Abstract
Diabetic retinopathy is one of the worst complications of diabetes and it is treated by invasive method. We prepared a surface modified poly (D, L-lactide-co-glycolide) i.e. PLGA nanoparticles for delivery of pioglitazone-a peroxisome proliferator-activated receptor-gamma agonist to posterior segment of the eye by topical administration. The present study investigated two grades of PLGA viz. 75:25 and 50:50. Surface modification was performed using polysorbate 80. Nanoparticles were prepared by single emulsion solvent evaporation method and optimized by using 3-factor 3-level Box-Behnken statistical design. Mean particle size, PDI and entrapment efficiency for optimized batch of PLGA 75:25 was found to be 163.23 nm, 0.286 and 91%, whereas; for PLGA 50:50 it was 171.7 nm, 0.280 and 93% respectively. DSC confirms the molecular dispersion of drug in polymer. In vitro release study showed biphasic drug release pattern with 58.48 ± 1.38% and 74.17 ± 1.38% cumulative drug release by PLGA 75:25 and 50:50 nanoparticles at the end of 10h. The release profile of pioglitazone from nanoparticles appeared to fit best with Higuchi model. In vivo study on rat showed dose dependent reduction in vascular endothelial growth factor concentration in vitreous fluid. The study reveals significance of peroxisome proliferator-activated receptor-gamma in management of diabetic retinopathy.
Keywords: Nanoparticle, PPAR-gamma, VEGF, Diabetic retinopathy, Box-Behnken statistical design, PLGA, Nanomaterials, Pharmaceutical science, Pharmacology, Ophthalmology, Alternative medicine
Nanoparticle; PPAR-gamma; VEGF; diabetic retinopathy; Box-Behnken statistical design; PLGA; Nanomaterials; Pharmaceutical Science; Pharmacology; Ophthalmology; Alternative Medicine.
1. Introduction
Diabetes mellitus is recognized as a global epidemic, has already affected 382 millions of people worldwide and it is projected that by year 2035 this number will be 559 millions [1]. Management of diabetes mellitus associated complications along with primary disease is the biggest challenge to researcher and health care provider [2]. Diabetic patients are at high risk of development of the most devastating microvascular complication known as diabetic retinopathy [3, 4]. Diabetic retinopathy (DR), leads to blindness in a patient aged from 20-65 years [5]. Nearly all patients with type 1 diabetes and more than 60% patients with type 2 diabetes are at risk of development of diabetic retinopathy after 10 years of incidence of diabetes [6]. Still, the complete cure is not available for diabetic retinopathy, for a sake the loss of vision can be prevented or extended either by laser treatment or by vitrectomy, which is associated with pain and further fear of loss of vision [7, 8]. Recently US-FDA has approved drugs like ranibizumab, aflibercept for the management of diabetic retinopathy, administered in the form of intravetrous injection [9, 10]. Although intravetrous injection helps to deliver therapeutic amount of drug at the same time, it results in pain and increased risk of bleeding as well as chances to have secondary infection. Moreover; this current treatment is applicable at advanced stage of diabetic retinopathy, therefore; there is surge to develop alternative method for the treatment of diabetic retinopathy in its early stage. Recently, several clinical studies proved the beneficial role of peroxisome proliferator-activated receptor-γ in management of diabetic retinopathy. Min K Song et al.(2012) reported the presence of peroxisome proliferator-activated receptor-γ (PPAR-γ) in the mammalian eye, prominently in the photoreceptor outer segments, retinal pigmented epithelium (RPE), choriocapillaris and retina. The PPAR-γ agonist is also well known to treat the diabetes and able to resolve crucial issues associated with diabetic retinopathy [4]. Increased level of VEGF can be acts as marker of progress of DR as reported by Saravia M. et al. (2017) [11]. It was found that the vitreous level of vascular endothelial growth factor (VEGF) in patient suffering from DR was markedly increased. Moreover; researchers from National Institute of Health and American Society of Retina Specialist concluded that formation of abnormal blood vessel, which results in blindness in diabetic retinopathy, is spurred by VEGF. Involvement of PPAR-γ receptors in diabetic retinopathy, current research involves preparation of topical formulation of PPAR-γ agonist with aim to regulate VEGF. Since the target is posterior segment of eye, the delivery of drug towards the back of eye is challenging task in view of protective barriers and defence mechanism of eye. We have prepared the surface modified nano-particles of pioglitazone which is a PPAR-γ agonist by using two different grades of PLGA [poly (D,L-lactide-co- glycolide)] viz. PLGA 75:25 and PLGA 50:50 by single emulsion solvent evaporation method with the application of Box-Behnken statistical design. PLGA is a biocompatible and biodegradable polymer, which degrades hydrolytically into nontoxic oligomer and monomer as lactic acid and glycolic acid respectively. Moreover; it is approved by US-FDA for clinical use [12]. Surface modification was achieved by using non-ionic surfactant polysorbate 80 (P80) to improve interaction with eye. We investigated the drug delivery by PLGA after topical administration with measurement of VEGF by ELISA based on in vivo study.
2. Materials and methods
2.1. Materials
Pioglitazone was obtained as a gift sample from Glenmark Pvt. Ltd. Nashik, India. PLGA 75:25 and 50:50 was gifted by Ltd., Evonik Mumbai, India. dichloromethane, polysorbate 80, Potassium dihydrogen orthophosphate and dipotassium hydrogen phosphate was obtained from SDFCL, Mumbai. Polyvinyl alcohol and mannitol was procured from Thomas baker, Mumbai, India. All other solvents and materials used were of analytical grade.
2.2. Analytical method development
To quantitate the content of pioglitazone from samples, the UV-Visible Spectrophotometry (UV-Vis Spectrophotometer 1800 Shimadzu Co., Japan) method was used and validated as per ICH guidelines Q2 (R1) in phosphate buffer pH 7.4. The absorbance was measured at 238 nm. Stock solution of concentration 100 μg/ml was prepared by dissolving 10 mg Pioglitazone in 1 ml methanol and volume was adjusted to 100 ml by phosphate buffer pH 7.4.
2.3. Preparation of PLGA nanoparticles
2.3.1. Preliminary studies
Trial batches were performed for selection of concentration of polymer, concentration of surface modifier (Polysorbate 80) and number of cycles of high pressure homogenizer given in Table 1. From these trial batches low, medium and high levels of individual parameters were fixed. Particle size and PDI were observed for selection of experimental batches of PLGA NPs.
Table 1.
Composition of trial batches.
| PLGA Concentration | P80 concentration (mg) |
Cycles (numbers) |
PVA Concentration (%) |
|---|---|---|---|
| 300 | 600 | 4 | 1 |
| 100 | 300 | 4 | 1 |
| 300 | 300 | 4 | 1 |
| 100 | 600 | 4 | 1 |
| 100 | 300 | 6 | 1 |
| 100 | 300 | 4 | 2 |
| 500 | 300 | 4 | 1 |
2.3.2. Procedure for preparation of nanoparticles
PLGA NPs with grade 75:25 were prepared by using single emulsion solvent evaporation method. The method involves preparation of an organic and aqueous phase separately. Organic phase is consists of PLGA dissolved in dichloromethane (DCM) and Pioglitazone in methanol while aqueous phase containing PVA and Polysorbate 80 dissolved in water. The organic phase was added drop by drop into the aqueous phase during high speed homogenization (T25 IKA Digital Ultra Turrax) at 15000 rpm. It results in formation of o/w emulsion which further broken down in nano-droplets. Further to achieve more smaller size prepared emulsion was subjected to High pressure homogenizer (NS 1001L Panda Gia Niro Soavi, Italy) at 600 bar pressure. Particle size and PDI of each batch was determined by using Zeta-sizer ZS 90, Ver. 7.01 Malvern Instruments. Further all batches were subjected for solvent evaporation by heating the suspension on magnetic stirrer (1 MLH Remi equipments, India). This suspension was then passed through 0.45 um membrane filters (Millipore, Bedford, MA, USA). All fltrate were centrifuged at 15,000 rpm at 4 °C for 20 min by using cooling centrifuge. The clear supernatant was collected and evaluated for entrapment efficiency [13]. One batch was also prepared by using same procedure but without addition of polysorbate 80 in order to check impact of surface modifier on nano formulation.
2.4. Formulation of PLGA nanoparticles by applying 33 Box-Behnken statistical design (experimental design)
A 3-factor 3-level Box-Behnken statistical design was applied to reconnoiter the variables and experimental trials performed at all 15 possible combinations. Design was applied to enumerate the impact of independent variables viz. polymer concentration, surface modifier concentration and number of cycles on the dependent variables viz. particle size, polydispersity index (PDI) and percent entrapment efficiency (%EE) of nanoparticles. The resulting data were fitted into Design Expert software and analysed statistically using analysis of variance (ANOVA). The statistical significance of the data was performed in terms of regression coefficients [14, 15].
The general polynomial equation for 33 Box-Behnken statistical design is:
| Y = β0+ β1∗A+ β2∗B+ β3∗C+ β12∗AB+ β13∗AC+ β23∗BC+ β11∗A2 +β22∗B2+ β33∗C2 |
The equation was applied to the responses, to describe the principal effects and interaction among the identified independent variables A, B, C with coded levels as -1, 0 and +1 (Table 2). These limits were selected on the basis of previous studies and the optimization was carried out within this domain. In general polynomial equation, Y is associated with the predicted responses; β0 is the intercept; β1, β2, β3 are coefficients for variables A, B, C respectively; β12, β13, β14 representing coefficients for interaction of variables A and B, A and C, B and C respectively; β11, β22, β33 representing coefficients for interaction A and A, B and B, C and C respectively.
Table 2.
Translation of the coded levels in actual units.
| Independent variables | Coded levels |
||
|---|---|---|---|
| -1 | 0 | +1 | |
| A = Polymer concentration (mg) | 100 | 200 | 300 |
| B= Surface modifier concentration (mg) | 300 | 450 | 600 |
| C= Number of cycles | 6 | 7 | 8 |
| Coded levels | Indicates |
|---|---|
| -1 | Low level of independent variable |
| 0 | Medium level of independent variable |
| +1 | High level of independent variable |
The three-dimensional (3D) response surface plots were used to show graphically the relationship and interaction between the coded variables and the response. The optimum concentrations of independent variables based on the responses constrained in their minimum levels were selected. Furthermore, formulations were selected by point prediction and chosen experimental domain as well as polynomial equation was validated by comparing the experimental responses with the predicted values. Then, finally optimized batch was prepared and evaluated for the particle size, PDI and entrapment efficiency. A 33 Box-Behnken statistical design layout is shown in Table 3.
Table 3.
33 Box-Behnken statistical design layout, experimental runs and their combinations.
| Runs | Batch no. | Factor 1 A |
Factor 2 B |
Factor 3 C |
|---|---|---|---|---|
| 1 | B1 | 0 | 0 | 0 |
| 2 | B2 | -1 | 0 | +1 |
| 3 | B3 | 0 | +1 | +1 |
| 4 | B4 | -1 | 0 | -1 |
| 5 | B5 | +1 | -1 | 0 |
| 6 | B6 | +1 | +1 | 0 |
| 7 | B7 | 0 | 0 | 0 |
| 8 | B8 | +1 | 0 | -1 |
| 9 | B9 | 0 | -1 | -1 |
| 10 | B10 | 0 | +1 | -1 |
| 11 | B11 | 0 | 0 | 0 |
| 12 | B12 | 0 | -1 | +1 |
| 13 | B13 | -1 | +1 | 0 |
| 14 | B14 | +1 | 0 | +1 |
| 15 | B15 | -1 | -1 | 0 |
The results obtained were analysed by Design Expert Software 11.0.5.0. All the formulations were prepared as per the procedure used for the preliminary formulations.
Based on optimization, a batch which was found to be optimized, the residue of same batch was subjected for spray drying (Labultima LU222, India) after 2–3 times washing with distilled water. Prior to spray drying residue was dispersed in distilled water containing manitol which act as cryoprotectant. During spray drying inlet and outlet temperature was set at 150 °C and 90 °C respectively. Aspirator rate was adjusted at 35Nm3/hr while sample feed rate was 2 ml/min [16]. With same procedure one batch of PLGA 50:50 was also prepared at optimized condition of PLGA 75:25 and it was also evaluated for same parameters simultaneously along with 75:25 grade nanoparticles.
2.5. Characterization of nanoparticles
2.5.1. Particle size and polydispersity index (PDI)
Average particle size and size distribution are important parameters because they influence the physicochemical properties and fate of NPs after in-vivo administration. The particle size and polydispersity index of nanoparticles were measured using dynamic light scattering (Zetasizer Nano ZS 90, Malvern Ltd., UK). Dynamic light scattering measures the fluctuations in light due to Brownian motion of particles, which is then correlated with the size of particles. The analysis was performed at 25 °C with an angle of detection 90° [15].
2.5.2. Entrapment efficiency
To determine the amount of Pioglitazone entrapped in nanoparticles, an indirect method was used. The total percentage of drug entrapped (% EE) was determined by spectrophotometric analysis. Before spray drying, the nanoparticles suspension was centrifuged at 15,000 rpm at 4 °C for 20 min using cooling centrifuge. The clear supernatant was collected and the concentration of Pioglitazone in the supernatant was determined spectrophotometrically at 238nm [17].
2.5.3. Zeta potential measurement
Zeta potential is the electrostatic potential that exists at the share plane of a particle, which is related to both; surface charge and the local environment of the particle. The zeta potential of the optimized batch of PLGA 75:25 (with and without surface modification) and PLGA 50:50 was determined by dynamic light scattering [14, 15].
2.5.4. Drug loading efficiency
Drug loading efficiency was calculated by using following equation after weighing the spray dried nanoparticles (both grade).
2.5.5. Product yield
Product yield were calculated as the weight of the final product after spray drying with respect to the initial total amount of the polymer and drug used for preparation [15].
The percent yield was calculated as:
2.5.6. Total drug content
Weigh accurately spray dried PLGA NPs equivalent to 10 mg of pioglitazone. It was dissolved in 100ml of phosphate buffer pH 7.4 to give 100 ug/ml. From prepared stock solution, 1ml solution was withdrawn and further serially diluted to make 10ug/ml. Concentration was measured at 238nm by UV-visible spectrophotometer.
2.5.7. In-vitro drug release
The in vitro release study of surface modified PLGA nanoparticles of pioglitazone (PLGA 75:25 and 50:50) was performed in triplicate as per procedure given by R. Kesarla et al. (2016) and Salama, Mahmoud and Kamel (2016) [18,19]. Phosphate buffer pH 7.4 was used as dissolution medium. A dialysis membrane previously soaked overnight in the diffusion medium was tied from one end and 1ml of formulation containing equivalent to 1mg of drug was accurately pipetted into dialysis sac and it's another end was closed tightly. The dialysis membrane sac was suspended in 100 ml diffusion medium in a beaker and temperature was maintained at 37 ± 0.5 °C. This assembly was kept on magnetic stirrer at 50 rpm. The 5ml of aliquots was withdrawn at specified time interval during 8h and analysed by spectrophotometrically at 238 nm. Fresh release medium was added to replenish withdrawn samples. Cumulative percentage drug released was calculated. Drug release from optimize batch was also subjected to describe drug release kinetics.
2.5.8. Shape and surface morphology
Morphological analysis of optimized batch of pioglitazone loaded surface modified PLGA nanoparticles (PLGA 75:25 and 50:50) were examined by scanning electron microscopy (JEOL model JSM-6390LV) operated at an accelerated voltage of 15 kV [20].
2.5.9. Thermal analysis
Thermal behaviour of pioglitazone, polyemer and spray dried drug loaded nano particles (both grade) was conducted using differential scanning calorimeter (PerkinElmer 4000) at rate of heating 10 °C/min.
2.5.10. In vivo evaluation
In vivo evaluation of formulation was carried out on Wistar rats (200–250 gm). The animals were acclimatized and maintained on a normal food and water ad libitum for a week. The experimental protocol was approved by Animal Ethical Committee. Experiment was designed and conducted in accordance with the guidelines laid by CPCSEA, New Delhi. Nanoparticles dispersion was placed in UV chamber with 100 μJ/cm2 dose (1.5 h) for sterilization prior to instillation. After a week the rats were weighed and tail-snip baseline blood glucose was measured with biochemical method using ready mix kit (Erba diagnostic kits, India). The animals were divided in 6 groups (n = 6). One group as control, second group as diabetes control, third group as with diabetes and treated with Pioglitazone PLGA 75:25 nano-suspension (4 mg/ml), fourth group as with diabetes and treated with Pioglitazone PLGA 50:50 nano-suspension (4 mg/ml), fifth group as with diabetes and treated with Pioglitazone PLGA 50:50 nano-suspension (2 mg/ml) and sixth group as with diabetes and treated with Pioglitazone PLGA 50:50 nano-suspension (6 mg/ml). Animals were treated with single intraperitoneal injection of 65 mg/kg STZ or control vehicle buffer pH 4.5. After 3 days tail snip blood glucose was again verified to ensure hyperglycaemia in STZ treated rats (glucose level more than 250 mg/dl). After a week of diabetes, treatment was initiated when STZ-treated diabetic rats demonstrate significantly elevated VEGF expression. The non-diabetic control rats treated with distilled water while STZ treated animals were treated with one drop of sterile nano formulation prepared in distilled water as mentioned above. After 4 weeks of continuous treatment, VEGF protein concentrations in the retina (vitreous fluid) from each group was determined [21, 22].
Analysis of VEGF level: Rats were sacrificed using CO2 anaesthesia. The vitreous fluid from both eyes was rapidly isolated and kept in deep freezer. VEGF concentration in vitreous fluid was then determine by using Rat ELISA Kit (R&D Systems, Inc Minneapolis) and analysed on ELISA reader (PowerWave XS, Biotek, India).
3. Results and discussion
3.1. Preliminary studies
Preliminary studies were performed in order select the concentration of polymer, concentration of surface modifier and number of cycles of high pressure homogenizer. Based on literature survey, PLGA concentration was varied in the range of 100 mg–500 mg, whereas; surface modifier concentration and HPH cycles were changed as 300 mg–600 mg and 4 to 6 respectively [12]. Prepared trial batches were evaluated for particle size, PDI and EE. Results of the preliminary studies are represented in Table 4.
Table 4.
Result of the preliminary studies.
| PLGA concentration (mg) | P80 concentration (mg) | Cycles (numbers) | PVA concentration (%) | Particle Size (nm) | PDI | Entrapment Efficiency (%) |
|---|---|---|---|---|---|---|
| 300 | 600 | 4 | 1 | 600 | 0.546 | 92 |
| 100 | 300 | 4 | 1 | 412 | 0.408 | 81 |
| 300 | 300 | 4 | 1 | 488 | 0.489 | 89 |
| 100 | 600 | 4 | 1 | 465 | 0.454 | 86 |
| 100 | 300 | 6 | 1 | 323 | 0.378 | 83 |
| 100 | 300 | 4 | 2 | 578 | 0.562 | 82 |
| 500 | 300 | 4 | 1 | 700 | 0.621 | 90 |
From results of preliminary study it was clear that, as concentration of polymer increases particle size and entrapment efficiency also increases. Increase in PLGA concentration led to an increase in the viscosity of the organic phase, thereby reducing the net shear stress and promoting the formation of droplets with larger size and uneven surface. NPs prepared with lower concentration of PLGA presented spherical shape without any agglomeration. On the other hand as the concentration of surface modifier increases, particle size and entrapment efficiency also increases. Molecules of the Polysorbate 80 adsorb onto the surface of PLGA nanoparticles and hence it increases the particle size. However; particle size does not increases significantly as compared to PLGA concentration. It was also observed that as the number of cycles of HPH increases particle size decreases along with PDI. Increase in number of cycles of HPH causes reduction in particle size due to very high shear stress which causing the formation of very fine droplets with uniform distribution of particles. Moreover; it has been observed that as the concentration of the PVA increases, there is increase in particle size as well as PDI.
Based on the preliminary studies result above mentioned factors were selected with three levels for optimization by the statistical design. Similar kind of results were obtained to Wagh and Apar, 2014; Jose et al, 2016 [15,23].
3.2. Experimental design
From the results of preliminary batches, 33 Box-Behnken Statistical Design was selected for optimization of surface modified PLGA nanoparticles. The amount of drug in the formulation was fixed to 50 mg and speed and duration of high speed homogenizer was fixed to 15000 rpm for 35 min. Prepared system was then immediately passed through the high pressure homogenizer at 600 bar pressure with different number of cycles as per optimization design. Total 15 runs with the three center points were prepared and shown in Table 5.
Table 5.
Experimental runs.
| Runs | Batch No. | Factor 1 A: PLGA (mg) | Factor 2 B: P80 (mg) | Factor 3 C: cycles (numbers) | Response1 Particle size (nm) | Response 2 PDI | Response 3 E.E. (%) |
|---|---|---|---|---|---|---|---|
| 1 | B1 | 200 | 450 | 7 | 182 | 0.323 | 85 |
| 2 | B2 | 100 | 450 | 8 | 163 | 0.286 | 91 |
| 3 | B3 | 200 | 600 | 8 | 179 | 0.359 | 86 |
| 4 | B4 | 100 | 450 | 6 | 172 | 0.310 | 82 |
| 5 | B5 | 300 | 300 | 7 | 189 | 0.388 | 91 |
| 6 | B6 | 300 | 600 | 7 | 186 | 0.364 | 94 |
| 7 | B7 | 200 | 450 | 7 | 182 | 0.323 | 85 |
| 8 | B8 | 300 | 450 | 6 | 192 | 0.387 | 90 |
| 9 | B9 | 200 | 300 | 6 | 185 | 0.289 | 87 |
| 10 | B10 | 200 | 600 | 6 | 186 | 0.321 | 88 |
| 11 | B11 | 200 | 450 | 7 | 185 | 0.368 | 86 |
| 12 | B12 | 200 | 300 | 8 | 173 | 0.283 | 88 |
| 13 | B13 | 100 | 600 | 7 | 169 | 0.267 | 82 |
| 14 | B14 | 300 | 450 | 8 | 186 | 0.354 | 93 |
| 15 | B15 | 100 | 300 | 7 | 166 | 0.288 | 81 |
3.2.1. Statistical evaluation of experimental design
The best fit model obtained by design expert for each response was evaluated on the basis of ANOVA by calculating the F value and Statistical significance of the data was performed in terms of regression coefficients. It was observed that the best fitted models were linear for the PDI and EE whereas quadratic for the particle size. The regression analysis for responses R1, R2, R3 and ANOVA of each model has shown in Tables 6 and 7 respectively.
Table 6.
Summary of results of regression analysis for responses.
| Response | Model | Sequential p-value |
Lack of fit p-value | Adjusted R2 | Predicted R2 |
|---|---|---|---|---|---|
| Particle size (R1) | Quadratic | 0.0001 | 0.2484 | 0.8880 | 0.8468 |
| Polydispersity index (R2) | Linear | 0.0053 | 0.5924 | 0.5807 | 0.3685 |
| Entrapment efficiency (R3) | Linear | 0.0001 | 0.1371 | 0.8819 | 0.8305 |
Table 7.
Analysis of variance of calculated model for responses.
| Result of ANOVA | Particle size (nm) |
Polydispersity index (PDI) | Entrapment efficiency (%) |
|---|---|---|---|
| Sum of squares | 1094.08 | 0.0152 | 202.25 |
| Degree of freedom (df) | 9 | 3 | 3 |
| Mean squares | 121.56 | 0.0051 | 67.42 |
| F value | 39.86 | 7.46 | 35.85 |
| P value | 0.0004 | 0.0053 | 0.0001 |
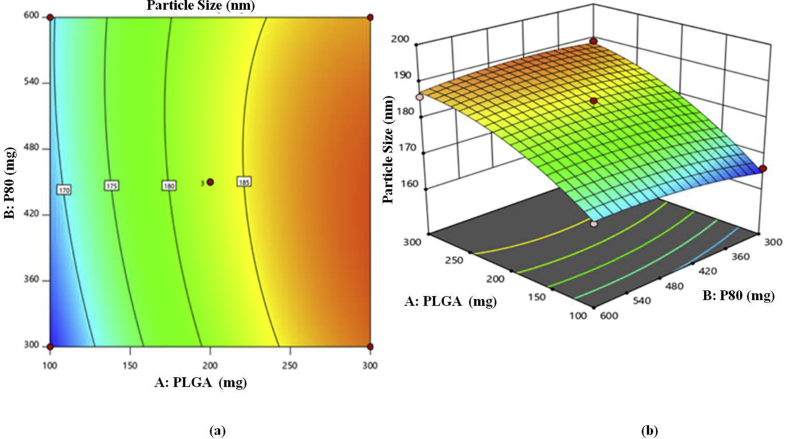
3.2.2. Particle size
Observed particle size for prepared batches is shown in Table 5. From results of the optimization studies it was observed that, as concentration of polymer and surface modifier increases, the particle size also increases. On the other hand as number of cycles of HPH increases there is decrease in particle size [15, 23]. The quadratic polynomial equation generated for particle size is given as follows:
| R1 = 183 + 10.37∗A+0.8750∗B-4.25∗C-1.50∗AB+0.7500∗AC+1.25∗BC-4∗A2-1.50∗B2-0.7500∗C2 | (1) |
The model F value of 39.86 implies the model is significant and values of probability less than 0.05 indicates that model terms are significant. In this case, A, B, C, AB, AC, BC, A2, B2, C2 are significant model terms. The polynomial Eq. (1) of response R1 showing the combined effect of PLGA concentration, polysorbate 80 concentration and number of cycles of HPH on particle size. In this equation, impact A and B is positive while C is negative. However; magnitude of A significantly high in comparison to B. Therefore PLGA significantly affects to size in comparison to concentration of P80. Magnitude of A and B is high as compare to A & C and B & C but it showing the negative effect. Magnitude of A and C is high but as compare to B and C is low. Magnitude of exponential of A is high as compare to B2 and C2, it showing the negative effect on particle size.
The impact of A, B and C can be further interpreted with the help of counter plot and 3D response surface plots (Figure 1).
Figure 1.
(a) Two dimensional counter plot, (b) three dimensional (3 D) response surface plot for response R1 (Particle Size).
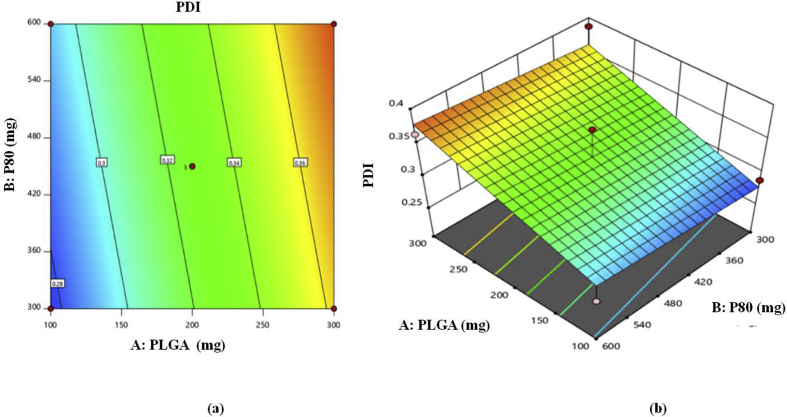
3.2.3. Polydispersity index
Polydispersity Index (PDI) which is alternatively known as the heterogeneity index is correlated with the uniformity of particle size distribution. It ranges from 0.0 to 1.0 which represents uniform to polydrisperse sample in context to particle size. Ideally, nanoparticles should possess PDI below or equal to 0.3. Particle size and size distribution are important factors for evaluating the stability of a colloidal dosage form upon storage [24]. PDI for prepared batches is shown in Table 5.
The linear polynomial equation generated for Polydispersity index is given as follows:
| R2 = 0.327 + 0.0427∗A + 0.0079∗B - 0.0031∗C | (2) |
Above Eq. (2) indicates positive impact of A and B on PDI. While; the impact of C was found to be negative on PDI. However; impact of A slightly more significant than B. Therefore; as concentration of polymer increases there is increase in PDI; as concentration of surface modifier increases there is slight increase in PDI and as number of cycles of HPH increases there is decrease in PDI [14]. The model F value of 7.46 implies the model is significant and values of probability less than 0.05 indicates that model terms are significant. The impact of A, B and C on PDI can be further interpreted with the help of counter plot and 3D response surface plots shown in Figure 2.
Figure 2.
(a) Two dimensional counter plot, (b) three dimensional (3 D) response surface plot for response R2 (PDI).
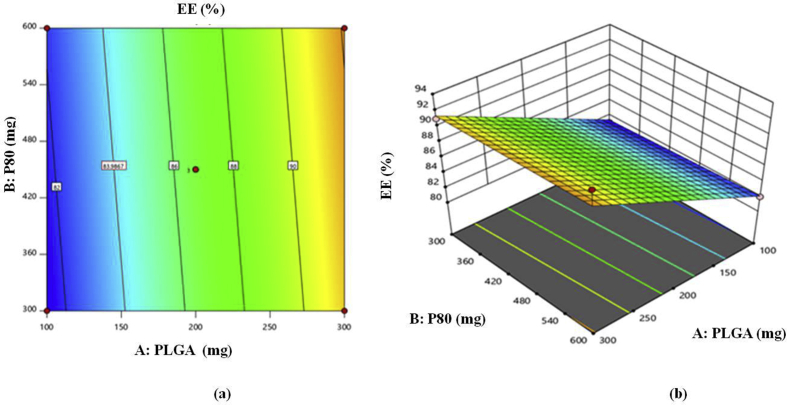
3.2.4. Entrapment efficiency
Results of entrapment efficiency for prepared batch is shown in Table 5. From results of the optimization studies it was observed that, as concentration of polymer increases there is increase in EE; as concentration of surface modifier increases there is increase in EE and as number of cycles of HPH increases there is increase in EE [23]. The linear polynomial equation generated for entrapment efficiency is given as follows:
| R3 = 86.73 + 5∗A + 0.3750∗B + 0.3750∗C | (3) |
The model F value of 35.85 implies the model is significant and values of probability less than 0.05 indicates that model terms are significant. In this case, A, B, C are significant model terms. Above Eq. (3) clearly indicates the significant impact of A on entrapment efficiency than B and C. The impact of A, B and C on entrapment efficiency can be further interpreted with the help of counter plot and 3D response surface plots given in Figure 3.
Figure 3.
(a) Two dimensional counter plot, (b) three dimensional (3 D) response surface plot for response R3 (EE).
3.2.5. Development of the optimize batch
Based on the statistical evaluations the software gave further 31 possible solutions for the optimization of the batches. The minimum particle size and PDI and higher percent entrapment efficiency were the selection criteria for optimized batch. Formulation B2 was selected as an optimized batch having lesser particle size, less PDI and more EE. A new optimized formulation was prepared according to the predicted model and evaluated for the responses as shown in Table 8.
Table 8.
Results of predicted batch and experimental batch with residual error.
| Response | Target | Predicted value | Experimental value | Residual error (%) |
|---|---|---|---|---|
| Particle size | 150 | 162 | 163.23 | 0.759 |
| PDI | 0.250 | 0.284 | 0.286 | 0.70 |
| Entrapment Efficiency | 95 | 90 | 91 | 1.11 |
The experimental values were compared with the predicted values & residual error calculated from Equation which given below and it was seen that the low percentage error which indicates that result given by software are validated.
3.3. Characterization of optimized batch
3.3.1. Particle size and PDI
The average particle size and polydispersity index of nano particles of PLGA 75:25 (with and without surface modification) and PLGA 50:50 (with surface modification) were determined. Results are summarized in below Table 9. Results clearly indicates size of nano particle is less than 180 nm and comparatively same for both grade of polymers.
Table 9.
Particle size and PDI of nano-particles.
| Batch | Particle Size (nm) | PDI |
|---|---|---|
| Surface modified nano-particles of PLGA 75:25 | 163.23 | 0.286 |
| Surface unmodified nano-particles of PLGA 75:25 | 174.36 | 0.287 |
| Surface modified nano-particles of PLGA 50:50 | 171.7 | 0.280 |
3.3.2. Zeta potential measurement
Zeta potential measurement provides information about charges on particle and magnitude of it can be correlated with stability of nanoparticles. Surface properties as well as eventual modification of nanopartilces can be evaluated by Zeta potential measurement. Zeta potentials indicate existence of repulsion between suspended particles due to which particles remain in Brownian motion. Zeta potential within in the range of is -30 to +30mv indicates the stability of the formulation [15].
Zeta potential of unmodified PLGA 75:25 nano particles was found to be -6.22 mV because of the terminal carboxyl acid end groups of PLGA molecules located on the surface of nanoparticles. The zeta potential of the surface modified PLGA 75:25 NPs was found to be -10.8 mV. This change in zeta potential confirms the surface modification of nano particle by Polysorbate 80. Zeta potential of surface modified PLGA 50:50 nano-particles was found to be -7.49 mV. Although the zeta potential of surface modified PLGA nano particles is more negative as compared to unmodified particles but Tahara K, Yamamoto H, Kawashima Y. (2010) has already proved the use of P80 as surface modifier increases the cell association and ultimately uptake and movement of drug towards retina [25]. Literature also revealed that presence of surface modification is a key step to target nanoparticles to back of eye [12]. Moreover; unmodified PLGA nano particles suffer from major limitation as short residence time when applied on mucosal tissues. It may be due to negative charges on cornea which repels the negatively charged PLGA particles. However; surface modification of PLGA particles using functional materials such as P80 results into improvement in the interaction with eye tissues and ultimately increases delivery efficiency. Polysorbate 80 has already proved its involvement in improved cellular association at corneal site due to its mucoadhesive property. It has been also observed that P80 modified NPs have a higher uptake into cells as compared with unmodified NPs. Additionally Polysorbate 80 also acts as a surfactant which help to reduce the surface tension at the ocular site [12]. Therefore; surface modified PLGA 75:25 and 50:50 nanoparticles were subjected for further evaluation.
3.3.3. Entrapment efficiency and drug loading efficiency
Entrapment efficiency and drug loading efficiency of optimized surface modified PLGA 75:25 and PLGA 50:50 nano particles was determined. Results are listed in following Table 10. Similar results were observed by Salama, Mahmoud & Kamel, (2016) [18].
Table 10.
Entrapment efficiency and drug loading efficiency of optimized batch.
| Nano Particles | Entrapment efficiency (%) | Drug loading efficiency (%) |
|---|---|---|
| Surface modified PLGA 75:25 | 91 | 7.89 |
| Surface modified PLGA 50:50 | 93 | 8.12 |
3.3.4. Product yield and total drug content
Product yield and total drug content of the surface modified PLGA 75:25 and PLGA 50:50 was determined. The percentage yield and drug content of the PLGA 75:25 nano particles was found to be 67% and 93% respectively. On the other hand percentage yield and drug content of the PLGA 50:50 nano particles was found to be 66% and 90% respectively. This may be due to the loss of drug during the various operations of preparation of nano particles [18].
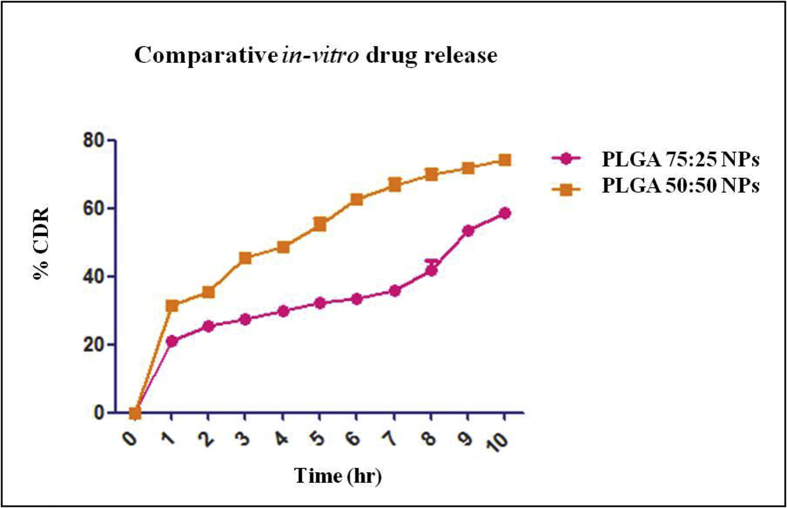
3.3.5. In-vitro drug release
Comparative in vitro drug release study between surface modified PLGA 75:25 and PLGA 50:50 nanoparticles was carried out for 10 h as per the procedure R. Kesarrla et al 2016 [19]. Surface modified PLGA 75:25 nanoparticles showed 58.481 ± 1.383% drug release at the end of 10 h. On the other hand surface modified PLGA 50:50 polymer showed 74.178 ± 1.384% drug release at the end of 10 h. In both case drug release pattern was found to be biphasic which might be due to initial burst release [26, 27]. Difference in drug release between two polymers reveals the more sustained release property of PLGA 75:25 polymer in comparison to PLGA 50:50. Drug release through different grades of PLGA polymer governed by the hydrophilicity and rate of degradation of polymer which depends on composition. PLGA 50:50 undergoes rapid degradation than PLGA 75:25 due to high proportion of glycolic acid which determines the hydrophilicity. Comparative plot of cumulative percent drug release by surface modified PLGA 75:25 and PLGA 50:50 nano particles are shown in Figure 4.
Figure 4.
Percentage drug release from PLGA 75:25 and PLGA 50:50 NPs (n = 3, mean ± SD).
Data obtained from in vitro drug release study was further subjected to mathematical treatment to determine drug release kinetic profile. The release constant was calculated from the slope of the appropriate plots and the regression coefficient (R2) was determined which is summarized in Table 11. The drug release from both grades of PLGA was best explained by Higuchi kinetic with highest R2 value. Korsmeyer-Peppas equation indicated a good linearity of regression coefficient (R2) for nano particles of both grades of PLGA with N value above 0.5 (Table 12) indicating the drug transport mechanism is quasi fickian. Similar results were observed by Wagh and Apar, 2014 [15].
Table 11.
Model fitting for release profile.
| Nano particles | Coefficient of determination (R2) |
Best fit model | |||
|---|---|---|---|---|---|
| First order | Zero order | Higuchi | Hixon-Crowel cube root | ||
| Surface modified PLGA 75:25 | 0.0275 | 0.9369 | 0.9654 | 0.9583 | Higuchi |
| Surface modified PLGA 50:50 | 0.9578 | 0.9262 | 0.9979 | 0.9762 | Higuchi |
Table 12.
Korsmeyer-Peppas drug release kinetics.
| Nano particles | R2 | N value | Mechanism |
|---|---|---|---|
| Surface modified PLGA 75:25 | 0.9895 | 0.5150 | Quasi fickian |
| Surface modified PLGA 50:50 | 0.9895 | 0.5103 | Quasi fickian |
3.3.6. Shape and surface morphology
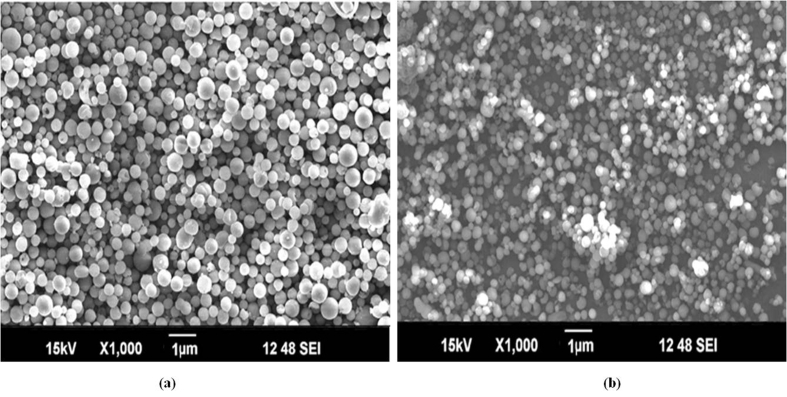
The morphology of the surface modified PLGA 75:25 and PLGA 50:50 nano particles was studied by scanning electron microscopy. Results for both grades of PLGA are shown in Figure 5. SEM analysis of particles reveals that all particles were spherical and possessed smooth surface without any fracture. This morphology further helps in uniform deposition of particles in ocular site and also facilitate movement of particles towards the retina.
Figure 5.
SEM image of surface modified Pioglitazone loaded nano particles of a) PLGA 75:25 and b) PLGA 50:50.
3.3.7. Thermal analysis
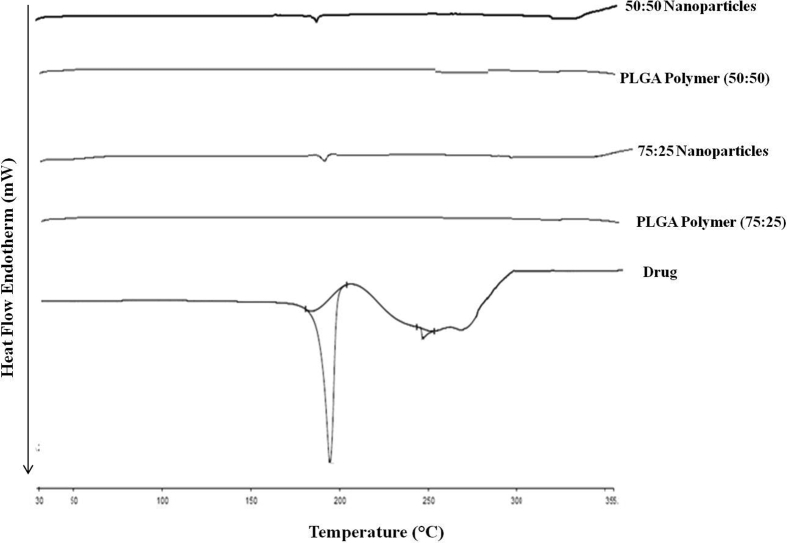
The DSC thermogram of Pioglitazone showed sharp characteristic endothermic peak at 190.58 °C which is also reported by Faruksha & Vetrichelvan, 2013 [28]. Characteristic peak of drug was disappeared in thermogram of surface modified PLGA 75:25 as well as PLGA 50:50 nano particles given in Figure 6. This study further confirmed the molecular dispersion of drug in polymer.
Figure 6.
Comparative DSC
3.3.8. In vivo evaluation
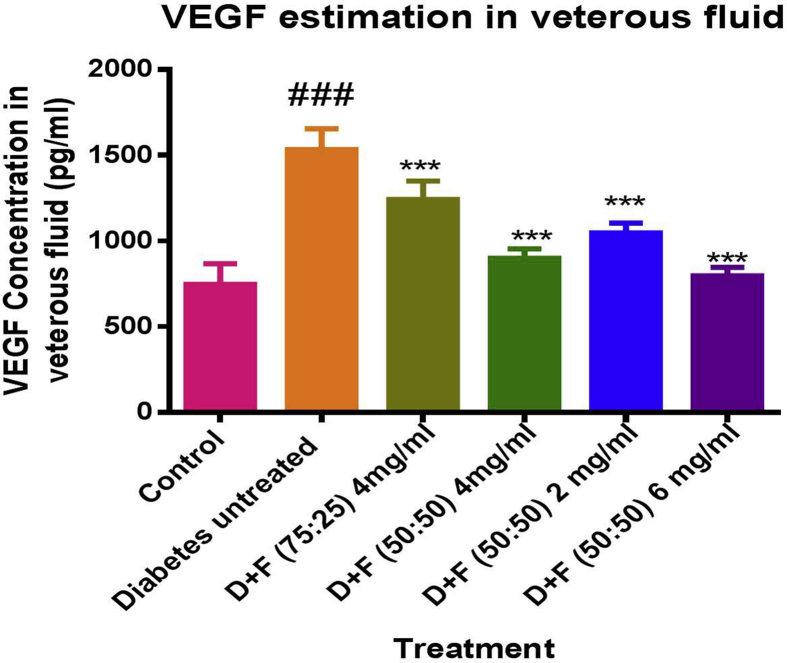
The effect of prolong treatment of surface modified Pioglitazone nano particles of PLGA 75:25 and 50:50 on VEGF protein in vitreous fluid of STZ-induced diabetic rats was determined using ELISA. Animals were grouped as non-diabetic, diabetic without treatment, diabetic with administration PLGA 75:25 Nano suspension (4 mg/ml), three groups as diabetic with treatment of PLGA 50:50 nanosuspension with different concentration viz. 2 mg/ml, 4 mg/ml and 6 mg/ml. In-vitro release study showed the more slow release of drug from PLGA 75:25 nanoparticles in comparison with PLGA 50:50 polymer. Therefore; drug loaded PLGA 50:50 nano suspension was evaluated at three different level of concentration to check impact of dose on VEGF level. After 4 weeks of study the VEGF level in vitreous was found to be less in entire treatment rats as compared to untreated rat. Moreover; the VEGF level was significantly reduced in PLGA 50:50 nano suspension treated animal than in PLGA 75:25 nano suspensions. This difference might be due to slight difference in permeation of particle towards the targeted size and further more slow release of drug by PLGA 75:25. The group treated with 6 mg/ml of nanosuspension showed decreased in VEGF as compared to 4 mg/ml. Whereas; level of VEGF was more in 2 mg/ml treated group than in 4 mg/ml treated group which clearly indicates the dose dependent activity of formulation given in Figure 7. In vivo study reveals the movement of drug loaded surface modified nano particles towards back of eye and its significance in management of diabetic retinopathy by topical instillation of formulation. Additionally; as the study proves the down regulation of VEGF by a PPAR-γ agonist, there is also hope to cure diabetic retinopathy by some other possible mechanisms which are associated with PPAR-γ receptors like inhibition of formation of AGE, decrease in angiogenesis, inflammation, retinal leakage and retinal leukostais as well as protection from apoptosis and oxidative stress on RPE.
Figure 7.
Effect of formulation on VEGF in rats.
4. Conclusion
Surface modified PLGA nanoparticles of Pioglitazone were successfully prepared and characterized for various parameters. Two grades of PLGA (75:25 and 50:50) were used to investigate ability to deliver drug towards posterior segment of eye after topical administration. Both grade nanoparticles showed small particle size as 163.23 nm (PLGA 75:25) and 171.7 nm (PLGA 50:50) which may help in penetration of particle and further haulage towards the retina site by different pathways of transportation. Zeta potential of surface modified PLGA 75:25 nanoparticle was different (-10.8 mV) in comparison to unmodified PLGA 75:25 nanoparticles (-6.22 mV) which confirmed the surface modification by polysorbate 80. Zeta potential of surface modified PLGA 50:50 nanoparticles was found to be -7.49 mV. SEM study showed all particles were spherical and with smooth surface. DSC study confirmed the molecular dispersion of drug in polymer. In vitro release study showed the initial burst release of drug which was later on controlled due to less solubility of polymer.In vitro study also reveals the more controlled and slow release by PLGA 75:25 in comparison to PLGA 50:50. Further effectiveness of formulation in treatment of DR was evaluated in diabetes induced rat. After 4 weeks of study the VEGF level in vitreous was found to be less in entire treatment rats as compared to untreated rat. Moreover; VEGF level was significantly less in PLGA 50:50 nano suspension than PLGA 75:25 nano suspension. PLGA 50:50 nano suspension further proved dose dependent reduction in VEGF level. Since; increased VEGF is indicator of DR, its down regulation proves movement of surface modified nano particle toward the posterior segment of eye. These results indicates that pioglitazone loaded surface modified PLGA nano particles possesses great potential to treat DR. It can be consider as feasible breakthrough avenue in management of DR which is free from any invasive and painful process.
Declarations
Author contribution statement
U. Laddha and S. Kshirsagar: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Das A., Stroud S., Mehta A., Rangasamy S. New treatments for diabetic retinopathy. Diabetes Obes. Metabol. 2015;17:219–230. doi: 10.1111/dom.12384. [DOI] [PubMed] [Google Scholar]
- 2.Gologorsky D., Thanos A., Vavvas D. Therapeutic interventions against inflammatory and angiogenic mediators in proliferative diabetic retinopathy. Mediat. Inflamm. 2012:1–10. doi: 10.1155/2012/629452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowluru R.A., Zhong Q., Santos J.M., Thanddampallayam M., Putt D., Gierhart D.L. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr. Metab. 2014;11:1–10. doi: 10.1186/1743-7075-11-8. http://www.nutritionandmetabolism.com/content/11/1/8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song M.K., Roufogalis B.D., Huang T.H.W. Modulation of diabetic retinopathy pathophysiology by natural medicines through PPAR-gamma-related pharmacology. Br. J. Pharmacol. 2012;165:4–19. doi: 10.1111/j.1476-5381.2011.01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdulrahman A., Alghadyan M.D. Diabetic retinopathy: an update. Saudi Journal of Ophthalmology. 2011;25:99–111. doi: 10.1016/j.sjopt.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarr J.M., Kaul K., Chopra M., Kohner E.M., Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmology. 2013:1–13. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciudin A., Hernandez C., Simo R. PPAR Research; 2013. Molecular Implication of the PPARs in Diabetic Eye; pp. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyd K. Published by American Academy of ophthalmology; 2019. Retinopathy Treatment.https://www.aao.org/eye-health/diseases/diabetic-retinopathy-treatment [Google Scholar]
- 9.The electronic Medicines Compendium (eMC) https://www.medicines.org.uk/emc/product/307/smpc
- 10.European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/eylea
- 11.Saravia M., Zeman L., Ingolotti M., Schlaen A. The VEGF paradox: dose diabetic retinopathy protect from age related macular degeneration? Med. Hypotheses. 2017;109:156–161. doi: 10.1016/j.mehy.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Tahara K., Karasawa K., Onodera R., Takeuchi H. Feasibility of drug delivery to the eye’s posterior segment by topical instillation of PLGA nanoparticles. Asian J. Pharm. Sci. 2017;12:394–399. doi: 10.1016/j.ajps.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rafati H., Mirzajani F., Atyabi F. Fabrication of biodegradable Poly (d,llactide- co-glycolide) nanoparticles containing tamoxifen citrate. Iran. Polym. J. (Engl. Ed.) 2010;19:437–446. [Google Scholar]
- 14.Pandit J., Sultana Y., Aqil M. Chitosan coated PLGA nanoparticles of bevacizumab as novel drug delivery to target retina: optimization, characterization and in-vitro toxicity evaluation, Artificial cells. Nanomedicine and Biotechnology. 2016:1–11. doi: 10.1080/21691401.2016.1243545. [DOI] [PubMed] [Google Scholar]
- 15.Wagh V., Apar D. Cyclosporine a loaded PLGA nanoparticles for dry eye disease: in vitro characterization studies. Journal of Nanotechnology. 2014:1–10. [Google Scholar]
- 16.Bhambere D., Shirivastava B., Sharma P., Gide P. Effect of polymer and formulation variables on properties of self-assembled polymeric micellar nanoparticles. J. Nanomed. Biother. Discov. 2014;4:1–11. [Google Scholar]
- 17.Sah A.K., Suresh P.K., Verma V.K. PLGA nanoparticles for ocular delivery of loteprednol etabonate: a corneal penetration study, Artificial cells. nanomedicine, and biotechnology. 2016:1–9. doi: 10.1080/21691401.2016.1203794. [DOI] [PubMed] [Google Scholar]
- 18.Salama A., Mahmoud A., Kamel R. A novel method for preparing surface- modified Fluocinolone Acetonide loaded PLGA nanoparticles for ocular use: in vitro and in vivo evaluations. AAPS PharmSciTech. 2015;17:1159–1172. doi: 10.1208/s12249-015-0448-0. [DOI] [PubMed] [Google Scholar]
- 19.Kesarla R., Tank T., Vora P.A., Shah T., Parmar S., Omri A. Preparation and evaluation of nanoparticles loaded ophthalmic in situ gel. Drug Deliv. 2016;23:2363–2370. doi: 10.3109/10717544.2014.987333. [DOI] [PubMed] [Google Scholar]
- 20.Lapez G.P., Iglesias I., Beneda J., Lozano R., Teijan J.M. Paclitaxel-loaded polyester nanoparticles prepared by spray-drying technology: in vitro bioactivity evaluation. J. Microencapsul. 2011;28:417–429. doi: 10.3109/02652048.2011.576785. [DOI] [PubMed] [Google Scholar]
- 21.Kusari J., Sheila X., Edwin P., Kenneth G., Clarke, Daniel W.G. Inhibition of vitreoretinal VEGF elevation and blood–retinal barrier breakdown in streptozotocin-induced diabetic rats by brimonidine. Retina IOVS. 2010;51:1044–1051. doi: 10.1167/iovs.08-3293. [DOI] [PubMed] [Google Scholar]
- 22.Angela K.W.L., Amy C.Y.L. Animal models of diabetic retinopathy: summary and comparison. Journal of Diabetes Research. 2013:1–29. doi: 10.1155/2013/106594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jose S., Juna B., Cinu T., Jyoti H., Aleykutty N. Carboplatin loaded Surface modified PLGA nanoparticles: optimization, characterization and in vivo brain targeting studies. Colloids Surf. B Biointerfaces. 2016;142:307–314. doi: 10.1016/j.colsurfb.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Danaei M., Dehghankhold M., Ataei S., Davarani F.H., Javanmard R., Dokhani A., Khorasani S., Mozafari M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:1–17. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tahara K., Yamamoto H., Kawashima Y. Cellular uptake mechanisms and intracellular distributions of polysorbate 80-modified poly (D,L-Lactide-Co-Glycolide) nanospheres for gene delivery. Eur. J. Pharm. Biopharm. 2010;75:218–224. doi: 10.1016/j.ejpb.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D., Tan T., Gao L., Zhao W., Wang P. Preparation of azithromycin nanosuspensions by high pressure homogenization and its physicochemical characteristics studies. Drug Dev. Ind. Pharm. 2007:569–575. doi: 10.1080/03639040600975147. [DOI] [PubMed] [Google Scholar]
- 27.Kondo M., Niwa T., Okamoto H., Danjo K. Particle characterization of poorly water-soluble drugs using a spray freeze drying technique. Chem. Pharm. Bull. 2009;57:657–662. doi: 10.1248/cpb.57.657. [DOI] [PubMed] [Google Scholar]
- 28.Faruksha A.U., Vectrichelvan T. Formulation, characterization and optimization of pioglitazone hydrochloride nanoparticles by solvent displacement method using 32 factorial design. International Journal of PharmTech Research. 2013;5:754–766. [Google Scholar]