Abstract
Background
Observational data suggests that low vitamin D status is associated with an increased incidence of pulmonary tuberculosis (TB) and mortality among people living with HIV. The primary aims of this study were to assess the effect of vitamin D3 supplementation on the risk of mortality and incidence of pulmonary TB among adults initiating antiretroviral therapy (ART).
Methods
We conducted a randomized, double-blind, placebo-controlled trial of vitamin D3 supplementation among adults living with HIV who initiated ART with low serum 25-hydroxyvitamin D (25(OH)D) levels at four large HIV care and treatment centers in Dar es Salaam, Tanzania. The vitamin D group received weekly 50,000 IU vitamin D3 supplements for the first month of ART followed by daily 2,000 IU vitamin D3 supplements. The placebo arm received a matching weekly and daily placebo regimen. The trial follow-up duration was one year and the primary efficacy outcomes were death and incident pulmonary TB. An intent-to-treat analysis was followed. This study is registered with ClinicalTrials.gov, NCT01798680, and is completed.
Findings
Between 24 February 2014 and 7 March 2018, 4,000 adults initiating ART were enrolled in the trial and followed-up for one year. A total of 415 deaths were recorded; 211 in the vitamin D3 group and 204 in the placebo group. Among all randomized participants, there was no overall effect of vitamin D3 supplementation on the risk of mortality (hazard ratio (HR): 1·04; 95% CI: 0·85–1·25; P=0·73). There was also no difference in the overall incidence of pulmonary TB between the vitamin D3 (50 events) and placebo groups (67 events) (HR: 0·78; 95% CI: 0·54–1·13; P=0·19). In terms of adverse events, the vitamin D3 regimen did not increase the risk of hypercalcemia (3 events vitamin D3 group, 2 events placebo group, relative risk: 1.25; 95% CI: 0.43–3.66; Fisher’s exact P =1·00). There were 101 hospitalizations reported in the vitamin D3 group and 94 in the placebo group (incidence rate ratio: 1.06; 95% CI: 0.80–1.41; P=0·66).
Interpretation
Additional research is needed before vitamin D3 supplementation should be considered for implementation in HIV care and treatment programs for the prevention of pulmonary TB or mortality.
Introduction
In 2018, it is estimated that 23·3 million people living with HIV (PLWHIV) worldwide were receiving antiretroviral therapy (ART) (1). The remarkable global scale-up of access to ART for PLWHIV has produced significant population-level health and economic benefits (2, 3), yet adults initiating ART in sub-Saharan Africa continue to experience disproportionally high rates of mortality during the initial months of treatment (4, 5). Tuberculosis (TB) remains the leading cause of death for PLWHIV and accounts for one-third of AIDS-related deaths (1, 6).
Vitamin D is an immunomodulatory micronutrient that has effects on both adaptive and innate immune responses (7). Vitamin D deficiency is common among PLWHIV and there is a large body of observational evidence in diverse populations that links low levels of vitamin D with increased risk of incident pulmonary TB and poor HIV treatment outcomes (8–11). In a prospective cohort study in Tanzania, we found that HIV-infected adults initiating ART with vitamin D deficiency (serum 25(OH)D concentrations <20 ng/mL) had approximately three times the risk of developing pulmonary TB and two times the risk of death as compared to those with serum vitamin D >30 ng/mL (10, 11). Studies have also documented that vitamin D deficiency in the context of HIV is associated with poorer CD4 T-cell recovery (12) and greater risk HIV disease progression among HIV-infected adults on ART (13, 14). Trials of vitamin D supplementation for treatment of TB have shown marginal to no effect on time to sputum culture or smear conversion and other TB treatment outcomes (15, 16). However, no randomized trials to date have examined the effect of vitamin D supplementation for prevention of pulmonary TB or mortality for PLWHIV.
We present the first efficacy trial of vitamin D supplementation for prevention of pulmonary TB and mortality among HIV-infected adults initiating ART. We also examined the effect of the vitamin D3 supplementation regimen on serum 25-hydroxyvitamin D (25(OH)D) response and the safety outcome of hypercalcemia.
Methods
Study design and participants
The Trial of Vitamins-4 (ToV4) was an individually randomized, parallel group, double-blind, placebo-controlled trial of vitamin D3 (cholecalciferol) supplementation for HIV-infected adults initiating ART that was conducted from 24 February 2014 to 7 March 2018 in Dar es Salaam, Tanzania. The trial protocol has been published previously (17). The trial protocol was approved by the Harvard T. H. Chan School of Public Health Institutional Review Board (IRB13–0231), the Tanzanian National Health Research Ethics Sub-Committee (NIMR/HQ/R.8a/Vol.IX/1658), and the Tanzania Food and Drug Authority (TFDA13/CTR/0005/3) and was monitored by an independent data and safety board. Written informed consent using Kiswahili forms was obtained from all participants. The trial funder had no role in the trial design, data collection and analysis, or interpretation of the results.
Investigators enrolled Tanzanian HIV-infected adults who were initiating ART with low serum 25(OH)D levels from four large HIV care and treatment centers. The trial inclusion criteria was i) adult men or women aged ≥18 years old, ii) living with HIV, iii) had a serum 25(OH)D concentration <30 ng/mL at the time of screening, iv) were initiating ART at the time of randomization, v) intended to stay in Dar es Salaam for at least 1 year, and vi) provided written informed consent. The exclusion criteria was: i) pregnant at the time of randomization or ii) enrolled in any other clinical trial. Women who became pregnant during follow-up discontinued the trial regimen and were discharged from the trial.
The screening visit for trial enrollment was integrated into the HIV treatment program ART eligibility visit. Serum 25(OH)D concentration at the screening visit was quantified with a commercial enzyme immunoassay (EIA) (Immunodiagnostics, Boldon, UK). Before the start of the trial we determined that the IDS EIA linearly overestimated 25(OH)D concentration as compared to the gold standard HPLC-MS/MS.(17) Accordingly, in order to enroll individuals with 25(OH)D <30 ng/mL, we set the inclusion threshold for trial eligibility with the IDS EIA test kit to 25(OH)D <37 ng/mL (see protocol (17) for details). Serum 25(OH)D results needed to be returned before ART initiation in order to be eligible for randomization.
During the trial, the Dar es Salaam HIV treatment program changed the ART eligibility criteria. At the start of trial enrollment in 2014, the ART initiation criteria for adults was a CD4 T-cell count <350 cells per μL or WHO HIV stage 3 or 4 disease. In January 2016, the ART initiation criteria changed to a CD4 T-cell count <500 cells per μL or WHO HIV stage 3 or 4 disease and in June 2016 the program gradually switched to a test-and-treat strategy (all individuals with HIV are eligible for ART). In order to maintain generalizability of the trial, we enrolled individuals initiating ART, regardless of the initiation criteria at the time. All study participants were provided with HIV care and treatment that adhered to Tanzanian national guidelines. Efavirenz/lamivudine/tenofovir (EFV/3TC/TDF) was the preferred first-line ART regimen throughout the trial. Participants received co-trimoxazole prophylaxis if their CD4 cell count was <200 cells per μL. All HIV/TB patients received directly observed therapy (DOT) with a 6-month short course regimen that included a 2-month intensive phase of daily rifampicin / isoniazid / pyrazinamide / ethambutol (RHZE), followed by a 4-month continuation phase of daily rifampicin and isoniazid (RH). Psychological and nutritional counselling (no food or nutritional supplements directly provided) was also provided as standard of care.
Randomization and Interventions
Participants were randomized to either a vitamin D3 supplementation or placebo group. The randomization list was computer-generated by a non-study statistician with sequence blocks of ten that were stratified study clinic. The vitamin D3 supplementation group received weekly 50,000 IU vitamin D3 oral supplements that were taken under direct observation at the randomization visit and for the following 3 weeks (four total 50,000 IU doses) at the study clinics. At one-month post-randomization, participants in the vitamin D3 group then received daily oral 2,000 IU vitamin D3 supplements that were to be taken daily until trial discharge at 1-year post randomization. Participants randomized to the placebo group received matching oral placebo supplements taken identically under direct observation for the first month followed by daily oral placebo supplements from one month until trial discharge. The vitamin D3 and placebo supplements were produced in the United States by Tischcon Corp. (Salisbury, MD) and there was no discernible difference between vitamin D3 and placebo supplements in any aspect, including appearance, taste, smell or weight. Complete allocation concealment was ensured with the use of regimen bottles that were pre-labeled with sequentially numbered participant identification numbers.
Procedures
At the randomization visit, all enrolled trials participants received a full clinical examination by study physicians, including WHO HIV disease staging and TB screening. Study nurses administered a standardized questionnaire on socio-demographic characteristics, morbidity and measured height and weight. All participants were followed-up at weekly clinic visits for 3 weeks (week 1, 2, 3) followed by monthly clinic visits starting at one month of follow-up until trial discharge at one year. At all follow-up clinic visits, research nurses administered a questionnaire on morbidity within the last 28 days and recorded anthropometric measurements. Nurses also recorded participant reported hospitalization (admitted for at least one night) since the last visit at follow-up visits. Physicians also performed a full clinical examination including TB screening and WHO HIV disease staging at each visit.
Outcomes
Participants who did not come for their scheduled clinic appointments were contacted via mobile phone or visited at home to assess vital status. For participants who died, a standardized verbal autopsy was administered to relatives or other individuals that were present around the time of death. Participants that died at a health facility also had events before the time of death documented from hospital records. Two senior HIV clinicians defined the cause of death by consensus and a third clinician served as a tiebreaker.
Participants were screened for pulmonary TB at all study visits and were suspected of having pulmonary TB if they presented with any of the following five clinical signs and symptoms: cough for 2 weeks or more, fever, 3 kg weight loss in one month or noticeable weight loss for new ART patients, night sweats, or hemoptysis. All individuals suspected of potentially having pulmonary TB provided a sputum specimen on the day TB was suspected and were given a sputum container for an early morning sputum to be collected the next day. A third sputum specimen was then collected under supervision by a nurse at the clinic the following day. The sputum smears were stained using the Ziehl-Nielsen technique and examined for acid-fast bacilli (AFB) by laboratory technologists. All individuals suspected of having pulmonary TB also received a chest X-ray. Pulmonary TB was diagnosed when at least one sputum smear was positive for AFB or when there were radiological features suggestive of TB in the absence of a positive sputum smear. During the trial the GeneXpert System (Cepheid, Sunnyvale, CA, USA) which used the cartridge-based Xpert MTB/RIF assay became available at three of the four study clinics. These clinics used a positive GeneXpert test to diagnose pulmonary TB among smear-negative individuals.
All participants had blood drawn at randomization, 1, 6, and 12 months post-randomization. Serum calcium and albumin concentrations were assessed with the Roche Cobas Integra 400 Plus (Roche Diagnostics). Hypercalcemia was defined as albumin-adjusted calcium >2·6 mmol/L. Serum 25(OH)D concentrations over time were assessed among a randomly selected sample of 400 participants who had a serum sample available from the randomization visit and at least one follow-up time point. 25(OH)D concentrations in this selected group were quantified at Children’s Hospital Boston by high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS). Day-to-day precision at various levels of 25(OH)D ranged from 5.6% to 8.5%. Additional secondary outcomes included in the trial protocol (appendix p 2) are planned to be reported in the second half of 2020 after completion of lab and statistical analyses.
Statistical Analysis
The sample size target was 4,000 participants and power calculations assumed 1:1 randomization to either vitamin D3 or placebo, a nominal Type I error rate (alpha) of 0·05 and a 10% loss to follow-up rate.(17) The trial had 90% power to detect a relative risk of 0·70 if the mortality rate was 10% in the placebo group. As for pulmonary TB, if the incidence was 5% in the placebo group, the trial had 86% power to detect a relative risk of 0·60.
An intent-to-treat analysis was used as the primary analytic strategy for all analyses. The primary efficacy outcomes were (i) all-cause mortality and (ii) incident pulmonary TB. The log-rank test was used to evaluate differences in the incidence of death and pulmonary TB between randomized treatment groups. We also used proportional hazard regression models to produce hazard ratios that included a fixed effect for clinic to account for stratified randomization. Participants who were diagnosed with pulmonary TB at the time of randomization, were suspected to have TB at the randomization visit that were later confirmed, or were receiving anti-TB treatment on the date of randomization were excluded from analyses of pulmonary TB incidence. We conducted sensitivity analyses excluding pulmonary TB cases occurring in the first 14, 30 and 90 days of follow-up to assess the potential of undetected prevalent TB and unmaking TB-IRIS to affect results. In exploratory analyses, we also examined the effect of vitamin D3 on sputum smear-positive pulmonary TB, sputum smear-negative pulmonary TB, microbiologically confirmed pulmonary TB (sputum smear or GeneXpert positive), and high bacillary load (≥ 2+) sputum smear-positive pulmonary TB. Pre-specified subgroup analyses for the primary outcomes of death and pulmonary TB were defined in the protocol based on biologic plausibility and included: baseline vitamin D status (25(OH)D <20 ng/mL and ≥ 20 ng/mL), sex (male and female), age (<40 versus ≥ 40 years), BMI (<18.5 kg/m2 vs ≥18.5 kg/m2), CD4-T cell count (<200 vs ≥200 cells per μL), hemoglobin (Hb) concentration, WHO HIV disease stage (I/II, III and IV), baseline pulmonary TB, use of isoniazid preventive therapy (IPT), ART regimen, and trial regimen compliance (high - 100% on weekly doses and ≥90% on daily doses and low - <100% on weekly doses and <90% on daily doses)(17). We used the Wald test for risk-ratio homogeneity to assess statistical significance of effect modification. We did not control for multiplicity of comparisons for effect modifiers since these were pre-specified independent hypotheses. We also conducted sensitivity analyses that adjusted for baseline factors which showed some degree of imbalance between randomization arms based on a P<0·20.
The effect of vitamin D3 supplementation on post-randomization 25(OH)D concentration was examined using linear mixed-effects models with a random intercept, a compound symmetric covariance structure, and robust standard errors. The models accounted for within-subject correlation and provided robust inference for fixed effects, even if the covariance structure was misspecified. As for adverse events, the Fisher’s exact test was used to evaluate differences in proportion of participants with incident hypercalcemia between treatment arms. We used Poisson regression to assess the incidence of hospitalization between treatment arms since some participants reported being hospitalized multiple times. All statistical analyses were performed with SAS, version 9.3 (SAS Institute). The study was registered at ClinicalTrial.gov, NCT01798680.
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
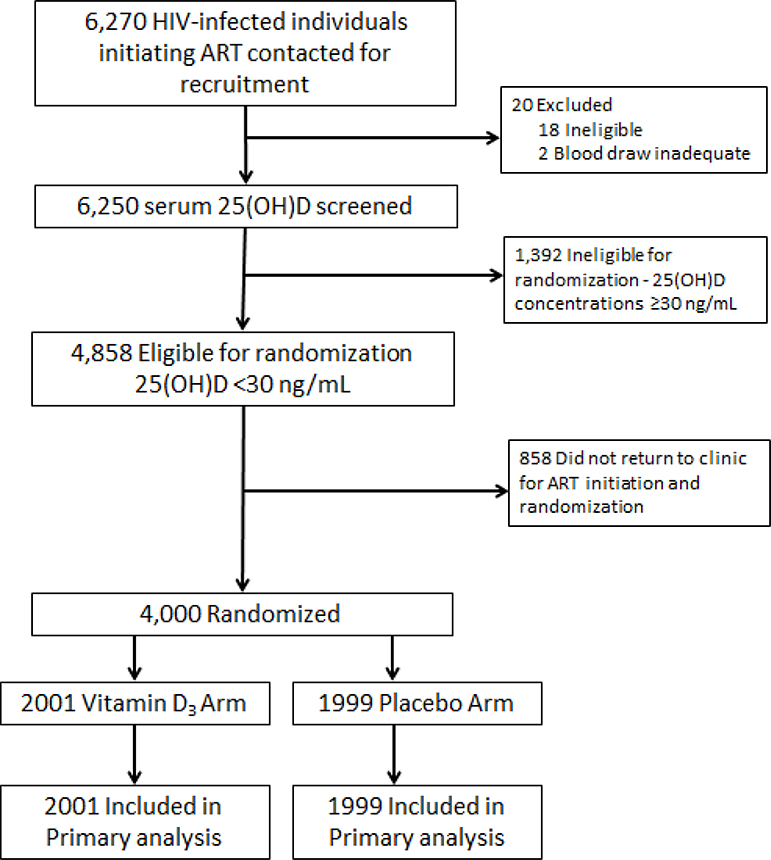
The trial began enrolling participants on 24 February 2014 and follow-up of all participants was completed on 7 March 2018. The trial flow diagram is presented in Figure 1. A total of 6,250 HIV-infected individuals initiating ART were contacted for participation and consented to serum 25(OH)D screening. Of individuals screened, 4,848 (77·7%) had a 25(OH)D concentration <30 ng/mL and were therefore eligible for randomization and 4,000 participants were randomized (2,001 to vitamin D3 and 1,999 to placebo). Baseline characteristics were similar across randomized groups (Table 1). During the trial follow-up period, 19 (0.5%) participants withdrew consent and 94 (2·4%) participating women became pregnant and were censored. At trial discharge, 75 (1·9%) participants had unknown vital status (2·1% of vitamin D group and 1·7% of placebo group).
Figure 1.
Trial flow diagram
Table 1.
Baseline characteristics of the intention-to-treat population (n=4,000)
| Vitamin D3 (n=2,001) | Placebo (n=1,999) | |
|---|---|---|
| Sex | ||
| Female | 1367 (68%) | 1368 (68%) |
| Male | 634 (32%) | 631 (32%) |
| Age (years) | 38.6 (9.8) | 38.8 (10.0) |
| Education* | ||
| No formal education | 308 (15%) | 327 (16%) |
| Primary | 1294 (64%) | 1294 (65%) |
| Secondary/ advanced | 398 (20%) | 377 (19%) |
| Body mass index (kg/m2)* | ||
| <18.5 | 440 (22%) | 404 (20%) |
| 18.5–24.9 | 1038 (52%) | 1064 (53%) |
| ≥25.0 | 521 (26%) | 531 (27%) |
| CD4 T-cell count (cells per μL) | ||
| < 200 | 866 (43%) | 845 (42%) |
| 200–349 | 461 (23%) | 445 (22%) |
| 350–499 | 300 (15%) | 333 (17%) |
| ≥ 500 | 284 (14%) | 278 (14%) |
| Missing | 90 (5%) | 98 (5%) |
| WHO HIV disease stage | ||
| I / II | 744 (37%) | 760 (38%) |
| III | 1161 (58%) | 1143 (57%) |
| IV | 96 (5%) | 96 (5%) |
| Baseline pulmonary TB | 189 (10%) | 175 (9%) |
| Isoniazid preventive therapy (IPT) | 35 (2%) | 27 (1%) |
| ART regimen | ||
| Efavirenz/lamivudine/tenofovir (EFV/3TC/TDF) | 1940 (97%) | 1943 (97%) |
| Other ART regimen | 61 (3%) | 56 (3%) |
| Mean days from vitamin D screening to randomization | 10.1 (8%) | 10.2 (9%) |
| Vitamin D status at screening visit | ||
| Insufficient, 25(OH)D 20.0–30.0 ng/mL | 955 (48%) | 972 (49%) |
| Moderate deficiency, 25(OH)D 10.0–19.9 ng/mL | 920 (46%) | 865 (43%) |
| Severe deficiency, 25(OH)D 0–9.9 ng/mL | 126 (6%) | 162 (8%) |
Data are n (%), mean (SD).
Data not available for all randomized participants
Adherence to the directly observed weekly randomized doses (50,000 IU vitamin D3 or placebo) was similarly high in both groups; 81·6% and 81·4% of participants took all the weekly doses and 98·1% and 96·0% only missed one weekly dose or took all doses in the vitamin D and placebo groups, respectively. Adherence to the daily regimen supplements (2,000 IU vitamin D3 or placebo) was also similar between groups (mean adherence of 80·8% and 80·6% for the vitamin D and placebo groups, respectively).
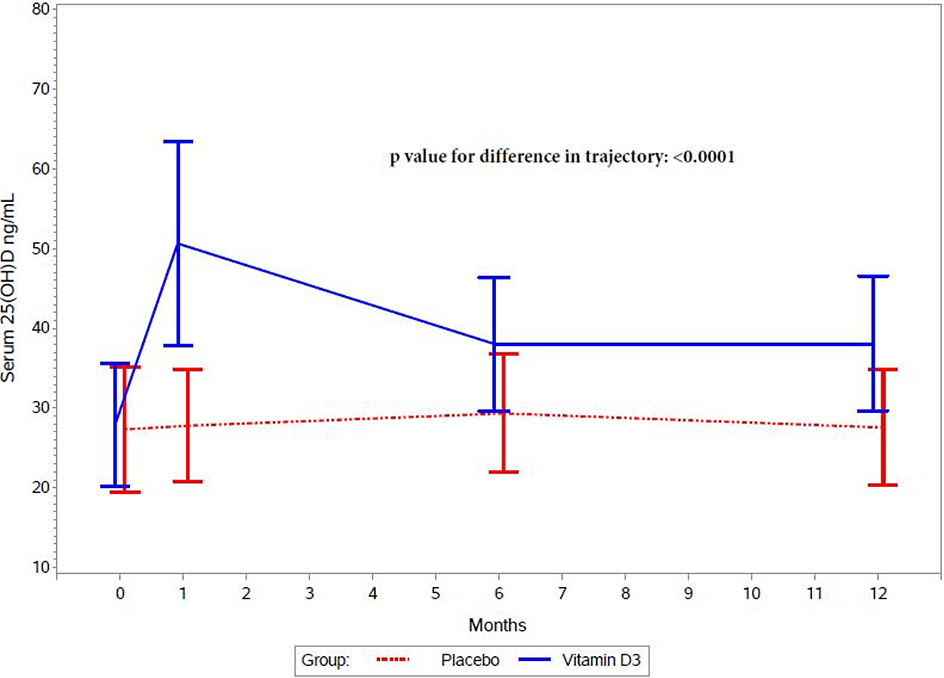
Figure 2 presents the effect of randomized treatment arm on serum 25(OH)D concentrations during follow-up among a random sample of 400 participants. Vitamin D3 supplementation increased post-randomization serum 25(OH)D concentrations as compared to the placebo (P-value for difference in trajectory: <0·0001). 25(OH)D levels were significantly higher in the vitamin D3 group at the 1, 6, and 12 month time points (appendix p 3).
Figure 2.
Serum 25(OH)D concentrations at baseline, 1 month, 6 months, and 12 months by randomized treatment arm
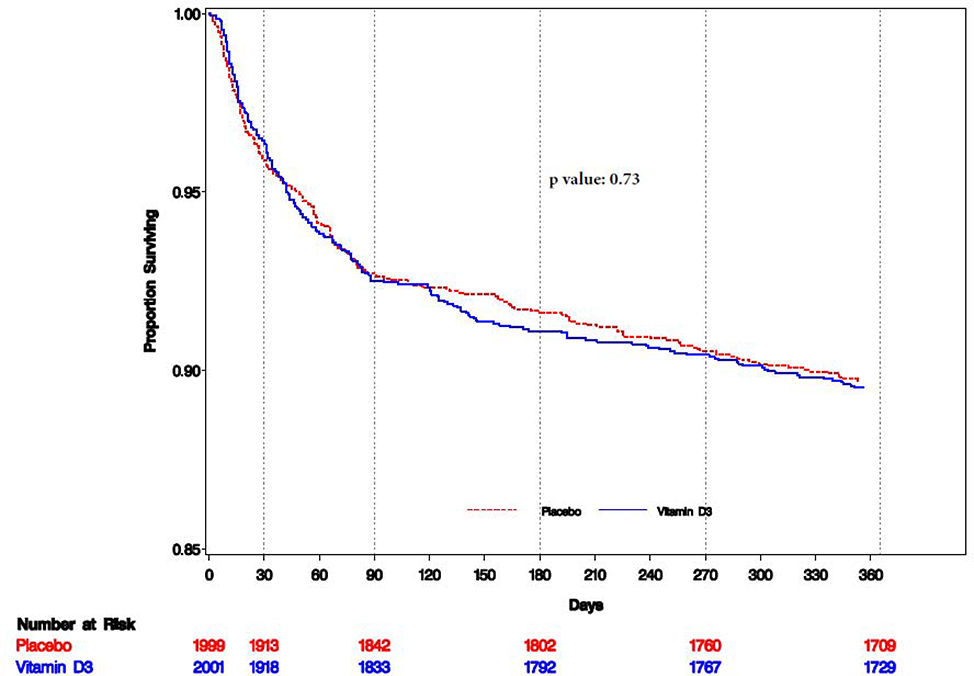
Among all randomized participants, there was no difference in the risk of mortality between randomized groups (P= 0·73) (Table 2 and Figure 3). Cause-specific mortality by randomized group is presented in appendix Table 2 (appendix p 4). There was no effect of vitamin D on the risk of pulmonary TB death between treatment arms (HR: 0·98; 95% CI: 0·64–1·50). Table 3 presents the effect of vitamin D3 on mortality stratified by predefined baseline factors. The effect of vitamin D3 was found to differ by baseline WHO HIV disease stage (P = 0.0053) and pulmonary TB disease status (P = 0.025). There were larger beneficial effects among individuals initiating ART with WHO HIV Stage IV disease and among participants with pulmonary TB disease at baseline. In sensitivity analyses, there was no qualitative change in findings overall or for subgroup effects after adjusting for potential baseline imbalance in 25(OH)D and CD4 T-cell count between randomized groups (appendix p 5–7).
Table 2.
Effect of vitamin D3 supplementation on all-cause mortality and incident pulmonary tuberculosis outcomes
| Vitamin D No. events / No. randomized at risk# (%) |
Placebo n / N (%) No. events / No. randomized at risk (%) |
Hazard ratio (95% CI) | p-value* | |
|---|---|---|---|---|
| All-cause mortality | 211 / 2001 (10.5) | 204 / 1999 (10.2) | 1.04 (0.85–1.25) | 0.73 |
| Incident pulmonary TB | 50 / 1812 (2.8) | 64 / 1827 (3.5) | 0.78 (0.54–1.13) | 0.19 |
| Incident sputum smear-positive pulmonary TB | 20 / 1812 (1.1) | 37 / 1827 (2.0) | 0.54 (0.31–0.92) | 0.026 |
| Incident sputum smear-negative pulmonary TB | 30 / 1812 (1.6) | 27 / 1827 (1.5) | 1.12 (0.66–1.88) | 0.68 |
| Incident microbiologically confirmed TB (sputum or GeneXpert positive) | 22 / 1812 (1.2) | 38 / 1827 (2.1) | 0.58 (0.34–0.97) | 0.039 |
| Incident high bacillary load (≥ 2+) sputum smear-positive pulmonary TB | 10 / 1812 (0.6) | 27 / 1827 (1.5) | 0.37 (0.18–0.76) | 0.0067 |
Log-rank test
Incident pulmonary TB analyses exclude participants who had pulmonary TB at baseline
Figure 3.
Kaplan-Meir plot for time to all-cause mortality (n=4,000)
Table 3.
Effect modification of vitamin D3 supplementation on mortality by pre-specified baseline factors
| Vitamin D n / N (%) |
Placebo n / N (%) |
Hazard Ratio (95% CI) | p-value for effect modification | |
|---|---|---|---|---|
| All participants | 211 / 2001 (10.5) | 204 / 1999 (10.2) | 1.04 (0.85–1.25) | - |
| Subgroups | ||||
| Sex | ||||
| Male | 105 / 634 (16.6) | 93 / 631 (14.7) | 1.13 (0.85–1.49) | 0.39 |
| Female | 106 / 1367 (7.8) | 111 /1368 (8.1) | 0.96 (0.73–1.25) | |
| Age | ||||
| < 40 years | 101 / 1176 (8.6) | 90 / 1130 (8.0) | 1.08 (0.82–1.44) | 0.73 |
| ≥ 40 years | 110 / 825 (13.3) | 114 / 755 (13.1) | 1.01 (0.78–1.32) | |
| CD4 T-cell count | ||||
| < 200 cells per μL | 134 / 866 (15.5) | 141 / 845 (16.7) | 0.91 (0.72–1.15) | 0.18 |
| ≥ 200 cells per μL | 60 / 1045 (5.7) | 44 / 1056 (4.2) | 1.40 (0.95–2.06) | |
| Missing | 17 / 90 (18.9) | 19 / 98 (19.4) | 0.98 (0.51–1.90) | |
| WHO HIV disease stage | ||||
| I / II | 29 / 744 (3.9) | 38 / 760 (5.0) | 0.78 (0.48–1.26) | 0.0053 |
| III | 160 / 1161 (13.8) | 128 / 1143 (11.2) | 1.24 (0.98–1.57) | |
| IV | 22 / 96 (22.9) | 38 / 96 (39.6) | 0.52 (0.31–0.89) | |
| Baseline pulmonary TB | ||||
| Yes | 23 / 189 (12.2) | 35 / 175 (20.0) | 0.59 (0.35–1.00) | 0.025 |
| No | 188 / 1812 (10.4) | 169 / 1824 (9.3) | 1.12 (0.91–1.38) | |
| Body mass index | ||||
| <18.5 kg/m2 | 94 / 440 (21.4) | 83 / 404 (20.5) | 1.13 (0.85–1.49) | 0.70 |
| ≥ 18.5 kg/m2 | 115 / 1559 (7.4) | 121 / 1595 (7.6) | 0.96 (0.73–1.25) | |
| Vitamin D status at screening visit | ||||
| Deficient 25(OH)D 0–19.9 ng/mL | 104 / 1046 (9.9) | 98 / 1027 (9.5) | 1.05 (0.80–1.38) | 0.88 |
| Insufficient 25(OH)D 20.0–29.9 ng/mL | 107 / 955 (11.2) | 106 / 972 (10.9) | 1.02 (0.78–1.33) | |
| Adherence to randomized trial regimen | ||||
| High – 100% on weekly doses and ≥90% on daily doses | 103 / 876 (11.8) | 110 / 889 (12.4) | 0.95 (0.72–1.24) | 0.35 |
| Low – <100% on weekly doses or < 90% on daily doses | 108 / 1125 (9.6) | 94 / 1110 (8.5) | 1.14 (0.87–1.50) | |
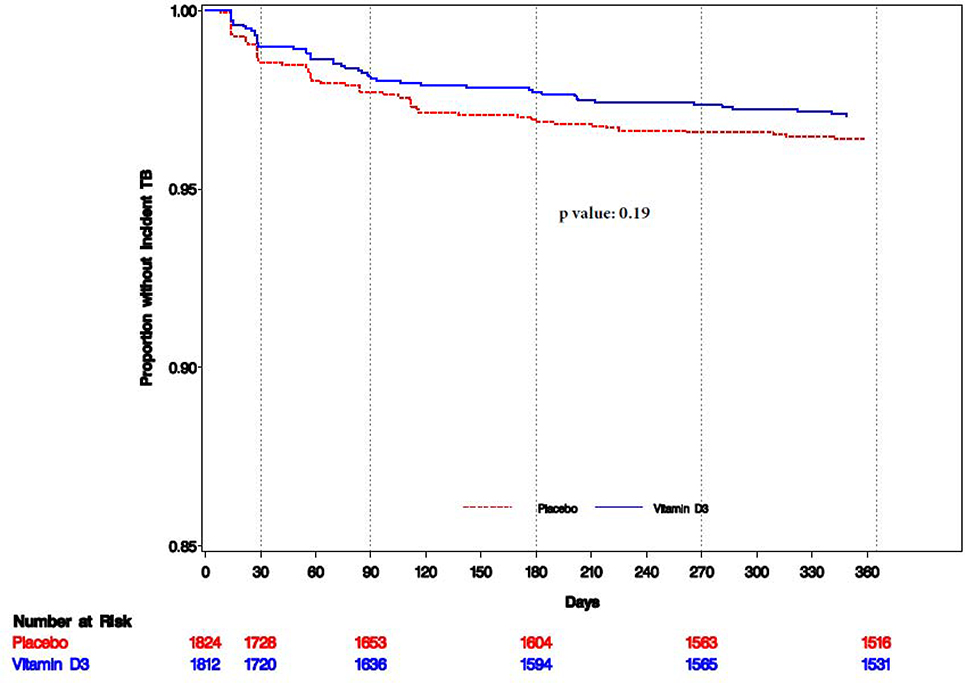
A total of 114 incident pulmonary TB cases were recorded, of which 57 (50%) were sputum smear-positive. Among sputum smear-negative TB cases, three were GeneXpert (5.3%) positive, while the remaining 54 cases were not tested with GeneXpert. There was no difference in the incidence of pulmonary TB between randomized groups (P=0·19) (Table 2 and Figure 4). However, in an exploratory analysis, vitamin D3 supplementation reduced the incidence of sputum smear-positive pulmonary TB, but there was no effect on sputum smear-negative pulmonary TB (Table 2). Further, vitamin D3 reduced the incidence of microbiologically confirmed TB and high bacillary load (≥ 2+) sputum smear-positive pulmonary TB (Table 2).
Figure 4.
Kaplan-Meir plot for time to incident pulmonary tuberculosis (n=3,636)
There was no evidence the overall effect of vitamin D on pulmonary TB was modified by baseline factors (appendix p 8) and there was no meaningful change in estimates after adjusting for baseline 25(OH)D and CD4 T-cell count (appendix p 6 and 9). We also assessed the potential for undetected prevalent TB cases at baseline and unmasking TB-IRIS to affect the results. The magnitude of the effect of vitaminD3 on the overall incidence of pulmonary TB remained consistent when excluding cases that occurred in the first 14, 30 and 90 days of follow-up (appendix p 10); however, there was some indication that vitamin D3 may have provided a particularly strong beneficial effect on incident sputum smear-positive pulmonary TB cases occurring after 90 days of ART.
A summary of the adverse events by treatment arm is included the appendix (appendix p 11). Five participants were diagnosed with incident hypercalcemia during follow-up; three participants in the vitamin D and two in the placebo group (relative risk: 1·25; 95% CI: 0·43–3·66; Fisher’s exact P =1·00). There were 101 hospitalizations reported in the vitamin D3 group and 94 in the placebo group (incidence rate ratio: 1·06; 95% CI: 0·80–1·41; P=0·66). There were no allergic reactions to the trial supplements recorded in the vitamin D3 or placebo groups.
Discussion
Vitamin D3 supplementation did not have an overall effect on mortality or incidence of pulmonary TB among HIV-infected Tanzanian adults initiating ART with low serum vitamin D levels. However, vitamin D3 may have reduced mortality for the subgroups of participants with pulmonary TB disease or WHO stage IV disease at ART initiation. In addition, exploratory analyses suggested that vitamin D3 supplementation may have reduced the incidence of sputum smear-positive pulmonary TB and high bacillary load sputum smear-positive pulmonary TB. The vitamin D3 supplementation regimen quickly increased and then maintained serum 25(OH)D levels as intended. There was no evidence that the vitamin D3 supplementation regimen increased the risk of hypercalcemia or other adverse events.
Our finding of no effect of vitamin D3 supplementation on mortality among all randomized participants is not completely in line with evidence from observational cohort studies which have consistently found that low serum 25(OH)D concentrations were associated with increased risk of death and HIV disease progression (10, 13, 18). This difference may partially be due to low 25(OH)D acting as a marker of infection and HIV disease severity that led to residual confounding in observational studies. Immune activation and infections can reduce serum vitamin 25(OH)D concentrations and therefore observational studies are unable to completely disentangle whether low 25(OH)D is either a marker of HIV disease severity or an indicator of potential to benefit from vitamin D supplementation (19, 20).
Nevertheless, we identified a beneficial effect of vitamin D3 among adults initiating ART with pulmonary TB disease and advanced HIV disease that may in part be explained by positive effects of supplementation on immune function for these individuals. Vitamin D has range of effects on innate and adaptive immune responses that may lead to better control of HIV, Mycobacterium tuberculosis (Mtb), and other viral and bacterial infections (19, 21). Specifically, vitamin D3 supplementation may improve immune responses to TB by enhancing cathelicidin-mediated macrophage killing of mycobacteria (22), interferon-gamma (IFN-γ)-mediated activity of macrophages (23), stimulation of phagolysosome fusion (24), and other antimicrobial pathways (7, 25). Vitamin D supplementation may also reduce HIV replication (26, 27) and hasten immune reconstitution (12, 20, 28). Prior randomized trials of vitamin D3 supplementation adjunct to TB treatment have found modest to no effect on sputum culture or smear conversion but were underpowered to evaluate mortality and only a few trials included HIV-infected individuals (15, 16). Additional trials are needed to confirm our mortality findings and studies are also needed to elucidate the biological mechanisms.
We also found no overall effect of vitamin D3 on the incidence of pulmonary TB. In an exploratory analysis, this appeared to be the balance of a large reduction in the incidence of sputum smear positive TB and minimal to no effect on sputum smear negative TB. Further in exploratory analyses, we observed a particularly strong beneficial effect on high bacillary load (≥2+) sputum smear-positive pulmonary TB. The beneficial effect of vitamin D3 supplementation on the incidence of sputum smear-positive pulmonary is in line with the large body of observational evidence linking low 25(OH)D concentrations with development of pulmonary TB (8, 9). The mechanisms may be positive TB-specific immunologic effects that reduce progression from latent to active sputum smear positive pulmonary TB or those that reduce the risk of TB acquisition (25). In addition, vitamin D3 supplementation may result in better control of HIV or underlying risk factors for sputum smear positive pulmonary TB (11, 19, 21). Nevertheless, it is also important to consider that the difference in effect by sputum positivity in our trial may be due to over-diagnosis of sputum smear-negative pulmonary TB based on clinical diagnosis using radiographic presentation. An effect on sputum smear-negative may have been diluted by including pneumonia, bacteremia and other infections that were not affected by vitamin D3 supplementation (29). Similarly, some individuals with paucibacillary sputum findings (scanty or 1+ AFB) may have been misclassified as pulmonary TB cases. As a result, future trials of vitamin D for pulmonary TB prevention should consider using more sensitive and specific methods for diagnosis of pulmonary TB such as culture, GeneXpert (including the Xpert MTB/RIF Ultra assay), urine lipoarabinomannan assay (LAM), and other more sensitive or specific TB diagnostics, particularly in the context of HIV. Further, we used the WHO-endorsed TB symptom screener that includes cough ≥2 weeks; however, use of cough of any duration in TB screening may identify more potential pulmonary TB cases (30).
Our trial has several other important limitations. First, the cumulative incidence of pulmonary TB in the placebo group (3.5%) was lower than expected in power calculations. The lower incidence may partially be due to the Dar es Salaam treatment program switching to a test-and-treat strategy during the course of the trial. As a result, we had lower power than expected to detect an effect on pulmonary TB. Second, we did not have data to define paradoxical TB-IRIS or unmasking TB-IRIS in direct alignment with International Network for the Study of HIV-associated IRIS (INSHI) consensus definitions (31). Nevertheless, the magnitude of the effect of vitamin D3 on overall incidence of pulmonary TB appeared to be robust to exclusion of pulmonary TB cases in the first 14, 30, and 90 days of follow-up. In addition, although we screened individuals for low serum 25(OH)D concentrations, few participants in our trial had very low levels of serum 25(OH)D <10 ng/mL (<5%). As a result, studies in populations with more severe forms of vitamin D deficiency should be considered. In addition, although we a priori defined subgroups of interest for mortality and pulmonary TB analyses, type I errors (false positives) are possible.
There was no overall effect of vitamin D3 supplementation on pulmonary TB or death. However, supplementation may have improved survival among the subgroups of HIV-infected adults initiating ART with pulmonary TB disease or advanced HIV disease. In addition, vitamin D3 may have reduced the incidence of sputum smear-positive pulmonary TB and high bacillary load pulmonary TB, which, if confirmed, suggests that vitamin D3 supplementation may be a public health intervention to reduce TB transmission. Nevertheless, these secondary and exploratory findings need to be assessed in future trials before considering integration of vitamin D supplementation into HIV care and treatment programs.
Supplementary Material
Research in Context.
Evidence before this study
We searched PubMed from database inception to December 1, 2019, using the search terms “vitamin D” and (“HIV” or “tuberculosis”) for studies published in English. Vitamin D is an immunomodulatory micronutrient and deficiency is common among PLWHIV. There is a relatively large observational evidence base that has found low levels of vitamin D are associated with increased risk of pulmonary tuberculosis disease among PLWHIV as well as uninfected individuals in diverse settings. Further, cohort studies among PLWHIV have also documented that low levels of vitamin D are associated with increased risk of HIV disease progression and mortality. No published randomized trials to date have examined the effect of vitamin D supplementation on prevention of pulmonary TB disease in adults or the effect on mortality among PLWHIV.
Added value of this study
This is the first randomized trial of vitamin D supplementation for prevention of pulmonary TB and mortality among PLWHIV. We found no overall effect of vitamin D3 supplementation on the risk of mortality among all randomized participants, but supplementation appeared to improve survival for the subgroups of adults initiating ART with concurrent pulmonary TB disease or WHO stage IV disease. In addition, vitamin D3 did not reduce the overall incidence of pulmonary TB; however, in exploratory analyses vitamin D3 appeared to reduce the incidence of sputum smear positive pulmonary TB, microbiologically confirmed TB, and incident high bacillary load (≥ 2+) sputum smear-positive pulmonary TB.
Implications of the available evidence
Vitamin D supplementation did not improve survival or reduce the overall incidence of TB. However, there was some indication that subgroups of PLWHIV initiating ART may experience survival benefits. In addition, vitamin D3 may the incidence of smear-positive and high bacillary load pulmonary TB. Nevertheless, our findings are not conclusive and additional research is needed to confirm these subgroup and exploratory findings before considering vitamin D supplementation as a public health program to reduce the risk of pulmonary TB or mortality in the context of HIV.
Acknowledgements
We thank the field teams (including physicians, nurses, supervisors, and laboratory staff), administrative staff, and the study participants, all of whom made this study possible. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK.
Funding: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Footnotes
Declaration of interest: None to declare
Data Sharing: De-identified individual participant data that underlie the results reported in this Article (text, tables, figures, and appendices) may be shared upon request. Data will be available by request from 12 months after publication. Data sharing would be done on a collaborative basis and require approval by study institutions and ethical boards. Proposals for data use should be directed to csudfeld@hsph.harvard.edu.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Joint United Nations Programme on HIV/AIDS. Communities at the centre: defending rights, breaking barriers, reaching people with HIV services2019. Available from: Available from: http://www.unaids.org/en/resources/documents/2018/global-aids-update.
- 2.Eaton JW, Menzies NA, Stover J, Cambiano V, Chindelevitch L, Cori A, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Glob Health. 2014;2(1):e23–34. [DOI] [PubMed] [Google Scholar]
- 3.Bor J, Herbst AJ, Newell ML, Barnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339(6122):961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and highincome countries. Lancet. 2006;367(9513):817–24. [DOI] [PubMed] [Google Scholar]
- 5.Anderegg N, Johnson LF, Zaniewski E, Althoff KN, Balestre E, Law M, et al. All-cause mortality in HIV-positive adults starting combination antiretroviral therapy: correcting for loss to follow-up. AIDS. 2017;31 Suppl 1:S31–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29(15):1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hewison M Vitamin D and innate and adaptive immunity. Vitam Horm. 2011;86:23–62. [DOI] [PubMed] [Google Scholar]
- 8.Huang SJ, Wang XH, Liu ZD, Cao WL, Han Y, Ma AG, et al. Vitamin D deficiency and the risk of tuberculosis: a meta-analysis. Drug Des Devel Ther. 2017;11:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aibana O, Huang CC, Aboud S, Arnedo-Pena A, Becerra MC, Bellido-Blasco JB, et al. Vitamin D status and risk of incident tuberculosis disease: A nested case-control study, systematic review, and individual-participant data meta-analysis. PLoS Med. 2019;16(9):e1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudfeld CR, Wang M, Aboud S, Giovannucci EL, Mugusi FM, Fawzi WW. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS One. 2012;7(6):e40036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sudfeld CR, Giovannucci EL, Isanaka S, Aboud S, Mugusi FM, Wang M, et al. Vitamin D status and incidence of pulmonary tuberculosis, opportunistic infections, and wasting among HIV-infected Tanzanian adults initiating antiretroviral therapy. J Infect Dis. 2013;207(3):378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezeamama AE, Guwatudde D, Wang M, Bagenda D, Kyeyune R, Sudfeld C, et al. Vitamin-D deficiency impairs CD4+T-cell count recovery rate in HIV-positive adults on highly active antiretroviral therapy: A longitudinal study. Clin Nutr. 2016;35(5):1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viard JP, Souberbielle JC, Kirk O, Reekie J, Knysz B, Losso M, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25(10):1305–15. [DOI] [PubMed] [Google Scholar]
- 14.Havers F, Smeaton L, Gupte N, Detrick B, Bollinger RC, Hakim J, et al. 25-Hydroxyvitamin D insufficiency and deficiency is associated with HIV disease progression and virological failure post-antiretroviral therapy initiation in diverse multinational settings. J Infect Dis. 2014;210(2):244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu HX, Xiong XF, Zhu M, Wei J, Zhuo KQ, Cheng DY. Effects of vitamin D supplementation on the outcomes of patients with pulmonary tuberculosis: a systematic review and meta-analysis. BMC Pulm Med. 2018;18(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolliffe DA, Ganmaa D, Wejse C, Raqib R, Haq MA, Salahuddin N, et al. Adjunctive vitamin D in tuberculosis treatment: meta-analysis of individual participant data. Eur Respir J. 2019;53(3). [DOI] [PubMed] [Google Scholar]
- 17.Sudfeld CR, Mugusi F, Aboud S, Nagu TJ, Wang M, Fawzi WW. Efficacy of vitamin D3 supplementation in reducing incidence of pulmonary tuberculosis and mortality among HIV-infected Tanzanian adults initiating antiretroviral therapy: study protocol for a randomized controlled trial. Trials. 2017;18(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepherd L, Souberbielle JC, Bastard JP, Fellahi S, Capeau J, Reekie J, et al. Prognostic value of vitamin D level for all-cause mortality, and association with inflammatory markers, in HIV-infected persons. J Infect Dis. 2014;210(2):234–43. [DOI] [PubMed] [Google Scholar]
- 19.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15(14):2579–85. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez-Sousa MA, Martinez I, Medrano LM, Fernandez-Rodriguez A, Resino S. Vitamin D in Human Immunodeficiency Virus Infection: Influence on Immunity and Disease. Front Immunol. 2018;9:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villamor E A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64(5 Pt 1):226–33. [DOI] [PubMed] [Google Scholar]
- 22.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179(4):2060–3. [DOI] [PubMed] [Google Scholar]
- 23.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3(104):104ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hmama Z, Nandan D, Sly L, Knutson KL, Herrera-Velit P, Reiner NE. 1alpha,25-dihydroxyvitamin D(3)-induced myeloid cell differentiation is regulated by a vitamin D receptor-phosphatidylinositol 3-kinase signaling complex. J Exp Med. 1999;190(11):1583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brighenti S, Bergman P, Martineau AR. Vitamin D and tuberculosis: where next? J Intern Med. 2018. [DOI] [PubMed] [Google Scholar]
- 26.Coussens AK, Naude CE, Goliath R, Chaplin G, Wilkinson RJ, Jablonski NG. High-dose vitamin D3 reduces deficiency caused by low UVB exposure and limits HIV-1 replication in urban Southern Africans. Proc Natl Acad Sci U S A. 2015;112(26):8052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguilar-Jimenez W, Villegas-Ospina S, Gonzalez S, Zapata W, Saulle I, Garziano M, et al. Precursor Forms of Vitamin D Reduce HIV-1 Infection In Vitro. Journal of acquired immune deficiency syndromes (1999). 2016;73(5):497–506. [DOI] [PubMed] [Google Scholar]
- 28.Eckard AR, O’Riordan MA, Rosebush JC, Lee ST, Habib JG, Ruff JH, et al. Vitamin D supplementation decreases immune activation and exhaustion in HIV-1-infected youth. Antivir Ther. 2018;23(4):315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colebunders R, Bastian I. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4(2):97–107. [PubMed] [Google Scholar]
- 30.Field SK, Escalante P, Fisher DA, Ireland B, Irwin RS, Adams TM, et al. Cough due to TB and other chronic infections: CHEST guideline and expert panel report. Chest. 2018;153(2):467–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.