Abstract
A set of common-acting iron-responsive 5′untranslated region (5′UTR) motifs can fold into RNA stem loops that appear significant to the biology of cognitive declines of Parkinson's disease dementia (PDD), Lewy body dementia (LDD), and Alzheimer's disease (AD). Neurodegenerative diseases exhibit perturbations of iron homeostasis in defined brain subregions over characteristic time intervals of progression. While misfolding of Aβ from the amyloid-precursor-protein (APP), alpha-synuclein, prion protein (PrP) each cause neuropathic protein inclusions in the brain subregions, iron-responsive-like element (IRE-like) RNA stem–loops reside in their transcripts. APP and αsyn have a role in iron transport while gene duplications elevate the expression of their products to cause rare familial cases of AD and PDD. Of note, IRE-like sequences are responsive to excesses of brain iron in a potential feedback loop to accelerate neuronal ferroptosis and cognitive declines as well as amyloidosis. This pathogenic feedback is consistent with the translational control of the iron storage protein ferritin. We discuss how the IRE-like RNA motifs in the 5′UTRs of APP, alpha-synuclein and PrP mRNAs represent uniquely folded drug targets for therapies to prevent perturbed iron homeostasis that accelerates AD, PD, PD dementia (PDD) and Lewy body dementia, thus preventing cognitive deficits. Inhibition of alpha-synuclein translation is an option to block manganese toxicity associated with early childhood cognitive problems and manganism while Pb toxicity is epigenetically associated with attention deficit and later-stage AD. Pathologies of heavy metal toxicity centered on an embargo of iron export may be treated with activators of APP and ferritin and inhibitors of alpha-synuclein translation.
Iron balance influences healthy cognition throughout the human lifespan
Levels of dietary heavy metal exposure, especially from iron, have long been linked to cognitive problems in neonates and children as much as in neurodegenerative diseases of the aging population (Carlson et al. 2009; Brunette et al. 2010; Chiou et al. 2020). Michael Georgieff and his colleagues reported that neuronal iron uptake by the divalent metal ion transporter DMT-1 (Slc11a2) was essential for normal neuronal development of the hippocampus while Slc11a2 expression was induced by spatial memory training. Deletion of Slc11a2 was shown to disrupt neuronal development in the hippocampus and to alter spatial memory behavior in rodent models while remedies are sought to prevent childhood a dietary anemia that can limit learning and cognitive well-being of children (Auerbach and Georgieff 2020; Geng et al. 2020). Supporting the consensus that anemia thwarts cognitive skills, genetic manipulation of the dietary iron uptake protein DMT-1 in the hippocampus reflected early stage forgetfulness reminiscent of Alzheimer's disease (AD) phenotypes, including changes to the AD specific amyloid precursor protein (APP) and APP binding proteins (Carlson et al. 2008).
We review the impact of iron in the posttranscriptional regulation of the genes products of neurodegenerative cognitive decline, namely, AD as well as the major alpha-synucleinopathies of Parkinson's disease (PD), PD with dementia (PDD), and Lewy body dementia (LBD). Maintenance of cognitive health and memory retention lasts over a lifetime (Smolen et al. 2019) and may involve breakdowns of iron homeostasis linked to the loss of normal function of APP, alpha-synuclein (αsyn) and Prion protein (PrP) when accelerating neurogenerative processes (Lumsden et al. 2018; Zhang et al. 2020a). Neurodegenerative dementia can be modeled not only via plaque and Lewy body pathologies, but also through the path leading to accelerated perturbations of iron homeostasis and ferroptosis as causes of neural death (Bartzokis et al. 2010, 2011; Zhang et al. 2020a).
A balance exits such that too little iron is associated with anemia in newborn babies and has frequently been linked to cognitive liabilities in young children (Geng et al. 2020). Too much iron underpins hemochromatosis, a pathology that was evaluated by James Connor and colleagues as a fundamental factor in neurodegeneration in amyotrophic lateral sclerosis patients as well as a means to explain oxidative and cognitive decline during aging (Nandar and Connor 2011; Nandar et al. 2014; Meadowcroft et al. 2018; Song et al. 2020). Systemic conditions of iron overload include genetic mutations in the genes that cause hemochromatosis (HFE, HFE2), hepcidin (HAMP) and transferrin receptor-2 (TFR2) (Piperno et al. 2020). Reduced hepcidin synthesis leads to increased intestinal iron absorption and an increase of release of Fe from macrophages. This results in tissue iron overload (Piperno et al. 2020). Excess brain Fe and changed energy production may accelerate AD and also PD as well as be a driver of oxidative stress and ferroptosis in vascular dementias resulting from mini strokes and microhemorrhages (Maynard et al. 2002; Barnham and Bush 2008; Bush and Tanzi 2008; Ayton et al. 2015a; Corriveau et al. 2016; Jiang et al. 2017; Stockwell et al. 2017; Newman et al. 2019; Piperno et al. 2020; Zhang et al. 2020a)
Ferroptosis is actually an iron regulated necrosis, which differs from apoptosis by exhibiting shrunken/condensed mitochondria caused by excess intracellular REDOX active iron and the absence of the antioxidant glutathione (Liddell 2015; Abdalkader et al. 2018). Associated with this ferroptosis, neurons and pancreatic islet cells exhibit increases in REDOX-active lipids in diseases such as in diabetes, AD, PD (Abdalkader et al. 2018; Bruni et al. 2018; Zhang et al. 2020a). Ferrostatin-1 is an antioxidant that traps peroxyl radicals, and thus serves as a potent inhibitor of ferroptosis while rescuing nerve cells as a neuroprotectant against Huntington's disease and preventing neurotoxicity from excess glutamate in cell models (Skouta et al. 2014). Brain iron loading was reported to impair DNA methylation and alter GABAergic function in mice, again reflecting the impact of iron load as an effector of ferroptosis to undermine behavioral with cognitive outcomes (Ye et al. 2019). Small molecules have been suggested as useful agents to rescue afflicted iron uptake deficits in defined patient sets (Grillo et al. 2017).
The classic paradigm of cellular iron sensing is the canonical interaction between iron regulatory Proteins (IRP1 and IRP2) and iron responsive element (IRE) RNA stem loops (IRP/IRE interaction) that controls iron homeostasis (as shown in Figs. 1, 2; Thomson et al. 1999; Goforth et al. 2010). IRP1 and IRP2 binding to IREs is under tight control responding to changes in intracellular iron levels in a coordinate manner by differentially regulating ferritin mRNA translation rates and transferrin receptor (TfR) mRNA stability (Anderson et al. 2012). Iron sensing is governed by an IRE/IRP interaction that increase TfR mRNA mRNA stability in the absence of iron to stimulate the cell's requirement for Fe-import. In a negative feedback loop, iron influx causes release of IRPs from the 5 IRE stem loops in the 3′UTR of TfR mRNA to enable ribonuclease digestion of TfR mRNAs to reduce excessive Fe. import (Thomson et al. 1999). There are many activators of iron homeostasis, including thyroid hormone linked to energy metabolism, that operates via IRP1 and IRP2 interactions with 5′UTR specific IRE stem loops in ferritin L and H as well as and Hif-2-alpha mRNAs (Figs. 1, 2; Leedman et al. 1996; Rogers 1996; Goforth et al. 2010; Sanchez et al. 2011; Anderson et al. 2012, 2013; Ghosh et al. 2013, 2018; Anderson and Leibold 2014). Ferritin expresses a cytoprotective ferroxidase activity in its H-chain, accounting for an added Fe storage capacity in mice that prevents MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) neurotoxicity (Kaur et al. 2003). In fact, ferritin replenishment prevents ferroptosis as a target for protection against cardiomyopathy (Fang et al. 2019, 2020; Hernandez-Gallardo and Missirlis 2020).
Figure 1.
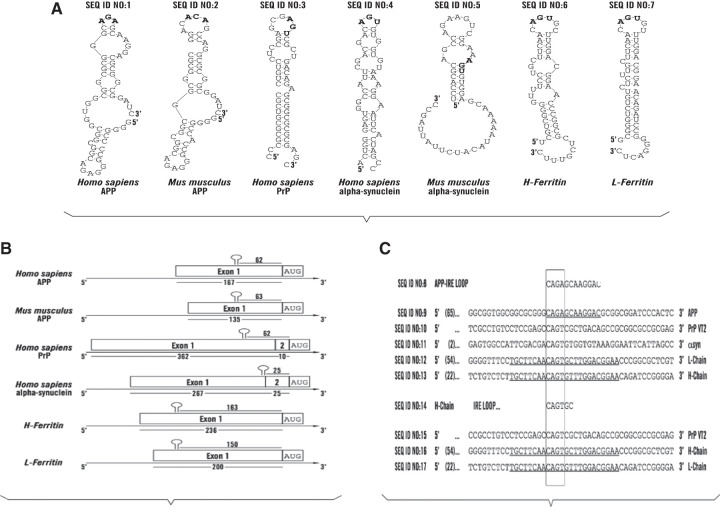
RNA stem loops common to the 5′UTRs in the mRNAs for L- and H-ferritin and the key neurodegenerative transcripts for the amyloid precursor protein, alpha synuclein and Prion Protein. (A) IRE-like AGU/AGA tri-loops and potential iron-regulatory protein binding motifs in key neurodegenerative disease transcripts for human and mouse APP and SNCA mRNAs and in the human PrP and ferritin- L and H transcripts; further links between iron and AD and PD. (B) Organization of the positions of the IRE-like RNA stem loops in the 5′untranslated regions of human and mouse APP mRNAs and in the human PrP, SNCA and ferritin- L and H transcripts. (C) Alignment of the central AGU and AGA motifs in the 5′UTRs of human APP, αsyn and PrP transcripts relative to those for H- and H-ferritin.
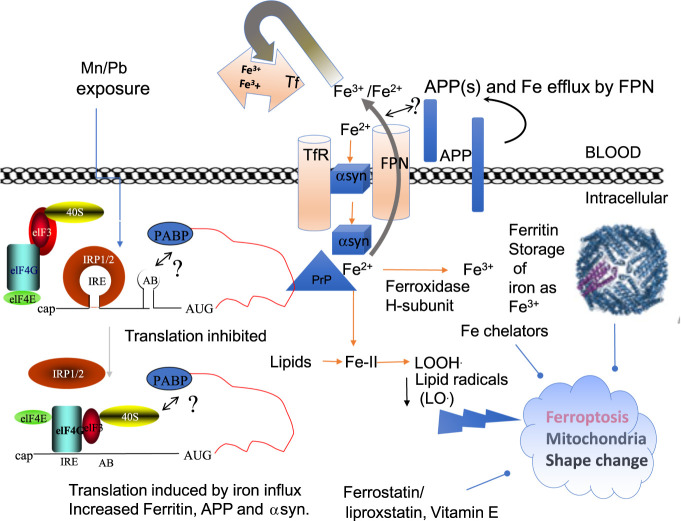
Figure 2.
A Model that merges the canonical IRE/IRP dependent pathways of translation of ferritin and APP to generate balanced iron homeostasis with a depiction of the reported roles of APP, αsyn, and PrP in iron transport (Singh et al. 2014; Baksi et al. 2016; Venkataramani et al. 2018; Tsatsanis et al. 2020). IRP1 and IRP2 are translation repressors in the absence of cellular iron and under conditions of iron chelation (Rogers et al. 2008). Iron influx releases IRPs from the IRE RNA stem loops in the 5′UTRs of L- and H-ferritin, Hif-2α and APP mRNAs to enhance the translation of these neuroprotective proteins (Cho et al. 2010; Goforth et al. 2010; Anderson et al. 2013; Chung et al. 2014). IRP2 in degraded from cells in conditions of iron influx while its deficiency has been associated with neurodegeneration (Zumbrennen-Bullough et al. 2014). Neurotoxic exposures to Mn and Pb repress IRP dependent translation of APP and ferritin H-chain (Venkataramani et al. 2018), a pathological path that we propose can enhance Fe levels to cause ferroptosis. Amyloidosis and α-synucleinopathy advance plaque and Lewy body formations while ferroptosis and neuronal viability appear to accelerate neurodegeneration with cognitive declines. There are testable links between by Nrf2 dependent gene expression of heme oxygenase and H-ferritin and glutathione production as a means to limit ferroptosis (Abdalkader et al. 2018; Song et al. 2020). Mn and Pb inhibition of APP can be predicted to promote ferroptosis and Fe buildup by interfering with APP/FPN complexes for Fe efflux while also toxically blocking ferritin translation, as associated with the production of cytotoxic reactive oxygen species (Rogers et al. 2016; Venkataramani et al. 2018; Fang et al. 2020), iron overload impairs epigenetic DNA methylation patterns to disrupt GABA neurotransmission (Ye et al. 2019). Ultimately the generation of highly dangerous lipid radicals resulting from iron overload can be scavenged by ferrostatin, the standard diagnostic inhibitor of ferroptosis, as well as by ferritin (Balla et al. 1992), activators of hepcidin (Yin et al. 2018), and by the application of nanochelators (Kang et al. 2019).
The endocrinal peptide hepcidin can be regulated to control iron overload by modulating FPN sequestration and export of iron from tissues into the blood stream (Yin et al. 2018). On the other hand, loss of IRP1 is linked to polycythemia as well as having a role in the pulmonary and cardiovascular systems, essential for erythropoiesis by regulating the IRE in the 5′UTR Hif-2-alpha mRNA (Ghosh et al. 2015). IRP2(−/−) mice develop microcytic anemia, erythropoietic protoporphyria and a progressive neurological disorder while both IRP2 and IRP1 have a key role for securing mitochondrial iron sufficiency (LaVaute et al. 2001; Galy et al. 2010; Zumbrennen-Bullough et al. 2014). Of note, the regulatory action of the downstream IL-1 responsive “acute box” motif extends the contribution of this classic paradigm of RNA mediated control of iron homeostasis by ferritin causing iron sequestration to also include the poly C binding proteins (PCBPs) (Thomson et al. 2005). Iron sequestration is a feature of the anemia of chronic disease. The cytosolic 5′UTR specific RNA binding proteins IRP1 as well as PCBPs not only control ferritin translation in the cytosol, these RNA binding proteins can enter the nucleus and regulate transcriptional events in adaptive iron homeostasis (Makayev and Liebhaber 2002; Thomson et al. 2005; Hernandez-Gallardo and Missirlis 2020).
We will discuss how environmental Mn and Pb accumulation in the body can be toxic to neurons potentially via ferroptosis (Kim et al. 2013). See the section Iron regulatory paths of ferritin, APP translation to mitigate metal neurotoxicity by manganese and lead exposures. Mn/Pb exposure to cells induces IRP1 dependent repression of translation of APP and the ferritin-H chain mRNAs (Fig. 4). Loss of APP translation limits the cell's capacity to form Fe-export complexes comprising of APP(s) and ferroportin (FPN) to increase intracellular toxic REDOX active iron (Venkataramani et al. 2018). Mn and Pb also inhibited IRP1 dependent translation of the heavy subunit of iron storage in multimer ferritin (Rogers et al. 2016; Khan et al. 2017).
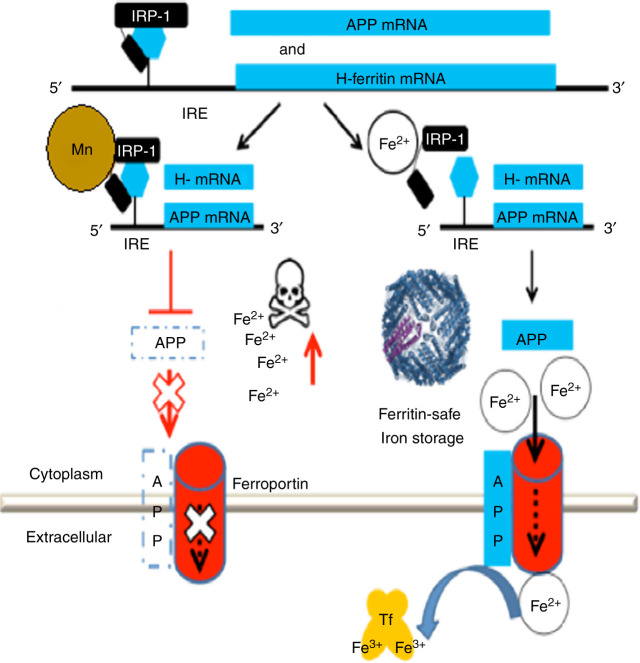
Figure 4.
Model for manganese neurotoxicity invoking activation of APP and ferritin translation by iron-regulatory Proteins. (Right) In healthy individuals, APP facilitates FPN dependent iron export from neurons. (Left) Mn, like Pb exposure, interferes with APP and ferritin by blocking the translation of their mRNAs when causing super-repression by IRP1. Reduced FPN driven iron export by loss of APP(s) and the loss of Fe storage (ferritin) was shown to induce an embargo of iron inside the cell causing reactive oxygen species and REDOX active cell death/ferroptosis (Rogers et al. 2016; Venkataramani et al. 2018). Excess iron forms in magnetic nanoparticles throughout the amyloid plaque during AD (Plascencia-Villa et al. 2016) while ferritin was increased in brains and cerebrospinal fluid of AD patients (Ayton et al. 2015a; Ayton et al. 2016). Of note, amyloid form APP is protective in rodents burdened from acute bacterial infection/oxidative neurotoxicity (Kumar et al. 2016).
Common role of iron in the translational expression and function of neurodegenerative disease proteins
Iron related function for APP, PrP, and αsyn central to Alzheimer's disease, Creutzfeldt-Jakob disease (CJD), and Parkinson's disease
There has been considerable support that the neurodegenerative proteins APP and alpha-synuclein (αsyn) as well as the prion protein (PrP) have a role to maintain cellular iron homeostasis in healthy individuals (Singh et al. 2009, 2013, 2014; Duce et al. 2010; Baksi et al. 2016; Baksi and Singh 2017; Bailey and Kosman 2019; Lahiri et al. 2019) (see model in Fig. 2). The last decade has introduced discoveries that APP and its secreted APP(s) ectodomain can facilitate FPN dependent cellular iron export to provide a novel central antioxidant defense to prevent iron overload in neuronal and endothelial cells (Duce et al. 2010; McCarthy et al. 2014; Rogers et al. 2016; Dlouhy et al. 2019; Tsatsanis et al. 2019, 2020). In these studies, appraisal of cellular iron status was reflected by complementary changes in cellular ferritin and transferrin receptor levels, thus supporting the function of APP695α to facilitate the export of iron from neurons. APP was originally shown to bind to FPN and then stimulate FPN-dependent iron release from neurons (Duce et al. 2010, 2013). This work also showed, unlike normal mice, app−/− mice were more vulnerable to dietary iron exposure, which caused Fe2+ accumulation and oxidative stress in cortical neurons.
Mounting evidence suggests that, like APP, the key neurodegenerative proteins αsyn of PD and PrP of CJD also harbor a key physiological role in iron homeostasis. First, alpha-synuclein is a cytosolic ferrireductase (Davies et al. 2011), which facilitates the uptake of transferrin-bound iron in retinal cells with implications for visual manifestations of PD in some patients (Baksi et al. 2016). Excess αsyn fibrilization impairs ferritinophagy in the outer retina in vivo and in retinal-pigment-epithelial (RPE) cells, action that results in the accumulation of iron-rich ferritin (Baksi and Singh 2017). Second, PrP was modleled to have a role in iron homeostasis since PrP knockout mice exhibited reduced iron uptake and transport (Singh et al. 2009, 2013, 2014). Supporting a common link between PrP, APP, and αsyn in iron metabolism, we identified a significant homology in the 5′UTRs of each of their transcripts (see Fig. 1; Singh et al. 2009). A molecular crosstalk does exist between misfolded proteins in AD and prion diseases (Morales et al. 2012). Finally, the importance of iron homeostasis extends to amyotrophic lateral sclerosis (ALS) since the familial ALS transcript “C9ORF72” encodes an IRE-like RNA stem loop in its 5′untranslated region that resembles the iron-responsive element-like RNA stem loop in the human cis-aconitase mRNA (Lu et al. 2016).
The overall role of αsyn in neurons has yet to be established although this protein is critical for synaptic dopamine neurotransmission (Oaks and Sidhu 2011) and represents1% of all proteins while15% of αsyn is associated with mitochondrial respiration (Ellis et al. 2005; Bendor et al. 2013). αsyn was found to promote dilation of the exocytotic fusion pore with implications for the synapse and neurotransmission (Logan et al. 2017; Pathak et al. 2017); this process may well yet depend on iron as scribed by the findings of N. Singh and her colleagues (Baksi et al. 2016; Baksi and Singh 2017). Of note, GATA transcription factors directly regulate the Parkinson's disease-linked SNCA gene while the heme metabolism genes including erythroid aminolevulinic acid synthase, ferrochelatase, and biliverdin reductase form a block of correlated gene expression in 113 samples of human blood, where αsyn naturally abounds (Scherzer et al. 2007). Supporting current SNCA 5′UTR directed therapeutic strategies for the treatment of PD, lowering the steady state levels of αsyn caused no defects to mitochondrial bioenergetics or loss of viability to neurons, typical of ferroptosis (Figs. 2, 3; Dixon et al. 2012, 2014; Pathak et al. 2017; Stockwell et al. 2017).
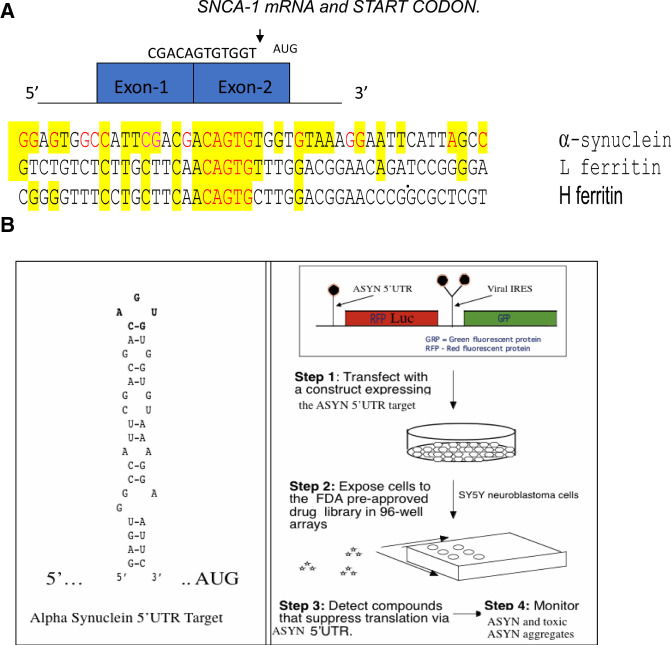
Figure 3.
The 5′Untranslated region of alpha synuclein mRNA was the screening target for identifying αsyn translation blockers to address the need for Parkinson's disease and PDD and LBD therapies. The SNCA 5′UTR can be tested also to alleviate conditions of excess environmentally induced α-synucleinopathy (Friedlich et al. 2007; Rogers et al. 2011; Mikkilineni et al. 2012; Harischandra et al. 2019b). (A) The unique RNA target in the 5′UTR of the SNCA transcript. Depicted is the SNCA 5′UTR with its two exons immediately in front of the SNCA gene start codon (Olivares et al. 2009). Shown is the linear alignment of the SNCA 5′UTR with those for L- and H-ferritin mRNAs in the vicinity of their IRE RNA stem loops. (B) High-throughput screen for small molecule inhibitors of the RNA stem loop folded from the major neuronal neurodegenerative SNCA mRNA (MULTIFOLD, as described in Cho et al. 2010). Shown is a version of the transfection-based screen to identify SNCA 5′UTR directed translation inhibitors of a downstream luciferase reporter gene. During typical screens, inhibitors selectively maintained translation of a control GFP gene driven from an internal ribosome entry site (IRES) RNA structure. This RNA targeting technology was used to identify translation inhibitors of APP mRNA for AD therapy (Rogers et al. 2002a,b). The list of key HTS inhibitors of the SNCA 5′UTR are shown in PUBCHEM website as AID 1813. (Collaboration between Neurochemistry Laboratory at MGH and the Broad Inst. Cambridge, MA, https://pubchem.ncbi.nlm.nih.gov/bioassay/1813#section=Description
Like APP mRNA, The 5′UTR of the alpha-synuclein transcript was predicted to encode an iron responsive-like element (IRE-like) RNA stem loop (Fig. 1; Rogers et al. 2002a,b, 2011; Friedlich et al. 2007; Cho et al. 2010). This finding and subsequent confirmations were consistent with fact that αsyn itself facilitates the uptake of transferrin-bound iron in retinal cells (Baksi et al. 2016; Baksi and Singh 2017). In models of PD, alpha-synuclein associated iron buildup has been linked to ferroptosis and morphological changes to mitochondria as an early preapoptotic event in the neurodegeneration of dopaminergic neurons (Fig. 2; Zhang et al. 2020a). In addition, induction of αsyn infectivity and spread was reported to result from excess Mn exposures that increases cellular Fe driven oxidative stresses and can induce Parkinsonism phenotypes (Rogers et al. 2016; Harischandra et al. 2018, 2019a; Venkataramani et al. 2018; Zhang et al. 2020b). Indeed we will discuss how translation activators of APP mRNA can be therapeutically used to prevent Mn toxic consequences to generate ferroptosis as much as αsyn translation inhibitors can be advanced to prevent Lewy body formation and thus PD/DLB pathology (Zhang et al. 2020b). See the section Therapies to limit αsyn expression. Specific iron-associated damage can be offset by the use of novel chelation treatment options for PD with small molecules or transferrin based iron chelation (Ayton et al. 2015b; Finkelstein et al. 2017).
APP cleavage by α-, β-, and γ-secretases, provides a hypothesis to describe how these proteases may contribute to AD pathogenesis and how rare missense mutations in APP and γ- secretase may cause familial AD (De Strooper and Karran 2016; Walsh and Selkoe 2020). However, the common role of iron homeostasis linked to the biology of APP αsyn and PrP may yet yield therapies that address pathologies related to perturbed iron homeostasis in the neurodegenerative diseases previously associated with this altered processing misfolding (Jin et al. 2018). Increases in the genetic copy numbers of APP and αsyn genes generate familial AD and PD, respectively (Huang et al. 2017, 2019) while genetic variations in the APP promoter correlate with AD risk (Lahiri et al. 2005b; Maloney et al. 2010), and APP gene duplications cause early-onset AD (Rovelet-Lecrux et al. 2006) These findings show that APP dose and levels of expression are indeed important for AD pathogenesis. The finding that Apo-E increases APP transcription 4–sixfold in human neurons by activation of cFos-containing AP-1 transcription factors suggests that it may be possible to influence APP transcription pharmacologically as an approach to AD prevention (Huang et al. 2017, 2019). It has already been possible to target APP mRNA translation in order to diminish APP and limit amyloid production as a therapeutic option for AD. Some of these latter studies include the use of repurposed iron chelators and anticholinesterases, some of which were tested in clinicals of efficacy trials for AD (Crapper McLachlan et al. 1991; Shaw et al. 2001; Bush and Tanzi 2008; Winblad et al. 2010; Rogers et al. 2019)
Iron-regulatory proteins (IRP) control of translation of APP, alpha-synuclein (αsyn), and prion protein (PrP) transcripts in conditions of iron influx
The iron-regulatory proteins (IRP-1 and -2) are the RNA-binding proteins that iron-dependently interact with IRE RNA stem loops that control the 5′UTR driven translation of the light and heavy subunits of ferritin, a genetic activity to enhance the iron storage capacity of all cell types, inclusive of promoting viability of neurons undergoing oxidative stress (Cho et al. 2010; Venkataramani et al. 2018). IRP1 and IRP2 determine rates of cellular iron acquisition by controlling the mRNA stability of the transferrin receptor (TfR) via 3′UTR specific RNA protein interactions. (Rogers et al. 2008; Cho et al. 2010; Sanchez et al. 2011)
Collaborative work demonstrated that the 5′-UTR of APP mRNA, like ferritin, controls APP expression at the level of translation in response to both iron and interleukin-1 (Thomson et al. 1999; Rogers et al. 1999, 2002a). This applies to modulate β-amyloid production in astrocytes (Rogers et al. 1999) while APP itself facilitates the central iron exporter FPN to efflux excess iron from neurons and endothelial cell (Duce et al. 2010; Kosman 2019; Rogers et al. 2019; Tsatsanis et al. 2020). The fact that IRP1 controls rates of APP translation in neuronal cell lines and rodent models is consistent with the major physiological function of APP to facilitate FPN dependent export of iron from Fe burdened neurons (Cho et al. 2010; Duce et al. 2010; Ayton et al. 2015c; Venkataramani et al. 2018). Debomoy Lahiri and his colleagues collaboratively reported microRNA-346 and iron are positive regulators of APP translation via the IRE RNA stem loops in 5′UTR of the mRNA (Long et al. 2019).
Excess brain iron confers suboptimal lethality to neurons by common pathways that toxically accelerate IRE/IRP disruption of ferritin translation and intracellular iron homeostasis to cause ferroptosis while glutathione acts as opposing antioxidant (Faucheux et al. 2002; Lee et al. 2009; Zhou and Tan 2017). We discuss the use of APP and alpha-synuclein inhibitors of translation when targeted to iron responsive-like elements in each of their transcripts to prevent αsyn buildup and its fibrilization into Lewy bodies. However, αsyn and PrP modulators can be modeled also to provide medicinal agents to prevent ferroptosis in vulnerable neurons, i.e., from iron and heavy metal oxidative death (Zhang et al. 2020a). The model in Figure 2 merges the IRE/IRP dependent ferritin regulatory pathways of translation with iron transport roles of αsyn, APP, and PrP. These pathways combine to generate ferroptosis in the event of neurotoxic exposures of cells to excess Fe, Mn, and Pb (Figs. 2, 4). Certainly, ferroptosis undermines neuronal viability and is associated with neurodegenerative disease of cognitive decline as much as by amyloidogenesis in AD and Lewy body formations via α-synucleinopathy in PD (Zhang et al. 2020a). Related efficacies exist between the synthesis antioxidant glutathione by Nrf2 inducible gene products as much as by the uses of modulators of APP and αsyn translation to adjust cellular Fe transport. Each of these paths can be tested to prevent ferroptosis early in the onset of neurodegeneration (Abdalkader et al. 2018; Song et al. 2020).
Increases of intracellular iron are associated with the dopaminergic neurons of the substantia nigra (SN) in the midbrain of PD patients (Jellinger et al. 1990; Oakley et al. 2007). This elevation of the SN iron pool has the potential to release IRP1/IRP2 from repressing IRE stem loop dependent translation of ferritin. The lack of IRP2 in knockout mice does accelerate ferritin expression in defined neurons (LaVaute et al. 2001). Elevated iron also can induce misexpression of the markers such as tyrosine hydroxylase and dopamine, which are hallmarks of the PD brain (Salvatore et al. 2005). Iron acting through IRE/IRP systems certainly induces αsyn production as well as that of APP in the PD brains (Rogers et al. 2011; Ayton et al. 2015c). The IRE-like AGU sequences are on the apex of the 5′UTR specific stem loop of αsyn mRNA, as is shown in Figures 1 and 3. This SNCA mRNA stem loop is uniquely folded to offer a new therapeutic RNA target to control αsyn translation (Rogers et al. 2011; Zhang et al. 2020b).
Small molecules targeted to the APP, αsyn, and PrP 5′UTRs to improve iron homeostasis
APP gene expression is regulated at the level of translation in astrocytes and some neuronal lines by the inflammatory cytokine IL-1 and by iron influx while transcriptional induction is clear in endothelial and some neuronal lines (Goldgaber et al. 1989; Rogers et al. 1999, 2002a; Lahiri et al. 2005a). This regulatory circuit contributes to known acute phase events in AD that compliment apo-lipoprotein-E and alpha-anti-chymotrypsin pathogenic chaperoning of amyloidogenic progression in an inflammatory cascade mounted by astrocytes and microglia (Nilsson et al. 2004; Potter and Chial 2019). APP mRNA translation is controlled by the same iron-dependent 5′UTR specific IRP/IRE RNA protein interactions that set rates of ferritin and FPN mRNA translation and the mRNA stabilization of DMT-1 and transferrin receptor (Qureshi et al. 2008; Rogers et al. 2008; Olivares et al. 2009; Cho et al. 2010; Duce et al. 2010; Venkataramani et al. 2018). To maintain homeostasis, iron influx into neurons releases binding of IRP1 from the IRE folded from 5′UTR sequences in APP mRNA, activity that relieves the precursor from translational repression while IL-1 coinduces alpha-secretase and APP(s) secretion to enhance Fe efflux (Tsatsanis et al. 2020).
Mechanistically, the fact that iron controls rates of APP translation is consistent with gene knockout studies by Sh-RNA in neural cell lines where a 20-fold reduction of intracellular IRP1 caused a fivefold increase of APP expression in neural cells (Cho et al. 2010). These findings are consistent with the model that neurodegeneration in AD is not only the result of protein fibrilization, however perturbations iron homeostasis run parallel to amyloidosis. In fact, the physiological role of APP in iron homeostasis centers on its contribution to FPN dependent export of excess iron from Fe burdened neurons, as yet to be fully characterized in terms of REDOX activity (Dlouhy et al. 2019). Both amyloid and Mn exposures certainly caused a reduction of intracellular APP (APPs) in cells that reflected a blockade of iron export and loss of viability (Venkataramani et al. 2018; Tsatsanis et al. 2020). Using transfection based studies, this embargo Fe export was shown to be lifted when translation of the human APP was uncoupled from its repression in the absence of IREs (Venkataramani et al. 2018).
The consequences of overexpression of the APP in the onset of AD, includes that of duplications of the APP gene (Sleegers et al. 2006) and transcriptional activations (Geller and Potter 1999; Theuns et al. 2006; Potter et al. 2016). These events cause familial AD via amyloidosis and congophilic amyloid angiopathy (CAA) in families, the so called pathology in “Dup-APP” forms of familial AD (Rovelet-Lecrux et al. 2006; Buss et al. 2016; Rogers et al. 2019). Chromosome 21 nondisjunction with its APP gene has been elegantly modeled in cases of spontaneous AD (Geller and Potter 1999). By analogy, gene duplication and triplication of alpha-synuclein also causes a rare familial PD with later onset dementia (PDD) (Singleton and Gwinn-Hardy 2004; Nishioka et al. 2006). Alpha-synuclein fibrilization is a feature of sporadic PD as well as Lewy body dementia (LBD), the second most common cognitive liability after AD and vascular dementia (Cantuti-Castelvetri et al. 2005).
We described the practicable means by which inhibition of APP expression at the level of translation of its mRNA can be used as an anti-amyloid strategy to treat Down syndrome with amyloidosis as well as during the pathology in “Dup-APP” (Buss et al. 2016; Rogers et al. 2019). Simple abundance of the APP and αsyn genes, and therefore their transcripts and protein levels, can cause neurodegeneration. Therefore, novel agents under investigation include 5′untranslated region inhibitors of both APP and alpha-synuclein translation. In the first case these provide anti amyloid efficacy for older AD patients (Bandyopadhyay et al. 2013) and, second, prevent α-synucleinopathy in PD and LBD patients (Ross et al. 2010b, Kuo et al. 2019). The existence and uses of highly selective 5′untranslated region inhibitors and activators that had been high-throughput screened (HTS) to modulate APP, αsyn, and PrP translation are summarized as follows:
Since APP mRNA translation is up-regulated by iron and Interleukin-1 via 5′UTR sequences (Rogers et al. 1999, 2002a; Long et al. 2019), it is highly feasible that APP 5′UTR directed translation blockers provide anti-amyloid efficacy for AD patients (Bandyopadhyay et al. 2013). Indeed, the anti-cholinesterase phenserine was shown to target APP 5′UTR sequences (Shaw et al. 2001) and then underwent clinical trials for AD efficacy (Greig et al. 2005; Yu et al. 2013). Its enantiomer posiphen is currently undergoing clinical trials as a therapeutic agent to prevent AD (Teich et al. 2018). The compound JTR-009 is a low molecular weight benzimidazole modeled to intercalate into the IRE-type-II RNA stem loop in the 5′UTR of APP mRNA, an action that irreversibly replaces IRP1 to chemically block APP mRNA translation (Rogers et al. 2002a; Bandyopadhyay et al. 2013; Teich et al. 2018). Table 1 summarizes data from these papers showing JTR-009 has a planar aromatic structure capable of reducing Aβ levels in neural cell lines (*IC-50 ∼ 50 nM) and may also generate a capacity to limit amyloid plaques in mouse models of AD and in aging AD patients similar to the clinically tested anti-amyloid associated APP mRNA translation blocker posiphen (Teich et al. 2018).
Manganese and lead exposure to children and working adults increases toxicity to neurons and the risk of cognitive problems in defined brain subregions during development and after aging, for example, Lowered IQ (Vollet et al. 2016) (iron regulatory paths of ferritin, APP translation to mitigate metal neurotoxicity by manganese and lead exposures section). To thwart the toxicity of these metals in the absence of long-term accumulation of amyloid, both APP and ferritin 5′UTR activators can be predicted to be trophic to such metal exposed neurons. The identity of a high-throughput screen to identify such APP 5′UTR translation activators is outlined in PUBCHEM-AID-1276. In addition, the known M1 selective muscarinic agonist AF102B is an existing FDA approved agent with the property to induce APP 5′UTR driven translation (Rogers et al. 2019). Induced levels of APP(s) may drive increased formation and distribution of FPN into intracellular and membrane-associated complexes with APP (Tsatsanis et al. 2019). A FPN-targeting sequence, (K/R)EWEE, present in APP and APLP2, but not APLP1, may indeed help modulate FPN-dependent iron efflux in the presence of an intrinsic or alternate active multicopper ferroxidase such as in haephestin and/or ceruloplasmin (Dlouhy et al. 2019). These molecular events can be modeled to increase Fe efflux sufficient to relieve cells of iron surfeit and prevent Fenton conversion of ROS into lethal hydroxyl radicals (Figs. 2, 4; Rogers et al. 2005; Fisher 2007; Fisher et al. 2016; Rogers et al. 2019; Mou et al. 2020) (iron regulatory paths of ferritin, APP translation to mitigate metal neurotoxicity by manganese and lead exposures section).
The prion specific PrP 5′UTR translation blocker - BL-1- was described in the high-throughput screen (HTS) described in PubChem AID: 488862. This benzimidazole was selected as an inhibitor of intracellular expression of prion protein after HTS conducted in cultured SHSY5Y neurons. In fact, the use of BL-1 to prevent prion accumulation can be used for treating amyloid mediated damage in AD since PrP is known to be an Aβ receptor while prion antibodies are anti-amyloid in their profile of efficacy in rodent models (Lauren et al. 2009; Barry et al. 2011). Additional to this anti-prion therapeutic property of BL-1, the capacity of this agent to reduce PrP translation may also remediate perturbed iron uptake into the brain. PrP has a role in iron uptake (Fig. 2; Singh et al. 2009); Inhibitors of PrP translation, such as demonstrated by BL-1, can activate translation of H-ferritin via its canonical IRE while increases of ferritin. This action is to be tested to rescue cells from iron overload, ferroptosis, and thereby improve neuronal viability from oxidative assault.
Several agents have been screened via 5′UTR dependent transfection-based assays to limit SNCA mRNA translation to treat PD, PDD, and LBD. The clinical trial drug posiphen was reported to inhibit translation of both brain and intestinal αsyn (Kuo et al. 2019). Similar to pharmacological increases translation of APP and ferritin as a therapy to prevent ferroptosis-associated damage to cultured neurons, SNCA 5′UTR blockers as enlisted in PUBCHEM-AID 1827, are to be used to limit alpha-synuclein expression to prevent ferroptosis in the onset of PD (Fig. 2; Zhang et al. 2020a). These SNCA 5′UTR blockers will have the added benefit of preventing Mn accelerated spread of αsyn fibrils (Fig. 4; Harischandra et al. 2018, 2019a).
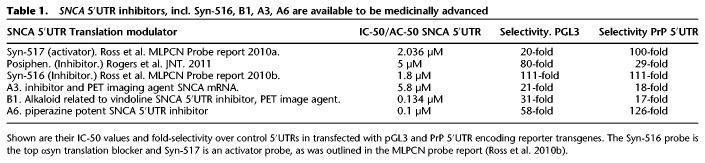
Table 1.
SNCA 5′UTR inhibitors, incl. Syn-516, B1, A3, A6 are available to be medicinally advanced
Therapies to limit αsyn expression
The biology and expression of αsyn: strategies to limit α-synucleinopathy to prevent PD and Lewy body dementia
Alpha-synuclein (αsyn) and PD pathology: αsyn is the ∼15 kDa protein implicated in pathogenesis of α-synucleinopathies, especially in the substantia nigra (SN) in the mid brain of patients with Parkinson's disease, the most prevalent movement disorder in humans (Ueda et al. 1993; Burre et al. 2018). Prodromal symptoms exist early in the onset of PD, including constipation and rapid eye movement sleep behavior disorder (RBD) (Stocchi and Torti 2017; Taguchi et al. 2020a,b). In the case of Dementia with Lewy bodies (DLB), Lewy body variant of Alzheimer's disease. and multiple system atrophy, αsyn undergoes a conformational change and oligomerization to cause a toxic gain of function and neurodegenerative deposition of aggregates commonly in Lewy bodies, also in dystrophic neurites and glial cytoplasmic inclusions (Lee et al. 2004) (LBD). Defined α-synucleinopathies are a central cause of dopaminergic cell death and PD pathology (Fanning et al. 2019; Kuo et al. 2019). Other familial PD associated genes include LRRK2 (Bakshi et al. 2019). Neurodegenerative REDOX active iron and αsyn fibrils are a coordinate feature of the SN in PD progression (Halliday et al. 2005; Jiang et al. 2017; Stockwell et al. 2017).
The intertwined links between iron and αsyn is supported by several lines of evidence: (i) the iron-binding pigment in neuromelanin is an MRI imaging target that accompanies neuronal depletion in the PD brain (Double et al. 2002; Halliday et al. 2005; Oakley et al. 2007; Chen et al. 2017). (ii) αsyn Itself, in health, has an integrative role in cellular iron transport (Baksi et al. 2016; Baksi and Singh 2017; Haining and Achat-Mendes 2017; Sulzer et al. 2018). As noted, increased expression of αsyn impairs ferritinophagy in the retinal pigment epithelium with Implications for retinal iron dys-homeostasis in PD and in dopaminergic neurotransmission (Baksi and Singh 2017). (iii) The presence of iron colocalized with αsyn in Lewy bodies, the increases of iron in the SN and the correlation between polymorphisms of the several genes implicated in iron metabolism and PD support a role for iron in the neurodegeneration (Oakley et al. 2007; Jiang et al. 2017). (iv) there is a uniquely folded version of an IRE-like RNA stem loop in the 5′UTR of SNCA mRNA (Figs. 1, 3; Mikkilineni et al. 2012). This SNCA 5′UTR potentially regulates brain αsyn by iron while the SNCA 5′UTR binds to IRP1 in a different manner than for IREs that control ferritin translation (Friedlich et al. 2007). The Ferritin L- and H-mRNAs encode a classic IRE RNA stem loop in their 5′UTRs that interacts with both IRP1 and IRP2 translational repressors in low iron conditions while the operational range of the SNCA 5′UTR in response to iron has yet to be recorded (Thomson et al. 2005; Wang et al. 2007a,b; Rogers et al. 2011).
The Braak stages of PD include later stage cognitive impairment that overlaps with AD and is present in PD with dementia (PDD) (Jellinger 2000). Alpha-synuclein and amyloid Aβ pathomechanisms converge via interaction of these two amyloidogenic proteins; coprecipitating into β-pleated oligomers and insoluble fibrils (Han et al. 1995; Jensen et al. 1997; Conway et al. 1998) although Aβ and αsyn rarely colocalize in brain amyloid deposits (Trojanowski and Lee 2001). Lewy body dementia (LBD) brains exhibit lowered SNCA mRNA but higher insoluble αsyn protein, suggesting misregulated translation additional to its clearance by chaperones (Cantuti-Castelvetri et al. 2005; Rogers et al. 2011). Alpha-synuclein and its mRNA were discordantly expressed relative to each other in LBD patients compared to controls (Cantuti-Castelvetri et al. 2005). In this case, the spread of αsyn is a feature of memory impairment and neuronal death (Adamowicz et al. 2017). Certainly alpha-synuclein mRNA is recruited to polysomes in response to exposures of HEK293 cells to ferric ammonium citrate, indicating translational control circuits at work (Febbraro et al. 2012). Published data on the reduction of αsyn levels shows that limiting levels of this protein does not adversely affect mitochondrial bioenergetics in rodent neurons (Logan et al. 2017; Pathak et al. 2017). In support, the phenotype of mice expressing the SNCA gene knockout are viable and this fact further justifies the therapeutic benefit to limit αsyn expression in the brain as much as to accelerate and target its clearance.
Clinicians currently have little choice other than to treat PD patients with standard disease symptomatic therapies such as rasagaline, a monoamine oxygenase inhibitor (Youdim and Lavie 1994) that prevents L-dopamine degradation (Youdim and Weinstock 2001; Trudler et al. 2014). For this reason, PD, PDD, and LBD are all α-synucleinopathies that provide an excellent justification for the use of SNCA 5′UTR directed translation blockers as a rationale for our strategy to limit αsyn translation in both PD and manganism patients. We will describe the therapeutic action of selective translation blockers of the SNCA transcript via its uniquely folded IRE in its 5′UTR (Rogers et al. 2011; Mikkilineni et al. 2012; Kuo et al. 2019; Zhang et al. 2020b). The model in Figure 3 shows that αsyn mRNA encodes 5′UTR specific stem loop with a CAGUGU loop motif in the apex of the IRE-like stem loop which can be regulated by iron via IRP1 and IRP2. This is a relevant translational regulatory circuit at play during the etiology of PD. The SNCA mRNA encodes this RNA motif as a drug target to prevent PD and PDD either dependently or independently of IRP1 and IRP2 (Zhang et al. 2008).
Parkinson's disease and PD with dementia (PDD)
As stated in the Common role of iron in the translational expression and function of neurodegenerative disease proteins section, duplication or triplication of the SNCA gene can lead to familial PD where increased dose of this pathogenic protein is correlated to severity of symptoms (i.e., “triple repeat SNCA patients” show dementia) (Singleton et al. 2003; Chartier-Harlin et al. 2004; Singleton and Gwinn-Hardy 2004; Ahn et al. 2008; Ikeuchi et al. 2008). Polymorphic variability in the promoter of the SNCA gene was also linked to its increased expression as a risk factor for PD (Chiba-Falek et al. 2006). Since increased expression of αsyn can genetically cause and progress PD, high-throughput screens were conducted to advance efficacies with selective lead 5′UTR directed translation blockers of SNCA mRNA (Table 1). As described in the RNA based therapies used as α-syn translation inhibitors for PD, PDD, LBD section, the αsyn translation blocker probe “Syn-516” had been published from this screening campaign to provide proof-of concept for this strategy (Fig. 3; Ross et al. 2010b).
Dementia with Lewy bodies (DLB)
Consistent with strategies to pharmacologically control αsyn translation rates and prevent its overexpression neuropathologically, DLB is characterized by the accumulation of aggregated alpha-synuclein protein in Lewy bodies and Lewy neurites similar to Parkinson's disease. Extrapyramidal motor features characteristic of PD are common in DLB patients, but are not essential for the clinical diagnosis of DLB (Outeiro et al. 2019). In fact, αsyn fibrilization in several parts of the brain generates the third most common dementia as contributed by LBD patients in the population (Cantuti-Castelvetri et al. 2005, 2007). LBD is often preceded by prodromal “REM (rapid eye movement) sleep behavior disorder (RBD)” (Posturna and Berg 2019). The exact cause of DLB is unknown it but involves widespread deposits of abnormal deposits of αsyn that form Lewy bodies or Lewy neurites of the diseased brain stem (Walker et al. 2015). DLB and PDD, jointly known as Lewy body dementias, are subject to clinical trials and meta-analyses that provide the basis for the treatment of cognitive, neuropsychiatric, and motor symptoms in patients with LBD. There is expert support for the application of treatments for related conditions, such as PD, for the management of common symptoms (e.g., autonomic dysfunction) in patients with Lewy body dementia. However future clinical trials need to focus on the treatment of symptoms specific to patients with LBD (McKeith et al. 2020; Taylor et al. 2020). Association between αsyn blood transcripts and neuroimaging indicated a presence of PD/DLB at work in the brains of those to be affected with both PD and DLB (Locascio et al. 2015). All of these clinical features remain consistent with RNA based therapeutic approaches that target and inhibit the RNA secondary structure of SNCA 5′UTR outlined in the RNA based therapies used as α-syn translation inhibitors for PD, PDD, LBD section and in Figure 3.
RNA based therapies used as α-syn translation inhibitors for PD, PDD, LBD
Introduction
There is compelling support to pharmacologically limit expression of αsyn with translation blockers of the SNCA transcript (Fig. 1). This is supported by the use of immunotherapeutic strategies in the brains to limit αsyn in PD patients (Spencer et al. 2016, 2017; Kuo et al. 2019). As noted above, duplication or triplication of the SNCA gene leads to familial PD and dementia (PDD) (Chartier-Harlin et al. 2004; Singleton and Gwinn-Hardy 2004; Ahn et al. 2008; Ikeuchi et al. 2008). Experimental siRNA infusions demonstrated it is possible to limit αsyn in animal models (Lewis et al. 2008) although small molecules are more amenable to medicinal chemical optimizations and development for an oral drug therapies. Dopaminergic toxicity is diminished in mice with genetic deletion of brain αsyn levels (Dauer et al. 2002; Song et al. 2004; Alvarez-Fischer et al. 2008; Chen et al. 2013, 2017). We reported the use of the 5′UTR of SNCA mRNA as a uniquely structured RNA target to identify novel high-throughput screened potent and selective αsyn translation inhibitors for future therapeutic development (Table 1; Ross et al. 2010b). We will discuss the proof of concept that the drug posiphen was shown to limit αsyn expression in the cortical neurons expressed in PAC transgenic mice that express the complete 145 kb human SNCA gene (Friedlich et al. 2007; Kuo et al. 2010, 2019; Rogers et al. 2011). The abundant αsyn is subject to Mn induced toxic spread and αsyn fibrilization from exosomes to cause neighboring inflammation as a factor on the etiology of PD (Venkataramani et al. 2018; Harischandra et al. 2019b). This consideration further supports our small molecule-oriented therapies to suppress αsyn translation as a therapy for PD.
In comparison to the L- and H ferritin mRNAs, which encode a classic IRE RNA stem loops that interact with both the IRP1 and IRP2 translation repressors, the IRE-like stem loop of SNCA mRNAs is formed at the splice junction of the first two exons in SNCA gene (Figs. 1, 3; Thomson et al. 2005; Wang et al. 2007a,b, Zhang et al. 2008, Olivares et al. 2009, Rogers et al. 2011). The 5′UTR of SNCA mRNA is a uniquely folded RNA target to identify relatively safe drugs, such as posiphen, that selectively and therapeutically limit αsyn expression in midbrain and cortical neurons, as expressed in PAC transgenic mice with the complete 145 kb SNCA gene (Rogers et al. 2011; Mikkilineni et al. 2012; Kuo et al. 2019; Taguchi et al. 2020a) This SNCA 5′UTR RNA target has been used to identify potential intercalators that bind and interact with the specificity into the predicted RNA stem loop to interfere with SNCA mRNA translation relative to ferritin mRNA (Ross et al. 2010b; Zhang et al. 2020b). SNCA 5′UTR modulators are modeled to leave the cellular Fe storage capacity unchanged, thus maintaining iron homeostasis and viability of dopaminergic neurons (Table 1; Figs. 1, 3; Jiang et al. 2017).
FDA‐approved glycosides provide proof that the SNCA 5′UTR is a valid target for PD
We stably transfected H4 neural cells with a human SNCA 5′UTR–luciferase construct, and then screened 780 natural products to identify top inhibitors of αsyn translation (Rogers et al. 2011). Of 17 hit SNCA 5′UTR directed natural product (NP) translation inhibitors*, four natural products, the cardiac glycosides strophanthidine, digoxigenin, sarmentogenin plus mycophenolic-acetate, exhibited IC-50 sec at <5 µM in dose-response. Western blotting confirmed strophanthidine reduced αsyn expression in SK-N-SN and SHSY5Y neural cell lines (IC-50 < 1 µM). Strophanthidine maintained cellular β-actin levels as well as levels of H-ferritin (IRE encoding mRNA), ensuring specificity toward SNCA mRNA; gitoxigenin exerted no αsyn inhibition. In the future It will be possible to advance such SNCA 5′UTR inhibitors, for example, by establishing efficient adeno-associated viral (AAV) vector constructs, which include the SNCA 5′UTR element, and use their neuron-specific (e.g., synapsin-1) promoters to drive the expression of human wild-type αsyn and mutant missense versions of associated with αsyn fibrilization (Cai et al. 2018).
Posiphen inhibits the SNCA 5′UTR as a valid in vivo therapeutic drug target for PD
The clinically tested anti-cholinesterase (AChe) phenserine is a physostigamine analog that reached phase III clinical assessment for AD and had been characterized to inhibit APP mRNA translation though its 5′UTR (Shaw et al. 2001; Venti et al. 2004; Utsuki et al. 2006). Posiphen (PS) is its enantiomer and is a well-tolerated drug and has been administered at doses of 75 mg versus 20 mg/kg/day in mice to suppress APP (Aβ) translation in vivo (Farlow 2004; Lahiri et al. 2007). Posiphen does not itself exhibit AChe activity and has been approved for clinical trials for AD while it also exhibited anti-αsyn efficacy in murine models of PD (Teich et al. 2018; Kuo et al. 2019). Because there was noted to be a 50% sequence similarity between the APP and SNCA 5′UTRs (Friedlich et al. 2007), we had originally predicted overlap in the spectrum of drugs that would suppress APP mRNA translation through its 5′UTR with those that might also suppress steady state levels of neuronal αsyn (Rogers et al. 2011).
Posiphen was first shown to have potential as a PD therapy when observed to repress the translation of αsyn (IC-50–5 µM) in primary neurons explanted for the brains of the SNCA 5′UTR competent PAC-Tg(SNCA) mouse model of PD (Rogers et al. 2011; Mikkilineni et al. 2012). An in vivo study extended posiphen's potential as a PD therapy since the drug reduced αsyn levels in both the brains and gut neurons of these mice (Kuo et al. 2019). In this study, posiphen normalized colonic motility reflecting prodromal gastrointestinal dysfunction in early PD before the onset of motor disturbances. Three-month PS treatment restored stool motility in PAC-Tg-SNCA mice overexpressing double familial PD mutations while biochemical determination for αsyn/β-actin was measured in the brain cortices postsacrifice. Indeed α-syn levels were lowered in the brain and guts of this mouse model of PD (Kuo et al. 2019). Untreated mice were compared to counterparts treated at doses of 10, 25, 50, 75 mg/kg/day PS for 3 wk (Kuo et al. 2019). Consistent was the fact that PS therapeutically limited APP and SNCA 5′UTRs ex vivo in neurons explanted from PAC/Tg(SNCA) mice (Mikkilineni et al. 2012). A cautionary note is that the metabolic byproducts of posiphen from the liver could include derivatives that cause cholinergic excitability (Mikkilineni et al. 2012). This attractive AD drug may therefore present as somewhat of challenge for usage in PD patients where a gait problems already exist (Mikkilineni et al. 2012).
The high‐throughput screened (HTS) small molecules Syn‐516 and Syn‐517 as proof of selectivity of SNCA 5′untranslated region as a drug target for PD, PDD, and LBD
Because of its unique RNA stem loop structure, the SNCA 5′UTR provides an excellent drug target to screen and identify suppressors of αsyn translation via an IRE-like stem loop not found in β and γ-syn mRNAs (Figs. 1, 3; Rogers et al. 2011; Mikkilineni et al. 2012; Zhang et al. 2020b). RNA targeted intercalators screened to bind the SNCA 5′UTR may well therapeutically interfere with the translation of SNCA mRNAs to a greater extent than for L- and H- ferritin mRNAs. This criterion should maintain normal iron homeostasis and viability of dopaminergic neurons while limiting α-synucleinopathy (Jiang et al. 2017). We will discuss the properties of the αsyn translation blocker probe “Syn-516” that was been published from a HTS to provide proof-of concept for our strategy (Ross et al. 2010b).
Based on the HTS screening campaign for αsyn translation blockers, 35 novel 5′-untranslated directed agents were identified after an exhaustive screen/counter-screen campaign from the Broad Inst., Cambridge, MA (Table 1). The compound “Syn-516” was published in 2012 as a highly selective and potent agent in an official probe report of the Molecular Libraries Probe Production Centers Network (MLPCN) of the NIH and designated as “ML-150” (Ross et al. 2010a,b). A representative four further top SNCA 5′UTR modulators from this MLPCN screen are shown in Table 1 with their listed IC50 and AC50 values. These AC50s were found on PUBCHEM to be even lower and more selective than Syn-516. It is feasible to test medicinal analogs of the best SNCA 5′UTR blocker from this list and determine its IC-50 profile for αsyn inhibition in induced pluripotent stem cells (ipSCs) differentiated to dopaminergic neurons from a patient with familial PD for triple copies of the SNCA gene (Bakshi et al. 2018, 2019). Useful information about the MLPCN probe and αsyn inhibitor Syn-516 is listed in PUBCHEM-AID 1827. During this same screen, the agent Syn-517 was cherry-picked as the most potent/selective translation activator of the 5′UTR in SNCA mRNA (bioassay AID 1814) (Ross et al. 2010a,b).
During screen/counter-screening, the lead αsyn translation blocker Syn-516 exhibited a “hit” cutoff of ≥62% inhibition at 7.5 µM where 2498 compounds were originally identified as inhibitors of SNCA 5′UTR driven luciferase expression. Of these, 2193 compounds had been retested at eight-point conc. series (20–0.16 µM) in the primary screening cell line. Compounds that had IC-50 values <1 µM were successfully retested (1381/2193, 63% rate). Syn-516 was advanced after two counter-screens (Table 1). First, the H4-2a neural cell line with the SNCA 5′-UTR stem–loop removed (H4-C) was screened (Z′ = 0.65) while precluding cytotoxic agents to cause no global reductions in transcription/translation. Second, a similar exclusion of PrP 5′UTR inhibitors was undertaken (Ross et al. 2010b). Cultured H4 neuronal cells were transfected with a construct containing the PrP 5′UTR upstream of luciferase (H4-PRP) and were assayed to confirm the compound Syn-516 was specific for the SNCA 5′UTR RNA stem–loop (Z′ = 0.75). Syn-516 inhibited 5′UTR driven translation of αsyn with an IC-50 of 1.8 µM and was initially found >34-fold selective over transfectants lacking this 5′UTR (H4-C) and from the 5′UTR of PrP mRNA (H4 H4-PrP) (counter-screens). N.B. inhibition commenced in the 50 nM range while the IC-50 value is shown Table 1.
Syn-516 was advanced as an SNCA 5′UTR directed inhibitor probe over the other SNCA 5′UTR directed inhibitors listed in Table 1 since this agent was the first compound to be assayed that displayed a metabolic tolerance and predicted suitability as required by the MLPCN for future in vivo studies. The chemical structure of the Syn-516 probe is N,N-Dimethyl-6-({[1-(1-naphthyl)-1H-tetrazol-5-yl]thio}methyl)-1,3,5-triazine-2,4-diamine and it is commercially available and bears a prominent di-amino thiazine structure similar to that present in lamotrigine (a drug used by bipolar patients). Lamotrigine is rapidly absorbed after oral administration with bioavailability at 98% with a plasma Cmax from 1.4 to 4.8 h. Similarly, western blotting and ELISAs showed the MLPCN probe Syn-517 dose responsively increased neural αsyn expression in SHSY5Y cells without change to steady state levels of β-actin (AID 2627).
To monitor the capacity of Syn-516 to increase IRE/IRP binding in a therapeutic mode, RNA protein binding measurements will be required. Scatchard analysis has been successfully used for these kinds of studies when establishing the dissociation (Kd) for the APP–IRE interaction with IRP1 (30 pM compared to Kd = 40 pM for the H-ferritin IRE and IRP1) (Allerson et al. 1999; Cho et al. 2010). In the future, efficacies to limit αsyn will be assessed by AAV-αsyn constructs expressing wt. and mutant alpha-synuclein from cDNA inserts with a bonafide SNCA 5′UTR cassette in front of the αsyn start codon (Cai et al. 2018). It will be necessary to determine which of the four focused SNCA 5′UTR inhibitors shown in Table 1 is most effective at an IC-50 in the 10–100 nM range compared to posiphen. This level of potency will be required to medicinally establish the in vivo efficacy for Syn-516 compared to posiphen since αsyn has a 48–72 h. half-life. αsyn exists in an equilibrium between being an in situ tetramer while it is the monomer form that favors fibrilization (Bartels et al. 2011; Dettmer et al. 2013, 2015a,b; Selkoe et al. 2014; Luth et al. 2015; Nuber et al. 2018; Fanning et al. 2019). For favorable blood brain barrier determinations, the use of in vitro membrane permeability assays can be conducted with endothelial cells (Lok et al. 2009). Optimal formulations of Syn-516 will be used for in vivo assays so as to correlate its capacity to increase the RNA binding of IRP1 to the SNCA 5′UTR with repression of SNCA mRNA translation.
Table 1 illustrates the point that there are currently no PET tracers available for imaging α-synuclein fibrils or SNCA mRNA in the human brain. This table shows the first generation of PET radiotracers targeting SNCA mRNA. There are in the pipeline probes preidentified to target the SNCA 5′UTR that will provide a useful tool for PET imaging and prediction of α-synucleinopathies in PD and PD-related dementias. Using radiolabeled derivatives of SNCA 5′UTR directed small molecules, we plan to define the localization of SNCA mRNA in anatomic human brain during the etiology of PD and conduct in vivo PET imaging of how SNCA mRNA accumulates in the brains of animal models and in human patients at the Martinos Center (MGH). As an example, the agent B1 (analog of vindoline) demonstrated excellent labeling and ADME properties when used as a PET imaging agent to monitor the brain distribution of SNCA mRNA in mice (C. Wang, personal communications; Gilbert et al. 2019).
Mn was shown to increase the infective spread of alpha-synuclein pathology from the gut to the brain (Harischandra et al. 2018). In fact, alpha-synuclein is neuroprotective at low doses of Mn exposure to neurons (Harischandra et al. 2015). This progression of Mn pathology supports the therapeutic strategy of Figures 2–4 for RNA targeting with APP and ferritin translation activators compared with the possibility of utilizing SNCA 5′UTR directed inhibitors. Certainly, pharmacological conditioning with αsyn inhibitors may generate therapeutic efficacy as a novel means to mitigate cognitive deficits of Mn associated α-synucleinopathy.
Iron regulatory paths of ferritin, APP translation to mitigate metal neurotoxicity by manganese and lead exposures
Manganese and lead (Pb) as neurotoxins
Manganese (Mn) and lead (Pb) exposure is associated with a range of brain developmental defects leading to lowered IQ (Vollet et al. 2016) and to the onset of memory loss as people age (Zawia and Basha 2005; Rogers et al. 2016; Venkataramani et al. 2018). Later-life risk for AD/PD after childhood exposures to lead are discussed (Basha et al. 2005b). Excess iron causes hemochromatosis whereas manganese and manganism are a behavioral/ movement crisis during working lives (Rogers et al. 2019). Mn in children leads to neuropsychological underscoring (Carpenter 2001; Rodan et al. 2018; Sarkar et al. 2018). Pb exposure from paint in the western world, and Pb in the industrial settings in the third world, leads to ADHD and then to later risks for ferroptosis and associated neural death from iron oxidation of lipid radicals, resulting in neurodegenerative disease such as AD (Bolin et al. 2006; Ashok et al. 2015; Rogers et al. 2016; Venkataramani et al. 2018). Several metals, including Pb, can trigger fibrilization of methionine-oxidized alpha-synuclein specific to an increased risk for PD (Yamin et al. 2003) while adult Mn toxicity is already a form of Parkinsonism (Peres et al. 2016). There are seminal works on early-life exposure of environmental factors such as Pb on late life disorders as per the LEARn (latent early-life associated regulation) pathway (Lahiri et al. 2009). This work provides a perspective for a disease particularly as complex as AD. Dementiacan be viw4ed as a transformation from many environmental effectors rather than only being considered as being a genetic a state (Maloney and Lahiri 2016).
Manganism
Biology and clinical pathological features of Mn toxic exposure
Mn exposure from welding settings affects all age groups to cause psychiatric, cognitive, and motor disturbances (Chia et al. 1993) including, bradykinesia, rigidity, tremor, and gait problems. Elevated environmental exposure of Mn causes the neurological disorder of manganism with disrupted movement features resembling idiopathic PD. Mn treated rats display motor deficits and striatal oxidative stress (Cordova et al. 2013) with iron flow from the blood to cerebral spinal fluid (Zheng et al. 1999; Tong et al. 2014). Motor disturbances in PD respond to Levadopa therapy while manganism patients treated with levodopa do not exhibit clinical benefit (Lu et al. 1994). In PD, motor disturbances are caused by a loss of neurons in the SN and to dopaminergic nerve terminals while Mn overexposure additionally incudes the loss of other monoaminergic neurons in the striatal globus pallidus (Cordova et al. 2013; Chen et al. 2014, 2015). Depending on exposure, Mn2+ and Mn3+ induce gait disturbances and toxic reactive oxygen species in rats neuronal Fe burden (Malecki et al. 1999).
Manganism has been documented for ∼150 yr while Mn is an essential dietary element and a key cofactor in many enzymatic reactions, including the antioxidant enzyme superoxide dismutase (Keen and Hurley 1987). These devastating gait disorders extend to young adult workers while environmental Mn exposure enhances prion like αsyn spread and synthesis (Venkataramani et al. 2018; Harischandra et al. 2019b). Excess Mn promotes the aggregation and prion-like cell-to-cell exosomal transmission of αsyn that can coalesce into the fibrils that generate Lewy bodies (Harischandra et al. 2019a,b). One familial form of PD is caused by recessive mutations to “Park-9,” a lysosomal Type-5 ATPase cation pump that regulates Mn efflux from neurons while also serving in the same molecular pathway of αsyn in Mn homeostasis (Gitler et al. 2009; Chesi et al. 2012; Guilarte 2013; Kong et al. 2015; Medici et al. 2015).
Mn and iron transport and the potential for neuronal death by ferroptosis
Mutations in the Mn exporter SLC39A14 causes Mn-induced toxicity in the liver and a brain Parkinsonian dysfunction (Zheng et al. 1999; Tuschl et al. 2016; Hutchens et al. 2017). However, while SLC39A14 (ZIP14) has a critical role in maintaining Mn homeostasis in mice, this transporter has a role in iron and zinc transport and ferroptosis when misregulated (Xin et al. 2017). Certainly, Mn exposure and toxicity to neurons appears operate via ferroptosis by interfering with IRE/IRP regulation of translation of APP where APP(s) directly can interact with FPN to accelerate iron efflux (Venkataramani et al. 2018; Tsatsanis et al. 2020). This loss of APP generates enhanced intracellular REDOX active Fe2+ in the event of reduced levels of APP/PPN Fe-export complexes as well as resulting from reductions of the iron storage protein ferritin (Khan et al. 2017; Venkataramani et al. 2018). Mn exposure to cells certainly blocks IRE-driven translation of not only APP but also the H-subunit of ferritin, the cell's central neuroprotective iron storage multimer (see Fig. 4; Lumsden et al. 2018).
Iron-transporter proteins are linked to the maintenance of physiologic Mn levels and balance, including that of divalent metal ion transporter-1 (DMT1) for iron import (Garrick et al. 2006; Salazar et al. 2008) and FPN for iron export (Mitchell et al. 2014). In fact, FPN plays a central role in regulating both iron and Mn efflux from neurons (Madejczyk and Ballatori 2012). Mice expressing dominant negative mutations to FPN encoding the flatiron phenotype exhibited tissue iron-overload similar to hemochromatosis in humans (Johnson and Wessling-Resnick 2007) and these were shown also deficient in Mn export (Seo and Wessling-Resnick 2015). Transfection based overexpression of the wild-type FPN transgene eliminated excess toxic Mn burden and protected neural cells from oxidative overload while the flatiron mutated FPN failed to protect Mn treated neurons (Seo and Wessling-Resnick 2015). As noted above, APP belongs to a family of proteins that physically interact by FPN, as initiated in pathway by IRP1 dependent translation sufficient to promote APP/FPN dependent neuronal efflux of excess iron in conditions of Mn exposure (Duce et al. 2010; Hahl et al. 2013; Ayton et al. 2015c; Venkataramani et al. 2018; Tsatsanis et al. 2020). Lei et al. (2012) found that a microtubule-associated tau deficiency induced Parkinsonism with dementia by impairing APP-mediated iron export by FPN (Lei et al. 2012).
APP is a copper-zinc metalloprotein (Multhaup et al. 1996; White et al. 1999) whose expression, and that of ferritin, are eliminated as an early event immediately before Mn is most toxic to neurons (Venkataramani et al. 2018). Figure 4 incorporates the published model that, while iron increased both APP and ferritin-H protein levels in SH-SY5Y cells, Mn exposure dose-responsively inhibited expression of these key iron-associated protective proteins. Toxicity only became evident after 24 h when levels of APP and ferritin has been reduced via 5′UTR directed translation inhibition (Venkataramani et al. 2018). As shown in Figure 4, Mn interfered with IRE/IRP repression pathways to regulate APP and ferritin so as to deprive primary neural cells of two key proteins that contribute to healthy Fe metabolism (Balla et al. 1992; Rogers et al. 2002a,b; Bandyopadhyay et al. 2006, 2007; Cho et al. 2010; Duce et al. 2010). Clearly, Mn exposure can activate IRP1 to ablate APP and ferritin in cells, a property that causes an increase in the accumulation of iron-II as a REDOX active and neuro-destructive agent (Fig. 4). In sum, increases of the APP(s) ectodomain sufficient to bind and facilitate FPN dependent cellular iron export may thereby provide an antiferroptotic therapy to mitigate the consequences of heavy metal exposure (Rogers et al. 2019; Tsatsanis et al. 2020). Alpha-synuclein blockers also offer potential neurotherapeutic agents that serve to protect neurons from ferroptosis. Certainly, oleic acid accumulation accelerates neurotoxicity when αsyn is overexpressed (Gitler et al. 2009; Fanning et al. 2019). Oleic acid peroxidation is toxic apparently as a consequence of αsyn dependent iron accumulation (Singh et al. 2014; Baksi et al. 2016).
For the purpose of therapies, perturbations of Mn transport and load are physiologically complex while being directly linked to movement and cognitive disorders such as for PDD (Lin et al. 2017; Xia et al. 2017; Jiang et al. 2020). Under the age of 40, pharmacological increases of IRE independent APP expression and APP(s) secretion offers a novel strategy to prevent Mn interference of translation of the APP and ferritin, suggesting the possible means to prevent cognitive problems for younger adult (Fig. 4; Nitsch et al. 2000; Rogers et al. 2019). For example, these authors showed an intervention with the known FDA drug AF120B was shown to activate APP 5′UTR directed translation and cleavage to APP(s) to potentially enhance FPN dependent excess iron and Mn export from prone neurons. AF102B is an alpha secretase activator that limits amyloid production (Beach et al. 2001). As a second example, yohimbine is a safe supplement as an alpha-2 adrenergic antagonist that limited cortico-amygdala circuit abnormalities in a genetic mouse strain with impaired fear extinction behavior (Hefner et al. 2008). Since yohimbine is a H-ferritin translation activator (Tibodeau et al. 2006; Rogers et al. 2016, 2019), this agent can be modeled to prevent Mn induced cell death via these ferroptosis pathways (Fig. 4; Morse et al. 2004; Stockwell et al. 2017).
Lead neurotoxicity
Lead poisoning is a problem to children in the third world as well as remaining a significant risk in the water supply and in preexisting lead paint and water supplies in the USA (Flint, Michigan) (Hanna-Attisha et al. 2016; Hanna-Attisha and Kuehn 2016). Acute environmental lead exposure generates nerve cell death with inflammatory encephalopathy and hemorrhage (Struzynska et al. 2001; Hossain et al. 2004). Early acute Pb exposure may well promote epigenetic changes that cause AD later in life (Basha et al. 2005a,b; Bakulski et al. 2012; Bihaqi et al. 2012; Eid et al. 2016). In the US and Canada, endangered populations would appreciate the relevance of the proposed “4R's” (remediation, renovation, reallocation, and research) against the Pb crisis (Maloney et al. 2018).
Pb is toxic to neurons, potentially via ferroptosis, by interfering with IRE/IRP driven translation of APP and the ferritin-H chain (Fig. 4; Rogers et al. 2016). By this pathway Pb, like Mn, has the capacity to cause heightened intracellular REDOX active Fe2+ is caused by reduced levels of APP/PPN Fe-export complexes, also loss of iron storage in ferritin (Khan et al. 2017). Neurotoxicity depends on Apo E3/ ApoE4 genotypes in rodents with a more pronounced effect in females (Engstrom et al. 2017). Early-life exposure to Pb activates microRNAs to target AD proteins (Masoud et al. 2016) and human tau phosphorylation (Dash et al. 2016). Pb exerts neurotoxicity when transported into cells by the central iron import protein DMT-1 (Zhu et al. 2012; An et al. 2015). Redox-active ferrous iron accumulates in neurons of the brains of Pb-mice exposed (Zhu et al. 2013; Zhou et al. 2014) As is the case for manganese neurotoxicity, the presence of Pb exposure at chronic sublethal doses to neurons can be directly assessed for its capacity to embargo iron export leading to its toxic excess (Zhu et al. 2012; An et al. 2015; Rogers et al. 2016).
Pb caused IRP1 translationally repressed the L- and H-chains of the assembled multimer of ferritin via IRE RNA stem loops in the 5′UTRs of their mRNAs, thus disabling the cell's Fe storage capacity (Figs. 1, 4; Rogers et al. 2016). At the same time, Pb repressed IRP-1 dependent translation of the APP, the key facilitator of the export of excess iron by FPN (Duce et al. 2010; Ayton et al. 2015c). In fact, epigenetic changes induced by early Pb exposure caused long-term induction APP transcription while the precursor is cleaved by β- and γ-secretase to generate the 40–42 residue amyloid peptide which increases the risk of AD at a later stage in life (Walsh and Selkoe 2016).
High dose Pb poisoning is clinically treated with the metal chelator succimer though this treatment presents side-effects (Horowitz 2001; Stangle et al. 2007; Rahman et al. 2014; Wirbisky et al. 2014). The testable model shown in Figure 4 proposes 5′UTR dependent activators of ferritin/APP translational axis of iron homeostasis indeed will mitigate Pb neurotoxicity at sublethal chronic dose exposure range (Rogers et al. 2016; Long et al. 2019). It is possible to characterize the druggable biological and therapeutic properties of small molecule αsyn blockers versus ferritin/APP activators in each case to prevent toxic metal induced ferroptosis and neurodegeneration (Duce et al. 2010; Abdalkader et al. 2018; Venkataramani et al. 2018; Tsatsanis et al. 2020). To treat Pb-based neurotoxicity, FDA preapproved and high affinity small-molecule modulators of IRE/IRP dependent translational induction might induce APP translation and APP(s) sufficient to form enough adaptive APP/FPN iron efflux complexes to efflux toxic overload of Fe. This is also the case for Mn exposures and L- and H ferritin subunits translation activators are also a desirable therapeutic route to provide protection from toxicity from both heavy metals (Tibodeau et al. 2006).
Conclusions
Mn and Pb toxicity to cultured neurons is associated with disrupted translation of APP whose loss causes disruption of neuronal iron export by FPN to generate conditions pf embargoed REDOX active intracellular iron (Rogers et al. 2016; Venkataramani et al. 2018). The model shown in Figure 4 depicts that APP mRNA is translationally controlled by IRP1 while APP and APP(s) binds to FPN to accelerate Fe and Mn efflux from cells (Duce et al. 2010; Rogers et al. 2019; Tsatsanis et al. 2020). IRP2 as well as IRP1 are the RNA binding proteins that set a healthy physiological balance for iron homeostasis when interacting with IREs in the untranslated regions of ferritin and transferrin receptors transcripts to control their intracellular steady state levels (Thomson et al. 1999, 2005; Cho et al. 2010). We originally showed that the influx of excess iron into neurons increased APP translation via 5′UTR specific IRE/IRP interactions (Rogers et al. 2002a). Subsequent alpha-secretase cleaved APP(s) favored conditions for a neuroprotective efflux of excess iron after its interaction with FPN, particularly in conditions of heightened brain iron overload (Cho et al. 2010; Duce et al. 2010; McCarthy et al. 2014).
The alpha-synuclein and APP 5′UTRs have been useful mRNA targets for identifying and advancing translation inhibitors such as the anti-cholinesterase phenserine and its enantiomer posiphen. Clinical trials have tested their action as bonafide anti-amyloid and anti-alpha synuclein agents respectively for treatment of AD and PD (Teich et al. 2018; Kuo et al. 2019). Extending from these agents, AF102B is a neuroprotective m-1 muscarinic agonist used to treat Sjogren's syndrome and can also induce APP(s) secretion resulting from alpha-secretase activation of the nonamyloidogenic pathway of APP expression (Fisher 2000; Fisher et al. 2000). AF102B, similar to high through put screened APP 5′UTR dependent translation activators, is a starting point for medicinal treatments to mitigate acute Pb and Mn toxicity. This drug can be tested for its capacity to enhance APP/FPN complexes to efflux toxically embargoed iron and prevent ROS accumulation (Figs. 2, 4). That is, this therapy my well apply especially in the younger population suffering from environmental/industrial heavy metal exposures at an age long before such Mn or Pb exposed subjects are at risk from the onset of amyloidogenic cascade as observed in older AD subjects.
Since Mn induces the spread of alpha-synuclein (Harischandra et al. 2015, 2018, 2019a), we propose that αsyn inhibitors represent another potential set of therapeutic agents for offsetting Mn toxicity as well as in treating neuronal death in the course of dementia-associated diseases such as and PDD and LBD. APP 5′UTR activators might be used to pharmacologically restore APP translation even in the event of Pb and/or Mn interference via APP 5′UTR sequences (Rogers et al. 2016; Long et al. 2019). The clinical the use of alpha-synuclein inhibitors, in contrast, might promote iron homeostasis when preventing alpha-synucleinopathies in addition to a promoting efficacy from excess iron load in conditions of αsyn overexpression (Ross et al. 2010b; Mikkilineni et al. 2012; Zhang et al. 2020b). In future, translation activators of ferritin include the drugs yohimbine and the novel agent BL-1 as candidates for neuroprotection against heavy metal stresses (Tibodeau et al. 2006)
Acknowledgments
This work was supported by a grant from the Michel J. FOX Foundation Pipeline grants program. J.T.R. was a coinvestigator on an R01AG056614 and recipient of NIH R21s that led to 5′UTR drug discovery R21NS059434 and R21NS064853 and on a regular R21NS077079.
Author contributions: J.T.R. and C.M.C. conceptualized and carried out this work and wrote the documentary review.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.052282.120.
References
- Abdalkader M, Lampinen R, Kanninen KM, Malm TM, Liddell JR. 2018. Targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration. Front Neurosci 12: 466 10.3389/fnins.2018.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamowicz DH, Roy S, Salmon DP, Galasko DR, Hansen LA, Masliah E, Gage FH. 2017. Hippocampal alpha-synuclein in dementia with Lewy bodies contributes to memory impairment and is consistent with spread of pathology. J Neurosci 37: 1675–1684. 10.1523/JNEUROSCI.3047-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn TB, Kim SY, Kim JY, Park SS, Lee DS, Min HJ, Kim YK, Kim SE, Kim JM, Kim HJ, et al. 2008. alpha-synuclein gene duplication is present in sporadic Parkinson disease. Neurology 70: 43–49. 10.1212/01.wnl.0000271080.53272.c7 [DOI] [PubMed] [Google Scholar]
- Allerson CR, Cazzola M, Rouault TA. 1999. Clinical severity and thermodynamic effects of iron-responsive element mutations in hereditary hyperferritinemia-cataract syndrome. J Biol Chem 274: 26439–26447. 10.1074/jbc.274.37.26439 [DOI] [PubMed] [Google Scholar]
- Alvarez-Fischer D, Henze C, Strenzke C, Westrich J, Ferger B, Hoglinger GU, Oertel WH, Hartmann A. 2008. Characterization of the striatal 6-OHDA model of Parkinson's disease in wild type and alpha-synuclein-deleted mice. Exp Neurol 210: 182–193. 10.1016/j.expneurol.2007.10.012 [DOI] [PubMed] [Google Scholar]
- An DZ, Ai JT, Fang HJ, Sun RB, Shi Y, Wang LL, Wang Q. 2015. Influence of iron supplementation on DMT1 (IRE)-induced transport of lead by brain barrier systems in vivo. Biomed Environ Sci 28: 651–659. [DOI] [PubMed] [Google Scholar]
- Anderson CP, Leibold EA. 2014. Mechanisms of iron metabolism in Caenorhabditis elegans. Front Pharmacol 5: 113 10.3389/fphar.2014.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CP, Shen M, Eisenstein RS, Leibold EA. 2012. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta 1823: 1468–1483. 10.1016/j.bbamcr.2012.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Nizzi CP, Chang YI, Deck KM, Schmidt PJ, Galy B, Damnernsawad A, Broman AT, Kendziorski C, Hentze MW, et al. 2013. The IRP1-HIF-2alpha axis coordinates iron and oxygen sensing with erythropoiesis and iron absorption. Cell Metab 17: 282–290. 10.1016/j.cmet.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok A, Rai NK, Tripathi S, Bandyopadhyay S. 2015. Exposure to As-, Cd-, and Pb-mixture induces Abeta, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol Sci 143: 64–80. 10.1093/toxsci/kfu208 [DOI] [PubMed] [Google Scholar]
- Auerbach M, Georgieff MK. 2020. Guidelines for iron deficiency in pregnancy: hope abounds: Commentary to accompany: UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol 188: 814–816. 10.1111/bjh.16220 [DOI] [PubMed] [Google Scholar]
- Ayton S, Faux NG, Bush AI, Alzheimer's Disease Neuroimaging Initiative. 2015a. Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE. Nat Commun 6, 6760 10.1038/ncomms7760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S, Lei P, Bush AI. 2015b. Biometals and their therapeutic implications in Alzheimer's disease. Neurotherapeutics 12: 109–120. 10.1007/s13311-014-0312-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S, Lei P, Hare DJ, Duce JA, George JL, Adlard PA, McLean C, Rogers JT, Cherny RA, Finkelstein DI, et al. 2015c. Parkinson's disease iron deposition caused by nitric oxide-induced loss of beta-amyloid precursor protein. J Neurosci 35: 3591–3597. 10.1523/JNEUROSCI.3439-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S, Faux NG, Bush AI. 2016. Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE. Nat Commun 6: 6760 10.1038/ncomms7760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DK, Kosman DJ. 2019. Is brain iron trafficking part of the physiology of the amyloid precursor protein? J Biol Inorg Chem 24: 1171–1177. 10.1007/s00775-019-01684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksi S, Singh N. 2017. alpha-Synuclein impairs ferritinophagy in the retinal pigment epithelium: Implications for retinal iron dyshomeostasis in Parkinson's disease. Sci Rep 7: 12843 10.1038/s41598-017-12862-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksi S, Tripathi AK, Singh N. 2016. Alpha-synuclein modulates retinal iron homeostasis by facilitating the uptake of transferrin-bound iron: Implications for visual manifestations of Parkinson's disease. Free Radic Biol Med 97: 292–306. 10.1016/j.freeradbiomed.2016.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi R, Xu Y, Mueller KA, Chen X, Granucci E, Paganoni S, Sadri-Vakili G, Schwarzschild MA. 2018. Urate mitigates oxidative stress and motor neuron toxicity of astrocytes derived from ALS-linked SOD1(G93A) mutant mice. Mol Cell Neurosci 92: 12–16. 10.1016/j.mcn.2018.06.002 [DOI] [PubMed] [Google Scholar]
- Bakshi R, Macklin EA, Logan R, Zorlu MM, Xia N, Crotty GF, Zhang E, Chen X, Ascherio A, Schwarzschild MA. 2019. Higher urate in LRRK2 mutation carriers resistant to Parkinson disease. Ann Neurol 85: 593–599. 10.1002/ana.25436 [DOI] [PubMed] [Google Scholar]
- Bakulski KM, Rozek LS, Dolinoy DC, Paulson HL, Hu H. 2012. Alzheimer's disease and environmental exposure to lead: the epidemiologic evidence and potential role of epigenetics. Curr Alzheimer Res 9: 563–573. 10.2174/156720512800617991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. 1992. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem 267: 18148–18153. [PubMed] [Google Scholar]
- Bandyopadhyay S, Huang X, Cho H, Greig NH, Youdim MB, Rogers JT. 2006. Metal specificity of an iron-responsive element in Alzheimer's APP mRNA 5′untranslated region, tolerance of SH-SY5Y and H4 neural cells to desferrioxamine, clioquinol, VK-28, and a piperazine chelator. J Neural Transm Suppl 71: 237–247. 10.1007/978-3-211-33328-0_25 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Goldstein LE, Lahiri DK, Rogers JT. 2007. Role of the APP non-amyloidogenic signaling pathway and targeting alpha-secretase as an alternative drug target for treatment of Alzheimer's disease. Curr Med Chem 14: 2848–2864. 10.2174/092986707782360060 [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Cahill C, Balleidier A, Huang C, Lahiri DK, Huang X, Rogers JT. 2013. Novel 5′ untranslated region directed blockers of iron-regulatory protein-1 dependent amyloid precursor protein translation: implications for down syndrome and Alzheimer's disease. PLoS One 8: e65978 10.1371/journal.pone.0065978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnham KJ, Bush AI. 2008. Metals in Alzheimer's and Parkinson's diseases. Curr Opin Chem Biol 12: 222–228. 10.1016/j.cbpa.2008.02.019 [DOI] [PubMed] [Google Scholar]
- Barry AE, Klyubin I, Mc Donald JM, Mably AJ, Farrell MA, Scott M, Walsh DM, Rowan MJ. 2011. Alzheimer's disease brain-derived amyloid-beta-mediated inhibition of LTP in vivo is prevented by immunotargeting cellular prion protein. J Neurosci 31: 7259–7263. 10.1523/JNEUROSCI.6500-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels T, Choi JG, Selkoe DJ. 2011. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477: 107–110. 10.1038/nature10324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tishler TA, Peters DG, Kosenko A, Barrall KA, Finn JP, Villablanca P, Laub G, Altshuler LL, et al. 2010. Prevalent iron metabolism gene variants associated with increased brain ferritin iron in healthy older men. J Alzheimers Dis 20: 333–341. 10.3233/JAD-2010-1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Peters DG, Amar CP, Tishler TA, Finn JP, Villablanca P, Altshuler LL, Mintz J, et al. 2011. Gender and iron genes may modify associations between brain iron and memory in healthy aging. Neuropsychopharmacology 36: 1375–1384. 10.1038/npp.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha MR, Murali M, Siddiqi HK, Ghosal K, Siddiqi OK, Lashuel HA, Ge YW, Lahiri DK, Zawia NH. 2005a. Lead (Pb) exposure and its effect on APP proteolysis and Abeta aggregation. FASEB J 19: 2083–2084. 10.1096/fj.05-4375fje [DOI] [PubMed] [Google Scholar]
- Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge YW, Lahiri DK, Zawia NH. 2005b. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J Neurosci 25: 823–829. 10.1523/JNEUROSCI.4335-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Walker DG, Potter PE, Sue LI, Fisher A. 2001. Reduction of cerebrospinal fluid amyloid beta after systemic administration of M1 muscarinic agonists. Brain Res 905: 220–223. 10.1016/S0006-8993(01)02484-2 [DOI] [PubMed] [Google Scholar]
- Bendor JT, Logan TP, Edwards RH. 2013. The function of alpha-synuclein. Neuron 79: 1044–1066. 10.1016/j.neuron.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihaqi SW, Schumacher A, Maloney B, Lahiri DK, Zawia NH. 2012. Do epigenetic pathways initiate late onset Alzheimer disease (LOAD): towards a new paradigm. Curr Alzheimer Res 9: 574–588. 10.2174/156720512800617982 [DOI] [PubMed] [Google Scholar]
- Bolin CM, Basha R, Cox D, Zawia NH, Maloney B, Lahiri DK, Cardozo-Pelaez F. 2006. Exposure to lead and the developmental origin of oxidative DNA damage in the aging brain. FASEB J 20: 788–790. 10.1096/fj.05-5091fje [DOI] [PubMed] [Google Scholar]
- Brunette KE, Tran PV, Wobken JD, Carlson ES, Georgieff MK. 2010. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev Neurosci 32: 238–248. 10.1159/000314341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni A, Pepper AR, Pawlick RL, Gala-Lopez B, Gamble AF, Kin T, Seeberger K, Korbutt GS, Bornstein SR, Linkermann A, et al. 2018. Ferroptosis-inducing agents compromise in vitro human islet viability and function. Cell Death Dis 9: 595 10.1038/s41419-018-0506-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burre J, Sharma M, Sudhof TC. 2018. Cell biology and pathophysiology of alpha-synuclein. Cold Spring Harb Perspect Med 8: a024091 10.1101/cshperspect.a024091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush AI, Tanzi RE. 2008. Therapeutics for Alzheimer's disease based on the metal hypothesis. Neurotherapeutics 5: 421–432. 10.1016/j.nurt.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss L, Fisher E, Hardy J, Nizetic D, Groet J, Pulford L, Strydom A. 2016. Intracerebral haemorrhage in Down syndrome: protected or predisposed? F1000Res 5: F1000 10.12688/f1000research.7819.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Feng D, Schwarzschild MA, McLean PJ, Chen X. 2018. Bimolecular fluorescence complementation of alpha-synuclein demonstrates its oligomerization with dopaminergic phenotype in mice. EBioMedicine 29: 13–22. 10.1016/j.ebiom.2018.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantuti-Castelvetri I, Klucken J, Ingelsson M, Ramasamy K, McLean PJ, Frosch MP, Hyman BT, Standaert DG. 2005. Alpha-synuclein and chaperones in dementia with Lewy bodies. J Neuropathol Exp Neurol 64: 1058–1066. 10.1097/01.jnen.0000190063.90440.69 [DOI] [PubMed] [Google Scholar]
- Cantuti-Castelvetri I, Keller-McGandy C, Bouzou B, Asteris G, Clark TW, Frosch MP, Standaert DG. 2007. Effects of gender on nigral gene expression and parkinson disease. Neurobiol Dis 26: 606–614. 10.1016/j.nbd.2007.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ES, Magid R, Petryk A, Georgieff MK. 2008. Iron deficiency alters expression of genes implicated in Alzheimer disease pathogenesis. Brain Res 1237: 75–83. 10.1016/j.brainres.2008.07.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ES, Tkac I, Magid R, O'Connor MB, Andrews NC, Schallert T, Gunshin H, Georgieff MK, Petryk A. 2009. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr 139: 672–679. 10.3945/jn.108.096354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter DO. 2001. Effects of metals on the nervous system of humans and animals. Int J Occup Med Environ Health 14: 209–218. [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, et al. 2004. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364: 1167–1169. 10.1016/S0140-6736(04)17103-1 [DOI] [PubMed] [Google Scholar]
- Chen X, Burdett TC, Desjardins CA, Logan R, Cipriani S, Xu Y, Schwarzschild MA. 2013. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc Natl Acad Sci 110: 300–305. 10.1073/pnas.1217296110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Parmalee N, Aschner M. 2014. Genetic factors and manganese-induced neurotoxicity. Front Genet 5: 265 10.3389/fgene.2014.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Chakraborty S, Mukhopadhyay S, Lee E, Paoliello MM, Bowman AB, Aschner M. 2015. Manganese homeostasis in the nervous system. J Neurochem 134: 601–610. 10.1111/jnc.13170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen H, Cai W, Maguire M, Ya B, Zuo F, Logan R, Li H, Robinson K, Vanderburg CR, et al. 2017. The melanoma-linked “redhead” MC1R influences dopaminergic neuron survival. Ann Neurol 81: 395–406. 10.1002/ana.24852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesi A, Kilaru A, Fang X, Cooper AA, Gitler AD. 2012. The role of the Parkinson's disease gene PARK9 in essential cellular pathways and the manganese homeostasis network in yeast. PLoS One 7: e34178 10.1371/journal.pone.0034178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia SE, Foo SC, Gan SL, Jeyaratnam J, Tian CS. 1993. Neurobehavioral functions among workers exposed to manganese ore. Scand J Work Environ Health 19: 264–270. 10.5271/sjweh.1475 [DOI] [PubMed] [Google Scholar]
- Chiba-Falek O, Lopez GJ, Nussbaum RL. 2006. Levels of alpha-synuclein mRNA in sporadic Parkinson disease patients. Mov Disord 21: 1703–1708. 10.1002/mds.21007 [DOI] [PubMed] [Google Scholar]
- Chiou B, Neely EB, McDevitt DS, Simpson IA, Connor JR. 2020. Transferrin and H-ferritin involvement in brain iron acquisition during postnatal development: impact of sex and genotype. J Neurochem 152: 381–396. 10.1111/jnc.14834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HH, Cahill CM, Vanderburg CR, Scherzer CR, Wang B, Huang X, Rogers JT. 2010. Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1. J Biol Chem 285: 31217–31232. 10.1074/jbc.M110.149161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Anderson SA, Gwynn B, Deck KM, Chen MJ, Langer NB, Shaw GC, Huston NC, Boyer LF, Datta S, et al. 2014. Iron regulatory protein-1 protects against mitoferrin-1-deficient porphyria. J Biol Chem 289: 7835–7843. 10.1074/jbc.M114.547778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KA, Harper JD, Lansbury PT. 1998. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med 4: 1318–1320. 10.1038/3311 [DOI] [PubMed] [Google Scholar]
- Cordova FM, Aguiar AS, Peres TV, Lopes MW, Gonçalves FM, Pedro DZ, Lopes SC, Pilati C, Prediger RD, Farina M, et al. 2013. Manganese-exposed developing rats display motor deficits and striatal oxidative stress that are reversed by Trolox. Arch Toxicol 87: 1231–1244. 10.1007/s00204-013-1017-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau R, Bosetti F, Emr M, Gladman JT, Koenig J, Moy C, Pahigiannis K, Waddy S, Koroshetz W. 2016. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol 36: 281–288. 10.1007/s10571-016-0334-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapper McLachlan DR, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W, Andrews DF. 1991. Intramuscular desferrioxamine in patients with Alzheimer's disease. Lancet 337: 1304–1308. 10.1016/0140-6736(91)92978-B [DOI] [PubMed] [Google Scholar]
- Dash M, Eid A, Subaiea G, Chang J, Deeb R, Masoud A, Renehan WE, Adem A, Zawia NH. 2016. Developmental exposure to lead (Pb) alters the expression of the human tau gene and its products in a transgenic animal model. Neurotoxicology 55: 154–159. 10.1016/j.neuro.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, et al. 2002. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci 99: 14524–14529. 10.1073/pnas.172514599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P, Moualla D, Brown DR. 2011. Alpha-synuclein is a cellular ferrireductase. PLoS One 6: e15814 10.1371/journal.pone.0015814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Karran E. 2016. The Cellular Phase of Alzheimer's Disease. Cell 164: 603–615. 10.1016/j.cell.2015.12.056 [DOI] [PubMed] [Google Scholar]
- Dettmer U, Newman AJ, Luth ES, Bartels T, Selkoe D. 2013. In vivo cross-linking reveals principally oligomeric forms of alpha-synuclein and beta-synuclein in neurons and non-neural cells. J Biol Chem 288: 6371–6385. 10.1074/jbc.M112.403311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer U, Newman AJ, Soldner F, Luth ES, Kim NC, von Saucken VE, Sanderson JB, Jaenisch R, Bartels T, Selkoe D. 2015a. Parkinson-causing alpha-synuclein missense mutations shift native tetramers to monomers as a mechanism for disease initiation. Nat Commun 6: 7314 10.1038/ncomms8314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer U, Newman AJ, von Saucken VE, Bartels T, Selkoe D. 2015b. KTKEGV repeat motifs are key mediators of normal alpha-synuclein tetramerization: Their mutation causes excess monomers and neurotoxicity. Proc Natl Acad Sci 112: 9596–9601. 10.1073/pnas.1505953112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. 2012. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149: 1060–1072. 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS, et al. 2014. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3: e02523 10.7554/eLife.02523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlouhy AC, Bailey DK, Steimle BL, Parker HV, Kosman DJ. 2019. Fluorescence resonance energy transfer links membrane ferroportin, hephaestin but not ferroportin, amyloid precursor protein complex with iron efflux. J Biol Chem 294: 4202–4214. 10.1074/jbc.RA118.005142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Double KL, Ben-Shachar D, Youdim MB, Zecca L, Riederer P, Gerlach M. 2002. Influence of neuromelanin on oxidative pathways within the human substantia nigra. Neurotoxicol Teratol 24: 621–628. 10.1016/S0892-0362(02)00218-0 [DOI] [PubMed] [Google Scholar]
- Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, Leong SL, Perez K, Johanssen T, Greenough MA, et al. 2010. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell 142: 857–867. 10.1016/j.cell.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duce JA, Ayton S, Miller AA, Tsatsanis A, Lam LQ, Leone L, Corbin JE, Butzkueven H, Kilpatrick TJ, Rogers JT, et al. 2013. Amine oxidase activity of beta-amyloid precursor protein modulates systemic and local catecholamine levels. Mol Psychiatry 18: 245–254. 10.1038/mp.2011.168 [DOI] [PubMed] [Google Scholar]
- Eid A, Bihaqi SW, Renehan WE, Zawia NH. 2016. Developmental lead exposure and lifespan alterations in epigenetic regulators and their correspondence to biomarkers of Alzheimer's disease. Alzheimers Dement (Amst) 2: 123–131. 10.1016/j.dadm.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barcelo-Coblijn GC, Nussbaum RL. 2005. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol Cell Biol 25: 10190–10201. 10.1128/MCB.25.22.10190-10201.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom AK, Snyder JM, Maeda N, Xia Z. 2017. Gene-environment interaction between lead and Apolipoprotein E4 causes cognitive behavior deficits in mice. Mol Neurodegener 12: 14 10.1186/s13024-017-0155-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, et al. 2019. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci 116: 2672–2680. 10.1073/pnas.1821022116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Cai Z, Wang H, Han D, Qi C, Zhang P, Gao F, Yingying Y, Song Z, Wu Q, et al. 2020. Loss of cardiac ferritin H facilitates cardiomyopathy via Slc7a11-mediated ferroptosis. Circ Res 127: 486–501. 10.1161/CIRCRESAHA.120.316509 [DOI] [PubMed] [Google Scholar]
- Fanning S, Haque A, Imberdis T, Baru V, Barrasa MI, Nuber S, Termine D, Ramalingam N, Ho GPH, Noble T, et al. 2019. Lipidomic Analysis of alpha-synuclein neurotoxicity identifies stearoyl CoA desaturase as a target for Parkinson treatment. Mol Cell 73: 1001–1014 e1008. 10.1016/j.molcel.2018.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow M. 2004. Utilizing combination therapy in the treatment of Alzheimer's disease. Expert Rev Neurother 4: 799–808. 10.1586/14737175.4.5.799 [DOI] [PubMed] [Google Scholar]
- Faucheux BA, Martin ME, Beaumont C, Hunot S, Hauw JJ, Agid Y, Hirsch EC. 2002. Lack of up-regulation of ferritin is associated with sustained iron regulatory protein-1 binding activity in the substantia nigra of patients with Parkinson's disease. J Neurochem 83: 320–330. 10.1046/j.1471-4159.2002.01118.x [DOI] [PubMed] [Google Scholar]
- Febbraro F, Giorgi M, Caldarola S, Loreni F, Romero-Ramos M. 2012. alpha-Synuclein expression is modulated at the translational level by iron. Neuroreport 23: 576–580. 10.1097/WNR.0b013e328354a1f0 [DOI] [PubMed] [Google Scholar]
- Finkelstein DI, Billings JL, Adlard PA, Ayton S, Sedjahtera A, Masters CL, Wilkins S, Shackleford DM, Charman SA, Bal W, et al. 2017. The novel compound PBT434 prevents iron mediated neurodegeneration and alpha-synuclein toxicity in multiple models of Parkinson's disease. Acta Neuropathol Commun 5: 53 10.1186/s40478-017-0456-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. 2000. Therapeutic strategies in Alzheimer's Disease: m1 Muscaric Agonists. Jpn. J. Pharmacol 84: 101–112. 10.1254/jjp.84.101 [DOI] [PubMed] [Google Scholar]
- Fisher A. 2007. M1 muscarinic agonists target major hallmarks of Alzheimer's disease–an update. Curr Alzheimer Res 4: 577–580. 10.2174/156720507783018163 [DOI] [PubMed] [Google Scholar]
- Fisher A, Michaelson DM, Brandeis R, Haring R, Chapman S, Pittel Z. 2000. M1 muscarinic agonists as potential disease-modifying agents in Alzheimer's disease. Rationale and perspectives. Ann N Y Acad Sci 920: 315–320. 10.1111/j.1749-6632.2000.tb06941.x [DOI] [PubMed] [Google Scholar]
- Fisher A, Bezprozvanny I, Wu L, Ryskamp DA, Bar-Ner N, Natan N, Brandeis R, Elkon H, Nahum V, Gershonov E, et al. 2016. AF710B, a novel M1/sigma1 agonist with therapeutic efficacy in animal models of Alzheimer's disease. Neurodegener Dis 16: 95–110. 10.1159/000440864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlich AL, Tanzi RE, Rogers JT. 2007. The 5′-untranslated region of Parkinson's disease alpha-synuclein messengerRNA contains a predicted iron responsive element. Mol Psychiatry 12: 222–223. 10.1038/sj.mp.4001937 [DOI] [PubMed] [Google Scholar]
- Galy B, Ferring-Appel D, Sauer SW, Kaden S, Lyoumi S, Puy H, Kolker S, Grone HJ, Hentze MW. 2010. Iron regulatory proteins secure mitochondrial iron sufficiency and function. Cell Metab 12: 194–201. 10.1016/j.cmet.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Garrick MD, Kuo HC, Vargas F, Singleton S, Zhao L, Smith JJ, Paradkar P, Roth JA, Garrick LM. 2006. Comparison of mammalian cell lines expressing distinct isoforms of divalent metal transporter 1 in a tetracycline-regulated fashion. Biochem J 398: 539–546. 10.1042/BJ20051987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller LN, Potter H. 1999. Chromosome missegregation and trisomy 21 mosaicism in Alzheimer's disease. Neurobiol Dis 6: 167–179. 10.1006/nbdi.1999.0236 [DOI] [PubMed] [Google Scholar]
- Geng F, Mai X, Zhan J, Xu L, Georgieff M, Shao J, Lozoff B. 2020. Timing of iron deficiency and recognition memory in infancy. Nutr Neurosci 1–10. 10.1080/1028415X.2019.1704991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh MC, Zhang DL, Jeong SY, Kovtunovych G, Ollivierre-Wilson H, Noguchi A, Tu T, Senecal T, Robinson G, Crooks DR, et al. 2013. Deletion of iron regulatory protein 1 causes polycythemia and pulmonary hypertension in mice through translational derepression of HIF2alpha. Cell Metab 17: 271–281. 10.1016/j.cmet.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh MC, Zhang DL, Rouault TA. 2015. Iron misregulation and neurodegenerative disease in mouse models that lack iron regulatory proteins. Neurobiol Dis 81: 66–75. 10.1016/j.nbd.2015.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh MC, Zhang DL, Ollivierre H, Eckhaus MA, Rouault TA. 2018. Translational repression of HIF2alpha expression in mice with Chuvash polycythemia reverses polycythemia. J Clin Invest 128: 1317–1325. 10.1172/JCI97684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T, Zürcher N, Wu C, Bhanot A, Hightower BG, Kim M, Albrecht DS, Wey HY, Schroeder FA, Rodriguez-Thompson A, et al. 2019. PET neuroimaging reveals histone deacetylase dysregulation in schizophrenia. J Clin Invest 129: 364–372. 10.1172/JCI123743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet JC, et al. 2009. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet 41: 308–315. 10.1038/ng.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goforth JB, Anderson SA, Nizzi CP, Eisenstein RS. 2010. Multiple determinants within iron-responsive elements dictate iron regulatory protein binding and regulatory hierarchy. RNA 16: 154–169. 10.1261/rna.1857210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RJ, Jacobsen JS, Vitek MP, Gajdusek DC. 1989. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci 86: 7606–7610. 10.1073/pnas.86.19.7606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig NH, Ruckle J, Comer P, Brownell L, Holloway HW, Flanagan DR Jr, Canfield CJ, Burford RG. 2005. Anticholinesterase and pharmacokinetic profile of phenserine in healthy elderly human subjects. Curr Alzheimer Res 2: 483–492. 10.2174/156720505774330564 [DOI] [PubMed] [Google Scholar]
- Grillo AS, SantaMaria AM, Kafina MD, Cioffi AG, Huston NC, Han M, Seo YA, Yien YY, Nardone C, Menon AV, et al. 2017. Restored iron transport by a small molecule promotes absorption and hemoglobinization in animals. Science 356: 608–616. 10.1126/science.aah3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR. 2013. Manganese and Parkinson's disease: a critical review and new findings. Environ Health Perspect 118: 1071–1080. 10.1289/ehp.0901748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahl P, Davis T, Washburn C, Rogers JT, Smith A. 2013. Mechanisms of neuroprotection by hemopexin: modeling the control of heme and iron homeostasis in brain neurons in inflammatory states. J Neurochem 125: 89–101. 10.1111/jnc.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haining RL, Achat-Mendes C. 2017. Neuromelanin, one of the most overlooked molecules in modern medicine, is not a spectator. Neural Regen Res 12: 372–375. 10.4103/1673-5374.202928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Ophof A, Broe M, Jensen PH, Kettle E, Fedorow H, Cartwright MI, Griffiths FM, Shepherd CE, Double KL. 2005. Alpha-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson's disease. Brain 128: 2654–2664. 10.1093/brain/awh584 [DOI] [PubMed] [Google Scholar]
- Han H, Weinreb PH, Lansbury PT Jr. 1995. The core Alzheimer's peptide NAC forms amyloid fibrils which seed and are seeded by beta-amyloid: is NAC a common trigger or target in neurodegenerative disease? Chem Biol 2: 163–169. 10.1016/1074-5521(95)90071-3 [DOI] [PubMed] [Google Scholar]
- Hanna-Attisha M, Kuehn BM. 2016. Pediatrician sees long road ahead for flint after lead poisoning crisis. JAMA 315: 967–969. 10.1001/jama.2016.1034 [DOI] [PubMed] [Google Scholar]
- Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. 2016. Elevated blood lead levels in children associated with the flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health 106: 283–290. 10.2105/AJPH.2015.303003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harischandra DS, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG. 2015. alpha-Synuclein protects against manganese neurotoxic insult during the early stages of exposure in a dopaminergic cell model of Parkinson's disease. Toxicol Sci 143: 454–468. 10.1093/toxsci/kfu247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harischandra DS, Ghaisas S, Rokad D, Zamanian M, Jin H, Anantharam V, Kimber M, Kanthasamy A, Kanthasamy AG. 2018. Environmental neurotoxicant manganese regulates exosome-mediated extracellular miRNAs in cell culture model of Parkinson's disease: relevance to alpha-synuclein misfolding in metal neurotoxicity. Neurotoxicology 64: 267–277. 10.1016/j.neuro.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harischandra DS, Ghaisas S, Zenitsky G, Jin H, Kanthasamy A, Anantharam V, Kanthasamy AG. 2019a. Manganese-induced neurotoxicity: new insights into the triad of protein misfolding, mitochondrial impairment, and neuroinflammation. Front Neurosci 13: 654 10.3389/fnins.2019.00654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harischandra DS, Rokad D, Neal ML, Ghaisas S, Manne S, Sarkar S, Panicker N, Zenitsky G, Jin H, Lewis M, et al. 2019b. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of alpha-synuclein. Sci Signal 12: eaau4543 10.1126/scisignal.aau4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. 2008. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci 28: 8074–8085. 10.1523/JNEUROSCI.4904-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Gallardo AK, Missirlis F. 2020. Loss of ferritin in developing wing cells: apoptosis and ferroptosis coincide. PLoS Genet 16: e1008503 10.1371/journal.pgen.1008503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz BZ MD. 2001. Lead poisoning and chelation in a mother-neonate pair. J Toxicol Clin Toxicol 39: 727–731. 10.1081/CLT-100108514 [DOI] [PubMed] [Google Scholar]
- Hossain MA, Russell JC, Miknyoczki S, Ruggeri B, Lal B, Laterra J. 2004. Vascular endothelial growth factor mediates vasogenic edema in acute lead encephalopathy. Ann Neurol 55: 660–667. 10.1002/ana.20065 [DOI] [PubMed] [Google Scholar]
- Huang YA, Zhou B, Wernig M, Sudhof TC. 2017. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and abeta secretion. Cell 168: 427–441 e421. 10.1016/j.cell.2016.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YA, Zhou B, Nabet AM, Wernig M, Sudhof TC. 2019. Differential signaling mediated by ApoE2, ApoE3, and ApoE4 in human neurons parallels Alzheimer's disease risk. J Neurosci 39: 7408–7427. 10.1523/JNEUROSCI.2994-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens S, Liu C, Jursa T, Shawlot W, Chaffee BK, Yin W, Gore AC, Aschner M, Smith DR, Mukhopadhyay S. 2017. Deficiency in the man- ganese efflux transporter SLC30A10 induces severe hypo-thyroidism in mice. J Biol Chem 292: 9760–9773. 10.1074/jbc.M117.783605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi T, Kakita A, Shiga A, Kasuga K, Kaneko H, Tan CF, Idezuka J, Wakabayashi K, Onodera O, Iwatsubo T, et al. 2008. Patients homozygous and heterozygous for SNCA duplication in a family with parkinsonism and dementia. Arch Neurol 65: 514–519. 10.1001/archneur.65.4.514 [DOI] [PubMed] [Google Scholar]
- Jellinger KA. 2000. Morphological substrates of mental dysfunction in Lewy body disease: an update. J Neural Transm Suppl 59: 185–212. 10.1007/978-3-7091-6781-6_21 [DOI] [PubMed] [Google Scholar]
- Jellinger K, Paulus W, Grundke-Iqbal I, Riederer P, Youdim MB. 1990. Brain iron and ferritin in Parkinson's and Alzheimer's diseases. J Neural Transm Park Dis Dement Sect 2: 327–340. 10.1007/BF02252926 [DOI] [PubMed] [Google Scholar]
- Jensen PH, Hojrup P, Hager H, Nielsen MS, Jacobsen L, Olesen OF, Gliemann J, Jakes R. 1997. Binding of Abeta to alpha- and beta-synucleins: identification of segments in alpha-synuclein/NAC precursor that bind Abeta and NAC. Biochem J 323: 539–546. 10.1042/bj3230539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang J, Rogers J, Xie J. 2017. Brain iron metabolism dysfunction in Parkinson's disease. Mol Neurobiol 54: 3078–3101. 10.1007/s12035-016-9879-1 [DOI] [PubMed] [Google Scholar]
- Jiang J, Wang F, Wang L, Xiao J, Guo D. 2020. Manganese chloride exposure causes disorder of energy metabolism and induces oxidative stress and autophagy in chicken liver. Biol Trace Elem Res 197: 254–261. 10.1007/s12011-019-01960-8 [DOI] [PubMed] [Google Scholar]
- Jin M, O'Nuallain B, Hong W, Boyd J, Lagomarsino VN, O'Malley TT, Liu W, Vanderburg CR, Frosch MP, Young-Pearse T, et al. 2018. An in vitro paradigm to assess potential anti-Abeta antibodies for Alzheimer's disease. Nat Commun 9: 2676 10.1038/s41467-018-05068-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EE, Wessling-Resnick M. 2007. Flatiron mice and ferroportin disease. Nutr Rev 65: 341–345. 10.1111/j.1753-4887.2007.tb00312.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Han M, Xue J, Baek Y, Chang J, Hu S, Nam H, Jo MJ, El Fakhri G, Hutchens MP, et al. 2019. Renal clearable nanochelators for iron overload therapy. Nat Commun 10: 5134 10.1038/s41467-019-13143-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo JQ, Boonplueang R, Viswanath V, Jacobs R, Yang L, Beal MF, et al. 2003. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron 37: 899–909. 10.1016/S0896-6273(03)00126-0 [DOI] [PubMed] [Google Scholar]
- Keen CL, Hurley LS. 1987. Effects of zinc deficiency on prenatal and postnatal development. Neurotoxicology 8: 379–387. [PubMed] [Google Scholar]
- Khan MA, Walden WE, Theil EC, Goss DJ. 2017. Thermodynamic and kinetic analyses of iron response element (IRE)-mRNA binding to iron regulatory protein, IRP1. Sci Rep 7: 8532 10.1038/s41598-017-09093-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Buckett PD, Wessling-Resnick M. 2013. Absorption of manganese and iron in a mouse model of hemochromatosis. PLoS One 8: e64944 10.1371/journal.pone.0064944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong SM, Chan BK, Park JS, Hill KJ, Aitken JB, Cottle L, Farghaian H, Cole AR, Lay PA, Sue CM, et al. 2015. Parkinson's disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes alpha-Synuclein externalization via exosomes. Hum Mol Genet 23: 2816–2833. 10.1093/hmg/ddu099 [DOI] [PubMed] [Google Scholar]
- Kosman DJ. 2019. Reply to Lahiri et al.: appealing for a role in cellular iron efflux. J Biol Chem 294: 9366 10.1074/jbc.RL119.009249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, Lefkowitz A, McColl G, Goldstein LE, Tanzi RE, et al. 2016. Amyloid-beta peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci Transl Med 8: 340ra372 10.1126/scitranslmed.aaf1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK, Orrison BM, Bruneau BG, Giasson BI, Smeyne RJ, Gershon MD, et al. 2010. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet 19: 1633–1650. 10.1093/hmg/ddq038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Nwankwo EI, Nussbaum RL, Rogers J, Maccecchini ML. 2019. Translational inhibition of alpha-synuclein by Posiphen normalizes distal colon motility in transgenic Parkinson mice. Am J Neurodegener Dis 8: 1–15. [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Ge YW, Maloney B. 2005a. Characterization of the APP proximal promoter and 5'-untranslated regions: identification of cell type-specific domains and implications in APP gene expression and Alzheimer's disease. Faseb J 19: 653–655. 10.1096/fj.04-2900fje [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Ge YW, Maloney B, Wavrant-De Vrieze F, Hardy J. 2005b. Characterization of two APP gene promoter polymorphisms that appear to influence risk of late-onset Alzheimer's disease. Neurobiol Aging 26: 1329–1341. 10.1016/j.neurobiolaging.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Chen D, Maloney B, Holloway HW, Yu QS, Utsuki T, Giordano T, Sambamurti K, Greig NH. 2007. The experimental Alzheimer's disease drug posiphen [(+)-phenserine] lowers amyloid-beta peptide levels in cell culture and mice. J Pharmacol Exp Ther 320: 386–396. 10.1124/jpet.106.112102 [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B, Zawia NH. 2009. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry 14: 992–1003. 10.1038/mp.2009.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B, Wang R. 2019. APPealing for a role in cellular iron efflux. J Biol Chem 294: 9365 10.1074/jbc.L119.009216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren J, Gimbel D, Nygaard H, Gilbert J, Strittmatter S. 2009. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature 457: 1128–1132. 10.1038/nature07761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVaute T, Smith S, Cooperman S, Iwai K, Land W, Meyron-Holtz E, Drake SK, Miller G, Abu-Asab M, Tsokos M, et al. 2001. Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat Genet 27: 209–214. 10.1038/84859 [DOI] [PubMed] [Google Scholar]
- Lee VM, Giasson BI, Trojanowski JQ. 2004. More than just two peas in a pod: common amyloidogenic properties of tau and alpha-synuclein in neurodegenerative diseases. Trends Neurosci 27: 129–134. 10.1016/j.tins.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Lee DW, Kaur D, Chinta SJ, Rajagopalan S, Andersen JK. 2009. A disruption in iron-sulfur center biogenesis via inhibition of mitochondrial dithiol glutaredoxin 2 may contribute to mitochondrial and cellular iron dysregulation in mammalian glutathione-depleted dopaminergic cells: implications for Parkinson's disease. Antioxid Redox Signal 11: 2083–2094. 10.1089/ars.2009.2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leedman PJ, Stein AR, Chin WW, Rogers JT. 1996. Thyroid hormone modulates the interaction between iron regulatory proteins and the ferritin mRNA iron-responsive element. J Biol Chem 271: 12017–12023. 10.1074/jbc.271.20.12017 [DOI] [PubMed] [Google Scholar]
- Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA, Cherny RA, Lam LQ, et al. 2012. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med 18: 291–295. 10.1038/nm.2613 [DOI] [PubMed] [Google Scholar]
- Lewis J, Melrose H, Bumcrot D, Hope A, Zehr C, Lincoln S, Braithwaite A, He Z, Ogholikhan S, Hinkle K, et al. 2008. In vivo silencing of alpha-synuclein using naked siRNA. Mol Neurodegener 3: 19 10.1186/1750-1326-3-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell JR. 2015. Targeting mitochondrial metal dyshomeostasis for the treatment of neurodegeneration. Neurodegener Dis Manag 5: 345–364. 10.2217/nmt.15.19 [DOI] [PubMed] [Google Scholar]
- Lin W, Vann DR, Doulias PT, Wang T, Landesberg G, Li X, Ricciotti E, Scalia R, He M, Hand NJ, et al. 2017. Hepatic metal ion transporter ZIP8 regulates manganese homeostasis and manganese-dependent enzyme activity. J Clin Invest 127: 2407–2417. 10.1172/JCI90896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locascio JJ, Eberly S, Liao Z, Liu G, Hoesing AN, Duong K, Trisini-Lipsanopoulos A, Dhima K, Hung AY, Flaherty AW, et al. 2015. Association between alpha-synuclein blood transcripts and early, neuroimaging-supported Parkinson's disease. Brain 138: 2659–2671. 10.1093/brain/awv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan T, Bendor J, Toupin C, Thorn K, Edwards RH. 2017. alpha-Synuclein promotes dilation of the exocytotic fusion pore. Nat Neurosci 20: 681–689. 10.1038/nn.4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok J, Sardi SP, Guo S, Besancon E, Ha DM, Rosell A, Kim WJ, Corfas G, Lo EH. 2009. Neuregulin-1 signaling in brain endothelial cells. J Cereb Blood Flow Metab 29: 39–43. 10.1038/jcbfm.2008.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, Maloney B, Rogers JT, Lahiri DK. 2019. Novel upregulation of amyloid-beta precursor protein (APP) by microRNA-346 via targeting of APP mRNA 5′-untranslated region: implications in Alzheimer's disease. Mol Psychiatry 24: 345–363. 10.1038/s41380-018-0266-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Huang C, Chu N, Cane D. 1994. Levadopa failure in chronic manganism. Neurology 44: 1600–1602. 10.1212/WNL.44.9.1600 [DOI] [PubMed] [Google Scholar]
- Lu M, Rajanala S, Mikkilineni S, Cahill C, Brown RE, Berry JD, Rogers J. 2016. The 5′-untranslated region of the C9orf72 mRNA exhibits a phylogenetic alignment to the cis-aconitase iron-responsive element; novel therapies for amytrophic lateral sclerosis. Neurosci Med 7: 15–26. 10.4236/nm.2016.71003 [DOI] [Google Scholar]
- Lumsden AL, Rogers JT, Majd S, Newman M, Sutherland GT, Verdile G, Lardelli M. 2018. Dysregulation of neuronal iron homeostasis as an alternative unifying effect of mutations causing familial Alzheimer's disease. Front Neurosci 12: 533 10.3389/fnins.2018.00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luth ES, Bartels T, Dettmer U, Kim NC, Selkoe DJ. 2015. Purification of alpha-synuclein from human brain reveals an instability of endogenous multimers as the protein approaches purity. Biochemistry 54: 279–292. 10.1021/bi501188a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madejczyk MS, Ballatori N. 2012. The iron transporter ferroportin can also function as a manganese exporter. Biochim Biophys Acta 1818: 651–657. 10.1016/j.bbamem.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makayev AV, Liebhaber SA. 2002. The Poly(C)-binding proteins: a multiplicity of functions and a search for mechanisms. RNA 8: 265–278. 10.1017/S1355838202024627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malecki EA, Devenyi AG, Beard JL, Connor JR. 1999. Existing and emerging mechanisms for transport of iron and manganese to the brain. J Neurosci Res 56: 113–122. [DOI] [PubMed] [Google Scholar]
- Maloney B, Lahiri DK. 2016. Epigenetics of dementia: understanding the disease as a transformation rather than a state. Lancet Neurol 15: 760–774. 10.1016/S1474-4422(16)00065-X [DOI] [PubMed] [Google Scholar]
- Maloney B, Ge YW, Petersen RC, Hardy J, Rogers JT, Perez-Tur J, Lahiri DK. 2010. Functional characterization of three single-nucleotide polymorphisms present in the human APOE promoter sequence: differential effects in neuronal cells and on DNA-protein interactions. Am J Med Genet B Neuropsychiatr Genet 153B: 185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney B, Bayon BL, Zawia NH, Lahiri DK. 2018. Latent consequences of early-life lead (Pb) exposure and the future: addressing the Pb crisis. Neurotoxicology 68: 126–132. 10.1016/j.neuro.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoud AM, Bihaqi SW, Machan JT, Zawia NH, Renehan WE. 2016. Early-life exposure to lead (Pb) alters the expression of microRNA that target proteins associated with Alzheimer's disease. J Alzheimers Dis 51: 1257–1264. 10.3233/JAD-151018 [DOI] [PubMed] [Google Scholar]
- Maynard CJ, Cappai R, Volitakis I, Cherny RA, White AR, Beyreuther K, Masters CL, Bush AI, Li QX. 2002. Overexpression of Alzheimer's disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J Biol Chem 277: 44670–44676. 10.1074/jbc.M204379200 [DOI] [PubMed] [Google Scholar]
- McCarthy RC, Park YH, Kosman DJ. 2014. sAPP modulates iron efflux from brain microvascular endothelial cells by stabilizing the ferrous iron exporter ferroportin. EMBO Rep 15: 809–815. 10.15252/embr.201338064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Ferman TJ, Thomas AJ, Blanc F, Boeve BF, Fujishiro H, Kantarci K, Muscio C, O'Brien JT, Postuma RB, et al. 2020. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 94: 743–755. 10.1212/WNL.0000000000009323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadowcroft MD, Wang J, Purnell CJ, Eslinger PJ, Neely EB, Yang QX, Connor JR. 2018. Reduced cerebral white matter integrity assessed by DTI in cognitively normal H63D-HFE polymorphism carriers. J Neuroimaging 28: 126–133. 10.1111/jon.12461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici S, Peana M, Delogu LG, Zoroddu MA. 2015. Mn(II) and Zn(II) interactions with peptide fragments from Parkinson's disease genes. Dalton Trans 41: 4378–4388. 10.1039/c2dt12168a [DOI] [PubMed] [Google Scholar]
- Mikkilineni S, Cantuti-Castelvetri I, Cahill CM, Balliedier A, Greig NH, Rogers JT. 2012. The anticholinesterase phenserine and its enantiomer posiphen as 5′untranslated-region-directed translation blockers of the Parkinson's alpha synuclein expression. Parkinsons Dis 2012: 142372 10.1155/2012/142372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CJ, Shawki A, Ganz T, Nemeth E, Mackenzie B. 2014. Functional properties of human ferroportin, a cellular iron exporter reactive also with cobalt and zinc. Am J Physiol Cell Physiol 306: C450–C459. 10.1152/ajpcell.00348.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales R, Estrada LD, Diaz-Espinoza R, Morales-Scheihing D, Jara MC, Castilla J, Soto C. 2012. Molecular cross talk between misfolded proteins in animal models of Alzheimer's and prion diseases. J Neurosci 30: 4528–4535. 10.1523/JNEUROSCI.5924-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse LJ, Payton SM, Cuny GD, Rogers JT. 2004. FDA-preapproved drugs targeted to the translational regulation and processing of the amyloid precursor protein. J Mol Neurosci 24: 129–136. 10.1385/JMN:24:1:129 [DOI] [PubMed] [Google Scholar]
- Mou X, Pilozzi A, Tailor B, Yi J, Cahill C, Rogers J, Huang X. 2020. Exposure to CuO nanoparticles mediates NFkappaB activation and enhances amyloid precursor protein expression. Biomedicines 8: 45 10.3390/biomedicines8030045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhaup G, Schlicksupp A, Hesse L, Beher D, Ruppert T, Masters C, Beyreuther K. 1996. The amyloid precursor protein of Alzheimer's disease in the reduction of copper(II) to copper(I). Science 271: 1406–1409. 10.1126/science.271.5254.1406 [DOI] [PubMed] [Google Scholar]
- Nandar W, Connor JR. 2011. HFE gene variants affect iron in the brain. J Nutr 141: 729S–739S. 10.3945/jn.110.130351 [DOI] [PubMed] [Google Scholar]
- Nandar W, Neely EB, Simmons Z, Connor JR. 2014. H63D HFE genotype accelerates disease progression in animal models of amyotrophic lateral sclerosis. Biochim Biophys Acta 1842: 2413–2426. 10.1016/j.bbadis.2014.09.016 [DOI] [PubMed] [Google Scholar]
- Newman M, Hin N, Pederson S, Lardelli M. 2019. Brain transcriptome analysis of a familial Alzheimer's disease-like mutation in the zebrafish presenilin 1 gene implies effects on energy production. Mol Brain 12: 43 10.1186/s13041-019-0467-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LN, Arendash GW, Leighty RE, Costa DA, Low MA, Garcia MF, Cracciolo JR, Rojiani A, Wu X, Bales KR, et al. 2004. Cognitive impairment in PDAPP mice depends on ApoE and ACT-catalyzed amyloid formation. Neurobiol Aging 25: 1153–1167. 10.1016/j.neurobiolaging.2003.12.011 [DOI] [PubMed] [Google Scholar]
- Nishioka K, Hayashi S, Farrer MJ, Singleton AB, Yoshino H, Imai H, Kitami T, Sato K, Kuroda R, Tomiyama H, et al. 2006. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson's disease. Ann Neurol 59: 298–309. 10.1002/ana.20753 [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Deng M, Tennis M, Schoenfeld D, Growdon JH. 2000. The selective muscarinic M1 agonistAF102B decreases levels of totalabeta in the cerebrospinal fluid of patients with Alzheimer's disease. Ann Neurol 48: 913–918. [DOI] [PubMed] [Google Scholar]
- Nuber S, Rajsombath M, Minakaki G, Winkler J, Muller CP, Ericsson M, Caldarone B, Dettmer U, Selkoe DJ. 2018. Abrogating native alpha-synuclein tetramers in mice causes a L-DOPA-responsive motor syndrome closely resembling Parkinson's disease. Neuron 100: 75–90 e75. 10.1016/j.neuron.2018.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Collingwood JF, Dobson J, Love G, Perrott HR, Edwardson JA, Elstner M, Morris CM. 2007. Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology 68: 1820–1825. 10.1212/01.wnl.0000262033.01945.9a [DOI] [PubMed] [Google Scholar]
- Oaks AW, Sidhu A. 2011. Synuclein modulation of monoamine transporters. FEBS Lett 585: 1001–1006. 10.1016/j.febslet.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares D, Huang X, Branden L, Greig NH, Rogers JT. 2009. Physiological and pathological role of alpha-synuclein in Parkinson's disease through iron mediated oxidative stress; the role of a putative iron-responsive element. Int J Mol Sci 10: 1226–1260. 10.3390/ijms10031226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Koss DJ, Erskine D, Walker L, Kurzawa-Akanbi M, Burn D, Donaghy P, Morris C, Taylor JP, Thomas A, et al. 2019. Dementia with Lewy bodies: an update and outlook. Mol Neurodegener 14: 5 10.1186/s13024-019-0306-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D, Berthet A, Bendor JT, Yu K, Sellnow RC, Orr AL, Nguyen MK, Edwards RH, Manfredsson FP, Nakamura K. (2017) Loss of alpha-synuclein does not affect mitochondrial bioenergetics in rodent neurons. eNeuro 4 10.1523/ENEURO.0216-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres TV, Schettinger MR, Chen P, Carvalho F, Avila DS, Bowman AB, Aschner M. 2016. Manganese-induced neurotoxicity: a review of its behavioral consequences and neuroprotective strategies. BMC Pharmacol Toxicol 17: 57 10.1186/s40360-016-0099-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno A, Pelucchi S, Mariani R. 2020. Inherited iron overload disorders. Transl Gastroenterol Hepatol 5: 25 10.21037/tgh.2019.11.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plascencia-Villa G, Ponce A, Collingwood JF, Arellano-Jimenez MJ, Zhu X, Rogers JT, Betancourt I, Jose-Yacaman M, Perry G. 2016. High-resolution analytical imaging and electron holography of magnetite particles in amyloid cores of Alzheimer's disease. Sci Rep 6: 24873 10.1038/srep24873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posturna R, Berg D. 2019. Prodromal Parkinson's disease: the decade past, the decade to come. Mov Disord 34: 665–675. 10.1002/mds.27670 [DOI] [PubMed] [Google Scholar]
- Potter H, Chial HJ. 2019. Targeting the interaction between apolipoprotein E and amyloid precursor protein: a novel Alzheimer's disease therapy. Biol Psychiatry 86: 169–170. 10.1016/j.biopsych.2019.06.005 [DOI] [PubMed] [Google Scholar]
- Potter H, Granic A, Caneus J. 2016. Role of trisomy 21 mosaicism in sporadic and familial Alzheimer's disease. Curr Alzheimer Res 13: 7–17. 10.2174/156720501301151207100616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi MM, Brown RH, Rogers JT, Cudkowicz M. 2008. Serum ferrtin and metal levels as risk factors for ALS. Open Neurol J 2: 51–54. 10.2174/1874205X00802010051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Khan KM, Al-Khaledi G, Khan I, Attur S. 2014. Early postnatal lead exposure induces tau phosphorylation in the brain of young rats. Acta Biol Hung 63: 411–425. 10.1556/ABiol.63.2012.4.1 [DOI] [PubMed] [Google Scholar]
- Rodan LH, Hauptman M, D'Gama AM, Qualls AE, Cao S, Tuschl K, Al-Jasmi F, Hertecant J, Hayflick SJ, Wessling-Resnick M, et al. 2018. Novel founder intronic variant in SLC39A14 in two families causing Manganism and potential treatment strategies. Mol Genet Metab 124: 161–167. 10.1016/j.ymgme.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT. 1996. Ferritin translation by interleukin-1and interleukin-6: the role of sequences upstream of the start codons of the heavy and light subunit genes. Blood 87: 2525–2537. 10.1182/blood.V87.6.2525.bloodjournal8762525 [DOI] [PubMed] [Google Scholar]
- Rogers JT, Leiter LM, McPhee J, Cahill CM, Zhan SS, Potter H, Nilsson LN. 1999. Translation of the Alzheimer amyloid precursor protein mRNA is up-regulated by interleukin-1 through 5′-untranslated region sequences. J Biol Chem 274: 6421–6431. 10.1074/jbc.274.10.6421 [DOI] [PubMed] [Google Scholar]
- Rogers JT, Randall JD, Cahill CM, Eder PS, Huang X, Gunshin H, Leiter L, McPhee J, Sarang SS, Utsuki T, et al. 2002a. An iron-responsive element type II in the 5′-untranslated region of the Alzheimer's amyloid precursor protein transcript. J Biol Chem 277: 45518–45528. 10.1074/jbc.M207435200 [DOI] [PubMed] [Google Scholar]
- Rogers JT, Randall JD, Eder PS, Huang X, Bush AI, Tanzi RE, Venti A, Payton SM, Giordano T, Nagano S, et al. 2002b. Alzheimer's disease drug discovery targeted to the APP mRNA 5′untranslated region. J Mol Neurosci 19: 77–82. 10.1007/s12031-002-0014-6 [DOI] [PubMed] [Google Scholar]
- Rogers J, Greig NH, Lahiri D, Fisher A. (2005) Translation and processing of the amyloid precursor protein in response to an m1 muscaric agonist and an acetyl cholinesterase inhibitor. In Therapeutic strategies in neurodegenerative diseases (ed. Fisher A, Hanin I), pp. 267–278. Paul Dunitz, NY. [Google Scholar]
- Rogers JT, Bush AI, Cho HH, Smith DH, Thomson AM, Friedlich AL, Lahiri DK, Leedman PJ, Huang X, Cahill CM. 2008. Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer's disease. Biochem Soc Trans 36: 1282–1287. 10.1042/BST0361282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Mikkilineni S, Cantuti-Castelvetri I, Smith DH, Huang X, Bandyopadhyay S, Cahill CM, Maccecchini ML, Lahiri DK, Greig NH. 2011. The alpha-synuclein 5′untranslated region targeted translation blockers: anti-alpha synuclein efficacy of cardiac glycosides and Posiphen. J Neural Transm 118: 493–507. 10.1007/s00702-010-0513-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Venkataramani V, Washburn C, Liu Y, Tummala V, Jiang H, Smith A, Cahill CM. 2016. A role for amyloid precursor protein translation to restore iron homeostasis and ameliorate lead (Pb) neurotoxicity. J Neurochem 138: 479–494. 10.1111/jnc.13671 [DOI] [PubMed] [Google Scholar]
- Rogers JT, Xia N, Wong A, Bakshi R, Cahill CM. 2019. Targeting the iron-response elements of the mRNAs for the Alzheimer's amyloid precursor protein and ferritin to treat acute lead and manganese neurotoxicity. Int J Mol Sci 20: 994 10.3390/ijms20040994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross NT, Metkar SR, Le H, Burbank J, Cahill C, Germain A, MacPherson L, Bittker J, Palmer M, Rogers J, et al. (2010a) Identification of a small molecule that selectively activates alpha-synuclein translational expression. In Probe Reports from the NIH Molecular Libraries Program, Bethesda, MD. [PubMed] [Google Scholar]
- Ross NT, Metkar SR, Le H, Burbank J, Cahill C, Germain A, MacPherson L, Bittker J, Palmer M, Rogers J, et al. (2010b) Identification of a small molecule that selectively inhibits alpha-synuclein translational expression. In Probe Reports from the NIH Molecular Libraries Program, Bethesda, MD. [PubMed] [Google Scholar]
- Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerrière A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, et al. 2006. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 38: 24–26. 10.1038/ng1718 [DOI] [PubMed] [Google Scholar]
- Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V, Zhao L, Garrick LM, et al. 2008. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proc Natl Acad Sci 105: 18578–18583. 10.1073/pnas.0804373105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore MF, Fisher B, Surgener SP, Gerhardt GA, Rouault T. 2005. Neurochemical investigations of dopamine neuronal systems in iron-regulatory protein 2 (IRP-2) knockout mice. Brain Res Mol Brain Res 139: 341–347. 10.1016/j.molbrainres.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Sanchez M, Galy B, Schwanhaeusser B, Blake J, Bahr-Ivacevic T, Benes V, Selbach M, Muckenthaler MU, Hentze MW. 2011. Iron regulatory protein-1 and -2: transcriptome-wide definition of binding mRNAs and shaping of the cellular proteome by iron regulatory proteins. Blood 118: e168–e179. 10.1182/blood-2011-04-343541 [DOI] [PubMed] [Google Scholar]
- Sarkar S, Malovic E, Harischandra DS, Ngwa HA, Ghosh A, Hogan C, Rokad D, Zenitsky G, Jin H, Anantharam V, et al. 2018. Manganese exposure induces neuroinflammation by impairing mitochondrial dynamics in astrocytes. Neurotoxicology 64: 204–218. 10.1016/j.neuro.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer CR, Eklund AC, Morse LJ, Liao Z, Locascio JJ, Fefer D, Schwarzschild MA, Schlossmacher MG, Hauser MA, Vance JM, et al. 2007. Molecular markers of early Parkinson's disease based on gene expression in blood. Proc Natl Acad Sci 104: 955–960. 10.1073/pnas.0610204104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D, Dettmer U, Luth E, Kim N, Newman A, Bartels T. 2014. Defining the native state of alpha-synuclein. Neurodegener Dis 13: 114–117. 10.1159/000355516 [DOI] [PubMed] [Google Scholar]
- Seo YA, Wessling-Resnick M. 2015. Ferroportin deficiency impairs manganese metabolism in flatiron mice. FASEB J 29: 2726–2733. 10.1096/fj.14-262592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KT, Utsuki T, Rogers J, Yu QS, Sambamurti K, Brossi A, Ge YW, Lahiri DK, Greig NH. 2001. Phenserine regulates translation of beta -amyloid precursor protein mRNA by a putative interleukin-1 responsive element, a target for drug development. Proc Natl Acad Sci 98: 7605–7610. 10.1073/pnas.131152998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Kong Q, Luo X, Petersen RB, Meyerson H, Singh N. 2009. Prion protein (PrP) knockout mice show altered iron metabolism: a functional role for PrP in iron uptake and transport. PLoS One 4: e6115 10.1371/journal.pone.0006115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Haldar S, Horback K, Tom C, Zhou L, Meyerson H, Singh N. 2013. Prion protein regulates iron transport by functioning as a ferrireductase. J Alzheimers Dis 35: 541–552. 10.3233/JAD-130218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Haldar S, Tripathi AK, McElwee MK, Horback K, Beserra A. 2014. Iron in neurodegenerative disorders of protein misfolding: a case of prion disorders and Parkinson's disease. Antioxid Redox Signal 21: 471–484. 10.1089/ars.2014.5874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton A, Gwinn-Hardy K. 2004. Parkinson's disease and dementia with Lewy bodies: a difference in dose? Lancet 364: 1105–1107. 10.1016/S0140-6736(04)17117-1 [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, et al. 2003. alpha-Synuclein locus triplication causes Parkinson's disease. Science 302: 841 10.1126/science.1090278 [DOI] [PubMed] [Google Scholar]
- Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, et al. 2014. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136: 4551–4556. 10.1021/ja411006a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleegers K, Brouwers N, Gijselinck I, Theuns J, Goossens D, Wauters J, Del-Favero J, Cruts M, van Duijn CM, Van Broeckhoven C. 2006. APP duplication is sufficient to cause early onset Alzheimer's dementia with cerebral amyloid angiopathy. Brain 129: 2977–2983. 10.1093/brain/awl203 [DOI] [PubMed] [Google Scholar]
- Smolen P, Baxter DA, Byrne JH. 2019. How can memories last for days, years, or a lifetime? Proposed mechanisms for maintaining synaptic potentiation and memory. Learn Mem 26: 133–150. 10.1101/lm.049395.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DD, Shults CW, Sisk A, Rockenstein E, Masliah E. 2004. Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp Neurol 186: 158–172. 10.1016/S0014-4886(03)00342-X [DOI] [PubMed] [Google Scholar]
- Song I, Snyder AM, Kim Y, Neely EB, Wade QW, Connor JR. 2020. The Nrf2-mediated defense mechanism associated with HFE genotype limits vulnerability to oxidative stress-induced toxicity. Toxicology 441: 152525 10.1016/j.tox.2020.152525 [DOI] [PubMed] [Google Scholar]
- Spencer B, Desplats PA, Overk CR, Valera-Martin E, Rissman RA, Wu C, Mante M, Adame A, Florio J, Rockenstein E, et al. 2016. Reducing endogenous alpha-synuclein mitigates the degeneration of selective neuronal populations in an Alzheimer's disease transgenic mouse model. J Neurosci 36: 7971–7984. 10.1523/JNEUROSCI.0775-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, Valera E, Rockenstein E, Overk C, Mante M, Adame A, Zago W, Seubert P, Barbour R, Schenk D, et al. 2017. Anti-alpha-synuclein immunotherapy reduces alpha-synuclein propagation in the axon and degeneration in a combined viral vector and transgenic model of synucleinopathy. Acta Neuropathol Commun 5: 7 10.1186/s40478-016-0410-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangle DE, Smith DR, Beaudin SA, Strawderman MS, Levitsky DA, Strupp BJ. 2007. Succimer chelation improves learning, attention, and arousal regulation in lead-exposed rats but produces lasting cognitive impairment in the absence of lead exposure. Environ Health Perspect 115: 201–209. 10.1289/ehp.9263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocchi F, Torti M. 2017. Constipation in Parkinson's Disease. Int Rev Neurobiol 134: 811–826. 10.1016/bs.irn.2017.06.003 [DOI] [PubMed] [Google Scholar]
- Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, et al. 2017. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171: 273–285. 10.1016/j.cell.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struzynska L, Bubko I, Walski M, Rafalowska U. 2001. Astroglial reaction during the early phase of acute lead toxicity in the adult rat brain. Toxicology 165: 121–131. 10.1016/S0300-483X(01)00415-2 [DOI] [PubMed] [Google Scholar]
- Sulzer D, Cassidy C, Horga G, Kang UJ, Fahn S, Casella L, Pezzoli G, Langley J, Hu XP, Zucca FA, et al. 2018. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson's disease. NPJ Parkinsons Dis 4: 11 10.1038/s41531-018-0047-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T, Ikuno M, Hondo M, Parajuli LK, Taguchi K, Ueda J, Sawamura M, Okuda S, Nakanishi E, Hara J, et al. 2020a. alpha-Synuclein BAC transgenic mice exhibit RBD-like behaviour and hyposmia: a prodromal Parkinson's disease model. Brain 143: 249–265. 10.1093/brain/awz380 [DOI] [PubMed] [Google Scholar]
- Taguchi T, Ikuno M, Yamakado H, Takahashi R. 2020b. Animal model for prodromal Parkinson's disease. Int J Mol Sci 21: 1961 10.3390/ijms21061961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JP, McKeith IG, Burn DJ, Boeve BF, Weintraub D, Bamford C, Allan LM, Thomas AJ, O'Brien JT. 2020. New evidence on the management of Lewy body dementia. Lancet Neurol 19: 157–169. 10.1016/S1474-4422(19)30153-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teich AF, Sharma E, Barnwell E, Zhang H, Staniszewski A, Utsuki T, Padmaraju V, Mazell C, Tzekou A, Sambamurti K, et al. 2018. Translational inhibition of APP by Posiphen: efficacy, pharmacodynamics, and pharmacokinetics in the APP/PS1 mouse. Alzheimers Dement (N Y) 4: 37–45. 10.1016/j.trci.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theuns J, Brouwers N, Engelborghs S, Sleegers K, Bogaerts V, Corsmit E, De Pooter T, van Duijn CM, De Deyn PP, Van Broeckhoven C. 2006. Promoter mutations that increase amyloid precursor-protein expression are associated with Alzheimer disease. Am J Hum Genet 78: 936–946. 10.1086/504044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Rogers JT, Leedman PJ. 1999. Iron-regulatory proteins, iron-responsive elements and ferritin mRNA translation. Int J Biochem Cell Biol 31: 1139–1152. 10.1016/S1357-2725(99)00080-1 [DOI] [PubMed] [Google Scholar]
- Thomson AM, Cahill CM, Cho HH, Kassachau KD, Epis MR, Bridges KR, Leedman PJ, Rogers JT. 2005. The acute box cis-element in human heavy ferritin mRNA 5′-untranslated region is a unique translation enhancer that binds poly(C)-binding proteins. J Biol Chem 280: 30032–30045. 10.1074/jbc.M502951200 [DOI] [PubMed] [Google Scholar]
- Tibodeau JD, Fox PM, Ropp PA, Theil EC, Thorp HH. 2006. The up-regulation of ferritin expression using a small-molecule ligand to the native mRNA. Proc Natl Acad Sci 103: 253–257. 10.1073/pnas.0509744102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Yang H, Tian X, Wang H, Zhou T, Zhang S, Yu J, Zhang T, Fan D, Guo X, et al. 2014. High manganese, a risk for Alzheimer's disease: high manganese induces amyloid-beta related cognitive impairment. J Alzheimers Dis 42: 865–878. 10.3233/JAD-140534 [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Lee VM. 2001. Parkinson's disease and related neurodegenerative synucleinopathies linked to progressive accumulations of synuclein aggregates in brain. Parkinsonism Relat Disord 7: 247–251. 10.1016/S1353-8020(00)00065-1 [DOI] [PubMed] [Google Scholar]
- Trudler D, Weinreb O, Mandel SA, Youdim MB, Frenkel D. 2014. DJ-1 deficiency triggers microglia sensitivity to dopamine toward a pro-inflammatory phenotype that is attenuated by rasagiline. J Neurochem 129: 434–447. 10.1111/jnc.12633 [DOI] [PubMed] [Google Scholar]
- Tsatsanis A, Dickens S, Kwok JCF, Wong BX, Duce JA. 2019. Post translational modulation of beta-amyloid precursor protein trafficking to the cell surface alters neuronal iron homeostasis. Neurochem Res 44: 1367–1374. 10.1007/s11064-019-02747-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsanis A, Wong BX, Gunn AP, Ayton S, Bush AI, Devos D, Duce JA. 2020. Amyloidogenic processing of Alzheimer's disease beta-amyloid precursor protein induces cellular iron retention. Mol Psychiatry 10.1038/s41380-020-0762-0. [DOI] [PubMed] [Google Scholar]
- Tuschl K, Clayton P, Gospe SJEA. 2016. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hyperman-ganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am J Hum Genet 99: 521 10.1016/j.ajhg.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. 1993. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci 90: 11282–11286. 10.1073/pnas.90.23.11282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsuki T, Yu QS, Davidson D, Chen D, Holloway HW, Brossi A, Sambamurti K, Lahiri DK, Greig NH, Giordano T. 2006. Identification of novel small molecule inhibitors of amyloid precursor protein synthesis as a route to lower Alzheimer's disease amyloid-beta peptide. J Pharmacol Exp Ther 318: 855–862. 10.1124/jpet.106.103309 [DOI] [PubMed] [Google Scholar]
- Venkataramani V, Doeppner TR, Willkommen D, Cahill CM, Xin Y, Ye G, Liu Y, Southon A, Aron A, Au-Yeung HY, et al. 2018. Manganese causes neurotoxic iron accumulation via translational repression of amyloid precursor protein and H-Ferritin. J Neurochem 147: 831–848. 10.1111/jnc.14580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venti A, Giordano T, Eder P, Bush AI, Lahiri DK, Greig NH, Rogers JT. 2004. The integrated role of desferrioxamine and phenserine targeted to an iron-responsive element in the APP-mRNA 5′-untranslated region. Ann N Y Acad Sci 1035: 34–48. 10.1196/annals.1332.003 [DOI] [PubMed] [Google Scholar]
- Vollet K, Haynes EN, Dietrich KN. 2016. Manganese exposure and cognition across the lifespan: contemporary review and argument for biphasic dose-response health effects. Curr Environ Health Rep 3: 392–404. 10.1007/s40572-016-0108-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Z, Possin KL, Boeve BF, Aarsland D. 2015. Lewy body dementias. Lancet 386: 1683–1697. 10.1016/S0140-6736(15)00462-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. 2016. A critical appraisal of the pathogenic protein spread hypothesis of neurodegeneration. Nat Rev Neurosci 17: 251–260. 10.1038/nrn.2016.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. 2020. Amyloid beta-protein and beyond: the path forward in Alzheimer's disease. Curr Opin Neurobiol 61: 116–124. 10.1016/j.conb.2020.02.003 [DOI] [PubMed] [Google Scholar]
- Wang J, Fillebeen C, Chen G, Biederbick A, Lill R, Pantopoulos K. 2007a. Iron-dependent degradation of apo-IRP1 by the ubiquitin-proteasome pathway. Mol Cell Biol 27: 2423–2430. 10.1128/MCB.01111-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Di X, D'Agostino RB Jr, Torti SV, Torti FM. 2007b. Excess capacity of the iron regulatory protein system. J Biol Chem 282: 24650–24659. 10.1074/jbc.M703167200 [DOI] [PubMed] [Google Scholar]
- White AR, Multhaup G, Maher F, Bellingham S, Camakaris J, Zheng H, Bush AI, Beyreuther K, Masters CL, Cappai R. 1999. The Alzheimer's disease amyloid precursor protein modulates copper-induced toxicity and oxidative stress in primary neuronal cultures. J Neurosci 19: 9170–9179. 10.1523/JNEUROSCI.19-21-09170.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Giacobini E, Frolich L, Friedhoff LT, Bruinsma G, Becker RE, Greig NH. 2010. Phenserine efficacy in Alzheimer's disease. J Alzheimers Dis 22: 1201–1208. 10.3233/JAD-2010-101311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirbisky SE, Weber GJ, Lee JW, Cannon JR, Freeman JL. 2014. Novel dose-dependent alterations in excitatory GABA during embryonic development associated with lead (Pb) neurotoxicity. Toxicol Lett 229: 1–8. 10.1016/j.toxlet.2014.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Wei J, Li Y, Wang J, Li W, Wang K, Hong X, Zhao L, Chen C, Min J, et al. 2017. Zebrafish slc30a10 deficiency revealed a novel compensatory mechanism of Atp2c1 in maintaining manganese homeostasis. PLoS Genet 13: e1006892 10.1371/journal.pgen.1006892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Y, Gao H, Wang J, Qiang Y, Imam MU, Li Y, Wang J, Zhang R, Zhang H, Yu Y, et al. 2017. Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov 3: 17025 10.1038/celldisc.2017.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamin G, Glaser CB, Uversky VN, Fink AL. 2003. Certain metals trigger fibrillation of methionine-oxidized alpha-synuclein. J Biol Chem 278: 27630–27635. 10.1074/jbc.M303302200 [DOI] [PubMed] [Google Scholar]
- Ye Q, Trivedi M, Zhang Y, Bohlke M, Alsulimani H, Chang J, Maher T, Deth R, Kim J. 2019. Brain iron loading impairs DNA methylation and alters GABAergic function in mice. FASEB J 33: 2460–2471. 10.1096/fj.201801116RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Wu Q, Monga J, Xie E, Wang H, Wang S, Zhang H, Wang ZY, Zhou T, Shi Y, et al. 2018. HDAC1 governs iron homeostasis independent of histone deacetylation in iron-overload murine models. Antioxid Redox Signal 28: 1224–1237. 10.1089/ars.2017.7161 [DOI] [PubMed] [Google Scholar]
- Youdim MB, Lavie L. 1994. Selective MAO-A and B inhibitors, radical scavengers and nitric oxide synthase inhibitors in Parkinson's disease. Life Sci 55: 2077–2082. 10.1016/0024-3205(94)00388-2 [DOI] [PubMed] [Google Scholar]
- Youdim MB, Weinstock M. 2001. Molecular basis of neuroprotective activities of rasagiline and the anti-Alzheimer drug TV3326 [(N-propargyl-(3R)aminoindan-5-YL)-ethyl methyl carbamate]. Cell Mol Neurobiol 21: 555–573. 10.1023/A:1015131516649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu QS, Reale M, Kamal MA, Holloway HW, Luo W, Sambamurti K, Ray B, Lahiri DK, Rogers JT, Greig NH. 2013. Synthesis of the Alzheimer drug Posiphen into its primary metabolic products (+)-N1-norPosiphen, (+)-N8-norPosiphen and (+)-N1, N8-bisnorPosiphen, their inhibition of amyloid precursor protein, alpha-Synuclein synthesis, interleukin-1beta release, and cholinergic action. Antiinflamm Antiallergy Agents Med Chem 12: 117–128. 10.2174/1871523011312020003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawia NH, Basha MR. 2005. Environmental risk factors and the developmental basis for Alzheimer's disease. Rev Neurosci 16: 325–337. 10.1515/REVNEURO.2005.16.4.325 [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang W, Tsuji Y, Torti SV, Torti FM. 2008. Post-transcriptional modulation of iron homeostasis during p53-dependent growth arrest. J Biol Chem 283: 33911–33918. 10.1074/jbc.M806432200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Chen L, Zhao Q, Du X, Bi M, Li Y, Jiao Q, Jiang H. 2020a. Ferroptosis was more initial in cell death caused by iron overload and its underlying mechanism in Parkinson's disease. Free Radic Biol Med 152: 227–234. 10.1016/j.freeradbiomed.2020.03.015 [DOI] [PubMed] [Google Scholar]
- Zhang P, Park HJ, Zhang J, Junn E, Andrews RJ, Velagapudi SP, Abegg D, Vishnu K, Costales MG, Childs-Disney JL, et al. 2020b. Translation of the intrinsically disordered protein alpha-synuclein is inhibited by a small molecule targeting its structured mRNA. Proc Natl Acad Sci 117: 1457–1467. 10.1073/pnas.1905057117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano JH. 1999. Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res 833: 125–132. 10.1016/S0006-8993(99)01558-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZD, Tan EK. 2017. Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol Neurodegener 12: 75 10.1186/s13024-017-0218-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Chen Y, Fan G, Feng C, Du G, Zhu G, Li Y, Jiao H, Guan L, Wang Z. 2014. Lead-induced iron overload and attenuated effects of ferroportin 1 overexpression in PC12 cells. Toxicol In Vitro 28: 1339–1348. 10.1016/j.tiv.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Zhu G, Fan G, Feng C, Li Y, Chen Y, Zhou F, Du G, Jiao H, Liu Z, Xiao X, et al. 2012. The effect of lead exposure on brain iron homeostasis and the expression of DMT1/FP1 in the brain in developing and aged rats. Toxicol Lett 216: 108–123. 10.1016/j.toxlet.2012.11.024 [DOI] [PubMed] [Google Scholar]
- Zhu G, Fan G, Feng C, Li Y, Chen Y, Zhou F, Du G, Jiao H, Liu Z, Xiao X, et al. 2013. The effect of lead exposure on brain iron homeostasis and the expression of DMT1/FP1 in the brain in developing and aged rats. Toxicol Lett 216: 108–123. 10.1016/j.toxlet.2012.11.024 [DOI] [PubMed] [Google Scholar]
- Zumbrennen-Bullough KB, Becker L, Garrett L, Hölter SM, Calzada-Wack J, Mossbrugger I, Quintanilla-Fend L, Racz I, Rathkolb B, Klopstock T, et al. 2014. Abnormal brain iron metabolism in Irp2 deficient mice is associated with mild neurological and behavioral impairments. PLoS One 9: e98072 10.1371/journal.pone.0098072 [DOI] [PMC free article] [PubMed] [Google Scholar]