Abstract
目的
通过粪便代谢组学,探索纳米二氧化钛(titanium dioxide nanoparticles,TiO2 NPs)经口染毒90 d对大鼠肠道及肠道菌群代谢的影响及其相关机制。
方法
12只清洁级雄性Sprague Dawley(SD)大鼠按体质量随机分成两组,分别以0和50 mg/kg体质量的TiO2 NPs持续灌胃90 d,对TiO2 NPs的粒径、晶型、纯度、比表面积进行表征,并在第90天收集大鼠的新鲜粪便。经过冻干、亲水相萃取等前处理后,使用超高效液相色谱-轨道阱高分辨质谱仪联用系统(ultra performance liquid chromatography-Q-exactive orbitrap-high-resolution mass spectrometry system,UPLC-QEMS)对粪便代谢物进行非靶向测定,鉴定标注检测得到的代谢物,并进行代谢组学分析。
结果
与对照组相比,TiO2 NPs染毒组大鼠体质量显著降低(P<0.05)。粪便代谢组学共发现22种代谢物浓度发生显著改变,其中黄嘌呤、甲基腺嘌呤、羟基吡啶、蛋氨酸亚砜等15种代谢物浓度显著上升,乙酰组胺、派可林酸、咪唑乳酸、缬氨酸等7种代谢物浓度显著下降。N-乙酰组胺、缬氨酸和蛋氨酸亚砜的改变倍数大于16倍。京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)通路分析发现,精氨酸生物合成通路和氨酰基-tRNA生物合成通路这两个代谢通路发生显著改变(错误发现率<0.05,通路受影响程度>0.10)。
结论
TiO2 NPs经口染毒90 d可扰乱肠道及肠道菌群代谢,并导致大鼠粪便中代谢物及代谢通路发生显著改变,提示TiO2 NPs经口暴露对大鼠的毒性作用可能与肠道及肠道菌群代谢改变密切相关。
Keywords: 纳米二氧化钛, 代谢组学, 粪便, 大鼠, Sprague-Dawley
Abstract
Objective
To explore the effects and related mechanisms of oral exposure titanium dioxide nanoparticles (TiO2 NPs) for 90 days on the intestinal and the gut microbiota of rats, through fecal metabolomics.
Methods
Twelve 4-week-old clean-grade Sprague Dawley (SD) rats were randomly de-vided into 2 groups by body weight, treated with TiO2 NPs at dose of 0 or 50 mg/kg body weight everyday respectively for 90 days. The solution of each infection was freshly prepared and shocked fully by ultrasonic. Characterization of the particle size, crystal form, purity, and specific surface area of TiO2 NPs was conducted. And the fresh feces of the rats were collected on the 90th day. After lyophilized and hydrophilic phase extraction, ultra performance liquid chromatography-Q-exactive orbitrap-high-resolution mass spectrometry system (UPLC-QEMS) was utilized for non-targeted determination of fecal meta-bolites. The metabolites were identified and labeled through Compound Discoverer 3.0 software, and used for subsequent metabolomics analysis. Bioinformatics analysis was carried out including unsupervised principal component analysis and supervised orthogonal projection to latent structure discriminant analysis for the differential metabolites between the two groups. The differential metabolites were followed-up for Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis.
Results
Compared with the control group, the body weight of the rats was significantly reduced (P<0.05) in the treatment group. A total of 22 metabolites in fecal metabolomics showed significant changes. Among them, xanthine, 1-methyladenine, 3-hydroxypyridine, methionine sulfoxide, pyridoxine, 1,5-isoquinolinediol, N-acetylornithine, N-acetyl-D-galactosamine, L-citrulline, L-methionine, leucine, DL-tryptophan, L-ornithine, 4-methyl-5-thiazoleethanol, and L-glutamic acid totaled 15 metabolites increased significantly. N-acetylhistamine, D-pipecolinic acid, imidazolelactic acid, L-valine, 2,3,4,6-tetramethylpyrazine, caprolactam, and histamine totaled 7 metabolites decreased significantly. N-acetylhistamine, L-valine and methionine sulfoxide were changed more than 16 times. Analysis of KEGG pathway revealed that the two metabolic pathways arginine biosynthesis and aminoacyl-tRNA biosynthesis were significantly changed (false discover rate < 0.05, pathway impact > 0.1).
Conclusion
Oral exposure to TiO2 NPs for 90 days could disrupt the metabolism of the intestine and gut microbiota, causing significant changes in metabolites and metabolic pathways which were related to inflammatory response, oxidative stress, glucose homeostasis, blood system and amino acid homeostasis in rat feces. It is suggested that the toxic effect of TiO2 NPs on rats may be closely related to intestinal and gut microbiota metabolism.
Keywords: Titanium dioxide nanoparticles, Metabolomics, Feces, Rats, Sprague-Dawley
食品级二氧化钛,欧盟授权编号E171,通常作为增白剂被广泛应用于食品工业中[1],包括奶酪、调味酱、牛奶、冰激凌、糖果以及糕点等等[2]。据Weir等[3]估计,美国人群中儿童(<10岁)通过饮食暴露二氧化钛的量为每日1~2 mg/kg,而其他年龄段的暴露量为每日0.2~0.7 mg/kg。最近有研究发现,食品级二氧化钛中直径<100 nm的纳米二氧化钛(titanium dioxide nanoparticles,TiO2 NPs)颗粒数目占总颗粒数的17%~35%[4]。相比于常规尺寸,TiO2 NPs具有更强的反应活性和渗透性[5],其安全性问题受到人们的广泛关注。
目前,TiO2 NPs的经口毒性研究发现,肝脏可能是其毒作用的靶器官[5]。Wang等[6]发现急性经口染毒80 nm TiO2 NPs可导致大鼠谷丙转氨酶/谷草转氨酶升高并出现肝脏病理损伤,Shukla等[7]通过对HepG2细胞进行染毒,发现TiO2 NPs可以导致人肝细胞出现DNA氧化损伤和凋亡。这些经口暴露的TiO2 NPs仅约有0.06%经小肠吸收,其余随粪便排出体外[8],相比于被吸收入血的极少部分,更多的TiO2 NPs直接作用于肠道,并对肠道和肠道菌群产生影响,但关于TiO2 NPs对肠道代谢物产生的影响及其相关机制方面的研究仍然十分有限。粪便代谢组学作为一种探索肠道和肠道菌群这个复杂的生物系统的研究方法越来越受到人们的关注[9]。利用代谢组学这一高灵敏度的分析方法[10],通过对粪便样本中的总体代谢物进行分析,可以敏感地检测到染毒引起的肠道及肠道菌群代谢通路改变,能够为理解毒物作用机制和发现生物标志物提供线索。为了更深入和全面地探索TiO2 NPs经口暴露对肠道的影响,本研究使用非靶向代谢组学的方法对大鼠的粪便代谢进行分析,探讨TiO2 NPs对肠道的影响及其相关机制。
1. 材料与方法
1.1. 主要试剂和仪器
超高效液相色谱仪使用的Amide色谱柱购自美国Waters公司,超高效液相色谱级乙腈和水购自美国Thermo Fisher Scientific公司,MIKRO22R型低温高速离心机购自德国Hettich公司,BP221S型电子天平购自德国Sartorious公司,Vortex Genie 2型涡旋振荡器购自美国Scientific Industries公司,KQ3200型超声仪购自中国昆山超声仪器厂,T10 basic S25型内切式组织匀浆机购自德国IKA公司,U3000型超高效液相色谱仪购自美国Thermo Fisher Scientific公司,Orbitrap Q-Exactive HF型轨道阱高分辨质谱仪购自美国Thermo Fisher Scientific公司。
1.2. TiO2 NPs的理化表征
TiO2 NPs购自上海麦克林试剂有限公司,颗粒多呈球形,表面光滑,晶型为锐钛矿,颗粒纯度超过99.99%,与产品标签描述相一致。详细的表征方法和结果参考本课题组前期发表的研究[11]。
1.3. 实验动物
12只4周龄清洁级雄性Sprague Dawley(SD)大鼠,体质量62.3~70.7 g,由北京大学医学部实验动物科学部提供[实验动物生产许可证:SCXK(京)2016-001;实验动物使用许可证:SYXK(京)2016-0041],适应性喂养1周后,按照随机数字表法分为两组,每组6只,饲养于清洁级动物房内(室温22~24 ℃,湿度60%~70%,光照12/12 h明暗交替)。为使染毒剂量能反映人群的最大暴露剂量,我们选择美国儿童日暴露剂量的中值(1.5 mg/kg),乘以E171中TiO2 NPs的占比(约占1/3),并使用100作为安全系数,计算得50 mg/kg体质量作为大鼠灌胃染毒的最高剂量。每日经口染毒TiO2 NPs的剂量分别为0(对照组,仅包含去离子水)和50 mg/kg体质量,并在每次染毒前分别依据每只大鼠体质量配制相应剂量的染毒液1 mL,超声分散30 min、涡旋混匀30 s后进行染毒,持续90 d。本研究通过北京大学生物医学伦理委员会审批(批准号LA2017073)。
1.4. 样品前处理
第90天用离心管收集大鼠新鲜粪便,混匀分装后保存于-80 ℃超低温冰箱等待后续测定。粪便样本使用冻干机(LYOQUEST-85型,日本Telstar公司)去除水分,电子天平称取30 mg样本加入900 μL预冷的甲醇/水(1 ∶1)溶液,用内切式组织匀浆机匀浆(5档,转速3 000 r/min);匀浆后使用涡旋振荡器充分混匀20 s,-20 ℃冰箱过夜。次日,离心机(16 000×g,4 ℃)离心10 min,取上清液850 μL在低温真空浓缩仪中浓缩悬干4 h。悬干后加入50 μL水/乙腈(1 ∶1)溶液复溶,涡旋混匀,从每个样品中吸取5 μL混合并均分成3份,制备3个质量控制(quality control,Qc)样本作为技术重复,样本制备好后上机分析。
1.5. 非靶向代谢组学测定
采用超高效液相色谱-轨道阱高分辨质谱仪联用系统(ultra performance liquid chromatography-Q-exactive orbitrap-high-resolution mass spectrometry system,UPLC-QEMS)进行非靶向代谢组学分析,待测样品打乱顺序后随机进样,且在待测样品分析前、测定6个样品后和样品分析结束后各测定一次Qc样品。采用QEMS进行质谱测定,高能碰撞解离方式进行碎裂获取子离子,标准化碰撞能分别为15、30和45 eV。分别采用正离子模式和负离子模式对结果进行测定,质量扫描范围50~1 100 m/z(质荷比),母离子全扫分辨率为60 K,采用数据依赖分析法扫描子离子,子离子二级扫描分辨率为30 K,轮廓分析最大注射进样时间60 ms。
1.6. 质谱数据解析及注释
仪器分析得到的原始结果文件导入Compound Discoverer 3.0(Thermo Fisher Scientific,美国)软件进行峰对齐、去卷积、滤除噪声、质荷比校正和基线校正,通过软件关联的mzCloud数据库和mzVault数据库进行代谢物的注释与鉴定工作, 筛选一级图谱母离子质荷比与理论值相对误差小于百万分之五且二级图谱强度质荷比与理论图谱中峰高、相对比值最为一致的代谢物。使用Metaboanalyst 4.0网站寻找所得代谢物在京都基因与基因组百科全书(Kyoto Encyclopedia of Genes and Genomes,KEGG)数据库中对应的KEGG ID,完成注释鉴定。
1.7. 数据分析
按照80%原则[12]剔除各个样品中检出率低于80%的代谢物,将筛选出的不同样本的代谢物浓度进行整合、归一,采用广义对数转换法对浓度进行标准化和归一化计算。
预处理完成后,使用欧氏距离对代谢物浓度进行完全聚类并绘制热图,展示各个样品中代谢物的浓度差异。采用主成分分析法(principal component analysis,PCA)对原始数据进行降维并观察组间样本的差异趋势及离群值,利用代谢物主成分得分中能将不同组别样品最大区分的两个主成分得分进行组合,绘制主成分分析得分图,展示代谢物在不同样品中的原始数据分布差异。使用隐结构正交投影判别分析(orthogonal projection to latent structure discriminant analysis,OPLS-DA)模型筛选TiO2 NPs染毒后发生改变的代谢物,以第一个预测成分和第一个正交成分绘制OPLS-DA模型得分图,根据各个变量在第一主成分的协方差大小p[1]和可靠程度p(corr)[1]绘制模型V得分图(V-plot),选取V-plot中p(corr)1绝对值>0.3的代谢物作为差异代谢物。通过Metaboanalyst 4.0对差异代谢物进行KEGG代谢通路分析,采用褐家鼠代谢通路数据库中的通路数据进行通路拓扑学分析,采用超几何分布检验算法对代谢通路富集的显著性进行检验;采用度中心性作为衡量差异代谢物对通路影响程度的标准,对差异代谢物进行代谢通路拓扑学分析,选择错误发现率(false discover rate,FDR)<0.05且通路影响程度>0.10的通路作为显著改变的通路。
2. 结果
2.1. 实验动物的体质量和一般情况
染毒组动物状态良好,毛发光泽活动正常,未见动物死亡。在第7~13周之间,染毒组动物体质量相比对照组显著降低,差异具有统计学意义(P<0.05,图1)。
1.
TiO2 NPs(50 mg/kg)染毒组和对照组体质量变化
Changes in body weight of TiO2 NPs (50 mg/kg) treatment and control group
*P<0.05, #P<0.01, compared with control group.
2.2. 非靶向代谢物的鉴定
使用Compound Discoverer软件共提取到8 829个峰,其中63个峰的一级、二级质谱匹配结果较好,对其进一步筛选后得到42种符合前述条件的代谢物,不同代谢物的浓度在两组间存在差异(图2)。主成分分析结果如图3A所示,第一主成分和第二主成分能够解释的方差占总方差比例分别为28.1%和24.6%,并且从图中可以看出TiO2 NPs染毒组和对照组在第二主成分存在清晰的分离趋势,提示染毒会造成两组间代谢物变化,可以进一步采用有监督的OPLS-DA模型进行后续分析。
2.
TiO2 NPs染毒组与对照组代谢物浓度热图
Heat map of metabolite concentrations in the TiO2 NPs treatment and control group
Each row represents a metabolite, each column represents a sample, and the color of each grid represents the relative concentration of metabolites in each row. This figure shows the distribution of metabolites in the treatment and control group after TiO2 NPs intervention.
3.
TiO2 NPs染毒组与对照组样品中代谢物的PCA得分图(A)和OPLS-DA得分图(B)
PCA score (A) and OPLS-DA score (B) graphs of metabolites in samples of the TiO2 NPs treatment group and the control group
2.3. 差异代谢物的筛选
OPLS-DA模型得分图显示,不同组之间样本完全分离(图3B),表示模型能够对不同组样本进行有效区分。OPLS-DA模型的V-plot见图4,以p(corr)[1]>0.3为标准筛选组间差异表达的代谢物,结果一共得到22种差异代谢物,其中有10种有机杂环化合物,10种有机酸及其衍生物,1种有机氧化合物,1种有机氮化合物。相比对照组,TiO2 NPs染毒组中黄嘌呤、甲基腺嘌呤、羟基吡啶、蛋氨酸亚砜等15种代谢物浓度显著上升,乙酰组胺、派可林酸、咪唑乳酸、缬氨酸等7种代谢物浓度显著下降(表1)。
4.
代谢物OPLS-DA模型的V得分图
V-score plot of the metabolite in OPLS-DA model
The abscissa represents the size of the difference between the groups, and the ordinate represents the reliability of the difference between the groups. Based on p (corr) [1]>0.3, The red dot indicates that the metabolite concentration is significantly higher in the treatment group than the control group, and the blue dot indicates that the metabolite concentration is significantly lower.
1.
差异代谢物详细信息
Details of differential metabolites
| Super class | Metabolite name | Molecular formula | Relative molecular mass |
HMDB ID | KEGG ID | Log change fold (log2N) |
| KEGG, Kyoto Encyclopedia of Genes and Genomes; HMDB, Human Metabolome Database; Log change fold was calculated by the metabolites concentration of treatment group/control group, and then taken logarithm. | ||||||
| Organoheterocyclic compounds | Xanthine | C5H4N4O2 | 152.110 9 | HMDB0000292 | C00385 | 2.857 1 |
| Organoheterocyclic compounds | 1-methyladenine | C6H7N5 | 149.153 3 | HMDB0011599 | C02216 | 2.045 8 |
| Organoheterocyclic compounds | 3-hydroxypyridine | C5H5NO | 95.099 3 | 2.191 9 | ||
| Organoheterocyclic compounds | Pyridoxine | C8H11NO3 | 169.177 8 | HMDB0000239 | C00314 | 2.149 2 |
| Organic acids and derivatives | Methionine sulfoxide | C5H11NO3S | 165.210 0 | HMDB0002005 | C02989 | 7.581 8 |
| Organoheterocyclic compounds | 1,5-isoquinolinediol | C9H7NO2 | 161.160 0 | 2.502 5 | ||
| Organic acids and derivatives | N-acetylornithine | C7H14N2O3 | 174.197 7 | HMDB0003357 | C00437 | 0.564 7 |
| Organic oxygen compounds | N-acetyl-D-galactosamine | C8H15NO6 | 221.207 8 | HMDB0000853 | C05021 | 2.558 6 |
| Organic acids and derivatives | L-citrulline | C6H13N3O3 | 175.185 7 | HMDB0000904 | C00327 | 1.895 0 |
| Organic acids and derivatives | L-methionine | C5H11NO2S | 149.211 0 | HMDB0000696 | C00073 | 1.370 4 |
| Organic acids and derivatives | Leucine | C6H13NO2 | 131.172 9 | HMDB0000687 | C00123 | 0.754 9 |
| Organoheterocyclic compounds | DL-tryptophan | C11H12N2O2 | 204.225 2 | HMDB0013609 | C00525 | -0.140 4 |
| Organic acids and derivatives | L-ornithine | C5H12N2O2 | 132.161 0 | HMDB0000214 | C00077 | 0.503 8 |
| Organoheterocyclic compounds | 4-methyl-5-thiazoleethanol | C6H9NOS | 143.207 0 | HMDB0032985 | C04294 | 1.259 6 |
| Organic acids and derivatives | L-glutamic acid | C5H9NO4 | 147.129 3 | HMDB0000148 | C00025 | 0.714 0 |
| Organic nitrogen compounds | Histamine | C5H9N3 | 111.145 1 | HMDB0000870 | C00388 | -1.004 3 |
| Organoheterocyclic compounds | Caprolactam | C6H11NO | 113.157 6 | HMDB0062769 | C06593 | -8.288 4 |
| Organoheterocyclic compounds | 2,3,5,6-tetramethylpyrazine | C8H12N2 | 136.194 3 | HMDB0036584 | -1.740 2 | |
| Organic acids and derivatives | L-valine | C5H11NO2 | 117.146 3 | HMDB0000883 | C00183 | -4.354 5 |
| Organoheterocyclic compounds | Imidazolelactic acid | C6H8N2O3 | 156.139 3 | HMDB0002320 | C05132 | -2.948 3 |
| Organic acids and derivatives | D-pipecolinic acid | C6H11NO2 | 129.157 0 | HMDB0005960 | -1.898 0 | |
| Organic acids and derivatives | N-acetylhistamine | C7H11N3O | 153.181 7 | HMDB0013253 | C05135 | -8.991 5 |
2.4. 代谢通路分析
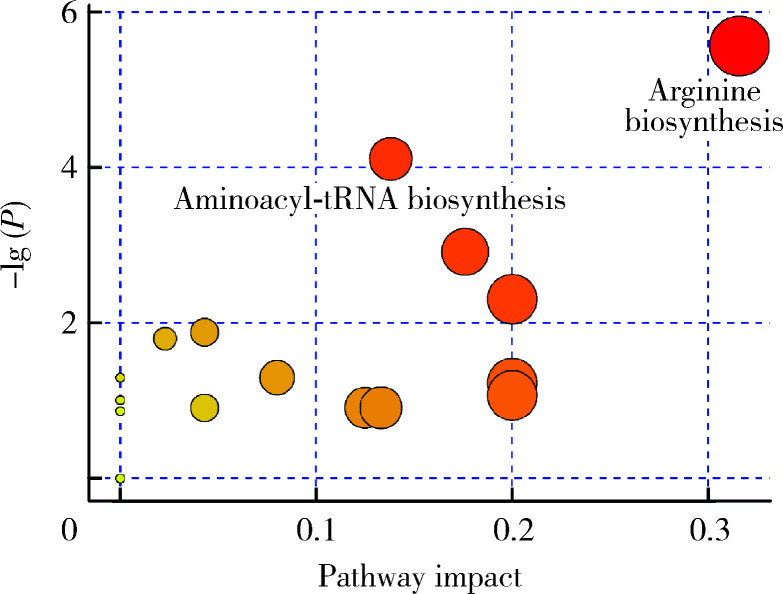
将上述22种差异代谢物进行KEGG通路分析和通路拓扑分析,共有两条通路出现显著改变,分别为精氨酸生物合成通路和氨酰基-tRNA生物合成通路(图5)。
5.
差异代谢物的KEGG通路分析结果
KEGG pathway analysis results of differential metabolites
The abscissa represents the degree of pathway impact obtained from the topology analysis. The size of the point is positively related to the pathway impact value of the path. The ordinate represents the negative logarithm of the P value obtained from the path enrichment analysis, and the yellow-red color gradient of the point is positively related to the negative logarithm of the P value of the pathway change. Significantly changed pathway names are marked in the figure.
3. 讨论
现有TiO2 NPs对肠道影响的相关研究发现,经口暴露TiO2 NPs可在肠道细胞内蓄积,并干扰维持肠道上皮结构相关基因的表达[13],减弱肠道屏障功能,影响多种营养物质的吸收[14],改变肠道菌群稳态[15]。
TiO2 NPs产生毒性的主要机制是其可以产生活性氧和自由基,进而非特异性地引起机体氧化应激、脂质蛋白过氧化、炎症反应、线粒体功能紊乱以及基因毒性[16]。本课题组前期研究发现,亚急性经口暴露TiO2 NPs可导致大鼠小肠微绒毛损伤并降低小肠对葡萄糖的吸收进而影响机体的葡萄糖稳态[17]。肠道菌群是指存在于消化道的微生物群,作为机体的一个重要组成部分,它从多个方面影响着机体的健康,包括代谢、营养、生理功能和免疫机能[18]。为进一步探索TiO2 NPs对肠道及肠道菌群的影响,本研究选择代谢组学的方法,通过对粪便代谢物进行分析,从代谢改变的角度解析TiO2 NPs染毒90 d对肠道的作用机制。通过OPLS-DA模型,我们发现共有22种代谢物浓度在TiO2 NPs染毒后发生显著改变,其中N-乙酰组胺、缬氨酸和蛋氨酸亚砜的改变倍数大于16倍(log2N>4)。
N-乙酰组胺是组胺乙酰化的产物,由于其几乎没有药理生理活性,被认为是机体失活组胺的一种途径[19]。本实验中TiO2 NPs染毒后,N-乙酰组胺的浓度相比对照组显著降低,表明TiO2 NPs干扰肠道组胺代谢,可能通过抑制组胺的失活从而加重肠道的炎症反应。缬氨酸是一种与胰岛素抵抗密切相关的必需氨基酸,Xiao等[20]发现缬氨酸缺乏将会导致小鼠胰岛素敏感性增加并降低血液葡萄糖水平。此外,还有动物实验表明,缺乏缬氨酸时骨髓造血干细胞将无法增殖[21]。现有研究表明,TiO2 NPs经口染毒可以扰乱葡萄糖稳态并导致凝血系统的改变[17, 22]。本研究中缬氨酸的显著降低可能是TiO2 NPs干扰血糖以及影响血液系统的一种机制。蛋氨酸亚砜是蛋氨酸的氧化产物,染毒组中蛋氨酸亚砜浓度的升高提示机体遭受氧化应激[23]。Guo等[14]进行的体外研究发现,TiO2 NPs染毒导致小肠上皮细胞中的活性氧生成显著增加,表明氧化应激是TiO2 NPs对消化道产生损伤的机制之一。粪便代谢组作为肠道菌群和宿主相互作用的中间媒介,是一个用来解读肠道菌群功能的有力工具[24]。本研究发现粪便中多种氨基酸浓度出现改变,这与肠道菌群引起的蛋白质发酵有密切关联。脱氨基作用产生的氨气以及含硫氨基酸(如甲硫氨酸)的降解会产生硫化氢,从而引起毒性效应并进一步改变肠道代谢[25]。但因缺乏菌群丰度的相关检测,本研究未能证实代谢与菌群改变的关系。从显著改变的差异代谢物中,我们发现TiO2 NPs染毒可导致肠道炎症反应加剧,产生氧化应激,干扰机体葡萄糖稳态并影响血液系统。
KEGG通路分析结果发现,精氨酸合成和氨酰基t-RNA生物合成通路发生显著改变。其中精氨酸合成通路的代谢物相比对照组上升,提示该通路在染毒后上调。精氨酸缺乏将导致机体活性氧生成增加[26],Tiwari等[27]研究发现,精氨酸缺乏可导致结核分枝杆菌活性氧产生增加并造成广泛的DNA损伤。精氨酸合成通路的上调,意味着肠道抗氧化机制的启动,肠道菌群会通过提高精氨酸的合成来对抗TiO2 NPs的杀菌作用。氨酰基t-RNA生物合成通路包括21种α氨基酸在翻译过程中与t-RNA发生氨酰基结合的反应,该通路出现显著改变主要是由于TiO2 NPs染毒后导致瓜氨酸、亮氨酸、蛋氨酸和缬氨酸浓度发生改变,提示TiO2 NPs对肠道氨基酸稳态产生影响。
综上所述,TiO2 NPs 经口染毒90 d,可导致大鼠粪便中22种代谢物出现显著改变,并导致精氨酸生物合成和氨酰基t-RNA生物合成两条通路发生改变,提示TiO2 NPs染毒后在炎症反应、葡萄糖稳态、血液系统、氧化应激和氨基酸稳态这五个方面对肠道及肠道菌群代谢产生影响,这对于TiO2 NPs对肠道可能的毒性机制以及未来的研究方向具有一定的提示作用。然而,非靶向代谢组学高灵敏的方法可能会造成结果的假阳性,相关结果还有待进一步验证。
Funding Statement
国家重点研发计划重点专项(2017YFC1600204); 国家自然科学基金(81703257)
National Science and Technology Major Project of the Ministry of Science and Technology of China(2017YFC1600204); National Natural Science Foundation of China(81703257)
References
- 1.Ropers MH, Terrisse H, Mercier-Bonin M, et al. Titanium dio-xide as food additive. Magdalena Janus: InTech Rijeka. 2017. [Google Scholar]
- 2.Baranowska-Wójcik E, Szwajgier D, Oleszczuk P, et al. Effects of titanium dioxide nanoparticles exposure on human health: A review. Biol Trace Elem Res. 2020;193(1):118–129. doi: 10.1007/s12011-019-01706-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weir A, Westerhoff P, Fabricius L, et al. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46(4):2242–2250. doi: 10.1021/es204168d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Doudrick K, Bi X, et al. Characterization of food-grade titanium dioxide: the presence of nanosized particles. Environ Sci Technol. 2014;48(11):6391–6400. doi: 10.1021/es500436x. [DOI] [PubMed] [Google Scholar]
- 5.Winkler HC, Notter T, Meyer U, et al. Critical review of the safety assessment of titanium dioxide additives in food. J Nanobiotechnology. 2018;16(1):51. doi: 10.1186/s12951-018-0376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Zhou G, Chen C, et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett. 2007;168(2):176–185. doi: 10.1016/j.toxlet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Shukla RK, Kumar A, Gurbani D, et al. TiO2 nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology. 2013;7(1):48–60. doi: 10.3109/17435390.2011.629747. [DOI] [PubMed] [Google Scholar]
- 8.Bachler G, von Goetz N, Hungerbuhler K. Using physiologically based pharmacokinetic (PBPK) modeling for dietary risk assessment of titanium dioxide (TiO2) nanoparticles. Nanotoxicology. 2015;9(3):373–380. doi: 10.3109/17435390.2014.940404. [DOI] [PubMed] [Google Scholar]
- 9.Karu N, Deng L, Slae M, et al. A review on human fecal metabolomics: Methods, applications and the human fecal metabolome database. Anal Chim Acta. 2018;(1030):1–24. doi: 10.1016/j.aca.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: Beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016;17(7):451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.周迪, 陈章健, 胡贵平, 等. 纳米二氧化钛亚急性经口暴露对大鼠氧化/抗氧化生物标志和炎性因子的影响[J/OL]. 北京大学学报(医学版), (2018-11-09) [2019-12-28].http://kns.cnki.net/kcms/detail/11.4691.R.20181108.1357.010.html.
- 12.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 13.Brun E, Barreau F, Veronesi G, et al. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part Fibre Toxicol. 2014;(11):13. doi: 10.1186/1743-8977-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Z, Martucci NJ, Moreno-Olivas F, et al. Titanium dioxide nanoparticle ingestion alters nutrient absorption in an in vitro model of the small intestine. NanoImpact. 2017;(5):70–82. doi: 10.1016/j.impact.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinget G, Tan J, Janac B, et al. Impact of the food additive titanium dioxide (E171) on gut microbiota-host interaction. Front Nutr. 2019;(6):57. doi: 10.3389/fnut.2019.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Z, Shuo H, Tenglong Y, et al. Toxicity of titanium dioxide nanoparticles induced by reactive oxygen species. Reactive Oxygen Species. 2019;8(23):267–275. [Google Scholar]
- 17.Chen Z, Wang Y, Wang X, et al. Effect of titanium dioxide nanoparticles on glucose homeostasis after oral administration. J Appl Toxicol. 2018;38(6):810–823. doi: 10.1002/jat.3589. [DOI] [PubMed] [Google Scholar]
- 18.Bull MJ, Plummer NT. Part 1: The human gut microbiome in health and disease. Integr Med (Encinitas) 2014;13(6):17–22. [PMC free article] [PubMed] [Google Scholar]
- 19.Weissbach H, Redfield BG, Axelrod J. The exzymic acetylation of serotonin and other naturally occurring amines. Biochim Biophys Acta. 1961;(54):190–192. doi: 10.1016/0006-3002(61)90954-4. [DOI] [PubMed] [Google Scholar]
- 20.Xiao F, Yu J, Guo Y, et al. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism. 2014;63(6):841–850. doi: 10.1016/j.metabol.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Taya Y, Ota Y, Wilkinson AC, et al. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science. 2016;354(6316):1152–1155. doi: 10.1126/science.aag3145. [DOI] [PubMed] [Google Scholar]
- 22.Duan Y, Liu J, Ma L, et al. Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice. Biomaterials. 2010;31(5):894–899. doi: 10.1016/j.biomaterials.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Lee BC, Dikiy A, Kim HY, et al. Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta. 2009;1790(11):1471–1477. doi: 10.1016/j.bbagen.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zierer J, Jackson MA, Kastenmüller G, et al. The fecal metabolome as a functional readout of the gut microbiome. Nat Genet. 2018;50(6):790–795. doi: 10.1038/s41588-018-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vernocchi P, Del Chierico F, Putignani L. Gut microbiota profiling: Metabolomics based approach to unravel compounds affecting human health. Front Microbiol. 2016;(7):1144. doi: 10.3389/fmicb.2016.01144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia Y, Dawson VL, Dawson TM, et al. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci U S A. 1996;93(13):6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiwari S, van Tonder AJ, Vilchèze C, et al. Arginine-deprivation-induced oxidative damage sterilizes Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2018;115(39):9779–9784. doi: 10.1073/pnas.1808874115. [DOI] [PMC free article] [PubMed] [Google Scholar]