Abstract
Mounting preclinical and clinical evidence indicates that rewiring the host immune system in favor of an antitumor microenvironment achieves remarkable clinical efficacy in the treatment of many hematological and solid cancer patients. Nevertheless, despite the promising development of many new and interesting therapeutic strategies, many of these still fail from a clinical point of view, probably due to the lack of prognostic and predictive biomarkers. In that respect, several data shed new light on the role of the tumor suppressor phosphatase and tensin homolog on chromosome 10 (PTEN) in affecting the composition and function of the tumor microenvironment (TME) as well as resistance/sensitivity to immunotherapy. In this review, we summarize current knowledge on PTEN functions in different TME compartments (immune and stromal cells) and how they can modulate sensitivity/resistance to different immunological manipulations and ultimately influence clinical response to cancer immunotherapy.
Keywords: PTEN, cancer, immune cells, immunoevasion, immunotherapy
1. Introduction
Tumorigenesis is a genetic/epigenetic process driven by oncogene activation and tumor suppressor gene inactivation [1]. Phosphatase and tensin homolog on chromosome 10 (PTEN) is one of the tumor suppressors most frequently inactivated in human cancer, due to genetic alterations or transcriptional/post-transcriptional inhibition; moreover, even a partial loss of its function (haploinsufficiency) may cause neoplastic transformation [2,3,4]. Hence, we will refer to either genetic mutations or protein lost as “PTEN-loss” [5]. The PTEN protein mainly acts as a lipid phosphatase, which converts phosphatidylinositol 3, 4, 5 trisphosphate (PIP3) into phosphatidylinositol 4, 5-bisphosphate (PIP2), counteracting the activity of the phosphoinositide 3-kinase (PI3K) and resulting in the inhibition of cell proliferation, survival and migration [2,6].
The regulation of PTEN expression and function in cancer cells is extremely complex and the recognition of its role as a predictive/prognostic biomarker is hampered by the lack of unequivocal methods to ascertain whether the protein is non-functional, or present [4,6,7]. A further, emerging level of complexity is related to the possibility that the loss of PTEN function may directly or indirectly influence not only cancer cell behavior, but also the tumor microenvironment (TME) and immune-infiltrate composition and function [8]. Since the interaction between cancer and stromal/immune cells may result in a tumor-permissive or non-permissive TME, PTEN activity is in a crucial position to control the overall effects of such interactions.
Immune escape represents one of the hallmarks of cancer and can be determined by a combination of relatively low cancer cell immunogenicity and tumor-dependent immunosuppression [9]. The recognition of the role of immune checkpoints, particularly cytotoxic T-lymphocyte antigen (CTLA)-4 and programmed cell death (PD)-1, has shed new light on the mechanisms of negative regulation of the immune system and opened a new era in the clinical application of immunotherapy in cancer [10,11]. In such a complex scenario, the continuous bidirectional interactions between cancer cells and infiltrating immune/inflammatory cells are dictated, at least in part, by the genetic background of the different cancerous and non-cancerous components. Such interactions, in turn, may crucially determine the sensitivity or resistance to immunotherapeutic approaches and provide novel targets for an effective treatment.
In this review, we focus on the possible implications of PTEN expression and function within different TME compartments (non-cancer cells and soluble factors), in order to better understand potential mechanisms underlying immunotherapy resistance.
2. Current Status of Clinical Cancer Immunotherapy
Immunotherapy is approved for the treatment of an ever-expanding array of advanced malignancies of different histological origin [12]. According to their mode of action, immunotherapies can be categorized into strategies that stimulate the effector machinery or neutralize immunosuppression mechanisms (summarized in Table 1) [13].
Table 1.
Immunotherapy strategies against cancer.
| Strategy | Biological Target(s)/Aim | Drug | Combination(s) | Effects on Tumor Growth | Ref | |
|---|---|---|---|---|---|---|
| Effector mechanisms stimulation | Vaccine | DCs | Sipuleucel-T (2010) | − | [14] | |
| Tumor antigens | n.a. | [15,16] | ||||
| Nucleic acids | n.a. | [15,16] | ||||
| Oncolytic virus | Cancer cells lysis and increased immune response | Talimogene laherparepvec (T-VEC) | − | [17,18] | ||
| Adoptive T cells | TILs | ± | [19,20] | |||
| CAR-T | Kymriah and Yescarta (2017) | − | [21] | |||
| Immunosuppressive mechanisms counteraction | Tregs inhibition | IL-2/CD25/chemotherapeutics | Cyclophosphamide | ± | [22,23,24] | |
| Inhibitory checkpoint blockade | CTLA-4 PD-1/PD-L1 |
Ipilimumab plus nivolumab (2018) | CTLA-4 plus PD-1 | − | [25] | |
| BMS-986207 plus nivolumab (NCT02913313) | II generation immunotherapy targets (LAG-3/TIM-3/TIGIT) | u.i. | [26,27] | |||
| Costimulatory receptors (4-1BB) |
± | [28,29,30] | ||||
| Conventional therapies | − | [31,32] | ||||
| Epigenetic modulators | u.i. | [33] | ||||
+, tumor progression; −, tumor regression; CAR-T, chimeric antigen receptor T cells; CTLA-4, cytotoxic T-lymphocyte antigen 4; DCs, dendritic cells; IL, interleukin; LAG, lymphocyte activation gene; n.a., not assessed; PD-1, programmed cell death-1; PD-L1, program death ligand; TIGIT, T cell immunoglobulin and ITIM domain; TILs, tumor-infiltrating lymphocytes; TIM, T-cell immunoglobulin mucin; Tregs, regulatory T cells; u.i., under investigation.
2.1. Stimulation of the Effector Machinery
Conventional preventive vaccines are used in healthy individuals to prevent infectious diseases. Conversely, therapeutic cancer vaccines are used in patients already affected by the disease, to prevent cancer progression, by boosting the action of the immune system [13]. Despite the failure of many cancer vaccine trials, two types of cancer vaccines are currently approved, an autologous dendritic cell (DC)-based vaccine for prostate cancer (Sipuleucel-T) and an oncolytic herpesvirus-based vaccine for metastatic melanoma (T-VEC) (revised in [15,16]). Adoptive T-cell transfer represents an alternative therapeutic strategy which employs ex vivo-activated or genetically engineered immune system cells, such as tumor-infiltrating lymphocytes (TILs) and chimeric antigen receptor T-cells (CAR-T) [34]. While the potential of TILs is currently limited to melanoma, CAR-T have shown great promise, particularly in hematological malignancies; conversely, their use to treat solid tumors has been limited so far, possibly due to immunosuppressive interactions between the TME and CAR-T [19,20,35,36].
2.2. Counteract the Immunosuppressive Mechanisms
The recognition of the biological role and therapeutic potential of immune checkpoint blockade (through CTLA-4 and PD-1 targeting) has revolutionized our approach to cancer immunotherapy, as also highlighted by the 2018 Nobel Prize award [11,37]. Many different monoclonal antibodies (mAb) targeting CTLA-4, PD-1, or PD ligand (PD-L)1, such as ipilumumab and nivolumab, pembrolizumab, atezolizumab, durvalumab and avelumab, have been approved for the treatment of specific cancers [25,38,39]. Although at higher risk of important immune-related toxicity, the combined inhibition of CTLA-4 and PD-1 signaling, using ipilimumab and nivolumab, has shown increased clinical efficacy in melanoma, kidney and lung cancer. Moreover, a second generation of immune checkpoint inhibitors (revised in [40]) is underway: among others, lymphocyte activation gene (LAG)-3, T-cell immunoglobulin mucin (TIM)-3, and T cell immunoglobulin and ITIM domain (TIGIT) represent the most promising cancer therapeutic targets, expressed by natural killer (NK), CD4+ and CD8+ T cells, as redundant co-inhibitory receptors [26,41,42,43]. Another combinatorial therapeutic strategy aims at both blocking inhibitory signals and stimulating activation/co-stimulatory signals [44]. For example, preclinical studies reported encouraging data for the simultaneous engagement of the co-stimulatory receptor 4-1BB (also known as CD137 or TNFSF9) in combination with an anti-PD-1 blockade [45].
3. PTEN in Immunoevasion
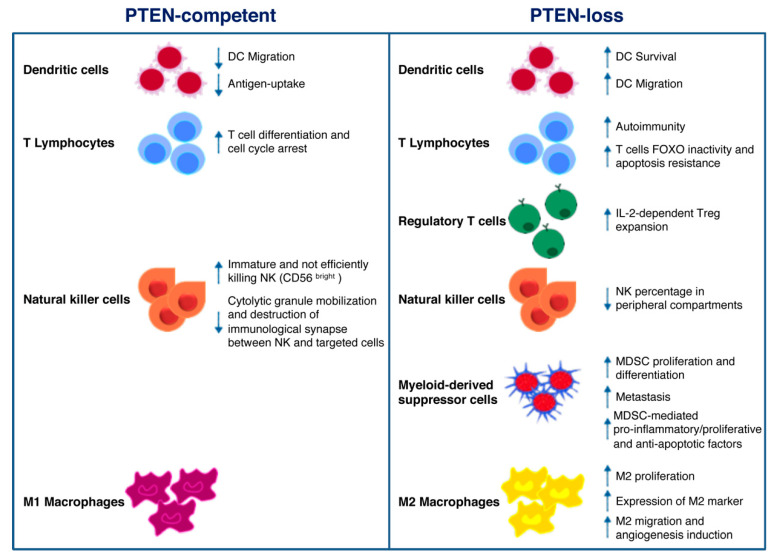
Mounting evidence suggests that PI3K signaling may influence the composition and functionality of the TME, thereby modulating immune response in cancer [46]. In particular, PTEN expression (or lack thereof) in cancer cells attracts different immune cell populations to the TME; on the other hand, PTEN function in immune cells regulates their activation status [8]. As schematically depicted in Figure 1, the overall effect of PTEN loss of function in different cellular compartments shifts the balance towards an immunosuppressive TME [47,48,49]. Here, we give a comprehensive overview of the potential role of PTEN in the regulation of the immunosuppressive aspects of the TME (Figure 1).
Figure 1.
Schematic illustration of phosphatase and tensin homolog on chromosome 10 (PTEN) function in immune cells. PTEN modulates several microenvironmental stimuli and immune cells processes, according to the cell type in which it is expressed (left panel) or not. As for cancer cells, the lack of PTEN activity mainly correlates with immune escape mechanisms and protumoral immune cells infiltration/expansion (right panel).
3.1. PTEN Role in Immune Cells
DCs are professional antigen-presenting cells (APCs) implicated in adaptive immunity, through foreign antigens processing and the subsequent major histocompatibility complex (MHC)-dependent presentation. This mechanism results in the stimulation of naïve T cells and cytotoxic effector cells (i.e., macrophages, NKs) function [50,51]. Failure to effectively present antigens or functional deficiencies in infiltrating DCs contributes to immune suppression [51]. The PI3K pathway plays a key role in DC functions. Indeed, DCs derived from PI3Kγ−/− mice display reduced ability to migrate in response to chemoattractants; similarly, antigen-loaded DCs also show decreased ability to move to lymph nodes [52]. Higher PTEN levels were observed in DCs of elderly, as compared to young, subjects, resulting in reduced AKT activation, antigen-uptake, and DC migration [53]. Based on the above evidence, targeting PTEN in DC-based cancer vaccines could represent a promising approach in immunotherapy. The advantageous effects exerted by the use of PTEN-silenced DCs were related not only to increased DC survival and CCR7-dependent migration, but also to enhanced CD8+ numbers [54]. Pan and collaborators demonstrated that PTEN cooperates in the negative regulation of Dectin-1 and FcεRI γ-chain (FcRγ)-mediated signaling. FcRγ represents an adapter of immunoreceptor tyrosine-based activation motif (ITAM) on DCs and Dectin-1 is a receptor containing an ITAM-like domain involved in DC function in the immune response. Though mostly implicated in antifungal response and leucocyte recognition, the authors underline the importance of PTEN-mediated negative regulation and hypothesize that PTEN targeting may be used as a strategy for the development of new cancer therapy approaches [55].
By modulating antigen receptor expression and cytokine release, PI3K signaling represents a central hub in the regulation of normal T cells differentiation into either cytotoxic CD8+ or helper CD4+. As a consequence of PIP3 production and mammalian target of rapamycin (mTOR) complex 2 phosphorylation, the transcription factor forkhead box (FOX)O translocates from the nucleus to the cytoplasm, resulting in the decreased expression of genes involved in cell cycle arrest and T cell differentiation [56]. PTEN-loss results in a persistent FOXO inactivation and resistance to apoptotic cytokine-mediated signaling, as often detected in hemangiomas and leukemias [57]. Moreover, PTEN heterozygous (PTEN+/−) mice are deficient in Fas-mediated apoptosis: as the binding of Fas (also known as CD95) to its ligand (FasL) is the main mechanism by which CD8+ cytotoxic T-cells kill non-self-cancer cells, PTEN haploinsufficiency results in lymphoid hyperplasia and tumor growth [58,59]. In CD4+ helper T cells, on the other hand, PTEN expression patterns result in opposite effects, according to the timing of PTEN gene inactivation. Indeed, thymocyte-specific PTEN deletion causes lymphomas and autoimmunity, whereas activated T cells hyper-proliferate and over-express cytokines in the presence of PTEN-loss [60,61].
PI3K signaling also regulates CD4+ regulatory T cell (Treg) functions. After T cell receptor engagement and cell activation, PTEN is down-regulated, and this results in interleukin (IL)-2 dependent PI3K signaling and Tregs expansion. On the other hand, IL-2 is able to promote PI3K pathway activation, despite high levels of PTEN, leading to T cell anergy. Overall, the loss of PTEN function cooperates with CD25 stimulation by IL-2 to promote Treg proliferation in a clinical setting [62,63]. PTEN expression may also inhibit T cell response through intercellular binding of PD-1/neurophilin-1 (Nrp-1) and PDL-1/semaphorin-4 (Sema4a) on Tregs and effector cells surface, respectively. As demonstrated by Francisco and coworkers, PD-1 upregulation attenuates PI3K activation during Tregs maturation [64]. It has also been demonstrated that the Sema4a/Nrp1 axis attenuates Tregs-mediated antitumor immune response: the binding of the ligand Sema4a, expressed on immune cells, prevents AKT activation through PTEN-dependent FOXO3a nuclear localization [65]. Another immunosuppressive mechanism, potentially influenced by PTEN, is mediated by the indoleamine 2, 3-dioxigenase (IDO) enzyme, which prevents tryptophan-mediated immunological stimulus and promotes kynurenine-dependent immune effector cells disruption. Intricate feedback signals exist, by which IDO cooperates with PD-1 to promote an immunosuppressive Tregs phenotype. IDO expression in DCs and APCs activates PTEN signaling in Tregs, thus blocking the PI3K cascade and increasing the activity of FOXO1 and FOXO3a, which in turn upregulate PD-1 and PTEN [66]. PTEN inactivation after PD-1 blockade abrogates FOXO-dependent immunosuppression and results in cancer regression [67,68,69].
CD56+/CD3− large granular NK cells recognize and destroy both infected and transformed cells. A variety of activating stimuli is necessary to fully activate the NK cytotoxicity: once activated, NK cells release perforin, granzymes, and antitumoral immune response-promoting cytokines (e.g., IFN-γ and tumor necrosis factor (TNF)-α) and induce apoptosis of targeted cells, through FasL/Fas or TNF-related apoptosis-inducing ligand (TRAIL)/TRAIL receptor interactions [70]. Consistently, a marked depletion of their number/activity correlates with increased risk of developing cancer and cancer progression [60]. Immature CD56bright NK cells express higher levels of PTEN, as compared to cytotoxic, CD56dim cells, suggesting a specific role of PTEN in NK cytotoxicity. In this context, PTEN disrupts the immunological synapses between NKs and targeted cells, by decreasing actin accumulation, polarization of microtubules and cytolytic granule mobilization [71]. Another group demonstrated that PTEN is also involved in NK activity by affecting their trafficking and localization in vivo. Indeed, the authors showed a decreased percentage of mature NK cells in peripheral compartments in PTEN-knock out (ko) NK mice [72]. Finally, PTEN is a central player in NK cells activation, through inhibition of the PIP3-mediated signaling cascade (extensively revised in [73]).
Myeloid-derived suppressor cells (MDSCs) are among the key actors involved in mediating tumor escape mechanisms in the TME, due to their specific ability to abolish T cell and NK functions [74,75]. It has been demonstrated that a complex network of micro-RNAs (miRs) regulates MDSCs activation and functions in the TME, thus opening new scenarios for novel immunotherapy approaches [76]. Tumor-derived transforming growth factor (TGF)-β1 upregulates miR-494, which in turn regulates MDSCs activity and localization. PTEN is a direct target of miR-494 and its downregulation, with the consequent activation of PI3K pathway, is involved not only in C-X-C chemokine receptor (CXCR)4-mediated MDSCs chemotaxis towards tumors, but also in metastases formation due to upregulation of metalloproteinases (MMP) (e.g., MMP2, MMP13) [77]. Moreover, TGF-β is also involved in modulating MDSCs expansion through the inhibition of PTEN and SH-2 containing inositol 5′ polyphosphatase (SHIP)1. TGF-β-treated bone marrow-MDSCs display lower levels of PTEN and SHIP-1, regulated by miR-21 and miR-155 respectively, and higher levels of signal transducer and activator of transcription (STAT)3: all these perturbations result in an increased number of MDSCs [78]. Several authors also elucidated the importance of the PTEN mRNA 3′UTR as the “seed sequence” recognized by specific miRs, in the regulation of MDSC proliferation and activity [79,80]. Mei and collaborators demonstrated that, among the soluble factors produced by tumor cells, the granulocyte–macrophage colony-stimulating factor is the main inducer of miR-200c. This miR is involved in the upregulation of MDSC-immunosuppressive functions and proliferation, by directly targeting the 3′UTR of both PTEN and friend of Gata 2 (FOG2). Inhibition of PTEN and FOG2, in turn, activates PI3K leading to the MDSC differentiation, and STAT3, involved in the production of pro-inflammatory, pro-proliferative and anti-apoptotic factors [79]. Interestingly, tumor-derived exosomes may exert similar effects on PTEN regulation in MDSCs. Indeed, miR-107 delivered by gastric cancer-derived exosomes exerts its activity by inhibiting the functions of PTEN and Dicer1, through the binding to the 3′UTR region, thus resulting in MDSC proliferation and acquisition of ARG-1-mediated suppressive function [80,81]. Similarly, glioma-derived exosomes act as shuttles for miR-21: the consequent inhibition of PTEN and activation of STAT3 promote bone marrow-derived MDSC proliferation and differentiation. Moreover, PTEN-silenced MDSCs upregulate the production of the immunosuppressive cytokine IL-10 [81].
Macrophages are conventionally classified into two categories according to the membrane receptors and their functions: M1 and M2 (or tumor-associated macrophages—TAM), endowed with tumor-suppressor and tumor-promoting properties, respectively. M2 macrophages are the most represented leukocytes in cancer stroma, representing up to 50% of the immune cells in the TME [46]. Li and colleagues showed that PTEN-silencing in macrophages causes an increased release of C-C motif chemokine ligand (CCL)-2 and vascular endothelial growth factor (VEGF)-A, promoting the M2 phenotype switch [82]. Moreover, PTEN deletion results in the expression of specific M2 markers, such as the immunomodulating protein arginase I [83]. By enhancing arginase I, M2 macrophages reduce T cell proliferation, hence promoting T cell anergy [84]. A recent paper demonstrated that PTEN expression, regulated by miR networks, can modulate macrophage polarization. Supernatants derived from human glioblastoma cells upregulated miR-32, which in turn interacts and suppresses PTEN in an in vitro model of human monocytes, thus promoting the M2 phenotype and resulting in an enhanced cell proliferation [85]. miR-21 reduces the expression of PTEN and its upstream positive regulator miR-200c in primary macrophages, promoting their differentiation into M2 [86]. Exosomes derived from pancreatic cancer cells promote hypoxia-inducible factors (HIF)-1α/HIF-2α-dependent M2 phenotype through PTEN regulation: miR-301a-3p converts stromal macrophages into M2 macrophages and promotes lung metastases formation by blocking PTEN transcription [87]. Hypoxic lung tumors release miR-103a which blocks PTEN activity: the consequent PI3K/AKT activation induces M2 polarization, with high ability to migrate and regulate angiogenesis [88]. In a very intricate bidirectional crosstalk between cancer cells and the surrounding TME, under hypoxic conditions, epithelial ovarian cancer TAMs release miR-223, which in turn regulates PTEN, promoting ovarian cancer cell proliferation and drug resistance, through PI3K activation [89].
3.2. PTEN Role in Stromal Cells
PTEN genetic inactivation in fibroblasts has extensively been studied in breast cancer [90,91,92]. Trimboli and coworkers demonstrated that PTEN-loss in fibroblasts results in an increased incidence of HER2-driven breast tumor, innate immune cells infiltration and VEGF-dependent angiogenesis. Indeed, PTEN-loss results in Ets2 phosphorylation and inactivation, thus allowing for the activation of a specific transcriptional program, associated with a more aggressive tumor behavior [90]. Moreover, the lack of PTEN in mammary stromal fibroblasts is associated with modulation of both fibroblasts and other surrounding TME cells. PTEN-loss modifies the oncogenic secretome through the downregulation of miR-320, hence reprogramming both endothelial and epithelial cells towards a more malignant phenotype [91]. The specific deletion of PTEN in murine fibroblasts promotes collagen deposition and parallel alignment of cellular matrix (a feature observed also in patients with breast cancer) even in the absence of cancer cells. Furthermore, PTEN-loss in fibroblasts fosters cancer-associated fibroblasts-like behaviors, such as upregulation of α-smooth muscle actin and MMP activity [92]. Mechanisms of PTEN inactivation have been investigated in pancreatic ductal adenocarcinoma (PDAC), using high throughput techniques. A recent study showed a surprisingly high percentage (40%) of PTEN-loss (due to partial deletion of chromosome 10) in stromal cells, while PTEN loss of heterozygosity was observed in 46.6% of tumors and juxta-tumoral stroma in PDAC patients. In addition, miR-21 overexpression in juxta-tumoral stroma cooperates with PTEN inactivation, even in the absence of PTEN mutations. These observations correlate with aggressive tumor features and worse prognosis [93]. PTEN-loss in PDAC fibroblasts is usually associated with the ablation of the smoothened (Smo) gene, resulting in PTEN degradation by the E3 ubiquitin ligase RNF5: proteasome-dependent PTEN inactivation leads to increased proliferation and reduced overall survival (OS) [94].
As widely recognized, PTEN also modulates angiogenesis [95]. Tian and collaborators demonstrated that transfection of HepG2 cells with wild type (wt) PTEN suppresses the expression of VEGF in a HIF-1-dependent manner. Transfection with a PTEN construct lacking the C2 phosphatase domain also resulted in downregulation of both VEGF and angiogenesis in vitro and in vivo, albeit to a lesser extent as compared with the complete PTEN construct. This evidence suggests a possible cooperation between phosphatase-dependent and -independent PTEN functions in regulating angiogenesis [96]. The tight link between PI3K/PTEN activation and VEGF was also investigated by our group: indeed, we demonstrated that the hyperactivation of PI3K pathway leads to HIF-1/2 translation and subsequent expression of VEGF [97]. Dong and collaborators showed that PTEN-defective melanoma cell lines express several cytokines, including VEGF, and their expression is transcriptionally inhibited by the PI3K inhibitor LY294002 [98]. A similar correlation was identified also for another pro-angiogenic factor: de la Iglesia and co-workers demonstrated that STAT3-dependent IL-8 expression occurs only in PTEN-loss glioblastoma contexts [99].
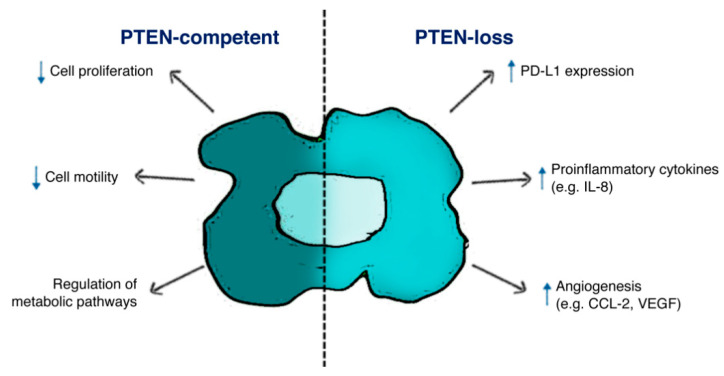
3.3. Tumor PTEN Affects Immune Infiltrate
A wealth of evidence suggests a central role of tumor PTEN status in modulating the TME immune infiltrate and cancer cells/TME interactions in different tumor histotypes (Figure 2).
Figure 2.
Schematic illustration of PTEN function in tumor cells. PTEN-status in cancer cells affects both biological features in cancer cells survival and tumor microenvironment (TME) composition, according to the expression of specific ligands (i.e., PD-L1) and soluble factors (i.e., IL-8, CCL-2, VEGF).
In mice and human lung squamous cell carcinoma, the TME of PTEN-loss samples is characterized by the specific accumulation of tumor-associated neutrophils and Tregs, involved in different processes such as angiogenesis and immunosuppression. Moreover, low levels of TAMs, NKs, T and B cells during tumor progression were observed according to increasing tumor burden [100]. In vitro experiments in PTEN-silenced triple negative breast cancer (TNBC) show that low levels of infiltrating CD4+ and CD8+ T cells are due to the induction of apoptotic mechanisms associated with PD-L1 expression and PTEN-loss [101]. In many instances, tumor genetic background, PTEN-loss in particular, shapes an immunosuppressive TME through the production of specific soluble factors, which in turn modify stromal/immune cells infiltration. Data from our and other groups suggest that PTEN-loss in prostate epithelium, glioblastoma and colorectal cancer (CRC) cells promotes a selective increase in IL-8 expression [99,102,103,104,105]. IL-8 production, in turn, correlates with an immunosuppressive, myeloid-enriched, TME and unequivocally with an adverse cancer prognosis, particularly for patients undergoing immunotherapy. In CRC patients, serum IL-8 levels are associated with activation of a gene expression program which enforces a monocyte-/macrophage-like phenotype in CD4+ T cells [106]. FOXP3 CD4+ Tregs markedly express the IL-8 receptor CXCR1, in order to respond to tumor-derived IL-8 and migrate in cancer tissue, and CD4+ T cells produce IL-8 themselves [107,108]. On the other hand, CXCR1 expression is downregulated on CD8+ T cells after antigen presentation, thereby reducing the number of cytotoxic T cells [109]. Interestingly, a recent paper reported that mesenchymal stem cells release IL-8, which activates c-Myc via STAT3 and mTOR signaling in gastric cancer cells, hence resulting in membrane PD-L1 overexpression and blockade of cytotoxic effects of CD8+ T cells. In this complex crosstalk between different cell population, neutralizing IL-8 could enhance the efficacy of PD-L1 antibodies [110]. In a cohort of 168 resected CRC patients, our group recently demonstrated that PTEN-loss in cancer cells significantly correlates with high levels of tumor-derived IL-8 and low levels of IL-8+ infiltrating mononuclear cells (Conciatori 2020, unpublished data, [103]).
Toso and coworkers showed that in PTEN-loss prostate cancer activation of JAK2 and phosphorylation of STAT3 induce the production of specific chemokines, which recruit an increased number of infiltrating MDSCs [111]. Similarly, in PTEN-loss melanoma xenograft models the upregulated production of soluble factors (e.g., CCL2, VEGF) recruits and/or regulates the function of suppressing immune cells (e.g., immature DCs, MDSCs), thus promoting an immunosuppressive TME. In metastatic melanoma patients, nanostring-based analysis of the TME inflammatory cells showed an 83% reduction in inflammation-related genes associated with the PTEN-loss status of cancer cells [112]. In a HRASG12V/PTEN−/− follicular thyroid carcinoma mouse model immunosuppressive Tregs and M2 macrophages were significantly upregulated. Moreover, cell lines isolated by HRASG12V/PTEN−/− express higher levels of chemotactic factors for MDSCs, T cells and macrophages as compared to BRAFV600E/PTEN−/− tumors, highlighting the tight association between genetic background and tumor immune infiltrate [113]. Tumor genetic background influences immune/inflammatory infiltrate into the TME not only in the primary tumor, but also in metastatic lesions, as demonstrated by Vidotto et al. Indeed, the authors showed that in PTEN-loss prostate cancer, higher levels of Tregs were observed in liver metastases as compared to the primary lesion, and high levels of CD8+ cells were present in bone metastases [114].
4. PTEN and Immunotherapy
4.1. PTEN and Conventional Immune Checkpoint Inhibitors
In recent years, many steps have been taken to increase the number of patients who may benefit from immunotherapy by understanding the mechanisms behind the primary and acquired resistance [115]. PTEN expression and function may contribute to the regulation of immune homeostasis and sensitivity/resistance to immune checkpoint inhibition-based therapeutic strategies. An inverse correlation between PD-L1 and PTEN expression has been demonstrated, both in vitro, using PTEN-silenced cellular models, and in vivo. In a series of 404 CRC patients PTEN-loss and PD-L1 overexpression significantly correlated with poor OS and higher tumor, node, metastasis (TNM) stage [116]. Conversely, treatment with an anti-PD-L1 antibody decreases tumor growth by upregulating PTEN levels in both pancreatic cancer tissue and liver metastases in mice models [117]. In vitro experiments show that PTEN-loss or PI3K pathway activation increase PD-L1 levels in glioma cell lines, resulting in decreased sensitivity to T cell-mediated killing [118]. Moreover, Waldron and colleagues correlated PTEN status with autologous T cell apoptosis in glioblastoma patients. PTEN-loss in glioblastoma cells fosters CD3+ T cells apoptosis upon cell-cell contact and such effect can be reverted by pre-treatment with PI3K/AKT pathway inhibitors, suggesting a possible therapeutic strategy encompassing PI3K/AKT-targeted inhibitors in combination with immunotherapy [119]. Along these lines, a recent case report analyzed drug response of a metastatic non-small-cell lung cancer (NSCLC) patient, whose tumor lacked PTEN expression: the authors described immunotherapy-resistance despite high levels of PD-L1 expression, while the tumor responded to rapamycin analogs [120]. Consistently, treatment with either AKT or mTOR inhibitor resulted in decreased PD-L1 expression in PTEN-loss TNBC: PD-L1 reduction, and the consequent increased T cell proliferation and apoptosis, could enhance antitumor immunity [101].
Despite the presence of high levels of PD-L1 in PTEN-loss tumor, a paradoxical resistance to PD-1 blockade often occurs in PTEN-loss contexts [121]. A recent study in a cohort of glioblastoma patients treated with an anti-PD-1 mAb (nivolumab or pembrolizumab) upon recurrence revealed that a greater percentage of PTEN mutations is observed in non-responders (72%), as compared to responders (23%) and that PTEN-loss is also associated with a specific immunosuppressive signature. In particular, in PTEN-loss patients the immunosuppressive TME is characterized by both high levels of CD68+/HLA-/DR- macrophages and the absence of immune infiltration [122]. Similar results were recently reported by Chen and coworkers: they analyzed all somatic genetic mutational status of 113 NSCLC patients in response to CTLA-4/PD-1 blockade and detected PTEN mutations exclusively in non-responders, albeit at a low frequency (6.8%) [123]. Failure to respond to the immune checkpoint blockade in the presence of PTEN-loss was widely described also in metastatic melanoma. A recent analysis conducted in metastatic melanoma patients treated with an anti-CTLA-4 followed by an anti-PD-1 mAb, identified recurrent loss of different tumor suppressor genes, including PTEN, in double non-responder patients. Among the tumor suppressor genes analyzed, PTEN-loss appeared to drive resistance to CTLA-4 blockade in particular [124]. However, published data conflict in regard to the correlation between PTEN status and anti-CTLA-4 response; in that respect, it is essential to keep in mind that cellular signaling cascades are closely intertwined with each other. For example, PTEN regulates the Src family: the combination of the Src family kinases inhibitor dasatinib and anti-CTLA-4 mAb reduces the number of MDSCs and Tregs, increases CD8+ T cells and thus results synergistic only in TGF-βR1/PTEN-loss mice [125,126]. A further example of crosstalk between PTEN and other pathways in regulating sensitivity/resistance to therapeutic immune checkpoint targeting is the interaction between PTEN expression and DNA mismatch repair (MMR): in a report focusing on PTEN immunohistochemistry (IHC) as a diagnostic tool for the screening of MMR-proficient breast cancer, PTEN-integrity seems to represent a protective factor against MMR deficiency in breast cancer [127].
Dynamic clonal tumor heterogeneity, often due to the selective pressure of specific drug treatments, further complicates the dissection of how the tumor genetic landscape influences immune homeostasis and response to immunotherapy. In that respect, a recent study on melanoma patients analyzed biopsies derived from patients who initially responded to either anti-PD-1 or anti-PD-1 plus anti-CTLA-4 mAb. The authors identified an association between PTEN mutations and patients who no longer respond to immunotherapy (28%). Among negative checkpoint modulators, V-domain Ig suppressor of T cell activation (VISTA) represents an interesting inhibitory immune-checkpoint protein, highly expressed on tumor-infiltrating leucocytes [128]. Resistance to anti-PD-1 was associated not only to decreased levels of the MHC components HLA-A and HLA-DPB1, but also to the accumulation of VISTA+ lymphocytes in biopsies taken at progression, as compared to pre-treatment biopsies [129]. The discrepancy between the percentage of PTEN-loss cases, as analyzed by IHC, and the high percentage of PD-L1 and VISTA+ lymphocytes (28% vs. 61% and 67%, respectively) in melanoma biopsies at progression suggests that the PTEN protein might be inactive, albeit present, in this context. More recently, Trujillo and his group carried out a similar analysis in order to define the characteristics of treatment-resistant metastases after combined anti-CTLA-4 plus anti-PD-1 therapy. This study shows that acquired immune resistance is mediated by: (i) T cell response insufficiency or tumor escape mechanisms (i.e., loss of antigens expression); (ii) overcoming of a specific memory-response against tumor cells, due to biallelic PTEN mutation and poor T cells infiltration [130]. In this regard, acquired immune resistance to anti PD-1 therapy is associated with changes in neoantigen presentation in the presence of biallelic PTEN-loss in patients affected by metastatic uterine leiomyosarcoma [131].
4.2. PTEN and Non-Conventional Immunotherapy
Even responses to the new immunotherapy approaches being developed (Table 1) might be influenced by the tumor genetic background, in particular by PTEN status, although data available are limited to in vitro and mice models [132]. One emerging approach exploits the use of OX40 agonist-based combinations, as investigated by Peng and collaborators. Based on results obtained in PTEN-loss/BRAF-mut melanoma, the authors demonstrated that a combination of OX40 receptor agonists and PI3K inhibitors successfully improves tumor immune response by promoting the proliferation and cytotoxicity of CD8+ T cells at the tumor site [133]. Similar results were also obtained by Kroon et al., who demonstrated that concomitant targeting of PD-1 and CD137 improves radiotherapy efficacy, in a BRAFV600E/PTEN-loss melanoma mouse model. This promising effect is mediated by an increased percentage of infiltrating CD8+ and CD4+ T cells, as demonstrated by high levels of granzyme B-expressing cells [134]. Such preclinical evidence could collectively represent a rationale for the treatment of PTEN-loss melanoma, highlighting the possibility to tailor treatment based on the best predicted response. An anti-TIM-3 mAb was investigated for its efficacy in TGF-βR/PTEN-loss head and neck squamous cell carcinoma transgenic mice. In the absence of treatment, these mice are characterized by both high percentage of TIM-3 Tregs and M2 macrophages; conversely, anti-TIM-3 mAb treatment significantly reduces the Tregs population but not M2 macrophages, hence enhancing the antitumor immune response [135].
CD20 represents a B cells marker expressed during B cells differentiation and rituximab, an anti-CD20 mAb approved by food and drug administration (FDA) in 1998, is used in cancer and autoimmune disease treatment for B cells depletion [136]. Gupta and collaborators demonstrated a link between PTEN and PI3K pathway activation and the response of human lymphoma cell lines to hexavalent antibodies (HexAbs) (anti-CD20, anti-CD22 and anti-CD22-20). HexAbs upregulate p38 and PTEN levels, leading to the activation of pro-apoptotic signals; moreover, the use of the PI3K inhibitor LY294002 increases apoptosis induced by HexAbs [137]. More recently, Baritaki and coworkers observed a similar response using the anti-CD20 mAb LFB-R603 in lymphoma cell lines: LFB-R603 inhibits both SNAIL and NF-κB and induces RKIP and PTEN, which in turn restores TRAIL-dependent apoptosis in TRAIL resistant non-Hodgin’s B-cell lymphoma [138].
Recent data in PTEN-loss mice developing head and neck tumors show that the upregulation of LAMTOR5 (a novel oncoprotein belonging to PI3K pathway) is associated with not only poor OS and lymph node grade, but also with F4/80 (a marker of mice macrophages) and VISTA levels. Consistently, the expression of VISTA as a T lymphocyte-activation inhibitor is associated with immunosuppressive microenvironment [139]. VISTA+ lymphocyte accumulation is also observed in melanoma patients with acquired resistance to immune checkpoint inhibitors and PTEN mutation. Such evidence could constitute the rationale of novel therapeutic strategies targeting VISTA in selected PTEN-loss melanoma patients [129].
5. Conclusions
Recent advances in immunotherapy (particularly the clinical use of immune checkpoint inhibitors) represent a turning point in the fight against cancer. However, mechanisms of immune escape and acquired resistance to immunotherapy hamper therapeutic efficacy resulting in treatment failure in a substantial proportion of patients. In that respect, certain genetic tumor characteristics, such as the lack of expression of the tumor suppressor PTEN, are of particular interest, due to their involvement in modulating both the tumor and the immune cell compartments of the TME towards an aggressive, therapy-resistant phenotype. Comprehensive PTEN status analysis could be crucial to refine the prediction of response to therapy in individual patients and to define new combinatorial approaches, in order to tailor the right therapies to patients who can actually benefit the most.
Abbreviations
| APCs | Antigen-Presenting Cells |
| CAR-T | Chimeric Antigen Receptor T cells |
| CCL | C-C motif chemokine Ligand |
| CRC | ColoRectal Cancer |
| CXCR | C-X-C chemokine Receptor |
| CTLA-4 | Cytotoxic T-Lymphocyte Antigen 4 |
| DCs | Dendritic Cells |
| FcRγ | FcεRI γ-chain |
| FDA | Food and Drug Administration |
| FOG2 | Friend Of Gata 2 |
| FOX | FOrkhead boX |
| HIF | Hypoxia-Inducible Factor |
| IDO | Indoleamine 2,3-DiOxigenase |
| IHC | ImmunoHistoChemistry |
| IL | InterLeukin |
| ITAM | Immunoreceptor Tyrosine-based Activation Motif |
| Ko | Knock out |
| LAG | Lymphocyte Activation Gene |
| mAb | Monoclonal Antibodies |
| MDSCs | Myeloid-Derived Suppressor Cells |
| MHC | Major Histocompatibility Complex |
| miR | microRNA |
| MMP | Metalloproteinase |
| MMR | MisMatch Repair |
| mTOR | mammalian Target Of Rapamycin |
| NK | Natural Killer |
| Nrp-1 | Neurophilin-1 |
| NSCLC | Non-Small-Cell Lung Cancer |
| OS | Overall Survival |
| PD-1 | Programmed cell Death-1 |
| PD-L | Program Death Ligand |
| PDAC | Pancreatic Ductal AdenoCarcinoma |
| PI3K | PhosphoInositide 3-Kinase |
| PIP2 | PhosphatidylInositol 4,5-bisPhosphate |
| PIP3 | PhosphatidylInositol 3,4,5 trisPhosphate |
| PTEN | Phosphatase and TENsin homolog on chromosome 10 |
| Sema4 | Semaphorin-4 |
| SHIP1 | SH-2 containing inositol 5′ polyphosphatase 1 |
| Smo | Smoothened |
| STAT | Signal Transducer and Activator of Transcription |
| TAM | Tumor-Associated Macrophage |
| TGF | Transforming Growth Factor |
| TIGIT | T cell ImmunoGlobulin and ITIM domain |
| TILs | Tumor-Infiltrating Lymphocytes |
| TIM | T-cell Immunoglobulin Mucin |
| TME | Tumor MicroEnvironment |
| TNBC | Triple Negative Breast Cancer |
| TNF | Tumor Necrosis Factor |
| TNM | Tumor, Node, Metastasis |
| TRAIL | TNF-Related Apoptosis-Inducing Ligand |
| Tregs | regulatory T cells |
| VEGF | Vascular Endothelial Growth Factor |
| VISTA | V-domain Ig Suppressor of T cell Activation |
| wt | Wild Type |
Author Contributions
Conceptualization, F.C. (Fabiana Conciatori), C.B. and M.M.; writing-original draft preparation, F.C. (Fabiana Conciatori), C.B. and I.F.; writing-review and editing, F.C. (Fabiana Conciatori), C.B., I.F., L.C., S.V. and V.F.; supervision, G.F., F.C. (Francesco Cognetti) and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by IFO Sperimentazioni OM1 and the Italian Association for Cancer Research (AIRC) (grant number L.C. IG 18622). Chiara Bazzichetto was supported by an AIRC fellowship for Italy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Croce C.M. Oncogenes and cancer. N. Engl. J. Med. 2008;358:502–511. doi: 10.1056/NEJMra072367. [DOI] [PubMed] [Google Scholar]
- 2.Milella M., Falcone I., Conciatori F., Cesta Incani U., Del Curatolo A., Inzerilli N., Nuzzo C.M., Vaccaro V., Vari S., Cognetti F., et al. PTEN: Multiple Functions in Human Malignant Tumors. Front. Oncol. 2015;5:24. doi: 10.3389/fonc.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y.R., Chen M., Pandolfi P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018;19:547–562. doi: 10.1038/s41580-018-0015-0. [DOI] [PubMed] [Google Scholar]
- 4.Bazzichetto C., Conciatori F., Pallocca M., Falcone I., Fanciulli M., Cognetti F., Milella M., Ciuffreda L. PTEN as a Prognostic/Predictive Biomarker in Cancer: An Unfulfilled Promise? Cancers. 2019;11:435. doi: 10.3390/cancers11040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milella M., Falcone I., Conciatori F., Matteoni S., Sacconi A., De Luca T., Bazzichetto C., Corbo V., Simbolo M., Sperduti I., et al. PTEN status is a crucial determinant of the functional outcome of combined MEK and mTOR inhibition in cancer. Sci. Rep. 2017;7:43013. doi: 10.1038/srep43013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciuffreda L., Falcone I., Incani U.C., Del Curatolo A., Conciatori F., Matteoni S., Vari S., Vaccaro V., Cognetti F., Milella M. PTEN expression and function in adult cancer stem cells and prospects for therapeutic targeting. Adv. Biol. Regul. 2014;56:66–80. doi: 10.1016/j.jbior.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Pulido R., Mingo J., Gaafar A., Nunes-Xavier C.E., Luna S., Torices L., Angulo J.C., Lopez J.I. Precise Immunodetection of PTEN Protein in Human Neoplasia. Cold Spring Harb. Perspect. Med. 2019;9 doi: 10.1101/cshperspect.a036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piro G., Carbone C., Carbognin L., Pilotto S., Ciccarese C., Iacovelli R., Milella M., Bria E., Tortora G. Revising PTEN in the Era of Immunotherapy: New Perspectives for an Old Story. Cancers. 2019;11:1525. doi: 10.3390/cancers11101525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Seidel J.A., Otsuka A., Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobosz P., Dzieciatkowski T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019;10:2965. doi: 10.3389/fimmu.2019.02965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christofi T., Baritaki S., Falzone L., Libra M., Zaravinos A. Current Perspectives in Cancer Immunotherapy. Cancers. 2019;11:1472. doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farkona S., Diamandis E.P., Blasutig I.M. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheever M.A., Higano C.S. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 2011;17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 15.Guo C., Manjili M.H., Subjeck J.R., Sarkar D., Fisher P.B., Wang X.Y. Therapeutic cancer vaccines: Past, present, and future. Adv. Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Q., Zhang C.D., Wu X.H. Therapeutic cancer vaccines: From initial findings to prospects. Immunol. Lett. 2018;196:11–21. doi: 10.1016/j.imlet.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Greig S.L. Talimogene Laherparepvec: First Global Approval. Drugs. 2016;76:147–154. doi: 10.1007/s40265-015-0522-7. [DOI] [PubMed] [Google Scholar]
- 18.Conry R.M., Westbrook B., McKee S., Norwood T.G. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum. Vaccin. Immunother. 2018;14:839–846. doi: 10.1080/21645515.2017.1412896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S., Margolin K. Tumor-infiltrating lymphocytes in melanoma. Curr. Oncol. Rep. 2012;14:468–474. doi: 10.1007/s11912-012-0257-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohaan M.W., van den Berg J.H., Kvistborg P., Haanen J. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: A viable treatment option. J. Immunother. Cancer. 2018;6:102. doi: 10.1186/s40425-018-0391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Z., Chen Y., Francisco N.M., Zhang Y., Wu M. The application of CAR-T cell therapy in hematological malignancies: Advantages and challenges. Acta Pharm. Sin. B. 2018;8:539–551. doi: 10.1016/j.apsb.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leonard W.J., Depper J.M., Uchiyama T., Smith K.A., Waldmann T.A., Greene W.C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982;300:267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- 23.Flynn M.J., Hartley J.A. The emerging role of anti-CD25 directed therapies as both immune modulators and targeted agents in cancer. Br. J. Haematol. 2017;179:20–35. doi: 10.1111/bjh.14770. [DOI] [PubMed] [Google Scholar]
- 24.Ghiringhelli F., Menard C., Puig P.E., Ladoire S., Roux S., Martin F., Solary E., Le Cesne A., Zitvogel L., Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J. Exp. Clin. Cancer Res. 2019;38:255. doi: 10.1186/s13046-019-1259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson A.C., Joller N., Kuchroo V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin S., Xu L., Yi M., Yu S., Wu K., Luo S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer. 2019;18:155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S., Lee L.F., Fisher T.S., Jessen B., Elliott M., Evering W., Logronio K., Tu G.H., Tsaparikos K., Li X., et al. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol. Res. 2015;3:149–160. doi: 10.1158/2326-6066.CIR-14-0118. [DOI] [PubMed] [Google Scholar]
- 29.Shindo Y., Yoshimura K., Kuramasu A., Watanabe Y., Ito H., Kondo T., Oga A., Ito H., Yoshino S., Hazama S., et al. Combination immunotherapy with 4-1BB activation and PD-1 blockade enhances antitumor efficacy in a mouse model of subcutaneous tumor. Anticancer Res. 2015;35:129–136. [PubMed] [Google Scholar]
- 30.McKee S.J., Doff B.L., Soon M.S., Mattarollo S.R. Therapeutic Efficacy of 4-1BB Costimulation Is Abrogated by PD-1 Blockade in a Model of Spontaneous B-cell Lymphoma. Cancer Immunol. Res. 2017;5:191–197. doi: 10.1158/2326-6066.CIR-16-0249. [DOI] [PubMed] [Google Scholar]
- 31.Asna N., Livoff A., Batash R., Debbi R., Schaffer P., Rivkind T., Schaffer M. Radiation therapy and immunotherapy-a potential combination in cancer treatment. Curr. Oncol. 2018;25:e454–e460. doi: 10.3747/co.25.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kordbacheh T., Honeychurch J., Blackhall F., Faivre-Finn C., Illidge T. Radiotherapy and anti-PD-1/PD-L1 combinations in lung cancer: Building better translational research platforms. Ann. Oncol. 2018;29:301–310. doi: 10.1093/annonc/mdx790. [DOI] [PubMed] [Google Scholar]
- 33.Sun W., Lv S., Li H., Cui W., Wang L. Enhancing the Anticancer Efficacy of Immunotherapy through Combination with Histone Modification Inhibitors. Genes. 2018;9:633. doi: 10.3390/genes9120633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geukes Foppen M.H., Donia M., Svane I.M., Haanen J.B. Tumor-infiltrating lymphocytes for the treatment of metastatic cancer. Mol. Oncol. 2015;9:1918–1935. doi: 10.1016/j.molonc.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez M., Moon E.K. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front. Immunol. 2019;10:128. doi: 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma S., Li X., Wang X., Cheng L., Li Z., Zhang C., Ye Z., Qian Q. Current Progress in CAR-T Cell Therapy for Solid Tumors. Int. J. Biol. Sci. 2019;15:2548–2560. doi: 10.7150/ijbs.34213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotte A., D’Orazi G., Bhandaru M. Nobel committee honors tumor immunologists. J. Exp. Clin. Cancer Res. 2018;37:262. doi: 10.1186/s13046-018-0937-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stamper C.C., Zhang Y., Tobin J.F., Erbe D.V., Ikemizu S., Davis S.J., Stahl M.L., Seehra J., Somers W.S., Mosyak L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- 39.Marshall H.T., Djamgoz M.B.A. Immuno-Oncology: Emerging Targets and Combination Therapies. Front. Oncol. 2018;8:315. doi: 10.3389/fonc.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marin-Acevedo J.A., Dholaria B., Soyano A.E., Knutson K.L., Chumsri S., Lou Y. Next generation of immune checkpoint therapy in cancer: New developments and challenges. J. Hematol. Oncol. 2018;11:39. doi: 10.1186/s13045-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long L., Zhang X., Chen F., Pan Q., Phiphatwatchara P., Zeng Y., Chen H. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer. 2018;9:176–189. doi: 10.18632/genesandcancer.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He Y., Cao J., Zhao C., Li X., Zhou C., Hirsch F.R. TIM-3, a promising target for cancer immunotherapy. Oncol. Targets Ther. 2018;11:7005–7009. doi: 10.2147/OTT.S170385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solomon B.L., Garrido-Laguna I. TIGIT: A novel immunotherapy target moving from bench to bedside. Cancer Immunol. Immunother. 2018;67:1659–1667. doi: 10.1007/s00262-018-2246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tai Y., Wang Q., Korner H., Zhang L., Wei W. Molecular Mechanisms of T Cells Activation by Dendritic Cells in Autoimmune Diseases. Front. Pharmacol. 2018;9:642. doi: 10.3389/fphar.2018.00642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartkowiak T., Curran M.A. 4-1BB Agonists: Multi-Potent Potentiators of Tumor Immunity. Front. Oncol. 2015;5:117. doi: 10.3389/fonc.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conciatori F., Bazzichetto C., Falcone I., Pilotto S., Bria E., Cognetti F., Milella M., Ciuffreda L. Role of mTOR Signaling in Tumor Microenvironment: An Overview. Int. J. Mol. Sci. 2018;19:2453. doi: 10.3390/ijms19082453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cetintas V.B., Batada N.N. Is there a causal link between PTEN deficient tumors and immunosuppressive tumor microenvironment? J. Transl. Med. 2020;18:45. doi: 10.1186/s12967-020-02219-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eissing M., Ripken L., Schreibelt G., Westdorp H., Ligtenberg M., Netea-Maier R., Netea M.G., de Vries I.J.M., Hoogerbrugge N. PTEN Hamartoma Tumor Syndrome and Immune Dysregulation. Transl. Oncol. 2019;12:361–367. doi: 10.1016/j.tranon.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor H., Laurence A.D.J., Uhlig H.H. The Role of PTEN in Innate and Adaptive Immunity. Cold Spring Harb. Perspect. Med. 2019;9 doi: 10.1101/cshperspect.a036996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran Janco J.M., Lamichhane P., Karyampudi L., Knutson K.L. Tumor-infiltrating dendritic cells in cancer pathogenesis. J. Immunol. 2015;194:2985–2991. doi: 10.4049/jimmunol.1403134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veglia F., Gabrilovich D.I. Dendritic cells in cancer: The role revisited. Curr. Opin. Immunol. 2017;45:43–51. doi: 10.1016/j.coi.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Del Prete A., Vermi W., Dander E., Otero K., Barberis L., Luini W., Bernasconi S., Sironi M., Santoro A., Garlanda C., et al. Defective dendritic cell migration and activation of adaptive immunity in PI3Kgamma-deficient mice. EMBO J. 2004;23:3505–3515. doi: 10.1038/sj.emboj.7600361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agrawal A., Tay J., Ton S., Agrawal S., Gupta S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J. Immunol. 2009;182:1138–1145. doi: 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J.H., Kang T.H., Noh K.H., Kim S.H., Lee Y.H., Kim K.W., Bae H.C., Ahn Y.H., Choi E.Y., Kim J.S., et al. Enhancement of DC vaccine potency by activating the PI3K/AKT pathway with a small interfering RNA targeting PTEN. Immunol. Lett. 2010;134:47–54. doi: 10.1016/j.imlet.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Pan Y.G., Yu Y.L., Lin C.C., Lanier L.L., Chu C.L. FcepsilonRI gamma-Chain Negatively Modulates Dectin-1 Responses in Dendritic Cells. Front. Immunol. 2017;8:1424. doi: 10.3389/fimmu.2017.01424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newton R.H., Turka L.A. Regulation of T cell homeostasis and responses by pten. Front. Immunol. 2012;3:151. doi: 10.3389/fimmu.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paik J.H., Kollipara R., Chu G., Ji H., Xiao Y., Ding Z., Miao L., Tothova Z., Horner J.W., Carrasco D.R., et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Cristofano A., Kotsi P., Peng Y.F., Cordon-Cardo C., Elkon K.B., Pandolfi P.P. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 59.Peter M.E., Hadji A., Murmann A.E., Brockway S., Putzbach W., Pattanayak A., Ceppi P. The role of CD95 and CD95 ligand in cancer. Cell Death Differ. 2015;22:549–559. doi: 10.1038/cdd.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki A., Yamaguchi M.T., Ohteki T., Sasaki T., Kaisho T., Kimura Y., Yoshida R., Wakeham A., Higuchi T., Fukumoto M., et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/S1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 61.Soond D.R., Garcon F., Patton D.T., Rolf J., Turner M., Scudamore C., Garden O.A., Okkenhaug K. Pten loss in CD4 T cells enhances their helper function but does not lead to autoimmunity or lymphoma. J. Immunol. 2012;188:5935–5943. doi: 10.4049/jimmunol.1102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walsh P.T., Buckler J.L., Zhang J., Gelman A.E., Dalton N.M., Taylor D.K., Bensinger S.J., Hancock W.W., Turka L.A. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J. Clin. Investig. 2006;116:2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bensinger S.J., Walsh P.T., Zhang J., Carroll M., Parsons R., Rathmell J.C., Thompson C.B., Burchill M.A., Farrar M.A., Turka L.A. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J. Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K., Sharpe A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Delgoffe G.M., Woo S.R., Turnis M.E., Gravano D.M., Guy C., Overacre A.E., Bettini M.L., Vogel P., Finkelstein D., Bonnevier J., et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munn D.H., Sharma M.D., Johnson T.S. Treg Destabilization and Reprogramming: Implications for Cancer Immunotherapy. Cancer Res. 2018;78:5191–5199. doi: 10.1158/0008-5472.CAN-18-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharma M.D., Baban B., Chandler P., Hou D.Y., Singh N., Yagita H., Azuma M., Blazar B.R., Mellor A.L., Munn D.H. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Investig. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma M.D., Shinde R., McGaha T.L., Huang L., Holmgaard R.B., Wolchok J.D., Mautino M.R., Celis E., Sharpe A.H., Francisco L.M., et al. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci. Adv. 2015;1:e1500845. doi: 10.1126/sciadv.1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Munn D.H., Sharma M.D., Johnson T.S., Rodriguez P. IDO, PTEN-expressing Tregs and control of antigen-presentation in the murine tumor microenvironment. Cancer Immunol. Immunother. 2017;66:1049–1058. doi: 10.1007/s00262-017-2010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y., Sun R. Tumor immunotherapy: New aspects of natural killer cells. Chin. J. Cancer Res. 2018;30:173–196. doi: 10.21147/j.issn.1000-9604.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Briercheck E.L., Trotta R., Chen L., Hartlage A.S., Cole J.P., Cole T.D., Mao C., Banerjee P.P., Hsu H.T., Mace E.M., et al. PTEN is a negative regulator of NK cell cytolytic function. J. Immunol. 2015;194:1832–1840. doi: 10.4049/jimmunol.1401224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leong J.W., Schneider S.E., Sullivan R.P., Parikh B.A., Anthony B.A., Singh A., Jewell B.A., Schappe T., Wagner J.A., Link D.C., et al. PTEN regulates natural killer cell trafficking in vivo. Proc. Natl. Acad. Sci. USA. 2015;112:E700–E709. doi: 10.1073/pnas.1413886112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mace E.M. Phosphoinositide-3-Kinase Signaling in Human Natural Killer Cells: New Insights from Primary Immunodeficiency. Front. Immunol. 2018;9:445. doi: 10.3389/fimmu.2018.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diaz-Montero C.M., Finke J., Montero A.J. Myeloid-derived suppressor cells in cancer: Therapeutic, predictive, and prognostic implications. Semin. Oncol. 2014;41:174–184. doi: 10.1053/j.seminoncol.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gabrilovich D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su Y., Qiu Y., Qiu Z., Qu P. MicroRNA networks regulate the differentiation, expansion and suppression function of myeloid-derived suppressor cells in tumor microenvironment. J. Cancer. 2019;10:4350–4356. doi: 10.7150/jca.35205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu Y., Lai L., Chen Q., Song Y., Xu S., Ma F., Wang X., Wang J., Yu H., Cao X., et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J. Immunol. 2012;188:5500–5510. doi: 10.4049/jimmunol.1103505. [DOI] [PubMed] [Google Scholar]
- 78.Li L., Zhang J., Diao W., Wang D., Wei Y., Zhang C.Y., Zen K. MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. J. Immunol. 2014;192:1034–1043. doi: 10.4049/jimmunol.1301309. [DOI] [PubMed] [Google Scholar]
- 79.Mei S., Xin J., Liu Y., Zhang Y., Liang X., Su X., Yan H., Huang Y., Yang R. MicroRNA-200c Promotes Suppressive Potential of Myeloid-Derived Suppressor Cells by Modulating PTEN and FOG2 Expression. PLoS ONE. 2015;10:e0135867. doi: 10.1371/journal.pone.0135867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ren W., Zhang X., Li W., Feng Q., Feng H., Tong Y., Rong H., Wang W., Zhang D., Zhang Z., et al. Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer. Cancer Manag. Res. 2019;11:4023–4040. doi: 10.2147/CMAR.S198886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo X., Qiu W., Liu Q., Qian M., Wang S., Zhang Z., Gao X., Chen Z., Xue H., Li G. Immunosuppressive effects of hypoxia-induced glioma exosomes through myeloid-derived suppressor cells via the miR-10a/Rora and miR-21/Pten Pathways. Oncogene. 2018;37:4239–4259. doi: 10.1038/s41388-018-0261-9. [DOI] [PubMed] [Google Scholar]
- 82.Li N., Qin J., Lan L., Zhang H., Liu F., Wu Z., Ni H., Wang Y. PTEN inhibits macrophage polarization from M1 to M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer Biol. Ther. 2015;16:297–306. doi: 10.1080/15384047.2014.1002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sahin E., Haubenwallner S., Kuttke M., Kollmann I., Halfmann A., Dohnal A.M., Chen L., Cheng P., Hoesel B., Einwallner E., et al. Macrophage PTEN regulates expression and secretion of arginase I modulating innate and adaptive immune responses. J. Immunol. 2014;193:1717–1727. doi: 10.4049/jimmunol.1302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Munder M., Schneider H., Luckner C., Giese T., Langhans C.D., Fuentes J.M., Kropf P., Mueller I., Kolb A., Modolell M., et al. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108:1627–1634. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 85.Bao L., Li X. MicroRNA-32 targeting PTEN enhances M2 macrophage polarization in the glioma microenvironment and further promotes the progression of glioma. Mol. Cell Biochem. 2019;460:67–79. doi: 10.1007/s11010-019-03571-2. [DOI] [PubMed] [Google Scholar]
- 86.Li N., Qin J.F., Han X., Jin F.J., Zhang J.H., Lan L., Wang Y. miR-21a negatively modulates tumor suppressor genes PTEN and miR-200c and further promotes the transformation of M2 macrophages. Immunol. Cell Biol. 2018;96:68–80. doi: 10.1111/imcb.1016. [DOI] [PubMed] [Google Scholar]
- 87.Wang X., Luo G., Zhang K., Cao J., Huang C., Jiang T., Liu B., Su L., Qiu Z. Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kgamma to Promote Pancreatic Cancer Metastasis. Cancer Res. 2018;78:4586–4598. doi: 10.1158/0008-5472.CAN-17-3841. [DOI] [PubMed] [Google Scholar]
- 88.Hsu Y.L., Hung J.Y., Chang W.A., Jian S.F., Lin Y.S., Pan Y.C., Wu C.Y., Kuo P.L. Hypoxic Lung-Cancer-Derived Extracellular Vesicle MicroRNA-103a Increases the Oncogenic Effects of Macrophages by Targeting PTEN. Mol. Ther. 2018;26:568–581. doi: 10.1016/j.ymthe.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu X., Shen H., Yin X., Yang M., Wei H., Chen Q., Feng F., Liu Y., Xu W., Li Y. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J. Exp. Clin. Cancer Res. 2019;38:81. doi: 10.1186/s13046-019-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trimboli A.J., Cantemir-Stone C.Z., Li F., Wallace J.A., Merchant A., Creasap N., Thompson J.C., Caserta E., Wang H., Chong J.L., et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bronisz A., Godlewski J., Wallace J.A., Merchant A.S., Nowicki M.O., Mathsyaraja H., Srinivasan R., Trimboli A.J., Martin C.K., Li F., et al. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat. Cell Biol. 2011;14:159–167. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones C.E., Hammer A.M., Cho Y., Sizemore G.M., Cukierman E., Yee L.D., Ghadiali S.N., Ostrowski M.C., Leight J.L. Stromal PTEN Regulates Extracellular Matrix Organization in the Mammary Gland. Neoplasia. 2019;21:132–145. doi: 10.1016/j.neo.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wartenberg M., Centeno I., Haemmig S., Vassella E., Zlobec I., Galvan J.A., Neuenschwander M., Schlup C., Gloor B., Lugli A., et al. PTEN alterations of the stromal cells characterise an aggressive subpopulation of pancreatic cancer with enhanced metastatic potential. Eur. J. Cancer. 2016;65:80–90. doi: 10.1016/j.ejca.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 94.Pitarresi J.R., Liu X., Avendano A., Thies K.A., Sizemore G.M., Hammer A.M., Hildreth B.E., 3rd, Wang D.J., Steck S.A., Donohue S., et al. Disruption of stromal hedgehog signaling initiates RNF5-mediated proteasomal degradation of PTEN and accelerates pancreatic tumor growth. Life Sci. Alliance. 2018;1:e201800190. doi: 10.26508/lsa.201800190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodriguez S., Huynh-Do U. The Role of PTEN in Tumor Angiogenesis. J. Oncol. 2012;2012:141236. doi: 10.1155/2012/141236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian T., Nan K.J., Wang S.H., Liang X., Lu C.X., Guo H., Wang W.J., Ruan Z.P. PTEN regulates angiogenesis and VEGF expression through phosphatase-dependent and -independent mechanisms in HepG2 cells. Carcinogenesis. 2010;31:1211–1219. doi: 10.1093/carcin/bgq085. [DOI] [PubMed] [Google Scholar]
- 97.Del Bufalo D., Ciuffreda L., Trisciuoglio D., Desideri M., Cognetti F., Zupi G., Milella M. Antiangiogenic potential of the Mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 2006;66:5549–5554. doi: 10.1158/0008-5472.CAN-05-2825. [DOI] [PubMed] [Google Scholar]
- 98.Dong Y., Richards J.A., Gupta R., Aung P.P., Emley A., Kluger Y., Dogra S.K., Mahalingam M., Wajapeyee N. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene. 2014;33:4632–4642. doi: 10.1038/onc.2013.409. [DOI] [PubMed] [Google Scholar]
- 99.De la Iglesia N., Konopka G., Lim K.L., Nutt C.L., Bromberg J.F., Frank D.A., Mischel P.S., Louis D.N., Bonni A. Deregulation of a STAT3-interleukin 8 signaling pathway promotes human glioblastoma cell proliferation and invasiveness. J. Neurosci. 2008;28:5870–5878. doi: 10.1523/JNEUROSCI.5385-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu C., Fillmore C.M., Koyama S., Wu H., Zhao Y., Chen Z., Herter-Sprie G.S., Akbay E.A., Tchaicha J.H., Altabef A., et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell. 2014;25:590–604. doi: 10.1016/j.ccr.2014.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mittendorf E.A., Philips A.V., Meric-Bernstam F., Qiao N., Wu Y., Harrington S., Su X., Wang Y., Gonzalez-Angulo A.M., Akcakanat A., et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol. Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maxwell P.J., Coulter J., Walker S.M., McKechnie M., Neisen J., McCabe N., Kennedy R.D., Salto-Tellez M., Albanese C., Waugh D.J. Potentiation of inflammatory CXCL8 signalling sustains cell survival in PTEN-deficient prostate carcinoma. Eur. Urol. 2013;64:177–188. doi: 10.1016/j.eururo.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Conciatori F., Bazzichetto C., Falcone I., Sperduti I., Amoreo C., Diodoro M., Cognetti F., Milella M., Ciuffreda L. PO-294 BRAFV600E/PTEn-loss status is associated with interleukin (IL)−8 expression in preclinical models of colorectal cancer (CRC) ESMO Open. 2018;3:A343. doi: 10.1136/esmoopen-2018-EACR25.808. [DOI] [Google Scholar]
- 104.Schalper K.A., Carleton M., Zhou M., Chen T., Feng Y., Huang S.P., Walsh A.M., Baxi V., Pandya D., Baradet T., et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 2020;26:688–692. doi: 10.1038/s41591-020-0856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yuen K.C., Liu L.F., Gupta V., Madireddi S., Keerthivasan S., Li C., Rishipathak D., Williams P., Kadel E.E., 3rd, Koeppen H., et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 2020;26:693–698. doi: 10.1038/s41591-020-0860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Komura T., Yano M., Miyake A., Takabatake H., Miyazawa M., Ogawa N., Seki A., Honda M., Wada T., Matsui S., et al. Immune Condition of Colorectal Cancer Patients Featured by Serum Chemokines and Gene Expressions of CD4+ Cells in Blood. Can. J. Gastroenterol. Hepatol. 2018;2018:7436205. doi: 10.1155/2018/7436205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Eikawa S., Ohue Y., Kitaoka K., Aji T., Uenaka A., Oka M., Nakayama E. Enrichment of Foxp3+ CD4 regulatory T cells in migrated T cells to IL-6- and IL-8-expressing tumors through predominant induction of CXCR1 by IL-6. J. Immunol. 2010;185:6734–6740. doi: 10.4049/jimmunol.1000225. [DOI] [PubMed] [Google Scholar]
- 108.Gesser B., Deleuran B., Lund M., Vestergard C., Lohse N., Deleuran M., Jensen S.L., Pedersen S.S., Thestrup-Pedersen K., Larsen C.G. Interleukin-8 induces its own production in CD4+ T lymphocytes: A process regulated by interleukin 10. Biochem. Biophys. Res. Commun. 1995;210:660–669. doi: 10.1006/bbrc.1995.1711. [DOI] [PubMed] [Google Scholar]
- 109.Hess C., Means T.K., Autissier P., Woodberry T., Altfeld M., Addo M.M., Frahm N., Brander C., Walker B.D., Luster A.D. IL-8 responsiveness defines a subset of CD8 T cells poised to kill. Blood. 2004;104:3463–3471. doi: 10.1182/blood-2004-03-1067. [DOI] [PubMed] [Google Scholar]
- 110.Sun L., Wang Q., Chen B., Zhao Y., Shen B., Wang H., Xu J., Zhu M., Zhao X., Xu C., et al. Gastric cancer mesenchymal stem cells derived IL-8 induces PD-L1 expression in gastric cancer cells via STAT3/mTOR-c-Myc signal axis. Cell Death Dis. 2018;9:928. doi: 10.1038/s41419-018-0988-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toso A., Revandkar A., Di Mitri D., Guccini I., Proietti M., Sarti M., Pinton S., Zhang J., Kalathur M., Civenni G., et al. Enhancing chemotherapy efficacy in Pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep. 2014;9:75–89. doi: 10.1016/j.celrep.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 112.Peng W., Chen J.Q., Liu C., Malu S., Creasy C., Tetzlaff M.T., Xu C., McKenzie J.A., Zhang C., Liang X., et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jolly L.A., Massoll N., Franco A.T. Immune Suppression Mediated by Myeloid and Lymphoid Derived Immune Cells in the Tumor Microenvironment Facilitates Progression of Thyroid Cancers Driven by Hras(G12V) and Pten Loss. J. Clin. Cell Immunol. 2016;7 doi: 10.4172/2155-9899.1000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vidotto T., Saggioro F.P., Jamaspishvili T., Chesca D.L., Picanco de Albuquerque C.G., Reis R.B., Graham C.H., Berman D.M., Siemens D.R., Squire J.A., et al. PTEN-deficient prostate cancer is associated with an immunosuppressive tumor microenvironment mediated by increased expression of IDO1 and infiltrating FoxP3+ T regulatory cells. Prostate. 2019;79:969–979. doi: 10.1002/pros.23808. [DOI] [PubMed] [Google Scholar]
- 115.Sharma P., Allison J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Song M., Chen D., Lu B., Wang C., Zhang J., Huang L., Wang X., Timmons C.L., Hu J., Liu B., et al. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS ONE. 2013;8:e65821. doi: 10.1371/journal.pone.0065821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao L., Li C., Liu F., Zhao Y., Liu J., Hua Y., Liu J., Huang J., Ge C. A blockade of PD-L1 produced antitumor and antimetastatic effects in an orthotopic mouse pancreatic cancer model via the PI3K/Akt/mTOR signaling pathway. Oncol. Targets Ther. 2017;10:2115–2126. doi: 10.2147/OTT.S130481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Parsa A.T., Waldron J.S., Panner A., Crane C.A., Parney I.F., Barry J.J., Cachola K.E., Murray J.C., Tihan T., Jensen M.C., et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 119.Waldron J.S., Yang I., Han S., Tihan T., Sughrue M.E., Mills S.A., Pieper R.O., Parsa A.T. Implications for immunotherapy of tumor-mediated T-cell apoptosis associated with loss of the tumor suppressor PTEN in glioblastoma. J. Clin. Neurosci. 2010;17:1543–1547. doi: 10.1016/j.jocn.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parikh A.R., Ali S.M., Schrock A.B., Albacker L.A., Miller V.A., Stephens P.J., Crilley P., Markman M. Response to rapamycin analogs but not PD-1 inhibitors in PTEN-mutated metastatic non-small-cell lung cancer with high tumor mutational burden. Lung Cancer Auckl. 2018;9:45–47. doi: 10.2147/LCTT.S161738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cretella D., Digiacomo G., Giovannetti E., Cavazzoni A. PTEN Alterations as a Potential Mechanism for Tumor Cell Escape from PD-1/PD-L1 Inhibition. Cancers. 2019;11:1318. doi: 10.3390/cancers11091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao J., Chen A.X., Gartrell R.D., Silverman A.M., Aparicio L., Chu T., Bordbar D., Shan D., Samanamud J., Mahajan A., et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 2019;25:462–469. doi: 10.1038/s41591-019-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen H., Chong W., Teng C., Yao Y., Wang X., Li X. The immune response-related mutational signatures and driver genes in non-small-cell lung cancer. Cancer Sci. 2019;110:2348–2356. doi: 10.1111/cas.14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roh W., Chen P.L., Reuben A., Spencer C.N., Prieto P.A., Miller J.P., Gopalakrishnan V., Wang F., Cooper Z.A., Reddy S.M., et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aah3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dey N., Crosswell H.E., De P., Parsons R., Peng Q., Su J.D., Durden D.L. The protein phosphatase activity of PTEN regulates SRC family kinases and controls glioma migration. Cancer Res. 2008;68:1862–1871. doi: 10.1158/0008-5472.CAN-07-1182. [DOI] [PubMed] [Google Scholar]
- 126.Yu G.T., Mao L., Wu L., Deng W.W., Bu L.L., Liu J.F., Chen L., Yang L.L., Wu H., Zhang W.F., et al. Inhibition of SRC family kinases facilitates anti-CTLA4 immunotherapy in head and neck squamous cell carcinoma. Cell Mol. Life Sci. 2018;75:4223–4234. doi: 10.1007/s00018-018-2863-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lopez G., Noale M., Corti C., Gaudioso G., Sajjadi E., Venetis K., Gambini D., Runza L., Costanza J., Pesenti C., et al. PTEN Expression as a Complementary Biomarker for Mismatch Repair Testing in Breast Cancer. Int. J. Mol. Sci. 2020;21:1461. doi: 10.3390/ijms21041461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lines J.L., Sempere L.F., Broughton T., Wang L., Noelle R. VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol. Res. 2014;2:510–517. doi: 10.1158/2326-6066.CIR-14-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kakavand H., Jackett L.A., Menzies A.M., Gide T.N., Carlino M.S., Saw R.P.M., Thompson J.F., Wilmott J.S., Long G.V., Scolyer R.A. Negative immune checkpoint regulation by VISTA: A mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod. Pathol. 2017;30:1666–1676. doi: 10.1038/modpathol.2017.89. [DOI] [PubMed] [Google Scholar]
- 130.Trujillo J.A., Luke J.J., Zha Y., Segal J.P., Ritterhouse L.L., Spranger S., Matijevich K., Gajewski T.F. Secondary resistance to immunotherapy associated with beta-catenin pathway activation or PTEN loss in metastatic melanoma. J. Immunother. Cancer. 2019;7:295. doi: 10.1186/s40425-019-0780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.George S., Miao D., Demetri G.D., Adeegbe D., Rodig S.J., Shukla S., Lipschitz M., Amin-Mansour A., Raut C.P., Carter S.L., et al. Loss of PTEN Is Associated with Resistance to Anti-PD-1 Checkpoint Blockade Therapy in Metastatic Uterine Leiomyosarcoma. Immunity. 2017;46:197–204. doi: 10.1016/j.immuni.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kruger S., Ilmer M., Kobold S., Cadilha B.L., Endres S., Ormanns S., Schuebbe G., Renz B.W., D’Haese J.G., Schloesser H., et al. Advances in cancer immunotherapy 2019—latest trends. J. Exp. Clin. Cancer Res. 2019;38:268. doi: 10.1186/s13046-019-1266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Peng W., Williams L.J., Xu C., Melendez B., McKenzie J.A., Chen Y., Jackson H.L., Voo K.S., Mbofung R.M., Leahey S.E., et al. Anti-OX40 Antibody Directly Enhances The Function of Tumor-Reactive CD8(+) T Cells and Synergizes with PI3Kbeta Inhibition in PTEN Loss Melanoma. Clin. Cancer Res. 2019;25:6406–6416. doi: 10.1158/1078-0432.CCR-19-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kroon P., Gadiot J., Peeters M., Gasparini A., Deken M.A., Yagita H., Verheij M., Borst J., Blank C.U., Verbrugge I. Concomitant targeting of programmed death-1 (PD-1) and CD137 improves the efficacy of radiotherapy in a mouse model of human BRAFV600-mutant melanoma. Cancer Immunol. Immunother. 2016;65:753–763. doi: 10.1007/s00262-016-1843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu J.F., Wu L., Yang L.L., Deng W.W., Mao L., Wu H., Zhang W.F., Sun Z.J. Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J. Exp. Clin. Cancer Res. 2018;37:44. doi: 10.1186/s13046-018-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boross P., Leusen J.H. Mechanisms of action of CD20 antibodies. Am. J. Cancer Res. 2012;2:676–690. [PMC free article] [PubMed] [Google Scholar]
- 137.Gupta P., Goldenberg D.M., Rossi E.A., Chang C.H. Multiple signaling pathways induced by hexavalent, monospecific, anti-CD20 and hexavalent, bispecific, anti-CD20/CD22 humanized antibodies correlate with enhanced toxicity to B-cell lymphomas and leukemias. Blood. 2010;116:3258–3267. doi: 10.1182/blood-2010-03-276857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Baritaki S., Militello L., Malaponte G., Spandidos D.A., Salcedo M., Bonavida B. The anti-CD20 mAb LFB-R603 interrupts the dysregulated NF-kappaB/Snail/RKIP/PTEN resistance loop in B-NHL cells: Role in sensitization to TRAIL apoptosis. Int. J. Oncol. 2011;38:1683–1694. doi: 10.3892/ijo.2011.984. [DOI] [PubMed] [Google Scholar]
- 139.Yang Q.C., Wu C.C., Cao L.Y., Xiao Y., Li H., Liu B., Sun Z.J. Increased Expression of LAMTOR5 Predicts Poor Prognosis and Is Associated with Lymph Node Metastasis of Head and Neck Squamous Cell Carcinoma. Int. J. Med. Sci. 2019;16:783–792. doi: 10.7150/ijms.33415. [DOI] [PMC free article] [PubMed] [Google Scholar]