Abstract
Background
Postextubation distress is detrimental to the prognosis of critically ill patients with successful spontaneous breathing trial. The known risk factors of failed weaning are associated with the heart, lungs, and diaphragm. The aim of this study was to explore the role of a combined model including indicators of heart, lung, and diaphragm ultrasound in predicting the weaning outcome.
Material/Methods
Patients’ clinical data and ultrasonic features of heart, lungs, and diaphragm were recorded. Patients were included in either the failed weaning group (n=24) or the successful weaning group (n=81). The association of potential variables with the risk of weaning failure was determined using multivariate logistic regression analysis. The accuracy of potential indicators for predicting the weaning outcome were evaluated and a multiindicator combined model was established to improve the predictive accuracy.
Results
Brain natriuretic peptide (odds ratio [OR]=1.120, P=0.004), left-atrial pressure (LAP) (OR=1.333, P=0.005), lung ultrasound score (LUS) (OR=1.736, P=0.001), and hemidiaphragm dysfunction (OR=3.942, P=0.014) were associated with an increased risk of weaning failure. However, all of these indicators could not accurately predict the weaning outcome independently (all areas under the curve [AUCs] <0.9). The combination of LAP, LUS, and hemidiaphragm dysfunction showed the highest AUC (AUC=0.919).
Conclusions
The combined model including LAP, LUS, and hemidiaphragm dysfunction were the most accurate method for the prediction.
MeSH Keywords: Echocardiography; Respiration, Artificial; Ventilator Weaning
Background
Mechanical ventilation is a necessary life support technology for critically ill patients [1,2]. The weaning outcome affects the morbidity and mortality of patients when their primary disease improves [3]. Therefore, accurate prediction of the weaning outcome is of great importance. The spontaneous breathing trial (SBT) is a routinely used assessment before extubation [4]. However, Deab and Bellani [5] pointed out that the predictive accuracy of the weaning outcome is limited by only relying on the result of SBT. Studies indicated that the incidence of reintubation ranges between 3% and 30% after a successful SBT [6,7]. Since the assessment of cardiopulmonary reserve is difficult by SBT, it is difficult to predict the weaning failure due to cardiopulmonary decompensation.
When shifting from positive-pressure ventilation to spontaneous breathing, a series of changes (e.g. intrathoracic pressure) in the body may cause heart, lung, and diaphragm dysfunctions, which may lead to a weaning failure [8,9]. Portable ultrasound, because of its popularity, has been used to determine the risk factors of weaning failure [10–12]. The assessment of left ventricular diastolic function can help identify a potential weaning failure. Lung ultrasound can accurately predict respiratory distress after extubation by assessing lung aeration loss. Previous studies have further demonstrated that the combined echocardiography and lung ultrasound can improve the predictive accuracy of weaning outcomes [13]. In addition, for some patients with normal cardiorespiratory state but diaphragmatic dysfunction, the evaluation of the diaphragmatic state can help accurately predict weaning outcomes [14].
However, the reasons for weaning failure are very complicated. The known risk factors include dysfunction of the heart, lungs, and diaphragm. The predicting accuracy of weaning outcomes is limited by the assessment of a single organ [15,16]. Research on ultrasonic assessment for multiple organs to increase the predictive accuracy are limited. The present study establishes a multiindex combination model by logistic regression for prediction of weaning outcomes. The aim of this study is to identify its role in the weaning outcomes for patients receiving mechanical ventilation.
Material and Methods
Research participants
From July 2016 to June 2019 in the intensive care unit (ICU) of our hospital, 105 patients ages >18 years (71 males and 34 females, ages 57.98±15.20 years) who were mechanically ventilated for ≥48 h with successful SBT and considered ready for extubation were recruited. Patients were excluded if they had a diagnosis of central respiratory depression, severe arrhythmia, history of diaphragmatic paralysis, or if they were ventilating through a tracheostomy. All patients and their families signed informed consents. This study was approved by the ethics committee of Ordos Central Hospital (no. 2016-083).
Clinical data collection
Clinical data including gender, age, body mass index, primary disease, length of stay in ICU, duration of mechanical ventilation, acute physiology and chronic health evaluation II, and sequential organ failure assessment were collected.
Spontaneous breathing trail
Patients underwent a 30-min SBT when they met all the defined criteria; rapid shallow breathing index (RSBI), respiratory rate, heart rate, and tidal volume were recorded. Brain natriuretic peptide (BNP) was measured using a Cobas E601 immunoassay analyzer (Roche Diagnostics, Basel, Switzerland) at the end of the SBT.
Ultrasound examination
GE vivid E9 (GE Healthcare, USA) and Resona 7, M9 (Mindray, Shenzhen, China) ultrasound diagnostic instruments were used in this study and the ultrasonic findings were recorded by the sonographer well trained in point-of-care ultrasound at the end of the SBT.
Echocardiography
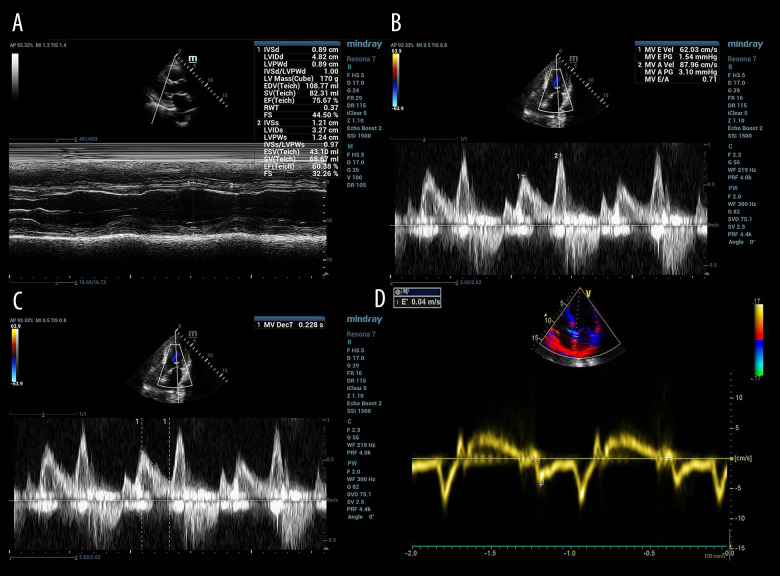
All patients were evaluated in a left or supine position with the probe in a standard parasternal long-axis with an apical four- or two-chamber view. Left ventricular ejection fraction (Figure 1A), the peak early (E) and peak atrial (A) velocity transmitral flow from pulsed-wave Doppler (Figure 1B), deceleration time of E wave (Figure 1C), and early diastolic mitral annulus velocity (e’) based on tissue Doppler imaging (Figure 1D) were measured. The E/A ratio, E/e’ ratio and left-atrial pressure (LAP) were calculated on the basis of the measured indicators [17].
Figure 1.
Echocardiographic assessment of left ventricular systolic and diastolic function in patients with mechanical ventilation ready for extubation. (A) Left ventricular ejection fraction (LVEF) measured for left ventricular systolic function; (B) peak early (E) and peak atrial (A) velocity transmitral flow from pulsed-wave Doppler measured for left ventricular diastolic function; (C) deceleration time of E wave (DTE) measured for diastolic function; (D) early diastolic mitral annulus velocity (e’) based on tissue Doppler imaging measured for the calculation of E/e’ ratio and left atrial pressure (LAP).
Lung ultrasound
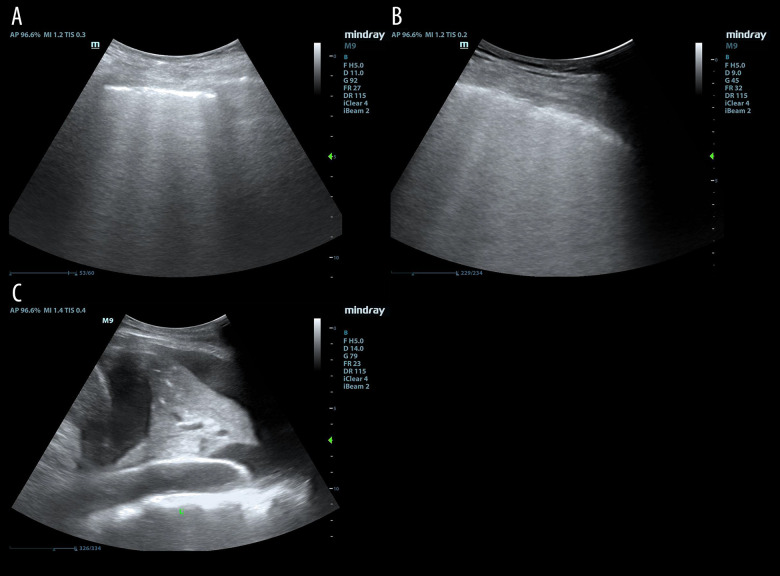
Lung ultrasound was performed according to the operation procedure of Soumer et al. [12] and lung ultrasound score (LUS) was calculated thereafter according to the observed worst ultrasound pattern: normal aeration, LUS=0; moderate loss of lung aeration (multiple, well-defined B lines), LUS=1 (Figure 2A); severe loss of lung aeration (multiple coalescent B lines), LUS=2 (Figure 2B); lung consolidation, LUS=3 (Figure 2C). Finally, the LUS of each part was accumulated to obtain the total LUS for each patient (maximum 36 points).
Figure 2.
Lung ultrasound assessment in patients with mechanical ventilation ready for extubation. (A) For moderate loss of lung aeration (multiple, well-defined B lines), lung ultrasound score (LUS)=1; (B) for severe loss of lung aeration (multiple coalescent B lines), LUS=2; (C) for lung consolidation, LUS=3.
Diaphragmatic ultrasound
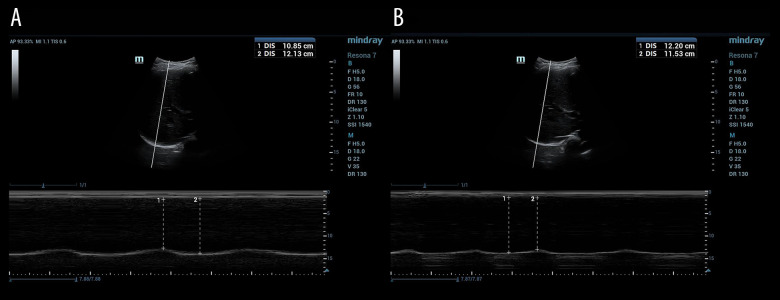
Patients were kept in a supine position as well for diaphragmatic ultrasound examination. The probe was placed laterally and perpendicularly on the lower intercostal spaces of the lateral chest wall between mid- and posterior axillary lines to observe the movement of left and right hemidiaphragm. The M-mode scan line was as perpendicular to the diaphragm image as possible (inclined angle not exceeding 20°). At least 3 consecutive respiratory cycles of the hemidiaphragm movement were recorded in M mode after stable breathing. The movements between the end of inspiration and the end of expiration were measured and averaged. Normal function of the diaphragm was defined when a movement was ≥1 cm (Figure 3A). Hemidiaphragmatic dysfunction was defined as when a unilateral hemidiaphragm movement was <1.0 cm (Figure 3B). Bilateral diaphragmatic dysfunction was defined as movement of <1.0 cm in both hemidiaphragms [18].
Figure 3.
Diaphragmatic ultrasound assessment in patients with mechanical ventilation ready for extubation. (A) For normal function of the diaphragm (movement ≥1 cm); (B) for hemidiaphragmatic dysfunction (movement <1.0 cm).
Grouping criterion
After successfully passing the SBT, the treating intensivist reviewed the patient for final assessment and then extubated the patient. The treating intensivist was blinded to the ultrasound findings. After extubation, the patient’s respiratory, oxygenation, consciousness, and vital signs were closely observed. Failed extubation was defined as the requirement for noninvasive positive-pressure ventilation (NPPV) or reintubation within 48 h. Patients with failed extubation were included in the failed weaning group, whereas the remaining patients were included in the successful weaning group.
Statistical methods
All data were processed using Statistical Product and Service Solutions (Chicago, IL, USA) software (version 22.0) and plotted by R package version 3.6.2 and MedCalc version 12 (MedCalc Software, Ostend, Belgium). The categorical variables were expressed in n and%, and the chi-square test was used for comparison. The numerical data were expressed as mean±standard deviation or median (interquartile range) when appropriate, and independent sample t tests or Mann-Whitney U tests were used for comparison. The association of potential variables with the risk of weaning failure was performed using multivariate logistic regression analysis in which all variables with P<0.05 from the univariate analysis were entered into the stepwise model. Receiver operating characteristic (ROC) curves were established to evaluate the accuracy of potential indicators for predicting the weaning outcome. A multiindicator combined model was established with logistic regression to explore the predictive value in the weaning outcome.
Results
Comparison of clinical and biochemical variables
Of the 105 patients, 81 (77%) who were successfully extubated were included in the successful weaning group. Twenty-four patients (23%) with failed extubation over the subsequent 48 h were included in the failed weaning group. Nine of the 24 patients were reintubated and the remaining 15 received NPPV. The reasons for reintubation included expectoration and poor airway protection (n=4), changes in consciousness (n=2), and worsened primary cardiopulmonary disease (n=3). The duration of mechanical ventilation, RSBI, and BNP in the failed weaning group were longer and larger than in the successful weaning group (P<0.05, Table 1).
Table 1.
Comparison of clinical and biochemical variables between the successful and failed weaning groups.
| Variables | Successful weaning group (n=81) | Failed weaning group (n=24) | P value | |
|---|---|---|---|---|
| Age (years) | 57.32±11.15 | 59.05±14.13 | 0.532 | |
| Male (n (%)) | 53 (65.4%) | 18 (75.0%) | 0.379 | |
| BMI (kg/m2) | 26 (22.5, 28) | 25.5 (24, 28) | 0.504 | |
| Primary disease(n (%)) | Pulmonary disease | 27 (33.3%) | 8 (33.3%) | 0.984 |
| Cardiovascular disease | 24 (29.6%) | 8 (33.3%) | ||
| Septic shock | 11 (13.6%) | 3 (12.5%) | ||
| Others | 19 (23.5%) | 5 (20.8%) | ||
| Length of stay in ICU (d) | 10 (7, 14) | 11.5 (9, 15) | 0.185 | |
| Duration of mechanical ventilation (h) | 125 (90, 180) | 155 (100, 225) | 0.023 | |
| APACHE II | 20 (15, 23) | 21 (17, 24) | 0.136 | |
| SOFA | 7 (4,10) | 8 (6, 11) | 0.154 | |
| RSBI (breath/min/l) | 34.8 (23.7, 54.5) | 43.4 (33.1, 68.8) | 0.028 | |
| BNP (pg/ml) | 183 (83, 402) | 275 (142, 993) | 0.026 | |
APACHE II – acute physiology and chronic health evaluation II; BMI – body mass index; BNP – brain natriuretic peptide; RSBI – rapid shallow breathing index; SOFA – sequential organ failure assessment.
Comparison of heart, lung, and diaphragm ultrasound variables
The E/e’ ratio, LAP, LUS, and proportion of hemidiaphragm dysfunction in the failed weaning group were larger than in the successful weaning group (P<0.05, Table 2).
Table 2.
Comparison of heart, lung, and diaphragm ultrasound variables between the successful and failed weaning groups.
| Variables | Successful weaning group (n=81) | Failed weaning group (n=24) | P value | |
|---|---|---|---|---|
| Echocardiographic indicators | LVEF (%) | 58.93±8.83 | 54.93±9.94 | 0.061 |
| E/A ratio | 1.35±0.33 | 1.27±0.34 | 0.303 | |
| E/e’ ratio | 7.4 (6.1, 10.2) | 10.5 (8.4, 17.5) | 0.019 | |
| DTE | 185 (160, 220) | 177 (130, 260) | 0.658 | |
| LAP | 12.4 (7.2, 16.8) | 15.9 (10.8, 21.3) | 0.017 | |
| LUS | 11 (7, 14) | 15 (11, 21) | 0.027 | |
| Diaphragmatic function n (%) | Normal function | 55 (67.9%) | 10 (41.7%) | 0.020 |
| Hemidiaphragm dysfunction | 26 (32.1%) | 14 (58.3%) | ||
| Bilateral diaphragmatic dysfunction | 0 | 0 | ||
A – peak atrial velocity transmitral flow; DTE – deceleration time of E wave; E – the peak early velocity transmitral flow; e’ – early diastolic mitral annulus velocity; LAP – left-atrial pressure; LUS – lung ultrasound score; LVEF – left ventricular ejection fraction; APACHE II – acute physiology and chronic health evaluation II; BMI – body mass index; BNP – brain natriuretic peptide; ICU – Intensive Care Unit; RSBI – rapid shallow breathing index; SOFA – sequential organ failure assessment
Multivariate logistic regression for risk of weaning failure
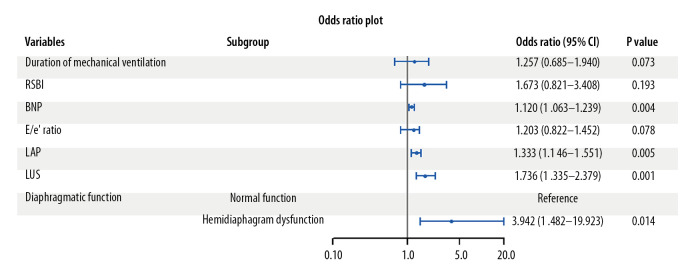
Multivariate logistic regression revealed that BNP (odds ratio [OR]=1.120, P=0.004), LAP (OR=1.333, P=0.005), LUS (OR=1.736, P=0.001), and hemidiaphragm dysfunction (OR=3.942, P=0.014) were associated with an increased risk of weaning failure (Figure 4).
Figure 4.
Multivariate logistic regression for the risk of weaning failure. Brain natriuretic peptide (BNP), left-atrial pressure (LAP), lung ultrasound score (LUS), and hemidiaphragm dysfunction were associated with an increased risk of weaning failure.
Predictive accuracy of potential variables for the weaning outcome
The weaning outcome could not be accurately predicted by BNP (area under curve [AUC]=0.680, <0.9), despite its highest sensitivity (100%). The AUCs of LAP and LUS were higher than that of BNP, but they were also difficult for predicting the weaning outcome independently (both AUCs <0.9). It was worth noting that the specificity of hemidiaphragm dysfunction in predicting weaning outcome (67.9%) was higher than those of BNP (43.2%), LAP (53.09%), and LUS (50.62%), but the sensitivity was lower (58.3%). Therefore, this study combined these potential variables through logistic regression to take advantage of their strength in predicting weaning outcome. It revealed that the combination of LAP, LUS, and hemidiaphragm dysfunction showed the highest AUC (AUC=0.919), with sensitivity and specificity of 91.7% and 82.7%, respectively. The fitting equation was logit (P)=−21.303+0.245×LAP+1.046×LUS+3.009×hemidiaphragm dysfunction (Table 3, Figure 5).
Table 3.
Predictive accuracy of potential variables for the weaning outcome.
| Variables | AUC | 95% CI | Cut-off point | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| BNP | 0.680* | 0.581–0.767 | 170.6 | 100.0 | 43.2 |
| LAP | 0.764* | 0.671–0.842 | 11.8 | 91.7 | 53.1 |
| LUS | 0.781* | 0.690–0.856 | 8.9 | 95.8 | 50.6 |
| Hemidiaphragm dysfunction | – | – | – | 58.3 | 67.9 |
| Combined model | 0.919 | 0.850–0.963 | 0.26 | 91.7 | 82.7 |
95% CI – 95% confidence interval; AUC – area under curve; BNP – brain natriuretic peptide; LAP – left-atrial pressure; LUS – lung ultrasound score; compared with the combined model,
P<0.0.
Figure 5.
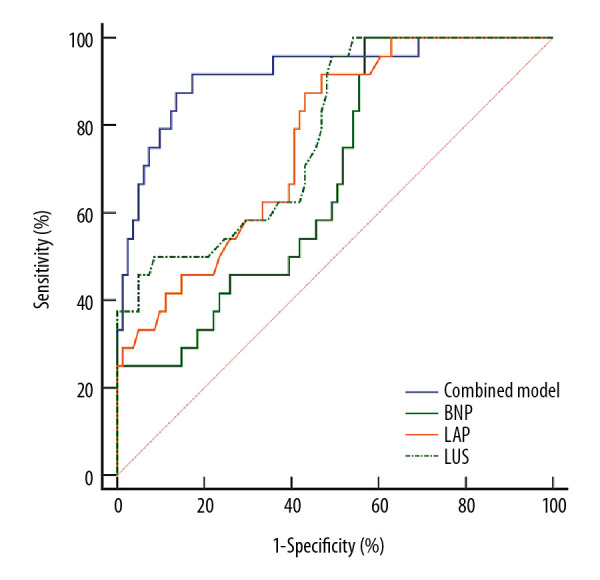
Accuracy of potential variables in predicting the weaning outcome by receiver operating characteristic (ROC) curves.
Discussion
Role of clinical indicators in predicting weaning outcomes
The present study showed that compared with the failed weaning group, the duration of mechanical ventilation was longer and the RSBI and BNP were larger in the successful weaning group. The duration of mechanical ventilation is usually related to the incidence of ventilator-related complications. Prolonged mechanical ventilation can easily lead to ventilator dependence, even weaning failure [19].
The oxygen consumption of the myocardial and respiratory muscle will gradually increase during SBT, which may lead to heart and lung dysfunction [20]. BNP and RSBI are known markers for myocardial and respiratory muscle function during SBT to assess the feasibility of weaning [21]. However, the multivariate analysis in this study suggested that only BNP in the clinical indicators was associated with an increased risk of weaning failure. It is reasonable to assume that the detection of BNP is more stable compared with RSBI and duration of mechanical ventilation, which are easily affected in the clinical setting [22,23].
Role of echocardiography in predicting weaning outcome
Left ventricular diastolic and systolic function is closely related to cardiogenic weaning difficulty, and the diastolic dysfunction is dominant [24,25]. Our study showed that the LAP estimated by E/e’ ratio was a good indicator of left ventricular diastolic function, and the multivariate analysis showed that LAP was an independent influencing factor of weaning failure. The possible reason may be that patients with raised LAP may be more likely to develop pulmonary venous congestion as the hemodynamic stress associated with weaning increases.
Role of lung ultrasound in predicting weaning outcomes
During the weaning process, pulmonary inflammation and cardiac insufficiency may cause an aeration loss and even lead to weaning failure. Lung ultrasound can noninvasively assess the location and extent of aeration loss, thereby helping to diagnose respiratory disorders [26]. In this study, we used LUS to quantify the degree of aeration loss after extubation. It revealed that LUS was an independent influencing factor of weaning failure, and it was a reliable indicator for the quantitative evaluation of respiratory function during weaning. It implied that patients with high LUS were not suitable for weaning from mechanical ventilation. Soummer et al. [12] further pointed out that after SBT, monitoring changes in the LUS can be used to adjust the treatment strategy in time and to decide whether to perform extubation.
Role of diaphragm ultrasound in predicting weaning outcomes
Diaphragm atrophy or dysfunction can be observed after mechanical ventilation for 24 h [27]. Diaphragm ultrasound can be used to evaluate diaphragm function by measuring diaphragm movement. Boussuges et al. [18] proposed that the cutoff value for diaphragm movement in healthy people be 1 cm for men and 0.9 cm for women. Therefore, in this study, diaphragm movement <1 cm was defined as hemidiaphragm dysfunction. By comparing diaphragm function between the two groups, hemidiaphragm dysfunction was shown to be significantly associated with an increased risk of weaning failure. The estimated OR of failure was approximately 3 times higher for patients with hemidiaphragm dysfunction than for those with normal function. Therefore, monitoring the diaphragm function by ultrasound can help to detect diaphragm dysfunction earlier and aid in decision making for extubation.
Combined model for predicting weaning outcomes
The ROC curves in this study showed that the indicators of cardiopulmonary function (BNP, LAP, and LUS) were more sensitive, whereas the specificity of the diaphragm function was better in predicting the weaning outcome. All of these indicators could not accurately predict the weaning outcome independently (all AUCs <0.9). Therefore, we combined these indicators utilizing their advantages in predicting outcomes based on the logistic regression model to improve the predictive accuracy. The results showed that the AUC of the combined model increased significantly (>0.9), with sensitivity and specificity of 91.67% and 82.72%, respectively. It implied that the combined model including the indicators of heart, lungs, and diaphragm was more accurate in the prediction of weaning outcomes than the individual indicators. In the clinical setting, physicians can calculate the probability of successful weaning using the combined prediction model and perform subsequent management.
Limitation and perspective
The reliability of the combined prediction model depends on the sample volume included in the model. The volume in our study is limited and the results need to be verified in a larger study. With the further development of big data and artificial intelligence, more mathematical models are being introduced in clinical settings to improve the diagnosis and management of various diseases. Our plans are to carry out a multicenter cooperative study with a larger volume to fit a more reliable combined prediction model to reduce the morbidity and mortality of critically ill patients.
Conclusions
The ultrasonic features of heart, lungs, and diaphragm provided critical information about cardiopulmonary and diagram function during a SBT. Failed extubation was more prevalent if increased BNP, LAP, LUS, and hemidiaphragm dysfunction were present. We are hopeful that the combined prediction model will help physicians optimize cardiac and respiratory function before weaning patients from ventilation.
Footnotes
Availability of data and materials
The datasets during the current study are available from the corresponding author upon request.
Conflict of interest
None.
Source of support: National Natural Science Foundation of China (NO.81771842)
References
- 1.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Resp Crit Care Med. 2017;195:438–42. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 2.Rittayamai N, Brochard L. Recent advances in mechanical ventilation in patients with acute respiratory distress syndrome. Eur Resp Rev. 2015;24:132–40. doi: 10.1183/09059180.00012414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jubran A, Lawm G, Duffner LA, et al. Post-traumatic stress disorder after weaning from prolonged mechanical ventilation. Intensive Care Med. 2010;36:2030–37. doi: 10.1007/s00134-010-1972-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns KEA, Soliman I, Adhikari NKJ, et al. Trials directly comparing alternative spontaneous breathing trial techniques: A systematic review and meta-analysis. Crit Care. 2017;21:127. doi: 10.1186/s13054-017-1698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deab SA, Bellani G. Extubation failure after successful spontaneous breathing trial: prediction is still a challenge! Resp Care. 2014;59:301–2. doi: 10.4187/respcare.03037. [DOI] [PubMed] [Google Scholar]
- 6.Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Resp J. 2007;29:1033–56. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 7.Gajic O, Tobin MJ. Principles and practice of mechanical ventilation. Crit Care. (2nd ed) 2007;11:315. [Google Scholar]
- 8.Lamia B, Maizel J, Ochagavia A, et al. Echocardiographic diagnosis of pulmonary artery occlusion pressure elevation during weaning from mechanical ventilation. Crit Care Med. 2009;37:1696–701. doi: 10.1097/CCM.0b013e31819f13d0. [DOI] [PubMed] [Google Scholar]
- 9.Haji K, Haji D, Canty DJ, et al. The impact of heart, lung and diaphragmatic ultrasound on prediction of failed extubation from mechanical ventilation in critically ill patients: A prospective observational pilot study. Crit Ultrasound J. 2018;10:13. doi: 10.1186/s13089-018-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farghaly S, Hasan AA. Diaphragm ultrasound as a new method to predict extubation outcome in mechanically ventilated patients. Aust Crit Care. 2017;30:37–43. doi: 10.1016/j.aucc.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Konomi I, Tasoulis A, Kaltsi I, et al. Left ventricular diastolic dysfunction – an independent risk factor for weaning failure from mechanical ventilation. Anaesth Intens Care. 2016;44:466–73. doi: 10.1177/0310057X1604400408. [DOI] [PubMed] [Google Scholar]
- 12.Soummer A, Perbet S, Brisson H, et al. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med. 2012;40:2064–72. doi: 10.1097/CCM.0b013e31824e68ae. [DOI] [PubMed] [Google Scholar]
- 13.Mongodi S, Via G, Bouhemad B, et al. Usefulness of combined bedside lung ultrasound and echocardiography to assess weaning failure from mechanical ventilation: A suggestive case. Crit Care Med. 2013;41:e182–5. doi: 10.1097/CCM.0b013e31828e928d. [DOI] [PubMed] [Google Scholar]
- 14.DiNino E, Gartman EJ, Sethi JM, McCool FD. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax. 2014;69:423–27. doi: 10.1136/thoraxjnl-2013-204111. [DOI] [PubMed] [Google Scholar]
- 15.Stieff KV, Lim F, Chen L. Factors influencing weaning older adults from mechanical ventilation: An integrative review. Crit Care Nurs Q. 2017;40:165–77. doi: 10.1097/CNQ.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 16.Haji K, Haji D, Canty DJ, et al. The impact of heart, lung and diaphragmatic ultrasound on prediction of failed extubation from mechanical ventilation in critically ill patients: A prospective observational pilot study. Crit Ultrasound J. 2018;10:13. doi: 10.1186/s13089-018-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohn DW, Song JM, Zo JH, et al. Mitral annulus velocity in the evaluation of left ventricular diastolic function in atrial fibrillation. J Am Soc Echocardiogr. 1999;12:927–31. doi: 10.1016/s0894-7317(99)70145-8. [DOI] [PubMed] [Google Scholar]
- 18.Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: Methods, reproducibility, and normal values. Chest. 2009;135:391–400. doi: 10.1378/chest.08-1541. [DOI] [PubMed] [Google Scholar]
- 19.Kollef MH. Ventilator-associated complications, including infection-related complications: The way forward. Crit Care Clin. 2013;29:33–50. doi: 10.1016/j.ccc.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Teboul JL. Weaning-induced cardiac dysfunction: Where are we today? Intensive Care Med. 2014;40:1069–79. doi: 10.1007/s00134-014-3334-4. [DOI] [PubMed] [Google Scholar]
- 21.Lara TM, Hajjar LA, de Almeida JP, et al. High levels of B-type natriuretic peptide predict weaning failure from mechanical ventilation in adult patients after cardiac surgery. Clinics (Sao Paulo) 2013;68:33–38. doi: 10.6061/clinics/2013(01)OA05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mekontso-Dessap A, de Prost N, Girou E, et al. B-type natriuretic peptide and weaning from mechanical ventilation. Intensive Care Med. 2006;32:1529–36. doi: 10.1007/s00134-006-0339-7. [DOI] [PubMed] [Google Scholar]
- 23.Vieira S, Savi A, Teixeira C, et al. Predicting success in weaning from mechanical ventilation. Crit Care. 2008;12:P328. [Google Scholar]
- 24.de Meirelles Almeida CA, Nedel WL, Morais VD, et al. Diastolic dysfunction as a predictor of weaning failure: A systematic review and meta-analysis. J Crit Care. 2016;34:135–41. doi: 10.1016/j.jcrc.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Saleh M, Vieillard-Baron A. On the role of left ventricular diastolic function in the critically ill patient. Intensive Care Med. 2012;38:189–91. doi: 10.1007/s00134-011-2448-1. [DOI] [PubMed] [Google Scholar]
- 26.Haddam M, Zieleskiewicz L, Perbet S, et al. Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med. 2016;42:1546–56. doi: 10.1007/s00134-016-4411-7. [DOI] [PubMed] [Google Scholar]
- 27.Vassilakopoulos T. Ventilator-induced diaphragm dysfunction: The clinical relevance of animal models. Intensive Care Med. 2008;34:7–16. doi: 10.1007/s00134-007-0866-x. [DOI] [PubMed] [Google Scholar]