Truncating variants in the gene encoding titin (TTNtv) are the most commonly identified pathogenic variants in cross-sectional studies of patients with dilated cardiomyopathy or atrial fibrillation (1,2). In principle, gene sequencing to identify individuals who harbor a TTNtv prior to disease onset could enable early diagnosis or preventive therapy. In practice, the clinical importance of identifying a TTNtv in an asymptomatic individual with respect to relative and absolute risks of future cardiovascular disease is largely unknown.
We analyzed gene sequencing data in 45,346 UK Biobank participants without a prior diagnosis of congestive heart failure, atrial fibrillation, or coronary artery disease (3). Mean age at enrollment was 58 years, and 52% were female. Cardiac magnetic resonance imaging was available in 12,553 of these participants, invited as part of the study protocol without consideration of disease status.
Regression models for all analyses were adjusted for the cubic spline of age, sex, and genetic ancestry as quantified by the first five principal components. Estimates were similar in unadjusted analyses.
TTNtv were identified using a previously validated algorithm, restricted to exons present in >90% of cardiac-specific transcripts as previously recommended (1, 4). A TTNtv was identified in 183 of 45,346 (0.40%) of individuals in the overall cohort and 54 of 12,553 individuals (0.43%) in the subset of individuals with cardiac imaging available.
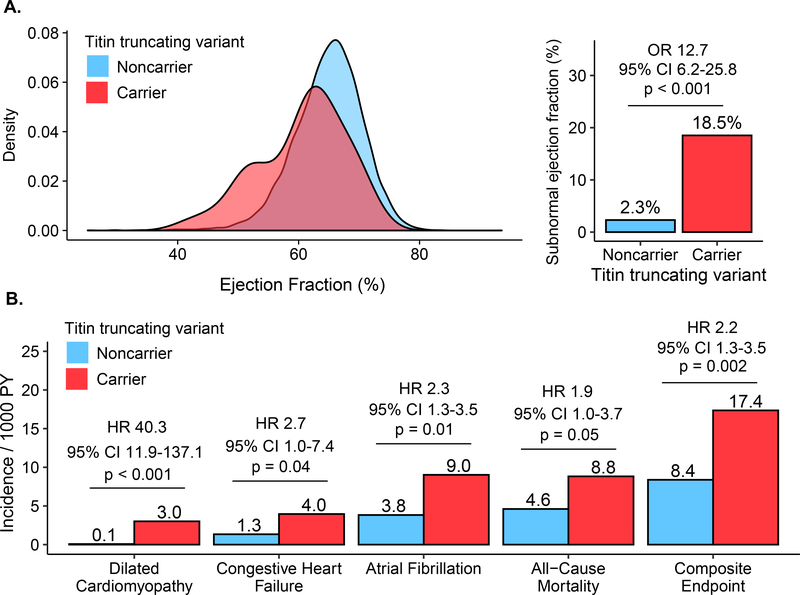
Within the subset of individuals with cardiac imaging available, TTNtv carriers had no significant difference in left ventricular end diastolic volume—mean volumes 139.6 mL and 138 mL for carriers and noncarriers respectively (adjusted difference 3.7mL; 95% CI −2.4 to 9.9mL; P= 0.23). However, consistent with impaired contractility, end-systolic volume was increased in carriers—mean volumes 56.4 and 49.3 mL for carriers and noncarriers respectively (adjusted difference 8.5mL; 95% CI 4.8 to 12.1mL; P< 0.001). Mean left ventricular ejection fraction was 60.5% in TTNtv carriers versus 64.9% in noncarriers (adjusted absolute difference −4.7%; 95% CI −3.3% to −6.1%; P< 0.001; Figure 1). A subnormal ejection fraction—defined as <52% for men and <54% for women—was present in 18.5% of TTNtv carriers versus 2.3% of noncarriers, adjusted odds ratio 12.7 (95% CI 6.2 to 25.8; P< 0.001). These observations confirm and extend those previously noted within hospital-based biobanks (5).
Figure 1: Left ventricular ejection fraction and incident cardiovascular disease in TTNtv carriers versus noncarriers.
(A) Shown is the distribution of left ventricular ejection fraction and prevalence of subnormal ejection fraction among 54 TTNtv carriers and 12,499 noncarriers. In a logistic regression model adjusted for the cubic spline of age, sex, and genetic ancestry, TTNtv carriers had 12.7-fold (95%CI 6.2 – 25.8; p< 0.001) increased risk of a subnormal ejection fraction. (B) Shown are the incidence rates per 1,000 person-years for the composite endpoint and each of its components. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated using a Cox regression model adjusted for the cubic spline of age, sex, and genetic ancestry.
We next determined the relationship of TTNtv carrier status with incident cardiovascular disease in all 45,346 individuals, using a composite primary endpoint of a physician diagnosis code of dilated cardiomyopathy, congestive heart failure, atrial fibrillation, and all-cause mortality. Over a median follow-up of 6.9 years, the composite endpoint occurred in 17 of 183 (9.3%) TTNtv carriers and 2,106 of 45,163 (4.6%) noncarriers, adjusted hazard ratio of 2.2 (95% CI 1.3 to 3.5; P= 0.002); Figure.
Our study has several limitations. First, outcomes were detected using hospital admission diagnosis codes rather than detailed endpoint adjudication. Second, participants were recruited between the ages of 40 and 69 years, raising the possibility of survivorship or ‘healthy volunteer’ bias. Third, >90% of the participants in our study were of European ancestry, limiting our ability to assess previously reported effect heterogeneity across ancestries (5).
In conclusion, 0.4% of asymptomatic adults harbor a TTNtv conferring substantially increased risk of subnormal ejection fraction and incident cardiovascular disease. However, despite several-fold increased relative risk, the majority of TTNtv carriers remained free of a new clinical diagnosis in prospective follow up. Prior studies suggest that superimposed clinical or environmental stressors—including chemotherapy, alcohol use and pregnancy—may unmask disease among those genetically predisposed by a TTNtv. Additional study is needed to further elucidate genetic and nongenetic determinants governing which TTNtv carriers will manifest clinical disease to better inform clinical management of asymptomatic individuals who harbor a TTNtv.
Acknowledgements
Our analysis of data from the UK Biobank was approved by the Partners HealthCare institutional review board (protocol 2013P001840) and was performed under UK Biobank application #7089.
Funding: Dr. Pirruccello is supported by the John S. LaDue Memorial Fellowship from Harvard Medical School. Dr. Aragam is supported by the American Heart Association Institute for Precision Cardiovascular Medicine under award number 17IFUNP33840012. Dr. Lubitz is supported by National Institutes of Health grant award number 1R01HL139731 and American Heart Association award number 18SFRN34250007. Dr. Ellinor is supported by the Fondation Leducq under award number 14CVD01, the National Institutes of Health under award numbers 1RO1HL092577, R01HL128914, and K24HL105780, and the American Heart Association under award number 18SFRN34110082. Dr. Kathiresan is supported by the Ofer and Shelly Nemirovsky Research Scholar Award from Massachusetts General Hospital and the National Human Genome Research Institute under award number 5UM1HG008895. Dr. Khera is supported by an institutional grant from the Broad Institute of MIT and Harvard (BroadIgnite), award numbers 1K08HG010155 and 5UM1HG008895 from the National Human Genome Research Institute, a Hassenfeld Scholar Award from Massachusetts General Hospital, and a sponsored research agreement from IBM Research.
Disclosures: Dr. Pirruccello has served as a consultant for Maze Therapeutics. Dr. Bick has served as a consultant for Maze Therapeutics. Dr. Lubitz receives sponsored research support from Bristol Myers Squibb / Pfizer, Bayer AG, and Boehringer Ingelheim, and has consulted for Bristol Myers Squibb / Pfizer and Bayer AG. Dr. Philippakis is a Venture Partner at GV, a subsidiary of Alphabet Corporation. Dr. Ellinor is supported by a grant from Bayer AG to the Broad Institute focused on the genetics and therapeutics of cardiovascular diseases, and has served on advisory boards or consulted for Bayer AG, Quest Diagnostics, and Novartis. Dr. Kathiresan is an employee of Verve Therapeutics, and holds equity in Verve Therapeutics, Maze Therapeutics, Catabasis, and San Therapeutics; he is a member of the scientific advisory boards for Regeneron Genetics Center and Corvidia Therapeutics, and he has served as a consultant for Acceleron, Eli Lilly, Novartis, Merck, Novo Nordisk, Novo Ventures, Ionis, Alnylam, Aegerion, Haug Partners, Noble Insights, Leerink Partners, Bayer Healthcare, Illumina, Color Genomics, MedGenome, Quest, and Medscape. Dr. Kathiresan also reports patents related to a method of identifying and treating a person having a predisposition to or afflicted with cardiometabolic disease (20180010185) and a genetics risk predictor (20190017119). Dr. Khera has served as a consultant or received honoraria from Color Genomics, Illumina, Novartis, Maze Therapeutics, and Navitor Pharmaceuticals, and has received grant support from the Novartis Institute for Biomedical Research. Dr. Khera also reports a patent related to a genetic risk predictor (20190017119). The remaining authors have nothing to disclose.
Abbreviation
- TTNtv
titin truncating variants
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roberts AM, Ware JS, Herman DS, et al. Integrated allelic, transcriptional, and phenomic dissection of the cardiac effects of titin truncations in health and disease. Sci. Transl. Med. 2015;7:270ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi SH, Weng L-C, Roselli C, et al. Association Between Titin Loss-of-Function Variants and Early-Onset Atrial Fibrillation. JAMA 2018;320:2354–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hout CVV, Tachmazidou I, Backman JD, et al. Whole exome sequencing and characterization of coding variation in 49,960 individuals in the UK Biobank. bioRxiv 2019:572347. [Google Scholar]
- 4.Karczewski KJ, Francioli LC, Tiao G, et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv 2019:531210. [Google Scholar]
- 5.Haggerty CM, Damrauer SM, Levin MG, et al. Genomics-First Evaluation of Heart Disease Associated With Titin-Truncating Variants. Circulation 2019;140:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]