Dear Sirs,
Since December 2019, severe acute respiratory syndrome (SARS) cases related to the coronavirus disease-2019 (COVID-19) emerged from Wuhan, Hubei Province, China [1–3] and spread all around the world contaminating more than 17 million people (> 174,000 cases in France) [4]. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) leads to a wide spectrum of mild disease most of the time with respiratory system being the most commonly affected organ. Still, coronaviruses have an established neuroinvasive propensity [5, 6], and MRI findings associated with acute neurological manifestations in COVID-19 patients have been recently reported [7, 8] to explain to some extent the neurological symptoms of patients infected with the SARS-CoV-2.
CLOCCs (previously termed Mild Encephalitis/Encephalopathy with Reversible Splenial lesion: MERS) is a rare infection-associated encephalopathy, involving cell-cytokine interactions [9], commonly described in the context of virus infections, metabolic disturbances, or antiepileptic drug. This syndrome presenting with great clinical heterogeneity [10], and complete regression of symptoms [11]. We hereby report the first case of cytotoxic lesion of the corpus callosum (CLOCCs) as presenting neuroradiological manifestation of COVID-2019 infection confirmed by chest computed tomography and nasopharyngeal swab sample test.
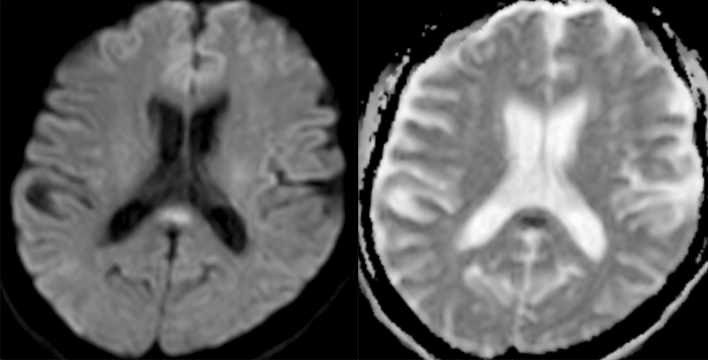
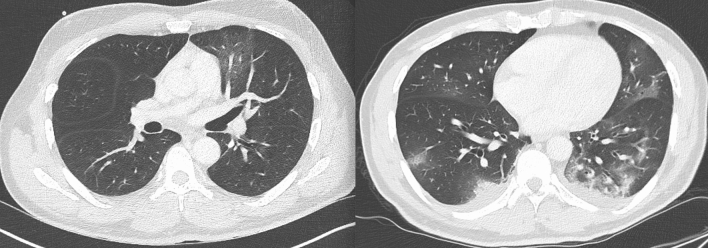
In the context of the SARS-CoV-2 pandemic, a 55-year-old man with no relevant medical history (no history of seizure) was admitted to the emergency department in France for recent headache. On first examination (Mar-20), it revealed body temperature of 38.2 °C (100.76 °F) with normal clinical examination and no other complaints except minor headache. Blood sampling revealed slight C-reactive protein increase at 8.1 mg/l (normal < 6 mg/l) and lymphocytes in the normal range. Blood glucose, hepatic and renal function were normal, as well as natremia and troponin. Patient had no respiratory symptoms. Chest radiograph was normal. During the evening, patient developed fainting sensations on standing with dizziness and impaired consciousness. There were no others neurological symptoms (no cervical rigidity). On a hospital day 2, echocardiography, nasopharyngeal swab for SARS-CoV-2-RNA and influenza were negative, as well as Legionella pneumophila and Streptococcus pneumoniae urinary tests. The laboratory results showed only significant elevated Creactive protein (37 mg/l; normal < 6 mg/l) and the symptoms were stable. On Mar-23 Brain MRI demonstrated findings compatible with a Cytotoxic lesion of the corpus callosum (CLOCCs) (Fig. 1), without other anomalies on MRI scan. Patient also underwent thoraco-abdominopelvic CT showing diffuse ground-glass opacities (Fig. 2), compatible with SARS-CoV-2 infection. On the same day a lumbar puncture was performed. The cerebrospinal fluid (CSF) was unnoticeable: clear with normal glycorrhachia, mild elevated proteins = 0.46 g/l (normal = 0–0.40 g/l), no pleocytosis, no red cells and negative cultures. No reverse transcription-polymerase chain reaction (RT-PCR) assay of the CSF sample was conducted. A repeated blood sampling revealed only increasing C-reactive protein blood levels (65 mg/l; normal < 6 mg/l). On Mar-27, patient respiratory function suddenly deteriorated and repeat chest CT scan (day 7) showed bilateral pulmonary lesions with arising crazypaving pattern (Fig. 2). On the same day, patient required mechanical ventilation for an acute respiratory distress syndrome in the context of a COVID19 with real-time RT-PCR assay of a nasopharyngeal swab specimen returning positive. There was no interval modification of the neurological symptoms. Patient was extubated 17 days later, and repeat MRI 3 days later showed complete regression of the corpus callosum lesion.
Fig. 1.
Axial brain MRI images demonstrate increased diffusion weighted signal in the splenium of the corpus callosum (left), confirmed as low signal on the ADC map (right)
Fig. 2.
Chest CT-scan demonstrates ground glass opacities in the left lower pulmonary lobe (left) and repeat CT-scan (right) showing bilateral pulmonary lesions with crazy-paving pattern
To our knowledge, this is the first reported case of CLOCCs as presenting neuroradiological manifestation of COVID-2019 infection proved by nasopharyngeal swab for SARS-CoV-2-RNA and CT chest. This report confirms the need for neuroimaging in the context of SARS-CoV-2 infection and neurological symptoms as the pandemics evolves and patients may develop wide spectrum of neurological complications.
Compliance with ethical standards
Conflicts of interest
All authors report no disclosures.
Ethical approval
This article does not contain any studies involving human participants performed by any of the Authors.
Informed consent
Written informed consent was obtained from the sister of patient for publication of this case report and any accompanying images.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Z, Guo D, Li C, et al. Coronavirus disease 2019: initial chest CT findings. Eur Radiol. 2020 doi: 10.1007/s00330-020-06816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO (2020) Coronavirus disease 2019 (COVID-19) https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 5.Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2020;12:14. doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74:8913–8921. doi: 10.1128/JVI.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chougar L, Shor N, Weiss N, et al. Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS-CoV-2 infection and neurological manifestations. Radiology. 2020 doi: 10.1148/radiol.2020202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kremer S, Lersy F, Anheim M, et al. Neurologic and neuroimaging findings in COVID-19 patients: a retrospective multicenter study. Neurology. 2020 doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- 9.Starkey J, Kobayashi N, Numaguchi Y, Moritani T. Cytotoxic lesions of the corpus callosum that show restricted diffusion: mechanisms, causes, and manifestations. RadioGraphics. 2017;37(2):562–576. doi: 10.1148/rg.2017160085. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Y, Zheng J, Zhang L, et al. Reversible splenial lesion syndrome associated with encephalitis/encephalopathy presenting with great clinical heterogeneity. BMC Neurol. 2016;16(1):49. doi: 10.1186/s12883-016-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan J, Yang S, Wang S, et al. Mild encephalitis/encephalopathy with reversible splenial lesion (MERS) in adults-a case report and literature review. BMC Neurol. 2017;17(1):103. doi: 10.1186/s12883-017-0875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]