Abstract
RAB proteins (RABs) represent the largest subfamily of Ras-like small GTPases that regulate a wide variety of endosomal membrane transport pathways. Their aberrant expression has been demonstrated in various malignancies and implicated in pathogenesis. Using The Cancer Genome Atlas (TCGA) database, we analyzed the differential expression and clinicopathological association of RAB genes in pancreatic ductal adenocarcinoma (PDAC). Of the 62 RAB genes analyzed, five (RAB3A, RAB26, RAB25, RAB21, and RAB22A) exhibited statistically significant upregulation, while five (RAB6B, RAB8B, RABL2A, RABL2B, and RAB32) were downregulated in PDAC as compared to the normal pancreas. Racially disparate expression was also reported for RAB3A, RAB25, and RAB26. However, no clear trend of altered expression was observed with increasing stage and grade, age, and gender of the patients. PDAC from occasional drinkers had significantly higher expression of RAB21 compared to daily or weekly drinkers, whereas RAB25 expression was significantly higher in social drinkers, compared to occasional ones. The expression of RABL2A was significantly reduced in PDAC from diabetic patients, whereas RAB26 was significantly lower in pancreatitis patients. More importantly, a significant association of high expression of RAB21, RAB22A, and RAB25, and low expression of RAB6B, RABL2A, and RABL2B was observed with poorer survival of PC patients. Together, our study suggests potential diagnostic and prognostic significance of RABs in PDAC, warranting further investigations to define their functional and mechanistic significance.
Keywords: RAB family genes, pancreatic cancer, UALCAN, clinicopathological, The Human Protein Atlas
1. Introduction
Pancreatic cancer (PC) is the seventh leading cause of cancer mortalities globally, and the third leading cause in the United States [1,2]. According to an estimate made by the American Cancer Society, 57,600 new PC cases will be diagnosed and nearly 47,050 deaths will occur in 2020 in the United States. Indeed, PC is predicted to become the second leading cause of cancer-related death in the United States by 2030 or earlier [3].
Pancreatic ductal adenocarcinoma (PDAC) is the most common and lethal subtype of PC, accounting for over 90% of total diagnoses. Most PDACs are diagnosed at late stages (III or IV) due to the lack of recognizable clinical symptoms, leaving limited therapeutic options for treatment [4]. Further, PDAC is a highly aggressive disease that does not respond well to existing therapies due to inherent or acquired resistance, resulting in poor clinical outcomes [5,6,7]. Several genetic aberrations, including KRAS mutation, P53 mutation or deletion, and SAMD4/DPC4 deletion among several others, have been identified and shown to drive malignant progression [8,9]. However, we have not succeeded in translating this information into effective clinical management approaches. Therefore, we must continue to search for novel molecular targets that could improve early detection, predict the patient’s prognosis, and facilitate the development of mechanism-based therapies.
RAB proteins (RABs) belong to the “RAS superfamily” of small G proteins and comprise the largest subfamily of small GTPases [10,11,12]. A total of 70 RAB proteins have been identified thus far in humans that play indispensable roles in the regulation of vesicle trafficking, including vesicle formation, transport, and the docking and fusion of transport vesicles [12,13,14,15,16]. In particular, RABs regulate the specificity and directionality of membrane-bound cargo traffic to ensure that cargo is delivered to its correct destination [17]. Significant structural homology exists between RAB proteins; however, they have distinct cellular functions and localization [13]. The functions of RABs are controlled by the recruitment of effector or regulatory proteins specific to individual RABs that facilitate the exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP) [18]. Some RABs are ubiquitously present, while some have cell type- or compartment-specific functions [19]. Aberrant expression or alterations in the RAB genes has been reported in several diseases, including cancer [19,20,21,22,23]. The deregulation of RABs results in the disruption of the regulatory network of vesicle trafficking, which affects cellular growth and behavior and thus facilitates the malignant progression of cancer cells.
The present study examined the expression and clinicopathological association of RAB genes in PDAC. Genetic alterations and protein–protein interaction networks of selected upregulated or downregulated RABs were also studied. We identified several differentially expressed RABs in PDAC, some of which also showed association with the patient’s race as well as diabetes and pancreatitis diagnoses. Furthermore, a significant association of RAB genes with patient survival was also found. Finally, we identified low-frequency genetic mutations, amplifications, and deep deletions of RABs and their interacting protein networks that suggest their various pathobiological functions in PDAC progression.
2. Results
2.1. Differential Expression of RAB Transcripts in Pancreatic Tumor Tissues and Association with Clinical Progression
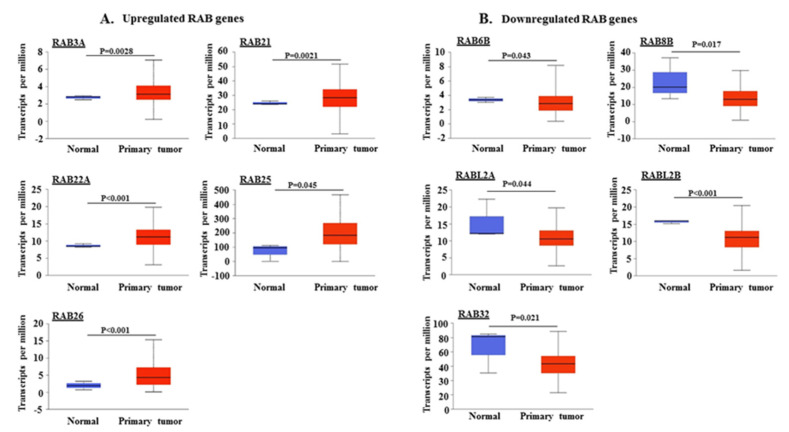
We analyzed the mRNA expression profiles of 62 RAB genes in PDAC using The Cancer Genome Atlas (TCGA) dataset through the UALCAN web portal (http://ualcan.path.uab.edu). A total of 10 RABs exhibited differential expression in primary tumor tissues as compared to the normal pancreas. Five RABs were significantly upregulated, including RAB3A (p = 0.0028), RAB21 (p = 0.0021), RAB22A (p < 0.01), RAB25 (p = 0.045), and RAB26 (p < 0.001) (Figure 1A), while the expression of the other five, RAB6B (p = 0.043), RAB8B (p = 0.017), RABL2A (p = 0.044), RABL2B (p < 0.001), and RAB32 (p = 0.021), was significantly downregulated (Figure 1B). The highest transcripts per million were detected for RAB25 in both normal and cancerous pancreatic tissues, whereas the lowest transcript levels were observed for RAB3A, RAB6B, and RAB26.
Figure 1.
Expression analysis of different RAB genes in pancreatic adenocarcinoma patient samples. Expression of different RAB genes was analyzed in normal pancreas and primary pancreatic ductal adenocarcinoma (PDAC) samples by using The Cancer Genome Atlas (TCGA) gene expression database on an interactive web portal, UALCAN. Based on the significant (p < 0.05) alteration of RAB genes in PDAC tissues (n = 178) relative to normal tissues (n = 4), the RAB genes were divided into two subsets: (A) upregulated RAB genes (RAB3A, RAB21, RAB22A, RAB25, and RAB26) and (B) downregulated RAB genes (RAB6B, RAB8B, RABL2A, RABL2B, and RAB32).
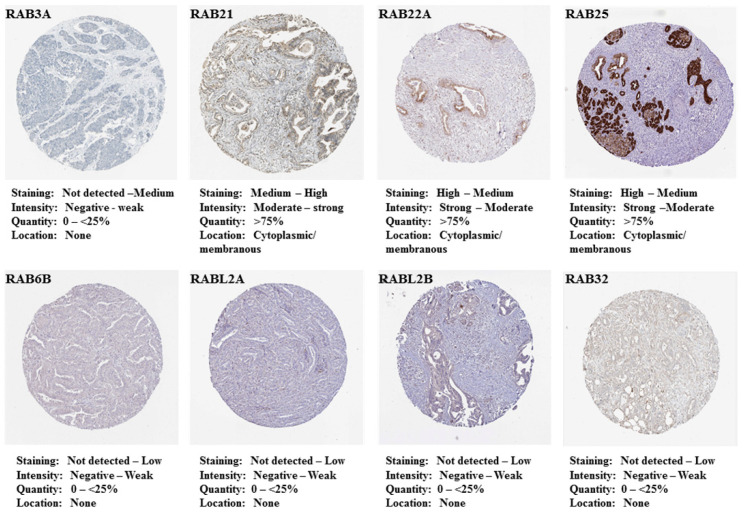
We further examined the in situ expression of these RAB genes at the protein level using immunohistochemistry data available in The Human Protein Atlas database (https://www.proteinatlas.org/). Correlating with the higher expression at the transcript level, RAB25 was found to have strong immunostaining in PC tissues, while RAB21 and RAB22A have medium to high staining (Figure 2). Negligible to weak staining, however, is reported for RAB3A, RAB6B, RABL2A, RABL2B, and RAB32 in PDAC tissues. No immunohistochemistry data are available for RAB26 and RAB8B in the database.
Figure 2.
In situ expression of different RABs in pancreatic adenocarcinoma specimens. Expression of upregulated (RAB3A, RAB21, RAB22A, and RAB25) and downregulated RAB genes (RAB6B, RABL2A, RABL2B, and RAB32) was analyzed in PDAC tissues at the protein level using immunohistochemistry data available in The Human Protein Atlas.
We also analyzed the correlation of differentially expressed RABs with PDAC stage and histological grade. Although stage- and grade-specific differences in the expression of some RABs were observed, no clear trend of altered RAB expression with increasing stage and grade could be established (Figure S1).
2.2. Association of RABs Expression with Race, Gender, and Age of Pancreatic Cancer Patients
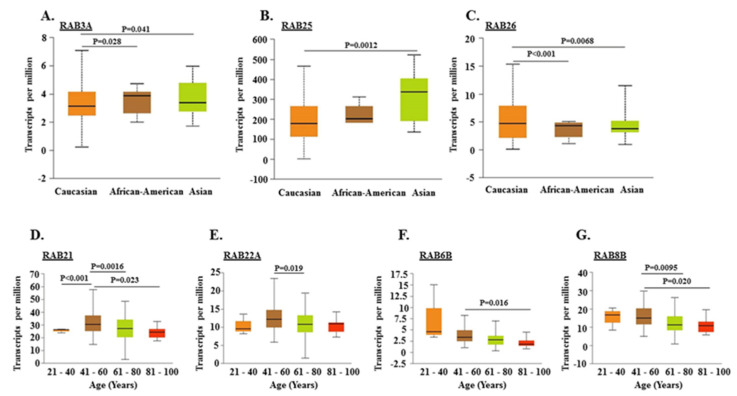
Although PDAC appears to afflict both males and females equally, race-specific differences in incidence and mortality are reported [24,25]. Therefore, we examined if the aberrant expression of RAB genes in PDAC has any association with patients’ gender, race, and age. We observed racially disparate expression for RAB3A, RAB25, and RAB26 in PDAC cases. RAB3A and RAB26 have significantly higher and lower expression, respectively, in PDAC tissues of African American (AA) and Asian racial backgrounds as compared to Caucasian Americans (CA). RAB25 has significantly elevated expression in PDACs from Asian patients as compared to those from CA patients (Figure 3A–C).
Figure 3.
Correlation of differential expression of RAB genes with race and age of pancreatic adenocarcinoma patients. Gene expression analysis of RAB3A, RAB25, and RAB26 in Caucasian (n = 156), African American (n = 6), and Asian (n = 11) PDAC patients (A–C). The expression pattern of RAB21, RAB22A, RAB6B, and RAB8B was analyzed in different age groups of PDAC patients starting from a younger age range (21–40 years) to older age groups (81–100 years) (D–G).
No statistically significant correlation of any of the downregulated RAB genes with the patient’s race was observed (data not shown). Similarly, no significant gender-specific association of any of the upregulated or downregulated RAB genes was observed (Figure S2). RAB21 and RAB22A showed significantly higher expression in middle-aged patients (41–60 years) compared to younger (21–40 years) and older (61–80 years) patients. The expression of RAB6B and RAB8B was significantly downregulated in older age groups as compared to the middle-aged patients (Figure 3D–G).
2.3. Association of RABs Expression in Pancreatic Tumors with Drinking Habits and Diagnoses of Diabetes and Pancreatitis in Patients
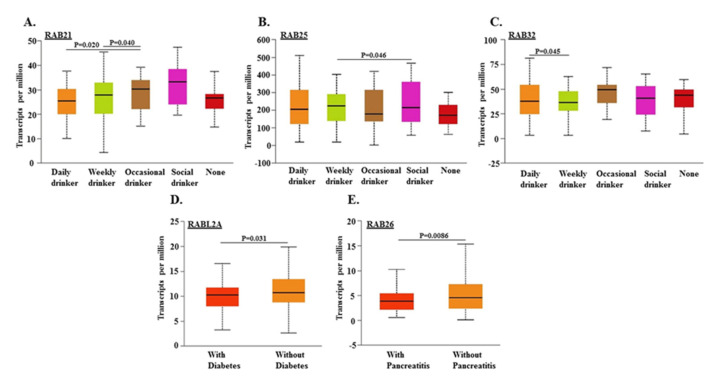
Lifestyle factors, such as drinking habits, and prior diagnoses of diabetes and pancreatitis have been suggested as risk factors for pancreatic cancer [1,26,27]. Therefore, we investigated if there was any association of aberrant RAB expression in PDACs with these behavioral and pathological aspects. PDAC cases from occasional drinkers had significantly higher expression of RAB21 compared to daily or weekly drinkers; however, its expression was the highest (albeit not significant) in social drinkers (Figure 4A).
Figure 4.
Relationship between differential expression of RAB genes in pancreatic tumors with patient’s drinking habits, and diagnoses of diabetes and pancreatitis. Transcript levels of different RAB genes (RAB21, RAB25, RAB32, RABL2A, and RAB26) were correlated with drinking habit (A–C), diabetes status (D), and chronic pancreatitis (E) in PDAC patients.
RAB25 expression was significantly higher in weekly drinkers as compared to social drinkers, and the expression was the lowest in occasional and non-drinkers but not significant (Figure 4B). Statistically significant differences in transcript levels of RAB32 were also found in weekly and daily drinkers, but the differences were not significant with occasional and social drinkers (Figure 4C). The expression of RABL2A was significantly reduced in PDACs from diabetic patients (Figure 4B), whereas RAB26 was significantly downregulated in patients with pancreatitis (Figure 4D,E).
2.4. Prognostic Potential of RABs Expression in Pancreatic Ductal Adenocarcinoma
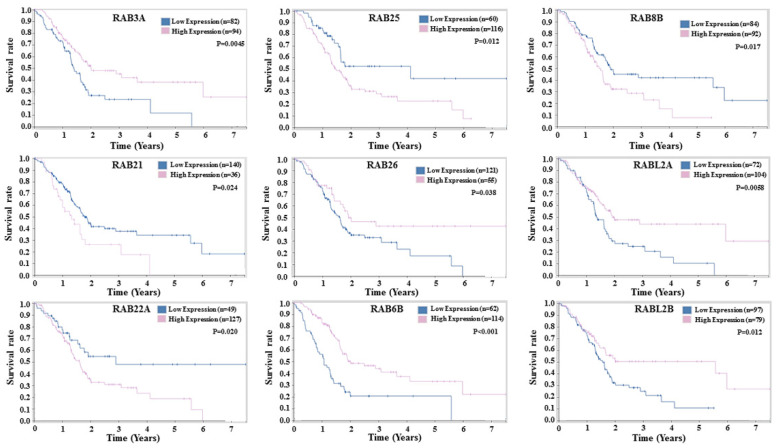
Since dysregulated expression of RABs can impact tumor cell growth and aggressiveness, we investigated its association with patient’s survival. The overall survival (OS) of patients was compared between patients having the high or low expression of various differentially expressed RAB genes. Kaplan–Meier plots drawn from the TCGA datasets demonstrated a significant association of several RAB genes with the survival of PDAC patients (Figure 5).
Figure 5.
Kaplan–Meier plots for the survival of pancreatic adenocarcinoma patients stratified by the expression level of RAB genes. The overall survival curves of the differential expression of upregulated (RAB3A, RAB21, RAB22A, RAB25, and RAB26) and downregulated (RAB6B, RAB8B, RABL2A, and RABL2B) RAB genes were analyzed in PDAC patients and correlated with the survival of the patients.
Among the 10 differentially expressed RABs, 9 had a significant association with the patient’s survival. High transcript levels of RAB21, RAB8B, RAB22A, and RAB25 exhibited significant association with a reduced overall survival of PDAC patients. In contrast, high transcript levels of RABL2A, RABL2B, RAB6B, RAB3A, and RAB26 significantly associated with better overall survival of PDAC patients. No significant association of RAB32 was detected with the patient’s survival (data not shown). It is interesting to note that although RAB3A and RAB26 have an upregulated expression in PC and RAB8B has downregulated expression, they unexpectedly exhibit positive and negative associations, respectively, with patient survival.
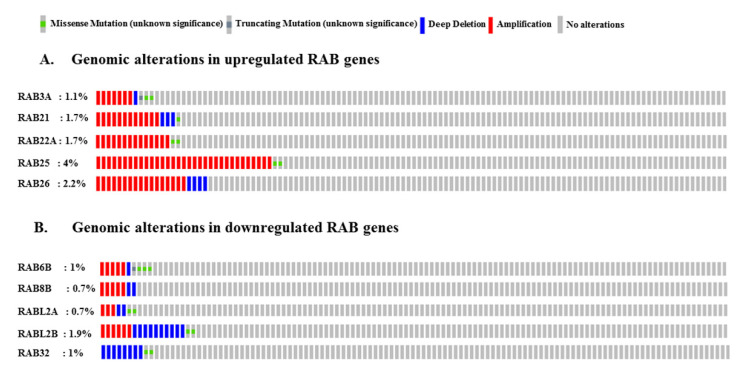
2.5. Genomic Alterations in RAB Genes Associated with Pancreatic Cancer
Altered expression of genes can result from gene amplification or deletion besides aberrant transcriptional regulation. Further, altered gene function can also result from gene mutations. Therefore, we analyzed these genomic alterations in differentially expressed RAB genes by using cBioPortal (https://www.cbioportal.org). Among the upregulated RAB genes, RAB25 showed a 4% genetic alteration rate, followed by RAB26 (2.2%), RAB21 (1.7%), RAB22A (1.7%), and RAB3A (1.1%). Most of these alterations were associated with gene amplification; however, deep deletions (RAB3A, RAB21, and RAB26), missense mutations (RAB3A, RAB21, RAB22A, and RAB25), and truncating mutation (RAB3A) were also reported (Figure 6A).
Figure 6.
Genomic alterations in differentially expressed RAB genes identified in pancreatic adenocarcinoma: OncoPrint analysis of upregulated (A) and downregulated (B) RAB genes using the c-BioPortal revealed extent of gene amplification, deep deletions, and nucleotide substitutions associated with pancreatic cancer.
Interestingly, gene amplification was also reported for some of the downregulated RAB genes (RAB6B, RAB8B, RABL2A, and RABL2B) (Figure 6). RABL2B exhibits the highest rate of deep deletions (1%), followed by RAB32 (0.85%), RABL2A (0.2%), RAB8B (0.2%), and RAB6B (0.1%). Furthermore, missense mutations (RAB6B, RABL2A, RABL2B, and RAB32) and truncating mutations (RAB6B) were also reported (Figure 6B).
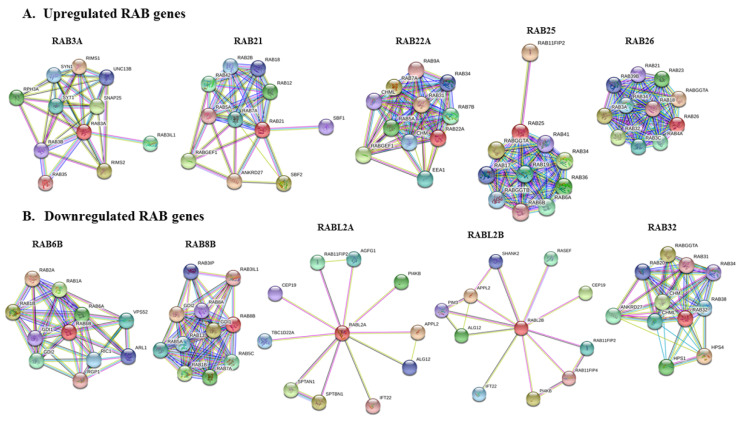
2.6. Protein–Protein Interaction Network of Selected RABs
Protein–protein interaction (PPI) networks were constructed for differentially expressed RABs using STRING (https://string-db.org/) to predict their potential pathobiological significance. Analysis of both known and predicted interactions of upregulated and downregulated RABs revealed several interacting partners, including other RAB GTPase and associated effector and regulatory proteins (Figure 7A,B). A common interaction of Rab GTPase-associated effector proteins involved in the regulation of GDP/GTP reaction exchange (RIMS1, RIMS2, RABGEF1, ANKRD27, SBF1, SBF2, RIC1, RGP1, RAB3IP, RAB3IL1, HPS1, and HPS4) was observed for RAB3A, RAB21, RAB22A, Rab6B, RAB8B, and RAB32.
Figure 7.
The protein–protein interaction networks of (A) upregulated or (B) downregulated RAB proteins constructed using STRING.
Similarly, proteins involved in the geranylgeranylation of RAB protein such as CHML, CHM, RABGTA, and RABGTB were also identified as interacting partners for RAB22A, RAB25, RAB26, and RAB32. Of note, some of the downregulated RAB proteins were identified to interact with the same partners that also interact with the upregulated RABs, suggesting complex molecular crosstalk within the RAB functional network. We have summarized the predicted functions of different RAB proteins (Table 1).
Table 1.
Function and localization of upregulated or downregulated RAB genes in pancreatic cancer.
| RAB Genes | Location | Function | References |
|---|---|---|---|
| Upregulated RAB Genes | |||
| RAB3A | Synaptotagmins and secretory vesicles | Regulation of exocytosis (Ca+2 dependent), secretory vesicle docking, fusion and transport of synaptic vesicles | [28,29] |
| RAB21 | Early endosomes, trans Golgi network, cytoplasmic vesicles | Endosomal transport of integrins, cell adhesion and migration, endolysosomal transport | [30,31] |
| RAB22A | Early endosomes, phagosomal membrane, and trans Golgi network | Protein trafficking from early endosomes to late endosomes, phagosome maturation, recycling endosomal biogenesis | [32,33] |
| RAB25 | Recycling endosomes | Protein trafficking through recycling endosomes to the plasma membrane, integrin transport | [34,35] |
| RAB26 | Synaptic and secretory vesicles | Regulated exocytosis of secretory granules and synaptic vesicles | [36,37] |
| Downregulated RAB Genes | |||
| RAB6B | Golgi | Retrograde transport from late endosomes via Golgi to ER | [38,39] |
| RAB8B | Golgi, plasma membrane, phagosome membrane | Apical transport pathways, adherens junction dynamics, from trans Golgi network and recycling endosomes to plasma membrane | [40,41,42] |
| RABL2A/RABL2B | Primary cilia, centriole | Intraflagellar transport and ciliary assembly | [43,44] |
| RAB32 | Mitochondria, melanosomes, autophagosomes | Mitochondrial dynamics regulation, transport from trans Golgi network to melanosomes, autophagy | [45,46] |
3. Discussion
Cancer is a progressive genetic disease that begins with the amplification or activating mutations in the oncogenes or deletion or inactivating mutations in the tumor suppressor genes [47,48]. As cancer cells evolve, they accumulate additional genetic aberrations as well as transcriptionally activate or suppress other functionally relevant tumor promoter or suppressor genes that support their malignant progression [49,50]. RAB proteins are an indispensable component of the membrane trafficking system that controls secretion, transport, recycling, and degradation of many tumor-associated proteins such as beta-integrins, epidermal growth factor receptor (EGFR), and matrix metalloproteinases (MMPs), among several others [51,52,53,54]. Further, the role of RABs in the shedding of extracellular vesicles has also been reported [55,56,57,58]. Aberrant expression of RABs is reported in various cancers, and they are shown to act either as a tumor promoter or suppressor in a context-dependent manner [59,60,61,62,63].
Among the upregulated RAB genes identified in PDAC, a higher expression of RAB3A is also reported in glioblastoma multiforme, where it promotes cell proliferation [64]. Moreover, its effector protein RAB3IP is involved in insulin exocytosis from pancreatic β cells [65] and promotes cell proliferation via inhibiting autophagy in gastric cancer [66]. RAB21 is known to regulate adhesion molecules and endosomal traffic of β-integrins [67] and promote the proliferation of glioblastoma [68] and breast cancer cells [69]. Further, RAB21 mediates acute pancreatitis through interaction with the TRAF3-MKK3 complex [70]. RAB22A is reported to promote the biogenesis of recycling endosomes [71] and regulate the sorting of membrane proteins such as transferrin to recycling endosomes [32]. RAB22A acts as a prognostic marker in breast cancer [72] and inhibits invasion and metastasis of breast cancer cells through secretion of exosomes [73]. It is also shown to be involved in the pathogenesis of colon cancer [74], renal cell carcinoma [75], and osteosarcoma [76]. RAB25 is involved in the recycling of proteins from late endosomes to the plasma membrane during cell migration [77]. The tumorigenic role of RAB25 is reported in non-small cell lung cancer [78], gastric cancer [79], and ovarian cancer [80], whereas some report its tumor suppressor function in breast [81] and colon cancers [82]. RAB26 is generally found in secretory cells such as pancreatic acinar cells and controls lysosomal traffic and mitochondrial localization and trafficking of synaptic vesicles [36,37].
Cancer-related information is scarce for the RAB genes that we found to be downregulated in PDAC; however, these RABs are reported to play essential roles in cellular physiology. RAB6B is expressed in a cell-type-specific manner and is involved in retrograde (Golgi to Endoplasmic reticulum) trafficking of proteins [83]. RAB8B is essential for the apical transport pathways and helps in intracellular vesicle docking and fusion to their target membrane [40,84]. Both RABL2A and RABL2B are known for intra-flagellar ciliary transport and control ciliary G-protein-coupled receptor (GPCR) trafficking [43,44]. Further, RAB32 is reported to be expressed in secretory cells, regulate autophagy and mitochondrial dynamics, and reside on lysosomes [45,85,86].
Racial disparities in cancer incidence and clinical outcomes are reported for many cancers, including PC [87]. While cancer health disparities could result from multiple factors, molecular and biological differences have been suggested to be involved as well [88]. Along those lines, we observed racially disparate expression of three RAB genes (RAB3A, RAB25, and RAB26). Therefore, it will be interesting to further investigate their functional significance in PC using appropriate model systems. Age, alcohol consumption, and prior diagnoses of diabetes and pancreatitis are suggested to be risk factors for developing PC [1]. Most PCs are diagnosed in patients over 60 years old, and they rarely develop in younger adults (less than 40 years old) [2,89]. In our study, we found significant differences in levels of some upregulated (RAB21 and RAB22A) and downregulated (RAB6B and RAB8B) RABs between age groups, but the pattern is not distinct enough to predict an etiological association. High alcohol consumption is also suggested as a risk factor for PC development [90]. We, however, did not observe a clear-cut suggestive association of RAB dysregulation with drinking habits, although statistically significant differences in levels were observed between daily, weekly, occasional, and social drinkers for some RABs. Type I and II diabetes and pancreatitis status are considered as potential risk factors of PC as well [1]. We found that RABL2A had significantly lower expression in PC patients with diabetes. It would be interesting to investigate its functional significance. We also found a significantly lower expression of RAB26 in PC patients with pancreatitis, which could suggest its regulation by inflammatory mediators.
We found a significant association of differentially expressed RABs with the survival of PDAC patients. Interestingly, some of the RABs (RAB3A, RAB26, and RAB8B) displayed an unexpected association with patients’ survival. Despite being overexpressed in PC, high RAB3A and RAB26 have a positive association with patient survival, while low levels of the downregulated RAB8B have a negative association with PC patient survival. This could be due to the altered functions of these RABs due to genetic alterations or their secondary regulation affecting their protein levels. Supporting this notion, we observed a lack of RAB3A immunostaining in PC tissues, suggesting its post-transcriptional regulation by yet unknown mechanisms [91,92]. It is also likely that the functional pathobiological impact of these RABs depends on other interacting proteins that may also be aberrantly expressed in PC. Therefore, functional and mechanistic insights regarding the roles of RABs are needed through laboratory research to precisely understand their pathobiological significance in PC. In summary, our study provides initial data to support further investigations on RAB functions and associated mechanisms to explore their utility as therapeutic targets and clinical biomarkers of diagnostic and prognostic significance.
4. Materials and Methods
4.1. Gene Expression Analysis Using UALCAN
UALCAN (http://ualcan.path.uab.edu/) is a publicly available web portal that uses TCGA level 3 RNAseq and clinical data to provide the differential expression status of the gene of interest in normal and cancerous tissue and its clinicopathological association. To assess the potential role of RAB proteins in the progression of PC, UALCAN was used to examine the relative transcript levels of various RAB genes in normal pancreas and pancreatic tissue samples. RAB genes with significant (p < 0.05) overexpression or downregulation in PDAC tissues were further examined for their clinicopathological significance. The UALCAN server was also used to analyze the association of upregulated or downregulated RAB genes with tumor grade and stage, and patient’s race, gender, age, drinking habits, diabetes, and pancreatitis status.
4.2. Prognostic Significance and In Situ Expression of Differentially Expressed RAB Genes
The association of differential expression status of RAB genes with patient survival was examined using TCGA database information via The Human Protein Atlas (https://www.proteinatlas.org/). Kaplan–Meir survival plots were drawn for prognostic values of RAB genes that had significant up- or downregulation in PC at the mRNA level. RAB genes with significant (p < 0.05) correlation of changes in survival rate with either higher or lower expression of RAB genes were chosen. The Human Protein Atlas stores plenty of information about thousands of proteins regarding their expression and distribution in a variety of cancer tissues. Using this database, we analyzed the protein expression and localization status of selected RAB genes in the pancreatic adenocarcinoma tissues.
4.3. Genomic Alterations Analysis and Protein–Protein Interaction Network of Selected RAB Genes
c-BioPortal (https://www.cbioportal.org/) is a readily accessible platform for the analysis of various cancer genomics datasets such as TCGA, GEO, and ICGC that provides information related to somatic mutation spectrum (gene amplification, deep deletion, missense mutations, etc.) and changes in mRNA and microRNAs [93,94]. We analyzed the genetic alterations in PC of upregulated and downregulated RAB genes using c-BioPortal, and results obtained in ‘Oncoprint’ for each RAB gene were downloaded and presented. Further, we used the STRING web interface (https://string-db.org/) [95] to examine the interacting partners of potential RAB genes through the construction of protein–protein interaction networks.
Abbreviations
| AA | African American |
| CA | Caucasian American |
| PDAC | Pancreatic ductal adenocarcinoma |
| TCGA | The Cancer Genome Atlas |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/15/5580/s1, Figure S1: Association of transcript levels of RAB3A, RAB21, RABL2A, RABL2B and RAB32 with tumor grade and stages of pancreatic adenocarcinoma, Figure S2: Gender-based differential expression of RAB genes.
Author Contributions
Conceptualization, S.A. and A.P.S.; methodology, S.S., A.P.S., and M.A.K.; software, S.A. and M.A.K.; validation, S.A., M.A.K., S.D., and A.P.S.; investigation, S.A., M.A.K., and A.P.S.; data curation, S.A. and M.A.K.; writing—original draft preparation, S.A., M.A.K., M.K., S.D., S.S., and A.P.S.; supervision, A.P.S.; funding acquisition, S.S. and A.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge funding from NIH/NCI [R01CA175772, R01CA224306, U01CA185490 (to A.P.S.) and R01CA204801, R01CA231925 (to S.S.)] and USAMCI.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rawla P., Sunkara T., Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Wolfgang C.L., Herman J.M., Laheru D.A., Klein A.P., Erdek M.A., Fishman E.K., Hruban R.H. Recent progress in pancreatic cancer. CA Cancer J. Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chand S., O’Hayer K., Blanco F.F., Winter J.M., Brody J.R. The Landscape of Pancreatic Cancer Therapeutic Resistance Mechanisms. Int. J. Biol. Sci. 2016;12:273–282. doi: 10.7150/ijbs.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capurso G., Sette C. Drug resistance in pancreatic cancer: New player caught in act. EBiomed. 2019;40:39–40. doi: 10.1016/j.ebiom.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan M.A., Srivastava S.K., Zubair H., Patel G.K., Arora S., Khushman M., Carter J.E., Gorman G.S., Singh S., Singh A.P. Co-targeting of CXCR4 and hedgehog pathways disrupts tumor-stromal crosstalk and improves chemotherapeutic efficacy in pancreatic cancer. J. Biol. Chem. 2020;295:8413–8424. doi: 10.1074/jbc.RA119.011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakravarthi B.V., Nepal S., Varambally S. Genomic and Epigenomic Alterations in Cancer. Am. J. Pathol. 2016;186:1724–1735. doi: 10.1016/j.ajpath.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan M.A., Azim S., Zubair H., Bhardwaj A., Patel G.K., Khushman M., Singh S., Singh A.P. Molecular Drivers of Pancreatic Cancer Pathogenesis: Looking Inward to Move Forward. Int. J. Mol. Sci. 2017;18:779. doi: 10.3390/ijms18040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rojas A.M., Fuentes G., Rausell A., Valencia A. The Ras protein superfamily: Evolutionary tree and role of conserved amino acids. J. Cell Biol. 2012;196:189–201. doi: 10.1083/jcb.201103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu L., Pan C., He S.M., Lang B., Gao G.D., Wang X.L., Wang Y. The Ras Superfamily of Small GTPases in Non-neoplastic Cerebral Diseases. Front. Mol. Neurosci. 2019;12:121. doi: 10.3389/fnmol.2019.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira-Leal J.B., Seabra M.C. Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 2001;313:889–901. doi: 10.1006/jmbi.2001.5072. [DOI] [PubMed] [Google Scholar]
- 13.Pylypenko O., Hammich H., Yu I.M., Houdusse A. Rab GTPases and their interacting protein partners: Structural insights into Rab functional diversity. Small GTPases. 2018;9:22–48. doi: 10.1080/21541248.2017.1336191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segev N. Ypt/rab gtpases: Regulators of protein trafficking. Sci. STKE. 2001 doi: 10.1126/stke.2001.100.re11. [DOI] [PubMed] [Google Scholar]
- 15.Zerial M., McBride H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 16.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 17.Zhen Y., Stenmark H. Cellular functions of Rab GTPases at a glance. J. Cell Sci. 2015;128:3171–3176. doi: 10.1242/jcs.166074. [DOI] [PubMed] [Google Scholar]
- 18.Hutagalung A.H., Novick P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzeng H.T., Wang Y.C. Rab-mediated vesicle trafficking in cancer. J. Biomed. Sci. 2016;23:70. doi: 10.1186/s12929-016-0287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J., Liu W., Lu X., Fu Y., Li L., Luo Y. High expression of small GTPase Rab3D promotes cancer progression and metastasis. Oncotarget. 2015;6:11125–11138. doi: 10.18632/oncotarget.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva P., Mendoza P., Rivas S., Diaz J., Moraga C., Quest A.F.G., Torres V.A. Hypoxia promotes Rab5 activation, leading to tumor cell migration, invasion and metastasis. Oncotarget. 2016;7:29548–29562. doi: 10.18632/oncotarget.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villagomez F.R., Medina-Contreras O., Cerna-Cortes J.F., Patino-Lopez G. The role of the oncogenic Rab35 in cancer invasion, metastasis, and immune evasion, especially in leukemia. Small GTPases. 2018:1–12. doi: 10.1080/21541248.2018.1463895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng K.W., Lahad J.P., Gray J.W., Mills G.B. Emerging role of RAB GTPases in cancer and human disease. Cancer Res. 2005;65:2516–2519. doi: 10.1158/0008-5472.CAN-05-0573. [DOI] [PubMed] [Google Scholar]
- 24.Arnold L.D., Patel A.V., Yan Y., Jacobs E.J., Thun M.J., Calle E.E., Colditz G.A. Are racial disparities in pancreatic cancer explained by smoking and overweight/obesity. Cancer Epidemiol. Biomarkers Prev. 2009;18:2397–2405. doi: 10.1158/1055-9965.EPI-09-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang B.Z., Stram D.O., Le Marchand L., Haiman C.A., Wilkens L.R., Pandol S.J., Zhang Z.F., Monroe K.R., Setiawan V.W. Interethnic differences in pancreatic cancer incidence and risk factors: The Multiethnic Cohort. Cancer Med. 2019;8:3592–3603. doi: 10.1002/cam4.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michaud D.S., Giovannucci E., Willett W.C., Colditz G.A., Fuchs C.S. Coffee and alcohol consumption and the risk of pancreatic cancer in two prospective United States cohorts. Cancer Epidemiol. Biomarkers Prev. 2001;10:429–437. [PubMed] [Google Scholar]
- 27.Andersen D.K., Korc M., Petersen G.M., Eibl G., Li D., Rickels M.R., Chari S.T., Abbruzzese J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes. 2017;66:1103–1110. doi: 10.2337/db16-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vieira O.V. Rab3a and Rab10 are regulators of lysosome exocytosis and plasma membrane repair. Small GTPases. 2018;9:349–351. doi: 10.1080/21541248.2016.1235004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Weering J.R., Toonen R.F., Verhage M. The role of Rab3a in secretory vesicle docking requires association/dissociation of guanidine phosphates and Munc18-1. PLoS ONE. 2007;2:616. doi: 10.1371/journal.pone.0000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X., He X., Fu X.Y., Chang Z. Varp is a Rab21 guanine nucleotide exchange factor and regulates endosome dynamics. J. Cell Sci. 2006;119:1053–1062. doi: 10.1242/jcs.02810. [DOI] [PubMed] [Google Scholar]
- 31.Pellinen T., Tuomi S., Arjonen A., Wolf M., Edgren H., Meyer H., Grosse R., Kitzing T., Rantala J.K., Kallioniemi O., et al. Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev. Cell. 2008;15:371–385. doi: 10.1016/j.devcel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Magadan J.G., Barbieri M.A., Mesa R., Stahl P.D., Mayorga L.S. Rab22a regulates the sorting of transferrin to recycling endosomes. Mol. Cell Biol. 2006;26:2595–2614. doi: 10.1128/MCB.26.7.2595-2614.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shakya S., Sharma P., Bhatt A.M., Jani R.A., Delevoye C., Setty S.R. Rab22A recruits BLOC-1 and BLOC-2 to promote the biogenesis of recycling endosomes. EMBO Rep. 2018;19:45918. doi: 10.15252/embr.201845918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casanova J.E., Wang X., Kumar R., Bhartur S.G., Navarre J., Woodrum J.E., Altschuler Y., Ray G.S., Goldenring J.R. Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol. Biol. Cell. 1999;10:47–61. doi: 10.1091/mbc.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caswell P.T., Spence H.J., Parsons M., White D.P., Clark K., Cheng K.W., Mills G.B., Humphries M.J., Messent A.J., Anderson K.I., et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev. Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Binotti B., Pavlos N.J., Riedel D., Wenzel D., Vorbruggen G., Schalk A.M., Kühnel K., Boyken J., Erck C., Martens H., et al. The GTPase Rab26 links synaptic vesicles to the autophagy pathway. Elife. 2015;4:05597. doi: 10.7554/eLife.05597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin R.U., Mills J.C. RAB26 coordinates lysosome traffic and mitochondrial localization. J. Cell Sci. 2014;127:1018–1032. doi: 10.1242/jcs.138776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Opdam F.J., Echard A., Croes H.J., van den Hurk J.A., van de Vorstenbosch R.A., Ginsel L.A., Goud B., Fransen J.A. The small GTPase Rab6B, a novel Rab6 subfamily member, is cell-type specifically expressed and localised to the Golgi apparatus. J. Cell Sci. 2000;113:2725–2735. doi: 10.1242/jcs.113.15.2725. [DOI] [PubMed] [Google Scholar]
- 39.Martinez O., Schmidt A., Salamero J., Hoflack B., Roa M., Goud B. The small GTP-binding protein rab6 functions in intra-Golgi transport. J. Cell Biol. 1994;127:1575–1588. doi: 10.1083/jcb.127.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato T., Iwano T., Kunii M., Matsuda S., Mizuguchi R., Jung Y., Hagiwara H., Yoshihara Y., Yuzaki M., Harada R., et al. Rab8a and Rab8b are essential for several apical transport pathways but insufficient for ciliogenesis. J. Cell Sci. 2014;127:422–431. doi: 10.1242/jcs.136903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau A.S., Mruk D.D. Rab8B GTPase and junction dynamics in the testis. Endocrinology. 2003;144:1549–1563. doi: 10.1210/en.2002-220893. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi S., Suzuki T., Kawaguchi A., Phongphaew W., Yoshii K., Iwano T., Harada A., Kariwa H., Orba Y., Sawa H. Rab8b Regulates Transport of West Nile Virus Particles from Recycling Endosomes. J. Biol. Chem. 2016;291:6559–6568. doi: 10.1074/jbc.M115.712760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dateyama I., Sugihara Y., Chiba S., Ota R., Nakagawa R., Kobayashi T., Itoh H. RABL2 positively controls localization of GPCRs in mammalian primary cilia. J. Cell Sci. 2019;132:jcs.224428. doi: 10.1242/jcs.224428. [DOI] [PubMed] [Google Scholar]
- 44.Kanie T., Abbott K.L., Mooney N.A., Plowey E.D., Demeter J., Jackson P.K. The CEP19-RABL2 GTPase Complex Binds IFT-B to Initiate Intraflagellar Transport at the Ciliary Base. Dev. Cell. 2017;42:22–36.e12. doi: 10.1016/j.devcel.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alto N.M., Soderling J., Scott J.D. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J. Cell Biol. 2002;158:659–668. doi: 10.1083/jcb.200204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wasmeier C., Romao M., Plowright L., Bennett D.C., Raposo G., Seabra M.C. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J. Cell Biol. 2006;175:271–281. doi: 10.1083/jcb.200606050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albertson D.G. Gene amplification in cancer. Trends Genet. 2006;22:447–455. doi: 10.1016/j.tig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki M.S., Kato M., Toguchida J., Yamaguchi T., Ejima Y., Ishizaki K., Kaneko A., Tanooka H. Somatic and germinal mutations of tumor-suppressor genes in the development of cancer. J. Radiat. Res. 1991;32:266–276. doi: 10.1269/jrr.32.SUPPLEMENT2_266. [DOI] [PubMed] [Google Scholar]
- 49.Baylin S.B., Jones P.A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 2016;8:019505. doi: 10.1101/cshperspect.a019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez-Tapia C., Wan F.Y. Fastest time to cancer by loss of tumor suppressor genes. Bull. Math. Biol. 2014;76:2737–2784. doi: 10.1007/s11538-014-0027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dozynkiewicz M.A., Jamieson N.B., Macpherson I., Grindlay J., van den Berghe P.V.E., von Thun A., Morton J.P., Gourley C., Timpson P., Nixon C., et al. Rab25 and CLIC3 collaborate to promote integrin recycling from late endosomes/lysosomes and drive cancer progression. Dev. Cell. 2012;22:131–145. doi: 10.1016/j.devcel.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caswell P.T., Chan M., Lindsay A.J., McCaffrey M.W., Boettiger D., Norman J.C. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J. Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bravo-Cordero J.J., Marrero-Diaz R., Megias D., Genis L., Garcia-Grande A., Garcia M.A., Arroyo A.G., Montoya M.C. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007;26:1499–1510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hendrix A., Maynard D., Pauwels P., Braems G., Denys H., Van den Broecke R., Lambert J., Van Belle S., Cocquyt V., Gespach C., et al. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J. Natl. Cancer Inst. 2010;102:866–880. doi: 10.1093/jnci/djq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Recchi C., Seabra M.C. Novel functions for Rab GTPases in multiple aspects of tumour progression. Biochem. Soc. Trans. 2012;40:1398–1403. doi: 10.1042/BST20120199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hooper S., Gaggioli C., Sahai E. A chemical biology screen reveals a role for Rab21-mediated control of actomyosin contractility in fibroblast-driven cancer invasion. Br. J. Cancer. 2010;102:392–402. doi: 10.1038/sj.bjc.6605469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peinado H., Aleckovic M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., Ghajar C., et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfeffer S.R. Two Rabs for exosome release. Nat. Cell Biol. 2010;12:3–4. doi: 10.1038/ncb0110-3. [DOI] [PubMed] [Google Scholar]
- 59.Cheng K.W., Lahad J.P., Kuo W.L., Lapuk A., Yamada K., Auersperg N., Liu J., Smith-McCune K., Lu K.H., Fishman D., et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat. Med. 2004;10:1251–1256. doi: 10.1038/nm1125. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J., Wei J., Lu J., Tong Z., Liao B., Yu B., Zheng F., Huang X., Chen Z., Fang Y., et al. Overexpression of Rab25 contributes to metastasis of bladder cancer through induction of epithelial-mesenchymal transition and activation of Akt/GSK-3beta/Snail signaling. Carcinogenesis. 2013;34:2401–2408. doi: 10.1093/carcin/bgt187. [DOI] [PubMed] [Google Scholar]
- 61.Ren P., Yang X.Q., Zhai X.L., Zhang Y.Q., Huang J.F. Overexpression of Rab27B is correlated with distant metastasis and poor prognosis in ovarian cancer. Oncol. Lett. 2016;12:1539–1545. doi: 10.3892/ol.2016.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seven D., Dogan S., Kilic E., Karaman E., Koseoglu H., Buyru N. Downregulation of Rab25 activates Akt1 in head and neck squamous cell carcinoma. Oncol. Lett. 2015;10:1927–1931. doi: 10.3892/ol.2015.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang M., Wang W., Ding J., Wang J., Zhang J. Downregulation of Rab17 promotes cell proliferation and invasion in non-small cell lung cancer through STAT3/HIF-1alpha/VEGF signaling. Thorac. Cancer. 2020;11:379–388. doi: 10.1111/1759-7714.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim J.K., Lee S.Y., Park C.W., Park S.H., Yin J., Kim J., Park J.B., Lee J.Y., Kim H., Kim S.C. Rab3a promotes brain tumor initiation and progression. Mol. Biol. Rep. 2014;41:5903–5911. doi: 10.1007/s11033-014-3465-2. [DOI] [PubMed] [Google Scholar]
- 65.Iezzi M., Regazzi R., Wollheim C.B. The Rab3-interacting molecule RIM is expressed in pancreatic beta-cells and is implicated in insulin exocytosis. FEBS Lett. 2000;474:66–70. doi: 10.1016/S0014-5793(00)01572-6. [DOI] [PubMed] [Google Scholar]
- 66.Guo W., Chen Z., Chen Z., Yu J., Liu H., Li T., Lin T., Chen H., Zhao M., Li G., et al. Promotion of Cell Proliferation through Inhibition of Cell Autophagy Signalling Pathway by Rab3IP is Restrained by MicroRNA-532-3p in Gastric Cancer. J. Cancer. 2018;9:4363–4373. doi: 10.7150/jca.27533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pellinen T., Arjonen A., Vuoriluoto K., Kallio K., Fransen J.A., Ivaska J. Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J. Cell Biol. 2006;173:767–780. doi: 10.1083/jcb.200509019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ge J., Chen Q., Liu B., Wang L., Zhang S., Ji B. Knockdown of Rab21 inhibits proliferation and induces apoptosis in human glioma cells. Cell Mol. Biol. Lett. 2017;22:30. doi: 10.1186/s11658-017-0062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye F., Tang H., Liu Q., Xie X., Wu M., Liu X., Chen B., Xie X. miR-200b as a prognostic factor in breast cancer targets multiple members of RAB family. J. Transl. Med. 2014;12:17. doi: 10.1186/1479-5876-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang N., Meng W., Jia R., Xiang S. Rab GTPase 21 mediates caerulin-induced TRAF3-MKK3-p38 activation and acute pancreatitis response. Biochem. Biophys. Res. Commun. 2019;518:50–58. doi: 10.1016/j.bbrc.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 71.Weigert R., Yeung A.C., Li J., Donaldson J.G. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol. Biol. Cell. 2004;15:3758–3770. doi: 10.1091/mbc.e04-04-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He M., Shen L., Jiang C., Gao G., Wang K., Jiao Y., Sun L., Cui Y., Ke Z., Yang Z. Rab22a is a novel prognostic marker for cell progression in breast cancer. Int. J. Mol. Med. 2020;45:1037–1046. doi: 10.3892/ijmm.2020.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang T., Gilkes D.M., Takano N., Xiang L., Luo W., Bishop C.J., Chaturvedi P., Green J.J., Semeza G.L. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA. 2014;111:3234–3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fang Z., Li C., Li S. MicroRNA-193b acts as a tumor suppressor in colon cancer progression via targeting RAB22A. Exp. Ther. Med. 2019;17:3921–3928. doi: 10.3892/etm.2019.7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong F., Liu K., Zhang F., Sha K., Wang X., Guo X., Huang N. MiR-204 inhibits the proliferation and invasion of renal cell carcinoma by inhibiting RAB22A expression. Oncol. Rep. 2016;35:3000–3008. doi: 10.3892/or.2016.4624. [DOI] [PubMed] [Google Scholar]
- 76.Yang D., Liu G., Wang K. miR-203 Acts as a Tumor Suppressor Gene in Osteosarcoma by Regulating RAB22A. PLoS ONE. 2015;10:e0132225. doi: 10.1371/journal.pone.0132225. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Scapin S.M., Carneiro F.R., Alves A.C., Medrano F.J., Guimaraes B.G., Zanchin N.I. The crystal structure of the small GTPase Rab11b reveals critical differences relative to the Rab11a isoform. J. Struct. Biol. 2006;154:260–268. doi: 10.1016/j.jsb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 78.Wang J., Zhou P., Wang X., Yu Y., Zhu G., Zheng L., Xu Z., Li F., You Q., Yang Q., et al. Rab25 promotes erlotinib resistance by activating the β1 integrin/AKT/β-catenin pathway in NSCLC. Cell Prolif. 2019;52:12592. doi: 10.1111/cpr.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiong J., Li S., Zeng X. High Rab25 expression associates with Ki67/TP53/CD133/VEGFR expression predicts poor prognosis in gastric cancer. Int. J. Clin. Exp. Pathol. 2017;10:7792–7800. [PMC free article] [PubMed] [Google Scholar]
- 80.Gomez-Roman N., Sahasrabudhe N.M., McGregor F., Chalmers A.J., Cassidy J., Plumb J. Hypoxia-inducible factor 1 alpha is required for the tumourigenic and aggressive phenotype associated with Rab25 expression in ovarian cancer. Oncotarget. 2016;7:22650–22664. doi: 10.18632/oncotarget.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng J.M., Volk L., Janaki D.K., Vyakaranam S., Ran S., Rao K.A. Tumor suppressor function of Rab25 in triple-negative breast cancer. Int. J. Cancer. 2010;126:2799–2812. doi: 10.1002/ijc.24900. [DOI] [PubMed] [Google Scholar]
- 82.Goldenring J.R., Nam K.T. Rab25 as a tumour suppressor in colon carcinogenesis. Br. J. Cancer. 2011;104:33–36. doi: 10.1038/sj.bjc.6605983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hopkins K.J., Wang G., Schmued L.C. Temporal progression of kainic acid induced neuronal and myelin degeneration in the rat forebrain. Brain Res. 2000;864:69–80. doi: 10.1016/S0006-8993(00)02137-5. [DOI] [PubMed] [Google Scholar]
- 84.Chen D., Guo J., Gahl W.A. RAB GTPases expressed in human melanoma cells. Biochim. Biophys. Acta. 1997;1355:1–6. doi: 10.1016/S0167-4889(96)00169-3. [DOI] [PubMed] [Google Scholar]
- 85.Wang C., Liu Z., Huang X. Rab32 is important for autophagy and lipid storage in Drosophila. PLoS ONE. 2012;7:e32086. doi: 10.1371/journal.pone.0032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Drizyte-Miller K., Chen J., Cao H., Schott M.B., McNiven M.A. The small GTPase Rab32 resides on lysosomes to regulate mTORC1 signaling. J. Cell Sci. 2020;133 doi: 10.1242/jcs.236661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Esnaola N.F., Ford M.E. Racial differences and disparities in cancer care and outcomes: Where’s the rub. Surg. Oncol. Clin. N. Am. 2012;21:417–437. doi: 10.1016/j.soc.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deshmukh S.K., Azim S., Ahmad A., Zubair H., Tyagi N., Srivastava S.K., Bhardwaj A., Singh S., Rocconi R.P., Singh A.P. Biological basis of cancer health disparities: Resources and challenges for research. Am. J. Cancer Res. 2017;7:1–12. [PMC free article] [PubMed] [Google Scholar]
- 89.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y.T., Gou Y.W., Jin W.W., Xiao M., Fang H.Y. Association between alcohol intake and the risk of pancreatic cancer: A dose-response meta-analysis of cohort studies. BMC Cancer. 2016;16:212. doi: 10.1186/s12885-016-2241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dragomir M., Calin G.A. Circular RNAs in Cancer—Lessons Learned From microRNAs. Front. Oncol. 2018;8:179. doi: 10.3389/fonc.2018.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hansen T.B., Kjems J., Damgaard C.K. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73:5609–5612. doi: 10.1158/0008-5472.CAN-13-1568. [DOI] [PubMed] [Google Scholar]
- 93.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6 doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Hureta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.