Abstract
Aim:
Digoxin is considered contraindicated in light-chain (AL) amyloidosis, given reports of increased toxicity published 30–50 years ago. We sought to determine the frequency of digoxin toxicity in patients with AL.
Methods:
We identified 107 patients with AL amyloidosis who received digoxin between 2000 and 2015.
Results:
The median age was 65 and the median digoxin dose and estimated glomerular filtration rate were 0.125 mg/d and 55 ml/min/1.73 m2, respectively. Digoxin dose was reduced in 16% of the patients, mainly due to high serum drug concentration or worsening renal function. The median duration of therapy was 5 months, with half of the patients stopping treatment, primarily due to physician preference. Significant arrhythmias developed in 11% of patients, almost exclusively in newly diagnosed patients. Arrhythmias presented as terminal events in five patients; four with bradycardia followed by pulseless electrical activity (PEA) with ventricular tachycardia/fibrillation (VT/VF) during resuscitation; all patients had acute renal failure and severe, decompensated heart failure. One patient had ventricular tachycardia as a terminal event. Only one patient was treated with digoxin antibody therapy.
Conclusions:
Digoxin may be cautiously utilized in AL amyloidosis patients. We suggest its use in lower doses and frequent drug concentration monitoring along with close monitoring of electrolytes and renal function. Nonetheless, toxicity at low serum concentration cannot be excluded due to potential for toxic concentration at the tissue level and should be taken under consideration when prescribing digoxin for these patients. Studies with higher-level evidence are needed to confirm these findings.
Keywords: Digoxin, amyloid, arrhythmia, atrial fibrillation, toxicity
Introduction
Systemic immunoglobulin light chain (AL) amyloidosis is caused by deposition of amyloidogenic light-chain proteins secreted by monoclonal plasma cells. Deposition occurs in various organs, leading to organ dysfunction and death. The most commonly involved organ is the heart, seen in over two-thirds of patients [1]. The leading cause of death in AL amyloidosis is cardiac causes, including sudden cardiac death due to pulseless electrical activity, lethal arrhythmias or refractory heart failure [2].
Atrial fibrillation is detected in approximately 12% of AL patients and is commonly associated with symptomatic heart failure [3]. Treatment of atrial fibrillation is challenging and hemodynamic deterioration may rapidly develop with the onset of tachycardia. Beta blockers and calcium channel blockers are often poorly tolerated given the hypotensive and negative inotropic effects in the setting of predominant diastolic dysfunction with fixed cardiac output. The use of amiodarone is problematic in AL patients with hepatic and/or thyroid dysfunction [4].
Cardiac glycosides were reported as case reports to have increased toxicity in cardiac amyloidosis, including reports of sudden cardiac death presumably due to lethal arrhythmias. However, reports were few, most more than 50 years ago, and included a limited number of patients [5,6]. Concern for the increased toxicity of cardiac glycosides also stemmed from an in vitro study in which digoxin was found to bind to AL amyloid fibrils [7]. This ex vivo observation suggested an increased risk of digoxin toxicity in amyloidosis, even when the serum drug concentration is within the normal range. No further data has emerged on the use of digoxin in amyloidosis for over 30 years, probably due to the reluctance to use this agent in amyloidosis, as well as its diminishing use for the management of arrhythmias and heart failure. However, clinicians managing patients with AL amyloidosis and atrial fibrillation needing rate control continue to face a most challenging management dilemma, which often comes down to the “lesser of evils” in the choice of therapy.
Patients and methods
All patients with AL amyloidosis seen at our institution between 1 January 2000 and 31 August 2015 were included in the screening population. We electronically searched for any mention of the following terms in the clinical notes of this cohort: “digoxin”, “lanoxin”, “digitek” or “digitalis”. The clinical record of all patients with a mention of any of the search words was reviewed to ascertain the use of digoxin during or after the diagnosis of AL amyloidosis. All patients gave informed consent to have their medical records reviewed for research purposes. The study was approved by the institutional review board.
Patients were grouped based on the timing of digoxin initiation in relation to the diagnosis of AL amyloidosis. Patients who initiated digoxin within 6 months of diagnosis and patients who were already on digoxin when diagnosed with AL amyloidosis were included in the early user group. Patients who were placed on digoxin >6 months following the diagnosis of AL amyloidosis formed the late user group. Patients in which digoxin was discontinued upon their initial evaluation for AL amyloidosis at our institution (n = 26) were reported but were not further analysed.
Digoxin levels, when available, were recorded. The estimated glomerular filtration rate (eGFR) was calculated using the modification of diet in renal disease equation. The medical records were reviewed for evidence of significant arrhythmia, defined as: ventricular fibrillation, sustained ventricular tachycardia, symptomatic non-sustained ventricular tachycardia, junctional rhythm, and high-grade atrio-ventricular block and or bradycardia. Records were reviewed regarding the need for adjustment of digoxin dose or pacemaker placement.
The Kruskal–Wallis test and the Pearson χ2 test were used to ascertain differences between continuous and categorical variables, respectively. p values less than .05 were considered significant. Statistical analysis was performed using JMP software (SAS, Cary, NC).
Results
Of 2638 patients with AL amyloidosis screened, 133 patients were treated with digoxin while seen at our institution (5% of the screened cohort). Indications for digoxin use were atrial fibrillation/flutter in 121 (91%) of the patients and heart failure in the remaining 12 (9%). The use of digoxin was similar across the study period (43 patients from 2000–2004 period; 49 patients from 2005–2009 period and 41 patients from the 2010–2015 time period).
Digoxin was initiated in proximity to the AL amyloidosis diagnosis in 43 patients, and in 27 patients it was initiated at an earlier time point and was continued following the diagnosis of amyloidosis (early users group; n = 70). Thirty-seven patients initiated digoxin later in their amyloidosis disease course (>6 months from diagnosis, late users group; n = 37). Twenty-six patients who were on digoxin treatment upon arrival at Mayo were advised to stop its use due to an elevated serum concentration (>2.0 ng/mL; n = 5) or for its perceived toxicity in amyloidosis (n = 21). Of note, 107 patients (4% of the screening population) who were not treated with digoxin were advised in their initial evaluation against the use of digoxin for the management of their disease.
Baseline characteristics and cardiac status
Baseline characteristics of the study cohort and by digoxin use groups are presented in Table 1. The median age at AL amyloidosis diagnosis was 65 years, higher in the early user group compared to the late user group (median 67 vs. 62 years; p = .04). The median number of involved organs was 2.
Table 1.
Baseline characteristics of the study cohort and by subgroups according to timing of digoxin use.
| Characteristics | Whole cohort (n = 107) | Early user group (n = 70) | Late user group (n = 37) | p |
|---|---|---|---|---|
| Age at diagnosis, median (IQR) | 65 (57–71) | 67 (59–74) | 62 (55–67) | .04 |
| Male gender, n (%) | 78 (73%) | 50 (71%) | 28 (76%) | .63 |
| Number of involved organs, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | .74 |
| Cardiac involvement | 97 (91%) | 66 (94%) | 31(84%) | .08 |
| ASCT use at any time | 34 (32%) | 21 (30%) | 13 (35%) | .58 |
| dFLC at diagnosis, mg/dL, median (IQR) (n = 82) | 33 (16–84) | 40 (17–99) | 28 (13–72) | .36 |
| Mayo 2004 stage III at diagnosis, n (%) (n = 67) | 37 (55%) | 27 (61%) | 10 (44%) | .16 |
ASCT: autologous stem cell transplantation; dFLC: difference between involved light-chains; IQR: interquartile range.
Bold value signifies the statistical significance at p < .05.
Baseline cardiac evaluation is presented in Table 2. Median left ventricular ejection fraction (EF) and cardiac index were within the lower limit of normal (53% and 2.6 l/min/m2, respectively). Cardiac biomarkers at the time of diagnosis were significantly elevated above normal reference [median troponin T 0.06 ng/mL (normal <0.01); median NT-proBNP 5738 pg/mL (normal values are age and gender dependent; for men age 65 < 89 pg/mL, for women age 65 < 190 pg/mL). Nineteen per cent of the patients had a pacemaker placed prior to initiation of digoxin and 10% had an implantable cardioverter defibrillator. Prior cardioversion for atrial fibrillation/flutter was performed in 14% of the patients.
Table 2.
Baseline cardiac characteristics of the study cohort and by the whole cohort and by timing subgroups.
| Characteristic, median (IQR)a | Whole cohort (n = 107) | Early user group (n = 70) | Late user group (n = 37) | p |
|---|---|---|---|---|
| Concomitant heart failure medications | ||||
| Diuretics, n (%) | 75 (70%) | 50 (71%) | 25 (68%) | .67 |
| Beta blockers, n (%) | 60 (56%) | 39 (56%) | 21 (57%) | .91 |
| Calcium channel blockers, n (%) | 14 (13%) | 10 (14%) | 4 (11%) | .6 |
| ACE inhibitors/ARBs, n (%) | 25 (23%) | 20 (29%) | 5 (14%) | .07 |
| Amiodarone, n (%) | 7 (7%) | 2 (3%) | 5 (14%) | .04 |
| Electrocardiogram rhythm (n = 99) | ||||
| Sinus rhythm | 39 (39%) | 24 (36%) | 15 (45.5%) | .76 |
| Atrial flutter/fibrillation | 47 (48%) | 32 (48%) | 15 (45.5%) | |
| Paced | 9 (9%) | 7 (11%) | 2 (6%) | |
| Other | 4 (4%) | 3 (5%) | 1 (3%) | |
| Echocardiogram | ||||
| IVS (mm) | 14 (12–16) | 14 (12–16) | 14 (11–16) | .66 |
| EF (%), median (IQR) | 53 (36–62) | 50 (33–61) | 55 (39–63) | .23 |
| Cardiac index, L/min/m2, median (IQR) (n = 93) | 2.6 (2.1–3.1) | 2.5 (2.1–3.1) | 2.6 (2.0–2.9) | .79 |
| LV Stroke volume index, cc/m2, median (IQR) (n = 85) | 35 (26–41) | 34 (24–40) | 36 (28–43) | .38 |
| Global average LV strain, (%), median (IQR) (n = 43) | −11[(−8)–(−15)] | −11 [(−9)–(−14)] | −12 [(−8)–(−15)] | .78 |
| Hemodynamic parameters | ||||
| Heart rate, beat/min | 84 (67–109) | 81 (67–111) | 88 (65–105) | .81 |
| SBP, mmHg, median (IQR) | 105 (93–118) | 104 (92–118) | 105 (94–117) | .77 |
| DBP, mmHg, median (IQR) | 64 (59–71) | 63 (60–70) | 68 (58–75) | .59 |
| Cardiac biomarkers | ||||
| Troponin T, ng/mL, median (IQR) (n = 89) | 0.06 (0.02–0.13) | 0.07 (0.02–0.15) | 0.05 (<0.01–0.11) | .2 |
| NT-proBNP, pg/mL, median (IQR) (n = 70) | 5738 (2203–11152) | 6241 (2209–13652) | 5423 (2128–8292) | .55 |
obtained at time of AL amyloidosis diagnosis for the early user group and at the time of digoxin initiation in the late user group.
ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blocker; DBP: diastolic blood pressure; EF: ejection fraction; IQR: interquartile range; IVS: interventricular septum; LV: left ventricle; NT-proBNP: N-terminal pro b-type natriuretic peptide; SBP: systolic blood pressure.
Bold value signifies the statistical significance at p < .05.
Digoxin dose and duration of therapy
The median initial dose of digoxin was 0.125 mg daily, with no difference between the early user and late user groups (p = .3). The estimated glomerular filtration rate at treatment initiation was 55 ml/min/1.73 m2 (IQR 37–76). Seventeen patients required a dose reduction, seen almost exclusively in early users than in the late users (16 patients and 1 patient, respectively). The new dose was 50% of the initial dose in 16/17 patients. Reasons for the dose reduction were high drug level (n = 5), worsening renal function (n = 4), unknown reason (n = 4), physician preference (n = 3) and toxicity (n = 1, visual disturbance). Two patients had dose escalation for inadequate heart rate control.
The median duration of therapy was 5 months (IQR 1–14 months), similar between the early user group (median 4 months, IQR 1–12) and the late user group (median 7 months, IQR 2–16; p = .43). Fifty-one per cent of patients were treated until last follow-up or death, whereas 49% discontinued therapy while alive. Reasons for digoxin discontinuation were: physician preference (n = 31); toxicity (n = 10); unknown (n = 9) and lack of benefit (n = 2).
Serum digoxin concentration
Serum concentration level(s) was available in 53% of the patients. However, the time from last dose was not available. The median measurements per patient with available testing were 2 (IQR 1–4). The median highest level was 1.0 ng/mL (0.9–1.8), with no difference between groups (p = .31). The corresponding daily digoxin dose at the time of highest level measure was 0.125 mg and the corresponding eGFR was 50 ml/min/1.73 m2 (IQR 28–67). Fifty-six per cent of the patients with measurement of digoxin serum concentration had at least one measure above 0.9 ng/mL, and 18% had at least one measurement above the high normal reference (>2 ng/mL).
Of the 26 patients who stopped digoxin upon arrival at Mayo, 10 patients had serum digoxin concentration measurement, 5 had serum concentration above 2 ng/mL (range 2.3–5 ng/mL). Four of these patients received a daily digoxin dose of 0.25 mg and one patient received 0.125 mg. The median eGFR for these 5 patients was 45 ml/min/1.73 m2 (range 42–65).
Toxicity
Significant arrhythmias during digoxin use
Twelve patients were found to have significant arrhythmias while on digoxin treatment, representing 11% of all patients (Table 3). These arrhythmias were more commonly seen in the early users than the later users (16% vs. 3%; p = .03). Of these, five were associated with terminal events, all of which occurred in the early user group. One patient received digoxin-specific antibody (Fab) during resuscitation.
Table 3.
Summary table of significant arrhythmia observed during digoxin treatment.
| Gender, age | Event primary/secondary rhythm | Acute renal failure | Creatinine event/baseline | Associated conditions, notes | Cardiac severity/NYHA | Outcomea; months from diagnosis |
|---|---|---|---|---|---|---|
| M, 67 | Cardiac/respiratory arrest Sinus bradycardia/asystole |
Present | 2.5/1.7 | End-stage HF,? tamponade | Severe Class IV |
Dieda; 4 |
| F, 58 | Cardiac arrest PEA junctional rhythm, VT, VF |
Present | 5.6/1.6 | Cardiogenic shock, hyperkalaemia (K = 5.7) | Severe Class IV |
Dieda; 1.5 |
| Received one dose of digoxin-specific antibody (Digibind) | ||||||
| M, 83 | Respiratory arrest PEA Sinus, VT, junctional rhythm |
Present | 2.8/1.8 | Severe HF, severe hyperkalaemia (K = 7.4) | Severe Class IV |
Dieda; 2 |
| Possible respiratory arrest | ||||||
| M, 33 | Unknown VT (death certificate) |
Unknown | Unknown | Pacemaker | Severe Class IV |
Dieda; 4 |
| 68, F | Cardiac arrest PEA paced rhythm, VT |
Present | 2.4/1.4 | Profound weakness, hypoxia, severe hyperkalaemia (K = 7.2) | Severe, Class IV |
Dieda; 72 |
| On digoxin +6 years | ||||||
| M, 57 | Junctional bradycardia (without definite symptoms) | Absent | 2.0/2.0 | Decompensated heart failure, Bradycardia improved off of beta-blocker, digoxin continued; concomitant amiodarone, |
Severe Class IV |
Died 6 months later; 24 |
| M, 69 | Cardiac arrest-3rd degree AV block (one dose digoxin)/bradycardia | Present | 4.7/1.2 | Dialysis initiation, permanent pacemaker placed, recurrent bradycardia after digoxin discontinued | Severe Class IV |
Died 2 months later, off digoxin; 2 |
| M, 70 | AF with slow VR, junctional (persistent after digoxin discontinued) | Present | 2.4/1.7 | Right heart failure (indication for digoxin) | Severe Class IV |
Died, 9 months later, off digoxin; 9 |
| M, 73 | Cardiac arrest, asystole, bradycardia | Absent | 1.0/1.0 | HF unclear, probably class III | Severe NYHA unknown |
Died 8 months later; 8 |
| M, 77 | Syncope, NSVT | Absent | 1.1/1.1 | ICD implanted after event, digoxin discontinued Heart failure; recurrent VT, PEA off dig |
Unclear, Class III |
Died, despite ICD, off digoxin 4 months later; 4 |
| F, 60 | Cardiac arrest, VF | Present | 2.4/1.1 | Day#15 post autologous stem cell transplantation; Severe HF; sepsis, ICD after event; recurrent cardiac arrest due to VT, 2 weeks off of digoxin | Severe, Class IV |
Alive, 84 |
| M, 59 | Syncope Electrophysiology study: inducible VT, sinus node dysfunction, prolonged HV |
Absent | 1.0 | ICD after electrophysiology study; No heart failure, Relapse prior to death | Moderate, Class II |
Died 60 months later, off digoxin; 84 |
AF: atrial fibrillation; HF: herat failure; HV: His-ventricular conduction time; ICD: implantable cardioverter defibrillator; NSVT: non-sustained ventricular tachycardia; PEA: pulseless electrical activity; VF: ventricular fibrillation; VR: ventricular response; VT: ventricular tachycardia.
Acute death associated with event.
Other toxicities
One patient complained of photopsia (perception of flashing lights) in his peripheral visual fields with a digoxin level of 1.7 ng/mL. The digoxin dose was decreased by one-half and the photopsia resolved.
Electrolyte disturbances
Hyperkalaemia (>5 mEq/L) was documented in 25 patients (23%), in seven of them at a level >5.5 mEq/L. Hypokalaemia (<3.5 mEq/L) was seen in 25% patients, in 10 with a level below 3.0 mEq/L.
Use of digoxin in the subpopulation of patients undergoing autologous stem cell transplant (ASCT)
Twenty-two patients (21% of the study cohort) were given digoxin during ASCT therapy for AL amyloidosis, 19 patients in the early user group and three patients in the late user group. Seven patients were on digoxin prior to ASCT and continued on with this drug during ASCT, and 15 patients initiated therapy during ASCT (7 patients only during ASCT and 8 patients continued with digoxin after their discharge). All but one patient received digoxin for control of atrial fibrillation/flutter. Cardiac involvement was present in 19 of these patients (86%).
The median initial digoxin dose at ASCT was 0.1875 mg per day (range 0.0625–0.25). The median eGFR at day of stem cell infusion was 66 ml/min/1.73 m2 (range 22–120). Seventeen patients (77%) had a serum digoxin level measured at least one time, with a median of 2 measurements (range 1–8). The median highest level was 0.9 ng/mL (range 0.3–4.2). Levels above 2 ng/mL occurred in two patients (4.2, 2.8 ng/mL), who were on digoxin prior to ASCT at a dose of 0.25 mg a day, and without significant impairment in their eGFR from baseline when the high digoxin levels were obtained (49 and 55 ml/min/1.73 m2, respectively). One patient experienced VF while on digoxin on day +15 (Table 3) with a recurrent episode of VF 15 days after digoxin discontinuation.
Seven patients had a reduction in digoxin dosage during the ASCT period (high serum level, n = 3; reduction in renal function, n = 2; physician preference n = 2). Digoxin was stopped in nine patients during ASCT period at a median of 20 days from day 0 (range 4–41). Reasons for digoxin discontinuation were: physician preference to avoid excess toxicity (n = 7); toxicity (n = 1); and lack of benefit (n = 1). One patient died 41 days following ASCT due to septic shock, 10 days after discontinuation of digoxin.
Discussion
AL amyloidosis is a challenging disease, both in the management of the underlying clonal plasma cell disorder and in the management of the organ dysfunction caused by the amyloid deposits. The disease involves on average 2 dominant organs [8], but at times 4–5 organs can be significantly affected. This by itself interferes with any drug therapy due to impaired absorption, metabolism, excretion and/or drug tolerance. Systolic hypotension due to cardiac and autonomic dysfunction limits the use of cardiac medications, particularly beta-blockers and calcium channel antagonists which are poorly tolerated and commonly lead to worsening heart failure [2]. The clinician is faced with a particularly difficult scenario in the management of atrial arrhythmias in AL amyloidosis. Hemodynamics are usually unstable with the onset of atrial fibrillation and rapid deterioration, including cardiogenic shock, may occur. The reports suggesting digoxin is contraindicated due to increased toxicity are of poor quality [5–7], and lack control comparator since cardiac sudden death is common in cardiac AL amyloidosis. Moreover, this needs to be counterbalanced by the risks of beta-blockers and calcium channel blockers, especially in those with significant cardiac involvement.
To our knowledge, this study represents the only large series of AL patients treated with digoxin. Digoxin was used almost exclusively to treat atrial fibrillation/flutter and was more commonly used soon after diagnosis. The study population had significant morbidity with the majority having advanced stage cardiac involvement. Baseline renal function was mildly impaired and acute renal failure and decompensated heart failure were present in most of those with arrhythmias that suffered terminal events. Electrolyte disturbances were common, with hypokalaemia/hyperkalaemia seen in over 50% of patients. This is particularly important as hypokalaemia, frequently seen due to diuretic use, potentiates digoxin toxicity. Therefore, close electrolyte monitoring is required when prescribing digoxin for AL amyloidosis patients treated with digoxin. Treatment duration was on average less than 6 month, due to both death from the disease and treatment discontinuation. Over half of the patients in this series received beta-blocker therapy, for rate control of atrial fibrillation, highlighting the challenges of medical management in AL amyloidosis with advanced cardiac involvement.
Digoxin dose was relatively modest with dose reduction required in close to 15% of patients, almost exclusively in patients with newly diagnosed disease. Digoxin drug concentration data in this series should be interpreted with caution, given non-standardized drug level monitoring which was available in only half of the patients. The high incidence of hypothyroidism in AL amyloidosis, reaching approximately 20% of the newly diagnosed patients [4] may result in altered metabolism, as hypothyroidism causes slower clearance of digoxin from the serum [9]. Drug–drug interaction can also result in altered serum drug concentration. Among those are: amiodarone, verapamil, quinidine, erythromycin and tetracycline (increase digoxin concentration); cholestyramine, antacids and bupropion (decrease digoxin concentration) [10]. Moreover, as there is potentially in vivo binding of digoxin to amyloid fibrils (following in vitro evidence), serum drug concentration may not accurately reflect local effects of digoxin in cardiac tissue. Conduction system disease is common in cardiac amyloidosis and may be worsened by digoxin. Review of the baseline electrocardiogram in sinus rhythm, if available, is recommended. Patients with significant sinus or atrioventricular block should not be treated with digoxin unless a pacemaker is present.
Significant arrhythmias, potential signs of digoxin toxicity, occurred in 11% of patients in this series. However, half of these occurred as terminal events associated with cardiac arrest due to PEA, a condition now recognized as a common cause of sudden death in AL [11,12]. PEA is not generally considered to be a manifestation of digoxin toxicity and we found similar rates of PEA in AL patients not receiving digoxin (2.5% in searching the medical records of non-digoxin patients). Although digoxin toxicity was considered in several patients, only one was treated with anti-digoxin antibodies and only one had reduction in dose due to toxicity. In the patients with non-terminal arrhythmias, 4/7 had recurrence of the same arrhythmia despite digoxin discontinuation. Although we cannot exclude digoxin therapy as a contributing factor in the arrhythmic events observed, our overall findings do not support a significantly increased risk of digoxin toxicity in AL amyloidosis, especially as a mechanism of sudden cardiac death. Historical data suggested sudden cardiac death to be accounted for approximately a third of causes of early death [2,13]. We have not observed such a high incidence of sudden cardiac death in our cohort. This may reflect a referral bias to our centre.
Comparison of arrhythmia risk and survival of AL patients with and without digoxin therapy is challenging, given the different patient populations in the study cohorts (newly diagnosed, previously treated) the variable duration of digoxin treatment (till last follow-up, drug discontinuation before end of follow-up), and the significantly increased proportion of patients with cardiac involvement in the digoxin group compared to the non-digoxin group.
This study has several important limitations. The retrospective and non-randomized treatment limits the assessment of potential excess digoxin toxicity. A case–control study and/or prospective trials are needed to achieve more valid data on digoxin safety and efficacy in cardiac AL amyloidosis. Variability in follow-up due to the referral nature of our practice limits our data regarding digoxin continuation in patients who died and details concerning the circumstances and cause of death.
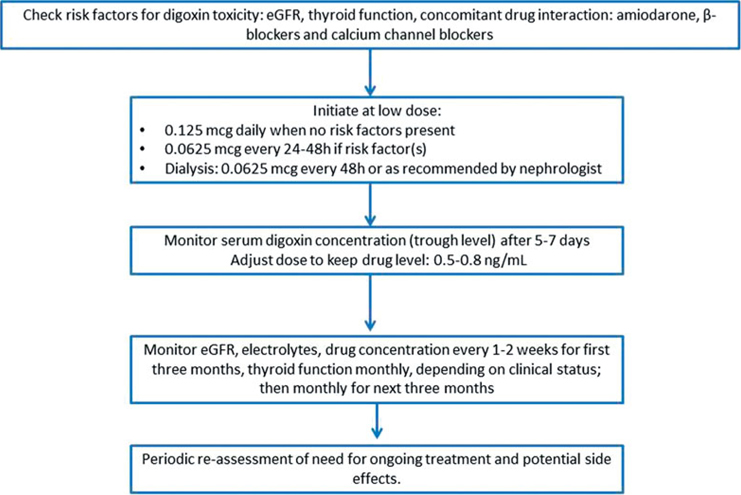
In summary, we have provided the largest contemporary experience on the use of digoxin in AL amyloidosis. Given the limited options for the management of atrial arrhythmias in AL amyloidosis, we conclude that digoxin use may be feasible with an acceptable safety profile, when administered cautiously and at a low daily dose. Drug serum concentration should be monitored along with close monitoring of electrolytes and renal function. We recommend maintaining a trough digoxin level between 0.5–0.8 ng/ml, in keeping with current guidelines for the use of digoxin in heart failure [14], in order to avoid excess toxicity (Figure 1). Physicians should be aware of potential tissue toxic drug concentration irrespective of serum drug concentration, which should prompt periodic reassessment of digoxin use.
Figure 1.
Proposed algoryhtm for the use of digoxin in AL amyloidosis for the management of atrial fibrillation.
Abbreviations:
- AL
light chain amyloidosis
- ASCT
autologous stem cell transplant
- EF
ejection fraction
- eGFR
estimated glomerular filtration rate
- IQR
interquartile range
- NT-proBNP
N-terminal of pro b natriuretic peptide
- PEA
pulseless electrical activity
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Grogan M, Dispenzieri A, Gertz MA. Light-chain cardiac amyloidosis: strategies to promote early diagnosis and cardiac response. Heart. 2017;103:1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Grogan M, Dispenzieri A. Natural history and therapy of AL cardiac amyloidosis [Review]. Heart Fail Rev. 2015; 20:155–162. [DOI] [PubMed] [Google Scholar]
- [3].Longhi S, Quarta CC, Milandri A, et al. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid. 2015;22:147–155. [DOI] [PubMed] [Google Scholar]
- [4].Muchtar E, Dean DS, Dispenzieri A, et al. Prevalence and predictors of thyroid functional abnormalities in newly diagnosed AL amyloidosis. J Intern Med. 2017;281:611–619. [DOI] [PubMed] [Google Scholar]
- [5].Cassidy JT. Cardiac amyloidosis. Two cases with digitalis sensitivity [Case Reports]. Ann Intern Med. 1961;55:989–994. [DOI] [PubMed] [Google Scholar]
- [6].Pomerance A Senile cardiac amyloidosis. Br Heart J. 1965; 27:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rubinow A, Skinner M, Cohen AS. Digoxin sensitivity in amyloid cardiomyopathy. Circulation. 1981;63:1285–1288. [DOI] [PubMed] [Google Scholar]
- [8].Muchtar E, Dispenzieri A, Kumar SK, et al. Immunoparesis in newly diagnosed AL amyloidosis is a marker for response and survival. Leukemia. 2017;31:92–99. [DOI] [PubMed] [Google Scholar]
- [9].Doherty JE, Perkins WH. Digoxin metabolism in hypo- and hyperthyroidism. Studies with tritiated digoxin in thyroid disease. Ann Intern Med. 1966;64:489–507. [DOI] [PubMed] [Google Scholar]
- [10].Marcus FI. Pharmacokinetic interactions between digoxin and other drugs. J Am Coll Cardiol. 1985;5(5 Suppl A):82A–90A. [DOI] [PubMed] [Google Scholar]
- [11].Hamon D, Algalarrondo V, Gandjbakhch E, et al. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol. 2016;222:562–568. [DOI] [PubMed] [Google Scholar]
- [12].Sayed RH, Rogers D, Khan F, et al. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J. 2015;36:1098–1105. [DOI] [PubMed] [Google Scholar]
- [13].Lubitz SA, Goldbarg SH, Mehta D. Sudden cardiac death in infiltrative cardiomyopathies: sarcoidosis, scleroderma, amyloidosis, hemachromatosis. Prog Cardiovasc Dis. 2008;51:58–73. [DOI] [PubMed] [Google Scholar]
- [14].Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]