Abstract
Vitamin D is a nutrient whose active form affects tissues as a hormone and possibly enhances performance. One plausible mechanism is by increasing testosterone concentration, which is established as an important factor for athletic performance. Therefore the aim of the study was to examine the relationship between plasma concentration of 25(OH)D and testosterone in Polish elite track and field athletes depending on vitamin D status, season, training period, body composition, sex, type of training, sun exposure and vitamin D supplementation. Plasma concentrations of 25(OH)D and testosterone were measured in all seasons within two years in athletes (70 females, 79 males) who represent strength (n = 103) and endurance (n = 46) kinds of sports, in the preparatorycompetitive season and transition period. There were no differences in 25(OH)D concentration between male and female athletes, insufficiency [25(OH)D < 30 ng/ml] was observed in 32.9%, whereas deficiency [25(OH)D < 20 ng/ ml] in 3.2%. Circannual rhythm was noted for vitamin D but not for testosterone concentration; no correlations between them were found either in strength or endurance athletes or between 25(OH)D and body composition. Testosterone concentration was higher in the transition period than in the preparatory-competition period only in male athletes. Higher 25(OH)D was observed in athletes who trained during winter in Africa (higher sun exposure) or used oral supplementation, whereas the respective testosterone levels were unchanged. In athletes, testosterone concentration did not reflect vitamin D status. The widespread of inadequate vitamin D status among athletes, makes it vital to recommend them the regular monitoring of 25(OH)D concentration and use of reasonable supplementation.
Keywords: Vitamin D status, Testosterone, Exercise, Athletes, Seasonal rhythm
INTRODUCTION
Vitamin D is a nutrient whose active form – calcitriol [1,25(OH)D] – affects tissues as a hormone not only responsible for calcium homeostasis but in many studies its pleiotropic, extraskeletal actions were described, constituting a link between its deficiency and many diseases and mortality [1–3]. Vitamin D status was evaluated on the basis of blood concentrations of 25(OH)D – 25-hydroxycholecalciferol – which is substrate for synthesis of the active form of vitamin D. Biological effects of vitamin D are mediated by the vitamin D receptor (VDR), a transcription factor that belongs to the same nuclear receptor family as testosterone. The presence of VDR and CYP27B1 (⍺-1 hydroxylase – the enzyme responsible for synthesis of the active form of vitamin D in kidneys) have been found in many cells of the reproductive system [4, 5]. Therefore, a potential influence of vitamin D on fertility is currently under debate [6]. In several studies involving older people, the relationship between vitamin D and testosterone concentration has been confirmed, but the association is strongest in deficient individuals [7–10].
The discovery of the presence of VDR in skeletal muscles raised interest in the possible role of vitamin D in muscle strength and function. Most randomized, controlled studies have confirmed such a relationship, but mainly in older subjects [11, 12]. For this purpose, vitamin D became popular in athletes, since some studies have confirmed its ergogenic effects on strength and endurance [13, 14]. Nevertheless, the majority of studies showed no influence of vitamin D on performance and more studies are needed to clarify that [15–18].
The main effect of vitamin D in muscles might be linked with satellite cell activity. Activation of VDR in satellite cells regulates cell fate decisions, whether it differentiates and divides or remains in the stem cell pool. In consequence the amount of type II muscle fibres is increased, and it transfers into greater speed and strength [19, 20]. The expression of IGF-1 is also increased after VDR activation, producing the hypertrophy and remodelling of myocytes [20]. The other mechanism of influence of VDR on muscles is a rapid nongenomic action by VDRs located in the muscle cell membrane and is linked with regulation of calcium ions influx into the muscle cell [19, 20].
Another potential influence of vitamin D on performance might be related to testosterone concentration [21, 22]. Testosterone undoubtedly enhances performance by increasing muscle growth, reducing body fat and improving psychological aspects (aggression, motivation, etc.), but its exogenous administration is prohibited in sport and clearly defined as doping [23–26]. Therefore, any practices which might legally promote endogenous testosterone concentration are cordially welcome by athletes. Despite lacking evidence, vitamin D is very popular among athletes as an ergogenic aid, frequently overdosed or prescribed by self-appointed experts [1, 27, 28]. The outcome of such practice is difficult to predict nowadays.
The data concerning the interaction between vitamin D and testosterone in athletes are scanty and equivocal. For example, in 45 soccer players the relationship between 25(OH)D and testosterone concentrations was confirmed [29], whereas in 50 hockey players it was not found [30].
The aim of the present study was to assess the relationship between plasma concentration of 25(OH)D and testosterone in Polish elite track and field athletes according to the vitamin D status, season, training period, body composition, sex, type of event performed, higher sun exposure in winter and vitamin D supplementation.
MATERIALS AND METHODS
Participants
The participants were recruited from the Polish national track and field team. In this retrospective, observational study, 284 samples were taken from 149 male and female elite athletes. The basic characteristics is presented in Table 1. All the athletes were Caucasians with skin type I-III according to Fitzpatrick’s scale [31].
TABLE 1.
Descriptive information of the athletes (n = 149).
| Females (n = 70) |
Males (n = 79) |
|||
|---|---|---|---|---|
| Strength (n = 47) | Endurance (n = 23) | Strength (n = 56) | Endurance (n = 23) | |
| Age [years] | 25.0 ± 0.3 | 25.0 ± 0.5 | 26.0 ± 0.3 | 26.0 ± 0.7 |
| Height [m] | 1.73 ± 0.01 | 1.70 ± 0.01 | 1.87 ± 0.01 | 1.81 ± 0.01 |
| Body mass [kg] | 64 ± 1 | 56 ± 1 | 90 ± 2 | 68 ± 1 |
| Body mass index [kg/m2] | 20.9 ± 0.5 | 19.1 ± 0.2 | 25.5 ± 0.5 | 20.7 ± 0.3 |
| Muscle mass [kg] | 27.2 ± 1.2 | 24.5 ± 0.9 | 40.2 ± 1.8 | 33.2 ± 1.5 |
| Fat mass [kg] | 15.3 ± 1.4 | 9.4 ± 1.0 | 13.8 ± 1.2 | 7.8 ± 0.5 |
| Fat percentage [%] | 20.9 ± 1.1 | 16.4 ± 1.1 | 18.4 ± 1.6 | 11.9 ± 1.1 |
The study was conducted according to the Declaration of Helsinki and was approved by the Committee on Bioethics at the Medical University of Warsaw (permission AKBE/111/15). Each subject had signed the consent form for routine medical monitoring, including the statement of agreement for the use of the results for scientific purposes. Because the study was retrospective neither written nor verbal consent for this particular study was obtained.
Training periods
Two training periods were distinguished: the transition period, when athletes do not train or compete (Sep-Nov), and the preparatorycompetitive period, when athletes train hard and compete (Dec-Aug). The usual training hours were 10 a.m. – 1 p.m. and 4 – 7 p.m. with weather-dependent sun exposure. During training abroad the daily sunshine exposure was longer than the one which provides recommended daily skin vitamin D synthesis [32].
Type of sports
The athletes were divided according to different types of athletic events based on character of training into strength (sprinters, jumpers and throwers) and endurance (distance runners and race walkers) groups.
Factors influencing vitamin D status
The following groups were distinguished for the purposes of the study: 0 (n = 110) – athletes who trained in Poland at the latitude of 49– 54° N; Sun (n = 42) – athletes exposed to sun during Polish winter (Jan-Mar) in the Republic of South Africa (RSA), at the latitude of 27° S and on Tenerife, at the latitude of 28° N, who were sampled within 1–4 weeks after their return to Poland; Supl (n = 51) – athletes with a deficit or insufficient vitamin D status who trained in Poland and were orally supplemented. The deficient athletes received individually adjusted vitamin D (cholecalciferol) supplementation of 4000–8000 IU daily, depending on the scale of deficiency, whereas the dose for the insufficient ones was established at 2000 IU in accordance with the experts’ guidelines for the general population in Poland and Central Europe and in athletes [28, 33]. The effectiveness of supplementation was evaluated after 8–12 weeks of treatment and only fully compliant athletes who confirmed strict follow-up of the prescribed doses were included in the Supl group.
Blood measurements
Fasting blood samples were collected in the morning from the antecubital vein into the clot activator tubes. Vitamin D status was evaluated on the basis of blood concentrations of 25(OH)D. The serum concentration of 25(OH)D was determined with the Liaison diagnostic system (DiaSorin, Stillwater, MN, USA) by a chemiluminescent immunoassay (CLIA); range of detection 4–150 ng/ml, precision 5.0% CV, accuracy SD 1.2.
In the present study the following serum 25(OH)D concentration criteria were assumed: < 20 ng/ml was defined as deficiency, 21–29 ng/ml as insufficiency, 30–50 ng/ml as normal, 50–100 ng/ml as high and > 100 ng/ml as toxic [32–34]. Deficiency and insufficiency were classified as inadequate vitamin D status.
Testosterone concentration was assessed with the electrochemiluminescent method on a Cobas analyser using Roche commercial kits.
Statistical analyses
The obtained data were analysed for normality of distribution with Shapiro-Wilk’s test and when the data were not normally distributed Wilcoxon’s test was used to compare groups. The within-subject (repeated measures, ANOVA) approach was used to examine the effect of the training periods and circannual rhythm on concentrations of 25(OH)D and testosterone. Analyses in pairs were conducted in subjects who were measured before and after the training camp abroad, during summer in Poland and after winter sun exposure abroad and before and after oral supplementation. The relationship between concentrations of 25(OH)D and testosterone were analysed on combined data using the Pearson correlation coefficient. The values are shown as mean ± SE and a P value less than 0.05 was considered statistically significant. Statistical analyses were performed using the Statistica 6 software.
RESULTS
There were no differences in 25(OH)D concentration between male and female athletes. The annual average 25(OH)D concentration was 36.0 ± 12.4 ng/ml, vitamin D insufficiency [25(OH)D < 30 ng/ml] was observed in 32.9% of athletes, whereas deficiency [25(OH)D < 20 ng/ml] in 3.2%.
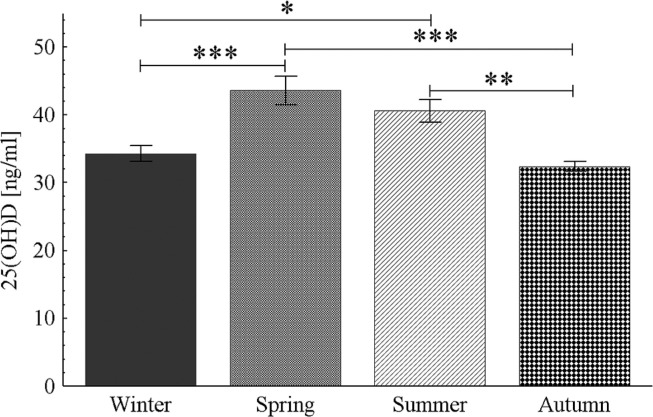
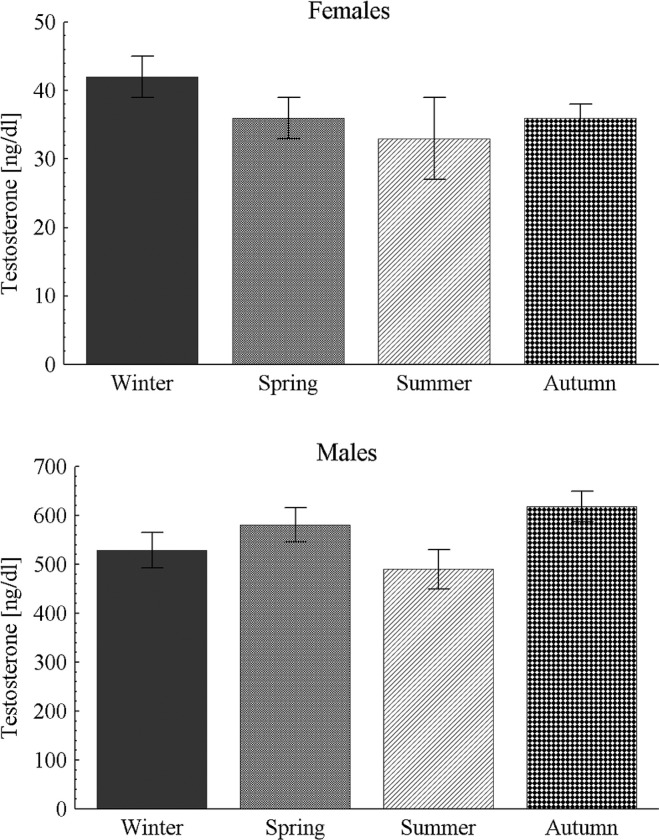
The seasonal pattern of 25(OH)D concentration was confirmed – significantly higher in summer than in autumn (p < 0.01) and winter (p < 0.05) and in spring than in autumn (p < 0.001) and winter (p < 0.01, Figure 1). There were no seasonal differences in plasma testosterone concentrations in both female and male athletes (Figure 2).
FIG. 1.
Seasonal pattern of 25(OH)D concentration in blood in all athletes;
* p < 0.05, ** p < 0.01, *** p < 0.001.
FIG. 2.
Seasonal pattern of testosterone concentration in blood in female and male athletes.
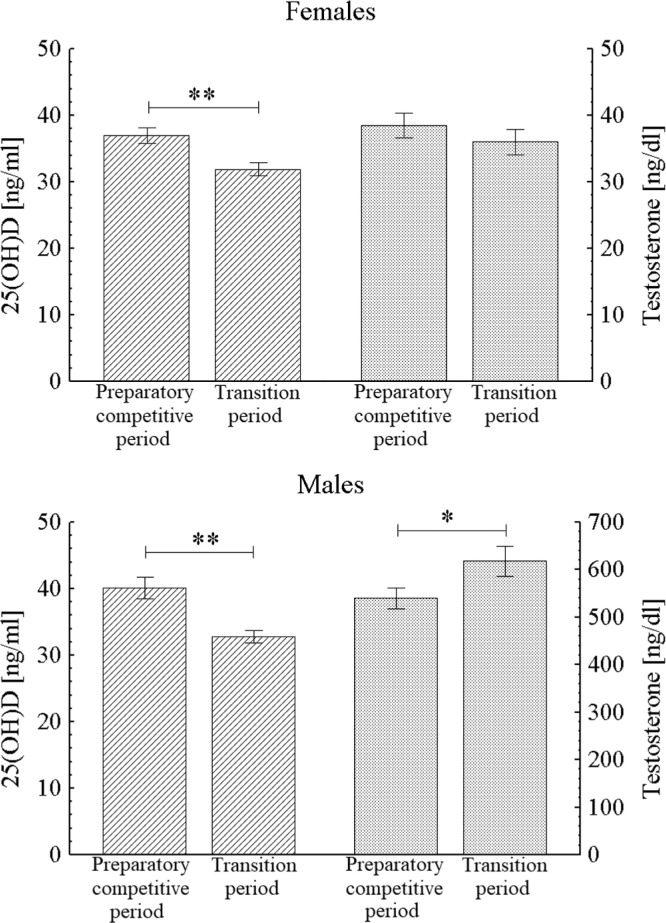
Plasma concentration of 25(OH)D was significantly higher in the samples collected during the training and competition months Dec-Aug (preparatory-competitive period) than recovery months Sep-Nov (transition period) in both male (40.1 ± 1.6 ng/ml vs. 32.8 ± 0.9 ng/ml, p < 0.01) and female (37.0 ± 1.2 ng/ml vs. 31.9 ± 1.0 ng/ml, p < 0.01) athletes. The testosterone concentration was significantly lower during the preparatory-competitive period only in male athletes, 539.8 ± 21.7 ng/dl vs. 618.1 ± 31.2 ng/dl in transition period, p < 0.05, whereas in females it was 38.5 ± 1.9 ng/dl vs. 36.0 ± 1.9 ng/dl, respectively, n.s. (Figure 3).
FIG. 3.
Blood concentration of 25(OH)D and testosterone during the training and competition months Dec-Aug (preparatorycompetitive period) and recovery months Sep-Nov (transition period) in male and female athletes; * p < 0.05, ** p < 0.01.
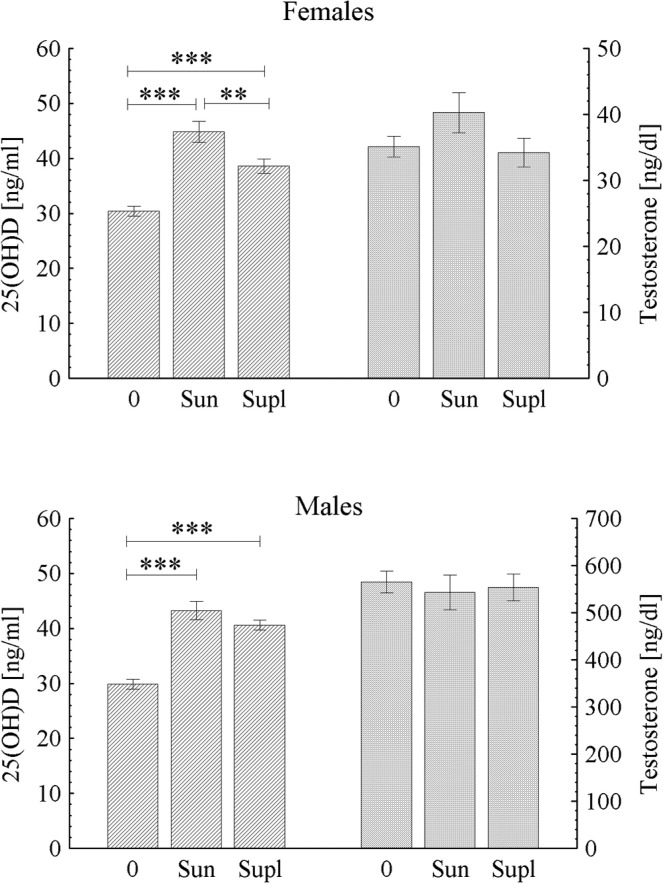
A significantly higher 25(OH)D concentration was observed both after the sun exposure in winter (Sun) and oral supplementation (Supl) than in non-supplemented athletes who trained during winter in Poland (0), whereas testosterone levels were not influenced (Figure 4).
FIG. 4.
Blood concentration of 25(OH)D and testosterone in female and male athletes who trained during winter in Poland (0), after the sun exposure in winter (Sun) and oral supplementation (Supl); ** p < 0.01, *** p < 0.001.
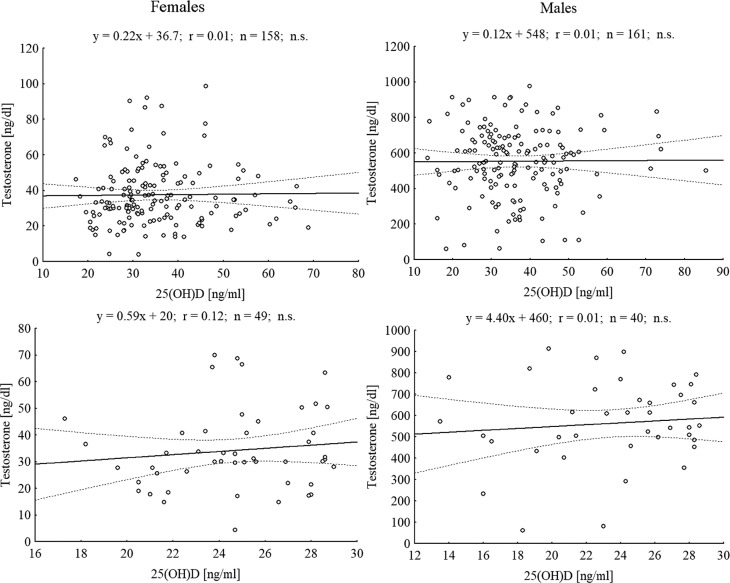
For the whole year in both female and male athletes there were no significant correlations between 25(OH)D and testosterone, as well as for the athletes with inadequate vitamin D status [25(OH)D below 30 ng/ml] separately (Figure 5). There were also no significant correlations between 25(OH)D and testosterone concentrations in either female or male athletes in each season separately, in strength or endurance disciplines separately, according to BMI and muscle mass, in athletes with proper vitamin D status [25(OH)D over 30 ng/ml] only and both in preparatory-competitive and transition periods. No correlation between percentage of body fat or muscle mass and testosterone concentration was found in either female or male athletes.
FIG. 5.
Correlations between 25(OH)D and testosterone for the whole year in female and male athletes in all subjects (top) and in athletes with inadequate vitamin D status [25(OH)D below 30 ng/ml] separately (bottom).
DISCUSSION
The recent changes of lifestyle, namely more time spent indoors and use of clothes and sunscreens, resulted in common vitamin D deficiency all over the world [35, 36]. The results obtained in the present study revealed an inadequate status of vitamin D in 30% of Polish elite athletes (annual average), which is similar to the values described in the sedentary population and in other athletes [37–40].
In the present study the seasonal rhythm of 25(OH)D concentration was observed with higher values in spring and summer. It is consistent with the data available in the literature for countries situated above the latitude of 40° N (Poland is situated in a moderate climate/latitude between 49° and 54° N) for both athletes and the rest of the population [39, 41–45]. The significant seasonal differences in vitamin D status emphasize the importance of the availability of ultraviolet B (UVB) radiation originating from the sun, which is mostly influenced by the latitude. The higher 25(OH)D concentration observed in the present study in spring results from the training camps at the lower latitude in RSA and Tenerife in which most athletes participate. Those locations are closer to the equator than Poland by approx. 21°, which proved to be a much stronger stimulus for vitamin D synthesis than the sun during summer in Poland. This is consistent with most studies in which the inverse correlation between latitude and 25(OH)D concentration was described (the average 25(OH)D concentration near the equator is 40 ng/ml, whereas in the far north or south it is just 15 ng/ml) [46, 47] and with our previous data [39]. The mechanism of more efficient vitamin D synthesis at lower latitudes results from better availability of UVB radiation due to a more favourable zenith angle.
In the present paper no seasonal pattern in testosterone concentration was observed. Similar findings were described in Finland (24 young adults sampled monthly), Belgium (5028 men aged 50–70 sampled over 3 years), California (915 old men), Boston (121 men, different races, 6 samples from each subject) and Southwest US (11000 patients grouped by months and seasons) [48–52]. Nevertheless, in several articles a seasonal pattern of testosterone concentration was identified, but there was no consistency in the months of peak and nadir [53–58]. Other authors have concluded that in the general population testosterone concentration depends more on age of subjects, sleep patterns (diurnal manner that is dependent on sleep) and region (the length of day and temperature) than on seasonal rhythm [59, 60]. Sim et al. even linked the higher testosterone observed in Koreans in winter with seasonal dietary variation in consumption of seafood, affecting via zinc the male reproductive function [58]. Summing up the available literature, any seasonal variations in testosterone can only be characterized as inconsistent and occur in a specific population when highly influenced by one factor [61]. It is also hypothesized that higher testosterone observed in summer is linked with greater physical activity undertaken at that time.
Exercise is a potent stimulus for the production of testosterone and the hypothalamo-pituitary-gonadal axis was defined as more sensitive in younger men due to their higher level of physical activity [59, 62].
Studies on the seasonal rhythm of testosterone in athletes are very limited. Martinez et al. studied 12 Spanish basketball players and sampled testosterone in pre-season (October), while training (December) and in competition (March-April). A higher concentration of testosterone was observed in the competition period, when athletes have decreased the training volume [63]. Lombardi et al. in 167 Italian soccer players have found the peak testosterone concentration in June, when athletes were on holiday, supporting the hypothesis that testosterone depends mostly on exercise load [29].
In the present study testosterone concentration was higher only in male athletes during the transition period with no training or competition (September-November), which also supports the above-mentioned hypothesis that in athletes the testosterone concentration is mostly affected by the training loads and this effect overlaps (if any are present) seasonal fluctuations. It can be concluded that leisure time physical activity promotes testosterone production, whereas strenuous professional training decreases it. Similar findings comparing the influence of exercise were obtained in several other studies [64–70].
Studies on women’s seasonal variations in testosterone are rare and show conflicting results; most of the available literature refers to male subjects [71–73]. The lack of significant differences observed in female athletes in the present study might be due to high variations within the menstrual cycle and use of hormonal contraceptives [74–75]. Those factors were not considered at the time of blood sampling and the majority of female subjects do not menstruate on a regular basis.
The relationship between vitamin D status and concentration of androgens and fertility was studied by both observational and interventional approaches, mainly in older subjects [6]. The European Male Ageing Study (3369 men in eight European countries) showed a positive correlation between 25(OH)D concentrations and total and free testosterone [76]. In a large cross-sectional study of 2299 men, higher total testosterone concentration and free androgen index were significantly higher in vitamin D-sufficient compared with vitamin D-insufficient or -deficient men [9]. Data from other cross-sectional studies have also shown a positive association between 25(OH)D and testosterone concentrations [7, 8, 77]. The opposite results were obtained by Sim et al., who confirmed the seasonal pattern for vitamin D but not for testosterone in 1559 Korean males. Moreover, no relationship between 25(OH)D and testosterone was found [58].
Authors of all the studies mentioned above discuss the limitation of heterogeneity of subjects and multiple factors affecting the androgen level: age, BMI, smoking, alcohol, physical activity and chronic diseases. It is possible that vitamin D deficiency and low testosterone coincide as epiphenomenon in subjects with poor health condition or even the reverse causality theory could be considered [78].
Interventional studies which assessed the relationship between vitamin D supplementation and testosterone concentration also showed inconsistent results. In studies where positive results were obtained vitamin D supplementation was accompanied by body mass reduction by diet and physical activity, which themselves are strong factors increasing the testosterone concentration [10, 79]. It is speculated that the effect of supplementation depends on the primary 25(OH)D concentration and is more pronounced in deficient subjects. In other studies no relationship was observed, either in sedentary or physically active subjects [80–82]. There is some evidence of the positive influence of vitamin D status on sperm motility [83, 84], as well as the effect of its supplementation on semen quality and male fertility [85, 86].
The two studies available in athletes also show the opposite results. In 50 young male ice hockey players examined in October at the latitude 50° N 25(OH)D concentration did not correlate with testosterone. Serum 25(OH)D concentration was also not associated with testosterone after adjusting for age, fat free mass and fat mass [30]. In Italian professional soccer players in latitude 41° to 42° N a seasonal pattern for both vitamin D and testosterone was found with a peak in August and June, respectively. Moreover, a weak but significant correlation between 25(OH)D and testosterone was detected [29]. The peak in testosterone occurred in the off-competition season. Therefore, the relationship could be accidental, as the seasonal rhythm of vitamin D depends on sun, whereas testosterone depends mostly on the training loads.
In the present study no relationship between 25(OH)D and testosterone was observed, either in athletes with adequate vitamin D status or in deficient ones, or according to the BMI or body composition. The seasonal pattern of vitamin D concentration was not reflected in testosterone and there was also no such relationship in athletes exposed to sunshine in winter and supplemented with vitamin D. Therefore, it supports the hypothesis that vitamin D status does not influence the testosterone concentration and any performanceenhancing effects of vitamin D in athletes are unlikely to be primarily mediated through testosterone. If vitamin D improves performance, it is more likely achieved by genomic and nongenomic signalling through the VDR in cardiac and skeletal muscles [30].
Concerning the current position of vitamin D in sport, primarily main health benefits should be considered, such as maintenance of immune function and musculoskeletal protection, and not its ergogenic effects. The results of the present study did not provide evidence that vitamin D influences testosterone concentration. The use of extreme doses by athletes does not improve performance; moreover, it can produce opposite effects, since there is evidence that not only low, but also high vitamin D concentrations are associated with impaired health, for example increased fall frequency, mortality, decreased sperm quality and suppression of calcitriol synthesis [87–90]. The proper understanding of the significance of the vitamin D status is a great challenge for sport medicine practitioners and nutritionists, who should provide athletes with personalized, safe supplementation protocols based on the guidelines established by real experts, not on myths and opinions of self-appointed experts. The results of the present study might be helpful to achieve that.
CONCLUSIONS
A circannual rhythm was present for vitamin D but not for testosterone. No relationship between vitamin D status and testosterone was found in track and field athletes.
Vitamin D insufficiency was present in 33% of Polish elite athletes, irrespectively from sex. The significant widespread of inadequate vitamin D status among athletes, who trained in Poland at the latitude of 49-54˚N and mostly outdoor, makes it vital to recommend to all athletes the regular monitoring of 25(OH)D concentration and use of reasonable supplementation.
Conflict of interest
The authors report no conflict of interest.
REFERENCES
- 1.Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, Shoenfeld Y, Lerchbaum E, Llewellyn DJ, Kienreich K, Soni M. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality – a review of recent evidence. Autoimmun Rev. 2013;12(10):976–89. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Gholami F, Moradi G, Zareei B, Rasouli MA, Nikkhoo B, Roshani D, Ghaderi E. The association between circulating 25-hydroxyvitamin D and cardiovascular diseases: a meta-analysis of prospective cohort studies. BMC Cardiovasc Disord. 2019;19(1):248. doi: 10.1186/s12872-019-1236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, Faramand A. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:I4673. doi: 10.1136/bmj.l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blomberg Jensen M, Nielsen JE, Jorgensen A, Rajpert-De Meyts E, Kristensen DM, Jorgensen N, Skakkebaek NE, Juul A, Leffers H. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010;25(5):1303–11. doi: 10.1093/humrep/deq024. [DOI] [PubMed] [Google Scholar]
- 5.Anagnostis P, Karras S, Goulis DG. Vitamin D in human reproduction: A narrative review. Int J Clin Pract. 2013;67:225–235. doi: 10.1111/ijcp.12031. [DOI] [PubMed] [Google Scholar]
- 6.Bosdou JK, Konstantinidou E, Anagnostis P, Kolibianakis EM, Goulis DG. Vitamin D and Obesity: Two Interacting Players in the Field of Infertility. Nutrients. 2019;11(7):1455. doi: 10.3390/nu11071455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anic GM, Albanes D, Rohrmann S, Kanarek N, Nelson WG, Bradwin G, Rifai N, McGlynn KA, Platz EA, Mondul AM. Association between serum 25-hydroxyvitamin D and serum sex steroid hormones among men in NHANES. Clin Endocrinol. 2016;85(2):258–66. doi: 10.1111/cen.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimptsch K, Platz EA, Willett WC, Giovannucci E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin Endocrinol. 2012;77(1):106–12. doi: 10.1111/j.1365-2265.2012.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wehr E, Pilz S, Boehm BO, Marz W, Obermayer-Pietsch B. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol. 2010;73(2):243–8. doi: 10.1111/j.1365-2265.2009.03777.x. [DOI] [PubMed] [Google Scholar]
- 10.Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, Obermayer-Pietsch B, Wehr E, Zittermann A. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011;43:223–225. doi: 10.1055/s-0030-1269854. [DOI] [PubMed] [Google Scholar]
- 11.Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, Petermans J, Reginster JY, Bruyère O. The effects of vitamin D on skeletal muscle strength, muscle mass and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2014;99(11):4336–45. doi: 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- 12.Rejnmark L. Effects of vitamin D on muscle function and performance: a review of evidence from randomized controlled trials. Ther Adv Chronic Dis. 2011;2(1):25–37. doi: 10.1177/2040622310381934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Close GL, Russell J, Cobley JN, Owens DJ, Wilson G, Fraser WD, Morton JP. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: implications for skeletal muscle function. J Sports Sci. 2013;31:344–53. doi: 10.1080/02640414.2012.733822. [DOI] [PubMed] [Google Scholar]
- 14.Koundourakis NE, Androulakis NE, Malliaraki N, Margioris AN. Relation of vitamin D level to maximal oxygen uptake in adults. Am J Cardiol. 2011;107:1246–9. doi: 10.1016/j.amjcard.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Close GL, Leckey J, Patterson M, Bradley W, Owens DJ, Fraser WD, Morton JP. The effects of vitamin D3 supplementation on serum total 25[OH]D concentration and physical performance: a randomised dose-response study. Br J Sports Med. 2013;47:692–6. doi: 10.1136/bjsports-2012-091735. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald J, Peterson B, Warpeha J, Wilson P, Rhodes G, Ingraham S. Vitamin D status and VO2peak during a skate treadmill graded exercise test in competitive ice hockey players. J Strength Cond Res. 2014;28:3200–5. doi: 10.1519/JSC.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 17.Forney L, Earnest CC, Henagan T, Johnson L, Castleberry T, Stewart L. Vitamin D Status, Body Composition, and Fitness Measures in College-Aged Students. J Strength Cond Res. 2014;28:814–24. doi: 10.1519/JSC.0b013e3182a35ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todd JJ, McSorley EM, Pourshahidi LK, Madigan SM, Eamon Laird E, Healy M, Magee PJ. Vitamin D3 supplementation using an oral spray solution resolves deficiency but has no effect on VO2 max in Gaelic footballers: results from a randomised, double-blind, placebocontrolled trial. Eur J Nutr. 2017;56(4):1577–87. doi: 10.1007/s00394-016-1202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton B. Vitamin D and Human Skeletal Muscle. Scand J Med Sci Sports. 2010;20(2):182–190. doi: 10.1111/j.1600-0838.2009.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsson K, Saini A, Stromberg A, Alam S, Lilja M, Rullman E, Gustafsson T. Evidence for vitamin D receptor expression and direct effects of 1alpha,25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology. 2016;157:98–111. doi: 10.1210/en.2015-1685. [DOI] [PubMed] [Google Scholar]
- 21.Dahlquist DT, Dieter BP, Koehle MS. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J Int Soc Sports Nutr. 2015;12:33. doi: 10.1186/s12970-015-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pojednic RM, Ceglia L. The emerging biomolecular role of vitamin D in skeletal muscle. Exerc Sport Sci Rev. 2014;42:76–81. doi: 10.1249/JES.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood RI, Stanton SJ. Testosterone and sport: current perspectives. Horm Behav. 2012;61(1):147–55. doi: 10.1016/j.yhbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J, Casaburi R, Bhasin S. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88(4):1478–85. doi: 10.1210/jc.2002-021231. [DOI] [PubMed] [Google Scholar]
- 25.Hermans EJ, Putman P, Baas JM, Gecks NM, Kenemans JL, van Honk J. Exogenous testosterone attenuates the integrated central stress response in healthy young women. Psychoneuroendocrinology. 2007;32(8–10):1052–61. doi: 10.1016/j.psyneuen.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA. Testosterone release and social context: when it occurs and why. Front Neuroendocrinol. 2009;30(4):460–69. doi: 10.1016/j.yfrne.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Owens DJ, Allison R, Close GL. Vitamin D and the athlete: Current perspectives and new challenges. Sports Med. 2018;48:3–16. doi: 10.1007/s40279-017-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, Rawson ES, Walsh NP, Garthe I, Geyer H, Meeusen R, van Loon L, Shirreffs SM, Spriet LL, Stuart M, Vernec A, Currell K, Ali VM, Budgett RGM, Ljungqvist A, Mountjoy M, Pitsiladis Y, Soligard T, Erdener U, Engebretsen L. IOC consensus statement: Dietary supplements and the high-performance athlete. Br J Sports Med. 2018;52:439–55. doi: 10.1136/bjsports-2018-099027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lombardi G, Vitale JA, Logoluso S, Logoluso G, Cocco N, Cocco G, Banfi G. Circannual rhythm of plasmatic vitamin D levels and the association with markers of psychophysical stress in a cohort of Italian professional soccer players. Chronobiol Int. 2017;34(4):471–9. doi: 10.1080/07420528.2017.1297820. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald JS, Orysiak J, Wilson PB, Mazur-Różycka J, Obminski Z. Association between vitamin D status and testosterone and cortisol in ice hockey players. Biol Sport. 2018;35(3):207–13. doi: 10.5114/biolsport.2018.74631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–71. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 32.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97:1153–58. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- 33.Płudowski P, Karczmarewicz E, Bayer M, Carter G, Chlebna-Sokół D, Czech-Kowalska J, Dębski R, Decsi T, Dobrzańska A, Franek E, Głuszko P, Grant WB, Holick MF, Yankovskaya L, Konstantynowicz J, Książyk JB, Księżopolska-Orłowska K, Lewiński A, Litwin M, Lohner S, Lorenc RS, Lukaszkiewicz J, Marcinowska-Suchowierska E, Milewicz A, Misiorowski W, Nowicki M, Povoroznyuk V, Rozentryt P, Rudenka E, Shoenfeld Y, Socha P, Solnica B, Szalecki M, Tałałaj M, Varbiro S, Żmijewski MA. Practical guidance for the supplementation of vitamin D and the treatment of deficits in Central Europe – recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol Pol. 2013;64:319–27. doi: 10.5603/ep.2013.0012. [DOI] [PubMed] [Google Scholar]
- 34.Romagnoli E, Pepe J, Piemonte S, Cipriani C, Minisola S. Management of endocrine disease: value and limitations of assessing vitamin D nutritional status and advised levels of vitamin D supplementation. Eur J Endocrinol. 2013;169(4):59–69. doi: 10.1530/EJE-13-0435. [DOI] [PubMed] [Google Scholar]
- 35.Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, Pierroz DD, Weber P, Hoffmann K. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111(1):23–45. doi: 10.1017/S0007114513001840. [DOI] [PubMed] [Google Scholar]
- 36.Cashman KD, Dowling KG, Skrabakova Z, Gonzalez-Gross M, Valtueña J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Mølgaard C, Jorde R, Grimnes G, Moschonis G, Mavrogianni C, Manios Y, Thamm M, Mensink GB, Rabenberg M, Busch MA, Cox L, Meadows S, Goldberg G, Prentice A, Dekker JM, Nijpels G, Pilz S, Swart KM, van Schoor NM, Lips P, Eiriksdottir G, Gudnason V, Cotch MF, Koskinen S, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT, Kiely M. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103:1033–44. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farrokhyar F, Tabasinejad R, Dao D, Peterson D, Ayeni O, Hadioonzadeh R, Bhandari M. Prevalence of vitamin D inadequacy in athletes: A systematic- review and meta-analysis. Sport Med. 2014;5:365–78. doi: 10.1007/s40279-014-0267-6. [DOI] [PubMed] [Google Scholar]
- 38.Bezuglov E, Tikhonova A, Zueva A, Khaitin V, Waśkiewicz Z, Gerasimuk D, Żebrowska A, Rosemann T, Nikolaidis P, Knechtle B. Prevalence and Treatment of Vitamin D Deficiency in Young Male Russian Soccer Players in Winter. Nutrients. 2019;11(10):2405. doi: 10.3390/nu11102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krzywanski J, Mikulski T, Krysztofiak H, Mlynczak M, Gaczynska E, Ziemba A. Seasonal Vitamin D Status in Polish Elite Athletes in Relation to Sun Exposure and Oral Supplementation. PLoS One. 2016;11(10):e0164395. doi: 10.1371/journal.pone.0164395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morton JP, Iqbal Z, Drust B, Burgess D, Close GL, Brukner PD. Seasonal variation in vitamin D status in professional soccer players of the English Premier League. Appl Physiol Nutr Metab. 2012;37:798–802. doi: 10.1139/h2012-037. [DOI] [PubMed] [Google Scholar]
- 41.Yeum K-J, Song BC, Joo N-S. Impact of Geographic Location on Vitamin D Status and Bone Mineral Density. Int J Environ Res and Public Health. 2016;13(2):184. doi: 10.3390/ijerph13020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sorensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br J Nutr. 2001;86(Suppl 1):S97–103. doi: 10.1079/bjn2001345. [DOI] [PubMed] [Google Scholar]
- 43.Barcal J, Thomas J, Hollis B, Austin K, Alexander B, Larson-Meyer D. Vitamin D and Weight Cycling: Impact on Injury, Illness, and Inflammation in Collegiate Wrestlers. Nutrients. 2016;8(12):E775. doi: 10.3390/nu8120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galan F, Ribas J, Sanchez-Martinez PM, Calero T, Sanchez AB, Munoz A. Serum 25-hydroxyvitamin D in early autumn to ensure vitamin D sufficiency in mid-winter in professional football players. Clin Nutr. 2012;31(1):132–6. doi: 10.1016/j.clnu.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Backx E, van der Avoort C, Tieland M, Maase K, Kies A, van Loon L, de Groot L, Mensink M. Seasonal variation in vitamin D status in elite athletes: a longitudinal study. Int J Sport Nutr Exerc Metab. 2017;27:6–10. doi: 10.1123/ijsnem.2016-0177. [DOI] [PubMed] [Google Scholar]
- 46.Grigalavicius M, Moan J, Dahlback A, Juzeniene A. Vitamin D and ultraviolet phototherapy in Caucasians. J Photochem Photobiol B. 2015;147:69–74. doi: 10.1016/j.jphotobiol.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Wacker M, Holick MF. Sunlight and Vitamin D: A global perspective for health. Dermatoendocrinol. 2013;5(1):51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martikainen H, Tapanainen J, Vakkuri O, Leppäluoto J, Huhtaniemi I. Circannual concentrations of melatonin, gonadotrophins, prolactin and gonadal steroids in males in a geographical area with a large annual variation in daylight. Acta Endocrinol (Copenh) 1985;109(4):446–50. doi: 10.1530/acta.0.1090446. [DOI] [PubMed] [Google Scholar]
- 49.Tancredi A, Reginster JY, Luyckx F, Legros JJ. No major month to month variation in free testosterone levels in aging males. Minor impact on the biological diagnosis of ‘andropause’. Psychoneuroendocrinol. 2005;30(7):638–46. doi: 10.1016/j.psyneuen.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Svartberg J, Barrett-Connor E. Could seasonal variation in testosterone levels in men be related to sleep? Aging Male. 2004;7(3):205–10. doi: 10.1080/13685530412331284696. [DOI] [PubMed] [Google Scholar]
- 51.Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Lack of seasonal variation in serum sex hormone levels in middle-aged to older men in the Boston area. J Clin Endocrinol Metab. 2007;92(11):4224–9. doi: 10.1210/jc.2007-1303. [DOI] [PubMed] [Google Scholar]
- 52.Moskovic DJ, Eisenberg ML, Lipshultz LI. Seasonal fluctuations in testosterone: estrogen ratio in men from the Southwest United States. J Androl. 2012;33(6):1298–304. doi: 10.2164/jandrol.112.016386. [DOI] [PubMed] [Google Scholar]
- 53.Meriggiola MC, Noonan EA, Paulsen CA, Bremner WJ. Annual patterns of luteinizing hormone, follicle stimulating hormone, testosterone and inhibin in normal men. Hum Reprod. 1996;11(2):248–52. doi: 10.1093/humrep/11.2.248. [DOI] [PubMed] [Google Scholar]
- 54.Valero-Politi J, Fuentes-Arderiu X. Annual rhythmic variations of follitropin,lutropin, testosterone and sex-hormone-binding globulin in men. Clin Chim Acta. 1998;271(1):57–71. doi: 10.1016/s0009-8981(97)00239-8. [DOI] [PubMed] [Google Scholar]
- 55.Svartberg J, Jorde R, Sundsfjord J, Bonaa KH, Barrett-Connor E. Seasonal variation of testosterone and waist to hip ratio in men: the Tromso study. J Clin Endocrinol Metab. 2003;88(7):3099–104. doi: 10.1210/jc.2002-021878. [DOI] [PubMed] [Google Scholar]
- 56.Andersson AM, Carlsen E, Petersen JH, Skakkebaek NE. Variation in levels of serum inhibin B, testosterone, estradiol, luteinizing hormone, follicle stimulating hormone and sex hormone-binding globulin in monthly samples from healthy men during a 17-month period: possible effects of seasons. J Clin Endocrinol Metab. 2003;88(2):932–7. doi: 10.1210/jc.2002-020838. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez-Sales M, Barriere O, Tremblay PO, Nekka F, Desrochers J, Tanguay M. Modeling testosterone circadian rhythm in hypogonadal males: effect of age and circannual variations. AAPS J. 2016;18:217–27. doi: 10.1208/s12248-015-9841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sim MY, Kim SH, Kim KM. Seasonal Variations and Correlations between Vitamin D and Total Testosterone Levels. Korean J Fam Med. 2017;38(5):270–5. doi: 10.4082/kjfm.2017.38.5.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stokes KA, Gilbert KL, Hall GM, Andrews RC, Thompson D. Different responses of selected hormones to three types of exercise in young men. Eur J Appl Physiol. 2013;113(3):775–83. doi: 10.1007/s00421-012-2487-5. [DOI] [PubMed] [Google Scholar]
- 60.Wittert G. The relationship between sleep disorders and testosterone in men. Asian J Androl. 2014;16(2):262–5. doi: 10.4103/1008-682X.122586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith RP, Coward RM, Kovac JR, Lipshultz LI. The evidence for seasonal variations of testosterone in men. Maturitas. 2013;74:208–12. doi: 10.1016/j.maturitas.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Tenover JS, Bremner WJ. The effects of normal aging on the response of the pituitary-gonadal axis to chronic clomiphene administration in men. J Androl. 1991;12(4):258–63. [PubMed] [Google Scholar]
- 63.Martínez AC, Seco Calvo J, Tur Marí JA, Abecia Inchaurregui LC, Orella EE, Biescas AP. Testosterone and cortisol changes in professional basketball players through a season competition. J Strength Cond Res. 2010;24(4):1102–8. doi: 10.1519/JSC.0b013e3181ce2423. [DOI] [PubMed] [Google Scholar]
- 64.Jensen AE, Arrington LJ, Turcotte LP, Kelly KR. Hormonal balance and nutritional intake in elite tactical athletes. Steroids. 2019;152:108504. doi: 10.1016/j.steroids.2019.108504. [DOI] [PubMed] [Google Scholar]
- 65.Cadegiani FA, Kater CE. Hypothalamicpituitary-adrenal (HPA) axis functioning in overtraining syndrome: findings from endocrine and metabolic responses on overtraining syndrome (EROS) – EROSHPA axis. Sports Med Open. 2017;3(1):45. doi: 10.1186/s40798-017-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hackney AC, Fahrner CL, Stupnicki R. Reproductive hormonal responses to maximal exercise in endurance trained men with low testosterone levels. Exp Clin Endocrinol Diabetes. 1997;105:291–5. doi: 10.1055/s-0029-1211767. [DOI] [PubMed] [Google Scholar]
- 67.Wheeler GD, Sing M, Pierce WD, Epling WF, Cumming DC. Endurance training decreases serum testosterone levels in men without change in luteinizing hormone pulsatile release. J Clin Endocrinol Metab. 1991;72:422–5. doi: 10.1210/jcem-72-2-422. [DOI] [PubMed] [Google Scholar]
- 68.Hackney AC. The male reproductive system and endurance exercise. Med Sci Sports Exerc. 1996;28:180–9. doi: 10.1097/00005768-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Duclos M, Corcuff JB, Rashedi M, Fougere V, Manier G. Does functional alteration of the gonadotropic axis occur in endurance trained athletes during and after exercise: a preliminary study. Eur J Appl Physiol. 1996;73:427–33. doi: 10.1007/BF00334419. [DOI] [PubMed] [Google Scholar]
- 70.Lucía A, Díaz B, Hoyos J, Fernández C, Villa G, Bandrés F, Chicharro JL. Hormone levels of world class cyclists during the Tour of Spain stage race. Br J Sports Med. 2001;35(6):424–30. doi: 10.1136/bjsm.35.6.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stanton SJ, Mullette-Gillman OA, Huettel SA. Seasonal variation of salivary testosterone in men, normally cycling women, and women using hormonal contraceptives. Physiol Behav. 2011;104(5):804–8. doi: 10.1016/j.physbeh.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Anders SM, Hampson E, Watson NV. Seasonality, waist-to-hip ratio, and salivary testosterone. Psychoneuroendocrinology. 2006;31(7):895–899. doi: 10.1016/j.psyneuen.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Moffat SD, Hampson E. Salivary testosterone concentrations in left-handers: an association with cerebral language lateralization? Neuropsychology. 2000;14(1):71–81. [PubMed] [Google Scholar]
- 74.Edwards DA, O’Neal JL. Oral contraceptives decrease saliva testosterone but do not affect the rise in testosterone associated with athletic competition. Hormones and Behavior. 2009;56(2):195–8. doi: 10.1016/j.yhbeh.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 75.Farland LV, Mu F, Eliassen AH, Hankinson SE, Tworoger SS, Barbieri RL, Dowsett M, Pollak MN, Missmer SA. Menstrual cycle characteristics and steroid hormone, prolactin, and growth factor levels in premenopausal women. Cancer Causes Control. 2017;28(12):1441–52. doi: 10.1007/s10552-017-0971-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee DM, Tajar A, Pye SR, Boonen S, Vanderschueren D, Bouillon R, O’Neill TW, Bartfai G, Casanueva FF, Finn JD, Forti G, Giwercman A, Han TS, Huhtaniemi IT, Kula K, Lean ME, Pendleton N, Punab M, Wu FC, EMAS study group Association of hypogonadism with vitamin D status: The European Male Ageing Study. Eur J Endocrinol. 2012;166(1):77–85. doi: 10.1530/EJE-11-0743. [DOI] [PubMed] [Google Scholar]
- 77.Rehman R, Lalani S, Baig M, Nizami I, Rana Z, Gazzaz ZJ. Association between vitamin D, reproductive hormones and sperm parameters in infertile male subjects. Front Endocrinol (Lausanne) 2018;9:607. doi: 10.3389/fendo.2018.00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Autier P, Mullie P, Macacu A, Dragomir M, Boniol M, Coppens K, Pizot C, Boniol M. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986–1004. doi: 10.1016/S2213-8587(17)30357-1. [DOI] [PubMed] [Google Scholar]
- 79.Canguven O, Talib RA, El Ansari W, Yassin DJ, Al Naimi A. Vitamin D treatment improves levels of sexual hormones, metabolic parameters and erectile function in middle-aged vitamin D deficient men. Aging Male. 2017;20:9–16. doi: 10.1080/13685538.2016.1271783. [DOI] [PubMed] [Google Scholar]
- 80.Heijboer AC, Oosterwerff M, Schroten NF, Eekhoff EM, Chel VG, Boer RA, Blankenstein MA, Lips P. Vitamin D supplementation and testosterone concentrations in male human subjects. Clin Endocrinol. 2015;83(1):105–10. doi: 10.1111/cen.12711. [DOI] [PubMed] [Google Scholar]
- 81.Jorde R, Grimnes G, Hutchinson MS, Kjærgaard M, Kamycheva E, Svartberg J. Supplementation with vitamin D does not increase serum testosterone levels in healthy males. Horm Metab Res. 2013;45:675–81. doi: 10.1055/s-0033-1345139. [DOI] [PubMed] [Google Scholar]
- 82.Scholten SD, Sergeev IN, Song Q, Birger CB. Effects of vitamin D and quercetin, alone and in combination, on cardiorespiratory fitness and muscle function in physically active male adults. Open Access J Sports Med. 2015;6:229–39. doi: 10.2147/OAJSM.S83159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tirabassi G, Cutini M, Muscogiuri G, Delli Muti N, Corona G, Galdiero M, Pivonello R, Colao A, Balercia G. Association between vitamin D and sperm parameters: Clinical evidence. Endocrine. 2017;58:194–8. doi: 10.1007/s12020-016-1198-9. [DOI] [PubMed] [Google Scholar]
- 84.Zhu CL, Xu QF, Li SX, Wei YC, Zhu GC, Yang C, Shi YC. Investigation of serum vitamin D levels in Chinese infertile men. Andrologia. 2016;48:1261–6. doi: 10.1111/and.12570. [DOI] [PubMed] [Google Scholar]
- 85.Blomberg Jensen M, Lawaetz JG, Petersen JH, Juul A, Jorgensen N. Effects of vitamin D supplementation on semen quality, reproductive hormones and live birth rate: a randomized clinical trial. J Clin Endocrinol Metab. 2018;103:870–81. doi: 10.1210/jc.2017-01656. [DOI] [PubMed] [Google Scholar]
- 86.Deng XL, Li YM, Yang XY, Huang JR, Guo SL, Song LM. Efficacy and safety of vitamin D in the treatment of idiopathic oligoasthenozoospermia. Zhonghua Nan Ke Xue. 2014;20:1082–5. [PubMed] [Google Scholar]
- 87.Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176:175–83. doi: 10.1001/jamainternmed.2015.7148. [DOI] [PubMed] [Google Scholar]
- 88.Gaksch M, Jorde R, Grimnes G, Joakimsen R, Schirmer H, Wilsgaard T, Mathiesen EB, Njølstad I, Løchen M-L, März W, Kleber ME, Tomaschitz A, Grübler M, Eiriksdottir G, Gudmundsson EF, Harris TB, Cotch MF, Aspelund T, Gudnason V, Rutters F, Beulens JW, van’t Riet E, Nijpels G, Dekker JM, Grove-Laugesen D, Rejnmark L, Busch MA, Mensink GB, Scheidt-Nave C, Thamm M, Swart KM, Brouwer IA, Lips P, van Schoor NM, Sempos CT, Durazo-Arvizu RA, Škrabáková Z, Dowling KG, Cashman KD, Kiely M, Pilz S. Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One. 2017;12:1–15. doi: 10.1371/journal.pone.0170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hammoud AO, Meikle AW, Peterson CM, Stanford J, Gibson M, Carrell DT. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J Androl. 2012;14:855–9. doi: 10.1038/aja.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Owens DJ, Tang JC, Bradley WJ, Sparks AS, Fraser WD, Morton JP, Close GL. Efficacy of high-dose vitamin D supplements for elite athletes. Med Sci Sports Exerc. 2017;49:349–56. doi: 10.1249/MSS.0000000000001105. [DOI] [PubMed] [Google Scholar]