Abstract
目的
探讨抗类瓜氨酸化抗体(anti-carbamylated fibrinogen antibodies, 抗CarP抗体)在系统性红斑狼疮(systemic lupus erythematosus,SLE)中的意义,SLE是否与类风湿关节炎(rheumatoid arthritis, RA)一样,存在大量针对类瓜氨酸的自身抗体,抗CarP抗体与关节损伤及疾病活动度高度相关。
方法
利用酶联免疫吸附法(enzyme-linked immunosorbent assay,ELISA)检测105例确诊的SLE患者和73例健康对照者血清中抗CarP抗体水平,收集其他SLE相关临床和实验室数据,采用SPSS 24.0软件进行相应统计学分析。
结果
SLE中存在抗CarP抗体,且明显高于健康对照组(P<0.05)。抗CarP抗体阳性组与阴性组SLE患者相比,在以下临床及实验室指标方面差异有统计学意义:病程、血沉(erythrocyte sedimentation rate,ESR)、C-反应蛋白(C-reactive protein,CRP)、类风湿因子(rheumatoid factor,RF)、抗心磷脂抗体、抗dsDNA抗体、D-二聚体、IgA、IgG、补体C3、补体C4、外周血红细胞计数和血红蛋白水平(P<0.05)。与SLE其他自身抗体相比,抗CarP抗体的阳性率(21.9%)高于抗Sm抗体(15.24%),与抗核糖体P蛋白抗体(22.86%)阳性率相似;在SLE特异性抗体阴性的患者中可检测到抗CarP抗体,其阳性率分别为: 抗Sm(-)组20.2%(18/89),抗dsDNA(-)组9.3%(4/43),抗核小体抗体(-)组12.5%(6/48)和抗核糖体P蛋白抗体(-)组20.9%(17/81)。而且,抗CarP抗体水平与病程、补体C3、补体C4、红细胞及血红蛋白呈明显负相关(P<0.05),与ESR、CRP、IgA、IgG、RF、抗心磷脂抗体、抗dsDNA抗体及D-二聚体呈明显正相关(P<0.05)。
结论
SLE患者血清中抗CarP抗体水平升高,抗CarP抗体与SLE患者临床及实验室指标具有相关性。
Keywords: 系统性红斑狼疮, 自身抗体, 类瓜氨酸化抗体(抗CarP抗体)
Abstract
Objective
Antibodies against carbamylated protein (anti-CarP) were found to be a promising marker to evaluate joint damage and disease activity in patients with rheumatoid arthritis (RA). However, whether anti-CarP antibodies were present in systemic lupus erythematosus (SLE) remained ambiguity. We have therefore undertaken this study to assess the levels of serum anti-CarP antibodies and to evaluate their clinical value in SLE.
Methods
Serum levels of antibodies against carbamylated-fibrinogen (anti-CarP) were measured by enzyme-linked immunosorbent assay (ELISA) in 105 SLE patients and 73 healthy controls. Other clinical and laboratory measurements of the SLE patients were collected from medical records. Data analyses between anti-CarP antibodies and other laboratory measurements were performed using SPSS software for Windows 24.0.
Results
The levels of serum anti-CarP antibodies in the patients with SLE were significantly higher than those in the healthy controls (P<0.05). There were significant differences between the anti-CarP-positive group and anti-CarP-negative group in many clinical features. The disease duration, values of ESR, CRP, RF, anti-cardiolipin, anti-dsDNA, D-dipolymer, IgA and IgG were significantly higher in the anti-CarP-positive group compared with the negative group (P<0.05). Conversely, the values of complement 3, complement 4, peripheral blood RBC, and hemoglobin were significantly lower in anti-CarP-positive group than in the negative group(P<0.05). Moreover, the incidence of increase of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), D-dipolymer, decrease of peripheral blood RBC, hemoglobin, complement 3, complement 4, and positive rate of anti-dsDNA were significant different between the two groups(P<0.05). The positive rate of anti-CarP (21.9%) was higher than that of anti-Sm (15.24%), and close to anti-ribosomal P protein (22.86%) in our SLE patients. In addition, anti-CarP antibody was present in the SLE patients lacking the disease specific antibodies, including anti-Sm (anti-CarP positive rate 20.2%, 18/89), anti-dsDNA (anti-CarP positive rate 9.3%, 4/43), anti-nucleosome (anti-CarP positive rate 12.5%, 6/48), and anti-ribosomal P protein antibody (anti-CarP positive rate 20.9%, 17/81). Moreover, the high levels of anti-CarP antibodies were correlated with short disease duration, low C3, C4, RBC, and hemoglobin (P<0.05), high ESR, CRP, IgA, IgG, RF, anti-cardiolipin, anti-dsDNA, and D-dipolymer (P<0.05).
Conclusion
The level of anti-CarP antibody was increased in the serum of patients with SLE. There were correlations between anti-CarP antibodies and clinical and laboratory indicators of SLE patients.
Keywords: Systemic lupus erythematosus, Antoantibodies, Anti-carbamylated fibrinogen antibodies (anti-CarP)
系统性红斑狼疮(systemic lupus erythematosus,SLE)是一种慢性、系统性自身免疫病,累及全身多个器官,并伴有大量自身抗体产生[1,2]。外周血中的高浓度自身抗体,如抗核抗体(antinuclear anti-body,ANA)、抗dsDNA抗体、抗核小体抗体及抗Sm抗体在SLE中具有较高的特异性和敏感性,可作为SLE疾病诊断及病情监测的重要指标[3,4,5]。然而,现有的自身抗体的检测不能完全满足临床需求,仍然存在部分现有血清学诊断抗体阴性的SLE患者,因此,发现新的自身抗体将有助于提高SLE的诊疗水平。
氨基甲酰化反应是一种蛋白质翻译后修饰,其化学反应过程为赖氨酸的氨基端与氰酸根在无需酶辅助的条件下自然地发生化学反应,赖氨酸被甲酰化后转变为在结构上与瓜氨酸高度相似的类瓜氨酸[6,7]。类瓜氨酸虽然在结构上与瓜氨酸类似,但其功能完全不同于瓜氨酸。抗类瓜氨酸抗体(anti-carbamylated fibrinogen antibodies, 抗CarP抗体)最早是在肾病患者中被发现[8],随后被发现存在于多种自身免疫疾病中。45%的RA患者体内抗CarP抗体升高,其可作为抗瓜氨酸蛋白抗体(anti-citrullinated protein Ab,ACPA)阴性RA患者的特异性血清学指标[9]。在原发性干燥综合征中,抗CarP抗体与淋巴细胞增生、唾液腺生发中心形成以及唾液腺功能损伤相关[10]。然而,抗CarP抗体在SLE中的研究甚少见,且目前已有的报道研究结果不一致[11,12,13,14]。
本研究检测了SLE患者血清中抗CarP抗体水平,并探讨了其与SLE患者临床和实验室指标之间的相关性。
1. 资料与方法
1.1. 临床资料
SLE组:选择2013年8月至2014年12月就诊于北京大学人民医院风湿免疫科的SLE住院患者105例,平均年龄(34.12±11.76)岁,其中男性10例,女性95例。所有入选患者均符合1997年美国风湿病学会(American College of Rheumatology, ACR)的SLE分类标准[15],其临床及实验室资料完善,同时排除合并心血管病、感染及其他自身免疫病。健康对照组:在北京大学人民医院体检的健康体检者73名,性别、年龄与SLE组匹配。本研究开始前获得了北京大学人民医院伦理委员会审查批准(2015PHB056-01)。
1.2. 研究方法
收集入组患者的临床资料和实验室检查资料,临床资料包括性别、病程、年龄、皮疹、口腔溃疡、浆膜炎、脱发、关节炎、24 h蛋白尿、肌炎、管型尿、发热、神经精神症状;实验室资料包括ESR、免疫球蛋白A/G/M、补体C3、补体C4、C-反应蛋白(C-reactive protein, CRP)、血沉(erythrocyte sedimentation rate, ESR)、D-二聚体、24 h尿蛋白、血肌酐、血尿素、尿酸、红细胞、白细胞、血小板、血红蛋白、类风湿因子(rheumatoid factor,RF)、ANA、抗RNP抗体、抗Sm抗体、抗SSA抗体、抗SSB抗体和抗心磷脂抗体、抗dsDNA抗体、抗核小体抗体和抗核糖体P蛋白抗体。分析比较抗CarP抗体与上述各项指标之间的相关性。
1.3. 检测方法
1.3.1 氨基甲酰化反应 人纤维蛋白原(fibrinogen)购自Sigma公司,参考文献[16]报道,在37 ℃条件下,50 μg人纤维蛋白原与0.1 mol/L氰酸钾在10 mL的磷酸盐缓冲液(PBS,pH7.4)中反应17 h,随后在4 ℃环境下用蒸馏水对反应液透析36 h,所得产物经过凝胶电泳证实为氨基甲酰化的人纤维蛋白原(CarP), 用于后续抗CarP抗体检测。
1.3.2 血清中抗CarP抗体的检测 采用ELISA法检测105例SLE患者和73例正常对照血清抗CarP抗体:ELISA 96孔板内加入100 μL CarP,37 ℃孵育2 h。用含0.05%(质量分数)吐温20的磷酸盐缓冲液(PBST)洗板3次,每孔加入300 μL 5%(质量分数)牛血清白蛋白(bovine serum albumin,BSA),室温封闭2 h,PBST洗板3次。在已包被CarP的每微孔内加入100 μL 1 :100 PBS稀释的血清[稀释液为含1%(体积分数)BSA的PBS],37 ℃孵育30 min,PBST洗板3次;每孔加入100 μL 1 :10 000 PBS稀释的HRP-羊抗人IgG,37 ℃孵育30 min,PBST洗板3次;每孔加入100 μL显色液TMB,室温避光反应10 min;每孔加入100 μL 2 mol/L H2SO4终止反应。在波长450 nm(参考波长570 nm)条件下检测光密度(D)值。每板设置空白对照(含1%BSA的PBS), 同时为减少板间差异,设置标准血清对照,标准血清由10份SLE患者血清混合组成,所得D值换算成AU值:AU值=[( -D空白)待检血清/( -D空白)标准血清]×100。
1.4. 统计学分析
采用SPSS 24.0进行统计学分析,计量资料服从正态分布的数据采用 x±s表示,组间差异采用t检验,相关性采用Pearson相关性分析;非正态分布数据采用中位数(最小值~最大值)表示,组间差异采用Mann-Whitney检验,相关性采用Spearman相关性分析,同时采用partial Spearman法进行年龄、病程矫正后相关性分析;计数资料采用卡方检验。P<0.05认为差异具有统计学意义。
2. 结果
2.1. 105例SLE患者一般资料
105例SLE患者中女性占90.5%,平均病程为6年(8个月至27年);SLE患者出现不同频率的临床症状,如发热、皮疹、脱发、口腔溃疡、24 h蛋白尿、关节炎、神经症状(表1)。
1.
SLE患者一般临床资料
Clinical and demographic features of SLE patients
| Items | Data (n=105) |
| Age/years, x±s | 34.12±11.76 |
| Women, n (%) | 95 (90.5) |
| Disease duration/years, median (range) | 6 (0-27) |
| Fever (>38 ℃), n (%) | 51 (48.6) |
| Rash, n (%) | 39 (37.1) |
| Alopecia, n (%) | 43 (41) |
| Oral ulcer, n (%) | 22 (21) |
| Proteinuria (>0.5 g/24 h), n (%) | 53 (50.5) |
| Arthritis, n (%) | 23 (21.9) |
| Neuropsychiatric symptom, n (%) | 7 (6.7) |
2.2. SLE患者血清抗CarP抗体的水平
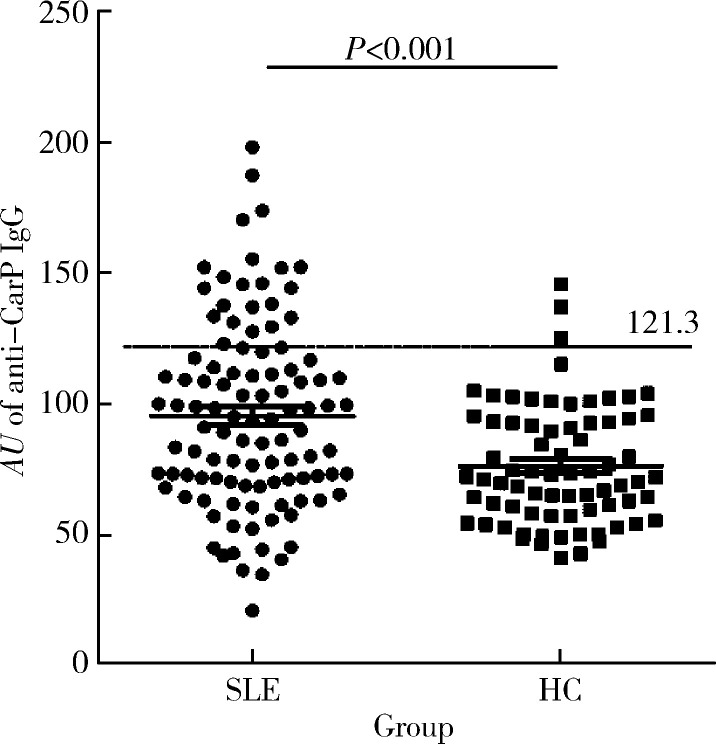
SLE患者血清中抗CarP抗体AU均值为95.16±35.68,高于正常对照组75.97±22.68,差异具有统计学意义(t=4.061,P<0.000 1)。正常对照组抗CarP抗体x±2s值定义为阳性阈值(即121.3),抗CarP抗体AU值大于121.3为阳性。抗CarP抗体在SLE患者中的阳性率明显高于正常对照组(21.9% vs. 4.1%,P<0.000 1,图1)。
1.
SLE与HC中抗CarP抗体水平的比较
Prevalence of anti-CarP antibodies in patients with SLE
SLE, systemic lupus erythematosus; HC, health control.
2.3. SLE患者血清抗CarP抗体阳性组与阴性组间临床及实验室指标的比较
105例SLE患者中,抗CarP抗体阳性组与抗CarP抗体阴性组间在多个实验室指标中存在差异,而在器官受累方面差异没有统计学意义(表2),抗CarP抗体阳性组SLE患者的病程明显长于阴性组,而且阳性组患者血清ESR、CRP、RF、抗心磷脂抗体、抗dsDNA抗体、D-二聚体、IgA及IgG明显高于阴性组(P<0.05);而在RBC、血红蛋白、补体C3和补体C4中,抗CarP抗体阳性组患者明显低于阴性组(P<0.05)。进一步比较以上具有统计学差异的临床及实验室指标的差异率,两组间除CRP和抗心磷脂抗体外,其余指标的差异率均存在明显的统计学意义(P<0.05,表3)。
2.
抗CarP抗体阳性组与抗CarP抗体阴性组SLE患者临床及实验室指标差异
Comparison of clinical and laboratory features between patients with anti-CarP and those without anti-CarP antibodies
| Items | Anti-CarP IgG(+), n=23 | Anti-CarP IgG(-), n=82 | P |
| PLT, platelet; WBC, white blood cell; RBC, red blood cell. ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; C3, complement 3; C4, complement 4; RF, rheumatoid factor; ANA, antinuclear antibody; SLEDAI, systemic lupus erythematosus disease activity index. | |||
| Women, n (%) | 21(91.3) | 74(90.2) | - |
| Age/years, x±s | 34.86±12.82 | 33.91±11.52 | 0.733 |
| Disease duration/ years, median (range) | 2(0-22) | 0.08(6-27) | 0.034 |
| Fever (>38℃), n (%) | 15(65.2) | 36(43.9) | 0.058 |
| Rash, n (%) | 8(34.8) | 31(37.8) | 0.496 |
| Alopecia, n (%) | 10(43.5) | 33(40.2) | 0.481 |
| Oral ulcer, n (%) | 2(8.7) | 20(24.4) | 0.084 |
| Arthritis, n (%) | 5(21.7) | 17(20.7) | 0.561 |
| Serositis, n (%) | 1(4.3) | 4(4.9) | 0.716 |
| Neuropsychiatric symptom, n (%) | 1(4.3) | 6(7.3) | 0.521 |
| ESR/(mm/h), median (range) | 78(11-126) | 23(1-137) | <0.000 1 |
| CRP/(mg/L), median (range) | 6.9(1-69) | 2.9(1-89) | 0.034 |
| C3/(g/L), x±s | 0.41±0.24 | 0.68±0.29 | <0.000 1 |
| C4/(g/L), median (range) | 0.05(0.02-0.34) | 0.13(0-0.34) | <0.000 1 |
| RF/(IU/mL), median (range) | 27.2(20-603) | 20(18-201) | <0.000 1 |
| Anti-Sm, n (%) | 4(17.4) | 9(10.9) | 0.671 |
| ANA, n (%) | 22(95.7) | 75(91.5) | 1.0 |
| SLEDAI, median (range) | 8(3-13) | 8(0-50) | 0.537 |
| Anti-cardiolipin/(RU/mL), median (range) | 9.6(0.1-133.6) | 5.7(0.1-108.9) | 0.003 |
| Anti-dsDNA/(IU/mL), median (range) | 416.6 (51.6-1 012.7) | 133.8 (0.1-1 040.4) | 0.002 |
| Anti-nuclesome/(IU/mL), median (range) | 41.65(5-200) | 42.99(1-200) | 0.287 |
| Anti-ribosomal P protein/(IU/mL), median (range) | 12.8(2-143) | 8.8(0-200) | 0.801 |
| D-dipolymer/(μg/L), median (range) | 779(38-6 920) | 197(14-2 398) | 0.001 |
| IgA/(g/mL), x±s | 3.42±1.96 | 2.27±1.19 | 0.001 |
| IgG/(g/mL), x±s | 24.53±10.57 | 12.25±5.43 | <0.000 1 |
| IgM/(g/mL), median (range) | 0.74(0.08-2.39) | 0.65(0.11-3.96) | 0.151 |
| PLT/(×109/L), x±s | 186.59±99.67 | 163.41±72.95 | 0.219 |
| WBC/(×109/L), x±s | 5.63±2.75 | 5.83±2.78 | 0.770 |
| RBC/ (×1012/L), x±s | 3.17±0.58 | 3.66±0.80 | 0.007 |
| Hemoglobin/(g/mL), x±s | 95.60±14.17 | 113.99±20.82 | <0.000 1 |
| Uric acid/(μmol/L), x±s | 326.5±151.3 | 340.7±116.0 | 0.639 |
| Blood urea/(mmol/L), median (range) | 4.65(2.02-25.37) | 5.35 (1.98-27.14) | 0.731 |
| Blood creatinine/(μmol/L), median (range) | 59(33-260) | 57(32-2 499) | 0.875 |
| Proteinuria for 24 h/(g/d), median (range) | 0.72(0.04-6.87) | 0.53(0-95.70) | 0.433 |
3.
抗CarP抗体阳性组与抗CarP抗体阴性组SLE患者实验室指标异常的比较
Comparison of abnormal laboratory features between patients with anti-CarP and those without anti-CarP antibodies
| Items | Anti-CarP IgG(+)(n=23) | Anti-CarP IgG(-)(n=82) | P |
| ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; C3, complement 3; C4, complement 4; IgA, immunoglobulin A; RF, rheumatoid factor; IgG, immunoglobulin G; RBC, red blood cell. | |||
| ESR high levels (>20 mm/h), n (%) | 20 (87) | 42 (54.5) | 0.005 |
| CRP high levels (>7.9 mg/L), n (%) | 8 (34.8) | 18 (21.9) | 0.111 |
| C3, low levels (<0.88 g/L), n (%) | 21 (91.3) | 58 (70.7) | 0.046 |
| C4, low levels (<0.15 g/L), n (%) | 19 (82.6) | 46 (56.1) | 0.013 |
| IgA, elevated levels (>3.78 g/mL), n (%) | 8 (34.8) | 7 (8.5) | 0.002 |
| RF, elevated levels (>30 IU/mL), n (%) | 8 (34.8) | 12 (14.6) | 0.046 |
| Anti-cardiolipin, elevated levels (>12 RU/mL), n (%) | 7 (30.4) | 11 (13.4) | 0.099 |
| IgG, elevated levels (>16.18 g/mL), n (%) | 17 (73.9) | 13 (15.9) | <0.001 |
| Anti-dsDNA, elevated levels (>100 IU/mL), n (%) | 19 (82.6) | 39 (47.6) | 0.001 |
| D-dipolymer, elevated levels (>250 μg/L), n (%) | 17 (73.9) | 34 (41.5) | 0.001 |
| RBC, low levels (<3.5×109/L), n (%) | 15 (65.2) | 28 (34.1) | 0.004 |
| Hemoglobin, low levels (<110 g/L), n (%) | 17 (73.9) | 33 (40.2) | 0.002 |
2.4. SLE患者中抗CarP抗体与其他自身抗体的比较
抗Sm抗体采用免疫印迹法检测,其结果以阴、阳性表示。抗dsDNA抗体、抗核小体抗体及抗核糖体P蛋白抗体采用ELISA法检测,其检测值阈值分别为100 IU/mL、20 IU/mL、20 IU/mL。在105例SLE患者中,抗CarP抗体阳性率(21.9%)高于抗Sm抗体(15.24%),但差异无统计学意义(P =0.143), 接近抗核糖体P蛋白抗体的阳性率(22.86%, P =0.065),低于抗dsDNA抗体(59.05%, P <0.001)和抗核小体抗体(54.29%, P <0.001)。在抗Sm抗体、抗dsDNA抗体、抗核小体抗体及抗核糖体P蛋白抗体阴性的SLE中,抗CarP抗体的阳性率分别为20.2%、9.3%、12.5%和20.9%,表明在SLE其他自身抗体血清学阴性时,抗CarP抗体可以作为补充,提高SLE诊断的敏感性。
2.5. SLE患者血清抗CarP抗体的水平与临床和实验室指标之间的相关性
对抗CarP抗体阳性组与抗CarP抗体阴性组间存在明显差异的临床和实验室指标进行相关性分析,发现抗CarP抗体与病程、补体C3、补体C4、红细胞、血红蛋白存在明显负相关(P<0.05),与ESR、CRP、IgA、IgG、RF、抗心磷脂抗体、抗dsDNA抗体、D-二聚体存在明显正相关(P<0.05)。经过年龄和病程矫正后,除病程和CRP外,抗CarP抗体与以上实验室指标之间仍然存在相关性(表4)。
4.
SLE患者血清抗CarP抗体与临床和实验室指标间的相关性
Correlation between anti-CarP IgG and the laboratory parameters in patients with SLE
| Items | Spearman rank test | Spearman partial correlation | ||
| r | P | R | P | |
| RF, rheumatoid factor; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; C3, complement 3; C4, complement 4; IgA, immunoglobulin A; IgG, immunoglobulin G; RBC, red blood cell. | ||||
| Disease duration | -0.210 | 0.032 | -0.060 | 0.572 |
| Hemoglobin | -0.438 | <0.001 | -0.323 | 0.002 |
| Anti-cardiolipin | 0.349 | <0.001 | 0.302 | 0.004 |
| Anti-dsDNA | 0.384 | <0.001 | 0.256 | 0.015 |
| RF | 0.466 | <0.001 | 0.475 | <0.001 |
| ESR | 0.502 | <0.001 | 0.440 | <0.001 |
| CRP | 0.195 | 0.048 | -0.004 | 0.970 |
| C3 | -0.351 | <0.001 | -0.377 | <0.001 |
| C4 | -0.356 | <0.001 | -0.366 | <0.001 |
| IgA | 0.380 | <0.001 | 0.365 | <0.001 |
| IgG | 0.601 | <0.001 | 0.651 | <0.001 |
| RBC | -0.36 | <0.001 | -0.263 | 0.012 |
| D-dipolymer | 0.357 | <0.001 | 0.539 | <0.001 |
3. 讨论
在西方人群中,Scinocca等[11]以类瓜氨酸化fibrinogen作为抗原检测37例SLE患者血清中抗CarP抗体,结果无一阳性。在另外两个西方人群的研究中,以类瓜氨酸化FBS作为抗原检测到SLE患者血清中抗CarP抗体阳性率分别为 9.8%和10.8%[12,13]。在日本SLE患者中抗CarP抗体阳性率为22%[14],这与本研究结果近似,本研究抗CarP抗体在SLE患者中的阳性率为21.9%,推测导致不同人群或者不同研究组获得的SLE患者中抗CarP抗体阳性率差异较大的原因,一方面可能因为各研究组所使用的包被抗原不同(CⅡ或FBS或fibrinogen)导致抗体检测的灵敏度不同,如在Shi等[17]的研究中可以看到,使用类瓜氨酸化的FBS和类瓜氨酸化的fibrinogen作为抗原检测相同RA患者中抗CarP抗体,所得结果是完全不同的;另一方面可能与不同种族人群中抗CarP抗体的水平不同有关。
抗CarP抗体阳性组与阴性组在多项临床和实验室指标之间存在明显差异,例如年龄、 ESR、CRP、IgG、RF、抗心磷脂抗体、D-二聚体、补体C3、IgG、红细胞和血红蛋白,而且抗CarP抗体与补体C3、补体C4、红细胞、血红蛋白存在明显负相关,与ESR、IgA、IgG、RF、抗心磷脂抗体、抗dsDNA抗体、D-二聚体存在明显正相关。虽然SLEDAI(systemic lupus erythematosus disease activity index)评分在抗CarP抗体阴性和阳性两组间并无差异,但是CRP与ESR作为反映病情的指标,存在明显差异,表明抗CarP抗体与疾病严重程度相关。本研究发现在临床症状如发热、皮疹、脱发、口腔溃疡、关节炎、神经症状方面,两组间差异未见统计学意义,而国外文献报道[18],抗CarP抗体在SLE合并关节炎及关节痛的患者中明显增高,推测导致这一不同的原因,可能与所使用的包被抗原不同有关,同时也可能因为本研究未纳入关节痛的病例,这一问题有待进一步对SLE患者进行更细致的分层研究。
目前普遍认为,SLE的发病是在遗传易感基础上,机体受到外在或内在环境因素刺激,机体免疫系统被激活,最终导致大量B细胞增殖和自身抗体产生[19]。在RA的研究中发现,蛋白质在翻译后进行的修饰(如瓜氨酸化或氨基甲酰化)可以激活机体免疫系统,产生大量针对这类蛋白质的自身抗体,氨基甲酰化反应将赖氨酸转变为类瓜氨酸,使蛋白质产生新的抗原表位,进而打破机体免疫耐受,诱导自身抗体产生并最终引发RA[20]。可以推测类瓜氨酸化蛋白参与SLE发病的作用机制与类瓜氨酸化蛋白在RA中的作用机制类似,但这尚有待进一步研究证实。
本研究还对比分析了抗CarP抗体与其他自身抗体在SLE中的阳性率,虽然差异无统计学意义,但抗CarP抗体阳性率(21.9%)高于抗Sm抗体(15.24%), 接近抗核糖体P蛋白抗体。同时,在抗Sm抗体、抗dsDNA抗体、抗核小体抗体及抗核糖体P蛋白抗体阴性的SLE中,抗CarP抗体的阳性率均高于9%。因此,其他自身抗体阴性时,抗CarP抗体可作为补充指标之一,用于提示SLE诊断及病情评估。
综上所述,SLE患者血清中存在抗CarP抗体,其水平明显高于健康对照者,抗CarP抗体与多项临床和实验室指标之间存在相关性。
Funding Statement
国家自然科学基金(81671604); 国家自然科学基金(81302554); 国家自然科学基金(31530020); 北京市科技新星计划(Z181100006218044); 北京大学临床医学+X青年项目-中央高校基本科研业务费(PKU2019LCXQ018)
Supported by the National Natural Science Foundation of China (81671604, 81302554, 31530020)(81671604); Supported by the National Natural Science Foundation of China (81671604, 81302554, 31530020)(81302554); Supported by the National Natural Science Foundation of China (81671604, 81302554, 31530020)(31530020); the Beijing Nova Program(Z181100006218044); the Fundamental Research Funds for Central Universities: Peking University Clinical Medcine Plus X-Young Scholars Project(PKU2019LCXQ018)
References
- 1.Lee SJ, Silverman E, Bargman JM. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat Rev Nephrol. 2011;7(12):718–729. doi: 10.1038/nrneph.2011.150. [DOI] [PubMed] [Google Scholar]
- 2.Yaniv G, Twig G, Shor DB, et al. A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients. Autoimmun Rev. 2015;14(1):75–79. doi: 10.1016/j.autrev.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Sherer Y, Gorstein A, Fritzler MJ, et al. Autoantibody explosion in systemic lupus erythematosus: more than 100 different anti-bodies found in SLE patients. Semin Arthritis Rheum. 2004;34(2):501–537. doi: 10.1016/j.semarthrit.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Betancur JF, Gómez-Puerta JA. Antinuclear antibodies mitotic patterns and their clinical associations [J/OL].Ann Rheum Dis (2019-04-29)[2019-08-01].https://ard.bmj.com/content/early/2019/04/26/annrheumdis-2019-215428.long.
- 5.Bizzaro N, Villalta D, Giavarina D, et al. Are anti-nucleosome antibodies a better diagnostic marker than anti-dsDNA antibodies for systemic lupus erythematosus? A systematic review and a study of metanalysis. Autoimmun Rev. 2012;12(2):97–106. doi: 10.1016/j.autrev.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Schreier SM, Steinkellner H, Jirovetz L, et al. S-carbamoylation impairs the oxidant scavenging activity of cysteine: its possible impact on increased LDL modification in uraemia. Biochimie. 2011;93(4):772–777. doi: 10.1016/j.biochi.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Gross ML, Piecha G, Bierhaus A, et al. Glycated and carbamylated albumin are more “nephrotoxic” than unmodified albumin in the amphibian kidney. Am J Physiol Renal Physiol. 2011;301(3):476–485. doi: 10.1152/ajprenal.00342.2010. [DOI] [PubMed] [Google Scholar]
- 8.Trepanier DJ, Thibert RJ. Carbamylation of erythrocyte membrane aminophospholipids: an in vitro and in vivo study. Clin Biochem. 1996;29(4):333–345. doi: 10.1016/0009-9120(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, Knevel R, Suwannalai P, et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc Natl Acad Sci USA. 2011;108(42):17372–17377. doi: 10.1073/pnas.1114465108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergum B, Koro C, Delaleu N, et al. Antibodies against carbamylated proteins are present in primary Sjögren’s syndrome and are associated with disease severity. Ann Rheum Dis. 2016;75(8):1494–1500. doi: 10.1136/annrheumdis-2015-207751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scinocca M, Bell DA, Racapé M, et al. Antihomocitrullinated fibrinogen antibodies are specific to rheumatoid arthritis and frequently bind citrullinated proteins/peptides. J Rheumatol. 2014;41(2):270–279. doi: 10.3899/jrheum.130742. [DOI] [PubMed] [Google Scholar]
- 12.López-Hoyos M, Álvarez-Rodríguez L, Mahler M, et al. Anti-carbamylated protein antibodies in patients with ageing associated inflammatory chronic disorders. Rheumatology (Oxford) 2016;55(4):764–766. doi: 10.1093/rheumatology/kev391. [DOI] [PubMed] [Google Scholar]
- 13.Ziegelasch M, van Delft MA, Wallin P, et al. Antibodies against carbamylated proteins and cyclic citrullinated peptides in systemic lupus erythematosus: results from two welldefined European cohorts. Arthritis Res Ther. 2016;18(1):289. doi: 10.1186/s13075-016-1192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakabo S, Yoshifuji H, Hashimoto M, et al. Anti-carbamylated protein antibodies are detectable in various connective tissue dis-eases. J Rheumatol. 2017;44(9):1384–1388. doi: 10.3899/jrheum.161432. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 16.Turunen S, Koivula MK, Risteli L, et al. Anticitrulline antibodies can be caused by homocitrulline-containing proteins in rabbits. Arthritis Rheum. 2010;62(11):3345–3352. doi: 10.1002/art.27644. [DOI] [PubMed] [Google Scholar]
- 17.Shi J, van de Stadt LA, Levarht EWT, et al. Anti-carbamylated protein (anti-CarP) antibodies precede the onset of rheumatoid arthritis. Ann Rheum Dis. 2014;73(4):780–783. doi: 10.1136/annrheumdis-2013-204154. [DOI] [PubMed] [Google Scholar]
- 18.Ceccarelli F, Perricone C, Colasanti T, et al. Anti-carbamylated protein antibodies as a new biomarker of erosive joint damage in systemic lupus erythematosus. Arthritis Res Ther. 2018;20(1):126. doi: 10.1186/s13075-018-1622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceccarelli F, Perricone C, Cipriano E, et al. Joint involvement in systemic lupus erythematosus: from pathogenesis to clinical assessment. Semin Arthritis Rheum. 2017;47(1):53–64. doi: 10.1016/j.semarthrit.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Mastrangelo A, Colasanti T, Barbati C, et al. The role of post-translational protein modifications in rheumatological diseases: Focus on rheumatoid arthritis [J/OL]. J Immunol Res, 2015, 2015: 712490(2015-05-18)[2019-08-01]. https://www.hindawi.com/journals/jir/2015/712490/.