Abstract
目的
探讨全腹膜外途径膀胱瓣肾盂吻合自体肾移植术治疗上尿路尿路上皮癌的可行性及有效性,总结自体肾移植术在上尿路尿路上皮癌治疗中的应用经验。
方法
报道1例行全腹膜外途径膀胱瓣肾盂吻合自体肾移植术治疗上尿路尿路上皮癌的病例,并对相关文献进行回顾总结。本例患者为64岁男性,1年前因右输尿管癌行根治性右肾输尿管切除术,现诊断左输尿管癌(G2,高级别)。为保留患者肾功能,同时考虑到常用保留肾单位手术的局限性,本中心创新性地为该患者行全腹膜外途径腹腔镜左肾切取、左输尿管切除、自体肾移植、膀胱瓣肾盂吻合术。
结果
手术过程顺利,无围术期并发症。术后1周肾功能即恢复至术前水平,随访期内肾功能正常,术后3个月行膀胱镜检查未见局部肿瘤复发征象。
结论
全腹膜外途径膀胱瓣肾盂吻合自体肾移植术是治疗上尿路尿路上皮癌可行、有效的方法。本创新性术式较以往术式有一定优势,全腹膜外途径手术具有创伤小、并发症少、恢复时间短等优势,且不增加肾热缺血时间;膀胱瓣肾盂吻合具有便于随访、发现早期病变及利于局部治疗等优势。通过本例特点分析及文献回顾,我们认为自体肾移植术对孤立肾上尿路尿路上皮癌或双侧上尿路尿路上皮癌患者来说,是一种可供选择的治疗方式,其具有保留肾功能且能完全切除肿瘤等优点,但目前自体肾移植术治疗上尿路尿路上皮癌缺乏长期随访和大样本研究,对术后肾功能及肿瘤复发的远期评估仍待完善。
Keywords: 尿路上皮癌, 肾, 移植, 自体, 全腹膜外腹腔镜, 吻合术, 外科
Abstract
Objective
To evaluate the feasibility and effectiveness of the totally extraperitoneal renal autotransplantation with boari flap-pelvis anastomosis in the treatment of upper urinary tract urothelial carcinoma (UTUC), and to review the experience of renal autotransplantation for UTUC treatment.
Me-thods
One case of applying the totally extraperitoneal renal autotransplantation with boari flap-pelvis anastomosis to the UTUC treatment was reported, and related literature was reviewed. The patient was a sixty-four-year old man who received right radical nephroureterectomy for right ureteral carcinoma 1 year before and diagnosed as left ureteral carcinoma(G2, high grade) this time. In order to preserve his renal function and avoid the shortness of common kidney-sparing surgery, a totally extraperitoneal procedure, including retroperitoneoscopic nephrectomy, ureterectomy, renal autotransplantation and Boari flap-pelvis anastomosis, was performed to the patient.
Results
The operation was completed successfully without perioperative complications. The renal function recovered to preoperative level within 1 week. No deterioration of renal function during the follow-up and no tumor recurrence was observed under cystoscopy at the 3-month postoperative consult.
Conclusion
The totally extraperitoneal renal autotransplantation with Boari flap-pelvis anastomosis is a feasible and effective treatment for UTUC. The innovative procedure has several advantages compared to the former ones. The extraperitoneal procedure results in significantly less pain, shorter hospital stay, decreased overall time to recovery and lower bowel complications risk without warm ischemia time extension. Meanwhile, the Boari flap-pelvis anastomosis simplifies the follow-up protocols and creates an easy route for cystoscopy and topical therapy. From the systematic clinical analysis, as well as the related literature review, it’s been concluded that the renal autotransplantation can be a reasonable option for the patients who have UTUC in solitary kidney or have bilateral UTUC. This type of treatment possesses advantages of preservation of renal function and total resection of malignant lesions. But long-term data and large cohort study on renal function or tumor recurrence are still absent which will be necessary to confirm the advantages of this approach.
Keywords: Urothelial carcinoma, Kidney, Transplantation, autologous, Totally extraperitoneal laparoscopy, Anastomosis, surgical
上尿路尿路上皮癌(upper urinary tract urothelial carcinoma,UTUC)占尿路上皮癌的5%~10%[1,2]。对于局限性UTUC,治疗方式包括保留肾单位的手术(包括腔内治疗、经皮内镜手术、输尿管部分切除术等)、根治性肾输尿管切除术和其他辅助治疗(化疗、放疗)。根据2019年欧洲泌尿外科学会指南,有肾积水、肿瘤直径>2 cm、尿细胞学或输尿管镜活检提示高级别肿瘤、存在多种组织学类型、多灶性病变或曾因膀胱癌行膀胱全切的患者被定义为高危UTUC患者,根治性肾输尿管切除术是其标准治疗方式[3]。而对于孤立肾(先天性或功能性)UTUC或双侧UTUC患者,接受根治性肾输尿管切除术意味着患者术后必须进行透析治疗或肾移植,将大大降低生存质量。现将北京大学第一医院开展的1例应用自体肾移植术治疗UTUC的病例进行总结报道,并结合病例进行文献回顾。
1. 资料与方法
1.1. 病历资料
64岁男性,主因“右侧输尿管癌术后1年余,确诊左侧输尿管癌10天”于2019年1月21日入院。患者1年余前因右侧输尿管癌行腹腔镜根治性右肾输尿管切除术,术后病理提示:右侧输尿管乳头状浸润性移行细胞癌,G2,部分G3(高级别尿路上皮癌),部分区域可见尿路上皮原位癌,侵犯黏膜固有层,PT1,未见淋巴结转移。术后每3个月规律复查膀胱镜,每半年规律复查泌尿系增强计算机断层扫描(computed tomography,CT)。8个月前因膀胱复发行经尿道膀胱肿瘤电切术治疗,术后病理提示:移行细胞癌,G2(高级别尿路上皮癌),未见明确浸润性生长,考虑PTa。术后行表柔比星规律膀胱灌注化疗。
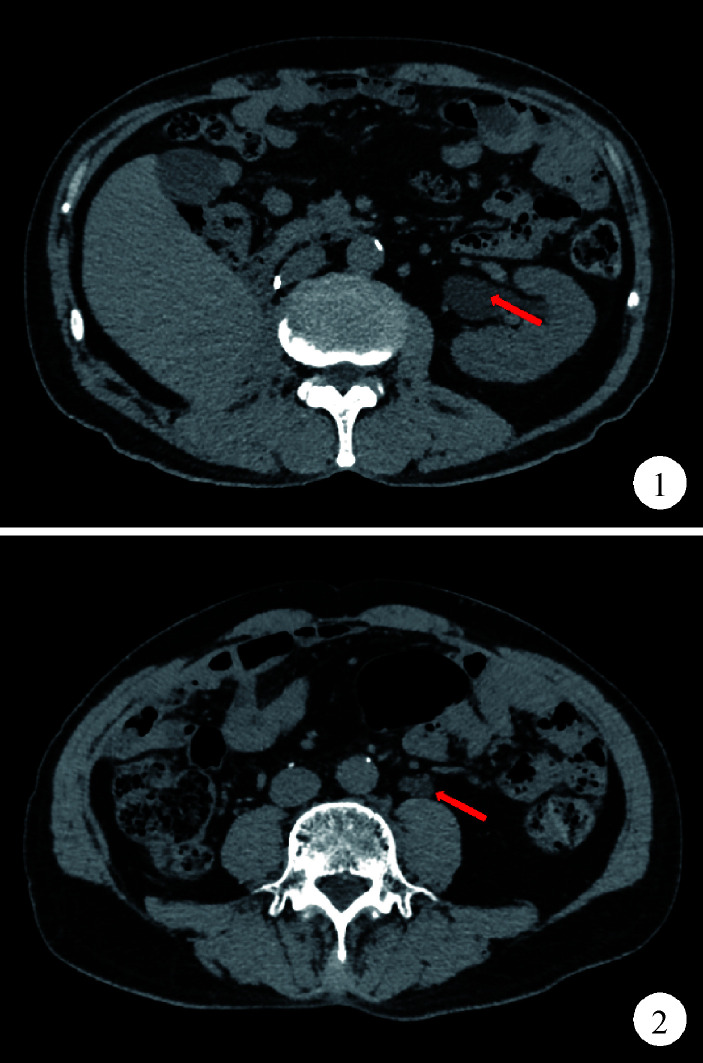
2个月前复查腹部和盆腔CT平扫示,左侧输尿管中段腔内可见一软组织密度灶,CT值约31 Hu,长度约2.3 cm,继发上方输尿管及左肾轻度积水(图1、2)。尿荧光原位杂交检查提示阳性,输尿管镜活检病理提示左侧输尿管炎伴细胞浸润。10天前行左侧输尿管镜检查+活检术+膀胱肿物诊断性电切术,术中发现左侧输尿管新生物,膀胱颈部见大小约0.5 cm新生物,术后病理示:左侧输尿管乳头状移行细胞癌,G2(高级别尿路上皮癌)。术前血肌酐104.81 μmol/L,肾小球滤过率64.453 mL/(min·1.7 m2)。
1.
CT平扫示左肾积水
CT scan demonstrates left hydronephrosis
2.
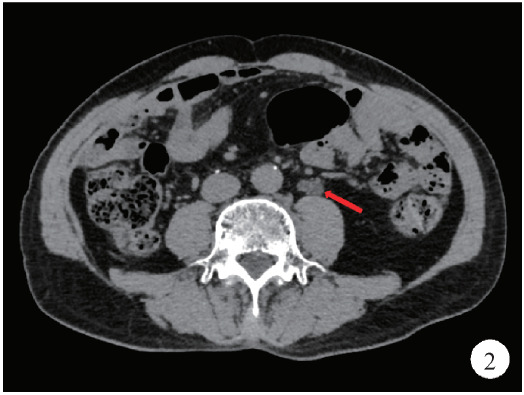
CT平扫示左侧输尿管中段软组织密度影
CT scan demonstrates a mass with soft tissue density in middle left ureter
1.2. 手术方法
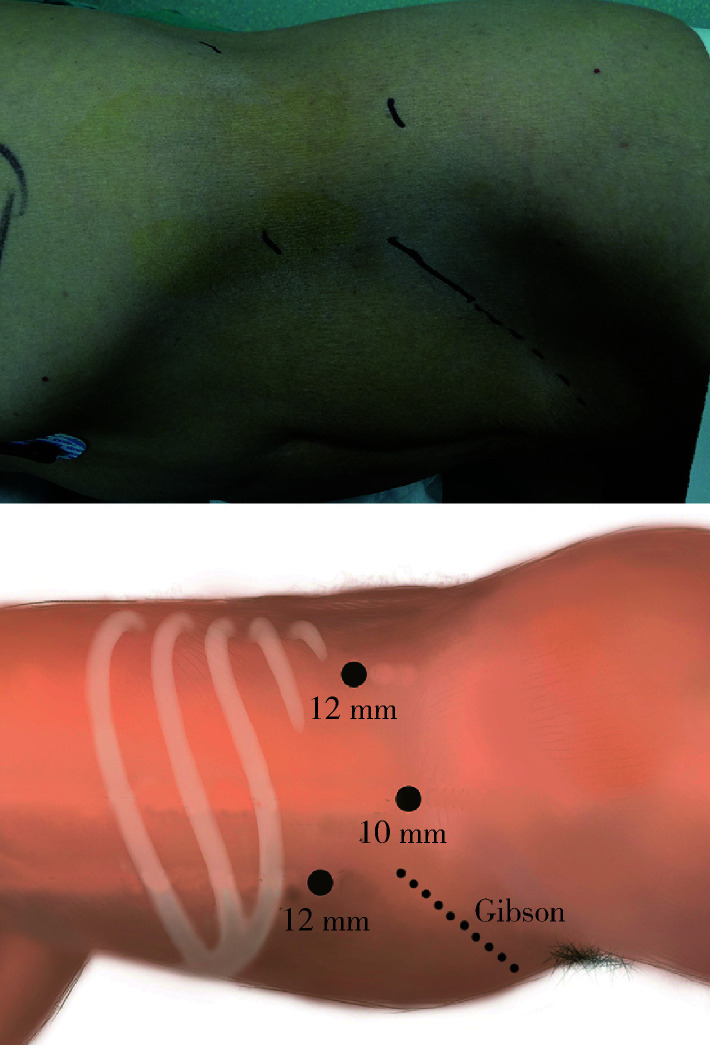
全身麻醉后,患者取右侧卧位,建立腹膜后腔后分别置入3个Trocar(套管针),于10 mm Trocar引入30°电子腹腔镜,分别于两个12 mm Trocar引入分离钳及超声刀(图3)。手术步骤主要分为:全腹膜外途径腹腔镜左肾切取、左侧输尿管切除、自体肾移植、膀胱瓣肾盂吻合术。
3.
腹腔镜Trocar布局及Gibson切口
Trocar distribution and Gibson incision
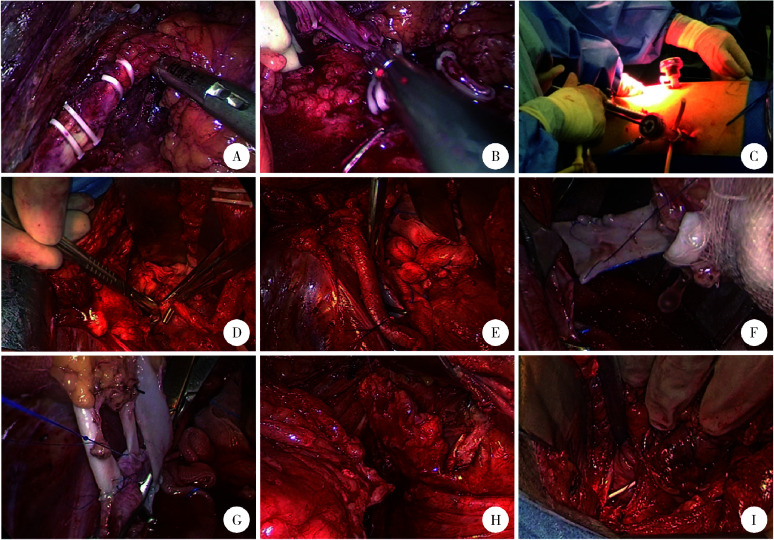
腹膜外途径腹腔镜左肾切取术:锐性分离扩大腹膜后间隙,沿后腹壁向肾上极游离,直达膈脚,充分游离显露肾背侧、上极、腹侧、下极及肾动静脉。然后显露左侧输尿管中、上段,向下游离至髂脊上缘,Hem-o-lock钳夹4次后离断输尿管(图4A),随后充分游离肾动脉和肾静脉。做左下腹斜切口(Gibson切口),手辅助下用Hem-o-lock分别钳夹肾动脉及肾静脉2次后离断,取出左肾(图4B、C)。台下将4 ℃ 保存用枸橼酸盐嘌呤溶液经肾动脉注入至肾静脉流出清亮液体,持续灌注肾及修建肾血管。输尿管切缘送冰冻病理,回报阴性。
4.
全腹膜外途径膀胱瓣肾盂吻合自体肾移植术的关键手术步骤
Key steps of the totally extraperitoneal renal autotransplantation with Boari flap-pelvis anastomosis
A, transecting the ureter; B, clamping and transecting the renal vessels; C, hand-assisted retroperitoneoscopic nephrectomy; D, ureterectomy with bladder cuff excision; E, dissecting left external iliac vessels; F, the renal vein was anastomosed end-to-side to the external iliac vein; G, the renal arteries were anastomosed end-to-side to the external iliac artery; H, Boari flap; I, Boari flap-pelvic anastomosis.
左侧输尿管切除术:改为平卧位,扩大左下腹切口,于腹膜外充分游离显露左侧输尿管,行输尿管全长切除及膀胱袖状切除(图4D),清扫左髂外淋巴结并显露左髂外动静脉(图4E)。
自体肾移植术:将一根肾静脉与髂外静脉行端侧吻合(图4F),两根肾动脉分别与髂外动脉行端侧吻合(图4G)。
膀胱瓣肾盂吻合术:输尿管腹侧劈开至肾盂,于膀胱左侧壁处制作膀胱瓣(图4H),与劈开输尿管及肾盂做宽大斜行吻合(图4I)。肾盂内留置F5支架管,充分止血后肾窝留置F20引流管,固定引流管并缝合各切口。
2. 结果
手术过程顺利,手术时间536 min,术中出血20 mL,无输血。术后住院9天,术后3周拔除尿管。术后病理回报:左输尿管移行细胞癌,G2(高级别尿路上皮癌),肿瘤微小,仅镜下可见,局灶可疑浸润黏膜固有层,考虑分期为PT1,切缘净;左髂外淋巴结(0/2)未见转移。
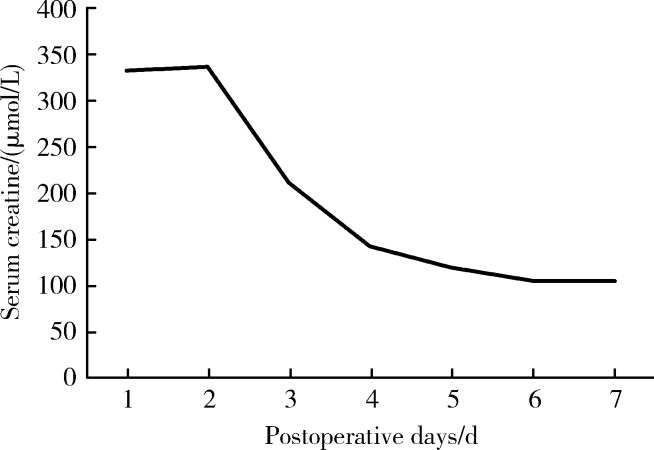
患者术后1周肾功能即恢复到术前水平(图5),术后3个月内血肌酐维持在96~115 μmol/L。术后3个月行膀胱镜检查,可通过膀胱镜清晰地观察到肾盂(图6),未见局部肿瘤复发征象,同期拔除肾盂内支架管。
5.
术后1周血肌酐变化情况
Postoperative serum creatinine level
6.
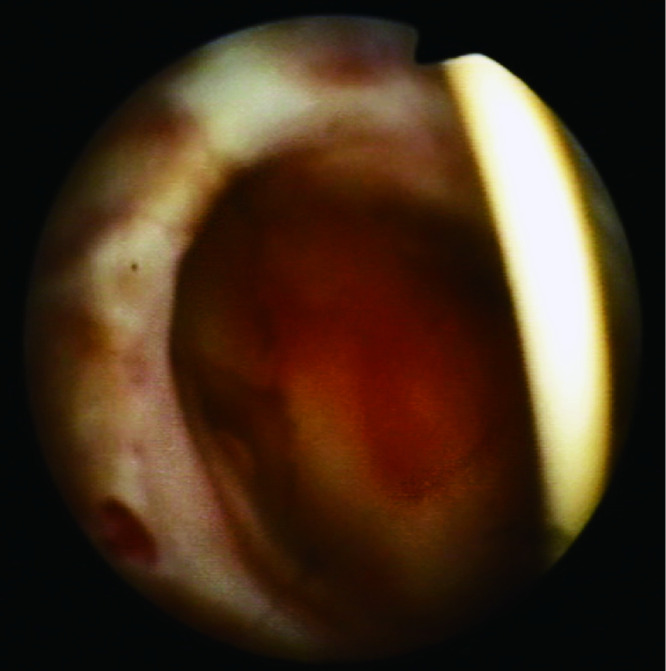
膀胱镜下的左侧肾盂
Direct view of the left pelvis under cystoscope
3. 讨论
对于孤立肾UTUC以及曾接受过对侧根治性肾输尿管切除术的患者来说,行根治性肾输尿管切除术意味着患者将完全失去肾功能,必须接受透析治疗或肾移植,这将给患者带来高昂的医疗花费和较差的生存质量,同时也将限制系统性化疗等进展期肿瘤治疗方式的开展[4]。对于这类患者,虽然保留肾单位手术是可以考虑的治疗方式,但均存在一定的局限性。腔内治疗存在低估分期和分级的风险[3],同时有较高的复发率,据报道84%的低危患者在进行腔内治疗后出现了至少一次的同侧复发[5],因此需要十分密切的随访。输尿管部分切除术则难以实施于多灶病变或者长段病变的患者,虽然膀胱瓣和肠代输尿管等尿路重建技术的出现可以弥补这一弊端,但这些尿路重建技术本身也存在一定局限性,如膀胱瓣尿路重建不适用于中、上段输尿管癌及膀胱容量小的患者,而肠代输尿管的缺点在于黏液分泌增多、电解质紊乱、慢性菌尿及结石形成等,这些都会导致肾功能的恶化,同时肠代输尿管还会带来肠梗阻等肠道相关的并发症[6]。
本例患者患有双侧输尿管癌,既往因右侧输尿管癌接受右侧根治性肾输尿管切除术,左侧输尿管癌活检病理为乳头状移行细胞癌,G2(高级别尿路上皮癌),肿瘤位于输尿管中部,直径2.3 cm,同时合并肾积水,属于高危UTUC患者,应接受根治性肾输尿管切除术。但为了保留患者肾功能,同时考虑到腔内治疗及输尿管部分切除术并尿路重建的局限性,我们创新地应用自体肾移植术治疗本例患者。
自体肾移植术在1963年由Hardy[7]首次报道,应用于输尿管损伤的治疗,现已广泛应用于治疗输尿管损伤、复杂结石病、肾动静脉畸形、肾肿瘤、腰痛血尿综合征等疾病。第一例应用自体肾移植术治疗UTUC的案例于1972年报道[8],至今全世界共有不足60例报道,本文将对部分文献进行回顾总结[9,10,11,12,13,14,15,16,17](表1)。
1.
自体肾移植治疗上尿路尿路上皮癌文献回顾
Published reports of renal autotransplantation for UTUC treatment
| Study | Year | Case | Approach | Procedure | Follow-up/ months |
Renal function | Tumor recurrence |
| Pettersson, et al[9] | 1984 | 8 | Open | Nephroureterectomy,extracorporeal total ureterectomy, renal autotransplantation by Gibson incision and pyelocystostomy | 14-53 | Normal in 7 cases | 3/8 |
| Gill, et al[13] | 2000 | 1 | Retroperitoneal laparoscopy |
Nephroureterectomy,extracorporeal ureterectomy, renal autotransplantation by Gibson incision and pyeloureterostomy | 6 | Normal | 0 |
| Jarrett, et al[14] | 2001 | 1 | Transperitoneal laparoscopy |
Nephrectomy, intracorporeal ureterectomy and renal autotransplan-tation by extraperitoneal incision | 6 | Normal | 0 |
| Kang, et al[15] | 2004 | 1 | Open | Nephroureterectomy,extracorporeal total ureterectomy,renal autotransplantation by Gibson incision and pyelocystostomy | 70 | Normal | 1/1 |
| Holmang, et al[10] | 2005 | 23 | Not reported | Not reported | 84-240 | Not reported | 10/23 |
| Iida, et al[16] | 2009 | 1 | Retroperitoneal laparoscopy |
Nephroureterectomy,extracorporeal ureterectomy, renal autotransplantation by Gibson incision and pyeloureterostomy | 20 | Normal | 0 |
| Cheng, et al[11] | 2014 | 12 | Retroperitoneal laparoscopy |
Hand-assisted nephroureterectomy, extracorporeal total ureterectomy, renal autotransplantation by Gibson incision and pyelocystostomy | 3-24 | Normal | 3/12 |
| Bourgi, et al[17] | 2018 | 2 | Not reported | Not reported | 30 (average) | Normal | 0 |
1984年Pettersson 等[9]报道了8例接受自体肾移植术治疗的UTUC患者,其中孤立肾UTUC或双侧UTUC共3例,在14~53个月的随访中, 5例无肿瘤复发,7例肾功能保持正常。该研究认为,对于低分期、低级别的孤立肾UTUC或双侧UTUC患者可以考虑进行自体肾移植术。2005年Holmang等[10]报道了23例接受自体肾移植术治疗的UTUC患者,移植失败率为12%, 9例孤立肾UTUC或双侧UTUC患者在7~20年的随访期内,3例无肿瘤复发且肾功能正常,2例无肿瘤复发但需透析治疗,4例出现了局部复发或远处转移。该研究认为,对侧肾功能正常的UTUC患者不应该考虑自体肾移植术,但对于孤立肾UTUC或双侧UTUC而又不适于腔内治疗或输尿管部分切除的患者,自体肾移植术不失为一种可供选择的治疗方式,同时,该研究还认为术后辅助卡介苗灌注治疗可能提高生存率。2014年Cheng等[11]报道了12例原发性输尿管癌患者成功实施自体肾移植术,其中有2例曾接受对侧根治性肾输尿管切除术,在平均(12.1±6.7)个月的随访期内,12例肾功能均正常,2例膀胱肿瘤复发(pTa),1例肾盂复发。短期内肿瘤复发率和肾功能恢复情况都比较乐观,该研究指出自体肾移植术具有完全切除肿瘤、保留肾功能、便于内镜随诊等优势。
自体肾移植术治疗UTUC的手术方式也在不断改进。1970年第一例手术采用了开放输尿管部分切除、开放肾切取、输尿管膀胱吻合术[8],1979年Pettersson等[9, 12]对尿路重建步骤进行了改良,在切除输尿管全长及大部分肾盂后,将肾盂剩余部分与膀胱直接吻合,即“肾盂膀胱吻合术”,这一改良在保留肾实质的同时,能最大程度切除尿路上皮组织。2000年Gill等[13]报道了第一例腹腔镜辅助的自体肾移植术, 2014年Cheng等[11]报道了手辅助经腹膜外途径腹腔镜肾输尿管切取、体外输尿管全长切除、自体肾移植、肾盂膀胱吻合术,该研究指出腹膜外途径腹腔镜肾切取术相较于之前的方式具有创伤小、腹腔内并发症少、肿瘤播散率低、术后恢复时间短等优势。
结合本病例特点及文献回顾,我们认为对于孤立肾UTUC或双侧UTUC患者,尤其是不适合进行腔内治疗或输尿管部分切除术的患者来说,自体肾移植术是一种值得选择的治疗方式。首先,自体肾移植术的开展并不受肿瘤位置的限制,因此,可以实现肿瘤的完全切除;其次,自体肾移植术可以保留肾功能,避免了患者必须接受透析治疗或肾移植的窘境。就手术方式的选择而言,本病例所采用的全腹膜外途径手术具有创伤小、外形美观、腹腔并发症少、肿瘤播散率低、住院日短等优势;同时腹膜外途径腹腔镜活体取肾术相较于传统的经腹途径腹腔镜活体取肾术并不增加肾热缺血时间,不会影响移植肾的功能[18],我们更进一步地在这一过程中采用了手辅助技术,可有效缩短手术时间及肾热缺血时间,较单纯腹腔镜活体取肾术可能更加安全。Ranch等[19]的研究表明,在长达4~8年(平均5.5年)的随访中,肾盂膀胱吻合不会因为反流而影响肾功能,泌尿系感染也并未明显增多,因此肾盂与膀胱直接进行吻合是一种合理有效的术式。膀胱瓣肾盂吻合使得术后随访更加简便,膀胱镜可以直接观察膀胱及肾盂内情况,能够发现早期复发灶并对其进行局部治疗[15];同时还可以提高灌注治疗的效果,化疗药物将通过宽大吻合口进入肾盂,这对于改善患者预后也有一定作用[20]。但目前自体肾移植术治疗UTUC缺乏长期随访数据和大样本、多中心的研究,对于术后肾功能及肿瘤复发的远期评估仍待完善。
Contributor Information
李 学松 (Xue-song LI), Email: pineneedle@sina.com.
周 利群 (Li-qun ZHOU), Email: zhoulqmail@sina.com.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000;164(5):1523–1525. [PubMed] [Google Scholar]
- 3.Rouprêt M, Babjuk M, Compérat E, et al. European Association of Urology Guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol. 2015;68(5):868–879. doi: 10.1016/j.eururo.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Kaag MG, O’Malley RL, O’Malley P. Changes in renal function following nephroureterectomy may affect the use of peri-operative chemotherapy. Eur Urol. 2010;58(4):581–587. doi: 10.1016/j.eururo.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadzinski AJ, Roberts WW, Faerber GJ, et al. Long-term outcomes of nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma. J Urol. 2010;183(6):2148–2153. doi: 10.1016/j.juro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Verduyckt FJ, Heesakkers JP, Debruyne FM. Long-term results of ileum interposition for ureteral obstruction. Eur Urol. 2002;42(2):181–187. doi: 10.1016/s0302-2838(02)00266-x. [DOI] [PubMed] [Google Scholar]
- 7.Hardy JD. High ureteral injuries. Management by autotransplantation of the kidney. JAMA. 1963;184(1):97–101. doi: 10.1001/jama.1963.03700150051008. [DOI] [PubMed] [Google Scholar]
- 8.Murphy GP, Staubitz WJ, Kenny GM. Renal autotransplantation for rehabilitation of a patient with multiple urinary tumors. J Urol. 1972;107(2):199–202. doi: 10.1016/s0022-5347(17)60982-0. [DOI] [PubMed] [Google Scholar]
- 9.Pettersson S, Brynger H, Henriksson C, et al. Treatment of urothelial tumors of the upper urinary tract by nephrouretherectomy, renal autotransplantation, and pyelocystostomy. Cancer. 1984;54(3):379–386. doi: 10.1002/1097-0142(19840801)54:3<379::aid-cncr2820540302>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Holmang S, Johansson SL. Tumours of the ureter and renal pelvis treated with resection and renal autotransplantation: a study with up to 20 years of follow-up. BJU Int. 2005;95(9):1201–1205. doi: 10.1111/j.1464-410X.2005.05505.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheng YT, Flechner SM, Chiang PH. The role of laparoscopy-assisted renal autotransplantation in the treatment of primary ureteral tumor. Ann Surg Oncol. 2014;21(11):3691–3697. doi: 10.1245/s10434-013-3382-y. [DOI] [PubMed] [Google Scholar]
- 12.Pettersson S, Brynger H, Johansson S, et al. Extracorporeal surgery and autotransplantation for carcinoma of the pelvis and ureter. Scand J Urol Nephrol. 1979;13(1):89–93. doi: 10.3109/00365597909180005. [DOI] [PubMed] [Google Scholar]
- 13.Gill IS, Uzzo RG, Hobart MG, et al. Laparoscopic retroperitoneal live donor right nephrectomy for purposes of allotransplantation and autotransplantation. J Urol. 2000;164(5):1500–1504. [PubMed] [Google Scholar]
- 14.Jarrett TW, Potter SR, Girrotto JA, et al. Laparoscopic assisted autotransplantation for treatment of transitional cell carcinoma of the mid ureter. J Urol. 2001;165(5):1625–1626. [PubMed] [Google Scholar]
- 15.Kang CH, Yu TJ, Hsieh HH, et al. Synchronous bilateral primary transitional cell carcinoma of the upper urinary tracts: ten patients with more than five years of follow-up. Urology. 2004;63(2):380–382. doi: 10.1016/j.urology.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 16.Iida T, Kawa G, Matsuda T. A case of preserving renal function by renal autotransplantation for bilateral urothelial carcinoma of the ureter. Int J Urol. 2009;16(6):587. doi: 10.1111/j.1442-2042.2009.02310.x. [DOI] [PubMed] [Google Scholar]
- 17.Bourgi A, Aoun R, Ayoub E, et al. Experience with renal autotransplantation: typical and atypical indications [J/OL]. Adv Urol, (2018-03-26) [2019-01-05]. https://doi.org/10.1155/2018/3404587.
- 18.Kortram K, Ijzermans JN, Dor FJ. Perioperative events and complications in minimally invasive live donor nephrectomy: A systematic review and meta-analysis. Transplantation. 2016;100(11):2264–2275. doi: 10.1097/TP.0000000000001327. [DOI] [PubMed] [Google Scholar]
- 19.Ranch T, Granerus G, Henriksson C, et al. Renal function after autotransplantation with direct pyelocystostomy. Long-term follow-up. Br J Urol. 1989;63(3):233–238. doi: 10.1111/j.1464-410x.1989.tb05181.x. [DOI] [PubMed] [Google Scholar]
- 20.Steffens J, Humke U, Alloussi S, et al. Partial nephrectomy and autotransplantation with pyelovesicostomy for renal urothelial carcinoma in solitary kidneys: a clinical update. BJU Int. 2007;99(5):1020–1023. doi: 10.1111/j.1464-410X.2007.06753.x. [DOI] [PubMed] [Google Scholar]