 Pancreatic cancer (PC), with a 5-year survival of <7%, is one of the most fatal of all human cancers. There is an urgent need to develop more effective therapies to move beyond the current dire condition and paucity of PC treatment options.
Pancreatic cancer (PC), with a 5-year survival of <7%, is one of the most fatal of all human cancers. There is an urgent need to develop more effective therapies to move beyond the current dire condition and paucity of PC treatment options.
Abstract
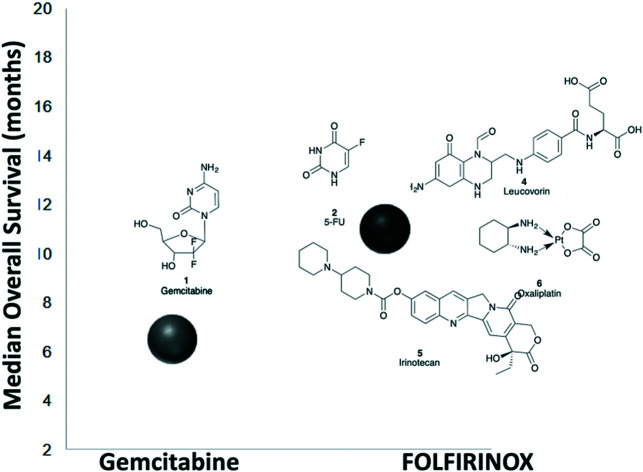
Pancreatic cancer (PC), with a 5 year survival of <7%, is one of the most fatal of all human cancers. The highly aggressive and metastatic character of this disease poses a challenge that current therapies are failing, despite significant efforts, to meet. This review examines the current status of the 35 small molecule inhibitors targeting pancreatic cancer in clinical trials and the >50 currently under investigation. These compounds inhibit biological targets spanning protein kinases, STAT3, BET, HDACs and Bcl-2 family proteins. Unsurprisingly, protein kinase inhibitors are overrepresented. Some trials show promise; a phase I combination trial of vorinostat 11 and capecitabine 17 gave a median overall survival (MoS) of 13 months and a phase II study of pazopanib 15 showed a MoS of 25 months. The current standard of care for metastatic pancreatic ductal adenocarcinoma, fluorouracil/folic acid (5-FU, Adrucil®), and gemcitabine (GEMZAR®) afforded a MoS of 23 and 23.6 months (EPAC-3 study), respectively. In patients who can tolerate the FOLFIRINOX regime, this is becoming the standard of treatment with a MoS of 11.1 months. Clinical study progress has been slow with limited improvement in patient survival relative to gemcitabine 1 monotherapy. A major cause of low PC survival is the late stage of diagnosis, occurring in patients who consider typical early stage warning signs of aches and pains normal. The selection of patients with specific disease phenotypes, the use of improved efficient drug combinations, the identification of biomarkers to specific cancer subtypes and more effective designs of investigation have improved outcomes. To move beyond the current dire condition and paucity of PC treatment options, determination of the best regimes and new treatment options is a challenge that must be met. The reasons for poor PC prognosis have remained largely unchanged for 20 years. This is arguably a consequence of significant changes in the drug discovery landscape, and the increasing pressure on academia to deliver short term ‘media’ friendly short-term news ‘bites’. PC research sits at a pivotal point. Perhaps the greatest challenge is enacting a culture change that recognises that major breakthroughs are a result of blue sky, truly innovative and curiosity driven research.
Introduction
In western countries, pancreatic cancer (PC) is the fourth leading cause of cancer death and is predicted to become the second most common cause of cancer related mortality in the USA by 2030.1,2 The prognosis for PC patients is dire, with a 5 year overall survival of <7%.3 The rate of PC is rising globally with more than 330 000 new cases diagnosed every year and a devastatingly comparable mortality rate.4
Histopathologically, PC exists as two main tumour types: exocrine, which is derived from the cells of the exocrine pancreas and accounts for more than 95% of all PC diagnoses, and endocrine, which arises from hormone producing endocrine cells. Pancreatic ductal adenocarcinoma (PDAC) accounts for about 90% of all exocrine tumours.6 More recently, PC has been sub-classified as squamous, pancreatic progenitor, immunogenic and aberrantly differentiated endocrine exocrine (ADEX) by genomic analyses.5
Surgical resection remains the only potentially curative treatment option for PC patients. However, less than 20% of patients are in a suitable disease state for this procedure because of either late diagnosis, tumour resistance to current drugs or no effective early detection biomarkers.7 Metastases are the primary cause of death for most cancer patients. Approximately 30% of patients succumb to metastatic PC within 12 months of surgical resection, even if clear resection margins are achieved during surgery.8,9 While adjuvant chemo- and radiotherapy have been used, this has resulted in no enhancements in patient survival in the past two decades.10,11 The highly aggressive, metastatic and heterogeneous character of this disease poses a critical challenge for the improvement of patient outcomes.
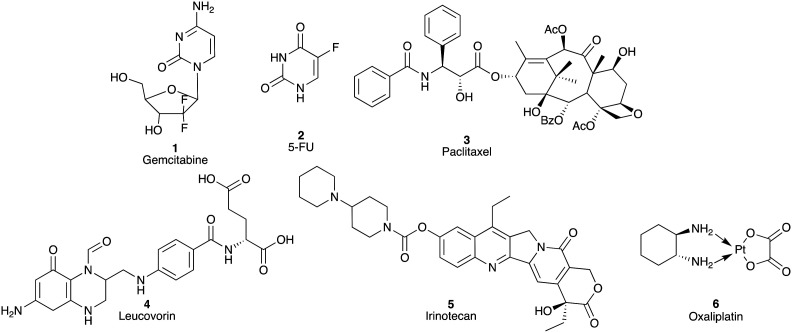
The 1997 introduction of gemcitabine (1; GEMZAR®) represented a significant enhancement in the treatment of PC over fluorouracil (2; 5-FU, Adrucil®; a 1957 introduction) with an increase in median overall survival (MoS) from 4.4 to 5.6 months (Fig. 1).1,12 The most recent update on the ESPAC-3 trial, post-surgical resection, details a >20 month MoS with 5-FU/folic acid adjuvant therapy.13,14 Subsequent use of combination approaches have enhanced therapeutic outcomes with nab-paclitaxel (3)/gemcitabine (1) and FOLFIRINOX (5-fluorouracil (2), leucovorin (4), irinotecan (5) and oxaliplatin (6)) with a MoS of 8.5 and 11.1 months, respectively.15,16
Fig. 1. Chemical structures of gemcitabine (1), 5-fluorouracil (5-FU; 2), paclitaxel (3), leucovorin (4), irinotecan (5) and oxaliplatin (6).
However, the median overall survival is still considered as less than a year (no surgical resection), and the combination chemotherapy regimens based on the gemcitabine (1) platform also failed to show any substantial survival advantage compared with single agent gemcitabine (1).17 The development of targeted therapies offers a new avenue to develop potentially more effective strategies.2,18 Herein, the development of targeted approaches using small molecules to treat pancreatic cancer spanning current clinical trial compounds and exploratory compounds is presented and discussed.
Small molecule inhibitors of PC in clinical trials
The majority of clinical trials have failed to demonstrate a clinically meaningful survival benefit, explainable via two key phenomena indicative of PDAC: intertumoral heterogeneity and the desmoplastic tumour microenvironment. Landmark genomic sequencing studies have revealed that apart from known high frequency mutations in pancreatic tumours,5,19–21 such as KRAS, TP53, CDKN2A, and SMAD4, most occur at a frequency of less than 5%.19,21,22 Further, there are no therapeutic agents clinically available that target driver mutations occurring at >20% prevalence, thereby hampering clinical trial efficiency as the responsive phenotype of a therapeutic regimen would fall below the detection threshold of most conventional randomized-controlled trial designs.23
This deeper understanding of the molecular pathology of PDAC has led to the identification of multiple additional therapeutic targets, such as those within the tumour microenvironment, in particular the desmoplastic stroma. The desmoplastic stroma is a component of the potent immunosuppressive tumour microenvironment in which many distinct cells appear to collaborate toward tumour growth and metastasis.24,25 Here, neoplastic, immune, and stromal cells reside in a dense extracellular matrix (ECM), contributing to the crosstalk between the tumour microenvironment and the epithelial tumour cells to promote growth, invasion and metastasis. Understanding the intricate pathways that are critical in these collaborative interactions is key to the design of innovative therapeutic approaches. Hyaluronan (HA) is a large linear glycosaminoglycan abundant within the stroma, which is a poor prognostic indicator and has been the target of recent therapeutic activity.26 Poor tumour vascularity and high interstitial fluid pressure, concomitant with high HA expression, compromise the efficient delivery of chemotherapeutic agents.27,28 To date, no small molecule inhibitors have been developed targeting HA; however, in preclinical mouse models, treatment with hyaluronidase decreased both tumour HA content and interstitial fluid pressure and re-expanded the microvasculature, leading to a survival benefit in mouse models of PDAC, which is supported by the recent phase II clinical trials with the pegylated recombinant human hyaluronidase (PEGPH20) in combination with nab-paclitaxel and gemcitabine.29
The continued expansion of mouse models representing the myriad of clinical PDAC phenotypes will most certainly continue to advance therapeutic development and improve outcomes for PDAC patients. There are currently many PDAC mouse models that mimic the human course of disease, with the majority built on the Pdx1-Cre/KrasG12D backbone;30 however, due to the recent advancement in our understanding of the significant molecular heterogeneity of PDAC, as well as the intricacies of the tumour microenvironment, we are still seemingly playing catch up. Clearly, the understanding revealed by these evolving models of PDAC will lead to the development of improved therapeutic strategies; however unfortunately, we may not see the benefits of these effects for many years to come.
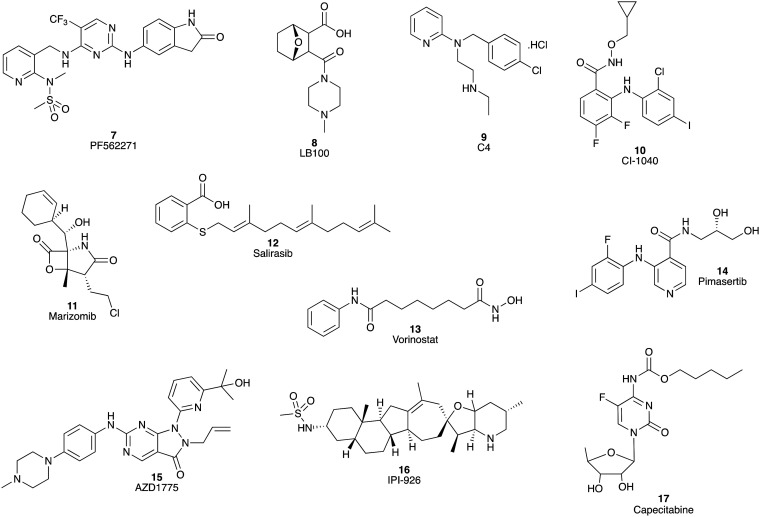
With the expanding understanding of PC biology and molecular characteristics, the development of drugs targeting various specific tumour signalling pathways has been recognized as a promising method to improve the treatment options of PC patients.17–31 Over the past ten years, an increasing number of clinical trials of small molecule inhibitors of PC have been undertaken. There are currently about 10 small molecules targeting PC in phase I clinical trials (Fig. 2). These include the dual inhibitor of focal adhesion kinase and pyruvate kinase 2 (PF-562271 7)31 and inhibitors of protein phosphatase 2A (LB-100 8),32 focal adhesion kinase (C4 9),31 mitogen-activated protein kinase (CI-1040 10),33 proteasome (marizomib, NPI-0052, 11),34 the RAS signalling pathway (salirasib 12),35 histone deacetylase (HDAC) (vorinostat, 13),36 MEK1/2 (pimasertib, AS-703026, 14),37 Wee1 kinase (AZD1775, 15),38 and Hedgehog signalling (saridegib, IPI-926, 16).39 To date these phase I clinical trials have shown promising safety and tolerability, as well as encouraging antitumor activity, which support further investigation for these agents as potential PC treatments. Of note, the vorinostat (13) and capecitabine (17) combination phase I clinical trial showed an encouraging MoS of 1.1 year.36 However, as a number of promising phase I trials have failed to translate the initial promise to phase II and beyond, these findings should be treated with caution.
Fig. 2. Chemical structures of the small molecule inhibitors (7–16) of PC with positive outcomes in phase I clinical trials, and capecitabine (17). Of note, the vorinostat (13) and capecitabine (17) combination phase I clinical trial showed an encouraging MoS of 1.1 year.34.
Despite some of these phase I trials showing promising outcomes as single agents, neither pimasertib (14) plus gemcitabine (1) nor IPI-926 (16) plus FOLFIRINOX provided any additional patient benefits, with the latter study closing early when a separate phase II trial indicated detrimental effects of this combination.39
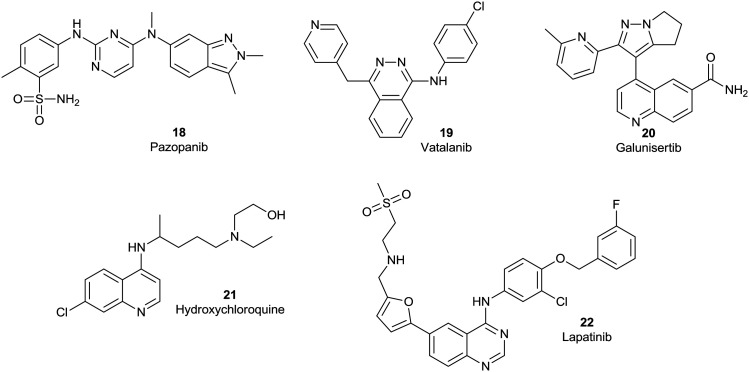
A number of small molecule inhibitors are in phase II clinical trials (Fig. 3), with reports of quite promising outcomes. In a phase II study of 32 patients with advanced, well-differentiated pancreatic neuroendocrine tumours, the tyrosine kinase receptor inhibitor pazopanib (18), an inhibitor of VEGF tyrosine kinase receptors 1, 2, and 3, showed a median progression-free survival (mPFS) of 14.4 months and a MoS of 25 months.40 A phase II trial of vatalanib (19), a polytyrosine kinase inhibitor with high affinity for PDGF and VEGF receptors, as a second-line therapy resulted in a favourable 6-month overall survival rate of 29% in patients with metastatic PC. This study revealed that the baseline IL-6 and serum LDH levels were predictive of patient survival. Vatalanib (19) may have an on-going role in maintenance therapies and/or in a biomarker-defined subtype of PC patients.41
Fig. 3. Chemical structures of the small molecule inhibitors pazopanib (18), vatalanib (19), galunisertib (20), hydroxychloroquine (21) and lapatinib (22) in phase II clinical trials targeting PC.
A phase II study of galunisertib (20), an ALK5 (transforming growth factor-beta receptor serine/threonine kinase) inhibitor, plus gemcitabine (1) as a first line therapy for patients with unresectable PC demonstrated a MoS of 8.9 months. High levels of macrophage inflammatory protein-1-alpha (MIP-1α) and interferon-gamma-induced protein 10 (IP-10) were predictive of poorer patient outcomes, with lower MoS observed, identified as the predictive markers for the potency of galunisertib plus gemcitabine. It was confirmed that patients with higher levels of MIP-1α or IP-10 had a significantly shorter MoS.42 The autophagy inhibitor, hydroxychloroquine (21), in combination with pre-operative short course chemoradiation, displayed a MoS of 23.3 months in a phase II study for patients with early, resectable PDAC. These studies are on-going.43 Lapatinib (22), a dual receptor tyrosine kinase inhibitor of HER2/neu and EGF, as the second-line therapy in a phase II clinical trial of 17 patients with metastatic PC, resulted in a mPFS of 2.6 months and a MoS of 5.2 months. In patients with stable disease, the corresponding outcomes were a mPFS of 4.0 months and a MoS of 8.3 months. These results suggest a critical role in identifying clinical biomarkers in the selection of potential treatment regimes.44
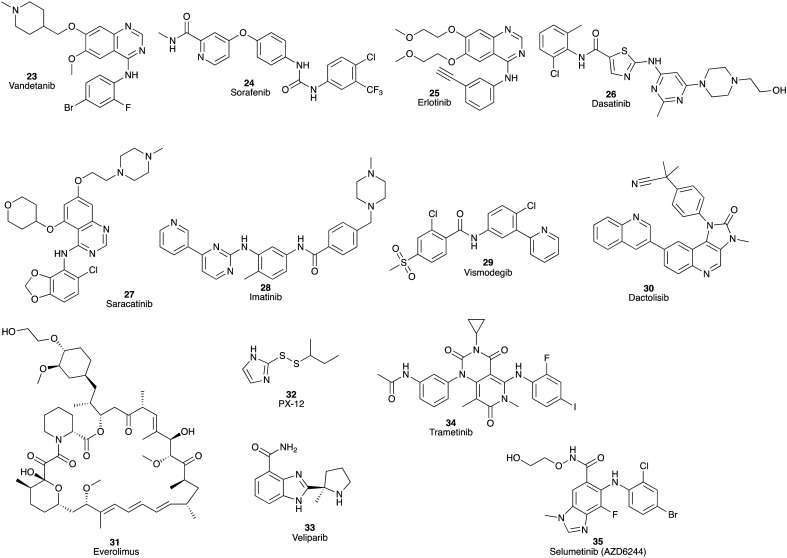
Poor phase II outcomes were noted for a wide range of initially promising compounds (Fig. 4). These include: vandetanib (23), a tyrosine kinase inhibitor of VEGFR2, RET, and EGFR, and gemcitabine (1), a combination that failed to improve MoS in advanced PC; again these studies were hampered by a lack of biomarkers to identify cancer trial patient subsets.45 The combination therapy of sorafenib (24), an inhibitor of VEGF receptor tyrosine kinase, with erlotinib (25) failed to meet the trial primary endpoint.46 The combination of dasatinib (26), a competitive inhibitor of SRC (a non-receptor tyrosine kinase protein) and ABL receptor tyrosine kinases, and gemcitabine (1) failed to show improved MoS or mPFS. Unexpectedly, this combination produced enhanced toxicity compared with single agent gemcitabine (1).47 Saracatinib (AZD0530, 27), an SRC receptor tyrosine kinase inhibitor, in combination with gemcitabine (1) also did not increase efficacy compared with gemcitabine (1) monotherapy.48 A phase II combination trial of imatinib (28), a receptor tyrosine kinase inhibitor of PDGFRs, and gemcitabine (1) for first-line treatment of advanced PC failed to provide statistical improvements over gemcitabine (1) monotherapy with a mPFS of 3.9 and a MoS of 6.3 months.49 A similar lack of statistical improvement was noted for vismodegib (29), an inhibitor of the Hedgehog signalling pathway, plus gemcitabine (1) with a MoS of 6.9 months in patients with metastatic PC.50
Fig. 4. Phase II pancreatic cancer clinical trial candidates that failed to show statistically significant improvements in MoS or failed at phase II for other reasons as either single agents or in combination with gemcitabine (1): vandetanib (23), sorafenib (24), erlotinib (25), dasatinib (26), saracatinib (27), imatinib (28) vismodegib (29), dactolisib (30), everolimus (31), PX-12 (32), veliparib (33), trametinib (34) and selumetinib (35). Note that erlotinib (25) in combination with gemcitabine (1) was approved for locally advanced, unresectable or metastatic PC in 2005.60.
Dactolisib (NVP-BEZ235) (30), a dual inhibitor of phosphatidylinositol 3-kinase (PI3K) and the mammalian target of rapamycin (mTOR), was poorly tolerated by patients with resistance to the macrocyclic drug everolimus (31), in pancreatic neuroendocrine tumours (pNETs) during a phase II trial. Both dactolisib (30) and everolimus (31) target the PI3K pathway, resulting in potential overlap of their toxicity profiles; a strict dose decrease design was performed to control adverse effects of the treatment, which consequently confined the duration of the treatment.51
A phase II monotherapy using PX-12 (32), an inhibitor of the proto-oncogene thioredoxin, showed a mPFS of 0.9 months and a MoS of 3.2 months leading to early termination of the study.52 The phase II trial of veliparib (33), a poly (ADP ribose) polymerase (PARP) inhibitor, as a single agent for patients with BRCA-mutated PDAC, showed a similarly disappointing outcome with a mPFS of 1.7 months and a MoS of 3.1 months. No favourable responses were identified.53 The combination of gemcitabine (1) with trametinib (34), an oral mitogen-activated protein kinase inhibitor (MEK), showed no overall survival benefits in patients with previously untreated metastatic PC in a phase II study. The failure to improve the overall survival may be ascribed to increased toxicity and the need to modify the dose. Moreover, a proper administration sequence of these drugs may need to replace the concurrent administration in this study.54 Selumetinib (35; AZD6244), a potent inhibitor of MEK1/2, as a second-line monotherapy was well tolerated, but with a MoS of 5.4 months offered no statistical benefit relative to gemcitabine (1) monotherapy.55
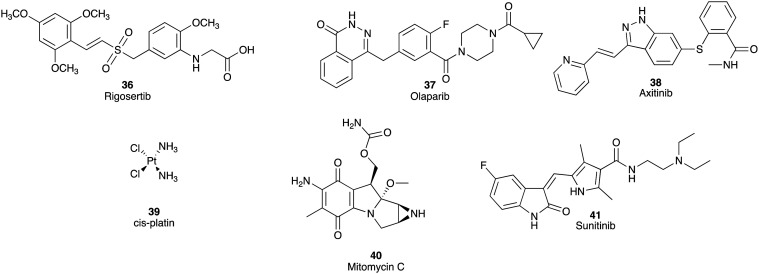
Rigosertib (36), a first-in-class Ras mimetic inhibitor of polo-like kinase 1 and phosphoinositide 3-kinase,56 olaparib (37), an inhibitor of PARP,57,58 and axitinib (38), an inhibitor of receptor tyrosine kinase of VEGFR, progressed to phase III clinical trials.59 Unfortunately, all failed to meet the trial endpoints, with no observable difference in survival coupled with an increase in adverse events over gemcitabine (1) as a single agent treatment. Despite 40 of 67 tumour samples showing KRAS oncogenic mutations, the rigosertib (36) plus gemcitabine (1) combination MoS was 6.1 months versus 6.4 months in the gemcitabine (1) monotherapy in patients with metastatic PDAC.56 The combination of olaparib (37) with irinotecan (5) and cisplatin (39) or irinotecan (5), cisplatin (39), and mitomycin C (40) led to significant toxicity, especially hematologic toxicity. This study was terminated as a consequence of poor risk versus benefit profiles.58 No significant survival benefit from the combination of axitinib (38) and gemcitabine (1) was observed over gemcitabine (1) monotherapy in patients with advanced PC.59
Erlotinib (Tarceva®; 25), a tyrosine kinase inhibitor of EGFR, in combination with gemcitabine (1) was approved by the FDA for treatment of locally advanced, unresectable, or metastatic pancreatic cancer in 2005,60 but other clinical trials with this compound are still on-going.46 Sunitinib (Sutent®; 41), a multi-targeted receptor tyrosine kinase inhibitor including VEGFR and PDGFR, was also approved to treat patients with progressive, well-differentiated pNETs in 2011 (Fig. 5).61
Fig. 5. Chemical structures of rigosertib (36), olaparib (37), axitinib (38), cisplatin (39) and mitomycin C (40) that failed to meet the phase III PC endpoint or failed at phase II due to toxicity issues. Sunitinib (41) was approved to treat patients with progressive, well-differentiated pNETs in 2011.61.
Although clinical trials have resulted in some promising outcomes, the overall progression is slow and unsatisfactory. The current status of these compounds and their biological targets is summarised in Table 1. New strategies for further development of clinical trials are needed in order to improve survival of PC patients.
Table 1. Small molecules that have entered clinical trials (phase I, II and III) and their effects on overall survival (OS), median disease-free progression (mPFS), median overall survival (MoS) and outcomes of the trial where known. Compounds aligned as either monotherapy or combination therapy.
| Pancreatic cancer monotherapy | ||||
| Compound | Target | Survival | Comment | Ref. |
| Gemcitabine (1) | Thymidylate synthase and dihydrofolate reductase | MoS 5.6 months | 3 | |
| 5-FU (2) | Thymidylate synthase | MoS 4.4 months | 3 | |
| Nab-paclitaxel (3) | Topoisomerase I | MoS 8.5 months | ||
| PF-562271 (7) | Focal adhesion kinase and pyruvate kinase 2 | Generally well tolerated and most adverse events were of grade 1 or 2 and reversible | 31 | |
| LB-100 (8) | Protein phosphatase 2A | Continued development alone and in combination with other therapies | 32 | |
| CI-1040 (10) | Mitogen-activated protein kinase | Well tolerated and both target suppression and anti-tumour activity were demonstrated in this phase I study | 33 | |
| Selumetinib (35) | Mitogen-activated protein kinase | MoS 5.4 months | No statistical benefit over gemcitabine monotherapy | 55 |
| Marizomib (11) | Proteasome | 61% of evaluable patients demonstrated stable disease with 39% having decreases in tumour measurements. | 34 | |
| Salirasib (12) | RAS signalling pathway | MoS 6.2 months | The combination of gemcitabine and salirasib appears well-tolerated. | 35 |
| Pazopanib (18) | VEGFR tyrosine kinase | mPFS 25 monthsMoS 14.4 months | 40 | |
| Vatalanib (19) | Polytyrosine kinase | MoS 6 months | 41 | |
| Hydroxychloroquine (21) | Autophagy | MoS 23.3 months | 43 | |
| Vismodegib (29) | Hedgehog signalling pathway | MoS 6.9 months | No statistical benefit over gemcitabine monotherapy | 50 |
| Dactolisib (30) | Phosphatidylinositol 3-kinase and the mammalian target of rapamycin | Poorly tolerated by patients in phase II study | 51 | |
| PX-12 (32) | Proto-oncogene thioredoxin | mPFS 0.9 months | Trial terminated | 52 |
| MoS 3.2 months | ||||
| Veliparib (33) | Poly (ADP ribose) polymerase | mPFS 1.7 months | No statistical benefit over gemcitabine monotherapy | 53 |
| MoS 3.1 months | ||||
| Erlotinib (25) | EGFR Tyrosine kinase | Approved by the FDA for treatment of locally advanced, unresectable, or metastatic pancreatic cancer in 2005 | 60 | |
| Sunitinib (41) | VEGFR and PDGFR tyrosine kinase | Approved to treat patients with progressive, well-differentiated pNETs in 2011 | 61 | |
| Pancreatic cancer combination therapy |
||||
| FOLFIRINOX | Thymidylate synthase, dihydrofolate reductase, Topoisomerase and DNA | MoS 11.1 months | 16 | |
| Vorinostat (13) + capecitabine (17) | Histone deacetylase | MoS 13 months | 36 | |
| Pimasertib (14) + gemcitabine (1) | Mitogen-activated protein kinase | MoS 7.3 months | No clinical benefit with 7.6 month placebo group survival | 37 |
| Trametinib (34) + gemcitabine (1) | No statistical benefit over gemcitabine monotherapy (phase II) | 54 | ||
| Saridegib (16) + FOLFIRINOX | Hedgehog signalling pathway | Study closed early | 39 | |
| Galunisertib (20) + gemcitabine (1) | Transforming growth factor-beta receptor serine/threonine kinase | MoS 8.9 months | 42 | |
| Lapatinib (22) + capecitabine (17) | HER2/neu and EGF receptor tyrosine kinase | mPFS 2.6 months | Progressive disease | 44 |
| Progressive disease | ||||
| MoS 5.2 months | ||||
| Stable disease | ||||
| mPFS 4.0 | ||||
| MoS 8.3 months | Stable disease | |||
| Vandetanib (23) + gemcitabine (1) | VEGFR2, RET, and EGFR tyrosine kinase | MoS 8.8 months | MoS of 9.0 months in the vandetanib group and in the placebo group | 45 |
| Sorafenib (24) + erlotinib (25) | VEGFR tyrosine kinase | Failed to meet the primary end point | 46 | |
| Axitinib (38) + gemcitabine (1) | Failed to meet endpoints (phase III) | 59 | ||
| Dasatinib (26) + gemcitabine (1) | SRC (a non-receptor tyrosine kinase protein) and ABL receptor tyrosine kinases | No statistical benefit over gemcitabine monotherapy | 47 | |
| Saracatinib (27) + gemcitabine (1) | SRC receptor tyrosine kinases | No statistical benefit over gemcitabine monotherapy | 48 | |
| Imatinib (28) + gemcitabine (1) | PDGFR tyrosine kinase | mPFS 3.9 months | No statistical benefit over gemcitabine monotherapy | 49 |
| MoS 6.3 months | ||||
| Rigosertib (36) + gemcitabine (1) | Polo-like kinase 1 and phosphoinositide 3-kinase | MoS 6.1 months | Failed to demonstrate an improvement in survival with MoS 6.4 months with gemcitabine monotherapy | 56 |
| Olaparib (37) + irinotecan (5) + cisplatin (39) | Poly (ADP ribose) polymerase | Significant toxicity, trial terminated | 58 | |
Investigational compounds
Inhibitors of protein kinases and tyrosine kinases
Tyrosine kinases are a key subtype of protein kinase that play a critical role in intracellular signal transduction. Tyrosine kinases include receptor tyrosine kinases (RTKs) and non-receptor cytoplasmic tyrosine kinases. RTKs are activated by diverse growth factor receptors including epidermal growth factor (EGF), vascular endothelial growth factor (VEGF) and fibroblast growth factor receptor 1 (FGFR1).17 Elevated signalling from these receptors from mutation or over-expression is responsible for a number of human cancers such as pancreatic, lung, head and neck, breast, brain and ovarian cancers.62,63 Enhanced expression of EGFR or its ligand also contributes to poor clinical outcomes in various epithelial cancers including breast, pancreatic, colorectal, and head and neck cancers.64,65 Of note, over-expression of EGFR signalling is known to result in the increased proliferation, chemoresistance and invasiveness of PC.64 In contrast, cytoplasmic tyrosine kinases do not have an extracellular receptor and are located in the cytoplasm, nucleus or plasma membrane. They comprise eight families in which the major ones include SRC, JAK, ABL and FAK.17
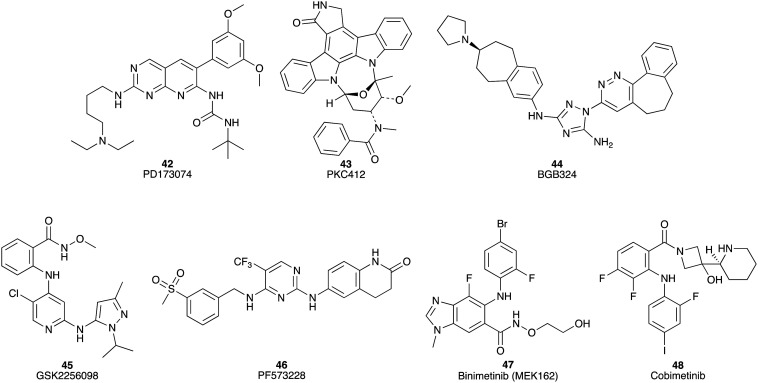
PD173074 (42) is an ATP-competitive inhibitor of (FGFR1) tyrosine kinase with an IC50 of ca. 25 nM (where IC50 = compound concentration required to inhibit 50% of the protein's activity) and also suppresses VEGFR2 with an IC50 of 100–200 nM in cell-free assays (including cell extract-based and purified enzyme-based).66 PD173074 (42) inhibits cell growth in AsPC-1, Capan-1, HPAF-II, MiaPaCa-2, and Panc 1 pancreatic cancer cell lines with IC50 values of 2.5 to 15 μM (drug exposure 48 h). In vivo, in HPAF-II and MiaPaCa-2 xenograft mouse models, PD173074 (42) also displayed potent inhibitory activity of tumour growth.67 PKC412 (43) exhibits a wide range of inhibitory activities against Ser/Thr and tyrosine kinases, including fms-like tyrosine kinases, protein kinase C, VEGFR2, tyrosine-protein kinase KIT, PDGFRα, and PDGFRβ.68 PKC412 (43) was screened in sixteen pancreatic cancer cell lines with IC50 values of 0.25 to 20 μM recorded (Fig. 6). In vivo, PKC412 (43) therapy resulted in significant tumour growth suppression (survival rates of PKC412 (43) and control following 100 days of treatment were about 60% and 15%, respectively) in an AsPC-1 murine xenograft model.68
Fig. 6. Chemical structures of small molecule inhibitors of protein kinases under investigation for their efficacy against pancreatic cancer: PD173074 (42), PKC412 (43), BGB324 (44), GSK2256098 (45), PF573228 (46), binimetinib (47) and cobimetinib (48).
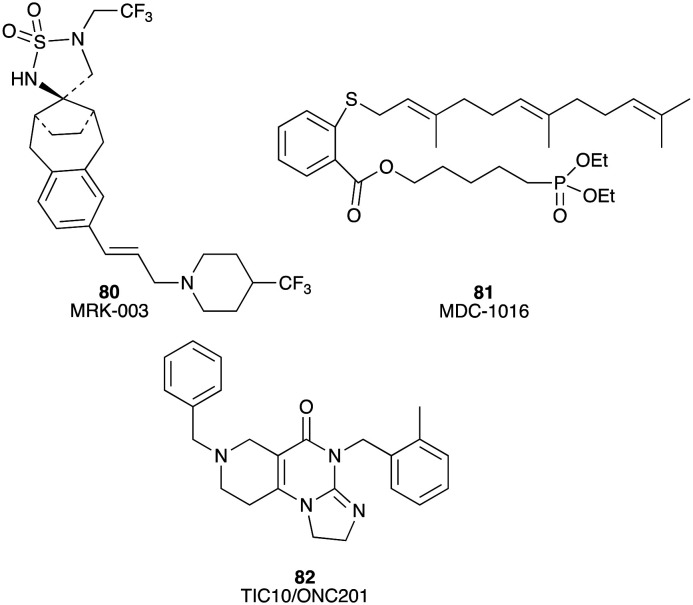
Activation of the receptor tyrosine kinase Axl is related to poor outcomes for pancreatic cancer patients.69 BGB324 (44), a small molecule inhibitor of Axl, was observed to display a 15-fold selectivity towards Axl (IC50 14 nM) relative to 133 tyrosine and serine/threonine kinases examined (Fig. 6).70In vitro, it enhanced the efficacy of gemcitabine (1) with IC50 values from 49 nM to 16 nM and 20 nM to 6.9 nM against the AsPC-1 and MiaPaCa-2 cell lines, respectively. In vivo, the combination therapy of BGB324 (44) and gemcitabine (1) improved the MoS to 83.5 days compared to gemcitabine (1) alone (65 days) in a mouse PC xenograft study.69
Inhibitors of focal adhesion kinase
Focal adhesion kinase (FAK) is commonly hyperactivated in pancreatic ductal adenocarcinoma. The kinase activity requires the phosphorylation of FAK at Y397 to induce down-stream events including activation of Akt and ERK.71 The inhibitory activity of GSK2256098 (45) against FAK, with an apparent Ki of 0.4 nM, was evaluated in six human PDAC cell lines: MiaPaCa-2, PANC-1, Hs766T, AsPC-1, L3.6P1, and BXPC-3.72 After GSK2256098 (45) treatments (0.1–10 μM), the responses of FAK Y397 phosphorylation in the six cell lines varied from low (<20% inhibition) to high (>90% inhibition). In addition, GSK2256098 (45) suppressed the growth of L3.6P1 and Panc-1 cells with IC50 values of 25 μM and 29 μM, respectively.71 PF573228 (46), an ATP-competitive inhibitor of FAK with IC50 of 4 nM in a cell-free assay,73 substantially sensitized the cells to apoptosis induced by lexatumumab (an agonistic human monoclonal antibody of death receptor 5). The combination of the monoclonal antibody lexatumumab and PF573228 (46) exhibited remarkable growth inhibition with at least a 3-fold decrease compared with untreated control mice in pancreatic tumour xenografts (Fig. 6).74
Inhibitors of mitogen-activated protein kinase kinase
The mitogen-activated protein kinase kinase (MEK) belongs to the mitogen-activated protein kinase (MAPK) signalling pathway with crucial roles in regulating various cellular functions, such as cell proliferation, survival, differentiation and motility.75 Binimetinib (MEK162) (47), a potent inhibitor of MEK1/2 with an IC50 of 12 nM in a cell-free assay,76 was examined for growth inhibitory effects against a panel of 29 pancreatic cancer cell lines. Fifteen of these cell lines returned IC50 values of <500 nM, e.g. MiaPaCa-2, AsPC-1, and CAPAN-2 with IC50 values of 92, 280 and 316 nM, respectively.77 Cobimetinib (48) is a potent and selective inhibitor of MEK with a biochemical IC50 of 0.9 nM towards MEK1.75 Cobimetinib (48) in combination with trametinib (34) inhibited tumour growth in a gemcitabine-resistant PC patient-derived orthotopic mouse xenograft model more effectively than gemcitabine monotherapy (Fig. 6).78
Inhibitors of protein kinase R-like endoplasmic reticulum kinase
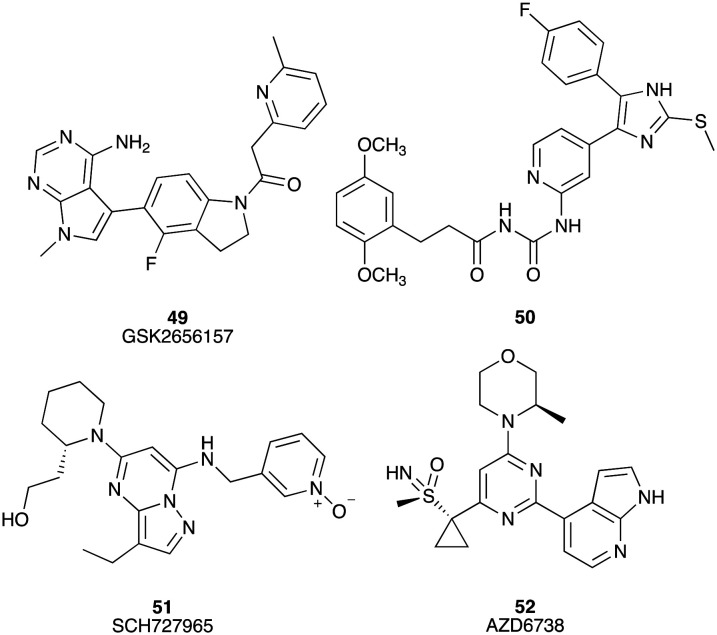
GSK2656157 (49) is an ATP competitive PERK (protein kinase R (PKR)-like endoplasmic reticulum kinase) inhibitor with an IC50 of 0.9 nM.79 Twice daily dosing of GSK2656157 leads to a dose dependent inhibition in human tumour xenograft models of PC (Fig. 7).79 Pyridine (50) inhibits the kinase activity of protein kinase CK1δ (IC50 = 4 nM) and has been assessed against PC cell lines Colo357 (EC50 = 3.5 μM) and Panc89 (EC50 = 1.5 μM).80 SCH727965 (dinaciclib) (51) is a potent small molecule cyclin-dependent kinase (CDK) inhibitor for CDK2, CDK5, CDK1 and CDK9 with IC50 values of 1, 1, 3 and 4 nM, respectively.81 Aberrant activation of CDKs and dysregulation of cell cycle progression is a hallmark of many human cancers. SCH727965 inhibited the growth of the MiaPaCa-2 and Pa20C pancreatic cancer cell lines in a dose-dependent manner showing GI50 values of 10 and 20 nM (GI50 = compound concentration required to elicit 50% of cell death relative to an untreated control), respectively. In vivo, the growth of a panel of low-passage PC xenografts was inhibited by SCH727965 (51) (Fig. 7).82
Fig. 7. Chemical structures of small molecule inhibitors of protein kinase R-like endoplasmic reticulum kinase under investigation for their efficacy against pancreatic cancer: GSK2656157 (49), 3-(2,5-dimethoxyphenyl)-N-((4-(5-(4-fluorophenyl)-2-(methylthio)-1H-imidazol-4-yl)pyridin-2-yl) carbamoyl)-propanamide (50), SCH727965 (51), and AZD6738 (52).
Ataxia telangiectasia and Rad3-related protein (ATR) is a serine/threonine protein kinase with the role of protecting cells from replication stress and also blocks the effect of anti-metabolite drug therapies. AZD6738 (52) is an ATP competitive inhibitor of ATR with an in vitro enzyme IC50 of 1 nM,83 which displayed a GR50 inhibitory efficacy (the concentration of drug required to reduce cell growth by 50%) against human pancreatic cell lines SW1990 (GR50 = 0.9 μM), Capan-1 (GR50 = 1.57 μM), AsPC-1 (GR50 = 1.98 μM), HPAF-II (GR50 = 3.2 μM), Capan-2 (GR50 = 4.8 μM), MiaPaCa-2 (GR50 = 9.8 μM) and Panc-1 (GR50 = 32.3 μM). In vitro and in vivo, AZD6738 synergizes with gemcitabine to induce the regression of pancreatic ductal adenocarcinoma (Fig. 7).84
Inhibitors of signal transducer and activator of transcription 3
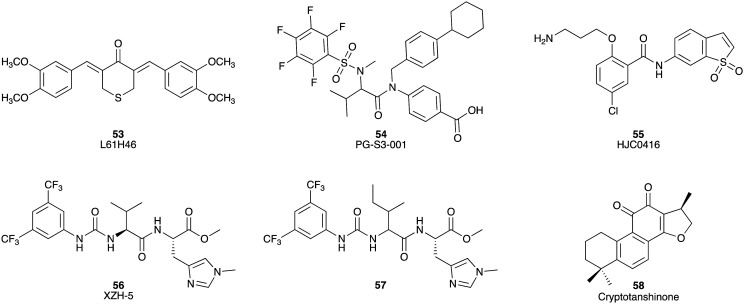
Signal transducer and activator of transcription3 (STAT3) is a transcription factor and oncogenic driver, which can promote the growth of malignant tumours. The STAT3 inhibitor L61H46 (53) showed potent inhibitory activity against human pancreatic cell lines BXPC-3 and Panc-1 with IC50 values of 0.86 and 2.83 μM, respectively.85 L61H46 (53) induced the reduction of STAT3 phosphorylation in a dose-dependent manner for both the Panc-1 and BXPC-3 cell lines. Additionally, in vivo, L61H46 (53) potently blocked pancreatic tumour growth in a BXPC-3 xenograft model.85 PG-S3–001 (54) is also a potent STAT3 binder (Kd = 324 nM), which induced cell death, with an ED50 value of 2.4 ± 0.2 μM (ED50 = compound concentration (dose) required to elicit an effect in 50% of cells) in the PDAC cell line Panc10.05, as well as inhibiting STAT3 activation with a Ki of 72 ± 6 μM.86 The benzamide analogue, HJC0416 (55), was identified as a STAT3 inhibitor and displayed significant antiproliferative activity against pancreatic cancer cell lines AsPC-1 and Panc-1 with IC50 values of 40 nM and 1.88 μM, respectively.87 XZH-5 (56) is a more recent addition to the STAT3 inhibitor family with inhibitory activity against Panc-1, HPAC, and SW1990 cells with IC50 values of 24.7, 17.4 and 17.9 μM, respectively.88,89 The related compound (57) was found to be more potent against Panc-1, HPAC, and SW1990 with IC50 values of 10.1, 7.6, and 8.3 μM, respectively.89 Cryptotanshinone (58), a STAT3 inhibitor with an IC50 of 4.6 μM,90 inhibited the growth of BxPC-3 cells with half maximal inhibitory concentration values of 29.4 and 22.8 μM after 24 h and 48 h treatment, respectively (Fig. 8).91
Fig. 8. Chemical structures of small molecule inhibitors of signal transducer and activator of transcription 3: L61H46 (53), PG-S3-001 (54), HJC0416 (55), XZH-5 (56), methyl N-(((3,5-bis(trifluoromethyl)phenyl)-carbamoyl)isoleucyl)-N‘-methyl-l-histidinate (57) and cryptotanshinone (58).
Inhibitors of bromodomain and extra terminal proteins
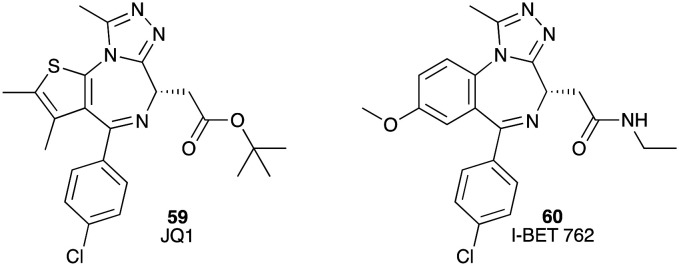
Bromodomain and extra-terminal (BET) proteins, such as BRD2, BRD3 and BRD4, mediate many critical cell cycle, apoptosis and inflammation genes. BET proteins contain two bromodomains (BD1 and BD2, respectively) and an extra-terminal domain (ET). Two bromodomain inhibitors JQ1 (59) (IC50 77 nM and 33 nM for BRD4 (BD1) and BRD4 (BD2), respectively)92 and I-BET 762 (60) with IC50 values from 32.5 to 42.5 nM showed excellent inhibitory activity against BRDs.93 Both 59 and 60 also inhibited growth of the human pancreatic cell lines AsPC-1, Panc-1 and CAPAN-1 with IC50 values of 37 ± 4, 720 ± 34 and 190 ± 25 nM for JQ1 (59) and 231 ± 39, 2550 ± 75 and 990 ± 5 nM for I-BET 762 (60). Reduced levels of c-Myc and p-Erk 1/2 proteins in vitro were observed on treatment of these cell lines with 59 and 60 (Fig. 9).94 Although bromodomain inhibitors demonstrated promising activity against human pancreatic cell lines, it was reported that pancreatic cancer cells can develop resistance to BET inhibitors, which can be regulated by GLI2.95
Fig. 9. Chemical structures of small molecule inhibitors of bromodomain and extra-terminal proteins: JQ1 (59) and I-BET 762 (60).
Inhibitors of histone deacetylases
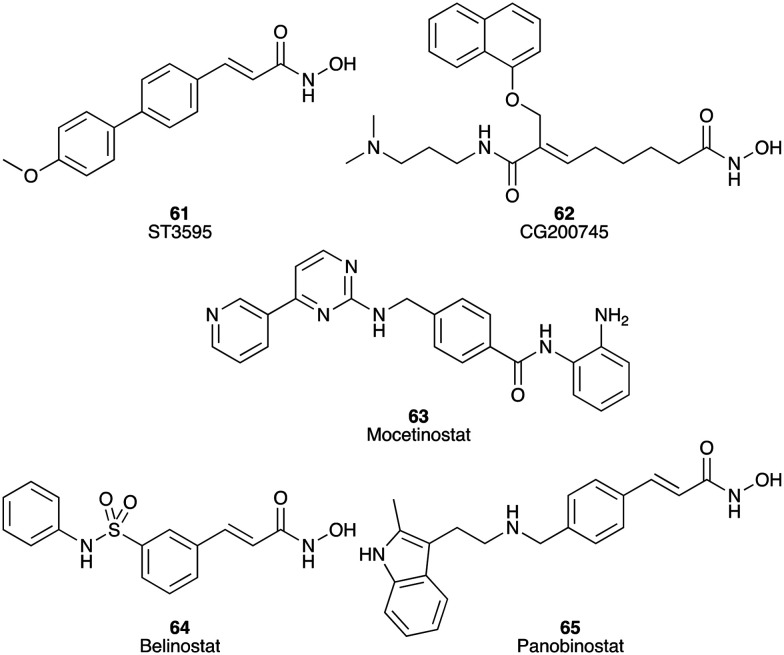
Histone deacetylases (HDACs) are divided into four classes, which are involved in multiple different stages of cancer. Aberrant expression of HDACs including classes I, II, and IV has been linked to various malignancies. In PC, HDAC2, 6 and 7 are overexpressed and associated with poor prognosis.96 Numerous HDAC inhibitors have been studied as potential treatments for PC (Fig. 10). For example, ST-3595 (61) (HDAC IC50 136.0 ± 11.6 nM)97 reduced the viability of Panc-1, AsPC-1, and MiaPaCa-2 cells in a dose-dependent manner in vitro and in vivo, as well as inhibiting HDAC activity. ST-3595 (61) also enhanced the efficacy of gemcitabine in vitro and in vivo.98 CG200745 (62), a hydroxamate-based HDAC inhibitor, showed significant inhibition against BxPC3, CFPAC1 and HPAC cells with IC50 values of 2.4, 10.7 and 7.4 μM, respectively. In vivo, it displayed synergistic anti-tumour effects with gemcitabine (1)/erlotinib (25) and markedly increased the sensitivity of PC to gemcitabine (1), improved antitumour results on gemcitabine-resistant PC cells, and reduced the tumour volume up to 50%.99 MGCD0103 (Mocetinostat) (63), which potently targeted HDAC1 (IC50 = 0.15 μM) but also showed inhibitory activity towards HDAC2, HDAC3, and HDAC11 in vitro,100 displayed activity against the AsPC-1, BxPC-3, MiaPaca-2, and Panc-1 cell lines with IC50 values of 3.9, 1.1, 0.6 and 1.8 μM, respectively.101 Another two new HDAC inhibitors, belinostat (64) and panobinostat (65), displayed potent inhibition against the growth of six human PC cell lines in a dose dependent manner, with EC50 values of belinostat (64) against AsPC1, BxPC3, Panc0327, Panc0403, Panc1005 and MiaPaCa-2 being 0.3, 0.7, 0.5, 1.1, 1.1 and 0.7 μM, respectively, and EC50 values of panobinostat (65) against BxPC3, Panc0327, Panc0403, Panc1005 and MiaPaCa-2 being 284, 0.46, 7.7, 261 and 13 nM, respectively. In particular, belinostat (64) alone, or in combination with gemcitabine, significantly inhibited the growth of human pancreatic tumours in immunodeficient mice, which may be a promising drug for the treatment of PC (Fig. 10).102
Fig. 10. Chemical structures of small molecule inhibitors of histone deacetylases: ST3595 (61), CG200745 (62), mocetinostat (63), belinostat (64) and panobinostat (65).
Inhibitors of Bcl-2 family proteins
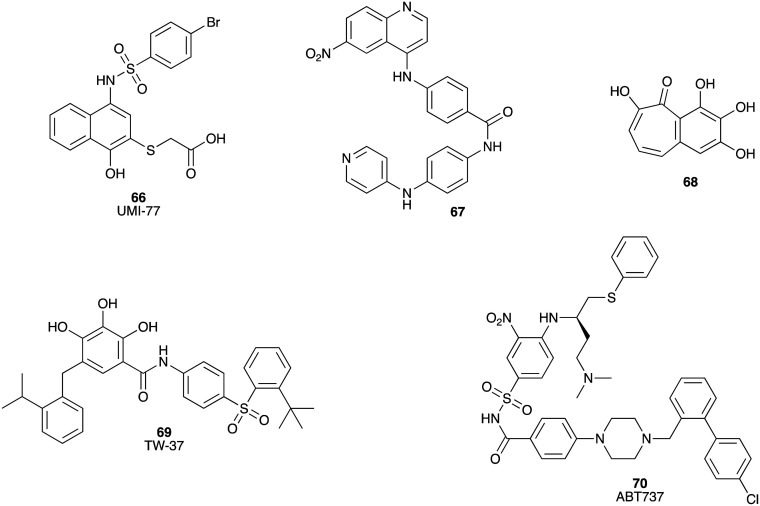
Apoptosis occurs predominately via two key pathways: extrinsic and intrinsic. The Bcl-2 family (Bcl-2, Bcl-xL, Bcl-w, and Mcl-1) participates in intrinsic apoptosis by promoting cell survival, thereby inhibiting cell death, via blocking of the pro-apoptotic proteins Bax and Bak.103 Myeloid cell leukemia-1 (Mcl-1) is a potent anti-apoptotic protein in the Bcl-2 family, which plays the role of a key survival factor in a wide range of human cancers, including pancreatic cancer. UMI-77 (66) is a selective inhibitor of Mcl-1 (Ki = 0.49 μM, IC50 = 0.31 μM),104 with demonstrated efficacy in initiating apoptosis in the pancreatic cancer cell lines AsPC-1, BxPC-3, and Capan-2 with IC50 values of 3.4, 4.4 and 5.5 μM, respectively. In a BxPC-3 xenograft model UMI-77 (66) significantly suppressed tumour growth.104 Two compounds (67 and 68) from US National Cancer Institute (NCI) diversity set IV were identified as binding inhibitors of Mcl-1 and mNoxa (an apoptosis regulating protein) with Ki values of 1.09 and 0.80 μM, respectively. Both compounds were active against MiaPaCa-2 (67, IC50 = 88.8 μM; 68, IC50 17.6 μM) and BxPC-3 (67, IC50 = 15.1 μM; 68, IC50 > 100 μM) cell lines (Fig. 11).105
Fig. 11. Chemical structures of small molecule inhibitors of Bcl-2 family proteins: UMI-77 (66), 4-((6-nitroquinolin-4-yl)amino)-N-(4-(pyridin-4-ylamino)phenyl)benzamide (67), 2,3,4,6-tetrahydroxy-5H-benzoannulen-5-one (68), TW-37 (69) and ABT737 (70).
The Bcl-2 family proteins are over-expressed in multiple metastatic human tumours, including pancreatic cancer. TW-37 (69), a small-molecule inhibitor of Bcl-2, Bcl-xL and Mcl-1 with Ki values of 0.29 μM, 1.11 μM and 0.26 μM, respectively,106 induced a dose- and time-dependent inhibition of cell growth of BxPC-3, HPAC and Colo-357 cell lines. TW-37 also inhibited tumour growth in a Colo-357 xenograft model.107,108 ABT737 (70) inhibits the Bcl-2, Bcl-xL and Bcl-w proteins, and sensitized paclitaxel induced cell death of the pancreatic cancer cell lines Panc-1, MiaPaCa-2, PK-8 (Bcl-xL high expression) and PK-59 (Bcl-2/Bcl-xL high expression) (Fig. 11).109
Small molecule inhibitors targeting other aberrant signalling pathways in PC
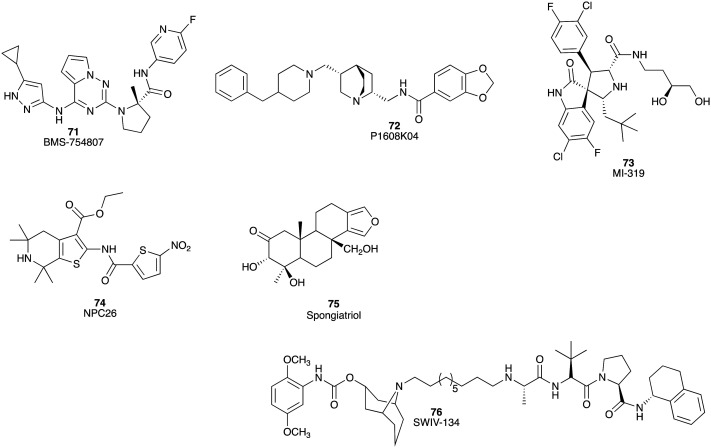
The type I insulin-like growth factor-I receptor (IGF-1R) belongs to the transmembrane tyrosine kinase growth factor receptor which plays a crucial role in the establishment and maintenance of the transformed phenotype and leads to mitogenesis and survival of cancer cells via activation. Pyrrolotriazine BMS-754807 (71) (Fig. 12) is an effective inhibitor (Ki < 2 nM) of IGF-1R and other insulin receptor (IR) family kinases.110In vitro, BMS-754807 (71) combined with gemcitabine (1) substantially decreased the IC50 values of gemcitabine in PC cell lines (AsPC: 19.7 μM to 75 nM, Panc-1: 3 μM to 70 nM, MiaPaCa-2: 72 to 16 nM, and BxPC-3: 28 to 16 nM).111 In murine xenografts, the combination of BMS-754807 (71) and gemcitabine (1) increased median animal survival from 28 days (gemcitabine monotherapy) to 41 days (Fig. 12).111
Fig. 12. Chemical structures of small molecule inhibitors of other aberrant signalling pathways linked to pancreatic cancer: BMS-754807 (71), P1608K04 (72), MI-319 (73), NPC26 (74), spongiatriol (75) and SWIV-134 (76).
There are nine members of the protein arginine methyltransferase (PRMT) family which catalyse the transfer of methyl groups to obtain methylated arginine. Of the PRMT family, PRMT5 is upregulated in breast, liver, colon, lung, bladder, and especially in pancreatic cancers.112 P1608K04 (72) inhibits PRMT5 activity by blocking PRMT5-mediated nuclear factor kappa B (NF-κB) methylation and is effective at reducing cell viability in the Panc-1, MiaPaCa-2, and AsPC1 PC cell lines with IC50 values of 8.5, 14, and 20 μM, respectively (Fig. 12).112
Murine double minute 2 (MDM2) protein plays a critical role in promoting ubiquitination and proteasome-dependent degradation of p53. Inhibition by small molecule inhibitors has the potential to restore the apoptotic and cell cycle regulatory functions of p53 by perturbing the interaction of MDM2 and p53.113 The MDM2 inhibitor MI-319 (73) showed in vitro synergistic outcomes in combination with cisplatin, inducing apoptosis to significantly decrease tumour growth in both Capan-2 and BxPC-3 tumour xenograft models with 50% of Capan-2 xenografted animals found to be tumour free (Fig. 12).113
It is well established that the structure and function of mitochondria are different in cancer cells relative to their non-cancerous counterparts. The mitochondria play an active role in metabolic reprogramming in cancer cells. NPC-26 (74), a novel mitochondrion interfering compound, inhibited Panc-1 cell growth at doses of 1 to 50 μM, as well as showing inhibitory effects in MiaPaCa-2 and AsPC-1 cells at 0.1 μM. In Panc1 xenografts treated with NPC-26 (74) significant tumour suppression and enhancement of gemcitabine (1) activity were reported (Fig. 12).114
The signal transducer NFκB promotes cell survival, proliferation and angiogenesis.115 The marine furanoditerpenoid spongiatriol (75) inhibited transcriptional activity of NFκB (IC50 3.4 ± 0.6 μM), with a concomitant decrease in the phosphorylation of NFκB in the AsPC-1 cell line and displayed cytotoxicity against AsPC-1, BxPC-3, MiaPaCa-2 and Panc-1 cell lines with IC50 values of 13 ± 2, 8 ± 3, 6 ± 1 and 13 ± 5 μM, respectively (Fig. 12).115
The sigma-2 receptor is known to be over-expressed in a number of proliferating tumour cells including PC. The ligands to this receptor can be internalized rapidly once they bind to the cancer cells, suggesting that the sigma-2 receptor is an attractive target for PC drug intervention.116 SW IV-134 (76), a small molecule drug conjugate of SW IV-32, and the sigma-2 ligand SW43 displayed potent binding affinity to the sigma-2 receptor (Ki 22.6 ± 1.8 nM).116 SW IV-134 showed potent cell killing characteristics against the CFPAC-1, BxPC-3, AsPC-1, Panc-1 and MiaPaCa-2 PC cell lines with IC50 values of 7.4 ± 0.3, 6.8 ± 0.2, 9.2 ± 0.4, 6.3 ± 0.1 and 7.8 ± 0.3 μM, respectively (Fig. 12).116
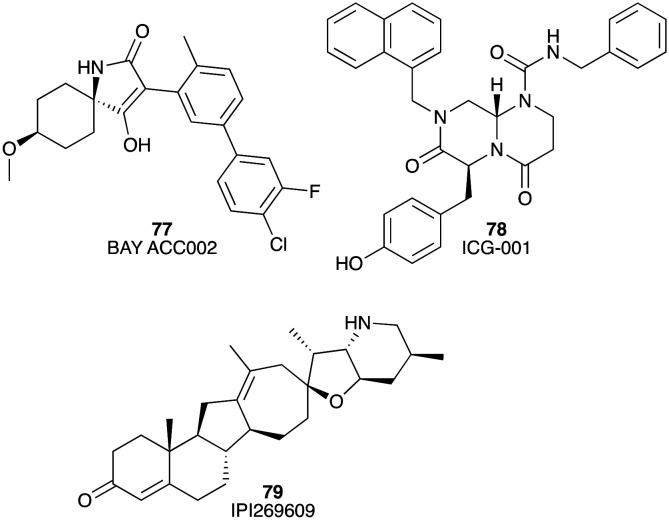
Acetyl-CoA carboxylase (ACC) is a rate-limiting enzyme in the de novo synthesis of lipids. BAY ACC002 (77) selectively inhibited ACC1 and ACC2 with IC50 values of 0.10 and 1.4 μM, respectively.117 Inhibition of ACC by BAY ACC002 (77) resulted in reduced signalling of the critical oncogenic drivers, Hedgehog and WNT, in PC cells.117 It was also found that the proliferation of DanG, Capan-2, BxPC-3, and Panc-1 PC cells was blocked by BAY ACC002 (77) via modulation of Hedgehog and WNT signalling. Further, in a Capan-2 PC mouse model BAY ACC002 (77) treatment led to marked tumour growth inhibition (TGI 42.6%) (Fig. 13).117
Fig. 13. Chemical structures of small molecule inhibitors of Bcl-2 family proteins: BAY ACC002 (77), ICG-001 (78), and IPI269609 (79).
Recent studies have highlighted the importance of ligand-mediated Wnt/β-catenin signalling during the start and progression of PC. CREB-binding protein (CBP) is capable of activating Wnt/β-catenin mediated transcription. ICG-001 (78) is a CBP inhibitor that blocks β-catenin/TCF-mediated transcription (IC50 = 3 μM).118 It suppressed the growth of AsPC-1, L3.6pl, Panc-1 and MiaPaCa-2 cell lines in a dose-dependent manner showing IC50 values of 5.48, 14.07, 3.43 and 3.31 μM, respectively. ICG-001 (78) also substantially extended survival in an orthotopic xenograft model of AsPC-1 (Fig. 13).119
There is some evidence that inhibition of abnormal Hedgehog signalling is a potentially viable therapeutic target in PC.118 IPI-269609 (79) inhibits the Hedgehog signalling pathway impeding the reporter activity of ShhN-stimulated Light II cells at a concentration of 6 μmol L–1.120 The in vitro efficacy of IPI-269609 (79) showed considerable growth inhibition variation from 0 to 100% at 6 μM across a panel of 21 PC cell lines. As a monotherapy, IPI-269609 significantly impeded tumour metastases in an orthotopic E3LZ10.7 pancreatic cell line xenograft mouse model (Fig. 13).120
Miscellaneous inhibitors
MRK-003 (80), an inhibitor of gamma secretase, significantly blocked tumour growth in 5 of 9 (56%) PDAC xenografts in a preclinical trial;121 MDC-1016 (81), a RAS inhibitor, greatly decreased tumour growth by 62% and 65% in a MiaPaCa-2 tumour xenograft mouse model at concentrations of 50 and 100 mg kg–1;122 TIC10/ONC201 (82), a small molecule inducer of tumour necrosis (TNF)-related apoptosis-inducing ligand (TRAIL), was studied in a preclinical trial (Fig. 14). The results revealed that TIC10 significantly inhibited tumour growth in a Panc-1 tumour xenograft mouse model and enhanced the anti-cancer activity of gemcitabine.123
Fig. 14. Chemical structures of miscellaneous inhibitors under preclinical evaluation for potential use in the treatment of PC: MRK-003 (80), MDC-1016 (81), and TIC10/ONC201 (82).
Conclusions
Over the past decade, significant research efforts have been directed towards the development of small molecule inhibitors as potential PC treatments. Although there have been multiple promising clinical trials, overall progress has been slow with limited enhancement in patient survival relative to gemcitabine (1) monotherapy. Within the myriad of chemical scaffolds reaching clinical trials, the high number of protein kinase inhibitors results in the pyrimidine (1, 2, 7, 17, 18, 22, 23, 25–28, 42, 46, 49, 51, 52, 63 and 82) and related (19 and 44) nuclei being an overrepresented motif. The presence of this motif does not guarantee activity against pancreatic cancer, being a reflection of the binding requirements within the kinase inhibitor family.
Numerous factors contribute to the poor treatment outcomes of PC. A primary characteristic of PC is excessive extracellular matrix production, with desmoplastic fibrotic stroma able to occupy up to 80% of tumour volume. The desmoplasia leads to the poor perfusion of pancreatic tumours, limiting the delivery of therapeutic agents into the tumours and correlates with poor prognosis of patients.7,124 In addition, PDAC is a late onset disease with a median diagnosis age of 71 years.125 This population of patients is accustomed to aches and pains, which results in the neglect of the earliest signs of malignancy. Additionally, the pancreas is positioned too deep within the human body to palpate. Thus early diagnosis of PC is difficult compared with other cancers like breast cancer.126 Furthermore, the efficacy of local therapies is limited. Surgical resection remains the only current potentially curative treatment option for PC patients. However, only a minority of patients are in an amenable disease state for this procedure due to the late diagnosis.7 Finally, the dismal outcomes of the clinical trials show that the response of PC to chemotherapy is poor. This is in part a function of validated reliable biomarkers capable of identifying PC subtypes and thus guides clinical trials.
Other factors for poor progress in this area include poor pre-clinical models and failure to identify responsive patients across an array of therapies that would enable improved trial design. Clearly PC trials could be improved by a more complete understanding of the molecular pathways involved. This is critical, especially in those cases where PC resistance is innate, not acquired, which is the case with most of the other cancers.126
Therefore, current challenges for clinical trials involve better design of preclinical models, enhancing patient management and selection, discerning efficient combinations of multiple types of agents, identification of biomarkers that specifically distinguish responsive cancer subtypes and improving study designs. Multiple inhibitors acting against multiple protein targets have been investigated (Table 2). This gives an unrefined indication of the scope of the problem in treating PC, with few well defined and validated targeted approaches possible, but this also provides hope for a more positive future outcome, with a ‘glass half-full approach’ struggling to see how all the current targets under investigation can all fail to enhance the treatment options for PC. Advances in sequencing technologies will ultimately aid in the identification of the most appropriate drug target or more likely multiple drug targets as the PC drug pipeline continues to be developed. Other initiatives such as ProCan127 that seeks to sequence the protein complement of human cancers may offer an alternative approach enabling rapid re-purposing of current clinical agents to protein targets identified during assessment of the diverse array of pancreatic cancer subtypes.
Table 2. Small molecule inhibitors and their identified targets under current investigation for the treatment of pancreatic cancer.
| Compound | IC50 (μM) | Target |
| PD173074 (42) | 2.5 to 15 (AsPC-1, Capan-1, HPAF-II, MiaPaCa-2, and Panc 1) | FGFR1 tyrosine kinase; VEGFR2 |
| PKC412 (43) | 0.25 to 20 (16 pancreatic cancer cell lines) | Ser/Thr and tyrosine kinases |
| BGB324 (44) + gemcitabine (1) | 0.016, 0.0069 (AsPC-1, MiaPaCa-2) | Receptor tyrosine kinase Axl |
| GSK2256098 (45) | 25, 29 (L3.6P1, Panc-1) | Focal adhesion kinase |
| PF573228 (46) | NA a | |
| Binimetinib (MEK162) (47) | 0.092, 0.28 and 0.316 (MiaPaCa-2, AsPC-1, and CAPAN-2 | Mitogen-activated protein kinase kinase |
| Cobimetinib (48) | NA | |
| GSK2656157 (49) | NA a | Protein kinase R (PKR)-like endoplasmic reticulum kinase |
| 3-(2,5-dimethoxyphenyl)-N-((4-(5-(4-fluorophenyl)-2-(methylthio)-1H-imidazol-4-yl)pyridin-2-yl)carbamoyl)-propanamide (50) | EC50: 3.5, 1.5 (Colo357, Panc89) | Protein kinase CK1δ |
| SCH727965 (dinaciclib) (51) | GI50: 0.01, 0.02 (MiaPaCa-2,Pa20C) | Cyclin-dependent kinase (CDK; CDK1,2,5,9) |
| AZD6738 (52) | GR50: 0.9 μM, 1.57, 1.98, 3.2, 4.8, 9.8, 32.3 (SW1990, Capan-1, AsPC-1, HPAF-II, Capan-2, MiaPaCa-2, and Panc-1) | Ataxia telangiectasia and Rad3-related protein |
| L61H46 (53) | 0.86, 2.83 (BXPC-3, Panc-1) | Signal transducer and activator of transcription3 |
| PG-S3-001 (54) | ED50: 2.4 (Panc10.05) | |
| HJC0416 (55) | 0.04, 1.88 (AsPC-1, Panc-1) | |
| XZH-5 (56) | 24.7, 17.4 and 17.9 (Panc-1, HPAC, and SW1990) | |
| Methyl Nα-(((3,5-bis(trifluoromethyl)phenyl)-carbamoyl)isoleucyl)-Nτ-methyl-l-histidinate (57) | 10.1, 7.6, 8.3 (Panc-1, HPAC, and SW1990) | |
| Cryptotanshinone (58) | NA a | |
| JQ1 (59) | 0.037, 0.72 and 0.19 (AsPC-1, Panc-1, and CAPAN-1) | Bromodomain and extra terminal proteins: BRD2, BRD3 and BRD4 |
| I-BET 762 (60) | 0.231, 2.55 and 0.99 (AsPC-1, Panc-1, and CAPAN-1) | |
| ST-3595 (61) | NA a | Histone deacetylases |
| CG200745 (62) | 2.4, 10.7 and 7.4 (BxPC3, CFPAC1 and HPAC) | |
| MGCD0103 (63) | 3.9, 1.1, 0.6 and 1.8 (AsPC-1, BxPC-3, MiaPaca-2, and Panc-1) | |
| Belinostat (64) | EC50: 0.3, 0.7, 0.5, 1.1, 1.1 and 0.7 (AsPC1, BxPC3, Panc0327, Panc0403, and Panc1005, MiaPaCa-2) | |
| Panobinostat (65) | 0.284, 0.00046, 0.0077, 0.261, 0.013 (BxPC3, Panc0327, Panc0403, Panc1005, and MiaPaCa-2) | |
| UMI-77 (66) | 3.4, 4.4 and 5.5 (AsPC-1, BxPC-3, and Capan-2) | Bcl-2 family proteins |
| 4-((6-nitroquinolin-4-yl)amino)-N-(4-(pyridin-4-ylamino) phenyl benzamide (67) | 15.1 (BxPC-3) | |
| 2,3,4,6-tetrahydroxy-5H-benzo[7]annulen-5-one (68) | 17.6 (MiaPaCa-2) | |
| TW-37 (69) | NA a | |
| ABT737 (70) | NA a | |
| BMS-754807 (71) + gemcitabine (1) | 0.075, 0.070, 0.016, 0.016 (AsPC, Panc-1, MiaPaCa-2, and BxPC-3) | IGF-1R and insulin receptor (IR) family kinases |
| P1608K04 (72) | 8.5, 14, and 20 (Panc-1, MiaPaCa-2, and AsPC1) | Protein arginine methyltransferases |
| MI-319 (73) | NA a | Murine double minute 2 protein |
| NPC-26 (74) | NA a | Mitochondrion interfering |
| Spongiatriol (75) | 13, 8, 6 and 13 (AsPC-1, BxPC-3, MiaPaCa-2 and Panc-1) | Nuclear factor kappa B |
| SW IV-134 (76) | 7.4, 6.8, 6.3 and 7.8 (CFPAC-1, BxPC-3, AsPC-1, Panc-1 and MiaPaCa-2) | Sigma-2 receptor |
| BAY ACC002 (77) | NA a | Acetyl-CoA carboxylase 1 and 2; Hedgehog signalling pathway |
| ICG-001 (78) | 5.48, 14.07, 3.43 and 3.31 (AsPC-1, L3.6pl, Panc-1 and MiaPaCa-2) | CREB-binding protein |
| IPI-269609 (79) | NA a | Hedgehog signalling pathway |
| MRK-003 (80) | NA a | Gamma secretase |
| MDC-1016 (81) | NA a | RAS inhibitor |
| TIC10/ONC201 (82) | NA a | Tumour necrosis (TNF)-related apoptosis-inducing ligand |
aNo IC50 value was reported in the primary literature.
The number of clinical trials investigating small molecule inhibitors for the treatment of PC is expanding rapidly, in part as a consequence of a greater understanding of cancer cell and receptor biology, and the inherent molecular heterogeneity, in particular, the understanding of the intricate molecular pathology of PDAC improved by genomic analyses.128 It is clear that a multifaceted approach across medicinal chemistry, chemical biology, proteomics and oncology is required to enhance treatment options, and ultimately patient outcomes. Of the progress made to date, the advent of combination approaches appears to have made the greatest inroads in disease treatment with FOLFIRINOX and variations on gemcitabine (1) combination therapy showing modest improvements in patient survival. However, correct identification of potential combinations is complicated by the lack of clear biomarkers to guide clinical trial study design.
Many of the issues associated with targeting pancreatic cancer have been discussed and the reasons for key difficulties known for the past 20 years. With this being the case, a reviewer rightly questioned, why has progress been so slow? This is an almost impossible question to answer beyond speculation, but there have been major changes in both the academic and industry drug development landscapes in this time. How much of this is a function of the parties involved being risk adverse? Is the increasing pressure in academia to provide rapid short-term fixes to problems that can be delivered in short media friendly ‘bites’ stifling creativity, exploration of the biology and interconnectivity of the human system enabling a true multifaceted approach to novel outcomes? Or is this a consequence of lack of novelty, inventiveness and interest? It is not possible in this review to provide solutions, but we clearly sit at a pivotal point in PC drug development: medicinal chemistry is extraordinarily capable of developing highly specific molecules; there is an absolute need for high specificity probe molecules; the corresponding validated protein targets; a more complete understanding of the complex nature of PC and the associated signalling (and resistance mechanisms) underpins these approaches. Team assembly and resourcing will ultimately drive the developments in this area. Academia resourced by industry and government, removed from the short term funding cycles is extremely capable of delivering the required innovation in this space. Perhaps the greatest challenge is in enacting a culture change that recognises that major breakthroughs are a result of blue sky, truly innovative and curiosity driven research.
The current status quo is not a viable option.
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
J. F. S. is the recipient of a scholarship from University of Newcastle, Australia and Binzhou Medical University, China, and this project has received funding from the UON Priority Research Centre for Drug Development.
Biographies

Jufeng Sun
Jufeng Sun was awarded a master's degree in medicinal chemistry by Tianjin University, China in 2005. She worked at Binzhou Medical University, China from 2006 to 2016 engaging in synthesis and screening of small molecules with anti-cancer activity. She moved to the University of Newcastle, Australia as a PhD candidate in 2017. Her PhD research focused on potential small molecule inhibitors of S100A2-p53 for anti-pancreatic cancer drug development under the supervision of Professor Adam McCluskey. Her research interests include cancer drug discovery as well as the applications of molecular modelling docking in medicinal chemistry.

Cecilia C Russell
Cecilia C Russell was awarded a PhD in medicinal chemistry by the University of Newcastle in 2006. She then worked in industry for Jurox Pty Ltd in both API manufacturing and pharmacokinetics – residue analysis. In 2011 she took up a research associate position in the McCluskey group at the University of Newcastle (Australia) where her primary research focus is the synthesis and development of small molecule modulators of proteins within antibiotic and cancer drug discovery as well as the application of flow chemistry to synthetic and medicinal chemistry.

Christopher Scarlett
Christopher Scarlett was awarded a PhD in pancreatic cancer biology by the University of Sydney in 2007. He took up a postdoctoral position at the Garvan Institute of Medical Research, Sydney as an NHMRC and Cancer Institute NSW Fellow with Professor Andrew Biankin investigating the role of bone marrow-derived cells in pancreatic carcinogenesis. In 2012 he took up an academic position at the University of Newcastle (Australia) where his primary research focus is the development and assessment of novel therapeutic agents targeting specific pancreatic cancer molecular phenotypes.

Adam McCluskey
Adam McCluskey was awarded a Ph.D. in organic chemistry by the University of Strathclyde in 1988. He moved to the University of Queensland as a postdoctoral fellow in reactive intermediates chemistry with Professor Curt Wentrup. In 1991 he undertook a change in research focus to medicinal chemistry with Professor Ronald J Quinn at Griffith University (Australia). He then took up an academic position at the University of Newcastle at the end of 1995 where his primary focus is on the development of small molecule modulators of proteins and protein–protein interactions.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c9md00447e
References
- Teague A., Lim K.-H., Wang-Gillam A. Ther. Adv. Med. Oncol. 2015;7:68–84. doi: 10.1177/1758834014564775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan A. V., Biankin A. V., Parish C. R., Levon M. Oncotarget. 2018;9:21613–21627. doi: 10.18632/oncotarget.25085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink D. M., Steele M. M., Hollingsworth M. A. Cancer Lett. 2016;381:217–236. doi: 10.1016/j.canlet.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A. L., Malvezzi M., Carioli G., Negri E., La Vecchia C., Boffetta P., Bosetti C. Clin. Gastroenterol. Hepatol. 2016;14:1452–1462. doi: 10.1016/j.cgh.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P., Chang D. K., Nones K., Johns A. L., Patch A. M., Gingras M. C., Miller D. K., Christ A. N., Bruxner T. J., Quinn M. C., Nourse C., Murtaugh L. C., Harliwong I., Idrisoglu S., Manning S., Nourbakhsh E., Wani S., Fink L., Holmes O., Chin V., Anderson M. J., Kazakoff S., Leonard C., Newell F., Waddell N., Wood S., Xu Q., Wilson P. J., Cloonan N., Kassahn K. S., Taylor D., Quek K., Robertson A., Pantano L., Mincarelli L., Sanchez L. N., Evers L., Wu J., Pinese M., Cowley M. J., Jones M. D., Colvin E. K., Nagrial A. M., Humphrey E. S., Chantrill L. A., Mawson A., Humphris J., Chou A., Pajic M., Scarlett C. J., Pinho A. V., Giry-Laterriere M., Rooman I., Samra J. S., Kench J. G., Lovell J. A., Merrett N. D., Toon C. W., Epari K., Nguyen N. Q., Barbour A., Zeps N., Moran-Jones K., Jamieson N. B., Graham J. S., Duthie F., Oien K., Hair J., Grützmann R., Maitra A., Iacobuzio-Donahue C. A., Wolfgang C. L., Morgan R. A., Lawlor R. T., Corbo V., Bassi C., Rusev B., Capelli P., Salvia R., Tortora G., Mukhopadhyay D., Petersen G. M., Initiative Australian Pancreatic Cancer Genome, Munzy D. M., Fisher W. F., Karim S. A., Eshleman J. R., Hruban R. H., Pilarsky C., Morton J. P., Sansom O. J., Scarpa A., Musgrove E. A., Bailey U. M., Hofmann O., Sutherland R. L., Wheeler D. A., Gill A. J., Gibbs R. A., Pearson J. V., Waddell N., Biankin A. V., Grimmond S. M. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- Types of Pancreatic Cancer Fact Sheet, Pancreatic Cancer UK, 2014.
- Kota J., Hancock J., Kwon J., Korc M. Cancer Lett. 2017;391:38–49. doi: 10.1016/j.canlet.2016.12.035. [DOI] [PubMed] [Google Scholar]
- Chang D. K., Johns A. L., Merrett N. D., Gill A. J., Colvin E. K., Scarlett C. J., Nguyen N. Q., Leong R. W., Cosman P. H., Kelly M. I., Sutherland R. L., Henshall S. M., Kench J. G., Biankin A. V. J. Clin. Oncol. 2009;27:2855–2862. doi: 10.1200/JCO.2008.20.5104. [DOI] [PubMed] [Google Scholar]
- Neoptolemos J. P., Dunn J. A., Stocken D. D., Almond J., Link K., Beger H., Bassi C., Falconi M., Pederzoli P., Dervenis C., Fernandez-Cruz L., Lacaine F., Pap A., Spooner D., Kerr D. J., Friess H., Buchler M. W. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- Chin V., Nagrial A., Sjoquist K., O'Conner C. A., Chantrill L., Biankin A. V., Scholten R. J., Yip D. Cochrane Database Syst Rev. 2018;3:1–165. doi: 10.1002/14651858.CD011044.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha A. S., Khoo A., Aliru M. L., Arora H. K., Gunther J. R., Krishnan S. Semin. Radiat. Oncol. 2016;26:320–337. doi: 10.1016/j.semradonc.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Burris H. A., Moore M. J., Andersen J., Green M. R., Rothenberg M. L., Modiano M. R., Cripps M. C., Portenoy R. K., Stroniolo A. M., Tarassoff P., Nelson R., Dorr F. A., Stephens C. D., Von Hoff D. D. J. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- Neoptolemos J. P., Stocken D. D., Tudur Smith C., Bassi C., Ghaneh P., Owen E., Moore M., Padbury R., Doi R., Smith D., Buchler M. W. Br. J. Cancer. 2009;100:246–250. doi: 10.1038/sj.bjc.6604838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoptolemos J. P., Palmer D. H., Ghaneh P., Psarelli E. E., Valle J. W., Halloran C. M., Faluyi O., O'Reilly D. A., Cunningham D., Wadsley J., Darby S., Meyer T., Gillmore R., Anthoney A., Lind P., Glimelius B., Falk S., Izbicki J. R., Middleton G. W., Cummins S., Ross P. J., Wasan H., McDonald A., Crosby T., Ma Y. T., Patel K., Sherriff D., Soomal R., Borg D., Sothi S., Hammel P., Hackert T., Jackson R., Büchler M. W. Lancet. 2017;389:1011–1024. doi: 10.1016/S0140-6736(16)32409-6. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Ervin T., Arena F. P., Chiorean E. G., Infante J., Moore M., Seay T., Tjuladin S. A., Ma W. W., Saleh M. N., Harris M., Reni M., Dowden S., Laheru D., Bahary N., Ramanathan R. K., Tabernero J., Hidalgo M., Goldstein D., Van Cutsem E., Wei X., Iglesias J., Renschler M. F. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrone C. R., Marchegiani G., Hong T. S., Ryan D. P., Deshpande V., McDonnell E. I., Sabbatino F., Santos D. D., Allen J. N., Blaszkowsky L. S., Clark J. W., Faris J. E., Goyal L., Kwak E. L., Murphy J. E., Ting D. T., Wo J. Y., Zhu A. X., Warshaw A. L., Lillemoe K. D., Fernández-del Castillo C. Ann. Surg. 2015;261:12–17. doi: 10.1097/SLA.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., El-Rayes B. F. Biologics. 2008;2:707–715. doi: 10.2147/btt.s3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanam I., Chung V. Cancers. 2018;10:36. doi: 10.3390/cancers10020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biankin A. V., Waddell N., Kassahn K. S., Gingras M. C., Muthuswamy L. B., Johns A. L., Miller D. K., Wilson P. J., Patch A. M., Wu J., Chang D. K., Cowley M. J., Gardiner B. B., Song S., Harliwong I., Idrisoglu S., Nourse C., Nourbakhsh E., Manning S., Wani S., Gongora M., Pajic M., Scarlett C. J., Gill A. J., Pinho A. V., Rooman I., Anderson M., Holmes O., Leonard C., Taylor D., Wood S., Xu Q., Nones K., Fink J. L., Christ A., Bruxner T., Cloonan N., Kolle G., Newell F., Pinese M., Mead R. S., Humphris J. L., Kaplan W., Jones M. D., Colvin E. K., Nagrial A. M., Humphrey E. S., Chou A., Chin V. T., Chantrill L. A., Mawson A., Samra J. S., Kench J. G., Lovell J. A., Daly R. J., Merrett N. D., Toon C., Epari K., Nguyen N. Q., Barbour A., Zeps N., Initiative Australian Pancreatic Cancer Genome, Kakkar N., Zhao F., Wu Y. Q., Wang M., Muzny D. M., Fisher W. E., Brunicardi F. C., Hodges S. E., Reid J. G., Drummond J., Chang K., Han Y., Lewis L. R., Dinh H., Buhay C. J., Beck T., Timms L., Sam M., Begley K., Brown A., Pai D., Panchal A., Buchner N., De Borja R., Denroche R. E., Yung C. K., Serra S., Onetto N., Mukhopadhyay D., Tsao M. S., Shaw P. A., Petersen G. M., Gallinger S., Hruban R. H., Maitra A., Iacobuzio-Donahue C. A., Schulick R. D., Wolfgang C. L., Morgan R. A., Lawlor R. T., Capelli P., Corbo V., Scardoni M., Tortora G., Tempero M. A., Mann K. M., Jenkins N. A., Perez-Mancera P. A., Adams D. J., Largaespada D. A., Wessels L. F., Rust A. G., Stein L. D., Tuveson D. A., Copeland N. G., Musgrove E. A., Scarpa A., Eshleman J. R., Hudson T. J., Sutherland R. L., Wheeler D. A., Pearson J. V., McPherson J. D., Gibbs R. A., Grimmond S. M. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S., Zhang X., Parsons D. W., Lin J. C., Leary R. J., Angenendt P., Mankoo P., Carter H., Kamiyama H., Jimeno A., Hong S. M., Fu B., Lin M. T., Calhoun E. S., Kamiyama M., Walter K., Nikolskaya T., Nikolsky Y., Hartigan J., Smith D. R., Hidalgo M., Leach S. D., Klein A. P., Jaffee E. M., Goggins M., Maitra A., Iacobuzio-Donahue C., Eshleman J. R., Kern S. E., Hruban R. H., Karchin R., Papadopoulos N., Parmigiani G., Vogelstein B., Velculescu V. E., Kinzler K. W. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell N., Pajic M., Patch A. M., Chang D. K., Kassahn K. S., Bailey P., Johns A. L., Miller D., Nones K., Quek K., Quinn M. C., Robertson A. J., Fadlullah M. Z., Bruxner T. J., Christ A. N., Harliwong I., Idrisoglu S., Manning S., Nourse C., Nourbakhsh E., Wani S., Wilson P. J., Markham E., Cloonan N., Anderson M. J., Fink J. L., Holmes O., Kazakoff S. H., Leonard C., Newell F., Poudel B., Song S., Taylor D., Waddell N., Wood S., Xu Q., Wu J., Pinese M., Cowley M. J., Lee H. C., Jones M. D., Nagrial A. M., Humphris J., Chantrill L. A., Chin V., Steinmann A. M., Mawson A., Humphrey E. S., Colvin E. K., Chou A., Scarlett C. J., Pinho A. V., Giry-Laterriere M., Rooman I., Samra J. S., Kench J. G., Pettitt J. A., Merrett N. D., Toon C., Epari K., Nguyen N. Q., Barbour A., Zeps N., Jamieson N. B., Graham J. S., Niclou S. P., Bjerkvig R., Grützmann R., Aust D., Hruban R. H., Maitra A., Iacobuzio-Donahue C. A., Wolfgang C. L., Morgan R. A., Lawlor R. T., Corbo V., Bassi C., Falconi M., Zamboni G., Tortora G., Tempero M. A., Initiative Australian Pancreatic Cancer Genome, Gill A. J., Eshleman J. R., Pilarsky C., Scarpa A., Musgrove E. A., Pearson J. V., Biankin A. V., Grimmond S. M. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou A., Waddell N., Cowley M. J., Gill A. J., Chang D. K., Patch A. M., Nones K., Wu J., Pinese M., Johns A. L., Miller D. K., Kassahn K. S., Nagrial A. M., Wasan H., Goldstein D., Toon C. W., Chin V., Chantrill L., Humphris J., Mead R. S., Rooman I., Samra J. S., Pajic M., Musgrove E. A., Pearson J. V., Morey A. L., Grimmond S. M., Biankin A. V. Genome Med. 2013;5:78. doi: 10.1186/gm482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer S. B., Chang D. K., Bailey P., Biankin A. V. Clin. Cancer Res. 2017;23:1638–1646. doi: 10.1158/1078-0432.CCR-16-2411. [DOI] [PubMed] [Google Scholar]
- Manji G. A., Olive K. P., Saenger Y. M., Oberstein P. Clin. Cancer Res. 2017;23:1670–1678. doi: 10.1158/1078-0432.CCR-16-2319. [DOI] [PubMed] [Google Scholar]
- Garrido-Laguna I., Hidalgo M. Nat. Rev. Clin. Oncol. 2015;12:319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- Cheng X. B., Sato N., Kohi S., Yamaguchi K. PLoS One. 2013;8:e80765. doi: 10.1371/journal.pone.0080765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano P. P., Cuevas C., Chang A. E., Goel V. K., Von Hoff D. D., Hingorani S. R. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive K. P., Jacobetz M. A., Davidson C. J. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani S. R., Zheng L., Bullock A. J., Seery T. E., Harris W. P., Sigal D. S., Braiteh F., Ritch P. S., Zalupski M. M., Bahary N., Oberstein P. E., Wang-Gillam A., Wu W., Chondros D., Jaing P., Khelifa S., Pu J., Aldrich C., Hendifar A. E. J. Clin. Oncol. 2018;36:359–366. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- Weng C. C., Lin Y. C., Cheng K. H. J. Clin. Med. 2019;8:E1369. [Google Scholar]
- Schultze A., Fiedler W. Anti-Cancer Agents Med. Chem. 2011;11:593–599. doi: 10.2174/187152011796817727. [DOI] [PubMed] [Google Scholar]
- Chung V., Mansfield A. S., Braiteh F., Richards D., Durivage H., Ungerleider R. S., Johnson F., Kovac J. S. Clin. Cancer Res. 2017;23:3277–3284. doi: 10.1158/1078-0432.CCR-16-2299. [DOI] [PubMed] [Google Scholar]
- LoRusso P. M., Adjei A. A., Varterasian M., Gadgeel S., Reid J., Mitchell D. Y., Hanson L., DeLuca P., Bruzek L., Piens J., Asbury P., Van Becelaere K., Herrera R., Sebolt-Leopold J., Meyer M. B. J. Clin. Oncol. 2005;23:5281–5293. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- Millward M., Price T., Townsend A., Sweeney C., Spencer A., Sukumaran S., Longenecker A., Lee L., Lay A., Sharma G., Gemmill R. M., Drabkin H. A., Lloyd G. K., Neuteboom S. T., McConkey D. J., Palladino M. A., Spear M. A. Invest. New Drugs. 2012;30:2303–2317. doi: 10.1007/s10637-011-9766-6. [DOI] [PubMed] [Google Scholar]
- Laheru D., Shah P., Rajeshkumar N. V., McAllister F., Taylor G., Goldsweig H., Le D. T., Donehower R., Jimeno A., Linden S., Zhao M., Song D., Rudek M. A., Hidalgo M. Invest. New Drugs. 2013;30:2391–2399. doi: 10.1007/s10637-012-9818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E., Arlinghaus L. R., Cardin D. B., Goff L., Berlin J. D., Parikh A., Abramson R. G., Yankeelov T. E., Hiebert S., Merchant N., Bhaskara S., Chakravarthy A. B. Radiother. Oncol. 2016;119:312–318. doi: 10.1016/j.radonc.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cutsem E., Hidalgo M., Canon J. L., Macarulla T., Bazin I., Poddubskaya E., Manojlovic N., Radenkovic D., Verslype C., Raymond E., Cubillo A., Schueler A., Zhaoi C., Hammel P. Int. J. Cancer. 2018;143:2053–2064. doi: 10.1002/ijc.31603. [DOI] [PubMed] [Google Scholar]
- Cuneo K. C., Morgan M., Schipper M. J., Maybaum J., Al-Hawary M., Simeone D. M., Sahai V., Zalupski M., Lawrence T. S. J. Clin. Oncol. 2017;35(4_suppl):TPS512. [Google Scholar]
- Ko A. H., LoConte N., Tempero M. A., Walker E. J., Kate Kelly R., Lewis S., Chang W. C., Kantoff E., Vannier M. W., Catenacci D. V., Venook A. P., Kindler H. L. Pancreas. 2016;45:370–375. doi: 10.1097/MPA.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A. T., Halperin D. M., Chan J. A., Fogelman D. R., Hess K. R., Malinowski P., Regan E., Ng C. S., Yao J. C., Kulke M. H. Lancet Oncol. 2015;16:695–703. doi: 10.1016/S1470-2045(15)70136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragovich T., Laheru D., Dayyani F., Bolejack V., Smith L., Seng J., Burris H., Rosen P., Hidalgo M., Rich P., Baker A. F., Raghunand N., Crowley J., Van Hoff D. D. Cancer Chemother. Pharmacol. 2014;74:379–387. doi: 10.1007/s00280-014-2499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melisi D., Garcia-Carbonero R., Macarulla T., Pezet D., Deplanque G., Fuchs M., Trojan J., Oettle H., Kozloff M., Cleverly A., Smith C., Estrem S. T., Gueorguieva I., Lahn M. M. F., Blunt A., Benhadji K. A., Tabernero J. Br. J. Cancer. 2018;119:1208–1214. doi: 10.1038/s41416-018-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T. S., Yon-Li Wo J., Jiang W., Yeap B. Y., Clark J. W., Ryan D. P., Blaszkowsky L. S., Drapek L. C., Mamon H. J., Murphy J. E., Faris J. E., Kwak E. L., Allen J. N., Zhu A. X., Goyal L., Lillemoe K. D., Ferrone C., DeLaney T. F., Fernandez-del Castillo C., Kimmelman A. J. Clin. Oncol. 2017;35:4118. [Google Scholar]
- Wu Z., Gabrielson A., Hwang J. J., Pishvaian M. J., Weiner L. M., Zhuang T., Ley L., Marshall J. L., He A. R. Cancer Chemother. Pharmacol. 2015;76:1309–1314. doi: 10.1007/s00280-015-2855-z. [DOI] [PubMed] [Google Scholar]
- Middleton G., Palmer D. H., Greenhalf W., Ghaneh P., Jackson R., Cox T., Evans A., Shaw V. E., Wadsley J., Valle J. W., Propper D., Wasan H., Falk S., Cunningham D., Coxon F., Ross P., Madhusudan S., Wadd N., Corrie P., Hickish T., Costello E., Campbell F., Rawcliffe C., Neoptolemos J. P. Lancet Oncol. 2017;18:486–499. doi: 10.1016/S1470-2045(17)30084-0. [DOI] [PubMed] [Google Scholar]
- Cardin D. B., Goff L., Li C. I., Shyr Y., Winkler C., DeVore R., Schlabach L., Holloway M., McClanahan P., Meyer K., Grigorieva J., Berlin J., Chan E. Cancer Med. 2014;3:572–579. doi: 10.1002/cam4.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. R. J., Van Cutsem E., Moore M. J., Bazin I. S., Rosemurgy A., Bodoky G., Deplanque G., Harrison M., Melichar B., Pezet D., Elekes A., Rock E., Lin C., Strauss L., O'Dwyer P. J. Ann. Oncol. 2017;28:354–361. doi: 10.1093/annonc/mdw607. [DOI] [PubMed] [Google Scholar]
- Renouf D. J., Moore M. J., Hedley D., Gill S., Jonker D., Chen E., Walde D., Goel R., Southwood B., Gauthier I., Walsh W., McIntosh L., Seymour L. Invest. New Drugs. 2012;30:779–786. doi: 10.1007/s10637-010-9611-3. [DOI] [PubMed] [Google Scholar]
- Moss R. A., Moore D., Mulcahy M. F., Nahum K., Saraiya B., Eddy S., Leber M., Poplin E. A. Gastrointest. Cancer Res. 2012;5:77–83. [PMC free article] [PubMed] [Google Scholar]
- Catenacci D. V., Junttila M. R., Karrison T., Bahary N., Horiba M. N., Nattam S. R., Marsh R., Wallace J., Kozloff M., Rajdev L., Cohen D., Wade J., Sleckman B., Lenz H. J., Stiff P., Kumar P., Xu P., Henderson L., Takebe N., Salgia R., Wang X., Stadler W. M., de Sauvage F. J., Kindler H. L. J. Clin. Oncol. 2015;33:4284–4292. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio N., Buzzoni R., Baudin E., Antonuzzo L., Hubner R. A., Lahner H., De Herder W. W., Raderer M., Teule A., Capdevila J., Libutti S. K. Anticancer Res. 2016;36:713–719. [PMC free article] [PubMed] [Google Scholar]
- Ramanathan R. K., Abbruzzese J., Dragovich T., Kirkpatrick L., Guillen J. M., Baker A. F., Pestano L. A., Green S., Van Hoff D. D. Cancer Chemother. Pharmacol. 2011;67:503–509. doi: 10.1007/s00280-010-1343-8. [DOI] [PubMed] [Google Scholar]
- Lowery M. A., Kelsen D. P., Capanu M., Smith S. C., Lee J. W., Stadler Z. K., Moore M. J., Kindler H. L., Golan T., Segal A., Maynard H., Hollywood E., Moynahan M., Salo-Mullen E. E., Do R. K. G., Chen A. P., Yu K. H., Tang L. H., O'Reilly E. M. Eur. J. Cancer. 2018;89:19–26. doi: 10.1016/j.ejca.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante J. R., Somer B. G., Park J. O., Li C. P., Scheulen M. E., Kasubhai S. M., Oh D. Y., Liu Y., Redhu S., Steplewski K., Le N. Eur. J. Cancer. 2014;50:2072–2081. doi: 10.1016/j.ejca.2014.04.024. [DOI] [PubMed] [Google Scholar]
- Bodoky G., Timcheva C., Spigel D. R., La Stella P. J., Ciuleanu T. E., Pover G., Tebbutt N. C. Invest. New Drugs. 2012;30:1216–1223. doi: 10.1007/s10637-011-9687-4. [DOI] [PubMed] [Google Scholar]
- O'Neil B. H., Scott A. J., Ma W. W., Cohen S. J., Aisner D. L., Menter A. R., Tejani M. A., Cho J. K., Granfortuna J., Coveler A. L., Olowokure O. O., Baranda J. C., Cusnir M., Phillip P., Boles J., Nazemzadeh R., Rarick M., Cohen D. J., Radford J., Fehrenbacher L., Bajaj R., Bathini V., Fanta P., Berlin J., McRee A. J., Maguire R., Wilhelm F., Maniar M., Jimeno A., Gomes C. L., Messersmith W. A. Ann. Oncol. 2016;27:1180–1180. doi: 10.1093/annonc/mdw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks E. D. Drugs. 2015;75:231–240. doi: 10.1007/s40265-015-0345-6. [DOI] [PubMed] [Google Scholar]
- Yarchoan M., Myzak C., Johnson B. A., De Jesus-Acosta A., Le D. T., Jaffee E. M., Azad N. S., Donehower R. C., Zheng L., Obeerstein P. E., Fine R. L., Laheru D. A., Goggins M. Oncotarget. 2017;8:44073–44081. doi: 10.18632/oncotarget.17237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioka T., Okusaka T., Ohkawa S., Boku N., Sawaki A., Fujii Y., Kamei Y., Takahashi S., Namazu K., Umeyama Y., Bycott P., Furuse J. Jpn. J. Clin. Oncol. 2015;45:439–448. doi: 10.1093/jjco/hyv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R. K., Ko A. H. Biologics. 2008;2:83–95. doi: 10.2147/btt.s1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal G. M., Cortazar P., Zhang J. J., Tang S., Sridhara R., Murgo A., Justice R., Pazdur R. Oncologist. 2012;17:1108–1113. doi: 10.1634/theoncologist.2012-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Sliwkowski M. X. Nat. Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Hubbard S. R. Cell. 2006;125:1029–1031. doi: 10.1016/j.cell.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Papageorgio C., Perry M. C. Cancer Invest. 2007;25:647–657. doi: 10.1080/07357900701522653. [DOI] [PubMed] [Google Scholar]
- Oliveira-Cunha M., Newman W. G., Siriwardena A. K. Cancers. 2011;3:1513–1526. doi: 10.3390/cancers3021513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M., Froum S., Hamby J. M., Schroeder M. C., Panek R. L., Lu G. H., Eliseenkova A. V., Green D., Schlessinger J., Hubbard S. R. EMBO J. 1998;17:5896–5904. doi: 10.1093/emboj/17.20.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchler P., Reber H. A., Roth M. M., Shiroishi M., Friess H., Hines O. J. Neoplasia. 2007;9:119–127. doi: 10.1593/neo.06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Fitori J., Su Y., Büchler P., Ludwig R., Giese N. A., Büchler M. W., Quentmeier H., Hines O. J., Herr I., Friess H. Cancer. 2007;110:1457–1468. doi: 10.1002/cncr.22931. [DOI] [PubMed] [Google Scholar]
- Ludwig K. F., Du W., Sorrelle N. B., Wnuk-Lipinska K., Topalovski M., Toombs J. E., Cruz V. H., Yabuuchi S., Rajeshkumar N. V., Maitra A., Lorens J. B., Brekken R. A. Cancer Res. 2018;78:246–255. doi: 10.1158/0008-5472.CAN-17-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S. J., Pan A., Franci C., Hu Y., Chang B., Li W., Duan M., Tornoreos A., Yu J., Heckrodt T. J., Zhang J., Ding P., Apartira A., Chua J., Brandt R., Pine P., Goff D., Singh R., Payan D. G., Hitoshi Y. Cancer Res. 2010;70:1544–1554. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- Zhang J., He D. H., Zajac-Kaye M., Hochwald S. N. Cell Cycle. 2014;13:3143–3149. doi: 10.4161/15384101.2014.949550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger K. R., Smitheman K. N., Korenchuk S., Mchugh C., Kruger R., Van Aller G. S., Smallwood A., Gondtarek R. R., Faitg T., Johnson N. Eur. J. Cancer. 2012;48:118. doi: 10.1016/S0959-8049(12)72185-8. [DOI] [Google Scholar]
- Slack-Davis J. K., Martin K. H., Tilghman R. W., Iwanicki M., Ung E. J., Autry C., Luzzio M. J., Cooper B., Kath J. C., Roberts W. G., Patersons J. T. J. Biol. Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- Zhao X., Sun W., Puszyk W. M., Wallet S., Hochwald S., Robertson K., Liu C. Tumour Biol. 2017;39:1010428317699120. doi: 10.1177/1010428317699120. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Tian H. Molecules. 2017;22:E1551. doi: 10.3390/molecules22101551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pheneger J., Wallace E., Marlow A., Hurley B., Lyssikatos J., Bendele A. M. and Lee P. A., ACR Annual Scientific Meeting, 2006, pp. 2–3.
- Hamidi H., Lu M., Chau K., Anderson L., Fejzo M., Ginther C., Linnartz R., Zubel A., Slamon D. J., Finn R. S. Br. J. Cancer. 2014;111:1788–1801. doi: 10.1038/bjc.2014.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K., Igarashi K., Murakami T., Kiyuna T., Lwin T. M., Hwang H. K., Delong J. C., Clary B. M., Bouvet M., Unno M., Hoffman R. M. Oncotarget. 2017;8:47490–47496. doi: 10.18632/oncotarget.17667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins C., Liu Q., Minthorn E., Zhang S. Y., Figueroa D. J., Moss K., Stanley T. B., Sanders B., Goetz A., Gaul N., Choudhry A. E., Alsaid H., Jucker B. M., Axten J. M., Kumar R. Cancer Res. 2013;73:1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
- Halekotte J., Witt L., Lanes C., Krüger M., Bührmann M., Rauh D., Pichlo C., Burnstein E., Luxenburger A., Baumann U., Knippschild U., Bischof J., Peifer C. Molecules. 2017;22:522. doi: 10.3390/molecules22040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry D., Guzi T., Shanahan F., Davis N., Prabhavalkar D., Wiswell D., Seghezzi W., Paruch K., Dwyer M. P., Doll R., Nomeir A., Windsor W., Fischmann T., Wang Y., Oft M., Chen T., Kirschmeier P., Lees E. M. Mol. Cancer Ther. 2010;9:2344–2354. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- Feldmann G., Mishra A., Bisht S., Karikari C., Garrido-Laguna I., Rasheed Z., Ottenhof N. A., Dadon T., Alvarez H., Fendrich V., Rajeshkumar N. V., Matsui W., Brossart P., Hidalgo M., Bannerji R., Maitra A., Nelkin B. D. Cancer Biol. Ther. 2011;12:598–609. doi: 10.4161/cbt.12.7.16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendetti F. P., Lau A., Schamus S., Conrads T. P., O'Connor M. J., Bakkenist C. J. Oncotarget. 2015;6:44289–44305. doi: 10.18632/oncotarget.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallez Y., Dunlop C. R., Johnson T. I., Koh S. B., Fornari C., Yates J. W. T., de Quios Fernandez S. Bernaldo, Lau A., Richards F. M., Jodrell D. I. Mol. Cancer Ther. 2018;17:1670–1682. doi: 10.1158/1535-7163.MCT-18-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai B., Yang L., Yang Y., Hu W., Li C., Lin J., Dai X., Lang G., Jin R., Zhao C. Cancer Manage. Res. 2018;10:565–581. doi: 10.2147/CMAR.S159090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpin C. C., Mac S., Jiang Y., Cheng H., Grimard M., Page B. D. G., Kamocka M., Haftchenary S., Su H., Ball D. P., Rosa D. A., Lai P. S., Gomez-Biagi R. F., Ali A. M., Rana R., Hanenberg H., Kerman K., McElyea K. C., Sudusky G., Gunning P. T., Fishel M. Mol. Cancer Ther. 2016;15:794–805. doi: 10.1158/1535-7163.MCT-15-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Yang Z., Ding C., Xiong A., Wild C., Wang L., Ye N., Cai G., Flores R. M., Ding Y., Shen Q., Zhou J. Eur. J. Med. Chem. 2014;82:195–203. doi: 10.1016/j.ejmech.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A., Liu Y., Jin Z., Hu Q., Lin L., Jou D., Yang J., Xu Z., Wang H., Li C., Lin J. PLoS One. 2012;7:e46624. doi: 10.1371/journal.pone.0046624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daka P., Liu A., Karunaratne C., Csatary E., Williams C., Xiao H., Lin J., Xu Z., Page R. C., Wang H. Bioorg. Med. Chem. 2015;23:1348–1355. doi: 10.1016/j.bmc.2015.01.025. [DOI] [PubMed] [Google Scholar]
- Shin D. S., Kim H. N., Shin K. D., Yoon Y. J., Kim S. J., Han D. C., Kwon B. M. Cancer Res. 2009;69:193–202. doi: 10.1158/0008-5472.CAN-08-2575. [DOI] [PubMed] [Google Scholar]
- Ge Y., Yang B., Chen Z., Cheng R. Mol. Med. Rep. 2015;12:7782–7788. doi: 10.3892/mmr.2015.4379. [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W. B., Fedorov O., Morse E. M., Keates T., Hickman T. T., Felletar I., Philpott M., Munro S., McKeown M. R., Wang Y., Christie A. L., West N., Cameron M. J., Schwartz B., Heightman T. D., La Thangue N., French C. A., Wiest O., Kung A. L., Knapp S., Bradner J. E. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodeme E., Jeffery K. L., Schaefer U., Beinke S., Dewell S., Chung C. W., Chandwani R., Marazzi I., Wilson P., Coste H., White J., Kirilovsky J., Rice C. M., Lora J. M., Prinjha R. K., Lee K., Tarakhovsky A. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal A. S., Williams C. R., Royce D. B., Pioli P. A., Sporn M. B., Liby K. T. Cancer Lett. 2017;394:76–87. doi: 10.1016/j.canlet.2017.02.021. [DOI] [PubMed] [Google Scholar]
- Kumar K., Raza S. S., Knab L. M., Chow C. R., Kwok B., Bentrem D. J., Popovic R., Ebine K., Licht J. D., Munshi H. G. Sci. Rep. 2015;5:9489. doi: 10.1038/srep09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Seto E. Cold Spring Harbor Perspect. Med. 2016;6:pii: a026831. doi: 10.1101/cshperspect.a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick V., Simões-Pires C., Cuendet M. J. Enzyme Inhib. Med. Chem. 2016;31:209–214. doi: 10.1080/14756366.2016.1180595. [DOI] [PubMed] [Google Scholar]
- Minjie S., Defei H., Zhimin H., Weiding W., Yuhua Z. Tumor Biol. 2015;36:9015–9022. doi: 10.1007/s13277-015-3537-5. [DOI] [PubMed] [Google Scholar]
- Lee H. S., Park S. B., Kim S. A., Kwon S. K., Cha H., Lee D. Y., Ro S., Cho J. M., Song S. Y. Sci. Rep. 2017;7:41615. doi: 10.1038/srep41615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournel M., Bonfils C., Hou Y., Yan P. T., Trachy-Bourget M. C., Kalita A., Liu J., Lu A. H., Zhou N. Z., Robert M. F., Gillespie J., Wang J. J., Ste-Croix H., Rahil J., Lefebvre S., Moradei O., Delorme D., Macleod A. R., Besterman J. M., Li Z. Mol. Cancer Ther. 2008;7:759–768. doi: 10.1158/1535-7163.MCT-07-2026. [DOI] [PubMed] [Google Scholar]
- Sung V., Richard N., Brady H., Maier A., Kelter G., Heise C. Cancer Sci. 2011;102:1201–1207. doi: 10.1111/j.1349-7006.2011.01921.x. [DOI] [PubMed] [Google Scholar]
- Chien W., Lee D. H., Zheng Y., Wuensche P., Alvarez R., Wen D. L., Aribi A. M., Thean S. M., Doan N. B., Said J. W., Koeffler H. P. Mol. Carcinog. 2014;53:722–735. doi: 10.1002/mc.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbridge A. R., Grabow S., Strasser A., Vaux D. L. Nat. Rev. Cancer. 2016;16:99–109. doi: 10.1038/nrc.2015.17. [DOI] [PubMed] [Google Scholar]
- Abulwerdi F., Liao C., Liu M., Azmi A. S., Aboukameel A., Mady A. S., Gulappa T., Cierpicki T., Owens S., Zhang T., Sun D., Stuckey J. A., Mohammad R. M., Nikolovska-Coleska Z. Mol. Cancer Ther. 2014;13:565–575. doi: 10.1158/1535-7163.MCT-12-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman A. M., O'Connell M. A., Howell L. A. ChemMedChem. 2016;11:840–844. doi: 10.1002/cmdc.201500488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad R. M., Goustin A. S., Aboukameel A., Chen B., Banerjee S., Wang G., Nikolovska-Coleska Z., Wang S., Al-Katib A. Clin. Cancer Res. 2007;13:2226–2235. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- Wang Z., Azmi A. S., Ahmad A., Banerjee S., Wang S., Sarkar F. H., Mohammad R. M. Cancer Res. 2009;69:2757–2765. doi: 10.1158/0008-5472.CAN-08-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Song W., Aboukameel A., Mohammad M., Wang G., Banerjee S., Kong D., Wang S., Sarkar F. H., Mohammad R. M. Int. J. Cancer. 2008;123:958–966. doi: 10.1002/ijc.23610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kasai S., Sasaki T., Watanabe A., Nishiya M., Yasuhira S., Shibazaki M., Maesawa C. Oncol. Lett. 2017;14:903–908. doi: 10.3892/ol.2017.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni J. M., Wittman M., Yang Z., Lee F., Greer A., Hurlburt W., Hillerman S., Cao C., Cantor G. H., Dell-John J., Chen C., Discenza L., Menard K., Li A., Trainor G., Vyas D., Kramer R., Attar R. M., Gottardis M. M. Mol. Cancer Ther. 2009;8:3341–3349. doi: 10.1158/1535-7163.MCT-09-0499. [DOI] [PubMed] [Google Scholar]
- Awasthi N., Zhang C., Ruan W., Schwarz M. A., Schwarz R. E. Mol. Cancer Ther. 2012;11:2644–2653. doi: 10.1158/1535-7163.MCT-12-0447. [DOI] [PubMed] [Google Scholar]
- Prabhu L., Chen L., Wei H., Demir Ö., Safa A., Zeng L., Amaro R. E., O'Neil B. H., Zhang Z. Y., Lu T. Mol. BioSyst. 2017;13:2509–2520. doi: 10.1039/c7mb00391a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmi A. S., Aboukameel A., Banerjee S., Wang Z., Mohammad M., Wu J., Wang S., Yang D., Philip P. A., Sarkar F. H., Mohammad R. M. Eur. J. Cancer. 2010;46:1122–1131. doi: 10.1016/j.ejca.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y. Y., Zhuang Y. H., Cai W. J., Liu Y., Zou W. B. Tumor Biol. 2016;37:15053–15063. doi: 10.1007/s13277-016-5403-5. [DOI] [PubMed] [Google Scholar]
- Guzmán E., Maher M., Temkin A., Pitts T., Wright A. Mar. Drugs. 2013;11:1140–1151. doi: 10.3390/md11041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim Y. M., Spitzer D., Vangveravong S., Hornick M. C., Garg G., Hornick J. R., Goedegebuure P., Mach R. H., Hawkins W. G. Mol. Oncol. 2014;8:956–967. doi: 10.1016/j.molonc.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova E., Scholz A., Paul J., Sturz A., Haike K., Siegel F., Mumberg D., Liu N. Oncotarget. 2017;8:48660–48670. doi: 10.18632/oncotarget.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami K. H., Nguyen C., Ma H., Kim D. H., Jeong K. W., Eguchi M., Moon R. T., Teo J. L., Kim H. Y., Moon S. H., Ha J. R., Khan M. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arensman M. D., Telesca D., Lay A. R., Kershaw K. M., Wu N., Donahue T. R., Dawson D. W. Mol. Cancer Ther. 2014;13:2303–2314. doi: 10.1158/1535-7163.MCT-13-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann G., Fendrich V., McGovern K., Bedja D., Bisht S., Alvarez H., Koorstra J. B., Habbe N., Karikari C., Mullendore M., Gabrielson K. L., Sharma R., Matsui W., Maitra A. Mol. Cancer Ther. 2008;7:2725–2735. doi: 10.1158/1535-7163.MCT-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuma M., Rasheed Z. A., Yabuuchi S., Omura N., Campbell N. R., de Wilde R. F., De Oliveira E., Zhang Q., Puig O., Matsui W., Hidalgo M., Maitra A., Rajeshkumar N. V. Mol. Cancer Ther. 2012;11:1999–2009. doi: 10.1158/1535-7163.MCT-12-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie G. G., Bartels L. E., Xie G., Papayannis I., Alston N., Vrankova K., Ouyang N., Rigas B. Neoplasia. 2013;15:1184–1195. doi: 10.1593/neo.131368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wang H., Ran L., Zhang Z., Jiang R. Biochem. Biophys. Res. Commun. 2016;476:260–266. doi: 10.1016/j.bbrc.2016.05.106. [DOI] [PubMed] [Google Scholar]
- Farrow B., Albo D., Berger D. H. J. Surg. Res. 2008;328:319–328. doi: 10.1016/j.jss.2007.12.757. [DOI] [PubMed] [Google Scholar]
- Gordon-Dseagu V. L., Devesa S. S., Goggins M., Stolzenberg-Solomon R. Int. J. Epidemiol. 2018;47:427–439. doi: 10.1093/ije/dyx232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstein P. E., Olive K. P. Ther. Adv. Gastroenterol. 2013;6:321–337. doi: 10.1177/1756283X13478680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.cmri.org.au/ProCan .
- Dreyer S. B., Chang D. K., Bailey P., Biankin A. V. Clin. Cancer Res. 2017;23:1638–1647. doi: 10.1158/1078-0432.CCR-16-2411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.