Abstract
Epigenetic modification is considered a major mechanism of the inactivation of tumor suppressor genes that finally contributes to carcinogenesis. LIM homeobox transcription factor 1α (LMX1A) is one of the LIM-homeobox-containing genes that is a critical regulator of growth and differentiation. Recently, LMX1A was shown to be hypermethylated and functioned as a tumor suppressor in cervical cancer, ovarian cancer, and gastric cancer. However, its role in lung cancer has not yet been clarified. In this study, we used public databases, methylation-specific PCR (MSP), reverse transcription PCR (RT-PCR), and bisulfite genomic sequencing to show that LMX1A was downregulated or silenced due to promoter hypermethylation in lung cancers. Treatment of lung cancer cells with the demethylating agent 5-aza-2’-deoxycytidine restored LMX1A expression. In the lung cancer cell lines H23 and H1299, overexpression of LMX1A did not affect cell proliferation but suppressed colony formation and invasion. These suppressive effects were reversed after inhibition of LMX1A expression in an inducible expression system in H23 cells. The quantitative RT-PCR (qRT-PCR) data showed that LMX1A could modulate epithelial mesenchymal transition (EMT) through E-cadherin (CDH1) and fibronectin (FN1). NanoString gene expression analysis revealed that all aberrantly expressed genes were associated with processes related to cancer progression, including angiogenesis, extracellular matrix (ECM) remodeling, EMT, cancer metastasis, and hypoxia-related gene expression. Taken together, these data demonstrated that LMX1A is inactivated through promoter hypermethylation and functions as a tumor suppressor. Furthermore, LMX1A inhibits non-small cell lung cancer (NSCLC) cell invasion partly through modulation of EMT, angiogenesis, and ECM remodeling.
Keywords: LMX1A, DNA methylation, lung cancer, tumor suppressor, epithelial mesenchymal transition
1. Introduction
Lung cancer is the most common cause of cancer-related death worldwide, and survival is largely dependent on the tumor stage [1,2]. Localized disease has a five-year survival rate of approximately 55%, whereas distant disease has a five-year survival rate of approximately only 4%. Unfortunately, approximately 57% of patients are diagnosed with distant disease [2]. Therefore, major efforts are being made to identify molecular markers, including aberrant DNA methylation, circulating cell-free tumor DNA, noncoding RNA, and proteomic markers, to facilitate early detection of lung cancer to improve prognosis and survival [3,4,5,6,7].
The progression of lung cancer is a complicated process, involving a series of genetic and epigenetic changes [8,9,10]. Recent research has demonstrated a promising advance in targeted therapy for lung cancers with epidermal growth factor (EGFR) mutations [11]. An increasing number of genes, such as p16, RASSF1A, APC, DAPK, MGMT, and FHIT, have demonstrated abnormal methylation in lung cancers [12,13,14,15,16,17]. In addition to genetic mutations, aberrant DNA methylation is another mechanism for inactivation of tumor suppressor genes to promote the occurrence and progression of lung cancer [18,19,20]. The identification of genetic mutations and epigenetic changes will further improve biomarker-driven precision treatment for lung cancer patients.
Homeobox genes include a large family of developmental regulators that are crucial for growth and differentiation. LIM homeobox genes, which encode LIM-homeodomain (LIM-HD) proteins, are one of the most important subfamilies of homeobox genes. They have two LIM domains in the amino termini and an HD domain for interacting with specific DNA sequences. Recently, research has shown that LIM homeobox genes play an important role in cancer development [21,22,23,24,25,26,27,28,29].
The LIM homeobox transcription factor 1α (LMX1A) gene is one of the LIM-homeobox-containing genes [30]. LMX1A has been reported to be a critical regulator of cell fate decisions and of the differentiation of human embryonic stem cells into midbrain dopaminergic neurons [31,32]. Recently, LMX1A has been found to be hypermethylated in cervical cancer, gastric cancer, bladder cancer, ovarian cancer, and colorectal cancer [25,26,28,29,33]. Moreover, LMX1A was reported to function as a tumor suppressor in cervical, gastric, and ovarian cancers [25,28,29], and its methylation was significantly associated with recurrence in bladder cancer [26] and survival in stage I and II colorectal cancer patients [33]. However, the role of LMX1A in lung cancer remains unclear. The purpose of this study was to investigate the epigenetic regulation and biological function of LMX1A in lung cancer. Our data demonstrated that LMX1A is epigenetically regulated and functions as a tumor suppressor in non-small cell lung cancer (NSCLC).
2. Results
2.1. Promoter Methylation of LMX1A in Non-Small Cell Lung Cancer
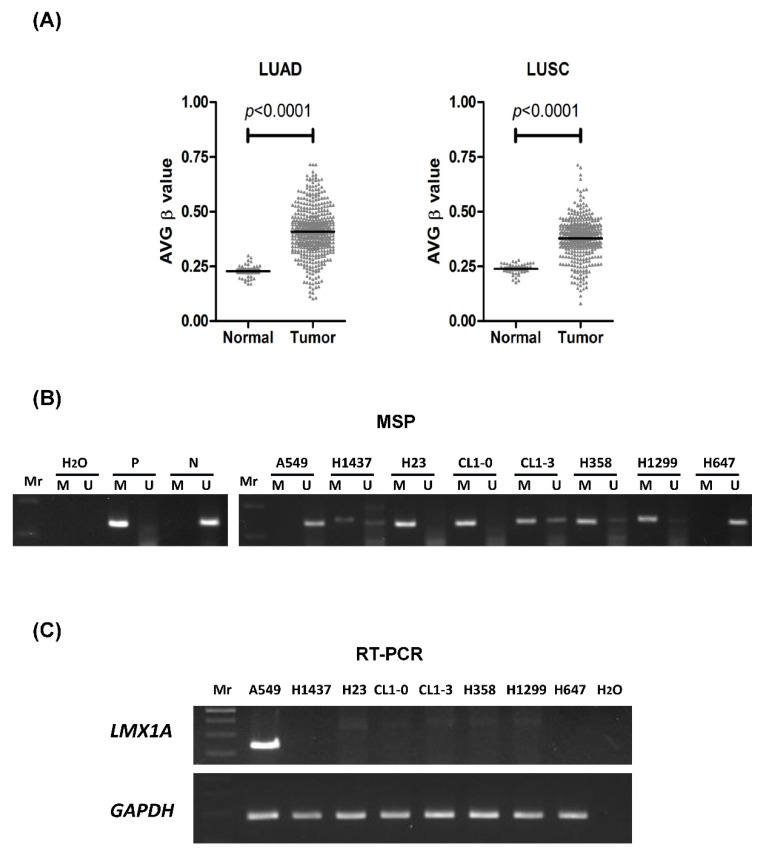
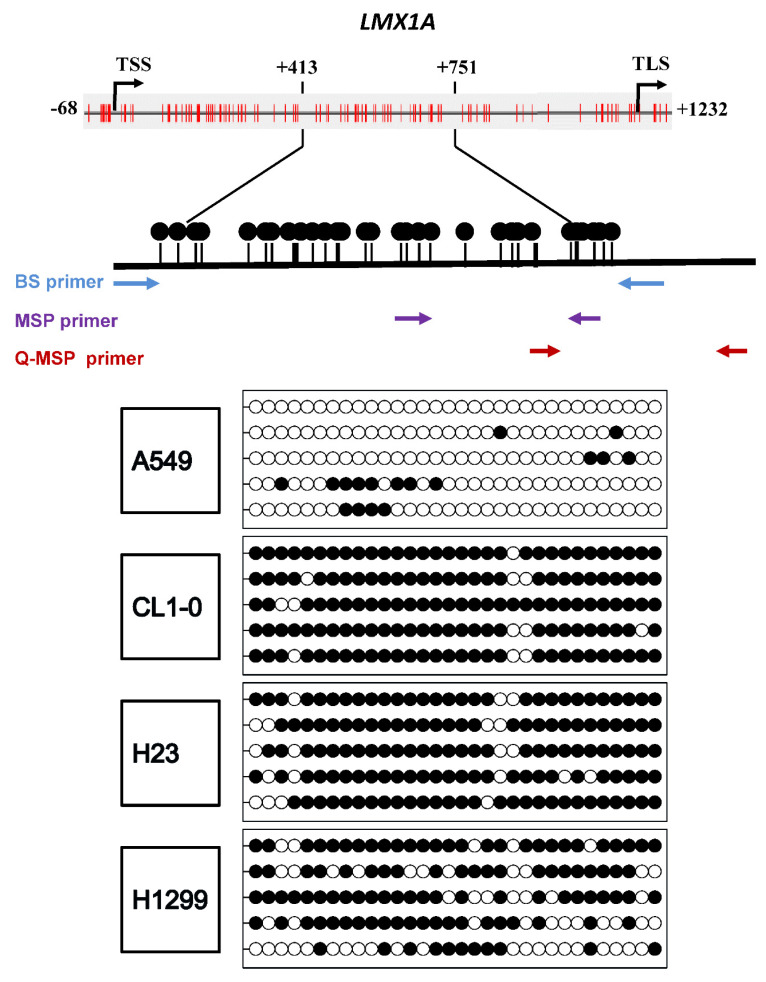
To determine whether promoter methylation of the LMX1A gene is a common epigenetic event in non-small cell lung cancer, we first used DNA methylation data from the SMART App (http://www.bioinfo-zs.com/smartapp) [34] to analyze the methylation levels of LMX1A in 458 lung adenocarcinoma (LUAD) and 364 lung squamous cell carcinoma (LUSC) samples. As shown in Figure 1A, the average β value was higher in the LUAD group than in the normal control group (p < 0.0001). A similar phenomenon was observed in LUSC (p < 0.0001). Then, we analyzed the methylation level and mRNA expression of LMX1A in lung cancer cell lines (Figure 1B,C). The data showed that LMX1A was frequently hypermethylated and downregulated in NSCLC cells. To further confirm the methylation-specific polymerase chain reaction (MSP) results, we applied bisulfite genomic sequencing to assess the methylation level in the promoter region of LMX1A. The results of bisulfate genomic sequencing revealed that LMX1A was poorly methylated in A549 and H647 cells (<20%) and highly methylated in H1299 (65%), H358 (70%), CL1-0 (93%), and H23 (88%) cells (Figure 2 and Figure S1).
Figure 1.
Promoter methylation levels of LMX1A in non-small cell lung cancer (NSCLC). (A) DNA methylation array data for LMX1A in 41 tissue samples from healthy individuals, 458 tissue samples from lung adenocarcinoma (LUAD) patients, and 364 tissue samples from lung squamous cell carcinoma (LUSC) patients from the Smart App (http://www.bioinfo-zs.com/smartapp) are shown. The results are shown as average (AVG) β values for the probes. Black lines indicate the mean AVG β value. The p-values for LMX1A methylation levels among the groups (normal versus tumor) were determined by the Mann–Whitney U test. (B) The promoter methylation statuses of LMX1A in eight NSCLC cell lines were analyzed by methylation-specific PCR (MSP) with methylated and unmethylated-specific primers. P: Positive control; N: Negative control. (C) Gene expression levels of LMX1A and the internal reference GAPDH in eight NSCLC cell lines were analyzed by reverse transcription polymerase chain reaction (RT-PCR). Mr: Molecular marker, or DNA ladder.
Figure 2.
Bisulfite genomic sequencing analysis of the CpG sites located at the promoter region of LMX1A. The LMX1A methylation status in four NSCLC cell lines was analyzed by bisulfite genomic sequencing. Each clone is represented by a row, and 32 CpG sites are represented as circles. Black circles and white circles represent methylated and unmethylated CpG sites, respectively. Arrows indicate the locations of the BS primers, MSP primers, and Q-MSP. TSS: Transcriptional start site; TLS: Translational start site; BS: Bisulfate sequencing; MSP: Methylation-specific PCR; Q-MSP: quantitative methylation-specific PCR.
2.2. Correlation between Methylation Status and Gene Expression Level of LMX1A in NSCLC Cell Lines
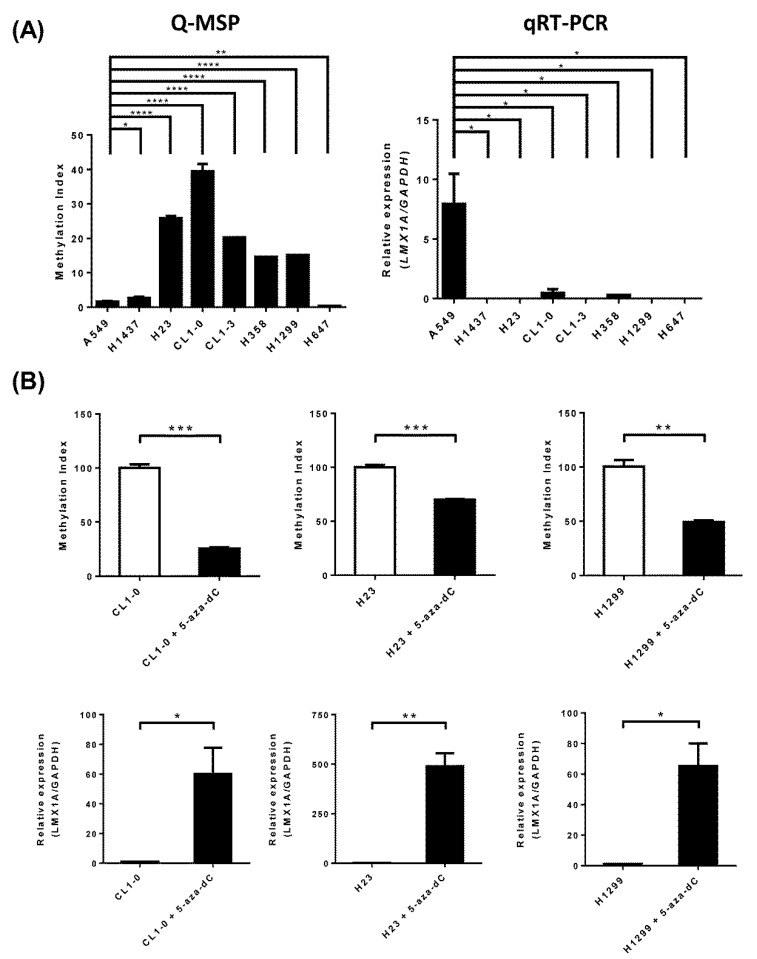
To assess the correlation between LMX1A methylation and gene expression, we analyzed the messenger RNA (mRNA) expression level and DNA methylation status of LMX1A in eight NSCLC cell lines by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and quantitative MSP (Q-MSP). The Q-MSP results (Figure 3A) showed a similar trend to the results from MSP gels and bisulfite sequencing (Figure 1 and Figure 2). The qRT-PCR data showed that LMX1A expression was detectable in A549 cells but undetectable in the other NSCLC cells (Figure 3A). To confirm the inverse correlation of these parameters, we treated CL1-0, H23, and HT1299 cells with a DNA methylation inhibitor (5-aza-2’-deoxycytidine; DAC). The reverse transcription polymerase chain reaction (RT-PCR) data revealed that LMX1A expression was restored in CL1-0, H23, and HT1299 cells after treatment with DAC (Figure 3B). In addition, methylated DNA was decreased after these cells were treated with a DNA methylation inhibitor (5-aza-2ʹ-deoxycytidine; DAC) (Figure 3B).
Figure 3.
Correlation between methylation status and gene expression level of LMX1A in NSCLC cell lines. (A) Quantitative DNA methylation levels of LMX1A in eight NSCLC cell lines were analyzed by Q-MSP. Gene expression levels of LMX1A and the internal reference GAPDH were analyzed by quantitative RT-PCR. (B) Quantitative DNA methylation levels of LMX1A in CL1-0, H23, and H1299 cells treated with 5 µM 5-aza-2ʹ-deoxycytidine (DAC) or left untreated were determined by Q-MSP. The results are represented as differences in the methylation index (MI). Gene expression levels of LMX1A and the internal reference GAPDH in CL1-0, H23, and H1299 cells treated with 5 µM DAC or left untreated were analyzed by quantitative RT-PCR. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 (Student’s t-test).
2.3. Overexpression of LMX1A Inhibits the Colony Formation and Invasion of NSCLC Cells in a Constitutive Expression System
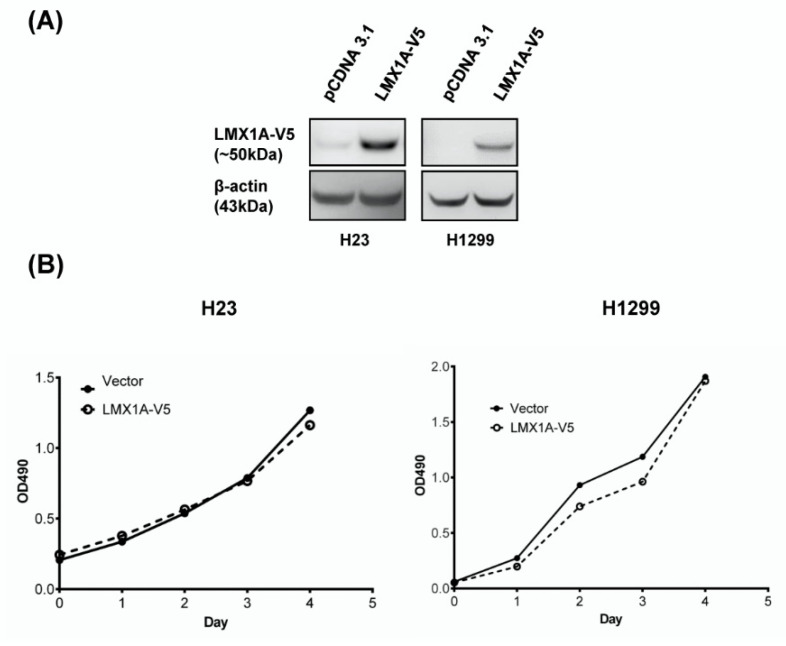
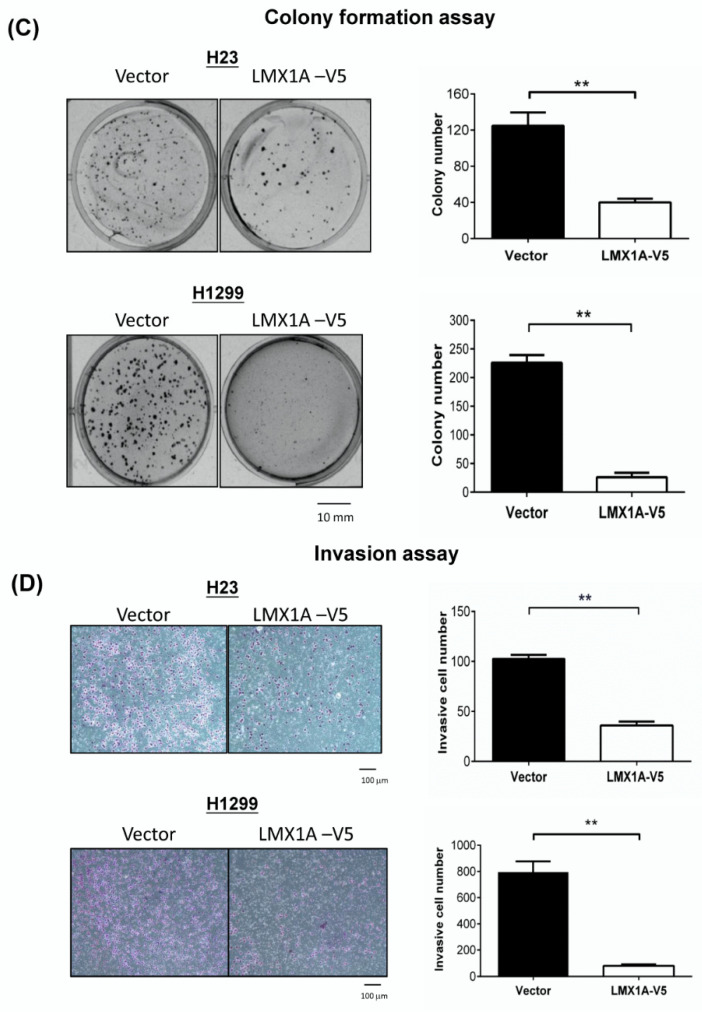
To elucidate the role of LMX1A in lung cancer, we analyzed the A549, H1299, H23, CL1-0, CL1-3, H358, and H647 cell lines for endogenous LMX1A expression. The results showed that the LMX1A protein was undetectable in all cell lines, except for A549 cells and normal lung tissues. LMX1A expression was downr egulated in cancer cell lines compared to normal lung tissue (Figure S2). LMX1A-expressing stable clones were generated using H23 and H1299 cells, and LMX1A expression was confirmed by Western blot analysis of the V5 tag protein (Figure 4A and Figure S2). The overexpression of LMX1A did not significantly alter cell viability (Figure 4B), but it did strongly repress the colony formation, indicating suppression of cell transformation (Figure 4C). Moreover, the stable overexpression of LMX1A decreased the number of invading cells (Figure 4D). Representative images of colony formation and invasion assays are shown in Figure 4C,D.
Figure 4.
Ectopic expression of LMX1A suppresses the colony formation and invasion of cancer cells in a constitutive expression system. (A) Expression of LMX1A in H23 and H1299 NSCLC cell lines stably transfected with LMX1A-V5 or the empty vector (pCDNA3.1) was analyzed by Western blots using an anti-V5 antibody. β-Actin was used as an internal control. (B) Cell proliferation (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS)) assays were performed using H23 and H1299 cells expressing LMX1A-V5 or the empty vector. The absorbance values are presented as the mean ± SEM from four independent experiments. (C) Colony formation assays were performed using H23 and H1299 cells expressing LMX1A-V5 or the empty vector. (D) Matrigel invasion assays were performed using H23 and H1299 cells expressing LMX1A-V5 or the empty vector. These results are presented as the mean ± SEM from three independent experiments in duplicate. ** p < 0.01 (Mann–Whitney U test).
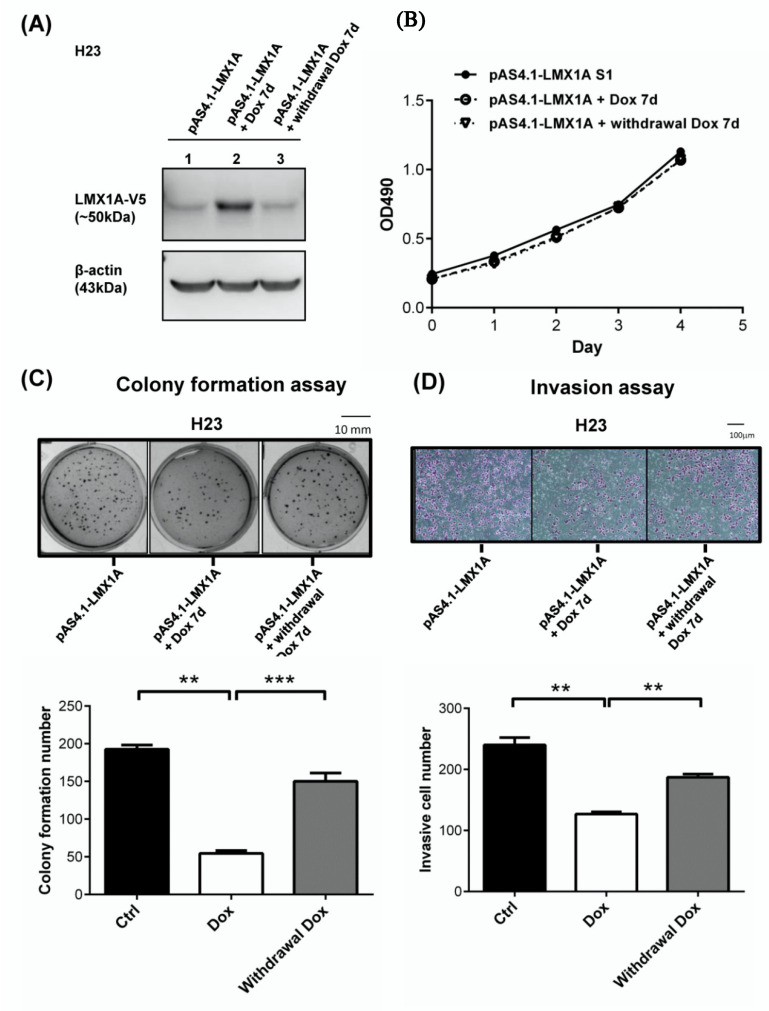
2.4. LMX1A Represses the Colony Formation and Invasion of NSCLC Cells in an Inducible Expression System
To further analyze the suppressive effects of LMX1A on tumorigenesis, we established an inducible expression system in NSCLC (H23) cells. Then, we treated the cells with 1 μg/mL doxycycline (Dox) to induce LMX1A expression. Western blotting analysis confirmed that Dox treatment increased the LMX1A protein levels (Figure 5A and Figure S2). We assessed the effects of LMX1A overexpression on cell growth, colony formation, and cell invasion after induction for 7 days. Although the viability of the LMX1A-overexpressing cells was not notably different from that of the controls (Figure 5B), LMX1A overexpression repressed colony formation (Figure 5C) and invasion (Figure 5D). These results were consistent with the data obtained using cells stably expressing LMX1A. In addition, the suppressive effects of LMX1A on colony formation and invasion were reversed after knockdown of LMX1A expression by withdrawal of Dox. Taken together, these data demonstrated that LMX1A suppresses cell transformation and the invasive phenotype.
Figure 5.
LMX1A suppresses the colony formation and invasion of cancer cells in an inducible expression system. (A) Doxycycline (Dox; 1 ng/μL)-inducible LMX1A expression was established in H23 cells and was assessed by Western blot analysis. β-Actin was used as an internal control. (B–D) Cells treated with Dox (1 ng/μL) for 7 days or withdrawn from Dox treatment for another 7 days were tested for cell proliferation (MTS) (B), colony formation (C), and invasion (D). The results are presented as the mean ± SEM from three independent experiments in duplicate. ** p < 0.01 and *** p < 0.001 (Mann–Whitney U test or Student’s t-test).
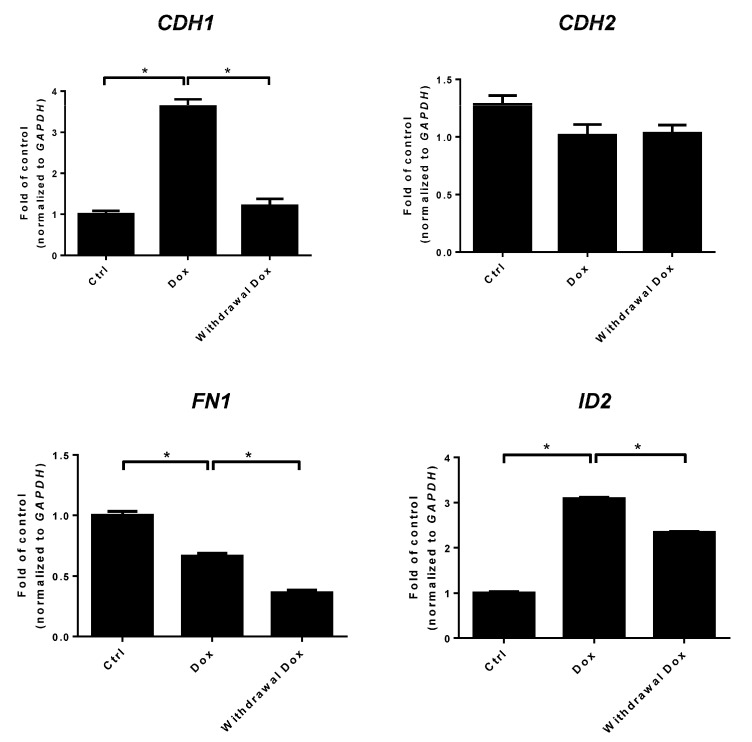
2.5. LMX1A Inhibits NSCLC Cell Invasion Partly through Modulation of Epithelial Mesenchymal Transition (EMT)
The epithelial mesenchymal transition (EMT) is a crucial developmental process that is often activated during cancer invasion and metastasis [35]. In a previous study, we demonstrated that LMX1A could suppress cervical cancer invasion through an incomplete EMT [29]. Here, we assessed whether LMX1A inhibited cancer invasion through EMT using qRT-PCR in H23 cells (Figure 6). Our data showed that LMX1A increased epithelial markers such as E-cadherin (CDH1) and decreased the mesenchymal markers fibronectin (FN1). Knockdown of LMX1A by withdrawal of doxycycline reversed CDH1 expression but not FN1 expression. The expression of N-cadherin (CDH2) remained high despite the overexpression of LMX1A. EMT-related transcription factors, such as SNAIL, SLUG, and TWIST, were not affected by overexpression of LMX1A (Figure S3A). The protein expression of Twist and Snail was not altered with manipulation of LMX1A (Figure S3B). Moreover, restoration of LMX1A significantly increased the expression of inhibitor of DNA-binding gene 2 (ID2) in H23 cells. The protein results of E-cadherin (CDH1), N-cadherin (CDH2), and inhibitor of DNA-binding gene 2 (ID2) were similar to the RNA expression data (Figure S4). These results suggest that LMX1A suppresses NSCLC invasion partly through EMT-related mechanisms. To further explore the potential mechanism responsible for LMX1A suppression of cancer cell invasion, we performed a NanoString gene expression analysis to investigate differentially expressed genes related to cancer progression upon LMX1A overexpression. Differentially expressed genes in the LMX1A -V5 H23 cell line are summarized in Table 1 and Table S1. Fourteen genes, including SRGN, SCG2, HGF, AMH, and FN1, were downregulated (fold change < –2). However, only three genes (THBS1, LEFTY1, and ID2) were upregulated (fold change > 2). LMX1A could repress four genes that belong to mesenchymal (Mes) cell markers (Table 1). All of the aberrantly expressed genes were related to processes involved in cancer progression, including angiogenesis, extracellular matrix (ECM) remodeling, cancer invasion, and cancer metastasis. These data suggested that LMX1A inhibits NSCLC cell invasion partly through modulation of EMT, angiogenesis, ECM remodeling, and hypoxia-related genes.
Figure 6.
Effects of LMX1A on epithelial mesenchymal transition (EMT)-related genes in NSCLC cells. Changes in EMT-related genes (CDH1, CDH2, FN1, and ID2) after induction of LMX1A in H23 cells. Data are shown as fold changes of mRNA expression relative to control cells without doxycycline. Values are expressed as the mean ± SEM from three independent experiments, each conducted in duplicate. * p < 0.05 (Mann–Whitney U test or Student’s t-test). CDH1: E-cadherin; CDH2: N-cadherin; FN1: Fibronectin; ID2: The inhibitor of DNA-binding gene 2.
Table 1.
Summary of differentially expressed genes in the LMX1A-V5 H23 cell line by NanoString gene expression analysis.
| Probe Name | Accession Number | Fold Change: LMX1A vs. Control | p Value of LMX1A vs. Control | EMT Spectrum | Progression Categories |
|---|---|---|---|---|---|
| SRGN | NR_036430.1 | −4.32693624 | 0.01084103 | Mes | Angiogenesis, ECM Layers, EMT |
| SCG2 | NM_003469.3 | −3.88797712 | 0.01710246 | Angiogenesis, Tumor Growth | |
| HGF | NM_000601.4 | −2.82349849 | 0.00009102 | Angiogenesis, ECM Layers, EMT, Tumor Growth, Tumor Invasion | |
| AMH | NM_000479.3 | −2.80556583 | 0.04690248 | Transcription Factor, Tumor Growth | |
| FN1 | NM_212482.1 | −2.80556583 | 0.04547928 | Mes | Angiogenesis, ECM Layers, ECM Remodeling, EMT, Tumor Invasion |
| APOE | NM_000041.2 | −2.73198891 | 0.02757248 | ECM Layers, Tumor Growth | |
| CSF2RB | NM_000395.2 | −2.59863329 | 0.0019465 | Mes | ECM Layers, EMT |
| MYH11 | NM_001040113.1 | −2.59863329 | 0.0019465 | Metastasis | |
| PIK3R6 | NM_001010855.3 | −2.59863329 | 0.0019465 | Angiogenesis | |
| CLU | NM_203339.2 | −2.56240726 | 0.02621217 | Angiogenesis, ECM Layers, Tumor Growth | |
| CRIP2 | NM_001270837.1 | −2.32546878 | 0.03496239 | ECM Layers, Tumor Growth | |
| TIMP1 | NM_003254.2 | −2.28131437 | 0.01276203 | Angiogenesis, ECM Layers, ECM Remodeling, Hypoxia, Metastasis, Tumor Growth | |
| CKMT1A | NM_001015001.1 | −2.19464755 | 0.01458759 | EMT | |
| CXCR4 | NM_003467.2 | −1.99022377 | 0.04348437 | Mes | ECM Layers, EMT, Hypoxia |
| THBS1 | NM_003246.2 | 3.25843048 | 0.0395245 | Angiogenesis, ECM Layers, ECM Remodeling, Hypoxia, Tumor Growth, Tumor Invasion | |
| LEFTY1 | NM_020997.2 | 2.91027427 | 0.04374892 | Tumor Growth | |
| ID2 | NM_002166.4 | 1.99489844 | 0.00435035 | Transcription Factor, Tumor Growth |
All overexpressed genes are represented as values >1 and all underexpressed genes are represented as values <−1; Mes: Mesenchymal.
3. Discussion
Lung carcinogenesis involves a multistage stepwise process with the accumulation of genetic and epigenetic alterations [36]. Epigenetic alterations include DNA methylation, histone modifications, and noncoding RNA expression [20]. Among them, aberrant DNA methylation-induced silencing of tumor suppressor genes is a hallmark and an early event in lung carcinogenesis [37,38]. Multiple genes, such as CDKN2A, DAPK, MGMT, RARB, RASSF1A, and TERT, have been found to be methylated early in lung premalignant lesions, and the level of methylation increases with tumor progression from premalignant lesions to neoplasms [39,40,41,42,43]. These methylated genes have crucial functions in cell cycle control, apoptotic signaling, DNA repair, cell growth, and immortalization. In this study, we found that LMX1A was methylation-silenced in non-small cell lung cancer (Figure 1, Figure 2 and Figure 3 and Figure S1). In addition, LMX1A served crucial functions in tumor invasion and epithelial-mesenchymal transition (Table 1). To our knowledge, there are no data reporting epigenetic regulation of LMX1A expression and its function in lung cancer.
DNA methylation is not only important in lung carcinogenesis but also serves as a biomarker for early lung cancer detection [7,44,45,46,47,48,49]. We analyzed the data from The Cancer Genome Atlas (TCGA) database through the UALCAN (http://ualcan.path.uab.edu) web portal and showed that methylation of LMX1A in non-small cell lung cancer occurred early in stage I patients and was then maintained throughout all the stages of non-small cell lung cancer (Figure S5) [50]. This result implied that LMX1A methylation could be used as a diagnostic biomarker for the early detection of lung cancer.
LMX1A, as a transcription factor, is one of the LIM-homeobox genes that plays a crucial role during development [22]. Recently, LMX1A has also been reported to play an important role in cancer. Our research group has reported that LMX1A was methylation-silenced and that the expression of LIMX1A inhibited colony formation and invasion in cervical cancer [29]. We also found that LMX1A, in combination with NKX6.1, SOX1, and ZNF177, could serve as a stage-independent prognostic marker in colorectal cancer [33]. In ovarian cancers, LMX1A was reported to be hypermethylated, and its expression inhibited cell proliferation, migration, invasion, and colony formation [25]. In addition, Zhao and his colleagues found that methylation of LMX1A could be used as an early diagnostic biomarker and was associated with recurrence in bladder cancer [26]. Dong and his colleagues found that methylation-mediated inactivation of LMX1A and restoration of LMX1A induced cell apoptosis and suppressed anchorage-independent growth in gastric cancer [28]. All of the above findings identify LMX1A as a tumor suppressor. However, LMX1A did not function as a tumor suppressor in other types of cancer. Tsai and his colleagues reported that the higher expression of LMX1A shown by immunohistochemical staining correlated with the WHO grade of meningioma and glioma [27]. Tsai and his colleagues reported that the higher expression of LMX1A was strongly correlated with the histologic grade and pathologic stage of pancreatic ductal adenocarcinomas [23]. LMX1A functions as an oncogene instead in these cancers.
In this study, we demonstrated that promoter methylation of LMX1A was frequent in NSCLC cell lines and tumor tissues (Figure 1, Figure 2 and Figure 3 and Figure S1). The methylation-induced silencing of LMX1A could be reversed after cells were treated with a DNA methylation inhibitor in the three cell lines (Figure 3B). Indeed, when we treated H647 cells with a histone deacetylase inhibitor (trichostatin A (TSA)), LMX1A expression was restored (Figure S6). These data suggested that histone modifications are also involved in the epigenetic silencing of LMX1A in lung cancer cells. Overexpression of LMX1A did not affect cell proliferation but inhibited colony formation and invasion. These results were similar to the suppressive effect of LMX1A in cervical cancer [29].
Activation of the EMT program is a well-known critical mechanism for the acquisition of invasiveness and metastasis in cancers [35]. Chao and his colleagues reported that LMX1A could inhibit the EMT pathway by upregulating the epithelial marker CDH1 and downregulating the mesenchymal markers CDH2 and FN1 by repressing the EMT-related transcription factors SNAIL, SLUG, and SIP1 in ovarian cancer [25]. In a previous study, we showed that LMX1A could suppress the EMT pathway partially by inhibition of the EMT-related transcription factors SNAIL, SLUG, and TWIST in cervical cancer [29]. In this study, we found that LMX1A suppressed NSCLC invasion partly through an EMT-related mechanism without influencing the EMT-related transcription factors SNAIL, SLUG, and TWIST (Figure 2 and Figure 6 and Figure S3). This finding suggests two possibilities. First, the regulation of SNAIL, SLUG, and TWIST might occur through post-translational modification instead of transcriptional regulation. Previous reports showed that no permanent genetic alterations in most EMT transcription factors were found in cancer, but transient changes were detected. These post-translational modifications or epigenetic modulation of EMT transcription factors could directly regulate their function by affecting protein stability, nuclear localization, protein–protein interactions, and ubiquitination. EMT transcription factors can drive tumor cell switching between epithelial traits for tumorigenic proliferation and a mesenchymal phenotype for invasion and migration. These transcription factors exert their function in a more flexible and reversible manner, [51,52]. Moreover, growing evidence suggests the presence of a transitional status between epithelial and mesenchymal phenotypes, i.e., the “hybrid epithelial-mesenchymal (hybrid E/M)” condition, which provides a possible reason for these conflicting results. Second, the effect on EMT was mediated through pathways other than SNAIL, SLUG, and TWIST. This hypothesis is supported by the finding that the EMT regulators SRGN, HGF, FN1, CSF2RB, CKMT1A, and CXCR4 were downregulated in the LMX1A-overexpressing H23 cell lines (Table 1). All the mentioned genes have been reported to be EMT regulators and play important roles in the migration, invasion, sphere formation, or drug resistance of cancer cells [53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71]. All together, these data suggested that LMX1A functions as a tumor suppressor partly by regulating EMT-related genes.
LMX1A has been reported to affect pathways other than EMT. Qian and his colleagues demonstrated that LMX1A represses C-MYC expression by activating ANGPTL4 to function as a tumor suppressor in gastric cancer [21]. In lung cancer, C-MYC is frequently dysregulated, important for cancer stem cell (CSC) properties, and associated with unfavorable patient survival [72], and its expression was significantly correlated with programmed death-ligand 1 (PD-L1) expression in NSCLC [73]. However, in the Nanostring gene expression analysis, angiopoietin-like 4 (ANGPL4) and C-MYC were not affected after overexpression of LMX1A in H23 cells. After overexpression of LMX1A in H23 cells, ID2 protein levels were increased. Knockdown of LMX1A by doxycycline (DOX) depletion reversed the ID2 protein level. Moreover, using the Eukaryotic Promoter Database (EPD) (http://epd.vital-it.ch) , we discovered four putative homeobox binding sites in the promoter of 2.5 kb of the ID2 gene. These data support ID2 as a potential target of LMX1A. The TGF-ß pathway induces EMT transition through many downstream signaling pathways. ID2 was significantly decreased by TGF-ß, and expression of ID2 was restored after treatment with a TGF-ß inhibitor [74]. It has been reported that ID2 inhibited cancer invasion and metastasis [75], and the mRNA expression levels of ID2 were inversely correlated with the tumor metastasis stage in clinical samples [76]. Moreover, transient overexpression of ID2 in the parental H23 cell line also represses cancer invasion (Figure S7). Taken together, these data provide evidence to further elucidate how LMX1A regulates ID2, and this is linked with its function.
In this study, we evaluated the role and function of LMX1A only in human NSCLC cells. Based on these in vitro studies, a xenograft animal model should be established to clarify LMX1A’s tumor suppressor role in NSCLC in vivo. To further explore the downstream signaling pathways related to the tumor suppressor function of LMX1A, researchers should use RNA sequencing technology for comprehensive analysis. Our results need further investigations in future studies.
In brief, promoter methylation leads to the silencing of LMX1A and contributes to cancer progression. LMX1A inhibits NSCLC cell invasion partly through modulation of EMT, angiogenesis, and ECM remodeling.
4. Materials and Methods
4.1. SMART (Shiny Methylation Analysis Resource Tool) App
SMART (Shiny Methylation Analysis Resource Tool) App (http://www.bioin fo-zs.com/smart app) is a web application for analyzing the DNA methylation of human cancers [34]. In this study, we used DNA methylation data in the SMART App for analysis of the methylation levels of LMX1A in 41 tissue samples from normal controls and 458 tissue samples from lung adenocarcinoma (LUAD) patients; 364 tissue samples from lung squamous cell carcinoma (LUSC) patients are shown.
4.2. Cell Lines
A total of eight human lung cancer cell lines (A549, H1437, H23, CL1-0, CL1-3, H358, H1299, and H647) were used in this study. They were obtained from Professor Yi-Ching Wang (National Cheng Kung University, Taiwan). We cultured cells in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Gaithersburg, MD, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and other components as previously described [77]. All cell cultures were maintained in an incubator containing 5% CO2 at 37 °C. Detailed descriptions are available in the Supplementary Materials and Methods.
4.3. DNA Methylation and Gene Expression Analysis
Genomic DNA of NSCLC cell lines was extracted and bisulfite-converted as previously described [78]. CpG-methylated human genomic DNA (Thermo Fisher Scientific, San Diego, CA, USA) and DNA extracted from normal peripheral blood lymphocytes were modified by sodium bisulfite to generate positive and negative controls, respectively. MSP, bisulfate sequencing, and Q-MSP were performed as previously described. For Q-MSP, the DNA methylation levels were assessed by determining the methylation index (MI) using the following formula: 100 × 2−[(Cp °f Gene) − (Cp °f COL2A)]. For gene expression analysis, RNA extraction and reverse transcription polymerase chain reaction (RT-PCR) were conducted as previously described [78]. The primer sequences were described previously [79] and are shown in Table S2. Detailed descriptions are available in the Supplementary Materials and Methods.
4.4. Plasmids Construction
The full-length LMX1A open-reading frame (ORF) was cloned into the pcDNA3.1-V5-His-TOPO constitutive expression vector (Invitrogen, Waltham, MA, USA) (termed pcDNA3.1-LMX1A) or the inducible expression vector pAS4.1w. Pbsd-aOn (Figure S8) (purchased from National RNAi Core Facility of Taiwan) (termed pAS4.1-LMX1A).
4.5. Generation of Cells Overexpressing Stable Clones or Inducible Expression Stable Clones
Assays for the generation of cells overexpressing stable clones or inducible expression stable clones were conducted as described previously [79]. Detailed descriptions are available in Supplementary Materials and methods.
4.6. Assays for Western Blot, Cell Viability, Anchorage-Independent Growth, and Invasion
Assays for Western blot, cell viability by MTS(3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, Inner Salt), anchorage-independent growth, and invasion were conducted as described previously [29].
4.7. NanoString Gene Expression Analysis
Total RNA was isolated from NSCLC cells using TRIzol reagent (Invitrogen) according to the manufacturer’s handbook. Purity and quantity were assessed by the NanoDrop 8000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). NanoString gene expression analysis was performed on total RNA from isolated cultured cells using the nCounter Human PanCancer Progression Panel (NanoString Technologies, Seattle, WA, USA) according to the manufacturer’s instructions [80,81,82]. Briefly, 200 ng of total RNA was hybridized with reporter and capture probes in a thermocycler (65 °C) for 24 h. Samples were then loaded onto the nCounter Cartridge by using the nCounter Prep Station, and RNA was quantified by using the Digital Analyzer. After quality control analysis, normalized RNA counts were generated by negative control subtraction and the geometric mean of the housekeeping genes TUBB, PGK1, GUSB, HPRT1, CLTC, and GAPDH with nSolver Analysis Software v2.5. Genes were considered not expressed if the normalized RNA counts were less than twice the SD of the negative control counts in both the LMX1A-V5 cells and the control cells.
4.8. Statistical Analysis
Statistical analyses were performed using GraphPad Prism software (version 5; GraphPad Software, La Jolla, CA, USA) and SPSS software (IBM SPSS Statistics 21; Asia Analytics Taiwan, Taipei, Taiwan). All values are expressed as the mean ± SEM. The Mann–Whitney U test was used to determine differences between gene methylation levels and disease status. The Student’s t-test and Mann–Whitney test were used to compare colony number, cell invasion, and relative RNA expression in the different stable transfectants.
5. Conclusions
In summary, the current study demonstrated that LMX1A functions as a tumor suppressor partly by repressing EMT, angiogenesis, and ECM remodeling in lung cancer. Epigenetic inactivation of LMX1A abolishes the tumor suppressor function. Our data identified a methylation biomarker for lung cancer progression and suggest a potential combination of epigenetic drugs in future therapeutic approaches.
Acknowledgments
We thank Ming-De Yan for advice on paper-writing, and Chia-Hsin Lin and Shin-Ping Lin for technical assistance. We thank the National RNAi Core Facility at Academia Sinica in Taiwan for providing shRNA reagents and related services.
Abbreviations
| LMX1A | LIM homeobox transcription factor 1α |
| RT-PCR | reverse transcription-polymerase chain reaction |
| MSP | methylation-specific PCR |
| EMT | epithelial mesenchymal transition |
| NSCLC | non-small cell lung cancer |
| CDH1 | E-cadherin |
| FN1 | fibronectin |
| CDH2 | N-cadherin |
| ID | DNA-binding gene 2 |
| ANGPL4 | angiopoietin-like 4 |
| ECM | extracellular matrix |
| EGFR | epidermal growth factor receptor |
| LIM-HD | LIM-homeodomain |
| LUAD | lung adenocarcinoma |
| LUSC | lung squamous cell carcinoma |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
| Q-MSP | quantitative methylation-specific PCR |
| DAC | 5-aza-2ʹ-deoxycytidine |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/15/5425/s1, Figure S1. Bisulfite genomic sequencing analysis of the CpG sites located at the promoter region of LMX1A. Figure S2. Protein expression of LMX1A in human NSCLC cells, normal lung tissues, and NSCLC cells with expression of LMX1A-V5. Figure S3. Effects of LMX1A on EMT-related transcription factors in NSCLC cells. Figure S4. Effects of LMX1A on EMT-related genes in NSCLC cells. Figure S5. The methylation of LMX1A in NSCLC patients according to tumor stages, analyzed from The Cancer Genome Atlas (TCGA) database through the UALCAN (http://ualcan.path.uab.edu) web portal. Figure S6. Reexpression of LMX1A after treatment of histone deacetylase (HDAC) inhibitors in H647 lung cancer cells. Figure S7. Transient overexpression of ID2 suppresses the invasion of cancer cells. Table S1. Summary of differentially expressed genes that has statistical significance in LMX1A-V5 H23 cell lines by NanoString gene expression. Table S2. Primer sequences used for MSP, Q-MSP, bisulfite sequencing, and qRT-PCR. Table S3. Raw data for colony formation assay and invasion assay.
Author Contributions
T.-H.W. and Y.-W.L. designed the research, performed all of the experiments, analyzed the data, and wrote the manuscript. S.-Y.C. and Y.-L.S. helped to analyze the data. Y.-L.S., C.-F.C. and H.C. provided experimental materials and helped to modify the manuscript. Y.-W.L. critically revised the manuscript. All authors discussed the results and commented on the manuscript. All authors have read and approved the final manuscript.
Funding
This work was supported in part by the following grants: MOST 108-2314-B-016-043-MY2 and MOST 105-2320-B-016-017-MY3 from the Ministry of Science and Technology, Taiwan, Republic of China; MAB-109-036, MAB-108-12, MAB-108-013, MAB-108-014, and MAB-108-015 from the Ministry of National Defense, Taiwan, Republic of China; TSGH-C108-072 and TSGH-C109-076 from Tri-Service General Hospital, Taiwan, Republic of China.
Conflicts of Interest
The authors declare no conflict of interest related to this work.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N., Noone A., Krapcho M., Miller D., Bishop K., Altekruse S., Kosary C., Yu M., Ruhl J., Tatalovich Z., et al. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD, USA: 2016. [(accessed on 24 May 2020)]. Available online: https://seer.cancer.gov/archive/csr/1975_2013/ [Google Scholar]
- 3.Aravanis A.M., Lee M., Klausner R.D. Next-Generation Sequencing of Circulating Tumor DNA for Early Cancer Detection. Cell. 2017;168:571–574. doi: 10.1016/j.cell.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Belinsky S.A., Klinge D.M., Dekker J.D., Smith M.W., Bocklage T.J., Gilliland F.D., Crowell R.E., Karp D.D., Stidley C.A., Picchi M.A. Gene promoter methylation in plasma and sputum increases with lung cancer risk. Clin. Cancer Res. 2005;11:6505–6511. doi: 10.1158/1078-0432.CCR-05-0625. [DOI] [PubMed] [Google Scholar]
- 5.Han W., Wang T., Reilly A.A., Keller S.M., Spivack S.D. Gene promoter methylation assayed in exhaled breath, with differences in smokers and lung cancer patients. Respir. Res. 2009;10:86. doi: 10.1186/1465-9921-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X.J., Hayward C., Fong P.Y., Dominguez M., Hunsucker S.W., Lee L.W., McLean M., Law S., Butler H., Schirm M., et al. A blood-based proteomic classifier for the molecular characterization of pulmonary nodules. Sci. Transl. Med. 2013;5:207ra142. doi: 10.1126/scitranslmed.3007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topaloglu O., Hoque M.O., Tokumaru Y., Lee J., Ratovitski E., Sidransky D., Moon C.S. Detection of promoter hypermethylation of multiple genes in the tumor and bronchoalveolar lavage of patients with lung cancer. Clin. Cancer Res. 2004;10:2284–2288. doi: 10.1158/1078-0432.CCR-1111-3. [DOI] [PubMed] [Google Scholar]
- 8.Breuer R.H., Pasic A., Smit E.F., van Vliet E., Vonk Noordegraaf A., Risse E.J., Postmus P.E., Sutedja T.G. The natural course of preneoplastic lesions in bronchial epithelium. Clin. Cancer Res. 2005;11:537–543. [PubMed] [Google Scholar]
- 9.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 2007;8:286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 10.Risch A., Plass C. Lung cancer epigenetics and genetics. Int. J. Cancer. 2008;123:1–7. doi: 10.1002/ijc.23605. [DOI] [PubMed] [Google Scholar]
- 11.Noor Z.S., Cummings A.L., Johnson M.M., Spiegel M.L., Goldman J.W. Targeted Therapy for Non-Small Cell Lung Cancer. Semin. Respir. Crit. Care Med. 2020;41:409–434. doi: 10.1055/s-0039-1700994. [DOI] [PubMed] [Google Scholar]
- 12.Hu H., Zhou Y., Zhang M., Ding R. Prognostic value of RASSF1A methylation status in non-small cell lung cancer (NSCLC) patients: A meta-analysis of prospective studies. Biomarkers. 2019;24:207–216. doi: 10.1080/1354750X.2019.1583771. [DOI] [PubMed] [Google Scholar]
- 13.Tuo L., Sha S., Huayu Z., Du K. P16(INK4a) gene promoter methylation as a biomarker for the diagnosis of non-small cell lung cancer: An updated meta-analysis. Thorac. Cancer. 2018;9:1032–1040. doi: 10.1111/1759-7714.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng H., Zhang Z., Qing X., Wang X., Liang C., Liu D. Promoter methylation of APC and RAR-β genes as prognostic markers in non-small cell lung cancer (NSCLC) Exp. Mol. Pathol. 2016;100:109–113. doi: 10.1016/j.yexmp.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J., Yu X.L., Zheng G.F., Zhao F. DAPK promoter methylation status correlates with tumor metastasis and poor prognosis in patients with non-small cell lung cancer. Cancer Biomark. 2015;15:609–617. doi: 10.3233/CBM-150501. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y., Lan Q., Siegfried J.M., Luketich J.D., Keohavong P. Aberrant promoter methylation of p16 and MGMT genes in lung tumors from smoking and never-smoking lung cancer patients. Neoplasia. 2006;8:46–51. doi: 10.1593/neo.05586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J.S., Kim H., Shim Y.M., Han J., Park J., Kim D.H. Aberrant methylation of the FHIT gene in chronic smokers with early stage squamous cell carcinoma of the lung. Carcinogenesis. 2004;25:2165–2171. doi: 10.1093/carcin/bgh217. [DOI] [PubMed] [Google Scholar]
- 18.Duruisseaux M., Esteller M. Lung cancer epigenetics: From knowledge to applications. Semin. Cancer Biol. 2018;51:116–128. doi: 10.1016/j.semcancer.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Mehta A., Dobersch S., Romero-Olmedo A.J., Barreto G. Epigenetics in lung cancer diagnosis and therapy. Cancer Metastasis Rev. 2015;34:229–241. doi: 10.1007/s10555-015-9563-3. [DOI] [PubMed] [Google Scholar]
- 20.Langevin S.M., Kratzke R.A., Kelsey K.T. Epigenetics of lung cancer. Transl. Res. 2015;165:74–90. doi: 10.1016/j.trsl.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian P., Li J., Zhang X., Li F., Bei S., Li H., Sun Q., Feng L. LMX1A inhibits C-Myc expression through ANGPTL4 to exert tumor suppressive role in gastric cancer. PLoS ONE. 2019;14:e0221640. doi: 10.1371/journal.pone.0221640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X., He C., Hu X. LIM homeobox transcription factors, a novel subfamily which plays an important role in cancer (review) Oncol. Rep. 2014;31:1975–1985. doi: 10.3892/or.2014.3112. [DOI] [PubMed] [Google Scholar]
- 23.Tsai W.C., Lin C.K., Yang Y.S., Chan D.C., Gao H.W., Chang F.N., Jin J.S. The correlations of LMX1A and osteopontin expression to the clinicopathologic stages in pancreatic adenocarcinoma. Appl. Immunohistochem. Mol. Morphol. 2013;21:395–400. doi: 10.1097/PAI.0b013e318277d9de. [DOI] [PubMed] [Google Scholar]
- 24.Lin W.C., Yan M.D., Yu P.N., Li H.J., Kuo C.C., Hsu C.L., Lin Y.W. The role of Sp1 and EZH2 in the regulation of LMX1A in cervical cancer cells. Biochim. Biophys. Acta. 2013;1833:3206–3217. doi: 10.1016/j.bbamcr.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Chao T.K., Yo Y.T., Liao Y.P., Wang Y.C., Su P.H., Huang T.S., Lai H.C. LIM-homeobox transcription factor 1, alpha (LMX1A) inhibits tumourigenesis, epithelial-mesenchymal transition and stem-like properties of epithelial ovarian cancer. Gynecol. Oncol. 2013;128:475–482. doi: 10.1016/j.ygyno.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y., Guo S., Sun J., Huang Z., Zhu T., Zhang H., Gu J., He Y., Wang W., Ma K., et al. Methylcap-seq reveals novel DNA methylation markers for the diagnosis and recurrence prediction of bladder cancer in a Chinese population. PLoS ONE. 2012;7:e35175. doi: 10.1371/journal.pone.0035175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai W.C., Lee H.S., Lin C.K., Chen A., Nieh S., Ma H.I. The association of osteopontin and LMX1A expression with World Health Organization grade in meningiomas and gliomas. Histopathology. 2012;61:844–856. doi: 10.1111/j.1365-2559.2012.04277.x. [DOI] [PubMed] [Google Scholar]
- 28.Dong W., Feng L., Xie Y., Zhang H., Wu Y. Hypermethylation-mediated reduction of LMX1A expression in gastric cancer. Cancer Sci. 2011;102:361–366. doi: 10.1111/j.1349-7006.2010.01804.x. [DOI] [PubMed] [Google Scholar]
- 29.Liu C.Y., Chao T.K., Su P.H., Lee H.Y., Shih Y.L., Su H.Y., Chu T.Y., Yu M.H., Lin Y.W., Lai H.C. Characterization of LMX-1A as a metastasis suppressor in cervical cancer. J. Pathol. 2009;219:222–231. doi: 10.1002/path.2589. [DOI] [PubMed] [Google Scholar]
- 30.Hobert O., Westphal H. Functions of LIM-homeobox genes. Trends Genet. 2000;16:75–83. doi: 10.1016/S0168-9525(99)01883-1. [DOI] [PubMed] [Google Scholar]
- 31.Cai J., Donaldson A., Yang M., German M.S., Enikolopov G., Iacovitti L. The role of Lmx1a in the differentiation of human embryonic stem cells into midbrain dopamine neurons in culture and after transplantation into a Parkinson’s disease model. Stem Cells. 2009;27:220–229. doi: 10.1634/stemcells.2008-0734. [DOI] [PubMed] [Google Scholar]
- 32.Chizhikov V.V., Lindgren A.G., Mishima Y., Roberts R.W., Aldinger K.A., Miesegaes G.R., Currle D.S., Monuki E.S., Millen K.J. Lmx1a regulates fates and location of cells originating from the cerebellar rhombic lip and telencephalic cortical hem. Proc. Natl. Acad. Sci. USA. 2010;107:10725–10730. doi: 10.1073/pnas.0910786107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung H.H., Kuo C.C., Hsiao C.W., Chen C.Y., Hu J.M., Hsu C.H., Chou Y.C., Lin Y.W., Shih Y.L. A Novel Prognostic DNA Methylation Panel for Colorectal Cancer. Int. J. Mol. Sci. 2019;20:4672. doi: 10.3390/ijms20194672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y., Ge D., Lu C. The SMART App: An interactive web application for comprehensive DNA methylation analysis and visualization. Epigenetics Chromatin. 2019;12:71. doi: 10.1186/s13072-019-0316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lantuéjoul S., Salameire D., Salon C., Brambilla E. Pulmonary preneoplasia--sequential molecular carcinogenetic events. Histopathology. 2009;54:43–54. doi: 10.1111/j.1365-2559.2008.03182.x. [DOI] [PubMed] [Google Scholar]
- 37.Belinsky S.A. Gene-promoter hypermethylation as a biomarker in lung cancer. Nat. Rev. Cancer. 2004;4:707–717. doi: 10.1038/nrc1432. [DOI] [PubMed] [Google Scholar]
- 38.Esteller M. Epigenetics in cancer. N. Engl. J. Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 39.Belinsky S.A., Nikula K.J., Palmisano W.A., Michels R., Saccomanno G., Gabrielson E., Baylin S.B., Herman J.G. Aberrant methylation of p16(INK4a) is an early event in lung cancer and a potential biomarker for early diagnosis. Proc. Natl. Acad. Sci. USA. 1998;95:11891–11896. doi: 10.1073/pnas.95.20.11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsou J.A., Galler J.S., Siegmund K.D., Laird P.W., Turla S., Cozen W., Hagen J.A., Koss M.N., Laird-Offringa I.A. Identification of a panel of sensitive and specific DNA methylation markers for lung adenocarcinoma. Mol. Cancer. 2007;6:70. doi: 10.1186/1476-4598-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yim H.W., Slebos R.J., Randell S.H., Umbach D.M., Parsons A.M., Rivera M.P., Detterbeck F.C., Taylor J.A. Smoking is associated with increased telomerase activity in short-term cultures of human bronchial epithelial cells. Cancer Lett. 2007;246:24–33. doi: 10.1016/j.canlet.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Licchesi J.D., Westra W.H., Hooker C.M., Herman J.G. Promoter hypermethylation of hallmark cancer genes in atypical adenomatous hyperplasia of the lung. Clin. Cancer Res. 2008;14:2570–2578. doi: 10.1158/1078-0432.CCR-07-2033. [DOI] [PubMed] [Google Scholar]
- 43.Selamat S.A., Galler J.S., Joshi A.D., Fyfe M.N., Campan M., Siegmund K.D., Kerr K.M., Laird-Offringa I.A. DNA methylation changes in atypical adenomatous hyperplasia, adenocarcinoma in situ, and lung adenocarcinoma. PLoS ONE. 2011;6:e21443. doi: 10.1371/journal.pone.0021443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahrendt S.A., Chow J.T., Xu L.H., Yang S.C., Eisenberger C.F., Esteller M., Herman J.G., Wu L., Decker P.A., Jen J., et al. Molecular detection of tumor cells in bronchoalveolar lavage fluid from patients with early stage lung cancer. J. Natl. Cancer Inst. 1999;91:332–339. doi: 10.1093/jnci/91.4.332. [DOI] [PubMed] [Google Scholar]
- 45.Kim H., Kwon Y.M., Kim J.S., Lee H., Park J.H., Shim Y.M., Han J., Park J., Kim D.H. Tumor-specific methylation in bronchial lavage for the early detection of non-small-cell lung cancer. J. Clin. Oncol. 2004;22:2363–2370. doi: 10.1200/JCO.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 46.Belinsky S.A., Liechty K.C., Gentry F.D., Wolf H.J., Rogers J., Vu K., Haney J., Kennedy T.C., Hirsch F.R., Miller Y., et al. Promoter hypermethylation of multiple genes in sputum precedes lung cancer incidence in a high-risk cohort. Cancer Res. 2006;66:3338–3344. doi: 10.1158/0008-5472.CAN-05-3408. [DOI] [PubMed] [Google Scholar]
- 47.Leng S., Do K., Yingling C.M., Picchi M.A., Wolf H.J., Kennedy T.C., Feser W.J., Baron A.E., Franklin W.A., Brock M.V., et al. Defining a gene promoter methylation signature in sputum for lung cancer risk assessment. Clin. Cancer Res. 2012;18:3387–3395. doi: 10.1158/1078-0432.CCR-11-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikolaidis G., Raji O.Y., Markopoulou S., Gosney J.R., Bryan J., Warburton C., Walshaw M., Sheard J., Field J.K., Liloglou T. DNA methylation biomarkers offer improved diagnostic efficiency in lung cancer. Cancer Res. 2012;72:5692–5701. doi: 10.1158/0008-5472.CAN-12-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wrangle J., Machida E.O., Danilova L., Hulbert A., Franco N., Zhang W., Glöckner S.C., Tessema M., Van Neste L., Easwaran H., et al. Functional identification of cancer-specific methylation of CDO1, HOXA9, and TAC1 for the diagnosis of lung cancer. Clin. Cancer Res. 2014;20:1856–1864. doi: 10.1158/1078-0432.CCR-13-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandrashekar D.S., Bashel B., Balasubramanya S.A.H., Creighton C.J., Ponce-Rodriguez I., Chakravarthi B., Varambally S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu R., Won J.Y., Kim C.H., Kim D.E., Yim H. Roles of the Phosphorylation of Transcriptional Factors in Epithelial-Mesenchymal Transition. J. Oncol. 2019;2019:5810465. doi: 10.1155/2019/5810465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao T.T., Yang M.H. Hybrid Epithelial/Mesenchymal State in Cancer Metastasis: Clinical Significance and Regulatory Mechanisms. Cells. 2020;9:623. doi: 10.3390/cells9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X.J., Ong C.K., Cao Y., Xiang Y.Q., Shao J.Y., Ooi A., Peng L.X., Lu W.H., Zhang Z., Petillo D., et al. Serglycin is a theranostic target in nasopharyngeal carcinoma that promotes metastasis. Cancer Res. 2011;71:3162–3172. doi: 10.1158/0008-5472.CAN-10-3557. [DOI] [PubMed] [Google Scholar]
- 54.Guo J.Y., Hsu H.S., Tyan S.W., Li F.Y., Shew J.Y., Lee W.H., Chen J.Y. Serglycin in tumor microenvironment promotes non-small cell lung cancer aggressiveness in a CD44-dependent manner. Oncogene. 2017;36:2457–2471. doi: 10.1038/onc.2016.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z., Deng Y., Zheng G., Jia X., Xiong Y., Luo K., Qiu Q., Qiu N., Yin J., Lu M., et al. SRGN-TGFβ2 regulatory loop confers invasion and metastasis in triple-negative breast cancer. Oncogenesis. 2017;6:e360. doi: 10.1038/oncsis.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Y., Xu J., Yang Y., Zhu L., Li X., Zhao W. SRGN Promotes Colorectal Cancer Metastasis as a Critical Downstream Target of HIF-1α. Cell Physiol. Biochem. 2018;48:2429–2440. doi: 10.1159/000492657. [DOI] [PubMed] [Google Scholar]
- 57.Fajardo-Puerta A.B., Mato Prado M., Frampton A.E., Jiao L.R. Gene of the month: HGF. J. Clin. Pathol. 2016;69:575–579. doi: 10.1136/jclinpath-2015-203575. [DOI] [PubMed] [Google Scholar]
- 58.Fan H., Lv P., Mu T., Zhao X., Liu Y., Feng Y., Lv J., Liu M., Tang H. LncRNA n335586/miR-924/CKMT1A axis contributes to cell migration and invasion in hepatocellular carcinoma cells. Cancer Lett. 2018;429:89–99. doi: 10.1016/j.canlet.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 59.Han J.Y., Kim H.S., Lee S.H., Park W.S., Lee J.Y., Yoo N.J. Immunohistochemical expression of integrins and extracellular matrix proteins in non-small cell lung cancer: Correlation with lymph node metastasis. Lung Cancer. 2003;41:65–70. doi: 10.1016/S0169-5002(03)00146-6. [DOI] [PubMed] [Google Scholar]
- 60.Han S., Khuri F.R., Roman J. Fibronectin stimulates non-small cell lung carcinoma cell growth through activation of Akt/mammalian target of rapamycin/S6 kinase and inactivation of LKB1/AMP-activated protein kinase signal pathways. Cancer Res. 2006;66:315–323. doi: 10.1158/0008-5472.CAN-05-2367. [DOI] [PubMed] [Google Scholar]
- 61.Jakowlew S.B., Mariano J.M., You L., Mathias A. Differential regulation of protease and extracellular matrix protein expression by transforming growth factor-beta 1 in non-small cell lung cancer cells and normal human bronchial epithelial cells. Biochim. Biophys. Acta. 1997;1353:157–170. doi: 10.1016/S0167-4781(97)00068-7. [DOI] [PubMed] [Google Scholar]
- 62.Liu F., Song S., Yi Z., Zhang M., Li J., Yang F., Yin H., Yu X., Guan C., Liu Y., et al. HGF induces EMT in non-small-cell lung cancer through the hBVR pathway. Eur. J. Pharm. 2017;811:180–190. doi: 10.1016/j.ejphar.2017.05.040. [DOI] [PubMed] [Google Scholar]
- 63.Morgillo F., Della Corte C.M., Fasano M., Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open. 2016;1:e000060. doi: 10.1136/esmoopen-2016-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pankov R., Yamada K.M. Fibronectin at a glance. J. Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 65.Rintoul R.C., Sethi T. Extracellular matrix regulation of drug resistance in small-cell lung cancer. Clin. Sci. (Lond.) 2002;102:417–424. doi: 10.1042/cs1020417. [DOI] [PubMed] [Google Scholar]
- 66.Yin H., Wang Y., Chen W., Zhong S., Liu Z., Zhao J. Drug-resistant CXCR4-positive cells have the molecular characteristics of EMT in NSCLC. Gene. 2016;594:23–29. doi: 10.1016/j.gene.2016.08.043. [DOI] [PubMed] [Google Scholar]
- 67.Jäger B., Klatt D., Plappert L., Golpon H., Lienenklaus S., Barbosa P.D., Schambach A., Prasse A. CXCR4/MIF axis amplifies tumor growth and epithelial-mesenchymal interaction in non-small cell lung cancer. Cell Signal. 2020;73:109672. doi: 10.1016/j.cellsig.2020.109672. [DOI] [PubMed] [Google Scholar]
- 68.Tu Z., Xie S., Xiong M., Liu Y., Yang X., Tembo K.M., Huang J., Hu W., Huang X., Pan S., et al. CXCR4 is involved in CD133-induced EMT in non-small cell lung cancer. Int. J. Oncol. 2017;50:505–514. doi: 10.3892/ijo.2016.3812. [DOI] [PubMed] [Google Scholar]
- 69.Xu X., Cao L., Zhang Y., Lian H., Sun Z., Cui Y. MicroRNA-1246 inhibits cell invasion and epithelial mesenchymal transition process by targeting CXCR4 in lung cancer cells. Cancer Biomark. 2018;21:251–260. doi: 10.3233/CBM-170317. [DOI] [PubMed] [Google Scholar]
- 70.Zheng C.H., Chen X.M., Zhang F.B., Zhao C., Tu S.S. Inhibition of CXCR4 regulates epithelial mesenchymal transition of NSCLC via the Hippo-YAP signaling pathway. Cell Biol. Int. 2018;42:1386–1394. doi: 10.1002/cbin.11024. [DOI] [PubMed] [Google Scholar]
- 71.Zhu Q., Zhang Z., Lu C., Xu F., Mao W., Zhang K., Shou H., Liu Z., Gu J., Ge D. Gefitinib promotes CXCR4-dependent epithelial to mesenchymal transition via TGF-β1 signaling pathway in lung cancer cells harboring EGFR mutation. Clin. Transl. Oncol. 2020;22:1355–1363. doi: 10.1007/s12094-019-02266-w. [DOI] [PubMed] [Google Scholar]
- 72.Chanvorachote P., Sriratanasak N., Nonpanya N. C-myc Contributes to Malignancy of Lung Cancer: A Potential Anticancer Drug Target. Anticancer Res. 2020;40:609–618. doi: 10.21873/anticanres.13990. [DOI] [PubMed] [Google Scholar]
- 73.Kim E.Y., Kim A., Kim S.K., Chang Y.S. MYC expression correlates with PD-L1 expression in non-small cell lung cancer. Lung Cancer. 2017;110:63–67. doi: 10.1016/j.lungcan.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Kondo M., Cubillo E., Tobiume K., Shirakihara T., Fukuda N., Suzuki H., Shimizu K., Takehara K., Cano A., Saitoh M., et al. A role for Id in the regulation of TGF-beta-induced epithelial-mesenchymal transdifferentiation. Cell Death Differ. 2004;11:1092–1101. doi: 10.1038/sj.cdd.4401467. [DOI] [PubMed] [Google Scholar]
- 75.Agapova O.A., Person E., Harbour J.W. Id2 deficiency promotes metastasis in a mouse model of ocular cancer. Clin. Exp. Metastasis. 2010;27:91–96. doi: 10.1007/s10585-010-9304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsunedomi R., Iizuka N., Tamesa T., Sakamoto K., Hamaguchi T., Somura H., Yamada M., Oka M. Decreased ID2 promotes metastatic potentials of hepatocellular carcinoma by altering secretion of vascular endothelial growth factor. Clin. Cancer Res. 2008;14:1025–1031. doi: 10.1158/1078-0432.CCR-07-1116. [DOI] [PubMed] [Google Scholar]
- 77.Wu T.H., Chang S.Y., Shih Y.L., Huang T.W., Chang H., Lin Y.W. Emetine Synergizes with Cisplatin to Enhance Anti-Cancer Efficacy against Lung Cancer Cells. Int. J. Mol. Sci. 2019;20:5914. doi: 10.3390/ijms20235914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang S.Y., Kuo C.C., Wu C.C., Hsiao C.W., Hu J.M., Hsu C.H., Chou Y.C., Shih Y.L., Lin Y.W. NKX6.1 hypermethylation predicts the outcome of stage II colorectal cancer patients undergoing chemotherapy. Genes Chromosomes Cancer. 2018;57:268–277. doi: 10.1002/gcc.22529. [DOI] [PubMed] [Google Scholar]
- 79.Tsao C.M., Yan M.D., Shih Y.L., Yu P.N., Kuo C.C., Lin W.C., Li H.J., Lin Y.W. SOX1 functions as a tumor suppressor by antagonizing the WNT/beta-catenin signaling pathway in hepatocellular carcinoma. Hepatol. (Baltim. Md.) 2012;56:2277–2287. doi: 10.1002/hep.25933. [DOI] [PubMed] [Google Scholar]
- 80.Kulkarni M.M. Digital multiplexed gene expression analysis using the NanoString nCounter system. Curr. Protoc. Mol. Biol. 2011;25:Unit25B.10. doi: 10.1002/0471142727.mb25b10s94. [DOI] [PubMed] [Google Scholar]
- 81.Tsang H.F., Xue V.W., Koh S.P., Chiu Y.M., Ng L.P., Wong S.C. NanoString, a novel digital color-coded barcode technology: Current and future applications in molecular diagnostics. Expert Rev. Mol. Diagn. 2017;17:95–103. doi: 10.1080/14737159.2017.1268533. [DOI] [PubMed] [Google Scholar]
- 82.Warren S. Simultaneous, Multiplexed Detection of RNA and Protein on the NanoString® nCounter® Platform. Methods Mol. Biol. 2018;1783:105–120. doi: 10.1007/978-1-4939-7834-2_5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.