltration have not been fully elucidated in Th2-dominant AD. Guttman-Yassky et al. demonstrated that a blockade of Th2 signaling by dupilumab significantly decreased and normalized not only Th2 signatures, but also Th17 and Th22 signatures, in lesional skin of patients suffering from AD [44]. This suggests the possibility that the increased Th17 and Th22 signatures may be associated with Th2 dominance in AD. Initially, IL-22 was thought to be produced from Th17 cells, but recent human studies have revealed that Th22 cells, which do not produce IL-17A, are the main IL-22 producers [174,175]. Notably, IL-22 production from Th17/22, Th22, and innate lymphoid cells is dependent on AHR [176,177,178,179].
Recent clinical trials of the anti-IL-17A antibody secukinumab, a very potent therapeutic agent for treating psoriasis, did not report its satisfactory efficacy against AD (https://clinicaltrials.gov/ct2/show/results/NCT02594098?term=atopic&cond=secukinumab&draw=2&rank=1). In contrast to IL-17A, IL-22 may exert additional pathogenic effects apart from those mediated by IL-4/IL-13 because the anti-IL-22 antibody fezakinumab shows weak therapeutic potential for treating patients with severe AD [46]. Moreover, fezakinumab is more efficacious for patients with high pretreatment expression of IL-22 than for those with low IL-22 expression [180].
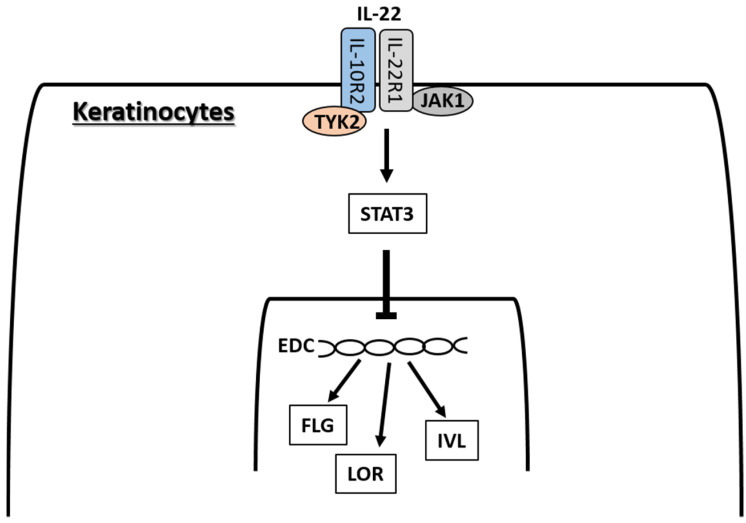
IL-22 signaling occurs via a heterodimeric receptor composed of IL-22R1 and IL-10R2, the expression of which is limited to epithelial cells, including those in skin [181,182] (Figure 5). The ligation of IL-22R1/IL-10R2 phosphorylates JAK1 and TYK2, and activates mainly STAT3 in keratinocytes [138,183], and STAT3, to a lesser extent, STAT1 and STAT5 in hepatoma cell lines [184]. IL-22 promotes keratinocyte proliferation and migration in association with STAT3 activation, and inhibits the terminal differentiation of keratinocytes [50,51,138,185,186,187]. IL-22 inhibits the expression of K1 [51,138,185], K10 [50,188], IVL [50,185], LOR [50,188], and FLG [50,51,138,186,188]. House dust mites may enhance the effects of IL-22 because they increase the IL-22R1 expression in keratinocytes [189].
Figure 5.
IL-22 binds IL-22R1/IL-10R2 complex, activates the JAK1/TYK2-STAT3 pathway and inhibits the expression of FLG, LOR, and IVL.
On the other hand, IL-22 upregulates the antimicrobial and proinflammatory EDC molecules, including S100A7, S100A8, and S100A9 in keratinocytes [50]. IL-4 and IL-13 are not significant inducers of S100A7, but its expression is enhanced in the lesional skin of AD probably because IL-17A and IL-22 upregulate S100A7 expression [138,190]. Similar to IL-4 and IL-13 [138,144], IL-22 also upregulates the IL-24 expression, which may inhibit FLG expression via JAK1-STAT3 activation [138,187]. Similar to IL-22, IL-24 is also responsible for keratinocyte proliferation and S100A7 upregulation [52]. Both IL-22 and IL-24 induce ROS production [191,192,193], and antioxidative AHR ligands may ameliorate the inflammatory outcome of IL-22 and IL-24. In fact, the antioxidant luteolin-7-glucoside decreases intracellular ROS levels and inhibits the IL-22-mediated activation of STAT3 [194].
8. Regulation of IVL, LOR, and FLG by IL-17A
Although the pathogenic significance of IL-17A is not fully understood in AD, IL-17A plays a critical role in the pathogenesis of psoriasis, as indicated by the excellent efficacy of anti-IL-17A biologics for psoriasis [195,196,197,198,199,200,201,202,203,204,205,206]. IL-17A alone may not be sufficient to fully activate the proinflammatory cascade, but it accelerates psoriatic inflammation with the help of other key pathogenic cytokines such as tumor necrosis factor-α (TNF-α), IL-23, and IL-22 [187,207,208,209].
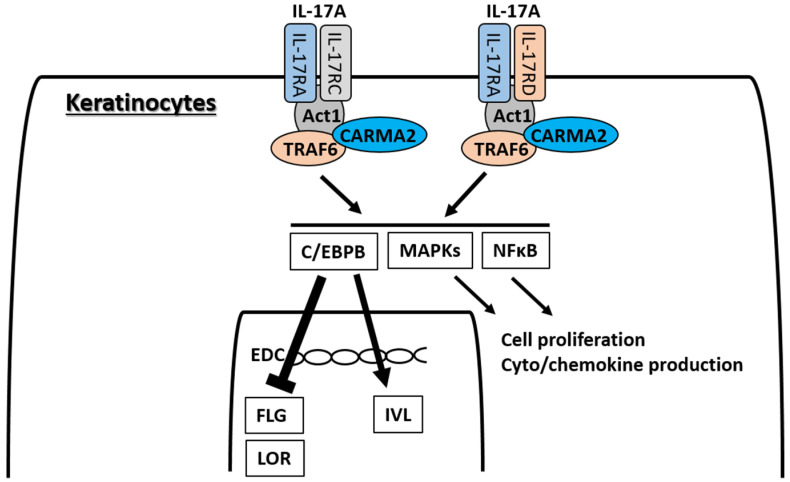
IL-17A signaling is known to occur via two heterodimeric receptors, IL-17RA/IL-17RC and IL-17RA/IL-17RD [210,211,212,213] (Figure 6). Keratinocytes express both IL-17RA/IL-17RC and IL-17RA/IL-17RD, and IL-17A ligation activates the transcription of different sets of genes [213]. Initial subcellular events in the ligation of IL-17RA/IL-17RC by IL-17A are the recruitment and activation of ACT1, TRAF6, and CARMA2 complexes, and the downstream activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and MAPKs [210,211,212,213]. The activation of NF-κB and MAPKs is involved in the IL-17A-mediated keratinocyte proliferation and cyto/chemokine production [208]. The ligation of IL-17RA/IL-17RC by IL-17A induces the activation of NF-κB, ERK, p38 MAPK, and JNK, while that of IL-17RA/IL-17RD mainly activates p38 MAPK and JNK and, to a lesser extent, NF-κB and ERK [213]. IL-17A promotes keratinocyte proliferation directly [208,214] and also indirectly by inducing the production of IL-19 from them [207,215,216,217]. IL-17A is unlikely to directly activate JAK-STAT pathways [138], but may activate STAT3 via IL-19 signaling [218].
Figure 6.
IL-17A has two receptor complexes, IL-17RA/IL-17RC and IL-17RA/IL-17RD, in keratinocytes. Signaling of both receptors occurs via downstream ACT1, TRAF6, and CARMA2 protein complexes, and activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and MAPKs. Activation of NF-κB and MAPKs induces cell proliferation and cyto/chemokine production in keratinocytes. IL-17A is not likely to directly activate JAK-STAT pathways. Another important transcription factor for IL-17A signaling is the C/CAAT-enhancer-binding protein β (C/EBPB) or C/EBPD. The IL-17A-C/EBPB pathway is likely to upregulate IVL expression, but to downregulate FLG and LOR expression.
Another important transcription factor for IL-17A signaling is the C/CAAT-enhancer-binding proteins (C/EBPs), particularly C/EBPB or C/EBPD [215]. In contrast, IL-17A is likely to inhibit the C/EBPA molecule [214]. The C/EBP family members are involved in epidermal keratinocyte differentiation [219] and are strongly upregulated in the lesional skin of psoriasis [215]. Together with the elevated CEBPB gene expression, the expression of keratinocyte terminal differentiation genes, such as IVL, FLG2, and TGM1, is upregulated in the lesional skin of psoriasis [215]. In the promoter region of the IVL gene, there is a binding site for C/EBP and the C/EBP transcription factor is necessary for the appropriate and continuous production of IVL protein [220]. In contrast to IVL, IL-17A is reported to downregulate the expression of K10 [51], LOR [51,215], and FLG [138,215,221]. In addition, the expression of S100A7 is upregulated by IL-17A [51,138,215]. Intriguingly, IL-4 and IL-13 are unlikely to affect the S100A7 expression or rather inhibit its expression in keratinocytes [138,222]. Notably, IL-17A is also a potent ROS producer in keratinocytes [192] and recent clinical trials have proven that topical treatment of the antioxidative AHR ligand tapinarof is efficacious for psoriasis [223,224].
9. Conclusions
The downregulation of EDC molecules, such as IVL, LOR, FLG, and FLG2, is the cardinal feature of the lesional skin of AD and is associated with skin barrier dysfunction. Although loss-of-function mutation of the FLG gene is the genetic abnormality most frequently associated with AD [95], an IL-4- and IL-13-deviated milieu is probably far more important in the pathogenesis of AD when we take into account the excellent efficacy of the anti-IL-4 receptor α antibody dupilumab for AD. IL-4 and IL-13 do inhibit the expression of IVL, LOR, FLG, and FLG2 in keratinocytes in vitro, and the blockade of IL-4 and IL-13 by dupilumab restores the decreased expression of FLG and LOR in the lesional skin of AD.
In addition to IL-4 and IL-13 produced from Th2 cells, IL-22 and IL-17A, produced from Th22 and Th17 cells, are also known to participate in the pathogenesis of AD. IL-4 and IL-13 downregulate the expression of EDC molecules via STAT6 and STAT3 activation. IL-22 also activates STAT3 and inhibits the expression of EDC molecules. IL-22 is probably more potent than IL-17A in downregulating the EDC molecules.
In contrast to cytokine-mediated downregulation, the expression of EDC molecules is upregulated by AHR signaling. In addition to IL-24 [225], IL-4, IL-13, IL-22, and IL-17A are all potent inducers of oxidative stress; therefore, antioxidative AHR ligands with an NRF2-activating profile are expected to be useful for the treatment of AD. Medicinal coal tar and soybean tar glyteer are such AHR and NRF2 dual activators and have been shown to be efficacious in AD. However, these crude agents contain various compounds and have a bad smell. A single chemical compound, tapinarof, is another AHR and NRF2 dual activator, the efficacy for AD of which was recently proven in clinical trials. Of course, exaggerated NRF2 activation seen in the NRF2-transgenic mouse is known to be associated with dysregulated epidermal terminal differentiation [36]. The mechanisms by which EDC molecules are regulated by cytokines, AHR, and NRF2 are not fully understood and there is significant scope for additional investigation. Future studies should open up new strategies for the development of drugs for AD.
Funding
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (H30-Shokuhin-Shitei-005) and Grant-in-Aid for Scientific Research (C, FAG0K08692) from the Ministry of Education, Culture, Sports, Science and Technology.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Ishitsuka Y., Roop D.R. Loricrin: Past, present, and future. Int. J. Mol. Sci. 2020;21:2271. doi: 10.3390/ijms21072271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalinin A., Marekov L.N., Steinert P.M. Assembly of the epidermal cornified cell envelope. J. Cell Sci. 2001;114:3069–3070. doi: 10.1242/jcs.114.17.3069. [DOI] [PubMed] [Google Scholar]
- 3.Karim N., Phinney B.S., Salemi M., Wu P.W., Naeem M., Rice R.H. Human stratum corneum proteomics reveals cross-linking of a broad spectrum of proteins in cornified envelopes. Exp. Dermatol. 2019;28:618–622. doi: 10.1111/exd.13925. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy L.H., Sutter C.H., Leon Carrion S., Tran Q.T., Bodreddigari S., Kensicki E., Mohney R.P., Sutter T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated production of reactive oxygen species is an essential step in the mechanism of action to accelerate human keratinocyte differentiation. Toxicol. Sci. 2013;132:235–249. doi: 10.1093/toxsci/kfs325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kypriotou M., Huber M., Hohl D. The human epidermal differentiation complex: Cornified envelope precursors, S100 proteins and the ‘fused genes’ family. Exp. Dermatol. 2012;21:643–649. doi: 10.1111/j.1600-0625.2012.01472.x. [DOI] [PubMed] [Google Scholar]
- 6.Proksch E., Brandner J.M., Jensen J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008;17:1063–1072. doi: 10.1111/j.1600-0625.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 7.Ishida-Yamamoto A., Takahashi H., Presland R.B., Dale B.A., Iizuka H. Translocation of profilaggrin N-terminal domain into keratinocyte nuclei with fragmented DNA in normal human skin and loricrin keratoderma. Lab. Investig. 1998;78:1245–1253. [PubMed] [Google Scholar]
- 8.Pearton D.J., Dale B.A., Presland R.B. Functional analysis of the profilaggrin N-terminal peptide: Identification of domains that regulate nuclear and cytoplasmic distribution. J. Investig. Dermatol. 2002;119:661–669. doi: 10.1046/j.1523-1747.2002.01831.x. [DOI] [PubMed] [Google Scholar]
- 9.Presland R.B., Kimball J.R., Kautsky M.B., Lewis S.P., Lo C.Y., Dale B.A. Evidence for specific proteolytic cleavage of the N-terminal domain of human profilaggrin during epidermal differentiation. J. Investig. Dermatol. 1997;108:170–178. doi: 10.1111/1523-1747.ep12333356. [DOI] [PubMed] [Google Scholar]
- 10.Chiba T., Nakahara T., Kohda F., Ichiki T., Manabe M., Furue M. Measurement of trihydroxy-linoleic acids in stratum corneum by tape-stripping: Possible biomarker of barrier function in atopic dermatitis. PLoS ONE. 2019;14:e0210013. doi: 10.1371/journal.pone.0210013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mischke D., Korge B.P., Marenholz I., Volz A., Ziegler A. Genes encoding structural proteins of epidermal cornification and S100 calcium-binding proteins form a gene complex (“epidermal differentiation complex”) on human chromosome 1q21. J. Investig. Dermatol. 1996;106:989–992. doi: 10.1111/1523-1747.ep12338501. [DOI] [PubMed] [Google Scholar]
- 12.Leśniak W., Graczyk-Jarzynka A. The S100 proteins in epidermis: Topology and function. Biochim. Biophys. Acta. 2015;1850:2563–2572. doi: 10.1016/j.bbagen.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin Z.A., de Guzman Strong C. Recent positive selection in genes of the mammalian epidermal differentiation complex locus. Front. Genet. 2017;7:227. doi: 10.3389/fgene.2016.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furue M., Uchi H., Mitoma C., Hashimoto-Hachiya A., Tanaka Y., Ito T., Tsuji G. Implications of tryptophan photoproduct FICZ in oxidative stress and terminal differentiation of keratinocytes. G. Ital. Dermatol. Venereol. 2019;154:37–41. doi: 10.23736/S0392-0488.18.06132-1. [DOI] [PubMed] [Google Scholar]
- 15.Kiyomatsu-Oda M., Uchi H., Morino-Koga S., Furue M. Protective role of 6-formylindolo[3,2-b]carbazole (FICZ), an endogenous ligand for arylhydrocarbon receptor, in chronic mite-induced dermatitis. J. Dermatol. Sci. 2018;90:284–294. doi: 10.1016/j.jdermsci.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Sutter C.H., Bodreddigari S., Campion C., Wible R.S., Sutter T.R. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases the expression of genes in the human epidermal differentiation complex and accelerates epidermal barrier formation. Toxicol. Sci. 2011;124:128–137. doi: 10.1093/toxsci/kfr205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buommino E., Baroni A., Papulino C., Nocera F.P., Coretti L., Donnarumma G., De Filippis A., De Martino L. Malassezia pachydermatis up-regulates AhR related CYP1A1 gene and epidermal barrier markers in human keratinocytes. Med. Mycol. 2018;56:987–993. doi: 10.1093/mmy/myy004. [DOI] [PubMed] [Google Scholar]
- 18.Furue M., Tsuji G., Mitoma C., Nakahara T., Chiba T., Morino-Koga S., Uchi H. Gene regulation of filaggrin and other skin barrier proteins via aryl hydrocarbon receptor. J. Dermatol. Sci. 2015;80:83–88. doi: 10.1016/j.jdermsci.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Yu J., Luo Y., Zhu Z., Zhou Y., Sun L., Gao J., Sun J., Wang G., Yao X., Li W. A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J. Allergy Clin. Immunol. 2019;143:2108–2119. doi: 10.1016/j.jaci.2018.11.036. [DOI] [PubMed] [Google Scholar]
- 20.Takei K., Mitoma C., Hashimoto-Hachiya A., Takahara M., Tsuji G., Nakahara T., Furue M. Galactomyces fermentation filtrate prevents T helper 2-mediated reduction of filaggrin in an aryl hydrocarbon receptor-dependent manner. Clin. Exp. Dermatol. 2015;40:786–793. doi: 10.1111/ced.12635. [DOI] [PubMed] [Google Scholar]
- 21.Furue M., Hashimoto-Hachiya A., Tsuji G. Antioxidative phytochemicals accelerate epidermal terminal differentiation via the AHR-OVOL1 pathway: Implications for atopic dermatitis. Acta Derm. Venereol. 2018;98:918–923. doi: 10.2340/00015555-3003. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto-Hachiya A., Tsuji G., Murai M., Yan X., Furue M. Upregulation of FLG, LOR, and IVL expression by Rhodiola crenulata root extract via aryl hydrocarbon receptor: Differential involvement of OVOL. Int. J. Mol. Sci. 2018;19:1654. doi: 10.3390/ijms19061654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirano A., Goto M., Mitsui T., Hashimoto-Hachiya A., Tsuji G., Furue M. Antioxidant Artemisia princeps extract enhances the expression of filaggrin and loricrin via the AHR/OVOL1 pathway. Int. J. Mol. Sci. 2017;18:1948. doi: 10.3390/ijms18091948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakahara T., Mitoma C., Hashimoto-Hachiya A., Takahara M., Tsuji G., Uchi H., Yan X., Hachisuka J., Chiba T., Esaki H., et al. Antioxidant Opuntia ficus-indica extract activates AHR-NRF2 signaling and upregulates filaggrin and loricrin expression in human keratinocytes. J. Med. Food. 2015;18:1143–1149. doi: 10.1089/jmf.2014.3396. [DOI] [PubMed] [Google Scholar]
- 25.Takei K., Mitoma C., Hashimoto-Hachiya A., Uchi H., Takahara M., Tsuji G., Kido-Nakahara M., Nakahara T., Furue M. Antioxidant soybean tar Glyteer rescues T-helper-mediated downregulation of filaggrin expression via aryl hydrocarbon receptor. J. Dermatol. 2015;42:171–180. doi: 10.1111/1346-8138.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuji G., Hashimoto-Hachiya A., Kiyomatsu-Oda M., Takemura M., Ohno F., Ito T., Morino-Koga S., Mitoma C., Nakahara T., Uchi H., et al. Aryl hydrocarbon receptor activation restores filaggrin expression via OVOL1 in atopic dermatitis. Cell Death Dis. 2017;8:e2931. doi: 10.1038/cddis.2017.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furue M., Fuyuno Y., Mitoma C., Uchi H., Tsuji G. Therapeutic agents with AHR inhibiting and NRF2 activating activity for managing chloracne. Antioxidants. 2018;7:90. doi: 10.3390/antiox7070090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furue M., Tsuji G. Chloracne and hyperpigmentation caused by exposure to hazardous aryl hydrocarbon receptor ligands. Int. J. Environ. Res. Public Health. 2019;16:4864. doi: 10.3390/ijerph16234864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ju Q., Fimmel S., Hinz N., Stahlmann R., Xia L., Zouboulis C.C. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters sebaceous gland cell differentiation in vitro. Exp. Dermatol. 2011;20:320–325. doi: 10.1111/j.1600-0625.2010.01204.x. [DOI] [PubMed] [Google Scholar]
- 30.Suskind R.R. Chloracne, “the hallmark of dioxin intoxication”. Scand. J. Work Environ. Health. 1985;11:165–171. doi: 10.5271/sjweh.2240. [DOI] [PubMed] [Google Scholar]
- 31.van den Bogaard E.H., Bergboer J.G., Vonk-Bergers M., van Vlijmen-Willems I.M., Hato S.V., van der Valk P.G., Schröder J.M., Joosten I., Zeeuwen P.L., Schalkwijk J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J. Clin. Investig. 2013;123:917–927. doi: 10.1172/JCI65642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Endo H., Sugioka Y., Nakagi Y., Saijo Y., Yoshida T. A novel role of the NRF2 transcription factor in the regulation of arsenite-mediated keratin 16 gene expression in human keratinocytes. Environ. Health Perspect. 2008;116:873–879. doi: 10.1289/ehp.10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee Y., Shin J.M., Jang S., Choi D.K., Seo M.S., Kim H.R., Sohn K.C., Im M., Seo Y.J., Lee J.H., et al. Role of nuclear factor E2-related factor 2 (Nrf2) in epidermal differentiation. Arch. Dermatol. Res. 2014;306:677–682. doi: 10.1007/s00403-014-1470-x. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa T., Ishitsuka Y., Inoue S., Nakamura Y., Saito A., Okiyama N., Fujisawa Y., Furuta J., Watanabe R., Fujimoto M. Nuclear factor erythroid 2-related factor 2 (Nrf2) regulates epidermal keratinization under psoriatic skin inflammation. Am. J. Pathol. 2020;190:577–585. doi: 10.1016/j.ajpath.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 35.Schäfer M., Farwanah H., Willrodt A.H., Huebner A.J., Sandhoff K., Roop D., Hohl D., Bloch W., Werner S. Nrf2 links epidermal barrier function with antioxidant defense. EMBO Mol. Med. 2012;4:364–379. doi: 10.1002/emmm.201200219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schäfer M., Willrodt A.H., Kurinna S., Link A.S., Farwanah H., Geusau A., Gruber F., Sorg O., Huebner A.J., Roop D.R., et al. Activation of Nrf2 in keratinocytes causes chloracne (MADISH)-like skin disease in mice. EMBO Mol. Med. 2014;6:442–457. doi: 10.1002/emmm.201303281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schäfer M., Werner S. Nrf2--A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015;88:243–252. doi: 10.1016/j.freeradbiomed.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Yang L., Fan X., Cui T., Dang E., Wang G. Nrf2 promotes keratinocyte proliferation in psoriasis through up-regulation of keratin 6, keratin 16, and keratin 17. J. Investig. Dermatol. 2017;137:2168–2176. doi: 10.1016/j.jid.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Vogel C.F.A., Van Winkle L.S., Esser C., Haarmann-Stemmann T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34:101530. doi: 10.1016/j.redox.2020.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuyuno Y., Uchi H., Yasumatsu M., Morino-Koga S., Tanaka Y., Mitoma C., Furue M. Perillaldehyde inhibits AHR signaling and activates NRF2 antioxidant pathway in human keratinocytes. Oxidative Med. Cell Longev. 2018;2018:9524657. doi: 10.1155/2018/9524657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takei K., Hashimoto-Hachiya A., Takahara M., Tsuji G., Nakahara T., Furue M. Cynaropicrin attenuates UVB-induced oxidative stress via the AhR-Nrf2-Nqo1 pathway. Toxicol. Lett. 2015;234:74–80. doi: 10.1016/j.toxlet.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka Y., Uchi H., Furue M. Antioxidant cinnamaldehyde attenuates UVB-induced photoaging. J. Dermatol. Sci. 2019;96:151–158. doi: 10.1016/j.jdermsci.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Uchi H., Yasumatsu M., Morino-Koga S., Mitoma C., Furue M. Inhibition of aryl hydrocarbon receptor signaling and induction of NRF2-mediated antioxidant activity by cinnamaldehyde in human keratinocytes. J. Dermatol. Sci. 2017;85:36–43. doi: 10.1016/j.jdermsci.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Guttman-Yassky E., Bissonnette R., Ungar B., Suárez-Fariñas M., Ardeleanu M., Esaki H., Suprun M., Estrada Y., Xu H., Peng X., et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019;143:155–172. doi: 10.1016/j.jaci.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 45.Krueger J.G., Wharton K.A., Jr., Schlitt T., Suprun M., Torene R.I., Jiang X., Wang C.Q., Fuentes-Duculan J., Hartmann N., Peters T., et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J. Allergy Clin. Immunol. 2019;144:750–763. doi: 10.1016/j.jaci.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 46.Guttman-Yassky E., Brunner P.M., Neumann A.U., Khattri S., Pavel A.B., Malik K., Singer G.K., Baum D., Gilleaudeau P., Sullivan-Whalen M., et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J. Am. Acad. Dermatol. 2018;78:872–881. doi: 10.1016/j.jaad.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashida S., Uchi H., Takeuchi S., Esaki H., Moroi Y., Furue M. Significant correlation of serum IL-22 levels with CCL17 levels in atopic dermatitis. J. Dermatol. Sci. 2011;61:78–79. doi: 10.1016/j.jdermsci.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Cordoro K.M., Hitraya-Low M., Taravati K., Sandoval P.M., Kim E., Sugarman J., Pauli M.L., Liao W., Rosenblum M.D. Skin-infiltrating, interleukin-22-producing T cells differentiate pediatric psoriasis from adult psoriasis. J. Am. Acad. Dermatol. 2017;77:417–424. doi: 10.1016/j.jaad.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon K.B., Blauvelt A., Papp K.A., Langley R.G., Luger T., Ohtsuki M., Reich K., Amato D., Ball S.G., Braun D.K., et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N. Engl. J. Med. 2016;375:345–356. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- 50.Boniface K., Bernard F.X., Garcia M., Gurney A.L., Lecron J.C., Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 2005;174:3695–3702. doi: 10.4049/jimmunol.174.6.3695. [DOI] [PubMed] [Google Scholar]
- 51.Nograles K.E., Zaba L.C., Guttman-Yassky E., Fuentes-Duculan J., Suárez-Fariñas M., Cardinale I., Khatcherian A., Gonzalez J., Pierson K.C., White T.R., et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br. J. Dermatol. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sa S.M., Valdez P.A., Wu J., Jung K., Zhong F., Hall L., Kasman I., Winer J., Modrusan Z., Danilenko D.M., et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J. Immunol. 2007;178:2229–2240. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 53.Dale B.A., Presland R.B., Lewis S.P., Underwood R.A., Fleckman P. Transient expression of epidermal filaggrin in cultured cells causes collapse of intermediate filament networks with alteration of cell shape and nuclear integrity. J. Investig. Dermatol. 1997;108:179–187. doi: 10.1111/1523-1747.ep12334205. [DOI] [PubMed] [Google Scholar]
- 54.Smith F.J., Irvine A.D., Terron-Kwiatkowski A., Sandilands A., Campbell L.E., Zhao Y., Liao H., Evans A.T., Goudie D.R., Lewis-Jones S., et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat. Genet. 2006;38:337–342. doi: 10.1038/ng1743. [DOI] [PubMed] [Google Scholar]
- 55.Nomura T., Sandilands A., Akiyama M., Liao H., Evans A.T., Sakai K., Ota M., Sugiura H., Yamamoto K., Sato H., et al. Unique mutations in the filaggrin gene in Japanese patients with ichthyosis vulgaris and atopic dermatitis. J. Allergy Clin. Immunol. 2007;119:434–440. doi: 10.1016/j.jaci.2006.12.646. [DOI] [PubMed] [Google Scholar]
- 56.Palmer C.N., Irvine A.D., Terron-Kwiatkowski A., Zhao Y., Liao H., Lee S.P., Goudie D.R., Sandilands A., Campbell L.E., Smith F.J., et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 57.Hanifin J.M., Rajka G. Diagnostic features of atopic eczema. Acta Derm. Venereol. 1980;92:44–47. [Google Scholar]
- 58.McLean W.H. Filaggrin failure from ichthyosis vulgaris to atopic eczema and beyond. Br. J. Dermatol. 2016;175:4–7. doi: 10.1111/bjd.14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Z., Hansmann B., Meyer-Hoffert U., Gläser R., Schröder J.M. Molecular identification and expression analysis of filaggrin-2, a member of the S100 fused-type protein family. PLoS ONE. 2009;4:e5227. doi: 10.1371/journal.pone.0005227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hasan M.Z., Kitamura M., Kawai M., Ohira M., Mori K., Shoju S., Takagi K., Tsukamoto K., Kawai Y., Inoue A. Transcriptional profiling of lactic acid treated reconstructed human epidermis reveals pathways underlying stinging and itch. Toxicol. In Vitro. 2019;57:164–173. doi: 10.1016/j.tiv.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 61.de Koning H.D., van den Bogaard E.H., Bergboer J.G., Kamsteeg M., van Vlijmen-Willems I.M., Hitomi K., Henry J., Simon M., Takashita N., Ishida-Yamamoto A., et al. Expression profile of cornified envelope structural proteins and keratinocyte differentiation-regulating proteins during skin barrier repair. Br. J. Dermatol. 2012;166:1245–1254. doi: 10.1111/j.1365-2133.2012.10885.x. [DOI] [PubMed] [Google Scholar]
- 62.Pendaries V., Le Lamer M., Cau L., Hansmann B., Malaisse J., Kezic S., Serre G., Simon M. In a three-dimensional reconstructed human epidermis filaggrin-2 is essential for proper cornification. Cell Death Dis. 2015;6:e1656. doi: 10.1038/cddis.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohamad J., Sarig O., Godsel L.M., Peled A., Malchin N., Bochner R., Vodo D., Rabinowitz T., Pavlovsky M., Taiber S., et al. Filaggrin 2 deficiency results in abnormal cell-cell adhesion in the cornified cell layers and causes peeling skin syndrome Type A. J. Investig. Dermatol. 2018;138:1736–1743. doi: 10.1016/j.jid.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishida-Yamamoto A., Iizuka H. Differences in involucrin immunolabeling within cornified cell envelopes in normal and psoriatic epidermis. J. Investig. Dermatol. 1995;104:391–395. doi: 10.1111/1523-1747.ep12665870. [DOI] [PubMed] [Google Scholar]
- 65.Loertscher J.A., Sattler C.A., Allen-Hoffmann B.L. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters the differentiation pattern of human keratinocytes in organotypic culture. Toxicol. Appl. Pharmacol. 2001;175:121–129. doi: 10.1006/taap.2001.9202. [DOI] [PubMed] [Google Scholar]
- 66.Seguchi T., Cui C.Y., Kusuda S., Takahashi M., Aisu K., Tezuka T. Decreased expression of filaggrin in atopic skin. Arch. Dermatol. Res. 1996;288:442–446. doi: 10.1007/BF02505232. [DOI] [PubMed] [Google Scholar]
- 67.Mitoma C., Mine Y., Utani A., Imafuku S., Muto M., Akimoto T., Kanekura T., Furue M., Uchi H. Current skin symptoms of Yusho patients exposed to high levels of 2,3,4,7,8-pentachlorinated dibenzofuran and polychlorinated biphenyls in 1968. Chemosphere. 2015;137:45–51. doi: 10.1016/j.chemosphere.2015.03.070. [DOI] [PubMed] [Google Scholar]
- 68.Mitoma C., Uchi H., Tsukimori K., Todaka T., Kajiwara J., Shimose T., Akahane M., Imamura T., Furue M. Current state of yusho and prospects for therapeutic strategies. Environ. Sci. Pollut. Res. Int. 2018;25:16472–16480. doi: 10.1007/s11356-017-0833-1. [DOI] [PubMed] [Google Scholar]
- 69.Tsuji G., Takahara M., Uchi H., Takeuchi S., Mitoma C., Moroi Y., Furue M. An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011;62:42–49. doi: 10.1016/j.jdermsci.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 70.Furue M., Takahara M., Nakahara T., Uchi H. Role of AhR/ARNT system in skin homeostasis. Arch. Dermatol. Res. 2014;306:769–779. doi: 10.1007/s00403-014-1481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kazlauskas A., Sundström S., Poellinger L., Pongratz I. The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor. Mol. Cell. Biol. 2001;21:2594–2607. doi: 10.1128/MCB.21.7.2594-2607.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lees M.J., Peet D.J., Whitelaw M.L. Defining the role for XAP2 in stabilization of the dioxin receptor. J. Biol. Chem. 2003;278:35878–35888. doi: 10.1074/jbc.M302430200. [DOI] [PubMed] [Google Scholar]
- 73.Esser C., Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015;67:259–279. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- 74.Mimura J., Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim. Biophys. Acta. 2003;1619:263–268. doi: 10.1016/S0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- 75.Loertscher J.A., Lin T.M., Peterson R.E., Allen-Hoffmann B.L. In utero exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin causes accelerated terminal differentiation in fetal mouse skin. Toxicol. Sci. 2002;68:465–472. doi: 10.1093/toxsci/68.2.465. [DOI] [PubMed] [Google Scholar]
- 76.Muenyi C.S., Carrion S.L., Jones L.A., Kennedy L.H., Slominski A.T., Sutter C.H., Sutter T.R. Effects of in utero exposure of C57BL/6J mice to 2,3,7,8-tetrachlorodibenzo-p-dioxin on epidermal permeability barrier development and function. Environ. Health Perspect. 2014;122:1052–1058. doi: 10.1289/ehp.1308045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ray S.S., Swanson H.I. Alteration of keratinocyte differentiation and senescence by the tumor promoter dioxin. Toxicol. Appl. Pharmacol. 2003;192:131–145. doi: 10.1016/S0041-008X(03)00277-1. [DOI] [PubMed] [Google Scholar]
- 78.Sutter C.H., Yin H., Li Y., Mammen J.S., Bodreddigari S., Stevens G., Cole J.A., Sutter T.R. EGF receptor signaling blocks aryl hydrocarbon receptor-mediated transcription and cell differentiation in human epidermal keratinocytes. Proc. Natl. Acad. Sci. USA. 2009;106:4266–4271. doi: 10.1073/pnas.0900874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van den Bogaard E.H., Podolsky M.A., Smits J.P., Cui X., John C., Gowda K., Desai D., Amin S.G., Schalkwijk J., Perdew G.H., et al. Genetic and pharmacological analysis identifies a physiological role for the AHR in epidermal differentiation. J. Investig. Dermatol. 2015;135:1320–1328. doi: 10.1038/jid.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kopf P.G., Walker M.K. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol. Appl. Pharmacol. 2010;245:91–99. doi: 10.1016/j.taap.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bargo P.R., Walston S.T., Chu M., Seo I., Kollias N. Non-invasive assessment of tryptophan fluorescence and confocal microscopy provide information on skin barrier repair dynamics beyond TEWL. Exp. Dermatol. 2013;22:18–23. doi: 10.1111/exd.12053. [DOI] [PubMed] [Google Scholar]
- 82.Gillies R., Zonios G., Anderson R.R., Kollias N. Fluorescence excitation spectroscopy provides information about human skin in vivo. J. Investig. Dermatol. 2000;115:704–707. doi: 10.1046/j.1523-1747.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- 83.Fritsche E., Schäfer C., Calles C., Bernsmann T., Bernshausen T., Wurm M., Hübenthal U., Cline J.E., Hajimiragha H., Schroeder P., et al. Lightening up the UV response by identification of the aryl hydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc. Natl. Acad. Sci. USA. 2007;104:8851–8856. doi: 10.1073/pnas.0701764104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katiyar S.K., Matsui M.S., Mukhtar H. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J. Investig. Dermatol. 2000;114:328–333. doi: 10.1046/j.1523-1747.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- 85.Mukhtar H., Del Tito B.J., Jr., Matgouranis P.M., Das M., Asokan P., Bickers D.R. Additive effects of ultraviolet B and crude coal tar on cutaneous carcinogen metabolism: Possible relevance to the tumorigenicity of the Goeckerman regimen. J. Investig. Dermatol. 1986;87:348–353. doi: 10.1111/1523-1747.ep12524446. [DOI] [PubMed] [Google Scholar]
- 86.Wei Y.D., Rannug U., Rannug A. UV-induced CYP1A1 gene expression in human cells is mediated by tryptophan. Chem. Biol. Interact. 1999;118:127–140. doi: 10.1016/S0009-2797(98)00118-5. [DOI] [PubMed] [Google Scholar]
- 87.Magiatis P., Pappas P., Gaitanis G., Mexia N., Melliou E., Galanou M., Vlachos C., Stathopoulou K., Skaltsounis A.L., Marselos M., et al. Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J. Investig. Dermatol. 2013;133:2023–2030. doi: 10.1038/jid.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin U.H., Karki K., Kim S.B., Safe S. Inhibition of pancreatic cancer Panc1 cell migration by omeprazole is dependent on aryl hydrocarbon receptor activation of JNK. Biochem. Biophys. Res. Commun. 2018;501:751–757. doi: 10.1016/j.bbrc.2018.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsuji G., Takahara M., Uchi H., Matsuda T., Chiba T., Takeuchi S., Yasukawa F., Moroi Y., Furue M. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: The basis of its anti-inflammatory effect. J. Investig. Dermatol. 2012;132:59–68. doi: 10.1038/jid.2011.194. [DOI] [PubMed] [Google Scholar]
- 90.Tsuji G., Ito T., Chiba T., Mitoma C., Nakahara T., Uchi H., Furue M. The role of the OVOL1-OVOL2 axis in normal and diseased human skin. J. Dermatol. Sci. 2018;90:227–231. doi: 10.1016/j.jdermsci.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 91.Furue K., Ito T., Tsuji G., Ulzii D., Vu Y.H., Kido-Nakahara M., Nakahara T., Furue M. The IL-13-OVOL1-FLG axis in atopic dermatitis. Immunology. 2019;158:281–286. doi: 10.1111/imm.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ito T., Tsuji G., Ohno F., Uchi H., Nakahara T., Hashimoto-Hachiya A., Yoshida Y., Yamamoto O., Oda Y., Furue M. Activation of the OVOL1-OVOL2 axis in the hair bulb and in pilomatricoma. Am. J. Pathol. 2016;186:1036–1043. doi: 10.1016/j.ajpath.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 93.Ito T., Tsuji G., Ohno F., Nakahara T., Uchi H., Furue M. Potential role of the OVOL1-OVOL2 axis and c-Myc in the progression of cutaneous squamous cell carcinoma. Mod. Pathol. 2017;30:919–927. doi: 10.1038/modpathol.2016.169. [DOI] [PubMed] [Google Scholar]
- 94.Nair M., Teng A., Bilanchone V., Agrawal A., Li B., Dai X. Ovol1 regulates the growth arrest of embryonic epidermal progenitor cells and represses c-myc transcription. J. Cell Biol. 2006;173:253–264. doi: 10.1083/jcb.200508196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paternoster L., Standl M., Waage J., Baurecht H., Hotze M., Strachan D.P., Curtin J.A., Bønnelykke K., Tian C., Takahashi A., et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat. Genet. 2015;47:1449–1456. doi: 10.1038/ng.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wei Y.D., Bergander L., Rannug U., Rannug A. Regulation of CYP1A1 transcription via the metabolism of the tryptophan-derived 6-formylindolo[3,2-b]carbazole. Arch. Biochem. Biophys. 2000;383:99–107. doi: 10.1006/abbi.2000.2037. [DOI] [PubMed] [Google Scholar]
- 97.Knutson J.C., Poland A. Response of murine epidermis to 2,3,7,8-tetrachlorodibenzo-p-dioxin: Interaction of the ah and hr loci. Cell. 1982;30:225–234. doi: 10.1016/0092-8674(82)90028-9. [DOI] [PubMed] [Google Scholar]
- 98.Furue M., Ulzii D., Vu Y.H., Tsuji G., Kido-Nakahara M., Nakahara T. Pathogenesis of atopic dermatitis: Current paradigm. Iran. J. Immunol. 2019;16:97–107. doi: 10.22034/IJI.2019.80253. [DOI] [PubMed] [Google Scholar]
- 99.Furue M., Chiba T., Tsuji G., Ulzii D., Kido-Nakahara M., Nakahara T., Kadono T. Atopic dermatitis: Immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol. Int. 2017;66:398–403. doi: 10.1016/j.alit.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Seo E., Yoon J., Jung S., Lee J., Lee B.H., Yu J. Phenotypes of atopic dermatitis identified by cluster analysis in early childhood. J. Dermatol. 2019;46:117–123. doi: 10.1111/1346-8138.14714. [DOI] [PubMed] [Google Scholar]
- 101.Arima K., Gupta S., Gadkari A., Hiragun T., Kono T., Katayama I., Demiya S., Eckert L. Burden of atopic dermatitis in Japanese adults: Analysis of data from the 2013 National Health and Wellness Survey. J. Dermatol. 2018;45:390–396. doi: 10.1111/1346-8138.14218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Igarashi A., Fujita H., Arima K., Inoue T., Dorey J., Fukushima A., Taguchi Y. Health-care resource use and current treatment of adult atopic dermatitis patients in Japan: A retrospective claims database analysis. J. Dermatol. 2019;46:652–661. doi: 10.1111/1346-8138.14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jung H.J., Bae J.Y., Kim J.E., Na C.H., Park G.H., Bae Y.I., Shin M.K., Lee Y.B., Lee U.H., Jang Y.H., et al. Survey of disease awareness, treatment behavior and treatment satisfaction in patients with atopic dermatitis in Korea: A multicenter study. J. Dermatol. 2018;45:1172–1180. doi: 10.1111/1346-8138.14540. [DOI] [PubMed] [Google Scholar]
- 104.Komura Y., Kogure T., Kawahara K., Yokozeki H. Economic assessment of actual prescription of drugs for treatment of atopic dermatitis: Differences between dermatology and pediatrics in large-scale receipt data. J. Dermatol. 2018;45:165–174. doi: 10.1111/1346-8138.14133. [DOI] [PubMed] [Google Scholar]
- 105.Takeuchi S., Oba J., Esaki H., Furue M. Non-corticosteroid adherence and itch severity influence perception of itch in atopic dermatitis. J. Dermatol. 2018;45:158–164. doi: 10.1111/1346-8138.14124. [DOI] [PubMed] [Google Scholar]
- 106.Elias P.M. Primary role of barrier dysfunction in the pathogenesis of atopic dermatitis. Exp. Dermatol. 2018;27:847–851. doi: 10.1111/exd.13693. [DOI] [PubMed] [Google Scholar]
- 107.Kim B.E., Leung D.Y.M. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol. Res. 2008;10:207–215. doi: 10.4168/aair.2018.10.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rinaldi A.O., Morita H., Wawrzyniak P., Dreher A., Grant S., Svedenhag P., Akdis C.A. Direct assessment of skin epithelial barrier by electrical impedance, spectroscopy. Allergy. 2019;74:1934–1944. doi: 10.1111/all.13824. [DOI] [PubMed] [Google Scholar]
- 109.Otsuka A., Doi H., Egawa G., Maekawa A., Fujita T., Nakamizo S., Nakashima C., Nakajima S., Watanabe T., Miyachi Y., et al. Possible new therapeutic strategy to regulate atopic dermatitis through upregulating filaggrin expression. J. Allergy Clin. Immunol. 2014;133:139–146. doi: 10.1016/j.jaci.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 110.Kim B.E., Leung D.Y.M., Boguniewicz M., Howell M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008;126:332–337. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Furue M., Iida K., Imaji M., Nakahara T. Microbiome analysis of forehead skin in patients with atopic dermatitis and healthy subjects: Implication of Staphylococcus and Corynebacterium. J. Dermatol. 2018;45:876–877. doi: 10.1111/1346-8138.14486. [DOI] [PubMed] [Google Scholar]
- 112.Iwamoto K., Moriwaki M., Miyake R., Hide M. Staphylococcus aureus in atopic dermatitis: Strain-specific cell wall proteins and skin immunity. Allergol. Int. 2019;68:309–315. doi: 10.1016/j.alit.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 113.Cascella R., Foti Cuzzola V., Lepre T., Galli E., Moschese V., Chini L., Mazzanti C., Fortugno P., Novelli G., Giardina E. Full sequencing of the FLG gene in Italian patients with atopic eczema: Evidence of new mutations, but lack of an association. J. Investig. Dermatol. 2011;131:982–984. doi: 10.1038/jid.2010.398. [DOI] [PubMed] [Google Scholar]
- 114.Thawer-Esmail F., Jakasa I., Todd G., Wen Y., Brown S.J., Kroboth K., Campbell L.E., O’Regan G.M., McLean W.H., Irvine A.D., et al. South African amaXhosa patients with atopic dermatitis have decreased levels of filaggrin breakdown products but no loss-of-function mutations in filaggrin. J. Allergy Clin. Immunol. 2014;133:280–282. doi: 10.1016/j.jaci.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sasaki T., Furusyo N., Shiohama A., Takeuchi S., Nakahara T., Uchi H., Hirota T., Tamari M., Shimizu N., Ebihara T., et al. Filaggrin loss-of-function mutations are not a predisposing factor for atopic dermatitis in an Ishigaki Island under subtropical climate. J. Dermatol. Sci. 2014;76:10–15. doi: 10.1016/j.jdermsci.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 116.Howell M.D., Kim B.E., Gao P., Grant A.V., Boguniewicz M., DeBenedetto A., Schneider L., Beck L.A., Barnes K.C., Leung D.Y. Cytokine modulation of atopic dermatitis filaggrin skin expression. J. Allergy Clin. Immunol. 2007;120:150–155. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jurakic Toncic R., Kezic S., Jakasa I., Ljubojevic Hadzavdic S., Balic A., Petkovic M., Pavicic B., Zuzul K., Marinovic B. Filaggrin loss-of-function mutations and levels of filaggrin degradation products in adult patients with atopic dermatitis in Croatia. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16232. [DOI] [PubMed] [Google Scholar]
- 118.Simpson E.L., Bieber T., Guttman-Yassky E., Beck L.A., Blauvelt A., Cork M.J., Silverberg J.I., Deleuran M., Kataoka Y., Lacour J.P., et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N. Engl. J. Med. 2016;375:2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 119.Tsoi L.C., Rodriguez E., Degenhardt F., Baurecht H., Wehkamp U., Volks N., Szymczak S., Swindell W.R., Sarkar M.K., Raja K., et al. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J. Investig. Dermatol. 2019;139:1480–1489. doi: 10.1016/j.jid.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wollenberg A., Howell M.D., Guttman-Yassky E., Silverberg J.I., Kell C., Ranade K., Moate R., van der Merwe R. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J. Allergy Clin. Immunol. 2019;143:135–141. doi: 10.1016/j.jaci.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 121.Jensen J.M., Fölster-Holst R., Baranowsky A., Schunck M., Winoto-Morbach S., Neumann C., Schütze S., Proksch E. Impaired sphingomyelinase activity and epidermal differentiation in atopic dermatitis. J. Investig. Dermatol. 2004;122:1423–1431. doi: 10.1111/j.0022-202X.2004.22621.x. [DOI] [PubMed] [Google Scholar]
- 122.Pellerin L., Henry J., Hsu C.Y., Balica S., Jean-Decoster C., Méchin M.C., Hansmann B., Rodriguez E., Weindinger S., Schmitt A.M., et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J. Allergy Clin. Immunol. 2013;131:1094–1102. doi: 10.1016/j.jaci.2012.12.1566. [DOI] [PubMed] [Google Scholar]
- 123.Broccardo C.J., Mahaffey S., Schwarz J., Wruck L., David G., Schlievert P.M., Reisdorph N.A., Leung D.Y. Comparative proteomic profiling of patients with atopic dermatitis based on history of eczema herpeticum infection and Staphylococcus aureus colonization. J. Allergy Clin. Immunol. 2011;127:186–193. doi: 10.1016/j.jaci.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Trzeciak M., Sakowicz-Burkiewicz M., Wesserling M., Dobaczewska D., Gleń J., Nowicki R., Pawelczyk T. Expression of cornified envelope proteins in skin and its relationship with atopic dermatitis phenotype. Acta Derm. Venereol. 2017;97:36–41. doi: 10.2340/00015555-2482. [DOI] [PubMed] [Google Scholar]
- 125.Guttman-Yassky E., Ungar B., Malik K., Dickstein D., Suprun M., Estrada Y.D., Xu H., Peng X., Oliva M., Todd D., et al. Molecular signatures order the potency of topically applied anti-inflammatory drugs in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2017;140:1032–1042. doi: 10.1016/j.jaci.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 126.Meisser S.S., Altunbulakli C., Bandier J., Opstrup M.S., Castro-Giner F., Akdis M., Bonefeld C.M., Johansen J.D., Akdis C.A. Skin barrier damage after exposure to paraphenylenediamine. J. Allergy Clin. Immunol. 2020;145:619–631. doi: 10.1016/j.jaci.2019.11.023. [DOI] [PubMed] [Google Scholar]
- 127.Amano W., Nakajima S., Kunugi H., Numata Y., Kitoh A., Egawa G., Dainichi T., Honda T., Otsuka A., Kimoto Y., et al. The Janus kinase inhibitor JTE-052 improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J. Allergy Clin. Immunol. 2015;136:667–677. doi: 10.1016/j.jaci.2015.03.051. [DOI] [PubMed] [Google Scholar]
- 128.Clarysse K., Pfaff C.M., Marquardt Y., Huth L., Kortekaas Krohn I., Kluwig D., Lüscher B., Gutermuth J., Baron J. JAK1/3 inhibition preserves epidermal morphology in full-thickness 3D skin models of atopic dermatitis and psoriasis. J. Eur. Acad. Dermatol. Venereol. 2019;33:367–375. doi: 10.1111/jdv.15301. [DOI] [PubMed] [Google Scholar]
- 129.Ranasinghe C., Trivedi S., Wijesundara D.K., Jackson R.J. IL-4 and IL-13 receptors: Roles in immunity and powerful vaccine adjuvants. Cytokine Growth Factor Rev. 2014;25:437–442. doi: 10.1016/j.cytogfr.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 130.Murata T., Taguchi J., Puri R.K., Mohri H. Sharing of receptor subunits and signal transduction pathway between the IL-4 and IL-13 receptor system. Int. J. Hematol. 1999;69:13–20. [PubMed] [Google Scholar]
- 131.Junttila I.S. Tuning the cytokine responses: An update on interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol. 2018;9:888. doi: 10.3389/fimmu.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Furue M., Ulzii D., Nakahara T., Tsuji G., Furue K., Hashimoto-Hachiya A., Kido-Nakahara M. Implications of IL-13Rα2 in atopic skin inflammation. Allergol. Int. 2020 doi: 10.1016/j.alit.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 133.Ulzii D., Kido-Nakahara M., Nakahara T., Tsuji G., Furue K., Hashimoto-Hachiya A., Furue M. Scratching counteracts IL-13 signaling by upregulating the decoy receptor IL-13Rα2 in keratinocytes. Int. J. Mol. Sci. 2019;20:3324. doi: 10.3390/ijms20133324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harada N., Higuchi K., Wakao H., Hamasaki N., Izuhara K. Identification of the critical portions of the human IL-4 receptor alpha chain for activation of STAT6. Biochem. Biophys. Res. Commun. 1998;246:675–680. doi: 10.1006/bbrc.1998.8696. [DOI] [PubMed] [Google Scholar]
- 135.Izuhara K., Heike T., Otsuka T., Yamaoka K., Mayumi M., Imamura T., Niho Y., Harada N. Signal transduction pathway of interleukin-4 and interleukin-13 in human B cells derived from X-linked severe combined immunodeficiency patients. J. Biol. Chem. 1996;271:619–622. doi: 10.1074/jbc.271.2.619. [DOI] [PubMed] [Google Scholar]
- 136.Murata T., Noguchi P.D., Puri R.K. Receptors for interleukin (IL)-4 do not associate with the common gamma chain, and IL-4 induces the phosphorylation of JAK2 tyrosine kinase in human colon carcinoma cells. J. Biol. Chem. 1995;270:30829–30836. doi: 10.1074/jbc.270.51.30829. [DOI] [PubMed] [Google Scholar]
- 137.Murata T., Husain S.R., Mohri H., Puri R.K. Two different IL-13 receptor chains are expressed in normal human skin fibroblasts, and IL-4 and IL-13 mediate signal transduction through a common pathway. Int. Immunol. 1998;10:1103–1110. doi: 10.1093/intimm/10.8.1103. [DOI] [PubMed] [Google Scholar]
- 138.Jin S.H., Choi D., Chun Y.J., Noh M. Keratinocyte-derived IL-24 plays a role in the positive feedback regulation of epidermal inflammation in response to environmental and endogenous toxic stressors. Toxicol. Appl. Pharmacol. 2014;280:199–206. doi: 10.1016/j.taap.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 139.Zhang W., Sakai T., Matsuda-Hirose H., Goto M., Yamate T., Hatano Y. Cutaneous permeability barrier function in signal transducer and activator of transcription 6-deficient mice is superior to that in wild-type mice. J. Dermatol. Sci. 2018;92:54–61. doi: 10.1016/j.jdermsci.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 140.Olsan E.E., West J.D., Torres J.A., Doerr N., Weimbs T. Identification of targets of IL-13 and STAT6 signaling in polycystic kidney disease. Am. J. Physiol. Renal. Physiol. 2018;315:F86–F96. doi: 10.1152/ajprenal.00346.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zissler U.M., Chaker A.M., Effner R., Ulrich M., Guerth F., Piontek G., Dietz K., Regn M., Knapp B., Theis F.J., et al. Interleukin-4 and interferon-γ orchestrate an epithelial polarization in the airways. Mucosal Immunol. 2016;9:917–926. doi: 10.1038/mi.2015.110. [DOI] [PubMed] [Google Scholar]
- 142.Masuoka M., Shiraishi H., Ohta S., Suzuki S., Arima K., Aoki S., Toda S., Inagaki N., Kurihara Y., Hayashida S., et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J. Clin. Investig. 2012;122:2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mitamura Y., Murai M., Mitoma C., Furue M. NRF2 activation inhibits both TGF-β1- and IL-13-mediated periostin expression in fibroblasts: Benefit of cinnamaldehyde for antifibrotic treatment. Oxidative Med. Cell Longev. 2018;2018:2475047. doi: 10.1155/2018/2475047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mitamura Y., Nunomura S., Nanri Y., Ogawa M., Yoshihara T., Masuoka M., Tsuji G., Nakahara T., Hashimoto-Hachiya A., Conway S.J., et al. The IL-13/periostin/IL-24 pathway causes epidermal barrier dysfunction in allergic skin inflammation. Allergy. 2018;73:1881–1891. doi: 10.1111/all.13437. [DOI] [PubMed] [Google Scholar]
- 145.Mitamura Y., Nunomura S., Furue M., Izuhara K. IL-24: A new player in the pathogenesis of pro-inflammatory and allergic skin diseases. Allergol. Int. 2020 doi: 10.1016/j.alit.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 146.Niess J.H., Hruz P., Kaymak T. The interleukin-20 cytokines in intestinal diseases. Front. Immunol. 2018;9:1373. doi: 10.3389/fimmu.2018.01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Furue M., Uchi H., Mitoma C., Hashimoto-Hachiya A., Chiba T., Ito T., Nakahara T., Tsuji G. Antioxidants for healthy skin: The emerging role of aryl hydrocarbon receptors and nuclear factor-erythroid 2-related factor-2. Nutrients. 2017;9:223. doi: 10.3390/nu9030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yasukawa S., Miyazaki Y., Yoshii C., Nakaya M., Ozaki N., Toda S., Kuroda E., Ishibashi K., Yasuda T., Natsuaki Y., et al. An ITAM-Syk-CARD9 signalling axis triggers contact hypersensitivity by stimulating IL-1 production in dendritic cells. Nat. Commun. 2014;5:3755. doi: 10.1038/ncomms4755. [DOI] [PubMed] [Google Scholar]
- 149.Eto H., Tsuji G., Chiba T., Furue M., Hyodo F. Non-invasive evaluation of atopic dermatitis based on redox status using in vivo dynamic nuclear polarization magnetic resonance imaging. Free Radic. Biol. Med. 2017;103:209–215. doi: 10.1016/j.freeradbiomed.2016.12.043. [DOI] [PubMed] [Google Scholar]
- 150.Bissonnette R., Poulin Y., Zhou Y., Tan J., Hong H.C., Webster J., Ip W., Tang L., Lyle M. Efficacy and safety of topical WBI-1001 in patients with mild to severe atopic dermatitis: Results from a 12-week, multicentre, randomized, placebo-controlled double-blind trial. Br. J. Dermatol. 2012;166:853–860. doi: 10.1111/j.1365-2133.2011.10775.x. [DOI] [PubMed] [Google Scholar]
- 151.Peppers J., Paller A.S., Maeda-Chubachi T., Wu S., Robbins K., Gallagher K., Kraus J.E. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2019;80:89–98. doi: 10.1016/j.jaad.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 152.Smith S.H., Jayawickreme C., Rickard D.J., Nicodeme E., Bui T., Simmons C., Coquery C.M., Neil J., Pryor W.M., Mayhew D., et al. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J. Investig. Dermatol. 2017;137:2110–2119. doi: 10.1016/j.jid.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 153.Takeuchi K., Ito K., Namikawa S. Anti-inflammatory activity of the dry distillation tar of delipidated soybean (Glyteer) (3). Effects on type I-type IV allergic reaction. Nihon Yakurigaku Zasshi. 1990;95:149–157. doi: 10.1254/fpj.95.4_149. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 154.Richardson W.H., Schmidt T.M., Nealson K.H. Identification of an anthraquinone pigment and a hydroxystilbene antibiotic from Xenorhabdus luminescens. Appl. Environ. Microbiol. 1988;54:1602–1605. doi: 10.1128/AEM.54.6.1602-1605.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zang Y.N., Jiang D.L., Cai L., Chen X., Wang Q., Xie Z.W., Liu Y., Zhang C.Y., Jing S., Chen G.H., et al. Use of a dose-response model to guide future clinical trial of Benvitimod cream to treat mild and moderate psoriasis. Int. J. Clin. Pharmacol. Ther. 2016;54:87–95. doi: 10.5414/CP202486. [DOI] [PubMed] [Google Scholar]
- 156.Bissonnette R., Vasist L.S., Bullman J.N., Collingwood T., Chen G., Maeda-Chubachi T. Systemic pharmacokinetics, safety, and preliminary efficacy of topical AhR agonist Tapinarof: Results of a phase 1 study. Clin. Pharmacol. Drug Dev. 2018;7:524–531. doi: 10.1002/cpdd.439. [DOI] [PubMed] [Google Scholar]
- 157.Hirakawa S., Saito R., Ohara H., Okuyama R., Aiba S. Dual oxidase 1 induced by Th2 cytokines promotes STAT6 phosphorylation via oxidative inactivation of protein tyrosine phosphatase 1B in human epidermal keratinocytes. J. Immunol. 2011;186:4762–4770. doi: 10.4049/jimmunol.1000791. [DOI] [PubMed] [Google Scholar]
- 158.Lu X., Malumbres R., Shields B., Jiang X., Sarosiek K.A., Natkunam Y., Tiganis T., Lossos I.S. PTP1B is a negative regulator of interleukin 4-induced STAT6 signaling. Blood. 2008;112:4098–4108. doi: 10.1182/blood-2008-03-148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sharma P., Chakraborty R., Wang L., Min B., Tremblay M.L., Kawahara T., Lambeth J.D., Haque S.J. Redox regulation of interleukin-4 signaling. Immunity. 2008;29:551–564. doi: 10.1016/j.immuni.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tanaka Y., Ito T., Tsuji G., Furue M. Baicalein inhibits benzo[a]pyrene-induced toxic response by downregulating Src phosphorylation and by upregulating NRF2-HMOX1 system. Antioxidants. 2020;9:507. doi: 10.3390/antiox9060507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hashimoto-Hachiya A., Tsuji G., Furue M. Antioxidants cinnamaldehyde and Galactomyces fermentation filtrate downregulate senescence marker CDKN2A/p16INK4A via NRF2 activation in keratinocytes. J. Dermatol. Sci. 2019;96:53–56. doi: 10.1016/j.jdermsci.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 162.Li H., Min J., Mao X., Wang X., Yang Y., Chen Y. Edaravone ameliorates experimental autoimmune thyroiditis in rats through HO-1-dependent STAT3/PI3K/Akt pathway. Am. J. Transl. Res. 2018;10:2037–2046. [PMC free article] [PubMed] [Google Scholar]
- 163.Park B., Lim J.W., Kim H. Lycopene treatment inhibits activation of Jak1/Stat3 and Wnt/β-catenin signaling and attenuates hyperproliferation in gastric epithelial cells. Nutr. Res. 2019;70:70–81. doi: 10.1016/j.nutres.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 164.Dao T.T.P., Song K., Kim J.Y., Kim Y.S. Igalan from Inula helenium (L.) suppresses the atopic dermatitis-like response in stimulated HaCaT keratinocytes via JAK/STAT3 signaling. Inflamm. Res. 2020;69:309–319. doi: 10.1007/s00011-020-01322-4. [DOI] [PubMed] [Google Scholar]
- 165.Lee J., Song K., Hiebert P., Werner S., Kim T.G., Kim Y.S. Tussilagonone ameliorates psoriatic features in keratinocytes and imiquimod-induced psoriasis-like lesions in mice via NRF2 activation. J. Investig. Dermatol. 2020;140:1223–1232. doi: 10.1016/j.jid.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 166.Takemura M., Nakahara T., Hashimoto-Hachiya A., Furue M., Tsuji G. Glyteer, soybean tar, impairs IL-4/Stat6 signaling in murine bone marrow-derived dendritic cells: The basis of its therapeutic effect on atopic dermatitis. Int. J. Mol. Sci. 2018;19:1169. doi: 10.3390/ijms19041169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Miake S., Tsuji G., Takemura M., Hashimoto-Hachiya A., Vu Y.H., Furue M., Nakahara T. IL-4 augments IL-31/IL-31 receptor alpha interaction leading to enhanced Ccl17 and Ccl22 production in dendritic cells: Implications for atopic dermatitis. Int. J. Mol. Sci. 2019;20:4053. doi: 10.3390/ijms20164053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tanaka G., Kanaji S., Hirano A., Arima K., Shinagawa A., Goda C., Yasunaga S., Ikizawa K., Yanagihara Y., Kubo M., et al. Induction and activation of the aryl hydrocarbon receptor by IL-4 in B cells. Int. Immunol. 2005;17:797–805. doi: 10.1093/intimm/dxh260. [DOI] [PubMed] [Google Scholar]
- 169.Furue M., Hashimoto-Hachiya A., Tsuji G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int. J. Mol. Sci. 2019;20:5424. doi: 10.3390/ijms20215424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tsuji G., Hashimoto-Hachiya A., Yen V.H., Miake S., Takemura M., Mitamura Y., Ito T., Murata M., Furue M., Nakahara T. Aryl hydrocarbon receptor activation downregulates IL-33 expression in keratinocytes via Ovo-like 1. J. Clin. Med. 2020;9:891. doi: 10.3390/jcm9030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Czarnowicki T., Esaki H., Gonzalez J., Malajian D., Shemer A., Noda S., Talasila S., Berry A., Gray J., Becker L., et al. Early pediatric atopic dermatitis shows only a cutaneous lymphocyte antigen (CLA)(+) TH2/TH1 cell imbalance, whereas adults acquire CLA(+) TH22/TC22 cell subsets. J. Allergy Clin. Immunol. 2015;136:941–951. doi: 10.1016/j.jaci.2015.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Esaki H., Brunner P.M., Renert-Yuval Y., Czarnowicki T., Huynh T., Tran G., Lyon S., Rodriguez G., Immaneni S., Johnson D.B., et al. Early-onset pediatric atopic dermatitis is T(H)2 but also T(H)17 polarized in skin. J. Allergy Clin. Immunol. 2016;138:1639–1651. doi: 10.1016/j.jaci.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 173.Gittler J.K., Shemer A., Suárez-Fariñas M., Fuentes-Duculan J., Gulewicz K.J., Wang C.Q., Mitsui H., Cardinale I., de Guzman Strong C., Krueger J.G., et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J. Allergy Clin. Immunol. 2012;130:1344–1354. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eyerich K., Dimartino V., Cavani A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur. J. Immunol. 2017;47:607–614. doi: 10.1002/eji.201646723. [DOI] [PubMed] [Google Scholar]
- 175.Nograles K.E., Zaba L.C., Shemer A., Fuentes-Duculan J., Cardinale I., Kikuchi T., Ramon M., Bergman R., Krueger J.G., Guttman-Yassky E. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J. Allergy Clin. Immunol. 2009;123:1244–1252. doi: 10.1016/j.jaci.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee J.S., Cella M., McDonald K.G., Garlanda C., Kennedy G.D., Nukaya M., Mantovani A., Kopan R., Bradfield C.A., Newberry R.D., et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 2011;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nikamo P., Cheuk S., Lysell J., Enerbäck C., Bergh K., Xu Landén N., Eidsmo L., Ståhle M. Genetic variants of the IL22 promoter associate to onset of psoriasis before puberty and increased IL-22 production in T cells. J. Investig. Dermatol. 2014;134:1535–1541. doi: 10.1038/jid.2014.5. [DOI] [PubMed] [Google Scholar]
- 178.Schiering C., Vonk A., Das S., Stockinger B., Wincent E. Cytochrome P4501-inhibiting chemicals amplify aryl hydrocarbon receptor activation and IL-22 production in T helper 17 cells. Biochem. Pharmacol. 2018;151:47–58. doi: 10.1016/j.bcp.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 179.Trifari S., Spits H. IL-22-producing CD4+ T cells: Middle-men between the immune system and its environment. Eur. J. Immunol. 2010;40:2369–2371. doi: 10.1002/eji.201040848. [DOI] [PubMed] [Google Scholar]
- 180.Brunner P.M., Pavel A.B., Khattri S., Leonard A., Malik K., Rose S., Jim On S., Vekaria A.S., Traidl-Hoffmann C., Singer G.K., et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J. Allergy Clin. Immunol. 2019;143:142–154. doi: 10.1016/j.jaci.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 181.Jones B.C., Logsdon N.J., Walter M.R. Structure of IL-22 bound to its high-affinity IL-22R1 chain. Structure. 2008;16:1333–1344. doi: 10.1016/j.str.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wolk K., Kunz S., Witte E., Friedrich M., Asadullah K., Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 183.Yang X., Zheng S.G. Interleukin-22: A likely target for treatment of autoimmune diseases. Autoimmun. Rev. 2014;13:615–620. doi: 10.1016/j.autrev.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lejeune D., Dumoutier L., Constantinescu S., Kruijer W., Schuringa J.J., Renauld J.C. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J. Biol. Chem. 2002;277:33676–33682. doi: 10.1074/jbc.M204204200. [DOI] [PubMed] [Google Scholar]
- 185.Avitabile S., Odorisio T., Madonna S., Eyerich S., Guerra L., Eyerich K., Zambruno G., Cavani A., Cianfarani F. Interleukin-22 promotes wound repair in diabetes by improving keratinocyte pro-healing functions. J. Investig. Dermatol. 2015;135:2862–2870. doi: 10.1038/jid.2015.278. [DOI] [PubMed] [Google Scholar]
- 186.Gutowska-Owsiak D., Schaupp A.L., Salimi M., Taylor S., Ogg G.S. Interleukin-22 downregulates filaggrin expression and affects expression of profilaggrin processing enzymes. Br. J. Dermatol. 2011;165:492–498. doi: 10.1111/j.1365-2133.2011.10400.x. [DOI] [PubMed] [Google Scholar]
- 187.Tohyama M., Hanakawa Y., Shirakata Y., Dai X., Yang L., Hirakawa S., Tokumaru S., Okazaki H., Sayama K., Hashimoto K. IL-17 and IL-22 mediate IL-20 subfamily cytokine production in cultured keratinocytes via increased IL-22 receptor expression. Eur. J. Immunol. 2009;39:2779–2788. doi: 10.1002/eji.200939473. [DOI] [PubMed] [Google Scholar]
- 188.Noh M., Yeo H., Ko J., Kim H.K., Lee C.H. MAP17 is associated with the T-helper cell cytokine-induced down-regulation of filaggrin transcription in human keratinocytes. Exp. Dermatol. 2010;19:355–362. doi: 10.1111/j.1600-0625.2009.00902.x. [DOI] [PubMed] [Google Scholar]
- 189.Jang M., Kim H., Kim Y., Choi J., Jeon J., Hwang Y., Kang J.S., Lee W.J. The crucial role of IL-22 and its receptor in thymus and activation regulated chemokine production and T-cell migration by house dust mite extract. Exp. Dermatol. 2016;25:598–603. doi: 10.1111/exd.12988. [DOI] [PubMed] [Google Scholar]
- 190.Gläser R., Meyer-Hoffert U., Harder J., Cordes J., Wittersheim M., Kobliakova J., Fölster-Holst R., Proksch E., Schröder J.M., Schwarz T. The antimicrobial protein psoriasin (S100A7) is upregulated in atopic dermatitis and after experimental skin barrier disruption. J. Investig. Dermatol. 2009;129:641–649. doi: 10.1038/jid.2008.268. [DOI] [PubMed] [Google Scholar]
- 191.Bansal G., Das D., Hsieh C.Y., Wang Y.H., Gilmore B.A., Wong C.M., Suzuki Y.J. IL-22 activates oxidant signaling in pulmonary vascular smooth muscle cells. Cell. Signal. 2013;25:2727–2733. doi: 10.1016/j.cellsig.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cho K.A., Suh J.W., Lee K.H., Kang J.L., Woo S.Y. IL-17 and IL-22 enhance skin inflammation by stimulating the secretion of IL-1β by keratinocytes via the ROS-NLRP3-caspase-1 pathway. Int. Immunol. 2012;24:147–158. doi: 10.1093/intimm/dxr110. [DOI] [PubMed] [Google Scholar]
- 193.Zhang J., Sun L., Li W., Wang Y., Li X., Liu Y. Overexpression of macrophage stimulating 1 enhances the anti-tumor effects of IL-24 in esophageal cancer via inhibiting ERK-Mfn2 signaling-dependent mitophagy. Biomed. Pharmacother. 2019;114:108844. doi: 10.1016/j.biopha.2019.108844. [DOI] [PubMed] [Google Scholar]
- 194.Palombo R., Savini I., Avigliano L., Madonna S., Cavani A., Albanesi C., Mauriello A., Melino G., Terrinoni A. Luteolin-7-glucoside inhibits IL-22/STAT3 pathway, reducing proliferation, acanthosis, and inflammation in keratinocytes and in mouse psoriatic model. Cell Death Dis. 2016;7:e2344. doi: 10.1038/cddis.2016.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Furue K., Ito T., Furue M. Differential efficacy of biologic treatments targeting the TNF-α/IL-23/IL-17 axis in psoriasis and psoriatic arthritis. Cytokine. 2018;111:182–188. doi: 10.1016/j.cyto.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 196.Furue K., Ito T., Tsuji G., Kadono T., Furue M. Psoriasis and the TNF/IL23/IL17 axis. G. Ital. Dermatol. Venereol. 2019;154:418–424. doi: 10.23736/S0392-0488.18.06202-8. [DOI] [PubMed] [Google Scholar]
- 197.Kamata M., Tada Y. Safety of biologics in psoriasis. J. Dermatol. 2018;45:279–286. doi: 10.1111/1346-8138.14096. [DOI] [PubMed] [Google Scholar]
- 198.Langley R.G., Elewski B.E., Lebwohl M., Reich K., Griffiths C.E., Papp K., Puig L., Nakagawa H., Spelman L., Sigurgeirsson B., et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N. Engl. J. Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 199.Gordon K.B., Armstrong A.W., Foley P., Song M., Shen Y.K., Li S., Muñoz-Elías E.J., Branigan P., Liu X., Reich K. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 Study. J. Investig. Dermatol. 2019;139:2437–2446. doi: 10.1016/j.jid.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 200.Lebwohl M., Strober B., Menter A., Gordon K., Weglowska J., Puig L., Papp K., Spelman L., Toth D., Kerdel F., et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N. Engl. J. Med. 2015;373:1318–1328. doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 201.Momose M., Asahina A., Umezawa Y., Nakagawa H. Long-term clinical efficacy and safety of secukinumab for Japanese patients with psoriasis: A single-center experience. J. Dermatol. 2018;45:318–321. doi: 10.1111/1346-8138.14145. [DOI] [PubMed] [Google Scholar]
- 202.Ogawa E., Sato Y., Minagawa A., Okuyama R. Pathogenesis of psoriasis and development of treatment. J. Dermatol. 2018;45:264–272. doi: 10.1111/1346-8138.14139. [DOI] [PubMed] [Google Scholar]
- 203.Komatsu-Fujii T., Honda T., Otsuka A., Kabashima K. Inverse responses of the skin and nail lesions of psoriatic arthritis to an anti-interleukin-17A antibody and an anti-tumor necrosis factor-α antibody. J. Dermatol. 2019;46:e440–e441. doi: 10.1111/1346-8138.15006. [DOI] [PubMed] [Google Scholar]
- 204.Lee M.G., Huang Y.H., Lee J.H., Lee S.C., Kim T.G., Aw D.C., Bao W., Dee C.M.A., Guana A., Tsai T.F. Secukinumab demonstrates superior efficacy and a faster response in clearing skin in Asian subjects with moderate to severe plaque psoriasis compared with ustekinumab: Subgroup analysis from the CLEAR study. J. Dermatol. 2019;46:752–758. doi: 10.1111/1346-8138.15004. [DOI] [PubMed] [Google Scholar]
- 205.Okubo Y., Ohtsuki M., Morita A., Yamaguchi M., Shima T., Tani Y., Nakagawa H. Long-term efficacy and safety of secukinumab in Japanese patients with moderate to severe plaque psoriasis: 3-year results of a double-blind extension study. J. Dermatol. 2019;46:186–192. doi: 10.1111/1346-8138.14761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Assefa G.T., Kaneko S., Oguro H., Morita E. Treatment of psoriasis and psoriatic arthritis with secukinumab after unsatisfactory response to ustekinumab in multiple sclerosis patient. J. Dermatol. 2019;46:e112–e113. doi: 10.1111/1346-8138.14619. [DOI] [PubMed] [Google Scholar]
- 207.Chiricozzi A., Guttman-Yassky E., Suárez-Fariñas M., Nograles K.E., Tian S., Cardinale I., Chimenti S., Krueger J.G. Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Investig. Dermatol. 2011;131:677–687. doi: 10.1038/jid.2010.340. [DOI] [PubMed] [Google Scholar]
- 208.Krueger J.G., Brunner P.M. Interleukin-17 alters the biology of many cell types involved in the genesis of psoriasis, systemic inflammation and associated comorbidities. Exp. Dermatol. 2018;27:115–123. doi: 10.1111/exd.13467. [DOI] [PubMed] [Google Scholar]
- 209.Zenobia C., Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000. 2015;69:142–159. doi: 10.1111/prd.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Brembilla N.C., Senra L., Boehncke W.H. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front. Immunol. 2018;9:1682. doi: 10.3389/fimmu.2018.01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.McGeachy M.J., Cua D.J., Gaffen S.L. The IL-17 family of cytokines in health and disease. Immunity. 2019;50:892–906. doi: 10.1016/j.immuni.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Okada S., Puel A., Casanova J.L., Kobayashi M. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clin. Transl. Immunol. 2016;5:e114. doi: 10.1038/cti.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Su Y., Huang J., Zhao X., Lu H., Wang W., Yang X.O., Shi Y., Wang X., Lai Y., Dong C. Interleukin-17 receptor D constitutes an alternative receptor for interleukin-17A important in psoriasis-like skin inflammation. Sci. Immunol. 2019;4:eaau9657. doi: 10.1126/sciimmunol.aau9657. [DOI] [PubMed] [Google Scholar]
- 214.Ma W.Y., Jia K., Zhang Y. IL-17 promotes keratinocyte proliferation via the downregulation of C/EBPα. Exp. Ther. Med. 2016;11:631–636. doi: 10.3892/etm.2015.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chiricozzi A., Nograles K.E., Johnson-Huang L.M., Fuentes-Duculan J., Cardinale I., Bonifacio K.M., Gulati N., Mitsui H., Guttman-Yassky E., Suárez-Fariñas M., et al. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS ONE. 2014;9:e90284. doi: 10.1371/journal.pone.0090284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Sun D.P., Yeh C.H., So E., Wang L.Y., Wei T.S., Chang M.S., Hsing C.H. Interleukin (IL)-19 promoted skin wound healing by increasing fibroblast keratinocyte growth factor expression. Cytokine. 2013;62:360–368. doi: 10.1016/j.cyto.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 217.Witte E., Kokolakis G., Witte K., Philipp S., Doecke W.D., Babel N., Wittig B.M., Warszawska K., Kurek A., Erdmann-Keding M., et al. IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J. Investig. Dermatol. 2014;134:2757–2767. doi: 10.1038/jid.2014.308. [DOI] [PubMed] [Google Scholar]
- 218.Lai X., Li X., Chang L., Chen X., Huang Z., Bao H., Huang J., Yang L., Wu X., Wang Z., et al. IL-19 up-regulates mucin 5AC production in patients with chronic rhinosinusitis via STAT3 pathway. Front. Immunol. 2019;10:1682. doi: 10.3389/fimmu.2019.01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.House J.S., Zhu S., Ranjan R., Linder K., Smart R.C. C/EBPalpha and C/EBPbeta are required for sebocyte differentiation and stratified squamous differentiation in adult mouse skin. PLoS ONE. 2010;5:e9837. doi: 10.1371/journal.pone.0009837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Crish J.F., Gopalakrishnan R., Bone F., Gilliam A.C., Eckert R.L. The distal and proximal regulatory regions of the involucrin gene promoter have distinct functions and are required for in vivo involucrin expression. J. Investig. Dermatol. 2006;126:305–314. doi: 10.1038/sj.jid.5700019. [DOI] [PubMed] [Google Scholar]
- 221.Gutowska-Owsiak D., Schaupp A.L., Salimi M., Selvakumar T.A., McPherson T., Taylor S., Ogg G.S. IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion. Exp. Dermatol. 2012;21:104–110. doi: 10.1111/j.1600-0625.2011.01412.x. [DOI] [PubMed] [Google Scholar]
- 222.Min H.J., Song H., Choi S.Y., Kim T.H., Cho H.J., Yoon J.H., Kim C.H. Th2 cytokines differentially regulate psoriasin expression in human nasal epithelia. Am. J. Rhinol. Allergy. 2014;28:449–453. doi: 10.2500/ajra.2014.28.4087. [DOI] [PubMed] [Google Scholar]
- 223.Bissonnette R., Bolduc C., Maari C., Nigen S., Webster J.M., Tang L., Lyle M. Efficacy and safety of topical WBI-1001 in patients with mild to moderate psoriasis: Results from a randomized double-blind placebo-controlled, phase II trial. J. Eur. Acad. Dermatol. Venereol. 2012;26:1516–1521. doi: 10.1111/j.1468-3083.2011.04332.x. [DOI] [PubMed] [Google Scholar]
- 224.Robbins K., Bissonnette R., Maeda-Chubachi T., Ye L., Peppers J., Gallagher K., Kraus J.E. Phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of plaque psoriasis. J. Am. Acad. Dermatol. 2019;80:714–721. doi: 10.1016/j.jaad.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 225.Li Y., Zhang H., Zhu X., Feng D., Gong J., Han T. Interleukin-24 induces neuroblastoma SH-SY5Y cell differentiation, growth inhibition, and apoptosis by promoting ROS production. J. Interferon Cytokine Res. 2013;33:709–714. doi: 10.1089/jir.2013.0004. [DOI] [PubMed] [Google Scholar]