Abstract
目的
总结原发性纵隔卵黄囊瘤诊治经验。
方法
选择北京大学第一医院胸外科2014年8月至2018年8月收治的7例原发性纵隔卵黄囊瘤患者临床及病理资料,进行回顾性分析。
结果
7例患者胸部CT显示前纵隔肿物,血清甲胎蛋白(alphafetoprotein, AFP) 水平均明显升高。5例术前接受穿刺活检,诊断为卵黄囊瘤,接受含铂双药或三药方案化疗后行肿瘤扩大切除,其中4例术后继续进行化疗;另2例患者术前未接受穿刺活检,术后病理诊断为卵黄囊瘤,并接受含铂方案化疗。7例患者均顺利完成手术,6例达到R0切除,1例为R1切除。2例出现术后并发症(1例肺炎和1例肺不张)。3例术后1年内出现肺转移,4例未出现复发和转移。
结论
原发性纵隔卵黄囊瘤临床罕见,恶性程度较高,经合理诊治后部分患者可获得长期生存。
Keywords: 原发性纵隔卵黄囊瘤, 化疗, 扩大切除
Abstract
Objective
Primary mediastinal yolk sac tumor, which is also known as endodermal sinus tumor, is a rare but lethal neoplasm and its prognosis is very dismal. The current treatment for this tumor is controversial, and chemotherapy combined with resection of residual lesions is adopted sometimes. We summarized the experience of seven primary mediastinal yolk sac tumors treated with platinum-based chemotherapy and extended resection in Peking University First Hospital.
Methods
Clinicopathological data of the patients with primary mediastinal yolk sac tumor who received operation in Peking University First Hospital between August 2014 and August 2018 were collected and analyzed retrospectively.
Results
We experienced seven primary mediastinal yolk sac tumors during this period. Computed tomography scan revealed an anterior mediastinal tumor in all the patients and all of them had markedly raised alphafetoprotein (AFP) and normal β-human chorion gonadotropin (β-HCG). Five patients underwent needle core biopsy before treatment, which showed a mediastinal yolk sac tumor. All of these patients received preoperative platinum-based chemotherapy and they all presented partial response according to computed tomography. Two other patients did not receive preoperative biopsy, so they directly underwent extended resection. All of the seven patients underwent operation successfully and two of them expe-rienced postoperative complications, including one with pneumonia and the other with atelectasis. R0 resection was achieved in six patients and R1 resection was achieved in the other patient. According to postoperative pathology, there were one microcyst subtype, one adenoid subtye, one giant capsule subtype and two hybrid subtypes. Surprisingly, there were no yolk sac tumor tissue in the other two patients after preoperative chemotherapy. All the patients received postoperative chemotherapy, excluded one patient who was unable to tolerate chemotherapy after operation. Three patients experienced postoperative pulmonary metastases within one year and two of them died soon. The other patient received chemotherapy and immunotherapy after recurrence and he was alive at the time of writing. Four other patients were alive without recurrence and metastasis.
Conclusion
Primary mediastinal yolk sac tumor is rare and its prognosis is poor. A multimodality approach including adjuvant chemotherapy and resection of residual lesions is the optimal treatment and it may lead to long-term survival.
Keywords: Primary mediastinal yolk sac tumor, Chemotherapy, Extended resection
卵黄囊瘤(yolk sac tumor, YST)亦称内胚窦瘤,是一种来源于原始生殖细胞的高度恶性肿瘤,主要发生于中轴器官,多见于性腺[1]。原发于纵隔的卵黄囊瘤临床罕见,可与其他纵隔生殖细胞肿瘤合并存在,亦可单独发生。目前国内外该病报道较少见,本研究通过分析北京大学第一医院胸外科收治的7例原发性纵隔卵黄囊瘤患者临床资料,总结诊治经验,以期提高临床医师对该病的认识。
1. 资料与方法
1.1. 临床资料
选择2014年8月至2018年8月北京大学第一医院胸外科收治的原发性纵隔卵黄囊瘤的临床及病理相关资料(术前症状、化疗方案、手术方式、术后并发症、病理和生存情况等)进行回顾性分析。共收集到病例7例,所有患者术前均接受胸部CT、血清甲胎蛋白(alphafetoprotein, AFP)及β-人绒毛膜促性腺激素(β-human chorion gonadotropin, β-HCG)等检查,5例患者术前接受穿刺活检,明确病理诊断后行含铂类方案化疗。
1.2. 手术方式
7例患者均接受全身麻醉,根据手术需要行单腔或双腔气管插管;手术入路根据肿瘤位置及累及范围采取侧开胸或正中开胸入路。所有患者均接受肿瘤扩大切除,包括联合部分心包、肺组织或膈神经切除等。
2. 结果
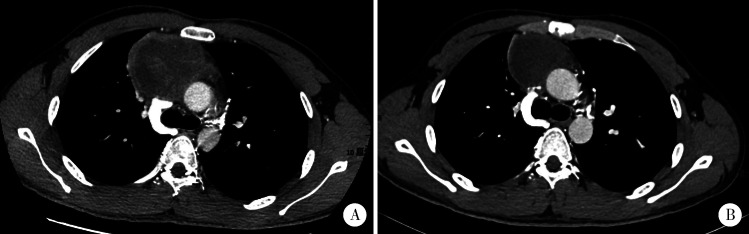
7例患者均为男性,平均年龄23岁(14~35岁)。5例患者术前存在胸闷或胸痛,其中2例合并发热,1例出现上腔静脉阻塞综合征。6例患者既往体健,1例合并克兰费尔特综合征(Klinefelter syndrome)。7例患者治疗前甲胎蛋白均明显升高(286~42 034 μg/L), β-人绒毛膜促性腺激素未见升高。5例患者术前接受穿刺活检并诊断为卵黄囊瘤,其中4例接受术前含铂双药或三药方案化疗,1例接受化疗及放疗(40 Gy),经术前治疗后肿瘤均缩小(图1);另2例术前未接受穿刺活检,术后病理显示为卵黄囊瘤。根据肿瘤位置及累及范围,4例采取腋下保留肌肉的小切口进行手术,其余3例经胸骨正中切口完成手术。7例患者均接受肿瘤扩大切除术,5例患者术后恢复良好;2例出现术后并发症,1例为肺部感染,另1例为肺不张(表1)。
1.
原发性纵隔卵黄囊瘤(病例7)化疗前后胸部CT图像
1.
7例原发性纵隔卵黄囊瘤临床资料
The clinical manifestations of seven primary mediastinal yolk sac tumor patients
| Case | Age/years | Symptom | AFP before chemotherapy/ (μg/L) |
Preoperative chemotherapy (cycle, drug) |
AFP after chemotherapy/ (μg/L) |
Surgical approach |
Excision extension |
Surgical margin |
Subtype | Postoperative chemotherapy (cycle, drug) |
Recurrence location |
Status* | Survival/ months |
| AFP, alpha fetoprotein; PEB, cisplatin+etoposide+bleomycin; EP, cis-platin+etoposide; R0, no cancer cells seen microscopically; R1, cancer cells present microscopically; SVCS, superior vena cava syndrome; LMSAT, left muscle-sparing axillary thoracotomy; MS, median sternotomy; RMSAT, right muscle-sparing axillary thoracotomy. *at the time of June 2019. | |||||||||||||
| 1 | 16 | Chest distress fever | 9 545.00 | 4×PEB | 4.84 | LMSAT | Tumor+partial pericardium+ phrenic nerve |
R0 | Not clear | 2×PEB | No | Alive | 48 |
| 2 | 17 | Chest distress chest pain | 285.50 | 4×EP | 13.31 | MS | Tumor+partial pericardium+ partial pulmonary |
R0 | Microcyst | 2×PEB | Pulmonary | Alive | 39 |
| 3 | 32 | No | 2 697.00 | - | - | RMSAT | Tumor+partial pericardium | R0 | Adenoid | 4×PEB | Pulmonary | Dead | 11 |
| 4 | 35 | Chest distress SVCS | 12 045.00 | 6×PEB | 1 117.00 | MS | Tumor+partial pericardium and pulmonary+superior vena cava |
R1 | Hybird | No | Pulmonary | Dead | 5 |
| 5 | 14 | Cough chest pain | 32 105.00 | 6×PEB | 10.91 | RMSAT | Tumor+thymoma | R0 | Giant capsule | 2×PEB | No | Alive | 23 |
| 6 | 22 | Cough | 1 210.00 | - | - | LMSAT | Tumor+ phrenic nerve | R0 | Hybird | 4×EP | No | Alive | 15 |
| 7 | 22 | Fever chest pain | 42 034.00 | 2×PEB | 261.60 | MS | Tumor+partial pericardium and pulmonary+superior vena cava |
R0 | Not clear | 4×PEB | No | Alive | 13 |
术后病理显示6例患者达到R0切除(显微镜下切缘阴性),另1例为R1切除(显微镜下切缘可见肿瘤组织)。5例接受术前化疗的患者中,1例术后标本内未见肿瘤残留,1例术后标本内未见卵黄囊瘤组织,仅见畸胎瘤组织,可知其化疗前为卵黄囊瘤和畸胎瘤混合存在,化疗后卵黄囊瘤成分消失。5例术后病理可见卵黄囊瘤成分,根据组织形态学分类,其中2例为混合型卵黄囊瘤,其余3例分别为微囊型、腺样型、巨囊型卵黄囊瘤。免疫组织化学染色显示7例患者AFP及CK(AE1/E3)均阳性,4例SALL4阳性,3例PLAP阳性,4例CD117阳性,1例CK7阳性,7例患者Ki-67增殖指数均大于或等于50%(表2及图2)。
2.
7例原发性纵隔卵黄囊瘤免疫组织化学检测结果
The immunohistochemistry of seven primary mediastinal sac tumor patients
| Case | Vimentin | CK(AE1/AE3) | AFP | SALL4 | PLAP | CD117 | CK7 | OCT4 | Ki-67/% |
| CK, cytokeratin; AFP, alpha fetoprotein; SALL4, spalt like transcription factor 4; PLAP, placental alkaline phosphatase; CD117, stem cell factor receptor molecule transcription factor-117; OCT4, octamer-binding transcription factor-4; Ki-67, proliferating antigen Ki-67. | |||||||||
| 1 | - | + | + | - | - | + | - | - | 70 |
| 2 | - | + | + | - | + | - | - | - | 50 |
| 3 | - | + | + | + | + | + | - | - | 70 |
| 4 | - | + | + | + | + | - | - | - | 70 |
| 5 | - | + | + | + | - | + | + | - | 60 |
| 6 | - | + | + | + | - | + | - | - | 60 |
| 7 | - | + | + | - | - | - | - | - | 60 |
2.
原发性纵隔卵黄囊瘤组织病理学检查结果
5例接受术前化疗患者中4例术后继续接受含铂双药或三药方案化疗,另1例(病例 5)因身体状况不佳未再继续接受化疗;2例(病例3和病例6)术前未接受化疗患者术后均接受含铂药物化疗。3例术后1年内出现肺转移,其中2例在出现肺转移后很快死亡,1例接受化疗联合免疫治疗后继续存活;另4例患者术后未出现复发及转移(表1)。
3. 讨论
生殖细胞肿瘤(germ cell tumors, GCTs)是一类起源于原始胚胎生殖细胞的肿瘤,多数发生于性腺(睾丸和卵巢),包括畸胎瘤、精原细胞瘤及非精原细胞GCTs等[2]。性腺外GCTs约占全部GCTs的2%~5%,常位于身体中轴部位,如颅内、纵隔、腹膜后及骶尾部[3]。GCTs常被认为是胚胎发育过程中未能迁移至性腺部位的原始生殖细胞残留在身体其他部位,进而恶变产生的肿瘤。纵隔是性腺外GCTs的常见发病部位,占所有性腺外GCTs的50%~70%。卵黄囊瘤属于非精原细胞性GCTs,原发于纵隔的卵黄囊瘤临床罕见,且多见于青年男性[4],预后较差。
原发性纵隔卵黄囊瘤多位于前纵隔,肿瘤常较大,且易侵犯邻近组织和器官,如上腔静脉及心包等。早期患者多无明显症状,当肿瘤较大或压迫周围脏器时可出现胸闷、胸痛及上腔静脉阻塞综合征(superior vena cava syndrome, SVCS)等,部分患者可合并发热[5]。本病易发生转移,常见转移部位为肺、肝、脑及骨等[6]。胸部CT或MRI检查通常表现为前纵隔肿块,且有助于明确肿瘤累及范围和可切除性,但不易与其他纵隔肿瘤(畸胎瘤和胸腺瘤等)相鉴别。卵黄囊瘤患者通常血清甲胎蛋白明显升高,可作为相对特异的诊断及预后判断指标[7],β-人绒毛膜促性腺激素多正常。本组5例接受术前化疗患者,在治疗后血清甲胎蛋白水平均有明显下降,胸部CT也显示肿瘤缩小。
原发性纵隔卵黄囊瘤多数包膜完整,切面呈囊性和实性混合形态,囊性结构多为蜂窝状,实性结构多呈鱼肉样。卵黄囊瘤的组织形态学多样,镜下见卵黄囊瘤呈网状、微囊状,囊腔内衬细胞胞浆透明、核浆比较高;常见核分裂象,间质为疏松结缔组织;其他结构变异包括实性结构、黏液瘤样结构、腺泡-腺管样结构、乳头状结构、巨囊性结构、多囊状结构和肝样分化等[8]。同一肿瘤内常可见至少两种生长方式,亦有呈单纯生长方式的卵黄囊瘤报道[9]。此外,肿瘤内可见特异性的Schiller-Duval小体和胞质内/胞质外透明小体,可据此与其他纵隔肿瘤进行鉴别。除组织学形态外,免疫组织化学检查是卵黄囊瘤诊断与鉴别诊断的重要手段。免疫组织化学检查常用抗体包括CK(AE1/AE3)、AFP及PLAP等,其中CK(AE1/AE3)常阳性,56%~86%病例AFP阳性,25%~58%病例PLAP阳性[10],但CK(AE1/AE3)特异性较低,AFP敏感性较低。最近有研究表明,SALL4是一种新型的生殖细胞抗体,其在生殖细胞肿瘤中具有较高特异性和敏感性,在卵黄囊瘤中阳性率较高,其他生殖细胞肿瘤内常呈弱阳性或阴性[11,12,13]。本组7例患者AFP及CK(AE1/E3)均阳性,4例SALL4阳性,3例PLAP阳性。结合组织学形态和免疫组织化学检查结果,通常可将纵隔卵黄囊瘤与其他纵隔肿瘤进行鉴别。该病可单独存在,也可与其他类型生殖细胞肿瘤混合存在。在接受治疗前,患者应先接受穿刺活检以便明确诊断。
原发性纵隔卵黄囊瘤对化疗敏感,放疗效果欠佳。目前常用化疗方案多以铂类药物(顺铂或卡铂)为基础联合依托泊苷、异环磷酰胺或博来霉素等,如VIP方案(顺铂+异环磷酰胺+依托泊苷)和PEB方案(顺铂+依托泊苷+博来霉素),一般治疗2~4个疗程[14,15,16]。本组患者均使用PEB或EP方案。大部分患者经过化疗后仍有肿瘤残余,因此手术在原发性纵隔卵黄囊瘤治疗方面占有重要地位。只要患者身体情况允许且技术上可行,患者化疗后均应接受手术以切除残余病灶。本组中2例患者因术前胸部CT显示其肿瘤切除难度不大,未接受术前穿刺活检而直接进行手术。目前推荐方案为术前化疗联合残余病灶切除,术后根据患者身体状况决定是否继续化疗。本组1例患者出现肺转移后接受化疗联合免疫治疗,效果较好,免疫治疗有望成为卵黄囊瘤新的治疗手段。
本病恶性程度高,预后较差,但经过合理治疗后仍有部分患者可获得长期生存[17]。Walsh等[18]报道9例原发性纵隔卵黄囊瘤经过化疗和手术切除,5例患者存活2年以上。目前认为以下因素与预后相关:(1)化疗前AFP水平;(2)化疗后的术后标本内是否存在残余肿瘤细胞;(3)是否出现肺转移[19]。随着治疗方案优化,该病治疗效果可望进一步提高。
综上所述,原发性纵隔卵黄囊瘤临床罕见,早期诊断困难,出现症状时肿瘤多较大或已侵犯邻近组织或器官。该病目前治疗多采用化疗联合手术切除残余病灶,经过合理治疗后部分患者能获得长期生存。
References
- 1.Isaacs H. Perinatal (fetal and neonatal) germ cell tumors. J Pediatr Surg. 2004;39(7):1003–1013. doi: 10.1016/j.jpedsurg.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 2.Akasbi Y, Najib R, Arifi S, et al. Complete histologic response to chemotherapy in a patient with a mediastinal yolk sac tumor: a case report. BMC Res Notes. 2014;7(1):803. doi: 10.1186/1756-0500-7-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.采 丽, 章 如松, 时 姗姗, et al. 纵隔原发生殖细胞肿瘤56例临床病理分析. 临床与实验病理学杂志. 2018;34(2):162–166. [Google Scholar]
- 4.Rodney AJ, Tannir NM, Siefker-Radtke AO, et al. Survival outcomes for men with mediastinal germ-cell tumors: the University of Texas M. D. Anderson Cancer Center experience. Urol Oncol. 2012;30(6):879–885. doi: 10.1016/j.urolonc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soriano PK, Iqbal MF, Siddiqui OM, et al. Non-seminomatous germ cell tumor presenting with superior vena cava syndrome. Am J Case Rep. 2017;18:902–907. doi: 10.12659/AJCR.904855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhry IU, Rahhal M, Khurshid I, et al. Radical surgical resection for giant primary mediastinal endodermal sinus tumor with pulmonary metastasis after chemotherapy: can be curative [J/OL]. BMJ Case Rep (2014-06-17)[2019-05-01].https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4069721/.
- 7.Saxman S, Nichols CR, Williams SD, et al. Mediastinal yolk sac tumor. The Indiana University experience, 1976 to 1988. J Thorac Cardiovasc Surg. 1991;102(6):913–916. [PubMed] [Google Scholar]
- 8.Kurman RJ. Blaustein’s pathology of the female genital tract. 5th ed.New York: Springer-Verlag. 2002. pp. 967–1033. [Google Scholar]
- 9.César AM, Suster S. Hepatoid yolk sac tumors of the mediastinum: a clinicopathologic and immunohistochemical study of four cases. Am J Surg Pathol. 1997;21(10):1210–1214. doi: 10.1097/00000478-199710000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Gao Y, Jiang J, Liu Q. Extragonadal malignant germ cell tumors: a clinicopathological and immunohistochemical analysis of 48 cases at a single Chinese institution. Int J Clin Exp Pathol. 2015;8(5):5650–5657. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F, Liu A, Peng Y, et al. Diagnostic utility of SALL4 in extragonadal yolk sac tumors: an immunohistochemical study of 59 cases with comparison to placental-like alkaline phosphatase, alpha-fetoprotein, and glypican-3. Am J Surg Pathol. 2009;33(10):1529–1539. doi: 10.1097/PAS.0b013e3181ad25d5. [DOI] [PubMed] [Google Scholar]
- 12.于 秀杰, 申 彦. SALL4与生殖细胞肿瘤. 天津医科大学学报. 2017;23(3):283–285. [Google Scholar]
- 13.Shojaei H, Hong H, Redline RW. High-level expression of divergent endodermal lineage markers in gonadal and extra-gonadal yolk sac tumors. Mod Pathol. 2016;29(10):1278–1288. doi: 10.1038/modpathol.2016.131. [DOI] [PubMed] [Google Scholar]
- 14.Tinica G, Butcovan D, Cimpeanu C, et al. A mediastinal germ cell tumor of yolk sac type: case report. Chirurgia. 2010;105(6):831. [PubMed] [Google Scholar]
- 15.Nakamura Y, Matsumura A, Katsura H, et al. Cisplatin-based chemotherapy followed by surgery for malignant nonseminomatous germ cell tumor of mediastinum: one institution's experience. Gen Thorac Cardiovasc Surg. 2009;57(7):363–368. doi: 10.1007/s11748-008-0375-z. [DOI] [PubMed] [Google Scholar]
- 16.佘 祥冬. 卵巢卵黄囊瘤诊疗进展. 国际妇产科学杂志. 2017;44(2):137–141. [Google Scholar]
- 17.Sudour-Bonnange H, Faure-Conter C, Martelli H, et al. Primary mediastinal and retroperitoneal malignant germ cell tumors in children and adolescents: results of the TGM95 trial, a study of the French Society of Pediatric Oncology (Société Francaise des Can-cers de l’Enfant) Pediatric Blood Cancer. 2017;64(9):e26294. doi: 10.1002/pbc.26494. [DOI] [PubMed] [Google Scholar]
- 18.Walsh GL, Taylor GD, Nesbitt JC, et al. Intensive chemotherapy and radical resections for primary nonseminomatous mediastinal germ cell tumors. Ann Thorac Surg. 2000;69(2):337–343. doi: 10.1016/s0003-4975(99)01472-1. [DOI] [PubMed] [Google Scholar]
- 19.Kesler KA, Rieger KM, Ganjoo KN, et al. Primary mediastinal nonseminomatous germ cell tumors: the influence of postchemotherapy pathology on long-term survival after surgery. J Thorac Cardiovasc Surg. 1999;118(4):692–700. doi: 10.1016/S0022-5223(99)70015-2. [DOI] [PubMed] [Google Scholar]