Abstract
Older AML patients have low remission rates and poor survival outcomes with standard chemotherapy. Microtransplantation (MST) refers to infusion of allogeneic hematopoietic stem cells without substantial engraftment. MST has been shown to improve clinical outcomes compared with chemotherapy alone. This is the first trial reporting on broad correlative studies to define immunologic mechanisms of action of MST in older AML patients. Older patients with newly diagnosed AML were eligible for enrollment, receiving induction chemotherapy with cytarabine and idarubicin (7+3). MST was administered 24 hours later. Patients with CR were eligible for consolidation with high dose cytarabine and a second cycle of MST. Responses were evaluated according to standard criteria per NCCN. Immune correlative studies were performed. Sixteen patients were enrolled and received 7+3 and MST (median age 73 years). Nine (56%) had high-risk and seven (44%) had standard-risk cytogenetics. Ten episodes of CRS were observed. No cases of GVHD or treatment-related mortality were reported. EFS was 50% at 6 months and 19% at 1 year. OS was 63% at 6 months and 44% at 1 year. Donor microchimerism was not detected. Longitudinal changes were noted in NGS, TCR sequencing, and cytokine assays. Addition of MST to induction and consolidation chemotherapy was well tolerated in older AML patients. Inferior survival outcomes in our study may be attributed to a higher proportion of very elderly patients with high-risk features. Potential immunologic mechanisms of activity of MST include attenuation of inflammatory cytokines and emergence of tumor-specific T cell clones.
Introduction
Older patients with Acute myeloid leukemia (AML) have low rates of remission and poor survival outcomes with standard chemotherapeutic approaches compared to younger patients.1 This relates to a higher frequency of adverse cytogenetic features and driver mutations, antecedent hematologic malignancies and prior chemotherapy or radiotherapy exposure that confer a worse prognosis.2–4 Older patients are also more likely to have medical co-morbidities and physical or cognitive functional impairment that may complicate management.5,6 While allogeneic stem cell transplantation offers the possibility of cure, it may be associated with severe graft-versus-host disease (GVHD), infections, and high rates of treatment-related mortality, leading to underutilization of this modality in older AML patients.7 Overall, this population has substantial, unmet medical needs and warrants novel, safer treatment approaches for disease control.
Microtransplantation (MST) refers to infusion of allogeneic hematopoietic stem cells without substantial long-term engraftment.8,9 MST has long been reported to revert tolerogenic states and confer resistance to leukemia challenge in murine models.10,11 Clinical trials of MST in high-risk AML and lymphoma have utilized conditioning chemotherapy to clear malignant cells while preserving host immune function, reducing the chance for donor engraftment and resulting in encouraging responses.9 Recently, Guo et al. demonstrated that among older AML patients in China, infusion of G-CSF mobilized leukapheresis product (MST) from related donors after mitoxantrone-based induction and consolidation chemotherapy could significantly improve hematopoietic recovery and remission rates compared with standard therapy alone.8 Two-year disease free survival was also significantly improved with addition of MST (38.9% v. 10%). GVHD has been observed in approximately 1% of cases with this approach and partial or full donor chimerism in approximately 3% of cases, although microchimerism (less than 1% donor chimerism) may occur more commonly and persist for weeks to years.12 The mechanisms by which MST may lead to improvement in outcomes via immune responses have not been clearly elucidated and may involve transient donor T cell or NK cell alloreactivity; rejection of donor lymphocytes and concomitant cytokine release; and/or enhanced host cytotoxic T cell responses.13,14
MST after frontline and consolidation chemotherapy in AML may trigger autologous antileukemia immune activity with an otherwise favorable safety profile, making this an attractive option for treatment of older patients. Availability of mismatched, related donors as a source of hematopoietic stem cells further supports the feasibility of this approach. To extend the clinical findings of recent international studies, we performed a single-center, phase I study evaluating the safety and efficacy of MST after induction and consolidation chemotherapy in older AML patients. As compared with studies performed in China, which have utilized a variety of induction regimens, including mitoxantrone-based chemotherapy, we exclusively used an anthracycline-based induction chemotherapy regimen for patients. This reflected a more conventional treatment approach for AML in the Western Hemisphere. To our knowledge, this is the first clinical trial reporting on broad correlative studies to better define the immunologic mechanisms of action of MST in older patients with AML.
Methods
Patients and Donors
Patients ≥ 55 years with newly diagnosed AML and one or more adverse features or ≥ 65 years regardless of adverse features were eligible for enrollment in this IRB approved single center, phase I study at Duke University Medical Center (IRB# Pro00043247; Clinicaltrials.gov NCT02046122). Adverse features included therapy-related AML, antecedent MDS or MPN, FLT3-ITD positive status or high-risk cytogenetic features by the Medical Research Council / National Cancer Research Institute (MRC/NCRI) revised risk classification system.15 Patients ≥ 65 years were eligible for enrollment regardless of adverse features given worse outcomes in this group historically, even among those with favorable cytogenetic features.16 Eligible patients were also required to have an HLA 3–5/6 haploidentical donor who could safely undergo stem cell mobilization and apheresis. Patients with acute promyelocytic leukemia were excluded. Histologic, chromosomal, and immunophenotypic analyses were performed in all patients prior to treatment. Specific molecular assays (FLT3-ITD and NPM1 mutation status) were evaluated in all patients at baseline.
Patients and donors received clinical and laboratory screening consistent with Foundation for the Accreditation of Cellular Therapy (FACT) accreditation guidelines for cellular therapy. HLA matching was performed at -A, -B and -DRB1 alleles by high resolution PCR. Donors received G-CSF (8 mcg/kg twice daily) for hematopoietic stem cell mobilization beginning 4 days prior to start of apheresis through the end of collection. A collection target of 2–3 × 108 CD3+ cells/kg recipient body weight was specified to provide enough cells for at least two infusions as below. Cells were freshly infused after collection or cryopreserved and stored for future infusions.
The final study protocol was approved by the Institutional Review Board at Duke University Medical Center (Durham, NC). Written informed consent for enrollment was obtained from all patients and donors or their legal guardians.
Treatment Design
Study schema is depicted in Figure 1. Patients received induction chemotherapy (IND1) with cytarabine (100 mg/m2) on days 1–7 and idarubicin (12 mg/m2) on days 1–3. Infusion of G-CSF mobilized donor leukapheresis product (MST) was performed 24–48 hours after IND1 at a minimum of 1 × 108 CD3+ cells/kg, as previously described.8,12 Patients who did not achieve complete remission with induction therapy were eligible to receive re-induction (IND2) with 7+3 and a second cycle of MST. Patients who achieved a CR with induction therapy received 2 cycles of consolidation with high dose cytarabine (HiDAC) and a final cycle of MST approximately 24–48 hours after the end of the second consolidation. This approach was selected given the possibility of insufficient cell numbers for multiple infusions and prioritizing MST after the second cycle of consolidation over the first cycle of consolidation. No GVHD prophylaxis or other maintenance therapy was administered, consistent with previous approaches.12 –The Duke patients in this pilot study did not receive G-CSF through neutrophil engraftment per our institutional practices. Prophylactic antibiotics, antiviral and antifungal agents were administered beginning after MST in the induction period. Diphenhydramine and/or hydrocortisone (n=14, 88%) were given as anti-inflammatory premedication prior to MST. Subjects who developed signs of CRS also received acetaminophen (n=5, 31%). All subjects responded and none required additional interventions with systemic steroids or tocilizumab
FIGURE 1.
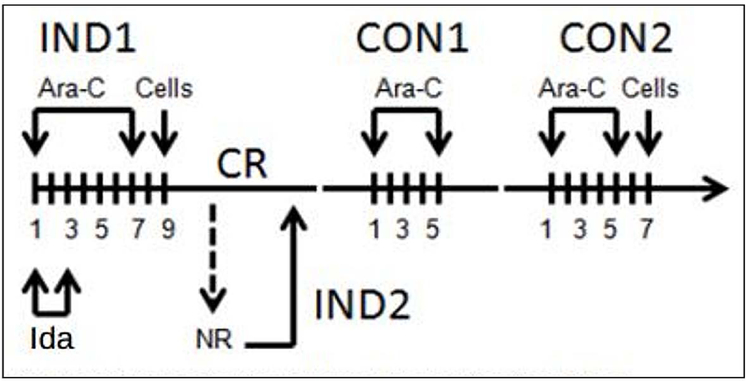
Study schema. Patients received conventional induction chemotherapy (IND1) with cytarabine (Ara–C) and idarubicin (Ida) followed by cell infusion (microtransplantation [MST]) 24–48 hours thereafter. Patients achieving a complete remission (CR) received up to 2 cycles of consolidation (CON1 and CON2) with Ara–C followed by cell infusion. Those patients not achieving a CR with IND1 could receive re–induction with the same regimen (IND2) followed by cell infusion
Safety
Patients were evaluated daily in the induction period through count recovery and daily during consolidation therapy. Clinical and laboratory evaluations were performed at serial timepoints thereafter for up to 2 years. Toxicities were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Engraftment was defined as ≥ 5% donor chimerism at count recovery. Unacceptable toxicities were defined as grade III GVHD of the liver or gut, grade IV GVHD of the skin, or any other grade IV toxicity attributed to MST lasting ≥ 7 days.
Response Criteria and Survival Measures
Responses were evaluated according to standard criteria defined by the National Comprehensive Cancer Network Guidelines for AML (www.nccn.org). Complete response was defined as recovery of trilineage hematopoiesis, with absolute neutrophil count (ANC) ≥ 1000 cells/μL, platelets ≥ 100,000/μl, normalization of marrow blasts (≤ 5%) and no evidence of extramedullary disease. Relapse was defined as reappearance of leukemic blasts in the peripheral blood, ≥ 5% marrow blasts without other cause, or evidence of extramedullary leukemia after a prior complete response.
Definitions of count recovery were consistent with those previously utilized. Neutrophil recovery was defined as the first of three consecutive days where the ANC was ≥ 500 cells/μl following induction chemotherapy and MST. Platelet recovery was defined as first of three consecutive days where platelets ≥ 30,000/μl after induction chemotherapy and MST.
Event-free survival (EFS) was defined as time from initial diagnosis until treatment failure after induction chemotherapy, leukemia relapse, or death from any cause. Overall survival (OS) was defined as time from initial diagnosis until death from any cause.
Targeted Next-Generation Sequencing
Amplicon based targeted DNA sequencing of gene regions commonly mutated in myeloid malignancies was performed. Pre-treatment bone marrow samples (day 0) were evaluated using the ThunderBolts™ Myeloid Panel (20–07218, RainDance Technologies) with 100 ng gDNA. Sequencing was performed using MiSeq V3 paired 300bp sequencing and human genome build v37 was used for alignment and filtering (NextGENe version 2.4.2.1, SoftGenetics, PA). Post-treatment bone marrow samples (day 30) were evaluated using a high-sensitivity NGS myeloid panel (AB0108, Myeloid VariantPlex, ArcherDX) using 50ng DNA and HiSeq 2500 paired 150bp sequencing. Data was analyzed using Archer Analysis version 5.1.3 software.
Peripheral Blood Mononuclear Cell (PBMC) Isolation and Storage
Peripheral blood was collected for immune correlative assays at selected time points (baseline, count recovery after induction chemotherapy, 8 weeks after consolidation therapy, and 3 months after consolidation therapy). PBMCs were isolated using standard density centrifugation techniques and cryopreserved until batch processing (Duke University, Durham, NC). PBMCs were thawed and viability was assessed prior to subsequent correlative assays.
Microchimerism
Genomic DNA (gDNA) was isolated from PBMCs and real time HLA-polymorphism specific quantitative PCR (qPCR) assay was performed to evaluate donor microchimerism (Fred Hutchinson Cancer Research Center, Seattle, WA). This assay was validated at a sensitivity of 1/100,000 and targeted a non-shared HLA-polymorphism between donor-recipient pairs.17 We were unable to get sufficient aspirate from bone marrow, therefore, the microchimerism assays were checked only in peripheral blood.
Lymphocyte Immunophenotypic Profiling
Multiparameter flow cytometry was performed using BD SORP Fortessa analyzer (Duke University, Durham, NC). T cell markers evaluated for subset profiling include CD3, CD4, CD8, CD25, and FOXP3. Surface markers of immune exhaustion that were evaluated include PD-1, PD-L1, PD-L2, LAG3, CTLA-4, CD28, CD40, TIM3, and CD278. Samples were immediately acquired after PBMCs were stained for surface, intracellular, and intranuclear markers as previously described.18
T Cell Receptor Sequencing
Genomic DNA was isolated from PBMCs at the RBM Immunology Unit at Duke University. Survey level sequencing of T cell receptor (TCR) genes was performed to evaluate T cell repertoire as previously described (Stanford University, Palo Alto, CA).19 Genomic DNA was amplified by real-time PCR with a set of primers targeting TCR V and J beta genes, collectively amplifying the CDR3 region of TCR beta. After cluster generation by bridge PCR, 200bp paired-end sequencing reactions were performed using MiSeq and HiSeq2000 sequencers. TRBV, TRBJ and TRBV-TRBJ usage frequencies were reported after removing background of low abundance clones (normalized to < 0.1% frequency of the most abundant clone’s frequency).
Cytokine Profiling
Plasma concentrations of candidate, inflammatory cytokines and chemokines were measured using the Meso Scale Diagnostic (MSD) system (Rockville, MD).20–22 V-PLEX Human Biomarker 54-Plex Multiplex Cytokine Plates (MSD, Cat#K15248D-1) were used to quantify the following markers: CRP, Eotaxin, Eotaxin-3, FGF (basic), GM-CSF, ICAM-1, IFN-γ, IL-10, IL-12/IL-23p40, IL-12p70, IL-13, IL-15, IL-16, IL-17A, IL-17A/F, IL-17B, IL-17C, IL-17D, IL-1RA, IL-1α, IL-1β, IL-2, IL-21, IL-22, IL-23, IL-27, IL-3, IL-31, IL-4, IL-5, IL-6, IL-7, IL-8, IL-8 (HA), IL-9, IP-10, MCP-1, MCP-4, MDC, MIP-1α, MIP-1β, MIP-3α, PlGF, SAA, TARC, Tie-2, TNF-α, TNF-β, TSLP, VCAM-1, VEGF-A, VEGF-C, VEGF-D, and VEGFR-1/Flt-1. Testing was performed at Duke University (Durham, NC).
Comprehensive Geriatric Assessment
Patients completed a comprehensive geriatric assessment previously utilized in CALGB 360401 prior to initiating chemotherapy.23 The assessment includes a healthcare professional questionnaire and self-administered patient questionnaires evaluating multiple domains, including functional status (Karnofsky Performance Status) and cognition (Blessed Orientation-Memory-Concentration Test). The Timed Up and Go Test (TUG) was used to assess mobility and fall risk.
Statistics
Survival curves were generated using the Kaplan-Meier method. Quantifiable measures from the comprehensive geriatric assessment (CALGB 360401) were compared before and after microtransplantation using the Student’s t test. Lymphocyte subset counts were also compared across timepoints using the Student’s t test. TCR oligoclonality was evaluated as the abundance of the top 1 and 5 sequences within the overall repertoire and was compared across timepoints using one-way ANOVA. GraphPad Prism Version 7.00 (Windows, GraphPad Software, La Jolla, California, USA) was used for the statistical analyses described.
All enrolled patients were included in the analysis of 54-plex immune/inflammatory response biomarkers (cytokines and chemokines). The Generalized Estimating Equations method was used to evaluate longitudinal changes in pre-treatment (baseline) biomarkers and post-treatment biomarkers (at count recovery and 8 weeks after consolidation).24 Pair-wise comparison was performed using the Wilcoxon signed-rank test.25 The association between changes in the 54-plex panel biomarkers and clinical outcomes were evaluated using the Wald test with df=1. 26 Clinical outcomes included presence of cytokine release syndrome, event-free survival and overall survival. Biomarker expression levels were log2-transformed and analyzed as continuous measures and box plots were used to illustrate the variability of the markers over time. Source code was tracked using the mercurial source code management program. The knitr extension package was used to produce dynamic reports. Effect size was assessed using hazard ratios (HR) assuming proportional hazards. Multiple comparisons were addressed within a framework of control of False Discovery Rate (FDR) using the method by Storey. 27–29 All analyses were performed using the R Statistical Environment [R] and extension packages from CRAN and the Bioconductor project. 27,30,31
Results
Patient and Donor Characteristics
Seventeen patients were enrolled (Table 1) between 2014 and 2017 with a median follow-up time of 299 days among all patients (range 42–1523) and 492 days among those achieving a complete response with induction therapy (range: 257–1523). The median age was 73 years (range 61–78). Five patients (29%) had an antecedent hematologic disorder and one (6%) had therapy-related AML. Ten patients (59%) had high-risk cytogenetics as per Medical Research Council / National Cancer Research Institute (MRC/NCRI) classification. Seven patients (41%) had standard-risk cytogenetics, including 3 (18%) with favorable-risk molecular features (NPM1 positive). No patients had the FLT3-ITD mutation (0%). One enrolled patient who developed sepsis during induction chemotherapy did not receive MST, and was excluded from final analysis of safety and efficacy.
Table 1.
Baseline Characteristics
| Characteristic | n (%) or Median (Range) |
|---|---|
| Age | 73 (61–78) |
| Sex | |
| Male | 11 (65%) |
| Female | 6 (35%) |
| Race | |
| Caucasian | 15 (88%) |
| African-American | 2 (12%) |
| Type of AML | |
| De Novo | 10 (59%) |
| Therapy-Related | 1 (6%) |
| AML-MDS/MPN | 5 (29%) |
| Erythroid Leukemia | 1 (6%) |
| Cytogenetics* | |
| Favorable | 0 (0%) |
| Standard | 7 (41%) |
| Adverse | 10 (59%) |
| Mutations† | |
| FLT3-ITD | 0/17 (0%) |
| NPM1 | 3/17 (19%) |
| Bi-allelic CEBPA | 0/11 (0%) |
| HLA Matched Loci among Donor/Recipient Pairs‡,‖ | |
| 4/10 | 1/16 (6%) |
| 5/10 | 11/16 (69%) |
| 6/10 | 4/16 (25%) |
Cytogenetic risk defined by Medical Research Council / National Cancer Research Institute (MRC/NCRI) revised classification in AML15
CEBPA mutational profiling not performed or available for all patients; results indicate total positive as a proportion of total tested
Includes HLA Class I (-A, -B, -C) and Class II (-DRB1, -DQB1) alleles
HLA loci matching not shown for one patient who did not receive MST after induction chemotherapy; this patient had de novo AML and adverse cytogenetics
As studies have shown improved outcomes among allogeneic stem cell transplant recipients with the use of younger donors and to limit exposure to possible multi-parous female donors, 32–35 we preferentially selected donors in the following order: younger and male children of the patient; female children; male siblings; and female siblings. Given the narrow windows between AML diagnosis, study enrollment and initiation of therapy, however, donor availability was the key determinant for selection
All haploidentical donors were adult children with a median age of 43 years (range 26–52). Accordingly, HLA matching was observed in 5/10 major loci in 11 patient-donor pairs (69%) and in 6/10 major loci in 4 patient-donor pairs (25%). Patients received a median of 2 cycles of donor cell infusion (MST) throughout the study period (range: 1–3). A target dose of 1 × 108 CD3+ cells/kg recipient weight was infused with each cycle of MST. Median doses of other cell populations infused was 35.7 × 109 (range: 13.82 – 81.31) for total nucleated cells and 0.31 × 109 (range: 0.13 – 0.58) for CD34+ cells with each cycle of MST.
Adverse Events
Median times to neutrophil and platelet recovery from induction chemotherapy were 29 and 32 days, respectively. Severe infections, defined as microbiologic or radiographic evidence of disseminated infection and/or significant vital signs derangements, were observed in 5/16 (31%) of patients during the induction phase. These were identified either before or more than 1 week after MST and were presumed to be unrelated to donor cell infusion. Hypoxia and/or respiratory failure warranting supplemental oxygen or intubation were observed in 4/16 (25%) of patients. These were also temporally unrelated to MST and developed before or more than 1 week after donor cell infusion. One patient developed multi-organ system dysfunction in the days following MST necessitating intensive care but had subsequent recovery. This was not clearly linked to MST, however. No patients developed donor engraftment or GVHD, and no treatment-related mortality was observed.
A subset of patients was observed to have signs and symptoms consistent with cytokine release syndrome soon after donor cell infusion, including fevers, chills, rigors, flushing, hypotension and/or hypoxia. These episodes occurred despite the use of standard pre-medications, including corticosteroids. They also appeared inconsistent with early-onset GVHD given the absence of diarrhea, rash, or liver injury in patients. Altogether, 10 episodes were observed in 9 patients across 26 cycles of MST (38%), with 6 characterized by very early symptom onset after cell infusion (~30–60 minutes), and another 4 characterized by onset of new fever within 6–12 hours after cell infusion. Patients received supportive care with generally rapid improvement in symptoms, while those with severe neutropenia and fevers initiated or broadened antibiotic coverage.
Treatment Responses and Survival Measures
Sixteen patients received induction therapy with 7+3 and MST. Ten patients (63%) achieved a complete response after 7+3+MST. This includes 6 of 7 (86%) patients with standard risk cytogenetics, 5 of whom required one round of 7+3+MST and 1 of whom required a second round (IND2). This also includes four of nine (44%) patients with high-risk cytogenetics; all four achieved CR after one round of 7+3+MST. Of the five patients with high-risk cytogenetics that did not respond to the initial round, three received re-induction chemotherapy without response and two went to hospice. Ultimately, of the ten patients who achieved a CR, eight completed two cycles of consolidation while two stopped after the first cycle of consolidation due to medical co-morbidities (one with cardiomyopathy, the other with poor performance status).
Of the ten patients who achieved a CR, nine had relapses at a median of 9 months from diagnosis and 1 has maintained a durable remission following conventional allogeneic stem cell transplantation. Of these 9 patients, one relapsed 14 months after 7+3 and MST and received consolidative allogeneic stem cell transplant with subsequent remission. Another relapsed after 11 months but achieved long-lasting disease stabilization with hypomethylating agents and is receiving ongoing therapy on a separate clinical trial. A third patient relapsed after 6 months but achieved disease control for 2 years on hypomethylating agents before succumbing to her disease. Among all patients treated with 7+3 and MST, estimated event-free survival was 50% at 6 months and 19% at 1 year (Figure 2).
FIGURE 2.
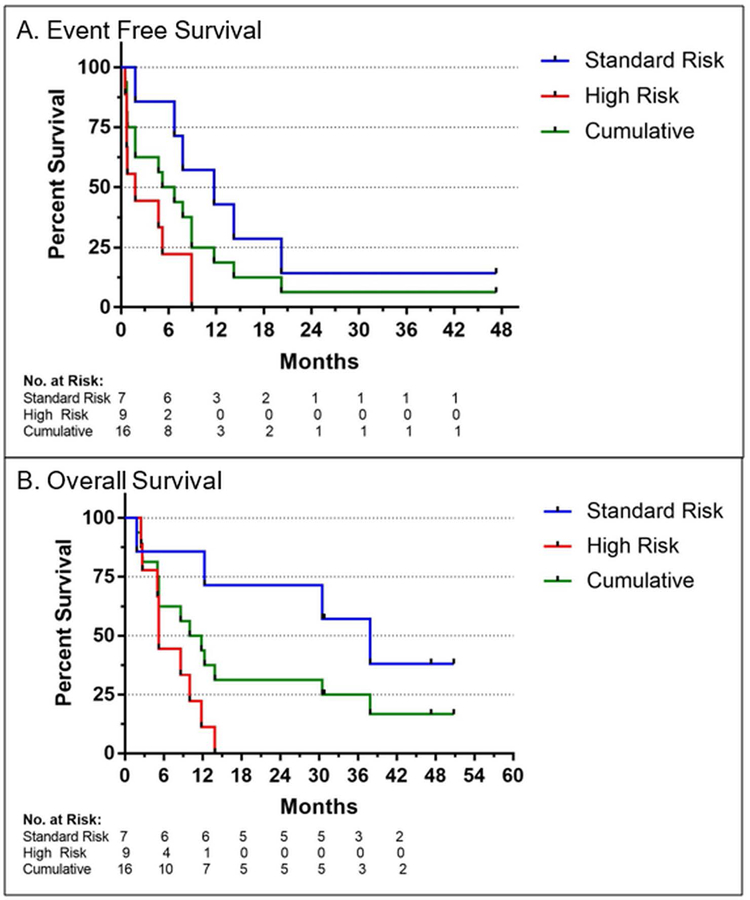
Event free survival and overall survival (OS) of older AML patients after induction and consolidation chemotherapy with microtransplantation (MST). Improved survival measures were observed in patients with standard vs high risk cytogenetic features as per MRC/NCRI classification
Patients with standard risk cytogenetics had significantly superior median overall survival relative to patients with high-risk cytogenetics (38 months v. 5 months). Estimated overall survival for the entire cohort at 6 months and 1 year was 63% and 44%, respectively. Three patients (19%) remain alive at 1195, 1634 and 1712 days, all of whom had standard risk cytogenetics at diagnosis.
Targeted Next-Generation Sequencing (NGS)
Myeloid NGS was performed in five patients prior to induction chemotherapy and MST and again at count recovery around day 30 (Figure 3 and Supplementary Table 1). Common variants identified included IDH1/2, NPM1, DNMT3A, TP53, EZH2, RUNX1, BCOR, CBL, CUX1, ETV6, JAK2, NRAS, and SRSF2. Three patients who had clearance of variants below the lower limit of detection or low-level persistence of variants at count recovery had a complete remission after induction chemotherapy and MST. Of these three, two had relapses at 5 and 18 months while one remains in remission. Two other patients had either stable, high-risk variant expression (TP53 mutation) or rising variant expression at count recovery. Both had refractory AML at count recovery.
FIGURE 3.
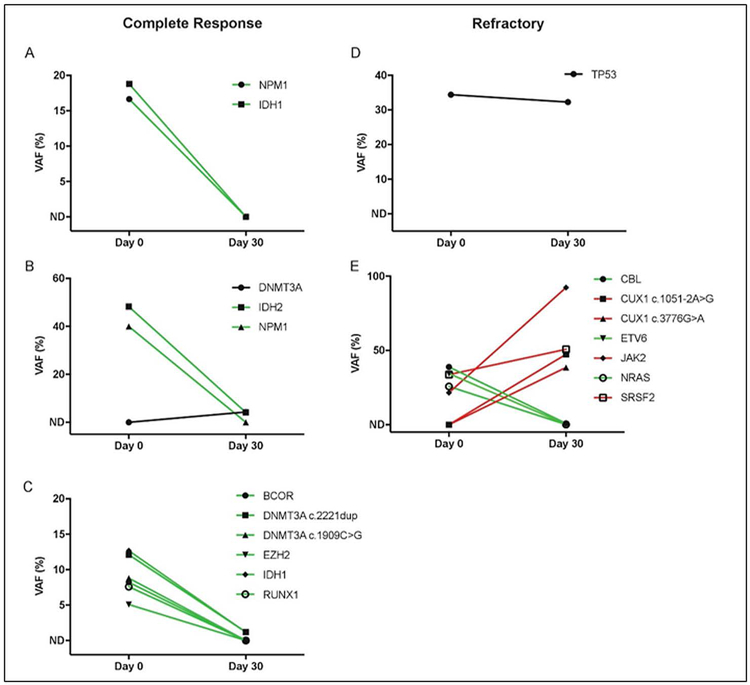
Variant allele frequencies (VAF) in bone marrow from baseline (Day 0) to count recovery (Day 30). Variants with allelic frequency less than 5% on Day 0 were excluded from analysis (non-detectable or ND). The lower-limit of detection of variants at Day 30 was ∼ 0.1% for those identified at baseline and ∼ 2.7% for those that were newly detected. Trendlines indicate variants increasing (red), decreasing (green), or remaining stable (black) through induction chemotherapy and MST. A–C, correspond with patients who developed a complete remission after induction therapy, while, D,E, correspond with patients who had refractory disease after induction therapy
Microchimerism
Microchimerism assays were performed in 10 patients across 22 timepoints, including at baseline, count recovery after induction chemotherapy, 8 weeks and 3 months after consolidation therapy. Although low cell counts were observed in many samples, gDNA quantity isolated from each sample was appropriate for assay performance. No evidence of peripheral microchimerism was detected at any timepoint at a sensitivity of approximately 1/100,000 donor alleles
Lymphocyte Immunophenotypic Profiling
Blood analytes were collected at the following timepoints to evaluate lymphocyte immunophenotypic profiling: before induction chemotherapy, count recovery after induction chemotherapy and MST, 8 weeks after completing consolidation therapy, 3 months after completing consolidation therapy, and before re-induction chemotherapy (when applicable). Peripheral blood mononuclear cells (PBMCs) were only sufficiently recovered and viable for these assays in approximately 20% of patient samples, however, likely owing to cryopreservation technique. Given limited samples, no statistically significant differences or correlation with clinical outcome were otherwise observed in T cell immuno-profiling following microtransplantation.
T Cell Receptor Sequencing
Peripheral T cell receptor (TCR) sequencing was performed in eight patients across serial timepoints and demonstrated varied clonal dynamics (Supplemental Figure 1). Patients were analyzed in two groups – those with favorable clinical responses (remission ≥ 6 months, n = 4) and unfavorable clinical responses (no remission or relapse within 6 months of therapy, n = 4). A trend towards increased abundance of the top five T cell clones was observed over time in favorable compared with unfavorable outcome patients. There was also an expansion in the abundance of the dominant T cell clone in two patients with favorable outcomes that was not observed in unfavorable outcome patients (Supplemental Figure 2).
Cytokine Profiling
With the assumption of an adjusted p-value (q-value) of 0.05 as the significance level, 13 genes had significant differences in longitudinal expression when comparing pre-treatment (baseline) and post-treatment samples (CR and 8-weeks) (Supplemental Figure 3). These differentially expressed biomarkers include: Eotaxin (CCL11, p = 1.0 × 10−5, q = 1.4 × 10−4), interleukin 8 (IL-8, p = 1.0 × 10−5, q = 1.4 × 10−4), serum amyloid A1 (SAA, p = 1.0 × 10−5, q = 1.4 × 10−4), tumor necrosis factor-beta (TNF-B, p = 1.0 × 10−5, q = 1.7 × 10−4), interleukin 31 (IL-31, p = 2.0 × 10−5, q = 1.8 × 10−4), interleukin 6 (IL-6, p = 7.0 × 10−5, q = 5.8 × 10−4), interleukin 12/interleukin 23-p40 subunit (IL-12/IL-23-p40, p = 8.0 × 10−5, q = 6.4 × 10−4), interleukin 13 (IL-13, p = 3.8 × 10−4, q = 2.5 × 10−3), thymus and activation-regulated chemokine (TARC/CCL17, p = 2.7 × 10−3, q = 1.6 × 10−2), macrophage-derived chemokine (MDC, p = 3.1 × 10−3, q = 1.6 × 10−2), interleukin 9 (IL-9, p = 3.4 × 10−3, q = 1.7 × 10−2), C-reactive protein (CRP, p = 5.2 × 10−3, q = 2.3 × 10−2), and monocyte chemotactic protein 1 (MCP1, p = 5.7 × 10−3, q = 2.3 × 10−2).
Correlations between baseline values and changes in 54-plex panel biomarkers with clinical outcomes (CRS, EFS, and OS) were also evaluated. Given small numbers, no statistically significant differences were observed using adjusted p-values. An association was observed between EFS and baseline values of two biomarkers: vascular endothelial growth factor D (VEGFD, HR= 0.25, 95% CI: 0.07 – 0.91) and interferon gamma (IFNG, HR= 0.67, 95% CI: 0.46 – 0.97). Associations also were observed between EFS and changes in three biomarkers from baseline to count recovery: Macrophage inflammatory protein-3 (MIP3A, HR= 3.2, 95% CI: 1.13 – 9.18, interleukin 2 (IL2, HR= 3.4, 95% CI: 1.09 – 10.497) and interleukin 1 alpha (IL1A, HR= 0.58, 95% CI: 0.35 – 0.98). Finally, associations were observed between EFS and changes in two biomarkers from baseline to 8 weeks after consolidation: MDC (HR= 0.31, 95% CI: 0.11 – 0.88) and IL1A (HR= 0.09, 95% CI: 0.008 – 0.876). No clear associations were observed in panel biomarkers and other clinical outcomes (CRS or OS).
Comprehensive Geriatric Assessment
All sixteen patients evaluable for response completed the comprehensive geriatric assessment prior to induction therapy. At baseline, patients had a median Karnofsky Performance Status (KPS) of 80 (range: 70–100), corresponding to “normal activity with effort, some signs or symptoms of disease.” Thirteen patients completed Timed “Up and Go” (TUG) with a median duration of 11 seconds (range: 6–34 seconds), slightly prolonged compared with reference ranges for healthy, age-matched adults.36 Five of these thirteen patients (38%) had TUG ≥ 12 seconds, corresponding to an increased risk of falls. The median Blessed Orientation-Memory-Concentration (BOMC) Test score among patients at baseline was 4 (range 0–10). Of note, three of sixteen patients (19%) had BOMC score of 10, suggesting underlying cognitive impairment.
Eight patients also completed the comprehensive geriatric assessment following consolidation therapy. No significant difference was observed in KPS, TUG, or BOMC scores between assessments at baseline and following consolidation therapy in these patients.
Discussion
Older patients with AML warrant novel treatment approaches with tolerable side effect profiles given high rates of medical co-morbidities and cognitive and functional impairment in this population. In this single-center, phase I study, we sought to characterize the safety of conventional induction and consolidation chemotherapy with microtransplantation (MST) in older patients with newly-diagnosed AML. MST is a unique clinical strategy that refers to the infusion of HLA-mismatched, haploidentical, G-CSF mobilized hematopoietic stem cells, T and NK cells after disease-directed therapy. Unlike conventional allogeneic hematopoietic stem cell transplantation, the goal of MST is for the early rejection of donor cells to stimulate and enhance a host-derived immune activity that improves anti-leukemia effects. In this study, the addition of MST to induction and consolidation chemotherapy was well-tolerated and without signals of major toxicity attributed to donor cell infusion. Adverse events included expected cytopenias and infection, attributed to the underlying disease process and conventional cytotoxic chemotherapy. No cases of donor engraftment or GVHD were observed in this study.
The median age of patients enrolled in the study was 73 years and approximately 60% had adverse cytogenetic features, indicating a population at high risk of poor response, relapse and death. The estimated median leukemia-free survival for the overall cohort was 5 months and overall survival was 10 months. Patients with adverse cytogenetic features had worse outcomes, with median overall survival of 5 months. This compares with historic cohorts of older AML patients > 65 receiving intensive therapy in retrospective and prospective series, where median overall survival durations of 6–10 months have been reported.1,6,37–39 Multicenter studies conducted in China have demonstrated the efficacy of chemotherapy and MST in older AML patients across age subgroups.8,12 Importantly, however, very elderly patients in these studies (> 75 years of age) had a median overall survival of 10 months, significantly worse when compared to patients < 75 years of age.12 This provides some rationale for inferior survival outcomes in our current study and suggests that the very elderly (> 75 years of age) may benefit from alternative approaches. Strategies to augment the efficacy of MST include alternative dosing schemes for haploidentical cells, modifying the cellular composition of the donor infusion, combination with novel agents or immune checkpoint inhibitors.
No evidence of microchimerism – defined as 0.001% to 1% peripheral donor chimerism in our study – was observed across serial timepoints. This is in contrast to other studies of MST, where donor chimerism may be transiently observed for days without sustained engraftment and microchimerism may persist from days to years.12,13 In one study evaluating induction and consolidation chemotherapy with MST in older AML patients, donor microchimerism was observed in 30.8% of patients, emerging at day 2 and peaking in 7–10 days.12 One potential explanation for the absence of microchimerism in our study may be the early clearance of this feature before count recovery, when our first post-treatment assays were performed. Many samples tested also had low cell quantities, making the detection of microchimerism less likely in these instances. Other than differences in the type of induction chemotherapy regimen used, treatment strategies in our study and multicenter studies conducted in China otherwise appeared highly concordant. This includes the timing and target dose of CD3+ donor T cells per infusion. Other unrecognized differences between the study populations may account for the differential detection of microchimerism between these cohorts, however.
AML features a dysregulated network of cytokines and chemokines which drive disease progression and individually correlate with clinical outcomes.40,41 In particular, pro-inflammatory cytokines like interleukin 1 beta (IL-1β), tumour necrosis factor alpha (TNF-α) and IL-6 have been associated with biologically aggressive disease whereas anti-inflammatory cytokines like interleukin 10 (IL-10) and transforming growth factor beta (TGF-β) have been associated with improved outcomes.41 In our study, patients were observed to have longitudinal changes in individual cytokine profiles before and after induction and consolidation chemotherapy and MST. In particular, several pro-inflammatory markers were observed to significantly decrease over time, including IL-6, IL-8, and CRP. Increased expression of these individual cytokines have been associated with adverse outcomes in AML.42,43 We also observed that increasing levels of IL-1α and decreasing levels of IL-2 following MST were associated with EFS, although this did not meet statistical significance. In contrast to our findings, IL-1 has previously been associated with myeloid progenitor expansion and disease progression in AML, and IL-2 has been associated with remission and prolonged survival in AML.40,44 Our findings suggest that MST may uniquely modify cytokine and chemokine signaling pathways and the adaptive immune repertoire in AML. Additional studies are warranted to define whether selected cytokines may serve as predictive biomarkers in this context.
The cytokine release syndrome (CRS) has been reported in previous studies of MST. In one study of patients receiving low-dose TBI and MST, a syndrome of CRS was observed consisting of fever, diarrhea, rash, and transaminitis in all 30 patients treated at cell dose levels of 1–2 × 108 cells / recipient kg.13 In our study, CRS was observed in approximately 40% of cases, typically mild, and resolving within 1–2 days. Most patients had fever as a manifestation of CRS, although given concomitant severe neutropenia and hospitalization, underlying infections or other etiologies may not be excluded. Overall, 5/8 patients (63%) with CRS achieved a complete response after induction therapy. Given small numbers, no significant differences were observed in measured cytokine levels between patients with or without CRS after MST.
Our study suggests that addition of MST to conventional, anthracycline-based induction chemotherapy in the West is well-tolerated by older AML patients, with no cases of engraftment or GVHD observed. While this pilot study does not demonstrate improved survival compared to historic controls receiving conventional chemotherapy, it does confirm high tolerability of this treatment strategy and low rates of severe toxicity. This is an important benchmark as older AML patients frequently experience functional compromise in multiple domains, including subsets of patients in our study who had an increased risk of falls and impaired cognition at baseline. High rates of CR (63%) were also observed in our overall study population, suggesting MST could serve as a bridge to conventional allogeneic stem cell transplantation in selected patients. MST may also offer other advantages as a treatment modality, including broad availability of haploidentical donors and cost-effectiveness. Finally, our study provides some insight into potential immune mechanisms of response following MST, including the emergence of T cell clones that may confer tumor-specific immunity in selected patients. This has been previously observed in older AML patients after MST and warrants further investigation in future studies.45 While no clear trends were observed in blood-based lymphocyte profiling assays, poor PBMC viability after thawing complicated the interpretation of our findings. Given the likely role T and NK cells play in antileukemia effects of MST, careful execution of these assays in the future may yield important insights and provide a rationale for combination approaches. Important limitations of our study include the small patient numbers and absence of a control arm. An international, multicenter, phase III study of induction and consolidation chemotherapy with MST in older AML patients is currently ongoing that would further address the efficacy of this approach.
Supplementary Material
Acknowledgments
This research was supported, in part, by the Intramural Research Program of the National Heart, Lung, and Blood Institute of National Institutes of Health.
Abbreviations:
- AML
Acute myeloid leukemia
- ANC
Absolute neutrophil count
- CRS
The cytokine release syndrome
- EFS
Event-free survival
- HR
Hazard ratios
- HiDAC
High dose cytarabine
- GVHD
Graft vs. Host Disease
- MST
Microtransplantation
- OS
Overall survival
- PBMC
Peripheral Blood Mononuclear Cell
- TCR
T cell receptor
Footnotes
Conflict of Interest Statement
CSH has received laboratory research funding from Merck and Sellas.
References
- 1.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97(12):1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Figueroa ME, Skrabanek L, Li Y, et al. MDS and secondary AML display unique patterns and abundance of aberrant DNA methylation. Blood. 2009;114(16):3448–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma - a Swedish population-based study. BMC Cancer. 2015;15:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao AV, Valk PJ, Metzeler KH, et al. Age-specific differences in oncogenic pathway dysregulation and anthracycline sensitivity in patients with acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(33):5580–5586. [DOI] [PubMed] [Google Scholar]
- 5.Buchner T, Berdel WE, Haferlach C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol. 2009;27(1):61–69. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106(5):1090–1098. [DOI] [PubMed] [Google Scholar]
- 7.Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015;94(7):1127–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo M, Hu KX, Yu CL, et al. Infusion of HLA-mismatched peripheral blood stem cells improves the outcome of chemotherapy for acute myeloid leukemia in elderly patients. Blood. 2011;117(3):936–941. [DOI] [PubMed] [Google Scholar]
- 9.Krakow EF, Ai HS, Shaffer B, Delisle JS, Hu KX, Sung AD. Do We Need Full Donor Chimerism? How Alloreactive Cell Therapies without Substantial Engraftment Might Treat Hematologic Cancers. Curr Drug Targets. 2017;18(3):281–295. [DOI] [PubMed] [Google Scholar]
- 10.Delorme EJ, Alexander P. Treatment of Primary Fibrosarcoma in the Rat with Immune Lymphocytes. Lancet. 1964;2(7351):117–120. [DOI] [PubMed] [Google Scholar]
- 11.Katz DH, Ellman L, Paul WE, Green I, Benacerraf B. Resistance of guinea pigs to leukemia following transfer of immunocompetent allogeneic lymphoid cells. Cancer Res. 1972;32(1):133–140. [PubMed] [Google Scholar]
- 12.Guo M, Chao NJ, Li JY, et al. HLA-Mismatched Microtransplant in Older Patients Newly Diagnosed With Acute Myeloid Leukemia: Results From the Microtransplantation Interest Group. JAMA Oncol. 2018;4(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colvin GA, Berz D, Ramanathan M, et al. Nonengraftment haploidentical cellular immunotherapy for refractory malignancies: tumor responses without chimerism. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15(4):421–431. [DOI] [PubMed] [Google Scholar]
- 14.Krakow EF, Bergeron J, Lachance S, Roy DC, Delisle JS. Harnessing the power of alloreactivity without triggering graft-versus-host disease: how non-engrafting alloreactive cellular therapy might change the landscape of acute myeloid leukemia treatment. Blood Rev. 2014;28(6):249–261. [DOI] [PubMed] [Google Scholar]
- 15.Grimwade D, Hills RK, Moorman AV, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. [DOI] [PubMed] [Google Scholar]
- 16.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert NC, Erickson TD, Yan Z, et al. Quantification of maternal microchimerism by HLA-specific real-time polymerase chain reaction: studies of healthy women and women with scleroderma. Arthritis and rheumatism. 2004;50(3):906–914. [DOI] [PubMed] [Google Scholar]
- 18.Murdoch DM, Staats JS, Weinhold KJ. OMIP-006: phenotypic subset analysis of human T regulatory cells via polychromatic flow cytometry. Cytometry A. 2012;81(4):281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer EH, Hsu AR, Liliental J, et al. A distinct evolution of the T-cell repertoire categorizes treatment refractory gastrointestinal acute graft-versus-host disease. Blood. 2013;121(24):4955–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nixon AB, Halabi S, Shterev I, et al. Identification of predictive biomarkers of overall survival (OS) in patients (pts) with advanced renal cell carcinoma (RCC) treated with interferon alpha (I) with or without bevacizumab (B): Results from CALGB 90206 (Alliance). Journal of Clinical Oncology. 2013;31(15_suppl):4520–4520.24220563 [Google Scholar]
- 21.Nixon AB, Pang H, Starr MD, et al. Prognostic and predictive blood-based biomarkers in patients with advanced pancreatic cancer: results from CALGB80303 (Alliance). Clin Cancer Res. 2013;19(24):6957–6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Starr MD, Brady JC, et al. Biomarker signatures correlate with clinical outcome in refractory metastatic colorectal cancer patients receiving bevacizumab and everolimus. Molecular cancer therapeutics. 2015;14(4):1048–1056. [DOI] [PubMed] [Google Scholar]
- 23.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29(10):1290–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M, Kong L, Li Z, Zhang L. Covariance estimators for generalized estimating equations (GEE) in longitudinal analysis with small samples. Statistics in medicine. 2016;35(10):1706–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitley E, Ball J. Statistics review 6: Nonparametric methods. Critical care (London, England). 2002;6(6):509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Samuelson FW, Gallas BD, Kang L, Sahiner B, Petrick N. On the assessment of the added value of new predictive biomarkers. BMC Med Res Methodol. 2013;13:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owzar K, Barry WT, Jung SH. Statistical Considerations for Analysis of Microarray Experiments. Cts-Clin Transl Sci. 2011;4(6):466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storey JD. A direct approach to false discovery rates. J Roy Stat Soc B. 2002;64:479–498. [Google Scholar]
- 29.Storey JD. False Discovery Rate In: Lovric M, ed. International Encyclopedia of Statistical Science. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011:504–508. [Google Scholar]
- 30.Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. In:2014. [Google Scholar]
- 31.Therneau TM. A Package for Survival Analysis in S. 2015.
- 32.Rezvani AR, Storer BE, Guthrie KA, et al. Impact of donor age on outcome after allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(1):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw BE, Logan BR, Spellman SR, et al. Development of an Unrelated Donor Selection Score Predictive of Survival after HCT: Donor Age Matters Most. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2018;24(5):1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bastida JM, Cabrero M, Lopez-Godino O, et al. Influence of donor age in allogeneic stem cell transplant outcome in acute myeloid leukemia and myelodisplastic syndrome. Leukemia Research. 2015;39(8):828–834. [DOI] [PubMed] [Google Scholar]
- 35.Vigorito AC, Campregher PV, Storer BE, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114(3):702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(2):64–68. [DOI] [PubMed] [Google Scholar]
- 37.Lowenberg B, Zittoun R, Kerkhofs H, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7(9):1268–1274. [DOI] [PubMed] [Google Scholar]
- 38.Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235–1248. [DOI] [PubMed] [Google Scholar]
- 39.Quintas-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood. 2012;120(24):4840–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kornblau SM, McCue D, Singh N, Chen W, Estrov Z, Coombes KR. Recurrent expression signatures of cytokines and chemokines are present and are independently prognostic in acute myelogenous leukemia and myelodysplasia. Blood. 2010;116(20):4251–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Binder S, Luciano M, Horejs-Hoeck J. The cytokine network in acute myeloid leukemia (AML): A focus on pro- and anti-inflammatory mediators. Cytokine Growth Factor Rev. 2018;43:8–15. [DOI] [PubMed] [Google Scholar]
- 42.Kupsa T, Vanek J, Zak P, Jebavy L, Horacek JM. Serum Levels of Cytokines and Cytokine Receptors Are Associated with Outcome in Newly Diagnosed AML Patients. Blood. 2016;128(22):5246–5246. [Google Scholar]
- 43.Artz AS, Logan B, Zhu X, et al. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica. 2016;101(11):1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carey A, Edwards DKt, Eide CA, et al. Identification of Interleukin-1 by Functional Screening as a Key Mediator of Cellular Expansion and Disease Progression in Acute Myeloid Leukemia. Cell Rep. 2017;18(13):3204–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo M, Hu KX, Liu GX, et al. HLA-Mismatched Stem-Cell Microtransplantation As Postremission Therapy for Acute Myeloid Leukemia: Long-Term Follow-Up. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(33):4084–4090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


