Abstract
Non-melanoma skin cancers (NMSCs) include basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and Merkel cell carcinoma (MCC). These neoplasms are highly diverse in their clinical presentation, as well as in their biological evolution. While the deregulation of the Hedgehog pathway is commonly observed in BCC, SCC and MCC are characterized by a strikingly elevated mutational and neoantigen burden. As result of our improved understanding of the biology of non-melanoma skin cancers, innovative treatment options including inhibitors of the Hedgehog pathway and immunotherapeutic agents have been recently investigated against these malignancies, leading to their approval by regulatory authorities. Herein, we review the most relevant biological and clinical features of NMSC, focusing on innovative treatment approaches.
Keywords: skin cancer, basal cell carcinoma, squamous cell carcinoma, Merkel cell carcinoma, Hedgehog pathway, immunotherapy, anti-PD1 monoclonal antibodies
1. Introduction
The incidence of skin cancers is increasing worldwide, as a result of the chronic exposure to sunlight, climatic changes and individual and social conditions [1,2]. As a whole, skin cancers include cutaneous melanoma (CM) and non-melanoma skin cancer (NMSC) that are mainly represented by basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). A peculiar chapter in the context of skin cancers is represented by Merkel cell carcinoma (MCC), which is historically classified among neuroendocrine tumors, although its behavior resembles, in most instances, CM for the high propensity to colonize lymph nodes [3,4].
NMSC originates from epidermal cells and shows common epidemiology (e.g., higher prevalence in Caucasian subjects). On the other hand, MCC is thought to arise from Merkel cells and its frequency increases in equatorial geographic areas, particularly among subjects of white ethnicity. The pathogenesis of BCC, SCC and MCC is multifactorial, but skin exposure to physical carcinogens is the prevalent risk factor. Indeed, ultraviolet radiation (UVR) has the potential to directly drive the malignant transformation of progenitor cells [5,6,7,8]. Other risk factors [9,10,11,12] for the development of BCC and SCC include concurrent diseases and dedicated treatments (i.e., psoriasis), chronic exposure to human papilloma virus, drug-induced immune suppression in transplanted patients and targeted agents for the treatment of other cancer types (notably, melanoma). Several studies have also demonstrated that low social-economic status positively influences NMSC development [13,14]. The integration of the Merkel cell polyomavirus (MCP-yV) within the genome of tumor cells is a common event in MCC, and the molecular features leading to the MCPyV-induced malignant transformation of Merkel cells have recently been elucidated [15]. The deregulation of the Wnt/Hedgehog pathway has been described as a pivotal mechanism in the development of BCC while SCC and MCC are characterized by a high neoantigen burden [11,15,16,17,18,19,20,21]. The knowledge of the basic events driving NMSC has provided the basis for the development of new therapeutic strategies that include both targeted agents and immunotherapy.
Herein, we review the most relevant findings in NMSC and MCC focusing on clinical and pathogenic features, as well as novel therapeutic strategies.
2. Normal Skin and the Mechanisms of Cancerogenesis
Normal skin is constituted by different layers: the epidermidis, papillary, reticulum dermis and subcutaneous fat. The epidermidis is composed by four sub-layers showing different functions, including the stratum corneum that provides a barrier function and protects the other layers. In addition, melanocytes of the basal stratum exert a protective role from UV radiation. Langherans cells (LCs) play a relevant role in the activation of the immune system, while Merkel cells control the light touch. The dermis includes fibroblasts and specialized cells as well as glands, blood vessels and nerves that are variably implicated in the physiologic regulation of skin functions [22].
The mechanisms leading to the development of NMSC as well as MCC are multifactorial and include the exposition to UVR, which also represents a risk factor for both melanoma and MCC [15,23,24]. UVR determines DNA damage and the development of somatic mutations, inflammation, oxidative stress and defective activity of the immune cells. These events are milestones for the development of skin cancers. However, UVA and UVB induce different skin alterations, inasmuch as UVA promotes deeper damages, while indirectly disrupting the DNA through free radical formation, whereas UVB induces erythema, thus directly damaging DNA. Many studies have suggested that UVR is mostly adsorbed by epidermal keratinocytes and induces immune-suppression through the dimerization of cyclobutane pyrimidine, mutations in p53 and other tumor suppressor genes, as well as directly inducing inflammation and apoptosis of keratinocytes [25,26,27]. Further relevant events in the carcinogenesis process induced by UVR include free radicals-mediated damage, as well as either mutations or single-nucleotide polymorphisms (SNPs) of the glutathione S-transferase enzyme [28]. In addition, several genetic syndromes (Table 1) have been associated with both BCC and SCC and, moreover, the nevoid BCC (NBCC) shed relevant light into the pathogenic mechanisms of BCC, including the development of dedicated targeted agents. The events driving the malignant transformation of Merkel cells as result of MCPyV infection are detailed in Section 5.1.
Table 1.
Genetic syndromes associated with NMSC.
| Syndrome | Type of NMSC | Clinical Features |
|---|---|---|
| Xeroderma Pigmentosus | BCC SCC | Autosomal recessive disorder characterized by defects in the mechanisms of DNA repair. NMSCs are frequently developed by younger (≤20 years). The risk is directly associated to UVR exposition. |
| Oculocutaneous Albinism | SCC | Autosomal recessive disease showing a pigmentary dilution of the skin, eyes, and hair. SCCs are frequently developed by young subjects exposed to UVR. A high incidence of metastatic SCC has been described in black population. |
| Epidermodysplasia Verruciformis | SCC | Rare and usually recessively inherited disorder characterized by a colonization of the skin by HPV. The majority of patients develop NMSC as adults, usually in sun-exposed regions, but earlier with respect to the general population. Aggressive biological behavior includes perineural spread, metastases and death. |
| Dystrophic epidermolysis bullosa | SCC | It is characterized by both dominant and recessive mutations of the type VII collagen gene. The majority of recessive patients develop SCCs, that occur during the third-fifth decade of life, frequently multiple and with high attitude to local recurrence and spreading to distant metastatic sites. |
| Basal Cell Nevous Syndrome (Gorlin) | BCC | It is an autosomal dominant disorder caused by inactivating mutations of PTCH1 or rarely PTCH2. Clinical features include early NMSC, involvement of multiple sites on course of life, odontogenic keratocysts of the jaw, palmo-plantar pits calcifications of the falx cerebri and abnormalities of the skeleton. Patients also develop multiple cancers of the SNC, ovary and heart, as well as other skin defects such as epidermoid cysts and facial milia. |
| Bazex-Dupré-Christol Syndrome | BCC | It is a rare condition characterized by follicular atrophoderma of the hands and feet, hypotrichosis, localized hypohidrosis, epidermoid cysts and multiple BCCs developed during the second decade of lifer showing a trichoepithelioma-like histology. The inheritance pattern is often X-linked. |
| Rombo Syndrome | BCC | Patients have an atrophoderma vermiculatum-like appearance on the cheeks with evidence of sweat duct proliferation. They often suffer of hypotrichosis, blepharitis, peripheral erythema, trichoepithelioma and skin cancer. |
3. Basal Cell Carcinoma
Basal cell carcinoma (BCC) is the most common cancer of the skin that, in as many as 80% of patients, develops in the head/neck region, often in the absence of pre-cancerous lesions. BCC rarely metastasizes, but frequently shows local invasion and tissue destruction, thus resulting in high morbidity [29]. Elderly Caucasian males, with fair skin chronically exposed to UVR, are more frequently affected in a fashion almost similar to females using sunbeds. Younger people are rarely affected, while the trunk is the most common primary site. Apart from UVR, external beam radiotherapy (RT) may also favor the occurrence of BCC, as well as arsenic, immune suppressive agents [4] and HIV infection, although a clear correlation with CD4 count has not been demonstrated. Many genetic syndromes may increase the risk of developing BCC [30] and, in this context, the nevoid BCC (NBCC) is an autosomal dominant disorder characterized by multiple lesions of the skin, pits of the palm and soles, jaw keratocysts and developmental defects [31].
3.1. The Genetic Landscape of BCC
A relevant topic in BCC concerns its genetic background. The deep knowledge of NBCC molecular features provides relevant information on the gene profile of BCC, thus revealing the primary involvement of the Patched 1 (PTCH1) gene. This is a transmembrane receptor that inhibits signals driven along the Hedgehog (HH) pathway [32,33,34,35]. In addition, smoothened (SMO) and glioma-associated (GLI) oncogenes have been investigated in sporadic BCC, thus revealing that loss-of-function mutations of SMO as well as alterations of the HH cascade are present in more than 90% of BCCs. The mechanisms implicated in the development of BCC are regulated by three ligands, namely Sonic- (SH), Indian- (IH) and Desert-Hedgehog (DH), whose high expression mostly occurs in the skin. They are bound by the receptor PTCH1 that, upon ligation, first activates SMO and then GLI-1, GLI-2 and GLI-3. These factors directly modulate genes implicated in tumorigenesis and angiogenesis, such as Cyclin-D1, Myc and Bcl-2. Apart from BCC, defects of the HH pathway also occur in medulloblastoma [36], breast [37], lung [38], prostate [39], colon [40] and pancreatic [41] cancers as well as lymphoproliferative disorders [42].
Unlike BCC and medulloblastoma, however, cells from solid cancers show a ligand-dependent activation of the HH pathway, although many alternative mechanisms have been described, including autocrine- (i.e., breast, lung and prostate cancer), paracrine- (i.e., colon and pancreatic cancer through both IL-6 and VEGF) and reverse paracrine-dependent HH activation, which is the consequence of soluble factors produced by stromal cells nearby tumor cells [43].
HH is critical in organogenesis, stem cell formation and tissue repair, whereas it directly controls the hair follicles and sebaceous glands of the skin [44]. Moreover, aberrant HH pathway drives a cancer stem cell phenotype, controls the primary cilium structure by triggering a complex signature that activates PTCH1 and SMO, the cytoplasmic release of GL1 proteins followed by their migration into the nucleus where upregulate genes implicated in the self-renewal, survival and angiogenesis [32,45,46,47]. The upregulation of the HH signaling is, therefore, a relevant pathogenic event occurring in more than 90% of BCC, whereas almost 10–20% of them bear SMO mutations. In addition, a non-canonical HH cascade has also been described and results mostly activated by kRAS, TGF-β and PI3K-AKT [48]. Beyond the HH pathway, other genetic alterations characterize BCC development, and they include members of the Ras family, TP53, Hyppo-YAP and TERT [43]. A “UV-signature” is frequently detected in BCC as consequence of the chronic exposition to UVA.
3.2. Epidemiology, Classification and Clinical Features
Data regarding the epidemiology of BCC are extremely heterogeneous, with a number of annual cases ranging from 88 to 164/100,000 persons-years across different countries. While it is possible that the real incidence of BCC is globally underestimated [8], the highest incidence rates are reported in Australia, followed by the US and Europe. The mortality of BCC is low, and it is mostly influenced by concurrent diseases, age and clinical complications, whereas it occasionally depends on extensive tissue infiltration and metastatic spreading that include either nodal or distant site involvement [49]. Development of advanced BCC mostly occurs in males and is associated with worse prognosis and younger age [50,51,52,53,54].
The clinical features of BCCs are extremely heterogeneous (Figure 1), and a universal classification is currently unavailable [55,56]. Clinical variants can be subdivided into: (i) nodular; (ii) superficial; (iii) dibroepithelial; and (iv) morpheaform. Some BCCs contain melanin, while nodular pattern may characterize any histologic variant. The nodular BCC shows high propensity to ulceration, as well as worse prognosis. Other variants include the cystic, mucinous, basosquamous and micronodular as well as multifocal BCC (Figure 2). In particular, the basosquamous BCC is a mixed variant characterized by histologic features of both BCC and SCC, showing high aggressiveness including its capability of local and distant metastasis [18].
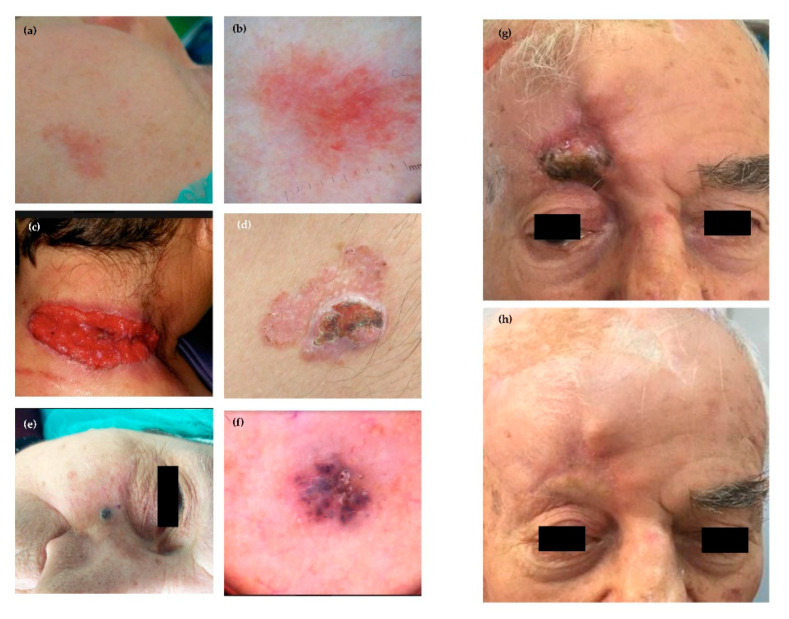
Figure 1.
Representative clinical patterns and dermatoscopy of BCCs: (a) clinical features and (b) dermatoscopy of superficial BCC of the cheek; (c) ulcerated and (d) multifocal BCC; (e) nodular pigmented BCC of the zigomatic area and (f) relative pattern by dermatoscopy; and (g,h) Effect of Hedgehog inhibitors in a patient with advanced BCC of the head.
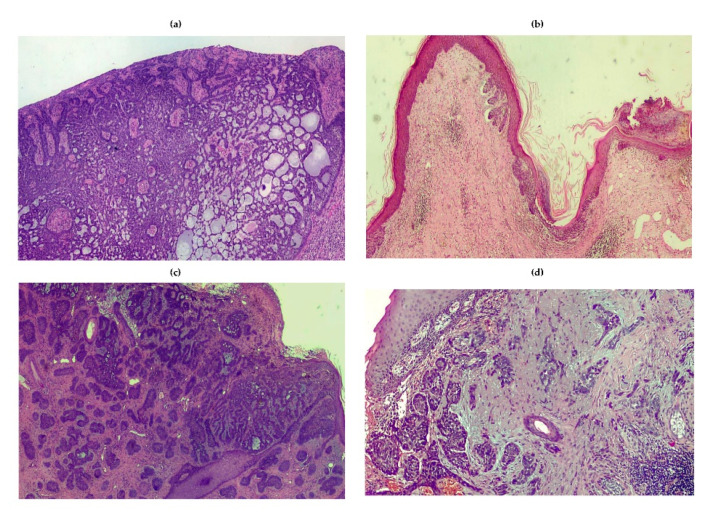
Figure 2.
Histologic patterns of BCC: (a) adenoid variant of nodular BCC showing island of tumor cells characterized by a cribriform pattern; (b) superficial BCC; (c) micronodular BCC; and (d) morpheaform variant showing malignant cells surrounded by a sclerotic stroma enriched in collagen. The infiltrative features are also shown.
3.2.1. Nodular
It is the most common variant accounting for 50–79% of BCCs [57]. Lesions are mainly characterized by a papule or a pearly nodule. The nodular BCC is often ulcerated and pigmented, or it shows a central depression and is frequently bleeding. The head/neck is the most common primary site.
3.2.2. Superficial
It is the second commonest clinical subtype [58]. Its prevalent feature is the appearance as macula, atrophic plaque, papula or erythema-like lesion that rarely results pigmented, well-defined, scaly and pinkish. Regression is a common feature of this type of BCC. The trunk and extremities of younger people as well as head/neck district are the most frequent primary sites. Multiple superficial BCCs may occur. The majority of superficial BCCs show a horizontal pattern of growth, rather than a vertical one, whereas ulceration, nodular features and invasive pattern are rarely observed. Notwithstanding a number of histologic variants and rare patterns, they have no relevant prognostic implications, apart from a modest propensity to local diffusion and distant metastasis [58].
3.2.3. Fibroepithelial
It is a rare form that mostly involves the trunk, mainly occurring as a pink-colored plaque, sessile or papula-like lesion [57]. It may include pigment.
3.2.4. Morpheaform
This is a rare variant of BCC (5–10%) characterized by an elevated or depressed pink/ivory and indurated plaque showing a smooth surface that often includes telangiectasias [59]. This form of BCC is highly aggressive with an elevated attitude to local invasion and distant metastasis.
3.2.5. Infiltrative
This variant is similar to morpheaform BCC and is mostly characterized by a heavy stromal fibrosis with dense collagen bundles; it grows in a poorly circumscribed fashion and often invades the subcutis, while tumor cells spread forming a large irregular nodule [59].
3.2.6. Micronodular
This form resembles the classical nodular BCC and seems characterized by a deep extension into the dermis, as well as sporadic infiltration of the subcutis with stromal proliferation [60].
3.2.7. Basosquamous
It shows infiltrating jagged clumps of tumor cells, some with a clear-cut basaloid morphology and cytoplasmic keratinization [61].
3.3. Therapeutic Options
The therapeutic strategy of BCC should be based on a multidisciplinary approach, although surgery (either curative or palliative) remains the primary option. Surgery requires a skin cancer board of experts and finality includes type of excision, adequate margins, appropriate techniques of reconstruction, tissue preservation and dedicated surgical approaches in certain difficult sites that require a topographic study of the of primary tumor as well as the early planning of adjuvant options that include both systemic therapy and RT [3].
3.3.1. Radiotherapy
RT should be regarded as the primary treatment in patients bearing BCC and considered not eligible for surgery due to advanced disease stage, comorbidities, risk of complications and location of primary sites (i.e., eyelid, nose or lip). A systematic review [62] reported an estimated recurrence rate of 3.5% after RT, with data similar to both surgery and micrographic technique developed by Mohs [63]. However, the principal indications for RT include: (i) inoperable tumors; (ii) the certainty of disfigurement that is not balanced by the certainty of clear margins; and (iii) incomplete resection with microscopic or macroscopic residual tumors [64].
Finally, external beam RT remains the most used treatment, although other options include brachytherapy that provides similar results in terms of recurrence-free survival and local complications [56,65].
3.3.2. Systemic Treatments
Early treatment of BCC was mainly based on the use of systemic or topic Imiquimod as well as 5-fluorouracil-based chemotherapeutic agent that unfortunately produced only modest improvements in terms of median OS [66]. Although it still represents an indication in a small group of patients, the knowledge of the mechanisms activated along the HH pathway has allowed developing and testing several targeted therapies. They include vismodegib, a small molecule optimized to inhibit SMO, and additional compounds including sonidegib, itraconazole and others at different stage of development. Both Vismodegib and Sonidegib have been approved for the treatment of locally advanced or metastatic disease, as well as for patients not candidate to surgery or radiotherapy [67,68,69,70]. In addition, itraconazole has shown efficacy and manageable safety in an exploratory phase 2 study (NCT01108094), while patidegib, glasdegib and talasdegib (LY2940680) are promising drugs under investigation that, by blocking HH-mediated signals, are indicated for the treatment of advanced disease [71,72]. The majority of HH inhibitors have proved able to improve OS and delay the recurrence in a high number of patients, while showing an acceptable safety profile, as demonstrated by the STEVIE clinical trial [73].
However, patients receiving HH inhibitors commonly experience adverse events of any grade that include alopecia, muscle spasms, fatigue, vomiting and dysgeusia, not taking into account the development of resistance [68,74]. Thus, intermittent, or dose escalation, schedules allow overcoming these complications; notably, Grade 3–4 toxicities causing definite treatment breaks are frequently associated with a poorer outcome [75,76]. Notwithstanding the experience from the real-life treated population is extremely puzzling, relevant results concerning clinical predictors of response have emerged from pivotal trials. In particular, the best response proved to be achieved in locally advanced disease, younger people, tumors smaller than 4 cm and absence of prior exposure to other HH inhibitors, whereas histology apparently does not influence outcome. Weekly interval dosing ameliorates adverse events, but a prolonged drug discontinuation has been associated with tumor progression. Moreover, intermittent schedules have been explored in the MIKIE phase 2 trial, which produced inconclusive results [76]. To date, there are no comparative studies analyzing potential difference between Vismodegib and Sonidegib, although indirect comparisons have been conducted using experimental trials’ results. The result of both the ERIVANCE [68] and BOLT trials [70,77] were similar, although Vismodegib showed an apparent advantage in the metastatic setting.
Re-treatment is an effective challenge in the majority of patients, whereas new indications are emerging, including neo-adjuvant treatment for locally advanced BCC, to be pursued until clinical response, followed by definitive surgery. This strategy is indicated for difficult lesions mostly located in peri-ocular and orbital areas.
3.3.3. Innovative Therapeutic Strategies
Based on the variability of response mostly due to the heterogeneous clinical presentation of these malignancies, recent data completed in other cancer models (e.g., medulloblastoma) show a high responsiveness to HH inhibitors, revealed a predictive gene profiling represented by deregulated genes such as GLI1, SPHK1, SHROOM2, PDL1M3 and OXT2 [3,74]. In addition, phase 1 studies demonstrated that GLI1 levels are decreased by treatment, and thus suggested its expression as a pharmacodynamics marker of treatment [75]. The BOLT trial [70], however, extended this observation to BCC, thus suggesting GLI1 as candidate master gene in BCC. Other biomarkers validated in pre-clinical models and phase 1 studies are MSI2, CCND2, PITCH1 and BAFF, as well as macrophage inflammatory protein-1 alpha, IL-8 and CCL19 deregulated expression [78].
Another interesting issue in the management of BCC concerns the mechanisms implicated in the acquired resistance. In this context, acquired mutations in SMO cause resistance, as well as amplification of downstream genes such as GLI2 and mutations of genes implicated in the control of ciliogenesis such as CFD1 and SNFN, which apparently promote resistance through SMO-independent pathways [79,80]. A relevant approach for overcoming resistance is to target different downstream mediators such as DYRK1B, whose antagonism is of great effort in decreasing GLI levels and thus restoring the sensitivity of malignant cells [81]. Furthermore, another strategy includes the use of mTOR inhibitors [79], itraconazole [82] or anti-PD1 monoclonal antibodies, which showed promising anti-proliferative activity in patients progressing after an HH inhibitor.
4. Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) is the second most common skin cancer that develops more frequently in Caucasian subjects exposed to environmental factors, as well as UVR, smoking, chronic infections and immune suppressors, or bearing peculiar genetic background. Detailed epidemiologic data are not available worldwide, and the incidence in the next decade in Europe is expected to approximately double, although the most reliable epidemiological information are collected in Australia, USA, and the Swedish Cancer Registry [83]. Mortality is correlated with the ability of malignant cells to spread toward distant sites, as well as with older age, male gender, site (i.e., lip, temple and ear), thickness, transplantation, treatment with BRAF inhibitors, HIV infection or chronic lymphatic leukemia (Table 2). Many studies have been published dealing with the increased risk of SCC following treatment with BRAF inhibitors in metastatic melanoma patients. These studies have clearly demonstrated that this targeted therapy induces a hyper-proliferation of keratinocytes, due to paradoxical activation of the mitogen-activated protein kinase (MAPK) pathway, in BRAF wild-type cells. This event mostly occurs in patients bearing oncogenic mutations of Ras and is, at least in part, reverted by the combination with MEK inhibitors [84].
Table 2.
Clinical and histologic risk factors associated with squamous cell carcinoma (NCCN Guidelines).
| Clinical Features | LOW RISK | HIGH RISK |
| Site and Size | Area L; <20 mm Area M; <10 mm |
Area L; ≥20 mm Area M; ≥10 mm Area H; |
| Margins | Well defined; R0 | Undefined or R1 |
| Immune Suppression | Absence | Presence |
| Exposition to radiotherapy or chronic inflammatory process | Absence | Presence |
| Rapid growth | Absence | Presence |
| Neurological symptoms | Absence | Presence |
| Histology | LOW RISK | HIGH RISK |
| Grading | G1 or G2 | G3 |
| Adenoid-squamous, Adenoid-cystic, Desmoplastic, Metaplastic, Carcinosarcoma |
Absence | Any Variant |
| Thickness or level of invasion | ≤6 mm and no subcutaneous invasion | >6 mm or subcutaneous invasion |
| Perineural, lymphatic or vascular invasion | Absence | Presence |
4.1. The Molecular Features of SCC
Studies on the genetic landscape of SCC demonstrated that genes altered by UVR exposition are TP53, CDKN2A, NOTCH1, NOTHC2, and p16 suppressor gene; epigenetic regulators such as KMTC2, KMT2A, ARID2, SETD2, CREBBP and TET2; mutations of TGF-β receptor; and mutations in DNA repair pathways including missense mutations in ATR, PIK3CA, ERRB4 and NF1 [85,86,87]. In the context of epigenetic modifications, a number of microRNAs (miRNAs) proved to be upregulated or downregulated in SCCs, and those exhibiting oncogenic functions (miRNA-21, -205, -181a, -125b, -34a, -148a, -214, -124 and -199a) have been found modified with respect to normal skin tissues. Thus, potential therapeutic strategies by antisense oligonucleotides are under investigation in pre-clinical studies [88,89]. In addition, such targeted therapies may induce PI3K and EGFR defects while chronic exposure to azathioprine has been associated with a peculiar hyperactivity of endogenous cytidine deaminases (APOBEC) in SCC developed in consequence of recessive epidermolysis bullosa [90]. Other studies discovered a number of SNPs associated with SCC as well as transcription factors and metastasis suppressor genes (i.e., CADM1 and AHR). Lastly, the microenvironment has a key role in enhancing or blocking the proliferative extent of cancer cells, and a major effect is played by HLA variants and PD1/PDL-1 interplay.
Finally, SCC has been found associated to hereditary syndromes as xeroderma pigmentosum, epidermolysis bullosa, oculocutaneous albinism, Fanconi anemia and Lynch syndrome [91,92].
4.2. The Clinical Characteristics of SCC
SCC includes many different subtypes, endowed by different clinical features ranging from an indolent behavior with slow growth to aggressive tumors showing invasive properties and high spreading toward distant sites (Figure 3) [93,94]. In patients receiving appropriate treatments, the risk of recurrence is about of 5%, while nodal and distant metastases occur in 4–6% of patients with a variability that almost depends on the histologic pattern and risk factors (Table 2). The clinical presentation is extremely heterogeneous in relation to the site, size, thickness and pigmentation. There is no accepted consensus on SCC classification, but superficial SCC is considered the most common variant that often develops from an actinic keratosis (AK) or a Bowen disease, although the risk of malignant transformation of these diseases is overall extremely low (Figure 4). However, the last edition of the WHO classification includes new variants including those SCC which develop from a cheratoacanthoma [84,95,96,97]. Notwithstanding, the majority of SCC are characterized by keratinocyte dysplasia that progressively involves the epidermis and derma, as well as the surrounding stromal tissues; furthermore, SCC may already be invasive at diagnosis. The majority of metastatic SCCs originate in the head and neck district, as well as in sun-exposed skin. In relation to grading, SCCs are classified into G1, G2 and G3.
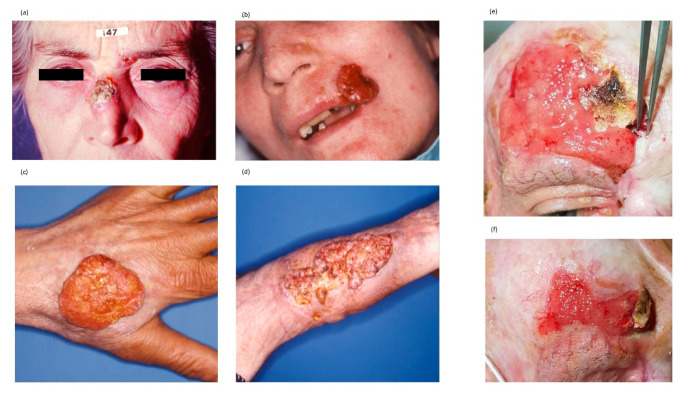
Figure 3.
Clinical features of SSCs. Panels are representative of clinical presentation and response to immunotherapy: (a) SCC of the nose that arises on photo-damaged skin in presence of actinic keratosis of the left eyebrow; (b) high grade SCC of the left commissura of the lower lip; (c) SCC of the hand in patients in active treatment for concomitant LLC; (d) SCC originated on previous burned skin; and (e,f) effect of six-months course of anti-PD-1 MoAb in a patient with locally advanced SCC unfit for further surgery.
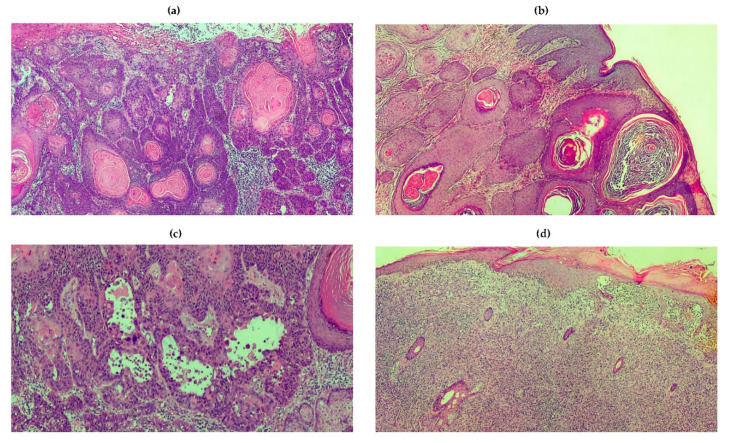
Figure 4.
Histologic variants of SSC. Panels are representative of histologic patterns from patients with SCC. (a) A moderately differentiated and ulcerated lesion showing enlarged, hyperchromatic and irregular nuclei. Corneal pearls in the middle reflect the keratinization ability. Malignant cells are surrounded by abundant inflammatory cells. (b) Verrucous SCC characterized by deeply invasive properties. (c) Spindle cell SCC showing elongate, fusiform cells that blend with the surrounding reactive fibroblastic component. (d) Acantholytic SCC characterized by a pseudoghiandolar pattern and dyskeratosis of tumor cells. The acantholytic phenomenon affects the inner portion of invasive nests and lobules.
Many SSCs develop as a plaque or a papule with a hyperkeratotic surface, often pigmented [96]. Based on the differentiation grade, the primary lesion may be verrucous, seborrhoic keratosis, crateriform, brown or light. Otherwise, it is ulcerated and red-fleshy non-keratotic lesion that in most instances may resemble an amelanotic melanoma, a cutaneous metastasis or a MCC. Poorly differentiated SSCs are larger tumors with induration, which may also involve surrounding skin and high propensity to infiltrate nearby structures. Metastatic SCCs are characterized by in-transit, nodal and visceral metastases. The majority of SCCs are diagnosed by means of a simple clinical examination, although dermatoscopy may be conclusive in the case of difficult patterns. Other non-invasive diagnostic techniques include in vivo reflectance confocal microscopy and optical coherence tomography, but their effective application in the clinical practice needs further confirmation.
4.3. The Immune System and SCC
The balance between cancer and immune system is a milestone for the malignant cell development and this event is considered critical for the SCC development [98,99]. Many studies have suggested that disruption of immune-editing phases is required for SCC, while both UV exposure and viral infections cooperate for the impairment of immune system response. Both innate and adaptive immunity apparently play a role in tumor antigen clearance in SCC [100] and small subsets of clonal cells are reprogrammed to favor immune suppression in favor of tumor cell proliferation [89]. The development of SCC is mostly based on a tight interplay between tumor and immune cells that leads to a favorable microenvironment [101,102]. It has been demonstrated that UV promote chronic inflammation that surrounds malignant squamous cells, by recruiting macrophages, and promoting both apoptosis and defective lymph node migration of specialized cutaneous dendritic cells (DCs), namely Langherans cells (LCs) as well as monocyte-derived dermal DCs [103,104,105]. Other immune populations actively deregulated in SCC development are natural killer cells (NKs) and innate lymphoid cells (ILCs) that are key regulators of Th1, Th2 and Th17 immune response. By contrast, modest data are known about myeloid-derived suppressor cells while two subsets of T-cells, namely y∂ and NK-T cells, apparently exert a major role. In this context, the dendritic epidermal y∂ T-cells (DETCs) are the best studied population. Moreover, the tumor microenvironment is engulfed of immune suppressive cytokines such as IL-6 and IL-10 as well as T-regulatory cells whose migration nearby tumor cells contribute to enhance the evasion from the immune system control. A recent mechanism described in SCC and activated by UVR exposition concerns specific photoreceptors such as urocanic acid, a molecule that is found at high concentration in the corneum stratum of the skin. The isomerization of the urocanic acid leads to the development of its cis-form that is critically involved in the transient alteration of the immune surveillance, thus favoring the immune system impairment. In addition, it has been demonstrated that urocanic acid directly interferes with T-cells, thus promoting the expansion of immune suppressive subsets [106].
The acquired immune response in SCC is at least in part dependent on the ability of T-cells to drive a Th1- or Th2- and Th17-mediated immune response. It has been demonstrated that tumor-specific T-cell response is a pre-requisite for preventing skin cancer, while IL-22 overproduction is a hallmark of high keratinocyte turnover. In addition, the SCC scenario is also influenced by the quality of B-cell response that may restrain the immune response through TNF-α and IL-10 overproduction, whereas a defective number of infiltrating CD20⁺ cells has been also demonstrated in humans. In this context, several cytokines and signaling molecules are variably involved in the immune evasion that characterizes SCC, and a peculiar association of IL-10 haplotypes, IL-4R and TNFR2 with an increased risk to develop SCC has clearly been demonstrated [107].
The interplay between malignant cells and surrounding stromal cells is a milestone for understanding the role of microenvironment in tumor progression [108]. In this context, an active network is regulated by signals driven by cytokines, chemokines, growth factors, inflammatory cells, immune cells and enzymes involved in stroma remodeling [109,110,111,112,113,114,115,116]. Moreover, the characteristics of tumor-infiltrating lymphocytes reflect the effective anti-cancer role of the immune system. In addition, the deep understanding of the tumor microenvironment has suggested that many somatic mutations in SCC acting as neo-antigens may be targeted by cytotoxic cells. Data from the early studies in melanoma using an anti-CTLA4 MoAb show that SCC is characterized by a mutational load of around 50 mutations per megabase of DNA. In addition, PD-1 expression was widely demonstrated on T-cells surrounding tumor cells that, conversely, showed a PD-1 level of about 30%. High PD-1 expression in the context of a “cold” microenvironment was associated, however, with an increased risk of metastases and was also identified in lymphatic disease, thus providing definitive evidence for the prognostic role of immune infiltration in advanced SCC. In this context, recent data highlight the role of mutational landscape and HLA haplotypes in modulating the pressure of the immunological profile of cancer cells, thus potentially favoring response to immunotherapy [89,92,117,118].
4.4. Systemic Treatments
The gold standard therapy for SCC is definitely surgery, with alternative options including laser dissection, intra-lesion drug injection and electrodissection. However, other strategies have been considered in those patients considered unfit for surgery in relation to comorbidities, site of primary tumor, risk of local infiltration or quality of curative margins. They include external beam radiotherapy (RT) and brachytherapy. Retrospective analyses revealed a high number of biases in RT-based studies, mostly due to the heterogeneity of randomization, although an improvement in terms of disease-specific survival and OS has emerged in the majority of studies. In addition, there are no reliable data for other adjuvant treatments of SCC and, therefore, the indication for RT include lesions of the head/neck with regional node metastases and extracapsular extension, patients with positive margins or those not candidate to surgery due to concurrent diseases or difficult primary sites [96].
The poor results obtained by conventional chemotherapy in patients with advanced SCC and the results of original studies showing the high number of somatic mutations and neo-antigen load suggested to plan clinical trials with immune checkpoint agents (mainly blocking PD-1) in patients excluded from other strategies. In a fashion almost similar to melanoma, PD-L1 levels did not correlate with clinical response to anti-PD1 mAbs). Therefore, the immune checkpoint inhibitors cemiplimab and pembrolizumab have been approved in US for the treatment of locally advanced or metastatic SCC [119,120,121]. Based on these data, other clinical phase 2 studies with neoadjuvant Cemiplimab in stage III–IV SCC of the head/neck demonstrated its potential benefit in preliminary studies. The R2810-ONC-1540 was a phase 2, open-label study accruing 193 patients with locally advanced or metastatic SCC (NCT02760498). The study demonstrated an overall response rate (ORR) of 49.2% with 17% complete responses, 32% partial responses and 15% stable diseases, whereas 17% developed progression that mainly occurred in the metastatic group (27%).
Apart from chemotherapy, other strategies are based on the high EGFR expression demonstrated in SCC; indeed, its levels correlated with outcomes. Thus, anti-EGFR MoAbs combined with chemotherapy or RT is considered an option in patients showing progression after first-line regimen with anti-PD1 agents. Currently, systemic chemotherapy has not been approved for SSC based on the modest results in terms of response, coupled with the cost of serious adverse events, especially in a fragile patient population. On the contrary, a relevant disease control and local response has been obtained by electrochemotherapy; indeed, the EURECA trial investigated this option in skin cancers achieving a 55% of response in SCC [122]. Several patients developing SCC are immune depressed by transplantation, whereas others are affected by HIV-1 infection or hematological disorders, and thus are often excluded from immunotherapy trials. In this context, adoptive cell transfer using chimeric antigen receptor T-cells (CAR-T cells) seems to represent a novel alternative strategy to be potentially pursued in this niche of patients [123,124,125].
5. Merkel Cell Carcinoma
Merkel cell carcinoma (MCC), also known as primary cutaneous neuroendocrine carcinoma, is a rare malignancy of the skin characterized by an aggressive clinical behavior [23]. Although MCC is rare, its incidence is rising steadily, probably both as a result of improvements in diagnosis, as well of the global ageing of the world population. According to data from the Surveillance, Epidemiology, and End Results (SEER) database, the incidence of MCC increased from 0.5/100,000 individuals in 2000 to 0.7/100,000 persons in 2013 [126], and similar trends have been reported across Europe and Australia [127,128,129]. The incidence of MCC progressively increases with every additional decade of life, and only 4% of MCC cases occur in patients under 50 years of age [130]. The malignancy predominantly affects subjects of white ethnicity, and its frequency is higher in geographic areas closer to the equator, thus suggesting an association between UV radiation and disease occurrence [131]. Furthermore, transplant recipients and patients with B cell malignancies have an increased risk of developing MCC. In particular, the standardized incidence ratio of MCC in patients with chronic lymphocytic leukemia has been estimated to be 15.7 (95% CI, 3.2–46) [132].
5.1. Pathogenesis of MCC
MCC is a poorly differentiated neuroendocrine carcinoma that lacks a recognized benign or dysplastic precursor. Traditionally thought to arise from Merkel cells, MCC more likely derives from a yet to be defined cellular population which underwent neuroendocrine differentiation before or during malignant transformation. Pro-B lymphocytes, pre-B lymphocytes, fibroblasts, dermal mesenchymal stem cells and epidermal progenitor cells are among the most investigated candidates as cell of origin, but the possibility that MCC may originate from multiple, distinct, cells cannot be ruled out at present [15]. Both viral and non-viral factors play a key role in the pathogenesis of MCC. Merkel cell polyomavirus is the causative agent of a substantial fraction of MCC cases. It was discovered in 2008 as a new member of the Polyomaviridae family of small, non-enveloped, double-stranded DNA viruses, and five geographically-related genotypic variants have been characterized thus far [133]. MCPyV is part of the human skin microbiome, being chronically shed from infected cells in the form of assembled virions. The virus determines asymptomatic infections of the skin and is highly prevalent in the population, with anti-MCPyV antibodies detected in as many as 50% of children and 80% of older individuals [134]. MCPyV-related oncogenesis follows a model of multi-step progression, in which a sequence of distinct events is required to induce the malignant transformation. First, the MCPyV genome is linearized and integrated into the host genome after a concurrent DNA-damaging event, such as UV exposure. Second, infected cells are forced to express two viral oncoproteins, namely small tumor antigen (sT) and large tumor antigen (LT). While sT has oncogenic activity per se, by inhibiting the proteasomal degradation of cyclin E and c-Myc, LT acquires pro-tumorigenic activity only when mutations of the 3′ end of the gene lead to the loss of the protein C-terminus. Indeed, truncated LT inactivates the tumor suppressor Rb, driving uncontrolled cell proliferation. Following tumor formation, multiple mechanisms contribute to cancer cell survival in the presence of a destructive immune response. In addition, MCPyV-specific T cell responses have been detected both locally and systemically in patients with MCC, but the frequent expression of PD-L1 by cancer cells disables their effects by inducing T cell exhaustion [15,135,136,137,138,139]. In this context, the defective expression of HLA class-I by tumor cells may hamper antigen presentation, further promoting immune evasion [140].
While the majority of MCC cases recorded across US and Europe are virus-positive, up to 80% of tumors diagnosed in Australia have negligible levels of MCPyV-associated antigens [141,142,143]. The mechanisms underlying the pathogenesis of MCPyV-negative MCC still need to be completely elucidated, but the observation that virus-negative MCCs are characterized by UV mutational signature supports the idea that UVR might play a pivotal role in the development of the neoplasm [144], which is definitely credible in a country such as Australia. Notably, virus-negative MCCs have a substantially higher mutational burden as compared with virus-positive tumors and harbor recurrent, clonal, inactivating mutations of TP53, RB1 and other genes involved in the Notch signaling that are not frequently observed in the MCPyV-positive counterpart [145,146,147,148].
5.2. The Clinical Presentation and Diagnosis of MCC
MCC typically presents as a solitary, painless, red or violaceous intracutaneous nodule rapidly growing on the sun-exposed skin of elderly, fair-skinned individuals. In an analysis of 9387 cases recorded in the US National Cancer Database between 1998 and 2012, MCC was diagnosed at local, locoregional, or metastatic stage in 65%, 26% and 8% of cases, respectively [149]. Skin, lungs, adrenals, liver, brain and skeleton are the preferred sites of metastasis. Nevertheless, in up to 15% of patients, lymph-node involvement is detected in the absence of a recognizable cutaneous tumor, possibly as result of the spontaneous regression of the primary tumor [150,151]. MCC regression is associated with improved prognosis [152], but little is known regarding the biological mechanisms leading to the disappearance of the primary tumor.
The diagnosis of MCC is clinically challenging, thereby relying almost entirely on histology examination. Morphologically, MCC is characterized by the aggregation of small, monomorphic, round cells with scant cytoplasm in the context of nodules or sheets located in the dermis or subcutaneous tissue (Figure 5) [151]. Metastatic small-cell lung cancer (SCLC), small-cell melanoma, Ewing’s sarcoma and some lymphomas can have pathological features similar to those of MCC, and immunohistochemistry (IHC) appears useful in the differential diagnosis of these entities. Classical IHC markers of MCC include chromogranin-A, synaptophysin, cytokeratin 20 (CK20) and MCPyV-associated antigens. The negativity of thyroid transcription factor 1 (TTF1) may enable the distinction between MCC and SCLC metastatic to the skin, while the assessment of the Ewing’s translocation may be necessary to rule out a cutaneous metastasis of Ewing’s sarcoma. Strikingly, MCC and SCC may co-exist in the same lesion, possibly as UV-induced unrelated malignancies or, intriguingly, as tumors originating from the same multi-potent stem cell, and diverging in their differentiation at a later stage [151].
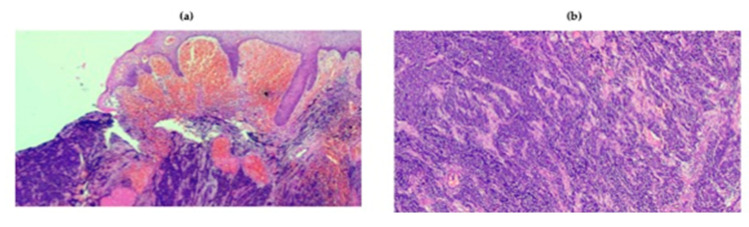
Figure 5.
Representative histologic patterns from MCC. (a,b) Histology of MCC characterized by deep infiltration of the dermis by lymphocytes at 4× (a) and 10× (b) magnification.
Following a pathological diagnosis of MCC, an accurate staging of the patient is mandatory. Both the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) recommend the use of the eighth edition of the TNM system in routine clinical practice [153]. Given the higher sensitivity shown by 18FDG-PET/CT imaging with respect to CT or MRI, functional imaging is presently considered the gold-standard procedure for the clinical assessment of MCC at diagnosis and follow-up [154]. In patients with radically resected MCC, the risk of recurrence is especially high within the first two years from the original diagnosis, and surveillance imaging should thus be performed every 3–6 months in this timeframe. The titers of antibodies against MCPyV T antigens have been shown to correlate with disease burden, and their increase is associated with tumor recurrence or progression, thus providing a non-invasive tool for follow-up individualization [155,156].
5.3. The Role of Sentinel Lymph Node Biopsy and of Systemic Treatments
The management of MCC patients primarily depends on the disease stage at presentation. In subjects without evidence of lymph-node involvement, sentinel lymph node biopsy (SLNB) is generally indicated. Patients with negative SLNB should undergo surgical excision with 1–2 cm margins, or definitive RT if surgery is not technically feasible [157]. By contrast, when the SLNB is positive, a careful assessment of occult metastatic disease should be carried out, and patients should be treated systemically if in stage IV, or with definitive surgery, RT or a sequence of surgery and RT in presence of regional lymph-node involvement [157]. Adjuvant RT can be recommended for patients with local MCC at high risk of relapse, including those who did not undergo SLNB [157,158,159]. While the role of adjuvant chemotherapy is highly debated, multiple trials are currently investigating immune checkpoint inhibitors, including pembrolizumab (NCT03712605), nivolumab (NCT03798639), ipilimumab (NCT03798639) or avelumab (NCT03271372), in the adjuvant setting and their results are awaited soon.
In this context, the phase 1/2 CheckMate 358 study [160] has recently tested nivolumab for the neoadjuvant treatment of 39 patients with stage IIA/IV, resectable MCC. Among the 36 patients who underwent surgery, 17 (46%) achieved a pathologic complete response, with tumor shrinkage being observed irrespective of MCP V detection, PD-L1 expression or tumor mutational burden. After a median follow-up of 20 months, both median recurrence-free survival and OS were not reached, in the presence of Grade 3/4 adverse events in just 8% of the whole cohort of patients. Notably, adverse events and tumor progression hindered the surgical intervention in two and one enrolled patients, respectively, thus emphasizing the importance of treatment tailoring in these subjects. Systemic therapeutic options for patients with stage IV are represented by either chemotherapy or immunotherapy.
Chemotherapeutic regimens including platinum-based combinations, etoposide, topotecan, taxanes and anthracyclines were widely used until 2016, leading to response rates in the range of 30–75%, and to median PFS and OS of approximately 3 and 10 months, respectively, in the first-line setting [161,162,163,164,165]. The immunosuppressive effect of chemotherapy is currently regarded as a possible mechanism accounting for the early development of resistance following treatment with cytotoxic agents in the context of a highly immunogenic cancer [166]. Therefore, cytotoxic chemotherapy is presently reserved to patients who are not candidates to immunotherapy (i.e., organ transplant recipients or patients with autoimmune diseases) or who have progressed to immune checkpoint inhibitors. The PD-1/PD-L1 axis is a key therapeutic target in MCC, and immunotherapy is currently recommended as the preferred first-line option in patients with advanced disease. Avelumab is a fully human IgG1 mAb directed against PD-L1, and its safety and efficacy have recently been investigated in the phase 2 JAVELIN Merkel 200 trial [167]. In Part A of this study, 88 patients with MCC progressive to at least one line of chemotherapy received avelumab at 10 mg/kg every two weeks. After a median follow-up of 29 months, objective responses were documented in 33% of cases, in the presence of 11% complete response rate. Strikingly, responses were durable, with 67% of them lasting over two years. The two-year PFS and OS rates were 26% and 36%, respectively. Notably, no substantial differences in terms of antitumor activity were seen between MCPyV-positive and -negative tumors, as well as between PD-L1-positive and -negative MCCs [168]. Part B of the JAVELIN Merkel 200 trial, aimed at investigating the safety and efficacy of first-line avelumab monotherapy in MCC, has recently completed the accrual of 112 patients. In a pre-planned interim analysis of 29 patients with at least three months of follow-up, the objective response rate was 62%, with a duration of response exceeding six months in the 83% of responding patients [169]. No Grade 4 toxicities have been reported in the JAVELIN trial, while Grade 3 adverse events have been documented in only 5% of patients enrolled in Part A of the study. In an analysis of 240 patients with advanced MCC receiving avelumab in the context of the expanded access program of the JAVELIN trial, the PD-L1 inhibitor confirmed a manageable safety profile [170]. Both FDA and EMA have approved avelumab for the treatment of adult patients with metastatic MCC.
The PD-1 blockers pembrolizumab and nivolumab have been recently tested in patients with advanced MCC. In a phase 2 study enrolling 50 patients with metastatic or recurrent locoregional MCC naïve to systemic therapy, pembrolizumab at 2 mg/kg every 21 days determined objective responses in 56% of cases, with a complete response rate of 24%. After a median follow-up of 14.9 months, the median PFS was 16.8 months, while the two-year OS rate was 69%. Again, no differences were detected according to either MCPyV or PD-L1 status in terms of response rate or duration of response. Grade 3 or greater adverse events were reported in 28% of patients, leading to treatment discontinuation in 14% of cases [171,172]. On this basis, the FDA granted accelerated approval to pembrolizumab for patients with recurrent locally advanced or metastatic MCC. Finally, the phase 1/2 CheckMate 358 trial has recently tested nivolumab at 240 mg every 14 days in 25 patients with advanced MCC. Among 22 evaluable patients, the overall response rate was 68%, with responses occurring in both treatment-naïve and pretreated patients, irrespective of the viral and PD-L1 status.
Innovative immunotherapeutic approaches for the treatment of advanced MCC patients include combinations of immune checkpoint inhibitors (including MoAbs against CTLA4 and LAG3), adoptive T cell or NK cell immunotherapies, as well as oncolytic viruses such as talimogene laherparepvec [139,173].
6. Conclusions and Future Perspectives
The progress in understanding the pathogenesis of BCC, SCC and MCC has allowed developing novel therapies, thus leading to great impact on survival and quality of life in many patients. To this regard, the knowledge of the genetic landscape of BCC has definitely proved that the cascade of signals driven through the HH pathway is crucial for the proliferation of cancer cells, and its inhibition by dedicated targeted agents restrains this property. However, other genetic defects have been discovered and new agents suggested for the treatment of this type of cancer, including blockers of PD-1 and PD-L1 signals. In this context, the immune system plays a key role in SCC pathogenesis and pre-clinical models have provided critical insights about alterations of immune cells that regulate the skin cancer biology. However, there is unlikely to be a single trigger for SCC development because the combination of genetic and environmental factors is of great effort for the malignant transformation of keratinocytes. Moreover, the comparison of premalignant with malignant skin tissues has permitted to reveal proteomic, genomic and immunological differences associated with cancer development. Other studies focused on microenvironment defects in NMSC, suggesting that dynamic interplay exists between malignant cells and those regulating either innate or adaptive immune system. The knowledge of these events has progressively changed the landscape of metastatic SCC treatment, thus providing novel options that are also under investigation in MCC and BCC. However, further information regarding the neoantigen load, the characteristics of the immune infiltrate and cells of the microenvironment are required to optimize the immunotherapy in NMSC. In this context, new techniques are under investigation to overcome actual limitations, with the purpose to tailor the treatment in relation to the continuous phenotypic and antigenic modifications that characterize cancer cells.
Acknowledgments
The authors are grateful to patients for their consent to publish clinical pictures.
Funding
This research was funded by Precision Medicine Project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Veisani Y., Jenabi E., Khazaei S., Nematollahi S. Global incidence and mortality rates in pancreatic cancer and the association with the Human Development Index: Decomposition approach. Public Health. 2018;156:87–91. doi: 10.1016/j.puhe.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Zaar O., Gillstedt M., Lindelöf B., Wennberg-Larkö A.-M., Paoli J. Merkel cell carcinoma incidence is increasing in Sweden. J. Eur. Acad. Derm. Venereol. 2016;30:1708–1713. doi: 10.1111/jdv.13698. [DOI] [PubMed] [Google Scholar]
- 3.Emerging trends in the treatment of advanced basal cell carcinoma. Public Health. 2017;64:1–10. doi: 10.1016/j.ctrv.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 4.García J.B., Suárez-Varela M.M., Vilata J.J., Marquina A., Pallardó L., Crespo J. Risk factors for non-melanoma skin cancer in kidney transplant patients in a Spanish population in the Mediterranean region. Acta Derm. Venereol. 2013;93:422–427. doi: 10.2340/00015555-1525. [DOI] [PubMed] [Google Scholar]
- 5.Chuang T.Y., Popescu N.A., Su W.P., Chute C.G. Squamous cell carcinoma. A population-based incidence study in Rochester, Minn. Arch. Derm. 1990;126:185–188. doi: 10.1001/archderm.1990.01670260055010. [DOI] [PubMed] [Google Scholar]
- 6.Samarasinghe V., Madan V. Nonmelanoma skin cancer. Oncotarget. 2012;5:3–10. doi: 10.4103/0974-2077.94323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuang T.Y., Popescu A., Su W.P., Chute C.G. Basal cell carcinoma. A population-based incidence study in Rochester, Minnesota. J. Am. Acad. Derm. 1990;22:413–417. doi: 10.1016/0190-9622(90)70056-N. [DOI] [PubMed] [Google Scholar]
- 8.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer. J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 9.Cribier B., Scrivener Y., Grosshans E. Tumors arising in nevus sebaceus: A study of 596 cases. Cell. 2000;42:263–268. doi: 10.1016/S0190-9622(00)90136-1. [DOI] [PubMed] [Google Scholar]
- 10.Euvrard S., Kanitakis J., Claudy A. Skin cancers after organ transplantation. N Engl. J. Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 11.Silverberg M.J., Leyden W., Warton E.M., Quesenberry C.P., Engels E.A., Asgari M.M. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J. Natl. Cancer Inst. 2013;105:350–360. doi: 10.1093/jnci/djs529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao S.S., Bolger P.M. Dietary arsenic intakes in the United States: FDA Total Diet Study, September 1991-December 1996. Mol. Nucleic. Acids. 2001;16:465–472. doi: 10.1080/026520399283759. [DOI] [PubMed] [Google Scholar]
- 13.Christenson L.J., Borrowman T.A., Vachon C.M., Tollefson M.M., Otley C.C., Weaver A.L., Roenigk R.K. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 14.Rigel D.S., Friedman R.J., Kopf A.W. Lifetime risk for development of skin cancer in the U.S. population: Current estimate is now 1 in 5. J. Am. Acad. Derm. 1996;35:1012–1013. doi: 10.1016/S0190-9622(96)90139-5. [DOI] [PubMed] [Google Scholar]
- 15.Harms P.W., Harms K.L., Moore P.S., DeCaprio J.A., Nghiem P., Wong M.K.K., Brownell I. The biology and treatment of Merkel cell carcinoma: Current understanding and research priorities. Nat. Rev. Clin. Oncol. 2018;15:763–776. doi: 10.1038/s41571-018-0103-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kripke M.L. Skin cancer, photoimmunology, and urocanic acid. Photo-dermatology. 1984;1:161–163. [PubMed] [Google Scholar]
- 17.Carroll R.P., Ramsay H.M., Fryer A.A., Hawley C.M., Nicol D.L., Harden P.N. Incidence and prediction of nonmelanoma skin cancer post-renal transplantation: A prospective study in Queensland, Australia. Oncotarget. 2003;41:676–683. doi: 10.1053/ajkd.2003.50130. [DOI] [PubMed] [Google Scholar]
- 18.Marzuka A.G., Book S.E. Basal cell carcinoma: Pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J. Biol. Med. 2015;88:167–179. [PMC free article] [PubMed] [Google Scholar]
- 19.Kripke M.L. Immunological unresponsiveness induced by ultraviolet radiation. Immunol. Rev. 1984;80:87–102. doi: 10.1111/j.1600-065X.1984.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 20.Kripke M.L. Effects of methoxsalen plus near-ultraviolet radiation or mid-ultraviolet radiation on immunologic mechanisms. Natl. Cancer Inst. Monogr. 1984;66:247–251. [PubMed] [Google Scholar]
- 21.Moloney F.J., Comber H., Conlon P.J., Murphy G.M. The role of immunosuppression in the pathogenesis of basal cell carcinoma. Br. J. Derm. 2006;154:790–791. doi: 10.1111/j.1365-2133.2006.07156.x. [DOI] [PubMed] [Google Scholar]
- 22.Madan V., Lear J.T., Szeimies R.-M. Non-melanoma skin cancer. Lancet. 2010;375:673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 23.Narayanan D.L., Saladi R.N., Fox J.L. Ultraviolet radiation and skin cancer. Int. J. Derm. 2010;49:978–986. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaskel P., Lange U., Sander S., Huber M.A., Utikal J., Leiter U., Krähn G., Meurer M., Kron M. Ultraviolet exposure and risk of melanoma and basal cell carcinoma in Ulm and Dresden, Germany. J. Eur. Acad. Derm. Venereol. 2014;29:134–142. doi: 10.1111/jdv.12488. [DOI] [PubMed] [Google Scholar]
- 25.Chang N.-B., Feng R., Gao Z., Gao W. Skin cancer incidence is highly associated with ultraviolet-B radiation history. Int. J. Hyg. Environ. Health. 2010;213:359–368. doi: 10.1016/j.ijheh.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Coelho S.G., Choi W., Brenner M., Miyamura Y., Yamaguchi Y., Wolber R., Smuda C., Batzer J., Kolbe L., Ito S., et al. Short- and long-term effects of UV radiation on the pigmentation of human skin. J. Investig. Derm. Symp. Proc. 2009;14:32–35. doi: 10.1038/jidsymp.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamin C.L., Melnikova V.O., Ananthaswamy H.N. P53 protein and pathogenesis of melanoma and nonmelanoma skin cancer. Adv. Exp. Med. Biol. 2008;624:265–282. doi: 10.1007/978-0-387-77574-6_21. [DOI] [PubMed] [Google Scholar]
- 28.Marshall S.E., Bordea C., Haldar N.A., Mullighan C.G., Wojnarowska F., Morris P.J., Welsh K.I. Glutathione S-transferase polymorphisms and skin cancer after renal transplantation. Kidney Int. 2000;58:2186–2193. doi: 10.1111/j.1523-1755.2000.00392.x. [DOI] [PubMed] [Google Scholar]
- 29.Cameron M.C., Lee E., Hibler B.P., Barker C.A., Mori S., Cordova M., Nehal K.S., Rossi A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Derm. 2018;80:303–317. doi: 10.1016/j.jaad.2018.03.060. [DOI] [PubMed] [Google Scholar]
- 30.de Sá T.R.C., Silva R., Lopes J.M. Basal cell carcinoma of the skin (part 1): Epidemiology, pathology and genetic syndromes. Future Oncol. 2015;11:3011–3021. doi: 10.2217/fon.15.246. [DOI] [PubMed] [Google Scholar]
- 31.Bresler S.C., Padwa B.L., Granter S.R. Nevoid Basal Cell Carcinoma Syndrome (Gorlin Syndrome) Head Neck Pathol. 2016;10:119–124. doi: 10.1007/s12105-016-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scales S.J., de Sauvage F.J. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharm. Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Wong S.Y., Reiter J.F. The primary cilium at the crossroads of mammalian hedgehog signaling. Curr. Top. Dev. Biol. 2009;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.di Magliano M.P., Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat. Rev. Cancer. 2004;3:903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 35.Otsuka A., Levesque M.P., Dummer R., Kabashima K. Hedgehog signaling in basal cell carcinoma. Trends Immunol. 2015;78:95–100. doi: 10.1016/j.jdermsci.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Raleigh D.R., Choksi P.K., Krup A.L., Mayer W., Santos N., Reiter J.F. Hedgehog signaling drives medulloblastoma growth via CDK6. Nat. Med. 2017;128:120–124. doi: 10.1172/JCI92710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monkkonen T., Lewis M.T. New paradigms for the Hedgehog signaling network in mammary gland development and breast Cancer. Biochim. Biophys. Acta Rev. Cancer. 2017;1868:315–332. doi: 10.1016/j.bbcan.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giroux-Leprieur E., Costantini A., Ding V.W., He B. Hedgehog Signaling in Lung Cancer: From Oncogenesis to Cancer Treatment Resistance. Int. J. Mol. Sci. 2018;19:2835. doi: 10.3390/ijms19092835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bushman W. Hedgehog Signaling in Prostate Development, Regeneration and Cancer. J. Dev. Biol. 2016;4:30. doi: 10.3390/jdb4040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song L., Li Z.-Y., Liu W.-P., Zhao M.-R. Crosstalk between Wnt/β-catenin and Hedgehog/Gli signaling pathways in colon cancer and implications for therapy. Cancer Biol. 2015;16:1–7. doi: 10.4161/15384047.2014.972215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai Y., Bai Y., Dong J., Li Q., Jin Y., Chen B., Zhou M. Hedgehog Signaling in Pancreatic Fibrosis and Cancer. Medicine (Baltimore) 2016;95:e2996. doi: 10.1097/MD.0000000000002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cortes J.E., Gutzmer R., Kieran M.W., Solomon J.A. Hedgehog signaling inhibitors in solid and hematological cancers. Cancer Treat. Rev. 2019;76:41–50. doi: 10.1016/j.ctrv.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Gonnissen A., Isebaert S., Haustermans K. Targeting the Hedgehog signaling pathway in cancer: Beyond Smoothened. Oncotarget. 2015;6:13899–13913. doi: 10.18632/oncotarget.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeng K.-S., Chang C.-F., Lin S.-S. Sonic Hedgehog Signaling in Organogenesis, Tumors, and Tumor Microenvironments. Int. J. Mol. Sci. 2020;21:758. doi: 10.3390/ijms21030758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta S., Takebe N., Lorusso P. Targeting the Hedgehog pathway in cancer. Adv. Med. Oncol. 2011;2:237–250. doi: 10.1177/1758834010366430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C., Chi S., Xie J. Hedgehog signaling in skin cancers. J. Control. Release. 2011;23:1235–1243. doi: 10.1016/j.cellsig.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonilla X., Parmentier L., King B., Bezrukov F., Kaya G., Zoete V., Seplyarskiy V.B., Sharpe H.J., McKee T., Letourneau A., et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat. Genet. 2016;48:398–406. doi: 10.1038/ng.3525. [DOI] [PubMed] [Google Scholar]
- 48.Pellegrini C., Maturo M.G., Di Nardo L., Ciciarelli V., García-Rodrigo C.G., Fargnoli M.C. Understanding the Molecular Genetics of Basal Cell Carcinoma. Int. J. Mol. Sci. 2017;18:2485. doi: 10.3390/ijms18112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCusker M., Basset-Seguin N., Dummer R., Lewis K., Schadendorf D., Sekulic A., Hou J., Wang L., Yue H., Hauschild A. Metastatic basal cell carcinoma: Prognosis dependent on anatomic site and spread of disease. Trends Pharm. Sci. 2014;50:774–783. doi: 10.1016/j.ejca.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 50.Wong S.L., Kattan M.W., McMasters K.M., Coit D.G. A nomogram that predicts the presence of sentinel node metastasis in melanoma with better discrimination than the American Joint Committee on Cancer staging system. Ann. Surg. Oncol. 2005;12:282–288. doi: 10.1245/ASO.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 51.Staples M.P., Elwood M., Burton R.C., Williams J.L., Marks R., Giles G.G. Non-melanoma skin cancer in Australia: The 2002 national survey and trends since 1985. Med. J. Aust. 2006;184:6–10. doi: 10.5694/j.1326-5377.2006.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 52.Richmond-Sinclair N.M., Pandeya N., Ware R.S., Neale R.E., Williams G.M., van der Pols J.C., Green A.C. Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: Longitudinal study of an Australian population. J. Investig. Derm. Symp. Proc. 2008;129:323–328. doi: 10.1038/jid.2008.234. [DOI] [PubMed] [Google Scholar]
- 53.Zoledronic acid prevents vertebral fractures in men with osteoporosis. Bonekey Rep. 2013;2:294. doi: 10.1038/bonekey.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lomas A., Leonardi-Bee J., Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Derm. 2012;166:1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 55.Trakatelli M., Morton C., Nagore E., Ulrich C., Del Marmol V., Peris K., Basset-Seguin N. Update of the European guidelines for basal cell carcinoma management. Eur. J. Derm. 2014;24:312–329. doi: 10.1684/ejd.2014.2271. [DOI] [PubMed] [Google Scholar]
- 56.Kauvar A.N.B., Cronin T., Roenigk R., Hruza G., Bennett R. Consensus for nonmelanoma skin cancer treatment: Basal cell carcinoma, including a cost analysis of treatment methods. Derm. Surg. 2015;41:550–571. doi: 10.1097/DSS.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 57.Di Stefani A., Chimenti S. Basal cell carcinoma: Clinical and pathological features. G Ital. Derm. Venereol. 2015;150:385–391. [PubMed] [Google Scholar]
- 58.Cullen R., Hasbún P., Campos-Villenas M. Superficial basal cell carcinoma. Exp. Hematol. 2016;149:140. doi: 10.1016/j.medcle.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 59.Abbas O., Richards J.E., Mahalingam M. Fibroblast-activation protein: A single marker that confidently differentiates morpheaform/infiltrative basal cell carcinoma from desmoplastic trichoepithelioma. Mod. Pathol. 2010;23:1535–1543. doi: 10.1038/modpathol.2010.142. [DOI] [PubMed] [Google Scholar]
- 60.Betti R., Menni S., Radaelli G., Bombonato C., Crosti C. Micronodular basal cell carcinoma: A distinct subtype? Relationship with nodular and infiltrative basal cell carcinomas. J. Derm. 2010;37:611–616. doi: 10.1111/j.1346-8138.2009.00772.x. [DOI] [PubMed] [Google Scholar]
- 61.Tan C.Z., Rieger K.E., Sarin K.Y. Basosquamous Carcinoma: Controversy, Advances, and Future Directions. Derm. Surg. 2016;43:23–31. doi: 10.1097/DSS.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 62.Drucker A.M., Adam G.P., Rofeberg V., Gazula A., Smith B., Moustafa F., Weinstock M.A., Trikalinos T.A. Treatments of Primary Basal Cell Carcinoma of the Skin: A Systematic Review and Network Meta-analysis. Ann. Intern. Med. 2018;169:456–466. doi: 10.7326/M18-0678. [DOI] [PubMed] [Google Scholar]
- 63.Connolly S.M., Baker D.R., Coldiron B.M., Fazio M.J., Storrs P.A., Vidimos A.T., Zalla M.J., Brewer J.D., Begolka W.S., Berger T.G., et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: A report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J. Control. Release. 2012;67:531–550. [Google Scholar]
- 64.Lazarevic D., Ramelyte E., Dummer R., Imhof L. Radiotherapy in Periocular Cutaneous Malignancies: A Retrospective Study. Dermatology (Basel) 2019;235:234–239. doi: 10.1159/000496539. [DOI] [PubMed] [Google Scholar]
- 65.Guinot J.L., Rembielak A., Perez-Calatayud J., Rodríguez-Villalba S., Skowronek J., Tagliaferri L., Guix B., Gonzalez-Perez V., Valentini V., Kovacs G. GEC-ESTRO ACROP recommendations in skin brachytherapy. Radiother. Oncol. 2018;126:377–385. doi: 10.1016/j.radonc.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 66.Peris K., Fargnoli M.C., Garbe C., Kaufmann R., Bastholt L., Seguin N.B., Bataille V., Del Marmol V., Dummer R., Harwood C.A., et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer. 2019;118:10–34. doi: 10.1016/j.ejca.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Dummer R., Guminski A., Gutzmer R., Dirix L., Lewis K.D., Combemale P., Herd R.M., Kaatz M., Loquai C., Stratigos A.J. The 12-month analysis from Basal Cell Carcinoma Outcomes with LDE225 Treatment (BOLT): A phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J. Am. Acad. Derm. 2016;75:113–125. doi: 10.1016/j.jaad.2016.02.1226. [DOI] [PubMed] [Google Scholar]
- 68.Sekulic A., Migden M.R., Oro A.E., Dirix L., Lewis K.D., Hainsworth J.D., Solomon J.A., Yoo S., Arron S.T., Friedlander P.A., et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dummer R., Guminksi A., Gutzmer R., Lear J.T., Lewis K.D., Chang A.L.S., Combemale P., Dirix L., Kaatz M., Kudchadkar R., et al. Long-term efficacy and safety of sonidegib in patients with advanced basal cell carcinoma: 42-month analysis of the phase II randomized, double-blind BOLT study. Br. J. Derm. 2019;182:1369–1378. doi: 10.1111/bjd.18552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Migden M.R., Guminski A., Gutzmer R., Dirix L., Lewis K.D., Combemale P., Herd R.M., Kudchadkar R., Trefzer U., Gogov S., et al. Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): A multicentre, randomised, double-blind phase 2 trial. Lancet Oncol. 2015;16:716–728. doi: 10.1016/S1470-2045(15)70100-2. [DOI] [PubMed] [Google Scholar]
- 71.Danial C., Sarin K.Y., Oro A.E., Chang A.L.S. An Investigator-Initiated Open-Label Trial of Sonidegib in Advanced Basal Cell Carcinoma Patients Resistant to Vismodegib. Clin. Cancer Res. 2015;22:1325–1329. doi: 10.1158/1078-0432.CCR-15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cortes J.E., Smith B.D., Wang E.S., Merchant A., Oehler V.G., Arellano M., DeAngelo D.J., Pollyea D.A., Sekeres M.A., Robak T., et al. Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: Phase 2 study results. Oncotarget. 2018;93:1301–1310. doi: 10.1002/ajh.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Basset-Séguin N., Hauschild A., Kunstfeld R., Grob J., Dréno B., Mortier L., Ascierto P.A., Licitra L., Dutriaux C., Thomas L., et al. Vismodegib in patients with advanced basal cell carcinoma: Primary analysis of STEVIE, an international, open-label trial. J. Control. Release. 2017;86:334–348. doi: 10.1016/j.ejca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 74.Sharpe H.J., Pau G., Dijkgraaf G.J., Basset-Seguin N., Modrusan Z., Januario T., Tsui V., Durham A.B., Dlugosz A.A., Haverty P.M., et al. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Trends Immunol. 2015;27:327–341. doi: 10.1016/j.ccell.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodon J., Tawbi H.A., Thomas A.L., Stoller R.G., Turtschi C.P., Baselga J., Sarantopoulos J., Mahalingam D., Shou Y., Moles M.A., et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin. Cancer Res. 2014;20:1900–1909. doi: 10.1158/1078-0432.CCR-13-1710. [DOI] [PubMed] [Google Scholar]
- 76.Dréno B., Kunstfeld R., Hauschild A., Fosko S., Zloty D., Labeille B., Grob J.-J., Puig S., Gilberg F., Bergström D., et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): A randomised, regimen-controlled, double-blind, phase 2 trial. Oncotarget. 2017;18:404–412. doi: 10.1016/S1470-2045(17)30072-4. [DOI] [PubMed] [Google Scholar]
- 77.Lear J.T., Migden M.R., Lewis K.D., Chang A.L.S., Guminski A., Gutzmer R., Dirix L., Combemale P., Stratigos A., Plummer R., et al. Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J. Eur. Acad. Derm. Venereol. 2017;32:372–381. doi: 10.1111/jdv.14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bridge J.A., Lee J.C., Daud A., Wells J.W., Bluestone J.A. Cytokines, Chemokines, and Other Biomarkers of Response for Checkpoint Inhibitor Therapy in Skin Cancer. Front. Immunol. 2018;5:351. doi: 10.3389/fmed.2018.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buonamici S., Williams J., Morrissey M., Wang A., Guo R., Vattay A., Hsiao K., Yuan J., Green J., Ospina B., et al. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci. Transl. Med. 2010;2:51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao X., Pak E., Ornell K.J., Pazyra-Murphy M.F., MacKenzie E.L., Chadwick E.J., Ponomaryov T., Kelleher J.F., Segal R.A. A Transposon Screen Identifies Loss of Primary Cilia as a Mechanism of Resistance to SMO Inhibitors. Cancer Discov. 2017;7:1436–1449. doi: 10.1158/2159-8290.CD-17-0281. [DOI] [PubMed] [Google Scholar]
- 81.Gruber W., Hutzinger M., Elmer D.P., Parigger T., Sternberg C., Cegielkowski L., Zaja M., Leban J., Michel S., Hamm S., et al. DYRK1B as therapeutic target in Hedgehog/GLI-dependent cancer cells with Smoothened inhibitor resistance. Oncotarget. 2016;7:7134–7148. doi: 10.18632/oncotarget.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim J., Aftab B.T., Tang J.Y., Kim D., Lee A.H., Rezaee M., Kim J., Chen B., King E.M., Borodovsky A., et al. Itraconazole and arsenic trioxide inhibit Hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leiter U., Keim U., Eigentler T., Katalinic A., Holleczek B., Martus P., Garbe C. Incidence, Mortality, and Trends of Nonmelanoma Skin Cancer in Germany. Eur. J. Cancer. 2017;137:1860–1867. doi: 10.1016/j.jid.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 84.Stratigos A.J., Garbe C., Dessinioti C., Bataille V., Bastholt L., Fargnoli M.C., Forsea A.M., Frenard C., Harwood C.A., Hauschild A., et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2. Treatment. J. Control. Release. 2020;128:60–82. doi: 10.1016/j.ejca.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Tate J.G., Bamford S., Jubb H.C., Sondka Z., Beare D.M., Bindal N., Boutselakis H., Cole C.G., Creatore C., Dawson E., et al. COSMIC: The Catalogue of Somatic Mutations In Cancer. Nucleic. Acids Res. 2018;47:D941–D947. doi: 10.1093/nar/gky1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y.Y., Hanna G.J., Laga A.C., Haddad R.I., Lorch J.H., Hammerman P.S. Genomic analysis of metastatic cutaneous squamous cell carcinoma. Clin. Cancer Res. 2015;21:1447–1456. doi: 10.1158/1078-0432.CCR-14-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ventura A., Pellegrini C., Cardelli L., Rocco T., Ciciarelli V., Peris K., Fargnoli M.C. Telomeres and Telomerase in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019;20:1333. doi: 10.3390/ijms20061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.García-Sancha N., Corchado-Cobos R., Pérez-Losada J., Cañueto J. MicroRNA Dysregulation in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019;20:2181. doi: 10.3390/ijms20092181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yilmaz A.S., Ozer H.G., Gillespie J.L., Allain D.C., Bernhardt M.N., Furlan K.C., Castro L.T.F., Peters S.B., Nagarajan P., Kang S.Y., et al. Differential mutation frequencies in metastatic cutaneous squamous cell carcinomas versus primary tumors. Cancer. 2016;123:1184–1193. doi: 10.1002/cncr.30459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cho R.J., Alexandrov L.B., Breems N.Y., Atanasova V.S., Farshchian M., Purdom E., Nguyen T.N., Coarfa C., Rajapakshe K., Prisco M., et al. APOBEC mutation drives early-onset squamous cell carcinomas in recessive dystrophic epidermolysis bullosa. Sci. Transl. Med. 2018;10:1126. doi: 10.1126/scitranslmed.aas9668. [DOI] [PubMed] [Google Scholar]
- 91.Pickering C.R., Zhou J.H., Lee J.J., Drummond J.A., Peng S.A., Saade R.E., Tsai K.Y., Curry J.L., Tetzlaff M.T., Lai S.Y., et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin. Cancer Res. 2014;20:6582–6592. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martincorena I., Roshan A., Gerstung M., Ellis P., Van Loo P., McLaren S., Wedge D.C., Fullam A., Alexandrov L.B., Tubio J.M., et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wahab A.A., Bener A., Teebi A.S. The incidence patterns of Down syndrome in Qatar. Clin. Genet. 2006;69:360–362. doi: 10.1111/j.1399-0004.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- 94.Chantrain C.F., Henriet P., Jodele S., Emonard H., Feron O., Courtoy P.J., DeClerck Y.A., Marbaix E. Mechanisms of pericyte recruitment in tumour angiogenesis: A new role for metalloproteinases. Nat. Med. 2006;42:310–318. doi: 10.1016/j.ejca.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 95.Dirschka T., Gupta G., Micali G., Stockfleth E., Dummer R., Jemec G.B.E., Malvehy J., Peris K., Puig S., Stratigos A.J., et al. Real-world approach to actinic keratosis management: Practical treatment algorithm for office-based dermatology. J. Dermatol. Treat. 2016;28:431–442. doi: 10.1080/09546634.2016.1254328. [DOI] [PubMed] [Google Scholar]
- 96.Stratigos A., Garbe C., Malvehy J., Del Marmol V., Pehamberger H., Peris K., Becker J.C., Zalaudek I., Saiag P., Middleton M.R., et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur. J. Cancer. 2015;51:1989–2007. doi: 10.1016/j.ejca.2015.06.110. [DOI] [PubMed] [Google Scholar]
- 97.Stratigos A.J., Garbe C., Dessinioti C., Bataille V., Bastholt L., Fargnoli M.C., Forsea A.M., Frenard C., Harwood C.A., Hauschild A., et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 1. epidemiology, diagnostics and prevention. J. Control. Release. 2020;128:60–82. doi: 10.1016/j.ejca.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 98.Riihilä P., Nissinen L., Knuutila J., Nezhad P.R., Viiklepp K., Kähäri V.-M. Complement System in Cutaneous Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019;20:3550. doi: 10.3390/ijms20143550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mittal D., Gubin M.M., Schreiber R.D., Smyth M.J. New insights into cancer immunoediting and its three component phases--elimination, equilibrium and escape. Curr. Opin. Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bernard J.J., Gallo R.L., Krutmann J. Photoimmunology: How ultraviolet radiation affects the immune system. Nat. Rev. Immunol. 2019;19:688–701. doi: 10.1038/s41577-019-0185-9. [DOI] [PubMed] [Google Scholar]
- 101.Motwani M.P., Gilroy D.W. Macrophage development and polarization in chronic inflammation. J. Control. Release. 2015;27:257–266. doi: 10.1016/j.smim.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 102.Ji A.L., Rubin A.J., Thrane K., Jiang S., Reynolds D.L., Meyers R.M., Guo M.G., George B.M., Mollbrink A., Bergenstråhle J., et al. Multimodal Analysis of Composition and Spatial Architecture in Human Squamous Cell Carcinoma. Cell. 2020;182:497–514. doi: 10.1016/j.cell.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tucci M., Ciavarella S., Strippoli S., Brunetti O., Dammacco F., Silvestris F. Immature dendritic cells from patients with multiple myeloma are prone to osteoclast differentiation in vitro. Exp. Hematol. 2011;39:773–783. doi: 10.1016/j.exphem.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 104.Fernandez T.L., Van Lonkhuyzen D.R., Dawson R.A., Kimlin M.G., Upton Z. Characterization of a human skin equivalent model to study the effects of ultraviolet B radiation on keratinocytes. Tissue Eng. Part. C Methods. 2014;20:588–598. doi: 10.1089/ten.tec.2013.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bald T., Quast T., Landsberg J., Rogava M., Glodde N., Lopez-Ramos D., Kohlmeyer J., Riesenberg S., van den Boorn-Konijnenberg D., Hömig-Hölzel C., et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature. 2014;507:109–113. doi: 10.1038/nature13111. [DOI] [PubMed] [Google Scholar]
- 106.Norval M., Simpson T.J., Ross J.A. Urocanic acid and immunosuppression. Photochem. Photobiol. 1989;50:267–275. doi: 10.1111/j.1751-1097.1989.tb04159.x. [DOI] [PubMed] [Google Scholar]
- 107.Freedman R.R., Woodward S., Sabharwal S.C. Alpha 2-adrenergic mechanism in menopausal hot flushes. Obs. Gynecol. 1990;76:573–578. [PubMed] [Google Scholar]
- 108.Szélig L., Kun S., Woth G., Molnár G.A., Zrínyi Z., Kátai E., Lantos J., Wittmann I., Bogár L., Miseta A., et al. Time courses of changes of para-, meta-, and ortho-tyrosine in septic patients: A pilot study. Redox Rep. 2016;21:180–189. doi: 10.1179/1351000215Y.0000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tucci M., Mannavola F., Passarelli A., Stucci L.S., Cives M., Silvestris F. Exosomes in melanoma: A role in tumor progression, metastasis and impaired immune system activity. Oncotarget. 2018;9:20826–20837. doi: 10.18632/oncotarget.24846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mannavola F., Tucci M., Felici C., Passarelli A., D’Oronzo S., Silvestris F. Tumor-derived exosomes promote the in vitro osteotropism of melanoma cells by activating the SDF-1/CXCR4/CXCR7 axis. J. Transl. Med. 2019;17:230. doi: 10.1186/s12967-019-1982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mannavola F., Pezzicoli G., Tucci M. DLC-1 down-regulation via exosomal miR-106b-3p exchange promotes CRC metastasis by the epithelial-to-mesenchymal transition. Mol. Nucleic. Acids. 2020;134:955–959. doi: 10.1042/CS20200181. [DOI] [PubMed] [Google Scholar]
- 112.Mannavola F., D’Oronzo S., Cives M., Stucci L.S., Ranieri G., Silvestris F., Tucci M. Extracellular Vesicles and Epigenetic Modifications Are Hallmarks of Melanoma Progression. Int. J. Mol. Sci. 2019;21:52. doi: 10.3390/ijms21010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mannavola F., Salerno T., Passarelli A., Tucci M., Internò V., Silvestris F. Revisiting the Role of Exosomes in Colorectal Cancer: Where Are We Now? Front. Oncol. 2019;9:521. doi: 10.3389/fonc.2019.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tucci M., Stucci L.S., Mannavola F., Passarelli A., D’Oronzo S., Lospalluti L., Giudice G., Silvestris F. Defective levels of both circulating dendritic cells and T-regulatory cells correlate with risk of recurrence in cutaneous melanoma. Clin. Transl. Oncol. 2019;21:845–854. doi: 10.1007/s12094-018-1993-2. [DOI] [PubMed] [Google Scholar]
- 115.Tucci M., Passarelli A., Mannavola F., Stucci L.S., Ascierto P.A., Capone M., Madonna G., Lopalco P., Silvestris F. Serum exosomes as predictors of clinical response to ipilimumab in metastatic melanoma. Oncoimmunology. 2017;7:e1387706. doi: 10.1080/2162402X.2017.1387706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gentles A.J., Newman A.M., Liu C.L., Bratman S.V., Feng W., Kim D., Nair V.S., Xu Y., Khuong A., Hoang C.D., et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Inman G.J., Wang J., Nagano A., Alexandrov L.B., Purdie K.J., Taylor R.G., Sherwood V., Thomson J., Hogan S., Spender L.C., et al. The genomic landscape of cutaneous SCC reveals drivers and a novel azathioprine associated mutational signature. Nat. Commun. 2018;9:3667. doi: 10.1038/s41467-018-06027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mueller S.A., Gauthier M.-E.A., Ashford B., Gupta R., Gayevskiy V., Ch’ng S., Palme C.E., Shannon K., Clark J.R., Ranson M., et al. Mutational Patterns in Metastatic Cutaneous Squamous Cell Carcinoma. J. Investig. Derm. 2019;139:1449–1458.e1. doi: 10.1016/j.jid.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 119.Migden M.R., Khushalani N.I., Chang A.L.S., Lewis K.D., Schmults C.D., Hernandez-Aya L., Meier F., Schadendorf D., Guminski A., Hauschild A., et al. Cemiplimab in locally advanced cutaneous squamous cell carcinoma: Results from an open-label, phase 2, single-arm trial. Lancet Oncol. 2020;21:294–305. doi: 10.1016/S1470-2045(19)30728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hernández-Guerrero T., Doger B., Moreno V. Cemiplimab for the treatment of advanced cutaneous squamous cell carcinoma. Drugs Today. 2019;55:485–494. doi: 10.1358/dot.2019.55.8.3005176. [DOI] [PubMed] [Google Scholar]
- 121.Migden M.R., Rischin D., Schmults C.D., Guminski A., Hauschild A., Lewis K.D., Chung C.H., Hernandez-Aya L., Lim A.M., Chang A.L.S., et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018;379:341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 122.Bertino G., Sersa G., De Terlizzi F., Occhini A., Plaschke C.C., Groselj A., Langdon C., Grau J.J., McCaul J.A., Heuveling D., et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur. J. Cancer. 2016;63:41–52. doi: 10.1016/j.ejca.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 123.Sackstein R., Schatton T., Barthel S.R. T-lymphocyte homing: An underappreciated yet critical hurdle for successful cancer immunotherapy. Lab. Investig. 2017;97:669–697. doi: 10.1038/labinvest.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simon B., Uslu U. CAR-T cell therapy in melanoma: A future success story? Exp. Derm. 2018;27:1315–1321. doi: 10.1111/exd.13792. [DOI] [PubMed] [Google Scholar]
- 125.Simon B., Harrer D.C., Schuler-Thurner B., Schaft N., Schuler G., Dörrie J., Uslu U. The siRNA-mediated downregulation of PD-1 alone or simultaneously with CTLA-4 shows enhanced in vitro CAR-T-cell functionality for further clinical development towards the potential use in immunotherapy of melanoma. Exp. Derm. 2018;27:769–778. doi: 10.1111/exd.13678. [DOI] [PubMed] [Google Scholar]
- 126.Paulson K.G., Park S.Y., Vandeven N.A., Lachance K., Thomas H., Chapuis A.G., Harms K.L., Thompson J.A., Bhatia S., Stang A., et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Derm. 2017;78:457–463.e2. doi: 10.1016/j.jaad.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fondain M., Dereure O., Uhry Z., Guizard A.V., Woronoff A.S., Colonna M., Molinie F., Bara S., Velten M., Marrer E., et al. Merkel cell carcinoma in France: A registries-based, comprehensive epidemiological survey. J. Eur. Acad. Derm. Venereol. 2018;32:1292–1296. doi: 10.1111/jdv.14798. [DOI] [PubMed] [Google Scholar]
- 128.Youlden D.R., Soyer H.P., Youl P.H., Fritschi L., Baade P.D. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993–2010. JAMA Derm. 2014;150:864–872. doi: 10.1001/jamadermatol.2014.124. [DOI] [PubMed] [Google Scholar]
- 129.Reichgelt B.A., Visser O. Epidemiology and survival of Merkel cell carcinoma in the Netherlands. A population-based study of 808 cases in 1993–2007. Mol. Nucleic. Acids. 2010;47:579–585. doi: 10.1016/j.ejca.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 130.Albores-Saavedra J., Batich K., Chable-Montero F., Sagy N., Schwartz A.M., Henson D.E. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: A population based study. J. Cutan. Pathol. 2009;37:20–27. doi: 10.1111/j.1600-0560.2009.01370.x. [DOI] [PubMed] [Google Scholar]
- 131.Stang A., Becker J.C., Nghiem P., Ferlay J. The association between geographic location and incidence of Merkel cell carcinoma in comparison to melanoma: An international assessment. J. Control. Release. 2018;94:47–60. doi: 10.1016/j.ejca.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Koljonen V., Kukko H., Pukkala E., Sankila R., Böhling T., Tukiainen E., Sihto H., Joensuu H. Chronic lymphocytic leukaemia patients have a high risk of Merkel-cell polyomavirus DNA-positive Merkel-cell carcinoma. Br. J. Cancer. 2009;101:1444–1447. doi: 10.1038/sj.bjc.6605306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feng H., Shuda M., Chang Y., Moore P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tolstov Y.L., Pastrana D.V., Feng H., Becker J.C., Jenkins F.J., Moschos S., Chang Y., Buck C.B., Moore P.S. Human Merkel cell polyomavirus infection II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int. J. Cancer. 2009;125:1250–1256. doi: 10.1002/ijc.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Longino N.V., Yang J., Iyer J.G., Ibrani D., Chow I.-T., Laing K.J., Campbell V.L., Paulson K.G., Kulikauskas R.M., Church C.D., et al. Human CD4+ T Cells Specific for Merkel Cell Polyomavirus Localize to Merkel Cell Carcinomas and Target a Required Oncogenic Domain. Cancer Immunol. Res. 2019;7:1727–1739. doi: 10.1158/2326-6066.CIR-19-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Iyer J.G., Afanasiev O.K., McClurkan C., Paulson K., Nagase K., Jing L., Marshak J.O., Dong L., Carter J., Lai I., et al. Merkel cell polyomavirus-specific CD8⁺ and CD4⁺ T-cell responses identified in Merkel cell carcinomas and blood. Clin. Cancer Res. 2011;17:6671–6680. doi: 10.1158/1078-0432.CCR-11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Afanasiev O.K., Yelistratova L., Miller N., Nagase K., Paulson K., Iyer J.G., Ibrani D., Koelle D.M., Nghiem P. Merkel polyomavirus-specific T cells fluctuate with merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin. Cancer Res. 2013;19:5351–5360. doi: 10.1158/1078-0432.CCR-13-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jing L., Ott M., Church C.D., Kulikauskas R.M., Ibrani D., Iyer J.G., Afanasiev O.K., Colunga A., Cook M.M., Xie H., et al. Prevalent and Diverse Intratumoral Oncoprotein-Specific CD8+ T Cells within Polyomavirus-Driven Merkel Cell Carcinomas. Cancer Immunol. Res. 2020;8:648–659. doi: 10.1158/2326-6066.CIR-19-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Colunga A., Pulliam T., Nghiem P. Merkel Cell Carcinoma in the Age of Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2017;24:2035–2043. doi: 10.1158/1078-0432.CCR-17-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Paulson K.G., Tegeder A., Willmes C., Iyer J.G., Afanasiev O.K., Schrama D., Koba S., Thibodeau R., Nagase K., Simonson W.T., et al. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol. Res. 2014;2:1071–1079. doi: 10.1158/2326-6066.CIR-14-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dabner M., McClure R.J., Harvey N.T., Budgeon C.A., Beer T.W., Amanuel B., Wood B.A. Merkel cell polyomavirus and p63 status in Merkel cell carcinoma by immunohistochemistry: Merkel cell polyomavirus positivity is inversely correlated with sun damage, but neither is correlated with outcome. Pathology. 2014;46:205–210. doi: 10.1097/PAT.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 142.Garneski K.M., Warcola A.H., Feng Q., Kiviat N.B., Leonard J.H., Nghiem P. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J. Investig. Derm. Symp. Proc. 2008;129:246–248. doi: 10.1038/jid.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mangana J., Dziunycz P., Kerl K., Dummer R., Cozzio A. Prevalence of Merkel cell polyomavirus among Swiss Merkel cell carcinoma patients. Dermatology (Basel) 2010;221:184–188. doi: 10.1159/000315067. [DOI] [PubMed] [Google Scholar]
- 144.Wong S.Q., Waldeck K., Vergara I.A., Schröder J., Madore J., Wilmott J.S., Colebatch A.J., De Paoli-Iseppi R., Li J., Lupat R., et al. UV-Associated Mutations Underlie the Etiology of MCV-Negative Merkel Cell Carcinomas. Cancer Res. 2015;75:5228–5234. doi: 10.1158/0008-5472.CAN-15-1877. [DOI] [PubMed] [Google Scholar]
- 145.Goh G., Walradt T., Markarov V., Blom A., Riaz N., Doumani R., Stafstrom K., Moshiri A., Yelistratova L., Levinsohn J., et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2015;7:3403–3415. doi: 10.18632/oncotarget.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Knepper T.C., Montesion M., Russell J.S., Sokol E.S., Frampton G.M., Miller V.A., Albacker L.A., McLeod H.L., Eroglu Z., Khushalani N.I., et al. The Genomic Landscape of Merkel Cell Carcinoma and Clinicogenomic Biomarkers of Response to Immune Checkpoint Inhibitor Therapy. Clin. Cancer Res. 2019;25:5961–5971. doi: 10.1158/1078-0432.CCR-18-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Harms K.L., de la Vega L.L., Hovelson D.H., Rahrig S., Cani A.K., Liu C.-J., Fullen D.R., Wang M., Andea A.A., Bichakjian C.K., et al. Molecular Profiling of Multiple Primary Merkel Cell Carcinoma to Distinguish Genetically Distinct Tumors from Clonally Related Metastases. JAMA Derm. 2017;153:505–512. doi: 10.1001/jamadermatol.2017.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Harms P.W., Vats P., Verhaegen M.E., Robinson D.R., Wu Y.-M., Dhanasekaran S.M., Palanisamy N., Siddiqui J., Cao X., Su F., et al. The Distinctive Mutational Spectra of Polyomavirus-Negative Merkel Cell Carcinoma. Cancer Res. 2015;75:3720–3727. doi: 10.1158/0008-5472.CAN-15-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harms K.L., Healy M.A., Nghiem P., Sober A.J., Johnson T.M., Bichakjian C.K., Wong S.L. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann. Surg. Oncol. 2016;23:3564–3571. doi: 10.1245/s10434-016-5266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vandeven N., Lewis C.W., Makarov V., Riaz N., Paulson K.G., Hippe D., Bestick A., Doumani R., Marx T., Takagishi S., et al. Merkel Cell Carcinoma Patients Presenting Without a Primary Lesion Have Elevated Markers of Immunity, Higher Tumor Mutation Burden, and Improved Survival. Clin. Cancer Res. 2017;24:963–971. doi: 10.1158/1078-0432.CCR-17-1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Becker J.C., Stang A., DeCaprio J.A., Cerroni L., Veness M., Nghiem P. Merkel cell carcinoma. Nat. Rev. Dis. Primers. 2017;3:17077. doi: 10.1038/nrdp.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen K.T., Papavasiliou P., Edwards K., Zhu F., Perlis C., Wu H., Turaka A., Berger A., Farma J.M. A better prognosis for Merkel cell carcinoma of unknown primary origin. J. Control. Release. 2013;206:752–757. doi: 10.1016/j.amjsurg.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 153.Matos L.L., Dedivitis R.A., Kulcsar M.A.V., de Mello E.S., Alves V.A.F., Cernea C.R. External validation of the AJCC Cancer Staging Manual, 8th edition, in an independent cohort of oral cancer patients. J. Control. Release. 2017;71:47–53. doi: 10.1016/j.oraloncology.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 154.Concannon R., Larcos G.S., Veness M. The impact of (18)F-FDG PET-CT scanning for staging and management of Merkel cell carcinoma: Results from Westmead Hospital, Sydney, Australia. Eur. J. Cancer. 2010;62:76–84. doi: 10.1016/j.jaad.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 155.Samimi M., Molet L., Fleury M., Laude H., Carlotti A., Gardair C., Baudin M., Gouguet L., Maubec E., Avenel-Audran M., et al. Prognostic value of antibodies to Merkel cell polyomavirus T antigens and VP1 protein in patients with Merkel cell carcinoma. Br. J. Derm. 2016;174:813–822. doi: 10.1111/bjd.14313. [DOI] [PubMed] [Google Scholar]
- 156.Paulson K.G., Carter J.J., Johnson L.G., Cahill K.W., Iyer J.G., Schrama D., Becker J.C., Madeleine M.M., Nghiem P., Galloway D.A. Antibodies to merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in merkel cell carcinoma patients. Cancer Res. 2010;70:8388–8397. doi: 10.1158/0008-5472.CAN-10-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Harrington C., Kwan W. Outcomes of Merkel cell carcinoma treated with radiotherapy without radical surgical excision. Ann. Surg. Oncol. 2014;21:3401–3405. doi: 10.1245/s10434-014-3757-8. [DOI] [PubMed] [Google Scholar]
- 158.Strom T., Carr M., Zager J.S., Naghavi A., Smith F.O., Cruse C.W., Messina J.L., Russell J., Rao N.G., Fulp W., et al. Radiation Therapy is Associated with Improved Outcomes in Merkel Cell Carcinoma. Ann. Surg. Oncol. 2016;23:3572–3578. doi: 10.1245/s10434-016-5293-1. [DOI] [PubMed] [Google Scholar]
- 159.Bhatia S., Storer B.E., Iyer J.G., Moshiri A., Parvathaneni U., Byrd D., Sober A.J., Sondak V.K., Gershenwald J.E., Nghiem P. Adjuvant Radiation Therapy and Chemotherapy in Merkel Cell Carcinoma: Survival Analyses of 6908 Cases from the National Cancer Data Base. J. Natl. Cancer Inst. 2016;108:djw042. doi: 10.1093/jnci/djw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Topalian S.L., Bhatia S., Amin A., Kudchadkar R.R., Sharfman W.H., Delord J.-P., Dunn L.A., Shinohara M.M., Kulikauskas R., Chung C.H., et al. Neoadjuvant Nivolumab for Patients with Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial. J. Clin. Oncol. 2020:JCO2000201. doi: 10.1200/JCO.20.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tai P.T., Yu E., Winquist E., Hammond A., Stitt L., Tonita J., Gilchrist J. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: Case series and review of 204 cases. J. Clin. Oncol. 2000;18:2493–2499. doi: 10.1200/JCO.2000.18.12.2493. [DOI] [PubMed] [Google Scholar]
- 162.Voog E., Biron P., Martin J.P., Blay J.Y. Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer. 1999;85:2589–2595. doi: 10.1002/(SICI)1097-0142(19990615)85:12<2589::AID-CNCR15>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 163.Garcia-Carbonero R., Marquez-Rodas I., de la Cruz-Merino L., Martinez-Trufero J., Cabrera M.A., Piulats J.M., Capdevila J., Grande E., Berrocal A. Recent Therapeutic Advances and Change in Treatment Paradigm of Patients with Merkel Cell Carcinoma. Oncologist. 2019;24:1375–1383. doi: 10.1634/theoncologist.2018-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cowey C.L., Mahnke L., Espirito J., Helwig C., Oksen D., Bharmal M. Real-world treatment outcomes in patients with metastatic Merkel cell carcinoma treated with chemotherapy in the USA. Future Oncol. 2017;13:1699–1710. doi: 10.2217/fon-2017-0187. [DOI] [PubMed] [Google Scholar]
- 165.Becker J.C., Lorenz E., Ugurel S., Eigentler T.K., Kiecker F., Pföhler C., Kellner I., Meier F., Kähler K., Mohr P., et al. Evaluation of real-world treatment outcomes in patients with distant metastatic Merkel cell carcinoma following second-line chemotherapy in Europe. Oncotarget. 2017;8:79731–79741. doi: 10.18632/oncotarget.19218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pommier Y., Sordet O., Antony S., Hayward R.L., Kohn K.W. Apoptosis defects and chemotherapy resistance: Molecular interaction maps and networks. Oncogene. 2004;23:2934–2949. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- 167.Kaufman H.L., Russell J., Hamid O., Bhatia S., Terheyden P., D’Angelo S.P., Shih K.C., Lebbé C., Linette G.P., Milella M., et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kaufman H.L., Russell J.S., Hamid O., Bhatia S., Terheyden P., D’Angelo S.P., Shih K.C., Lebbé C., Milella M., Brownell I., et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J. Immunother. Cancer. 2018;6:7. doi: 10.1186/s40425-017-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.D’Angelo S.P., Russell J., Lebbé C., Chmielowski B., Gambichler T., Grob J.-J., Kiecker F., Rabinowits G., Terheyden P., Zwiener I., et al. Efficacy and Safety of First-line Avelumab Treatment in Patients with Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol. 2018;4:e180077. doi: 10.1001/jamaoncol.2018.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Walker J.W., Grignani G., Nathan P., Dirix L., Fenig E., Ascierto P.A., Sandhu S., Munhoz R., Benincasa E., Flaskett S., et al. Efficacy and safety of avelumab treatment in patients with metastatic Merkel cell carcinoma: Experience from a global expanded access program. J. Immunother. Cancer. 2020;8:e000313. doi: 10.1136/jitc-2019-000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nghiem P., Bhatia S., Lipson E.J., Sharfman W.H., Kudchadkar R.R., Brohl A.S., Friedlander P.A., Daud A., Kluger H.M., Reddy S.A., et al. Durable Tumor Regression and Overall Survival in Patients with Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. J. Clin. Oncol. 2019;37:693–702. doi: 10.1200/JCO.18.01896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nghiem P.T., Bhatia S., Lipson E.J., Kudchadkar R.R., Miller N.J., Annamalai L., Berry S., Chartash E.K., Daud A., Fling S.P., et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lara K.M., In G.K., Matcuk G.R., Mehta A., Hu J.S. Talimogene laherparepvec in combination with pembrolizumab leads to a complete response in a patient with refractory Merkel cell carcinoma. JAAD Case Rep. 2018;4:1004–1006. doi: 10.1016/j.jdcr.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]