Abstract
Background
Asthma is a chronic disease that causes reversible narrowing of the airways due to bronchoconstriction, inflammation and mucus production. Asthma continues to be associated with significant avoidable morbidity and mortality. Self management facilitated by a healthcare professional is important to keep symptoms controlled and to prevent exacerbations.
Telephone and Internet technologies can now be used by patients to measure lung function and asthma symptoms at home. Patients can then share this information electronically with their healthcare provider, who can provide feedback between clinic visits. Technology can be used in this manner to improve health outcomes and prevent the need for emergency treatment for people with asthma and other long‐term health conditions.
Objectives
To assess the efficacy and safety of home telemonitoring with healthcare professional feedback between clinic visits, compared with usual care.
Search methods
We identified trials from the Cochrane Airways Review Group Specialised Register (CAGR) up to May 2016. We also searched www.clinicaltrials.gov, the World Health Organization (WHO) trials portal and reference lists of other reviews, and we contacted trial authors to ask for additional information.
Selection criteria
We included parallel randomised controlled trials (RCTs) of adults or children with asthma in which any form of technology was used to measure and share asthma monitoring data with a healthcare provider between clinic visits, compared with other monitoring or usual care. We excluded trials in which technologies were used for monitoring with no input from a doctor or nurse. We included studies reported as full‐text articles, those published as abstracts only and unpublished data.
Data collection and analysis
Two review authors screened the search and independently extracted risk of bias and numerical data, resolving disagreements by consensus.
We analysed dichotomous data as odds ratios (ORs) while using study participants as the unit of analysis, and continuous data as mean differences (MDs) while using random‐effects models. We rated evidence for all outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) approach.
Main results
We found 18 studies including 2268 participants: 12 in adults, 5 in children and one in individuals from both age groups. Studies generally recruited people with mild to moderate persistent asthma and followed them for between three and 12 months. People in the intervention group were given one of a variety of technologies to record and share their symptoms (text messaging, Web systems or phone calls), compared with a group of people who received usual care or a control intervention.
Evidence from these studies did not show clearly whether asthma telemonitoring with feedback from a healthcare professional increases or decreases the odds of exacerbations that require a course of oral steroids (OR 0.93, 95% confidence Interval (CI) 0.60 to 1.44; 466 participants; four studies), a visit to the emergency department (OR 0.75, 95% CI 0.36 to 1.58; 1018 participants; eight studies) or a stay in hospital (OR 0.56, 95% CI 0.21 to 1.49; 1042 participants; 10 studies) compared with usual care. Our confidence was limited by imprecision in all three primary outcomes. Evidence quality ratings ranged from moderate to very low. None of the studies recorded serious or non‐serious adverse events separately from asthma exacerbations.
Evidence for measures of asthma control was imprecise and inconsistent, revealing possible benefit over usual care for quality of life (MD 0.23, 95% CI 0.01 to 0.45; 796 participants; six studies; I2 = 54%), but the effect was small and study results varied. Telemonitoring interventions may provide additional benefit for two measures of lung function.
Authors' conclusions
Current evidence does not support the widespread implementation of telemonitoring with healthcare provider feedback between asthma clinic visits. Studies have not yet proven that additional telemonitoring strategies lead to better symptom control or reduced need for oral steroids over usual asthma care, nor have they ruled out unintended harms. Investigators noted small benefits for quality of life, but these are subject to risk of bias, as the studies were unblinded. Similarly, some benefits for lung function are uncertain owing to possible attrition bias.
Larger pragmatic studies in children and adults could better determine the real‐world benefits of these interventions for preventing exacerbations and avoiding harms; it is difficult to generalise results from this review because benefits may be explained at least in part by the increased attention participants receive by taking part in clinical trials. Qualitative studies could inform future research by focusing on patient and provider preferences, or by identifying subgroups of patients who are more likely to attain benefit from closer monitoring, such as those who have frequent asthma attacks.
Plain language summary
What are the benefits and harms of using technology to monitor people with asthma from home?
Take‐home message A wide range of technologies have been developed to connect people with asthma to their healthcare professionals between routine checkups. Studies that have tested these strategies have not proved that 'telemonitoring' leads to better symptom control or fewer attacks, and could not rule out the possibility that it may cause unintended harm by making people less likely to take action when it is needed. Telemonitoring may have small benefits for quality of life and lung function, but these results are very uncertain.
Background Regular contact with a doctor or an asthma nurse is important to keep on top of asthma symptoms and to change inhalers if necessary. Telephone and Internet technologies are now used for lots of long‐term health conditions as a way of monitoring symptoms between visits to a clinic. For asthma, lung function and other asthma symptoms can be measured at home and information sent electronically to the doctor or nurse, who can decide whether action needs to be taken before the person is due to come back to the clinic.
Review question We wanted to find out whether home telemonitoring including feedback from a healthcare professional offers added benefits for people with asthma compared with their usual monitoring.
Study characteristics We found 18 studies including a total of 2268 people: 12 included adults, five included children and one included individuals from both age groups. Most people included in the studies had mild to moderate persistent asthma, and studies generally lasted between three and 12 months. People in the intervention group were given one of a variety of technologies to record and share their symptoms (text messaging, Web systems or phone calls) and were compared with a group of people who received usual care, or a control group.
Main results and quality of the evidence We could not tell whether people in the telemonitoring groups had a higher or lower chance than people in the control group of having attacks that would require a course of oral steroids, a visit to the emergency department or a hospital stay. No reports described other potential harms of home telemonitoring. Studies used lots of different types of technology, and we couldn't tell whether some were better than others. Our confidence in the results ranged from moderate to very low, meaning that additional studies are likely to change some of these results and may influence how much we believe them.
Using technology to monitor people with asthma from home may offer benefits over usual care for overall quality of life, but the effect was small, and studies did not agree with each other. These interventions may provide benefits for lung function, but lots of people dropped out of the studies, so we couldn't be sure.
Summary of findings
Summary of findings for the main comparison. Summary of findings table 1.
| Home telemonitoring and feedback vs usual care for people with asthma | ||||||
| Patient or population: people with asthma Setting: home Intervention: home telemonitoring with remote feedback from a healthcare professional Comparison: usual monitoring | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual monitoring | Risk with home telemonitoring and feedback | |||||
|
Exacerbations requiring oral corticosteroids 7.3‐month follow‐up** |
399 per 1000 | 382 per 1000 (285 to 489) | OR 0.93 (0.60 to 1.44) | 466 (4 RCTs) | ⊕⊕⊝⊝ LOWa,b,c | 2 child studies, 2 adult studies. Subgroup differences not significant (P value = 0.78) 2 child studies and 6 adult studies in ED analysis agreed with the OCS analysis (OR 0.75, 95% CI 0.36 to 1.58) |
|
Exacerbations requiring hospital admission 7.8‐month follow‐up |
Children (< 16 years) | OR 1.38 (0.51 to 3.68) | 421 (4 RCTs) | ⊕⊕⊕⊝ MODERATEc,d | 4 child studies and 6 adult studies presented separately owing to significant subgroup differences (P value = 0.04) Telemonitoring beneficial for adults, but probably not for children |
|
| 38 per 1000 | 52 per 1000 (20 to 127) | |||||
| Adults (17 to 65 years) | OR 0.24 (0.06 to 0.94) | 621 (6 RCTs) | ⊕⊕⊕⊝ MODERATEc,d,e | |||
| 83 per 1000 | 21 per 1000 (5 to 79) | |||||
| Asthma control Follow‐up varied from 3 to 12 months | Asthma control was reported in 3 different ways across 4 studies Summary of results in Comments column | ‐ | (4 RCTs) | ⊕⊝⊝⊝ VERY LOWf,g,h | ACQi (MD ‐0.24, 95% CI ‐0.72 to 0.24) (2 adult studies); ACT 'well‐controlled' (29/60 vs 8/29) (1 adult study) (MD 0.09, 95% CI 0.92 to 1.10) (1 child study) | |
| Serious and non‐serious adverse events | None of the studies explicitly reported serious or non‐serious adverse events as an outcome separate from asthma exacerbation outcomes | ‐ | (0 RCTs) | N/A | No studies | |
|
Asthma‐related quality of life (AQLQ)i 9.6‐month follow‐up 1 to 7, higher = better |
Mean AQLQ score was 3.58*** | Mean AQLQ score in the intervention group was 0.23 better (0.01 better to 0.45 better) | ‐ | 796 (6 RCTs) | ⊕⊕⊝⊝ LOWf,i | |
|
Lung function % predicted trough FEV1 higher = better 7.6‐month follow‐up |
Mean predicted FEV1 was 68.4%*** | Mean % predicted FEV1 in the intervention group was 7.21% higher (1.52 higher to 12.89 higher) | ‐ | 149 (3 RCTs) | ⊕⊕⊕⊝ MODERATE j | ‐ |
|
Unscheduled healthcare visits 6.2‐month follow‐up |
332 per 1000 | 329 per 1000 (155 to 565) | OR 0.99 (0.37 to 2.62) | 430 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW c,k,l | Very unbalanced dropout in 1 study showing different effects from the other 2 (12% vs 57%) |
| * The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
** With the exception of the asthma control outcome, where a range is given, follow‐ups are given in months as a weighted mean duration of studies in the analysis. *** Risk with usual monitoring was calculated as a weighted mean of scores in the control groups of studies contributing to the analysis. For the AQLQ analysis, this did not include the 2 studies reporting change from baseline. ACQ = Asthma Control Questionnaire; ACT = Asthma Control Test; AQLQ = Asthma Quality of Life Questionnaire; CI = confidence interval; FEV1 = forced expiratory volume in 1 second; MD = mean difference; OR = odds ratio; RCT = randomised control trial; RR = risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aA couple of studies carrying < 20% of the overall weight had high attrition and uncertainty with selection bias, but this was not judged to be serious enough to downgrade (no downgrade)
bOne study could not be included because exacerbations were used as the unit of analysis rather than people with exacerbations, and the small number of studies in the analysis compared with the emergency department and hospital exacerbations analyses suggests that this may have been recorded and not reported (‐1 publication bias)
cConfidence intervals include important benefit of either treatment, so it is difficult to interpret the result (‐1 imprecision)
dRisk of bias was confined mostly to the blinding domains, which is unlikely to have affected this outcome. Uncertainty in the selection bias domains was not deemed serious enough to downgrade (no downgrade)
eHeterogeneity between studies in the adult subgroup was high but not statistically significant, and all but one of the point estimates lay in the same direction, favouring telemonitoring (no downgrade)
fStudies were generally at high risk of bias for the blinding domains, which may have affected results on subjective rating scales (‐1 risk of bias)
gSerious inconsistency between the two studies reporting the ACQ and results across asthma control outcomes did not give a clear direction of effect (‐1 inconsistency)
hImprecision varied across the 3 asthma control outcomes, but overall the effect was unclear owing to differences in direction, magnitude and confidence intervals (‐1 imprecision)
j Child and adult studies were pooled, and important heterogeneity was noted (I2 = 54%, P value = 0.06) (‐1 inconsistency)
kTwo studies carrying most of the weight were judged to be at high risk of bias owing to high dropout; in particular, Cingi 2015 had 57% dropout in the control group compared with 12% in the intervention group (‐1 risk of bias)
lCingi showed an effect in the opposite direction to that noted in the other two studies, which introduced important heterogeneity (I2 = 73%, P value = 0.03) (‐1 inconsistency)
iThe minimal clinically important difference (MCID) for both the Asthma Control Questionnaire (ACQ) and the Asthma Quality of Life Questionnaire (AQLQ) is 0.5 units
Background
Description of the condition
Asthma is a chronic disease of the airways that causes reversible inflammation and narrowing of the airways, along with mucus production (GINA 2014). These features commonly cause symptoms of wheezing, breathlessness, chest tightness and cough, although symptoms vary between people and over time in terms of presence, frequency and severity (GINA 2014).
Despite the emergence and updating of several national and international management guidelines recommending a range of cost‐effective treatments based on frequency and severity of symptoms and exacerbations (e.g. BTS/SIGN 2014; GINA 2014), the disease remains a significant cause of avoidable morbidity and mortality around the world (BTS/SIGN 2014; Global Asthma Report 2011; NRAD 2014). A national review of the 195 asthma deaths that occurred between February 2012 and January 2013 in the UK revealed that, in the year preceding their death, nearly one‐third of these individuals had no record of seeing a general practitioner (GP), and nearly two‐thirds had not had an asthma checkup in secondary care (NRAD 2014). The importance of self monitoring and regular checkups with a healthcare professional to monitor symptoms and encourage adherence to preventer inhalers is now well accepted (Gibson 2002; NRAD 2014), especially for those at high risk of severe asthma attacks.
Description of the intervention
Information and communication technologies have been proposed as a way for patients to record and share information regularly about their asthma control with a healthcare professional. This method of monitoring may identify worsening asthma between consultations, prompting action, such as a medication change, or an additional visit. Remote monitoring in this way is a form of 'telehealth', otherwise referred to as 'telecare', 'digital health', 'mHealth' or 'telemedicine', which involves "the use of information and communication technologies to deliver healthcare at a distance and to support patient self‐management through remote monitoring and personalised feedback" (Mclean 2013).
Communication technologies used in health care are varied, ranging from simple automated reminder systems for patients to take medication or attend their appointments (Gurol‐Urganci 2013) to more complex health communications sent via email (Atherton 2012), telephone systems (Cash‐Gibson 2012) or text messages (de Jongh 2012); however, feedback and personalised care from a healthcare professional are important components of what can be considered telehealth. Health services around the world are considering communication technologies in their various forms as a way of managing the rising number of people with long‐term health conditions, to improve health outcomes and reduce the burden on emergency and inpatient services (Steventon 2012; UK Department of Health 2012).
Governments and healthcare providers are increasingly adopting 'telehealth' and investing in research to pin down how and for whom it could be beneficial. Programmes include an initiative in the UK encouraging wide availability of 'e‐consultations' and home 'telemonitoring' (UK Department of Health 2013). A Telehealth Pilots Programme in Australia (Australian Government Department of Health 2016) offers widespread eHealth to chronically ill people and vulnerable elderly people in the Netherlands (Government of the Netherlands 2016), along with recognition of 'telehealth' monitoring by US Medicaid insurance (Medicaid 2016). Studies have assessed the role of a range of technology‐based consultations and monitoring in asthma and other health conditions, including telephone calls, email contacts, text messaging and video conferencing (Laver 2013; McLean 2010; McLean 2011).
Remote monitoring of asthma with technologies might include features such as recording symptoms online or automatically transferring home peak flow readings to a doctor or nurse. Regular recording and remote sharing of this information may trigger a response from a healthcare professional, who uses the information to provide personalised care. Researchers have assessed telehealth in several ways, including as an alternative for usual primary or secondary care clinic appointments (e.g. Rasmussen 2005); this was recently addressed by a related Cochrane review (Kew 2016). However, this review will consider evidence for home telemonitoring of asthma control between visits with personalised feedback from a healthcare professional (e.g. Ryan 2012).
How the intervention might work
In the context of asthma, a condition affecting more than 300 million people worldwide (Global Asthma Report 2011), which places a significant burden on healthcare systems, telehealth may represent an unobtrusive and efficient way of maintaining contact between patients and healthcare professionals. Regular monitoring with communication technologies may serve to enhance self management behaviours that have known benefits for morbidity and mortality, such as keeping personalised action plans up‐to‐date and adhering to maintenance medications (NRAD 2014). As an alternative to methods of monitoring that do not include feedback from a healthcare professional, telehealth may offer a more interactive method of supporting self management.
Although governments and health services have highlighted the potential for cost‐savings and improved clinical outcomes of telehealth used in this way, its use to monitor patients with potentially serious or life‐threatening conditions may not be without hazard. Focus groups have suggested that technology may be acceptable to patients and clinicians, but they have raised concerns that it could actually discourage self management, or might increase the likelihood of serious outcomes by instilling a false sense of security (Pinnock 2007a).
The feasibility of home telemonitoring using technology in different situations and populations may be hampered by barriers, including insufficient healthcare infrastructure and funding (Lustig 2012). However, this approach may reduce inequality in health care related to socioeconomic status and rural living by improving access to services (Jannett 2003; Lustig 2012).
Why it is important to do this review
The release of the UK National Health Service (NHS) mandate in 2013 has resulted in a push to advance the use of communication technologies for economic and clinical benefit. A recent overview of systematic reviews suggested that these benefits should not be assumed, and that people at highest risk of serious health outcomes are likely to show the biggest gains (Mclean 2013). For asthma, existing reviews have noted a large degree of variation in the way telehealth is delivered in studies and to whom and with what it is compared (Jaana 2009; McLean 2010), and have been limited for this reason in the conclusions that could be drawn. This review considers evidence for ongoing personalised feedback from a healthcare professional using home telemonitoring between visits, compared with monitoring without feedback. A related review has considered evidence for remote checkups as an alternative to face‐to‐face asthma consultations (Kew 2016).
Objectives
To assess the efficacy and safety of home telemonitoring with healthcare professional feedback between clinic visits, compared with usual care.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel randomised controlled trials (RCTs) of any duration. We included studies reported as full‐text articles, those published as abstracts only and unpublished data.
Types of participants
We included studies of adults or children with a diagnosis of asthma. We excluded studies recruiting participants with other long‐term health conditions, unless investigators presented data for people with asthma separately.
Types of interventions
We included studies comparing home telemonitoring of asthma between clinic visits, using any form of technology (e.g. telephone calls, emails, text messages, online software), with a form of monitoring that does not include ongoing remote professional feedback. We included studies that compared the two types of monitoring on top of education or another co‐intervention. We excluded studies using automated telehealth interventions that did not include personalised input from a healthcare professional.
Types of outcome measures
Primary outcomes
Exacerbations requiring oral corticosteroids*.
Asthma control (measured on a validated scale, e.g. the Asthma Control Questionnaire).
Serious adverse events (including mortality).
Secondary outcomes
Asthma‐related quality of life (measured on a validated scale, e.g. the Asthma Quality of Life Questionnaire (AQLQ)).
Unscheduled healthcare visits.
Lung function (trough forced expiratory volume in one second (FEV1) preferred).
Adverse events/side effects.
Reporting in the study of one of more of the outcomes listed here was not an inclusion criterion for the review.
*If studies reported exacerbations in a different way (e.g. requiring an emergency department (ED) visit), we analysed these separately.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. This Register contains trial reports identified through systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). We searched all records in the CAGR using the search strategy presented in Appendix 2.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/). We searched all databases from their inception to May 2016, and we imposed no restriction on language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information.
On 2 August 2016, we searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
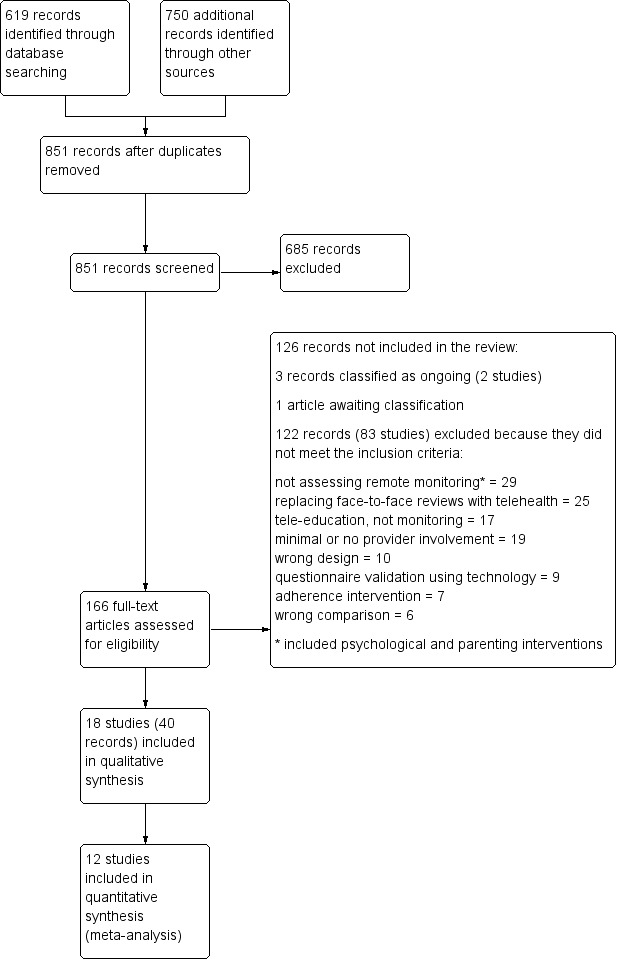
Two review authors (KMK and CJC) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publications, and two review authors (KMK and CJC) independently screened these documents, identified studies for inclusion and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion. We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram (Figure 1) and a Characteristics of excluded studies table (Moher 2009).
1.
Study flow diagram.
Data extraction and management
We used a data collection form for study characteristics and outcome data that had been piloted on at least one study in the review. One review author (KMK) extracted the following study characteristics from the included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (KMK and CJC) independently extracted outcome data from the included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a useable way. We resolved disagreements by consensus. One review author (KMK) transferred data into the Review Manager 5 (RevMan 2014) file. We double‐checked that data were entered correctly by comparing data presented in the systematic review with data provided in the study reports. A second review author (CJC) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (KMK and CJC) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion and assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low or unclear, and we provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias relates to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to those outcomes.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs), and continuous data as mean differences (MDs) or standardised mean differences (SMDs). We entered data presented as a scale with a consistent direction of effect.
We undertook meta‐analyses only when this was meaningful, i.e. when treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
We did not include in the meta‐analyses skewed data that were reported as medians and interquartile ranges, but described the results narratively instead.
When a single trial reported multiple trial arms, we included only the relevant arms. When two comparisons (e.g. drug A vs placebo, drug B vs placebo) were combined in the same meta‐analysis, we halved the control group to avoid double‐counting.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (i.e. number of adults admitted to hospital, rather than number of admissions per adult). However, if exacerbations were reported as rate ratios, we analysed them on this basis.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only). When this was not possible, and missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the studies in each analysis. If we identified substantial heterogeneity, we reported it and explored possible causes through prespecified subgroup analysis.
Assessment of reporting biases
When we were able to pool more than 10 studies, we created and examined a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We used a random‐effects model for all analyses, as we expected variation in effects due to differences among study populations and interventions. We performed sensitivity analyses by using a fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table by using the seven outcomes specified above. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies that contributed data to the meta‐analyses for prespecified outcomes. We followed methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) while using GRADEpro GDT 2015 software. We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we made comments to aid the reader's understanding of the review, when necessary.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses for the primary outcomes, provided at least one study was included for each subgroup.
Mean age (≤ 16 years, 17 to 65 years, > 65 years).
Type of technology (telephone calls, text messages, emails).
We used the formal test for subgroup interactions provided in Review Manager 5 (RevMan 2014).
Sensitivity analysis
We planned to carry out sensitivity analyses, while excluding the following from the primary analyses.
Studies recruiting people with severe or life‐threatening asthma.
Unpublished data (obtained from trial authors or from conference abstracts).
Studies at high risk of detection bias*.
*Inadequate selection procedures may result in unbalanced baseline characteristics between groups, which could skew the data. In light of the nature of the studies, we anticipated that all or most studies would be at high risk of performance or detection bias, so we have discussed the possible effects of lack of blinding, in particular for subjective outcomes.
Results
Description of studies
Results of the search
We identified 619 records in the main electronic database search. We identified a total of 750 additional records through a search conducted for an older teleheathcare review with a broader scope (McLean 2010) (n = 709), clinicaltrials.gov (n = 29) and the World Health Organization (WHO) trials portal (n = 11), as well as reference lists of other reviews (n = 1). Collating all searches revealed a total of 1369 records, of which 518 were duplicates. We screened the remaining 851 unique records and excluded 685 by looking at titles and abstracts alone. We reviewed full‐text articles for the remaining 166 records; 123 records related to 84 studies did not meet the inclusion criteria and were excluded (with reasons), three records related to two ongoing studies (Ahmed 2011; Perry 2015) and one record is awaiting classification (Ricci 2001). This left 18 studies, with 40 associated reports, which met the inclusion criteria for this review (see trial flow in Figure 1).
Included studies
Eighteen studies, including a total of 2268 participants, met the inclusion criteria for this review (Bateman 2000; Cingi 2015; Deschildre 2012; Donald 2008; Finkelstein 2005; Guendelman 2002; Jan 2007; Kokubu 1999; Kokubu 2000; Liu 2011; Ostojic 2005; Prabhakaran 2009; Ryan 2012; van der Meer 2009; Voorend‐van Bergen 2015; Willems 2008; Xu 2010; Young 2012). An overview of study, participant and intervention characteristics is given in Table 2, and more in‐depth information and risk of bias details can be found in the Characteristics of included studies table.
1. Summary of study and intervention characteristics.
| Study ID | N | Country | Duration (mo) | Age group | Mean age (y) | % male | Technology | Intervention | Control |
| Bateman 2000 | 135 | South Africa | 12 | Adults | NR | NR | Web system | Interactive Web system. Daily modem transfer of spirometry data, clinician decision support and participant education | Usual care, no additional monitoring |
| Cingi 2015 | 136 | Turkey | 3 | Adults | 32.8 | 47.2 | Smartphone app | Mobile phone application (POPET‐Asthma) to communicate with their physician, record health status and medication compliance | Usual care. Mobile phone app to record symptoms at beginning and end only. No feedback |
| Deschildre 2012 | 50 | France | 12 | Children | 11.1* | 74.0 | Web system | Interactive Web system. Daily modem transfer of spirometry data with treatment feedback from physician | Usual care, no additional monitoring |
| Donald 2008 | 71 | Australia | 12 | Adults | 36.2 | 23.9 | Phone calls | Six phone calls from the nurse to monitor symptoms and give advice. All received a PEF meter, education session and AAP | Usual care plus an education session, PEF meter and AAP |
| Finkelstein 2005 | 240 | USA | 12 | Adults | NR | NR | Portable device | Portable computer connected to home PEF meter to monitor symptoms and communicate with practitioner | Usual care, no additional monitoring |
| Guendelman 2002 | 134 | USA | 3 | Children | 12.1 | 57.5 | Portable device | Interactive 'Health Buddy' device for education and management. PEF, symptom and medication responses reviewed daily by nurse | Paper asthma diary with 2 follow‐up visits with nurse |
| Jan 2007 | 164 | Taiwan | 3 | Children | 10.4 | 38.4 | Web system and phone | Internet symptom and PEF diaries and individual AAP that could be shared with physician, who provided feedback via phone or email | Usual care with asthma education, PEF meter and AAP |
| Kokubu 1999 | 50 | Japan | 6 | Adults | 52.8 | 46.0 | Web system | Telemedicine system to monitor airway status at home with nurse instruction via phone | Usual care, no additional monitoring |
| Kokubu 2000 | 75 | Japan | 6 | Adults | 48.6 | 36.0 | Web system | Telemedicine system to monitor airway status at home with nurse instruction via phone | Usual care, no additional monitoring |
| Liu 2011 | 120 | Taiwan | 6 | Adults | 52.2 | 49.5 | Smartphone app | Mobile phone‐based software with online symptom, medication and lung function diary reviewed by medical staff | Written asthma diary and AAP |
| Ostojic 2005 | 16 | Croatia | 4 | Adults/teens | 24.7 | 56.5 | SMS | PEF, symptoms and medication use sent via SMS to asthma specialist, who gave weekly SMS advice for review or medications | PEF, symptoms and medication use diary reviewed at the end of the study |
| Prabhakaran 2009 | 120 | Singapore | 3 | Adults | 38.5 | 41.0 | SMS | SMS monitoring with advice on asthma control | Usual care, no additional monitoring |
| Ryan 2012 | 288 | UK | 6 | Adults/teens | 49.0 | 37.6 | Smartphone app | Symptom, PEF and medication data sent via mobile phone twice daily with immediate feedback according to AAP | Paper‐based monitoring with same guideline‐based care as the active group |
| van der Meer 2009 | 200 | Netherlands | 12 | Adults | 36.5 | 30.5 | Web system or SMS | Daily FEV1, weekly ACQ and symptom reporting via SMS or a website, which also held education and a treatment plan | Access to diary online ‐ not transmitted |
| Voorend‐van Bergen 2015 | 180 | Netherlands | 12 | Children | 10.4 | 66.0 | Web system | Web‐based monthly monitoring of asthma control according to scores on the ACT | Usual care, no additional monitoring |
| Willems 2008 | 109 | Netherlands | 12 | Adults/children | 27.8 | 51.4 | Web system | Asthma monitor with home modem transferring symptoms and medication use diaries to an asthma nurse | Usual care, no additional monitoring |
| Xu 2010 | 82 | Australia | 6 | Children | 7.0 | 51.2 | Phone calls or emails | Fortnightly calls or emails from a nurse specialist to collect symptom data and to offer education and advice | Usual care plus education session |
| Young 2012 | 98 | USA | 3 | Adults | 44.5 | 23.5 | Phone calls | Phone call with the pharmacist to assess self management and medication usage | Usual care including mail receipt of prescription refill and written instructions |
N is the total number of participants randomised to the intervention and control group(s) relevant to this review
% FEV1 is the baseline mean of predicted normal values
AAP = asthma action plan
ACQ = Asthma Control Questionnaire
ACT = Asthma Control Test
FEV1 = forced expiratory volume in one second
mo = months
NR = not reported
PEF = peak expiratory flow
POPET = Physician On Call Patient Engagement Trial
SMS = short message service
y = years
*Value is the mean of the median ages reported for the intervention and control groups in Deschildre 2012
All included studies were parallel RCTs. The number of participants in each study ranged from 16 to 288, and the median number was 120. As shown in Table 2, seven studies took place in Europe (the Netherlands, Croatia, France, Turkey and the UK), five in Asia (Japan, Singapore and Taiwan), three in the USA, two in Australia and one in South Africa. Eleven studies were run from respiratory clinics in hospitals or outpatient centres (Cingi 2015; Deschildre 2012; Donald 2008; Guendelman 2002; Jan 2007; Kokubu 2000; Liu 2011; Ostojic 2005; Prabhakaran 2009; Willems 2008; Xu 2010), and four from general practitioners' offices or medical centres (Bateman 2000; Kokubu 1999; Ryan 2012; van der Meer 2009). Young 2012 was run through pharmacies in an 11‐county region in the USA, and two studies that were reported only as conference abstracts did not reveal the setting in which they took place (Finkelstein 2005; Voorend‐van Bergen 2015).
Population characteristics and inclusion criteria
Twelve studies recruited adults or adults and adolescents (Bateman 2000; Cingi 2015; Donald 2008; Finkelstein 2005; Kokubu 1999; Kokubu 2000; Liu 2011; Ostojic 2005; Prabhakaran 2009; Ryan 2012; van der Meer 2009; Young 2012), five studies recruited only children (Deschildre 2012; Guendelman 2002; Jan 2007; Voorend‐van Bergen 2015; Xu 2010) and one study recruited both adults and children (Willems 2008). Two of the adult studies also recruiting adolescents over 12 (Ostojic 2005; Ryan 2012) and the one study recruiting both adults and children (Willems 2008) were classified as adult studies because the mean age of participants was over 18, and data for children were not reported separately. The overall weighted mean of population ages was 31.6 years (range, seven to 52.8). The mean age of child populations was 10.4 years (range, seven to 12.1) and the mean age in adult studies was 41.6 (range, 24.7 to 52.8). The mean percentage male indicated a relatively even split of males and females (45.6% male), although the percentage male in individual studies ranged from 23.5% to 74.0%.
Deschildre 2012, Kokubu 1999, Kokubu 2000 and Prabhakaran 2009 listed inclusion criteria that would have led to recruitment of people with severe asthma; all required that participants had at least a course of oral steroids, a visit to the ED or admission to hospital within the previous year, and these studies excluded participants with mild or intermittent asthma or specifically required them to meet the criteria for severe asthma. Otherwise, studies generally recruited people with mild to moderate persistent asthma, and common inclusion criteria included physician‐diagnosed or guideline‐diagnosed asthma, a recent prescription for asthma controller medications ‐ usually inhaled corticosteroid (ICS) or long‐acting beta agonist (LABA) + ICS ‐ access to and competency with relevant technologies and proficiency in the given language (usually English). Common exclusion criteria were pregnancy or breastfeeding, other chronic illnesses and current smoking. Two child studies (Deschildre 2012 and Voorend‐van Bergen 2015) specifically recruited children with allergic asthma, and Ryan 2012 required that participants score 1.5 or lower on the Asthma Control Questionnaire to indicate insufficient symptom control.
Interventions and comparisons
Six trials provided three‐ or four‐month interventions, five trials gave six‐month interventions and seven trials tested the interventions for a year (see Table 2). All monitoring interventions involved ways for participants or their parents to track their asthma control at home and to share this information with a healthcare professional to receive management advice between usual clinic visits. Nine studies used an Internet‐based device, programme or website for participants to record and transmit a range of symptom, medication or lung function data to the healthcare professional (Bateman 2000; Deschildre 2012; Finkelstein 2005; Guendelman 2002; Jan 2007; Kokubu 1999; Kokubu 2000; Voorend‐van Bergen 2015; Willems 2008). Healthcare professionals, often a specialist nurse, regularly reviewed the data and responded with management advice, often based on personalised asthma action plans. Six studies used a similar system of recording and response that was done primarily via short message service (SMS) or mobile phone software (Cingi 2015; Liu 2011; Ostojic 2005; Prabhakaran 2009; Ryan 2012; van der Meer 2009). Three studies involved regular calls or email contact with a nurse or pharmacist to monitor symptoms and advise on changes to medication (Donald 2008; Xu 2010; Young 2012).
Most included trials used usual care as their comparison group (Bateman 2000; Cingi 2015; Deschildre 2012; Donald 2008; Finkelstein 2005; Jan 2007; Kokubu 1999; Kokubu 2000; Prabhakaran 2009; Voorend‐van Bergen 2015; Willems 2008; Xu 2010; Young 2012). In three of these studies, people in the usual care group received a minimal intervention such as an education session, a personalised asthma action plan or a peak flow meter to encourage self monitoring at home (Donald 2008; Jan 2007; Xu 2010). Five studies gave participants in the control group an asthma diary or a peak flow meter to record their symptoms at home (Guendelman 2002; Liu 2011; Ostojic 2005; Ryan 2012; van der Meer 2009), but these data were not shared with a healthcare professional between usual visits.
Excluded studies
After reviewing the full texts, a total of 126 citations (86 studies) were not included in the review. It was often difficult to tell whether a study met the inclusion criteria for the review by reading the title and abstract alone, so we excluded 122 citations (83 studies) after viewing full texts. We classified three citations (two studies) as ongoing (Ahmed 2011; Perry 2015), and one citation as awaiting classification because we did not have enough details to confirm whether it met the review's inclusion criteria (Ricci 2001).
Of the 122 citations (83 studies) that were listed as excluded because they did not meet the inclusion criteria, we excluded 29 (23 studies) because closer inspection showed that investigators were assessing an intervention other than home telemonitoring, including psychological or parenting interventions (Aaron 2016; Chandler 1990; Chen 2013; Cicutto 2009; Clark 2007; Clarke 2014; Eakin 2012; Gustafson 2012; Halterman 2012; Huang 2013; Janevic 2012; Jerant 2003; Khan 2003; Kojima 2005; Lobach 2013; McCowan 2001; NCT01117805; Osman 1997; van den Berg 2002; van Gaalen 2012; van Reisen 2010; Wiecha 2007; Zachgo 2002). We excluded 25 citations (six studies) because researchers assessed the feasibility of replacing face‐to‐face reviews with reviews conducted using technology, which was a different question from the one we set out to answer in this review (Chan 2007; Gruffydd‐Jones 2005; Hashimoto 2011; Pinnock 2003; Pinnock 2007; Rasmussen 2005). We excluded 17 citations (14 studies) because investigators were assessing the use of information technologies to deliver asthma education (Barbanel 2003; Burbank 2012; De Vera 2014; Dwinger 2013; Garbutt 2010; McPherson 2006; NCT00562081; NCT00910585; NCT00964301; Pedram 2012; Peruccio 2005; Seid 2012; Shanovich 2009; Yun 2013), 19 citations (11 studies) because researchers assessed automated feedback interventions that did not include ongoing input from a healthcare professional (Andersen 2007; Bender 2010; Kattan 2006; Merchant 2016; Morrison 2014; Petrie 2012; Rikkers‐Mutsaerts 2012; Searing 2012; Vasbinder 2013; Vollmer 2006; Zairina 2015), nine citations (seven studies) because investigators were reporting validation of a technology‐delivered asthma questionnaire (Bender 2001; Bender 2007; Price 2007; Rand 2005; Rosenzweig 2008; Schatz 2010; Uysal 2013) and seven citations (seven studies) because the intervention was aimed purely at improving adherence rather than monitoring asthma control (Boyd 2014; Burkhart 2002; Bynum 2001; Chatkin 2006; Foster 2014; MacDonell 2015; Taitel 2014). Ten citations (nine studies) were not reports of RCTs and were recorded as using the wrong design for the review (Apter 2000; Araujo 2012; Claus 2004; Cruz‐Correia 2007; Fonseca 2006; Friedman 1999; Lam 2011; Murphy 2001; Raat 2007), and six citations (six studies) compared a home telemonitoring intervention with another active comparator that was not eligible for this review (Apter 2015; Baptist 2013; de Jongste 2008; NCT00149474; Schatz 2003; Sparrow 2005).
Risk of bias in included studies
Figure 2 presents an overview of risk of bias in the included studies, and we provide below a summary of possible bias related to each domain. We have given full details of the rationale for each judgement in each study's risk of bias table (see the Characteristics of included studies tables).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Much uncertainty surrounded the two selection bias domains, despite efforts to clarify procedures with study authors. Seven studies were at low risk of bias for random sequence generation (Cingi 2015; Ostojic 2005; Prabhakaran 2009; Ryan 2012; van der Meer 2009; Voorend‐van Bergen 2015; Willems 2008), and eight were at low risk for allocation concealment (Cingi 2015; Guendelman 2002; Jan 2007; Kokubu 1999; Kokubu 2000; Ryan 2012; Voorend‐van Bergen 2015; Young 2012); we considered only Cingi 2015, Ryan 2012 and Voorend‐van Bergen 2015 to be at low risk in both selection bias domains. We rated the remaining studies as unclear.
Blinding
It was not possible to blind participants and personnel to group allocation because of the nature of the interventions and comparisons, and this posed the most serious risk of bias for the evidence in this review. We assessed risk of performance bias separately for objective (e.g. exacerbations) and subjective (e.g. quality of life) outcomes to better represent how this bias was likely to have affected our confidence in the results. We considered all studies to be at low risk of performance bias for objective outcomes and at high risk of bias for subjective outcomes.
Although theoretically outcome assessors could have been independent from the study and blinded to allocation, we did not assume that this was the case unless it was explicitly described in the report, or unless study authors confirmed this through personal communication. Fifteen studies did not describe methods to blind outcome assessors and did not confirm that those measuring outcomes were not blinded to group allocation; we rated these as having high risk of bias (Bateman 2000; Cingi 2015; Deschildre 2012; Finkelstein 2005; Guendelman 2002; Jan 2007; Kokubu 1999; Kokubu 2000; Liu 2011; Ostojic 2005; Prabhakaran 2009; van der Meer 2009; Voorend‐van Bergen 2015; Willems 2008; Xu 2010). We rated the remaining three studies as having low risk of bias (Donald 2008; Ryan 2012; Young 2012). Researchers described Cingi 2015 as a double‐blind trial, but participants, who self rated their symptoms for the primary outcome, could have conceivably worked out which group they were in by noting what they received during the study.
Incomplete outcome data
We considered half of the included studies to be at low risk of attrition bias because attrition was low and balanced across intervention and control groups, or because we believed that methods used to replace data for participants who did not complete the study would have adequately controlled for bias (Guendelman 2002; Jan 2007; Kokubu 2000; Ostojic 2005; Prabhakaran 2009; Ryan 2012; Voorend‐van Bergen 2015; Xu 2010; Young 2012). We rated three studies as unclear because we could not tell how many people dropped out of the study. We considered six studies to be at high risk of bias, mostly because attrition was high or was much higher in one group than in another, or because analyses did not include those who dropped out before the end of the study. Willems 2008 reported that up to 28% of data for particular outcomes were missing owing to errors with data transmission or poor compliance with questionnaires, and Cingi 2015 analysed only data for participants who completed the questionnaire at the end of the study, which represented a much smaller proportion of the control group than the intervention group (42.6% and 88.2%, respectively).
Selective reporting
We rated 14 studies as having low risk of bias because we were satisfied that all planned outcomes had been fully reported in published reports, or because study authors provided us with additional information upon request. We rated Cingi 2015 as low risk, although we could not meta‐analyse some outcomes because of the way they were reported, and statistical methods used were appropriate for the study data. We were unsure of the risk of reporting bias in Kokubu 1999 because it was available only in Japanese, and it was difficult to confirm whether all intended outcomes had been reported, even with translation. For Kokubu 2000, the translation confirmed that not all named outcomes had been reported sufficiently to include them in meta‐analyses, so we rated this study as having high risk of bias. We rated two other studies (Bateman 2000 and Finkelstein 2005) as having high risk of bias because they were available only as conference abstracts, not in peer‐reviewed journals, and this meant that study authors provided very little information about the conduct of the studies or their numerical results.
Other potential sources of bias
We did not note any additional sources of bias, so we rated all studies as having low risk for 'other sources of bias'.
Effects of interventions
See: Table 1
Primary outcomes
Exacerbations requiring oral corticosteroids
Home telemonitoring with feedback might be better or worse than usual monitoring (odds ratio (OR) 0.93, 95% confidence Interval (CI) 0.60 to 1.44; 466 participants; four studies; I2 = 0%; Analysis 1.1). Over seven months, 399 people per thousand who were monitored in their usual way had an exacerbation compared with 382 per thousand if they received telemonitoring with remote feedback from a healthcare professional (95% CI 285 to 489 per 1000). We had low confidence in the estimate because only four studies could be included in the analysis (Deschildre 2012; Donald 2008; Ryan 2012; Xu 2010), indicating possible publication bias. In addition, confidence intervals were too wide to reveal whether one monitoring strategy is likely to be better than another.
1.1. Analysis.
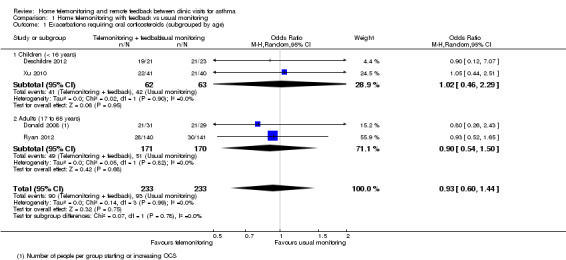
Comparison 1 Home telemonitoring with feedback vs usual monitoring, Outcome 1 Exacerbations requiring oral corticosteroids (subgrouped by age).
In addition to the four studies that were meta‐analysed, Voorend‐van Bergen 2015 reported the total number of exacerbations rather than the number of participants having at least one exacerbation, so we could not combine their data with data from other studies. Researchers observed 10 exacerbations among the 90 participants in the Web group and 17 exacerbations in the 87 participants receiving standard care.
Eight studies reported exacerbations that required a visit to the ED (Donald 2008; Guendelman 2002; Kokubu 2000; Liu 2011; Ryan 2012; van der Meer 2009; Willems 2008; Xu 2010), which supported the main analysis that home telemonitoring and feedback might be better or worse than control (OR 0.75, 95% CI 0.36 to 1.58; 1018 participants; eight studies; I2 = 47%; Analysis 1.2). We noted important heterogeneity in the ED overall analysis and within each child and adult subgroup. We had low confidence in the effect because confidence intervals were too wide to show whether one strategy is likely to be better than another, and because we noted important heterogeneity both within and across subgroups.
1.2. Analysis.
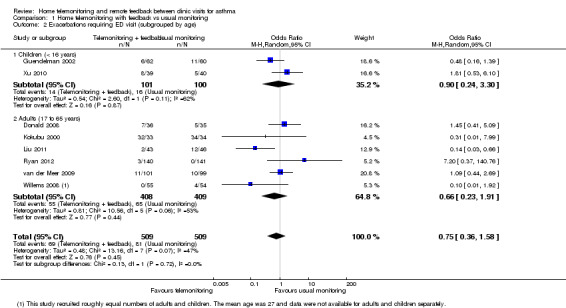
Comparison 1 Home telemonitoring with feedback vs usual monitoring, Outcome 2 Exacerbations requiring ED visit (subgrouped by age).
A look at exacerbations requiring hospital admission revealed that the overall pooled effect including child and adult studies showed uncertainty in relation to benefit or harm compared with usual monitoring (OR 0.56, 95% CI 0.21 to 1.49; 1042 participants; 10 studies; I2 = 45%; Analysis 1.3). However, the test for subgroup differences was statistically significant, suggesting possible benefit for adults in reducing the number of exacerbations requiring hospital admission (OR 0.24, 95% CI 0.06 to 0.94).
1.3. Analysis.

Comparison 1 Home telemonitoring with feedback vs usual monitoring, Outcome 3 Exacerbations requiring hospital admission (subgrouped by age).
Asthma control
Most studies did not use validated measures of asthma control, and the four that did could not be pooled in a single analysis (Cingi 2015; Ryan 2012; van der Meer 2009; Voorend‐van Bergen 2015). Two adult studies (Ryan 2012; van der Meer 2009) used the Asthma Control Questionnaire (ACQ), for which the minimal clinically important difference (MCID) is 0.5. These studies showed very different effects, which made the pooled result difficult to interpret (mean difference (MD) ‐0.24, 95% CI ‐0.72 to 0.24; 478 participants; two studies; I2 = 91%).
One child study (Voorend‐van Bergen 2015) using the Asthma Control Test (ACT) as a continuous variable found no difference in scores between the two types of monitoring (MD 0.09, 95% CI ‐0.92 to 1.10). One adult study (Cingi 2015) reported the number of people who were classed on the ACT as 'well controlled'. The effect favoured home telemonitoring and feedback, but the CI included the possibility that the effect may be the same as that for usual monitoring (OR 2.46, 95% CI 0.94 to 6.41).
Overall we had very low confidence in asthma control outcomes owing to inconsistency both within outcomes and between them in terms of direction and magnitude of effects. In addition, imprecision in most of the estimates made them difficult to interpret, and the nature of these subjective scales means that they may be subject to performance and detection biases associated with inability to blind the interventions.
Serious adverse events (including mortality)
None of the studies recorded serious adverse events separately from asthma exacerbations, and none reported whether anyone died during the study.
Subgroup analysis and investigation of heterogeneity
Mean age (< 16 years, 17 to 65 years, > 65 years)
Two child studies (Deschildre 2012; Xu 2010) and two adult studies (Donald 2008; Ryan 2012) contributed to the primary outcome of requiring oral corticosteroids, and the test for subgroup differences did not indicate a difference in effects (I2 = 0%, P value = 0.78). Two child studies (Guendelman 2002; Xu 2010) and six adult studies (Donald 2008; Kokubu 2000; Liu 2011; Ryan 2012; van der Meer 2009; Willems 2008) contributed to the exacerbation requiring ED visit analysis, and a large degree of heterogeneity was evident within each subgroup (I2 = 62%, P value = 0.11; I2 = 53%, P value = 0.06). As above, the test for subgroup differences for exacerbations requiring hospital admission analysis suggests that adults may fare better with home telemonitoring than children. It was not possible to subgroup the asthma control or serious adverse event outcomes to investigate the effects of age.
Type of technology (telephone calls, text messages, emails)
We divided studies by type of technology used, for the purpose of subgroup analysis, but we found nearly as many subgroups as studies, so it was not possible to draw any conclusions about whether the type of technology influenced the effect on exacerbations requiring oral steroids (Analysis 2.1; Analysis 2.2; Analysis 2.3).
2.1. Analysis.
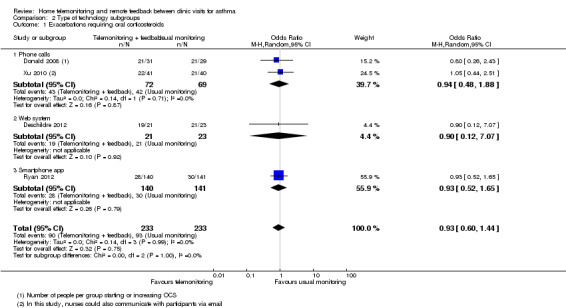
Comparison 2 Type of technology subgroups, Outcome 1 Exacerbations requiring oral corticosteroids.
2.2. Analysis.
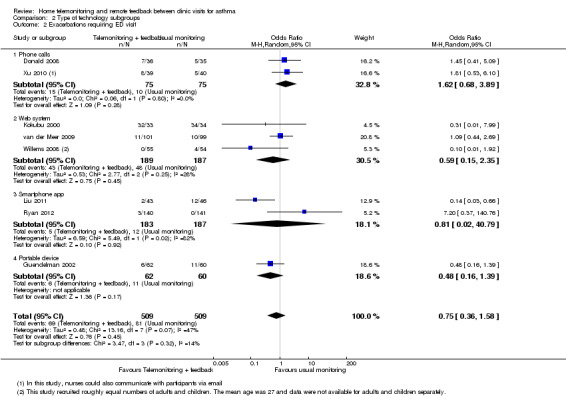
Comparison 2 Type of technology subgroups, Outcome 2 Exacerbations requiring ED visit.
2.3. Analysis.
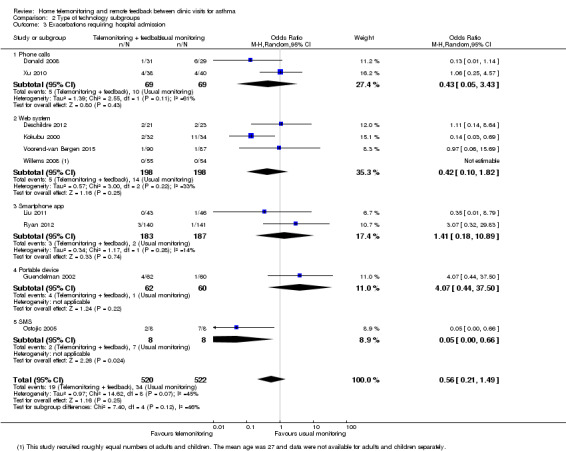
Comparison 2 Type of technology subgroups, Outcome 3 Exacerbations requiring hospital admission.
Sensitivity analyses
Studies recruiting people with severe or life‐threatening asthma
Deschildre 2012, Kokubu 2000, and Xu 2010 contributed to the primary analyses that listed inclusion criteria requiring populations with severe asthma. Deschildre 2012 and Xu 2010 were the only child studies contributing to Analysis 1.1, and when they were removed, the pooled effect was very similar (OR 0.93, 95% CI 0.60 to 1.44 with; OR 0.90, 95% CI 0.54 to 1.50 without). Removing Kokubu 2000 and Xu 2010 from the exacerbation requiring ED visit analysis did not change the interpretation (OR 0.75, 95% CI 0.36 to 1.58 with; OR 0.65, 95% CI 0.27 to 1.59 without). When all three studies (Deschildre 2012; Kokubu 2000; Xu 2010) were removed from the exacerbation requiring hospitalisation outcome, the overall effect showed a similar magnitude but became more imprecise (OR 0.56, 95% CI 0.21 to 1.49, to OR 0.58, 95% CI 0.13 to 2.56). These studies did not contribute to the asthma control or serious adverse events primary analyses.
Unpublished data (obtained from trial authors or from conference abstracts)
We obtained none of the data in the primary outcome analyses from trial authors or from conference abstracts, so this sensitivity analysis was not necessary.
Studies at high risk of detection bias
We considered only three studies (Donald 2008; Ryan 2012; Young 2012) to be at low risk for detection bias, and they contributed only to the exacerbations outcomes, which are unlikely to have been affected by this type of bias. For these reasons, we did not conduct the sensitivity analysis.
Secondary outcomes
Asthma‐related quality of life
People in the telemonitoring with feedback groups scored better on the AQLQ than those monitored in the usual way (MD 0.23, 95% CI 0.01 to 0.45; 796 participants; six studies; I2 = 54%). The MCID on the scale is 0.5 units. We downgraded our confidence in the result to low because of the potential for performance and detection bias in the measure, and because we noted important variation between study results.
Kokubu 2000 reported a change in the Japanese Ministry of Health and Welfare asthma quality of life score. We chose not to combine this with the AQLQ data using SMD, because this change score was obtained on a different scale, and we could not find details of properties of the measure.
Lung function
Home telemonitoring with feedback showed an overall benefit on lung function compared with usual monitoring, measured as percentage predicted pre‐bronchodilator forced expiratory volume in one second (FEV1) (MD 7.21, 95% CI 1.52 to 12.89; 149 participants; three studies; I2 = 0%) and change in peak expiratory flow (PEF) (MD 13.20, 95% CI 0.58 to 25.82; 66 participants; one study).
van der Meer 2009 reported FEV1 as litres change from baseline, which could not be pooled with results of the other studies. This study showed a 240 mL mean increase from baseline in the home telemonitoring group (SD 810 mL) and a 10 mL mean decrease in the control group (SD 752 mL). Additionally, Voorend‐van Bergen 2015 reported several lung function parameters as z‐scores in the paper; we could not use the absolute final scores, as we observed a significant baseline imbalance between groups.
Unscheduled healthcare visits
Variation between study results made the pooled effect difficult to interpret (OR 0.99, 95% CI 0.37 to 2.62; 430 participants; three studies; I2 = 73%), but telemonitoring did not lead to a clear increase or decrease in the number of people making unscheduled healthcare visits. We had very low confidence in the result owing to imprecision of the estimate, attrition bias and important heterogeneity.
Deschildre 2012 reported data as the mean number of unscheduled visits per participant, which could not be pooled with dichotomous data. The study showed a slightly higher rate of unscheduled visits in the home telemonitoring group (mean 5.24, SD 3.62) compared with the usual care group (mean 4.43, SD 4.13).
Adverse events/side effects
As with serious adverse events, none of the studies explicitly reported adverse events as an outcome separate from asthma‐related adverse outcomes.
Discussion
Summary of main results
Home telemonitoring with feedback might be better or worse than usual monitoring for exacerbations requiring a course of oral steroids (odds ratio (OR) 0.93, 95% confidence interval (CI) 0.60 to 1.44; 466 participants; four studies), a visit to the emergency department (OR 0.75, 95% CI 0.36 to 1.58; 1018 participants; eight studies) or a hospital stay (OR 0.56, 95% CI 0.21 to 1.49; 1042 participants; 10 studies). Our confidence in the results was reduced owing to wide confidence intervals, which meant that we could not rule out important benefits or harms of the intervention.
Evidence for measures of asthma control was patchy and did not show a consistent direction of effect, with most studies not using validated measures that could be pooled. We noted imprecision in the estimates, and the nature of these subjective scales means they may be subject to performance and detection bias associated with inability to blind the interventions.
None of the studies recorded serious or non‐serious adverse events separately from asthma exacerbations, and none reported whether anyone died during the studies; this is a limitation of the evidence. Researchers have been concerned that this type of increased monitoring can lead to a false sense of security, actually increasing adverse events and the need for emergency care, which does not seem to be the case. However, the benefits of home telemonitoring are modest at best, given the resources and infrastructure required to implement them.
With the exception of hospital admissions, adult and child studies showed similar findings, and too few studies with too much variation in the interventions used prevented any meaningful conclusions about which types of technology may offer the greatest benefit.
Within the secondary outcomes, people in the telemonitoring groups scored better on the Asthma Quality of Life Questionnaire (AQLQ) than those monitored in the usual way (mean difference (MD) 0.23, 95% CI 0.01 to 0.45; 796 participants; six studies; I2 = 54%), but study results showed important variation, and even the upper CIs did not reach what is considered to be a meaningful difference on the scale (0.5 units). Some benefit of home telemonitoring on lung function was apparent.
Overall completeness and applicability of evidence
The field of telehealthcare is rapidly growing, and national health systems are pushing to bring home telemonitoring interventions into widespread practice; this explains the number of studies that met our inclusion criteria. We deliberately refined the question in such a way that we would exclude studies of automated monitoring based on algorithms, monitoring as part of broad telehealth interventions that included all manner of education and adherence modules and sharing of symptom data to inform asthma management. That said, some of the included study interventions did involve components that may have confounded the comparison we set out to measure (e.g. an asthma education page within a monitoring website, clinician decision support to interpret monitoring data). We focused on telemonitoring interventions that required clinician input to make the evidence easier to apply to real clinical situations, but the number of excluded studies illustrates the breadth and complexity of this growing field of health care, along with the evidence that has not been considered in this review. Moreover, communication technologies for interventions with another primary focus such as education, compliance or inhaler technique have not been considered, and this splitting may cause difficulty for decision makers in a field that is as varied and dynamic as the technologies themselves (Rada 2015).
Some of the adult studies recruited people with severe or uncontrolled asthma (Deschildre 2012; Kokubu 1999; Kokubu 2000; Prabhakaran 2009; Ryan 2012), but most did not specify, and we found a mix both within and across analyses. This has implications for services that seek to implement monitoring strategies such as these, because we cannot be sure whether people who have regular exacerbations would derive the greatest benefit from extra monitoring, or in fact whether they are more likely to be harmed by the weakened responsibility patients feel in terms of their own health. A related issue is the small number of studies reporting what we considered to be the most important outcomes (i.e. those prespecified in our review protocol), in particular, lack of explicit reporting of adverse events, which may be related to the frequency of these events expected in different populations. Other sources of variation such as the nature of the control group (e.g. provision of an action plan in Ryan 2012) and the frequency of planned and actual feedback provided by healthcare professionals also make the results difficult to apply to practice. We did not seek to explore uptake, acceptability, equity of access and persistence in use, all of which are important determinants of how an intervention may be applied to a real‐life setting.
Quality of the evidence
Our confidence in the evidence based on GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) quality ratings ranged from moderate to very low, with none of the analyses thought to be of high quality.
When considering the effects of risk of bias on evidence quality, we noted that most of the high risk of bias judgements involved the blinding domains, which reduced our confidence only for subjective outcomes (asthma control and quality of life). We also noted some issues with high or unbalanced dropout in some studies, although we considered this to have affected only the lung function and unscheduled healthcare visits analyses, for which high‐risk studies carried a lot of weight. For one of the primary outcomes ‐ exacerbations requiring oral steroids ‐ a couple of the contributing studies had high attrition and uncertainty with selection bias, but they carried less than a fifth of the overall weight, so we did not judge this as serious enough to warrant a downgrade.
Inconsistency between study results was a problem in several of the analyses (asthma control, exacerbations requiring emergency department (ED) visits and hospital admissions, quality of life and unscheduled healthcare visits) and could not be explained in most cases by planned subgroup analyses for age or type of technology. This may reflect the variation in any number of factors related to the studies, such as baseline characteristics of recruited populations, countries in which studies were conducted, length or details of monitoring strategies used, the way outcomes were measured, the nature of the control group and the presence of action plan usage in the interventions. Picking apart each of these factors statistically was not possible, given the relatively small number of studies included in any one analysis.
We did not downgrade the quality of evidence for any outcome for its indirectness to the study question, because we were careful to implement the study inclusion criteria as planned. Therefore, we considered the populations, interventions, comparison groups and outcomes of the studies included in the review to reflect well the question we set out to answer.
Imprecision was perhaps the most limiting factor in this body of evidence, which made results difficult to interpret. This was due to the relatively small number of participants whose data contributed to most of the analyses. In several outcome analyses, confidence intervals (CIs) included the possibility that carrying on as usual was at least as good as and potentially better than providing additional telemonitoring and tailored feedback. In these cases, one cannot say for certain whether additional monitoring will mean patients will do better, or that it definitely will not make them worse, for example, by removing personal responsibility and making them less likely to take action when it is needed.
Several of the pooled estimates were made less precise mainly by variation in the direction and magnitude of individual study results that could not be explained by planned subgroup analyses; this was reflected in the downgrade decisions for inconsistency.
We suspected that publication bias might have affected only the exacerbations requiring oral steroids analysis, as the number of studies reporting this outcome was smaller than the number included in similar analyses of exacerbations requiring ED visits or hospital admissions, suggesting that oral steroid courses might have been recorded and not reported. Although several other analyses included only data from a small number of the 18 included studies, we were able to rule out publication bias in most cases by checking measured outcomes directly with study authors.
Potential biases in the review process
We recorded any deviations from the published protocol and explained why we believed it was not possible or meaningful to do what was planned. However, a degree of subjectivity in the application of study eligibility criteria was unavoidable. In the protocol, we tried to outline as best as possible the type of intervention and control that would answer the question we were examining, but we could not anticipate the complexity of the interventions and all the ways they would differ. Once a short list had been made via the title and abstract sift, we spent time discussing each title and paper for inclusion or exclusion and revisited previous decisions to ensure consistency. Thus, we have recorded a long list of excluded studies that we had to examine in detail, which we intend will help readers understand the sifting process. When devising the original short list, we sifted independently to reduce bias, and we collated a large number of additional references from trial registries and related works to make the list of included studies as complete as possible.
Once the list of included studies had been decided, we contacted study authors to clarify anything about the interventions and study methods that was uncertain, and to ask for additional unpublished data to reduce publication bias in the analyses. We translated non‐English language papers in duplicate with a structured data extraction form based on the one used for all other studies.
Agreements and disagreements with other studies or reviews
Systematic reviews of the evidence for remote or telehealth interventions have grappled with the clinical applicability of a narrow research question with the completeness of a broad one. Meta‐analyses and narrative syntheses generally focus on a given intervention for a broader population (e.g. respiratory disease) or all long‐term health conditions, or look broadly at interventions delivered via technology for a given condition regardless of their purpose. These broader reviews are difficult to compare with our own, as they compile evidence for interventions that often need to be assessed in quite different ways. For example, when assessing the feasibility of providing an annual asthma checkup over the phone, one wants to know whether patients are at risk of adverse outcomes by removing face‐to‐face contact (Kew 2016). This is different from assessing possible improvements in asthma control by using electronic devices to improve adherence (Craven 2015), and from using technology to monitor symptoms to minimise the need for rescue oral steroids or emergency treatment. The first approach is applied to look for equivalent efficacy, and the other approaches, as is the case in the current review, to look for superiority of technological interventions.
McLean 2010 looked at all 'telehealth' interventions for asthma regardless of their constituent parts or comparators used, and found 21 studies. McLean 2010 noted the clinical heterogeneity and concluded that telehealth interventions are not likely to be of benefit for patients with relatively mild asthma. Similarly, Zhao 2014, which included six studies, noted that asthma function scores were not improved by 'telemedicine' interventions. Despite differences in study inclusion criteria, the conclusions of these reviews are largely consistent with our own, with home telemonitoring or 'telehealth' interventions failing to show clear benefit over controls.
Jaana 2009 conducted a review of monitoring interventions more similar to what we set out to assess in this review; however, that review included people with other respiratory illnesses and outcomes focused on patient attitudes and receptiveness rather than effectiveness and safety. The review authors emphasized the "variations in study approaches and an absence of robust study designs and formal evaluations", which describes a common problem for syntheses in this rapidly evolving area.
Authors' conclusions
Implications for practice.
Current evidence does not support the widespread implementation of telemonitoring with healthcare provider feedback between asthma clinic visits. Studies have not yet proved that additional telemonitoring strategies lead to better symptom control or reduced need for oral steroids over usual asthma care, nor have they ruled out unintended harms. Investigators have reported small benefits in quality of life, but these are subject to a risk of bias, as the studies were unblinded. Similarly, some benefits for lung function are uncertain owing to possible attrition bias.
Implications for research.
Larger pragmatic studies in children and adults could better determine the real‐world benefits of these interventions for preventing exacerbations and avoiding harms; it is difficult to generalise results from this review because benefits may be explained at least in part by the increased attention participants receive when taking part in clinical trials. Qualitative studies could inform future research by focusing on patient and provider preferences or by identifying subgroups of patients who are more likely to derive benefit from closer monitoring, such as those who have frequent attacks.
Acknowledgements
We are very grateful to Cemal Cingi, Antoine Deschildre, Sylvia Guendelman, Nuray Bayar Muluk, Dermot Ryan, Hilary Pinnock, Lathy Prabhakaran and Daniëlle Willems for responding to our messages and providing additional data for their included studies, and to Akira Kuriyama and Kataoka Yuki for assisting in the translation and assessment of Kokubu 2000.
We are also grateful for comments and support provided by staff at the Cochrane Airways Group.
Rebecca Normansell (the Editor for the protocol) and Julia Walters (the Editor for the full review) commented critically on this review.
The methods section of this protocol is based on a standard template used by the Cochrane Airways Group. The Background shares similarities with another review, which has been co‐developed (Kew 2016).
The National Clinical Guideline Centre (NCGC) and the Cochrane Airways Group (CAG) undertook collaborative work pertaining to a systematic review of published evidence on telehealthcare for monitoring asthma control. The CAG reviews are restricted to interventions that involve a healthcare professional only. This is different from the larger question addressed by the NCGC (as part of the National Institute for Health and Care Excellence (NICE) asthma guideline commission). The NCGC review of evidence is published in the NICE clinical guideline on asthma diagnosis and monitoring and received funding from NICE.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Telemedicine Explode All
#6 telehealth* or tele‐health*
#7 telemedicine* or tele‐medicine*
#8 (internet* or computer* or web*):ti,ab,kw
#9 interactive* or telecommunication*
#10 (telephone or phone or SMS):ti,ab,kw
#11 tele‐monitor* or telemonitor*
#12 telemanagement or tele‐management
#13 teleconsultation or tele‐consultation
#14 telecare* or tele‐care*
#15 telematic*
#16 telepharmacy or tele‐pharmacy
#17 telenurs* or tele‐nurs*
#18 (video or email or e‐mail):ti,ab,kw
#19 remote NEXT consult*
#20 wireless or bluetooth
#21 tele‐homecare or telehomecare
#22 "remote care"
#23 tele‐support or telesupport
#24 mobile NEXT health*
#25 "computer mediated therapy"
#26 ehealth or e‐health
#27 mhealth or m‐health
#28 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27
#29 #4 and #28
#30 (#29) AND (INREGISTER)
[Note: in search line #1, MISC1 denotes the field in which the reference has been coded for condition, in this case, asthma]
Data and analyses
Comparison 1. Home telemonitoring with feedback vs usual monitoring.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations requiring oral corticosteroids (subgrouped by age) | 4 | 466 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.60, 1.44] |
| 1.1 Children (< 16 years) | 2 | 125 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.46, 2.29] |
| 1.2 Adults (17 to 65 years) | 2 | 341 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.54, 1.50] |
| 2 Exacerbations requiring ED visit (subgrouped by age) | 8 | 1018 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.36, 1.58] |
| 2.1 Children (< 16 years) | 2 | 201 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.24, 3.30] |
| 2.2 Adults (17 to 65 years) | 6 | 817 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.23, 1.91] |
| 3 Exacerbations requiring hospital admission (subgrouped by age) | 10 | 1042 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.21, 1.49] |
| 3.1 Children (< 16 years) | 4 | 421 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.51, 3.68] |
| 3.2 Adults (17 to 65 years) | 6 | 621 | Odds Ratio (M‐H, Random, 95% CI) | 0.24 [0.06, 0.94] |
| 4 Asthma control (ACQ) | 2 | 478 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.72, 0.24] |
| 5 Asthma control (ACT) | 1 | Mean Difference (Random, 95% CI) | 0.09 [‐0.92, 1.10] | |
| 6 ACT > 19 (well controlled) | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Asthma‐related quality of life (AQLQ) | 6 | 796 | Mean Difference (Random, 95% CI) | 0.23 [0.01, 0.45] |
| 8 Lung function (trough FEV1) | 3 | 149 | Mean Difference (IV, Random, 95% CI) | 7.21 [1.52, 12.89] |
| 9 Lung function (change in PEF L/min) | 1 | 66 | Mean Difference (IV, Random, 95% CI) | 13.20 [0.58, 25.82] |
| 10 Unscheduled healthcare visits | 3 | 430 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.37, 2.62] |
1.4. Analysis.
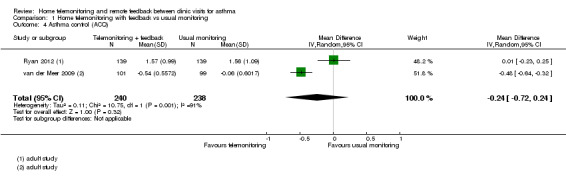
Comparison 1 Home telemonitoring with feedback vs usual monitoring, Outcome 4 Asthma control (ACQ).
1.5. Analysis.

Comparison 1 Home telemonitoring with feedback vs usual monitoring, Outcome 5 Asthma control (ACT).
1.6. Analysis.

Comparison 1 Home telemonitoring with feedback vs usual monitoring, Outcome 6 ACT > 19 (well controlled).
1.7. Analysis.

Comparison 1 Home telemonitoring with feedback vs usual monitoring, Outcome 7 Asthma‐related quality of life (AQLQ).
1.8. Analysis.

Comparison 1 Home telemonitoring with feedback vs usual monitoring, Outcome 8 Lung function (trough FEV1).
1.9. Analysis.

Comparison 1 Home telemonitoring with feedback vs usual monitoring, Outcome 9 Lung function (change in PEF L/min).
1.10. Analysis.

Comparison 1 Home telemonitoring with feedback vs usual monitoring, Outcome 10 Unscheduled healthcare visits.
Comparison 2. Type of technology subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations requiring oral corticosteroids | 4 | 466 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.60, 1.44] |
| 1.1 Phone calls | 2 | 141 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.48, 1.88] |
| 1.2 Web system | 1 | 44 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.12, 7.07] |
| 1.3 Smartphone app | 1 | 281 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.52, 1.65] |
| 2 Exacerbations requiring ED visit | 8 | 1018 | Odds Ratio (M‐H, Random, 95% CI) | 0.75 [0.36, 1.58] |
| 2.1 Phone calls | 2 | 150 | Odds Ratio (M‐H, Random, 95% CI) | 1.62 [0.68, 3.89] |
| 2.2 Web system | 3 | 376 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.15, 2.35] |
| 2.3 Smartphone app | 2 | 370 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.02, 40.79] |
| 2.4 Portable device | 1 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 0.48 [0.16, 1.39] |
| 3 Exacerbations requiring hospital admission | 10 | 1042 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.21, 1.49] |
| 3.1 Phone calls | 2 | 138 | Odds Ratio (M‐H, Random, 95% CI) | 0.43 [0.05, 3.43] |
| 3.2 Web system | 4 | 396 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.10, 1.82] |
| 3.3 Smartphone app | 2 | 370 | Odds Ratio (M‐H, Random, 95% CI) | 1.41 [0.18, 10.89] |
| 3.4 Portable device | 1 | 122 | Odds Ratio (M‐H, Random, 95% CI) | 4.07 [0.44, 37.50] |
| 3.5 SMS | 1 | 16 | Odds Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.66] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bateman 2000.
| Methods |
Study design: 12‐month parallel RCT Setting: practices in South Africa |
|
| Participants |
Population: 135 participants were randomised to telemonitoring (68) or to control (67) Baseline characteristics Mean age (SD): NR % male: NR Inclusion criteria: people with moderate or severe asthma who had direct asthma‐related expenditure of > USD 150 during the preceding year Exclusion criteria: NR |
|
| Interventions |
Intervention: PAP, a comprehensive computerised interactive guideline‐based clinical decision support system to which patients are linked telephonically by modem to permit daily monitoring of home spirometry and other clinical details by a healthcare coordinator. PAP provides the practitioner with regular status reviews and treatment recommendations, along with education for patients Control: Patients remained under the usual care of practitioners |
|
| Outcomes | Quality of life (Juniper scale), healthcare utilisation, direct costs of care | |
| Notes |
Funding: NR No full paper available, only conference abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method used to generate the random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | No information regarding allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It would have been possible to blind outcome assessors, but no information suggests this was done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout not reported |
| Selective reporting (reporting bias) | High risk | Only an abstract was available with minimal information about methods and no useable outcome data relevant to the review |
| Other bias | Low risk | None noted |
Cingi 2015.
| Methods |
Study design: 12‐month multi‐centre prospective, double‐blind parallel RCT Setting: conducted from June 2013 to December 2013 in pulmonary disease university departments and research hospitals in Turkey |
|
| Participants |
Population: 136 participants with asthma were randomised to telemonitoring (68) or to control (68), and 191 people with allergic rhinitis were randomised separately and not included in this review Baseline characteristics Mean age (SD): telemonitoring 32.0 (3.7); control 34.5 (8.2) % male: telemonitoring 50.0%; control 41.4% Inclusion criteria: diagnosis of mild to severe persistent asthma according to the Global Initiative for Asthma (GINA) classification upon presentation at outpatient clinics. Patients were required to own a smartphone and to consent to participation in a study researching the impact of mobile communication on disease management Exclusion criteria: pregnancy, breastfeeding, failure to provide consent |
|
| Interventions |
Intervention: mobile phone app allowing participants to fill out health status, send urgent messages to the physician or post information, medicine alerts and option to record adherence Control: Control groups received an application that allowed completion of the Asthma Control Test only at the beginning and end of the trial and did not include communication or health status or medication usage tracking. Physicians communicated with control group participants using only conventional methods upon participant request; these communications were recorded as study findings Participants in both groups received standard treatment during the study period, according to treatment guidelines |
|
| Outcomes | ACT (median scores and % scoring < 20 at endpoint), unplanned health visits, satisfaction scores, communication times | |
| Notes | Funding: "There was no funding source" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed by simple randomization using a random number generator" |
| Allocation concealment (selection bias) | Low risk | "The participating patient list was shared with POPET LLC (only the initials, age, gender, diagnosis, treatment plan of the patients), which randomized the patients daily to their respective groups" |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "Patients were blinded to the type of software (POPET or control) they would receive and were not trained in the use of the application in the clinic setting" This effort to blind would have controlled for some performance bias, but participants may have worked out that they were in an intervention or control group by what they had access to. It is unlikely that this would have introduced bias for the objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | "Patients were blinded to the type of software (POPET or control) they would receive and were not trained in the use of the application in the clinic setting" This effort to blind would have controlled for some performance bias, but participants may have worked out that they were in an intervention or control group by what they had access to. This may have affected subjective rating scales |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This trial was described as double‐blind, but the main outcome was a rating scale completed by participants who, by the nature of the intervention, could have worked out if they were in the intervention or control group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Patients who did not complete the final survey were excluded from the analysis and reported as attrition" "In total, 88.2% (n = 60) of the intervention group and 42.6% (n = 29) of the control group of asthma patients finished the trial" |
| Selective reporting (reporting bias) | Low risk | Named outcomes were reported, but some could not be included in meta‐analysis with the other studies owing to the statistical methods used (z‐scores and non‐parametric tests) |
| Other bias | Low risk | None noted |
Deschildre 2012.
| Methods |
Study design: 12‐month parallel RCT Setting: Paediatric Pulmonary Unit, Hospital Jeanne de Flandre, University Hospital, Lille, and 3 paediatric departments in the Nord‐Pas de Calais region Study ran from January 2003 to December 2007 |
|
| Participants |
Population: 50 children and adolescents randomised to telemonitoring (25) or to conventional treatment control (25) Baseline characteristics Median age: telemonitoring 11.0; control 11.2 % male: telemonitoring 72; control 76 Inclusion criteria: children aged 6 to 16 years with severe allergic asthma according to the Third Paediatric Asthma Consensus (i.e. frequent acute episodes requiring oral corticosteroid therapy, associated with moderate episodes (exercise‐induced asthma, chronic cough, sleep disturbances, treatment with short‐acting b2‐agonists 3 times per week) and airflow limitation). All children had uncontrolled asthma when taking long‐acting beta‐agonists and inhaled corticosteroids with frequent severe exacerbations. Reversibility in FEV1, defined as reversibility > 12% and/or an increase of ≥ 200 mL Exclusion criteria: congenital or acquired chronic illnesses other than asthma |
|
| Interventions |
Intervention: Children's treatment was managed with daily home spirometry transmitted to the physician via modem, along with medical feedback. The general practitioner was contacted, if needed, in the case of FEV1 values of 60% to 80% predicted. In cases of FEV1 < 60% predicted, the physician judged whether a course of oral corticosteroids was rapidly required and informed or contacted the general practitioner or the paediatrician who followed the child at the hospital Control: conventional treatment (i.e. no additional monitoring and feedback from physician) |
|
| Outcomes | Exacerbations requiring a course of oral steroids, unscheduled visit to a physician or ED or hospitalisation, number of days of corticosteroid treatment, daily dose of ICS, lung function measures, paediatric AQLQ Measured at the end of 12 months and at 4‐month checkups, spirometry every day |
|
| Notes | Funding: grant from the French Ministry of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation to HM or CT group was performed at inclusion, resulting in groups of 6 participants at each investigation centre |
| Allocation concealment (selection bias) | Unclear risk | No information regarding allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigators were not blind and appear to be those taking measurements |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was much higher in the telemonitoring group (40%) than in the usual care group (20%), and only those dropping out after 120 days were included in the final analysis, representing 88% of the randomised sample |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported but could not be included in the meta‐analysis, as non‐parametric tests were used (which was appropriate for the study data) |
| Other bias | Low risk | None noted |
Donald 2008.
| Methods |
Study design: 12‐month parallel RCT Setting: 2 teaching hospitals in Australia Pariticpants were recruited between 1 May 201 and 30 November 2003 |
|
| Participants |
Population: 71 participants were randomised to telemonitoring (36) or to control (35) Baseline characteristics Mean age (SD): telemonitoring 36.2 (NR) ‐ groups combined % male: telemonitoring 23.9 ‐ groups combined Inclusion criteria: aged 18 to 55 years and admitted to 1 or both of 2 teaching hospitals with a primary diagnosis of asthma Exclusion criteria: another chronic respiratory condition, an unstable medical condition, a cognitive or intellectual disability, psychiatric illness, unable to speak English |
|
| Interventions |
Intervention: 6 follow‐up telephone calls from the nurse educator to ask about current asthma symptoms and to give advice on their management. All participants received a PEF meter and instructions on how to record their results. All participants attended a face‐to‐face session with an asthma nurse educator and received advice on the pathophysiology of asthma, medications, triggers and self management, and were given an Asthma Action Plan Control: The control group was encouraged to continue with self management and usual GP care |
|
| Outcomes | Hospital admissions at recruitment, written plan and PEF monitor ownership, delivery of management sessions, healthcare utilisation, days lost from work or study, exacerbations requiring use of oral steroids, healthcare costs | |
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method used to generate the random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | No information regarding allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “All participants were telephoned weekly by a researcher (blinded to participant allocation)” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 71 participants were randomised; 44 replies were received at 6 months and 49 at 12 months. No description of how data were modified or imputed for those not contributing to the analyses |
| Selective reporting (reporting bias) | Low risk | Outcomes named in the methods were well reported in the results section, although this could not be verified with a protocol |
| Other bias | Low risk | None noted |
Finkelstein 2005.
| Methods |
Study design: 12‐month parallel RCT Setting: NR |
|
| Participants |
Population: 240 participants were randomised to telemonitoring (NR) or to control (NR) Baseline characteristics Mean age (SD): NR % male: NR Inclusion criteria: age 18 and older with mild persistent to severe asthma Exclusion criteria: NR |
|
| Interventions |
Intervention: "Home Automated Telemanagement" (HAT). Participants used portable computers connected with a peak flow meter to report their symptoms and to communicate with their provider. The HAT system monitored participants' asthma severity and assisted in carrying out individualised asthma action plans Control: usual care, not described |
|
| Outcomes | 'Clinical outcomes' ‐ Those reported in abstract include AQOL symptoms domain and activities domain, CSQ, depression on CESD‐D, number of ED visits (not people) per 2 months | |
| Notes |
Funding: US National Institutes of Health (NIH) and National Heart, Lung, and Blood Institute (NHLBI) No full paper available, only conference abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method used to generate the random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | No information regarding allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It would have been possible to blind outcome assessors, but no information suggests this was done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout not reported, only interim data for first 50 participants recruited |
| Selective reporting (reporting bias) | High risk | Only an abstract was available with minimal information about methods. Two conference abstracts from 2005 report data for the first 50 participants to complete 4‐month follow‐up. No data are available for the full population of 240 enrolled in the study, and none for the full 12 months of the trial |
| Other bias | Low risk | None noted |
Guendelman 2002.
| Methods |
Study design: 3‐month parallel RCT Setting: 1 clinic in California, USA Participants were recruited between April 1999 and July 2000 |
|
| Participants |
Population: 134 participants were randomised to telemonitoring (66) or to control (68) Baseline characteristics Mean age (SD): telemonitoring 12.0 (2.3); control 12.2 (2.9) % male: telemonitoring 61; control 54 Inclusion criteria: Children were eligible for inclusion in the study if they were 8 to 16 years of age and had an English‐speaking caregiver, a telephone to the house and persistent asthma Exclusion criteria: involved in other asthma or drug efficacy studies, involved in research that required behaviour modification, had mental or physical challenges that made it difficult to use the Health Buddy. Children with co‐morbid conditions that could affect their quality of life were also excluded |
|
| Interventions |
Intervention: Health Buddy device, a computerised interactive asthma self management and education programme that connected to the Internet and asked every day about asthma status, peak flow and medication. Responses were downloaded overnight to the nurse co‐ordinator. Devices were interactive and gave immediate feedback on questions regarding asthma symptoms, medications, PEF and other items Control: Paper asthma diary. All children returned for 2 follow‐up visits at 6 and 12 weeks, when they received further standardised teaching from the nurse co‐ordinator |
|
| Outcomes | Limitation in activity, asthma symptoms, missed school days, PEFR, healthcare utilisation including ED visits and hospitalisations. Measured at 0, 6 and 12 weeks | |
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information, "randomised" |
| Allocation concealment (selection bias) | Low risk | "Following baseline interview the nurse opened a sealed envelope containing the treatment assignment" |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self reported outcomes were assessed by the nurse co‐ordinator, no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Baseline characteristics of children who did and did not complete the trial did not differ. 62/66 participants in the intervention group and 60/68 participants randomised to the control group completed 12 weeks. Reasons for dropping out of the study included moving out of the area (n = 3) and life crisis (n = 4). Five familles who dropped out could not be contacted |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes are reported |
| Other bias | Low risk | None noted |
Jan 2007.
| Methods |
Study design: 3‐month parallel RCT Setting: 1 paediatric allergy and asthma clinic in Taiwan Study was conducted between January and December 2004 |
|
| Participants |
Population: 164 participants were randomised to home telemonitoring (88) or to control (76) Baseline characteristics Mean age (SD): monitoring 10.9 (2.5); control 9.9 (3.2) % male: monitoring 39.7; control 36.8 Inclusion criteria: Children were eligible for inclusion in the study if they were between the ages of 6 and 12 years, were given access to the Internet by their caregivers and had a physician’s diagnosis of asthma Exclusion criteria: other chronic conditions such as bronchopulmonary dysplasia |
|
| Interventions |
Intervention: “Blue Angel for Asthma Kids”, an Internet‐based paediatric asthma monitoring programme for asthmatic children and their parents. The system has symptom and peak flow diaries and individual Asthma Action Plan suggestions based on GINA (Global Initiative for Asthma) guidelines. These data can be shared with the patient’s physician, who can give feedback via telephone or email Control: traditional treatment in an outpatient allergy and asthma clinic accompanied by a PEF meter and diary. This group also received asthma education as part of usual care, including verbal and printed information. Individuals were given an Asthma Action Plan to aid decision making |
|
| Outcomes | PEF records, symptom diaries, paediatric QoL test, childhood asthma control test, caregiver survey of asthma knowledge, adherence to treatment, asthma diaries | |
| Notes | Funding: supported in part by a grant from the National Science Council (NSC 94‐2815‐C‐426‐005‐E) and by a grant from the Bureau of Health Promotion, Department of Health (DOH 93‐HP‐1124), Taiwan, R.O.C. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Children and their caregivers, who were randomised” No details of methods used to generate the sequence |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information is given as to how outcomes of the groups were collected, and whether outcome assessors were blinded to allocation of participants. Blinding would not have been possible for outcomes recorded by the Internet programme, but would have been possible for outcomes recorded via questionnaires at baseline and at 12 weeks. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 164 randomised, 82/88 in the intervention arm (93.2%) and 71/76 in the control arm (93.4%) completed the study, representing relatively low and balanced attrition |
| Selective reporting (reporting bias) | Low risk | Satisfaction questionnaires data not shown, but this was not one of the review's named outcomes |
| Other bias | Low risk | None noted |
Kokubu 1999.
| Methods |
Study design: 6‐month parallel RCT Setting: Japanese medical centres |
|
| Participants |
Population: 50 participants were randomised to home telemonitoring (24) or to control (26) Baseline characteristics Mean age (SD): monitoring 54.2 (14.3); control 51.5 (14.9) % male: monitoring 66.7; control 26.9 Participants had a mean duration of asthma of around 17 years, ICS mean dose of 1000 mcg/d and 11 ED visits in past year Inclusion criteria: Patients with high hospitalisation risk were enrolled in the study upon screening for those with multiple previous emergency room visits Exclusion criteria: not available from the translation |
|
| Interventions |
Intervention: telemedicine system to monitor airway status at home for participants with poorly controlled asthma, whereby a nurse provides instructions to individuals via telephone to help them manage exacerbation under the supervision of physicians Control: description not available from translation ‐ presumed to be usual care |
|
| Outcomes | Number of emergency room visits (reported only as the number per patient per year, which could not be pooled), activities of daily living, PEF | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but details of how the sequence was generated not described |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by telephone call to the registration centre |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial was open label (from translator) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information available |
| Selective reporting (reporting bias) | Unclear risk | Could not be determined from the translation |
| Other bias | Low risk | No other bias noted |
Kokubu 2000.
| Methods |
Study design: 6‐month parallel RCT Setting: 17 tertiary care hospitals in Japan |
|
| Participants |
Population: 75 participants were randomised to home telemonitoring (37) or to control (38) Baseline characteristics Mean age (SD): monitoring 49.9 (15.6); control 47.3 (13.6) % male: monitoring 37.5; control 44.1 Participants had a mean duration of asthma of around 17 years, ICS mean dose of 1000 mcg/d and 6 ER visits in past year Inclusion criteria: patients with asthma who were treated with sufficient inhaled steroid therapy and were admitted to emergency department more than 3 times in past year Exclusion criteria: COPD, chronic heart failure |
|
| Interventions |
Intervention: telemedicine system to monitor airway status at home for participants with poorly controlled asthma, whereby a nurse provides instructions to individuals via telephone to help them manage exacerbation under the supervision of their physicians. Participants measured their PEF twice daily and sent this information to the nurse via the system. Participants inhaled the corticosteroid when PEF was ≥ 80%. When inhaled B2‐stimuli with the best PEF was between 60% and 80%, and when PEF did not recover up to 80%, participants were instructed to inhale an increased dosage of corticosteroids or to take oral corticosteroids. When the best PEF was < 60%, participants were instructed to visit their physicians Control: conventional asthma therapy including twice‐daily measurement of PEF, which was recorded in a diary and was not shared |
|
| Outcomes | Hospitalisation, night and daytime ED visits, compliance with PEF measurements and medications, PEFR, QoL | |
| Notes | Funding: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but details of how the sequence was generated not described |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by telephone to the registration centre |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial was open label (from translator), and no information suggests outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 people in the intervention group (13.5%) and 4 people in the control group (10.5%) did not complete the trial. These people were not included in the analyses, but dropout was fairly low and balanced, so was not thought to pose significant risk of bias |
| Selective reporting (reporting bias) | High risk | Translator stated that some numerical data were underreported and were "not usable for meta‐analysis" |
| Other bias | Low risk | None noted |
Liu 2011.
| Methods |
Study design: 6‐month parallel RCT Setting: outpatient clinics of a teaching hospital in Taiwan |
|
| Participants |
Population: 120 participants were randomised to remote monitoring (60) or to control (60) Baseline characteristics Mean age (SD): monitoring 50.4 (12.9); control 54.0 (15.7) % male: monitoring 51.2; control 47.8 Inclusion criteria: participants with moderate to severe persistent asthma from outpatient clinics of Chang Gung Memorial Hospital Exclusion criteria: NR |
|
| Interventions |
Intervention: mobile telephone‐based interactive self care software: electronic diary provided to record participants' daily asthma symptom scores, use of relievers and lung function measures. Management advice was given via GPRS on the basis of uploaded data, in accordance with GINA guidelines. Participants and medical staff reviewed daily, weekly and monthly data on the website. Data were given to physicians to adjust their treatment plan when participants returned to their clinics Control: written asthma diary and action plan. All participants received asthma education, self management plan and standard treatment |
|
| Outcomes | Quality of life on the SF‐12, episodes of acute exacerbation and medications used for asthma control on return visit, FEV1, FVC, asthma symptom score; numbers of unscheduled clinic visits, emergency department visits and hospitalisations | |
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method used to generate the random sequence not described |
| Allocation concealment (selection bias) | Unclear risk | No information regarding allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It would have been possible to blind outcome assessors, but no information suggests this was done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropout was quite high and balanced between groups (28% and 23%). Outcomes are reported for the 43 and 46 participants completing the 6‐month follow‐up, not for the 60 and 60 randomised |
| Selective reporting (reporting bias) | Low risk | No mention of trial registration. Outcomes stated in the methods were well reported |
| Other bias | Low risk | None noted |
Ostojic 2005.
| Methods |
Study design: 4‐month parallel RCT Setting: 1 respiratory clinic in Croatia |
|
| Participants |
Population: 16 participants were randomised to home telemonitoring (8) or to control (8) Baseline characteristics Mean age (SD): monitoring 24.8 (6.3); control 24.5 (7.1) % male: monitoring 63; control 50 Inclusion criteria: moderate persistent asthma for ≥ 6 months and treated with ICS and LABA Exclusion criteria: no history of smoking, chronic bronchitis or emphysema. Patients without consistent access to a cell telephone or unable to use SMS were excluded |
|
| Interventions |
Intervention: Participants were told to note PEF, medication usage and symptoms in a paper diary. PEF was to be done 3 times a day, then those in the text group would send their results daily to a computer at the asthma centre. Both groups were treated according to GINA guidelines, but the text group received weekly instructions by text from an asthma specialist on adjustments to therapy as well as invitations, when required, to come in for an extra office visit Control: Controls also kept a daily diary of peak flow and symptoms, but their results were reviewed by the physician only at the end of the study period upon attending the physician’s office Both groups were treated according to GINA guidelines |
|
| Outcomes | Office pulmonary function test measurements, patient daily records of PEF and symptoms, details of asthma medication, PEF variability, cost, reliability of text. Measured at baseline and at 16 weeks | |
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomised by computer” |
| Allocation concealment (selection bias) | Unclear risk | No details about allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It would have been possible to blind outcome assessors, but no information suggests this was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data; no study withdrawals |
| Selective reporting (reporting bias) | Low risk | Outcomes stated in the methods were well reported, although a protocol was not available |
| Other bias | Low risk | None noted |
Prabhakaran 2009.
| Methods |
Study design: 3‐month parallel RCT Setting: 1 hospital in Singapore Participants were recruited between 1 August 2007 and 30 June 2008 |
|
| Participants |
Population: 120 participants were randomised to home telemonitoring (60) or to control (60) Baseline characteristics Mean age (SD): monitoring 37 (12); control 40 (13) % male: monitoring 35; control 47 Inclusion criteria: 21 years of age or older, admitted for an acute exacerbation of asthma, own a mobile phone, know how to use an SMS system, English speaking, willing to participate in the study and give written consent Exclusion criteria: significant co‐morbidity e.g. bronchiectasis, heart failure, diabetes mellitus with complications, stroke, renal impairment, COPD; did not know how to use an SMS system, had mild intermittent asthma |
|
| Interventions |
Intervention: The 60 participants in the intervention group had SMS monitoring to assist with management of their asthma control for the next 3 months Control: The 60 participants in the control group were left to self manage their asthma for 3 months All 120 participants recruited were seen by a trained asthma nurse educator, who assessed their asthma control, compliance with treatment and inhaler technique before providing individualised asthma education |
|
| Outcomes | Asthma Control Test, use of nebulisation, ED visits and hospital admissions for asthma since the last admission 12 weeks previously | |
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was done from an envelope with slips of paper. Participants had to draw from the envelope to discover their allocated group |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether it was possible to predict allocation from the slips used |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It would have been possible to blind outcome assessors, but no information suggests this was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Since all patients received the inpatient phase of asthma education, the intention‐to‐treat approach was used to analyse the secondary objective on clinical outcomes. We were not able to contact three subjects from the control group and two subjects from the intervention group to assess their asthma control and number of nebulizations. Nevertheless, information about the number of emergency visits and hospital admissions for asthma were retrieved for all patients from the hospital computer system" |
| Selective reporting (reporting bias) | Low risk | No study protocol found but named outcomes well reported |
| Other bias | Low risk | None noted |
Ryan 2012.
| Methods |
Study design: 6‐month parallel RCT Setting: 32 general practices in the UK |
|
| Participants |
Population: 288 participants were randomised to home telemonitoring (145) or to control (143) Baseline characteristics Mean age (SD): monitoring 46.6 (18); control 51.5 (17.7) % male: monitoring 33.8; control 41.3 Inclusion criteria: patients aged 12 and older who were registered with participating practices, had poorly controlled asthma (defined as score ≥ 1.5 on the ACQ) and had, or were willing to borrow, a compatible mobile phone handset and a contract with a compatible network Exclusion criteria: other lung disease, unable to communicate in English, receiving specialist care for severe/difficult asthma, general practitioner advised against inclusion for major social/clinical problems |
|
| Interventions |
Intervention: twice‐daily recording and mobile phone‐based transmission of symptoms, drug use and peak flow with immediate feedback prompting action according to an agreed plan Control: paper‐based monitoring. "To ensure that our trial specifically tested the impact of the technology, we opted to provide the paper group with the same clinical care as the intervention group, rather than using (probably less intensive) usual care as a comparator." Both groups also received a 30‐minute education session from the practice nurse before randomisation "The practices’ asthma nurse provided clinical care in accordance with the stepwise approach advocated by the BTS‐SIGN asthma guideline" |
|
| Outcomes | ACQ, KASE‐AQ, Mini‐AQLQ, costs, adverse events, asthma ED attendances, asthma hospitalisation, acute exacerbation, course of oral steroids, unscheduled healthcare attendances, withdrawal. Measures taken at 6 months after randomisation | |
| Notes | Funding Asthma UK (project ID 07/047) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All consenting participants were stratified by practice and centrally randomised (Health Services Research Unit, University of Aberdeen) to mobile phone or paper based monitoring with a 1:1 allocation with random block sizes of two or four" "All consenting participants were stratified by practice and centrally randomised (Health Services Research Unit, University of Aberdeen) to mobile phone or paper based monitoring with a 1:1 allocation with random block sizes of two or four" |
| Allocation concealment (selection bias) | Low risk | "Telephone randomisation ensured concealment until the treatment was assigned" |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | "The practice nurse informed the patient of allocation to ensure the researchers were blinded to allocation throughout data collection and analysis" Participants could not be blinded, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assessment of outcomes was blinded. A researcher blinded to allocation collected primary outcome data at the final trial visit; non‐attendees were sent the questionnaires by post" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Our main analysis was on an intention to treat (ITT) basis. We assumed that participants who did not attend the three or six month assessment had not improved their control and their previous results were therefore carried forward.30 A per protocol analysis was undertaken as a sensitivity analysis" |
| Selective reporting (reporting bias) | Low risk | "All analyses were agreed a priori. We did not plan, or undertake, any interim analysis" Registered on clinicaltrials.gov |
| Other bias | Low risk | None noted |
van der Meer 2009.
| Methods |
Study design: 12‐month parallel RCT Setting: 37 general practices in the Netherlands Study ran from September 2005 to September 2006 |
|
| Participants |
Population: 200 participants were randomised to home telemonitoring (101) or to control (99) Baseline characteristics Mean age (SD): monitoring 36; control 37 % male: monitoring 32; control 29 Inclusion criteria: ages 18 to 50, prescription of inhaled corticosteroids for ≥ 3 months in the previous year, access to the Internet at home, mastery of the Dutch language Exclusion criteria: not receiving maintenance oral glucocorticosteroid treatment, no serious co‐morbid conditions that interfered with asthma treatment |
|
| Interventions |
Intervention: Internet‐based self management program. Participants measured FEV1 daily and reported the highest of 3 measurements before taking their medication. They completed the Asthma Control Questionnaire (ACQ) once a week and reported symptoms via Internet or text. Participants monitored their asthma using the special website or via text on a mobile phone, then used an Internet‐based asthma treatment plan and online education, including asthma news, frequently asked questions and other information. Participants could also communicate with a specialised asthma nurse using the Web or telephone. The ACQ score was fed into an algorithm, and participants received 1 of 4 treatment messages Control: Control participants had access to the part of the website on which a diary of symptoms and exacerbations was kept |
|
| Outcomes | Consumer Asthma Knowledge Questionnaire, inhaler technique, medication changes per participant, healthcare utilisation, AQLQ, ACQ, symptom‐free days, trough FEV1, daily inhaled steroid dose, exacerbations | |
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned using a computer‐generated permuted‐block scheme" |
| Allocation concealment (selection bias) | Unclear risk | "Allocation took place by computer after collection of the baseline data ensuring concealment of allocation" It is not clear whether this was central allocation |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of intervention, outcome assessor or data analyser |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 200 adults were randomised; after 12 months 92 remained in the control group and 91 in the intervention group. 9 participants withdrew consent, and 8 were lost to follow‐up. Investigators analysed complete cases and did not impute missing values |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | None noted |
Voorend‐van Bergen 2015.
| Methods |
Study design: 12‐month parallel RCT Setting: multi‐centre trial in the Netherlands. Children recruited by their own paediatrician from general hospitals (n = 5) and tertiary referral centres (n = 2) in the Netherlands from February 2010 to November 2011 |
|
| Participants |
Population: 280 participants were randomised to home telemonitoring using ACT scores (91), to the usual care control group based on ACT without Web feedback (89) and to a group for which FeNO and the ACT were used to monitor asthma (92). We chose not to include the FeNO group, as the comparison between Web and control groups was a purer comparison of the effect of home telemonitoring than of use of FeNO Baseline characteristics Mean age (SD): Web 10.6 (2.8), usual care 10.2 (3.2) % male: Web 66, usual care 69 Inclusion criteria: children aged 4 to 18 years, with atopic asthma based on clinical symptoms, a previous bronchodilator response of > 9% increase in FEV1 of predicted (FEV1%) and/or previous airway hyperresponsiveness (AHR) to methacholine. Atopy was defined as a radioallergosorbent test class 2 or higher for ≥ 1 airborne allergen. Patients had been using inhaled corticosteroids (ICS) for ≥ 3 months before the start of the study Exclusion criteria: active smoking, pulmonary diseases other than asthma, recent (< 1 year) or multiple admissions to an intensive care unit for asthma, inability to perform FeNO measurements and/or use of omalizumab |
|
| Interventions |
Intervention: In the Web group, treatment was adapted monthly according to the Web‐based ACT score filled in online by the children. The researcher or asthma nurse emailed treatment advice within 3 working days Control: In the usual care group, treatment was adapted every 4 months according to the child's ACT score In the usual care and Web‐based groups, treatment was adapted according to the ACT score, respectively, at 4‐month and 1‐month intervals |
|
| Outcomes | Asthma control on the ACT or the C‐ACT; primary endpoint was proportion of symptom‐free days (SFD) based on a 4‐week Web‐based diary filled in at the start and after 1 year. Also measured daily ICS dose. Measured at the start and end of the study; children seen every 4 months during the 12‐month period | |
| Notes |
Funding: Lung Foundation Netherlands (grant no. 3.4.08.039), the Netherlands Organization for Health Research (ZonMW) (grant no. 171002101) and Fund Nuts Ohra (grant no. 0901‐023) Trial registration: NTR 1995 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Children were automatically and randomly allocated to one of the three groups by a randomisation programme on the study website, in a 1:1:1 ratio, stratified for age (<12 or ≥12 years), centre and dose of ICS (<400 or .400 mg budesonide or equivalent daily dose; figure 1)" |
| Allocation concealment (selection bias) | Low risk | "Automatically and randomly allocated" suggests that the allocation sequence could not be tampered with |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were not blinded to randomisation group. Treating physicians were blinded to randomisation group, FeNO and ACT. Local investigators, unblinded to ACT and FeNO, provided physicians with treatment advice based on study algorithms and on the treatment plan |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total number randomised was 280, and 268 completed the study (95.7%). Loss to follow‐up was low and balanced across groups |
| Selective reporting (reporting bias) | Low risk | Study was prospectively registered, and full outcome data were available in the published paper and in an online supplementary appendix |
| Other bias | Low risk | None noted |
Willems 2008.
| Methods |
Study design: 12‐month parallel RCT Setting: single outpatient centre in the Netherlands |
|
| Participants |
Population: 109 outpatients (56 children and 53 adults) were randomised to home telemonitoring (55) or to control (54) Baseline characteristics Mean age (SD): monitoring 27.2 (19.3); control 28.4 (21.0) % male: monitoring 58.2; control 44.4 Inclusion criteria: asthmatic outpatients from the Medical Respiratory Department and the Department of Paediatrics. Patients aged 7 and older with an asthma severity of stage I to III as described in the Global Initiative for Asthma (GINA) guidelines were potentially eligible. Patients had to be competent to use an asthma monitor, and had to possess a household phone connection Exclusion criteria: severe co‐morbidity (such as chronic obstructive pulmonary disease or heart failure), structural defects in the upper airways or lungs |
|
| Interventions |
Intervention: The intervention group used a hand‐held electronic asthma monitor connected to the home modem, which registered lung function and symptoms. Participants were asked to perform daily PEF measurements and to transfer monitor data monthly or more frequently if symptoms worsened. The asthma nurse classified the asthma following a stepwise intervention protocol based on GINA and the Dutch College of General Practitioners, and guided medication changes, usually over the phone. Caregivers assisted the children in monitor use and in contacts with the asthma nurse Control: regular outpatient care |
|
| Outcomes | Primary outcome was asthma‐specific quality of life on the AQLQ or the PAQLQ. Children aged 12 to 18 years were asked to complete the adolescent version of the questionnaire by themselves, and parents or caregivers filled in a proxy version for children aged 7 to 12 years. Secondary outcomes were lung function (PEF and FEV1 at baseline and endpoint), self reported symptoms on a 0 to 3 scale and asthma‐related medical consumption from diaries (medication use, visits or telephone contacts with health professionals and ED visits) | |
| Notes |
Funding: Dutch Health Care Insurance Board The paper reported adult and child baseline data separately and together, but reported efficacy data only for both age groups combined. Study authors were not able to provide separate efficacy data for adults and children |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation took place on participant level after stratification by age (ages 7 to 18 vs 18 years and older), as regular care differs between these age groups. The asthma nurse used a list of random numbers to allocate participants to 1 of the 2 treatment arms |
| Allocation concealment (selection bias) | Unclear risk | No details about whether or how allocation was concealed |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants could not be blinded, and nurse practitioners were not blinded to allocation of participants, as they received monthly transfers of monitor data. It is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | We noted no evidence of outcome assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 109 participants were randomised, 5 were lost to follow‐up. Technical problems occurred, and when data transfer was missed, the nurse practitioner attempted to contact participants by telephone; however this was not possible in 21% of missed data transfers. At baseline, compliance with filling in the questionnaires was 100%, for subsequent measurements response rate was 85% to 92% for questionnaires and 81% to 90% for diaries. 28% of PEF data transfers from adults and 18% from children were missed |
| Selective reporting (reporting bias) | Low risk | Outcomes stated in the Methods were well reported in the Results, but no trial protocol was available to verify |
| Other bias | Low risk | None noted |
Xu 2010.
| Methods |
Study design: 6‐month parallel RCT Setting: recruited from the Royal Children's Hospital Brisbane, and Caboolture, Gold Coast, and Ipswich hospitals in Queensland The trial started in August 2006 and was completed in September 2007 |
|
| Participants |
Population: 121 participants were randomised to home telemonitoring (41) or to control (41), or to 1 other group not relevant to this review Baseline characteristics Mean age (SD): monitoring 6.5 (3); control 7.4 (3) % male: monitoring 51.2; control 51.2 Inclusion criteria: children and young people aged between 3 and 16 years with doctor‐diagnosed asthma who had had an admission to hospital in the previous 12 months or had presented at least once in the previous 12 months to an emergency department or to their general practitioner or specialist with acute asthma requiring oral steroid rescue Exclusion criteria: NR |
|
| Interventions |
Intervention: The nurse support group received regular follow‐up calls from 1 Nurse Specialist every 2 weeks. When families preferred email contact, the nurse used email to collect the same data and to offer education and advice on asthma Control: Participants' primary care physicians were notified and continued to provide primary asthma care. All families received the same initial asthma education with the same Nurse Specialist. The control group received regular GP or hospital outpatient care |
|
| Outcomes | Primary outcomes were health resource utilisation such as GP visits, hospital ED presentations and hospital admissions. Other outcomes included use of oral steroids, PAQLQ, time of school or work for parents/carers. Measured at baseline and at the end of the study | |
| Notes | Funding: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised into 3 study groups. Block randomisation was used with random block sizes of 3 or 6 to create an allocation to 1 of the 3 groups for all study participants |
| Allocation concealment (selection bias) | Unclear risk | No details about allocation concealment |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No evidence of outcome assessor blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant in the control group was lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Outcomes were well reported for named outcomes, but no trial protocol was available to check |
| Other bias | Low risk | None noted |
Young 2012.
| Methods |
Study design: 3‐month parallel RCT Setting: 11‐county region in north central Wisconsin, USA |
|
| Participants |
Population: 98 participants were randomised to home telemonitoring (49) or to control (49) Baseline characteristics Mean age (SD): monitoring 45.4 (16.8); control 43.7 (14) % male: monitoring 26.5; control 20.4 Inclusion criteria: participation in Community Health Access (a charity programme sponsored by the Marshfield Clinic and supported by the FHC) or FHC programmes (federally funded programmes to assist underserved, uninsured and underinsured individuals in the northern Wisconsin area), 19 years of age or older, English speaking, receipt of ≥ 1 asthma medication(s) dispensed in the 6‐month period ending January 31, 2009, diagnosis of asthma Exclusion criteria: enrolment in the FHC Pharmacy medication auto‐refill programme |
|
| Interventions |
Intervention: telephone consultation from pharmacists regarding asthma self management and medication use. Five pharmacists incorporated the intervention into their usual practice Control: usual care, which included mail receipt of a prescription refill with written medication use instructions |
|
| Outcomes | Asthma Control Test, Patient Activation Measure, Morisky Medication Adherence Scale. Measured at 3‐month endpoint and at 6‐month follow‐up | |
| Notes | Funding: Grant 1UL1RR025011 from the Clinical & Translational Science Award programme of the National Center for Research Resources, National Institutes of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by the data manager. No details of how the sequence was generated |
| Allocation concealment (selection bias) | Low risk | Research assistants then forwarded participant contact information to a data manager for random assignment to the intervention or control group. Data manager forwarded intervention group participants’ contact information to the FHC Pharmacy Manager for allocation to pharmacists |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | It would not have been possible to hide allocation from participants and personnel, but it is unlikely that this would have introduced bias for objective outcomes (e.g. number of people having exacerbations) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Subjective outcomes such as quality of life and symptom scales filled in by participants or personnel may have been subject to performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants and researchers were blinded to allocation of participants to intervention and control groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout was balanced between groups (˜ 15% in both groups) |
| Selective reporting (reporting bias) | Low risk | No trial registration, but no evidence of selective reporting in the paper |
| Other bias | Low risk | None noted |
ACT = Asthma Control Test
ACQ = Asthma Control Questionnaire
AQLQ = Asthma Quality of Life Questionnaire
CSQ = Client Satisfaction Questionnaire
ED = emergency department
KASE‐AQ = Knowledge, Attitude and Self‐efficacy Asthma Questionnaire
NR = not reported
PAQLQ = Paediatric Asthma Quality of Life Questionnaire
PEF = peak expiratory flow
RCT = randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aaron 2016 | Intervention did not match inclusion criteria ‐ self regulation and goal attainment intervention delivered over the phone |
| Andersen 2007 | Intervention did not match inclusion criteria ‐ minimal or no provider involvement |
| Apter 2000 | Design did not match inclusion criteria ‐ not a trial report |
| Apter 2015 | Comparison did not match inclusion criteria ‐ telemedicine portal used with or without home visits (both groups used the portal) |
| Araujo 2012 | Design did not match inclusion criteria ‐ cross‐over RCT |
| Baptist 2013 | Comparison did not match inclusion criteria ‐ phone calls for asthma education vs non‐asthma education phone calls |
| Barbanel 2003 | Intervention did not match inclusion criteria ‐ asthma education intervention led by a pharmacist |
| Bender 2001 | Intervention did not match inclusion criteria ‐ study assessing validity of self reports |
| Bender 2007 | Intervention did not match inclusion criteria ‐ study assessing validity of self reports |
| Bender 2010 | Intervention did not match inclusion criteria ‐ minimal or no provider involvement |
| Boyd 2014 | Intervention did not match inclusion criteria ‐ pharmacist‐led intervention about adherence |
| Burbank 2012 | Intervention did not match inclusion criteria ‐ focus on asthma education, not on remote monitoring |
| Burkhart 2002 | Intervention did not match inclusion criteria ‐ intervention to improve adherence to home PEF measurements |
| Bynum 2001 | Intervention did not match inclusion criteria ‐ pharmacy‐led technology intervention to improve adherence |
| Chan 2007 | Intervention did not match inclusion criteria ‐ asthma reviews conducted remotely vs face‐to‐face |
| Chandler 1990 | Intervention did not match inclusion criteria ‐ specifically monitoring theophylline levels |
| Chatkin 2006 | Intervention did not match inclusion criteria ‐ phone calls to promote adherence, not monitoring |
| Chen 2013 | Intervention did not match inclusion criteria ‐ asthma behavioural intervention using technology, not monitoring |
| Cicutto 2009 | Intervention did not match inclusion criteria ‐ not asthma monitoring with remote feedback |
| Clark 2007 | Intervention did not match inclusion criteria ‐ counselling intervention, not asthma monitoring |
| Clarke 2014 | Intervention did not match inclusion criteria ‐ parenting intervention, not asthma monitoring |
| Claus 2004 | Design did not match inclusion criteria ‐ not an RCT |
| Cruz‐Correia 2007 | Design did not match inclusion criteria ‐ cross‐over RCT |
| de Jongste 2008 | Comparison did not match inclusion criteria ‐ comparing 2 types of electronic monitoring (FeNO vs symptoms) |
| De Vera 2014 | Intervention did not match inclusion criteria ‐ asthma education by a pharmacist, with some adherence monitoring |
| Dwinger 2013 | Intervention did not match inclusion criteria ‐ coaching/education intervention using technology for multiple chronic conditions |
| Eakin 2012 | Intervention did not match inclusion criteria ‐ not asthma monitoring with remote feedback |
| Fonseca 2006 | Design did not match inclusion criteria ‐ survey of RCT participants |
| Foster 2014 | Intervention did not match inclusion criteria ‐ intervention to improve adherence, not monitoring |
| Friedman 1999 | Design did not match inclusion criteria ‐ not an RCT report |
| Garbutt 2010 | Intervention did not match inclusion criteria ‐ asthma coaching/education intervention over the phone |
| Gruffydd‐Jones 2005 | Intervention did not match inclusion criteria ‐ asthma reviews conducted remotely vs face‐to‐face |
| Gustafson 2012 | Intervention did not match inclusion criteria ‐ self determination theory intervention, not remote monitoring |
| Halterman 2012 | Intervention did not match inclusion criteria ‐ multi‐faceted intervention, not just remote monitoring |
| Hashimoto 2011 | Intervention did not match inclusion criteria ‐ steroid tapering remotely vs face‐to‐face |
| Huang 2013 | Intervention did not match inclusion criteria ‐ support intervention, not remote monitoring |
| Janevic 2012 | Intervention did not match inclusion criteria ‐ management intervention for African American women |
| Jerant 2003 | Intervention did not match inclusion criteria ‐ mixed diagnosis study comparing models for delivering home care |
| Kattan 2006 | Intervention did not match inclusion criteria ‐ minimal or no provider involvement |
| Khan 2003 | Intervention did not match inclusion criteria ‐ 1 phone call at discharge |
| Kojima 2005 | Intervention did not match inclusion criteria ‐ not technology‐based |
| Lam 2011 | Design did not match inclusion criteria ‐ cross‐sectional analysis of an ongoing RCT, and mixed diagnosis |
| Lobach 2013 | Intervention did not match inclusion criteria ‐ not asthma monitoring with remote feedback |
| MacDonell 2015 | Intervention did not match inclusion criteria ‐ focus on improving adherence, not on monitoring |
| McCowan 2001 | Intervention did not match inclusion criteria ‐ computer‐aided decision support during consultation |
| McPherson 2006 | Intervention did not match inclusion criteria ‐ asthma education delivered via CD‐ROM and book vs book alone |
| Merchant 2016 | Intervention did not match inclusion criteria ‐ minimal or no provider involvement. Although data could be made available to patients' healthcare providers, feedback was provided primarily automatically through the Propeller Health system and by study researchers |
| Morrison 2014 | Intervention did not match inclusion criteria ‐ minimal or no provider involvement |
| Murphy 2001 | Design did not match inclusion criteria ‐ comment on RCT |
| NCT00149474 | Comparison did not match inclusion criteria ‐ 2 types of remote monitoring (PEF or symptoms) |
| NCT00562081 | Intervention did not match inclusion criteria ‐ focus on asthma education, not on monitoring |
| NCT00910585 | Intervention did not match inclusion criteria ‐ focus on asthma education, not on monitoring |
| NCT00964301 | Intervention did not match inclusion criteria ‐ focus on asthma education, not on monitoring |
| NCT01117805 | Intervention did not match inclusion criteria ‐ counselling intervention, not monitoring |
| Osman 1997 | Intervention did not match inclusion criteria ‐ post‐admission follow‐up |
| Pedram 2012 | Intervention did not match inclusion criteria ‐ main focus of the study was to educate patients on how to use a peak flow meter |
| Peruccio 2005 | Intervention did not match inclusion criteria ‐ treatment awareness education delivered over the phone, not monitoring |
| Petrie 2012 | Intervention did not match inclusion criteria ‐ minimal or no provider involvement |
| Pinnock 2003 | Intervention did not match inclusion criteria ‐ asthma reviews conducted remotely vs face‐to‐face |
| Pinnock 2007 | Intervention did not match inclusion criteria ‐ asthma reviews conducted remotely vs face‐to‐face |
| Price 2007 | Intervention did not match inclusion criteria ‐ validating the Asthma Control Test for Internet use |
| Raat 2007 | Design did not match inclusion criteria ‐ questionnaire, not RCT |
| Rand 2005 | Intervention did not match inclusion criteria ‐ study measuring validity of self report |
| Rasmussen 2005 | Intervention did not match inclusion criteria ‐ asthma reviews conducted remotely vs face‐to‐face |
| Rikkers‐Mutsaerts 2012 | Intervention did not match inclusion criteria ‐ minimal or no provider involvement |
| Rosenzweig 2008 | Intervention did not match inclusion criteria ‐ validation study |
| Schatz 2003 | Comparison did not match inclusion criteria ‐ phone calls on top of face‐to‐face review |
| Schatz 2010 | Intervention did not match inclusion criteria ‐ letter regarding validation of telephone delivery of the Asthma Control Questionnaire |
| Searing 2012 | Intervention did not match inclusion criteria ‐ minimal or no provider involvement |
| Seid 2012 | Intervention did not match inclusion criteria ‐ asthma education and motivational interviewing, not remote monitoring |
| Shanovich 2009 | Intervention did not match inclusion criteria ‐ focus on asthma education, not on remote monitoring |
| Sparrow 2005 | Comparison did not match inclusion criteria ‐ phone monitoring with or without asthma education |
| Taitel 2014 | Intervention did not match inclusion criteria ‐ pharmacy‐led compliance intervention, not remote monitoring |
| Uysal 2013 | Intervention did not match inclusion criteria ‐ validating the Asthma Control Test via text messaging |
| van den Berg 2002 | Intervention did not match inclusion criteria ‐ GP telephone access to paediatricians |
| van Gaalen 2012 | Intervention did not match inclusion criteria ‐ multi‐faceted intervention, not just remote monitoring |
| van Reisen 2010 | Intervention did not match inclusion criteria ‐ multi‐faceted intervention, not just remote monitoring |
| Vasbinder 2013 | Intervention did not match inclusion criteria ‐ minimal or no provider involvement. Medication reminder system |
| Vollmer 2006 | Intervention did not match inclusion criteria ‐ minimal or no provider involvement for most of the intervention group |
| Wiecha 2007 | Intervention did not match inclusion criteria ‐ multi‐faceted intervention, not just remote monitoring |
| Yun 2013 | Intervention did not match inclusion criteria ‐ asthma education via text |
| Zachgo 2002 | Intervention did not match inclusion criteria ‐ computer works out best inhaler type for patient |
| Zairina 2015 | Intervention did not match inclusion criteria ‐ minimal or no provider involvement. Although data could be made available to patients' healthcare providers, feedback was provided primarily automatically or by study researchers |
Characteristics of studies awaiting assessment [ordered by study ID]
Ricci 2001.
| Methods | Unclear whether this is a report of a randomised controlled trial (RCT). Presented as an oral communication ‐ cannot find additional information to clarify inclusion or exclusion |
| Participants | Children with bronchial asthma, unclear numbers or unclear specific inclusion/exclusion criteria and baseline characteristics |
| Interventions | "A system of teleassistance for in‐house monitoring" of respiratory function ‐ no other information about the intervention or comparison |
| Outcomes | Not available |
| Notes | None |
Characteristics of ongoing studies [ordered by study ID]
Ahmed 2011.
| Trial name or title | Facilitating patient self‐management in chronic disease: integrating electronic personal health records and ongoing communication into a web‐based self‐management tool |
| Methods |
Study design: 6‐month parallel multi‐centre 2‐arm pilot randomised controlled trial Setting: pulmonary clinics in 2 tertiary care hospitals in Montreal, Canada |
| Participants |
Population: adults with asthma, full population not yet recruited Baseline characteristics Full population not yet recruited, no baseline characteristics Inclusion criteria: males and females aged 18 to 69 years; physician diagnosis of asthma and prescribed ≥ 1 rescue medication; classified by doctor as having poor asthma control; access to the Internet; smoking < 20 pack‐years; can speak and understand English or French Exclusion criteria: diagnosis of COPD or other serious medical diagnoses (e.g. lung cancer) |
| Interventions |
Intervention: My Asthma Portal (MAP) includes tailored education, asthma medical information, tools to optimise self management and health behaviours and nurse case management support Control: participants receive ongoing asthma care from a respirologist. An asthma nurse provides education and follow‐up as needed. Topics such as the importance of avoiding triggers, taking all asthma medications as prescribed and using the written action plan as needed. Follow‐up phone calls between visits are provided by the asthma nurse, when appropriate (i.e. missed appointments, to clarify aspects of the action plan or prescribed asthma medications) |
| Outcomes | Primary outcomes are asthma control and asthma health‐related quality of life. Secondary outcomes are acceptance of the technology, usage rates, pattern usage, asthma self efficacy, medication adherence and healthcare utilisation |
| Starting date | 30/03/2009 |
| Contact information | Professor Sarah Ahmed 3654 Prom Sir‐William‐Osler Montreal H3G 1Y5 Canada +1 514 398 4400 ext. 00531 |
| Notes | Funding : Canadian Institutes of Health Research ID number(s): ISRCTN34326236 |
Perry 2015.
| Trial name or title | Breath connection: a school‐based telemedicine program for rural children with asthma |
| Methods | Cluster‐randomised trial |
| Participants | Rural children, ages 7 to 14 years |
| Interventions | Comparing a school‐based telemedicine intervention against usual care. The intervention provides comprehensive asthma education via telemedicine to rural children with asthma, their caregivers and school nurses; prospectively monitors asthma symptoms and lung function; and provides primary care providers with evidence‐based treatment prompts |
| Outcomes | Days wheezing, peak flow meter use, symptom‐free days |
| Starting date | Unclear |
| Contact information | University of Arkansas for Medical Sciences, Little Rock, AR |
| Notes | To date, 364/414 parent‐child dyads have been enrolled from 17 school districts in the rural Mississippi Delta region of Arkansas. Median age of children enrolled is 9.6 years, with 54.6% male, 81.8% African American, 80% with state‐issued insurance and 45.6% from a family with total household income < $15,000. At baseline, 72.2% of children were classified as patients with moderate to severe persistent asthma, and 72.1% had uncontrolled asthma according to national guidelines |
Differences between protocol and review
We chose to assess performance bias separately for objective and subjective outcomes. We added two exacerbation outcomes (those requiring ED visit and those requiring hospital admission) because the definition pre‐defined in the protocol (those requiring oral steroids) did not always match how exacerbations were categorised in the included studies.
We could not interpret the planned subgroup analysis conducted to assess types of technology because the number of different technologies was nearly as large as the number of included studies.
We did not conduct a sensitivity analysis upon removing studies at high risk of detection bias because we judged too few studies to be at low risk of bias contributing to outcomes, and because high‐risk studies contributed only to the exacerbations outcomes, which are unlikely to have been affected by this type of bias.
We changed the title of the review from "Asthma monitoring with remote feedback from a health professional" to "Home telemonitoring and remote feedback between clinic visits for people with asthma", following comments from the managing editor and the contact editor to better describe the intervention under study in line with the published literature. We carried this change through the objectives and the rest of the review for consistency.
Contributions of authors
Kayleigh Kew: background, methods, data extraction, risk of bias assessment, analysis, interpretation, results write‐up and discussion.
Christopher Cates: background, methods, data extraction, risk of bias assessment, interpretation of data, results write‐up and discussion.
Sources of support
Internal sources
-
Kayleigh Kew, UK.
Supported by St George's, University of London
-
Christopher Cates, UK.
Supported by St George's, University of London
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure (NIHR), Cochrane Programme Grant or Cochrane Incentive funding to the Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Kayleigh Kew: none.
Christopher Cates: none.
New
References
References to studies included in this review
Bateman 2000 {published and unpublished data}
- Bateman ED, Kruger MJ. A computer‐based home‐monitoring disease management programme, Pulmassist Plus® (PAP), achieves significant improvement in quality of life, and healthcare costs in moderate and severe asthma [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161:A457. [Google Scholar]
Cingi 2015 {published data only}
- Cingi C, Yorgancioglu A, Cingi CC, Oguzulgen K, Muluk NB, Ulusoy, S, et al. The "physician on call patient engagement trial" (POPET): measuring the impact of a mobile patient engagement application on health outcomes and quality of life in allergic rhinitis and asthma patients. International Forum of Allergy and Rhinology 2015;5(6):487‐97. [DOI] [PubMed] [Google Scholar]
Deschildre 2012 {published and unpublished data}
- Deschildre A, Beghin L, Salleron J, Iliescu C, Thumerelle C, Santos C, et al. Home telemonitoring (forced expiratory volume in 1 s) in children with severe asthma does not reduce exacerbations. European Respiratory Journal 2012;39:290‐6. [DOI] [PubMed] [Google Scholar]
Donald 2008 {published and unpublished data}
- Donald KJ, McBurney H, Teichtahl H, Irving L. A pilot study of telephone based asthma management. Australian Family Physician 2008;37:170‐3. [PubMed] [Google Scholar]
Finkelstein 2005 {published and unpublished data}
- Finkelstein J. Inhaled steroid adherence in moderate and severe asthma. CRISP (Computer Retrieval of Information on Scientific Projects) 2000; Vol. 31/07/2005 End date.
- Finkelstein J, Joshi A, Amelung P. Evaluation of home telemanagement in adult asthma patients [Abstract]. Journal of Allergy and Clinical Immunology 2005;115(2):S63. [Google Scholar]
- Finkelstein J, Joshi A, Arora M, Amelung P. Impact of home telemanagement in adult asthma [Abstract]. American Thoracic Society 2005 International Conference. 2005 May 20‐25; San Diego:[A40] [Poster: G14].
Guendelman 2002 {published and unpublished data}
- Guendelman S, Meade K, Benson M, Chen YQ, Samuels S. Improving asthma outcomes and self‐management behaviors of inner‐city children: a randomized trial of the Health Buddy interactive device and an asthma diary. Archives of Pediatrics and Adolescent Medicine 2002;156:114‐20. [DOI] [PubMed] [Google Scholar]
- Guendelman S, Meade K, Chen YQ, Benson M. Asthma control and hospitalizations among inner‐city children: results of a randomized trial. Telemedicine Journal and e‐Health 2004;10(Suppl 2):S6‐14. [PubMed] [Google Scholar]
Jan 2007 {published and unpublished data}
- Jan RL, Wang JY, Huang MC, Tseng SM, Su HJ, Liu LF, et al. An internet‐based interactive tele‐monitoring system for improving childhood asthma outcomes in Taiwan. Telemedicine Journal and e‐Health 2007;13:257‐68. [DOI] [PubMed] [Google Scholar]
Kokubu 1999 {published data only}
- Kokubu F, Suzuki H, Sano Y, Kihara N, Adachi M. [Tele‐medicine system for high‐risk asthmatic patients]. Arerugi [Allergy] 1999;48:700‐12. [PubMed] [Google Scholar]
Kokubu 2000 {published and unpublished data}
- Kokubu F, Nakajima S, Ito K, Makino S, Kitamura S, Fukuchi Y, et al. [Hospitalization reduction by an asthma tele‐medicine system]. Arerugi [Allergy] 2000;49:19‐31. [PubMed] [Google Scholar]
Liu 2011 {published and unpublished data}
- Liu WT, Huang CD, Wang CH, Lee KY, Lin SM, Kuo HP. A mobile telephone‐based interactive self‐care system improves asthma control. European Respiratory Journal 2011;37:310‐7. [DOI] [PubMed] [Google Scholar]
- Liu WT, Wang CH, Huang CD, Kuo HP, Liu WT, Kuo HP. A novel mobile phone‐based self‐care system improves asthma control [Abstract]. American Thoracic Society International Conference. 2007 May 18‐23; San Francisco:[A93].
Ostojic 2005 {published and unpublished data}
- Ostojic V, Cvoriscec B, Ostojic SB, Reznikoff D, Stipic‐Markovic A, Tudjman Z. Improving asthma control through telemedicine: a study of short‐message service. Telemedicine Journal and e‐Health 2005;11:28‐35. [DOI] [PubMed] [Google Scholar]
- Ostojic V, Cvoriscec B, Ostojic SB, Tudjman Z, Stipic‐Markovic A. Using of short message service (SMS) for long‐term management of asthmatic patients [abstract]. European Respiratory Society 12th Annual Congress. 2002 Sep 14‐18; Stockholm:[P1003].
Prabhakaran 2009 {published and unpublished data}
- Prabhakaran L, Chee J, Chua KC, Mun WW. The use of text messaging to improve asthma control: a study of short message service (SMS) [Abstract]. Respirology (Carlton, Vic.) 2009;14:A217 [PD 10‐01]. [Google Scholar]
- Prabhakaran L, Chee WY, Chua KC, Abisheganaden J, Wong WM. The use of text messaging to improve asthma control: a pilot study using the mobile phone short messaging service (SMS). Journal of Telemedicine and Telecare 2010;16:286‐90. [DOI] [PubMed] [Google Scholar]
Ryan 2012 {published and unpublished data}
- Ryan D, Pinnock H, Amanda L, Lionel T, Sheikh A, Musgrave S, et al. Can mobile phone technology improve asthma control? A randomised trial [Abstract]. European Respiratory Society 20th Annual Congress. 2010 Sep 18‐22; Barcelona:[2138].
- Ryan D, Pinnock H, Lee AJ, Tarassenko L, Ayansina D, Musgrave S, et al. Can your mobile phone help your asthma: preliminary results [Abstract]. Primary Care Respiratory Journal 2010;19:A15 [54]. [Google Scholar]
- Ryan D, Pinnock H, Lee AJ, Tarassenko L, Pagliari C, Sheikh A, et al. The CYMPLA trial. Mobile phone‐based structured intervention to achieve asthma control in patients with uncontrolled persistent asthma: a pragmatic randomised controlled trial. Primary Care Respiratory Journal 2009;18:343‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D, Pinnock H, Tarassenko L, Lee A, Sheikh A, Price D. Can your mobile phone improve your asthma? [Abstract]. Thorax 2010;65:S135. [Google Scholar]
- Ryan D, Price D, Musgrave SD, Malhotra S, Lee AJ, Ayansina D, et al. Clinical and cost effectiveness of mobile phone supported self monitoring of asthma: multicentre randomised controlled trial. BMJ (Online) 2012;344:e1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Meer 2009 {published and unpublished data}
- Meer V, Bakker MJ, Hout WB, Rabe KF, Sterk PJ, Kievit J, et al. Self‐management for asthma on the Internet: a randomized study. Nederlands Tijdschrift Voor Geneeskunde 2010;154:403‐9. [Google Scholar]
- Meer V, Stel HF, Bakker MJ, Roldaan AC, Assendelft WJJ, Sterk PJ, et al. Internet‐based self management improves short‐term asthma control: the SMASHING study [Abstract]. Primary Care Respiratory Journal 2008;17:124. [Google Scholar]
- Meer V, Bakker MJ, Rabe KF, Sterk PJ, Kievit J, Assendelft WJJ, et al. Improved quality of life by internet based self management versus usual care in asthma a randomized controlled trial [Abstract]. European Respiratory Society 18th Annual Congress. 2008 Oct 3‐7; Berlin:[1372].
- Meer V, Bakker MJ, Hout WB, Rabe KF, Sterk PJ, Kievit J, et al. Internet‐based self‐management plus education compared with usual care in asthma: a randomized trial. Annals of Internal Medicine 2009;151:110‐20. [DOI] [PubMed] [Google Scholar]
- Meer V, Stel HF, Bakker MJ, Roldaan AC, Assendelft WJ, Sterk PJ, et al. Weekly self‐monitoring and treatment adjustment benefit patients with partly controlled and uncontrolled asthma: an analysis of the SMASHING study. Respiratory Research 2010;11:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meer V, Hout WB, Bakker MJ, Rabe KF, Sterk PJ, Assendelft WJJ, et al. Cost‐effectiveness of internet‐based self‐management compared with usual care in asthma. PLoS One 2011;6:e27108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Voorend‐van Bergen 2015 {published and unpublished data}
- Beerthuizen T, Voorend‐van Bergen S, Hout WB, Vaessen‐Verberne AA, Brackel HJ, Landstra AM, et al. Cost‐effectiveness of FENO‐based and web‐based monitoring in paediatric asthma management: a randomised controlled trial. Thorax 2016;71(7):607‐13. [CENTRAL: 1139864; CRS: 4900132000017696; PUBMED: 27048197] [DOI] [PubMed] [Google Scholar]
- Bergen SV, Beerthuizen T, Hout W, Vaessen‐Verberne A, Brackel H, Landstra A, et al. Cost‐effectiveness of FeNO‐ and web‐based monitoring in pediatric asthma management. European Respiratory Journal 2015;46(Suppl 59):OA4776. [CENTRAL: 1126623; CRS: 4900132000012681; EMBASE: 72107805] [Google Scholar]
- Voorend‐van Bergen S, Vaessen‐Verberne A, Landstra A, Brackel H, Berg N, Jongste J, et al. FeNO and web‐based monitoring in paediatric asthma management; the BATMAN study [Abstract]. European Respiratory Journal 2013;42(Suppl 57):629s [3014]. [Google Scholar]
- Voorend‐van Bergen S, Vaessen‐Verberne AA, Brackel HJ, Landstra AM, Berg NJ, Hop WC, et al. Monitoring strategies in children with asthma: a randomised controlled trial. Thorax 2015;70(6):543‐50. [DOI] [PubMed] [Google Scholar]
Willems 2008 {published and unpublished data}
- NCT00411346. Patient Research In Self‐Management of Asthma (PRISMA). https://clinicaltrials.gov/NCT00411346 (accessed 2 June 2015).
- Willems DC, Joore MA, Hendriks JJ, Nieman FH, Severens JL, Wouters EF, et al. The effectiveness of nurse‐led telemonitoring of asthma: results of a randomized controlled trial. Journal of Evaluation in Clinical Practice 2008;14:600‐9. [DOI] [PubMed] [Google Scholar]
- Willems DC, Joore MA, Hendriks JJ, Duurling RA, Wouters EF, Severens JL. Process evaluation of a nurse‐led telemonitoring programme for patients with asthma. Journal of Telemedicine and Telecare 2007;13:310‐7. [DOI] [PubMed] [Google Scholar]
- Willems DCM, Joore MA, Hendriks JJE, Wouters EFM, Severens JL. Cost‐effectiveness of a nurse‐led telemonitoring intervention based on peak expiratory flow measurements in asthmatics: results of a randomised controlled trial. Cost Effectiveness and Resource Allocation 2007;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2010 {published and unpublished data}
- ACTRN12606000400561. Improving Childhood Asthma Management Through a Telemedicine Monitoring Network. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12606000400561 (accessed 2 June 2015).
- Xu C, Jackson M, Scuffham PA, Wootton R, Simpson P, Whitty J, et al. A randomized controlled trial of an interactive voice response telephone system and specialist nurse support for childhood asthma management. Journal of Asthma 2010;47:768‐73. [DOI] [PubMed] [Google Scholar]
Young 2012 {published and unpublished data}
- Young HN, Havican SN, Griesbach S, Thorpe JM, Chewning BA, Sorkness CA. Patient and pharmacist telephonic encounters (PARTE) in an underserved rural patient population with asthma: results of a pilot study. Telemedicine Journal and e‐Health 2012;18:427‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aaron 2016 {published data only}
- Aaron M, Nelson BW, Kaltsas E, Brown RW, Thomas LJ, Patel MR. Impact of goal setting and goal attainment methods on asthma outcomes: findings from an asthma self‐management intervention for African American women. Health Education & Behavior 2016;[Epub ahead of print]. [CRS: 4900132000021647; PUBMED: 27179290] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersen 2007 {published data only}
- Andersen UM. Does a www‐based interactive computer program change asthma outcomes, quality of life and asthma knowledge [Abstract]. Journal of Allergy and Clinical Immunology 2007;119:S9 [34]. [Google Scholar]
Apter 2000 {published data only}
- Apter A. Inhaled steroid adherence in moderate and severe asthma. CRISP (Computer Retrieval of Information on Scientific Projects) 2000; Vol. 31/08/2005 End date.
Apter 2015 {published data only}
- Apter AJ, Bryant‐Stephens, Morales KH, Wan F, Hardy S, Reed‐Wells S, et al. Using IT to improve access, communication, and asthma in African American and Hispanic/Latino adults: rationale, design, and methods of a randomized controlled trial. Contemporary Clinical Trials 2015;44:119‐28. [DOI] [PubMed] [Google Scholar]
Araujo 2012 {published data only}
- Araujo L, Jacinto T, Moreira A, Castel‐Branco MG, Delgado L, Costa‐Pereira A, et al. Clinical efficacy of web‐based versus standard asthma self‐management. Journal of Investigational Allergology and Clinical Immunology 2012;22:28‐34. [PubMed] [Google Scholar]
Baptist 2013 {published data only}
- Baptist AP, Ross JA, Yang Y, Song PX, Clark NM. A randomized controlled trial of a self‐regulation intervention for older adults with asthma. Journal of the American Geriatrics Society 2013;61:747‐53. [DOI] [PubMed] [Google Scholar]
Barbanel 2003 {published data only}
- Barbanel D, Eldridge S, Griffiths C. Can a self‐management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax 2003;58:851‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bender 2001 {published data only}
- Bender BG. Assessment mode and validity of self‐reports in children. CRISP (Computer Retrieval of Information on Scientific Projects) 2001; Vol. 31/01/2005 End date.
Bender 2007 {published data only}
- Bender BG, Bartlett SJ, Rand CS, Turner C, Wamboldt FS, Zhang L. Impact of interview mode on accuracy of child and parent report of adherence with asthma‐controller medication. Pediatrics 2007;120:e471‐7. [DOI] [PubMed] [Google Scholar]
Bender 2010 {published data only}
- Bender BG, Apter A, Bogen DK, Dickinson P, Fisher L, Wamboldt FS, et al. Test of an interactive voice response intervention to improve adherence to controller medications in adults with asthma. Journal of the American Board of Family Medicine 2010;23:159‐65. [DOI] [PubMed] [Google Scholar]
Boyd 2014 {published data only}
- Boyd MJ, Elliott RA, Barber N, Mehta R, Waring J, Chuter A, et al. The impact of the New Medicines Service (NMS) in England on patients' adherence to their medicines. International Journal of Pharmacy Practice 2014;22(S2):66. [Google Scholar]
Burbank 2012 {published data only}
- Burbank A, Rettiganti M, Brown RH, Jones S, Perry TT. Asthma education via telemedicine: effects on asthma knowledge and self‐efficacy [Abstract]. Journal of Investigative Medicine 2012;60:401. [Google Scholar]
Burkhart 2002 {published data only}
- Burkhart P. Promoting children's adherence to asthma self‐management. CRISP (Computer Retrieval of Information on Scientific Projects) 2002; Vol. 31/07/2004 End date.
Bynum 2001 {published data only}
- Bynum A, Hopkins D, Thomas A, Copeland N, Irwin C. The effect of telepharmacy counseling on metered‐dose inhaler technique among adolescents with asthma in rural Arkansas. Telemedicine Journal and e‐Health 2001;7:207‐17. [DOI] [PubMed] [Google Scholar]
Chan 2007 {published data only}
- Callahan CW. Asthma In‐Home Monitoring (AIM) Trial. http://www.clinicaltrials.gov/ct/show/NCT00282516 (accessed 4 August 2015).
- Callahan CW, Chan DS, Hatch‐Pigott G, Manning N, Proffitt L, Lawless A, et al. One year randomized controlled trial of home telemonitoring of children with persistent asthma using store‐and‐forward technology vs office‐based care [abstract]. American Thoracic Society 2005 International Conference. 2005 May 20‐25; San Diego:[C47] [Poster: A21].
- Chan DS, Callahan CW, Hatch‐Pigott VB, Lawless A, Proffitt HL, Manning NE, et al. Internet‐based home monitoring and education of children with asthma is comparable to ideal office‐based care: results of a 1‐year asthma in‐home monitoring trial. Pediatrics 2007;119:569‐78. [DOI] [PubMed] [Google Scholar]
- Chan DS, Callahan CW, Sheets SJ, Moreno CN, Malone FJ. An Internet‐based store‐and‐forward video home telehealth system for improving asthma outcomes in children. American Journal of Health‐System Pharmacy 2003;60:1976‐81. [DOI] [PubMed] [Google Scholar]
Chandler 1990 {published data only}
- Chandler MH, Clifton GD, Louis BA, Coons SJ, Foster TS, Phillips BA. Home monitoring of theophylline levels: a novel therapeutic approach. Pharmacotherapy 1990;10:294‐300. [PubMed] [Google Scholar]
Chatkin 2006 {published data only}
- Chatkin JM, Blanco DC, Scaglia N, Wagner MB, Fritscher CC, Chatkin J, et al. Impact of a low‐cost and simple intervention in enhancing treatment adherence in a Brazilian asthma sample. Journal of Asthma 2006;43:263‐6. [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen SH, Huang JL, Yeh KW, Tsai YF. Interactive support interventions for caregivers of asthmatic children. Journal of Asthma 2013;50:649‐57. [DOI] [PubMed] [Google Scholar]
Cicutto 2009 {published data only}
- Cicutto L, Ashby MN, Feldman D. Telephone intervention based strategies to increase the completion and use of asthma action plans for adults with asthma [Abstract]. American Thoracic Society International Conference. 2009 May 15‐20; San Diego:A1033.
Clark 2007 {published data only}
- Clark NM, Gong ZM, Wang SJ, Lin X, Bria WF, Johnson TR, et al. A randomized trial of a self‐regulation intervention for women with asthma. Chest 2007;132:88‐97. [DOI] [PubMed] [Google Scholar]
Clarke 2014 {published data only}
- Clarke SA, Calam R, Morawska A, Sanders M. Developing web‐based Triple P 'Positive Parenting Programme' for families of children with asthma. Child 2014;40:492‐7. [DOI] [PubMed] [Google Scholar]
Claus 2004 {published data only}
- Claus R, Michael H, Josef L, Jan‐Torsten T, Marion S. Internet based patient education evaluation of a new tool for young asthmatics [Abstract]. European Respiratory Society 14th Annual Congress. 2004 Sep 3‐7; Glasgow; Vol. 24:383s.
Cruz‐Correia 2007 {published data only}
- Cruz‐Correia R, Fonseca J, Lima L, Araujo L, Delgado L, Castel‐Branco MG, et al. A comparison of web‐based and paper‐based self management tools for asthma: patients' opinions and quality of data in a randomised crossover study. Journal on Information Technology in Healthcare 2007;5:357‐71. [PubMed] [Google Scholar]
- Cruz‐Correia R, Fonseca J, Lima L, Araújo L, Delgado L, Castel‐Branco MG, et al. Web‐based or paper‐based self‐management tools for asthma ‐ patients' opinions and quality of data in a randomized crossover study. Studies in Health Technology and Informatics 2007;127:178‐89. [PubMed] [Google Scholar]
de Jongste 2008 {published data only}
- Jongste JC, Carroro S, Hop WC, Baraldi E. Daily exhaled nitric oxide telemonitoring in the management of childhood asthma [Abstract]. American Thoracic Society International Conference. 2008 May 16‐21; Toronto:Poster #908.
De Vera 2014 {published data only}
- Vera MA, Sadatsafavi M, Tsao NW, Lynd LD, Lester R, Gastonguay L, et al. Empowering pharmacists in asthma management through interactive SMS (EmPhAsIS): study protocol for a randomized controlled trial. Trials 2014;15:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwinger 2013 {published data only}
- Dwinger S, Dirmaier J, Herbarth L, Konig HH, Eckardt M, Kriston L, et al. Telephone‐based health coaching for chronically ill patients: study protocol for a randomized controlled trial. Trials 2013;14:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eakin 2012 {published data only}
- Eakin MN, Rand CS, Bilderback A, Bollinger ME, Butz A, Kandasamy V, et al. Asthma in Head Start children: effects of the Breathmobile program and family communication on asthma outcomes. Journal of Allergy and Clinical Immunology 2012;129:664‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fonseca 2006 {published data only}
- Fonseca JA, Costa‐Pereira A, Delgado L, Fernandes L, Castel‐Branco MG. Asthma patients are willing to use mobile and web technologies to support self‐management. Allergy 2006;61:389‐90. [DOI] [PubMed] [Google Scholar]
Foster 2014 {published data only}
- Foster J, Smith L, Usherwood T, Sawyer S, Reddel H. Electronic reminders improve adherence with preventer inhalers in Australian primary care patients [Abstract]. Annual Scientific Meetings of the Thoracic Society of Australia and New Zealand and the Australian and New Zealand Society of Respiratory Science. 2014 April 4‐9; Adelaide:24 [TO 034].
Friedman 1999 {published data only}
- Friedman R. Impact of a telecommunication system in childhood asthma. CRISP (Computer Retrieval of Information on Scientific Projects) 1999; Vol. 29/09/2004 End date.
Garbutt 2010 {published data only}
- Garbutt JM, Banister C, Highstein G, Sterkel R, Epstein J, Bruns J, et al. Telephone coaching for parents of children with asthma: impact and lessons learned. Archives of Pediatrics & Adolescent Medicine 2010;164:625‐30. [DOI] [PubMed] [Google Scholar]
- Swerczek LM, Banister C, Bloomberg GR, Bruns JM, Epstein J, Highstein GR, et al. A telephone coaching intervention to improve asthma self‐management behaviors. Pediatric Nursing 2013;39:125‐30, 145. [PubMed] [Google Scholar]
Gruffydd‐Jones 2005 {published data only}
- Gruffydd‐Jones K, Hollinghurst S, Ward S, Taylor G. Targeted routine asthma care in general practice using telephone triage. British Journal of General Practice 2005;55:918‐23. [PMC free article] [PubMed] [Google Scholar]
- Gruffydd‐Jones K, Ward S. Targeted routine asthma care in general practice using the telephone triage [Abstract]. European Respiratory Journal 2005;26:Abstract No. 4264. [PMC free article] [PubMed] [Google Scholar]
Gustafson 2012 {published data only}
- Gustafson D, Wise M, Bhattacharya A, Pulvermacher A, Shanovich K, Phillips B, et al. The effects of combining web‐based eHealth with telephone nurse case management for pediatric asthma control: a randomized controlled trial. [References]. Journal of Medical Internet Research 2012;14:41‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise M, Gustafson DH, Sorkness CA, Molfenter T, Staresinic A, Meis T, et al. Internet telehealth for pediatric asthma case management: integrating computerized and case manager features for tailoring a web‐based asthma education program. Health Promotion Practice 2007;8:282‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halterman 2012 {published data only}
- Halterman JS, Fagnano M, Montes G, Fisher S, Tremblay P, Tajon R, et al. The school‐based preventive asthma care trial: results of a pilot study. Journal of Pediatrics 2012;161:1109‐1115.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halterman JS, Sauer J, Fagnano M, Montes G, Fisher S, Tremblay P, et al. Working toward a sustainable system of asthma care: development of the School‐Based Preventive Asthma Care Technology (SB‐PACT) trial. Journal of Asthma 2012;49:395‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hashimoto 2011 {published data only}
- Hashimoto S, Brinke A, Roldaan AC, Veen IH, Möller GM, Sont JK, et al. Internet‐based tapering of oral corticosteroids in severe asthma: a pragmatic randomised controlled trial. Thorax 2011;66:514‐20. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Brinke A, Roldaan AC, Veen IH, Moller GM, Sont JK, et al. Monitoring exhaled nitric oxide (FENO) to tailor the lowest effective dose of oral corticosteroids in severe asthma (MONOSA‐study) [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181:A3721. [Google Scholar]
Huang 2013 {published data only}
- Huang JL, Chen SH, Yeh KW. Health outcomes, education, healthcare delivery and quality [3056]. Constructed supporting program improves asthma treatment outcomes in children. World Allergy Organization Journal 2013;6:P224. [Google Scholar]
Janevic 2012 {published data only}
- Janevic MR, Sanders GM, Thomas LJ, Williams DM, Nelson B, Gilchrist E, et al. Study protocol for Women of Color and Asthma Control: a randomized controlled trial of an asthma‐management intervention for African American women. BMC Public Health 2012;12:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jerant 2003 {published data only}
- Jerant A. A randomized trial of home self‐efficacy enhancement. CRISP (Computer Retrieval of Information on Scientific Projects) 2003; Vol. 31/12/2007 End date.
Kattan 2006 {published data only}
- Kattan M, Crain EF, Steinbach S, Visness CM, Walter M, Stout JW, et al. A randomized clinical trial of clinician feedback to improve quality of care for inner‐city children with asthma. Pediatrics 2006;117:e1095‐103. [DOI] [PubMed] [Google Scholar]
Khan 2003 {published data only}
- Khan MSR, O'Meara M, Stevermuer TL, Henry RL. Randomized controlled trial of asthma education after discharge from an emergency department. Journal of Paediatrics and Child Health 2004;40:674‐7. [DOI] [PubMed] [Google Scholar]
- Khan S, O'Meara M, Hurst T, Henry RL. Randomised controlled trial of asthma education by telephone after discharge from an emergency department [Abstract]. European Respiratory Journal 2003;22:Abstract No: [P2294]. [Google Scholar]
Kojima 2005 {published data only}
- Kojima N, Takeda Y, Akashi M, Kamiya T, Matsumoto M, Ohya Y, et al. Interactive education during summer camp for children with asthma improved adherence of self‐management [Abstract]. Journal of Allergy and Clinical Immunology 2005;115:S115. [Google Scholar]
Lam 2011 {published data only}
- Lam A. Practice innovations: delivering medication therapy management services via videoconference interviews. Consultant Pharmacist 2011;26:764‐74. [DOI] [PubMed] [Google Scholar]
Lobach 2013 {published data only}
- Lobach DF, Kawamoto K, Anstrom KJ, Silvey GM, Willis JM, Johnson FS, et al. A randomized trial of population‐based clinical decision support to manage health and resource use for Medicaid beneficiaries. Journal of Medical Systems 2013;37:9922. [DOI] [PubMed] [Google Scholar]
MacDonell 2015 {published data only}
- MacDonell KK, Gibson‐Scipio WM, Lam P, Naar‐King S, Secord E. The Detroit young adult asthma project: feasibility and acceptability of a multi‐component, technology‐based intervention targeting adherence to controller medication. American Thoracic Society International Conference. 2015 May 15‐20; Denver:A3634.
McCowan 2001 {published data only}
- McCowan C, Neville RG, Ricketts IW, Warner FC, Hoskins G, Thomas GE. Computer assisted assessment and management of patients with asthma. American Thoracic Society 97th International Conference. 2001 May 18‐23; San Francisco.
McPherson 2006 {published data only}
- Glazebrook C, McPherson A, Forster D, James C, Crook I, Smyth A. The asthma files: randomised controlled trial of interactive multimedia program to promote self‐management skills in children [abstract]. American Thoracic Society 100th International Conference. 2004 May 21‐26; Orlando:D92 Poster 506.
- McPherson AC, Glazebrook C, Forster D, James C, Smyth A. A randomized, controlled trial of an interactive educational computer package for children with asthma. Pediatrics 2006;117:1046‐54. [DOI] [PubMed] [Google Scholar]
Merchant 2016 {published data only}
- Merchant R, Inamdar R, Quade R, Sickle D, Maenner M, Patmas M. Interim results from a randomized, controlled trial of remote monitoring of inhaled bronchodilator use on asthma control and management [Abstract]. American College of Chest Physicians Annual Meeting. 26‐31 October 2013; Chicago; Vol. 144:71A.
- Merchant RK, Inamdar R, Quade RC. Effectiveness of population health management using the propeller health asthma platform: a randomized clinical trial. Journal of Allergy and Clinical Immunology 2016;4(3):455‐63. [CENTRAL: 1135189; CRS: 4900132000015067; PUBMED: 26778246] [DOI] [PubMed] [Google Scholar]
Morrison 2014 {published data only}
- Morrison D, Wyke S, Saunderson K, McConnachie A, Agur K, Chaudhuri R, et al. Findings from a pilot Randomised trial of an Asthma Internet Self‐management Intervention (RAISIN). BMJ Open 2016;6(5):e009254. [CRS: 4900132000021653; PUBMED: 27173807] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D, Wyke S, Thomson NC, McConnachie A, Agur K, Saunderson K, et al. A Randomized trial of an Asthma Internet Self‐management Intervention (RAISIN): study protocol for a randomized controlled trial. Trials 2014;15:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2001 {published data only}
- Murphy JC. Telemedicine offers new way to manage asthma. American Journal of Health‐System Pharmacy 2001;58:1693, 1696. [DOI] [PubMed] [Google Scholar]
NCT00149474 {published data only}
- Peak Flow Monitoring in Older Adults With Asthma. https://clinicaltrials.gov/NCT00149474 (accessed 2 June 2015).
NCT00562081 {published data only}
- The Virtual Asthma Clinic. https://clinicaltrials.gov/NCT00562081 (accessed 2 June 2015).
NCT00910585 {published data only}
- Coaching in Childhood Asthma. https://clinicaltrials.gov/NCT00910585 (accessed 2 June 2015).
NCT00964301 {published data only}
- Telemedicine Education for Rural Children With Asthma. https://clinicaltrials.gov/NCT00964301 (accessed 2 June 2015).
NCT01117805 {published data only}
- Women of Color and Asthma Control. https://clinicaltrials.gov/NCT01117805 (accessed 2 June 2015).
Osman 1997 {published data only}
- Osman L. A controlled study evaluating relative benefits of two types of review after an A&E attendance. CRISP (Computer Retrieval of Information on Scientific Projects) 1997; Vol. 01/03/2001 End date.
- Osman L. A randomised controlled trial of benefits of specialist review or telephone follow up after an Accident & Emergency attendance for asthma. http://www.isrctn.com/ISRCTN11122844 (accessed 4 August 2015).
Pedram 2012 {published data only}
- Pedram RS, Piroozmand N, Zolfaghari M, Kazemnejad A, Firoozbakhsh S. Education of how‐to‐use peak flow meter and following up via SMS on asthma self‐management [Farsi]. HAYAT 2012;18:1‐9. [Google Scholar]
Peruccio 2005 {published data only}
- Peruccio D, Bauer B, Delaronde S. Improving asthma treatment and quality of life in a managed care population [Abstract]. Journal of Allergy & Clinical Immunology 2005;115:S63. [PubMed] [Google Scholar]
Petrie 2012 {published data only}
- Petrie KJ, Perry K, Broadbent E, Weinman J. A text message programme designed to modify patients' illness and treatment beliefs improves self‐reported adherence to asthma preventer medication. British Journal of Health Psychology 2012;17:74‐84. [DOI] [PubMed] [Google Scholar]
Pinnock 2003 {published data only}
- Pinnock H, Bawden R, Proctor S, Wolfe S, Scullion J, Price D, et al. Accessibility, acceptability, and effectiveness in primary care of routine telephone review of asthma: pragmatic, randomised controlled trial. BMJ (Clinical research ed.) 2003;326:477‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock H, Sheikh A, Bawden R, Proctor S, Wolfe S, Scullion J, et al. A randomised controlled trial comparing telephone review with fact to face consultations in the management fo adult asthmatics in UK primary care. Primary Care Respiratory Journal 2002;11:68‐9. [Google Scholar]
- Pinnock H, Sheikh A, Bawden R, Proctor S, Wolfe S, Scullion J, et al. Cost effectiveness of telephone vs face to face consultations for annual asthma review: randomised controlled trial in UK primary care [Abstract]. European Respiratory Journal 2003;22:Abstract No: [2250]. [Google Scholar]
- Pinnock H, Sheikh A, Bawden R, Proctor S, Wolfe S, Scullion J, et al. Telephone vs. face to face consultations in the management of adult asthmatics in UK primary care: a randomised controlled trial [abstract]. European Respiratory Society 12th Annual Congress. 2002 Sep 14‐18; Stockholm:Abstract no.: P593.
Pinnock 2007 {published data only}
- Pinnock H, Adlem L, Gaskin S, Harris J, Snellgrove C, Sheikh A. Accessibility, clinical effectiveness, and practice costs of providing a telephone option for routine asthma reviews: phase IV controlled implementation study. British Journal of General Practice 2007;57(542):714‐22. [PMC free article] [PubMed] [Google Scholar]
- Pinnock H, Adlem L, Gaskin S, Snellgrove C, Harris J, Sheikh A. Impact on access of providing a telephone option for primary care asthma reviews: controlled implementation study [Abstract]. European Respiratory Journal 2005;26:Abstract No. 1711. [Google Scholar]
- Pinnock H, Madden V, Snellgrove C, Sheikh A. Telephone or surgery asthma reviews? Preferences of participants in a primary care randomised controlled trial. Primary Care Respiratory Journal 2005;14:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock H, McKenzie L, Price D, Sheikh A. Cost‐effectiveness of telephone or surgery asthma reviews: economic analysis of a randomised controlled trial. British Journal of General Practice 2005;55:119‐24. [PMC free article] [PubMed] [Google Scholar]
- Pinnock H, Norman C, Bowden K, Sheikh A. Impact on asthma morbidity and patient enablement of providing a telephone option for primary care asthma reviews: phase IV controlled implementation study [Abstract]. Primary Care Respiratory Journal 2006;15:187. [Google Scholar]
- Pinnock H, Norman C, Bowden K, Sheikh A. Impact on asthma morbidity and patient enablement of providing a telephone option in a primary care asthma review service controlled implementation study [Abstract]. European Respiratory Journal 2006;28:571s [3361]. [Google Scholar]
Price 2007 {published data only}
- Price D. A validation of the UK English language Asthma Control Test (ACT) for internet use among adults with asthma. East Norfolk and Waveney Research Consortium (Norfolk & Norwich UH/ Norwich PCT/James Paget/NWMHP) 2007; Vol. 1/1/2008:Ongoing.
- Price D. Validating asthma control test on the Internet. Suffolk and Norfolk Research and Development Consortium (S.A.N.D.) 2007; Vol. 30/12/2007:Ongoing.
Raat 2007 {published data only}
- Raat H, Mangunkusumo RT, Mohangoo AD, Juniper EF, Lei J. Internet and written respiratory questionnaires yield equivalent results for adolescents. Pediatric Pulmonology 2007;42:357‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rand 2005 {published data only}
- Rand C. Assessment Mode and Validity of Self‐Reports in Adults. clinicaltrials.gov (accessed 4 August 2015).
- Rand CS. Adherence intervention for minority children with asthma. CRISP (Computer Retrieval of Information on Scientific Projects) 2000; Vol. 31/03/2005 End date.
Rasmussen 2005 {published data only}
- Phanareth K, Rasmussen L, Nolte H, Backer V. Using the Internet as a tool for the management of asthma disease. European Respiratory Journal 2002;20:54s. [Google Scholar]
- Rasmussen L, Backer V, Phanareth K. Preliminary data from a clinical trial: does use of an internet based asthma‐monitoring tool increase lung function and asthma control? [abstract]. American Thoracic Society 99th International Conference. 2003 May 16‐21; Seattle:A102 Poster D6.
- Rasmussen L, Phanareth K, Nolte H, Backer V. A long term randomized clinical study of 300 asthmatics. An internet based asthma monitoring tool improves quality of life significantly [Abstract]. European Respiratory Journal 2004;24:261s. [Google Scholar]
- Rasmussen L, Phanareth K, Nolte H, Backer V. Preliminary data from a clinical trial: an Internet based asthma monitoring tool increases lung function and decrease airway hyperresponsiveness [Abstract]. European Respiratory Journal 2003;22:Abstract No: [3351]. [Google Scholar]
- Rasmussen LM, Phanareth K, Nolte H, Backer V. Can internet‐based management improve asthma control? A long term randomised clinical study of 300 asthmatics [Abstract]. European Respiratory Journal 2005;26:Abstract No. 400. [Google Scholar]
- Rasmussen LM, Phanareth K, Nolte H, Backer V. Can internet‐based monitoring of asthma maintain asthma control over a 12 month period [Abstract]. European Respiratory Journal 2006;28:122s [783]. [Google Scholar]
- Rasmussen LM, Phanareth K, Nolte H, Backer V. Internet‐based monitoring of asthma: a long‐term, randomized clinical study of 300 asthmatic subjects. Journal of Allergy and Clinical Immunology 2005;115:1137‐42. [DOI] [PubMed] [Google Scholar]
Rikkers‐Mutsaerts 2012 {published data only}
- Rikkers‐Mutsaerts E, Winters AE, Bakker MJ, Stel HF, Meer V, Jongste JC, et al. Internet‐based self‐management compared with usual care in adolescents with asthma: a randomised controlled trial [Abstract]. European Respiratory Society 20th Annual Congress. 2010 Sep 18‐22; Barcelona:[5396].
- Rikkers‐Mutsaerts ER, Winters AE, Bakker MJ, Stel HF, Meer V, Jongste JC, et al. Internet‐based self‐management compared with usual care in adolescents with asthma: a randomized controlled trial. Pediatric Pulmonology 2012;47:1170‐9. [DOI] [PubMed] [Google Scholar]
Rosenzweig 2008 {published data only}
- Rosenzweig JC, Williams AE, Kite A, Yang M, Kosinski M. Reliabilty and validity of the asthma control test (ACT) paper and telephone formats [Abstract]. European Respiratory Society 18th Annual Congress. 2008 Oct 3‐7; Berlin:[P3501].
Schatz 2003 {published data only}
- Schatz M, Rodriguez E, Falkoff R, Zeiger RS. The relationship of frequency of follow‐up visits to asthma outcomes in patients with moderate persistent asthma. Journal of Asthma 2003;40:49‐53. [DOI] [PubMed] [Google Scholar]
Schatz 2010 {published data only}
- Schatz M, Zeiger RS. Ineffectiveness of telephone‐based environmental control intervention to improve asthma outcomes. Journal of Allergy and Clinical Immunology 2010;126:873‐5. [DOI] [PubMed] [Google Scholar]
Searing 2012 {published data only}
- Searing DA, Bender BG. Short message service (SMS) for asthma management: a pilot study utilizing text messaging to promote asthma self‐management [Abstract]. Annual Meeting of American Academy of Allergy, Asthma and Immunology (AAAAI). 2012 Mar 2‐6; Orlando; Vol. 129:AB142 [538].
Seid 2012 {published data only}
- Seid M, D'Amico EJ, Varni JW, Munafo JK, Britto MT, Kercsmar CM, et al. The in vivo adherence intervention for at risk adolescents with asthma: report of a randomized pilot study. Journal of Pediatric Psychology 2012;37:390‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shanovich 2009 {published data only}
- Shanovich KK, Pulvermacher AD, Hollman SJ, Richardson PA, Wise ME, Lee SH, et al. Nurse case management services provided to supplement a web‐based asthma education program [Abstract]. Journal of Allergy and Clinical Immunology 2008;121:S40 [155]. [Google Scholar]
- Shanovich KK, Sorkness CA, Wise ME, Pulvermacher AD, Bhattacharya A, Gustafson DH. Internet telehealth for pediatric nurse case management improves asthma control [Abstract]. Journal of Allergy and Clinical Immunology 2009;123:S43. [Google Scholar]
Sparrow 2005 {published data only}
- Sparrow D. Telecommunications System in Asthma. www.clinicaltrials.gov/NCT00232557 (accessed 4 August 2015).
Taitel 2014 {published data only}
- Taitel M, Jiang JZ, Rudkin K. Impact of pharmacist‐call intervention program on new‐to‐therapy patients' medication adherence. Value in Health 2014;17:A138‐9. [Google Scholar]
Uysal 2013 {published data only}
- Uysal MA, Mungan D, Yorgancioglu A, Yildiz F, Akgun M, Gemicioglu B, et al. Asthma control test via text messaging: could it be a tool for evaluating asthma control?. Journal of Asthma 2013;50:1083‐9. [DOI] [PubMed] [Google Scholar]
van den Berg 2002 {published data only}
- Berg NJ, Have WH, Bindels PJE, Palen J, Aalderen WMC. Is the availability of a 24 hour asthma‐telephone useful in the implementation of asthma treatment guidelines for children aged 6‐16 years, among general practitioners? [abstract]. European Respiratory Journal 2002;20:329s. [Google Scholar]
van Gaalen 2012 {published data only}
- Gaalen JL, Bakker MJ, Bodegom‐Vos L, Snoeck‐Stroband JB, Assendelft WJ, Kaptein AA, et al. Implementation strategies of internet‐based asthma self‐management support in usual care. Study protocol for the IMPASSE cluster randomized trial. Implementation Science 2012;7:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaalen JL, Beerthuizen T, Meer V, Reisen P, Redelijkheid GW, Snoeck‐Stroband JB, et al. Long‐term outcomes of internet‐based self‐management support in adults with asthma: randomized controlled trial. Journal of Medical Internet Research 2013;15:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Reisen 2010 {published data only}
- Reisen PAJ, Redelijkheid WG, Gaalen JL, Meer V, Bakker MJ, Beerthuizen T, et al. The long‐term outcomes of a one‐year Internet‐based self‐management support programme compared with usual care in asthma. Additional follow‐up 1.5 year after a randomized trial [Abstract]. European Respiratory Society 20th Annual Congress. 2010 Sep 18‐22; Barcelona:[E2160].
Vasbinder 2013 {published data only}
- Vasbinder E, Goossens L, Janssens H, Winter B, Dijk L, Vulto A, et al. E‐monitoring of asthma therapy to improve compliance in children (E‐MATIC). European Respiratory Journal 2015;46(Suppl 59):OA4771. [CENTRAL: 1126624; CRS: 4900132000015170; EMBASE: 72107800] [Google Scholar]
- Vasbinder EC, Goossens LMA, Janssens H, Winter BCM, Dijk L, Vulto AG, et al. E‐Monitoring of asthma therapy to improve compliance in children (E‐MATIC). Pharmacoepidemiology and Drug Safety 2015;24:489. [CENTRAL: 1126710; CRS: 4900132000012210; EMBASE: 72098979] [Google Scholar]
- Vasbinder EC, Janssens HM, Rutten‐van Molken MP, Dijk L, Winter BC, Groot RC, et al. e‐Monitoring of asthma therapy to improve compliance in children using a real‐time medication monitoring system (RTMM): the e‐MATIC study protocol. BMC Medical Informatics and Decision Making 2013;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vollmer 2006 {published data only}
- Vollmer WM, Kirshner M, Peters D, Drane A, Stibolt T, Hickey T, et al. Use and impact of an automated telephone outreach system for asthma in a managed care setting. American Journal of Managed Care 2006;12:725‐33. [PubMed] [Google Scholar]
- Vollmer WM, Peters D, Kirshner M, Naleway A, Buist AS, Hickey T, et al. Use of a telephone triage system for asthma management [Abstract]. American Thoracic Society International Conference. 2005 May 20‐25; San Diego:[A40] [Poster: G15].
Wiecha 2007 {published data only}
- Wiecha JM, Adams WG. BostonBreathes: an RCT to improve pediatric asthma care with a home‐based interactive website for patient education, monitoring, and clinical teamwork. AMIA Annual Symposium Proceedings. 2007:1154. [PubMed]
- Wiecha JM, Adams WG, Rybin D, Rizzodepaoli M, Heller J, Clay JM. Evaluation of a web‐based asthma self‐management system: a randomised controlled pilot trial. BMC Pulmonary Medicine 2015;15(1):epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yun 2013 {published data only}
- Yun TJ, Arriaga RI. A text message a day keeps the pulmonologist away. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. 2013:1769‐78.
Zachgo 2002 {published data only}
- Zachgo W, Juenemann R, Halbich C, Hofmann N, Eleljanova S, Reiss Plagemann C, et al. Optimized computer‐based inhaled steroid therapy in asthma. American Journal of Respiratory and Critical Care Medicine 2002;165:A766. [Google Scholar]
Zairina 2015 {published data only}
- Zairina E, Abramson M, Stewart K, Mcdonald C, Walker S, Rochford P, et al. Management of asthma with supportive telehealth of respiratory function in pregnancy (the mastery trial). Respirology 2015;20(Suppl 2):135. [Google Scholar]
- Zairina E, Abramson MJ, McDonald CF, Li J, Dharmasiri T, Stewart K, et al. Study protocol for a randomised controlled trial evaluating the efficacy of a telehealth program ‐ management of asthma with supportive telehealth of respiratory function in pregnancy. BMC Pulmonary Medicine 2015;15(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zairina E, Abramson MJ, McDonald CF, Li J, Dharmasiri T, Stewart K, et al. Telehealth to improve asthma control in pregnancy: a randomized controlled trial. Respirology (Carlton, Vic.) 2016;21(5):867‐74. [CENTRAL: 1139850; CRS: 4900132000017642; PUBMED: 27037722] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ricci 2001 {published data only}
- Ricci A, Barosi G, Gelmetti A, Esposito R, Tzialla C, Napoli A, et al. A system of teleassistance for in‐house monitoring respiratory function in children with bronchial asthma. Minerva Pediatrica 2001;53:466‐7. [PubMed] [Google Scholar]
References to ongoing studies
Ahmed 2011 {published data only}
- Ahmed S, Bartlett SJ, Ernst P, Lin CJ, Pare G, Perreault R, et al. My asthma portal: preliminary results of a web‐based self management intervention [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183:A5321. [Google Scholar]
- Ahmed S, Bartlett SJ, Ernst P, Pare G, Kanter M, Perreault R, et al. Effect of a web‐based chronic disease management system on asthma control and health‐related quality of life: study protocol for a randomized controlled trial. Trials 2011;12:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perry 2015 {published data only}
- Perry TT, Halterman JS, Brown RH, Hunter CR, Randle SM, Tilford JM, et al. Breath connection: a school‐based telemedicine program for rural children with asthma. Journal of Allergy and Clinical Immunology 2015;135(2 Suppl 1):AB169. [Google Scholar]
Additional references
Atherton 2012
- Atherton H, Sawmynaden P, Sheikh A, Majeed A, Car J. Email for clinical communication between patients/caregivers and healthcare professionals. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007978.pub2] [DOI] [PubMed] [Google Scholar]
Australian Government Department of Health 2016
- Australian Government Department of Health. Telehealth Pilots Programme. http://www.health.gov.au/internet/main/publishing.nsf/Content/ehealth‐nbntelehealth‐pilots (accessed 7 June 2016).
BTS/SIGN 2014
- British Thoracic Society/Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. https://www.brit‐thoracic.org.uk/guidelines‐and‐quality‐standards/asthma‐guideline/ (accessed 12 March 2015).
Cash‐Gibson 2012
- Cash‐Gibson L, Felix LM, Minorikawa M, Pappas Y, Gunn LH, Majeed A, et al. Automated telephone communication systems for preventive healthcare and management of long‐term conditions. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD009921] [DOI] [PMC free article] [PubMed] [Google Scholar]
Craven 2015
- Craven VE, Morton RW, Spencer S, Devadason SG, Everard ML. Electronic monitoring and reminding devices for improving adherence to inhaled therapy in patients with asthma. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD011554] [DOI] [Google Scholar]
de Jongh 2012
- Jongh T, Gurol‐Urganci I, Vodopivec‐Jamsek V, Car J, Atun R. Mobile phone messaging for facilitating self‐management of long‐term illnesses. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD007459.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 2002
- Gibson PG, Powell H, Wilson A, Abramson MJ, Haywood P, Bauman A, et al. Self‐management education and regular practitioner review for adults with asthma. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD001117] [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2014
- Global Initiative for Asthma. Global strategy for asthma management and prevention. www.ginaasthma.org/local/uploads/files/GINA_Report_2014_Aug12.pdf (accessed 12 March 2015).
Global Asthma Report 2011
- International Union Against Tuberculosis and Lung Disease. The Global Asthma Report 2011. http://theunion.org/what‐we‐do/publications/technical/global‐asthma‐report (accessed 12 March 2015).
Government of the Netherlands 2016
- Government of the Netherlands. Government encouraging use of eHealth. https://www.government.nl/topics/ehealth/contents/government‐encouraging‐use‐of‐ehealth (accessed 7 June 2016).
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime Inc). GRADEpro Guideline Development Tool. Ontario: McMaster University (developed by Evidence Prime Inc), 2015.
Gurol‐Urganci 2013
- Gurol‐Urganci I, Jongh T, Vodopivec‐Jamsek V, Atun R, Car J. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD007458.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jaana 2009
- Jaan M, Paré G, Sicotte C. Home telemonitoring for respiratory conditions: a systematic review. American Journal of Managed Care 2009;15(5):313‐20. [PubMed] [Google Scholar]
Jannett 2003
- Jennett PA, Affleck Hall L, Hailey D, Ohinmaa A, Anderson C, Thomas R, et al. The socio‐economic impact of telehealth: a systematic review. Journal of Telemedicine and Telecare 2003;9(6):311‐20. [DOI] [PubMed] [Google Scholar]
Kew 2016
- Kew K, Cates C. Remote versus face‐to‐face check‐ups for asthma. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011715.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laver 2013
- Laver KE, Schoene D, Crotty M, George S, Lannin NA, Sherrington C. Telerehabilitation services for stroke. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD010255.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lustig 2012
- Lustig TA, Board on Health Care Services, Institute of Medicine. The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary. Washington, DC: The National Academies Press, 2012. [PubMed] [Google Scholar]
McLean 2010
- McLean S, Chandler D, Nurmatov U, Liu JLY, Pagliari C, Car J, et al. Telehealthcare for asthma. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD007717.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McLean 2011
- McLean S, Nurmatov U, Liu JLY, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD007718.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mclean 2013
- McLean S, Sheikh A, Cresswell K, Nurmatov U, Mukherjee M, Hemmi A, et al. The impact of telehealthcare on the quality and safety of care: a systematic overview. PLoS One 2013;8(8):e71238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Medicaid 2016
- Medicaid.gov. Delivery systems: telemedicine. https://www.medicaid.gov/medicaid‐chip‐program‐information/by‐topics/delivery‐systems/telemedicine.html (accessed 7 June 2016).
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
NRAD 2014
- Royal College of Physicians. Why asthma still kills: the national review of asthma deaths. https://www.rcplondon.ac.uk/sites/default/files/why‐asthma‐still‐kills‐full‐report.pdf (accessed 12 March 2015).
Pinnock 2007a
- Pinnock H, Slack R, Pagliari C, Price D, Sheikh A. Understanding the potential role of mobile phone‐based monitoring on asthma self‐management: qualitative study. Clinical and Experimental Allergy 2007;37:794‐802. [DOI] [PubMed] [Google Scholar]
Rada 2015
- Rada G. Telemedicine: are we advancing the science?[editorial]. Cochrane Database of Systematic Reviews 2015; Vol. 9. [DOI: 10.1002/14651858.ED000105] [DOI] [PMC free article] [PubMed]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Steventon 2012
- Steventon A, Bardsley M, Billings J, Dixon J, Doll H, Hirani S, et al. Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomised trial. BMJ 2012;344:e3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
UK Department of Health 2012
- Department of Health. Long Term Conditions Compendium of Information: Third Edition (2012). https://www.gov.uk/government/publications/long‐term‐conditions‐compendium‐of‐information‐third‐edition (accessed 12 March 2015).
UK Department of Health 2013
- Department of Health. A mandate from the Government to the NHS Commisioning Board: April 2013 to March 2015. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/256497/13‐15_mandate.pdf (accessed 12 March 2015).
Zhao 2014
- Zhao J, Zhai YK, Zhu WJ, Sun DX. Effectiveness of telemedicine for controlling asthma symptoms: a systematic review and meta‐analysis. Telemedicine Journal and e‐Health 2014;21(6):484‐92. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kew 2015
- Kew KM, Cates C. Asthma monitoring with remote feedback from a health professional. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011714] [DOI] [Google Scholar]


