Over the decade from 2010–2019, the prevalence of opioid use and incidence of opioid-related overdose rose sharply in the United States.1,2 Fatal drug overdoses now exceed the peak mortality related to AIDS in 1995,3 with more than 70,000 overdose deaths in 2017, approximately 47,600 of which were attributed to opioids.4 Infectious complications of injection drug use have also risen during this time, including severe bacterial infections and chronic hepatitis C virus (HCV).5,6 The rise in misuse of prescription opioids7 and the role of illicit fentanyl and its analogues8 have often taken center stage in media coverage about the shifts in opioid epidemiology. However, a closer examination reveals a more complex picture, with multiple overlapping social and economic factors contributing to the increasing prevalence of opioid use and worsening vulnerability of people who use them.1,9 In particular, opioid misuse and use disorder are strongly associated with criminal justice involvement (CJI).9 Furthermore, the shifting demographics and epidemiology of opioid use have coincided with a public re-examination of systemic racism and mass incarceration, bringing renewed attention to the public health impact of CJI. Recent estimates suggest that 1.9 million Americans have an opioid use disorder (OUD),10 while 6.6 million are under the supervision of adult correctional systems.11 Because both CJI and OUD are highly prevalent and associated with co-morbid infections, the delivery of care for addiction and its infectious complications within criminal justice-based health systems has wide-reaching implications, including for community-based providers of care for infectious diseases.
Criminal justice institutions and public health
The “criminal justice system” is a network of separate yet interrelated public institutions, including: law enforcement agencies; courts; correctional institutions, which incarcerate individuals as part of pre-trial detention or sentencing for a criminal conviction; and community supervision agencies, which administer restrictions and enforce sanctions for criminal defendants who remain in the community (e.g. probation, parole and alternative sentencing programs). Among settings of incarceration, the term “jail” refers to institutions designed to hold criminal defendants in pre-trial detention, while awaiting adjudication of their charges. Jails are generally administered by a local county government or sheriff’s department. Prisons are usually state or federal institutions, which hold individuals who have been sentenced for a criminal conviction. In some jurisdictions, shorter sentences (typically of less than 1–2 years) may be served in local jails instead of prison.12
The distinction between correctional institutions necessitates different models of healthcare delivery. For example, clinicians in county jails typically care for a highly transient patient population, often with acute medical issues occurring around the time of arrest (e.g. withdrawal from substances, mental health crises, etc.). While medical service providers in jails have an opportunity to screen patients for chronic diseases (see Table 1), establishing a plan for referral to appropriate care in the community will be a central priority for the majority of individuals. In contrast, medical care in prison will commonly include population-wide chronic disease management, health screenings and preventive care. Regardless of the setting, however, populations with CJI are disproportionately affected by chronic health conditions, including: hypertension,13 cardiovascular disease,13 diabetes mellitus,13 severe mental illness,14 post-traumatic stress disorder (PTSD),15 OUD and other substance use disorders (SUDs),14 HIV/AIDS13 and viral hepatitis.13 Nationwide, an estimated 64.5% of those in settings of incarceration have a SUD, while 32.9% have been diagnosed with a mental health disorder.14
Table 1:
Recommendations for preventive care among people who use opioids in the criminal justice system
| Screening | Vaccination | Harm reduction | |||
|---|---|---|---|---|---|
| Disease | Recommendations | Disease | Recommendations | Disease | Recommendations |
| HIV | Opt-out screening on intake for all individuals55 | Tetanus | Td recommended every 10 years and TDaP once for all adults (especially important for PWID given ongoing risk)77 | Blood-borne pathogens | •Avoid sharing of needles and equipment (including cottons, cookers, etc.) • Avoid using syringes to avoid drugs • Smoke, snort, swallow or “booty bump” (rectal administration) drugs instead of injecting • Reduce number of sharing partners |
| HBV | HBsAg, HBsAb and HBcAb recommended for all PWID77 | HBV | Administer vaccine series to all PWID without evidence of HBV immunity (negative Hepatitis B surface Antibody)77 | Severe bacterial infections | • Use a new needle every time (avoid even personal reuse) • Wash hands, use gloves and sterilize injection sites with alcohol prior to every use • Avoid licking needles or other equipment prior to injection |
| HCV | Opt-out screening on intake for all individuals [authors’ opinion and USPSTF recommendation to screen all adults]76 | HAV | Administer vaccine with booster dose at 6 months to all PWID (serology not necessary prior to vaccination)77 | Overdose | • Naloxone distribution • Use a small test dose to assess drug potency prior to use • Avoid using alone • • Avoid mixing opioids with benzodiazepines or other sedating drugs • Counseling regarding overdose risk associated with loss of tolerance and cue-associated cravings upon release from incarceration |
| STDs | Screen all people who use drugs for chlamydia, gonorrhea, and syphilis77 | Other: HPV, Pneumococcus, Meningococcus, Pertussis | As indicated based on age and HIV status | ||
| Latent TB infection | Annual PPD or interferon-gamma release assay84 | Seasonal influenza | Indicated annually for all adults | ||
Because jails and prisons place individuals in confinement, they have a legal obligation to provide medical care to those under their custody. 16 A seminal 1976 U.S. Supreme Court case Estelle versus Gamble established that denying medical care was “deliberate indifference” to the health of incarcerated individuals and prohibited by the constitution.16 However, people under community supervision also carry a high burden of OUD and chronic infectious diseases but have less frequently been targeted for medical interventions. Those under community supervision typically continue to receive medical care in the community, though in some cases an individual may be mandated to participate in a particular treatment program for substance use or mental health.12
The relationship between CJI and chronic health conditions is complex and multi-directional. Because of drug prohibition and the occurrence of “acquisitive crime” to sustain substance use, people with SUDs experience ongoing risk of CJI. Furthermore, substance use and HIV have been described as being “syndemic” with interpersonal violence among economically disadvantaged communities, thereby increasing risk of CJI.17 Prior to arrest, people with CJI are thus more likely to have experienced violence, substance use and their infectious and psychiatric sequelae and often face significant barriers to healthcare access in the community. Incarceration also leads to loss of housing and employment and severs social ties, thereby destabilizing people with SUDs. Overall mortality is 8–12 times greater than that of the general population in the early post-release period.18 Among people who inject drugs (PWID), sharing of needles and equipment and the incidence of HIV and HCV increase following release from incarceration.19,20 Furthermore, a history of recent incarceration appears to be associated with increased intensity of opioid and other substance use.9
The unique challenges of providing care in correctional settings
Healthcare providers working in correctional settings face unique challenges. The movement of patients and staff between physical locations is constrained and resource limitations are common within correctional health systems. Maintaining confidentiality can be challenging, because security staff are necessarily involved in patient flows and remain in close proximity to potentially violent patients.21 The cost and discomfort associated with transporting patients to providers outside of a facility (typically in shackles and accompanied by at least two armed guards) also can present a barrier to receiving appropriate specialty care.22 In particular, for people using opioids, the risk of legal sanctions for possession or unsupervised opioid use may serve as a barrier to seeking OUD treatment during incarceration.
In these and other ways, priorities set by security staff can have a powerful impact on healthcare delivery. At times, such as when a physical altercation occurs, the security mission of the institution must take precedence over routine medical care to protect the safety of both staff and patients. However, the security mission can also challenge professional ethics through dual loyalty, meaning that healthcare staff must subjugate patient care needs to security staff mandates. For example, healthcare staff are commonly asked to medically “clear” individuals before they are transferred to administrative segregation (i.e. “solitary confinement”), which is associated with mental health risks. Addressing dual loyalty systematically with training to recognize and manage ethical conflicts, has been demonstrated to be feasible and acceptable.23
Prescribing decisions in correctional facilities, especially regarding medications for opioid use disorder (MOUD), are influenced by dual loyalty, security mandates and stigma. Medical care in correctional settings is often structured to minimize diversion of psychoactive substances such as opioid analgesics and MOUD (e.g. methadone and buprenorphine), even in the face of great medical need. Though data are limited, available reports describe a predominance of psychosocial treatments and peer recovery supports (e.g. alcoholics anonymous), while few correctional facilities offer MOUD.24 Even when MOUD is available, medications are often provided exclusively for the management of acute withdrawal, chronic pain or for maintenance treatment of OUD in pregnant women.24 A preference by criminal justice staff for psychosocial drug treatment is commonly cited as a barrier to offering MOUD.24,25 However, these attitudes contradict the community standard of care, in which psychosocial treatments combined with maintenance pharmacotherapy using methadone, buprenorphine or naltrexone is considered first line.26
Patient-centered OUD care for criminal justice-involved patients
The ideal approach to managing OUD during incarceration requires screening, withdrawal management, initiation or continuation of MOUD, and planning for reentry (see Figures 1 and 2). Most jails and prisons in the U.S. do not offer MOUD, but law suits, new legislation, and changes in attitudes toward MOUD are contributing to increased MOUD availability.27 Identifying people with OUD can be challenging during incarceration, due to fear of further criminal sanctions for ongoing use. The Rapid Opioid Dependence Screen (RODS) was recently developed and validated for use in criminal justice settings, and may be a helpful tool for integrating OUD engagement into routine medical care.28 Other validated screening instruments also exist. An initial assessment of people found to have OUD should include: 1) an assessment of co-morbid medical and psychiatric conditions, 2) a careful evaluation for withdrawal from opioids and other substances, 3) counseling regarding expectations for OUD management (either withdrawal management or maintenance treatment), and 4) screening for infectious and other complications of addiction. Offering the full array of MOUD options to people with OUD in criminal justice settings is feasible, safe and effective.29–31 Initiating and maintaining MOUD treatment during incarceration increases retention in treatment in the community after release, which results in long-term benefits including reduced injection behavior, heroin use and non-fatal overdose 12-months after release.30
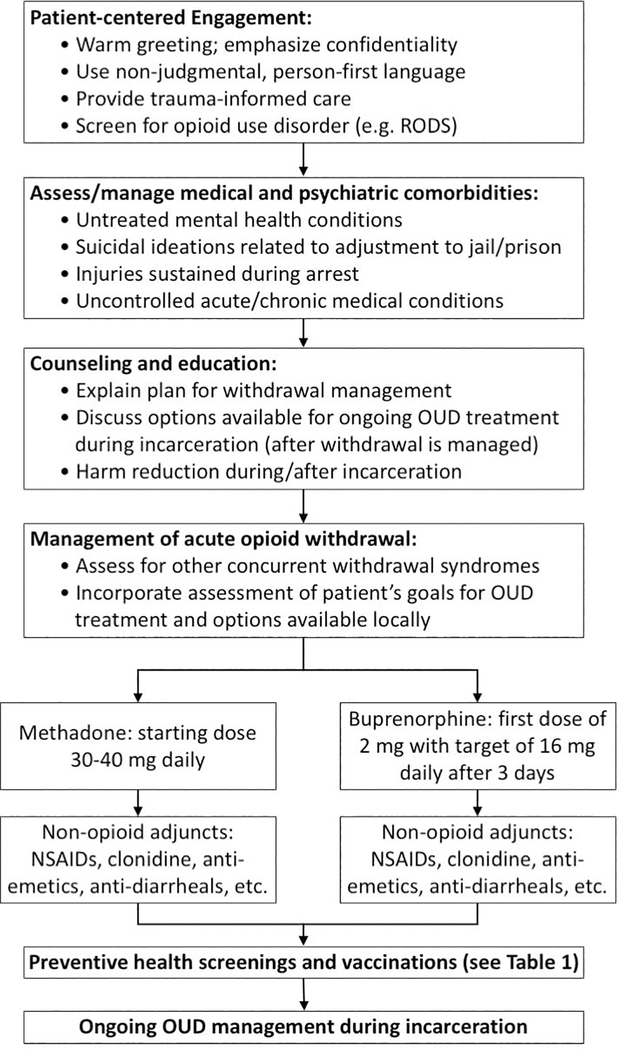
Figure 1: Approach to the patient with opioid use disorder (OUD) upon intake to a correctional facility.
Upon intake to a correctional facility, patients suspected of having OUD should undergo a comprehensive evaluation, including medical management of withdrawal symptoms; infectious disease screening; counseling and education; and the formulation of a plan for transition to ongoing care for OUD and its infectious complications. OUD = opioid use disorder; RODS = Rapid Opioid Dependence Screen (28); NSAIDs = non-steroidal anti-inflammatory drugs.
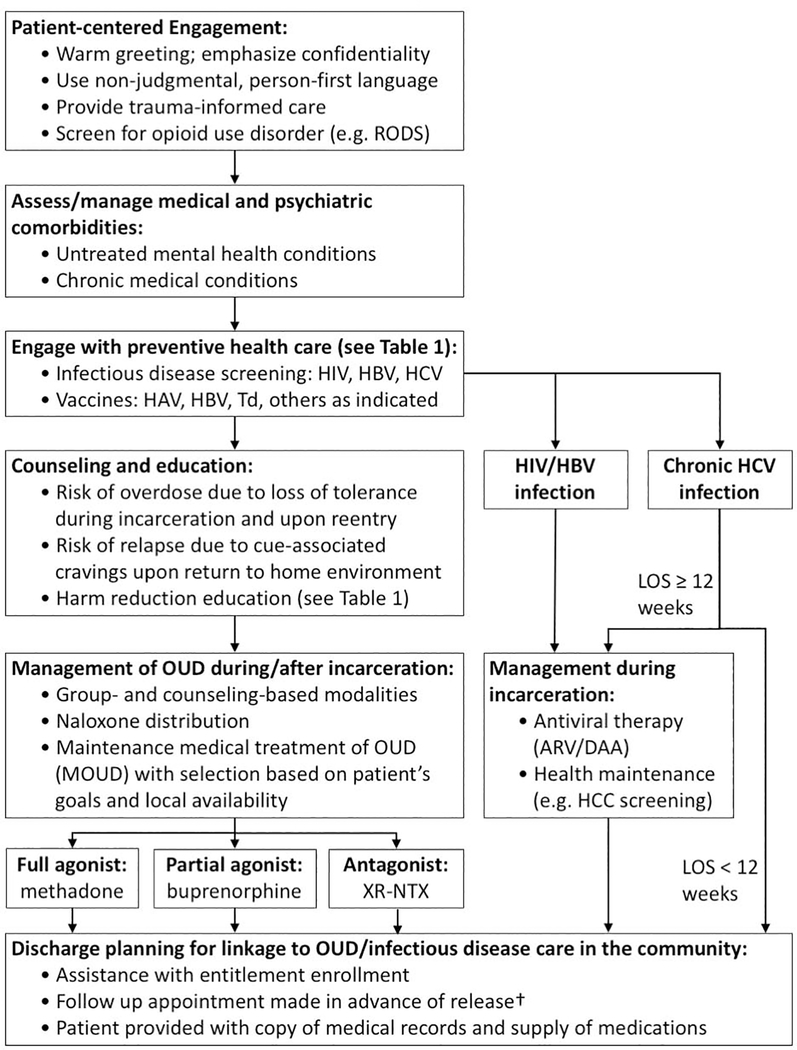
Figure 2: Approach to the patient with opioid use disorder (OUD) during incarceration and prior to community reentry.
During incarceration, patients with OUD should undergo a comprehensive evaluation. This evaluation should include: the assessment and management of psychiatric/medical comorbidities; infectious diseases screening and immunizations; medical treatment of OUD and chronic infections; and a plan for transition to ongoing care for OUD and its infectious complications in the community upon reentry. OUD = opioid use disorder; RODS = Rapid Opioid Dependence Screen;28 XR-NTX = extended-release naltrexone; HIV = human immunodeficiency virus; HBV = hepatitis B virus; HCV = hepatitis C virus; ARV = antiretroviral therapy for HIV; DAA = direct-acting antiviral therapy for viral hepatitis; LOS = Length of stay. †Patient navigation or follow-up in a transitions clinic, if available, may improve healthcare engagement.61,64 (Adapted from Masyukova MI., Hanna DB., Fox AD. HIV treatment outcomes among formerly incarcerated transitions clinic patients in a high prevalence setting. Health Justice 2018;6(1):16. Doi: 10.1186/s40352–018-0074–5; Cunningham WE., Weiss RE., Nakazono T., et al. Effectiveness of a Peer Navigation Intervention to Sustain Viral Suppression Among HIV-Positive Men and Transgender Women Released From Jail: The LINK LA Randomized Clinical Trial. JAMA Internal Medicine 2018;178(4):542; with permission.)
Maintenance treatment
A comprehensive OUD treatment program will provide access to full agonist (methadone), partial agonist (buprenorphine) and antagonist (extended-release naltrexone) pharmacotherapies in combination with psychosocial treatment (see Figure 2). In accordance with the community standard of care, the choice of medication involves shared decision-making, taking into account the patient’s preferences, treatment goals, and the risks and benefits of each option.26 In the first 18 months of a newly established program in the Rhode Island Department of Corrections, there was a 350% increase in MOUD uptake, with more than half of patients choosing methadone, a substantive minority (approximately 40–45%) choosing buprenorphine and ≤ 2.5% choosing naltrexone.29,32 Rollout of this program was associated with a decrease in opioid overdose deaths among community-dwelling individuals with recent CJI across the state of Rhode Island.33 These findings are consistent with international data demonstrating that receiving MOUD during incarceration is associated with large reductions in overall and overdose-related mortality during incarceration and post-release.31 In their review of this and other data, the National Academies of Science, Engineering and Medicine concluded that all FDA-approved medications for maintenance treatment of OUD should be made available in criminal justice settings.34
Medical management of opioid withdrawal
Under current circumstances, limited MOUD availability creates obstacles for providing the community standard of care to patients with OUD in criminal justice settings. When maintenance opioid agonist therapy (OAT) is unavailable, patients with OUD will almost universally experience withdrawal upon being taken into custody. Medically managed withdrawal (often referred to as “detoxification”) should be provided in these settings to avoid untreated symptoms, but clinicians must be aware this does not constitute long-term OUD treatment and is associated with an increased risk of subsequent overdose related to loss of tolerance.35 Comparable to the management of withdrawal syndromes upon hospital admission, several options are available for symptomatic treatment (see Figure 1).36 Where possible, withdrawal management with methadone or buprenorphine is preferable (sample protocols for buprenorphine induction are available at https://pcssnow.org/wp-content/uploads/2014/02/PCSS-MATGuidanceBuprenorphineInduction.Casadonte.pdf and https://www.asam.org/docs/default-source/education-docs/clinic-induction-protocol-example_it-matttrs_8-28-2017.pdf?sfvrsn=a30640c2_2). In general, slowly tapering opioids is preferable––longer periods of time for tapering will lessen opioid withdrawal symptoms. Non-opioid adjuncts for symptom control can be used in addition to opioids (or alone if opioids are not available for withdrawal management). These include clonidine, NSAIDs, anti-emetics, anti-diarrheal agents and the judicious use of benzodiazepines.36 Careful assessment for concurrent withdrawal from other substances is critical, because benzodiazepine and alcohol withdrawal can be fatal.
The chosen approach to OUD and withdrawal management in correctional settings can have lasting effects on patient engagement in the community. In particular, the prolonged withdrawal syndrome experienced by those who are forced to taper off methadone maintenance therapy during incarceration can result in a later aversion to methadone as a treatment modality.37 Conversely, emphasizing “detoxification” and avoidance of maintenance opioids can contribute to a perception of a period of abstinence in prison as “clean time.”38 While patients may prefer to avoid opioids and MOUD during periods of incarceration, the perspective that prolonged abstinence in correctional facilities can increase one’s chances of sustaining a long-term recovery in the community is at odds with data showing that approximately 65%−90% of people with OUD will relapse within 6 months of release in the absence of maintenance MOUD treatment, with at most a marginal benefit from prolonged abstinence.39,40 Furthermore, a focus on the purported benefits of prolonged abstinence during incarceration may provide unrealistic expectations about the severity of cue-associated cravings when patients with OUD return to their home environment. Forced withdrawal during incarceration also removes the protective effect of opioid tolerance and leaves individuals susceptible to overdose upon release if they return to using the amount that they were previously using.35 Return to opioid use can also occur during incarceration; hence it is critical that correctional healthcare systems create conditions for overdose preparedness. Necessary actions include ensuring naloxone availability in code kits, training nursing staff to administer naloxone and establishing nurse-driven protocols for its use during an emergency in the absence of an ordering provider. Security staff may even carry naloxone and be trained in its use.41 Correctional facilities can also distribute naloxone and provide training for its use to people re-entering the community.42
Care during reentry
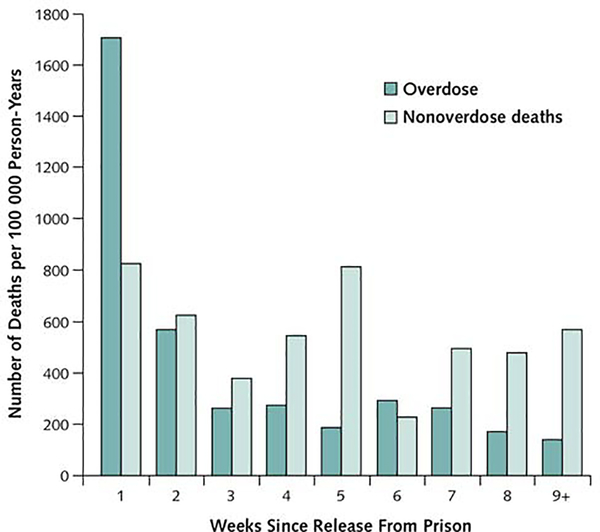
In the early post-release period, the risk of fatal overdose is more than 10 times that of the general population, and is greatest (more than 120 times the general population rate) during the first week (see Figure 3).18 Therefore, any medical encounter with a patient who uses opioids during the course of incarceration presents an opportunity to provide education about the post-release risk of overdose. Counseling can also suggest methods to reduce the risk of acquired infection in the event of relapse (see Table 1). Extended-release naltrexone (XR-NTX), a long-acting, monthly injection of an opioid antagonist reduces relapse and non-fatal overdose in people with OUD and CJI.40 It is particularly appealing to criminal justice institutions, because it has been marketed as supporting an abstinence-based recovery.43 However, emerging data suggests that XR-NTX may be less effective than OAT at protecting patients from overdose,44,45 and its relatively limited use outside of correctional institutions may be a barrier to continuing treatment after release. The initiation of OAT therapy upon release or during parole46 is also feasible and has been shown to improve rates of viral suppression among HIV-positive people with OUD.47 Initiating MOUD treatment at least 30 days prior to release has been recommended by the American Society for Addiction Medicine.26
Figure 3: Risk of overdose-related and all-cause mortality following release from prison.
From Binswanger IA., Blatchford PJ., Mueller SR., et al. Mortality After Prison Release: Opioid Overdose and Other Causes of Death, Risk Factors, and Time Trends From 1999 to 2009. Annals of Internal Medicine 2013;159(9):592; with permission.)
Integrating care for addiction and infectious diseases among criminal justice-involved populations
Though OUD treatment is challenging in correctional settings, incarceration nevertheless provides an opportunity to screen, prevent and treat the chronic infectious complications of OUD. Conversely, due to limited access to safe injection equipment, poorly controlled OUD may lead to acute infections during incarceration. Clinicians in criminal justice settings must therefore be vigilant for infections related to substance use.
Integration of OUD treatment into infectious disease specialty care is occurring in community settings and should be the ideal during incarceration.48 These efforts largely center around maximizing opportunities to offer MOUD during episodes of care for infections such as endocarditis, osteomyelitis, bacteremia, HIV and HCV.48 In correctional healthcare, however, specialists often have a somewhat circumscribed role. Patients cannot self-refer to specialty care, and specialist referral is often subject to strict controls to reduce the cost of care. Furthermore, the constrained movement of clinicians in correctional settings has facilitated the innovative use of telemedicine to manage chronic infections such as HIV and HCV (e.g. project ECHO).50 While telemedicine allows infectious disease specialists to provide expert care for patients who are geographically dispersed across multiple facilities, it also limits their role to consultation; each facility’s on-site provider is typically responsible for enacting recommendations and monitoring a patient’s response between scheduled telemedicine sessions. Hence, correctional health systems must identify opportunities to integrate screening, management and preventive care protocols for both OUD and infectious diseases at the primary care level.
Acute infections
Infectious disease specialists practicing in the community may care for incarcerated patients who become hospitalized. It is important for these providers to note that for many people with OUD and other SUDs, illicit drug use may continue during incarceration.51 Therefore, it is critical to take a careful substance use history, including recent injection for any patient presenting from correctional facilities with skin and soft tissue infections, osteomyelitis, endocarditis and other endovascular infections. In most correctional institutions, medical facilities will include an infirmary, in which round-the-clock nursing care can be provided. Typically, patients will be placed in an infirmary upon return from hospitalization or admitted directly to the infirmary for observation and management of acute medical issues requiring an intermediate level of care. In many facilities, patients needing ongoing intravenous antibiotics or wound care after hospitalization remain in the infirmary for the duration of their course, mirroring the function of an outpatient parenteral antimicrobial therapy (OPAT) service.52 It is essential to communicate recommendations for clinical monitoring and further management clearly, ideally through direct communication between facility-based providers and hospital-based infectious disease consultants. However, facility-based clinicians must be aware of the limited level of care that can be provided in an infirmary.53 Any patient with evidence of sepsis or who is suspected/found to have active bacteremia should be transferred to an emergency department immediately for further evaluation. If a patient is unstable, blood cultures can be drawn, and an initial dose of antibiotics administered on-site while arranging transfer, but diligence in timely communicating microbiologic results to hospital-based care teams is critically important.
Chronic infections
Apart from differences in the delivery system, managing HIV in correctional settings is not substantially different from doing so in the community (Figure 2).54 Policies regarding HIV screening vary according to jurisdiction but universal opt-out screening on intake is recommended by the U.S. Centers for Disease Prevention and Control (CDC) for all jails and prisons (Table 1) and is increasingly being implemented.55,56 Universal screening in jails is somewhat more challenging than in prisons or community supervision agencies, due to high turnover of individuals being admitted for stays of only a few days. Nearly all prisons and jails offer HIV testing in some circumstance, but in one survey, only 56% of prisons reported providing routine HIV screening at intake (including both opt-in and opt-out), while only 4% of jails provided routine screening.56
Once HIV is detected, either through screening or self-report, all incarcerated individuals with HIV in the United States should have access to safe and effective first-line antiretroviral (ARV) regimens, with regular monitoring of viral load and CD4+ T-cell count, vaccinations, and any indicated prophylaxis against opportunistic infections. However, limited data exist to assess how consistently these measures are available.54 Many state prison systems fund ARV provision through the 340b funding mechanism,57 avoiding the cost control hurdles (e.g. prior authorization) of health insurance plans in the community. While correctional health systems can generally provide consistent ARV access through established pharmacy relationships, interruptions can occur during transfers between institutions or trips to local courts for judicial proceedings. Healthcare providers responsible for HIV care in criminal justice settings must ensure adequate protocols are in place to prepare nursing and pharmacy staff for such events.
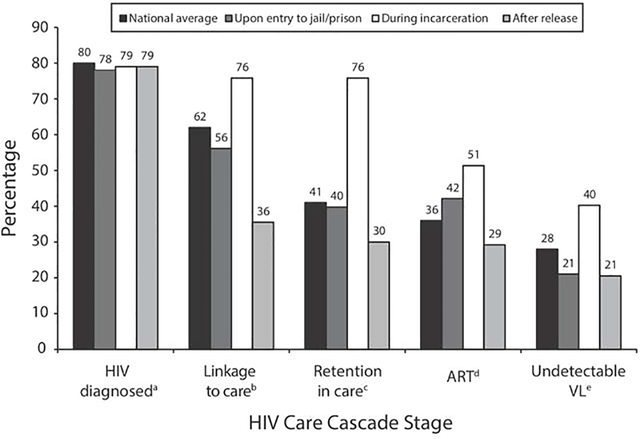
A substantial proportion of people living with HIV/AIDS (PLWHA) in correctional institutions were not engaged in HIV care prior to arrest.58 For these individuals, arrest and subsequent incarceration therefore represents an opportunity for engagement or re-engagement with ARV therapy and other indicated preventive measures. As a consequence, providers of HIV care in correctional settings must be vigilant for signs of immune reconstitution inflammatory syndrome (IRIS). PLWHA have relatively consistent access to ARVs while incarcerated, and adherence can be enhanced by administering ARVs under daily direct observation if requested by a provider. As a result, for PLWHA with CJI, rates of retention in HIV care and viral suppression during incarceration are significantly higher than in the community (Figure 4).58
Figure 4: Care cascade for HIV before, during and after incarceration.
(From Iroh PA., Mayo H., Nijhawan AE. The HIV Care Cascade Before, During, and After Incarceration: A Systematic Review and Data Synthesis. American Journal of Public Health 2015;105(7):e5–16; with permission.)
Unfortunately, however, the stability in HIV virologic control associated with incarceration proves fragile, and rates of viral suppression return to pre-arrest levels after release.58 Individuals reentering the community from jail or prison face a number of complex and simultaneous challenges, including: establishing stable housing, finding employment, reconnecting with friends and loved ones and meeting requirements for parole, all while re-engaging with community-based medical providers, and reactivating or re-enrolling health insurance coverage.59–61 These competing demands can provoke anxiety, exacerbating the cue-associated cravings that are often stimulated by returning to prior settings of substance use. Furthermore, the lack of patient autonomy in correctional settings can, in our experience, create a dependence on institutional support (e.g. directly observed medication administration) that is not available in the community. Thus, interruptions in ARV coverage are common following release from incarceration. In a landmark study of PLWHA being released from the state prison system in Texas during the period of 2004–2007, only 5.4% of patients succeeded in filling an ARV prescription within the first 10 days of release and 30.0% within 60 days.62 Enrolling in an AIDS Drug Assistance Program (ADAP) prior to release can reduce the chance of treatment interruption.
Peer navigation has emerged as one of the most effective interventions to improve retention in HIV care and ARV adherence upon release.63 In a large randomized trial of a structured, 12-session behavioral intervention administered by a peer-navigator among HIV-positive patients being released from the Los Angeles County Jail, viral suppression was maintained in 49.6% of patients receiving the intervention, compared with 36% of controls who receiving standard transitional case management alone.64 Of note, the intervention was equally effective in people who use substances and those who did not.64 Similar outcomes have been reported among HIV-positive patients receiving care in specialized “transitions clinics,” which integrate peer navigators with lived experience of the criminal justice system into comprehensive, patient-centered primary care.61 However, peer-navigation services are available in only a select few jurisdictions and community clinics around the United States. The CDC recommends that prior to release, correctional facilities make an appointment with a community healthcare provider, assist with enrollment in an entitlement program, and provide a copy of the medical record and a supply of HIV medications.55 Advocating for correctional systems to suspend, rather than terminate Medicaid enrollment for individuals on intake can also reduce interruptions in HIV care.65
Chronic HCV infection is substantially more prevalent than HIV, but screening for HCV is less common and access to treatment is currently very limited.66 The prevalence of HCV varies regionally, but estimates range between 40% and 80% among PWID.67,68 In state prison populations, HCV seroprevalence estimates range from 6% in Idaho to 40% in New Mexico.66 Novel direct-acting antiviral (DAA) therapies are well-tolerated and highly effective, but their cost is limiting or prohibitive for most correctional health systems (even if cost-effective for society as a whole).69 As the cost of treatment begins to decline with competition from newer treatment options, HCV treatment in jails and prisons is rapidly expanding.70 Following guidelines from the Federal Bureau of Prisons, DAA therapy is often prioritized for patients with higher degrees of hepatic fibrosis due to resource constraints.71 The degree of fibrosis can be assessed with easy to calculate clinical scores, such as the AST to Platelet Ratio Index or Fibrosis-4 score, or with more costly––but more precise––non-invasive measures such as transient elastography and direct biomarkers when available. Given the expense of the medications, administering them as directly observed therapy (DOT) is reasonable and may improve adherence.72
Treatment outcomes in correctional settings are comparable to published results from community treatment programs with reported rates of sustained viral response generally above 90%.70,73 However, release from incarceration or transfer to another correctional facility prior to completion of DAA therapy remains a barrier to successful treatment.70 As with HIV, post-release linkage to care for HCV is challenging and marked by high rates of loss-to-follow up.70,74 Early studies of peer navigation for HCV treatment following release from incarceration have been less promising than with HIV,74 and given these challenges, universal HCV screening at intake to correctional facilities remains relatively low.75 However, expanded screening may be on the horizon, given the recent recommendation by the United States Preventive Services Task Force that all adults be screened for HCV regardless of age or known risk factors.76
Opportunities for preventive care
Though it may have deleterious effects for individuals who are already made vulnerable by overlapping social and structural factors, an episode of CJI also presents an opportunity to provide preventive health needs that are often underutilized by people with OUD and other SUDs (Table 1). CDC guidelines recommend screening all PWID for sexually transmitted infections, HIV, chronic hepatitis B virus (HBV), chronic HCV and latent tuberculosis infection (LTBI).77 Universal screening for LTBI with either tuberculin skin testing or interferon-gamma release assays on intake is standard in most correctional facilities.78 All people with OUD who are not known to be either immune or actively infected should be vaccinated against hepatitis A and hepatitis B. All people with OUD who are hepatitis B surface antibody negative and not found to have evidence of chronic HBV infection should receive vaccination, including individuals with isolated hepatitis B core antibody who remain at risk of infection. Up-to-date booster dosing with tetanus and diphtheria toxoids every 10 years should be maintained for all incarcerated adults,79 and PWID are at increased risk of exposure to tetanus.
Patient-centered care
Due to fear of criminal sanctions for ongoing opioid use and widespread stigma experienced by people who use drugs in healthcare and criminal justice settings, patients with OUD may exhibit substantial mistrust toward medical providers.80,81 Approaching a patient with warmth, asking about their substance use in a non-judgmental fashion and reinforcing protections for the confidentiality of health information may help to overcome these barriers and facilitate meaningful engagement. Formal harm reduction services (e.g. syringe exchange, condom distribution, etc.) may be limited in correctional settings, but taking a harm reduction approach is possible with any patient (see Table 1). The provision of trauma-informed care is another key principle guiding healthcare engagement for patients with OUD. Trauma and PTSD are highly prevalent among people with addiction,17 as well as those with CJI.15,82 Training staff to recognize and de-escalate behavioral manifestations of trauma-related psychological symptoms can promote a healthcare environment that emphasizes safety and trust.83 Ultimately, providing comprehensive, patient-centered care for people with OUD in settings of incarceration requires a willingness to promote health within a system defined by deprivation and advocate for the community standard of care (see Figures 1 and 2).
Synopsis.
This article provides an overview of the diagnosis and management of opioid use disorder and its infectious complications among populations with criminal justice involvement. Opioid use disorder and chronic infections such as HIV and hepatitis C virus are highly prevalent among incarcerated individuals and some of the unique features of correctional facilities present challenges for their appropriate medical management. We outline evidence-based strategies for integrated, patient-centered treatment during incarceration and the potentially hazardous transition back to the community upon release.
Key points.
Opioid use disorder and its infectious complications are highly prevalent among criminal justice populations
Incarcerated patients with opioid use disorder, HIV and hepatitis C virus should be provided with medical treatment in line with the community standard of care
For opioid use disorder and chronic infections, screening practices and access to medications vary widely among correctional facilities
The transition from a correctional setting to the community represents a highly vulnerable period, marked by high rates of relapse to substance use, loss to medical follow up for HIV and other infections, and fatal overdose.
A patient-centered approach can promote health and improve engagement with medical treatment for opioid use disorder and its infectious complications.
Acknowledgments
Disclosures: The authors have no conflicts of interest to disclose. This work was supported in part by grants from the National Institute of Mental Health [T32 MH 019139-30] to DW and from the National Institute on Drug Abuse [R34 DA 045592] to AN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Longo DL., Compton WM, Jones CM, et al. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. New England Journal of Medicine 2016;374(2):154–63. Doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NIDA Overdose Death Rates. National Institute on Drug Abuse. Available at: NIDA. “Overdose Death Rates.” National Institute on Drug Abuse, 29 Jan. 2019, https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates Accessed 10 Dec. 2019 Accessed December 10, 2019. [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) HIV and AIDS--United States, 1981–2000. MMWR Morb Mortal Wkly Rep 2001;50(21):430–4. [PubMed] [Google Scholar]
- 4.Scholl L, Seth P, Kariisa M, et al. Drug and Opioid-Involved Overdose Deaths — United States, 2013–2017. MMWR Morb Mortal Wkly Rep 2018;67(5152). Doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zibbell JE., Asher AK, Patel RC, et al. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health 2018;108(2):175–81. Doi: 10.2105/AJPH.2017.304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schranz AJ., Fleischauer A, Chu VH, et al. Trends in Drug Use-Associated Infective Endocarditis and Heart Valve Surgery, 2007 to 2017: A Study of Statewide Discharge Data. Ann Intern Med 2019;170(1):31–40. Doi: 10.7326/M18-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szalavitz M What the media gets wrong about opioids. Columbia Journalism Review 2018. [Google Scholar]
- 8.Brico E A dangerous fentanyl myth lives on. Columbia Journalism Review 2019. [Google Scholar]
- 9.Winkelman TNA., Chang VW, Binswanger IA Health, Polysubstance Use, and Criminal Justice Involvement Among Adults With Varying Levels of Opioid Use. JAMA Netw Open 2018;1(3):e180558 Doi: 10.1001/jamanetworkopen.2018.0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han B, Compton WM, Blanco C, et al. Prescription Opioid Use, Misuse, and Use Disorders in U.S. Adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 2017;167(5):293–301. Doi: 10.7326/M17-0865. [DOI] [PubMed] [Google Scholar]
- 11.Kaeble D, Cowhig M Correctional Populations in the United States, 2016. 2016:14. [Google Scholar]
- 12.Bureau of Justice Statistics of the United States Department of Justice. Criminal Justice System Description. Available at: https://www.bjs.gov/content/justsys.cfm Accessed December 20, 2019.
- 13.Maruschak LM., Berzofsky M Medical Problems of State and Federal Prisoners and Jail Inmates, 2011–12. Bureau of Justice Statistics, United States Department of Justice; 2015. [Google Scholar]
- 14.CASA Columbia Behind Bars II: Substance Abuse and America’s Prison Population. New York, NY: The National Center on Addiction and Substance Abuse at Columbia University; 2010. [Google Scholar]
- 15.Wolff N, Huening J, Shi J, et al. Trauma exposure and posttraumatic stress disorder among incarcerated men. J Urban Health 2014;91(4):707–19. Doi: 10.1007/s11524-014-9871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rold WJ. Thirty Years After Estelle v. Gamble: A Legal Retrospective. J Correct Health Care 2008;14(1):11–20. Doi: 10.1177/1078345807309616. [DOI] [Google Scholar]
- 17.Singer M A Dose of Drugs, a Touch of Violence, a Case af AIDS: Conceptualizing the SAVA Syndemic. Free Inq Creat Sociol 1996;24(2):99–110. [Google Scholar]
- 18.Binswanger IA., Blatchford PJ, Mueller SR, et al. Mortality After Prison Release: Opioid Overdose and Other Causes of Death, Risk Factors, and Time Trends From 1999 to 2009. Annals of Internal Medicine 2013;159(9):592 Doi: 10.7326/0003-4819-159-9-201311050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood E, Li K, Small W, et al. Recent Incarceration Independently Associated with Syringe Sharing by Injection Drug Users. Public Health Reports (1974-) 2005;120(2):150–6. Doi: 10.2307/20056766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone J, Fraser H, Lim AG, et al. Incarceration history and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. The Lancet Infectious Diseases 2018;18(12):1397–409. Doi: 10.1016/S1473-3099(18)30469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen SA., Aburabi R When security and medicine missions conflict: confidentiality in prison settings. Intl Jnl of Prisoner Health 2016;12(2):73–7. Doi: 10.1108/IJPH-03-2016-0007. [DOI] [PubMed] [Google Scholar]
- 22.Rappaport ES., Reynolds HN, Baucom S, et al. Telehealth Support of Managed Care for a Correctional System: The Open Architecture Telehealth Model. Telemedicine and E-Health 2018;24(1):54–60. Doi: 10.1089/tmj.2016.0275. [DOI] [PubMed] [Google Scholar]
- 23.Glowa-Kollisch S, Graves J, Dickey N, et al. Data-Driven Human Rights: Using Dual Loyalty Trainings to Promote the Care of Vulnerable Patients in Jail. Health and Human Rights 2015;17(1):124 Doi: 10.2307/healhumarigh.17.1.124. [DOI] [PubMed] [Google Scholar]
- 24.Nunn A, Zaller N, Dickman S, et al. Methadone and buprenorphine prescribing and referral practices in US prison systems: Results from a Nationwide Survey. Drug and Alcohol Dependence 2009;105(1–2):83–8. Doi: 10.1016/j.drugalcdep.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedmann PD., Hoskinson R, Gordon M, et al. Medication-Assisted Treatment in Criminal Justice Agencies Affiliated with the Criminal Justice-Drug Abuse Treatment Studies (CJ-DATS): Availability, Barriers, and Intentions. Substance Abuse 2012;33(1):9–18. Doi: 10.1080/08897077.2011.611460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comer S, Cunningham C, Fishman MJ, et al. National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Rockville, MD: American Society of Addiction Medicine; 2015. [Google Scholar]
- 27.Taylor K Jail Ordered to Give Inmate Methadone for Opioid Addiction in Far-Reaching Ruling. The New York Times; 2018. [Google Scholar]
- 28.Wickersham JA., Azar MM, Cannon CM, et al. Validation of a Brief Measure of Opioid Dependence: The Rapid Opioid Dependence Screen (RODS). J Correct Health Care 2015;21(1):12–26. Doi: 10.1177/1078345814557513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke JG., Martin RA, Gresko SA, et al. The First Comprehensive Program for Opioid Use Disorder in a US Statewide Correctional System. Am J Public Health 2018;108(10):1323–5. Doi: 10.2105/AJPH.2018.304666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rich JD., McKenzie M, Larney S, et al. Methadone continuation versus forced withdrawal on incarceration in a combined US prison and jail: a randomised, open-label trial. The Lancet 2015;386(9991):350–9. Doi: 10.1016/S0140-6736(14)62338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedrich D, Alves P, Farrell M, et al. The effectiveness of opioid maintenance treatment in prison settings: a systematic review: Opioid maintenance in prison. Addiction 2012;107(3):501–17. Doi: 10.1111/j.1360-0443.2011.03676.x. [DOI] [PubMed] [Google Scholar]
- 32.Brinkley-Rubinstein L, Peterson M, Clarke J, et al. The benefits and implementation challenges of the first state-wide comprehensive medication for addictions program in a unified jail and prison setting. Drug and Alcohol Dependence 2019;205:107514 Doi: 10.1016/j.drugalcdep.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Green TC., Clarke J, Brinkley-Rubinstein L, et al. Postincarceration Fatal Overdoses After Implementing Medications for Addiction Treatment in a Statewide Correctional System. JAMA Psychiatry 2018;75(4):405 Doi: 10.1001/jamapsychiatry.2017.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Committee on Medication-Assisted Treatment for Opioid Use Disorder, Board on Health Sciences Policy, Health and Medicine Division, et al. Medications for Opioid Use Disorder Save Lives. Washington, D.C.: National Academies Press; 2019. [PubMed] [Google Scholar]
- 35.Strang J Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ 2003;326(7396):959–60. Doi: 10.1136/bmj.326.7396.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haber PS., Demirkol A, Lange K, et al. Management of injecting drug users admitted to hospital. Lancet 2009;374(9697):1284–93. Doi: 10.1016/S0140-6736(09)61036-9. [DOI] [PubMed] [Google Scholar]
- 37.Maradiaga JA., Nahvi S, Cunningham CO, et al. “I Kicked the Hard Way. I Got Incarcerated.” Withdrawal from Methadone During Incarceration and Subsequent Aversion to Medication Assisted Treatments. Journal of Substance Abuse Treatment 2016;62:49–54. Doi: 10.1016/j.jsat.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell SG., Kelly SM, Brown BS, et al. Incarceration and opioid withdrawal: the experiences of methadone patients and out-of-treatment heroin users. J Psychoactive Drugs 2009;41(2):145–52. Doi: 10.1080/02791072.2009.10399907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon MS., Kinlock TW, Schwartz RP, et al. A randomized clinical trial of methadone maintenance for prisoners: findings at 6 months post-release. Addiction 2008;103(8):1333–42. Doi: 10.1111/j.1360-0443.2008.002238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JD., Friedmann PD, Kinlock TW, et al. Extended-Release Naltrexone to Prevent Opioid Relapse in Criminal Justice Offenders. N Engl J Med 2016;374(13):1232–42. Doi: 10.1056/NEJMoa1505409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aloe J Vermont prison staff will carry opioid rescue medication Narcan after rise in overdoses. Burlington Free Press; Available at: https://www.burlingtonfreepress.com/story/news/2019/01/22/vermont-prisons-expand-access-overdose-reversal-drug-naloxone/2579110002/ Accessed December 17, 2019. [Google Scholar]
- 42.Zucker H, Annucci AJ, Stancliff S, et al. Overdose prevention for prisoners in New York: a novel-program and collaboration. Harm Reduct J 2015;12(1):51, s12954–015-0084–8. Doi: 10.1186/s12954-015-0084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodnough A, Zernike K Seizing on Opioid Crisis, a Drug Maker Lobbies Hard for Its Product. The New York Times; 2017. [Google Scholar]
- 44.Larochelle MR., Bernson D, Land T, et al. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality: A Cohort Study. Ann Intern Med 2018;169(3):137 Doi: 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Binswanger IA., Glanz JM Potential Risk Window for Opioid Overdose Related to Treatment with Extended-Release Injectable Naltrexone. Drug Saf 2018;41(10):979–80. Doi: 10.1007/s40264-018-0705-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon MS., Kinlock TW, Schwartz RP, et al. Buprenorphine Treatment for Probationers and Parolees. Substance Abuse 2015;36(2):217–25. Doi: 10.1080/08897077.2014.902787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Springer SA., Qiu J, Saber-Tehrani AS, et al. Retention on Buprenorphine Is Associated with High Levels of Maximal Viral Suppression among HIV-Infected Opioid Dependent Released Prisoners. PLoS ONE 2012;7(5):e38335 Doi: 10.1371/journal.pone.0038335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Springer SA., Korthuis PT, Del Rio C Integrating Treatment at the Intersection of Opioid Use Disorder and Infectious Disease Epidemics in Medical Settings: A Call for Action After a National Academies of Sciences, Engineering, and Medicine Workshop. Ann Intern Med 2018;169(5):335–6. Doi: 10.7326/M18-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young JD., Patel M, Badowski M, et al. Improved virologic suppression with HIV subspecialty care in a large prison system using telemedicine: an observational study with historical controls. Clin Infect Dis 2014;59(1):123–6. Doi: 10.1093/cid/ciu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arora S, Thornton K, Jenkusky SM, et al. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep 2007;122 Suppl 2:74–7. Doi: 10.1177/00333549071220S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moazen B, Saeedi Moghaddam S, Silbernagl MA, et al. Prevalence of Drug Injection, Sexual Activity, Tattooing, and Piercing Among Prison Inmates. Epidemiol Rev 2018;40(1):58–69. Doi: 10.1093/epirev/mxy002. [DOI] [PubMed] [Google Scholar]
- 52.Paladino JA., Poretz D Outpatient parenteral antimicrobial therapy today. Clin Infect Dis 2010;51 Suppl 2:S198–208. Doi: 10.1086/653520. [DOI] [PubMed] [Google Scholar]
- 53.Paris JE. Infirmary Care Correctional Medicine. St. Louis, MO: Mosby; 1998. p. 77–85. [Google Scholar]
- 54.Springer SA., Altice FL Managing HIV/AIDS in correctional settings. Curr HIV/AIDS Rep 2005;2(4):165–70. Doi: 10.1007/s11904-005-0011-9. [DOI] [PubMed] [Google Scholar]
- 55.Beckwith C, Bick J, Chow W, et al. HIV Testing Implementation Guidance for Correctional Settings. Atlanta, GA: Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 56.Solomon L, Montague BT, Beckwith CG, et al. Survey Finds That Many Prisons And Jails Have Room To Improve HIV Testing And Coordination Of Postrelease Treatment. Health Affairs 2014;33(3):434–42. Doi: 10.1377/hlthaff.2013.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huh K, Boucher A, McGaffey F, et al. Pharmaceuticals in State Prisons: How departments of corections purchase, use and monitor prescription drugs. Philadelphia, PA: The Pew Charitable Trusts; 2017. [Google Scholar]
- 58.Iroh PA., Mayo H, Nijhawan AE The HIV Care Cascade Before, During, and After Incarceration: A Systematic Review and Data Synthesis. American Journal of Public Health 2015;105(7):e5–16. Doi: 10.2105/AJPH.2015.302635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Olphen J, Freudenberg N, Fortin P, et al. Community Reentry: Perceptions of People with Substance Use Problems Returning Home from New York City Jails. J Urban Health 2006;83(3):372–81. Doi: 10.1007/s11524-006-9047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Visher C, LaVigne N, Travis J Returning Home: Understanding the Challenges of Prisoner Reentry: Maryland Pilot Study: Findings from Baltimore: (720382011–001) 2004. Doi: 10.1037/e720382011-001. [DOI] [Google Scholar]
- 61.Masyukova MI., Hanna DB, Fox AD HIV treatment outcomes among formerly incarcerated transitions clinic patients in a high prevalence setting. Health Justice 2018;6(1):16 Doi: 10.1186/s40352-018-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baillargeon J, Giordano TP, Rich JD, et al. Accessing Antiretroviral Therapy Following Release From Prison. JAMA 2009;301(8):848 Doi: 10.1001/jama.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Westergaard RP., Hochstatter KR, Andrews PN, et al. Effect of Patient Navigation on Transitions of HIV Care After Release from Prison: A Retrospective Cohort Study. AIDS Behav 2019;23(9):2549–57. Doi: 10.1007/s10461-019-02437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cunningham WE., Weiss RE, Nakazono T, et al. Effectiveness of a Peer Navigation Intervention to Sustain Viral Suppression Among HIV-Positive Men and Transgender Women Released From Jail: The LINK LA Randomized Clinical Trial. JAMA Internal Medicine 2018;178(4):542 Doi: 10.1001/jamainternmed.2018.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boutwell AE., Freedman J Coverage expansion and the criminal justice-involved population: implications for plans and service connectivity. Health Aff (Millwood) 2014;33(3):482–6. Doi: 10.1377/hlthaff.2013.1131. [DOI] [PubMed] [Google Scholar]
- 66.Spaulding AC., Anderson EJ, Khan MA, et al. HIV and HCV in U.S. Prisons and Jails: The Correctional Facility as a Bellwether Over Time for the Community’s Infections. AIDSRev 2017;19(3):301 Doi: 10.24875/AIDSRev.M17000006. [DOI] [PubMed] [Google Scholar]
- 67.Lelutiu-Weinberger C, Pouget ER, Des Jarlais DDC, et al. A meta-analysis of the hepatitis C virus distribution in diverse racial/ethnic drug injector groups. Social Science & Medicine 2009;68(3):579–90. Doi: 10.1016/j.socscimed.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Des Jarlais DC., Arasteh K, Feelemyer J, et al. Hepatitis C virus prevalence and estimated incidence among new injectors during the opioid epidemic in New York City, 2000–2017: Protective effects of non-injecting drug use. Drug and Alcohol Dependence 2018;192:74–9. Doi: 10.1016/j.drugalcdep.2018.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen JT., Rich JD, Brockmann BW, et al. A Budget Impact Analysis of Newly Available Hepatitis C Therapeutics and the Financial Burden on a State Correctional System. J Urban Health 2015;92(4):635–49. Doi: 10.1007/s11524-015-9953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chan J Outcomes of Hepatitis C Virus Treatment in a Jail Population: Successes and Challenges Facing Expansion. Las Vegas, NV; 2019. [Google Scholar]
- 71.Federal Bureau of Prisons Evaluation and Management of Chronic Hepatitis C Virus (HCV) Infection. Federal Bureau of Prisons; 2018. [Google Scholar]
- 72.McDermott CL., Lockhart CM, Devine B Outpatient directly observed therapy for hepatitis C among people who use drugs: a systematic review and meta-analysis. J Virus Erad 2018;4(2):118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sterling RK., Cherian R, Lewis S, et al. Treatment of HCV in the Department of Corrections in the Era of Oral Medications. J Correct Health Care 2018;24(2):127–36. Doi: 10.1177/1078345818762591. [DOI] [PubMed] [Google Scholar]
- 74.Akiyama MJ., Columbus D, MacDonald R, et al. Linkage to hepatitis C care after incarceration in jail: a prospective, single arm clinical trial. BMC Infect Dis 2019;19(1):703 Doi: 10.1186/s12879-019-4344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spaulding A, Chhatwal J, Thanthong-Knight S, et al. HepCorrections Available at: http://www.hepcorrections.org/ Accessed December 18, 2019.
- 76.United States Preventive Services Task Force Draft Recommendation Statement: Hepatitis C Virus Infection in Adolescents and Adults: Screening - US Preventive Services Task Force; Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement/hepatitis-c-screening1 Accessed January 14, 2020. [Google Scholar]
- 77.Centers for Disease Control and Prevention (CDC) Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the U.S. Department of Health and Human Services. MMWR Recomm Rep 2012;61(RR-5):1–40. [PubMed] [Google Scholar]
- 78.Nijhawan AE., Iroh PA, Brown LS, et al. Cost analysis of tuberculin skin test and the QuantiFERON-TB Gold In-tube test for tuberculosis screening in a correctional setting in Dallas, Texas, USA. BMC Infect Dis 2016;16(1):564 Doi: 10.1186/s12879-016-1901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang JL., Tiwari T, Moro P, et al. Prevention of Pertussis, Tetanus, and Diphtheria with Vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2018;67(2):1–44. Doi: 10.15585/mmwr.rr6702a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McNeil R, Small W, Wood E, et al. Hospitals as a “risk environment”: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med 2014;105:59–66. Doi: 10.1016/j.socscimed.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paquette CE., Syvertsen JL, Pollini RA Stigma at every turn: Health services experiences among people who inject drugs. International Journal of Drug Policy 2018;57:104–10. Doi: 10.1016/j.drugpo.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jäggi LJ., Mezuk B, Watkins DC, et al. The Relationship between Trauma, Arrest, and Incarceration History among Black Americans: Findings from the National Survey of American Life. Society and Mental Health 2016;6(3):187–206. Doi: 10.1177/2156869316641730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chaudhri S, Zweig KC, Hebbar P, et al. Trauma-Informed Care: a Strategy to Improve Primary Healthcare Engagement for Persons with Criminal Justice System Involvement. J GEN INTERN MED 2019;34(6):1048–52. Doi: 10.1007/s11606-018-4783-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Centers for Disease Control and Prevention (CDC), National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Prevention and control of tuberculosis in correctional and detention facilities: recommendations from CDC. Endorsed by the Advisory Council for the Elimination of Tuberculosis, the National Commission on Correctional Health Care, and the American Correctional Association. MMWR Recomm Rep 2006;55(RR-9):1–44. [PubMed] [Google Scholar]