Abstract
Background
Studies on the effect of high-intensity interval training (HIT) compared with moderate intensity continuous training (MICT) on health-related quality of life (HRQoL) after heart transplantation (HTx) is scarce. No available studies among de novo HTx recipients exists. This study aimed to investigate the effect of HIT vs. MICT on HRQoL in de novo recipients.
Methods
The HITTS study randomized eighty-one de novo HTx recipients to receive either HIT or MICT (1:1). The HIT intervention were performed with 2–4 interval bouts with an intensity of 85–95% of maximal effort. The MICT group exercised at an intensity of 60–80% of their maximal effort with a duration of 25 min. HRQoL was assessed by the Short Form-36 version 2 (SF-36v2) and the Hospital Anxiety and Depression Scale, mean 11 weeks after surgery and after a nine months’ intervention. The participants recorded their subjective effect of the interventions on their general health and well-being on a numeric visual analogue scale. Clinical examinations and physical tests were performed. Differences between groups were investigated with independent Student t-tests and with Mann-Whitney U tests where appropriate. Within-group differences were analyzed with Paired-Sample t-tests and Wilcoxon Signed Rank tests. Correlations between SF-36 scores and VO2peak were examined with Pearson’s correlations.
Results
Seventy-eight participants completed the intervention. Both exercise modes were associated with improved exercise capacity on the physical function scores of HRQoL. Mental health scores remained unchanged. No differences in the change in HRQoL between the groups occurred except for Role Emotional subscale with a larger increase in the HIT arm. Better self-reported physical function was associated with higher VO2peak and muscle strength.
Conclusion
HIT and MICT resulted in similar mean changes in HRQoL the first year after HTx. Both groups experienced significant improvements in the physical SF-36v2.
Trial registration
ClinicalTrials.gov number: NCT01796379 Registered 18 February 2013.
Keywords: Health-related quality of life; Heart transplantation; High-intensity interval training; Moderate intensity continuous training; Oxygen consumption; Muscle strength,self-reported physical function; Exercise
Background
Heart transplantation (HTx) is the preferred therapy for selected patients with end-stage heart failure [1]. To improve physical capacity and health-related quality of life (HRQoL), cardiac rehabilitation is an integrated component in most HTx programs.
HRQoL is impaired prior to transplantation [2–4]. Longitudinal studies have reported that HRQoL improves significantly after HTx, with the greatest improvement occurring during the first half year [2, 5, 6]. Most of the studies assessing long-term HRQoL after HTx have shown that HRQoL remains good up to five, [2, 7] ten [8] and up to 20 [9] years after transplant.
The physical domains in HRQoL are lower in HTx recipients than in the general population [1, 10], while the mental health domains has been found comparable to the general population [7, 8]. The physical functioning subscale in the Short-Form-36 (SF-36v2) is related to peak oxygen consumption (VO2peak), reflecting an association between self-reported physical function and objective measurements [11, 12]. The impact of exercise capacity on HRQoL has been studied at different time points after HTx [11–21]. Studies have found an association between improved exercise capacity and HRQoL [11, 19], but the effect of different exercise modes on HRQoL is unclear [1], mainly due to lack of high-quality studies. Only one small study has examined the effect of high-intensity interval training (HIT) vs. moderate intensity continuous training (MICT) on HRQoL in maintenance HTx recipients, but found no difference between the two groups [13]. The effect of HIT vs. MICT on HRQoL in newly heart transplanted recipients has not been studied, but these patients may have a greater potential for improvement in HRQoL [1].
The aim of this study was to investigate the effect of HIT vs. MICT the first year after heart transplantation. We hypothesized that HIT would improve HRQoL more than MICT in de novo HTx recipients.
Methods
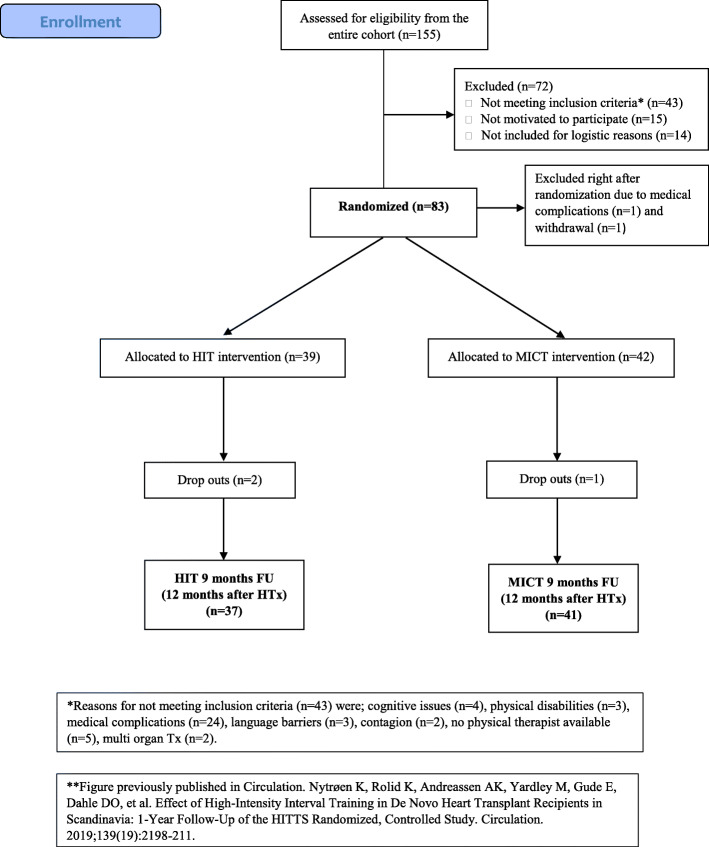
The study-design and other results has been described earlier [22, 23]. In short, it was a multi-center, randomized controlled trial comparing HIT vs. MICT in adult, consenting de novo HTx recipients. The trial was conducted at three transplant-centers in Scandinavia. The primary endpoint for the overall project was the change in VO2peak, while the prespecified endpoint for this substudy was the change in HRQoL. Eighty-one participants were included 7–16 weeks after HTx, and 78 were retested after nine months (Fig. 1). A permuted block randomization list was computer generated by a third party. Numbered sealed envelopes detailing the individual treatment allocation was prepared based on this list. Participants were assigned a randomization number at inclusion. After the CPET test at baseline, the envelope was opened and the patient was allocated to HIT or MICT.
Fig. 1.
Patient recruitment and follow-up
Exercise intervention
The intervention is described elsewhere [22, 23]. Briefly, the participants were randomized 1:1, to either nine months of HIT or MICT at 11 ± 2 weeks after HTx. Participants in both groups exercised 2–3 times per week in the 9-month long intervention. The HIT consisted of 2–4 interval bouts at an intensity of 85–95% of maximal effort (corresponding to a rated perceived exertion (RPE) of 16–18). Between the HIT bouts, there was an active rest period (RPE 11–13). The goal for the HIT group was to be able to perform 4 interval bouts of 4 min length in the last intervention period. The MICT group followed the standard-of care exercise recommendations in recently HTx recipients, with an exercise intensity of 60–80% of maximal effort (corresponding to an RPE of 12–15) for a duration of 25 min. Both interventions included a 10 min warm up and a cool-down period of 5 min at the end of the exercise session. In addition, both groups performed strength training. All exercise sessions were performed in the participants’ local communities, supervised by health personnel and all exercise sessions in both groups were logged and monitored with a heart rate monitor. Of 72 planned sessions, the HIT group completed median (interquartile range (IR)) 60 (28) sessions and the MICT group completed 56 (37) (p for difference 0.858).
Self-reported questionnaires
HRQoL was assessed by the generic questionnaire SF-36v2, [24] frequently used in HTx populations [1, 25]. The SF-36 is divided into eight subscales; Physical Functioning, Role Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role Emotional and Mental Health. The eight subscales aggregate into two summary scores; a Physical Component and a Mental Component; higher score indicating better HRQoL. In this study, all scores were transformed to norm-based values with a standardized mean of 50 and a standard deviation (SD) of 10. A change of 2–4 points on any item is considered to be of clinical significance [24].
Symptoms of anxiety and depression were measured with the Hospital Anxiety and Depression Scale (HADS) [26]. The participants’ socio-demographic background was assessed by a simple questionnaire at baseline and at follow-up. Additionally, at follow-up, all the participants recorded: “To what extent do you feel participation in this study had a positive impact on your general health and well-being” on a numeric visual analogue scale (VAS), ranging from “not at all” to “to a very great extent”.
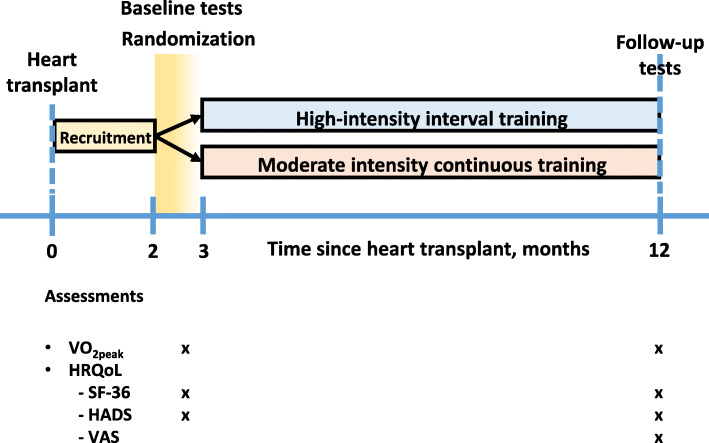
All the questionnaires were self-administered and filled out during the study visits at both time points (Fig. 2). The Physical Functioning subscale from SF-36v2 was selected to represent self-reported physical function.
Fig. 2.
Design of the study
Exercise testing
All participants underwent a cardiopulmonary exercise test (CPET) with measurements of VO2peak at baseline and at follow-up. Most of the Norwegian participants (n = 70) in were tested on a treadmill with breath-by-breath gas analysis (Jaeger® Masterscreen® CPX, Carefusion), and four of the particpants were tested on a bicycle (Schiller Cardiovit® CS-200 Excellence). The participants from Sweden and Denmark (n = 7) were tested on a bicycle (Jaeger®,Oxy Con Pro® and Jaeger® Vyntus® CPX). The CPET tests was performed with an individualized protocol with a gradual increase in workload until exhaustion [22, 27]. Isokinetic muscle strength and muscular exercise capacity in the lower limbs were measured with a dynamometer (Cybex 6000) [22, 23, 28].
Ethics
All participants provided written informed consent prior to inclusion. The study was approved by the regional ethic committees in Norway, Sweden and Denmark. The study is conducted according to the Helsinki Declaration. https://clinicaltrials.gov/ identifier NCT01796379.
Statistics
Continuous data are expressed as mean ± SD, or median (IR). Categorical data are presented as number and percentages. An intention-to-treat analysis were conducted. Differences between the two groups were investigated with independent Student t -tests and with Mann-Whitney U tests where appropriate. The change (delta value) for each participant between baseline and 1-year follow-up was calculated by subtracting the results at 1-year follow-up with the results at baseline [Change = 1-year follow-up – baseline]. The change was assessed by independent t-tests to calculate the mean difference in change between the two groups in normally distributed variables, and by Mann-Whitney U tests for variables with skewed distribution. Within-group differences were analyzed with Paired-Sample t-tests and Wilcoxon Signed Rank tests. We assessed associations between HRQoL scores and parameters reflecting exercise capacity using Pearson’s and Spearman’s correlations. Missing data in the SF-36v2 were handled by the “half-scale” rule, which means that a scale score was calculated if at least half of the items of that specific scale were answered [24]. For the two HADS scales, scores were calculated for those with complete data only. All data were analyzed using IBM SPSS 25.0 (IBM Corporation, United States). P values < 0.05 (two-sided) were considered statistically significant.
Results
Demographic data are provided in Table 1. There were no differences between the intervention arms regarding baseline socio-demographic or clinical characteristics.
Table 1.
Baseline characteristics in the HIT group and the MICT groupa
| Variables | HIT (n = 37) | MICT (n = 41) |
|---|---|---|
| Sex n (%) men | 28 (76) | 29 (71) |
| Age (years) | 50 ± 12 | 48 ± 14 |
| Body Mass Index kg/m2 | 24.8 ± 3.4 | 25.6 ± 3.9 |
| In a relationship (married/cohabitant) | 22 (61) | 30 (73) |
| Employed | 8 (22) | 9 (22) |
| Primary diagnosis n (%) | ||
| CM/CAD/Other | 21 (57) / 14 (38) / 2 (5) | 31 (75) / 6 (15) / 4 (10) |
| Donor age (years) | 37 ± 14 | 39 ± 14 |
| Ischaemic time (min) | 181 ± 77 | 184 ± 82 |
| Median (IR) years of HF duration pre HTx | 4.0 (9.1) | 4.5 (8.1) |
| Median (IR) days on waitlist | 85 (192) | 71 (167) |
| Smoking (n (%) No/Ex-smoker) | 18 (49) / 19 (51) | 21 (51) / 20 (49) |
| Biomarkers | ||
| Hemoglobin (g/dL) | 11.8 ± 1.8 | 11.7 ± 1.6 |
| Creatinine (μmol/L) | 116.1 ± 33.9 | 118.5 ± 28.0 |
| eGFR (mL/min/1.73m2) | 65.1 ± 20.9 | 62.7 ± 23.3 |
| HbA1c (%) | 5.7 ± 0.9 | 5.6 ± 0.7 |
| Medication at inclusion n (%) | ||
| Ciclosporine | 24 (65) | 31 (76) |
| Tacrolimus | 11 (30) | 10 (24) |
| Everolimus | 12 (32) | 13 (32) |
| Prednisolone | 37 (100) | 41 (100) |
| Mycophenolate | 34 (92) | 36 (88) |
| Statin | 36 (97) | 41 (100) |
| Beta blocker | 9 (24) | 12 (30) |
| Calcium blocker | 8 (22) | 12 (30) |
| ACE inhibitor | 0 | 2 (5) |
| ARB | 4 (11) | 3 (8) |
| Diuretics | 31 (84) | 32 (78) |
Variables are presented as mean ± standard deviation, median (interquartile range (IR)) or number (percentages). ACE angiotensin converting enzyme, ARB angiotensin II reseptor blocker, CAD coronary artery disease, CM cardiomyopathy, eGFR estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology Collaboration calculation), HbA1c hemoglobin A1c, HF heart failure, HIT High-intensity interval training, HTx heart transplantation, MICT moderate intensity contionuous training
aNo difference between groups
All HRQoL variables were similar in the two groups at baseline. Symptoms of depression and anxiety were low in both groups at baseline (Table 2).
Table 2.
Baseline and follow-up results in the HIT group and the MICT groupd
| Variables | Baseline | Follow-up HIT (n = 37) |
t-test, P value | Baseline | Follow-up MICT (n = 41) |
t-test, P value |
|---|---|---|---|---|---|---|
| Health-related quality of life SF-36v2 components summaries and subscales | ||||||
| Physical Component Summary (PCS) | 42.2 ± 7.6 | 48.4 ± 9.3 | < 0.001 | 43.2 ± 7.7 | 49.0 ± 8.4 | < 0.001 |
| Mental Component Summary (MCS) | 52.5 ± 12.9 | 53.4 ± 11.9 | 0.673 | 55.1 ± 8.2 | 52.5 ± 9.6 | 0.086 |
| Physical Functioning | 45.0 ± 7.0 | 50.8 ± 6.0 | < 0.001 | 46.4 ± 6.4 | 51.6 ± 6.6 | < 0.001 |
| Role Physical | 37.6 ± 10.4 | 48.1 ± 9.3 | < 0.001 | 40.8 ± 10.0 | 47.0 ± 10.0 | < 0.001 |
| Bodily Pain | 47.8 ± 9.3 | 50.5 ± 10.5 | 0.163 | 48.1 ± 9.2 | 49.1 ± 12.2 | 0.583 |
| General Health | 48.2 ± 9.4 | 50.8 ± 11.0 | 0.067 | 49.8 ± 7.3 | 51.2 ± 9.4 | 0.292 |
| Vitality | 50.6 ± 10.8 | 52.6 ± 12.7 | 0.196 | 51.2 ± 9.4 | 53.6 ± 9.0 | 0.031 |
| Social Functioning | 46.7 ± 9.9 | 50.2 ± 9.1 | 0.047 | 48.7 ± 8.7 | 50.7 ± 9.0 | 0.278 |
| Role Emotional | 46.8 ± 13.1 | 52.0 ± 9.1 | 0.027 | 50.7 ± 7.7 | 48.7 ± 10.5 | 0.246 |
| Mental Health | 53.1 ± 11.0 | 53.7 ± 9.7 | 0.684 | 55.4 ± 7.8 | 54.0 ± 9.7 | 0.232 |
| HADS Anxiety median (IR) | 3.0 (3.5) | 2.0 (4.5) | 0.310a | 3.0 (3.5) | 3.0 (4) | 0.400a |
| HADS Depression median (IR) | 2.0 (3.5) | 2.0 (4.8) | 0.331a | 1.0 (2.5) | 1.0 (3.8) | 0.866a |
| VAS scale (0–100) median (IR)b | 82.0 (20.5) | 75.5 (37.3) | 0.235c | |||
| Cardiopulmonary exercise test | ||||||
| VO2peak (mL/kg/min) | 19.5 ± 4.3 | 24.4 ± 6.5 | < 0.001 | 21.3 ± 5.3 | 24.4 ± 6.7 | < 0.001 |
| % of predicted VO2peak | 53.3 ± 11.6 | 66.6 ± 15.4 | < 0.001 | 58.4 ± 12.5 | 66.9 ± 14.8 | < 0.001 |
| RPE (Borg scale score) | 18.7 ± 0.5 | 18.8 ± 0.6 | 0.290 | 18.5 ± 1.1 | 18.8 ± 0.7 | 0.098 |
| RER | 1.17 ± 0.11 | 1.19 ± 0.1 | 0.314 | 1.22 ± 0.13 | 1.22 ± 0.1 | 0.751 |
| Muscular capacity | ||||||
| Maximal muscle strength extensors (Newton meter) | 184 ± 74 | 237 ± 81 | < 0.001 | 186 ± 73 | 222 ± 80 | < 0.001 |
| Muscular exercise capacity extensors (Joule) | 2154 ± 952 | 3170 ± 1267 | < 0.001 | 2319 ± 1201 | 2870 ± 1240 | < 0.001 |
Health-related quality of life, exercise capacity and muscular strength at baseline (~ 11 weeks after HTx and at 9 months intervention (first yearly annual follow-up). Variables are presented as mean ± standard deviation or median (Interquartile range (IR)).HADS Hospital Anxiety and Depression Scale, HIT High-intensity interval training, MICT moderate intensity contionuous training, RER Respiratory exchange ratio, RPE Rated perceived exertion, VAS visual analogue scale
aWilcoxon Signed Rank Test
bMeasured at follow-up only
cMann-Whitney U test (difference between groups at follow-up)
dNo difference between groups at baseline
During the intervention, the scores for the SF-36v2 subscales Physical Functioning and Role Physical improved significantly in both exercise arms (Table 2). The improvement in these scales exceeded two points, which is regarded a clinically important difference [24]. Accordingly, the Physical Component Summary scores improved significantly (Table 2). The Mental Component Summary scores were above 50 at baseline, while HADS scores were low. Neither the Mental Component Summary scores nor the HADS scores did change significantly during the intervention period (Table 2).
The participants’ general health and well-being was good, as shown on the VAS scale. At follow-up, the HIT group scored 82 points and the MICT group scored 76 (p for difference = 0.235) (Table 2).
As reported earlier, there was a significant between-group difference in increased VO2peak over the intervention period, in favor of HIT [23] (Table 3). However, there were no differences between the two exercise arms in HRQoL, the main endpoint of this substudy, except on the Role Emotional subscale, which covers the spectrum of mental health-related role constraints related to work or other daily activities [24] (Table 3). Maximal RPE (Borgs scale score) were equal between the two groups and did not change during the intervention period [23] (Table 3).
Table 3.
Comparison of change between the HIT group and the MICT group from baseline to follow-up
| Variables | Change within the HIT group Mean ± SD (n = 37) |
Change within the MICT group Mean ± SD (n = 41) |
Difference in mean change between groups mean [95% CI] |
P value Difference in change between groups |
|---|---|---|---|---|
| Health-related quality of life SF-36v2 components summaries and subscales | ||||
| Physical Component Summary (PCS) | 6.3 ± 8.2** | 5.7 ± 5.7** | 0.6 [− 3.1, 4.2] | 0.762 |
| Mental Component Summary (MCS) | 0.9 ± 12.5 | − 2.6 ± 9.3 | 3.4 [− 1.5, 8.5] | 0.170 |
| Physical Functioning | 5.8 ± 5.6** | 5.2 ± 5.6** | 0.6 [− 2.0, 3.2] | 0.653 |
| Role Physical | 10.5 ± 11.2** | 6.2 ± 10.0** | 4.3 [− 0.6, 9.1] | 0.082 |
| Bodily Pain | 2.7 ± 11.5 | 1.0 ± 11.4 | 1.7 [− 3.5, 6.9] | 0.509 |
| General Health | 2.6 ± 8.3 | 1.4 ± 8.6 | 1.1 [− 2.7, 4.9] | 0.555 |
| Vitality | 2.0 ± 9.2 | 2.6 ± 7.3* | − 0.6 [− 4.3, 3.2] | 0.760 |
| Social Functioning | 3.6 ± 10.5* | 2.0 ± 11.6 | 1.5 [− 3.5, 6.5] | 0.541 |
| Role Emotional | 5.2 ± 13.4* | − 2.0 ± 11 | 7.2 [1.6, 12.8] | 0.012 |
| Mental Health | 0.6 ± 9.0 | − 1.4 ± 7.3 | 2.0 [− 1.7, 5.7] | 0.284 |
| HADS Anxiety | −1.0a | −0.8a | 0.920c | |
| HADS Depression | −1.0b | −0.2a | 0.427c | |
| Cardiopulmonary exercise test | ||||
| VO2peak (mL/kg/min) | 4.8 ± 4.1** | 3.1 ± 3.5** | 1.8 [0.1, 3.5] | 0.044 |
| Improvement in mL/kg/min (%) | 25.2 ± 21.1** | 15.1 ± 17.8** | 10.1 [1.3, 19.0] | 0.025 |
| % of predicted VO2peak | 13.2 ± 10.7** | 8.5 ± 9.1** | 4.7 [0.2, 9.2] | 0.040 |
| RPE (Borg scale score) | 0.1 ± 0.8 | 0.3 ± 1.2 | 0.2 [−0.3, 0.7] | 0.424 |
| RER | 0.02 ± 0.1 | −0.01 ± 0.1 | 0.02 [− 0.03, 0.1] | 0.338 |
| Muscular capacity | ||||
| Maximal muscle strength extensors (Newton meter) | 54 ± 49** | 36 ± 34** | 178 [− 3, 39] | 0.094 |
| Muscular exercise capacity extensors (Joule) | 1016 ± 812** | 551 ± 780** | 464 [63, 863] | 0.024 |
Health-related quality of life, exercise capacity and muscular strength at baseline (~ 11 weeks after HTx and at 9 months intervention (first yearly annual follow-up). Variables are presented as mean ± standard deviation. CI Confidence Interval, HADS Hospital Anxiety and Depression Scale, HIT High-intensity interval training, MICT moderate intensity contionuous training, SD standard deviation, RER Respiratory exchange ratio, RPE Rated perceived exertion, VAS visual analogue scale
Within group: **p < 0.001, *p < 0.05
aBased on negative ranks
bBased on positive ranks
cMann-Whitney U test
There were no differences between groups regarding rejections or serious/adverse events during the intervention period [23].
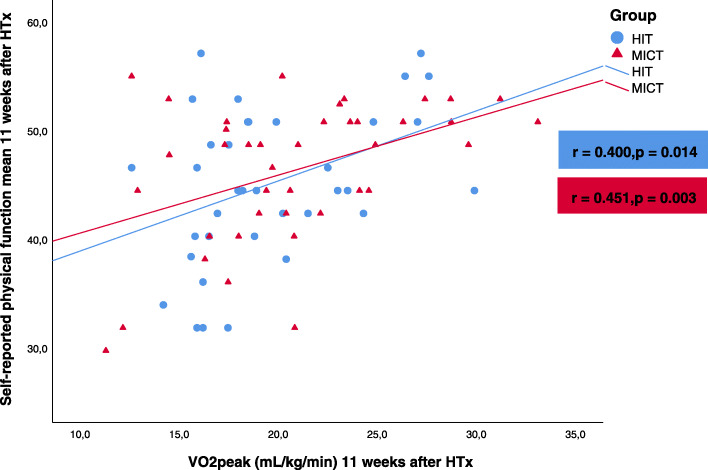
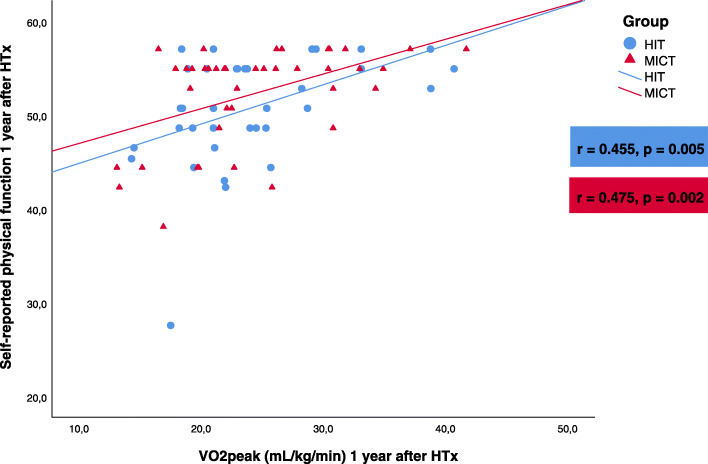
There was a positive correlation between VO2peak and the self-reported physical function in both groups, both at baseline (Fig. 3) and at follow-up (Fig. 4). In the HIT group, we found a modest correlation between the change from baseline to 1-year follow-up in self-reported physical function and the change in VO2peak (Pearson’s r = 0.35, p = 0.03). There was no correlation between the corresponding changes in the MICT group (Pearson’s r = − 0.13, p = 0.41).
Fig. 3.
Correlation between self-reported physical function and VO2peak in the high-intensity training (HIT) group and the moderate intensity continuous training (MICT) group mean 11 weeks after heart transplantation (HTx)
Fig. 4.
Correlation between self-reported physical function and VO2peak in the high-intensity training (HIT) group and the moderate intensity continuous training (MICT) group 1 year after heart transplantation (HTx)
The self-reported physical function also correlated with the extensors’ maximal muscle strength and muscle endurance at both time points in both groups (See Additional File 1, Figs. 1, 2, 3 and 4).
The SF-36 Role Physical scale correlated modestly with VO2peak in both groups at 1-year follow-up. Correlations between other CPET values (heart rate variables, O2 pulse, maximal ventilation, respiratory exchange ratio, RPE) and SF-36 subscales were weak in both groups at both time points. However, there was a moderate correlation between metabolic equivalents and self-reported physical function in both groups at both time points (data not shown).
Missing data in the questionnaires
There was little missing data. At baseline there were 1.3% missing for the following SF-36 subscales; Role Physical, Vitality and Mental Health and 2.6% missing for the Role Emotional subscale and each of the two SF-36 sum scores. At follow-up there were 1.3% missing for all of the SF-36 subscales except of General Health and Social Functioning, while there were 2.6% missing for each of the two SF-36 sum scores and for each of the HADS scores.
Discussion
The main findings in the present study were: 1) In patients who had recently undergone HTx, the Physical Component Scores improved significantly during the nine-months long intervention period, and 2) There were no differences in HRQoL between patients allocated to HIT or MICT, except on the Role Emotional subscale where the HIT group had a significantly higher score.
Maintenance HTx recipients tend to score lower than the general population on the physical function domains of HRQoL [7, 8]. Interventions to improve physical function in HTx recipients are of special interest since improved physical function is associated with better HRQoL [11, 17] and is a strong predictor for survival [12].
In exercise trials comparing HIT with a control group in maintenance HTx recipients, improvements in general health is higher in the intervention groups [14, 15]. These results suggest that exercise has a positive effect on HRQoL in the long term after HTx. In line with our findings, Hsu et al. [16] observed improved HRQoL in the physical function domains of SF-36 after cardiac rehabilitation early after HTx. It should be noticed that neither our study, nor the study by Hsu et al., [16] had a control group without an exercise program. The relatively high HRQoL observed at the end of our trial, and in the study by Hsu et al. [16] may reflect an overall improved health status during the first year after HTx, rather than an effect of exercise alone. For example, Ortega et al. [29] found improvements in SF-36 physical domains over the first year after HTx without an intervention.
To our knowledge, only one prior study has investigated the effect of HIT vs. MICT on HRQoL in HTx recipients [13]. In this crossover trial (n = 16), [13] there were no differences between the groups regarding HRQoL, symptoms of anxiety or depression, which is in line with our results. However, the same study found a significant decrease in symptoms of anxiety in the HIT group, and a significant decrease in symptoms of depression in both groups. This contrasts our study, where symptoms of depression and anxiety were low and stable throughout the intervention period in both groups.
The improvement in the Role Emotional subscale in our patients randomized to HIT may reflect an improved sense of achievement associated with exhaustive exercise, but may also be an incidental finding.
We found correlations between VO2peak and self-reported physical function at both time points, as previously reported in maintenance HTx recipients [19]. The correlation between the change in self-reported physical function and the change in VO2peak from baseline to 1-year follow-up was observed in the HIT group only. This may be due to the higher mean change in VO2peak in the HIT group compared to the MICT group. VO2peak and self-reported physical function are strong predictors for long-term survival after HTx [12]. Obtaining self-reported physical function is less resource-demanding than performing CPET with measurements of VO2peak. However, the correlation between the two is modest, and self-reported physical function cannot fully substitute VO2 measurements in the short and in the longer term after HTx.
Limitations
The high baseline HRQoL scores may reflect an above average healthy population and may also have affected the impact of the intervention on HRQoL. For obvious reasons, the sickest patients could not be enrolled in the trial. Thus, our results may not be valid for the entire HTx population. HRQoL was a secondary, but prespecified endpoint in the HITTS (High-intensity Interval Training in De Novo Heart Transplant Recipients in Scandinavia) study [22, 23]. With only 78 participants we may face a type II error due to insufficient statistical power.
A disease-specific HRQoL questionnaire could have been more sensitive to detect differences between groups. So far, no disease-specific questionnaires in Norwegian are available for the HTx population. In a prior HTx study, we experienced a ceiling effect using the heart failure-specific Kansas City Cardiomyopathy Questionnaire [30] and decided not to use this questionnaire in this study.
The HITTS trial [22, 23] was not designed to assess the participants´ daily activities and the roles they were hoping to assume. This limits our ability to explain the between-group difference in the Role Emotional scale.
Clinical implications and future directions
Interventions for good and stable HRQoL, both short- and long-term after HTx, are needed. Exercise yields better physical function and makes it easier to engage in various activities of everyday life. However, despite improved VO2peak with the HIT intervention, HRQoL was similar in both intervention arms. The development of an organ transplant-specific HRQoL questionnaire is warranted for future research in this field, [25] as it probably will be more accurate to detect changes in health status associated with organ transplant issues.
Conclusion
This randomized controlled trial demonstrated significant improvements in the physical function components in HRQoL over a nine-month long exercise intervention in de novo HTx recipients. However, despite a larger improvement in exercise capacity in the HIT group, there were no between-group differences regarding the change in HRQoL.
Supplementary information
Additional file 1: Figure 1. Correlation between self-reported physical function and maximal muscle strength in the high-intensity training group and the moderate intensity continuous training group 11 weeks after heart transplantation (HTx). Figure 2. Correlation between self-reported physical function and maximal muscle strength in the high-intensity training group and the moderate intensity continuous training group 1 year after heart transplantation (HTx). Figure 3. Correlation between self-reported physical function and muscle endurance in the high-intensity training group and the moderate intensity continuous training group 11 weeks after heart transplantation (HTx). Figure 4. Correlation between self-reported physical function and muscle endurance in the high-intensity training group and the moderate intensity continuous training group 1 year after heart transplantation (HTx).
Acknowledgements
We want to thank all the HTx recipients for participating in the HITTS study. Thanks to professor Finn Gustafsson at Rigshospitalet, Copenhagen and professor Eva Irene Bossano Prescott, Bispebjerg Frederiksberg Hospital for help with planning the study. We also thank physical therapist Julia Philip Wigh at Sahlgrenska University Hospital for help with the coordination of the Swedish population and Professor Stefan Grau and PhD student Andreas Lundberg Zachrisson for help with the muscle strength testing of the Swedish population.
Published abstract
Part of this work is earlier presented at the International Society for Heart and Lung Transplantation 38th and 39th Annual Meeting and Scientific Sessions in April 2018 [31] and in April 2019 [32, 33].
Authors` contributions
KR coordinated the study, collected data, analyzed and drafted the paper. AKA, EG and KB were principal investigator responsible for the participants in Norway and were involved in the inclusion of the participants. MY and EB collected data and contributed to coordination of the study. ARA and IG were engaged in both the inclusion process of the participants and the coordination in-hospital in Norway. CHD coordinated the exercise intervention and collected data in Denmark. KIP contributed especially to the HRQoL part of the study. KK was responsible for the study in Sweden. LG and KN were the principal investigators, designed the study, drafted and revised the paper. All authors have contributed in revisions and to the final version of this paper.
Abbreviations
- CPET
Cardiopulmonary exercise test
- HADS
Hospital Anxiety and Depression Scale
- HIT
High-intensity interval training
- HITTS
High-intensity Interval Training in De Novo Heart Transplant Recipients in Scandinavia
- HRQoL
Health-related quality of life
- HTx
Heart transplantation/Heart transplant
- IR
Interquartile range
- MICT
Moderate intensity continuous training
- RPE
Rated perceived exertion
- SD
Standard deviation
- SF-36v2
Short Form-36 version 2
- VAS
Visual analogue scale
- VO2peak
Peak oxygen consumption
Funding
This work was supported with a PhD grant from the Norwegian Health Association (grant number 12906), a post-doctoral grant from the South-Eastern Norway Regional Health Authority (grant number 2013111), and a grant from Scandiatransplant.
Availability of data and materials
The data generated and analyzed during the current study are not publicy available due to Norways strict guidelines for privacy policy and data sharing.
Ethics approval and consent to participate
All participants provided written informed consent prior to inclusion. The study was approved by the regional ethic committees in Norway, Sweden and Denmark. The study is conducted according to the Helsinki Declaration. https://clinicaltrials.gov/ identifier NCT01796379.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12955-020-01536-4.
References
- 1.Anderson L, Nguyen TT, Dall CH, Burgess L, Bridges C, Taylor RS. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst Rev. 2017;4:CD012264. doi: 10.1002/14651858.CD012264.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kugler C, Tegtbur U, Gottlieb J, Bara C, Malehsa D, Dierich M, et al. Health-related quality of life in long-term survivors after heart and lung transplantation: a prospective cohort study. Transplantation. 2010;90(4):451–457. doi: 10.1097/TP.0b013e3181e72863. [DOI] [PubMed] [Google Scholar]
- 3.Kobashigawa J, Olymbios M. Quality of life after heart transplantation. In: Kobashigawa J, editor. Clinical guide to heart transplantation. Cham: Springer International Publishing; 2017. pp. 185–191. [Google Scholar]
- 4.Tackmann E, Dettmer S. Health-related quality of life in adult heart-transplant recipients-a systematic review. Herz. 2018. 10.1007/s00059-018-4745-8https://link.springer.com/content/pdf/10.1007%2Fs00059-018-4745-8.pdf. [DOI] [PubMed]
- 5.Kugler C, Gottlieb J, Warnecke G, Schwarz A, Weissenborn K, Barg-Hock H, et al. Health-related quality of life after solid organ transplantation: a prospective, multiorgan cohort study. Transplantation. 2013;96(3):316–323. doi: 10.1097/TP.0b013e31829853eb. [DOI] [PubMed] [Google Scholar]
- 6.Myaskovsky L, Dew MA, McNulty ML, Switzer GE, DiMartini AF, Kormos RL, et al. Trajectories of change in quality of life in 12-month survivors of lung or heart transplant. Am J Transplant. 2006;6(8):1939–1947. doi: 10.1111/j.1600-6143.2006.01395.x. [DOI] [PubMed] [Google Scholar]
- 7.Saeed I, Rogers C, Murday A. Health-related quality of life after cardiac transplantation: results of a UK National Survey with norm-based comparisons. J Heart Lung Transplant. 2008;27(6):675–681. doi: 10.1016/j.healun.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Politi P, Piccinelli M, Poli PF, Klersy C, Campana C, Goggi C, et al. Ten years of “extended” life: quality of life among heart transplantation survivors. Transplantation. 2004;78(2):257–263. doi: 10.1097/01.tp.0000133537.87951.f2. [DOI] [PubMed] [Google Scholar]
- 9.Galeone A, Kirsch M, Barreda E, Fernandez F, Vaissier E, Pavie A, et al. Clinical outcome and quality of life of patients surviving 20 years or longer after heart transplantation. Transpl Int. 2014;27(6):576–582. doi: 10.1111/tri.12298. [DOI] [PubMed] [Google Scholar]
- 10.Nytrøen K, Gullestad L. Exercise after heart transplantation: an overview. World J Transplant. 2013;3(4):78–90. doi: 10.5500/wjt.v3.i4.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karapolat H, Eyigor S, Durmaz B, Yagdi T, Nalbantgil S, Karakula S. The relationship between depressive symptoms and anxiety and quality of life and functional capacity in heart transplant patients. ClinResCardiol. 2007;96(9):593–599. doi: 10.1007/s00392-007-0536-6. [DOI] [PubMed] [Google Scholar]
- 12.Yardley M, Havik OE, Grov I, Relbo A, Gullestad L, Nytrøen K. Peak oxygen uptake and self-reported physical health are strong predictors of long-term survival after heart transplantation. Clin Transpl. 2016;30(2):161–169. doi: 10.1111/ctr.12672. [DOI] [PubMed] [Google Scholar]
- 13.Dall CH, Gustafsson F, Christensen SB, Dela F, Langberg H, Prescott E. Effect of moderate- versus high-intensity exercise on vascular function, biomarkers and quality of life in heart transplant recipients: a randomized, crossover trial. J Heart Lung Transplant. 2015;34(8):1033–1041. doi: 10.1016/j.healun.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Nytrøen K, Rustad LA, Aukrust P, Ueland T, Hallen J, Holm I, et al. High-intensity interval training improves peak oxygen uptake and muscular exercise capacity in heart transplant recipients. Am J Transplant. 2012;12(11):3134–3142. doi: 10.1111/j.1600-6143.2012.04221.x. [DOI] [PubMed] [Google Scholar]
- 15.Christensen SB, Dall CH, Prescott E, Pedersen SS, Gustafsson F. A high-intensity exercise program improves exercise capacity, self-perceived health, anxiety and depression in heart transplant recipients: a randomized, controlled trial. J Heart Lung Transplant. 2012;31(1):106–107. doi: 10.1016/j.healun.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Hsu CJ, Chen SY, Su S, Yang MC, Lan C, Chou NK, et al. The effect of early cardiac rehabilitation on health-related quality of life among heart transplant recipients and patients with coronary artery bypass graft surgery. Transplant Proc. 2011;43(7):2714–2717. doi: 10.1016/j.transproceed.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Ulubay G, Ulasli SS, Sezgin A, Haberal M. Assessing exercise performance after heart transplantation. Clin Transpl. 2007;21(3):398–404. doi: 10.1111/j.1399-0012.2007.00658.x. [DOI] [PubMed] [Google Scholar]
- 18.Yardley M, Gullestad L, Bendz B, Bjørkelund E, Rolid K, Arora S, et al. Long-term effects of high-intensity interval training in heart transplant recipients: A 5-year follow-up study of a randomized controlled trial. Clin Transplant. 2017;31:e12868. doi: 10.1111/ctr.12868. [DOI] [PubMed] [Google Scholar]
- 19.Karapolat H, Eyigor S, Durmaz B, Nalbantgil S, Yagdi T, Zoghi M. The effect of functional performance, respiratory function and osteopenia on the quality of life after heart transplantation. Int J Cardiol. 2008;124(3):381–383. doi: 10.1016/j.ijcard.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 20.Wu YT, Chien CL, Chou NK, Wang SS, Lai JS, Wu YW. Efficacy of a home-based exercise program for orthotopic heart transplant recipients. Cardiology. 2008;111(2):87–93. doi: 10.1159/000119695. [DOI] [PubMed] [Google Scholar]
- 21.Buendia F, Almenar L, Martinez-Dolz L, Sanchez-Lazaro I, Navarro J, Aguero J, et al. Relationship between functional capacity and quality of life in heart transplant patients. Transplant Proc. 2011;43(6):2251–2252. doi: 10.1016/j.transproceed.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Nytrøen K, Yardley M, Rolid K, Bjørkelund E, Karason K, Wigh JP, et al. Design and rationale of the HITTS randomized controlled trial: effect of high-intensity interval training in de novo heart transplant recipients in Scandinavia. Am Heart J. 2016;172:96–105. doi: 10.1016/j.ahj.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Nytrøen K, Rolid K, Andreassen AK, Yardley M, Gude E, Dahle DO, et al. Effect of high-intensity interval training in De novo heart transplant recipients in Scandinavia: 1-year follow-up of the HITTS randomized, Controlled Study. Circulation. 2019;139(19):2198–2211. doi: 10.1161/CIRCULATIONAHA.118.036747. [DOI] [PubMed] [Google Scholar]
- 24.Ware JE, Jr, Kosinski M, Bjorner BJ, Turner-Bowker D, Gandek B, Maruish ME. User’s manual for the SF36V2© Health survey 2edition: QualityMetric Inc. 2008. pp. 1–310. [Google Scholar]
- 25.Shahabeddin Parizi A, Krabbe PFM, Buskens E, Bakker SJL, Vermeulen KM. A scoping review of key health items in self-report instruments used among solid organ transplant recipients. Patient. 2018;12(2):171–181. doi: 10.1007/s40271-018-0335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Working Group on Cardiac Rehabilitation & Excercise Physiology and Working Group on Heart Failure of the European Society of Cardiology Recommendations for exercise testing in chronic heart failure patients. Eur Heart J. 2001;22(1):37–45. doi: 10.1053/euhj.2000.2388. [DOI] [PubMed] [Google Scholar]
- 28.Rolid K, Andreassen AK, Yardley M, Bjørkelund E, Karason K, Wigh JP, et al. Clinical features and determinants of VO2peak in de novo heart transplant recipients. World J Transplant. 2018;8(5):188–197. doi: 10.5500/wjt.v8.i5.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ortega T, Diaz-Molina B, Montoliu MA, Ortega F, Valdes C, Rebollo P, et al. The utility of a specific measure for heart transplant patients: reliability and validity of the Kansas City cardiomyopathy questionnaire. Transplantation. 2008;86(6):804–810. doi: 10.1097/TP.0b013e318183eda4. [DOI] [PubMed] [Google Scholar]
- 30.Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7(2):235–242. doi: 10.1016/j.ejheart.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Rolid K, Andreassen AK, Yardley M, Bjørkelund E, Authen AR, Grov I, et al. Predictors for health related quality of life in De novo heart transplant recipients. J Heart Lung Transplant. 2018;37(4):S296. [Google Scholar]
- 32.Rolid K, Andreassen AK, Yardley M, Gude E, Bjørkelund E, Authen AR, et al. Effect of high intensity interval training on health related quality of life in De novo heart transplant recipients-the HITTS study. J Heart Lung Transplant. 2019;38(4):S196–S1S7. [Google Scholar]
- 33.Rolid K, Andreassen AK, Yardley M, Gude E, Bjørkelund E, Authen AR, et al. Associations between self-reported physical function and exercise capacity in De novo heart transplant recipients. J Heart Lung Transplant. 2019;38(4):S196. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure 1. Correlation between self-reported physical function and maximal muscle strength in the high-intensity training group and the moderate intensity continuous training group 11 weeks after heart transplantation (HTx). Figure 2. Correlation between self-reported physical function and maximal muscle strength in the high-intensity training group and the moderate intensity continuous training group 1 year after heart transplantation (HTx). Figure 3. Correlation between self-reported physical function and muscle endurance in the high-intensity training group and the moderate intensity continuous training group 11 weeks after heart transplantation (HTx). Figure 4. Correlation between self-reported physical function and muscle endurance in the high-intensity training group and the moderate intensity continuous training group 1 year after heart transplantation (HTx).
Data Availability Statement
The data generated and analyzed during the current study are not publicy available due to Norways strict guidelines for privacy policy and data sharing.