Abstract
Background
Probiotic therapy has been shown to be beneficial against some liver diseases. However, there is still uncertainty regarding the clinical efficacy of probiotics for the treatment of variceal rebleeding. This research explored the efficacy of probiotics in variceal rebleeding.
Material/Methods
This was a retrospective study of 704 consecutive patients with liver cirrhosis who recovered from esophagogastric variceal bleeding after endoscopic treatment. Patients were subdivided into a probiotics cohort (n=214) and a non-probiotics cohort (n=490) based on the cumulative defined daily dose (cDDD) of probiotics received during follow-up. Propensity score matching was utilized to obtain a relatively balanced cohort of 200 patients per group for the analysis. Patients were monitored for rebleeding during the one-year follow-up.
Results
Multivariate Cox regression analysis revealed that probiotic therapy (≥28cDDD) was an independent protector against rebleeding (AHR=0.623; 95% CI=0.488–0.795; P<0.001). After propensity score matching, Kaplan-Meier analysis revealed that the rebleeding rate was higher in the non-probiotics cohort (n=200) than in the probiotics cohort (n=200) (56.0% vs. 44.0%, P=0.002). The incidence of rebleeding decreased with increased probiotic dosage (56.0%, 48.5%, 43.3%, and 38.1% in <28 cDDD, 28–60 cDDD, 61–90 cDDD, and >90 cDDD groups, respectively; P=0.011). The median rebleeding interval in the probiotics cohort (n=95) was significantly longer than that in the non-probiotics cohort (n=261) (147.0 vs. 91.0 days; P<0.001).
Conclusions
Adjuvant probiotic therapy significantly reduced the incidence of variceal rebleeding and delayed rebleeding after endotherapy in patients with cirrhosis.
MeSH Keywords: Esophageal and Gastric Varices, Liver Cirrhosis, Probiotics, Recurrence
Background
Esophagogastric variceal bleeding (EGVB) is one of the main complications of portal hypertension in cirrhosis, and has a high risk of rebleeding and death [1]. The cost of hospitalization for recurrent bleeding is also significantly higher than that for other types of decompensations [1]. For patients without secondary prophylaxis, the rebleeding rate is as high as 60% in 1 or 2 years [2]. Thus, preventing the occurrence of rebleeding is critical. Currently, the first-line standard therapy after variceal bleeding is the combination of repeated endoscopic and nonselective β-blocker therapy [3,4]. Although there have been great advances in these therapies, the risk of rebleeding remains high [5]. Therefore, there is a need to further improve the management of these patients.
Probiotics are live microorganisms that can be used for the modulation of intestinal microflora [6]. An increasing number of benefits of probiotics have been recognized, mainly because they are non-pharmacological, ecological, and relatively inexpensive measures for the prevention and treatment of a variety of diseases [7]. The main features underlying their beneficial effects include their ability to modulate the inflammatory response, strengthen the intestinal barrier, and modify the intestinal flora [8,9]. In recent years, the relationship between intestinal flora and liver disease has been receiving increasing attention. Studies have shown that hepatitis, cirrhosis, and associated complications are linked to varying degrees of intestinal flora imbalance and bacterial translocation [10,11]. Numerous studies have demonstrated positive results following the use of probiotics in liver disease, including hepatic encephalopathy (HE), alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), and hepatocellular carcinoma (HCC) [12]. Each probiotic strain has unique functionalities in the treatment of liver disease [12]. For instance, Lactobacillus rhamnosus GG, as the most typical probiotic strain, has been demonstrated to prevent hepatic fibrosis by reducing hepatic bile acid synthesis and promoting bile acid excretion [13]. Bifidobacterium CECT7765 can help control sustained inflammation in decompensated cirrhosis [14]. Additionally, the use of Akkermansia muciniphila has been found to mitigate ethanol-induced liver injury and neutrophil infiltration owing to its promotional effect on mucus production, which strengthens intestinal barrier integrity [15]. Thus, probiotics are an attractive strategy for treating liver diseases [8,16].
In addition, some studies have shown a positive effect of probiotics in the treatment of variceal bleeding. De Santis et al. found that oral probiotics could lower portal pressure, decreasing the risk of bleeding in patients with cirrhosis and esophageal varices in a case study [17]. The beneficial effect of probiotics in this context has been supported by two independent studies. One showed that adjunctive probiotic treatment attenuated portal hypertension and the other found that probiotics can be used to assist in the primary prevention of variceal bleeding [18,19]. However, there have been few reports on the role of probiotics in the prevention of variceal rebleeding.
In the current study, we retrospectively analyzed the clinical data of 704 cirrhotic patients with variceal bleeding, with the aim of assessing the efficacy of probiotics in esophagogastric variceal rebleeding. Our results showed that probiotic therapy significantly deceased the variceal rebleeding rate and delayed rebleeding time. This study provides new ideas for the secondary prophylaxis of esophagogastric variceal bleeding.
Material and Methods
Patient selection
Approval was obtained from the Ethical Review Committee of Beijing Ditan Hospital (Beijing, China). This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki. Because this was an observational retrospective study, the ethics committee waived the need for informed consent.
The target population of this study was patients with cirrhosis who recovered from an esophagogastric variceal bleeding after endoscopic treatment at Capital Medical University affiliated Beijing Ditan Hospital, between August 2008 and October 2016. All patients included in the study were diagnosed with cirrhosis based on previous or current biopsy findings, laboratory data, or imaging findings [20]. EGVB was diagnosed based on one of the following endoscopic findings: active bleeding from a varix, clots overlying a varix, “white nipple” overlying a varix, and varices with no other potential source of bleeding [21]. Patients were said to have recovered from variceal bleeding if they had no hematemesis, and maintained a stable haemoglobin concentration (without transfusion) and hemodynamic condition for at least 5 d after endoscopy [22].
Exclusion criteria included: liver or other organ malignancies before or during the first admission period, pregnancy, other diseases that can cause bleeding (ulcerative diseases, gastric mucosal lesion, idiopathic thrombocytopenic purpura, or other hematological diseases), resected liver and spleen, follow-up period of less than 1 year, or incomplete information. In addition, we also excluded patients who had taken probiotics within 3 months prior to inclusion.
Treatments and group assignments
All patients were evaluated by physicians and received standard treatment according to the previous reports [3,4]. Endoscopic treatment of variceal bleeding (endoscopic variceal ligation for esophageal varices and N-butyl cyanoacrylate injection for gastric varices) was performed as soon as possible. If bleeding could not be controlled within 48 h after endoscopy, transjugular intrahepatic portosystemic shunt placement, balloon tamponade, or surgery was performed. These patients with uncontrolled bleeding requiring further measures were not within the scope of our study. Upon recovery from variceal bleeding, patients received repeated rounds of variceal ligation therapy or sclerotherapy in the follow-up period to prevent rebleeding. Moreover, depending on the clinical condition of the patient, contraindications, and drug tolerability, nonselective β-blockers were prescribed for some patients.
In addition, some patients were also given probiotics at varied doses as an adjuvant therapy to regulate intestinal microflora dysbiosis caused by decompensated cirrhosis during this process. The probiotics discussed in this study are commonly used and produced in China, including “Bacillus licheniformis Capsule, Live;” “Live Combined Bifidobacterium, Lactobacillus, and Enterococcus Capsules, Oral;” and “Combined Bifidobacterium, Lactobacillus, Enterococcus, and Bacillus cereus Tablets, Live”. The amount of probiotics was calculated using the defined daily dose (DDD). DDD is a unit of statistical measurement that is recommended by the World Health Organization for quantifying a prescribed dose of drugs, i.e., the average maintenance dose for an adult in a day would be called 1 DDD. The DDD information for each probiotic used in our study is shown in Supplementary Table 1. The cumulative defined daily dose (cDDD), which indicates the exposure duration of drug use, is the sum of the DDDs of any drug used during the follow-up period [23]. Considering clinically effective dosage of probiotics [24], patients who received ≥28 cDDD of probiotics were enrolled in the probiotics cohort, whereas those who took <28 cDDD of probiotics were included in the non-probiotics cohort.
Clinical evaluation
We collected basic information about the patients at admission, including age, sex, comorbidities, etiologies, previous variceal bleeding, and other information about the bleeding. Routine laboratory tests, such as hematologic parameters, blood chemistry, and hemagglutination index, were also performed. Child-Pugh classification and Model for End-Stage Liver Disease (MELD) score were used to evaluate hepatic dysfunction [25]. We also documented secondary prophylaxis measures taken during the follow-up period. Furthermore, to explore the effect of comorbidities on variceal rebleeding, we used the Deyo-Charlson Comorbidity Index [26]. In our study, the index comprised nine diagnostic categories: peripheral vascular disease, cerebrovascular disease, congestive heart failure, chronic pulmonary disease, myocardial infarction, dementia, hemiplegia or paraplegia, rheumatic disease, and renal disease.
The risk of rebleeding was assessed from the inception point to the point of development of variceal rebleeding and after 1 year, in the probiotics cohort and the non-probiotics cohort, respectively. To reduce bias between the two cohorts, propensity score matching was used to simulate a relatively randomized retrospective study. We used a logistics regression model to calculate the propensity score for each patient. The variables included in the model were those that may affect the risk of rebleeding, including etiologies, international normalized ratio (INR), glucose (GLU), total bilirubin (TBIL), Child-Pugh classification, MELD score, and other treatment measures.
Outcomes and follow-up
The outcome of our study was clinically significant esophagogastric variceal rebleeding. This diagnosis was based on clinical findings and endoscopic evaluation. First, there was clinically significant bleeding; this was defined as recurrent hematemesis or melena resulting in either hospital admission, a drop in the hemoglobin level of at least 3 g/L, or blood transfusion according to the Baveno Guidelines [3]. Second, endoscopic examination revealed esophagogastric varices with no other potential source of bleeding such as ulceration and portal hypertensive gastropathy. The follow-up period was from the inception point until after 1 year or within the year if rebleeding occurred.
Data on the use of probiotics
As this is a retrospective study, medical information, including the type and dosage of probiotics, was obtained from the outpatient and inpatient electronic medical record systems; moreover, telephone follow-up was conducted to understand the patient’s medication compliance. Cases of patients with poor drug compliance or unclear compliance were regarded as having incomplete information and thus excluded. To determine the dose-response relationship, we stratified the study population into four groups according to the levels of prescribed probiotics for the patients (<28, 28–60, 61–90, and >90 cDDD). The bacterial content of each of the three probiotics supplements used was recorded, as follows. (1) ‘Bacillus licheniformis Capsule, Live’: composed of Bacillus licheniformis only; the number of cells in a capsule was not less than 250 million. (2) ‘Live Combined Bifidobacterium, Lactobacillus, and Enterococcus Capsules, Oral’: composed of Bacillus bifidus, Lactobacillus acidophilus, and Enterococcus faecalis; the number of viable bacteria in a capsule was not less than 1.0×107 colony forming units (CFU). (3) ‘Combined Bifidobacterium, Lactobacillus, Enterococcus, and Bacillus cereus Tablets, Live: ‘ composed of Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus; the number of Bifidobacterium infantis, Lactobacillus acidophilus, and Enterococcus faecalis in a capsule was not less than 5×107 CFU and the number of Bacillus cereus in a capsule was not less than 5×106 CFU.
Statistical analyses
Statistical analyses were performed with the Statistical Package for Social Sciences version 22.0 (IBM Corp, Armonk, NY). Statistical significance was established at P<0.05. A Cox proportional hazards model was used to perform a multivariate analysis and the backward elimination procedure. P<0.05 was set as the cut-off value for the elimination. Clinical and demographic characteristics between patients with EGVB who used probiotics and those who did not use probiotics were analyzed using a Fisher’s exact or chi-square test for categorical variables and by Mann-Whitney U test or Student’s T-test for continuous variables. Numbers were used to describe categorical variables, mean±standard deviation (SD) values were used to describe normally distributed continuous variables, and medians with interquartile ranges (IQR) were used for continuous variables with skewed distributions. Propensity score matching was used to mitigate the differences in baseline characteristics between the two groups. The cumulative incidence of rebleeding was determined using the Kaplan-Meier method. The results were compared by modified log-rank test.
Results
Patients’ characteristics
Between August 2008 and October 2016, 3557 patients with EGVB were identified; among these, 704 fulfilled the inclusion and exclusion criteria and were recruited for this study. In total, 214 patients who received ≥28 cDDD of probiotics were enrolled in the probiotics cohort, whereas 490 patients who took less than 28 cDDD of probiotics were included in the non-probiotics cohort. After 1: 1 propensity score matching, the probiotics and non-probiotics cohorts comprised 200 matched patients (Figure 1).
Figure 1.
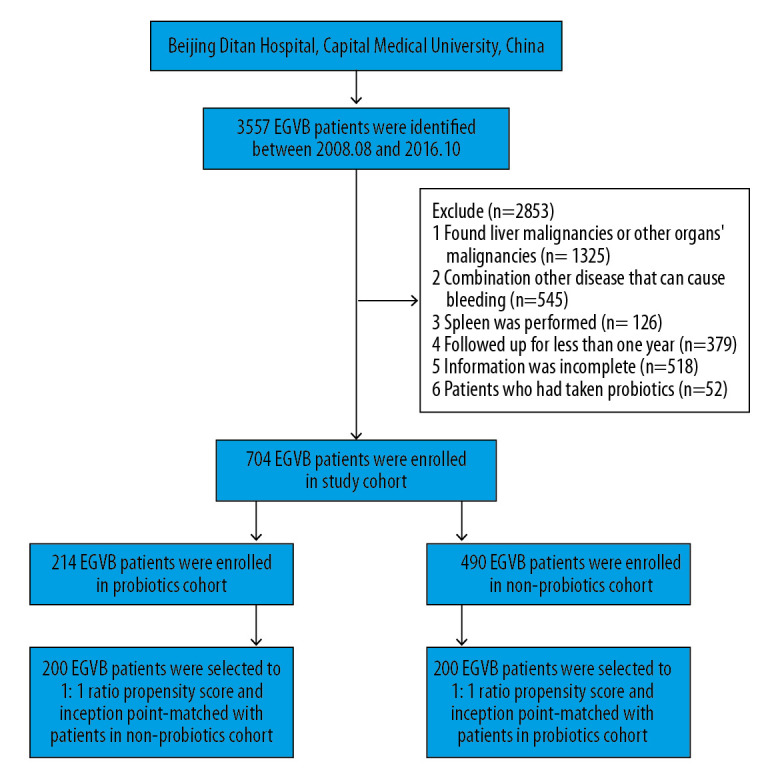
Flowchart of the enrollment of patients with EGVB into the probiotics and non-probiotics cohorts.
The clinical characteristics of the 704 patients included in this study are presented in Table 1. The median age was 52.0 years (interquartile ranges, 45.0–59.0) and 70.5% of the patients were male. Hepatitis B virus (HBV) infection (48.9%), ALD (18.5%), and hepatitis C virus (HCV) infection (8.9%) were the three main underlying causes of EGVB. Of these three major causes, ALD patients had the highest incidence of rebleeding (60.0%) (Supplementary Table 2). Most of the patients with EGVB (87.2%) were free of HE and a large proportion of the patients (55.1%) had experienced bacterial infections. A previous history of variceal bleeding was found in 253 (35.9%) patients. Most of the bleeding (79.1%) had occurred simultaneously in the esophageal and gastric varices; a small number of patients had bleeding in only the esophageal (18.5%) or gastric (2.4%) varices. Most patients had moderately impaired liver function: 443 patients (62.9%) had Child-Pugh class B liver disease, and the median MELD score was 10 points. The comorbidity index was not associated with rebleeding.
Table 1.
Baseline characteristics.
| Variables | Total (n=704) | Rebleeding group (n=356) | Non-rebleeding group (n=348) | P-value |
|---|---|---|---|---|
| Age (years) | 52.0 (45.0–59.0) | 51.5 (45.0–58.0) | 52.0 (44.0–60.0) | 0.955 |
| Sex (Male/Female) | 496/208 | 250/106 | 246/102 | 0.892 |
| Etiology (HBV/HCV/Alcohol/Alcohol+HBV/Other) | 344/63/130/53/114 | 147/34/78/36/61 | 197/29/52/17/53 | <0.001 |
| Comorbidity index (0/1/≥2) | 489/157/58 | 234/86/36 | 255/71/22 | 0.228 |
| Hepatic encephalopathy (yes/no) | 90/614 | 52/304 | 38/310 | 0.143 |
| Bacterial infection (yes/no) | 388/316 | 205/151 | 183/165 | 0.183 |
| Previous variceal bleeding (yes/no) | 253/451 | 130/226 | 123/235 | 0.746 |
| Location of varices (esophageal/gastric/esophageal and gastric) | 130/17/557 | 57/10/289 | 73/7/268 | 0.202 |
| Child-Pugh class (A/B/C) | 164/443/97 | 73/222/61 | 91/221/36 | <0.001 |
| MELD score | 10.0 (9.0–13.0) | 11.0 (9.0–14.0) | 10.0 (9.0–12.0) | 0.005 |
| ALT (U/L) | 25.5 (18.0–39.0) | 25.5 (17.7–39.1) | 25.1 (18.1–38.9) | 0.896 |
| AST (U/L) | 31.7 (22.3–48.1) | 32.9 (22.8–50.4) | 30.5 (22.1–46.9) | 0.282 |
| TBIL (μmol/L) | 20.0 (13.3–31.0) | 20.5 (13.0–33.1) | 19.7 (13.6–29.9) | 0.400 |
| GGT (U/L) | 26.5 (15.3–58.7) | 30.6 (16.6–70.9) | 24.1 (13.7–48.7) | 0.001 |
| ALB (g/L) | 30.0±5.8 | 29.8±5.7 | 30.2±5.8 | 0.345 |
| CREA (μmol/L) | 65.0 (53.3–77.6) | 65.8 (54.0–77.6) | 64.3 (52.1–77.6) | 0.474 |
| GLU (mmol/L) | 8.4 (6.7–11.2) | 8.9 (6.9–11.7) | 8.0 (6.4–10.3) | <0.001 |
| WBC (×109/L) | 4.3 (2.9–6.5) | 4.5 (3.0–6.7) | 4.0 (2.8–6.5) | 0.126 |
| NLR | 4.0 (2.7–6.4) | 4.2 (2.7–7.1) | 3.8 (2.6–5.8) | 0.029 |
| HGB (g/L) | 79.2 (62.2–95.2) | 78.2 (62.7–94.0) | 79.8 (62.1–97.0) | 0.607 |
| PLT (×109/L) | 59.5 (44.0–79.5) | 59.7 (43.4–78.7) | 59.5 (44.1–81.0) | 0.586 |
| INR | 1.3 (1.2–1.4) | 1.3 (1.2–1.5) | 1.3 (1.2–1.4) | 0.001 |
| Secondary prophylaxis* (yes/no) | 656 (93.2) | 324 (91.0) | 332 (95.4) | 0.021 |
HBV – hepatitis B virus; HCV – hepatitis C virus; MELD – model for end-stage liver disease; ALT – alanine aminotransferase; AST – aspartate aminotransferase; TBIL – total bilirubin; GGT-γ – glutamyl transferase; ALB – albumin; CREA – creatinine; GLU – glucose; WBC– white blood cell; NLR– neutrophil-lymphocyte ratio; HGB – hemoglobin; PLT– platelet; INR– international normalized ratio.
Secondary prophylaxis included endoscopic treatment (variceal ligation therapy or sclerotherapy) and/or nonselective beta-blockers.
Data are presented as number, mean±SD, or median (IQR).
Effect of probiotics on variceal rebleeding
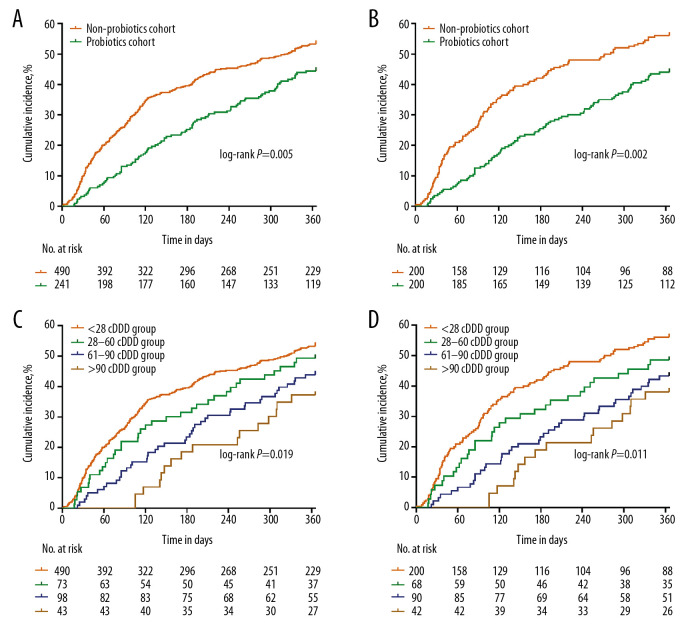
In total, 356 (50.6%) patients experienced rebleeding within 1 year. After adjusting for age, sex, etiologies, comorbidity index, HE, bacterial infection, previous variceal bleeding, Child-Pugh class, MELD score, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), TBIL, albumin (ALB), creatinine (CREA), GLU, neutrophil-lymphocyte ratio (NLR), white blood cell (WBC), platelet (PLT), hemoglobin (HGB), INR, and secondary prophylaxis using the Cox regression model, probiotic therapy (≥28 cDDD) was an independent protective factor in EGVB rebleeding (adjusted hazard ratio (AHR)=0.623, 95% confidence interval (CI)=0.488–0.795, P<0.001; Table 2). Of the 704 patients, patients in the non-probiotics cohort had a significantly higher cumulative incidence of rebleeding than those in the probiotics cohort (53.3% vs. 44.4%, P=0.005; Figure 2A). To further confirm the role of probiotics in patients with EGVB, the propensity scores were calculated for 214 patients in the probiotics cohort and 490 patients in the non-probiotics cohort. We successfully matched 200 patients in both the probiotics and non-probiotics cohorts. There was no significant difference between cohorts with respect to any key confounders at baseline (Table 3). After matching, the rebleeding rate in the non-probiotics cohort (n=200) was still found to be higher than that in the probiotics cohort (n=200) (56.0% vs. 44.0%, P=0.002; Figure 2B).
Table 2.
Univariate and multivariate analyses with esophagogastric variceal rebleeding.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age (years) | 1.002 (0.992–1.012) | 0.696 | ||
| Sex (Male vs. Female) | 1.048 (0.835–1.316) | 0.683 | ||
| Etiology | <0.001 | 0.002 | ||
| HCV vs. HBV | 1.400 (0.964–2.033) | 0.077 | 1.461 (1.003–2.129) | 0.048 |
| Alcohol vs. HBV | 1.643 (1.248–2.163) | <0.001 | 1.395 (1.044–1.864) | 0.024 |
| Alcohol+HBV vs. HBV | 1.919 (1.332–2.764) | <0.001 | 1.844 (1.278–2.661) | 0.001 |
| Other vs. HBV | 1.385 (1.028–1.868) | 0.032 | 1.542 (1.137–2.092) | 0.005 |
| Comorbidity index | 1.217 (1.044–1.420) | 0.012 | ||
| Hepatic encephalopathy (yes vs. no) | 1.240 (0.924–1.664) | 0.152 | ||
| Bacterial infection (yes vs. no) | 1.207 (0.978–1.489) | 0.080 | ||
| Previous variceal bleeding (yes vs. no) | 1.038 (0.836–1.288) | 0.737 | ||
| Child-Pugh class | 1.320 (1.108–1.572) | 0.002 | ||
| MELD score | 1.066 (1.037–1.095) | <0.001 | ||
| ALT (U/L) | 1.001 (1.000–1.002) | 0.001 | ||
| AST (U/L) | 1.000 (1.000–1.000) | 0.008 | ||
| TBIL (μmol/L) | 1.004 (1.002–1.006) | <0.001 | 1.003 (1.001–1.006) | 0.007 |
| GGT (U/L) | 1.001 (1.001–1.002) | 0.002 | ||
| ALB (g/L) | 0.989 (0.971–1.007) | 0.241 | ||
| CREA (μmol/L) | 1.004 (1.000–1.007) | 0.039 | ||
| GLU (mmol/L) | 1.031 (1.013–1.050) | 0.001 | 1.032 (1.013–1.050) | 0.001 |
| WBC (×109/L) | 1.038 (1.016–1.060) | <0.001 | ||
| NLR | 1.016 (1.000–1.033) | 0.053 | ||
| HGB (g/L) | 0.999 (0.994–1.003) | 0.559 | ||
| PLT (×109/L) | 1.000 (0.997–1.003) | 0.786 | ||
| INR | 2.122 (1.480–3.043) | <0.001 | 1.697 (1.167–2.469) | 0.006 |
| Secondary prophylaxis (yes vs. no) | 0.490 (0.340–0.704) | <0.001 | 0.554 (0.381–0.807) | 0.002 |
| Probiotics therapy (≥28cDDD vs. <28cDDD) | 0.713 (0.564–0.902) | 0.005 | 0.623 (0.488–0.795) | <0.001 |
HR – hazard ratio; CI – confidence interval; HBV – hepatitis B virus; HCV – hepatitis C virus; MELD – model for end-stage liver disease; ALT – alanine aminotransferase; AST – aspartate aminotransferase; TBIL – total bilirubin; GGT-γ – glutamyl transferase; ALB – albumin; CREA – creatinine; GLU – glucose; WBC – white blood cell; NLR – neutrophil-lymphocyte ratio; HGB – hemoglobin; PLT – platelet; INR – international normalized ratio.
Figure 2.
The cumulative incidence of rebleeding in patients with EGVB. Both cumulative incidence of rebleeding in the probiotics and non-probiotics cohorts before (A) and after (B) matching, and the cumulative incidence of rebleeding after consumption of different dosages of probiotics before (C) and after (D) matching are shown.
Table 3.
Comparison of demographic and clinical characteristics between patients with EGVB with probiotics cohort and non-probiotics cohort.
| Variables | Before propensity matching | After propensity matching | ||||
|---|---|---|---|---|---|---|
| Probiotics cohort (n=214) | Non-probiotics cohort (n=490) | P-value | Probiotics cohort (n=200) | Non-probiotics cohort (n=200) | P-value | |
| Age (years) | 53.1±10.9 | 51.6±10.7 | 0.098 | 53.4±11.0 | 51.2±10.9 | 0.048 |
| Sex (Male/Female) | 146/68 | 350/140 | 0.391 | 67/133 | 56/144 | 0.233 |
| Etiology (HBV/HCV/Alcohol/Alcohol+HBV/Other) | 94/21/47/9/43 | 250/42/83/44/71 | <0.001 | 92/20/41/8/39 | 81/19/48/14/38 | 0.082 |
| Comorbidity index (0/1/≥2) | 147/44/23 | 342/113/35 | 0.043 | 138/39/23 | 128/52/20 | 0.157 |
| Hepatic encephalopathy (yes/no) | 41/173 | 49/441 | 0.001 | 34/166 | 27/173 | 0.330 |
| Bacterial infection (yes/no) | 140/173 | 248/242 | <0.001 | 128/72 | 110/90 | 0.067 |
| Previous variceal bleeding (yes/no) | 58/156 | 195/295 | 0.001 | 54/146 | 82/118 | 0.003 |
| Location of varices (esophageal/gastric/esophageal and gastric) | 37/6/171 | 93/11/386 | 0.726 | 36/6/158 | 48/5/147 | 0.225 |
| Child-Pugh class (A/B/C) | 33/135/46 | 131/308/51 | <0.001 | 30/131/39 | 41/124/35 | 0.293 |
| MELD score | 11.0 (9.0–14.0) | 10.0 (9.0–13.0) | 0.033 | 11.0 (9.0–14.0) | 11.0 (9.0–14.0) | 0.394 |
| ALT (U/L) | 26.8 (18.7–40.4) | 24.9 (17.5–38.7) | 0.125 | 26.2 (18.8–39.9) | 24.6 (17.3–39.3) | 0.189 |
| AST (U/L) | 34.2 (24.6–53.6) | 30.4 (22.0–46.2) | 0.011 | 33.5 (24.5–52.7) | 31.2 (22.0–49.7) | 0.191 |
| TBIL (μmol/L) | 21.8 (14.7–37.1) | 19.2 (12.9–29.5) | 0.002 | 21.7 (14.5–33.9) | 22.1 (12.9–34.1) | 0.571 |
| GGT (U/L) | 28.3 (15.3–65.4) | 25.9 (15.3–52.8) | 0.189 | 27.2 (14.9–62.9) | 30.2 (17.0–59.1) | 0.553 |
| ALB (g/L) | 28.7±5.5 | 30.6±5.8 | <0.001 | 28.4 (24.2–32.4) | 29.8 (26.4–33.4) | 0.009 |
| CREA (μmol/L) | 5.2 (52.9–76.9) | 65.0 (53.6–78.0) | 0.898 | 64.3 (52.4–75.4) | 62.1 (50.9–77.9) | 0.620 |
| GLU (mmol/L) | 9.1 (7.0–12.0) | 8.2 (6.5–11.0) | 0.006 | 9.1 (7.0–11.8) | 8.3 (6.5–11.5) | 0.128 |
| WBC (×109/L) | 4.8 (3.2–7.0) | 4.1 (2.7–6.3) | 0.020 | 4.8 (3.2–7.0) | 4.3 (2.8–6.5) | 0.260 |
| NLR | 4.3 (3.0–6.7) | 3.9 (2.6–6.2) | 0.038 | 4.3 (3.0–6.7) | 4.1 (2.6–6.0) | 0.183 |
| HGB (g/L) | 79.2 (62.2–95.0) | 78.6 (62.2–96.1) | 0.714 | 79.2 (62.2–95.0) | 78.1 (59.3–95.8) | 0.790 |
| PLT (×109/L) | 59.0 (45.3–78.9) | 60.0 (43.4–80.0) | 0.927 | 59.0 (45.7–78.7) | 63.0 (43.4–81.8) | 0.570 |
| INR | 1.3 (1.2–1.5) | 1.3 (1.2–1.4) | 0.621 | 1.3 (1.2–1.5) | 1.3 (1.2–1.4) | 0.245 |
| Secondary prophylaxis (yes/no) | 200/14 | 456/34 | 0.848 | 186/14 | 190/10 | 0.400 |
HBV – hepatitis B virus; HCV – hepatitis C virus; MELD – model for end-stage liver disease; ALT – alanine aminotransferase; AST – aspartate aminotransferase; TBIL – total bilirubin; GGT-γ – glutamyl transferase; ALB – albumin; CREA – creatinine; GLU – glucose; WBC – white blood cell; NLR – neutrophil-lymphocyte ratio; HGB – hemoglobin; PLT – platelet; INR– international normalized ratio. Data are presented as number, mean±SD, or median (IQR).
Effect of probiotics dose on variceal rebleeding
There was a dose-dependent relationship between probiotic use and the risk of developing rebleeding. When compared with patients in the <28 cDDD group (non-probiotics cohort, n=490), the adjusted HRs were 0.755 (95% CI, 0.529–1.078), 0.629 (95% CI, 0.452–0.874), and 0.442 (95% CI, 0.265–0.737) for patients in the 28–60 cDDD, 61–90 cDDD, and >90 cDDD groups, respectively, and there was a trend toward risk reduction with increasing cDDD (P=0.001; Table 4). The incidence of variceal rebleeding decreased with an increase in probiotic dosage (unmatched: 53.3%, 49.3%, 43.9%, and 37.2% in <28 cDDD, 28–60 cDDD, 61–90 cDDD, and >90 cDDD groups, respectively; P=0.019; Figure 2C; after matching: 56.0%, 48.5%, 43.3%, and 38.1%, respectively; P=0.011; Figure 2D).
Table 4.
Risk factor of probiotics dose for esophagogastric variceal rebleeding.
| Probiotics dose group | n | Rebleeding n (%) | AHR* (95% CI) | P-value |
|---|---|---|---|---|
| <28 cDDD group | 490 | 261 (53.3) | 1.0 | – |
| 28–60 cDDD group | 73 | 36 (49.3) | 0.755 (0.529–1.078) | 0.123 |
| 61–90 cDDD group | 98 | 43 (43.9) | 0.629 (0.452–0.874) | 0.008 |
| >90 cDDD group | 43 | 16 (37.2) | 0.442 (0.265–0.737) | 0.002 |
| P for trends | 0.001 |
AHR – adjusted hazard ratio; CI – confidence interval.
AHR represents multivariate-adjusted hazard ratio: etiology, comorbidity index, Child-Pugh class, MELD score, ALT, AST, TBIL, GGT, CREA, GLU, WBC, INR, secondary prophylaxis are adjusted using a Cox proportional hazard regression model.
Probiotics delay the recurrence time of EGVB
Furthermore, we analyzed 356 patients who had experienced recurring EGVB. We divided them into the probiotics cohort (n=95) and non-probiotics cohort (n=261) according to whether the patients were advised to take probiotics during the follow-up period. We found that, although EGVB rebleeding occurred in all of these patients, the median time of recurrence in patients from the probiotics cohort (n=95) was 147.0 days, which was significantly longer than the recurrence period of 91.0 days recorded for patients from the non-probiotics cohort (n=261) (P<0.001; Figure 3).
Figure 3.
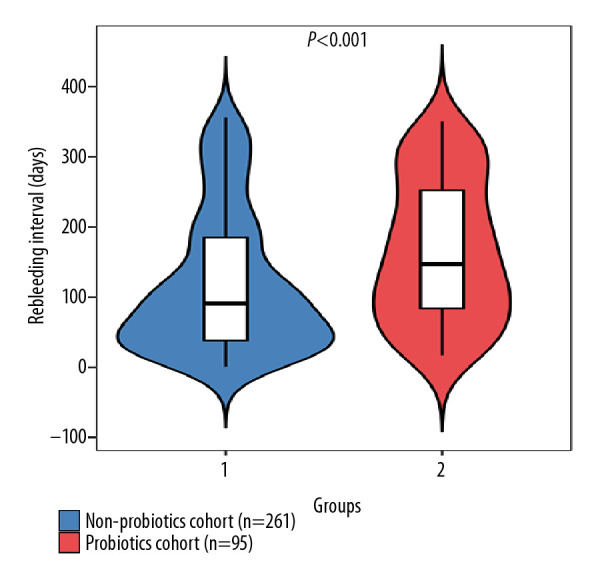
Use of probiotics and variceal rebleeding intervals in patients with recurrent bleeding.
Subgroup analysis of variceal rebleeding
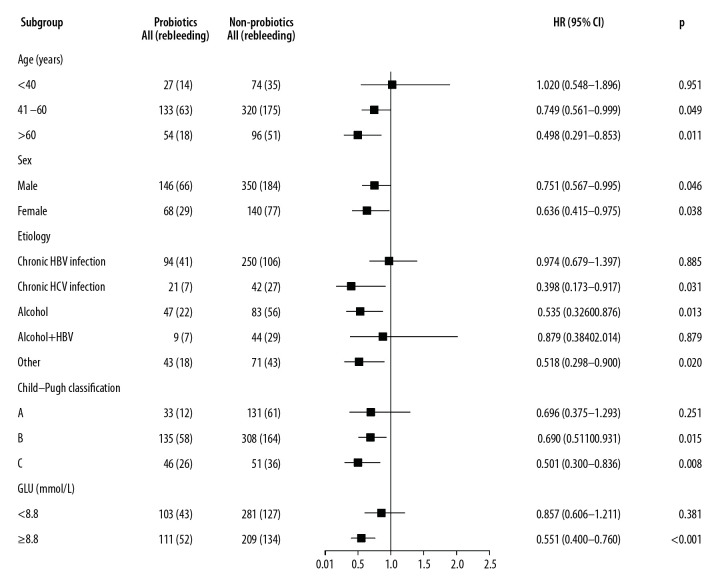
The relationship between variceal rebleeding and key baseline stratification factors associated with the rebleeding was analyzed using the Cox proportional-hazards model. This analysis showed that the most significant benefit of taking probiotics (≥28 cDDD) was seen in patients with the following characteristics: age >40 years of either sex, GLU ≥8.8 mmol/L and Child-Pugh class B/C. Furthermore, treatment with probiotics (≥28 cDDD) can significantly reduce the occurrence of rebleeding in patients with HCV infection (HR=0.398; 95% CI=0.173–0.917), ALD (HR=0.535; 95% CI=0.326–0.876), or other etiology (HR=0.518; 95% CI=0.298–0.900) (Figure 4). Details regarding other etiologies are given in Supplementary Table 2. Furthermore, we divided the patients with other etiologies into groups of patients with HBV and non-HBV infections. In these two subgroups, we observed that probiotics were more effective in the prevention of rebleeding in patients with non-HBV infections (Supplementary Table 3). Therefore, our findings support that probiotics can reduce rebleeding rate in patients without HBV infection.
Figure 4.
Forest plot showing variceal rebleeding risk of the probiotics (n=214) and non-probiotics (n=490) cohorts in different subgroups of EGVB patients.
Discussion
Our population-based study in patients with EGVB suggested that probiotics independently protect patients with EGVB against the occurrence of rebleeding in a dose-dependent manner. Our study is the first to reveal the effects of probiotics use on reducing variceal rebleeding in patients with EGVB, thus supporting the pleiotropic effects of probiotics in advanced liver disease. A propensity score-matching was used to mimic the randomization of a prospective study and reduce the bias caused by confounding variables, which further substantiates the credibility of these findings.
Although the mechanisms by which probiotics may have protective effects against rebleeding are not yet completely understood, they have been acknowledged to contribute to restoring intestinal permeability, microbiome composition, and the normal inflammatory response [27]. Previous studies have shown that Bifidobacterium strains can protect the host by preventing an increase in intestinal permeability and promoting a healthier microbial environment [28,29]. In addition, Lactobacillus strains can enhance immune defense mechanisms [30,31]. In our study, strains of Bifidobacterium and Lactobacillus were the main probiotics used, and their protective role observed in patients with EGVB may involve similar mechanisms. In patients with EGVB, complications are more common with bacterial infections; in the present study, about 55% of the patients had a bacterial infection. Treatment and prophylactic use of antibiotics are common, and previous studies have shown that the use of antibiotics is a major cause of intestinal flora imbalance [32,33]. Studies have shown that malnutrition also leads to intestinal flora imbalance, and in patients with EGVB, the decreases in liver nutrient synthesis and absorptive ability caused by bleeding further aggravate the intestinal flora imbalance. Reciprocal interactions between gut microbiota and the liver become highly dysfunctional with the progression of clinically significant portal hypertension, which is the primary factor underlying EGVB [34]. Cirrhosis patients with EGVB are prone to changes in intestinal flora, which can result in intestinal endotoxemia. These changes can also induce or aggravate the portal hypertension caused by esophageal and gastric vein injury, thereby resulting in increased bleeding. Intestinal probiotics can effectively improve the intestinal flora imbalance; they also help to reduce portal pressure in patients with cirrhosis and regulate the immune system by strengthening anti-inflammatory effects [35,36]. Therefore, the early and adequate use of probiotics may be able to reduce the incidence of rebleeding through enhanced regulation of intestinal flora.
It should be noted that, in our study, the non-probiotics cohort (n=490) included both those who did not consume probiotics at all (n=397) and those who took probiotics but for whom the cumulative dose was less than 28 cDDD (n=93; median 13, interquartile range 7–20). We also attributed the latter group of patients (n=93) to the non-probiotic cohort, mainly as the benefits of probiotics for patients with variceal bleeding may need to reach a threshold effective dose [37]. Similar definitions for drug cohorts are widely used in some retrospective cohort studies of drug efficacy evaluation [38–40]. In our study, the dose of 28 cDDD was a hypothesis based on the previous literature, which showed that taking probiotics for 28 days can improve some parameters involved in portal hemodynamics [24]. As three probiotic preparations were used in this study, we calculated their cumulative dose using the cDDD (not the number of days). Although some studies have explored the effect of probiotics on portal hypertension, there is still no clear evidence of the effective dose for probiotics to reduce portal hypertension [18,19,24,35]. Further studies are needed to resolve this question.
We also found that the most significant benefit of taking probiotics (≥28 cDDD) was seen in patients who were relatively older (age >40 years), and had higher GLU levels, more severe liver damage (Child-Pugh B/C), and no HBV infection. Liver is the major metabolic organ in the human body, and normal liver metabolism can maintain the relative stability of blood glucose levels in the body. However, in patients with liver cirrhosis, especially in its decompensation stage, serious damage to liver function can lead to disorders of glucose metabolism and an increase in blood sugars [41]. Elevated blood sugar level, in turn, speeds up the progression of cirrhosis and increases the risk of bleeding, as well as death [42,43], which is consistent with observations in our study. A previous study has shown that the modulation of intestinal microbiota by probiotics may be effective toward prevention and management of diabetes [44], thus reducing the patients’ risk of rebleeding. Another study showed that changes in gut microflora are affected by liver disease stage [45]. Patients with a more advanced Child-Pugh grade have greater perturbations in proinflammatory cytokines, intestinal permeability, and endotoxin levels [46,47]. Therefore, the higher the Child-Pugh grade is, the greater the benefit of probiotics.
Furthermore, we found that probiotics could also play a protective role in patients with HCV infection and ALD. Meanwhile, for HBV patients, the effect of probiotics on rebleeding within a year was not statistically significant. Clinical data indicated that the composition of intestinal bacteria was significantly different in patients with different diseases [48]. Therefore, the role of probiotics in each etiology is also different. A heat-treated Enterococcus faecalis strain can significantly reduce the blood concentration of transaminases in both short- and long-term HCV infection without any side effects [49]. A clinical study also suggested that the use of probiotics may show therapeutic effects in the treatment of patients with alcoholic hepatitis [50]. It has been reported that the effect of intestinal microbiota changes in patients with cirrhotic hepatitis B on hepatic fibrotic and neoplastic transformations is less than that in patients with CHC [51]. In contrast, in CHB cirrhosis, intestinal Bifidobacterium species might switch from being beneficial species to opportunistic pathogens [52].
Moreover, we observed a higher incidence of variceal rebleeding in ALD patients than in CHB and CHC patients. Previous studies have shown that, similar to other etiologies, patients with alcoholic cirrhosis develop portal hypertension and subsequent alterations in the hepatic, splanchnic, and systemic hemodynamics [53]. However, in alcohol-related cirrhosis, sinusoidal pressure is generally higher compared with that in other types of cirrhosis [53]. Moreover, alcohol consumption can acutely increase portal-collateral blood flow and portal pressure [53]. In this study, about 50.0% of patients with ALD did not undergo alcohol withdrawal. All of these factors resulted in the increased incidence of rebleeding in patients with ALD.
There are several potential limitations of this study. First, the results of this study cannot be generalized to all patients recovering from variceal bleeding because this study is a retrospective, single-center study. We are designing a prospective, multicenter, randomized study to confirm these findings. Second, as this was an observational study, not all patients received standard treatment; there may be differences in other treatment methods between the probiotic and non-probiotic cohort. However, we collected as much treatment information as possible and performed propensity score matching and multivariate Cox proportional-hazards analysis to reduce the impact of other treatment methods on the evaluation of the effect of probiotics. Third, in subgroup analyses, the number of patients in some subgroups were small, so the results should be interpreted cautiously for patients in these subgroups. Furthermore, there were no positive control drugs. This is because probiotics are commonly used in cirrhosis patients, and the purpose of this study was to investigate whether probiotics play a part in rebleeding but not to compare them with other effective drugs. Finally, to validate the long-term effectiveness of probiotics therapy, long-term follow-up is required to investigate not only cumulative bleeding event rates but also its cost and survival rate. Despite these limitations, we observed a protective effect of probiotics on esophagogastric variceal rebleeding. This is the first report of such an effect to our knowledge. Therefore, this may be a new way to enrich secondary prophylaxis measures to prevent esophagogastric variceal rebleeding. This study provides new insights to enrich the secondary prophylaxis scheme of EGVB.
Conclusions
In this population-based cohort study, our data suggested that adjuvant probiotic therapy significantly reduced the incidence of variceal rebleeding and delayed rebleeding after endoscopic treatment of patients with EGVB. This protective effect was more significant in patients with Child-Pugh class B/C and no HBV infection.
Supplementary Data
Supplementary Table 1.
Some details of the probiotics discussed in our study.
| Probiotics name | NMPN | The maintenance dose per day* | DDD |
|---|---|---|---|
| Bacillus licheniformis Capsule, Live | S10950019 | Three times a day and two tablets (0.5 g) at a time | 1.5 g |
| Live Combined Bifidobacterium, Lactobacillus, and Enterococcus Capsules, Oral | S10950032 | Two times a day and three tablets (0.63 g) at a time | 1.26 g |
| Combined Bifidobacterium, Lactobacillus, Enterococcus, and Bacillus cereus Tablets, Live | S20060010 | Three times a day and three tablets (1.5 g) at a time | 4.5 g |
NMPN –national medicine permission number; DDD – defined daily dose.
The maintenance dose per day of each probiotic preparation was obtained from the drug instructions.
Supplementary Table 2.
Detailed etiological distribution.
| Etiology | N (%) | Rebleeding rate | |
|---|---|---|---|
| Chronic HBV infection | 344 (48.9) | 42.7% | |
| Chronic HCV infection | 63 (8.9) | 54.0% | |
| Alcohol | 130 (18.5) | 60.0% | |
| Alcohol+HBV | 53 (7.5) | 67.9% | |
| Other HBV irrelevant | Alcohol+HCV | 9 (1.3) | 52.3% |
| Alcohol+PBC+AIH | 1 (0.1) | ||
| Alcohol+PBC | 3 (0.4) | ||
| PBC+AIH | 4 (0.6) | ||
| PBC | 28 (4.0) | ||
| AIH | 7 (1.0) | ||
| Unknown | 57 (8.1) | ||
| Other HBV correlation | HBV+HCV | 3 (0.4) | 80.0% |
| HBV+PBC | 2 (0.3) | ||
HBV – hepatitis B virus; HCV – hepatitis C virus; PBC – primary biliary cirrhosis; AIH – autoimmune hepatitis.
Supplementary Table 3.
Subgroup analysis of patients with other etiologies.
| Probiotics cohort all (rebleeding) | Non-probiotics cohort all (rebleeding) | P-value* | |
|---|---|---|---|
| Other HBV irrelevant (n=109) | 41 (17) | 68 (40) | 0.037 |
| Other HBV correlation (n=5) | 2 (1) | 3 (3) | 0.360 |
HBV – hepatitis B virus.
Subgroup analysis of esophagogastric variceal rebleeding in patients from the probiotics and non-probiotics cohorts using Cox regression.
Acknowledgements
We gratefully recognize the patients who participated in this study. We thank Yan Sang for her help with the data.
Footnotes
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest
None.
Source of support: This study was supported by the National Natural Science Foundation of China (Grant No. 81473641 and 81774234) and the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX201707)
References
- 1.Holster IL, Tjwa ET, Moelker A, et al. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy+beta-blocker for prevention of variceal rebleeding. Hepatology. 2016;63:581–89. doi: 10.1002/hep.28318. [DOI] [PubMed] [Google Scholar]
- 2.Shi KQ, Liu WY, Pan ZZ, et al. Secondary prophylaxis of variceal bleeding for cirrhotic patients: A multiple-treatments meta-analysis. Eur J Clin Invest. 2013;43:844–54. doi: 10.1111/eci.12115. [DOI] [PubMed] [Google Scholar]
- 3.de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding incirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–35. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Pagan JC, Villanueva C, Albillos A, et al. Nadolol plus isosorbide mononitrate alone or associated with band ligation in the prevention of recurrent bleeding: A multicentre randomised controlled trial. Gut. 2009;58:1144–50. doi: 10.1136/gut.2008.171207. [DOI] [PubMed] [Google Scholar]
- 6.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–78. [PubMed] [Google Scholar]
- 7.Soriano G, Guarner C. Probiotics in cirrhosis: Do we expect too much? Liver Int. 2013;33:1451–53. doi: 10.1111/liv.12288. [DOI] [PubMed] [Google Scholar]
- 8.Wiest R, Albillos A, Trauner M, et al. Targeting the gut-liver axis in liver disease. J Hepatol. 2017;67:1084–103. doi: 10.1016/j.jhep.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62:787–96. doi: 10.1136/gutjnl-2012-302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betrapally NS, Gillevet PM, Bajaj JS. Gut microbiome and liver disease. Transl Res. 2017;179:49–59. doi: 10.1016/j.trsl.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albillos A, de la Hera A, Gonzalez M, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208–17. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 12.Meng X, Li S, Li Y, et al. Gut microbiota’s relationship with liver disease and role in hepatoprotection by dietary natural products and probiotics. Nutrients. 2018;10:147. doi: 10.3390/nu10101457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Chen K, Li F, et al. Probiotic Lactobacillus rhamnosus GG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice. Hepatology. 2019;71:2050–66. doi: 10.1002/hep.30975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moratalla A, Caparros E, Juanola O, et al. Bifidobacterium pseudocatenulatum CECT7765 induces an M2 anti-inflammatory transition in macrophages from patients with cirrhosis. J Hepatol. 2016;64:135–45. doi: 10.1016/j.jhep.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Grander C, Adolph TE, Wieser V, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67:891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 16.Acharya C, Bajaj JS. Gut microbiota and complications of liver disease. Gastroenterol Clin North Am. 2017;46:155–69. doi: 10.1016/j.gtc.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Santis A, Famularo G, De Simone C. Probiotics for the hemodynamic alterations of patients with liver cirrhosis. Am J Gastroenterol. 2000;95:323–24. doi: 10.1111/j.1572-0241.2000.01726.x. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N, Kumar A, Sharma P, et al. Effects of the adjunctive probiotic VSL#3 on portal haemodynamics in patients with cirrhosis and large varices: A randomized trial. Liver Int. 2013;33:1148–57. doi: 10.1111/liv.12172. [DOI] [PubMed] [Google Scholar]
- 19.Rincon D, Vaquero J, Hernando A, et al. Oral probiotic VSL#3 attenuates the circulatory disturbances of patients with cirrhosis and ascites. Liver Int. 2014;34:1504–12. doi: 10.1111/liv.12539. [DOI] [PubMed] [Google Scholar]
- 20.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–61. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–38. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 22.de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–68. doi: 10.1016/j.jhep.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 23.WHO Collaborating Centre for Drug Statistics Methodology, Norwegian Institute of Public Health. Oslo, Norway: ATC/DDD Index; 2012. https://www.whocc.no/atc_ddd_index/ [Google Scholar]
- 24.Marlicz W, Wunsch E, Mydlowska M, et al. The effect of short term treatment with probiotic VSL#3 on various clinical and biochemical parameters in patients with liver cirrhosis. J Physiol Pharmacol. 2016;67:867–77. [PubMed] [Google Scholar]
- 25.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–49. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 26.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–19. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 27.Moratalla A, Gomez-Hurtado I, Santacruz A, et al. Protective effect of Bifidobacterium pseudocatenulatum CECT7765 against induced bacterial antigen translocation in experimental cirrhosis. Liver Int. 2014;34:850–58. doi: 10.1111/liv.12380. [DOI] [PubMed] [Google Scholar]
- 28.Caplan MS, Miller-Catchpole R, Kaup S, et al. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–83. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 29.Ruseler-van EJ, van Lieshout LM, Gosselink MJ, Marteau P. Inability of Lactobacillus casei strain GG, L. acidophilus, and Bifidobacterium bifidum to degrade intestinal mucus glycoproteins. Scand J Gastroenterol. 1995;30:675–80. doi: 10.3109/00365529509096312. [DOI] [PubMed] [Google Scholar]
- 30.Schiffrin EJ, Rochat F, Link-Amster H, et al. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J Dairy Sci. 1995;78:491–97. doi: 10.3168/jds.S0022-0302(95)76659-0. [DOI] [PubMed] [Google Scholar]
- 31.Bernet MF, Brassart D, Neeser JR, Servin AL. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483–89. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raymann K, Shaffer Z, Moran NA. Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees. PLoS Biol. 2017;15:e2001861. doi: 10.1371/journal.pbio.2001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becattini S, Taur Y, Pamer EG. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med. 2016;22:458–78. doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baffy G. Potential mechanisms linking gut microbiota and portal hypertension. Liver Int. 2019;39:598–609. doi: 10.1111/liv.13986. [DOI] [PubMed] [Google Scholar]
- 35.Tandon P, Moncrief K, Madsen K, et al. Effects of probiotic therapy on portal pressure in patients with cirrhosis: A pilot study. Liver Int. 2009;29:1110–15. doi: 10.1111/j.1478-3231.2009.02020.x. [DOI] [PubMed] [Google Scholar]
- 36.Lo RS, Austin AS, Freeman JG. Is there a role for probiotics in liver disease? Sci World J. 2014;2014 doi: 10.1155/2014/874768. 874768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62:787–96. doi: 10.1136/gutjnl-2012-302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang FM, Wang YP, Lang HC, et al. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: A population-based study. Hepatology. 2017;66:896–907. doi: 10.1002/hep.29172. [DOI] [PubMed] [Google Scholar]
- 39.Huang YW, Lee CL, Yang SS, et al. Statins reduce the risk of cirrhosis and its decompensation in chronic hepatitis B patients: A nationwide cohort study. Am J Gastroenterol. 2016;111:976–85. doi: 10.1038/ajg.2016.179. [DOI] [PubMed] [Google Scholar]
- 40.Tsan YT, Lee CH, Ho WC, et al. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31:1514–21. doi: 10.1200/JCO.2012.44.6831. [DOI] [PubMed] [Google Scholar]
- 41.Moscatiello S, Manini R, Marchesini G. Diabetes and liver disease: An ominous association. Nutr Metab Cardiovasc Dis. 2007;17:63–70. doi: 10.1016/j.numecd.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Majid S, Azam Z, Shah HA, et al. Factors determining the clinical outcome of acute variceal bleed in cirrhotic patients. Indian J Gastroenterol. 2009;28:93–95. doi: 10.1007/s12664-009-0034-z. [DOI] [PubMed] [Google Scholar]
- 43.Yang CH, Chiu YC, Chen CH, et al. Diabetes mellitus is associated with gastroesophageal variceal bleeding in cirrhotic patients. Kaohsiung J Med Sci. 2014;30:515–20. doi: 10.1016/j.kjms.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Gomes AC, Bueno AA, de Souza RG, Mota JF. Gut microbiota, probiotics and diabetes. Nutr J. 2014;13:60. doi: 10.1186/1475-2891-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Preveden T, Scarpellini E, Milic N, et al. Gut microbiota changes and chronic hepatitis C virus infection. Expert Rev Gastroenterol Hepatol. 2017;11:813–19. doi: 10.1080/17474124.2017.1343663. [DOI] [PubMed] [Google Scholar]
- 46.Rayes N, Seehofer D, Hansen S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: A controlled trial in liver transplant recipients. Transplantation. 2002;74:123–27. doi: 10.1097/00007890-200207150-00021. [DOI] [PubMed] [Google Scholar]
- 47.Rayes N, Seehofer D, Theruvath T, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation-a randomized, double-blind trial. Am J Transplant. 2005;5:125–30. doi: 10.1111/j.1600-6143.2004.00649.x. [DOI] [PubMed] [Google Scholar]
- 48.Haque TR, Barritt AT. Intestinal microbiota in liver disease. Best Pract Res Clin Gastroenterol. 2016;30:133–42. doi: 10.1016/j.bpg.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 49.Oo KM, Lwin AA, Kyaw YY, et al. Safety and long-term effect of the probiotic FK-23 in patients with hepatitis C virus infection. Biosci Microbiota Food Health. 2016;35:123–28. doi: 10.12938/bmfh.2015-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li F, Duan K, Wang C, et al. Probiotics and alcoholic liver disease: Treatment and potential mechanisms. Gastroenterol Res Pract. 2016;2016 doi: 10.1155/2016/5491465. 5491465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 52.Xu M, Wang B, Fu Y, et al. Changes of fecal Bifidobacterium species in adult patients with hepatitis B virus-induced chronic liver disease. Microb Ecol. 2012;63:304–13. doi: 10.1007/s00248-011-9925-5. [DOI] [PubMed] [Google Scholar]
- 53.Bolognesi M, Verardo A, Di Pascoli M. Peculiar characteristics of portal-hepatic hemodynamics of alcoholic cirrhosis. World J Gastroenterol. 2014;20:8005–10. doi: 10.3748/wjg.v20.i25.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Some details of the probiotics discussed in our study.
| Probiotics name | NMPN | The maintenance dose per day* | DDD |
|---|---|---|---|
| Bacillus licheniformis Capsule, Live | S10950019 | Three times a day and two tablets (0.5 g) at a time | 1.5 g |
| Live Combined Bifidobacterium, Lactobacillus, and Enterococcus Capsules, Oral | S10950032 | Two times a day and three tablets (0.63 g) at a time | 1.26 g |
| Combined Bifidobacterium, Lactobacillus, Enterococcus, and Bacillus cereus Tablets, Live | S20060010 | Three times a day and three tablets (1.5 g) at a time | 4.5 g |
NMPN –national medicine permission number; DDD – defined daily dose.
The maintenance dose per day of each probiotic preparation was obtained from the drug instructions.
Supplementary Table 2.
Detailed etiological distribution.
| Etiology | N (%) | Rebleeding rate | |
|---|---|---|---|
| Chronic HBV infection | 344 (48.9) | 42.7% | |
| Chronic HCV infection | 63 (8.9) | 54.0% | |
| Alcohol | 130 (18.5) | 60.0% | |
| Alcohol+HBV | 53 (7.5) | 67.9% | |
| Other HBV irrelevant | Alcohol+HCV | 9 (1.3) | 52.3% |
| Alcohol+PBC+AIH | 1 (0.1) | ||
| Alcohol+PBC | 3 (0.4) | ||
| PBC+AIH | 4 (0.6) | ||
| PBC | 28 (4.0) | ||
| AIH | 7 (1.0) | ||
| Unknown | 57 (8.1) | ||
| Other HBV correlation | HBV+HCV | 3 (0.4) | 80.0% |
| HBV+PBC | 2 (0.3) | ||
HBV – hepatitis B virus; HCV – hepatitis C virus; PBC – primary biliary cirrhosis; AIH – autoimmune hepatitis.
Supplementary Table 3.
Subgroup analysis of patients with other etiologies.
| Probiotics cohort all (rebleeding) | Non-probiotics cohort all (rebleeding) | P-value* | |
|---|---|---|---|
| Other HBV irrelevant (n=109) | 41 (17) | 68 (40) | 0.037 |
| Other HBV correlation (n=5) | 2 (1) | 3 (3) | 0.360 |
HBV – hepatitis B virus.
Subgroup analysis of esophagogastric variceal rebleeding in patients from the probiotics and non-probiotics cohorts using Cox regression.