Abstract
Background
Obesity is a global public health threat. Chromium picolinate (CrP) is advocated in the medical literature for the reduction of body weight, and preparations are sold as slimming aids in the USA and Europe, and on the Internet.
Objectives
To assess the effects of CrP supplementation in overweight or obese people.
Search methods
We searched The Cochrane Library, MEDLINE, EMBASE, ISI Web of Knowledge, the Chinese Biomedical Literature Database, the China Journal Fulltext Database and the Chinese Scientific Journals Fulltext Database (all databases to December 2012), as well as other sources (including databases of ongoing trials, clinical trials registers and reference lists).
Selection criteria
We included trials if they were randomised controlled trials (RCT) of CrP supplementation in people who were overweight or obese. We excluded studies including children, pregnant women or individuals with serious medical conditions.
Data collection and analysis
Two authors independently screened titles and abstracts for relevance. Screening for inclusion, data extraction and 'Risk of bias' assessment were carried out by one author and checked by a second. We assessed the risk of bias by evaluating the domains selection, performance, attrition, detection and reporting bias. We performed a meta‐analysis of included trials using Review Manager 5.
Main results
We evaluated nine RCTs involving a total of 622 participants. The RCTs were conducted in the community setting, with interventions mainly delivered by health professionals, and had a short‐ to medium‐term follow up (up to 24 weeks). Three RCTs compared CrP plus resistance or weight training with placebo plus resistance or weight training, the other RCTs compared CrP alone versus placebo. We focused this review on investigating which dose of CrP would prove most effective versus placebo and therefore assessed the results according to CrP dose. However, in order to find out if CrP works in general, we also analysed the effect of all pooled CrP doses versus placebo on body weight only.
Across all CrP doses investigated (200 µg, 400 µg, 500 µg, 1000 µg) we noted an effect on body weight in favour of CrP of debatable clinical relevance after 12 to 16 weeks of treatment: mean difference (MD) ‐1.1 kg (95% CI ‐1.7 to ‐0.4); P = 0.001; 392 participants; 6 trials; low‐quality evidence (GRADE)). No firm evidence and no dose gradient could be established when comparing different doses of CrP with placebo for various weight loss measures (body weight, body mass index, percentage body fat composition, change in waist circumference).
Only three studies provided information on adverse events (low‐quality evidence (GRADE)). There were two serious adverse events and study dropouts in participants taking 1000 µg CrP, and one serious adverse event in an individual taking 400 µg CrP. Two participants receiving placebo discontinued due to adverse events; one event was reported as serious. No study reported on all‐cause mortality, morbidity, health‐related quality of life or socioeconomic effects.
Authors' conclusions
We found no current, reliable evidence to inform firm decisions about the efficacy and safety of CrP supplements in overweight or obese adults.
Keywords: Adult, Humans, Dietary Supplements, Obesity, Obesity/drug therapy, Overweight, Overweight/drug therapy, Picolinic Acids, Picolinic Acids/administration & dosage, Randomized Controlled Trials as Topic, Resistance Training, Weight Lifting, Weight Loss
Plain language summary
Chromium picolinate supplementation for overweight or obese people
Review question
Are chromium supplements useful for reducing body weight in overweight or obese adults?
Background
Chromium is an essential nutrient (trace element) required for the normal metabolism of carbohydrate, protein and fat (i.e. the chemical reactions involved in breaking down these molecules to a form suitable for absorption by the body). Chromium increases the activity of insulin, and dietary supplementation with chromium has produced improvements in glucose metabolism which may lower blood glucose being important for overweight people with diabetes. It is generally believed that chromium may help to reduce a person's weight by decreasing the amount of fat in the body. Chromium is also said to suppress the appetite and stimulate the production of heat by the body, thus increasing energy expenditure. This may contribute to weight loss. Chromium picolinate is one of several chemical compounds of chromium sold as a nutritional supplement as a potential aid to weight loss.
Study characteristics
We included nine randomised controlled trials which compared the efficacy and safety of 8 to 24 weeks of chromium supplementation and placebo in overweight or obese adults (i.e. with a body mass index between 25 and 29.9 kg/m2 defining being overweight and a body mass index of 30kg/m2 or more defining obesity). A total of 622 participants took part in the studies, 346 participants received chromium picolinate and 276 received placebo. The evidence is current to December 2012.
Key results
When the results obtained from the doses of chromium picolinate investigated (200 µg, 400 µg, 500 µg, 1000 µg) were pooled, study participants lost around 1 kg of body weight more than participants receiving placebo. We were unable to find good evidence that this potential weight loss effect increased with increasing dose of chromium picolinate. Only three of nine studies provided information on adverse events, so we were unable to determine whether chromium picolinate supplements are safe and whether any potential harms may increase with dose. In addition, the length of studies included was rather short (maximum of 24 weeks), so we were unable to determine any long‐term effects of supplementation. No study reported whether supplementation was associated with increases in deaths from any cause or illnesses (such as myocardial infarction or stroke), or the health‐related quality of life or socioeconomic effects of supplementation.
Quality of the evidence
The overall quality of evidence was considered low and we have inadequate information from which to draw conclusions about the efficacy and safety of chromium picolinate supplementation in overweight or obese adults.
Summary of findings
for the main comparison.
| Chromium picolinate supplementation for overweight or obese adults | ||||
|
Population: overweight or obese adults Settings: community volunteers and outpatients Intervention: chromium picolinate Comparison: placebo | ||||
| Outcomes | Relative / absolute effect(s) (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Health‐related quality of life | See comment | See comment | See comment | Not investigated |
|
Adverse events Follow‐up: 8 weeks to 6 months |
2 serious adverse events and study dropouts after 1000 µg chromium picolinate (2/15 participants); 1 serious adverse event after 400 µg chromium picolinate (1/39 participants); 1 serious adverse event (1/18 participants) and 2 study dropouts on placebo (2/58 participants) | 189 (3) |
⊕⊕⊝⊝ lowa | Only 3/9 studies provided information on adverse events |
| Death from any cause | See comment | See comment | See comment | Not investigated |
| Morbidity | See comment | See comment | See comment | Not investigated |
|
Weight loss [kg] Follow‐up: 12 to 16 weeks |
‐1.1 (‐1.7 to ‐0.4) | 392 (6) |
⊕⊕⊝⊝ lowb | All chromium picolinate doses were pooled |
| Socioeconomic effects | See comment | See comment | See comment | Not investigated |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
aDowngraded by two levels owing to high risk of performance and detection bias, and inadequate reporting in most of the included studies
bDowngraded by two levels owing to indirectness and conflicting evidence between different studies of various doses of chromium picolinate and duration of treatment
Background
Description of the condition
Obesity and overweight are common global health conditions. The prevalence of obesity and overweight has increased considerably in both developing and developed countries. The World Health Organization (WHO) have estimated that, globally in 2005, approximately 1.6 billion adults (aged 15 years or older) were overweight and that at least 400 million adults were obese (WHO 2006). The WHO projects that, by 2015, approximately 2.3 billion adults will be overweight and more than 700 million will be obese. Obesity is defined as the degree of fat storage associated with elevated health risks. However, because fat mass is difficult to measure, the pragmatic definition of obesity is based on body mass index (BMI). The WHO guidelines define a BMI of 18.5 to 24.9 kg/m2 as normal, 25 to 29.9 kg/m2 as grade 1 overweight and greater than 30 kg/m2 as grade 2 overweight (obesity) (WHO 1995).
Obesity is a concern because of its implications for the health of an individual, as it increases the risk of many diseases and health conditions, including coronary heart disease (Rimm 1995; Whitlock 2002), type 2 diabetes (Colditz 1995), hypertension, dyslipidaemia (Denke 1994), sleep apnoea and respiratory problems (Naimark 1960).
Description of the intervention
Chromium is an essential trace element required for the normal metabolism of carbohydrate, protein and fat. Chromium is a cofactor necessary for the activity of insulin, and dietary supplementation with chromium has produced modest improvements in glucose metabolism, insulin sensitivity and body composition in human trials (Drake 2012). Organic chromium is a compound of trivalent chromium and it assists in efficient chromium absorption. Chromium picolinate (CrP) is advocated in the medical literature for the reduction of body weight (Murray 1998; Pizzorno 1999) and preparations are sold as slimming aids in the USA and Europe, and on the Internet.
Adverse effects of the intervention
In a narrative review, most of the reported side effects of CrP supplementation were non‐specific and the most frequent complaints were watery stools, weakness, dizziness, headaches, nausea and vomiting (Kleefstra 2006). Overall, chromium was well tolerated. There were no serious adverse events. Also, the number of individuals reporting adverse events in the supplemented groups was not significantly different from that in placebo groups (John 2007; Stephen 2008).
How the intervention might work
It is generally believed that chromium may exert its effects on weight loss by decreasing fat levels in the body and through insulin‐sensitising effects. CrP has been suggested to impact on neurotransmitters involved in the regulation of eating behaviour, mood and food cravings (Docherty 2005). Chromium may suppress the appetite and stimulate thermogenesis through sensitisation of insulin‐sensitive glucoreceptors in the brain (Wang 2007). Body fat distribution is related to insulin sensitivity; peripheral fat is more insulin‐sensitive than central fat found in the chest and abdomen (Kahn 2006).
Why it is important to do this review
Chromium may improve impaired glucose tolerance, reduce elevated blood lipid concentrations, and result in weight loss and improved body composition in some individuals, but results have been equivocal (Volpe 2001). A meta‐analysis of 10 double‐blind, placebo‐controlled trials provided evidence of a relatively small reduction in body weight in overweight and obese individuals receiving CrP (Pittler 2003). However, because of the limited number of trials and participants, the clinical relevance of this effect is debatable and a lack of robustness means that the results have to be interpreted with caution. Since the publication of this meta‐analysis, the results of many studies including large numbers of individual shave become available. A systematic review of all available randomised controlled trials (RCTs) is needed, which could help clinicians, individuals and others decide whether chromium is a useful weight loss tool for overweight and obese individuals.
Objectives
To assess the effects of CrP supplementation in overweight or obese people.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Adults (aged 18 years and older) defined as overweight or obese at baseline. We excluded studies including children, pregnant women or individuals with serious medical conditions.
Diagnostic criteria
Adults with a BMI between 25 and 29.9 kg/m2 were considered overweight; those with a BMI of 30 kg/m2 or higher were considered obese.
Types of interventions
We investigated the following comparisons of the intervention versus controls/comparators where the same letters indicate direct comparisons.
Intervention
(a) Chromium picolinate (CrP)
(b) CrP plus another treatment
Comparator
(a1) Placebo
(a2) Different CrP dosage
(b) Placebo plus another treatment
Concomitant treatments (e.g. diet or exercise) had to be identical between intervention and control groups.
Types of outcome measures
Primary outcomes
Weight loss (e.g. BMI, waist circumference, percentage body fat).
Adverse events (e.g. gastrointestinal, nervous system, metabolism).
Health‐related quality of life (measured with a validated instrument).
Secondary outcomes
Death from any cause.
Morbidity (e.g. cardiovascular outcomes such as myocardial infarction or stroke).
Blood pressure.
Lipids (e.g. total cholesterol, HDL‐C, LDL‐C and triglycerides).
Fasting blood glucose.
Socioeconomic effects.
Timing of outcome measurement
Short‐term: one to six weeks.
Medium‐term: more than 6 weeks to 12 weeks.
Long‐term: more than 12 weeks.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception to the specified date to identify trials:
The Cochrane Library (Issue 10, 2012).
MEDLINE (to December 2012).
EMBASE (to December 2012).
ISI Web of Knowledge (to December 2012).
Chinese Biomedical Literature Database (CBM) (to December 2012).
China Journal full‐text database (to December 2012).
Chinese Scientific Journals full‐text database (to December 2012).
We also searched databases of ongoing trials (www.ClinicalTrials.gov/) and the Current Controlled Trials metaRegister (www.controlled‐trials.com/).
For detailed search strategies please see Appendix 1 (searches were not older than six months at the moment the final review draft was checked into the Cochrane Information Management System for editorial approval). We used PubMed's 'My NCBI' (National Center for Biotechnology Information) email alert service for the identification of newly published studies using a basic search strategy (see Appendix 1).
If we detected additional key words of relevance during any of the electronic or other searches we planned to modify the electronic search strategies to incorporate these terms. We included studies published in any language.
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, (systematic) reviews, meta‐analyses and health‐technology assessment reports.
Data collection and analysis
Selection of studies
To identify the studies to be assessed further, two review authors (TH, GX) independently scanned the abstract or title, or both, of every record retrieved. We investigated the full text of all potentially relevant articles. Where there were differences in opinion between authors, these were resolved by a third author (ZZ). If resolution of disagreement was not possible, we intended to add the article to those 'awaiting assessment' and we contacted the trial authors for clarification. We present an adapted PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow‐chart showing the process of study selection (Figure 1) (Liberati 2009).
1.
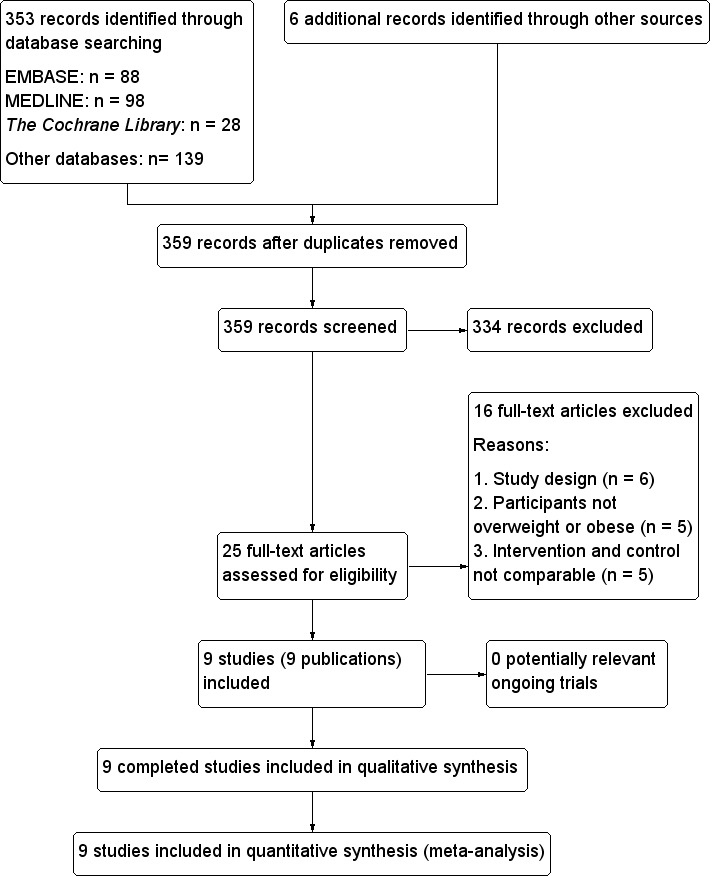
Study flow diagram.
Data extraction and management
For studies that fulfilled the inclusion criteria, two authors (TH, HZ) independently extracted relevant population and intervention characteristics using standard data extraction templates (for details see Table 2 and Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9); any disagreements were resolved by discussion or, if required, by a third author.
1. Overview of study populations.
|
Characteristic Study ID |
Intervention(s) and control(s) | [N] Screened / eligible | [N] Randomised | [N] Safety | [N] ITT | [N] Finishing study | [%] Randomised finishing study | Follow‐upa |
| 1. Kaats 1996 | I1: CrP 200 μg/day | 233 | 33 | 33 | ‐ | 33 | 100 | 72 days |
| I2: CrP 400 μg/day | 66 | 66 | ‐ | 66 | 100 | |||
| C: placebo | 55 | 55 | ‐ | 55 | 100 | |||
| total: | 154 | 154 | ‐ | 154 | 100 | |||
| 2. Kaats 1998 | I: CrP 400 μg/day | 130 | 62 | 62 | ‐ | 62 | 100 | 90 days |
| C: placebo | 60 | 60 | ‐ | 60 | 100 | |||
| total: | 122 | 122 | ‐ | 122 | 100 | |||
| 3. Joseph 1999 | I: CrP 1000 μg/day + RT | 35 | 17 | 17 | ‐ | 17 | 100 | 12 weeks |
| C: placebo + RT | 15 | 15 | ‐ | 15 | 100 | |||
| total: | 32 | 32 | ‐ | 32 | 100 | |||
| 4. Kleefstra 2006 | I1: CrP 500 μg/day | 60 | 19 | 19 | ‐ | 17 | 89 | 6 months |
| I2: CrP 1000 μg/day | 17 | 17 | ‐ | 14 | 82 | |||
| C: placebo | 17 | 17 | ‐ | 15 | 88 | |||
| total: | 53 | 53 | ‐ | 46 | 87 | |||
| 5. Iqbal 2009 | I: CrP 500 μg/day | 153 | 33 | 33 | 28 | 28 | 84 | 16 weeks |
| C: placebo | 30 | 30 | 29 | 29 | 96 | |||
| total: | 63 | 63 | 57 | 57 | 90 | |||
| 6. Volpe 2001 | I: CrP 400 μg/day + weight training | 44 | 22 | 22 | ‐ | 20 | 91 | 12 weeks |
| C: placebo + weight training | 22 | 22 | ‐ | 17 | 77 | |||
| total: | 44 | 44 | ‐ | 37 | 84 | |||
| 7. Anton 2008 | I: CrP 400 μg/day | 99 | 28 | 28 | ‐ | 19 | 68 | 8 weeks |
| C: placebo | 28 | 28 | ‐ | 21 | 75 | |||
| total: | 56 | 56 | ‐ | 40 | 71 | |||
| 8. Campbell 1999 | I: CrP 1000 μg/day + RT | 23 | 9 | 9 | ‐ | 9 | 100 | 13 weeks |
| C: placebo + RT | 9 | 9 | ‐ | 9 | 100 | |||
| total: | 18 | 18 | ‐ | 18 | 100 | |||
| 9. Yazaki 2010 | I: CrP 400 μg/day | 156 | 40 | 40 | ‐ | 30 | 75 | 24 weeks |
| C: placebo | 40 | 40 | ‐ | 28 | 70 | |||
| total: | 80 | 80 | ‐ | 58 | 72 | |||
| Grand total | All interventions | 346 | 320 | 93 | ||||
| All controls | 276 | 256 | 93 | |||||
| All interventions and controls | 622 | 576 | 93 | |||||
aDuration of intervention and/or follow‐up under randomised conditions until end of study
"‐" denotes not reported
C: control; CrP: chromium picolinate; I: intervention; ITT: intention‐to‐treat; RT: resistance training
We sent an email request to contact authors of published studies to enquire whether they were willing to answer questions regarding their trials. We published the results of this survey in Appendix 10. Thereafter, we sought relevant missing information on the trial from the original author(s) of the article, if required.
We planned to provide information, including the trial identifier, about potentially relevant ongoing studies in the table 'Characteristics of ongoing studies'. We also intended to include specific data from the protocol of each included study, obtained from databases of ongoing trials or from publications of study designs, or both, in Appendix 6 ('Matrix of study endpoints (protocol/trial documents)').
Dealing with duplicate publications and companion papers
In the case of duplicate publications and companion papers of a primary study, we tried to maximise the yield of information by the simultaneous evaluation of all available data.
Assessment of risk of bias in included studies
Two authors (TH, JL) assessed each trial independently. We resolved possible disagreements by consensus, or by consultation with a third author (ZZ). In cases of disagreement, we consulted the rest of the group and made a judgement based on consensus.
We assessed risk of bias using The Cochrane Collaboration’s tool (Higgins 2011; Higgins 2011a) and adopted the following bias criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding (performance bias and detection bias), separated for blinding of participants and personnel and blinding of outcome assessment.
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias) ‐ see Appendix 5.
Other bias.
We judged 'Risk of bias' criteria as low, high or unclear, and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We present a 'Risk of bias' figure and a 'Risk of bias summary' figure.
We assessed the impact of individual bias domains on study results at endpoint and study levels.
For performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessors) and attrition bias (incomplete outcome data) we intended to evaluate risk of bias separately for subjective and objective outcomes.
We defined the following endpoints as subjective outcomes.
Adverse events.
Health‐related quality of life.
We defined the following outcomes as objective outcomes.
Weight loss.
Death from any cause.
Blood pressure.
Lipids.
Fasting blood glucose.
Socioeconomic effects.
Measures of treatment effect
We expressed dichotomous data as odds ratios (ORs) or risk ratios (RRs) with 95% confidence intervals (CIs). We expressed continuous data as mean differences (MDs) with 95% CIs.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome.
Dealing with missing data
We tried our best to obtain relevant missing data from authors if feasible, and carefully performed evaluations of important numerical data, such as screened, randomised participants as well as intention‐to‐treat (ITT), as‐treated and per‐protocol (PP) populations. We investigated attrition rates (e.g. dropouts, losses to follow‐up and withdrawals), and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward (LOCF)).
Assessment of heterogeneity
In the event of substantial clinical, methodological or statistical heterogeneity, our intention was not to report study results as meta‐analytically pooled effect estimates.
We identified heterogeneity by visual inspection of the forest plots and by using a standard Chi2 test with a significance level of α = 0.1, in view of the low power of this test. We specifically examined heterogeneity using the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I2 statistic of 75% or more indicates a considerable level of inconsistency (Higgins 2011).
If heterogeneity was found, we intended to attempt to determine potential reasons for it by examining individual study and subgroup characteristics.
We expected the following characteristics to introduce clinical heterogeneity:
Sex.
Age.
Chromium doses.
Body mass index (BMI).
Duration of treatment.
Assessment of reporting biases
We planned to use funnel plots when we included 10 or more studies for a given outcome, in order to assess small study effects. As there could be several explanations for funnel plot asymmetry we planned to interpret results carefully (Sterne 2011).
Data synthesis
We planned, unless there was good evidence for homogeneity across studies, to primarily summarise data at low risk of bias by means of a random‐effects model (Wood 2008). We intended to interpret random‐effects meta‐analyses giving due consideration to the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual study (Riley 2011). In addition, we performed statistical analyses according to the statistical guidelines contained in the newest version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses of our primary outcome parameter(s) (see above) and investigate any interactions:
Dose (depending on data).
Duration of intervention (depending on data).
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors on effect sizes.
Restricting the analysis to published studies.
Restricting the analysis, taking into account risk of bias, as specified above.
Restricting the analysis to very long or large studies to establish how much they dominate the results.
Restricting the analysis to studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
We also planned to test the robustness of the results by repeating the analysis using different measures of effect size (RR, OR etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For a detailed description of studies, see the 'Characteristics of included studies' and 'Characteristics of excluded studies' sections.
Results of the search
The initial search identified 359 records; from these, 25 full text papers were identified for further examination. We excluded the other studies on the basis of their titles or abstracts because they did not meet the inclusion criteria, were not relevant to the question under study or were a duplicate report (see Figure 1). After screening the full text of the selected publications, nine studies (nine publications) met the inclusion criteria. All studies were published in English. We contacted all authors of included studies and received no reply.
Included studies
A detailed description of the characteristics of included studies is presented elsewhere (see 'Characteristics of included studies' and Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9).
The following is a succinct overview.
Comparisons
Three studies evaluated CrP plus resistance training (RT) or weight training versus placebo with RT or weight training (Campbell 1999; Joseph 1999; Volpe 2001). The other studies investigated CrP alone versus placebo.
Overview of study populations
A total of 622 participants were included in the nine trials, 346 participants were randomised to CrP and 276 to placebo. A total of 320 (93%) participants receiving CrP and 256 (93%) participants receiving placebo finished the study.The individual total sample sizes ranged from 18 to 154.
Study design
All studies were RCTs. All trials adopted a parallel‐group superiority design and all used a placebo control. No trial was multicentred. In terms of blinding, five studies were double‐blinded for participants and personnel (Joseph 1999; Kaats 1996; Kaats 1998; Kleefstra 2006; Yazaki 2010). Outcome assessors were blinded in four studies (Joseph 1999; Kaats 1996; Kaats 1998; Kleefstra 2006). Studies were performed between the years 1996 and 2010. The duration of interventions ranged from eight weeks to six months, with a mean study period of 12 weeks. Only two trials had a duration of intervention longer than 24 weeks (Kleefstra 2006; Yazaki 2010); durations in the other trials were 16 weeks (Iqbal 2009), 12 weeks (Campbell 1999; Joseph 1999; Volpe 2001), 10 weeks (Kaats 1996), 13 weeks (Kaats 1998) and 8 weeks (Anton 2008).
Settings
All of the studies were conducted in the USA. Two studies had an outpatient setting (Kleefstra 2006; Iqbal 2009); the other studies included community volunteers.
Participants
The participating population comprised overweight and obese adults only (see Appendix 3 and Appendix 4). Females were recruited more often than males in four trials (Iqbal 2009; Kaats 1996; Kaats 1998; Kleefstra 2006); one trial recruited more male than female participants (Joseph 1999). Two trials included only women (Anton 2008; Volpe 2001) and one trial only men (Campbell 1999). Four trials reported age as a range of values (Campbell 1999; Iqbal 2009; Volpe 2001; Yazaki 2010), whereas five trials reported age as a mean value (Anton 2008; Joseph 1999; Kaats 1996; Kaats 1998; Kleefstra 2006). All trials included participants from economically developed countries. Two trials reported the ethnic proportion of participants (Anton 2008; Iqbal 2009). One trial included participants with diabetes mellitus reporting insulin treatment before the start of the trial (Kleefstra 2006). Across all studies, mean baseline BMI at baseline ranged from 28.4 to 37.8 kg/m2.
No trial reported participant comorbidities, six trials provided detail about cointerventions in participants (Anton 2008; Campbell 1999; Joseph 1999; Kaats 1998; Volpe 2001; Yazaki 2010) and one trial provided details of the concomitant medications used by participants (Kleefstra 2006). Criteria for entry into the individual studies are outlined in the 'Characteristics of included studies' section.
Diagnosis
Participants were diagnosed as overweight or obese according to BMI criteria. In all the studies, participants had a BMI greater than 25 kg/m2.
Interventions
No study had a titration period. CrP was applied by the oral route and varied in dosing schedule between one and two times a day. The daily dose of chromium varied between 0.4 mg and 1 mg, with an average daily dose of 0.5 mg. All studies used a matching placebo as the control intervention.
Outcomes
All studies explicitly stated a primary endpoint in the publication; five studies also stated secondary endpoints (Anton 2008; Iqbal 2009; Kleefstra 2006; Volpe 2001; Yazaki 2010).
Reporting of endpoints
BMI was measured in four studies (Iqbal 2009; Joseph 1999; Kleefstra 2006; Yazaki 2010), weight was measured in six studies (Anton 2008; Campbell 1999; Joseph 1999; Kaats 1996; Kaats 1998; Volpe 2001). Body fat (as a percentage) was measured in six studies (Campbell 1999; Joseph 1999; Kaats 1996; Kaats 1998; Volpe 2001; Yazaki 2010). Waist circumference was measured in three studies (Iqbal 2009; Joseph 1999; Volpe 2001). Lipids were measured in four studies (Iqbal 2009; Kleefstra 2006; Volpe 2001; Yazaki 2010). Fasting glucose was measured in four studies (Anton 2008; Iqbal 2009; Volpe 2001; Yazaki 2010). Three studies reported adverse events (Anton 2008; Kleefstra 2006; Yazaki 2010). Two studies assessed food intake (Anton 2008; Volpe 2001), and two studies assessed muscle size, and strength or power development during the trial (Campbell 1999; Volpe 2001).
No studies investigated death from any cause, health‐related quality of life or the socioeconomic effects of treatment. For a summary of all outcomes assessed in each study, see Appendix 5.
Excluded studies
Sixteen publications were excluded after careful evaluation of the full‐text article (Albarracin 2008; Bunting 1994; Diaz 2008; Docherty 2005; Earle 1989; Geohas 2007; Hoeger 1998; Joyal 2004; Pasman 1997; Pittler 2004; Rabinowitz 1983; Stupar 1999; Trent 1995; Wang 2010; Wilson 1995; Zenk 2007) ‐ see Figure 1.
The reasons for exclusion were: intervention and control not comparable (Albarracin 2008; Diaz 2008; Geohas 2007; Hoeger 1998; Zenk 2007), study design (Bunting 1994; Joyal 2004; Pasman 1997; Pittler 2004; Stupar 1999; Wang 2010) and participants not being obese or overweight (Docherty 2005; Earle 1989; Rabinowitz 1983; Trent 1995; Wilson 1995). For further details, see 'Characteristics of excluded studies'.
Risk of bias in included studies
For details on the risk of bias of included studies see 'Characteristics of included studies'. For an overview of review authors' judgements about each 'Risk of bias' item for individual studies and across all studies, see Figure 2 and Figure 3. We investigated performance bias, detection bias and attrition bias separately for objective and subjective outcome measures. We defined weight loss (e.g. BMI, waist circumference, percentage body fat); blood pressure; lipids (e.g. total cholesterol, HDL‐C and LDL‐C; triglycerides); and fasting blood glucose as objective outcomes. We defined adverse events (e.g. gastrointestinal, nervous system, metabolism) and health‐related quality of life as subjective outcomes.
2.
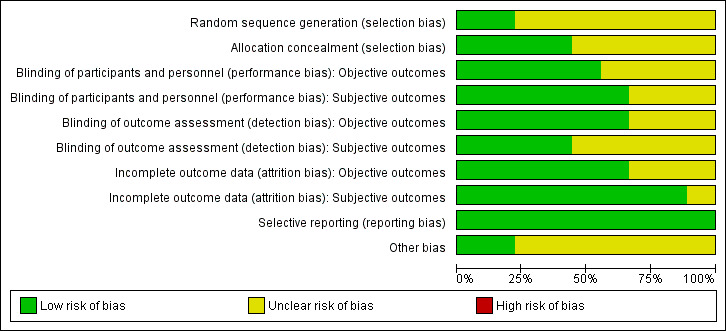
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
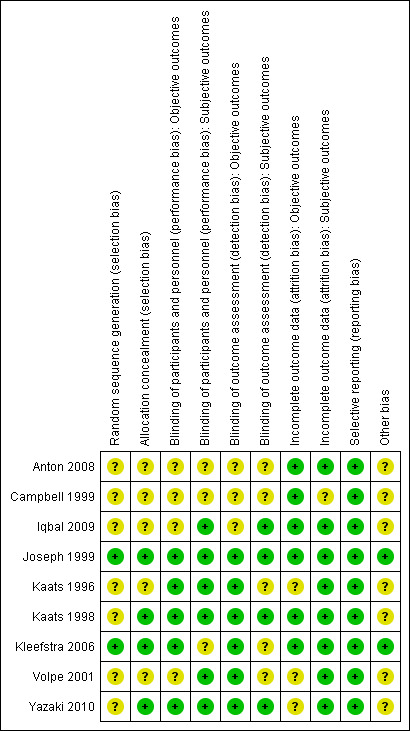
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four trials reported that allocation to groups was concealed (Joseph 1999; Kaats 1998; Kleefstra 2006; Yazaki 2010); the remainder did not explain how concealment was carried out, and were thus graded 'unclear' for the domain based on this criterion. Two trials provided details on random sequence generation (Joseph 1999; Kleefstra 2006).
Blinding
Five studies explicitly stated that blinding of participants and personnel was undertaken (Joseph 1999; Kaats 1996; Kaats 1998; Kleefstra 2006; Yazaki 2010). Four studies did not provide sufficient information about blinding procedures (Anton 2008; Campbell 1999; Iqbal 2009; Volpe 2001).
Incomplete outcome data
Numbers of study withdrawals were described in six studies that had losses to follow up (Anton 2008; Campbell 1999; Iqbal 2009; Kleefstra 2006; Volpe 2001; Yazaki 2010). Analysis was reported as ITT in one study (Iqbal 2009). No ITT analysis was undertaken in six trials (Anton 2008; Campbell 1999; Kaats 1996; Kleefstra 2006; Volpe 2001; Yazaki 2010). One study used PP analyses (Kleefstra 2006). Two studies did not report losses to follow up (Joseph 1999; Kaats 1998). Detailed descriptions of participants' withdrawals and reasons underpinning them were not provided in the study by Kaats 1996.
Selective reporting
All trials met a low 'Risk of bias' criteria for selective reporting, as they reported the prespecified primary outcomes and all expected outcomes.
Other potential sources of bias
Seven trials had a commercial source of funding possibly creating a risk of bias (Anton 2008; Campbell 1999; Iqbal 2009; Kaats 1996; Kaats 1998; Volpe 2001; Yazaki 2010).
Effects of interventions
See: Table 1
Baseline characteristics
For details of baseline characteristics, see Appendix 3 and Appendix 4.
Chromium picolinate (pooled doses versus placebo)
We focused this review on investigating which dose of CrP versus placebo would prove most effective and therefore specified the comparisons ranked according to CrP dose.
However, in order to find out whether CrP works in general, we also analysed the effect on body weight of the pooled CrP doses versus placebo. The MD in weight between CrP and placebo groups after 12 to 16 weeks of treatment was in favour of CrP (MD ‐1.1 kg (95% CI ‐1.7 to ‐0.4); P = 0.001; 392 participants; 6 trials; I2 = 0%; Analysis 1.1).
1.1. Analysis.
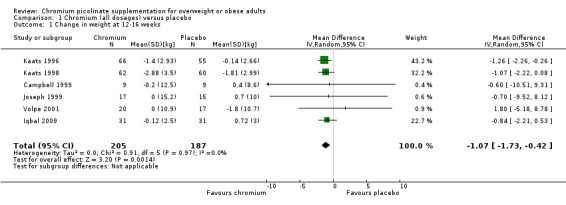
Comparison 1 Chromium (all dosages) versus placebo, Outcome 1 Change in weight at 12‐16 weeks.
Chromium picolinate 200 μg versus placebo
Primary outcomes
Weight change outcomes
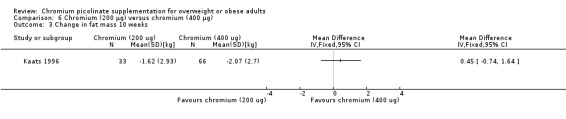
After 10 weeks of treatment, the one trial assessing weight loss (Kaats 1996) found no statistically significant differences in weight loss between the CrP 200 μg and placebo groups (MD ‐0.9 kg (95% CI ‐2.3 to 0.4); P = 0.18; 88 participants; Analysis 2.1). However, participants in the CrP groups lost a greater percentage of body fat (MD ‐1.1 kg (95% CI ‐2.0 to ‐0.2); P = 0.02; 88 participants; Analysis 2.2) and fat mass (MD ‐1.4 kg (95% CI ‐2.7 to ‐0.2); P = 0. 02; 88 participants; Analysis 2.3) than participants in the control groups.
2.1. Analysis.
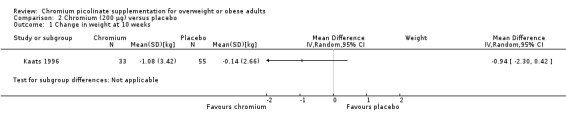
Comparison 2 Chromium (200 μg) versus placebo, Outcome 1 Change in weight at 10 weeks.
2.2. Analysis.
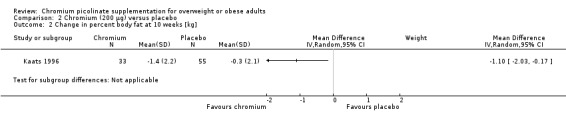
Comparison 2 Chromium (200 μg) versus placebo, Outcome 2 Change in percent body fat at 10 weeks [kg].
2.3. Analysis.
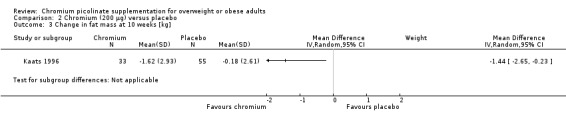
Comparison 2 Chromium (200 μg) versus placebo, Outcome 3 Change in fat mass at 10 weeks [kg].
Health‐related quality of life
Not investigated.
Adverse events
Not reported.
Secondary outcomes
Death from any cause
Not reported.
Socioeconomic effects
Not investigated.
Chromium picolinate 400 μg versus placebo
Primary outcomes
Weight change outcomes
Change in body mass index
There was no statistically significant difference between the two groups at six weeks (MD 0.2 kg/m2 (95% CI ‐2.4 to 2.8); P = 0.88; 42 participants; 1 trial; Analysis 3.1.1) and 12 weeks (MD 1 kg/m2 (95% CI ‐1.3 to 3.3); P = 0.39; 42 participants; 1 trial; Analysis 3.1.2).
3.1. Analysis.
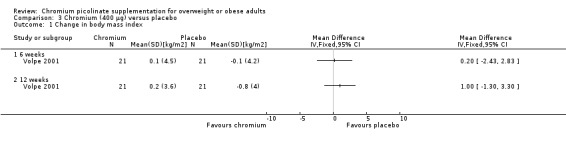
Comparison 3 Chromium (400 μg) versus placebo, Outcome 1 Change in body mass index.
Change in weight loss
In a short‐term, six‐week trial there were no statistically significant differences between the two groups (MD ‐0.7 kg (95% CI ‐7.5 to 6.1); P = 0.84; 42 participants; 1 trial; Analysis 3.2.1). Three trials presented weight loss outcomes at around 12 weeks (Kaats 1996; Kaats 1998; Volpe 2001): participants in the CrP groups lost more weight than participants in the control intervention (MD ‐1.1 kg (95% CI ‐1.9 to ‐0.4); P = 0.003; 280 participants; 3 trials; I2 = 0%; Analysis 3.2.2).
3.2. Analysis.
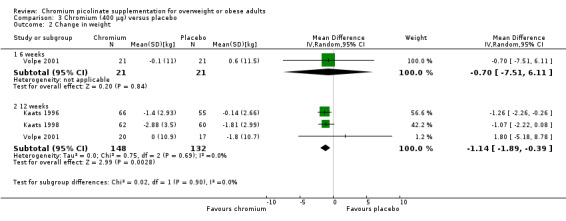
Comparison 3 Chromium (400 μg) versus placebo, Outcome 2 Change in weight.
Change in percentage body fat
No statistically significant differences were apparent at six weeks (MD ‐0.9% (95% CI ‐2 to 0.2); P = 0.12; 122 participants; 1 trial; Analysis 3.3.1) or at 12 weeks (MD ‐0.9% (95% CI ‐2 to 0.2); P = 0.10; 280 participants; 3 trials; I2 = 56%; Analysis 3.3.2).
3.3. Analysis.
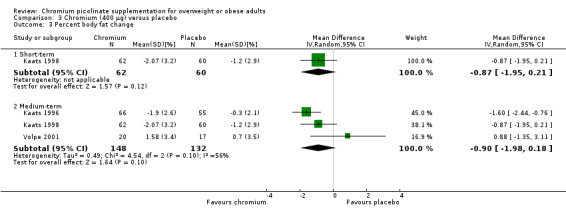
Comparison 3 Chromium (400 μg) versus placebo, Outcome 3 Percent body fat change.
Change in fat mass
No statistically significant differences were detected at six weeks (MD ‐0.4 kg (95% CI ‐4.6 to 3.8); P = 0.84; 42 participants; one trial; Analysis 3.4.1). At 12 weeks a decrease was observed in favour of CrP (MD ‐1.6 kg (95% CI ‐2.3 to ‐0.9); P < 0.0001; 280 participants; 3 trials; I2 = 0%; Analysis 3.4.2).
3.4. Analysis.
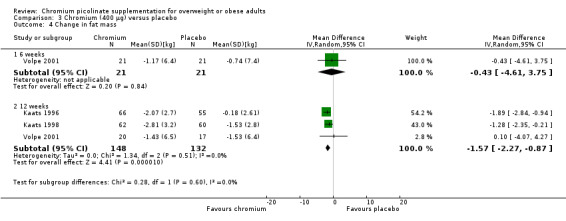
Comparison 3 Chromium (400 μg) versus placebo, Outcome 4 Change in fat mass.
Change in waist circumference
The change in waist circumference was not statistically significantly different between the two groups at six weeks (MD 0.2 cm (95% CI ‐5.8 to 6.2); P = 0.95; 42 participants; 1 trial; Analysis 3.5.1) or 12 weeks (MD ‐1.4 cm (95% CI ‐7.7 to 4.9); P = 0.66; 37 participants; 1 trial; Analysis 3.5.2).
3.5. Analysis.
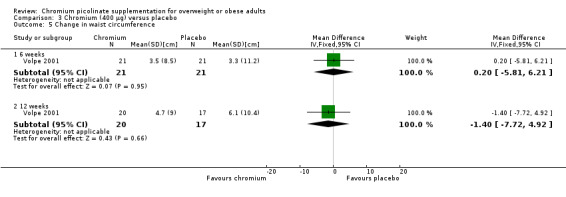
Comparison 3 Chromium (400 μg) versus placebo, Outcome 5 Change in waist circumference.
Health‐related quality of life outcomes
Not investigated.
Adverse events
One participant receiving CrP and one participant receiving placebo experienced a serious adverse event (see Appendix 8). Two participants receiving placebo left the study due to adverse events (see Appendix 9).
Secondary outcomes
Change in fasting glucose
Fasting glucose was examined in a single study (Volpe 2001). There were no statistically significant differences between the CrP and placebo groups at 12 weeks (MD ‐2 mg/dL (95% CI ‐12 to 8); P = 0.70; 37 participants; 1 trial; Analysis 3.6).
3.6. Analysis.
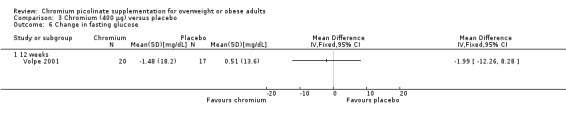
Comparison 3 Chromium (400 μg) versus placebo, Outcome 6 Change in fasting glucose.
Change in total cholesterol
There was no statistically significant difference between the CrP group and placebo group after 12 weeks of treatment (MD ‐0.5 mg/dL (95% CI ‐23 to 24); P = 0.97; 37 participants; 1 trial; Analysis 3.7) (Volpe 2001).
3.7. Analysis.
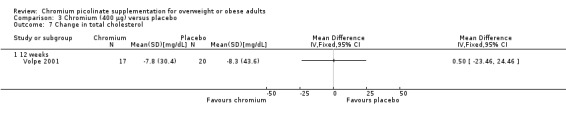
Comparison 3 Chromium (400 μg) versus placebo, Outcome 7 Change in total cholesterol.
Change in triacylglycerol
Change in triacylglycerol levels was not statistically significantly different between the two groups at 12 weeks (MD 2 mg/dL (95% CI ‐39 to 43); P = 0.92; 37 participants; 1 trial; Analysis 3.8) (Volpe 2001).
3.8. Analysis.
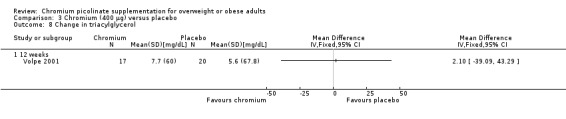
Comparison 3 Chromium (400 μg) versus placebo, Outcome 8 Change in triacylglycerol.
Death from any cause
Not reported.
Socioeconomic effects
Not investigated.
Chromium picolinate 500 μg versus placebo
Two studies (Iqbal 2009; Kleefstra 2006) with a combined total of 91 participants included data on the effect of CrP 500 μg versus placebo.
Primary outcomes
Weight change outcomes
Change in body mass index
One study (Kleefstra 2006) found no statically significant differences between the CrP and placebo groups at six months (MD 0.2 kg/m2 (95% CI ‐0.45 to 0.9); P = 0.56; 31 participants; Analysis 4.1). Results were similar at 16 weeks (MD ‐0.8 kg/m2 (95% CI ‐2.2 to 0.5); P = 0.23; 62 participants; Analysis 4.2).
4.1. Analysis.
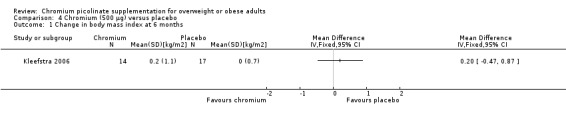
Comparison 4 Chromium (500 μg) versus placebo, Outcome 1 Change in body mass index at 6 months.
4.2. Analysis.
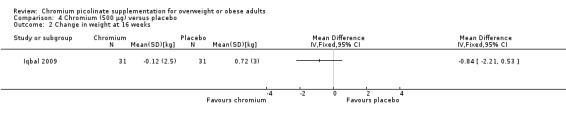
Comparison 4 Chromium (500 μg) versus placebo, Outcome 2 Change in weight at 16 weeks.
Change in waist circumference
The change in waist circumference at 16 weeks was not statistically significantly different between the two groups (MD 0.6 cm (95% CI ‐1 to 2.3); P = 0.45; 60 participants; 1 trial; Analysis 4.3).
4.3. Analysis.
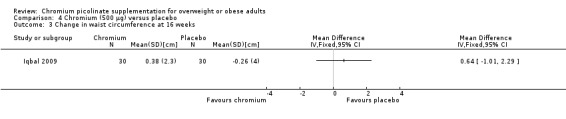
Comparison 4 Chromium (500 μg) versus placebo, Outcome 3 Change in waist circumference at 16 weeks.
Health‐related quality of life outcomes
Not investigated.
Adverse events
Not reported.
Secondary outcomes
Change in fasting glucose
No statistically significant differences were detected at 16 weeks between groups (MD 0.4 mg/dL (95% CI ‐0.2 to 0.9); P = 0.17; 60 participants; 1 trial; Analysis 4.4).
4.4. Analysis.
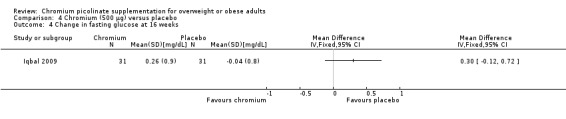
Comparison 4 Chromium (500 μg) versus placebo, Outcome 4 Change in fasting glucose at 16 weeks.
Change in blood pressure
Change in blood pressure at 16 weeks was not statistically significantly different between the two groups, for either systolic blood pressure (MD 0 mm Hg (95% CI ‐12 to 12); P = 1.00; 31 participants; 1 trial; Analysis 4.5) or diastolic blood pressure (MD 2 mm Hg (95% CI ‐5 to 9); P = 0.56; 31 participants; 1 trial; Analysis 4.6).
4.5. Analysis.
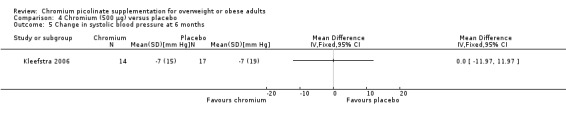
Comparison 4 Chromium (500 μg) versus placebo, Outcome 5 Change in systolic blood pressure at 6 months.
4.6. Analysis.
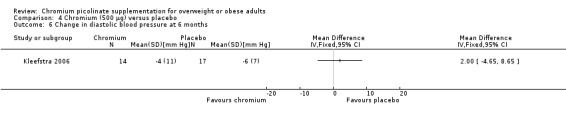
Comparison 4 Chromium (500 μg) versus placebo, Outcome 6 Change in diastolic blood pressure at 6 months.
Change in total cholesterol
Change in total cholesterol was reported in two studies (Iqbal 2009; Kleefstra 2006).There was no statistically significant difference between the intervention and placebo groups (MD ‐0.1 mg/dL (95% CI ‐0.5 to 0.4); P = 0.88; 91 participants; 1 trial; I2 = 0%; Analysis 4.7).
4.7. Analysis.
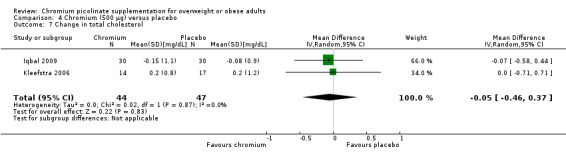
Comparison 4 Chromium (500 μg) versus placebo, Outcome 7 Change in total cholesterol.
Change in triacylglycerol
There was no statistically significant difference between the CrP and placebo groups (MD ‐0.3 (95% CI ‐0.8 to 0.2); P = 0.26; 93 participants; 2 trials; I2 = 0%; Analysis 4.8).
4.8. Analysis.
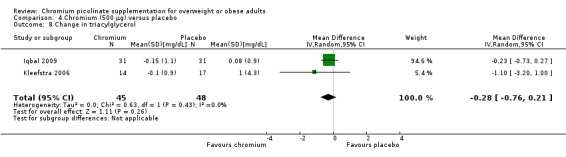
Comparison 4 Chromium (500 μg) versus placebo, Outcome 8 Change in triacylglycerol.
Death from any cause
Not reported.
Socioeconomic effects
Not investigated.
Chromium picolinate 1000 μg versus placebo
Five studies (Anton 2008; Campbell 1999; Joseph 1999; Kleefstra 2006; Yazaki 2010) with a combined total of 207 participants included data on the effects of CrP 1000 μg versus placebo.
Primary outcomes
Weight change outcomes
Change in weight loss
After 12 weeks of treatment, two trials(Campbell 1999; Joseph 1999) found that there was no statistically significant difference in weight loss between groups (MD ‐0.7 kg (95% CI ‐7.3 to 5.9); P = 0.85; 50 participants; 2 trials; I2 = 0%; Analysis 5.1.1). Also, there was no statistically significant difference in BMI change at 24 weeks (MD 0.11 kg/m2 (95% CI ‐0.1 to 0.3); P = 0.25; 90 participants; 2 trials; Analysis 5.2.1) or 12 weeks (MD 0.3 kg/m2 (95% CI ‐0.01 to 0.6); P = 0.06; 99 participants; I2 = 0%; 2 trials; Analysis 5.2.2).
5.1. Analysis.
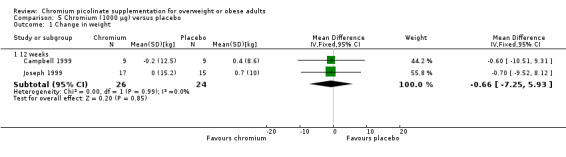
Comparison 5 Chromium (1000 μg) versus placebo, Outcome 1 Change in weight.
5.2. Analysis.
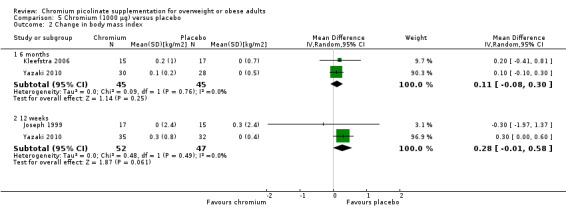
Comparison 5 Chromium (1000 μg) versus placebo, Outcome 2 Change in body mass index.
Change in percentage body fat
There was no statistically significant difference with regard to percentage body fat change between intervention and comparator groups at 24 weeks (MD 1% (95% CI ‐0.4 to 2.6); P = 0.14; 58 participants; 1 trial; Analysis 5.3.1) or 12 weeks (MD 0.9% (95% CI ‐0.4 to 2.2); P = 0.16; 117 participants; 3 trials; Analysis 5.3.2).
5.3. Analysis.
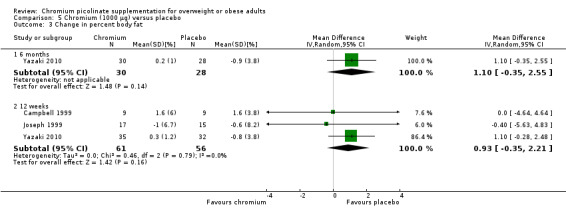
Comparison 5 Chromium (1000 μg) versus placebo, Outcome 3 Change in percent body fat.
Change in waist circumference
The change in waist circumference at 12 weeks did not differ statistically significantly between the two groups (MD ‐1.6 cm (95% CI ‐6.5 to 3.3); P = 0.52; 32 participants; 1 trial; Analysis 5.4).
5.4. Analysis.
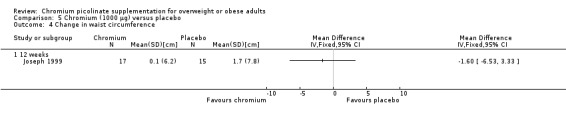
Comparison 5 Chromium (1000 μg) versus placebo, Outcome 4 Change in waist circumference.
Health‐related quality of life outcomes
Not investigated.
Adverse events
Two studies reported adverse events at six months and found no statistically significant differences between groups (RR 4.03 (95% CI 0.46 to 35.11); P = 0.21; 94 participants; I2 = 0%; Analysis 5.9.1); one study also found no statistically significant difference at 12 weeks (RR 0.30 (95% CI 0.01 to 7.02); P = 0.46; 40 participants; Analysis 5.9.2).
5.9. Analysis.
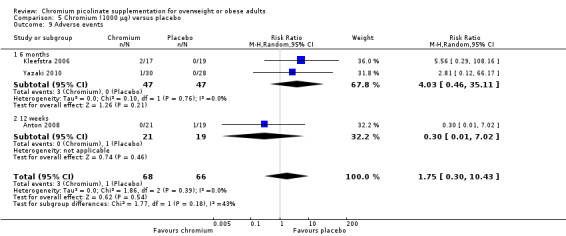
Comparison 5 Chromium (1000 μg) versus placebo, Outcome 9 Adverse events.
Two participants receiving CrP reported a serious adverse event (see Appendix 8) and left the study due to an adverse event (see Appendix 9).
Secondary outcomes
Change in fasting glucose
Fasting glucose was examined in two studies (Joseph 1999; Yazaki 2010) that found no statistically significant differences between groups at 12 weeks (MD 0.3 mg/dL (95% CI ‐1 to 1); P = 0.64; 99 participants; 2 trials; I2 = 43%; Analysis 5.5.1) or 6 months (MD 0 mg/dL (95% CI ‐2 to 2); P = 1.0; 58 participants; Analysis 5.5.2).
5.5. Analysis.
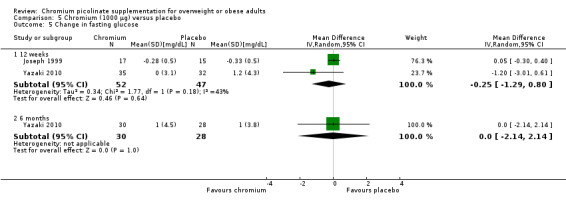
Comparison 5 Chromium (1000 μg) versus placebo, Outcome 5 Change in fasting glucose.
Change in total cholesterol
There was no statistically significant difference in total cholesterol between the two groups at 24 weeks (MD 0.1 mg/dL (95% CI ‐0.7 to 0.5); P = 0.81; 90 participants; 2 trials; I2 = 0%; Analysis 5.6.1) or 12 weeks (MD ‐0.1 mg/dL (95% CI ‐0.6 to 0.3); P = 0.57; 67 participants; 1 trial; Analysis 5.6.2).
5.6. Analysis.
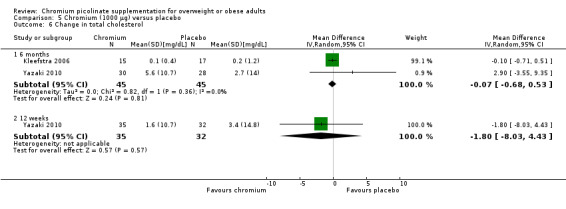
Comparison 5 Chromium (1000 μg) versus placebo, Outcome 6 Change in total cholesterol.
Change in triacylglycerol
Change in triacylglycerol levels did not differ statistically significantly between the two groups at 6 months (MD ‐1 mg/dL (95% CI ‐3 to 1); P = 0.26; 90 participants; 2 trials; Analysis 5.7.1) or 12 weeks (MD ‐4 mg/dL (95% CI 95% CI ‐13 to 6); P = 0.45; 67 participants; 1 trial; Analysis 5.7.2).
5.7. Analysis.
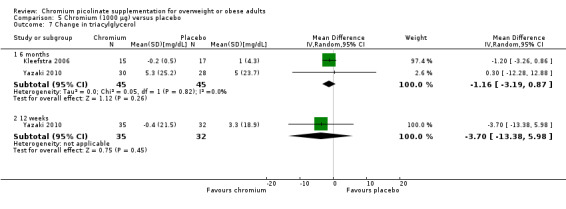
Comparison 5 Chromium (1000 μg) versus placebo, Outcome 7 Change in triacylglycerol.
Change in basal metabolic rate
Change in basal metabolic rate was not statistically significant between groups at 12 weeks (MD ‐0.4 MJ/day (95% CI 95% CI ‐1.4 to 0.6); P = 0.44; 18 participants; 1 trial; Analysis 5.8.1).
5.8. Analysis.
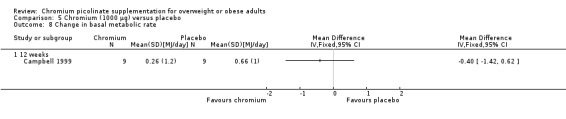
Comparison 5 Chromium (1000 μg) versus placebo, Outcome 8 Change in basal metabolic rate.
Change in blood pressure
Change in blood pressure did not differ statistically significantly between the two groups at 12 weeks (systolic blood pressure: MD 2 mm Hg (95% CI ‐1 to 5); P = 0.18; 67 participants; 1 trial; Analysis 5.10.1; diastolic blood pressure: MD 1 mm Hg (95% CI ‐2 to 4); P = 0.54; 67 participants; 1 trial; Analysis 5.11.1) or at 24 weeks (systolic blood pressure: MD 3 mm Hg (95% CI 95% ‐0.4 to 6); P = 0.08; 90 participants; 2 trials; I2 = 0%; Analysis 5.10.2; diastolic blood pressure: MD 3 mm Hg (95% CI ‐1 to 7); P = 0.13; 90 participants; 2 trials; I2 = 29%; Analysis 5.11.2).
5.10. Analysis.
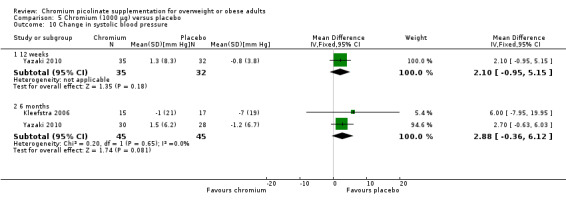
Comparison 5 Chromium (1000 μg) versus placebo, Outcome 10 Change in systolic blood pressure.
5.11. Analysis.
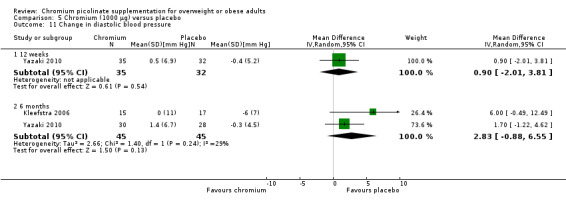
Comparison 5 Chromium (1000 μg) versus placebo, Outcome 11 Change in diastolic blood pressure.
Death from any cause
Not reported.
Socioeconomic effects
Not investigated.
Chromium picolinate 200 μg versus chromium picolinate 400 μg
One three‐arm study (Kaats 1996) with a combined total of 99 participants investigated the effects of 200 µg CrP versus 400 µg CrP.
Primary outcomes
Weight change outcomes
Change in weight loss
After 10 weeks of treatment, there was no statistically significant difference between the two groups (MD 0.3 kg (95% CI ‐1 to 1.7); P = 0.65; 99 participants; Analysis 6.1).
6.1. Analysis.
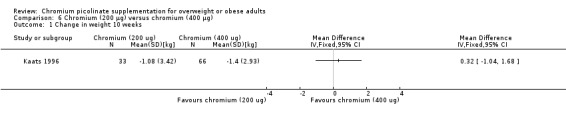
Comparison 6 Chromium (200 μg) versus chromium (400 μg), Outcome 1 Change in weight 10 weeks.
Health‐related quality of life outcomes
Not investigated.
Adverse events
Not reported.
Change in percentage body fat
No statistically significant difference between groups was apparent at 10 weeks (MD 0.5% (95% CI ‐0.5 to 1.5); P = 0.32; 99 participants; Analysis 6.2).
6.2. Analysis.
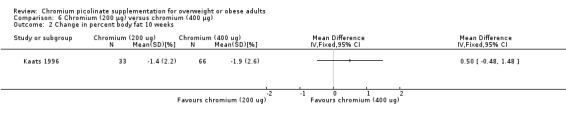
Comparison 6 Chromium (200 μg) versus chromium (400 μg), Outcome 2 Change in percent body fat 10 weeks.
Change in fat mass
No statistically significant difference between groups was observed at 10 weeks (MD 0.5 kg (95% CI ‐0.7 to 1.6); P = 0.46; 99 participants; one trial; Analysis 6.3).
6.3. Analysis.
Comparison 6 Chromium (200 μg) versus chromium (400 μg), Outcome 3 Change in fat mass 10 weeks.
Secondary outcomes
Death from any cause
Not reported.
Socioeconomic effects
Not investigated
Chromium picolinate 500 μg versus chromium picolinate 1000 μg
One three‐arm study (Kleefstra 2006) with 60 participants investigated the effects of 500 μg CrP versus 1000 μg CrP.
Primary outcomes
Weight change outcomes
After 24 weeks of treatment, one study found no statistically significant difference in change in BMI between groups (MD 0 kg/m2 (95% CI ‐0.8 to 0.8); P = 1.00; 29 participants; Analysis 7.1).
7.1. Analysis.
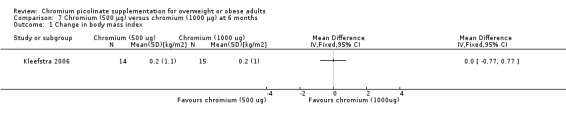
Comparison 7 Chromium (500 μg) versus chromium (1000 μg) at 6 months, Outcome 1 Change in body mass index.
Health‐related quality of life outcomes
Not investigated.
Adverse events
Adverse events did not differ significantly between groups at six months (RR 5.00 (95% CI 0.26 to 97); P = 0.29; 34 participants; Analysis 7.6).
7.6. Analysis.
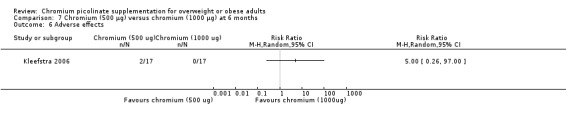
Comparison 7 Chromium (500 μg) versus chromium (1000 μg) at 6 months, Outcome 6 Adverse effects.
Secondary outcomes
Change in total cholesterol
Total cholesterol change at 24 weeks showed no statistically significant difference between groups (MD ‐0.3 mg/dL (95% CI ‐0.8 to 0.2); P = 0.21; 29 participants; Analysis 7.2).
7.2. Analysis.
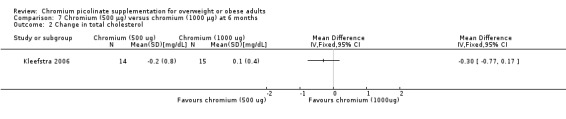
Comparison 7 Chromium (500 μg) versus chromium (1000 μg) at 6 months, Outcome 2 Change in total cholesterol.
Change in triacylglycerol
Triacylglycerol levels showed no statistically significant difference between groups at 24 weeks (MD 0.1 mg/dL (95% CI ‐0.4 to 0.6); P = 0.71; 29 participants; Analysis 7.3).
7.3. Analysis.
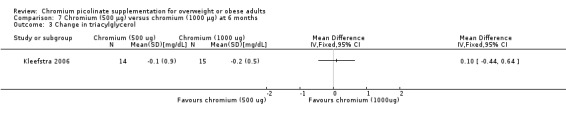
Comparison 7 Chromium (500 μg) versus chromium (1000 μg) at 6 months, Outcome 3 Change in triacylglycerol.
Change in blood pressure
There was no statistically significant change in systolic blood pressure (MD ‐6 mm Hg (95% CI ‐19 to 7); P = 0.37; 29 participants; one trial; Analysis 7.4) or diastolic blood pressure (MD ‐4 mm Hg (95% CI ‐12 to 4); P = 0.33; 29 participants; Analysis 7.5) between groups at 24 weeks.
7.4. Analysis.
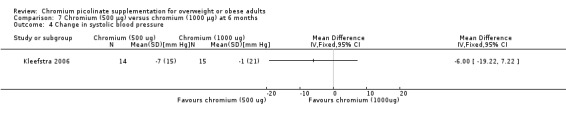
Comparison 7 Chromium (500 μg) versus chromium (1000 μg) at 6 months, Outcome 4 Change in systolic blood pressure.
7.5. Analysis.
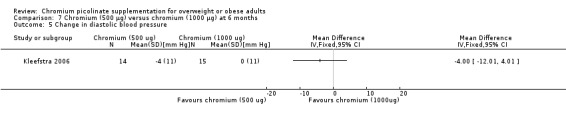
Comparison 7 Chromium (500 μg) versus chromium (1000 μg) at 6 months, Outcome 5 Change in diastolic blood pressure.
Death from any cause
Not reported.
Socioeconomic effects
Not investigated.
Subgroup analyses
As there was no statistical heterogeneity across the study results with regard to body weight, we did not analyse the data by subgroups.
Sensitivity analyses
We did not perform sensitivity analyses due to the low number of studies included.
Assessment of reporting bias
Not performed due to the low number of included trials.
Discussion
Summary of main results
Relatively few trials were identified that met the inclusion criteria for this review and most were relatively recent (published in the past 10 years). The trials were heterogeneous in nature, particularly in terms of interventions and outcomes, and sample sizes were small to medium, with 622 participants evaluated in total. The studies were conducted in the community setting, with interventions mainly delivered by health professionals, and provided outcome data at 12 to 16 weeks for weight and at 8 to 24 weeks for adverse events.
The findings of this review demonstrate that CrP supplements across all doses have some effect on weight loss after 12 to 16 weeks of treatment, but firm evidence for a specific dose could not be established.
Furthermore, there was no conclusive evidence for other outcomes of weight loss (e.g. BMI, waist circumference, percentage body fat), adverse events (e.g. gastrointestinal, nervous system, metabolism), blood pressure, lipids (e.g. total cholesterol, HDL‐C, LDL‐C, triglycerides) or fasting blood glucose.
Overall completeness and applicability of evidence
The duration of follow up of the included studies was a maximum of six months. Long‐term efficacy was not evaluated, and only three trials (Anton 2008; Kleefstra 2006; Yazaki 2010) reported data on adverse events in each group. Therefore, the efficacy and safety of CrP could not firmly be established. Whether CrP supplementation should be used in clinical practice for overweight or obese people depends on the evaluation of its effects established by large double‐blind RCTs investigating patient‐important outcome measures.
Quality of the evidence
There was an unclear risk of selection bias for the majority of the included trials. Five studies explicitly stated that blinding of the participants and personnel was undertaken. Four studies did not provide sufficient information about blinding procedures. Numbers of study withdrawals were described in seven studies that had losses to follow‐up. Analysis was reported as ITT in only one study. Two studies did not report losses to follow‐up. No study could be clearly associated with selective reporting. Five trials had a commercial source of funding which may create a potential source of bias.
Potential biases in the review process
We used well‐defined inclusion and exclusion criteria, independent data extraction by two assessors and the 'Risk of bias' assessment tool (Higgins 2009) in order to minimise potential biases in the review process. We conducted extensive electronic and manual searches to search for relevant articles. As we included only published data in our review, the possibility of publication bias cannot be ruled out. The major limitations of our review were that only a small number of studies met our inclusion criteria and a majority of these were of short‐to‐medium duration.
Agreements and disagreements with other studies or reviews
To date, one systematic review of 10 studies has been published that examined the effects of CrP in overweight or obese people (Pittler 2004). For body weight, a significant differential effect was found in favour of CrP (MD ‐1.1 kg (95% CI ‐1.8 to ‐0.4 kg); n = 489). This result is comparable to our pooled analysis of all CrP doses versus placebo. However, the clinical relevance of the effect is debatable. A definitive difference between our and Pittler's review is the fact that we included only participants who were overweight or obese at baseline.
Authors' conclusions
Implications for practice.
We identified nine studies that met our inclusion criteria and most were relatively recent (published in the past 10 years). The trials were heterogeneous in nature, particularly in terms of interventions and outcomes, and sample sizes were small to medium, with 622 participants evaluated in total. The studies were conducted in the community setting, with interventions mainly delivered by health professionals, and were of short‐to‐medium follow up (six months or less). We found no current reliable evidence to inform firm decisions about the efficacy or safety of CrP supplements in overweight or obese adults.
Implications for research.
An insufficient number of studies were included to enable us to examine the longer‐term impact of CrP supplements in overweight or obese people. Only one study had a follow‐up of six months. Further double‐blind RCTs of CrP are required to provide more conclusive evidence. Trials evaluating patient‐important outcomes, such as health‐related quality of life and morbidity endpoints, should be large and of reasonable duration. In addition, future prospective studies that carefully investigate the underlying mechanisms of the potential effects of CrP in preventing people from becoming overweight or obese are encouraged.
Acknowledgements
The review authors would like to thank the following people for commenting on the review: Lun Li and Jinhui Tian who gave good advice for this review.
Appendices
Appendix 1. Search strategies
| Search terms and databases |
| Unless otherwise stated, search terms are free text terms. Abbreviations: '$': stands for any character; '?': substitutes one or no character; adj: adjacent (i.e. number of words within range of search term); exp: exploded MeSH; MeSH: medical subject heading (MEDLINE medical index term); pt: publication type; sh: MeSH; tw: text word. |
| The Cochrane Library |
| #1 MeSH descriptor Obesity explode all trees #2 MeSH descriptor Weight Gain explode all trees #3 MeSH descriptor Weight Loss explode all trees #4 MeSH descriptor Body Mass Index explode all trees #5 (overweight in All Text or (over in All Text and weight in All Text) ) #6 (adipos* in All Text or (fat in All Text and overload in All Text and syndrom* in All Text)) #7 (overeat* in All Text or (over in All Text and eat* in All Text) ) #8 (overfeed* in All Text or (over in All Text and feed* in All Text) ) #9 (weight in All Text and (gain in All Text or chang* in All Text) ) #10 (body in All Text and mass in All Text and ind* in All Text) #11 MeSH descriptor Waist circumference explode all trees #12 MeSH descriptor Waist‐Hip Ratio explode all trees #13 MeSH descriptor Abdominal fat explode all trees #14 MeSH descriptor Body fat distribution explode all trees #15 MeSH descriptor Skinfold thickness explode all trees #16 MeSH descriptor Overweight explode all trees #17 ((weight in All Text near/6 cyc* in All Text) or (weight in All Text near/6 reduc* in All Text) or (weight in All Text near/6 los* in All Text) or (weight in All Text near/6 maint* in All Text) or (weight in All Text near/6 decreas* in All Text) ) #18 ((weight in All Text near/6 watch* in All Text) or (weight in All Text near/6 control* in All Text) or (weight in All Text near/6 chang* in All Text) or (weight in All Text near/6 gain* in All Text)) #19 BMI in All Text #20 (waist‐hip in All Text and ratio* in All Text) #21 (waist in All Text and circumferenc* in All Text) #22 (body in All Text and (fat in All Text near/6 distribution* in All Text) ) #23 ((abominal in All Text and fat in All Text) or (skinfold in All Text and thickness in All Text)) #24 (obes* in All Text or adipos* in All Text) #25 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12) #26 (#13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24) #27 (#25 or #26) #28 MeSH descriptor chromium picolinate explode all trees #29 chromium picolinate in All Text #30 (#28 or #29) #31(#27 and #30) |
| MEDLINE |
| 1 exp Obesity/ or exp Obesity hypoventilation syndrome/ or exp Obesity, abdominal/ or exp Obesity, morbid/ or exp Prader‐Willi Syndrome/ 2 exp Overweight/ 3 exp Adipose tissue/ 4 exp Weight gain/ or exp Weight loss/ 5 exp body fat distribution/ or exp body mass index/ or exp waist circumference/ or exp skinfold thickness/ or exp waist‐hip ratio/ 6 exp Body Composition/ 7 (overweight$ or over weight$).tw,ot. 8 fat overload syndrom$.tw,ot. 9 (overeat$ or over eat$).tw,ot. 10 (overfeed$ or over feed$).tw,ot. 11 (adipos$ or obes$).tw,ot. 12 (weight adj3 (cyc$ or reduc$ or los$ or maint$ or decreas$ or watch$ or control$ or gain$ or chang$)).tw,ot. 13 (body mass ind$ or waist‐hip ratio$).tw,ot. 14 skinfold thickness$.tw,ot. 15 abdominal fat$.tw,ot. 16 ((abdominal or subcutaneous or intra‐abdominal or visceral or retroperitoneal or retro peritoneal) adj3 fat*).tw,ot. 17 or/1‐16 18 exp chromium picolinate/ 19 chromium picolinate.tw,ot. 20 18 or 19 21 17 and 20 22 randomized controlled trial.pt. 23 controlled clinical trial.pt. 24 randomi?ed.ab. 25 placebo.ab. 26 drug therapy.fs. 27 randomly.ab. 28 trial.ab. 29 groups.ab. 30 or/22‐29 31 Meta‐analysis.pt. 32 exp Technology Assessment, Biomedical/ 33 exp Meta‐analysis/ 34 exp Meta‐analysis as topic/ 35 hta.tw,ot. 36 (health technology adj6 assessment$).tw,ot. 37 (meta analy$ or metaanaly$ or meta?analy$).tw,ot. 38 (search* adj10 (medical databas*or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content* or systemat*)).tw,ot. 39 or/31‐38 40 30 or 39 41 (comment or editorial or historical‐article).pt. 42 40 not 41 43 21 and 42 44 (animals not (animals and humans)).sh. 45 43 not 44 |
| EMBASE |
| 1 exp Obesity/ 2 exp weight change/ or exp weight control/ or exp weight gain/ or exp weight reduction/ 3 exp body mass/ or exp waist circumference/ or exp waist hip ratio/ 4 exp abdominal fat/ or exp body fat distribution/ 5 exp skinfold thickness/ 6 (obes$ or adipos* or overweight or over weight).tw,ot. 7 (overeat or over eat or overfeed or over feed or fat overload syndrom$).tw,ot. 8 (weight adj6 (cyc$ or reduc$ or los$ or maint$ or decreas$ or watch$ or control or chang$ or gain)).tw,ot. 9 (body mass ind$ or waist hip ratio or waist circumferenc$).tw,ot. 10 (body fat adj3 distribution*).tw,ot. 11 (abdominal fat or skinfold thickness).tw,ot. 12 or/1‐11 13 exp chromium picolinate/ 14 chromium picolinate.tw,ot. 15 13 or 14 16 12 and 15 17 exp Randomized Controlled Trial/ 18 exp Controlled Clinical Trial/ 19 exp Clinical Trial/ 20 exp Comparative Study/ 21 exp Drug comparison/ 22 exp Randomization/ 23 exp Crossover procedure/ 24 exp Double blind procedure/ 25 exp Single blind procedure/ 26 exp Placebo/ 27 exp Prospective Study/ 28 ((clinical or control$ or comparativ$ or placebo$ or prospectiv$ or randomi?ed) adj3 (trial$ or stud$)).ab,ti. 29 (random$ adj6 (allocat$ or assign$ or basis or order$)).ab,ti. 30 ((singl$ or doubl$ or trebl$ or tripl$) adj6 (blind$ or mask$)).ab,ti. 31 (cross over or crossover).ab,ti. 32 or/17‐31 33 exp meta analysis/ 34 (metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. 35 (search$ adj10 (medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. 36 exp Literature/ 37 exp Biomedical Technology Assessment/ 38 hta.tw,ot. 39 (health technology adj6 assessment$).tw,ot. 40 or/33‐39 41 32 or 40 42 (comment or editorial or historical‐article).pt. 43 41 not 42 44 16 and 43 45 limit 44 to human 46 44 not 45 |
| ISI Web of Knowledge |
| #1 Topic= (Obesity) OR Topic= (Overweight) OR Topic= (Weight Gain) OR Topic= (Weight Loss) OR Topic= (Body Mass Index) OR Topic= (Waist circumference) OR Topic= (Waist‐Hip Ratio) OR Topic= (Abdominal fat) OR Topic= (Body fat distribution) OR Topic= (Skinfold thickness) OR Topic= (BMI) #2 Topic= (chromium picolinate) #3 #1 AND #2 (201 citations) |
| Chinese Biomedical Database (CBM) |
| #1 "Obesity"[Mesh] #2 Obesity [ti/ab] #3 "Overweight"[Mesh] #4 "Overweight"[ti/ab] #5 Weight Gain [ti/ab] #6 "Weight Gain"[Mesh] #7 Weight Loss [ti/ab] #8 "Weight Loss"[Mesh] #9 Body Mass Index [ti/ab] #10 "Body Mass Index"[Mesh] #11 Waist circumference [ti/ab] #12"Waist circumference"[Mesh] #13 Waist‐Hip Ratio [ti/ab] #14"Waist‐Hip Ratio"[Mesh] #15 "Abdominal fat"[ti/ab] #16"Abdominal fat"[Mesh] #17 Body fat distribution [ti/ab] #18 "Body fat distribution"[Mesh] #19 Skinfold thickness [ti/ab] #20 Skinfold thickness [Mesh] #21 "BMI"[Mesh] #22 BMI [ti/ab] #23 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 #24 chromium picolinate[ti/ab] #25 "chromium picolinate"[Mesh] #26 #24 OR #25 #27 #23 AND #26 #28 limit 27 to human |
| China Journal Full‐text Database |
| #1 Obesity OR Overweight OR Weight Gain OR Weight Loss OR Body Mass Index OR Waist circumference OR Waist‐Hip Ratio OR Abdominal fat OR Body fat distribution OR Skinfold thickness OR BMI #2 chromium picolinate #3 #1 AND #2 |
| Chinese Scientific Journals Full‐text Database |
| #1 Obesity OR Overweight OR Weight Gain OR Weight Loss OR Body Mass Index OR Waist circumference OR Waist‐Hip Ratio OR Abdominal fat OR Body fat distribution OR Skinfold thickness OR BMI #2 chromium picolinate #3 #1 AND #2 |
| 'My NCBI' alert service |
| ("picolinic acid" [Supplementary Concept] OR "picolinic acid" [All Fields] OR "chromium picolinate" [All Fields]) AND Randomized Controlled Trial [ptyp] |
Appendix 2. Description of interventions
| Characteristic | Intervention(s) [route, frequency, total dose/day] | Comparator(s) [route, frequency, total dose/day] |
| Kaats 1996 | I1: chromium picolinate once a day, 200 μg/day | Placebo once a day |
| I2: chromium picolinate once a day, 400 μg/day | ||
| Kaats 1998 | Chromium picolinate once a day, 400 μg/day | Placebo once day |
| Joseph 1999 | Chromium picolinate twice daily, 1000 μg/day + RT (twice weekly for 12 weeks) | Placebo + RT (twice weekly for 12 weeks) |
| Kleefstra 2006 | I1: Chromium picolinate twice daily, 500 μg/day | Placebo capsule twice daily |
| I2: Chromium picolinate twice daily, 1000 μg/day | ||
| Iqbal 2009 | Chromium picolinate capsule twice daily, 500 μg/day | Placebo capsule twice daily |
| Volpe 2001 | Chromium picolinate once a day, 400 μg/day + a supervised weight‐training and walking program (twice weekly for 12 weeks) | Placebo once a day + a supervised weight‐training and walking program (twice weekly for 12 weeks) |
| Anton 2008 | Chromium picolinate 1000 μg/day | Placebo |
| Campbell 1999 | Chromium picolinate twice daily, 924 μg/day + RT (twice weekly for 12 weeks) | Placebo twice daily + RT (twice weekly for 12 weeks) |
| Yazaki 2010 | Chromium picolinate capsule twice daily, 500 μg | Placebo capsule twice daily, 815 mg |
|
Footnotes I: intervention; RT: resistance training | ||
Appendix 3. Baseline characteristics (I)
| Characteristic | Intervention(s) and comparator(s) | Duration of intervention | Participating population | Study period [year(s)] | Country | Setting | Duration of disease [mean/range years (SD), or as reported] |
| Kaats 1996 | I1: CrP 200 μg/day | 72 days | Participants were recruited from the first 233 volunteers who responded to a news story about the study run on the local central broad‐casting system | 1996 | USA | Community volunteer | ‐ |
| I2: CrP 400 μg/day | |||||||
| C: placebo | |||||||
| Kaats 1998 | I: CrP 400 μg/day | 90 days | Participants were recruited from a variety of fitness and athletic clubs in San Antonio and Houston, Texas | 1998 | USA | Community volunteer | ‐ |
| C: placebo | |||||||
| Joseph 1999 | I: CrP 1000 μg/day + resistance training | 12 weeks | Moderately overweight older men and women | 1999 | USA | Community volunteer | ‐ |
| C: placebo + resistance training | |||||||
| Kleefstra 2006 | I1: CrP 500 μg/day | 6 months | Participants with type 2 diabetes mellitus | 2006 | USA | Outpatients | ‐ |
| I2: CrP 1000 μg/day | |||||||
| C: placebo | |||||||
| Iqbal 2009 | I: CrP 500 μg/day | 16 weeks | Nondiabetic participants aged 18 to 75 years with metabolic syndrome and abdominal adiposity | 2009 | USA | Outpatients | ‐ |
| C: placebo | |||||||
| Volpe 2001 | I: CrP 400 μg/day + weight training | 12 weeks | Pre‐menopausal women with a BMI between 27 and 41 kg/m2 | 2001 | USA | Community volunteer | ‐ |
| C: placebo + weight training | |||||||
| Anton 2008 | I: CrP 400 μg/day | 8 weeks | Healthy, overweight adult women who reported craving for carbohydrates | 2008 | USA | Community volunteer | ‐ |
| C: placebo | |||||||
| Campbell 1999 | I: CrP 1000 μg/day + resistance training | 13 weeks | Older men | 1999 | USA | Community volunteer | ‐ |
| C: placebo + resistance training | |||||||
| Yazaki 2010 | I: CrP 400 μg/day | 24 weeks | Healthy overweight adults | 2010 | USA | Community volunteer | ‐ |
| C: placebo | |||||||
|
Footnotes "‐" denotes not reported BMI: body mass index; C: comparator: CrP: chromium picolinate; I: intervention; SD: standard deviation | |||||||
Appendix 4. Baseline characteristics (II)
| Characteristic | Intervention(s) and comparator(s) | Sex [female %] | Age [mean/range years (SD), or as reported] | FBG [mg/dl] | BP systolic/diastolic [mm Hg] | BMI [mean kg/m2] | Co‐medications / Co‐interventions | Co‐morbidities |
| Kaats 1996 | I1: CrP 200 μg/day | ‐ | 45.9 ± 11.9 | ‐ | ‐ | 30.3 ± 5.5 | ‐ | ‐ |
| I2: CrP 400 μg/day | ‐ | 45.7 ± 11.8 | ‐ | ‐ | 30.6 ± 5.1 | ‐ | ‐ | |
| C: placebo | ‐ | 44.3 ± 11.2 | ‐ | ‐ | 30.6 ± 5.5 | ‐ | ‐ | |
| Kaats 1998 | I: CrP 400 μg/day | ‐ | 41.1 ± 10.5 | ‐ | ‐ | 30.2 ± 7.1 | ‐ | ‐ |
| C: placebo | ‐ | 43.5 ± 7.6 | ‐ | ‐ | 28.4 ± 5.4 | ‐ | ‐ | |
| Joseph 1999 | I: CrP 1000 μg/day + resistance training | 47.1 | 63 ± 4 | 5.73 ± 0.43 mmol/L | ‐ | 28.9 ± 2.5 | Control diet + resistance training | ‐ |
| C: placebo + resistance training | 46.7 | 60 ± 4 | 5.73 ± 0.43 mmol/L | ‐ | 29.3 ± 2.4 | Control diet + resistance training | ‐ | |
| Kleefstra 2006 | I1: CrP 500 μg/day | 86.2 | 60 ± 8.8 | ‐ | 147 ± 24 / 85 ± 10 | 35 ± 7.2 | Insulin | ‐ |
| I2: CrP 1000 μg/day | 84.9 | 59 ± 6.4 | ‐ | 156 ± 25 / 84 ± 14 | 33 ± 4.2 | Insulin | ‐ | |
| C: placebo | 83.1 | 62 ± 7.5 | ‐ | 159 ± 20 / 83 ± 10 | 34 ± 4.3 | Insulin | ‐ | |
| Iqbal 2009 | I: CrP 500 μg/day | 60.6 | 47.7 ± 10 | 4.74 ± 0.8 mmol/L | 130 ± 12 / 81 ± 10 | 37.8 ± 9 | Insulin | ‐ |
| C: placebo | 30.0 | 51.1 ± 13 | 4.54 ± 0.6 mmol/L | 129 ± 15 / 79 ± 10 | 35.2 ± 6 | Insulin | ‐ | |
| Volpe 2001 | I: CrP 400 μg/day + weight training | 100 | 42.6 ± 6.5 | 42.6 ± 6.5 | 91 ± 13 | 27‐41 | Weight training | ‐ |
| C: placebo + weight training | 100 | 42.5 ± 4.2 | 42.5 ± 4.2 | 91 ± 6 | 27‐41 | Weight training | ‐ | |
| Anton 2008 | I: CrP 400 μg/day | 0 | 32 ± 10.2 | 87.1 ± 1.4 | 115 ± 13 / 74 ± 10 | 30.7 ± 4.2 | Control diet | ‐ |
| C: placebo | 0 | 34.5 ± 9.7 | 87.9 ± 6.8 | 114 ± 11 / 74 ± 10 | 31.9 ± 4.7 | Control diet | ‐ | |
| Campbell 1999 | I: CrP 1000 μg/day + resistance training | 0 | 50‐75 | ‐ | ‐ | 27‐34 | Resistance training | ‐ |
| C: placebo + resistance training | 0 | 50‐75 | ‐ | ‐ | 27‐34 | Resistance training | ‐ | |
| Yazaki 2010 | I: CrP 400 μg/day | 50 | 25‐75 | ‐ | 133 ± 17 / 80 ± 10 | 36.0 ± 6.7 | ‐ | ‐ |
| C: placebo | 50 | 25‐75 | ‐ | 137 ± 18 / 81 ± 11 | 36.1 ± 7.6 | ‐ | ‐ | |
|
Footnotes "‐" denotes not reported "±" denotes single standard deviation BP: blood pressure; BMI: body mass index; C: control; CrP: chromium picolinate; FBG: fasting blood glucose; I: intervention | ||||||||
Appendix 5. Matrix of study endpoints (publications)
|
Characteristic Study ID |
Endpoint | Time of measurementa | Outcome reportingb [analysed & reported as not significant (e.g. P > 0.05)] | Outcome reportingb [analysed but not reported] | Outcome reportingb [measured & not analysed or analysed but not reported because of non‐significant results ] | Outcome reportingb [not mentioned but likely to have been measured & analysed but not reported because of non‐significant results] |
| Kaats 1996 | Body composition improvement (P) | 0, 72 days | N/A | N/A | N/A | N/A |
| Body weight (P) | 0, 72 days | x | N/A | N/A | N/A | |
| Fat weight (P) | 0, 72 days | N/A | N/A | N/A | N/A | |
| Percentage body fat (S) | 0, 72 days | N/A | N/A | N/A | N/A | |
| Non‐fat mass (S) | 0, 72 days | x | N/A | N/A | N/A | |
| Kaats 1998 | Body weight (P) | 0, 90 days | N/A | N/A | N/A | N/A |
| Fat weight (P) | 0, 90 days | N/A | N/A | N/A | N/A | |
| Percentage body fat (S) | 0, 90 days | N/A | N/A | N/A | N/A | |
| Fat‐free mass (S) | 0, 90 days | x | N/A | N/A | N/A | |
| Joseph 1999 | Fasting glucose (P) | 1, 13 weeks | N/A | N/A | N/A | N/A |
| Fasting insulin (P) | 1, 13 weeks | x | N/A | N/A | N/A | |
| Fasting C‐peptide (S) | 1, 13 weeks | x | N/A | N/A | N/A | |
| Weight loss (S) | 1, 13 weeks | N/A | N/A | N/A | N/A | |
| BMI (S) | 1, 13 weeks | x | N/A | N/A | N/A | |
| Waist circumference (S) | 1, 13 weeks | x | N/A | N/A | N/A | |
| Waist to hip ratio (O) | 1, 13 weeks | x | N/A | N/A | N/A | |
| Kleefstra 2006 | A1c (P) | 0, 1, 3, 6 months | x | N/A | N/A | N/A |
| Lipid profile (S) | 0, 1, 3, 6 months | x | N/A | N/A | N/A | |
| BMI (S) | 0, 1, 3, 6 months | x | N/A | N/A | N/A | |
| Blood pressure (S) | 0, 1, 3, 6 months | x | N/A | N/A | N/A | |
| Plasma chromium concentration (S) | 0, 1, 3, 6 months | x | N/A | N/A | N/A | |
| Iqbal 2009 | Insulin sensitivity index (P) | 0, 16 weeks | x | N/A | N/A | N/A |
| Glucose metabolism (S) | 0, 16 weeks | N/A | N/A | N/A | N/A | |
| Oxidative stress (S) | 0, 16 weeks | x | N/A | N/A | N/A | |
| Fasting serum lipids (S) | 0, 16 weeks | x | N/A | N/A | N/A | |
| C‐reactive protein (S) | 0, 16 weeks | x | N/A | N/A | N/A | |
| Weight (O) | 0, 16 weeks | x | N/A | N/A | N/A | |
| Waist circumference (O) | 0, 16 weeks | x | N/A | N/A | N/A | |
| Volpe 2001 | Percentage body fat (P) | 0, 6, 12 weeks | N/A | N/A | N/A | N/A |
| Fat mass (P) | 0, 6, 12 weeks | N/A | N/A | N/A | N/A | |
| BMI (P) | 0, 6, 12 weeks | x | N/A | N/A | N/A | |
| Body weight (P) | 0, 6, 12 weeks | x | N/A | N/A | N/A | |
| Resting metabolic rate (P) | 0, 6, 12 weeks | x | N/A | N/A | N/A | |
| Biochemical parameters (S) | 0, 6, 12 weeks | x | N/A | N/A | N/A | |
| Anton 2008 | Food intake (P) | 0, 1, 8 weeks | N/A | N/A | N/A | N/A |
| Hunger levels (P) | 0, 1, 8 weeks | N/A | N/A | N/A | N/A | |
| Adverse events (P) | 0, 1, 8 weeks | N/A | N/A | N/A | N/A | |
| Body weight (S) | 0, 1, 8 weeks | N/A | N/A | N/A | N/A | |
| Fat cravings (S) | 0, 1, 8 weeks | N/A | N/A | N/A | N/A | |
| Glucose and insulin (O) | 0, 1, 8 weeks | x | N/A | N/A | N/A | |
| Campbell 1999 | Fat‐free mass (P) | 0, 6, 12 weeks | N/A | N/A | N/A | N/A |
| Body weight (P) | 0, 6, 12 weeks | x | N/A | N/A | N/A | |
| Urinary creatinine excretion (P) | 0, 6, 12 weeks | N/A | N/A | N/A | N/A | |
| Total body water (P) | 0, 6, 12 weeks | N/A | N/A | N/A | N/A | |
| Body muscle mass (P) | 0, 6, 12 weeks | N/A | N/A | N/A | N/A | |
| Vastus lateralis type II fibre area (S) | 0, 6, 12 weeks | N/A | N/A | N/A | N/A | |
| Skinfold thickness (S) | 0, 6, 12 weeks | N/A | N/A | N/A | N/A | |
| Resting metabolic rate (S) | 0, 6, 12 weeks | x | N/A | N/A | N/A | |
| Percentage body fat (S) | 0, 6, 12 weeks | N/A | N/A | N/A | N/A | |
| Fat mass (S) | 0, 6, 12 weeks | N/A | N/A | N/A | N/A | |
| Yazaki 2010 | BMI (P) | 0, 12, 24 weeks | x | N/A | N/A | N/A |
| Waist to hip ratio (P) | 0, 12, 24 weeks | x | N/A | N/A | N/A | |
| Percentage body fat (S) | 0, 12, 24 weeks | x | N/A | N/A | N/A | |
| Blood pressure (S) | 0, 12, 24 weeks | x | N/A | N/A | N/A | |
| Basic metabolic (S) | 0, 12, 24 weeks | x | N/A | N/A | N/A | |
| Urinalysis (O) | 0, 12, 24 weeks | x | N/A | N/A | N/A | |
|
Footnotes: "‐" denotes not reported "x" denotes "yes" aUnderlined times of measurement denote data as reported in the results section of the publication (other times represent planned but not reported points in time) bConstitutes 'high risk of bias' according to the Outcome Reporting Bias In Trials (ORBIT) study classification system for missing or incomplete outcome reporting in reports of randomised trials (Kirkham 2010) (P) primary or (S) secondary endpoint(s) refer to verbatim statements in the publication, (O) other endpoints relate to outcomes which were not specified as 'primary' or 'secondary' outcomes in the publication Endpoint in bold = review primary outcome A1c: HbA1c (glycosylated haemoglobin A1c); BMI: body mass index; N/A: not applicable | ||||||
Appendix 6. Matrix of study endpoints (protocol/trial documents)
|
Characteristic Study ID (trial identifier) |
Endpoint | Time of measurement |
| Kaats 1996 | ‐ | ‐ |
| Kaats 1998 | ‐ | ‐ |
| Joseph 1999 | ‐ | ‐ |
| Kleefstra 2006 | ‐ | ‐ |
| Iqbal 2009 | Insulin sensitivity index, glucose metabolism, oxidative stress, fasting serum lipids, C‐reactive protein, weight, waist circumference |
0, 16 weeks |
| Volpe 2001 | ‐ | ‐ |
| Anton 2008 | ‐ | ‐ |
| Campbell 1999 | ‐ | ‐ |
| Yazaki 2010 | ‐ | ‐ |
|
Footnotes "‐" denotes no protocol was detected | ||
Appendix 7. Definition of endpoint measurement
|
Characteristic Study ID |
Overweight and obesity | Cardiovascular mortality | Morbidity | Health‐related quality of life |
| Kaats 1996 | WHO guidelines | ‐ | ‐ | ‐ |
| Kaats 1998 | WHO guidelines | ‐ | ‐ | ‐ |
| Joseph 1999 | WHO guidelines | ‐ | ‐ | ‐ |
| Kleefstra 2006 | WHO guidelines | ‐ | ‐ | ‐ |
| Iqbal 2009 | WHO guidelines | ‐ | ‐ | ‐ |
| Volpe 2001 | WHO guidelines | ‐ | ‐ | ‐ |
| Anton 2008 | WHO guidelines | ‐ | ‐ | ‐ |
| Campbell 1999 | WHO guidelines | ‐ | ‐ | ‐ |
| Yazaki 2010 | WHO guidelines | ‐ | ‐ | ‐ |
|
Footnotes "‐" denotes not reported WHO: World Health Organization | ||||
Appendix 8. Adverse events (I)
|
Characteristic Study ID |
Intervention(s) and comparator(s) | Randomised / Safety [N] | Deaths [N] | All adverse events [N] | All adverse events [%] | Severe/serious adverse events [N] | Severe/serious adverse events [%] |
| Kaats 1996 | I1: CrP 200 μg/day | 33 | No participant died | ‐ | ‐ | ‐ | ‐ |
| I2: CrP 400 μg/day | 66 | No participant died | ‐ | ‐ | ‐ | ‐ | |
| C: placebo | 55 | No participant died | ‐ | ‐ | ‐ | ‐ | |
| Kaats 1998 | I: CrP 400 μg/day | 62 | No participant died | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 60 | No participant died | ‐ | ‐ | ‐ | ‐ | |
| Joseph 1999 | I: CrP 1000 μg/day + resistance training | 17 | No participant died | ‐ | ‐ | ‐ | ‐ |
| C: placebo + resistance training | 15 | No participant died | ‐ | ‐ | ‐ | ‐ | |
| Kleefstra 2006 | I1: CrP 500 μg/day | 17 | No participant died | 0 | 0 | 0 | 0 |
| I2: CrP 1000 μg/day | 17/15 | No participant died | 2 | 13 | 2 | 13 | |
| C: placebo | 19 | No participant died | 0 | 0 | 0 | 0 | |
| Iqbal 2009 | I: CrP 500 μg/day | 33 | No participant died | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 30 | No participant died | ‐ | ‐ | ‐ | ‐ | |
| Volpe 2001 | I: CrP 400 μg/day + weight training | 22 | No participant died | ‐ | ‐ | ‐ | ‐ |
| C: placebo + weight training | 20 | No participant died | ‐ | ‐ | ‐ | ‐ | |
| Anton 2008 | I: CrP 400 μg/day | 21 | No participant died | 0 | 0 | 0 | 0 |
| C: placebo | 19/18 | No participant died | 1 | 6 | 1 | 6 | |
| Campbell 1999 | I: CrP 1000 μg/day + resistance training | 9 | No participant died | ‐ | ‐ | ‐ | ‐ |
| C: placebo + resistance training | 9 | No participant died | ‐ | ‐ | ‐ | ‐ | |
| Yazaki 2010 | I: CrP 400 μg/day | 40/39 | No participant died | 1 | 3 | 1 | 3 |
| C: placebo | 40 | No participant died | 0 | 0 | 0 | 0 | |
|
Footnotes "‐" denotes not reported C: control; CrP: chromium picolinate; I: intervention | |||||||
Appendix 9. Adverse events (II)
| Characteristic | Intervention(s) and comparator(s) | Randomised / Safety [N] | Left study due to adverse events [N] | Left study due to adverse events [%] | Hospitalisation [N] | Hospitalisation [%] | Outpatient treatment [N] | Outpatient treatment [%] | Symptoms [N] | Symptoms [%] |
| Kaats 1996 | I1: CrP 200 μg/day | 33 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| I2: CrP 400 μg/day | 66 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C: placebo | 55 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Kaats 1998 | I: CrP 400 μg/day | 62 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 60 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Joseph 1999 | I: CrP 1000 μg/day + resistance training | 17 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo + resistance training | 15 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Kleefstra 2006 | I1: CrP 500 μg/day | 17 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| I2: CrP 1000 μg/day | 17/15 | 2 | 13 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C: placebo | 19 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Iqbal 2009 | I: CrP 500 μg/day | 33 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 30 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Volpe 2001 | I: CrP 400 μg/day + weight training | 22 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo + weight training | 20 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Anton 2008 | I: CrP 400 μg/day | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo | 19/18 | 1 | 6 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Campbell 1999 | I: CrP 1000 μg/day + resistance training | 9 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo + resistance training | 9 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Yazaki 2010 | I: CrP 400 μg/day | 40/39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo | 40 | 1 | 6 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
|
Footnotes "‐" denotes not reported C: control; CrP: chromium picolinate; I: intervention | ||||||||||
Appendix 10. Survey of authors' providing information on trials
We tried our best to obtain relevant missing data from all authors of included studies but received no reply.
Data and analyses
Comparison 1. Chromium (all dosages) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in weight at 12‐16 weeks | 6 | 392 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.73, ‐0.42] |
Comparison 2. Chromium (200 μg) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in weight at 10 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Change in percent body fat at 10 weeks [kg] | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Change in fat mass at 10 weeks [kg] | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 3. Chromium (400 μg) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body mass index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 6 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in weight | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 weeks | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.7 [‐7.51, 6.11] |
| 2.2 12 weeks | 3 | 280 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.89, ‐0.39] |
| 3 Percent body fat change | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Short‐term | 1 | 122 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.95, 0.21] |
| 3.2 Medium‐term | 3 | 280 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.98, 0.18] |
| 4 Change in fat mass | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 6 weeks | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐4.61, 3.75] |
| 4.2 12 weeks | 3 | 280 | Mean Difference (IV, Random, 95% CI) | ‐1.57 [‐2.27, ‐0.87] |
| 5 Change in waist circumference | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 weeks | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐5.81, 6.21] |
| 5.2 12 weeks | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐7.72, 4.92] |
| 6 Change in fasting glucose | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Change in total cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Change in triacylglycerol | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Chromium (500 μg) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body mass index at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Change in weight at 16 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in waist circumference at 16 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Change in fasting glucose at 16 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Change in systolic blood pressure at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Change in diastolic blood pressure at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Change in total cholesterol | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.46, 0.37] |
| 8 Change in triacylglycerol | 2 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.76, 0.21] |
Comparison 5. Chromium (1000 μg) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in weight | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 12 weeks | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐7.25, 5.93] |
| 2 Change in body mass index | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 90 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.08, 0.30] |
| 2.2 12 weeks | 2 | 99 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.01, 0.58] |
| 3 Change in percent body fat | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 6 months | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 1.1 [‐0.35, 2.55] |
| 3.2 12 weeks | 3 | 117 | Mean Difference (IV, Random, 95% CI) | 0.93 [‐0.35, 2.21] |
| 4 Change in waist circumference | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐1.60 [‐6.53, 3.33] | |
| 5 Change in fasting glucose | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 12 weeks | 2 | 99 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.29, 0.80] |
| 5.2 6 months | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐2.14, 2.14] |
| 6 Change in total cholesterol | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 6 months | 2 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.68, 0.53] |
| 6.2 12 weeks | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐8.03, 4.43] |
| 7 Change in triacylglycerol | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 6 months | 2 | 90 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐3.19, 0.87] |
| 7.2 12 weeks | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐3.7 [‐13.38, 5.98] |
| 8 Change in basal metabolic rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Adverse events | 3 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.30, 10.43] |
| 9.1 6 months | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 4.03 [0.46, 35.11] |
| 9.2 12 weeks | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.02] |
| 10 Change in systolic blood pressure | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 12 weeks | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 2.1 [‐0.95, 5.15] |
| 10.2 6 months | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | 2.88 [‐0.36, 6.12] |
| 11 Change in diastolic blood pressure | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 12 weeks | 1 | 67 | Mean Difference (IV, Random, 95% CI) | 0.9 [‐2.01, 3.81] |
| 11.2 6 months | 2 | 90 | Mean Difference (IV, Random, 95% CI) | 2.83 [‐0.88, 6.55] |
Comparison 6. Chromium (200 μg) versus chromium (400 μg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in weight 10 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Change in percent body fat 10 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in fat mass 10 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. Chromium (500 μg) versus chromium (1000 μg) at 6 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body mass index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Change in total cholesterol | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in triacylglycerol | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Change in systolic blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Change in diastolic blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anton 2008.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: (1) be a healthy female without any chronic disease, (2) be a carbohydrate craver, determined by self‐reported carbohydrate cravings on 2 or more days of the week, (3) be >18 and <50 years of age, (4) have a BMI between 25 and 39.9 kg/m2 and (5) be a non‐smoker. Exclusion criteria: participants were excluded if they had a diagnosable eating disorder or were taking any medications or dietary supplements (including CrP) that could influence appetite, hunger or satiety. Diagnostic criteria: BMI |
|
| Interventions |
Number of study centres: 1 Treatment before study: not stated Titration period: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: food intake at breakfast, lunch and dinner was directly measured; hunger levels, fat cravings and body weight | |
| Study details |
Run‐in period: 8 weeks Study terminated before regular end: not stated |
|
| Publication details |
Language of publication: English Non‐commercial funding: this research was supported by the Health and Performance Enhancement Division of the Pennington Biomedical Research Center Publication status: peer review journal |
|
| Stated aim for study | Quote: "To assess the effect of CrPic in modulating food intake in healthy, overweight, adult women who reported craving carbohydrates" | |
| Notes | Abbreviations: BMI: body mas index; CrP: chromium picolinate | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to receive either 1000 μg of chromium as CrP or placebo Comment: no detail is given on the methodology |
| Allocation concealment (selection bias) | Unclear risk | Comment: no detail is given |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: just mentions ''double‐blind"; detailed information not provided |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: just mentions ''double‐blind"; detailed information not provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: just mentions ''double‐blind"; detailed information not provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: just mentions ''double‐blind"; detailed information not provided |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Quote: "One participant in the placebo group dropped out of the study because of an adverse emotional reaction reportedly due to the study medication." No other adverse events were reported. Comment: nothing was detected |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Quote: "One participant in the placebo group dropped out of the study because of an adverse emotional reaction reportedly due to the study medication." No other adverse events were reported. Comment: nothing was detected |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available |
| Other bias | Unclear risk | Comment. the trial had a commercial source of funding possibly creating a risk of bias |
Campbell 1999.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: men, age range 50 to 75 years; BMI range 27 to 34 kg/m2; non‐diabetic; physically able to safely engage in all aspects of the study protocol; clinically normal cardiac function, blood pressure, liver function and kidney function Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: not stated Titration period: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: body weight, each man’s baseline maximal strength for each exercise was set as the greater of two one‐repetition‐maximum values obtained during the first two resistance exercise sessions; urinary creatinine excretion (P < 0.001), muscle strength (P < 0.001), arm‐pull muscle power, knee‐extension muscle power, fat‐free mass (P < 0.001), whole body muscle mass (P < 0.001) and vastus lateralis type II fibre area (P < 0.05) | |
| Study details |
Run‐in period: 13 weeks Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Commercial / non‐commercial / other funding: supported by National Institute on Aging Grants T32 AG‐0048, 1‐R29‐AG‐13409 and RO1‐AG‐11811, by General Clinical Research Center Grant MO1‐RR‐10732, and by an independent monetary gift from Nutrition 21 Publication status: peer review journal |
|
| Stated aim for study | Quote: "To assess the effect of high‐dose chromium picolinate supplementation on body composition, including body density, whole body muscle mass and muscle" | |
| Notes | Abbreviations: BMI: body mass index; Cr: creatinine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Each man was randomly assigned in a double‐blind fashion to either a chromium picolinate group or a placebo group" Comment: no detail is given on the methodology |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: no details provided |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Eighteen of 23 men successfully completed the study protocol. The reasons for the five men withdrawing included the following: 1) a request by a participant’s personal physician to avoid aggravation of a chronic hip injury; 2) an irritation of chronic elbow tendonitis, unrelated to resistance exercise; 3) a personal family commitment; 4) a resistance exercise‐induced aggravation of an existing knee condition; and 5) a shoulder injury caused by slipping on ice. Comment: The primary outcome data were all reported |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: no details provided |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available Comment: nothing was detected |
| Other bias | Unclear risk | Comment: the trial had a commercial source of funding possibly creating a risk of bias |
Iqbal 2009.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: non‐diabetic population aged 18 to 75 years with metabolic syndrome and abdominal adiposity; participants' eligibility required waist circumference ≥ 102 cm for men and ≥ 89 cm for women and at least two of the following: systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 85 mm Hg or taking ≥ 1 antihypertensive agent; fasting blood glucose ≥ 6.1 mmol/L, but < 7 mmol/L; fasting triglycerides ≥ 1.68, but ≤ 8.96 mmol/L; or HDL‐C ≤ 1 mmol/L for males and ≤ 1.29 mmol/L for females Exclusion criteria: 2‐hour plasma glucose value of ≥ 11.1 mmol/L, ASCVD, LDL‐C > 4.9 mmol/L, liver transaminases three times the upper limit of normal, renal insufficiency, fibrates or dietary supplements (excluding a multivitamin with < 100 μg chromium) Diagnostic criteria: waist circumference, BMI, according to the criteria of the National Cholesterol Education Program Adult Treatment Panel III |
|
| Interventions |
Number of study centres: 1 Treatment before study: not reported Titration period: not reported |
|
| Outcomes | Outcomes reported in abstract of publication: insulin sensitivity index derived from a frequently sampled intravenous glucose tolerance test. Prespecified secondary endpoints included changes in other measurements of glucose metabolism, oxidative stress, fasting serum lipids and high sensitivity C‐reactive protein | |
| Study details |
Run‐in period: 16 weeks Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Commercial and non‐commercial funding: this work was supported by the following grants: R21DK067241, K‐23 AT‐00058, and M01‐RR00040 (Translational Research Center [TRC]). Nutrition 21 provided active drug and placebo. Dr Boston is the principal author of the modelling software MinMod Millenium Publication status: peer review journal |
|
| Stated aim for study | Quote: "To determine the effects of chromium picolinate (CrP) on glucose metabolism in patients with metabolic syndrome" | |
| Notes | Abbreviations: BMI: body mass index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "conducted a randomised, double‐blind, placebo‐controlled study of the safety and efficacy of 16 weeks of CrPic therapy and randomised in a 1:1 double‐blind fashion to receive either CrPic or matching placebo." Comment: No other details given |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of concealment is not described |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Comment: method of blinding is not described |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: three participants withdrew for personal reasons |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment. no subjective outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available and there was no selective reporting |
| Other bias | Unclear risk | Comment: the trial had a commercial source of funding possibly creating a risk of bias |
Joseph 1999.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: moderately overweight older men and women (aged 54 to 71 years; BMI 26 to 36 kg/m2) who were not actively involved in any physical training volunteered to participate in this 13‐week study Exclusion criteria: included populations with any metabolic or cardiac abnormalities. When this study was performed, the 1979 recommendations of the National Diabetes Data Group (NDDG) 21 were used to exclude diabetics at screening. Populations with fasting plasma glucose greater than 7.77 mmol/L or 2‐hour OGTT plasma glucose > 11.1 mmol/L and one additional 0 to 120‐minute plasma glucose sample > 11.1 mmol/L were deemed diabetic and were excluded from the study Diagnostic criteria: according to the criteria of The National Diabetes Data Group (NDDG) |
|
| Interventions |
Number of study centres: 1 Treatment before study: not stated Titration period: not stated RT consisted of 12 weeks of progressive RT twice weekly with a minimum of days, rest between training sessions |
|
| Outcomes | Outcomes reported in abstract of publication: weight, body fat, fat mass, fat free mass, basal plasma glucose or insulin levels, glycosylated haemoglobin, triglycerides, total cholesterol, LDL‐C, HDL‐C | |
| Study details |
Run‐in period: 13 weeks Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Non‐commercial funding: Supported by National Institutes of Health Grants No. 1‐R29‐AG13409 and RO1‐AG11811, National Institute on Aging Grant No. T32‐AG00048, an independent monetary gift from Nutrition 21, San Diego, CA, and General Clinical Research Center Grant No. MO1‐RR10732. Publication status: peer review journal |
|
| Stated aim for study | Quote: "To assess the effect of 12 weeks of resistance training (RT) with or without chromium picolinate (CrP) supplementation on glucose tolerance in moderately overweight older men and women" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomised; computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Comment: hospital pharmacy |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "... a double‐blind fashion, all capsules, including placebo, were furnished by our hospital pharmacy and were indistinguishable from each other. Neither the researchers nor the patients knew into which group they had been randomised" |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "All capsules, including placebo, were furnished by our hospital pharmacy and were indistinguishable from each other. Neither the researchers nor the patients knew into which group they had been randomized. Independent pharmacists dispensed either chromium capsules or placebo in numbered containers according to a computer‐generated randomization list. No restrictions were used" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: no data missing |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all of the outcomes listed in the methods section were reported as results |
| Other bias | Low risk | Comment: no other details given to assess whether an important risk of bias exists |
Kaats 1996.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: adults consulting their personal physician Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: not stated Titration period: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: body composition, body weight, percentage body fat, non‐fat mass and body composition improvement | |
| Study details |
Run‐in period: 72 days Study terminated before regular end: not stated |
|
| Publication details |
Language of publication: English Commercial funding: "Funding for the study was provided by the Living at Goal Weight Center, San Antonio, Texas; Optimal Health Products, San Antonio, Texas; and Nutrition 21, Inc., San Diego, California." Publication status: peer review journal |
|
| Stated aim for study | Quote: "To examine the effect of chromium picolinate (CrP) on body composition" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised, but method not stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Comment: none of the investigators, research technicians dispensing the product or populations knew which code corresponded to the amount of CrP in the canister |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not details provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no subjective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Quote: "Some concerns may be raised about the relatively high dropout rate in our study ‐ 69 of 219 (31.5%). A comparison of their initial body composition scores revealed no significant difference between the three groups nor between any of the three groups of patients who completed or failed to complete the protocol" Comment: unclear influence of attrition rate |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment. no subjective outcome |
| Selective reporting (reporting bias) | Low risk | Comment: all of the outcomes listed in the methods section were reported as results |
| Other bias | Unclear risk | Comment: the trial had a commercial source of funding possibly creating a risk of bias |
Kaats 1998.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: adults consulting their personal physician Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: not stated Titration period: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: body weight, percentage body fat, fat mass and fat‐free mass | |
| Study details |
Run‐in period: 90 days Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Commercial funding: this study has been supported financially by Nutrition 21, Inc., San Diego, California Publication status: peer review journal |
|
| Stated aim for study | Quote: "To determining whether the body composition changes observed in the initial study could be replicated in this study" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: randomised, but method not stated |
| Allocation concealment (selection bias) | Low risk | Comment: independent local pharmacist |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "None of the investigators, research technicians dispensing the product, or participants knew which participant's number corresponded to the placebo or active product. An independent local pharmacist acted as trustee for the study and randomly assigned participant's numbers to bottles that had been prelabeled with either an “X” or “Y” to correspond with either active product or placebo" |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: not details provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: no dropouts |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: all of the outcomes listed in the methods section were reported as results |
| Other bias | Unclear risk | Comment: the trial had a commercial source of funding possibly creating a risk of bias |
Kleefstra 2006.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: Individuals with type 2 diabetes, glycosylated haemoglobin (A1c) ≥ 8%, daily use of insulin ≥ 50 units, creatinine ≤ 150 μmol/L for men and ≤ 120 μmol/L for women, creatinine clearance ≥ 50 ml/min, alanine aminotransferase ≤ 90 units/L and age < 75 years Exclusion criteria: included pregnancy, or intention to become pregnant during the study, and a history of liver or renal disease Diagnostic criteria: BMI, A1c |
|
| Interventions |
Number of study centres: 1 Treatment before study: not stated Titration period: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: A1c, BMI, blood pressure, lipid profile and insulin requirement. | |
| Study details |
Run‐in period: 6 months Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Commercial / non‐commercial / other funding: not stated Publication status: peer review journal |
|
| Stated aim for study | Quote: "To determine the effect of chromium treatment on glycemic control in a western population of insulin‐dependent patients with type 2 diabetes". | |
| Notes | Abbreviations: BMI: body mass index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Comment: independent pharmacists dispensed either chromium capsules or placebo in numbered containers according to a computer‐generated randomisation list. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "All capsules, including placebo, were furnished by our hospital pharmacy and were indistinguishable from each other. Neither the researchers nor the patients knew into which group they had been randomised" |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Quote: "All capsules, including placebo, were furnished by our hospital pharmacy and were indistinguishable from each other. Neither the researchers nor the patients knew into which group they had been randomised" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: no attrition bias was detected |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Quote: "Of 53 patients randomised, 1 patient was lost to follow‐up, and all attempts to locate this participant were in vain (telephone contact, letters, and visits). Six other individuals, one in the placebo group, three in the 500 µg group, and two in the 1,000 µg group, discontinued the study for the following reasons: one patient required a blood transfusion and was hospitalised, and three other patients were hospitalised due to percutaneous transluminal coronary angioplasty, chronic obstructive pulmonary disease, and glycemic dysregulation.Two patients discontinued the study due to possible adverse effects. Unfortunately, intention‐to‐treat analyses were not possible because for five of six excluded patients follow‐up data were lacking. However, the main conclusions of the per‐protocol analyses would likely not have been different in an intention‐to‐treat analyses" |
| Selective reporting (reporting bias) | Low risk | Comment: all of the outcomes listed in the methods section were reported as results |
| Other bias | Low risk | Comment: no other details given to assess whether an important risk of bias exists |
Volpe 2001.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: 44 female participants, with a BMI between 27 and 41 kg/m2, between 27 and 51 years of age, premenopausal, sedentary, not taking any dietary supplements, not taking any vitamin or mineral supplements containing chromium, not on a weight‐loss programme, not taking any weight loss supplements, non‐smoking and with no history of chronic diseases or recent acute illness. Participants were asked not to alter their dietary habits during the course of the study Exclusion criteria: not stated Diagnostic criteria: BMI |
|
| Interventions |
Number of study centres: 1 Treatment before study: not stated Titration period: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: body composition, resting metabolic rate, fasting plasma glucose, serum insulin, plasma glucagon, serum C‐peptide and serum lipid concentrations or iron and zinc indices, serum total cholesterol concentration, exercise training | |
| Study details |
Run‐in period: 12 weeks Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Commercial and non‐commercial funding: "the authors would like to thank Nutrition 21 (San Diego, CA) for funding this project. This research is based upon work partially supported by the Cooperative State Research Extension, Education Service, U.S. Department of Agriculture Experiment Station, under Project No. MAS00757 Manuscript No. 3280" Publication status: peer review journal |
|
| Stated aim for study | Quote: "To investigate the effect of chromium picolinate (CP) supplementation on body composition, resting metabolic rate (RMR), selected biochemical parameters and iron and zinc status in moderately obese women participating in a 12‐week exercise program" | |
| Notes | Abbreviations: BMI: body mass index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Forty‐four free‐living females were assigned, in a stratified randomised manner based on their BMI, to one of two groups" Comment: no other details given |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Quote from publication: "double‐blind" Comment: no other details given |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: no details provided |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Quote: "Thirty‐seven of the initial 44 people (84%) completed this study. Sixteen people reported forgetting to take their capsules and 13 people missed exercise training; however, no people reported forgetting to take the capsules more than five days (average 3 days) during the study period. Furthermore, none of these people missed exercise training more than four times (average 3) during the entire study. Two people did have minor problems with their knees or ankles, so they were unable to perform all of the specified weight training exercises (e.g. leg extension and calf raises)" |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: no subjective outcome |
| Selective reporting (reporting bias) | Low risk | Comment: all of the outcomes listed in the methods section were reported as results |
| Other bias | Unclear risk | Comment: the trial had a commercial source of funding possibly creating a risk of bias |
Yazaki 2010.
| Methods | Parallel randomised controlled clinical trial | |
| Participants |
Inclusion criteria: overweight (BMI > 25 kg/m2) non‐smoking adults aged 25 to 75 years with abdominal adiposity (waist circumference > 80 cm in females and > 100 cm in males) Exclusion criteria: contraindication to abdominal computed tomography scans (weight > 375 pounds, claustrophobia, unstable vital signs, or radiation procedure in past six months), diagnosed diabetes, diagnosed eating disorder, uncontrolled hypertension, emphysema, intestinal or stomach disease, kidney disease, substance abuse, pregnancy or intention to become pregnant during the study Diagnostic criteria: BMI, waist circumference measures |
|
| Interventions |
Number of study centres: 1 Treatment before study: not stated Titration period: not stated |
|
| Outcomes | Outcomes reported in abstract of publication: weight, height, blood pressure, percentage body fat, serum and urinary biomarkers | |
| Study details |
Run‐in period: 24 weeks Study terminated before regular end: no |
|
| Publication details |
Language of publication: English Commercial funding: supported by Nutrition 21, Inc Publication status: peer review journal |
|
| Stated aim for study | Quote: "Assess the effects of chromium picolinate supplementation, alone and combined with nutritional education, on weight loss in healthy overweight adults" | |
| Notes | Abbreviations: BMI: body mass index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "People were enrolled and randomized using balanced allocation within gender" Comment: no details provided |
| Allocation concealment (selection bias) | Low risk | Quote: "People and study personnel were blinded to the intervention. Chromium and placebo were prepackaged and shipped from the manufacturer to the study site. Bottles were labelled and coded by an unblinded individual unaffiliated with the study. Investigators thus only knew the treatment assignment (group A or B) of the people without knowledge of whether these contained chromium or placebo" |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote: "People and study personnel were blinded to the intervention. Chromium and placebo were prepackaged and shipped from the manufacturer to the study site. Bottles were labelled and coded by an unblinded individual unaffiliated with the study. Investigators thus only knew the treatment assignment (group A or B) of the people without knowledge of whether these contained chromium or placebo" |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no details provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: insufficient reporting of attrition/exclusions to permit judgement |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: no subjective outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is not available |
| Other bias | Unclear risk | Comment: the trial had a commercial source of funding possibly creating a risk of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albarracin 2008 | Combination therapy of chromium picolinate and biotin versus placebo |
| Bunting 1994 | Animal study |
| Diaz 2008 | Combination therapy of chromium picolinate and conjugated linoleic acid in tonalin oil versus placebo and canola oil |
| Docherty 2005 | Participants not obese or overweight |
| Earle 1989 | Participants not obese or overweight |
| Geohas 2007 | Combination therapy of chromium picolinate and biotin versus placebo |
| Hoeger 1998 | Combination therapy of chromium picolinate, inulin, capsicum, L‐phenylalanine and other lipotropic nutrients versus placebo |
| Joyal 2004 | Not a randomised trial |
| Pasman 1997 | Not a randomised trial |
| Pittler 2004 | Not a randomised trial |
| Rabinowitz 1983 | Participants not obese or overweight |
| Stupar 1999 | Not a randomised trial |
| Trent 1995 | Participants not obese or overweight |
| Wang 2010 | Not a randomised trial |
| Wilson 1995 | Participants not obese or overweight |
| Zenk 2007 | Combination therapy investigating 3‐acetyl‐7‐oxo‐dehydroepiandrosterone alone (7‐Keto) and in combination with calcium citrate, green tea extract, ascorbic acid, chromium nicotinate and cholecalciferol (HUM5007) versus placebo |
Differences between protocol and review
Timing of outcome measurement: changed from "Short‐term: 1 to 4 weeks, medium‐term: more than 4 weeks to 12 weeks" to "Short‐term: 1 to 6 weeks, medium‐term: more than 6 weeks to 12 weeks".
Contributions of authors
Hongliang Tian (TH): protocol drafting, search strategy development, trial selection, data interpretation and review drafting.
Xiaohu Guo (GX): protocol drafting, trial selection, data extraction, data analysis, data interpretation and review drafting.
Xiyu Wang (WX): protocol drafting, search strategy development, acquiring trial reports and review drafting.
Zhiyun He (HZ): acquiring trial reports, trial selection, data extraction, data analysis, data interpretation and review drafting.
Rao Sun (SR): protocol drafting, search strategy development, acquiring trial reports, trial selection and review drafting.
Sai GE (GS): protocol drafting, search strategy development, data extraction, data analysis, data interpretation and review drafting.
Zongjiu Zhang (ZZ): protocol drafting, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation and review drafting.
Declarations of interest
None known
New
References
References to studies included in this review
Anton 2008 {published data only}
- Anton SD, Morrison CD, Cefalu WT, Martin CK, Coulon S, Geiselman P, et al. Effects of chromium picolinate on food intake and satiety. Diabetes Technology & Therapeutics 2008;10(5):405‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1999 {published data only}
- Campbell WW, Joseph LJ, Davey SL, Cyr‐Campbell D, Anderson RA, Evans WJ. Effects of resistance training and chromium picolinate on body composition and skeletal muscle in older men. Journal of Applied Physiology 1999 ;86(1):29‐39. [DOI] [PubMed] [Google Scholar]
Iqbal 2009 {published data only}
- Iqbal N, Cardillo S, Volger S, Bloedon LT, Anderson RA, Boston R, et al. Chromium picolinate does not improve key features of metabolic syndrome in obese nondiabetic adults. Metabolic Syndrome and Related Disorders 2009;7(2):143‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph 1999 {published data only}
- Joseph LJ, Farrell PA, Davey SL, Evans WJ, Campbell WW. Effect of resistance training with or without chromium picolinate supplementation on glucose metabolism in older men and women. Metabolism 1999;48(5):546‐53. [DOI] [PubMed] [Google Scholar]
Kaats 1996 {published data only}
- Kaats GR, Blum K, Fisher JA, Adelman JA. Effects of chromium picolinate supplementation on body composition: a randomized, double‐masked, placebo‐controlled study. Current Therapeutic Research 1996;57(10):747‐56. [Google Scholar]
Kaats 1998 {published data only}
- Kaats GR, Blum K, Pullin D, Keith SC, Wood R. A randomized, double‐masked, placebo‐controlled study of the effects of chromium picolinate supplementation on body composition: a replication and extension of a previous study. Current Therapeutic Research 1998;59(6):379‐88. [Google Scholar]
Kleefstra 2006 {published data only}
- Kleefstra N, Houweling ST, Jansman FG, Groenier KH, Gans RO, Meyboom‐de Jong B. Chromium treatment has no effect in patients with poorly controlled, insulin‐treated type 2 diabetes in an obese western population: a randomized, double‐blind, placebo‐controlled trial. Diabetes Care 2006;29(3):521‐5. [DOI] [PubMed] [Google Scholar]
Volpe 2001 {published data only}
- Volpe SL, Huang HW, Larpadisorn K, Lesser II. Effect of chromium supplementation and exercise on body composition, resting metabolic rate and selected biochemical parameters in moderately obese women following an exercise program. Journal of the American College of Nutrition 2001;20(4):293‐306. [DOI] [PubMed] [Google Scholar]
Yazaki 2010 {published data only}
- Yazaki Y, Faridi Z, Ma Y, Ali A, Northrup V, Njike VY, et al. A pilot study of chromium picolinate for weight loss. Journal of Alternative and Complementary Medicine 2010;16(3):291‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Albarracin 2008 {published data only}
- Albarracin CA, Fuqua BC, Evans JL, Goldfine ID. Chromium picolinate and biotin combination improves glucose metabolism in treated, uncontrolled overweight to obese patients with type 2 diabetes. Diabetes/Metabolism Research and Reviews 2008;24(1):41‐51. [DOI] [PubMed] [Google Scholar]
Bunting 1994 {published data only}
- Bunting LD, Fernandez JM, Thompson DL Jr, Southern LL. Influence of chromium picolinate on glucose usage and metabolic criteria in growing Holstein calves. Journal of Animal Science 1994;72(6):1591‐9. [DOI] [PubMed] [Google Scholar]
Diaz 2008 {published data only}
- Diaz ML, Watkins BA, Li Y, Anderson RA, Campbell WW. Chromium picolinate and conjugated linoleic acid do not synergistically influence diet‐ and exercise‐induced changes in body composition and health indexes in overweight women. The Journal of Nutritional Biochemistry 2008;19(1):61‐8. [DOI] [PubMed] [Google Scholar]
Docherty 2005 {published data only}
- Docherty JP, Sack DA, Roffman M, Finch M, Komorowski JR. A double‐blind, placebo‐controlled, exploratory trial of chromium picolinate in atypical depression: effect on carbohydrate craving. Journal of Psychiatric Practice 2005;11(5):302‐14. [DOI] [PubMed] [Google Scholar]
Earle 1989 {published data only}
- Earle KE, Archer AG, Baillie JE. Circulating and excreted levels of chromium after an oral glucose challenge: influence of body mass index, hypoglycemic drugs, and presence and absence of diabetes mellitus. The American Journal of Clinical Nutrition 1989;49(4):685‐9. [DOI] [PubMed] [Google Scholar]
Geohas 2007 {published data only}
- Geohas J, Daly A, Juturu V, Finch M, Komorowski JR. Chromium picolinate and biotin combination reduces atherogenic index of plasma in patients with type 2 diabetes mellitus: a placebo‐controlled, double‐blinded, randomized clinical trial. The American Journal of the Medical Sciences 2007;333(3):145‐53. [DOI] [PubMed] [Google Scholar]
Hoeger 1998 {published data only}
- Hoeger WW, Harris C, Long EM, Hopkins DR. Four‐week supplementation with a natural dietary compound produces favorable changes in body composition. Advances in Therapy 1998;15(5):305‐14. [PubMed] [Google Scholar]
Joyal 2004 {published data only}
- Joyal SV. A perspective on the current strategies for the treatment of obesity. Current Drug Targets CNS and Neurological Disorders 2004;3(5):341‐56. [DOI] [PubMed] [Google Scholar]
Pasman 1997 {published data only}
- Pasman WJ, Westerterp‐Plantenga MS, Saris WH. The effectiveness of long‐term supplementation of carbohydrate, chromium, fibre and caffeine on weight maintenance. International Journal of Obesity and Related Metabolic Disorders 1997;21(12):1143‐51. [DOI] [PubMed] [Google Scholar]
Pittler 2004 {published data only}
- Pittler MH, Ernst E. Dietary supplements for body‐weight reduction: a systematic review. The American Journal of Clinical Nutrition 2004;79(4):529‐36. [DOI] [PubMed] [Google Scholar]
Rabinowitz 1983 {published data only}
- Rabinowitz MB, Gonick HC, Levin SR, Davidson MB. Effects of chromium and yeast supplements on carbohydrate and lipid metabolism in diabetic men. Diabetes Care 1983;6(4):319‐27. [DOI] [PubMed] [Google Scholar]
Stupar 1999 {published data only}
- Stupar J, Vrtovec M, Kocijancic A, Gantar A. Chromium status of tannery workers in relation to metabolic disorders. Journal of Applied Toxicology 1999;19(6):437‐46. [DOI] [PubMed] [Google Scholar]
Trent 1995 {published data only}
- Trent LK, Thieding‐Cancel D. Effects of chromium picolinate on body composition. The Journal of Sports Medicine and Physical Fitness 1995;35(4):273‐80. [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang ZQ, Cefalu WT. Current concepts about chromium supplementation in type 2 diabetes and insulin resistance. Current Diabetes Reports 2010;10(2):145‐51. [DOI] [PubMed] [Google Scholar]
Wilson 1995 {published data only}
- Wilson BE, Gondy A. Effects of chromium supplementation on fasting insulin levels and lipid parameters in healthy, non‐obese young subjects. Diabetes Research and Clinical Practice 1995;28(3):179‐84. [DOI] [PubMed] [Google Scholar]
Zenk 2007 {published data only}
- Zenk JL, Frestedt JL, Kuskowski MA. HUM5007, a novel combination of thermogenic compounds, and 3‐acetyl‐7‐oxo‐dehydroepiandrosterone: each increases the resting metabolic rate of overweight adults. The Journal of Nutritional Biochemistry 2007;18(9):629‐34. [DOI] [PubMed] [Google Scholar]
Additional references
Colditz 1995
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Annals of Internal Medicine 1995;122:481‐6. [DOI] [PubMed] [Google Scholar]
Denke 1994
- Denke MA, Sempos CT, Grundy SM. Excess body weight an under recognized contributor to dyslipidaemia in white American women. Archives of Internal Medicine 1994;154:401‐10. [DOI] [PubMed] [Google Scholar]
Docherty 2005
- Docherty JP, Sack DA, Roffman M, Finch M, Komorowski JR. A double‐blind, placebo‐controlled, exploratory trial of chromium picolinate in atypical depression: effect on carbohydrate craving. Journal of Psychiatric Practice 2005;11:302‐14. [DOI] [PubMed] [Google Scholar]
Drake 2012
- Drake TC, Rudser KD, Seaquist ER, Saeed A. Chromium infusion in hospitalised patients with severe insulin resistance: a retrospective analysis. Endodontic Practice 2012;31:1‐17. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
John 2007
- Zenk JL, Frestedt JL, Kuskowski MA. HUM5007, a novel combination of thermogenic compounds, and 3‐acetyl‐7‐oxo‐dehydroepiandrosterone: each increases the resting metabolic rate of overweight adults. The Journal of Nutritional Biochemistry 2007;18(9):629‐34. [DOI] [PubMed] [Google Scholar]
Kahn 2006
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006;444:840‐6. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Kleefstra 2006
- Kleefstra N, Houweling ST, Jansman FG, Groenier KH, Gans RO, Meyboom‐de Jong B, et al. Chromium treatment has no effect in patients with poorly controlled, insulin‐treated type 2 diabetes in an obese Western population: a randomized, double‐blind, placebo‐controlled trial. Diabetes Care 2006;29(3):521‐5. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 1999;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 1998
- Murray MT, Pizzorno JE (editors). Encyclopedia of Natural Medicine. 2nd Edition. Rocklin: Prima Publishing , 1998. [Google Scholar]
Naimark 1960
- Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. Journal of Applied Physiology 1960;15:377‐82. [DOI] [PubMed] [Google Scholar]
Pittler 2003
- Pittler MH, Stevinson C, Ernst E. Chromium picolinate for body weight reduction: Meta‐analysis of randomized trials. International Journal of Obesity and Related Metabolic Disorders 2003;27:522‐9. [DOI] [PubMed] [Google Scholar]
Pizzorno 1999
- Pizzorno JE, Murray MT, editors. Textbook of Natural Medicine. London: Churchill Livingstone, 1999. [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Rimm 1995
- Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA, et al. Body size and fat distribution as predictors of coronary heart disease among middle‐aged and older US men. American Journal of Epidemiology 1995;141(12):1117‐27. [DOI] [PubMed] [Google Scholar]
Stephen 2008
- Anton SD, Morrison CD, Cefalu WT, Martin CK, Coulon S, Geiselman P, et al. Effects of chromium picolinate on food intake and satiety. Diabetes Technology & Therapeutics 2008;10(5):405‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Volpe 2001
- Volpe SL, Huang HW, Larpadisorn K, Lesser II. Effect of chromium supplementation and exercise on body composition, resting metabolic rate and selected biochemical parameters in moderately obese women following an exercise program. The American College of Nutrition 2001;20(4):293‐306. [DOI] [PubMed] [Google Scholar]
Wang 2007
- Wang ZQ, Qin J, Martin J, Zhang XH, Sereda O, Anderson RA, et al. Phenotype of subjects with type 2 diabetes mellitus may determine clinical response to chromium supplementation. Metabolism 2007;56:1652‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitlock 2002
- Whitlock G, Lewington S, Ni Mhurchu C. Coronary heart disease and body mass index: a systematic review of the evidence from large prospective cohort studies. Seminars in Vascular Medicine 2002;2(4):369‐81. [DOI] [PubMed] [Google Scholar]
WHO 1995
- World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. Technical Report Series No. 854. www.who.int/childgrowth/publications/physical_status/en/index.html (accessed 16 July 2012). [PubMed]
WHO 2006
- World Health Organization. Fact sheet No 311: Obesity and overweight. www.who.int/mediacentre/factsheets/fs311/en/ (accessed 6 August 2010).
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


