The aim of cancer immunotherapy is to enhance the immune response against tumor cells. The emergence of immuno-oncology as the first broadly successful strategy to treat metastatic cancer will require clinicians to integrate this new type of medicine with chemotherapy, surgery, radiation therapy, and the use of targeted small molecules. Immuno-oncologic drugs include a broad range of agents, including antibodies, vaccines, adjuvant therapies, cytokines, oncolytic viruses, bispecific molecules, and cellular therapies.1 Vaccines have generally not proved to be efficacious unless they are used as a preventive agent against virally induced tumors.2 The selective targeting of neoantigens created by tumor-specific mutations3 may prove otherwise. Alternatively, adoptive cell-transfer–based therapies bypass the need for active immunization and therefore have potential efficacy in immunologically compromised patients with cancer.
Genetically engineered T cells constitute a powerful new class of therapeutic agents that offer hope for curative responses in patients with cancer. Chimeric antigen receptor (CAR) T cells were recently approved by the Food and Drug Administration (FDA) and are poised to enter the practice of medicine for the treatment of leukemia and lymphoma (see video). Synthetic biology approaches for cellular engineering provide a broadly expanded set of tools to program immune cells for enhanced function. Advances in T-cell engineering, genetic editing, the selection of the most functional lymphocytes, and cell manufacturing have the potential to broaden T-cell–based therapies and foster new applications beyond oncology in infectious diseases, organ transplantation, and autoimmunity. This review addresses the principles of T-cell engineering and synthetic immunity, with a focus on the efficacy and toxic effects of current CAR therapies.
IMMUNO-ONCOLOGY
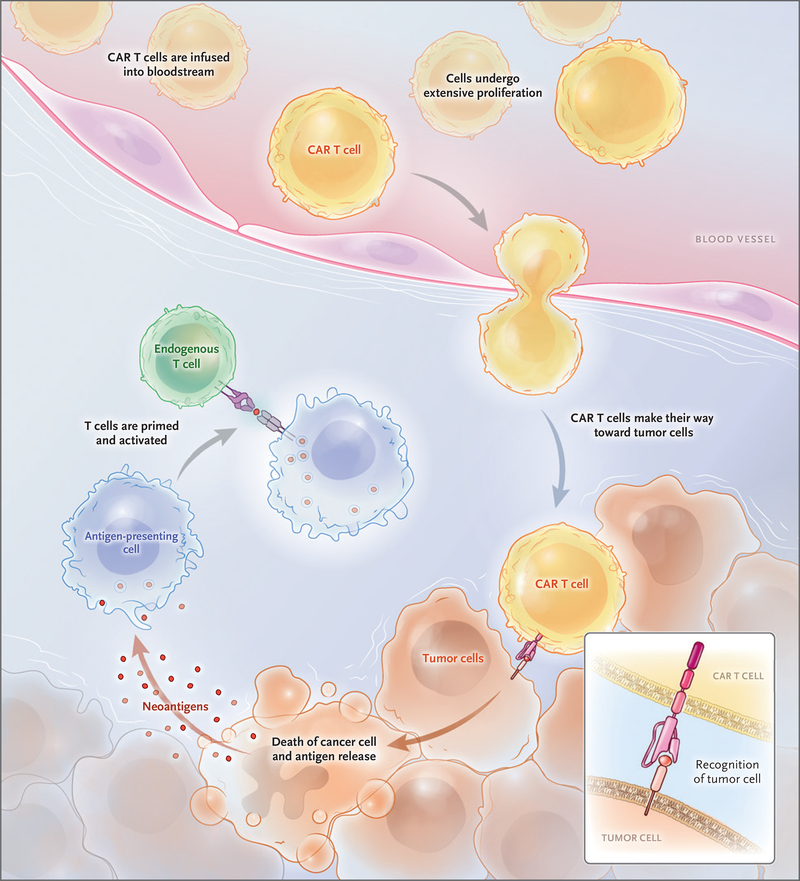
Adoptive cell transfer is a term that was first coined to describe the infusion of lymphocytes to mediate rejection of organ allografts and to treat tumors.4,5 The first successful clinical applications of adoptive cell transfer in the 1980s were based on the use of autologous tumor-infiltrating lymphocytes in patients with metastatic melanoma and allogeneic donor lymphocyte infusions in patients with relapsed leukemia.6,7 Gene-transfer techniques were developed in the 1990s to redirect the specificity of T cells with the use of T-cell receptors or CARs.8 CARs are engineered receptors that graft a defined specificity onto an immune effector cell, typically a T cell, and augment T-cell function.9 Once infused, CAR T cells engraft and undergo extensive proliferation in the patient (Fig. 1). Each CAR T cell can kill many tumor cells,10 and CAR T cells may promote immune surveillance to prevent tumor recur rence through antigen release, by assisting tumor-infiltrating lymphocytes to attack tumors, or by their own persistence.11,12
Figure 1. Chimeric Antigen Receptor (CAR) T Cells Engrafting, Trafficking to Tumor, and Proliferating Extensively after Infusion.
After infusion, CAR T cells leave the blood and travel to sites of tumor, where they identify and kill tumor cells. This can trigger extensive proliferation of CAR T cells and the release of tumor antigens, which activates the immune system to recruit non–CAR T cells, thus eliciitng further antitumor responses in a process known as cross priming.
Antitumor immunity comprises complementary innate and adaptive immune responses. The cellular components of innate immunity (natural killer cells and myeloid cells) recognize and destory virally infected cells and a range of tumor cells in a manner that is not restricted by the major histocompatibility complex. Adaptive immunity is antigen specific and is mediated by B lymphocytes and T lymphocytes that are controlled by antigen-presenting cells such as dendritic cells. More than a century ago, Paul Ehrlich proposed that the immune system is programmed to avoid the generation of autoreactive immune responses, and he termed this aversion to autoreactivity “horror autotoxicus.”13 The central challenge in immuno-oncology is that most tumor antigens are self-antigens that are also expressed on normal tissues.14 Thus, antitumor responses are often transient and ineffective, owing to host immune responses that evolved to prevent autoimmunity.15 T-cell engineering provides a means to overcome immune tolerance.
CD19 CAR T CELLS
CARs are synthetic receptors that redirect the specificity, function, and metabolism of T cells (Fig. 2). CARs consist of a T-cell activating domain (typically including the zeta chain of the CD3 complex) and extracellular immunoglobulin-derived heavy and light chains to direct specificity.16–18 These minimal structures, termed first-generation CARs,9 recognize antigen independently of HLA but do not direct sustained T-cell responses, owing to their limited signaling capability.19,20 Chimeric costimulatory receptors, which enhance proliferation and afford antiapoptotic functions in human primary T cells,21 paved the way for dual-signaling CARs that could effectively direct the expansion of functional T cells on repeated exposure to antigen.22 These receptors, termed second-generation CARs,9 enabled the generation of the persistent “living drugs” that are the foundation of current CAR T-cell therapy.
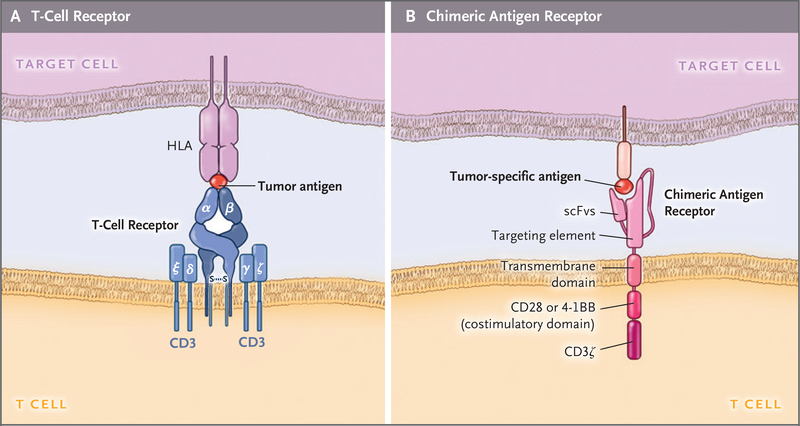
Figure 2. Structure of CARs and T-Cell Receptors.
Panel A shows the structure of a T-cell receptor, which consists of heterodimeric and antigen-specific α and β chains that closely associate with the invariant ε, δ, γ, and ζ chains of the CD3 complex. The T-cell receptor binds to the HLA allele that has a bound peptide derived from a tumor antigen on the target cell. Panel B shows the CAR, which includes the single-chain variable fragment (scFv) that binds to tumor antigens, fused to a spacer and transmembrane domain. The intracellular domain contains costimulatory domains, such as CD28 and 4–1BB and the CD3ζ chain, which drive signal activation and amplification of CAR T cells. S–S denotes disulfide bond.
We chose CD19 as our first target not only 5 because of its frequent expression in B-cell leukemias and lymphomas but also because of its broader and higher expression relative to other potential targets, such as CD20 or CD22.23,24 Its expression in normal tissues, which is confined to the B-cell lineage, predicted that on-target and off tumor activity would be limited to B-cell aplasia, a side effect that can be mitigated with immunoglobulin-replacement therapy. We further reasoned that B-cell depletion may preempt a potential antibody response to the CAR, especially its murine components. A single infusion of human peripheral-blood T cells engineered with a CD19-specific CAR was shown to eradicate established lymphomas and leukemias in mice,24 which prompted pursuit of the clinical translation of CD19 CAR therapy.
Clinical implementation required a reproducible T-cell manufacturing platform, which hinges on effective gene-transfer tools and T-cell culture conditions. Research to restore immune function in patients with human immunodeficiency virus infection or the acquired immunodeficiency syndrome led to the development of reproducible culture techniques.25,26 The FDA approved the first applications for an investigational new drug for CD19 CAR therapy in 2007. The first protocols used either gamma-retroviral or lentiviral vectors that encoded CARs that included either CD28 or 4–1BB costimulatory domains.22,27,28
Several clinical trials soon showed dramatic outcomes in patients with relapsed, refractory B-cell cancers, including non-Hodgkin’s lymphoma,29 chronic lymphocytic leukemia,30 and adult and pediatric acute lymphoblastic leukemia.31,32 These results were confirmed in larger series8,33–35 (Table 1). More than 1000 patients have received CD19-targeted CAR T cells in the United States alone. The defining characteristic of the responses is that they tend to be durable. Although head-to-head comparisons of various CAR T-cell designs are lacking, some inferences can be drawn from the available data. For instance, the inclusion of mouse sequences can trigger rejection of the CAR T cells by the host immune system, and many studies suggest that lack of immunogenicity, and hence persistence of CAR T cells, is associated with improved relapse-free survival among patients with leukemia. Thus, CAR designs that are composed of fully human sequences have become preferable.
Table 1.
Responses to CAR T-Cell Therapy.*
| Disease | Response Rate | Comments | Reference |
|---|---|---|---|
| percent | |||
| Leukemia | |||
| B-cell acute lymphoblastic leukemia (in adults) | 83–93 | High initial remission rates; unresolved issue is whether CAR T-cell therapy is definitive therapy or should be followed by allogeneic hematopoietic stem-cell therapy | Park et al.,35 Davila et al.,36 Turtle et al.37 |
| B-cell acute lymphoblastic leukemia (in children) | 68–90 | Approximately 25% of patients reported to have a relapse with CD19-negative or CD19-low leukemia; CD22 CAR T cells may improve survival among some patients with CD19 relapses | Maude et al.,34 Maude et al.,38 Fry et al.,39 Lee et al.40 |
| Chronic lymphocytic leukemia | 57–71 | Relapse is rare in patients who have a complete response; ibrutinib appears to increase response rates | Porter et al.,41 Turtle et al.42 |
| Lymphoma | |||
| Diffuse large B-cell lymphoma | 64–86 | Approximately 40–50% of patients reported to have a durable complete response | Turtle et al.,43 Kochenderfer et al.,44 Schuster et al.,45 Neelapu et al.46 |
| Follicular lymphoma | 71 | At a median follow-up of 28.6 mo, the response was maintained in 89% of patients who had a response | Schuster et al.45 |
| Transformed follicular lymphoma | 70–83 | A total of 3 of 3 patients with transformed follicular lymphoma had a complete response | Turtle et al.,43 Schuster et al.,45 Neelapu et al.46 |
| Refractory multiple myeloma | 25–100 | B-cell maturation antigen CAR T cells; stringent complete response in approximately 25% of patients | Ali et al.,47 Fan et al.,48 Berdeja et al.49 |
| Solid tumors | |||
| Glioblastoma | ND | In case report from phase 2 study, complete response on magnetic resonance imaging after intravenous and cerebrospinal fluid administration of CAR T cells; response lasted 7.5 mo | Brown et al.50 |
| Pancreatic ductal adenocarcinoma | 17 | In one patient with liver metastasis, CAR T-cell treatment produced a complete metabolic response in the liver but was ineffective against the primary pancreatic tumor | Beatty et al.51 |
ND denotes not determined.
TOXIC EFFECTS ASSOCIATED WITH CAR T CELLS
Adverse effects are associated with all cancer therapies, and CAR T cells are no exception. The spectrum of toic effects associated with CAR T cells (Table 2) differs from that of checkpoint antibodies targeting programmed cell death 1 and cytotoxic T-lymphocyte antigen 4, in which the primary toxic effects are colitis, rashes, and polyendocrinopathies.15,64 Many of the toxic effects that are reported with CAR T cells are on-target effects. Their spectrum depends on the specificity of antibody single-chain variable fragments and T-cell activation. These toxic effects are thus reversible when the target cell is eliminated or the engraftment of the CAR T cells is terminated. This reversibility contrasts with many of the toxic effects associated with cytotoxic chemotherapy, which are off-target effects and can cause permanent genetic modifications of cells in the entire organism, including stem cells. These permanent modifications can have long-term clinically significant consequences.65
Table 2.
Reported Toxic Effects of CAR T Cells.
| CAR Specificity and Adverse Effect | Reference |
|---|---|
| CD19 CAR | |
| B-cell aplasia and hypogammaglobulinemia | Kochenderfer et al.,52 Kalos et al.53 |
| Cytokine release syndrome | Davila et al.,36 Lee et al.,54 Teachey et al.55 |
| Dermatitis | Rubin et al.56 |
| Hematophagocytic lymphohistiocytosis and macrophage activation syndrome | Grupp et al.,32 Porter et al.,41 Teachey et al.55 |
| Neurologic effects such as ataxia and aphasia | Brudno and Kochenderfer57 |
| Cerebral edema | Gust et al.58 |
| B-cell maturation antigen CAR: the cytokine release syndrome | Riches et al.59 |
| Mesothelin CAR: anaphylaxis (antibody to murine single-chain variable fragments) | Maus et al.60 |
| Carbonic anhydrase IX CAR: cholangitis (on-target) | Lamers et al.61 |
| HER2/neu CAR: lethal cytokine release syndrome | Morgan et al.62 |
| Carcinoembryonic antigen–related cell-adhesion molecule 5 (CEACAM5) CAR: hemorrhagic colitis (on-target) | Thistlethwaite et al.63 |
B-cell aplasia was a predicted on-target, off-tumor adverse effect of CARs that target B-cell differentiation antigens such as CD19, CD20, and CD22.23 Clinical experience indicates that the B-cell aplasia induced by CD19 CARs is more complete than that observed after antibody therapy with rituximab. B-cell aplasia is rapidly reversed after CAR T cells are ablated.66 Guidelines for the clinical treatment of patients with CAR-induced B-cell aplasia are evolving and may differ for children and adults, since children may have an incomplete long-lived plasma-cell compartment and weaker humoral immunity. Most human plasma cells do not express CD19.67
In some patients, CAR T cells induce a clinical syndrome of fevers, hypotension, hypoxia, and neurologic changes associated with marked elevations of serum cytokine levels.36,54,55 This spectrum of clinical and laboratory findings has been termed the cytokine release syndrome. The occurrence of the cytokine release syndrome is associated with both CD19 and B-cell maturation antigen (BCMA, also known as CD269) CARs, and in the case of CD19 CARs, the severity of the cytokine release syndrome is associated with tumor burden as measured by blasts in bone marrow at the time of treatment.36,38 The cytokine release syndrome manifests with a noninfectious flulike syndrome and can progress to life-threatening capillary leakage with hypoxia and hypotension. The onset of the cytokine release syndrome correlates with the pharmacokinetic characteristics of the CAR T cells, with a temporal association between the syndrome and peak levels of CAR T cells. The cytokine release syndrome is an on-target toxic effect and is not common in patients who do not have a clinical response after CAR therapy. This syndrome is associated with T-cell activation and high levels of cytokines, including interleukin-6 and interferon-γ. Tocilizumab (Actemra), an anti–interleukin-6-receptor antagonist, is usually effective in the management of severe cytokine release syndrome induced by CAR T cells.32,36 The FDA recently approved tocilizumab for the treatment of CAR T-cell–induced cytokine release syndrome. Glucocorticoids are promptly administered if the patient does not have a rapid response to interleukin-6 receptor blockade. As of this writing, the use of interleukin-6 blockade and glucocorticoids has not been reported to interfere with the efficacy of tocilizumab, and an ongoing prospective study (ClinicalTrials.gov number, NCT02906371) is addressing whether prophylactic tocilizumab can be administered without compromising efficacy. In pediatric and adult acute pre–B-cell lymphoblastic leukemia, tumor burden at baseline predicts whether severe cytokine release syndrome will develop, and predictive biomarkers in the serum have been identified.35,36,55
All research groups testing CD19 and BCMA CAR T cells have reported neurotoxicity.36,38,43 This appears to be a class effect with CD19-directed therapies because the same spectrum of toxic effects has been reported with blinatumomab, a bispecific anti-CD19 and anti-CD3 monoclonal antibody.68 The cause of the neurotoxicity remains unknown; it is usually fully reversible and not related to spread of cancer to the central nervous system (CNS). Cerebral edema has been reported in trials by Gust et al.58 and Kite Pharma69 but has not been observed in trials conducted by Maude et al.34 and Park and colleagues.35 Until the pathophysiology of the neurologic syndromes is explained, management remains empirical.
Integration of viral vectors has been associated with safety concerns in clinical applications using genetically modified hematopoietic stem cells. For example, patients with X-linked severe combined immunodeficiency underwent gene transfer to restore expression of the gene encoding interleukin-2 receptor γ chain (IL2RG) with the use of gamma-retroviral vectors. Although 9 of 10 patients were successfully treated, T-cell leukemia developed in 4 of the 9 several years after gene therapy.70 In more than 1000 patients infused with T-cell receptors or CAR-modified T cells, no occurrence of an oncogenic transformation has yet been reported.
OTHER TYPES OF CAR THERAPY FOR OTHER HEMATOLOGIC CANCERS
CD19 CAR therapy is the most successful and best-known CAR therapy. Several important lessons with respect to target selection have emerged. The common occurrence of B-cell aplasia highlights the damage that CAR T cells can inflict on normal tissues that express the target antigen. Although this on-target side effect is not life-threatening in the case of CD19, other targets may result in death.62 Relapses after a complete remission may be CD19-negative71; this highlights the critical need to anticipate antigen escape. Targeting CD19 has proved to be more effective than targeting CD20 and CD22, which suggests that high density of CAR target expression is preferable, although other factors may be at work.39 Although there is no indication that CAR T-cell trafficking is rate-limiting in B-cell cancers, some extramedullary disease sites (e.g., retroperitoneal or CNS leukemia) have, on occasion, not had a response. Data are lacking from a formal assessment of response rates among patients with extramedullary disease as compared with those in whom lekukemia is confined to marrow. It remians unclear why responses to CD19 CAR therapy are more frequent and deeper in acute lymphoblastic leukemia than in chronic lymphocytic leukemia or non-Hodgkin’s lymphoma. Disease location and tumor microenvironment as well as host T-cell function probably account for these differences, given that the CD19 target density is similar.59
The efficacy of CAR therapy against B-cell cancers is a good omen for the treatment of other hematologic cancers. Several candidate targets for multiple myeloma have been explored preclinically; these include kappa light chain, CD138, Lewis Y antigen, BCMA, CS1 (cell-surface glycoprotein CD2 subset 1, also called signaling lymphocytic activation molecule F7 [SLAMF7] or CD319), CD38, and integrin β7. The results of recent clinical studies of the targeting of BCMA, albeit preliminary, are encouraging,47 and registration trials by several companies are ongoing. Several targets have also been suggested for acute myeloid leukemia: CD33, CLEC12A, CD44v6, EMR2, Tim3, CD70, Lewis Y antigen, CD123, and folate receptor β. Clinical trials that are designed to investigate the latter three targets have already been initiated. Although the mechanism is unclear, a fatal complication involving the capillary leak syndrome after administration of CD123 CAR T cells warrants further scrutiny of this target. Lacking targets with an expression profile as favorable as CD19, the targeting of two or more antigens (combinatorial targeting) may prove to be necessary to preempt antigen escape without exacerbating toxicity.72
CELL ENGINEERING AND SYNTHETIC BIOLOGY
The combination of genetic engineering and synthetic biology offers a wide range of possibilities to design T cells with enhanced functions. New prospects to increase the efficacy (prevention of antigen escape) and safety (reduction of on-target and off-tumor activity) of CAR therapy include combinatorial targeting and Boolean logic–gated T cells that may recognize either one of two antigens or the two antigens only.8,73 Engineered T cells can also be used as a launching pad to reach the tumor microenvironment by, for example, expressing costimulatory ligands on the surface of CAR T cells74 or secreting cytokines or other molecules.75,76 The safe use of T cells may be further increased with the use of controllable suicide switches such as inducible caspase 9 and truncated epidermal growth factor receptor.66,77
Genetic engineering techniques are evolving as well. The advent of targeted nucleases offers new prospects to either delete genes in T cells or insert transgenes into a selected locus.78,79 Adoptive transfer of genetically modified T cells will probably provide the initial proofs of concept for the emerging field of genome editing. Our research group recently found that expressing a CAR from the T-cell receptor locus enhances tumor elimination by sustaining T-cell function, through diminished tonic signaling and delayed T-cell exhaustion.80
CHALLENGES AND PERSPECTIVES
The FDA approvals of CD19 CAR T cells for relapsed and refractory acute lymphoblastic leukemia and for diffuse large B-cell lymphoma are noteworthy from several perspectives, but perhaps most important is that this is the first form of gene-transfer therapy to gain commercial approval by the FDA. Because of the risk of the cytokine release syndrome and neurologic toxic effects, these CAR T cells were approved contingent with a Risk Evaluation and Mitigation Strategy, whereby the FDA requires that physicians and hospital staff complete training for management of adverse effects.
The use of genetically engineered T cells as a precision medicine for leukemia and lymphoma has the potential to transform therapy for cancer. The principal scientific challenge in the field is the use of CAR therapy to treat solid tumors. T cells can eliminate solid tumors, as exemplified by checkpoint therapy and infusions of tumor-infiltrating lymphocytes for advanced cancers, including melanoma, cholangiocarcinoma, and colorectal cancer.6,81–83 As of this writing, there is a singular example of a striking regression of multifocal glioblastoma after intracranial administrations of CAR T cells targeting interleukin-13 receptor alpha 2 (IL13Rα2).50 The identification of suitable targets for CAR T cells in solid tumors requires further research to prevent or minimize off-tumor activity.8,72,73
Although autologous CAR T-cell therapies have immense therapeutic potential, the cost implications and complexity of autologous T-cell therapies remain problematic for broader applications. The development of off-the-shelf “universal” CAR T cells is possible with the use of a variety of 7 gene-editing techniques and has been successful in a few children with pre–B-cell acute lymphoblastic leukemia with a high degree of immuno-suppression.84 The major challenge in developing off-the-shelf T cells is avoidance of immune rejection in both host-versus-graft and graft-versus-host directions. T cells may also be generated from human embryonic stem cells and induced pluripotent stem cells. Thus, the combination of techniques involving induced pluripotent stem cells and synthetic biology may provide an opportunity to generate off-the-shelf T cells that uniquely combine favorable attributes, including antigen specificity, lack of alloreactivity, histocompatibility, and enhanced functional properties.85
The principles discussed here could also be used to design cell therapies targeted to treat other diseases. Autoimmunity, infection, inflammation, degeneration, wound healing, and fibrosis are all examples of conditions that could benefit from engineered effector T cells or regulatory T cells.86,87 CAR therapy is now global, with more than 250 trials listed on ClinicalTrials.gov, although most open trials are located in the United States and China, with relatively few in Europe and Japan.88 The reasons for this geographic disparity are probably complex and may in part relate to regional differences in the social acceptance of therapies involving genetic interventions. The momentum that has been generated by the approval of CD19 CAR T cells for oncology is likely to accelerate the translation of engineered cell therapies for a plethora of inflammatory and regenerative medicine applications.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Carl H. June, Center for Cellular Immunotherapies, Perelman School of Medicine, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia
Michel Sadelain, Center for Cell Engineering, Sloan Kettering Institute, Memorial Sloan Kettering Cancer Center, New York
REFERENCES
- 1.Hoos A. Development of immuno-oncology drugs — from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov 2016;15:235–47. [DOI] [PubMed] [Google Scholar]
- 2.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–43. [DOI] [PubMed] [Google Scholar]
- 3.Ott PA, Hu Z, Keskin DB, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017;547:217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billingham RE, Brent L, Medawar PB. Quantitative studies on tissue transplantation immunity. II. The origin, strength and duration of actively and adoptively acquired immunity. Proc R Soc Lond B Biol Sci 1954;143:58–80. [DOI] [PubMed] [Google Scholar]
- 5.Mitchison NA. Studies on the immunological response to foreign tumor transplants in the mouse. I. The role of lymph node cells in conferring immunity by adoptive transfer. J Exp Med 1955;102: 157–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: a preliminary report. N Engl J Med 1988;319:1676–80. [DOI] [PubMed] [Google Scholar]
- 7.Kolb HJ, Mittermüller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990;76:2462–5. [PubMed] [Google Scholar]
- 8.Sadelain M, Rivière I, Riddell S. Therapeutic T cell engineering. Nature 2017; 545:423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadelain M, Brentjens R, Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol 2009;21:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davenport AJ, Jenkins MR, Cross RS, et al. CAR-T cells inflict sequential killing of multiple tumor target cells. Cancer Immunol Res 2015;3:483–94. [DOI] [PubMed] [Google Scholar]
- 11.Stephan MT, Ponomarev V, Brentjens RJ, et al. T cell-encoded CD80 and 4–1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med 2007;13:1440–9. [DOI] [PubMed] [Google Scholar]
- 12.Scholler J, Brady T, Binder-Scholl G, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 2012;4: 132ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silverstein AM. Autoimmunity versus horror autotoxicus: the struggle for recognition. Nat Immunol 2001;2:279–81. [DOI] [PubMed] [Google Scholar]
- 14.Finn OJ. Cancer immunology. N Engl J Med 2008;358:2704–15. [DOI] [PubMed] [Google Scholar]
- 15.June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23: 540–7. [DOI] [PubMed] [Google Scholar]
- 16.Kuwana Y, Asakura Y, Utsunomiya N, et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem Biophys Res Commun 1987;149:960–8. [DOI] [PubMed] [Google Scholar]
- 17.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A 1993; 90:720–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brocker T, Peter A, Traunecker A, Karjalainen K. New simplified molecular design for functional T cell receptor. Eur J Immunol 1993;23:1435–9. [DOI] [PubMed] [Google Scholar]
- 19.Brocker T, Karjalainen K. Signals through T cell receptor-zeta chain alone are insufficient to prime resting T lymphocytes. J Exp Med 1995;181:1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong MC, Latouche JB, Krause A, Heston WD, Bander NH, Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia 1999;1:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krause A, Guo HF, Latouche JB, Tan C, Cheung NK, Sadelain M. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J Exp Med 1998;188: 619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol 2002;20:70–5. [DOI] [PubMed] [Google Scholar]
- 23.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood 2008;112:1570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med 2003;9:279–86. [DOI] [PubMed] [Google Scholar]
- 25.Levine BL, Cotte J, Small CC, et al. Large-scale production of CD4+ T cells from HIV-1-infected donors after CD3/ CD28 costimulation. J Hematother 1998; 7:437–48. [DOI] [PubMed] [Google Scholar]
- 26.Hollyman D, Stefanski J, Przybylowski M, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother 2009;32:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brentjens RJ, Santos E, Nikhamin Y, et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin Cancer Res 2007;13: 5426–35. [DOI] [PubMed] [Google Scholar]
- 28.Milone MC, Fish JD, Carpenito C, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 2009;17:1453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010; 116:4099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 2018;15: 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JH, Rivière I, Wang X, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018;378:449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014; 6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turtle CJ, Hanafi L-A, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016;126:2123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 2018;24:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turtle CJ, Hay KA, Hanafi LA, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol 2017;35:3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 2016;8:355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuster SJ, Svoboda J, Chong EA, et al. et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017;377:2545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016;128:1688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan F, Zhao WH, Liu J, et al. Durable remissions with BCMA-specific chimeric antigen receptor (CAR)-modified T cells in patients with refractory/relapsed mulple myeloma. J Clin Oncol 2017. June 5 (Epub ahead of print). [Google Scholar]
- 49.Berdeja JG, Lin Y, Raje NS, et al. First-in-human multicenter study of bb2121 anti-BCMA CAR T-cell therapy for relapsed/refractory multiple myeloma: updated results. J Clin Oncol 2017;35:Suppl: 3010.28715249 [Google Scholar]
- 50.Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 2016;375:2561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beatty GL, O’Hara MH, Lacey SF, et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 2018. March 20 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012;119:2709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalos M, Levine BL, Porter DL, et al. T cells expressing chimeric receptors establish memory and potent antitumor effects in patients with advanced leukemia. Sci Transl Med 2011;3:95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 2016;6:664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubin CB, Elenitsas R, Taylor L, et al. Evaluating the skin in patients undergoing chimeric antigen receptor modified T-cell therapy. J Am Acad Dermatol 2016; 75:1054–7. [DOI] [PubMed] [Google Scholar]
- 57.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016; 127:3321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov 2017;7:1404–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood 2013;121:1612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maus MV, Haas AR, Beatty GL, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res 2013;1:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamers CH, Sleijfer S, Vulto AG, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol 2006;24(13):e20–e22. [DOI] [PubMed] [Google Scholar]
- 62.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18: 843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thistlethwaite FC, Gilham DE, Guest RD, et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient preconditioning-dependent respiratory toxic-. ity. Cancer Immunol Immunother 2017; 66:1425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy — immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol 2017;13:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muller PY, Milton MN. The determination and interpretation of the therapeutic index in drug development. Nat Rev Drug Discov 2012;11:751–61. [DOI] [PubMed] [Google Scholar]
- 66.Paszkiewicz PJ, Fräßle SP, Srivastava S, et al. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J Clin Invest 2016;126:4262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhoj VG, Arhontoulis D, Wertheim G, et al. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood 2016;128:360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015;16:57–66. [DOI] [PubMed] [Google Scholar]
- 69.Harris J. Kite reports cerebral edema death in ZUMA-1 CAR T-cell trial. OncLive May 8, 2017. (https://www.onclive.com/web-exclusives/kite-reports-cerebral-edema-death-in-zuma1-car-tcell-trial).
- 70.Hacein-Bey-Abina S, Garrigue A, Wang GP, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest 2008;118:3132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sotillo E, Barrett DM, Black KL, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov 2015;5:1282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perna F, Berman SH, Soni RK, et al. Integrating proteomics and transcriptomics for systematic combinatorial chimeric antigen receptor therapy of AML. Cancer Cell 2017;32(4):506–519.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell 2017;168:724–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Z, Condomines M, van der Stegen SJC, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 2015;28:415–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 2012;119:4133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu B, Ren J, Luo Y, et al. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep 2017;20:3025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou X, Dotti G, Krance RA, et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood 2015;125:4103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 2014;370:901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaj T, Gersbach CA, Barbas CF III ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 2013;31:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eyquem J, Mansilla-Soto J, Giavridis T, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017;543:113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344:641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tran E, Robbins PF, Lu Y-C, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med 2016;375: 2255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med 2017;9: 9. [DOI] [PubMed] [Google Scholar]
- 85.Themeli M, Rivière I, Sadelain M. New cell sources for T cell engineering and adoptive immunotherapy. Cell Stem Cell 2015;16:357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lederman MM, Cannon PM, Currier JS, et al. A cure for HIV infection: “not in my lifetime” or “just around the corner”? Pathog Immun 2016;1:154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol 2016;16:149–63. [DOI] [PubMed] [Google Scholar]
- 88.June CH, O’Connor RS, Kawalekar OU, et al. CAR T cell immunotherapy for human cancer. Science 2018;359(6382): 1361–5. [DOI] [PubMed] [Google Scholar]