Abstract
Background
Tofacitinib (Tofa) has been approved for moderately to severely active ulcerative colitis (UC). To improve its therapeutic efficacy and limit dose-dependent toxicity, we developed a macromolecular prodrug of Tofa (P-Tofa). If the prodrug design improves the potency and duration of Tofa therapy, it would widen its therapeutic window, potentially leading to improved safety and better clinical management of UC.
Methods
P-Tofa was synthesized by conjugating Tofa to N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer via a cleavable carbamate linker. DSS-induced UC mouse model were treated with Tofa (daily oral gavage, from day 8), P-Tofa (single intravenous administration on day 8, dose equivalent to Tofa treatment) and saline. Healthy mice were used as a positive control. The therapeutic efficacy was evaluated using disease activity index (DAI), endoscopic score and end-point histology. The optical imaging, immunohistochemistry and flow cytometry were used to understand P-Tofa’s working mechanism.
Results
DAI results suggested that a single dose P-Tofa treatment was more efficacious than dose equivalent daily Tofa treatment. Endoscopic evaluation and histology analyses confirmed that while both P-Tofa and Tofa protected the colon, P-Tofa treated group was observed with better colon integrity with less tissue damage. Optical imaging, flow cytometry and immunohistochemistry results showed that P-Tofa passively targeted the inflamed colon and being retained via cellular sequestration.
Conclusions
Single intravenous administration of P-Tofa was more effective than dose equivalent daily oral Tofa gavage in ameliorating DSS-induced colitis. This observed superior therapeutic efficacy may be attributed to P-Tofa’s passive targeting to and retention by the inflamed colon.
Keywords: ulcerative colitis (UC), tofacitinib, inflammation targeting, ELVIS, prodrug
1. INTRODUCTION
Ulcerative colitis (UC) is type of inflammatory bowel disease (IBD) resulting in a chronic and relapsing inflammatory response in the colon, affecting approximately 1 million Americans, resulting in substantial disability. [1–4] Approximately 38,000 new cases of UC per year are reported in the United States, [4] with the related annual direct or indirect costs estimated to be $8.1–14.9 billion. [5] The current treatment of IBD includes anti-inflammatory agents (e.g., glucocorticoids and 5-aminosalicylates) and immunosuppressants (e.g., tumor necrosis factor (TNF) inhibitors and anti-integrin antibodies). [6, 7] Clinical use of these medications for moderate to severe UC, however, only result in sustained remission in less than 30% of the time. Around 20 to 30% of patients with UC eventually would need colectomy, which is a complex procedure with complications. [8] Currently, there is no cure for UC and there is an urgent need for new therapy to augment the natural progression of the disease.
Tofacitinib (Tofa) is a Janus Kinase inhibitor that has first been approved by US FDA for treatment of rheumatoid arthritis (moderate to severe) in patients who had an inadequate response or intolerance to methotrexate. Recently, it has been approved as a new treatment for moderate to severe active UC. As an immunosuppressant, however, its dose-dependent toxicities, which may be partially attributed to its ubiquitous systemic distribution, has raised concerns. [9] To limit its systemic exposure, Tofa was conjugated to an N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer via a carbamate bond to form a macromolecular prodrug. [10] We hypothesized that after systemic administration, the prodrug will passively target and subsequently be internalized by inflammatory cells at the intestinal inflammatory lesions, after which the prodrug may be activated subcellularly to provide a sustained local presence of Tofa, leading to effective resolution of the inflammatory processes with limited systemic immune suppression.
2. METHOD AND MATERIALS
2.1. Materials
N-(2-Hydroxypropyl) methacrylamide (HPMA), S,S′-bis(α,α′-dimethyl-α″-acetic acid)-trithiocarbonate were prepared as described previously. [11] Tofacitinib citrate and Tofacitinib base were provided by Jinlan Pharm-Drugs Technology Co., Ltd (Hangzhou, China). IRDye® 800CW carboxylate was provided by LI-COR, Inc. (Lincoln, NE, USA). Alexa Fluor® 647 NHS ester was provided by Life Technologies, Inc. (Eugene, OR, USA). P-Tofa and the fluorescence-labeled P-Tofa were prepared according to procedures described previously. [10] It has a weight-average molecular weight (Mw) of 30.4 kDa and a polydispersity index (PDI) of 1.32. The Tofa content in P-Tofa was found to be ∼13 wt %. Dextran sulfate sodium (DSS, Mw Ca 40,000) was provided by Alfa Aesar (Haverhill, MA, USA). All compounds were reagent grade and used without further purification.
Swiss Webster mice (8-week old) were purchased from Charles River Laboratories, fed a standard diet ad libitum and maintained under standard housing conditions in a University of Nebraska Medical Center (UNMC) animal facility. All animal experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of UNMC.
2.2. Instruments
1H and 13C NMR spectra were recorded on a 500 MHz NMR spectrometer (Varian, Palo Alto, CA, USA). ÄKTA Pure system (GE HealthCare, Chicago, IL, USA) equipped with UV detector and Superdex 200 increase column 10/300 GL, multi-angle light scattering (MALS) detector (Wyatt, Santa Barbara, CA, USA) and differential refractive index (dRI) detector (Wyatt, Santa Barbara, CA, USA) were used to determine the copolymers’ weight average molecular weight (Mw) and number average molecular weight (Mn). Drug content was analyzed on an Agilent 1100 HPLC system (Santa Clara, CA, USA) with a Hypersil™ ODS C18 Column (Thermo Scientific, Waltham, MA, USA). Paraffin-embedded tissue sectioning was done using a Leica RM2255 rotary microtome (Buffalo Grove, IL, USA). Histology slides was scanned by a VENTANA iScanner HT (Tucson, AZ, USA). Immuno-fluorescence labeled slides were analyzed using a ZEISS LSM 800 confocal microscope (Peabody, MA, USA). Live animals were imaged using LI-COR® Pearl imaging system (Lincoln, NE, USA). The endoscope used for the colitis scoring was purchased from Gradient Lens Corporation® (Rochester, NY, USA)
2.3. Treatment of dextran sulfate sodium (DSS)-induced ulcerative colitis mouse model
Forty 8-week old male Swiss Webster mice were randomly assigned into four groups. Group 1 was designed as a healthy control group, in which the animals received sterile water. Groups 2–4 were designed as saline, Tofa and P-Tofa treated group. The experimental groups received 2 cycles of 4% dextran sulfate sodium (DSS, Alfa Aesar, MW 40 kDa) as described previously. [12] Each cycle includes seven-day continuous 4% DSS followed by a seven-day continuous sterile water washout. In Group 2 (Saline), animals received saline injection on day 7 post induction. In Group 3 (Tofa treated), animals received Tofa from day 7 post induction to the end of the study for 21 days. Tofa was suspended in 0.5% methylcellulose and 0.025% Tween 20 solution and was given once daily by oral gavage at a dosing level of 10 mg/kg/day. [13] In Group 4 (P-Tofa treated), animals received a single i.v. injection of P-Tofa on day 7 post induction at a Tofa equivalent dose of 210 mg/kg. The mice’s body weight, stool consistency and presence of hematochezia (tested using ColoScreen™ FOB Test Kit, Helena Laboratories™, Texas, US) were examined and recorded daily. The disease activity index (DAI) was calculated as the sum of the change of body weight, diarrheal score and the bloody stool score (Table 1), according to the method adapted from literature. [14, 15]
Table 1.
The scoring system for the calculation of the disease activity index based on weight loss, stool consistency and the degree of intestinal bleeding [14, 15]
| Score | Weight loss | Stool consistency | Hemoccult (Blood stool) |
|---|---|---|---|
| 0 | none | Normal | Negative hemoccult |
| 1 | 1–5% | Mild soft stool | Positive hemoccult |
| 2 | 6–10% | Very soft; wet | Blood traces in stool visible |
| 3 | 11–20% | Watery diarrhea | Gross rectal bleeding |
Blood was collected using maxillary sampling method weekly starting a week before colitis induction until necropsy. Animals were sacrificed on day 28 after 24 hours fast. Blood and major organs (heart, liver, spleen, lung, kidney, small intestine, colon, and mesentery lymph nodes) were collected after perfusion. The length of colon was measured. About 1 cm of the distal colon was collected for FACs analyses of cell phenotypes from each animal. The remainder of the colon was assigned to histological evaluation.
2.5. Colonoscopy and colitis scoring
The endoscope used for colonoscopy with outside diameter 1.85 mm and LED light was purchased from Gradient Lens Corporation® (Rochester, NY, USA) equipped with a camera from Amscope (Irvine, CA, USA). The tip was sterilized and lubricated with surgical lubricant (Surgilube®, HR Pharmaceuticals, Inc. York, PA, USA) prior to imaging of each animal. Gentle air inflation of the colon was achieved by an external air pump (Variable-Speed Peristaltic Tubing Pump, Traceable® Products, TX, USA). Noninvasive images were taken weekly from each animal in all groups. The images were scored by a gastroenterologist (DDE) who was blinded to the treatment groups. The scoring was based on the scoring system (Table 2) adapted from the literature. [16]
Table 2.
Endoscopic colitis score based on the observed signs of inflammation. [16]
| Score | Thickening of the colon | Change of vascular pattern | Fibrin visible | Granularity of the mucosal surface |
|---|---|---|---|---|
| 0 | Transparent | Normal | None | None |
| 1 | Moderate | Moderate | Little | Moderate |
| 2 | Marked | Marked | Marked | Marked |
| 3 | Nontransparent | Bleeding | Extreme | Extreme |
2.6. Histological evaluation
Collected colon tissue was opened longitudinally and rolled colonic segments with mucosa outward into “Swiss rolls” [17], fixed in 4% PFA for 48 hr and then paraffin embedded. The 5 μm cross-sections were used for H&E staining. The section slides stained with H&E staining were scanned using a high-throughput bright-field slide scanner.
The H&E stained slides were histologically graded by a pathologist (SML, who was blinded to the group arrangement), using a scoring system (Table 3) adapted from literature. [18] The score for every histopathologic feature was also summed for each animal as a total histological score.
Table 3.
Histological grading criteria. [18]
| Parameters | Score | Histological features |
|---|---|---|
| Inflammation | 0 | None |
| 1 | Slight | |
| 2 | Moderate | |
| 3 | Severe | |
| Extent | 0 | None |
| 1 | Mucosa | |
| 2 | Mucosa and submucosa | |
| 3 | Transmural | |
| Regeneration | 0 | Complete regeneration or normal tissue |
| 1 | Almost complete regeneration | |
| 2 | Regeneration with crypt depletion | |
| 3 | Surface epithelium not intact | |
| 4 | No tissue repair | |
| Crypt damage | 0 | None |
| 1 | Basal 1/3 damage | |
| 2 | Basal 2/3 damage | |
| 3 | Only surface epithelium lost | |
| 4 | Entire crypt and epithelium lost | |
| Percent involvement | 1 | 1–25% |
| 2 | 26–50% | |
| 3 | 51–75% | |
| 4 | 76–100% | |
2.7. P-Tofa biodistribution analyses using optical imaging
To understand the biodistribution of P-Tofa, P-Tofa-IRDye® 800CW (0.2 mg polymer/mouse, IRDye content 8 × 10− 6 mol/g) was mixed with P-Tofa to a total Tofa equivalent dose of 210 mg/kg and intravenously injected into mice exposed to DSS (n = 3/time point) on day 7 post-induction. Healthy mice received the same injection that were used as the control (n=3). Mice were euthanized 1 and 3 days post-injection. Major organs (liver, spleen, kidney, small intestine, colon, and mesentery lymph nodes) were collected and imaged using a Pearl® Impulse small animal imaging system (LI-COR, Lincoln, NE). Image acquiring condition was dual channel (800nm and white light) with 85 μm resolution. The image for each mouse was normalized using the same intensity scale with a common minimum and maximum value.
2.8. Analysis of P-Tofa internalization and cellular retention with flow cytometry
To analyze cellular biodistribution of P-Tofa, Alexa Flour® 647 labeled P-Tofa (P-Tofa-Alexa 647 200 mg polymer/kg mouse) was mixed with P-Tofa to a total Tofa equivalent dose of 210 mg/kg and i.v. injected to mice with DSS exposure (n = 3/time point) on day 7 post-induction. Mice were euthanized 1 and 3 days after the administration of P-Tofa-Alexa 647. Blood and major organs (liver, spleen, kidney, colon, and mesenchymal lymph nodes) were collected and processed for flow cytometry analysis. Blood cells were marked by the following antibodies: BF650-labeled anti-mouse F4/80, BV711-labeled anti-mouse CD11b, BV786-labeled anti-mouse CD3e, and PE-Cy7-labeled CD19; BV421-labeled anti-mouse CD11c, APC-eFluor 780-labeled anti-mouse Ly-6G (BD Biosciences), PE/Dazzle™ 594 anti-mouse CD45 (Biolegend). The cells were analyzed using LSR II Green flow cytometer (BD Biosciences) and FlowJo software (Treestar, Inc., San Carlos, CA).
2.9. Immunohistochemistry analysis of P-Tofa cellular uptake at intestinal inflammatory site
Mice were fed with 4% DSS received P-Tofa-Alexa 647 (i.v. 200 mg polymer/kg mouse, mixed with P-Tofa to a total Tofa equivalent dose of 210 mg/kg) on day 7 and sacrificed 1 day later. Colons were collected and prepared for histologic examination as described above. Tissue sections (20 μm) were immunohistochemically stained after antigen retrieval using citrate buffer with the following antibodies: rabbit anti-IBA1 (Wako, 019–19741, dilution 1:250), rat anti-CD326(EpCAM) (eBioscience, 14–5791-85, dilution 1:250) and rabbit anti-CD146 (Abcam, ab75769, dilution 1/250), respectively, overnight at 4 °C after antigen retrieval and 10% normal goat serum blocking. Slides incubated with rat anti-CD326 were further incubated with Alexa Fluor 488-labeled goat anti-rat secondary antibody (Thermo Fisher scientific, A11006, dilution 1:1000) and slides incubated with rabbit anti-IBA1 and rabbit anti-CD146 were incubated with Alexa Fluor 488-labled goat anti-rabbit secondary antibody (Thermo Fisher scientific, A11008, dilution 1:1000) for another 1 hr at 20 °C in the dark. The stained slides were imaged using a ZEISS LSM 800 confocal microscope after mounted in ProLong® Gold antifade mountant with DAPI (Thermo Fisher scientific, P36931).
2.10. Immunocytochemistry
Mouse macrophage cell line (J774, ATCC, VA) and human epithelial cell line (Caco-2, ATCC, VA) were cultured in 30 mL of culture medium in a T-75 culture flask. Dulbecco’s Modified Eagle Medium (L-glutamine, high glucose) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat inactivated FBS was used. Cells were incubated in a humidified 5% CO2 incubator at 37 °C. The cell culture medium was changed every 3 days until the cells reach 80% confluent. J774 and Caco-2 cells were detached by trypsin (0.05% trypsin, 0.53mM EDTA, 6 mL), counted using automated counter Countess II (Invitrogen, MA) and seeded at 9×104 cells/well onto 12 mm diameter coverslips in 24-well plates. After cells adhere to 12 mm coverslips for 24 hr, the cells were challenged with 1 μg/mL lipopolysaccharides (LPSs) for 24 hr. After activation, cells were treated with Alexa Flour®647-labeled P-Tofa (1 mg/mL) in the presence of LPS (1 μg/mL) for 24 hr. The cells were incubated with LysoTracker™ Red DND-99 (Life technologies, MA) for 2 hr under complete culture medium. Cells were then washed with PBS, fixed with 4% paraformaldehyde in PBS and mounted with DAPI containing mounting media and observed using confocal microscope.
2.11. Statistical methods
One-way analysis of variance (ANOVA), followed by Tukey’s post hoc test to account for multiple comparisons, was used for data analysis using GraphPad Prism Software. P-values ≤ 0.05 were considered as statistically significant.
3. Result
3.1. Disease activity evaluation
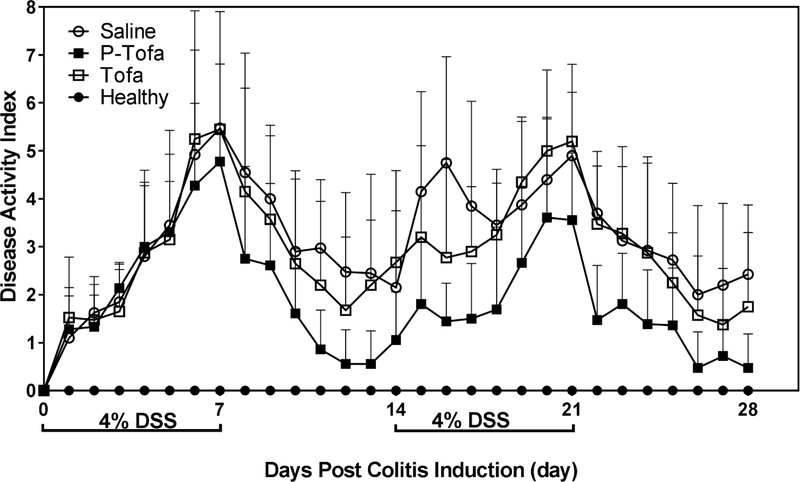
Rectal bleeding, stool consistency and body weight changes were monitored daily from the beginning of the study. From day 2 after DSS administration, stool hemoccult was observed in each group. From day 5 after DSS administration, rectal bleeding was observed in each group. There was resolution of rectal bleeding and stool hemoccult testing was negative after the first 7 day water cycle in almost all treatment groups. Rectal bleeding resumed with the initiation of the second DSS cycle in all animals with the exception of the P-Tofa treated group. In Figure 1, the data showed disease activity index (DAI) changes including body weight, stool consistency and hemoccult testing. The P-Tofa group showed lower DAI after drug administration at day 7 comparing to free Tofa treated group and saline treated groups. The DAI of the P-Tofa treated group was significantly lower compared to saline group (p<0.05) on day 11, 12, 13, 15, 16, 17, 18, 22, 28. Although, the DAI of P-Tofa treated group was not significant different from the healthy group from day 11 to day 17 and day 22 to day 28 except day 23. The DAI of P-Tofa treated group were significant lower that Tofa treated group on day 13, 14, 19, 21 and 22.
Figure 1. Disease activity index (DAI) of mice.
The DAI from each animal was calculated as the sum of the score of stool consistency, body weight change and hemoccult.
To evaluate colon fibrosis and damage, colon length of the animals was measured at necropsy on day 28 (Figure 2A). [19] P-Tofa treated animals were found to have significantly longer colon length than saline and Tofa treated animals as seen in the statistical analysis on Figure 2B. Additionally, there was no significant difference found between the Tofa and saline groups in relation to colon length.
Figure 2. Mice colon length at euthanasia.
A. The representative images of colons from each group. The colons were straight but not stretched and aligned at the ileocecal junction. The shortened length of the colon tissues from Saline group and Tofa treated group indicates the fibrosis resulting from inflammation. B. Quantitative analysis of the colon length (from ileocecal junction to distal end of rectum). ** P < 0.01, *** P < 0.001, **** P < 0.0001.
3.2. Colonoscopy evaluation
Before DSS administration, mice from each group were imaged using a colonoscope and representative images are shown in Figure 3A D0. After treating with 4% DSS for 7 days, all mice showed severe tissue damage as shown in Figure 3A D7 except those from the healthy control group. After treatment for 7 days, return of normal heatlhy visualized vasculature pattern was observed only in P-Tofa treated group and the healthy control group. In contrast, the Tofa treated group showed thickened colon and granularity on endoscopic grading. Individuals in the P-Tofa treated group showed mild to no damage in the colon which correlate to the disease activity index in Figure 1. After the second 4% DSS administration cycle, the P-Tofa treated group showed recurrence of inflammatory injury on endoscopic examination with noted colonic thickening and disappearance of regular vascular pattern. The Tofa treated group had continual colonic thickening and granularity through the course of the second DSS cycle through day 14. At the end point of the study (D28), most mice in P-Tofa group showed regular colonic mucosa or mildly inflamed mucosa on endoscopic imaging. At day 21 of the study, the P-Tofa and Tofa treated group showed significant improvement in colonic inflammation visualized on endoscopic image with the P-Tofa treated group having more resolution of inflammatory markers on imaging. In contrast the saline group showed persistent severe colonic inflammation on imaging. These findings correlated well with disease activity index scoring based on the body weight change and stool conditions (Figure 1) as well as histological evaluation shown in Figure 4. As seen in Figure 3B, the semi-quantitative scores from endoscopic observation were significantly varied between the various groups from week 2 to week 4 (P < 0.01) with the exception at week 4 when the P-Tofa treated group was not significantly different from the healthy control group. The colonoscopy results indicated that at the end of the experiment, P-Tofa treated group showed no statistically significant difference from the healthy control animals (Figure 3B).
Figure 3. Colonoscopy analyses of the animals.
A. Colonoscopy images of the mice from different treatment groups at designed time points. White arrow indicates colon surface irregularities; Green arrow indicates angiogenesis or bleeding. B. Semi-quantitative scoring of the colon according to parameters shown in Table 2. Significant difference (P < 0.01) was shown between each group from week 2 to week 4, except P-Tofa treated group, which was not significantly different from the healthy control group at week 4 (P > 0.05).
Figure 4. Histological analysis of colon tissue.
A. Representative images of H&E staining were shown. White arrow indicated mucosal erosion. Black arrow indicated loss of goblet cells and crypt architecture associated with epithelial damage. B. Semi-quantitative histological scores according to the grading system shown in Table 3. Significant difference was found between saline, Tofa treated group and healthy control group. * P < 0.05, ** P < 0.01
3.3. Histological evaluation
The severity of colonic inflammation and the therapeutic effects of the treatments were evaluated by H&E staining of colon sections. Colon tissues from each group were harvested at 28 days post colitis induction at necropsy. Severe goblet cell loss and crypt architecture damage were observed in the saline group as seen in Figure 4A. Mild crypt damages were observed in P-Tofa and Tofa group, respectively. Inflammatory cell infiltration were observed in saline, Tofa and P-Tofa treated groups. There was severe mucosal epithelium damage observed in saline group while only mild mucosal epithelium damaged were found in P-Tofa and Tofa treated groups. The H&E stained colon sections were scored by a professional pathologist who was blinded to the treatment group (Figure 4B). Significant difference was found in Tofa group and saline group when compared to healthy control; however, no significant difference was found between P-Tofa group and healthy control.
3.4. Optical imaging-based P-Tofa biodistribution study
To analyze the P-Tofa biodistribution and prove its inflammatory tissue specificity, optical imaging was used. The results are shown in Figure 5. Mice treated with 4% DSS or regular water for 7 days received P-Tofa-IRDye® 800CW administration through tail vein injection. Mice were sacrificed at day 1 and day 3 day after the drug administration. As shown in Figure 5A, isolated and perfused major organs had P-Tofa-IRDye infrared signal, especially in liver and kidney. DSS treated animals’ colon and mesenchymal lymph nodes (MLNs) had strong signals at 1 day after drug administration which was not found in healthy control group. At day 3, the signal decreased in all tissues except liver and kidney. Figure 5B showed the semi-quantified signal results. At day 1 post injection, no significant difference in signal intensity was observed in colon between DSS treated and healthy control group, but there is a trend (P = 0.1637) that DSS treated group has more prodrug accumulation. At day 3 post injection, prodrug signal was still stronger in DSS treated group which mean P-Tofa could target and be retained at the inflammation site.
Figure 5. Near infrared optical imaging-based analysis of P-Tofa biodistribution.
A. Representative ex vivo optical images of major organs, colon and small intestine from ulcerative colitis mice and healthy control mice at day 1 and day 3 post P-Tofa-IRDye® 800CW administration. B. P-Tofa was detected mainly at liver, kidney, colon and mesenchymal lymph nodes (MLNs) at 1 day post injection. At 3 days post injection, drug signal increased at liver and decreased in other organs. Higher infrared signal intensity was observed in colon of DSS treated than healthy control group but did not reach statistical significance (P = 0.1637).
3.6. Immunohistochemistry analyses of colon
The results of immunohistochemistry shown in Figure 6 illustrated the P-Tofa’s biodistribution on cellular level. The colocalization of Iba1 (a pan-macrophage biomarker which can stain macrophage and dendritic cells. [20]) positive cells and P-Tofa in lamina propria indicated P-Tofa was internalized by myeloid cells such like macrophages. The colocalization of P-Tofa and CD326+ cells or CD146+ cells were not as strong as Iba1+ cells. The distribution pattern of P-Tofa from lamina propria to luminal epithelium supports that the polymeric prodrug originated from blood stream and migrated to local colon tissue through passive targeting mechanism.
Figure 6. Representative images of immunohistochemistry from DSS treated mice.
Green signal showed different antibody staining (anti-Iba1, anti-CD326 and anti-CD146). Red signal showed P-Tofa distribution. Blue signal showed the nucleus. Anti-Iba1 stained colon tissue showed strong colocalization of P-Tofa and Iba1+ cells (yellow color). Anti-CD326 stained tissue did not show strong colocalization of CD326+ cells and P-Tofa. Some colocalization was observed in anti-CD146 stained slides.
3.7. P-Tofa internalization and retention assessed by flow cytometry
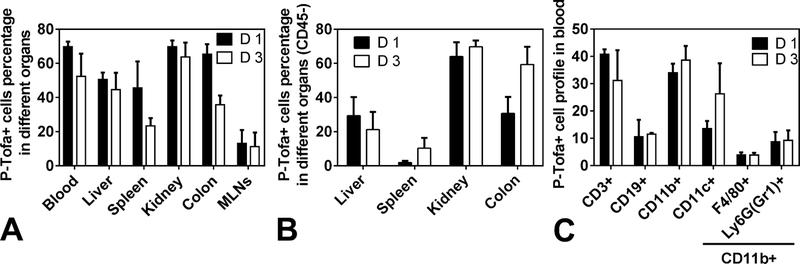
At 1 day after systemic administration, ~70% colon cells internalized P-Tofa and 3 days after injection the amount decreased to ~40% as shown in Figure 7A. This finding from flow cytometry correlated with optical imaging results. Large number of cells in blood, liver and kidney also internalized P-Tofa at 1 day and 3 days post injection. The percentage of cells in spleen internalizing P-Tofa decreased from day 1 to day 3. Figure 7B showed the P-Tofa+ cells percentage in different organs which were CD45-. This result showed the residential cells internalized P-Tofa in different organs. The percentage of residential colon cells increased from ~30% at day 1 to ~60% at day 3 which proved P-Tofa retained at inflamed colon. The same trend was observed in spleen. Figure 3C showed cell profile in blood internalizing P-Tofa. At both day 1 and day 3, ~30–40% CD3+ (T cells) and ~30–40% CD11b+ (myeloid cells) cells internalized and retained P-Tofa. Specifically, the percentage of CD11b+CD11c+ (dendritic cells) cells internalizing P-Tofa increased from day 1 to day 3. Macrophages (CD11b+F4/80+) showed less P-Tofa internalization in blood than dendritic cells. This result correlated with the immunohistochemistry findings of P-Tofa’s colocalization with Iba1+ cells.
Figure 7. Flow cytometry analysis of in vivo cellular sequestration of P-Tofa.
A. The percentage of P-Tofa+ cells in different organ at 1 day (D 1) and 3 days (D 3) after injection in mice with 7-day 4% DSS oral gavage. B The percentage of residential P-Tofa+ cells (CD45-) in different organs at 1 day (D 1) and 3 days (D 3) after injection. C. The percentage of P-Tofa+ cell phenotypes in the blood.
3.6. Immunocytochemistry
To further investigate the internalization and subcellular trafficking of P-Tofa, epithelial cells (Caco2) and macrophage (J774) were cultured in vitro. Alexa Flour®647 labeled P-Tofa was incubated with cells and imaged. P-Tofa-Alexa 647 signal was less in epithelial cells compared with macrophage. P-Tofa-Alexa 647 internalized by J774 was sequestered in lysosomes as shown in Figure 8 (colocalization of red signal and green signal). Within the lysosomal compartment, the environment is acidic and tofacitinib could be gradually released from P-Tofa polymer, which may lead to sustained amelioration of the inflammation.
Figure 8. P-Tofa-Alexa 647 internalization by macrophage (J774) and epithelial cells (Caco2).
Lysosomes stained with LysoTracker (Green), P-Tofa labeled with Alexa Flour®647 (Red), Nuclei (Blue). P-Tofa-Alexa 647 internalized by J774 was sequestered in lysosomes (colocalization). Less drug was internalized in epithelial cells comparing with macrophage.
4. Discussion
Tofacitinib, a pan-JAK inhibitor, was approved in 2012 for the treatment of rheumatoid arthritis and in 2017 it was approved for the treatment of psoriatic arthritis by US FDA. The approval was expanded in May 2018 for the treatment of moderate to severe ulcerative colitis. Tofacitinib is a small molecule administered orally that target cytokine signaling by preventing phosphorylation of Janus kinases associated cytokine receptor. Subsequently, phosphorylation of signal transducers and activators of transcription (STATs) that relay Janus kinase signaling and transcription of cytokines in the nucleus will be diminished. Key cytokines in the pathogenesis of ulcerative colitis such as IL-4, IL-5, IL-13 and IL-23 are targeted by Janus kinase inhibitors. [21, 22] Tofacitinib was found to be effective in phase 2 and 3 trials in moderate-severe ulcerative colitis at a dosage of 3mg, 5mg, 10mg and 15mg twice daily. Higher dosage showed faster and better remission, [23, 24] however, Tofacitinib, as a low molecular weight drug, frequently cause dose-dependent toxicities as a result of off-target side effect. Its immunosuppressive property contributes to the increased risk of infection, particularly herpetic infections. Understanding the expended application of Tofacitinib in the clinical management from rheumatoid arthritis to ulcerative colitis, and its dose-dependent toxicities limitations associated with the immune suppression, we proposed to develop a macromolecular prodrug nanomedicine of Tofacitinib in this study to incorporate disease associated inflammatory tissue specificity. We aimed to achieve disease associated organ/tissue-specific anti-inflammatory effects, which may potentiate the therapeutic efficacy and mitigate the systemic adverse effects associated with Tofa dosage, by enhancing its accumulation at active inflammation in colonic tissue.
For inflammatory bowel disease (IBD), loss of integrity of intestinal epithelium has been shown to play a key pathogenic role. [25] Tofa, as a JAK inhibitor, prevents epithelial damage by inhibiting Th2-type cytokines (IL-4, IL-5 and IL-13) expression. IL-4 plays an important role in naïve T cells differentiation into Th2 cells. IL-5 has been shown to be a key mediator in eosinophil activation and promotion of B cell growth. Additionally, IL-13 promotes fibrosis and induces apoptosis and degradation of tight junctions in epithelial cells. [21, 22] According to our previous study, IL-4 activated bone marrow macrophages were repressed for a longer time when pretreated by P-Tofa compared to Tofa. [10] The macrophage activation biomarker (Arg1, Ym1/2 and Fizz1) were significantly lower in P-Tofa group quantified by qPCR which indicated P-Tofa’s sustained anti-inflammatory activity in relation to tofacitinib.
Previously, the prodrug dexamethasone HPMA copolymer conjugate (P-Dex) and P-Tofa have been found to passively target and be retained at the sites of inflammation due to its extravasation through leaky vasculature and inflammatory cell-mediated sequestration (ELVIS) mechanism, providing sustained inflammation amelioration. [26] This observation has been validated in multiple inflammatory disease animal models, including rheumatoid arthritis, lupus nephritis, aseptic implant loosening and ulcerative colitis. [10, 27–30] P-Tofa showed a robust inflammation targeting effect as shown in optical imaging (Figure 5) and was retained at inflamed tissue as shown in Figure 7 with increased internalization by residential colon cells. P-Tofa showed strong targeting to inflamed colon comparing to healthy control at 1 day post injection. The spleen showed high signal intensity as shown in Figure 5 and Figure 7A. The spleen served as the main filter for blood-borne pathogens and antigens in the body. [31] It also served as a site for monocytes storage. When inflammation presents in the body, the spleen could deploy the monocytes rapidly to regulate inflammation. [32] When P-Tofa is injected to DSS treated mice, it was internalized by macrophages through endocytosis and retained at inflammation site, in this case the inflamed colon as shown in Figure 6 and 7. After P-Tofa passively targeted to the inflamed colon, it was sequestered in dendritic cells/macrophages as shown in Figure 6 and 7C. High signal of P-Tofa-IRDye ® 800CW was detected in the liver and kidney. There were two possible reasons for this high accumulation. One may be attributed to liver inflammation as reported in literature that DSS promoted hepatic inflammation. [33] Mild liver inflammation was also observed during histological evaluation (data not shown). The other possible explanation is that the liver and kidney are clearance organs which would process P-Tofa, leading to high P-Tofa presence as shown in Figure 5 and 7.
Since P-Tofa was injected through tail vein, its distribution should be from blood stream to the epithelium which correlated the findings shown in Figure 5. Epithelial or endothelial cells didn’t internalize P-Tofa as much as dendritic cells/macrophage. These findings further validated the ELVIS (extravasation through leaky vasculature and inflammatory cell-mediated sequestration) mechanism. [26] P-Tofa passively targeted and retained at the inflammation site mediated by dendritic cells/macrophages. As in vitro cell culture shows in Figure 8, P-Tofa trafficked into lysosomal compartment subcellularly. Due to the acidic pH in lysosomes, Tofa was released from polymer by cleaving the carbamate bond. Compared to Tofa’s ubiquitous distribution, P-Tofa could passively target to inflammation sites and release Tofa locally to achieve sustained inflammation amelioration.
From Figure 1, the DAI results combining body weight change, stool consistency and hemoccult showed significant difference between P-Tofa and saline group. P-Tofa showed superior therapeutic efficacy. Although Tofa did show significant difference compared with saline but did not show significant difference compared with healthy control at some days, the overall outcome of daily Tofa treatment was not as good as single P-Tofa injection. At necropsy at day 28, the colon length was measured as shown in Figure 2. Shortened colon length in saline and Tofa group indicating inflammation and colon damage which was not found in P-Tofa group compared with healthy control. Colonoscopy visualized the colonic inflammation in the mucosa throughout the study. The aforementioned results also correlated with histology staining.
In addition to amplifying the therapeutic efficacy, the macromolecular prodrug may also reduce typical side effects associated with parent drug treatment. [10, 29, 30] To assess the safety profile of P-Tofa, the hematology and liver function profiles of inflammatory rats treated with P-Tofa have been evaluated previously. [10] The lower levels of WBC count and ALP were detected in the P-Tofa group when compared to the Tofa treated group at the later stage of the treatment. These parameters were still within the normal range according to the vendor (Charles River Laboratories, Inc. MA). This reduction of WBC number may be interpreted as Tofa’s immunosuppressive effect or the attenuation of the systemic inflammation. More comprehensive toxicity studies are necessary to accurately define the P-Tofa’s safety profile in relevant experimental disease models.
In the present study, DSS-induced ulcerative colitis mouse model was chosen for providing proof of concept in investigation of a potential novel therapeutic intervention of ulcerative colitis. DSS-induced ulcerative colitis, as well as other chemical induced colitis models, exhibited several clinical and histological features of inflammatory bowel disease. [34–36] The inflammation was not directly induced by DSS. Rather it damages epithelial cells and expose lamina propria and induce subsequent immune cells infiltration. [37] Besides chemical induced colitis model, transgenic model such as the TRUC mouse model may better recapitulate the complex pathophysiology of human ulcerative colitis. The TRUC model disrupts transcriptional regulatory T-bet in the innate immune system of Rag2−/− mice. [38] To fully evaluate the therapeutic efficacy and safety profile of P-Tofa, it should be further evaluated on the TRUC mouse model. Tofacitinib as a pan-JAK inhibitor also showed promising therapeutic effects in Crohn’s Disease (CD) from clinical study. CD, as an immune disease, affecting multiple locations in the body which is not only located on the colon. Therefore, to assess the therapeutic potential of P-Tofa for CD, it needs be tested on CD-relevant preclinical models.
The water-soluble, biocompatible HPMA copolymer itself is non-degradable in the body which may pose a limitation of P-Tofa and raise potential safety concern associated with its long-term clinical utility. Although the molecular weight of P-Tofa is around 35kDa which could be excreted from the body eventually, a degradable HPMA copolymer may be further developed to address this concern. [39]
5. Conclusion
In this study, we validated the therapeutic efficacy of a macromolecular prodrug of a Janus Kinase (JAK) inhibitor, Tofacitinib (P-Tofa) in the treatment of DSS-induced chronic ulcerative colitis mouse model. A single systemic administration of P-Tofa provided superior and sustained therapeutic efficacy comparing to dose equivalent daily Tofa treatment. This improvement may be attributed to P-Tofa’s passive targeting to inflamed colon tissue and the subsequent sequestration by inflammatory cells at the pathology. Further efficacy and safety evaluation on other UC and Crohn’s disease models is necessary to fully establish the potential utility of P-Tofa as a new therapy for improved treatment of inflammatory bowel diseases (IBD).
6. Acknowledgements
This study was supported in part by the National Institute of Allergy and Infectious Diseases (R01 AI119090) of the National Institute of Health of the United States of America, and China Scholarship Council (XW, GZ, FZ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reference
- 1.Høivik ML, et al. , Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN Study. Gut, 2013. 62(3): p. 368–375. [DOI] [PubMed] [Google Scholar]
- 2.Molodecky NA, et al. , Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology, 2012. 142(1): p. 46–54. e42. [DOI] [PubMed] [Google Scholar]
- 3.Ng SC, et al. , Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet, 2017. 390(10114): p. 2769–2778. [DOI] [PubMed] [Google Scholar]
- 4.Loftus EV Jr, Update on the incidence and prevalence of inflammatory bowel disease in the United States. Gastroenterology & hepatology, 2016. 12(11): p. 704. [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen R, et al. , Systematic review: the costs of ulcerative colitis in Western countries. Alimentary pharmacology & therapeutics, 2010. 31(7): p. 693–707. [DOI] [PubMed] [Google Scholar]
- 6.Danese S, New therapies for inflammatory bowel disease: from the bench to the bedside. Gut, 2012. 61(6): p. 918–932. [DOI] [PubMed] [Google Scholar]
- 7.De Vries L, et al. , The Future of Janus Kinase Inhibitors in Inflammatory Bowel Disease. Journal of Crohn’s and Colitis, 2017: p. jjx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman S, Tofacitinib for Ulcerative Colitis—A Promising Step Forward. 2017, Mass Medical Soc [DOI] [PubMed] [Google Scholar]
- 9.Tanaka Y, Recent progress and perspective in JAK inhibitors for rheumatoid arthritis: from bench to bedside. The Journal of Biochemistry, 2015. 158(3): p. 173–179. [DOI] [PubMed] [Google Scholar]
- 10.Wei X, et al. , Development of a Janus Kinase Inhibitor Prodrug for the Treatment of Rheumatoid Arthritis. Molecular pharmaceutics, 2018. 15(8): p. 3456–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X-M, et al. , Synthesis and evaluation of a well-defined HPMA copolymer–dexamethasone conjugate for effective treatment of rheumatoid arthritis. Pharmaceutical research, 2008. 25(12): p. 2910–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chassaing B, et al. , Dextran sulfate sodium (DSS)‐induced colitis in mice. Current protocols in immunology, 2014: p. 15.25. 1–15.25. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vries L, et al. The Efficacy of Tofacitinib and a Selective Janus Kinase 1 Inhibitor in Dextran Sulphate Sodium Colitis Models. in JOURNAL OF CROHNS & COLITIS. 2016. OXFORD UNIV PRESS; GREAT CLARENDON ST, OXFORD OX2 6DP, ENGLAND. [Google Scholar]
- 14.Cooper HS, et al. , Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Laboratory investigation; a journal of technical methods and pathology, 1993. 69(2): p. 238–249. [PubMed] [Google Scholar]
- 15.Rumi G, et al. , Dual role of endogenous nitric oxide in development of dextran sodium sulfate-induced colitis in rats. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society, 2004. 55(4): p. 823–836. [PubMed] [Google Scholar]
- 16.Becker C, et al. , In vivo imaging of colitis and colon cancer development in mice using high resolution chromoendoscopy. Gut, 2005. 54(7): p. 950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moolenbeek C and Ruitenberg E, The ‘Swiss roll’: a simple technique for histological studies of the rodent intestine. Laboratory animals, 1981. 15(1): p. 57–60. [DOI] [PubMed] [Google Scholar]
- 18.Kim C, et al. , L-cysteine supplementation attenuates local inflammation and restores gut homeostasis in a porcine model of colitis. Biochimica et Biophysica Acta (BBA)-General Subjects, 2009. 1790(10): p. 1161–1169. [DOI] [PubMed] [Google Scholar]
- 19.Rieder F and Fiocchi C, Intestinal fibrosis in inflammatory bowel disease—Current knowledge and future perspectives. Journal of Crohn’s and Colitis, 2008. 2(4): p. 279–290. [DOI] [PubMed] [Google Scholar]
- 20.Pierezan F, et al. , Immunohistochemical expression of ionized calcium binding adapter molecule 1 in cutaneous histiocytic proliferative, neoplastic and inflammatory disorders of dogs and cats. Journal of comparative pathology, 2014. 151(4): p. 347–351. [DOI] [PubMed] [Google Scholar]
- 21.Fuss IJ, et al. , Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. The Journal of clinical investigation, 2004. 113(10): p. 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heller F, et al. , Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology, 2005. 129(2): p. 550–564. [DOI] [PubMed] [Google Scholar]
- 23.Sandborn WJ, et al. , Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. New England Journal of Medicine, 2012. 367(7): p. 616–624. [DOI] [PubMed] [Google Scholar]
- 24.Sandborn WJ, et al. , Tofacitinib as induction and maintenance therapy for ulcerative colitis. New England Journal of Medicine, 2017. 376(18): p. 1723–1736. [DOI] [PubMed] [Google Scholar]
- 25.Maloy KJ and Powrie F, Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature, 2011. 474(7351): p. 298. [DOI] [PubMed] [Google Scholar]
- 26.Yuan F, et al. , Development of macromolecular prodrug for rheumatoid arthritis. Advanced drug delivery reviews, 2012. 64(12): p. 1205–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, et al. , Novel dexamethasone-HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis research & therapy, 2007. 9(1): p. R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei X, et al. , Pharmacokinetic and biodistribution studies of HPMA copolymer conjugates in an aseptic implant loosening mouse model. Molecular pharmaceutics, 2017. 14(5): p. 1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan F, et al. , Dexamethasone prodrug treatment prevents nephritis in lupus‐prone (NZB× NZW) F1 mice without causing systemic side effects. Arthritis & Rheumatology, 2012. 64(12): p. 4029–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren K, et al. , Macromolecular glucocorticoid prodrug improves the treatment of dextran sulfate sodium-induced mice ulcerative colitis. Clinical Immunology, 2015. 160(1): p. 71–81. [DOI] [PubMed] [Google Scholar]
- 31.Bronte V and Pittet MJ, The spleen in local and systemic regulation of immunity. Immunity, 2013. 39(5): p. 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swirski FK, et al. , Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science, 2009. 325(5940): p. 612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gäbele E, et al. , DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. Journal of hepatology, 2011. 55(6): p. 1391–1399. [DOI] [PubMed] [Google Scholar]
- 34.Okayasu I, et al. , A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology, 1990. 98(3): p. 694–702. [DOI] [PubMed] [Google Scholar]
- 35.Morris GP, et al. , Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology, 1989. 96(2): p. 795–803. [PubMed] [Google Scholar]
- 36.Boirivant M, et al. , Oxazolone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. Journal of Experimental Medicine, 1998. 188(10): p. 1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Low D, Nguyen DD, and Mizoguchi E, Animal models of ulcerative colitis and their application in drug research. Drug design, development and therapy, 2013. 7: p. 1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garrett WS, et al. , Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell, 2007. 131(1): p. 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, et al. , FRET-trackable biodegradable HPMA copolymer-epirubicin conjugates for ovarian carcinoma therapy. Journal of Controlled Release, 2015. 218: p. 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]